Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver an opinion on iron hydroxide adipate tartrate as a novel food (NF) pursuant to Regulation (EU) 2015/2283 and as a source of iron in the context of Directive 2002/46/EC. The NF is intended to be used in food supplements up to a maximum dose of 100 mg per day, corresponding to a maximum daily intake of iron of 36 mg. The target population proposed by the applicant is the general population above 3 years of age. The NF which is the subject of the application is an engineered nanomaterial having primary particles, of almost spherical morphology, with a diameter typically smaller than 5 nm. The studies provided for absorption, distribution, metabolism and excretion (ADME) and bioavailability indicate that iron, once taken up into the epithelial cells of the gut, is subject to the same mechanisms of regulation and absorption as that of other forms of iron. Further studies provided in the context of the toxicological assessment indicate that the NF does not lead to iron bioaccumulation in tissues and organs at the doses tested. The Panel notes that the NF contains nickel at concentrations that may increase the risk of flare‐up reactions in nickel‐sensitised young individuals up to 10 years of age. In the 90‐day toxicity study, findings related to haematology, clinical biochemistry and organ weights were observed and the Panel defined a no observed adverse effect level (NOAEL) of 231 mg/kg body weight (bw) per day, that is, the mid‐dose used in the study. The Panel considers that the NF is a source from which iron is bioavailable and it is safe under the proposed conditions of use.
Keywords: novel foods, nutrient source, Iron, food supplement, iron hydroxide adipate tartrate, IHAT
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
On 9 December 2019, the company NEMYSIS Limited, submitted a request to the Commission in accordance with Article 10 of Regulation (EU) No 2015/2283 to place on the EU market iron hydroxide adipate tartrate (IHAT).
IHAT is intended to be used in food supplements. In addition, as IHAT is also a new source of iron, the opinion should also address the bioavailability of iron from this source in the context of Directive 2002/46/EC of the European Parliament and of the Council laying down requirements for food supplements.
In accordance with Article 10(3) of Regulation (EU) 2015/2283, the European Commission asks the European Food Safety Authority to provide a scientific opinion on the safety of IHAT as a novel food. In addition, as IHAT is also a new source of iron, the opinion should also address the bioavailability of iron from this source in the context of Directive 2002/46/EC on food supplements.
1.2. Information on existing evaluations and authorisations
In 2015, the EFSA published a scientific opinion on dietary reference values (DRVs) for iron (EFSA NDA Panel, 2015). Population reference intakes (PRIs) for iron were defined as ranging between 7 and 16 mg/day for different age and population groups. No tolerable upper intake level (UL) has been set for iron by SCF or EFSA.
The following substances have been authorised as sources of iron for addition to foods according to Regulation (EC) No 1925/20061: ferrous bisglycinate, ferrous carbonate, ferrous citrate, ferric ammonium citrate, ferrous gluconate, ferrous fumarate, ferric sodium diphosphate, ferrous lactate, ferrous sulfate, ferrous ammonium phosphate, ferric sodium EDTA, ferric diphosphate (ferric pyrophosphate), ferric saccharate and elemental iron.
The following substances have been authorised as a source of iron for additions to food for specific groups according to Regulation (EU) No 609/20132: ferrous carbonate, ferrous citrate, ferric ammonium citrate, ferrous gluconate, ferrous fumarate, ferric sodium diphosphate, ferrous lactate, ferrous sulfate, ferrous ammonium phosphate, ferric sodium EDTA, ferric diphosphate (ferric pyrophosphate), ferric saccharate, elemental iron, ferrous bisglycinate and ferrous l‐pidolate.
The following substances have been authorised as a source of iron for use in the manufacture of food supplements according to Directive 2002/46/EC3: ferrous carbonate, ferrous citrate, ferric ammonium citrate, ferrous gluconate, ferrous fumarate, ferric sodium diphosphate, ferrous lactate, ferrous sulfate, ferric diphosphate (ferric pyrophosphate), ferric saccharate, elemental iron, ferrous bisglycinate, ferrous l‐pidolate, ferrous phosphate, ferrous ammonium phosphate, ferric sodium EDTA, iron (II) taurate.
Eight health claims on iron pursuant to Article 13(1) and 14(1)b of Regulation 1924/20064 have been authorised by the European Commission.
Adipic acid and its sodium and potassium salts are authorised as food additives5 (E 355, E 356, E 357) in some food categories at maximum levels ranging from 1,000 mg/kg to 10,000 mg/L.
Tartaric acid (l(+)‐) is a food additive5 (E 334) authorised for quantum satis use in several food categories, with restrictions up to a maximum level of 5,000 mg/kg in some food categories.
2. Data and methodologies
2.1. Data
The safety assessment of this NF is based on data supplied in the application, information submitted by the applicant following EFSA’s requests for supplementary information and information provided by the EFSA Working group on nanomaterials.
Administrative and scientific requirements for NF applications referred to in Article 10 of Regulation (EU) 2015/2283 are listed in the Commission Implementing Regulation (EU) 2017/24696.
A common and structured format for the presentation of NF applications is described in the EFSA guidance on the preparation and presentation of a NF application (EFSA NDA Panel, 2016). As indicated in this guidance, it is the duty of the applicant to provide all of the available (proprietary, confidential and published) scientific data, (including both data in favour and not in favour) that are pertinent to the safety of the NF.
This NF application includes a request for the protection of proprietary data in accordance with Article 26 of Regulation (EU) 2015/2283. The data requested by the applicant to be protected comprise: In vitro mammalian cell micronucleus (MN) test (Study report 21.007709.0002); In vitro Mammalian Cell Gene Mutation Tests using the Thymidine Kinase Gene (Study report 19.016575.0002); 90‐day repeated dose oral toxicity study in rodents (Study report N25‐0021 and related annexes).
2.2. Methodologies
The assessment follows the methodology set out in the EFSA guidance on NF applications (EFSA NDA Panel, 2016) and the principles described in the relevant existing guidance documents from the EFSA Scientific Committee. The legal provisions for the assessment are laid down in Article 11 of Regulation (EU) 2015/2283 and in Article 7 of the Commission Implementing Regulation (EU) 2017/2469.
This assessment concerns only the risks that might be associated with consumption of the NF under the proposed conditions of use and is not an assessment of the efficacy of the NF with regard to any claimed benefit.
The evaluation of bioavailability of the nutrient iron from the source iron hydroxide adipate tartrate was conducted in line with the principles contained in the ‘Guidance on safety evaluation of sources of nutrients and bioavailability of nutrient from the sources’ (EFSA ANS Panel, 2018).
The evaluation of the NF as a nanomaterial was conducted in line with the principles of the ‘Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health’ (EFSA Scientific Committee, 2021).
3. Assessment
3.1. Introduction
The NF which is the subject of the application is IHAT. The NF is produced by chemical synthesis and consists of an engineered nanomaterial meant to be used as a source of iron. The NF is proposed to be used in food supplements. The target population is the general population above 3 years of age.
The NF falls in the following food category according to Regulation (EU) 2015/2283:
(ix) vitamins, minerals and other substances used in accordance with Directive 2002/46/EC, Regulation (EC) No 1925/2006 or Regulation (EU) No 609/2013, where:
a production process not used for food production within the Union before 15 May 1997 has been applied as referred to in point (a) (vii) of this paragraph; or they contain
or consist of engineered nanomaterials as defined in point (f) of this paragraph.
3.2. Identity of the NF
| Common name | Iron oxo‐hydroxide adipate tartrate |
| Other names | Iron hydroxide adipate tartrate, Iron oxyhydroxide adipate tartrate |
| Trade name | IHAT |
| CAS number | 2460638‐28‐0 |
| Molecular formula (calculated) |
FeOm(OH)n(H2O)x(C4H6O6)y(C6H10O4)z where: m and n are undefined as per accepted practice for ferric iron oxohydroxides (Cornell and Schwertmann, 2003) x = 0.28–0.88 y = 0.78–1.50 z = 0.04–0.19 Tartaric (C4H6O6) and adipic (C6H10O4) acid are represented in their protonated form |
| Molecular weight | Average molecular weight: 35,803.4 Da (lower–upper bound: 27,670.5–45,319.4 Da) |
The NF, iron oxo‐hydroxide adipate tartrate (referred to as iron hydroxide adipate tartrate, IHAT7), is an engineered analogue of the ferritin core. It is a tartrate‐modified, nanodisperse Fe(III) oxo‐hydroxide, formed in an adipate buffer, with functional properties and primary particle size similar to the ferritin core. The NF8 is intended to be used as a source of iron in food supplements.
The physicochemical properties of IHAT were first described in Powell et al. (2014). Inductively coupled plasma optical emission spectrometry (ICP‐OES) was used to assess the iron content and – after fractionation into precipitated, nanoparticulate and soluble Fe by centrifugation and ultrafiltration – the phase distribution of IHAT. Particle morphology and primary particle size were assessed by high‐angle annular dark‐field aberration‐corrected scanning transmission electron microscopy (HAADF‐STEM). Transmission electron microscopy (TEM) with electron energy‐loss spectroscopy (EELS) was used to evaluate the local iron environment of the tartrate‐modified ferrihydrite and confirm the ferric (Fe3+) form of the iron in the particles. Finally, Fourier transform infrared spectroscopy (FTIR) and X‐ray diffraction (XRD) were used to assess the presence of tartrate in the ferrihydrite particles and the modifications of the ferrihydrite lattice due to the interaction with tartaric acid. Taken together, XRD, STEM, FTIR, TEM and EELS data show that the tartrate‐modified ferrihydrite in IHAT is a disrupted or strained ferrihydrite structure where the tartrate ligand has been incorporated into the particles during co‐precipitation. In IHAT, the core ferrihydrite is smaller and less crystalline and has larger lattice spacings than in unmodified, synthetic ferrihydrite. Data are also consistent with ligand bonding between the tartrate and the surface of the ferrihydrite nanoparticles, which might play a role in inhibiting further crystallisation and growth and in facilitating nanodispersion in aqueous systems (Powell et al., 2014).
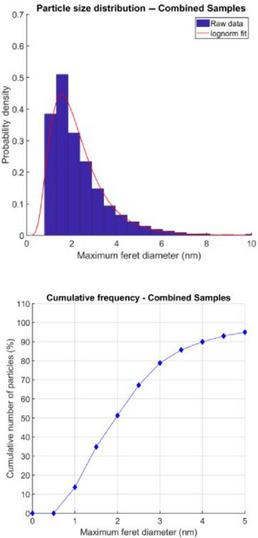
The applicant provided data showing that the elemental composition, physicochemical properties and nanospecific descriptive parameters of the industrially manufactured NF are consistent with those of the IHAT synthesised at laboratory scale (Powell et al., 2014), which was used in the ADME studies. Characterisation of two samples from batch #1 (see Section 3.4 Compositional data) by means of dynamic light scattering (DLS) and TEM with energy dispersion X‐ray (EDX) spectroscopy indicated a mean hydrodynamic diameter in the range of 4.1–4.2 nm and a median size of the primary particles in the range of 1.7–2.1 nm, respectively, with an almost spherical shape and an elemental composition consisting of iron, oxygen, and carbon. Upon request of the Panel, the applicant provided further analyses to show the consistency of the test material used in Powell et al. (2014) of a sample used for the scale‐up process and of samples from NF batches #1, #2, #3, #4 and #5 (see Section 3.5 Compositional data). Median and mean particle size investigated by HAADF‐STEM were ca. 2 nm for > 90% of particles (in number) and < 5 nm for all samples. TEM‐EELS and STEM‐EDX spectra of the different samples were coherent, confirming their consistency in terms of composition and lattice structure. Once dispersed in water, the samples showed a comparable phase distribution, with typically 94–97% of the material being nanoparticulate, 2–3% soluble, and 0–3% microparticulate. Dissolution behaviour in a model lysosomal assay (10 mmol/L citric acid, 0.9% NaCl, pH 5) was also similar, with 46–59% of the material remaining nanoparticulate after 6 h.
3.3. Production process
According to the information provided, the NF is produced in line to Good Manufacturing Practice (GMP) and Hazard Analysis Critical Control Points (HACCP) principles.
