Abstract
Background: Since the 1990s, there have been a lot of research on single-nucleotide polymorphism (SNP) and different diseases, including many studies on 5,10-methylenetetrahydrofolate reductase (MTHFR) polymorphism and essential hypertension (EH). Nevertheless, their conclusions were controversial. So far, six previous meta-analyses discussed the internal relationship between the MTHFR polymorphism and EH, respectively. However, they did not evaluate the credibility of the positive associations. To build on previous meta-analyses, we updated the literature by including previously included papers as well as nine new articles, improved the inclusion criteria by also considering the quality of the papers, and applied new statistical techniques to assess the observed associations. Objectives: This study aims to explore the degree of risk correlation between two MTHFR polymorphisms and EH. Methods: PubMed, EMBASE, the Cochrane Library, CNKI, and Wan Fang electronic databases were searched to identify relevant studies. We evaluated the relation between the MTHFR C677T (rs1801133) and A1298C (rs1801131) polymorphisms and EH by calculating the odds ratios (OR) as well as 95% confidence intervals (CI). Here we used subgroup analysis, sensitivity analysis, cumulative meta-analysis, assessment of publication bias, meta-regression meta, False-positive report probability (FPRP), Bayesian false discovery probability (BFDP), and Venice criterion. Results: Overall, harboring the variant of MTHFR C677T was associated with an increased risk of EH in the overall populations, East Asians, Southeast Asians, South Asians, Caucasians/Europeans, and Africans. After the sensitivity analysis, positive results were found only in the overall population (TT vs. CC: OR = 1.14, 95% CI: 1.00–1.30, P h = 0.032, I 2 = 39.8%; TT + TC vs. CC: OR = 1.15, 95% CI: 1.01–1.29, P h = 0.040, I 2 = 38.1%; T vs. C: OR = 1.14, 95% CI: 1.04–1.25, P h = 0.005, I 2 = 50.2%) and Asian population (TC vs. CC: OR = 1.14, 95% CI: 1.01–1.28, P h = 0.265, I 2 = 16.8%; TT + TC vs. CC: OR = 1.17, 95% CI: 1.04–1.30, P h = 0.105, I 2 = 32.9%; T vs. C: OR = 1.10, 95% CI: 1.02–1.19, P h = 0.018, I 2 = 48.6%). However, after further statistical assessment by FPRP, BFDP, and Venice criteria, the positive associations reported here could be deemed to be false-positives and present only weak evidence for a causal relationship. In addition, when we performed pooled analysis and sensitivity analysis on MTHFR A1298C; all the results were negative. Conclusion: The positive relationships between MTHFR C677T and A1298C polymorphisms with the susceptibility to present with hypertension were not robust enough to withstand statistical interrogation by FPRP, BFDP, and Venice criteria. Therefore, these SNPs are probably not important in EH etiology.
Keywords: MTHFR, Rs1801133, Rs1801131, Essential hypertension, FPRP, BFDP, Venice criteria
Introduction
Essential hypertension (EH) is a common disease in the world and a threat to the health of people. If blood pressure is not well controlled, it can lead to serious consequences, such as coronary heart disease, enlarged heart, heart failure, cerebral hemorrhage, optic papillary edema, and renal insufficiency. At the same time, chronic high blood pressure could lead to clinical symptoms such as dizziness, headache, chest pain, tinnitus, vomiting, palpitation, and blurred vision (O'Donnell et al., 1998). EH is also called primary hypertension because its etiology is not clear, and there is, so far, no complete understanding thereof (Bähr et al., 2003). It is the result of the interaction of various environmental factors and genetic factors. The former mainly includes diet, smoking, mental stress, and lifestyle. The latter includes contributors such as obesity, family history, and genetic variations or polymorphisms (Singh et al., 2016). In recent years, many genes related to hypertension have been reported, and relevant evidence suggested that genetic factors accounted for about 25–65% of the proportion in those with hypertension (Krzesinski and Saint-Remy, 2012). Therefore, genetic factors are critical to explore as a possible internal cause of this disease. In Europe, Newton-Cheh et al. conducted a genome-wide association study (GWAS) which used blood pressure as a continuous trait (Newton-Cheh et al., 2009). Through a two-stage meta-analysis, three loci associated with systolic blood pressure (MTHFR, CYP17A1, and PLCD3) and five diastolic blood pressure loci (FGF5, C10orf107, SH2B3, CYP1A2, and ZNF652) were identified at the genome-wide level. This is of great significance for the genetic research of EH (Rafiq et al., 2010).
