Abstract
Background
Carbapenem-resistant gram-negative bacteria (CRGNB) continue to present a global healthcare crisis. We aimed to identify emerging trends of CRGNB over nearly 2 decades and describe the impact of CRGNB on patient outcomes.
Methods
Patients from whom CRGNB were isolated between 2000 and 2017 were included in the study. Carbapenem resistance was defined by the most recent breakpoints and applied across the study period. Patient demographics, clinical characteristics, and outcomes were retrieved from the electronic health record.
Results
A total of 94 888 isolates from 64 422 patients were identified; 9882 (10%) isolates from 4038 patients were carbapenem-resistant. Pseudomonas aeruginosa was the most common CRGNB each year. The second most common CRGNB emerged in waves over time. Carbapenem daily defined doses increased in parallel with CRGNB rates (R2 = 0.8131). The overall 30-day mortality rate was 19%, which decreased from 24% in 2000 to 17% in 2017 (P = .003; R2 = .4330). Among patients with CRGNB bloodstream infections (n = 319), overall 30- and 90-day mortality rates were 27% and 38%, respectively. Charlson score (adjusted odds ratio [aOR], 1.11 per point), intensive care unit residence (aOR, 7.32), and severe liver disease (aOR, 4.8.4) were independent predictors of 30-day mortality, while receipt of transplantation was associated with lower rates of death (aOR, 0.39). Among patients admitted between 2011 and 2017 (n = 2230), 17% died during hospitalization, 32% were transferred to long-term care facilities, and 38% were discharged home.
Conclusions
CRGNB emerged in waves over time, causing high rates of mortality. Despite increasing rates of CRGNB, overall patient outcomes have improved, suggesting that recognition and novel therapeutics have made a major impact.
Keywords: carbapenem resistance, epidemiology, mortality
Rates of carbapenem-resistant gram-negative bacteria (CRGNB) increased over 2 decades. Pseudomonas aeruginosa was the most common CRGNB and other CRGNB emerged in waves. Isolation of Acinetobacter species was associated with the highest mortality, but rates of death decreased over time.
Carbapenem-resistant gram-negative bacteria (CRGNB) are a public health threat prioritized by the Centers for Disease Control and Prevention (CDC) and World Health Organization [1, 2]. Given the limited therapeutic options, rates of patient morbidity and mortality are increased disproportionately when compared to infections caused by carbapenem-susceptible gram-negative bacteria [3]. The global dissemination of carbapenem-resistant Enterobacterales (CRE) has raised awareness of CRGNB [4, 5] as an urgent threat to patients outlined in the latest CDC report [1]. Over time, CRE have become increasingly diverse, expanding beyond Klebsiella pneumoniae carbapenemase (KPC)–producing K. pneumoniae to other Enterobacterales species via both carbapenemase and noncarbapenemase mechanisms of carbapenem resistance [6–8]. Despite widespread attention paid to CRE, other carbapenem-resistant threats may have an even larger impact on outcomes of patients. Carbapenem-resistant Acinetobacter baumannii complex is the top-priority pathogen identified by a global survey of clinicians taking into account healthcare burden, patient mortality, and treatability [9], whereas carbapenem-resistant Pseudomonas aeruginosa remains the most prevalent CRGNB in the United States (US) [10–12].
Despite an increased understanding of the prevalence and underlying mechanisms of resistance associated with CRGNB over the past decade, studies demonstrating the impact and comparison of bacterial taxa on patient outcomes are notably absent. Large-scale epidemiologic investigations derived from secondary databases or billing records generally lack robust patient-level outcome data and are unable to report trends in CRGNB over time due to breakpoint changes and variable implementation at participating centers [4, 11, 13, 14]. On the other hand, smaller descriptive studies are subject to uncontrolled confounding and limited sample sizes and do not account for long-term outcomes [15]. To address these limitations, we performed a longitudinal observational study at our center to evaluate the changing epidemiology and impact on clinical outcomes of CRGNB over an 18-year period using a standardized definition of resistance.
METHODS
Patient Selection and Setting
Adult patients with a positive clinical culture from any site were evaluated at the University of Pittsburgh Medical Center (UPMC) Presbyterian Hospital from 1 January 2000 to 31 December 2017. Surveillance cultures from nares or perirectal swabs were excluded from the analysis. Microbiology records were extracted for the 6 most common gram-negative bacteria (GNB) over the study period [16]. Carbapenem resistance was defined as nonsusceptibility (intermediate or resistance) to any carbapenem according to the 2017 Clinical Laboratory and Standards Institute interpretative criteria [17]. These criteria were applied retrospectively to all isolates over the study period to account for changes to susceptibility breakpoints over time. The primary event was defined as the first positive culture per unique patient yielding CRGNB. Secondary events included isolation of a different CRGNB species or recurrence with the same CRGNB after 90 days. Primary and secondary events were either monomicrobial or polymicrobial (>1 CRGNB isolated at the specific event). Patients were reincluded in the analysis if another CRGNB species was identified at any point after the primary event, or if the same CRGNB was reisolated >90 days after the primary event. Pathogen-specific incidence rates included both primary and secondary events.
Statistical Analysis
Incidence rates were standardized per 1000 patient-days. Rates of antibiotic consumption were normalized by daily defined doses (DDDs) per year [18]. Patient outcomes included 30- and 90-day mortality, total and post-CRGNB isolation hospital length of stay, CRGNB recurrence within 1 year, and hospital disposition (further details provided in the Supplementary Materials).