IHAT is manufactured by a chemical synthesis. An acidic aqueous solution comprising iron (III) chloride, l‐(+)‐tartaric acid and adipic acid is neutralised through the addition of sodium hydroxide, resulting in the formation of IHAT (Figure 1). The product is then precipitated, recovered through a physical separation process (e.g. filtration or centrifugation) and dried.
Figure 1.
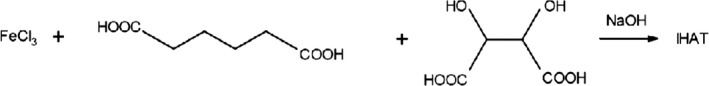
Synthetic route for the production of IHAT
In terms of IHAT formulation, the applicant highlighted that the material synthesised at laboratory scale (Powell et al., 2014) was conventionally tray‐dried, whereas the industrial NF is ethanol‐recovered. This results in the laboratory material having ‘excipients’ in its formulation (namely salt from the pH neutralisation, and the excess adipic and tartaric acids), whereas the one from commercial/ethanol‐recovered processes has remarkably lower levels of these ‘excipients’, as they are mostly washed out. This formulation difference does neither appear to affect the identity of the NF nor its physical‐chemical behaviour.
The Panel considers that the production process is sufficiently described and does not raise safety concerns.
3.4. Compositional data
The NF consists of iron, tartaric acid, adipic acid, sodium, chloride and water.
In order to confirm that the manufacturing process is reproducible and adequate to produce on a commercial scale, a product with the required characteristics, the applicant provided analytical information for five independent batches of the NF (Table 1).
Table 1.
Batch to batch analysis of the NF
| Parameter | Batch number | Method of analysis | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5a | ||
| Physical/chemical | ||||||
| Iron % w/w (dry matter) | 30.3 | 32.2 | 34.7 | 33.6 | 35.1 | ICP‐OES |
| Tartaric acid % w/w (dry matter) | 32.8 | 33.9 | 35.0 | 32.8 | 28.9 | HPLC‐DAD |
| Adipic acid % w/w (dry matter) | 2.1 | 2.5 | 1.9 | 2.1 | 2.0 | HPLC‐DAD |
| Sodium % w/w (dry matter) | 10.5 | 10.5 | 10.5 | 11.0 | 11.0 | ICP‐OES |
| Chloride % w/w (dry matter) | 3.3 | 3.9 | 2.6 | 4.1 | 4.0 | ICP‐OES |
| Dry mass balance % w/w | 79.0 | 83.0 | 84.7 | 83.6 | 81.0 | Calculated |
| Water (%) | 17.1 | 16.0 | 14.3 | 20.7 | 11.2a | Karl Fisher |
| Iron (%) % w/w (wet basis) | 25.2 | 27.0 | 29.8 | 26.7 | 31.2 | Considering water content |
| Tartaric acid % w/w (wet basis) | 27.8 | 28.3 | 31.0 | 26.7 | 27.1 | Considering water content |
| Physical/chemical | ||||||
| Adipic acid % w/w (wet basis) | 1.7 | 2.1 | 1.7 | 1.7 | 1.9 | Considering water content |
| Sodium % w/w (wet basis) | 8.7 | 8.8 | 9.0 | 8.8 | 9.7 | Considering water content |
| Chloride % w/w (wet basis) | 2.8 | 3.3 | 2.3 | 3.4 | 3.7 | Considering water content |
| Phase distribution (in water) | ||||||
| Soluble (%) | 3.2 | 3.5 | 2.2 | 3.3 | 2.7 | ICP‐OES |
| Nano (%) | 95.0 | 96.5 | 94.9 | 93.7 | 97.3 | ICP‐OES |
| Micro (%) | 1.8 | 0.0 | 2.9 | 2.9 | 0.0 | ICP‐OES |
| Primary particle size | ||||||
| Median diameter (nm) | 1.88 | 1.82 | 2.15 | 2.00 | 1.88 | HAADF‐STEMb |
| Mean diameter (nm) | 2.18 | 2.12 | 2.61 | 2.38 | 2.32 | HAADF‐STEMb |
| Dv(10) (nm) | 1.68 | 2.32 | 2.00 | 1.81 | 1.86 | DLS |
| Dv(50) (nm) | 3.25 | 3.47 | 2.99 | 2.89 | 2.90 | DLS |
| Dv(90) (nm) | 5.49 | 5.91 | 5.17 | 5.17 | 5.18 | DLS |
| Particle size distribution (volume‐based) of secondary microparticles in the dry powder | ||||||
| Dv(10) (µm) | 18.2 | 14.26 | 2.81 | 10.28 | 84.26 | Laser diffraction |
| Dv(50) (µm) | 569.14 | 598.70 | 498.21 | 457.86 | 614.65 | Laser diffraction |
| Dv(90) (µm) | 1,282.53 | 1,329.95 | 1,320.22 | 1,198.04 | 1,302.45 | Laser diffraction |
| Density (cm3) | 2.09 | 2.16 | 2.17 | 2.13 | 2.16 | Pycnometry |
| Microbiological | ||||||
| TAMC (CFU/g) | – | < 10 | < 10 | < 10 | – | Ph. Eur. 9.4 |
| TYMC (CFU/g) | – | < 10 | 10 | < 10 | – | Ph. Eur. 9.4 |
| Heavy metals | ||||||
| Ni (mg/kg) | 38.6 | 39.2 | 48.2 | 46.3 | 41.4 | ICP‐MSc |
| Cd (mg/kg) | < 0.04 | < 0.04 | < 0.04 | < 0.04 | < 0.04 | ICP‐MSd |
| Pb (mg/kg) | 0.17 | 0.16 | 0.19 | 0.20 | 0.18 | ICP‐MSc |
| Hg (mg/kg) | < 0.04 | < 0.04 | < 0.04 | < 0.04 | < 0.04 | ICP‐MSc |
| As (mg/kg) | 0.67 | 0.62 | 0.80 | 0.70 | 0.69 | ICP‐MSc |
| Cr (mg/kg) | 52.9 | 53.4 | 68.0 | 64.6 | 56.8 | ICP‐MSc |
| Residual solvents | ||||||
| Ethanol (mg/kg) | < 89 | < 89 | < 89 | < 89 | < 89 | HS‐GC |
| 2‐Propanol (mg/kg) | < 7.5 | < 7.5 | < 7.5 | < 7.5 | < 7.5 | HS‐GC |
CFU: colony forming units; DLS: dynamic light scattering; Dv: percentile of the volume‐based particle size distribution; HAADF‐STEM: high‐angle annular dark‐field aberration‐corrected scanning transmission electron microscopy; HPLC‐DAD: high‐performance liquid chromatography with a diode‐array detector; HS‐GC: headspace gas chromatography; ICP‐OES: inductively coupled plasma optical emission spectrometry; LOQ: limit of quantification; Ph. Eur.: European Pharmacopeia; TAMC: total aerobic microbial count; TYMC: total yeast and mould count; UV–VIS: ultraviolet–visible spectroscopy.
Batch number 5 was subject to a drying process lasting twice the time used for the other batches.
Data obtained from number‐based distributions.
LOQ 0.2 mg/kg.
LOQ 0.04 mg/kg.
Information was provided on the accreditation of the laboratories that conducted the analyses presented in the application.
The Panel considers that the information provided on the composition is sufficient for characterising the NF.
3.4.1. Stability
The applicant performed stability tests with three independently produced batches of the NF (batches # 2, 3 and 4). The tests were carried out at normal storage conditions (25 ± 2°C; RH: 60 ± 10%) and accelerated conditions (40 ± 2°C; RH: 75 ± 5%) for a period of 24 and 6 months, respectively. The batches were analysed for physical/chemical and microbial parameters, and for phase distribution (percentage of iron present as soluble, nano‐ and microparticulate Fe) and size of secondary microparticles in the dry powder.
No relevant changes in the parameters were observed after 24 months under normal conditions or 6 months under accelerated conditions.
The Panel considers that the data provided sufficient information with respect to the stability of the NF during 24 months.
3.5. Specifications
The specifications of the NF are indicated in Table 2.
Table 2.
Specifications of the NF
| Description: Iron hydroxide adipate tartrate (IHAT) is a red‐brown micro powder, odourless, insoluble in water | |
|---|---|
| Parameter | Specification |
| Physical/chemical | |
| Iron (% dry matter) | 24–36 |
| Adipate (% dry matter) | 1.5–4.5 |
| Tartrate (% dry matter) | 28–40 |
| Water (%) | 10–21 |
| Sodium (% dry matter) | 9–11 |
| Chloride (% dry matter) | 2.6–4.2 |
| Phase distribution (in water) | |
| Soluble | 2–4% |
| Nano | 92–98% |
| Micro | 0–3% |
| Primary particle size | |
| Median diameter1 | 1.5–2.3 nm |
| Mean diameter1 | 1.8–2.8 nm |
| Dv(10)2 | 1.5–2.5 nm |
| Dv(50)2 | 2.5–3.5 nm |
| Dv(90)2 | 5.0–6.0 nm |
| Heavy metals | |
| Arsenic | < 0.80 mg/kg |
| Nickel | < 50 mg/kg |
| Residual solvents | |
| Ethanol | < 5,000 mg/kg |
| Microbiological | |
| TAMC | < 10 CFU/g |
| TYMC | < 10 CFU/g |
CFU: colony forming units; Dv: percentile of the volume‐based particle size distribution; TAMC: total aerobic microbial count; TYMC: total yeast and mould count.
Number‐based (by TEM).
Volume‐based (hydrodynamic diameter by DLS).
The Panel considers that the information provided on the specifications of the NF is sufficient and does not raise safety concerns. The Panel, however, notes that the maximal content of nickel in the NF may pose a risk in nickel‐sensitised individuals (further discussed in Section 3.7.5).
3.6. History of use of the NF and/or of its source
There is no history of use of the NF.
The intake of iron in the human diet is discussed in Section 3.9 Nutritional information.
Tartaric acid and adipic acid are consumed as a part of the normal diet as constituents of some foods (e.g. respectively, in grapes, wine, bananas and beets, and in sugar cane) or as authorised food additives (E 334 and E 355–E 357).
3.7. Proposed uses and use levels and anticipated intake
3.7.1. Target population
The target population proposed by the applicant is the general population above 3 years of age.
3.7.2. Proposed uses and use levels
The applicant intends to market the NF for use in food supplements, up to a maximum dose of 100 mg per day, corresponding to a maximum daily intake of iron of 36 mg; the highest dose is to be defined in accordance with the equivalent maximum amount of iron supplementation permitted at the national level.
3.7.3. Anticipated intake of the NF
EFSA performed an intake assessment of the anticipated daily intake of the NF based on the applicant’s proposed uses and maximum proposed use levels. The proposed daily intake of the NF, i.e. 100 mg, would result in a maximum daily intake of its constituents corresponding to 36 mg iron, 4.5 mg adipate, 36 mg tartrate, 11 mg sodium and 4.2 mg chloride.
The anticipated daily intake of the NF and of iron (on a mg/kg body weight (bw) basis), are presented in Table 3.
Table 3.
Use of the NF in food supplements and resulting intake expressed as mg/kg bw per day
| Population group | Age (years) | Body weighta (kg) | Use level (mg/day) | Intake of NF (mg/kg bw per day)b | Intake of iron (mg/kg bw per day)b |
|---|---|---|---|---|---|
| Children | 3 to < 10 | 23.1 | 100 | 4.33 | 1.56 |
| Young adolescents | 10 to < 14 | 43.4 | 100 | 2.30 | 0.83 |
| Older adolescents | 14 to < 18 | 61.3 | 100 | 1.63 | 0.59 |
| Adults | ≥ 18 | 70 | 100 | 1.43 | 0.51 |
NF: novel food; bw: body weight.
Default and average body weights for each population group are available in EFSA Scientific Committee (2012).
Intake in ‘mg/kg bw per d’ is calculated by considering the use levels in ‘mg/d’ and default body weights defined in EFSA Scientific Commitee (2012).
3.7.4. Combined intake from the NF and other sources
The average iron intake ranges between 7.5 and 11.5 mg/day in children aged 3 to < 10 years, between 9.2 and 14.7 mg/day in children aged 10 to < 18 years and between 9.4 and 17.9 mg/day in adults (≥ 18 years) (EFSA NDA Panel, 2015). The combined intake from the NF and the background diet may result in the intake of iron up to 47.5 mg/day in children aged 3 to < 10 years, 50.7 mg/day in children aged 10 to < 18 years and 53.9 mg/day in adults.