Studies have shown that hyperhomocysteinemia (hHcy) is an important risk factor for hypertension. 5,10-Methylenetetrahydrofolate reductase (MTHFR) plays an important role in folate metabolism–methionine cycle. Together with methionine synthase reductase (MTRR), it maintains the normal metabolism of folate and participates in the maintenance of normal homocysteine (Hcy) levels in the body. Specific MTHFR gene mutations can lead to a decrease in the activity of key enzymes and disorders in folate metabolism, which increased the demand for folate to maintain Hcy methylation to methionine (Met) and ultimately lead to the increase of Hcy level and hHcy (Ren et al., 2018). The human MTHFR gene is located in autosomal 1p36.3. There are a variety of mutation types and multiple mutation sites in the MTHFR gene, of which C677T (rs1801133) and A1298C (rs1801131) are two common gene sites. The 677C-T gene mutation is located in exon 4 of the catalytic activity of the N-terminal region, where cytosine is replaced by thymine, and the corresponding protein is changed from alanine (Ala) to valine (Val). The 1298A-C mutation is located in the C-terminal regulatory region of exon 7, where adenosine mutates to cytosine, causing the glutamate (Glu) encoding to be replaced by alanine (Ala) (Weisberg et al., 2001; Chen et al., 2009; Nursal et al., 2018). Globally, the ethnic and geographic distribution of these two loci are significantly different. In 2010, approximately 1.39 billion people suffered from EH, especially in low- and middle-income countries (Kearney et al., 2005; Yang and Gu, 2016; Gheorghe et al., 2018; Mills et al., 2020). Therefore, EH was a major risk factor contributing to the global burden of disease (Falaschetti et al., 2014). Although many studies have elucidated the pathogenesis, the associations between the MTHFR SNPs and blood pressure were unclear (Doris, 2002; Staessen et al., 2003). Studies have shown that EH was related to the inheritance of a variety of susceptibility genes.
Researchers have explored the susceptibility conveyed via the MTHFR gene polymorphisms to develop EH. There are many published meta-analyses, but the conclusions were controversial (Qian et al., 2007; Niu et al., 2012; Yang B. et al., 2014; Yang K.-M. et al., 2014; Wu et al., 2014; Fu et al., 2019). Researchers have also continued to conduct various population-based studies wherein they explored the relationship between specific MTHFR gene variations and EH, but the results were different (Nishio et al., 1996; Sohda et al., 1997; Nakata et al., 1998; Gao et al., 1999; Powers et al., 1999; Zhan et al., 2000a; Zhan et al., 2000b; Kobashi et al., 2000; Li et al., 2000; Rajkovic et al., 2000; Zusterzeel et al., 2000; Benes et al., 2001; Kahleová et al., 2002; Wang et al., 2002; Rodríguez-Esparragón et al., 2003; Sun et al., 2003; Frederiksen et al., 2004; Heux et al., 2004; Liu et al., 2004; Yilmaz et al., 2004; Cesari et al., 2005; Liu et al., 2005; Tylicki et al., 2005; Demir et al., 2006; Hu et al., 2006; Kalita et al., 2006; Li and Huang, 2006; Lwin et al., 2006; Deng, 2007; Hu et al., 2007; Hui et al., 2007; Marinho et al., 2007; Markan et al., 2007; Nagy et al., 2007; Tang et al., 2007; Xing and Hua, 2007; Canto et al., 2008; Fridman et al., 2008; Ilhan et al., 2008; Lin et al., 2008; Luo et al., 2008; Soares et al., 2008; Cai and Gong, 2009; Deshmukh et al., 2009; Fakhrzadeh et al., 2009; Ng et al., 2009; Wang et al., 2010a; Wang et al., 2010b; Yu, 2010; Liu et al., 2011a; Liu et al., 2011b; Demirel et al., 2011; Jin et al., 2011; Ma and Yang, 2011; Mendilcioglu et al., 2011; Su et al., 2011; Alghasham et al., 2012; Cao, 2012; Fowdar et al., 2012; Yin et al., 2012; Zhang et al., 2012; Bayramoglu et al., 2013; Fridman et al., 2013; Yang et al., 2013; Yao et al., 2013; Cai et al., 2014; Husemoen et al., 2014; Vazquez-Alaniz et al., 2014; Bayramoglu et al., 2015; Nassereddine et al., 