RESULTS
Patients, Bacterial Species, and Recurrence of CRGNB Isolation
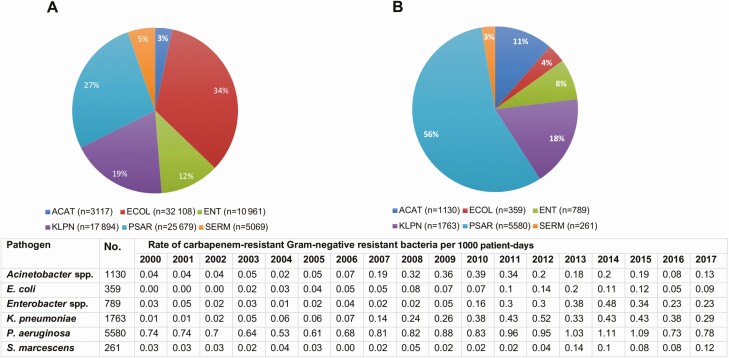
From 2000 to 2017, 94 888 isolates collected from 64 222 patients were evaluated. Escherichia coli was the most common pathogen (34%), followed by P. aeruginosa (27%), K. pneumoniae (19%), Enterobacter species (Enterobacter cloacae and Klebsiella aerogenes [formerly Enterobacter aerogenes]) (12%), Serratia marcescens (5%), and Acinetobacter species (3%) (Figure 1A). Ten percent (9882/94 888) of isolates were carbapenem-resistant, accounting for a total of 5450 cases from 4038 unique patients (Figure 1B, Table 1). Seventy-six percent (4115/5450) of cases were defined as primary events (initial CRGNB isolation, including 75 patients with 2 CRGNB species and 1 patient with 3 CRGNB species). The remaining 24% (1335/5450) of cases were secondary events. Secondary events included isolation of a different CRGNB species (43% [576/1335]) or recurrence with the same CRGNB after 90 days (57% [759/1335]). Recurrence with the same CRGNB was more common for P. aeruginosa (28% [589/2116]) than all other pathogens (9% [170/1999]) (P < .001; Supplementary Figure 1). Overall, 60% (805/1335) of secondary events were due to P. aeruginosa.
Figure 1.
Distribution of all gram-negative bacteria and carbapenem-related gram-negative bacteria, 2000–2017. A, All study organisms between 2000 and 2017 (n = 94 888). B, Total carbapenem-resistant gram-negative bacteria between 2000 and 2017 (n = 9882). Abbreviations: ACAT, Acinetobacter species; ECOL, Escherichia coli; E. coli, Escherichi coli; ENT, Enterobacter species; KLPN, Klebsiella pneumoniae; K. pneumonae, Klebsiella Pneumoniae; P. aeruginosa, Pseudomonas aeruginosa; PSAR, Pseudomonas aeruginosa; SERM, Serratia marcescensS. marcescens, Serratia marcescens; spp., species.
Table 1.
Patient Characteristics and Outcomes by Carbapenem-resistant Gram-negative Bacteria
| Variables | Total (n = 4038) |
Acinetobacter spp (n = 447) |
Escherichia coli
(n = 212) |
Enterobacter spp (n = 531) |
Klebsiella pneumoniae
(n = 576) |
Pseudomonas aeruginosa
(n = 2070) |
Serratia marcescens
(n = 126) |
Polymicrobiala (n = 76) |
|---|---|---|---|---|---|---|---|---|
| Age, y, median (IQR) | 60 (49–70) | 60 (50–69) | 61.5 (54–73) | 60 (51–69) | 61 (50–69) | 59 (47–70) | 58 (49–68) | 62 (46–71) |
| Male sex | 2313 (57.3) | 258 (57.7) | 92 (43.4) | 329 (62.0) | 301 (52.3) | 1204 (58.2) | 87 (69.1) | 42 (55.3) |
| Charlson score, median (IQR) | 5 (3–7) | 5 (3–7) | 6 (3–9) | 5 (3–7) | 5 (3–8) | 5 (2–7) | 5 (3–6) | 5 (3–7) |
| History of transplantation | 737 (18.3) | 70 (15.7) | 27 (12.7) | 52 (9.8) | 120 (20.8) | 437 (21.1) | 24 (19.1) | 7 (9.2) |
| Cystic fibrosis | 190 (4.7) | 2 (0.5) | 2 (0.9) | 0 (0) | 3 (0.5) | 182 (8.8) | 0 (0) | 1 (1.3) |
| Diabetes mellitus | 1530 (37.9) | 194 (43.5) | 90 (42.5) | 177 (33.3) | 222 (38.5) | 770 (37.2) | 39 (31.0) | 38 (50.0) |
| Chronic kidney disease | 1185 (29.4) | 148 (33.1) | 70 (33.0) | 137 (25.8) | 183 (31.8) | 584 (28.2) | 37 (29.4) | 27 (35.5) |
| Moderate to severe liver disease | 498 (12.3) | 49 (11.0) | 28 (13.2) | 65 (12.2) | 122 (21.2) | 208 (10.1) | 18 (14.3) | 8 (10.5) |
| ICU residence at time of culture | 1666 (41.3) | 211 (47.2) | 69 (32.6) | 222 (41.8) | 229 (39.8) | 847 (40.9) | 50 (39.7) | 38 (50.0) |
| Carbapenem-resistant | 2679 (66.3) | 337 (75.4) | 74 (34.9) | 165 (31.1) | 475 (79.3) | 1559 (75.3) | 33 (26.2) | 54 (71.1) |
| Carbapenem-intermediate | 1359 (33.7) | 110 (24.6) | 138 (65.1) | 366 (68.9) | 119 (20.7) | 511 (24.7) | 93 (73.8) | 22 (28.9) |
| Culture site | ||||||||
| Bloodstream | 319 (7.9) | 31 (6.9) | 27 (12.7) | 53 (10.0) | 95 (16.5) | 85 (4.1) | 26 (20.6) | 2 (2.6) |
| Respiratory | 2149 (53.2) | 287 (64.2) | 43 (20.3) | 230 (43.3) | 190 (33.0) | 1307 (63.1) | 43 (31.8) | 49 (64.5) |
| Urine | 689 (17.1) | 20 (4.5) | 101 (47.6) | 95 (17.9) | 185 (32.1) | 267 (12.9) | 13 (10.3) | 8 (10.5) |
| Abscess/deep wound | 776 (19.2) | 93 (20.8) | 40 (17.9) | 136 (25.6) | 95 (16.5) | 361 (17.4) | 43 (34.1) | 15 (19.7) |
| Superficial wound | 89 (2.2) | 15 (3.4) | 4 (1.8) | 17 (3.1) | 9 (1.6) | 39 (1.9) | 4 (3.2) | 1 (1.3) |
| Other | 16 (0.4) | 1 (0.2) | 1 (0.5) | 0 (0) | 2 (0.4) | 11 (0.5) | 0 (0) | 1 (1.3) |