The supplemental daily intake of adipate and tartrate resulting from consumption of the NF is considered negligible compared to their occurrence in food as natural constituents or as food additives.
3.7.5. Estimate of exposure to undesirable substances
The EFSA risk assessment on nickel (Ni) from food (EFSA CONTAM Panel, 2020) concluded that for acute effects a MOE of 30 or higher to the reference point of 4.3 µg Ni/kg bw for Ni‐sensitised individuals was considered as being indicative of a low health concern. The consumption of the NF at the maximal use level may lead to Ni intake up to 5 µg, which provides a MOE > 30, only in subjects above 10 years of age (Table 4). Furthermore, the Ni intake from other foods comes in addition. EFSA in 2020 concluded that ‘The MOE values for the mean upper bound acute dietary exposure and for the 95th percentile upper bound raises a health concern for nickel‐sensitised individuals’. (EFSA CONTAM Panel, 2020).
Table 4.
Intake of nickel and MOE from the consumption of the NF at the proposed use levels in children and adolescents
| Age range (years) | Body weighta range (kg) | Intake of Ni (µg)b | Intake range of Ni (µg/kg bw)c | MOEd range |
|---|---|---|---|---|
| 3–10 | 14.5–33.8 | 5 | 0.34–0.15 | 12–29 |
| 11–17 | 37.6–62.5 | 5 | 0.13–0.08 | 32–54 |
NF: novel food; bw: body weight; MOE: margin of exposure.
Average of males and females median body weights for children and adolescents as available in EFSA NDA Panel (2013).
Intake calculated using proposed use level of the NF, i.e. 100 mg and the maximum Ni content as per the NF specifications, i.e. 50 mg/kg NF.
Intake in ‘µg/kg bw per day’ is calculated by considering the maximum Ni intake and the average of the males and females median body weights as defined in EFSA NDA Panel (2013).
Margin of exposure against the reference point of 4.3 µg Ni/kg bw, as defined by the EFSA CONTAM Panel (2020).
The Panel notes that the maximal Ni concentration in the NF is > 20‐fold the mean concentration in foods with naturally high occurrence of Ni (i.e. legumes, nuts and oilseeds) and mineral supplements, i.e. the food supplement category with the highest Ni content (EFSA CONTAM Panel, 2015). On an absolute basis, the greatest Ni levels are found in the food category ‘Cocoa beans and cocoa products (solid)’ (EFSA CONTAM Panel, 2015); the maximal Ni concentration of the NF is > 5‐fold the mean concentrations in this food category.
The tolerable daily intake (TDI) for chronic Ni intake of 13 µg/kg bw will not be exceeded with the NF in addition to the rest of the diet.
The Panel concludes that the NF poses a risk to nickel‐sensitised individuals up to 10 years of age.
3.8. Absorption, distribution, metabolism and excretion (ADME)
In line with the testing strategy detailed in the EFSA Guidance on nanomaterials (EFSA Scientific Committee, 2021), the applicant provided:
three in vitro digestion studies to assess whether the NF degrades quickly and fully under simulated human gastrointestinal (GI) tract conditions (step 0 of the EFSA assessment strategy for nanomaterials),
a review of the existing physicochemical and toxicological information on the NF (step 1a of the EFSA assessment strategy for nanomaterials), and
an in vitro degradation study to assess the particulate state of the NF under simulated lysosomal conditions (step 1b of the EFSA assessment strategy for nanomaterials).
The first in vitro digestion study (University of Cambridge, 2019a) simulated GI conditions in a fasted state (i.e. without concomitant food ingestion) using a static model. With the assumption that the NF is not expected to be exposed to an oral phase (being encapsulated to swallow and disperse in the stomach) only the gastric phase (2 h, pH 3, pepsin solution, 37°C) and the intestinal phase (4 h, pH 7, pancreatin and bile extract solutions, 37°C) were studied according to the conditions of Minekus et al. (2014). The NF (batch #1, see Section 3.4 Compositional data) was tested alongside with ferrous sulfate (FeSO4, a standard iron supplement) and ferric chloride (FeCl3, as another standard iron salt) at 0.6 mmol/L and 1.8 mmol/L Fe (equivalent to 33.5 and 100.5 mg Fe/L, respectively, resulting in concentrations of 0.15 mmol/L and 0.45 mmol/L in the final intestinal stage, respectively), with the latter concentration considered to be representative of human exposure at the intended use level of the NF. The phase distribution (microparticulate, nanoparticulate and soluble) of the samples collected during the intestinal phase (at 0 min, 30 min, 120 min and 240 min, and also at 15 min and 60 min for the 0.6 mmol/L concentration) was determined by centrifugation and ultrafiltration followed by the analysis of total iron through ICP‐OES. The degradation rate profile in the intestinal phase at both concentrations did not show a decrease in the presence of particles over time; rather a tendency to formation of secondary particles in the micro‐range was found. These particles are likely to be represented by agglomerates, but no attempt was made to probe this (e.g. by sonication with different energy levels). It is concluded that the NF does not dissolve under human GI tract conditions. The study authors noted that the two iron salts were also predominantly present in particulate form in the intestinal phase and put forward precipitation of ferric iron particles at pH 7 as the likely explanation.
The second study was also conducted with a static two‐phase fasted in vitro digestion model, according to the conditions of Koch et al. (2013). Also, in this study, no time trend towards dissolution in the intestinal phase was noted and the NF was confirmed to be largely present in particulate form, in this case, almost exclusively as nanoparticles, with agglomerates sparingly present.
Following a request from the Panel, the applicant submitted an additional in vitro simulated GI digestion study of the NF in fed conditions, according to Minekus et al. (2014) (EcamRicert, 2021a). The NF and an ionic control (ferrous chloride) were tested in duplicate at three different concentrations (5.4, 10.7 and 21.4 mmol/L Fe) intended to correspond to a daily iron intake of 15 mg, 30 mg (i.e. the NF expected intake based on use levels) and 60 mg.
The model food was simulated by a mixture of powdered protein and lipid, corresponding to the protein‐to‐lipid ratio of 1.7 (about 116 mg of protein and 60 mg of lipids), equivalent to 1 g of chicken meat according to the study authors. The NF in fasted conditions (i.e. without the model food) was tested as well. Samples were collected at the end of the gastric phase and during the intestinal phase (at 0 min, 15 min, 30 min and 60 min) and the soluble and particulate iron were differentiated via 3 kDa ultrafiltration. In addition, the particulate fraction was subjected to TEM‐EDX analysis. The results showed that the NF is resistant to gastric degradation and that the degradation rate profile in the intestinal phase, independently on the NF concentration and the presence or absence of the model food, does not show an appreciable decrease in the presence of particles over time. The size distributions and median values of the NF particles in the intestinal phase are consistent with those of pristine NF and the particles are not agglomerated. The experiment with FeCl2 also showed the presence of small Fe‐containing particles in the intestinal phase, which, however, were largely agglomerated, generated by precipitation of ionic iron associated with the pH change and the decrease of iron solubility. It is concluded that the NF does not quickly dissolve under human GI tract in vitro conditions.
In an in vitro study to assess the dissolution under lysosomal conditions, according to Pelfrene et al. (2017) (University of Cambridge, 2019b), the NF (batch #1, see Section 3.4 Compositional data) was tested alongside with ferrous sulfate (FeSO4) and ferric chloride (FeCl3) as controls. Three iron concentrations of the NF were tested at pH 4.5 (0.15 mmol/L, 0.4 mmol/L and 1 mmol/L) with the middle concentration considered to be representative of human exposure at the intended use level of the NF. The phase distribution (microparticulate, nanoparticulate and soluble) of the samples collected during the assay at different time points (0, 6, 24, 48, 72, 96 h) was determined by centrifugation and ultrafiltration followed by the analysis of total iron through ICP‐OES.
The results show that the NF dissolves, with the fraction of soluble iron being greater at lower concentrations, but not to a degree qualifying it as not biopersistent according to the EFSA Guidance on nanomaterials (EFSA Scientific Committee, 2021). Even at the lowest concentration tested, ≥ 21% of the material (mass‐based) remains in a particulate form at 72 h compared to the initial concentration, which is higher than the threshold set at ≤ 12% (EFSA Scientific Committee, 2021). Since also for the two iron salts tested the threshold was exceeded at 72 h, the study authors argue that the static test performed is unable to mimic in vivo lysosomal conditions, where dissolved iron is expected to be shuttled out of the lysosome so that critical concentration levels leading to precipitation of iron in nanoform are not reached. The Panel considers that the test is inconclusive since it does not unambiguously show that the material is not biopersistent (which is required according to the EFSA Scientific Committee, 2021).
3.8.1.
3.8.1.1.
Mechanisms of absorption, distribution metabolism and excretion of the NF
The applicant provided in vitro and in vivo studies to elucidate the mechanisms of absorption, distribution, metabolism and excretion of the NF. The Panel noted that some of the studies provided (Pereira et al., 2013, 2014; Aslam et al., 2014; Latunde‐Dada et al., 2014; Powell et al., 2014) used as test item IHAT as described in Powell et al. (2014). In the light of the data reported in section 3.2 indicating that the proposed NF has analogous chemical and elemental composition, comparable morphological shape, size and nanoforms distribution as IHAT described in Powell et al. (2014), the Panel considers that it can be reasonably assumed that the NF has an uptake, absorption, degradation, and bioavailability comparable to IHAT as described in the above‐mentioned papers.
In vitro studies
Pereira et al. (2013) showed that the IHAT particles were readily adherent to the cell membrane of differentiated Caco‐2 cells and assessed internalisation using TEM. The authors presented evidence suggesting that uptake of Fe(III) from the nanoparticles was by endocytosis. Despite the uptake process of Fe(III) being different from the established DMT‐1 pathway for soluble Fe(II), the Fe was incorporated into ferritin in Caco‐2 cells, suggesting that it entered the same intracellular iron pool as Fe(II) taken up by normal physiological pathways.
Pereira et al. (2014) investigated the hydrodynamic particle size of IHAT by DLS and the non‐aquated primary particle size by TEM. Characterisation of the dispersion showed that > 90% of Fe was present in the nanoparticulate fraction. DLS measurements showed mono‐disperse particles (i.e. < 10 nm) that had not agglomerated/aggregated.
Further studies by Latunde‐Dada et al. (2014) examined the mechanisms of IHAT uptake and utilisation in HuTu 80 cell line and used other blockers of the different intracellular pathways of iron metabolism (i.e., divalent metal transporter 1 (DMT‐1) and duodenal cytochrome b). The results were consistent with uptake by endocytosis, hydrolysis in the lysosomes and the release of iron into the cytoplasm, where it became part of the normal intracellular iron pool.
The Panel notes that some uncertainties that remain regarding these studies include whether the cells used are good models for intestinal cells, whether the inhibitors and blockers used to interpret different sections of the uptake and metabolism pathways are specific to those pathways, and whether the forms of Fe used are the same as those that would be found in the intact gut. To a large extent, these uncertainties are addressed in the following sections on in vivo studies in animal models and in humans.
In vivo studies
A paper by Powell et al. (2014) describes an in vivo study on iron uptake and iron concentration in selected organs/tissues following administration of IHAT. Iron‐deficient male CD1 mice received by gavage as a single‐dose IHAT or Fe(III) nitrilotriacetate complex (Fe(III) NTA, soluble Fe(III) control) that was 59Fe‐labelled, providing 2 µmol Fe (100 µL of 20 mmol/L solution). No statistically significant differences were observed between the 59Fe content in the carcass of animals (gut removed) of the two groups. When treating the intestinal lumen with ferrozine, a strong chelator of soluble Fe(II), to inhibit any uptake of free Fe(II) by DMT‐1, no significant impact on absorption of the IHAT was observed, whereas Fe(III)NTA absorption was almost completely inhibited. The results indicate that uptake of IHAT, or uptake of Fe from IHAT, is independent of the reduction of Fe(III) to Fe(II) and the DMT‐1 pathway.