2015; Pérez-Razo et al., 2015; Wang et al., 2015; Wei et al., 2015; Wen et al., 2015; Amrani-Midoun et al., 2016; Fan et al., 2016; Ghogomu et al., 2016; Wu and Xu, 2016; Dwivedi and Sinha, 2017; Rios et al., 2017; Zhang et al., 2017; Zhao et al., 2017; Arina et al., 2019; Liu et al., 2019; Nong et al., 2019; Wu et al., 2019; Zhao et al., 2019; Cai et al., 2020; Candrasatria et al., 2020)—for example, three studies (Nakata et al., 1998; Wen et al., 2015; Fan et al., 2016) indicated that C677T was a risk gene locus for EH. However, other studies have found no correlation between them (Rodríguez-Esparragón et al., 2003; Amrani-Midoun et al., 2016). Similarly, two studies (Kahleová et al., 2002; Alghasham et al., 2012) reported the correlation between A1298C and EH, but others (Ng et al., 2009; Wei et al., 2015) believed that A1298C did not play a role in the pathogenesis of EH. Because of the controversies in the field, several meta-analyses incorporating relevant studies were conducted, but there were still some shortcomings in the methods of these systematic summaries with statistical analysis of the available literature. Firstly, there were obvious errors in incorporating certain studies that included hypertension experienced in pregnancy within two of the existing meta-analyses. The latter is problematic because extrapolating the results of these meta-analyses to apparently healthy individuals of the general population will not be permissible—for example, 11 studies on pregnancy-induced hypertension were mistakenly included in a meta-analysis (Fu et al., 2019), as described below (Powers et al., 1999; Kobashi et al., 2000; Li et al., 2000; Rajkovic et al., 2000; Zusterzeel et al., 2000; Yilmaz et al., 2004; Demir et al., 2006; Nagy et al., 2007; Canto et al., 2008; Vazquez-Alaniz et al., 2014; Rios et al., 2017). Similarly, authors of another meta-analysis (Yang B. et al., 2014) also mistakenly included four studies on hypertensive disorders of pregnancy (Sohda et al., 1997; Yu, 2010; Mendilcioglu et al., 2011; Su et al., 2011). Secondly, several meta-analyses authors did not carry out literature quality assessment (Qian et al., 2007; Niu et al., 2012). Finally, they did not assess the reliability of the statistical association and the levels of cumulative epidemiological evidence (Qian et al., 2007; Niu et al., 2012; Yang B. et al., 2014; Yang K.-M. et al., 2014; Wu et al., 2014; Fu et al., 2019). Therefore, we addressed the shortcomings of the previous meta-analyses investigating the relationships between the common MTHFR SNPs at loci 677 and 1298 with hypertension. Additionally, we included evidence from new case–control studies that were not included previously but increased the sample size and the reliability of our findings.
Materials and Methods
Search Strategy
Databases, including PubMed, EMBASE, the Cochrane Library, CNKI, and Wan Fang databases, were searched for evidence between the carrier status of the MTHFR gene variants and susceptibility to EH. The retrieval strategy was as follows: (MTHFR C677T OR rs1801133 OR Ala222Val) AND (MTHFR A1298C OR rs1801131 OR Glu429Ala) AND (polymorphism OR mutation OR variant OR genotype) AND (essential hypertension OR hypertension OR EH OR blood pressure). Two researchers who were familiar with the literature search process and meta-analysis methods conducted a literature search to identify all possible original studies. The search deadline was December 2020.
Selection Criteria
The inclusion criteria were as follows: (1) case–control study, (2) complete study of genotype data, and (3) study exploring the relationship between MTHFR polymorphisms and EH.
The exclusion criteria were as follows: (1) unrelated diseases or other diseases associated with hypertension or secondary hypertension, (2) incomplete study of genotype data and genotype frequency, (3) duplicate study, and (4) letters, reviews, animal experiments, other analysis.