| Patient outcomes | ||||||||
| 30-day mortality | 782 (19.4) | 111 (24.8) | 30 (14.2) | 94 (17.7) | 126 (21.9) | 370 (17.9) | 28 (22.2) | 23 (30.3) |
| 90-day mortality | 1266 (31.4) | 170 (38.0) | 52 (24.5) | 155 (29.2) | 190 (33.0) | 632 (30.5) | 38 (30.2) | 29 (38.2) |
| 1-year mortality | 1885 (46.7) | 252 (56.4) | 81 (38.2) | 211 (39.7) | 274 (47.6) | 975 (47.1) | 51 (40.5) | 41 (53.9) |
| Length of stayb, median (IQR) | 26 (10–58) | 29 (12–60) | 20 (8–52) | 26 (9–52) | 26 (11–59) | 28 (10–64) | 24 (9–44) | 25 (9–57) |
| Length of stay after isolation of CRGNBb, median (IQR) | 13 (5–33) | 17 (7–37) | 11 (5–29) | 13 (5–31) | 14 (5–31) | 13 (5–35) | 11 (4–29) | 16 (6–34) |
| Reisolation of any CRGNB within 1 year | 879 (21.8) | 123 (25.8) | 41 (18.2) | 75 (13.7) | 183 (29.5) | 430 (20.3) | 27 (20.8) | … |
| Recurrence with same CRGNB within 1 year | 424 (10.3) | 28 (5.9) | 0 (0) | 8 (12.3) | 85 (13.7) | 295 (13.9) | 8 (6.2) | … |
| Hospital dispositionb | ||||||||
| In-hospital mortality | 379 (17.0) | 50 (25.6) | 20 (13.6) | 74 (16.4) | 77 (19.9) | 133 (14.6) | 12 (13.5) | 13 (17.1) |
| Discharge to homec | 692 (37.4) | 31 (21.4) | 49 (38.6) | 146 (38.7) | 113 (36.3) | 305 (39.3) | 32 (41.6) | 16 (25.4) |
| Transfer to facilityc,d | 1103 (59.6) | 108 (74.5) | 75 (59.1) | 224 (59.4) | 184 (59.2) | 451 (58.1) | 43 (55.8) | 18 (47.3) |
| 30-day readmissionc | 261 (14.1) | 19 (13.1) | 12 (8.4) | 47 (12.5) | 43 (13.8) | 119 (15.3) | 5 (6.5) | 4 (10.5) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: CRGNB, carbapenem-resistant gram-negative bacteria; ICU, intensive care unit; IQR, interquartile range; spp., species.
aPolymicrobial group includes Acinetobacter species (n = 30), Escherichia coli (n = 11), Enterobacter species (n = 11), Klebsiella pneumoniae (n = 21), and Pseudomonas aeruginosa (n = 3).
bHospital disposition data available from all patients admitted from 2011 to 2017 (n = 2230).
cOutcomes among unique patients who survived in hospital stay from 2011 to 2017 (n = 1851).
dFacilities included long-term care facilities, subacute nursing facilities, and acute inpatient rehab.
Sixty-three percent (879/1335) of secondary events occurred within 1 year of initial CRGNB isolation. The 1-year recurrence rate for isolating the same CRGNB was 10% (424/4038; Table 1). One-year recurrence rates were highest for P. aeruginosa (14% [295/2070]) and K. pneumoniae (14% [85/576]) and lowest for Acinetobacter species (6% [28/447]) and E. coli (0% [0/212]). Of the 4038 unique patients, 15% (604/4038) had recurrent CRGNB isolation within 1 year of the initial index case.
Carbapenem Resistance Rates Over Time
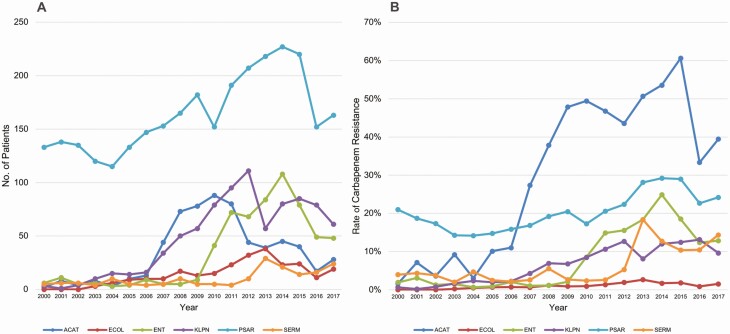
Overall rates of carbapenem resistance increased from 6% to 11% (P < .001, R2 = 0.7715), and were increased significantly for each pathogen over the study period (Figure 2B). Fifty-six percent of CRGNB were P. aeruginosa, followed by K. pneumoniae (18%), Acinetobacter species (11%), Enterobacter species (8%), E. coli (4%), and S. marcescens (3%; Figure 1B). Pseudomonas aeruginosa was the most common CRGNB in each year of the study; however, the proportion of P. aeruginosa decreased from 89% in 2000 to 47% in 2017 (P < .001). The second most common CRGNB varied over time, including Acinetobacter species from 2007 to 2010, K. pneumoniae from 2011 to 2012, and Enterobacter species from 2013 to 2014 (Figure 2A, Supplementary Table 1). Overall, the rate of CRGNB isolation increased from 0.81 to 1.65/1000 patient-days over the study period (P < .001, R2 = 0.7282). The incidence rate for each pathogen individually also increased (Acinetobacter species: P = .0429, R2 = 0.2322; E. coli: P < .001, R2 = 0.6312; Enterobacter species: P < .001, R2 = 0.6221; K. pneumoniae: P < .001, R2 = 0.7652; P. aeruginosa: P = .003, R2 = 0.4371; S. marcescens: P = .002, R2 = 0.4590).
Figure 2.
A, Temporal changes in carbapenem resistance rates among unique patients with carbapenem-resistant gram-negative bacteria, 2000–2017 (n = 4038). B, Temporal changes in carbapenem resistance rates, 2000–2017. Abbreviations: ACAT, Acinetobacter species; ECOL, Escherichia coli; ENT, Enterobacter species; KLPN, Klebsiella pneumoniae; PSAR, Pseudomonas aeruginosa; SERM, Serratia marcescens.