Latunde‐Dada et al. (2014) describes two in vivo studies, where IHAT was fed to male outbred Swiss CD1 mice.
In the first study, iron‐deficient mice were fed for 7 days with either an iron‐sufficient diet, a low‐iron diet or a low‐iron diet supplemented by gavage with 50 µg Fe/day as IHAT or FeSO4. At the study end, the haemoglobin concentration in animals fed IHAT or FeSO4 was statistically significantly higher than the iron‐deficient control and not different compared to the iron‐sufficient control. Animals fed IHAT or FeSO4 had statistically significantly lower splenic iron concentration compared to the iron‐sufficient control and not statistically significantly different from the iron‐deficient control. Non‐haem iron concentrations in the duodenum and liver did not show statistically significant differences between supplemented groups. No statistically significant differences were observed for relative hepatic Hamp1 mRNA expression between supplemented groups.
In the second study, the authors demonstrated that iron uptake from either 59Fe‐labelled IHAT or 59Fe‐labelled FeSO4 was higher in iron‐deficient animals than in controls. Over the 4‐h labelling period in iron‐deficient mice, 49 ± 17% of 59Fe was systemically transferred from 59Fe‐labelled IHAT versus 70 ± 11% from 59Fe‐labelled FeSO4, giving some information about the relative bioavailability of the two compounds.
Aslam et al. (2014) performed two in vivo studies to investigate how iron from IHAT is absorbed in the duodenum, and more specifically, whether (i) the transfer into the systemic circulation is mediated by ferroportin (Fpn), as suggested by the in vitro experiments, and ii) whether IHAT itself crosses the gut epithelium.
Ferroportin is the transporter through which iron is exported from the intestinal cell into the portal circulation. It is the site of control for iron absorption, being up‐ or downregulated according to the body’s requirements, a process controlled by hepcidin, produced by the liver. In the first study, intestine‐specific ferroportin‐1 knockout (Fpn‐KO) mice and litter‐mate wild‐type (WT) control mice received an iron‐deficient diet for 4 weeks (iron‐depletion period) followed by 4 weeks iron‐deficient diet with or without supplementation with ca. 20 mg iron/kg diet as IHAT or FeSO4. An iron‐sufficient control group received ca. 35 mg iron/kg diet as Fe(III) citrate monohydrate throughout the 8 weeks study duration. At the end of the study, WT mice showed comparable haemoglobin concentrations in animals receiving IHAT or FeSO4 and the iron‐sufficient control group, suggesting that both iron forms were efficiently absorbed in the gut. In the Fpn‐KO mice, IHAT and iron from the other sources were not able to reverse the iron deficiency, suggesting that transfer across the gut was through the ferroportin pathway.
In the second study, the authors assessed the absorption of IHAT in isolated duodenal loops of Fpn‐KO and wild‐type mice. The duodenal loops of iron‐deficient mice were directly infused with a saline solution providing IHAT or FeNTA2 (ferric nitrilotriacetate chelate; soluble Fe(III) control) (both 100 µL of 500 µM Fe) or no iron. All Fpn‐KO mice had statistically significantly lower serum iron concentrations than WT mice in the same test group. In WT mice, serum iron levels increased statistically significantly 30 min following infusion with IHAT as well as with FeNTA2. In the Fpn‐deficient mice, there was no observed difference in serum iron levels after intestinal instillation of IHAT as compared to a non‐iron containing saline solution. This provides support to the hypothesis that IHAT cannot be transferred across the gut directly, since should IHAT have been absorbed, serum iron level would have been increased. Differences in rates of absorption between IHAT and FeNTA2 were instead observed, with the iron level being significantly lower for IHAT. The authors attributed this to either the lack of passage to the stomach being required for efficient absorption of IHAT or because of the fact that uptake of iron from IHAT might require the endosomal/lysosomal breakdown prior to systemic release of iron.
Bioavailability
The bioavailability of IHAT was already addressed in some of the ADME studies described above. In particular, direct evidence for the absorption of IHAT relative to FeSO4 was provided by Latunde‐Dada et al. (2014), who showed that 49% IHAT was absorbed in 30 min, compared to 70% of FeSO4.
Animal studies
The bioavailability of iron from the NF was further assessed in two rat studies, where IHAT was supplemented with the diet for 14 days (Powell et al., 2014; Pereira et al., 2014). In a third rat study (Vivo Science, 2019), the applicant investigated the distribution and accumulation of iron in different tissues following supplementation of the NF to the diet for 28 or 90 days.
In the first study (Powell et al., 2014), iron‐deficient male Sprague–Dawley rats were administered orally (feed) for 14 days ad libitum either a low iron diet (control) or low iron diet fortified with approx. 30 mg Fe/kg diet as IHAT, synthetic ferrihydrite or FeSO4. At the end of the study, animals receiving IHAT and FeSO4 had comparable haemoglobin concentrations, which were statistically significantly higher than in the low‐iron control and synthetic ferrihydrite groups.
In the second study (Pereira et al., 2014), iron‐deficient male Sprague–Dawley rats were administered orally (feed) for 14 days ad libitum either an iron‐deficient diet supplemented with 20 mg Fe/kg diet as IHAT (corresponding to 1.8 mg Fe/kg bw per day)9 or as FeSO4, or an iron‐sufficient diet (50 mg Fe/kg diet as Fe(III)citrate) (corresponding to 4.5 mg Fe/kg bw per day).9 At the end of the study, animals receiving a diet supplemented with IHAT or FeSO4 showed increased haemoglobin concentrations compared to the baseline, comparable among the two groups but statistically significantly lower than the iron‐sufficient control group.
The applicant provided a 90‐day repeated dose toxicity study in Wistar rats (Vivo Science, 2019; discussed in Section 3.10.2 Subchronic toxicity) integrated with toxicokinetic assessment as per the EFSA Guidance on nanomaterials (EFSA Scientific committee, 2021). In the toxicokinetic arm of the study, two satellite groups of six animals/sex per group were administered the NF by gavage at doses of 0 or 115.5 mg/kg bw per day for 28 or 90 days to gain additional information about distribution and accumulation of iron in different tissues as well as the presence of particles in tissues with specific involvement in particle uptake and accumulation.
Maintenance of the two satellite groups was conducted under GLP conditions during in‐life phase (viability, clinical signs, behaviour, body weight gain, macroscopic examination of selected organs; no blood was taken and no histopathology performed). After 28 days and 90 days of treatment, either with the test item or the vehicle, one half of the group animals (6 males/6 females per treatment) was sacrificed and selected organs and tissues were analysed for total iron content and cellular iron levels (non‐GLP part of the study). Total iron content was analysed by ICP‐OES in the liver, duodenum and spleen of all animals as well as in the most proximal small intestine draining mesenteric lymph node of half of the animals, and in the second most proximal small intestine draining mesenteric lymph node of the other half of the animals. Cellular iron levels were assessed histochemically in the mesenteric lymph node and in the proximal small bowel (regular jejunal mucosa and Peyer’s patches) by in situ cytometry (ISC).
Results from the study indicate that there were no statistically significant differences regarding viability, clinical signs and behaviour, body weight and macroscopic findings in organs between the test group and the control. There were no statistically significant differences between the two NF satellite groups of different treatment duration (28 or 90 days, respectively) and control group regarding total iron concentrations (by ICP‐OES) in the selected organs/tissues (liver, duodenum, spleen and proximal small bowel mesenteric lymph nodes). The study did not detect any appreciable difference in intracellular iron levels (by ISC) or distribution in the selected organs/tissues, i.e. mesenteric lymph nodes and proximal small bowel (regular jejunal mucosa and Peyer’s patches).
Since the treatment duration (28 or 90 days) did not result in statistically significant differences in total iron concentration and distribution between the two satellite groups tested with the NF at the lower dose level used in the 90‐day study (115.5 mg/kg bw per day; no toxicologically relevant effects were noted), the Panel considers that there is no indication for bioaccumulation of the NF in gut wall epithelial cells, Peyer’s patches, mesenteric lymph nodes and the spleen.
Human studies
The applicant provided two single‐dose human studies on the bioavailability of iron from IHAT (MRC, 2013, 2020; Pereira et al., 2014). Details of the study design, study population, doses used and parameters related to bioavailability and safety are presented in Table 6. It is noted that these studies were conducted with IHAT providing 60 mg of iron per day in the adult study population as compared to a maximum of 36 mg of iron as the proposed use level for the NF.
Table 6.
Summary of studies in humans related to the NF
| Reference | Study design | Study population | Duration of study | Doses; route of administration if relevant | Parameters investigated related to bioavailability and safety |
|---|---|---|---|---|---|
| MRC (2013) | Single‐blind, single‐dose, cross‐over study comparing 15 ferric iron oxide organic acid preparations (Fe‐OA) (including IHAT) against ferrous sulfate. |
4 pre‐menopausal women (aged 18 to 45 years, from the UK) with mild iron deficiency (serum ferritin < 12 µg/L) or mild‐moderate iron deficiency anaemia (haemoglobin 10‐11.9 g/dL plus either serum ferritin < 20 µg/L or transferrin saturation < 10%) for each tested Fe‐OA preparation. In total, 67 finished the study. |
14 days |
1 × Fe‐OA (60 mg Fe equivalent) [IHAT tested at a dose of 66.8 mg Fe‐equivalent/person]. 1 × FeSO4 (60 mg Fe equivalent). Oral administration via methylcellulose capsule, on empty stomach or with light breakfast. |
Relative bioavailability of iron from Fe‐OA compared to FeSO4 No safety‐related parameters were tested except for reporting of adverse events. |
| MRC (2019) | Randomised, double‐blind, placebo‐controlled, parallel study with 3 arms (IHAT, FeSO4, placebo) | Per protocol population was 582 healthy young children. 189 subjects (aged 6‐35 months, both sexes, from Gambia) with iron deficiency and anaemia were included in the IHAT study arm. | 12 weeks |
IHAT: IHAT powder providing 20 mg Fe, 21 mg tartaric acid and 4.7 mg adipic acid (1 capsule orally)/child per day for 12 weeks (assumed to be bioequivalent to 12.5 mg Fe of FeSO4, assuming a 60% bioavailability of IHAT relative to FeSO4). FeSO4: 62.5 mg ferrous sulfate heptahydrate powder providing 12.5 mg Fe (1 capsule orally)/child per day for 12 weeks. |
Inflammation marker in the gut (faecal calprotectin) and blood (serum C‐reactive protein (CRP); alpha 1‐acid glycoprotein (AGP)). Diarrhoea‐related parameters Faecal microbiome Reporting of adverse events (including serious ones). |
| MRC (2020) | Double‐blind, single‐dose, randomised cross‐over study comparing the IHAT against ferrous sulfate. |
32 pre‐menopausal healthy women (aged 18–52 years, from Gambia), non‐pregnant, non‐lactating, with normal C‐reactive protein (CRP) at screening (CRP < 5 mg/L). 32 women completed the study – 10 non‐anaemic – 22 anaemic, whereas iron deficiency anaemia (IDA) was defined as haemoglobin 9‐11 g/dL and serum ferritin < 15 ng/mL. |
14 days |
Single oral dose of either IHAT(i) or IHAT(ii) as capsule (equivalent to 60 mg Fe) as well as a single oral dose of FeSO4 as capsule (equivalent to 60 mg Fe), 14 days apart. Administered as single oral dose of IHAT(i)(tray‐dried) or IHAT(ii) (ethanol precipitated and then tray‐dried) (capsule) and single oral dose of FeSO4 (capsule) 14 days apart. |
Relative bioavailability of iron from IHAT compared to FeSO4. Serum iron, transferrin saturation, plasma iron, hepcidin concentration in blood. Pathogen growth in blood samples. Reporting of serious adverse events and adverse events. |
| JM‐USDA (2019) | Randomised, double‐blind, placebo‐controlled, parallel study with 6 arms (IHAT, placebo, 3 FeSO4 groups differing in dose and one plus micronutrients and another Fe‐product under investigation) | Per protocol population was 160 subjects. 27 iron‐replete non‐anaemic post‐menopausal women and age‐comparable men (aged 50–77 years, 15 female, 12 male, mainly white or Caucasian, from US), were enrolled to the IHAT arm. | 28 days |
IHAT: 60 mg Fe/day as capsule. Three FeSO4 groups: (1) 60 mg Fe/day, (2) 420 mg Fe/week, (3) 60 mg Fe/day plus micronutrients. Another Fe‐product under investigation, 60 mg Fe/day. IHAT providing 60 mg Fe (1 capsule orally)/fasted (12 h) person per day for 28 days. |
Ex vivo malarial infectivity; Ex vivo bacterial proliferation potential (E. coli, A. baumannii, K. pneumonia, S. aureus, Salmonella Typhimurium). Gut inflammation markers (faecal calprotectin, myeloperoxidase, α‐1 antitrypsin, tumour necrosis factor‐α with LPS) and gut irritation questionnaire. Reporting of adverse events. |
The first study (MRC, 2013, Pereira et al., 2014) was a pilot study which investigated the bioavailability of IHAT as measured by erythrocyte incorporation of iron, and the production of non‐transferrin bound iron as measured by its maximum serum concentration, following the ingestion of IHAT. In addition, total iron absorption, serum iron, the rate of iron absorption and transferrin saturation following the ingestion of IHAT in comparison to FeSO4 were analysed. The study, carried out on four iron‐deficient women in the UK, indicated a relative bioavailability of iron from IHAT of 76% [95% CI 55–98] relative to that of iron from FeSO4. Furthermore, a lower increase of serum iron at 4 h compared to the same dose of iron from FeSO4 was observed.
