(See the Major Article by Hogan et al on pages e4568–77.)
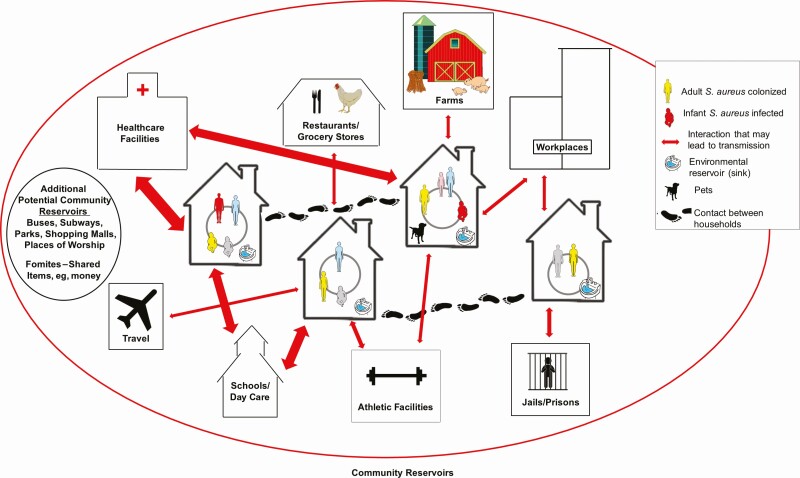
Methicillin-resistant Staphylococcus aureus (MRSA) is now a major community-based pathogen worldwide. The basis for this is multifactorial and includes the emergence of epidemic clones with enhanced virulence, colonization potential, and transmissibility [1]. MRSA infection results from a complex interplay that includes opportunity, S. aureus virulence determinants, and host vulnerabilities [2]. Several studies have demonstrated the household’s critical role as a reservoir for MRSA in the community [1]; epidemic clones “ping-pong” among family members [3, 4], resulting in high rates of recurrent infection. Following a MRSA infection, there is an increase in infections of household members [3, 4]; colonization of household members [5]; and contamination of environmental surfaces [6, 7]. This is likely due to the extensive degree of physical contact among household members and the large amount of time people spend at home [8]. Research on the spread of MRSA within households has revealed that transmission is influenced by household size, composition, types of contact, intrahousehold relationships, pets, colonization levels, strain types, and environmental contamination [1]. There is also a dynamic interaction between households and community sources of MRSA clones. Strains are transmitted bidirectionally between households and the community in different settings and through various activities [1]. Figure 1 illustrates some of the potential pathways for the spread of MRSA in a community. Interaction is shown among people within households, between households, and with community sites. The entry, diffusion, and dissemination of MRSA strains occur through the flow of people, animals, and shared objects, with infection playing an important role in transmission dynamics.
Figure 1.
Graphic display of how Staphylococcus aureus, including methicillin-resistant S. aureus, spreads through the community. Possible pathways for the spread of S. aureus in a neighborhood. Household members share close physical contact with each other, their household environments (eg, kitchen sinks), and their pets. These same people interact with members of other households, such as extended family members, friends, and neighbors. They also interact with community sites, such as healthcare facilities, athletic facilities, and schools or day-care facilities. Travel may also introduce new strains into the community. As ongoing transmission events, either directly from person to person or mediated through fomites, new strains are periodically introduced into households. This entry, diffusion, and dissemination of strains also occur at the community level through the flow of people, animals, and objects. Some community members are persistently colonized, while others are only temporarily colonized, sometimes long enough to transmit to another person and other times they clear colonization before transmission occurs. These dynamics are also affected by external factors, such as weather patterns. Infection also plays a role in S. aureus transmission dynamics. Based on a combination of exposure, host susceptibility, and strain virulence factors, infections occur among a relatively small percentage of community members. This, in turn, increases the risk of transmission and infection among other household members, as well as their contacts in the community. The arrows are weighted based on the relative likelihood of S. aureus transmission. Reprinted with permission from Trends in Microbiology [1].
In light of the household’s central role in MRSA transmission, several studies have evaluated strategies to reduce recurrent infections in households where a community-associated (CA)-MRSA infection has occurred [9, 10]. In this issue of Clinical Infectious Diseases, Hogan et al present the results of HOME2. This was a randomized noninferiority trial that compared 2 approaches, personalized decolonization and household decolonization, with the goal of reducing recurrent CA-MRSA infections in the households of children with a history of a medically attended CA-MRSA infection in the past year [11]. The personalized decolonization approach asked any household member with a self-reported skin and soft tissue infection (SSTI) in the past year to perform a 5-day decolonization regimen, which included hygiene education, twice-daily intranasal mupirocin, and daily bleach–water baths. The household decolonization approach asked all household members to perform the same 5-day decolonization regimen. By 3 months, recurrent SSTI was self-reported in 10% of household members in the personalized decolonization arm compared with 11% of household members in the household decolonization arm, a nonstatistically significant difference. Environmental contamination pressure was controlled for statistically in the primary analyses and was positively associated with longitudinal MRSA colonization of household members. HOME2 builds on an earlier trial published in 2011 [12] that compared decolonizing an entire household with decolonizing only the infected child in households of children with a CA-MRSA infection. By 12 months, recurrent SSTI were reported in 52% of cases in the household decolonization arm compared with 72% of cases in the index decolonization arm, a statistically significant difference.