Antibiotic Consumption Rates
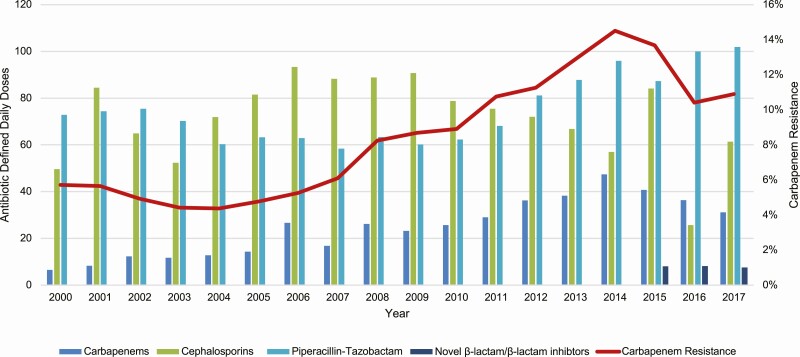
From 2000 to 2017, carbapenem DDDs per 1000 patient-days increased from 6.52 to 35.08 (P < .001, R2 = 0.8311). Carbapenem DDDs increased in parallel with CRGNB rates (Figure 3, Supplementary Figures 2 and 3; lag = 0 years, R2 = 0.9068). Correlations were stronger for lactose-fermenting organisms (R2 = 0.9311) than non-lactose-fermenting organisms (R2 = 0.7669; Figure 3). These findings were consistent when DDDs and incidence rates of CRGNB were analyzed by 3-month intervals or quarters (lag = 0 quarters, R2 = 0.8552). Carbapenem DDDs declined from 2014 to 2017 (P = .003, R2 = 0.993), which coincided with the introduction of ceftazidime-avibactam and ceftolozane-tazobactam at our center (2015—2017 mean combined DDDs = 7.88/year; P = .004, R2 = 0.4210). Rates of resistance did not correlate as strongly with other agents or classes (Figure 3, Supplementary Table 2).
Figure 3.
Temporal changes in carbapenem resistance rates among unique patients and antibiotic defined daily doses. Carbapenems: ertapenem, doripenem, imipenem, and meropenem. Cephalosporins: cefepime and ceftazidime. Fluoroquinolones: ciprofloxacin, levofloxacin, and moxifloxacin. Novel β-lactam/β-lactamase inhibitors: ceftazidime-avibactam and ceftolazane-tazobactam.
Patient Demographics and Clinical Characteristics
Fifty-seven percent (2313/4038) of patients were male, the median age was 60 years (interquartile range [IQR], 49–70 years), and the median Charlson comorbidity index [19] was 5 (IQR, 3–7). Eighteen percent (737/4038) of patients were transplant recipients. Five percent (190/4038) of patients had cystic fibrosis. At the time of CRGNB isolation, 41% (1666/4038) of patients resided in the intensive care unit (ICU), 38% (1525/4038) were on a medical ward, 11% (446/4038) were on an observational unit, and 10% (401/4038) were in inpatient rehabilitation. The most common culture source was respiratory tract (53% [2149/4038]), followed by deep wound/abscesses (19% [776/4038]), urinary tract (17% [689/4038]), bloodstream (8% [319/4038]), superficial wounds (2% [89/4038]), and other sources (0.4% [16/4038]). Over the study period, the percentage of patients with CRGNB who received a transplant decreased from 30.7% (43/140) in the year 2000 to 10.8% (30/278) in 2017 (P < .001, R2 = 0.7606). Increases over time occurred in median age (from 55 to 60 years; P < .001, R2 = 0.6659) and percentage of patients with chronic kidney disease (from 22% to 31%; P = .008, R2 = 0.3587), whereas the percentage of patients with severe liver disease decreased (from 17% to 9%; P < .001; R2 = 0.6203).
Across CRGNB, patient demographics and underlying diseases were similar with some notable exceptions (Table 1). Compared to all other CRGNB, patients with Acinetobacter species were more likely to be in the ICU (P < .05), patients with K. pneumoniae were more likely to have severe liver disease (P < .001), and cystic fibrosis was more common among patients with P. aeruginosa (P < .001). Overall, non-lactose-fermenting organisms were more commonly isolated from a respiratory source than were lactose-fermenting organisms (P < .001), while lactose-fermenting organisms were associated with higher rates of bacteremia compared to non–lactose fermenters (P < .001) (Table 1). Escherichia coli was more commonly isolated from the urine compared to other pathogens (P < .01).
Clinical Outcomes of Patients
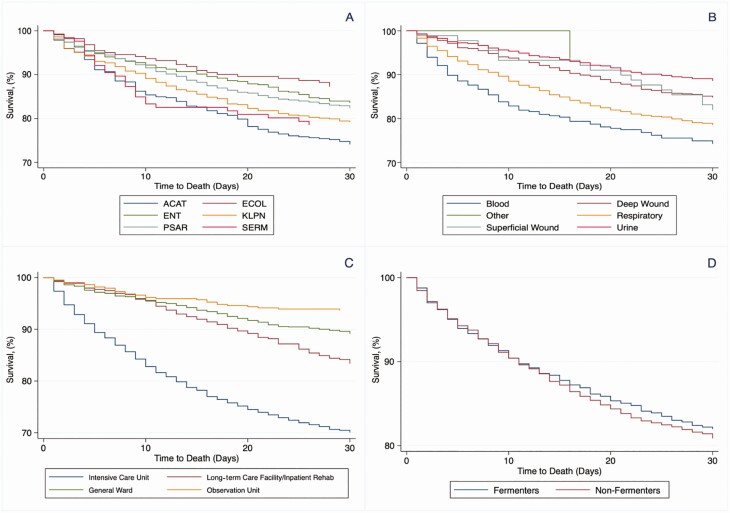
Rates of 30- and 90-day all-cause mortality were 19% (782/4038) and 31% (1266/4038), respectively. At 1 year after index CRGNB isolation, the mortality rate was 47% (1885/4038). Death within 30 days was highest following isolation of Acinetobacter species (25% [111/447]) and lowest for E. coli (14% [30/212]) (P < .001; Figure 4). By culture site, 30-day mortality was highest for CRGNB isolation from the bloodstream (27% [85/319]) and lowest for urine (12% [80/689]) (P < .001, Supplementary Figures 4 and 5). On univariate analysis, isolation of Acinetobacter species, bloodstream infection, ICU residence, severe liver disease, and chronic renal diseases were associated with higher rates of 30-day mortality. Rates of 30-day mortality did not vary by resistance definition (with or without inclusion of “intermediate” resistance) for any CRGNB except Acinetobacter species (28% [93/337] for resistant vs 18% [19/110] for intermediate (P = .02; Tables 1 and 2). Patients with cystic fibrosis and a history of transplantation demonstrated lower rates of 30-day mortality (Table 2, Supplementary Table 3). Mortality among transplant recipients with a CRGNB isolated from any site was 17% (129/764); the rate for bloodstream infection among transplant recipients was 25% (18/73). Overall, transplant patients were younger (P < .001) and more likely to have chronic kidney disease (P < .001), severe liver disease (P < .001), and cystic fibrosis (P < .001). Compared to nontransplant patients, isolation of CRGNB from a respiratory source occurred more frequently in transplant patients (P < .001); P. aeruginosa was the most common CRGNB isolated (60% [457/764]) (Supplementary Table 4).