Data Extraction
According to the abovementioned inclusion and exclusion criteria, two researchers searched independently the abovementioned databases and finally checked them to ensure a comprehensive search and information extraction of all relevant studies. If there was a difference in opinion, it would be discussed with a third researcher. The extraction will be based on the following information: last name of the first author, publication year, country, geographical location, ethnicity of the study subjects, source of the case group, source of the control group, matching, diagnostic criteria for EH, genotype data in cases and controls, sample size, genotype examination, and adjustments.
Quality Assessment
All studies were independently evaluated and verified by two investigators. According to previously published articles (Thakkinstian et al., 2011; Xue et al., 2014) together with the characteristics of this study, a quality assessment table was designed. The maximum score a study could achieve based on the quality assessment criteria was 20. We regarded studies achieving a score of 12 and higher as good-quality research. Those scoring below 12 were not eligible for inclusion based on the low quality thereof. The specific evaluation criteria are shown in Table 1.
TABLE 1.
Scale for the quality assessment of molecular association studies of essential hypertension.
| Criterion | Score |
|---|---|
| Source of case | |
| Selected from population | 2 |
| Selected from hospital | 1 |
| Not described | 0 |
| Source of control | |
| Population-based | 3 |
| Blood donors or volunteers | 2 |
| Hospital-based | 1 |
| Not described | 0 |
| Ascertainment of essential hypertension | |
| International diagnostic criteria | 2 |
| Regional diagnostic criteria | 1 |
| Not described | 0 |
| Ascertainment of control | |
| Controls were tested to screen out EH | 2 |
| Controls were subjects who did not report EH, no objective testing | 1 |
| Not described | 0 |
| Matching | |
| Controls matched with cases by age and sex | 2 |
| Controls matched with cases only by age or sex | 1 |
| Not matched or not described | 0 |
| Genotyping examination | |
| Genotyping done blindly and quality control | 2 |
| Only genotyping done blindly or quality control | 1 |
| Unblinded and without quality control | 0 |
| HWE | |
| HWE in the control group | 2 |
| Hardy–Weinberg disequilibrium in the control group | 1 |
| Not described | 0 |
| Association assessment | |
| Assess association between genotypes and EH with appropriate statistics and adjustment for confounders | 2 |
| Assess association between genotypes and EH with appropriate statistics without adjustment for confounders | 1 |
| Inappropriate statistics used | 0 |
| Total sample size | |
| >1,000 | 3 |
| 500–1,000 | 2 |
| 200–500 | 1 |
| <200 | 0 |
HWE, Hardy–Weinberg equilibrium; EH, essential hypertension.
Credibility Analysis
The reliability of the statistically significant association was evaluated by false-positive reporting probability (FPRP), Bayesian false discovery probability (BFDP) test, and Venice criterion (Ioannidis et al., 2008). When FPRP <0.2 and BFDP <0.8, based on a predetermined prior probability of 0.001, the results of the genetic association were valid, indicating that the association might be true (Wakefield, 2007). The Venice criterion evaluated the credibility of cumulative results in terms of validity of evidence, reproducibility of studies, and bias control. The evaluation indicators were as follows: (1) evidence validity (n: genotype sample size): A: n ≥ 1,000; B: 100 ≤ n < 1,000; C: n < 100; (2) reproducibility: A: I 2 <25%; B: I 2 >25% and I 2 < 50%; C: I 2 >50% or higher; and (3) bias control: A: no bias; B: no obvious bias but data is missing; C: obvious bias. A, B, and C represent strong, moderate, and weak cumulative epidemiological evidence, respectively (Wakefield, 2007; Ioannidis et al., 2008).