The strengths of HOME2 include that it uses a well-characterized cohort of households of children with a recent history of CA-MRSA infection. The study demonstrates that a potentially less burdensome decolonization approach was equally effective in reducing recurrent SSTI. The study also has limitations. There were differences in some baseline characteristics between groups, particularly, a higher prevalence of MRSA colonization among household members in the personalized decolonization arm. Neither decolonization approach addressed environmental contamination. The outcome relied on self-report of SSTI rather than culture-confirmed infections. Although the study had a 12-month observation period, the primary outcome was measured as cumulative SSTI at 3 months.
How can the findings from HOME2 be incorporated into clinical practice? Targeting a single potential source of exposure and subsequent infection among a multiplicity of potential exposures appears to reduce levels of recurrent infection, but recurrent infections still occur. The authors note this as a limitation; a 1-time decolonization regimen is inadequate to prevent SSTI regardless of who is targeted, and they suggest consideration of more prolonged or periodic decolonization interventions. Therefore, does the information from this new trial change what we should tell patients who experience a CA-MRSA infection? Miller posed a similar question in this same journal [13] in a Commentary on the 2011 study [11]. Two of the points raised remain especially relevant. First, it is hard to determine the full benefits of household decolonization approaches when we still do not have a true control condition to know what the rates of recurrent SSTI would be in households with no intervention. In an earlier longitudinal study that included infected adults (72%) and children (28%), we found that 43% of index patients reported recurrent SSTI by 6 months, 43% of whom required hospitalization [14]. In the longitudinal HOME study [15], the precursor to the current trial, 53% of index patients with at least 1 follow-up visit experienced an SSTI, along with 19% of their household contacts. The levels of recurrent SSTI were lower in HOME2, but at least 1 year had passed in these households since the index infection had been medically attended. Perhaps the lower levels of recurrent SSTI in HOME2 reflect the impact of previously received antimicrobial therapy, either 1 year ago for the index infection or more recently in instances where a household member had an infection that was medically attended [15]. Second, household decolonization efforts are burdensome. Would it be practical to implement them outside of the highly controlled settings of a clinic trial, and with patients who are likely to be less motivated? The results of the trial indicate that there will be potential challenges: only 71% of participants in the personalized approach and only 62% of participants in the household approach were 80% adherent to the decolonization regimen. Furthermore, 49% of participants reported an adverse effect, albeit mostly relatively minor events. Targeted decolonization may be less burdensome than decolonizing an entire household, but it might also be more complicated to implement if it requires accurately determining which members had a recent SSTI. Offering a more complicated regimen, especially after just a single infection, may still be underutilized unless a family is particularly motivated, along with their provider. A recent review article on this topic concluded that there is only limited evidence that these decolonization approaches are effective [16]. Given their limited effectiveness and considerable burden, are they superior to heightened vigilance and promptly treating recurrences, as needed? What other alternatives are we left with?
The development of more effective strategies to reduce CA-MRSA infections, initial and recurrent, will depend on greater insight into the factors that contribute to the success of emerging epidemic S. aureus clones. Most research on S. aureus and households has been limited to studies conducted after a household index infection has occurred and treatment has been administered. Thus, the source of infection and the directionality of transmission are difficult to ascertain. Analyses of transmission are also often limited to looking at the spread of the clinical isolate. Novel research methods that overcome this limitation and that apply rigorous research methods, such as social network analyses [17, 18], whole genomic sequencing [19–21], and mathematical modeling [22], are needed to provide a more nuanced understanding of S. aureus transmission and infection dynamics. In the meantime, the current study makes a contribution by providing evidence of noninferiority for an additional approach to reduce recurrent infections in the households of children with a recent history of a medically attended CA-MRSA infection.
Notes
Acknowledgments. The authors appreciate the assistance of Skye Peebles in the preparation of Figure 1.
Financial support. This work was supported by the National Institute on Drug Abuse to J. K. (T32DA031099) and the National Institute of Allergy and Infectious Diseases (NIAID) to A. C. U. and F. D. L. (R21AI152046).