Figure 4.
Kaplan-Meier plots of 30-day survival of unique patients with carbapenem-resistant gram-negative bacteria (CRGNB), 2000–2017 (n = 4038), by organism (A), culture site (B), hospital location at time of carbapenem-resistant pathogen isolation (C), and lactose fermentation status of CRGNB (D). Abbreviations: ACAT, Acinetobacter species; ECOL, Escherichia coli; ENT, Enterobacter species; KLPN, Klebsiella pneumoniae; PSAR, Pseudomonas aeruginosa; SERM, Serratia marcescens.
Table 2.
Variables Associated With 30-Day Mortality for Bloodstream Infections Only
| Variable | Dead (n = 85) |
Alive (n = 234) |
Univariate P Value |
Multivariate P Value |
aOR (95% CI) |
|---|---|---|---|---|---|
| Age, y, mean (SD) | 61.1 (13.6) | 58.3 (15.9) | .2113 | .239 | 1.01 (.99–1.03) |
| Charlson scorea, mean (SD) | 4.2 (3.2) | 3.2 (2.8) | .1705 | .039 | 1.11 (1.01–1.23) |
| Male sex | 37 (45.5) | 93 (39.7) | .543 | .325 | 0.75 (.42–1.34) |
| ICU residence | 60 (70.6) | 65 (27.8) | <.001 | <.001 | 7.32 (3.97–13.50) |
| Acinetobacter sppb | 10 (11.8) | 21 (8.8) | <.001 | .165 | 2.41 (.70–8.34) |
| Polymicrobial culture | 0 (0) | 2 (0.9) | .7311 | … | |
| Carbapenem-resistantc | 47 (55.3) | 122 (52.1) | .617 | .796 | 1.08 (.57–2.08) |
| History of transplantation | 18 (21.2) | 55 (23.5) | .559 | .021 | 0.39 (.18–0.87) |
| Cystic fibrosis | 0 (0) | 3 (1.3) | .294 | … | |
| Chronic kidney disease | 34 (40.0) | 79 (33.8) | .303 | .792 | 1.09 (.58–2.06) |
| Severe liver disease | 30 (21.6) | 39 (16.7) | <.001 | <.001 | 4.84 (2.23–10.49) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; ICU, intensive care unit; SD, standard deviation; spp, species.
aCharlson score adjusted for age and severe liver disease to avoid confounding.
bOdds of mortality calculated relative to carbapenem-resistant Escherichia coli.
cDefined as resistant, not including intermediate resistance, according to 2017 Clinical and Laboratory Standards Institute carbapenem breakpoints.
Index CRGNB isolated from the bloodstream (n = 319) were independently associated with higher rates of 30-day mortality (Table 2). Overall 30-day, 90-day, and 1-year mortality rates were 27% (85/319), 38% (121/319), and 57% (183/319), respectively. Among patients with bloodstream infections, multivariate logistic regression analysis showed that Charlson score adjusted for age (adjusted odds ratio [aOR], 1.11 per point [95% confidence interval {CI}, 1.01–1.23]), ICU residence (aOR, 7.32 [95% CI, 3.97–13.50]), and severe liver disease (aOR, 4.84 [95% CI, 2.23–10.49]) were independent predictors of 30-day mortality (Table 2). Additional covariates associated with 30-day mortality across all patients included isolation of Acinetobacter species (aOR, 1.95 [95% CI, 1.21–3.14]), chronic kidney disease (aOR, 1.28 [95% CI, 1.05–1.58]), and older age (aOR per year, 1.02 [95% CI, 1.02–1.03]) (Supplementary Table 3). In both models, transplantation was associated with lower odds of 30-day mortality (aOR, 0.37 [95% CI, .16–.83] and 0.63 [95% CI, .49–.80], respectively; Table 2, Supplementary Table 1). Findings were consistent for transplant recipients with and without the inclusion of cystic fibrosis as an underlying disease (data not shown).
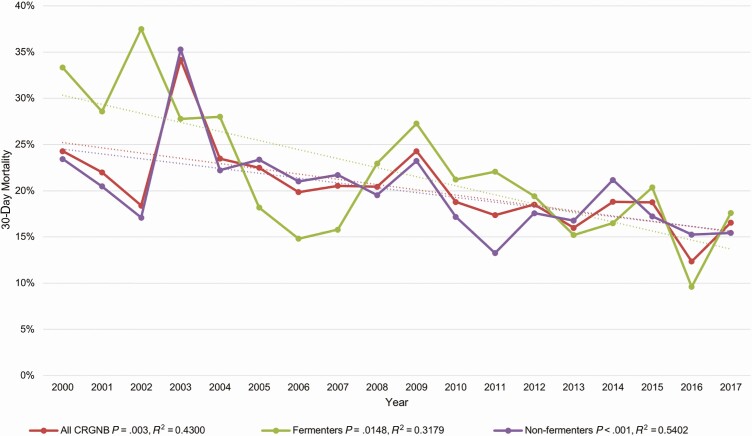
Rates of 30-day mortality decreased over time, from 24% (34/140) in the year 2000 to 17% (46/278) in 2017 (P = .0030, R2 = 0.4330). Correlations were stronger for non-lactose-fermenting vs lactose-fermenting CRGNB (R2 = 0.5402 vs R2 = 0.317; P < .001; Figure 5).
Figure 5.
Rates of 30-day mortality over time for all carbapenem-resistant gram-negative bacteria, fermenters and nonfermenters, 2000–2017. Abbreviation: CRGNB, carbapenem-resistant gram-negative bacteria.
Among a subset of patients identified from 2011 to 2017, isolation of CRGNB was categorized as healthcare-associated in 83% (1912/2230) [20]. Over this time period, the in-hospital mortality rate was 17% (379/1912). Sixty percent (1103/1851) and 38% (692/1851) of patients surviving hospital discharge were transferred to a long-term care facility/nursing home or discharged home, respectively. Within 30 days of hospital discharge, 14% were readmitted to the hospital. The median lengths of total and post-CRGNB isolation hospital stays were 26 days (IQR, 10–58 days) and 13 days (IQR, 5–33 days), respectively. Readmission rates following isolation of Acinetobacter species were associated with lower rates of survival and longer lengths of stay than other CRGNB (Table 1). Although transplant recipients had longer lengths of stay than nontransplant patients (P < .001), they had higher rates of discharge home (P < .001) and lower rates of transfer to long-term care facility/nursing homes (P < .001; Supplementary Table 4).