Statistical Analysis
Chi-square goodness-of-fit test was used to perform Hardy–Weinberg equilibrium (HWE) on the included control group and to select the studies conforming to HWE. We calculated the combined odds ratio (OR) and 95% confidence intervals (CI) to determine the association between MTHFR gene polymorphisms and EH. Evaluation of heterogeneity was by Cochran Q test and I 2 test. When P >0.10 and/or I 2 ≤50%, a fixed-effect model was selected to combine the effect sizes. When P ≤0.10 and/or I 2 >50%, a random-effects model was selected (Higgins et al., 2003). Allelic model, dominant model, recessive model, heterozygote model, and homozygote genetic model were used for the evaluation (Fu et al., 2019). We could identify the sources of heterogeneity in the following ways: (1) meta-regression was performed on the factors that might lead to heterogeneity in the study itself; (2) according to ethnicity, geographical distribution, and genetic testing quality, a subgroup analysis was conducted; and (3) we conducted a sensitivity analysis by excluding individual studies one by one and judged the publication bias by Begg’s funnel plot and Egger test. If there was publication bias, we would use the trim-and-fill method to correct it (Zhang et al., 2018). Cumulative meta-analysis of included literature was performed to dynamically observe the results (Lau et al., 1992). STATA12.0 software was used for statistical analysis, and P <0.05 was considered to be statistically significant.
Results
Description of the Included Studies
Based on STREGA and PRISMA (Swartz, 2011), we carried out this research. According to the retrieval method, we searched PubMed, Embase, the Cochrane Library, CNKI, and Wan Fang electronic databases and obtained 930 articles. According to strict inclusion and exclusion criteria, 66 original literatures were finally included in this study, including 63 articles on C677T polymorphism and 11 articles on A1298C polymorphism (see Figure 1 for the detailed flow chart of the included literature).
FIGURE 1.
Flow chart of literature selection for the current meta-analysis.
Basic Characteristics and Quality Evaluation of Literature
A total of 66 articles were included, of which 63 were about MTHFR C677T and EH and 11 were about MTHFR A1298C. The results of the quality assessment were 25 high-quality studies and 41 low-quality studies. There were 58 studies in the control group that complied with HWE. The specific features of the included literature are shown in Table 2. The detailed genotype distribution is shown in Table 3 and Table 4.
TABLE 2.
General characteristics of the included studies and literature quality scores.
| First author/Year | Country | Geographic region | Ethnicity | Source of cases | Source of controls | Matching | Sex | Quality scores |
|---|---|---|---|---|---|---|---|---|
| C677T | ||||||||
| Nishio [22] 1996 | Japan | EA | A | HB | HB | No | M | 7 |
| Nakata [24] 1998 | Japan | EA | A | HB | HB | Age, sex | – | 10 |
| Gao [25] 1999 | China | EA | EA | PB | PB | No | M/W | 13 |
| Zhan [30] 2000 | China | EA | A | PB | PB | No | M/W | 13 |
| Zhan [31] 2000 | China | EA | EA | PB | PB | No | M/W | 13 |
| Benes [33] 2001 | Czech Republic | E | C | HB | PB | No | M/W | 8 |