The second study (MRC, 2020) is an exploratory study in which two IHAT preparations were tested for iron bioavailability (one was obtained using laboratory‐scale tray‐drying of the entire solution after synthesis and one industrially manufactured using ethanol precipitation followed by tray‐drying step as before). Each compound was labelled with a stable iron isotope (i.e. 2 mg 58Fe for IHAT and 10 mg 57Fe for FeSO4), and their absorption was determined from the red blood cell incorporation of the stable isotope 14 days after the single dose. Additional analyses included the post‐ingestion rise in transferrin saturation and serum iron as well as serum hepcidin. Overall, across the entire study, the mean relative bioavailability value (RBV) for IHAT relative to FeSO4 was 36.7% [95% CI 29.1–44.2]; the RBV for subjects with iron deficiency anaemia was 42.4% [95% CI 33.4–51.4]. There were no statistically significant differences between the RBV of the IHAT recovered by the two ‘manufacture’ procedures. Increases in serum iron and transferrin saturation over 6 h following ingestion of preparations of IHAT were significantly lower (p < 0.05) in comparison to FeSO4 in both iron deficient and non‐iron deficient groups). Multivariate regression analysis showed that the bioavailability of each compound, i.e. the red blood cell incorporation of the stable iron isotope 14 days following dosage, was best predicted in a model including the maximum serum iron increase observed in the 6‐h post‐dosage samples (p < 0.0001 for both compounds). Hepcidin data following a single‐dose of IHAT or ferrous sulfate were not statistically different.
The Panel notes that the results of the two human studies show a bioavailability of iron from IHAT of 76% and 36.7%, respectively, relative to the iron absorption from FeSO4. The reason for this discrepancy in relative bioavailability between the two studies is unclear. Possibly the small number of subjects (n = 4) in the first study and differences in the FeSO4 substance (which were commercial FeSO4 tablets in the first study, while the FeSO4 labelled with a stable isotope of iron in the second study was freshly manufactured and encapsulated in gelatine capsules) may account for some of the difference in the results.
Two other human studies provided by the applicant reported on iron status parameters under prolonged supplementation with IHAT (MRC, 2019; JM‐USDA, 2019; for details of the studies see Section 3.10.3 Human data and Table 6).
The IHAT‐Gut (MRC, 2019), carried out in young children, aged 6–35 months with iron deficiency and anaemia, receiving either 20 mg Fe/day from IHAT (considered to be bioequivalent with 12.5 mg Fe of FeSO4), 12.5 mg Fe of FeSO4 or placebo, over 12 weeks. Results show, that in both iron supplementation groups, Hb improved by at least 1 g/dL, ferritin also improved and the proportion of children who remained iron deficient and iron‐deficient anaemic decreased, while there were no such improvements in either Hb or ferritin in the placebo group. Comparison for non‐inferiority for IHAT vs. FeSO4 supplementation using a logistic regression model revealed that IHAT was non‐inferior vs. FeSO4 for iron deficiency correction probability.
The study by JM‐USDA (2019) aimed to compare the safety of IHAT,10 administered at a 60 mg Fe/day dose level over four weeks with FeSO4 in healthy iron‐replete non‐anaemic post‐menopausal women and age‐comparable men. Among others, iron status parameters, including haemoglobin, transferrin, ferritin, iron, unsaturated iron‐binding capacity, total iron‐binding capacity and per cent transferrin saturation, were analysed in serum/blood. As a result, no changes in iron status parameters were observed over a period of 28 days in either the group receiving IHAT or the group receiving FeSO4 in this iron‐replete healthy population.
Conclusions on ADME
Based on the data on dissolution presented by the applicant, the Panel concludes that the NF does not dissolve under human GI conditions, but does so under lysosomal conditions. However, the dissolution is not to a degree that can qualify it as not biopersistent according to the EFSA Guidance on nanomaterials (EFSA Scientific Committee, 2021). Therefore, according to the EFSA assessment strategy for nanomaterials, in vitro toxicity studies, starting from genotoxicity, are needed to assess if nanospecific in vivo testing would be required to evaluate the safety of this nanomaterial.
Based on the literature provided by the applicant to elucidate, the mechanisms of absorption, distribution, metabolism and excretion of IHAT, the Panel considers that in vitro and in vivo studies support the fact that particles of the NF are absorbed independently from the reduction/DMT‐1 pathway, possibly by endocytosis, and that the iron from the NF is released from the lysosome to supplement the common enterocyte pool of dietary‐derived iron.
The Panel notes that the iron derived from IHAT does not circumvent systemic iron regulatory mechanisms as demonstrated by the fact that the intestinal export of iron from IHAT into the portal circulation is ferroportin‐mediated, similarly to that of soluble iron.
The Panel also notes that the data with ferroportin knockout mice suggest that IHAT does not translocate intact from the gut epithelium into the blood circulation. The Panel also notes that demonstration via direct analyses of IHAT particles in blood, organs and tissues is a substantial technical challenge.
Finally, the Panel notes that animal and human studies indicate that iron from IHAT is absorbed, even though at a lower rate relative to the iron absorbed from FeSO4.
The Panel concludes that iron from the NF is bioavailable, and once taken up into the epithelial cells of the gut, is subject to the same mechanisms of regulation and absorption as that of other forms of iron.
3.9. Nutritional information
The applicant provided a nutritional analysis of the NF. The NF is primarily constituted of iron (24–36% dry matter), adipate (1.5–4.5% dry matter) and tartrate (28–36% dry matter), together with sodium (9–11% dry matter) and chloride (2.6–4.2% dry matter). All these constituents are occurring in the food chain either as natural constituents of foods or as food additives. The proposed daily intake of the NF, i.e. 100 mg, would result in a maximum daily intake of its constituents corresponding to 36 mg iron, 4.5 mg adipate, 36 mg tartrate, 11 mg sodium and 4.2 mg chloride.
Population reference intakes (PRIs) for iron range between 7 and 16 mg/day for the different age and population groups (EFSA NDA Panel, 2015). Thus, the maximum iron intake via the NF may be about two to five times higher than the PRIs.
No tolerable upper intake level (UL) has been set for iron by SCF or EFSA (EFSA NDA Panel, 2015). Although adverse gastrointestinal effects have been reported after short‐term ingestion of non‐haem iron preparations at doses of 50–60 mg/day, particularly if taken without food, EFSA NDA Panel (2004) considered that these adverse gastrointestinal effects are not a suitable basis to establish an UL for iron from all sources. The Panel considers that taking into account the composition of the NF and the proposed conditions of use consumption of the NF is not nutritionally disadvantageous.
3.10. Toxicological information
The applicant provided three toxicological studies on the NF, which were conducted in compliance with OECD principles of GLP (OECD, 1998a) and in accordance with the relevant test guidelines from the OECD (OECD, 1998b,c; 2016a,b). These studies which are claimed proprietary by the applicant are listed in Table 5.
Table 5.
List of toxicological studies with the NF
| Reference | Type of study | Test system | Dose |
|---|---|---|---|
| CHELAB (2021) | In vitro mammalian cell micronucleus test (GLP, OECD 487:2016) | Chinese Hamster Ovary (CHO) cells | Up to 90.4 µg/mL without S9 mix. Up to 9.8 µg/mL with S9 mix. |
| CHELAB (2019) | L5178Y Tk+/− Mouse Lymphoma Mutation Assay (GLP, OECD 490:2016) | L5178 Tk+/− mouse lymphoma cells | Up to 2.5 mg/mL (with and without S9 mix) |
| Vivo Science (2019) | 90‐day repeated dose oral toxicity study (GLP, OECD TG 408 extended to some endocrine endpoints of OECD 407) | Wistar rats | Control and 3 doses up to 462.13 mg/kg bw/day (114.1 mg Fe/kg bw per day) |
3.10.1. Genotoxicity
Upon request of the Panel, the applicant provided an in vitro mammalian cell micronucleus (MN) test using Chinese hamster ovary (CHO) cells (CHELAB, 2021; unpublished, GLP, OECD 487:2016). The NF (batch #1, see Section 3.4 Compositional data) was dispersed in Ham’s culture medium enriched with 5% fetal bovine serum (FBS). The NF did not induce micronucleus formation in the test system employed when tested for 4 h and 24 h at doses up to 90.4 and 78.1 µg/mL, respectively (57% and 50% cytostasis) in the absence of the metabolic activation system S9, and for 4 h at doses up to 9.8 µg/mL in the presence of the metabolic activation system S9 (50% cytostasis). The results of the study indicate that the NF is neither clastogenic nor aneugenic at the above concentrations. Information on the size distribution and dispersion state of the test material in the culture medium is provided later in this section.
The NF (batch #1, see Section 3.4 Compositional data) was tested in an in vitro L5178 Tk+/− mouse lymphoma cells (MLA) assay (CHELAB, 2019; unpublished, GLP, OECD 490:2016) at doses up to 2.5 mg/mL for 4 h with and without S9 as well as for 24 h without the presence of the metabolic activation system S9. The NF was dispersed in Fisher’s medium, 5% heat‐inactivated Horse serum, 1 mmol/L sodium pyruvate, 1% Pen/Strep, 0.1% Pluronic F‐127. No statistically significant increases in mutant frequency compared to negative control were observed at concentrations of the NF up to 2.5 mg/mL NF under the test conditions applied, with and without S9 and in absence of cytotoxicity. The results of the study indicate that the NF does not induce gene mutation up to 2.5 mg/mL. Information on the size distribution and dispersion state of the test material in the culture medium is provided below.
Upon request of the Panel, the applicant provided two additional studies (EcamRicert, 2021b,c) evaluating the size distribution and dispersion state of the test material (batch #1, see Section 3.4 Compositional data) in the culture medium used in the two genotoxicity studies, as well as its cellular uptake in test conditions. TEM‐EDX and DLS analyses of the NF suspended in the culture medium and assessment of cellular uptake by TEM‐EDX were provided.
For the MN test (OECD TG 487), TEM results indicate that the NF under test conditions was detected within CHO cells in the cytoplasm and/or nucleus. The NF was present as primary particles with mean diameters of a few nm comparable with the NF in pristine form.
For the MLA assay (OECD TG 490), the results indicate that under the test conditions, the NF is stable and presents primary particles with an average mean size of a few nm. TEM‐EDX indicated that after exposing the cells to the NF for 4 and 24 h in the absence of S9, nanoparticles internalisation and their presence (as agglomerates) within the nucleus could be observed. In the presence of S9, no iron‐specific signal was detected using TEM‐EDX, though electron‐dense nanoparticles compatible with IHAT were visible within the cells.
Overall, the studies indicate that the NF is present in particulate form and does not extensively agglomerate under the cell culture conditions used in the genotoxicity studies above. Within the cells, the iron‐containing particles closely resembled those of the pristine particles in the NF.