DISCUSSION
Our study provides unique insights into the changing epidemiology of CRGNB over the past 2 decades at a major academic medical center in the US, and is one of the first to report both short- and long-term patient outcomes stratified by various CRGNB species. In doing so, we identified P. aeruginosa as the most common CRGNB in each year of the study, but importantly the second most common CRGNB varied over time as outbreaks of Acinetobacter species and CRE peaked in the late 2000s and early 2010s, respectively. Consistent with national trends [1], the overall number of CRGNB isolated at our center increased steadily over several years, but recently we have observed a downtrend across species that parallels consumption of carbapenems. Importantly, mortality rates have continued to decline over time. Isolation of CRGNB was common among vulnerable patients in our hospital, including transplant recipients and those with multiple comorbid conditions. Indeed, we found that established risk factors for poor outcomes, including severity of illness (ICU residence) and underlying diseases (older age, chronic liver and renal disease) were associated with higher rates of death. Moreover, we have shown that isolation of CRGNB is associated with a 1-year survival rate of 53%, which may reflect the underlying disease of patients with CRGNB or ongoing infectious complications. Survival rates and overall lengths of hospital stay were disproportionate for patients from whom Acinetobacter species were isolated compared to other CRGNB species [3, 11]. Acinetobacter species were most commonly isolated from the respiratory tract or abscess/deep wound cultures, and at the time of isolation patients were more likely to reside in the ICU. Finally, we found that isolation of CRGNB among transplant recipients was associated with lower odds of death compared to isolation of CRGNB from non–transplant recipients, a finding that supports future investigation.
Carbapenem resistance continues to be a major threat in the US [1, 4, 15, 21, 22], which is of great concern given the attributable mortality and economic burden imposed by CRGNB [1, 23]. Considerable attention and antibiotic development efforts have been devoted toward CRE, especially carbapenemase-producing Enterobacterales, in part due to their ability to disseminate carbapenemase enzymes via mobile genetic elements [24]. Nonetheless, our data underscore the importance of other CRGNB, namely P. aeruginosa and Acinetobacter species, which are associated with the greatest burden and highest mortality rates, respectively [11]. Pseudomonas aeruginosa alone resulted in 36% of all in-hospital deaths following monomicrobial CRGNB isolation. Other unique insights can be derived from the epidemiology of CRGNB at our center, including a significant shift in the types of CRGNB isolated over time [25, 26]. While rates of carbapenem resistance increased among nonlactose fermenters (Acinetobacter species and, to a lesser degree, P. aeruginosa), they were overshadowed by the rise in CRE [27, 28]. In the US, the initial wave of CRE was driven by K. pneumoniae [13]; however, as demonstrated in recent reports [6, 25, 26], we identified a second wave of CRE driven by Enterobacter species. Genomic analysis indicates this is associated with a high degree of clonal diversity among isolates, including clones harboring various carbapenemases [29].These observations stand in contrast to the global dissemination of K. pneumoniae, which occurred through successful dissemination of a single clone, sequence type 258, which predominates at our center [29, 30]. Despite the initial wave of CRE, our data mirror recent SENTRY databases and CDC estimates, which point to a decrease or stabilization of CRGNB rates [1, 13, 14, 31]. This may be due to increased vigilance and concerted efforts to address the spread to multidrug-resistant organisms nationwide [1].
Analyzing data from a single center with access to detailed medical records and application of uniform carbapenem breakpoints enabled us to correlate antimicrobial consumption with trends in CRGNB isolation over time. Carbapenem consumption correlated with, but did not precede, the increase in carbapenem resistance rates. This is consistent with our institutional approach to treat infections due to CRGNB with carbapenem combination therapy prior to the introduction of new agents in 2015 [32, 33]. Correlations were most pronounced among lactose-fermenting bacteria as noted previously [34]. Other studies have documented that an increase in carbapenem use generally precedes associated rates of increased carbapenem resistance by a few months [35]. It is possible that local antimicrobial stewardship and infection prevention measures may have also had an impact on the epidemiology of CRGNB [30].
Another key finding of our analysis is an overall trend toward decreased mortality rates over time. These findings may be attributable to sepsis campaigns, improvements in supportive therapy, expansion of routine surveillance allowing for earlier identification of at-risk patients, changing epidemiology of CRGNB, and the introduction of novel therapeutic options that limit use of more toxic, less efficacious therapies [36–39]. The data support strong correlations among non–lactose fermenters, which may highlight the importance of local synergistic combinations at our center for Acinetobacter species and the routine use of ceftolazane-tazobactam for P. aeruginosa infections [33, 36, 38, 40, 41]. These data also support our prior observation of lower mortality rates among patients with KPC-producing K. pneumoniae bloodstream infections who received ceftazidime-avibactam compared to other treatment options [38]. Among patients with bloodstream infections specifically, our patients’ 30-day mortality rate of 27% compares favorably to the recently reported range of 43%–50% among patients with bloodstream infections due to difficult-to-treat resistance (DTR) GNB [42, 43]. Importantly, these studies were conducted prior to the availability of new β-lactam/β-lactamase inhibitor combinations, define mortality based on in-hospital survival, and include higher percentages of Acinetobacter species than the current analysis, which makes direct comparisons challenging. It is plausible, however, that differentiating DTR from carbapenem resistance is associated with worse clinical outcomes given the lack of front-line therapeutic options [42, 43]. Indeed, it is likely that carbapenem-resistant Enterobacter species and P. aeruginosa retain susceptibility to other β-lactams or fluoroquinolones, which would define them as carbapenem resistant, but not DTR [43].
Transplant recipients, of whom 99% were solid organ transplant recipients, experienced lower rates of death following isolation of CRGNB from the bloodstream or other sites (Table 2, Supplementary Table 4). Patient characteristics, including younger age, attenuation of the septic inflammatory response with the immunosuppressive agents, aggressive medical attention, and a low threshold for admission together with active surveillance of CRGNB carriage status may have led to improved outcomes among these patients [44]. Similar findings have been reported in a large multicenter retrospective cohort and a propensity-matched case-control study [45, 46], and in smaller reports among patients infected with multidrug-resistant organisms [47, 48]. These data support future investigation to validate our hypothesis that mortality rates are lower among solid organ transplant recipients from whom CRGNB are isolated. Our group has initiated subsequent studies.