| Kahleova [34] 2002 | Czech Republic | E | C | HB | Volunteers | No | M/W | 9 |
| Wang [35] 2002 | China | EA | A | HB | HB | No | M/W | 7 |
| Rodríguez-Esparragón [36] 2003 | Spain | E | C | PB | PB | Age, sex | M/W | 14 |
| Sun [37] 2003 | China | EA | A | HB | HB | No | M/W | 8 |
| Heux [39] 2004 | Australia | O | C | NR | NR | Age, sex | M/W | 11 |
| Liu [41] 2004 | China | EA | A | PB | PB | No | M/W | 12 |
| Cesari [42] 2005 | Italy | E | C | NR | NR | No | M/W | 8 |
| Liu [43] 2005 | China | EA | EA | PB | PB | No | M/W | 12 |
| Tylicki [44] 2005 | Austria/Poland | O | C | HB | HB | Age, sex | – | 12 |
| Hu [46] 2006 | China | EA | A | PB | PB | Age, sex | M/W | 15 |
| Li [48] 2006 | China | EA | A | HB | HB | No | M/W | 7 |
| Lwin [49] 2006 | Japan | EA | A | PB | PB | No | M | 11 |
| Hu [50] 2007 | China | EA | EA | PB | PB | Age, sex | M/W | 15 |
| Markan [52] 2007 | India | SA | A | HB | HB | Age, sex | M/W | 12 |
| Tang [54] 2007 | China | EA | A | HB | HB | No | M/W | 10 |
| Xing [55] 2007 | China | EA | A | HB | PB | Age | M/W | 15 |
| Hui [56] 2007 | Japan | EA | A | PB | PB | No | M/W | 13 |
| Deng [57] 2007 | China | EA | A | HB | PB | Age, sex | M/W | 14 |
| Fridman [59] 2008 | Argentina | Af | M | HB | HB | No | M/W | 10 |
| Ilhan [60] 2008 | Turkey | WA | C | HB | HB | Age | M/W | 9 |
| Lin [61] 2008 | China | EA | A | HB | HB | Age, sex | M/W | 11 |
| Luo [62] 2008 | China | EA | A | HB | HB | No | M/W | 10 |
| Soares [63] 2008 | Brazil | SAm | M | NR | Volunteers | Age, sex | M/W | 11 |
| Deshmukh [65] 2009 | United States | NAm | C | PB | Volunteers | No | M/W | 9 |
| Fakhrzadeh [66] 2009 | Iran | WA | C | PB | PB | No | M/W | 11 |
| Ng [67] 2009 | Australia | E | C | PB | PB | No | M/W | 10 |
| Liu [69] 2011 | China | EA | A | HB | HB | No | M/W | 9 |
| Liu H [70] 2011 | China | EA | A | HB | HB | No | M/W | 10 |
| Jin [72] 2011 | China | EA | A | HB | HB | Age, sex | M/W | 12 |
| Ma [73] 2011 | China | EA | A | HB | HB | No | M/W | 8 |
| Cao [75] 2012 | China | EA | A | HB | HB | Age, sex | M/W | 12 |
| Fowdar [76] 2012 | Australia | O | C | PB | PB | Age, sex | M/W | 15 |
| Yin [77] 2012 | China | EA | A | PB | PB | Age, sex | M/W | 16 |
| Zhang [78] 2012 | China | EA | A | HB | PB | No | M/W | 12 |
| Alghasham [79] 2012 | Saudi Arabia | WA | C | HB | HB | No | M/W | 7 |
| Bayramoglu [80] 2013 | Turkey | WA | C | HB | HB | No | M/W | 7 |
| Fridman [81] 2013 | Argentina | Af | C | HB | HB | No | M/W | 10 |
| Yao [82] 2013 | China | EA | A | HB | HB | Age, sex | M/W | 10 |
| Yang [83] 2013 | China | EA | A | HB | HB | No | M/W | 11 |
| Cai [84] 2014 | China | EA | EA | PB | PB | No | M/W | 14 |
| Bayramoglu [87] 2015 | Turkey | WA | C | HB | HB | No | M/W | 7 |
| Nassereddine [88] 2015 | Morocco | Af | C | HB | HB | Age, sex | M/W | 12 |
| Wei [90] 2015 | Malaysia | SEA | A | HB | HB | No | M/W | 11 |
| Wen [91] 2015 | China | EA | A | NR | NR | No | M/W | 8 |
| Pérez-Razo [92] 2015 | Mexico | NAm | M | HB | Blood donors | No | M/W | 13 |
| PB | PB | Age, sex | M/W | 15 | ||||
| Amrani-Midoun [93] 2016 | Argentina | Af | C | HB | Blood donors | No | M/W | 9 |
| Fan [94] 2016 | China | EA | A | HB | HB | No | M/W | 11 |
| Dwivedi [95] 2017 | India | SA | A | HB | HB | No | – | 6 |