The applicant provided an additional cytotoxicity study to substantiate the cellular uptake of the NF nanoparticles (CHELAB, 2020). The viability of cells was tested comparing the NF (batch #1, see Section 3.4 Compositional data) versus ferrous sulfate. The study indicated that cell toxicity could be explained by iron concentration, irrespective of the source and the viability of cells was comparable for the two test items. The results suggest that cellular uptake of iron from NF nanoparticles and from soluble ferrous sulfate are comparable.
3.10.2. Subchronic toxicity
In a 90‐day repeated dose toxicity study in Wistar rats (Vivo Science, 2019; unpublished, GLP, OECD 408:1998 extended by some endocrine endpoints from OECD 407:1998), the NF was administered by gavage to 10 male and 10 female animals per dose group, at doses of 0 (control), 115.5 (low dose), 231.1 (medium dose) and 462.1 mg/kg bw per day (high dose), respectively, corresponding to 28.5, 57.0 and 114.1 mg Fe/kg bw per day.
The applicant provided additional information to demonstrate that at the highest dose of the NF tested (462.1 mg/kg bw per day), there was no substantial alteration in the physical‐chemical characteristics of the test item occurred due to alteration of the size distribution of the material, e.g. by agglomeration. The test item was prepared by suspending the powder in purified water each day of gavage administration and by using it within 5‐h post‐preparation; in this time frame, the test item was reported to be fully dispersed up to the highest dose tested.
The endocrine endpoints assessed according to OECD TG 407 included weight of testis, epididymis, prostate, ovary, uterus, thyroid, as well as, macroscopic and histopathologic investigation of these tissues from control and high dose.
There were no fatalities and no statistically significant differences to control regarding body weight, body weight gain, food consumption, apart from a decrease of body weight in high‐dose females (−7.7%) with no concomitant decrease in food consumption. No relevant findings were reported for general clinical signs and behaviour (including IRWIN test, beam walk, grip strength), and ophthalmology.
An increase in water consumption was observed in male rats in the majority of monitoring periods in the medium‐ (up to 31%) and high‐dose (up to 33%) groups compared to control. In females in the high dose at some time points also an increased water consumption up to 22% was noticed, while in the mid‐ and low‐dose water consumption was reduced by 22 and 18%, respectively.
As far as haematology is concerned, in high‐dose male rats the levels of haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration and reticulocytes were statistically significantly increased, which is in line with iron uptake. In addition, neutrophil granulocytes and monocytes were increased dose‐related and statistically significant at the highest dose (+57% and +85%, respectively) compared to control. Values for all these parameters were reported to be within historical control ranges, except for mean corpuscular haemoglobin and neutrophil granulocytes.
In high‐dose female rats, a statistically significant increase compared to control could be observed for neutrophil granulocytes (+139%; historical control data not provided) and mean corpuscular haemoglobin concentration.
In medium‐ and high‐dose male rats, prothrombin time was statistically significantly decreased compared to control, which was considered to be due to elevated prothrombin time of the concurrent control compared to historical controls (absence of significant changes in relative amounts of thrombocytes).
As far as clinical biochemistry is concerned, in high‐dose male rats, creatinine (−56%) (also in females but not significantly), glucose (−11%) and A/G ratio (−10%) were statistically significantly decreased and bile acids increased (+83%); in high‐dose female rats, sodium (+7%) and globulin (+8%) were statistically significantly increased and A/G ratio decreased (−10%) compared to control.
In terms of organ weights, in high‐dose male rats, there was a statistically significant increase in absolute weight of testis (left (+11%) and right (+10%); also observed in mid‐dose animals) and epididymis (left (+22%) and right (+18%)) and in the relative weight of epididymis (left (+15%)); there was a statistically significant decrease in relative prostate weight in low‐ (−13%) and high‐dose (−13%) males. The standard histopathological analysis did not reveal changes in these organs. The Panel notes that the control animals were in the low range regarding testis weight as compared to historical control data and all test animals had testis weight within the historical control range.
In high‐dose female rats, absolute liver weight was statistically significantly decreased (−9.7%) and relative brain weight increased (+6.4%) compared to control. The absolute and relative adrenal weight was statistically significantly decreased in the low‐dose group (−20% and −24%, respectively). The relative weight of uteri (corpus + cervix) were statistically significantly increased in all treated groups (+35%, +47% and +45% for low‐, mid‐ and high dose, respectively; also observed in absolute weight). Macroscopic analyses revealed a statistically significant increase in the incidence of fluid‐filled uterus at all doses (1/10, 5/10, 6/10 and 4/10 for control, low‐, mid‐ and high dose, respectively), which was reported by the authors to be possibly associated with the proestrus phase of the oestrus cycle. Individual data allowed confirmation that higher uterine weights were associated with fluid accumulation, but the Panel considers the evidence provided for a link to the proestrus phase to be inconclusive. No firm conclusions can be drawn on the adversity of these findings in the absence of oestrus cycle monitoring and hormonal level data.
The weight of evidence indicates possible endocrine effects (effects on testes, epididymides, prostate in males, uteri and adrenals in females, all hormone‐sensitive/producing organs) associated with the NF intake. The Panel notes that according to the literature, iron plays an important role in male serum reproductive hormones, fertility and sexual function, although the extent to which perturbations in body iron levels may affect male reproductive health in the general population remains to be elucidated (Gabrielsen et al., 2018). The Panel also notes that, in females, the role of iron in reproductive fitness throughout the lifespan is extensively documented in the literature (Miller, 2016), and recent studies suggest the existence of an interplay between iron levels and hormonal status (Rossi et al., 2016; Tonai et al., 2020).
Two high‐dose females showed either a swollen trachea or enlarged heart, both in the absence of histopathological findings. In the lungs, alveolar thickening was observed in the high‐dose group of both sexes (4/10 male, 3/10 females) and in the control of female rats (4/10), while minimal haemosiderin pigmentation was observed in one female rat in the high‐dose group. Haemosiderin pigmentation of the spleen was observed in all animals in high‐dose and control groups of both sexes. All these observations were considered by the Panel as non‐treatment related.
Based on the findings in haematology, clinical biochemistry and organ weights mainly in males in the high‐dose group, the Panel considers that the medium‐dose tested, i.e. 231.1 mg/kg bw per day corresponding to 57.0 mg Fe/kg bw per day, is the overall no observed adverse effect level (NOAEL) of this study.
3.10.3. Human data
The applicant provided four intervention studies with the NF, three of them in adults and one in children up to three years of age.
Study designs, IHAT supplemented doses, target populations and parameters investigated in the studies are summarised in Table 6. Upon request of the Panel, the applicant clarified how the NF as intended to be commercialised relates to the test material used in the provided human studies. The applicant provided evidence of consistency between the NF produced in the laboratory (including tray‐drying; used in the studies (MRC, 2013, 2020)) and the commercially produced NF (including an ethanol precipitation step; used in the study (MRC, 2019) and the study by JM‐USDA (2019)) with regard to physicochemical properties including nanospecific characteristics.
In the first study on pre‐menopausal women (MRC, 2013; see also Section 3.8 ADME), no study incidents related to the ingestion of IHAT were reported.
In the second study conducted on pre‐menopausal women (MRC, 2020; see also Section 3.8 ADME), based on study incidence reports, three adverse events (mild abdominal pain, lower abdominal pain, gastric pain and diarrhoea), possibly related to the treatment were reported, but no serious adverse events were reported. Lower growth of E. coli and Salmonella Typhimurium with IHAT compared to FeSO4 was observed at 2 and 4 h (p < 0.05) using ex vivo assays in serum (collected from each subject at 0, 2, 4 and 6 h following a single dose of each compound and incubated for 5 h).
The IHAT‐GUT study (MRC, 2019; Pereira et al., 2018) is a phase II trial conducted on children 6–35 months of age.
The results for safety‐related outcomes were as follows. Regarding diarrhoea, 65 episodes of moderate or severe diarrhoea (defined as three or more loose or watery stools) were recorded in the study during the 3x/week morbidity data collection (excludes episodes of diarrhoea reported as adverse events) which amounted to 8.8%, 8.4% and 13.1% and lasted 4, 2 and 2 days in the IHAT, FeSO4 and placebo groups, respectively. IHAT was neither superior vs. FeSO4 in relation to incidence density of moderate‐severe diarrhoea nor in relation to duration of moderate‐severe diarrhoea.
Children in the placebo group had lower calprotectin levels than children in both iron groups (padjusted = 0.039). There were no statistical differences between the iron groups. Systemic inflammation markers (AGP and CRP) did not differ between the iron groups. AGP was lower in the placebo group than in both iron groups (p = 0.039), but differences were small. There were no statistical differences between the groups in relation to proportion of days with diarrhoea or proportion of days with moderate‐severe diarrhoea. The placebo group had a higher prevalence of dysentery (bloody diarrhoea) than the IHAT group [ORplacebo/IHAT = 2.48 (90% CI 1.02, 6.05), p = 0.093]. Differences between IHAT and FeSO4 were not significant. There were no statistically significant differences in the composition of the faecal microbiome between any of the treatment groups, overall or when analysed individually by age group. There were no differences between groups in relation to adverse events such as hospitalisation, acute respiratory infection, diarrhoea (requiring nurse treatment) or fever.
The study by JM‐USDA (2019) is a Phase I trial aimed among others to compare the safety profile indicators between IHAT and FeSO4 in iron‐replete non‐anaemic post‐menopausal women and age‐comparable men. As for the results of this study, IHAT did neither affect the 28‐day change in ex vivo susceptibility of erythrocytes to malarial infectivity nor the ex vivo bacterial proliferation potential, similarly to the FeSO4. IHAT did not affect the 28‐day changes in gut inflammation markers. One adverse event (diarrhoea over 2 days) was reported by one subject.
The Panel concludes that the available human data obtained in adults ingesting IHAT corresponds to 60 mg of iron, either as a single dose or for 28 days, and in young children receiving IHAT corresponds to 20 mg of iron over 12 weeks, do not raise safety concerns.
3.11. Allergenicity
The Panel considers that owing to the absence of protein, the NF is unlikely to trigger allergic reactions by exogenous proteins in the target population under the proposed conditions of use.
The Panel noted that consumption of the NF at the proposed use levels, i.e. 100 mg per day, may result in an acute intake of nickel up to 5 µg. Nickel‐sensitised individuals orally exposed to Ni may experience flare‐up reactions, i.e. aggravation of eczema or develop maculopapular exanthema (Antico and Soana, 2015). EFSA CONTAM Panel (2020) identified a lowest‐observed‐adverse‐effect‐level of 4.3 µg Ni/kg bw which was selected as the reference point. The margin of exposure (MOE) approach was applied and a MOE of 30 or higher was considered as being indicative of a low health concern. The Panel notes that in young individuals up to 10 years of age the MOE is < 30 (see Section 3.7.5) and, therefore, for these subjects a risk of flare‐up reactions exists in nickel‐sensitised individuals.
4. Discussion
The NF which is the subject of the application is iron hydroxide adipate tartrate (IHAT). The NF is produced by chemical synthesis and consists of an engineered nanomaterial meant to be used as a source of iron.
The NF is intended to be used in food supplements up to a maximum dose of 100 mg per day, corresponding to a maximum daily intake of iron of 36 mg. The target population proposed by the applicant is the general population above 3 years of age.
No UL has been set for iron by the SCF or EFSA, while PRIs range between 7 and 16 mg/day for the different age and population groups (EFSA NDA Panel, 2015) Thus, the maximum iron intake via the NF may be about two to five times higher than the PRIs.
In aqueous suspension, the particles constituting the NF exist as dispersed primary particles, of almost spherical morphology, with a diameter typically smaller than 5 nm. Undissolved IHAT particles appear to reach the intestinal lumen and become absorbed independently from the reduction/DMT‐1 pathway, possibly by endocytosis. The iron contained in the particles appears to be released in the lysosome to supplement the common enterocyte pool of dietary‐derived iron. The Panel notes that the iron derived from IHAT does not circumvent systemic iron regulatory mechanisms and that there are no indications that IHAT particles may translocate intact from the gut epithelium into the blood circulation. The Panel considers IHAT as representative of the NF for the purpose of the assessment.