Potential conflicts of interest. F. D. L. reports royalties from UpToDate. A. C. U. reports grants from the National Institutes of Health/NIAID, GlaxoSmithKline, Allergan, and Merck outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Knox J, Uhlemann AC, Lowy FD. Staphylococcus aureus infections: transmission within households and the community. Trends Microbiol 2015; 23:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998; 339:520–32. [DOI] [PubMed] [Google Scholar]
- 3. Jones TF, Creech CB, Erwin P, Baird SG, Woron AM, Schaffner W. Family outbreaks of invasive community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2006; 42:e76–8. [DOI] [PubMed] [Google Scholar]
- 4. Cook HA, Furuya EY, Larson E, Vasquez G, Lowy FD. Heterosexual transmission of community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2007; 44:410–3. [DOI] [PubMed] [Google Scholar]
- 5. Cluzet VC, Gerber JS, Nachamkin I, et al. Duration of colonization and determinants of earlier clearance of colonization with methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2015; 60:1489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knox J, Uhlemann AC, Miller M, et al. Environmental contamination as a risk factor for intra-household Staphylococcus aureus transmission. PLoS One 2012; 7:e49900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uhlemann AC, Knox J, Miller M, et al. The environment as an unrecognized reservoir for community-associated methicillin resistant Staphylococcus aureus USA300: a case-control study. PLoS One 2011; 6:e22407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leech JA, Nelson WC, Burnett RT, Aaron S, Raizenne ME. It’s about time: a comparison of Canadian and American time-activity patterns. J Expo Anal Environ Epidemiol 2002; 12:427–32. [DOI] [PubMed] [Google Scholar]
- 9. Ellis MW, Schlett CD, Millar EV, et al. Hygiene strategies to prevent methicillin-resistant Staphylococcus aureus skin and soft tissue infections: a cluster-randomized controlled trial among high-risk military trainees. Clin Infect Dis 2014; 58:1540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaplan SL, Forbes A, Hammerman WA, et al. Randomized trial of “bleach baths” plus routine hygienic measures vs. routine hygienic measures alone for prevention of recurrent infections. Clin Infect Dis 2014; 58:679–82. [DOI] [PubMed] [Google Scholar]
- 11. Hogan PG, Parrish KL, Mork RL, et al. HOME2: household vs. personalized decolonization in households of children with methicillin-resistant Staphylococcus aureus skin and soft tissue infection— a randomized clinical trial. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fritz SA, Hogan PG, Hayek G, et al. Household versus individual approaches to eradication of community-associated Staphylococcus aureus in children: a randomized trial. Clin Infect Dis 2012; 54:743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller LG. Where we are with community-associated Staphylococcus aureus prevention— and in the meantime, what do we tell our patients? Clin Infect Dis 2012; 54:752–4. [DOI] [PubMed] [Google Scholar]
- 14. Knox J, Sullivan SB, Urena J, et al. Association of environmental contamination in the home with the risk for recurrent community-associated, methicillin-resistant Staphylococcus aureus infection. JAMA Intern Med 2016; 176:807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hogan PG, Mork RL, Thompson RM, et al. Environmental methicillin-resistant Staphylococcus aureus contamination, persistent colonization, and subsequent skin and soft tissue infection. JAMA Pediatr 2020; 174:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pittet LF, Curtis N. Are decontamination measures effective in preventing recurrent staphylococcal skin infection in children? Arch Dis Child 2020; 105:603–7. [DOI] [PubMed] [Google Scholar]
- 17. Lucet JC, Paoletti X, Demontpion C, et al. ; Staphylococcus aureus Resistant a la Meticilline en Hospitalisation A Domicile Study Group . Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med 2009; 169:1372–8. [DOI] [PubMed] [Google Scholar]
- 18. Nerby JM, Gorwitz R, Lesher L, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2011; 30:927–32. [DOI] [PubMed] [Google Scholar]
- 19. Uhlemann AC, Kennedy AD, Martens C, Porcella SF, Deleo FR, Lowy FD. Toward an understanding of the evolution of Staphylococcus aureus strain USA300 during colonization in community households. Genome Biol Evol 2012; 4:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uhlemann AC, Dordel J, Knox JR, et al. Molecular tracing of the emergence, diversification, and transmission of S. aureus sequence type 8 in a New York community. Proc Natl Acad Sci U S A 2014; 111:6738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uhlemann AC, McAdam PR, Sullivan SB, et al. Evolutionary dynamics of pandemic methicillin-sensitive Staphylococcus aureus ST398 and its international spread via routes of human migration. mBio 2017; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macal CM, North MJ, Collier N, et al. Modeling the transmission of community-associated methicillin-resistant Staphylococcus aureus: a dynamic agent-based simulation. J Transl Med 2014; 12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]