Our study represents one of few that combines robust, standardized CRGNB epidemiology with in-depth patient-level clinical outcomes over time. However, some limitations of our analysis should be noted. First, like prior studies [14], our case definition was predicated upon microbiology records, and therefore did not differentiate between CRGNB colonization or infection, which limits our ability to associate CRGNB isolation directly to patient outcomes and mortality. Of important note, however, our multivariate logistic regression analyses for patients with bloodstream infections were well-aligned to those of the overall patient cohort. We also acknowledge that reincluding patients with recurrence after 90 days may include some patients with long-term colonization; however, we have successfully applied such definitions previously [49, 50]. Second, the study was conducted at a single center and may not be applicable to other settings and patient populations. In exchange, our study design allowed in-depth access to patient data, antibiotic use, and microbiology records. These data allowed us to control for important clinical factors in multivariable logistic regression models, although residual confounding cannot be excluded. Third, we did not include a control group of patients from whom carbapenem-susceptible GNB were isolated, which is beyond the scope of this epidemiologic study; however, such studies have been completed previously [3, 44]. Inclusion of control subjects in future studies is valuable to control for baseline differences in patient populations that are infected by various CRGNB species—for instance, severe liver disease among patients with K. pneumoniae or ICU admission among patients with Acinetobacter species.
In conclusion, we have demonstrated the changing epidemiology of CRGNB over nearly 2 decades at our center that may be reflected in subsequent national data. The emergence of various waves of CRGNB suggests that future waves are imminent and continued surveillance will be essential in detecting emerging trends. In comparison to other CRGNB, isolation of carbapenem-resistant Acinetobacter species is associated with the worst prognostic indicators that may reflect the vulnerability of those infected with Acinetobacter species and the paucity of effective, well-tolerated treatment options. On an encouraging note, however, overall trends in CGRNB rates and all-cause mortality are decreasing, which supports the ongoing efforts in education and drug development to combat the continued public health crisis that has been imposed by CRGNB.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the Antimicrobial Stewardship and Transplant Infectious Diseases teams at University of Pittsburgh Medical Center (UPMC) for their continued review and feedback of these data. The authors are also indebted to James Mezynski, Michael Repcik, and Michael Riffle for their analytical and systems support at UPMC.
Financial support. This work was supported by the National Institutes of Health (career development and research grants K08AI114883 and R03AI144636 to R. K. S.).
Potential conflicts of interest. C. J. C. has been awarded investigator-initiated research grants from Astellas, Merck, Melinta, and Cidara for studies unrelated to this project; served on advisory boards or consulted for Astellas, Merck, The Medicines Company, Cidara, Scynexis, Shionogi, Qpex, and Needham & Company; and has received speaking honoraria from Merck and T2 Biosystems. M. H. N. has received grant support from the National Institutes of Health, Pulmocide, Scynexis, Astellas, Merck, and T2 Biosystems, outside the submitted work. R. K. S. has received grant support from Accelerate Diagnostics, Achaogen, Allergan, Merck, Melinta, Roche, Shionogi, Tetraphase, VenatoRx, has served on advisory boards for Accelerate Diagnostics, Achaogen, Allergan, Entasis, Summit, Merck, Nabriva, Shionogi, and VenatoRx; and has received speaking honoraria from Allergan, Menarini, Pfizer, and T2 Biosystems. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. Atlanta, GA: CDC, 2019. [Google Scholar]
- 2. World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discover, and development of new antibiotics. Geneva, Switzerland:WHO, 2017. [Google Scholar]
- 3. Kadri SS, Lai YLE, Ricotta EE, et al. ; NIH Antimicrobial Resistance Outcomes Research Initiative (NIH-ARORI) . External validation of difficult-to-treat resistance prevalence and mortality risk in gram-negative bloodstream infection using electronic health record data from 140 US hospitals. Open Forum Infect Dis 2019; 6:ofz110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012–2013. JAMA 2015; 314:1479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iovleva A, Doi Y. Carbapenem-resistant Enterobacteriaceae. Clin Lab Med 2017; 37:303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilson BM, El Chakhtoura NG, Patel S, et al. Carbapenem-resistant Enterobacter cloacae in patients from the US Veterans Health Administration, 2006–2015. Emerg Infect Dis 2017; 23:878–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Babiker A, Evans DR, Griffith MP, et al. Clinical and genomic epidemiology of carbapenem-non-susceptible Citrobacter spp. at a tertiary healthcare center over two decades. J Clin Microbiol 2020; 58:e00275-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Duin D, Arias CA, Komarow L, et al. ; Multi-Drug Resistant Organism Network Investigators . Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): a prospective cohort study. Lancet Infect Dis 2020; 20:731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tacconelli E, Carrara E, Savoldi A, et al. ; WHO Pathogens Priority List Working Group . Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18:318–27. [DOI] [PubMed] [Google Scholar]
- 10. Zilberberg MD, Kollef MH, Shorr AF. Secular trends in Acinetobacter baumannii resistance in respiratory and blood stream specimens in the United States, 2003 to 2012: a survey study. J Hosp Med 2016; 11:21–6. [DOI] [PubMed] [Google Scholar]
- 11. Cai B, Echols R, Magee G, et al. Prevalence of carbapenem-resistant gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis 2017; 4:ofx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gniadek TJ, Carroll KC, Simner PJ. Carbapenem-resistant non-glucose-fermenting gram-negative bacilli: the missing piece to the puzzle. J Clin Microbiol 2016; 54:1700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castanheira M, Deshpande LM, Mendes RE, Canton R, Sader HS, Jones RN. Variations in the occurrence of resistance phenotypes and carbapenemase genes among Enterobacteriaceae isolates in 20 years of the SENTRY antimicrobial surveillance program. Open Forum Infect Dis 2019; 6:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jernigan JA, Hatfield KM, Wolford H, et al. Multidrug-resistant bacterial infections in U.S. hospitalized patients, 2012–2017. N Engl J Med 2020; 382:1309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morbid Mortal Wkly Rep 2013; 62:165–70. [PMC free article] [PubMed] [Google Scholar]
- 16. Yount RJ, Vries JK, Councill CD. The medical archival system: an information retrieval system based on distributed parallel processing. Inf Process Manag 1991; 27:379–89. [Google Scholar]
- 17. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, M100-ED29. 29th informational supplement. Wayne, PA: CLSI, 2019. [Google Scholar]