The studies provided for ADME and bioavailability indicate that iron from the NF is bioavailable, and once taken up into the epithelial cells of the gut, is subject to the same mechanisms of regulation and absorption as that of other forms of iron. Further studies provided in the context of the toxicological assessment indicate that the NF does not lead to iron bioaccumulation in tissues and organs at the doses tested.
The Panel notes that the NF is intended to be commercialised in capsules. In the human studies provided, the NF was administered in capsules without added excipients. Should any excipient be added in the formulation of the NF, e.g. to improve flowability or to allow manufacturing of other forms, such as tablets, the Panel considers that the impact on the agglomeration state of the particles and the bioavailability of the nutrient source has to be assessed.
As the NF contains Ni at concentrations higher than that in commonly consumed food, the NF may increase the risk of flare‐up reactions in nickel‐sensitised young individuals up to 10 years of age.
In the 90‐day toxicity study, findings related to haematology, clinical biochemistry and organ weights were observed. The Panel considers that the effects observed are critical effects related to the NF. The NOAEL defined based on these findings was the mid‐dose used in the study, i.e. 231 mg/kg bw per day.
The ratio between this NOAEL and the maximum exposure to the NF based on the proposed conditions of use results in a MOE of 53 in children below 10 years of age, 100 in young adolescents, 143 in old adolescents and 163 in adults. Since the NF provides bioavailable iron that does not circumvent systemic iron regulatory mechanisms and there are effective mechanisms to prevent iron overload, the Panel considers the MOEs as sufficient.
5. Conclusions
The Panel considers that the NF, iron hydroxide adipate tartrate, is a source from which iron is bioavailable.
The Panel concludes that the NF, iron hydroxide adipate tartrate, is safe under the proposed conditions of use.
5.1. Protection of Proprietary data in accordance with Article 26 of Regulation (EU) 2015/2283
The Panel could not have reached the conclusion on the safety of the NF under the proposed conditions of use without the data claimed as proprietary by the applicant, i.e. in vitro mammalian cell micronucleus test, in vitro mammalian cell gene mutation tests using the thymidine kinase gene, 90‐day repeated dose oral toxicity study in rodents.
6. Steps taken by EFSA
On 03/07/2020 EFSA received a letter from the European Commission with the request for a scientific opinion on the safety of iron hydroxide adipate tartrate. Ref. Ares(2020)3501791.
On 01/07/2020, a valid application on iron hydroxide adipate tartrate, which was submitted by Nemysis Limited, was made available to EFSA by the European Commission through the Commission e‐submission portal (NF 2019/1417) and the scientific evaluation procedure was initiated.
On 07/08/2020, EFSA requested the applicant to provide additional information to accompany the application and the scientific evaluation was suspended.
On 26/08/2020, additional information was provided by the applicant through the Commission e‐submission portal and the scientific evaluation was restarted.
On 27/01/2021, EFSA requested the applicant to provide additional information to accompany the application and the scientific evaluation was suspended.
On 05/05/2021, additional information was provided by the applicant through the Commission e‐submission portal and the scientific evaluation was restarted.
On 18/06/2021, EFSA requested the applicant to provide additional information to accompany the application and the scientific evaluation was suspended.
On 15/10/2021, additional information was provided by the applicant through the Commission e‐submission portal and the scientific evaluation was restarted.
During its meeting on 27/10/2021, the NDA Panel, having evaluated the data, adopted a scientific opinion on the safety of iron hydroxide adipate tartrate as a NF pursuant to Regulation (EU) 2015/2283.
Abbreviations
- ADME
absorption, distribution, metabolism and excretion
- bw
body weight
- Caco‐2
colorectal adenocarcinoma cell line
- CHO
Chinese hamster ovary
- CFU
colony forming units
- CONTAM
Panel on Contaminants in the food chain
- DLS
dynamic light scattering
- DMT‐1
divalent metal transporter 1
- DRV
dietary reference value
- Dv
percentile of the volume‐based particle size distribution
- EDTA
Ethylenediaminetetraacetic acid
- EDX
energy dispersion X‐ray
- EELS
electron energy loss microscopy
- FAIM
Food Additive Intake Model
- Fpn
ferropontin
- FTIR
Fourier transform infrared spectroscopy
- GI
gastrointestinal
- GLP
good laboratory practice
- GMP
good manufacturing practice
- HACCP
hazard analysis and critical control points
- HAADF‐STEM
high‐angle annular dark‐field aberration‐corrected scanning transmission electron microscopy
- HPLC‐DAD
high‐performance liquid chromatography with diode‐array detector
- HS‐GC
headspace gas chromatography
- HT‐29
human colon cancer cell line
- HUTU 80
duodenum adenocarcinoma cell line
- HACCP
hazard analysis critical control points
- ICP‐MS
inductively coupled plasma mass spectrometry
- ICP‐OES
inductively coupled plasma optical emission spectrometry
- IHAT
iron hydroxide adipate tartrate
- ISC
in situ cytometry
- LOD
limit of detection
- LOQ
limit of quantification
- MN
mammalian cells micronucleus test
- MLA
mouse lymphoma cells assay
- MOE
margin of exposure
- NDA
Panel on Nutrition, Novel Foods and Food Allergens
- NOAEL
no observed adverse effect level
- NF
novel food
- OR
odds ratio
- Ph. Eur.
European Pharmacopeia
- PRI
population reference intake
- RBV
relative bioavailability value
- RH
relative humidity
- SCF
Scientific Committee on Food
- siRNA
small interfering RNA
- TAMC
total aerobic microbial count
- TDI
tolerable daily intake
- TEM
transmission electron microscopy
- TYMC
total yeast and mould count
- UL
tolerable upper intake level
- UV–VIS
ultraviolet–visible spectroscopy
- WT
wild type
- w/w
weight per weight
- XRD
X‐ray diffraction
Appendix A – Identity table according to EFSA Guidance on Nanomaterials (EFSA Scientific Committee, 2021)
1.
| Characteristic | Description/values | Method (when relevant) |
|---|---|---|
| Name |
Iron oxo‐hydroxide adipate tartrate IHAT |
n.a. |
| Description | IHAT is a tartrate‐modified, nano‐disperse Fe(III) oxohydroxide, formed in an adipate buffer, with similar functional properties and primary particle size as the iron found in the ferritin core. The tartrate‐modified ferrihydrite is precipitated from an Fe(III) chloride solution in the presence of sodium tartrate and adipate buffer. Fe(III)‐oxohydroxide nanocores are constrained from growth and crystallisation by being captured inside a corona of tartrate with some dispersion‐aiding adipic acid and tartaric acid mixed into the formulation. | ICP‐OES TEM and EDX XRD, STEM, FTIR, and EELS (see Powell et al., 2014) |
| Intended use | Dietary source of iron | n.a. |
| Material composition |
Hydrated matter: iron 28.0 (25.2–31.2) w/w %, tartaric acid 28.2 (26.7–31.0) w/w %, adipic acid 1.8 (1.7–2.1) w/w %, sodium 9.0 (8.7–9.7) w/w %, w/w %, chloride 3.1 (2.3–3.7) w/w %, and adsorbed water 15.9 (11.2–20.7) w/w %. The remainder (14%) is assumed to be structural hydrogen and oxygen, as per the accepted structure for 2‐line ferrihydrite (5Fe2O3 8H2O; Chappell et al., 2017). As dry matter: iron 33.2 (30.4–35.1) w/w %, tartaric acid 32.7 (28.9–35.0) w/w %, adipic acid 2.1 (1.9‐2.5) w/w %, sodium 10.7 (10.5–11.0) w/w %, chloride 3.6 (2.6–4.1) w/w %. |
Iron: ICP‐OES, UV.VIS Adipic and tartaric acids: HPLC‐DAD Sodium, chloride: ICP‐OES Water: Karl Fischer titration |
| Elemental composition | Primary nanoparticles have been shown to contain iron, oxygen, and carbon. | EDX |
| CAS number | 2460638‐28‐0 | n.a. |
| Molecular weight |
Average molecular weight: 35,803.4 Da (lower limit: 27,670.5 Da; upper limit: 45,319.4 Da). These results were obtained by modelling. The model was based on:
|
Modelled |
| Molecular formula |
FeOm(OH)n(H2O)x(C4H6O6)y(C 6 H 10 O 4 )z where: m and n are undefined as per accepted practice for ferric iron oxohydroxides (see Cornell and Schwertmann, 2003) x = 0.28–0.88 y = 0.78–1.50 z = 0.04–0.19 Tartaric acid (C4H6O6) and adipic acid (C6H10O4) are represented in their protonated form. |
Calculated |
| Constituent particle size |
Minimum external dimension by electron microscopy: Median diameter = 1.98 nm (uncertainty = 0.02 nm, 95% confidence level), width of distribution: mean absolute value (MAD) = 0.99 nm;
Mean diameter = 2.31 nm (uncertainty = 0.02 nm, 95% confidence level), width of distribution: standard deviation (SD) = 1.39 nm. |
Electron microscopy (HAADF‐STEM) |
| Particle shape |
Constituent particles of almost spherical shape. TEM micrographs at magnifications: (a) 250,000x (b) 400,000x. |
TEM |
| Structure |
Schematic molecular structure of IHAT (red: oxygen; white: hydrogen; black: carbon; brown: iron) |
n.a. |
| Specific surface area | Given that the median radius of IHAT particles is 1.55 nm (volume mean diameter 3.1 nm) and the density is 2.15 g/cm3, a value of 904 m2/g is obtained. | Calculated |
| Appearance | Red‐brown microsized powder. | n.a. |
| Density | 2.14 g/cm3 | Pycnometry |
| Surface charge | Zeta potential at pH 6.4: – 47.6 mV. | Electrophoretic light scattering |
| Solubility [g/L] | 2–4% in water (proportion of solute in solvent at room temperature, with regard to iron content). | n.a. |
| Agglomeration and/or aggregation state and size |
In water, the following phase distribution is observed by ultrafiltration and ICP‐OES analysis: 95.9% nanoparticulates, 2.8% soluble, 1.3% microparticulates. Nanoparticulates show the following size distribution by dynamic light scattering expressed as a function of volume (Dv nm; conversion from intensity of the scattering signal to volume [mean ± SD]): Dv(10): 1.9 ± 0.24 Dv(50): 3.1 ± 0.25 Dv(90): 5.4 ± 0.32 (refractive Index 1.920, Absorption = 0.10). Microparticulates show the following particle size distribution by laser diffraction (volume‐based particle size; µm [mean ± SD]): Dv(10): 26 ± 33.08 Dv(50): 547.7 ± 67.20 Dv(90): 1,286.6 ± 52.73. |
Ultrafiltration and ICP‐OES Dynamic light scattering Laser diffraction |
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Bohn T, Castenmiller J, De Henauw S, Ildico Hirsch‐Ernst K, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Frenzel T, Heinonen M, Prieto Maradona M, Marchelli R, Neuhäuser‐Berthold M, Poulsen M, Rudolf Schlatter J, van Loveren H, Germini A and Knutsen HK, 2021. Scientific Opinion on the safety of iron hydroxide adipate tartrate as a novel food pursuant to Regulation (EU) 2015/2283 and as a source of iron in the context of Directive 2002/46/EC. EFSA Journal 2021;19(12):6935, 31 pp. 10.2903/j.efsa.2021.6935
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00200
Panel members: Dominique Turck, Torsten Bohn, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: The EFSA NDA Panel wishes to thank the members of the EFSA Cross‐cutting WG nanotechnologies for the support provided to this scientific output.
Adopted: 27 October 2021
Notes
Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009.
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements.
EU Register on nutrition and health claims, accessible at https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives.
Commission Implementing Regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. OJ L 351, 30.12.2017, pp. 64–71.
In some of the studies assessed in the opinion, IHAT at different research stages is further referred to as: ligand‐modified Fe(III) poly oxo‐hydroxide, Fe : tartrate : adipate = 2:1:1; LM Fe(III) poly oxo‐hydroxide; nanoparticulate tartrate‐modified Fe(III) poly oxo‐hydroxide; nano Fe(III) [Nano Fe(III) poly oxo‐hydroxide : adipic acid : tartaric acid = 2:1:1]; nano Fe(III) poly oxo‐hydroxide adipate tartrate; nanodisperse fine ferrihydrite; nanoparticulate tartrate‐modified Fe(III) poly oxo‐hydroxide; nanoparticulate tartrate‐modified Fe(III) poly oxo‐hydroxide, Fe : tartrate : adipate = 2:1:1; tartrate‐modified ferrihydrite. For the purpose of this opinion, in the following sections, these products are referred to as IHAT and they are considered representative of the NF for the purpose of the assessment.