- 18. World Health Organization. WHO Collaborating Centre for Drug Statistics Methodology (WHOCC): DDD definition and general considerations. Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 19. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–9. [DOI] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention. CDC/NHSN surveillance definitions for specific types of infections. Atlanta, GA: CDC, 2019. [Google Scholar]
- 21. Braykov NP, Eber MR, Klein EY, Morgan DJ, Laxminarayan R. Trends in resistance to carbapenems and third-generation cephalosporins among clinical isolates of Klebsiella pneumoniae in the United States, 1999–2010. Infect Control Hosp Epidemiol 2013; 34:259–68. [DOI] [PubMed] [Google Scholar]
- 22. Thaden JT, Lewis SS, Hazen KC, et al. Rising rates of carbapenem-resistant Enterobacteriaceae in community hospitals: a mixed-methods review of epidemiology and microbiology practices in a network of community hospitals in the southeastern United States. Infect Control Hosp Epidemiol 2014; 35:978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bartsch SM, McKinnell JA, Mueller LE, et al. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect 2017; 23:48.e9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonomo RA, Burd EM, Conly J, et al. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis 2018; 66:1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Britt NS, Ritchie DJ, Kollef MH, et al. Clinical epidemiology of carbapenem-resistant gram-negative sepsis among hospitalized patients: shifting burden of disease? Am J Infect Control 2018; 46:1092–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCann E, Srinivasan A, DeRyke CA, et al. Carbapenem-nonsusceptible gram-negative pathogens in ICU and non-ICU settings in US hospitals in 2017: a multicenter study. Open Forum Infect Dis 2018; 5:ofy241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rhomberg PR, Jones RN. Summary trends for the meropenem yearly susceptibility test information collection program: a 10-year experience in the United States (1999–2008). Diagn Microbiol Infect Dis 2009; 65:414–26. [DOI] [PubMed] [Google Scholar]
- 28. Chamieh A, El-Hajj G, Zmerli O, Afif C, Azar E. Carbapenem resistant organisms: a 9-year surveillance and trends at Saint George University Medical Center [manuscript published online ahead of print 4 April 2019]. J Infect Public Health 2019. doi:10.1016/j.jiph.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 29. Annavajhala MK, Gomez-Simmonds A, Uhlemann AC. Multidrug-resistant Enterobacter cloacae complex emerging as a global, diversifying threat. Front Microbiol 2019; 10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marsh JW, Mustapha MM, Griffith MP, et al. Evolution of outbreak-causing carbapenem-resistant Klebsiella pneumoniae ST258 at a tertiary care hospital over 8 years. MBio 2019; 10:e01945–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Woodworth K, Walters M, Weiner L, et al. Vital signs: containment of novel multidrug-resistant organisms and resistance mechanisms—United States, 2006–2017. MMWR Morbid Mortal Wkly Rep 2018; 67:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2015; 2:ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shields RK, Clancy CJ, Gillis LM, et al. Epidemiology, clinical characteristics and outcomes of extensively drug-resistant Acinetobacter baumannii infections among solid organ transplant recipients. PLoS One 2012; 7:e52349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Federico MP, Furtado GH. Immediate and later impacts of antimicrobial consumption on carbapenem-resistant Acinetobacter spp., Pseudomonas aeruginosa, and Klebsiella spp. in a teaching hospital in Brazil: a 10-year trend study. Eur J Clin Microbiol Infect Dis 2018; 37:2153–8. [DOI] [PubMed] [Google Scholar]
- 35. McLaughlin M, Advincula MR, Malczynski M, Qi C, Bolon M, Scheetz MH. Correlations of antibiotic use and carbapenem resistance in Enterobacteriaceae. Antimicrob Agents Chemother 2013; 57:5131–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haidar G, Philips NJ, Shields RK, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis 2017; 65:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McConville TH, Sullivan SB, Gomez-Simmonds A, Whittier S, Uhlemann AC. Carbapenem-resistant Enterobacteriaceae colonization (CRE) and subsequent risk of infection and 90-day mortality in critically ill patients, an observational study. PLoS One 2017; 12:e0186195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agent Chemother 2017; 61:e00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Babiker A, Clarke L, Doi Y, Shields RK. Fosfomycin for treatment of multidrug-resistant pathogens causing urinary tract infection: a real-world perspective and review of the literature. Diagn Microbiol Infect Dis 2019; 95:114856. [DOI] [PubMed] [Google Scholar]
- 40. Shields RK, Nguyen MH, Potoski BA, et al. Doripenem MICs and ompK36 porin genotypes of sequence type 258, KPC-producing Klebsiella pneumoniae may predict responses to carbapenem-colistin combination therapy among patients with bacteremia. Antimicrob Agents Chemother 2015; 59:1797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oleksiuk LM, Nguyen MH, Press EG, et al. In vitro responses of Acinetobacter baumannii to two- and three-drug combinations following exposure to colistin and doripenem. Antimicrob Agents Chemother 2014; 58:1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huh K, Chung DR, Ha YE, et al. Impact of difficult-to-treat resistance in gram-negative bacteremia on mortality: retrospective analysis of nationwide surveillance data [manuscript published online ahead of print 29 January 2020]. Clin Infect Dis 2020. doi:10.1093/cid/ciaa084. [DOI] [PubMed] [Google Scholar]
- 43. Kadri SS, Adjemian J, Lai YL, et al. ; National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH–ARORI) . Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis 2018; 67:1803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis. BMC Infect Dis 2017; 17:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Donnelly JP, Locke JE, MacLennan PA, et al. Inpatient mortality among solid organ transplant recipients hospitalized for sepsis and severe sepsis. Clin Infect Dis 2016; 63:186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kalil AC, Syed A, Rupp ME, et al. Is bacteremic sepsis associated with higher mortality in transplant recipients than in nontransplant patients? A matched case-control propensity-adjusted study. Clin Infect Dis 2015; 60:216–22. [DOI] [PubMed] [Google Scholar]
- 47. Taglietti F, Di Bella S, Galati V, Topino S, Iappelli M, Petrosillo N. Carbapenemase-producing Klebsiella pneumoniae–related mortality among solid organ-transplanted patients: do we know enough? Transpl Infect Dis 2013; 15:E164–5. [DOI] [PubMed] [Google Scholar]
- 48. Venditti M, Falcone M, Micozzi A, et al. Staphylococcus aureus bacteremia in patients with hematologic malignancies: a retrospective case-control study. Haematologica 2003; 88:923–30. [PubMed] [Google Scholar]
- 49. Shields RK, McCreary EK, Marini RV, et al. Early experience with meropenem-vaborbactam for treatment of carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2020; 71:667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shields RK, Potoski BA, Haidar G, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2016; 63:1615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.