In the present scientific opinion, the term NF is used to describe the product as manufactured by the applicant, while IHAT is used to refer to the products used in the research studies prior to the industrialisation of the NF.
Conversion from ad libitum diet to mg/kg bw per day in rats as per (EFSA Scientific Committee, 2012).
Considered equivalent to the NF except for the iron content.
References
- Antico A and Soana R, 2015. Nickel sensitization and dietary nickel are a substantial cause of symptoms provocation in patients with chronic allergic‐like dermatitis syndromes. Allergy Rhinol (Providence), 6, 56–63. 10.2500/ar.2015.6.0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam MF, Frazer DM, Faria N, Bruggraber SF, Wilkins SJ, Mirciov C, Powell JJ, Anderson GJ and Pereira DI, 2014. Ferroportin mediates the intestinal absorption of iron from a nanoparticulate ferritin core mimetic in mice. The FASEB Journal, 28, 3671–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell HF, Thom W, Bowron DT, Faria N, Hasnip PJ and Powell JJ, 2017. Structure of naturally hydrated ferrihydrite revealed through neutron diffraction and first‐principles modeling. Physical Review Materials, 1. 036002. 10.1103/PhysRevMaterials.1.036002 [DOI] [Google Scholar]
- CHELAB , 2019. Study report N. 19.016575.0002. L5178Y Tk+/− Mouse Lymphoma Mutation Assay (MLA) according to OECD 490:2016 on IHAT (Iron Hydroxide Adipate Tartrate). CHELAB srl, Italy. Unpublished.
- CHELAB , 2020. Study report N. 20.512230.0002. Cytotoxicity test preparatory to Micronucleus Assay based on OECD 487:2016 on IHAT(Iron Hydroxide Adipate Tartrate). CHELAB srl, Italy. Unpublished.
- CHELAB , 2021. Study report 21.007709.0002. Test for in vitro mammalian Cell Micronucleus test according to OECD 487:2016 (Microscope Method) on IHAT (Iron Hydroxide Adipate Tartrate). CHELAB srl, Italy. Unpublished.
- Cornell RM and Schwertmann U, 2003. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses. 2nd Edition. Wiley. 10.1002/3527602097 [DOI] [Google Scholar]
- EcamRicert , 2021a. In vitro gastrointestinal dissolution of IHAT. Unpublished report. [Google Scholar]
- EcamRicert , 2021b. Physico‐chemical characterization of IHAT and its intracellular uptake in genotoxicity test conditions. 24/9/2021. Unpublished report.
- EcamRicert , 2021c. Physico‐chemical characterization of IHAT and its intracellular uptake in genotoxicity test conditions. 20/3/2021. Unpublished report.
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipic M, Frutos MJ, Galtier P, Gundert‐Remy U, Kuhnle GG, Lambre C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Di Domenico A, Fairweather‐Tait S, McArdle HJ, Smeraldi C and Gott D, 2018. Guidance on safety evaluation of sources of nutrients and bioavailability of nutrient from the sources (Revision 1). EFSA Journal 2021;19(3):6552, 39 pp. 10.2903/j.efsa.2021.6552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2015. Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA Journal 2015;13(2):4002, 202 pp. 10.2903/j.efsa.2015.4002 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Leblanc J‐C, Nebbia CS, Ntzani E, Petersen A, Schwerdtle T, Vleminckx C, Wallace H, Guérin T, Massanyi P, Van Loveren H, Baert K, Gergelova P and Nielsen E, 2020. Scientific Opinion on the update of the risk assessment of nickel in food and drinking water. EFSA Journal 2020;18(11):6268, 101 pp. 10.2903/j.efsa.2020.6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products Nutrition and Allergies) , 2004. Opinion of the Scientific Panel on Dietetic products, nutrition and allergies [NDA] related to the Tolerable Upper Intake Level of Iron. EFSA Journal 2004;2(11):125, 34 pp. 10.2903/j.efsa.2004.125 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2013. Scientific Opinion on Dietary Reference Values for energy. EFSA Journal 2013;11(1):3005, 112 pp. 10.2903/j.efsa.2013.3005 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products Nutrition and Allergies) , 2015. Scientific Opinion on Dietary Reference Values for iron. EFSA Journal 2015;13(10):4254, 115 pp. 10.2903/j.efsa.2015.4254 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2016. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA Journal 2016;14(11):4594, 24 pp. 10.2903/j.efsa.2016.4594 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 35 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , More S, Bampidis V, Benford D, Bragard C, Halldorsson T, Hernández‐Jerez A, Hougaard Bennekou S, Koutsoumanis K, Lambré C, Machera K, Naegeli H, Nielsen S, Schlatter J, Schrenk D, Silano V, Turck D, Younes M, Castenmiller J, Chaudhry Q, Cubadda F, Franz R, Gott D, Mast J, Mortensen A, Oomen AG, Weigel S, Barthelemy E, Rincon A, Tarazona J and Schoonjans R, 2021. Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health. EFSA Journal 2021;19(8):6768, 111 pp. 10.2903/j.efsa.2021.6768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen JS, Lamb DJ and Lipshultz LI, 2018. Iron and a man's reproductive health: the good, the bad, and the ugly. Current Urology Reports, 19, 60. 10.1007/s11934-018-0808-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- JM‐USDA , 2019. Safe iron study – Phase 1. Statistical analysis. Jean Mayer USDA Human Nutrition Research Center on aging (JM‐USDA HNRCA) at Tufts University, USA. Unpublished.
- Koch I, Reimer KJ, Bakker MI, Basta NT, Cave MR, Denys S, Dodd M, Hale BA, Irwin R, Lowney YW, Moore MM, Paquin V, Rasmussen PE, Repaso‐Subang T, Stephenson GL, Siciliano SD, Wragg J and Zagury GJ, 2013. Variability of bioaccessibility results using seventeen different methods on a standard reference material, NIST 2710. Journal of Environmental Science and Health, Part A, 48, 641–655. [DOI] [PubMed] [Google Scholar]
- Latunde‐Dada GO, Pereira DI, Tempest B, Ilyas H, Flynn AC, Aslam MF, Simpson RJ and Powell JJ, 2014. A nanoparticulate ferritin‐core mimetic is well taken up by HuTu 80 duodenal cells and its absorption in mice is regulated by body iron. Journal of Nutrition, 144, 1896–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EM, 2016. The reproductive ecology of iron in women. American Journal of Physical Anthropology, 159, 172–195. 10.1002/ajpa.22907 [DOI] [PubMed] [Google Scholar]
- Minekus M, Alminger M, Alvito P, Balance S, Bohn T, Bourlieu C, Carriere F, Boutrou R, Corredig M, Dupont D, Dufour C, Egger L, Golding M, Karakaya S, Kirkhus B, Le Feunteun S, Lesmes U, Macierzanka A, Mackie A, Marze S, McClements JS, Menard O, Recio I, Santos CN, Singh RP, Vegarud GE, Wickham MS, Weitschies W and Brodkorb A, 2014. A standardised static in vitro digestion method suitable for food ‐ an international consensus. Food Functions, 5, 1113–1124. [DOI] [PubMed] [Google Scholar]
- MRC (Medical Research Council) , 2013. Study report HNR6129. A pilot study to assess the influence of dietary organic acids on iron absorption. MRC, Human Nutrition Research, Cambridge, UK. Unpublished.
- MRC (Medical Research Council) , 2019. Study report SCC1489 clinical trial report. A novel nano‐iron supplement (IHAT) to safely combat iron deficiency and anaemia (IDA) in young children: a double‐blind randomised controlled trial. MRC, Unit The Gambia, London school of hygiene and tropical medicine, Unpublished. [Google Scholar]
- MRC (Medical Research Council) , 2020. Study report SCC1422. An exploratory study to determine bioavailability and transferrin saturation following a single dose of a novel iron supplement (IHAT) in Gambian women ‐ end of study report. MRC, Unit The Gambia, London school of hygiene and tropical medicine, Unpublished. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2016a. Test No. 487: In Vitro Mammalian Cell Micronucleus Test. In: OECD guidelines for the testing of chemicals, Section 4, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. 10.1787/9789264264861-en [DOI]
- OECD (Organisation for Economic Co‐operation and Development) , 2016b. Test No. 490: In Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. 10.1787/9789264264908-en [DOI]
- OECD (Organisation for Economic Co‐operation and Development) , 1998b. Test No. 407 Repeated Dose 28‐day Oral Toxicity Study in Rodents. In: Guideline for the Testing of Chemicals; Section 4: Health Effects. Revised version, adopted 21st September. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 1998c. Test No. 408: Repeated dose 90‐day oral toxicity study in rodents. In: OECD guidelines for the testing of chemicals, Section 4: Health effects. Revised version, adopted 21st September [Google Scholar]
- OECD(Organisation for Economic Co‐operation and Development , 1998a. OECD Principles of good laboratory practice (as revised in 1997). OECD series on principles of good laboratory practice and compliance monitoringnumber 1, ENV/MC/CHEM(98)17. 41 pp. [Google Scholar]
- Pelfrene A, Cave MR, Wragg J and Douay F, 2017. In vitro investigations of human bioaccessibility from reference materials using simulated lung fluids. International Journal of Environmental Research and Public Health, 14, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira DI, Bruggraber SF, Faria N, Poots LK, Tagmount MA, Aslam MF, Frazer DM, Vulpe CD, Anderson GJ and Powell JJ, 2014. Nanoparticulate iron(III) oxo‐hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomedicine, 10, 1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira DIA, Mohammed NI, Ofordile O, Camara F, Baldeh B, Mendy T, Sanyang C, Jallow AT, Hossain I, Wason J and Prentice AM, 2018. A novel nano‐iron supplement to safely combat iron deficiency and anaemia in young children: The IHAT‐GUT double‐blind, randomised, placebo‐controlled trial protocol. Gates Open Res, 2, 48. 10.12688/gatesopenres.12866.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira DI, Mergler BI, Faria N, Bruggraber SF, Aslam MF, Poots LK, Prassmayer L, Lonnerdal B, Brown AP and Powell JJ, 2013. Caco‐2 cell acquisition of dietary iron(III) invokes a nanoparticulate endocytic pathway. PLoS One, 8, e81250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JJ, Bruggraber SF, Faria N, Poots LK, Hondow N, Pennycook TJ, Latunde‐Dada GO, Simpson RJ, Brown AP and Pereira DI, 2014. A nano‐disperse ferritin‐core mimetic that efficiently corrects anemia without luminal iron redox activity. Nanomedicine, 10, 1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi EM, Marques VB, Nunes DO, Carneiro MTWD, Podratz PL, Merlo E, dos Santos L and Graceli JB, 2016. Acute iron overload leads to hypothalamic‐pituitary‐gonadal axis abnormalities in female rats. Toxicology Letters, 240, 196–213. 10.1016/j.toxlet.2015.10.027 [DOI] [PubMed] [Google Scholar]
- Tonai S, Kawabata A, Nakanishi T, Lee JY, Okamoto A, Shimada M and Yamashita Y, 2020. Iron deficiency induces female infertile in order to failure of follicular development in mice. Journal of Reproduction and Development, 66, 475–483. 10.1262/jrd.2020-074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Cambridge, Department of Veterinary Medicine , 2019a. Study to assess the particulate state of IHAT under the conditions of the upper gastrointestinal tract. Unpublished.
- University of Cambridge, Department of Veterinary Medicine , 2019b. Study to assess the particulate state of IHAT under lysosomal. Unpublished.
- Vivo Science , 2019. Study report N25‐001. Repeated dose toxicity study of Iron Hydroxide Adipate Tartrate [IHAT] (OECD 408) ‐ 90‐day oral toxicity test in Wistar rats extended by some endocrine endpoints of OECD 407. Vivo Science GmbH. Germany. Unpublished.