| Wu [98] 2016 | China | EA | A | HB | HB | No | M/W | 7 |
| Ghogomu [99] 2016 | Cameroun | Af | Af | HB | PB | No | M/W | 10 |
| Zhang [100] 2017 | China | EA | A | HB | HB | No | M/W | 8 |
| Zhao [101] 2017 | China | EA | A | HB | HB | No | M/W | 11 |
| Liu [102] 2019 | China | EA | A | PB | PB | No | M/W | 16 |
| Nong [103] 2019 | China | EA | A | PB | PB | No | M/W | 11 |
| Wu [104] 2019 | China | EA | A | HB | HB | No | M/W | 9 |
| Zhao [105] 2019 | China | EA | A | HB | PB | No | M/W | 10 |
| Candrasatria [106] 2020 | Indonesia | SEA | A | PB | PB | No | M/W | 13 |
| A1298C | ||||||||
| Kahleova [34] 2002 | Czech Republic | E | C | HB | Volunteers | No | M/W | 9 |
| Tylicki [44] 2005 | Austria/Poland | O | C | HB | HB | Age, sex | – | 12 |
| Markan [52] 2007 | India | SA | A | HB | HB | Age, sex | M/W | 11 |
| Ng [67] 2009 | Australia | E | C | PB | PB | No | M/W | 10 |
| Wang [107] 2010 | China | EA | A | PB | PB | No | M/W | 13 |
| Wang [108] 2010 | China | EA | A | PB | PB | No | M/W | 15 |
| Demirel [109] 2011 | Turkey | WA | C | PB | PB | No | M/W | 8 |
| Fowdar [76] 2012 | Australia | O | C | PB | PB | Age, sex | M/W | 15 |
| Alghasham [79] 2012 | Saudi Arabia | WA | C | HB | HB | No | M/W | 7 |
| Wei [90] 2015 | Malaysia | SEA | A | HB | HB | No | M/W | 11 |
| Liu [102] 2019 | China | EA | A | PB | PB | No | M/W | 16 |
HB, hospital-based study; PB, population-based study; EA, East Asia; SA, South Asia; WA, Western Asia; SEA, Southeast Asia; O, Oceania; E, Europe; Af, Africa; SAm, South America; NAm, North America; A, Asian; C, Caucasian; M, mixed; M/W, men/women.
TABLE 3.
The genotype distribution and HWE of MTHFR C677T polymorphism in this meta-analysis. The bold value is C/T T/T A/C C/C genotype data is incomplete, and HWE evaluation cannot be performed.
| First author/Year | Number of samples | Genotype of cases | Genotype of controls | HWE | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cases/controls | C/C | C/T | T/T | C/C | C/T | T/T | Chi | p | |
| Nishio [22] 1996 | 47/82 | 16 | 26 | 5 | 29 | 44 | 9 | 1.631 | 0.2015 |
| Nakata [24] 1998 | 173/184 | 63 | 91 | 19 | 65 | 83 | 36 | 1.031 | 0.3100 |
| Gao [25] 1999 | 127/170 | 44 | 68 | 15 | 62 | 84 | 24 | 0.275 | 0.6001 |
| Zhan [30] 2000 | 127/170 | 44 | 68 | 15 | 62 | 84 | 24 | 0.275 | 0.6001 |
| Zhan [31] 2000 | 127/170 | 44 | 68 | 15 | 62 | 84 | 24 | 0.275 | 0.6001 |
| Benes [33] 2001 | 193/209 | 73 | 93 | 27 | 86 | 106 | 17 | 4.005 | 0.0454 |
| Kahleova [34] 2002 | 164/173 | 82 | 55 | 27 | 86 | 69 | 18 | 0.553 | 0.4570 |
| Wang [35] 2002 | 105/46 | 17 | 51 | 37 | 14 | 23 | 9 | 0.007 | 0.9354 |
| Rodríguez-Esparragón [36] 2003 | 232/215 | 83 | 115 | 34 | 95 | 100 | 20 | 0.751 | 0.3861 |
| Sun [37] 2003 | 55/46 | 6 | 22 | 27 | 14 | 23 | 9 | 0.007 | 0.9354 |
| Heux [39] 2004 | 247/249 | 87 | 125 | 35 | 105 | 119 | 25 | 1.080 | 0.2988 |
| Liu [41] 2004 | 100/100 | 29 | 45 | 26 | 31 | 50 | 19 | 0.021 | 0.8838 |
| Cesari [42] 2005 | 100/101 | 36 | 39 | 25 | 32 | 49 | 19 | 0.001 | 0.9748 |
| Liu [43] 2005 | 100/100 | 29 | 45 | 26 | 31 | 50 | 19 | 0.021 | 0.8838 |
| Tylicki [44] 2005 | 90/90 | 40 | 39 | 11 | 42 | 38 | 10 | 0.100 | 0.7517 |
| Hu [46] 2006 | 157/115 | 75 | 55 | 33 | 61 | 42 | 12 | 1.330 | 0.2488 |
| Li [48] 2006 | 26/30 | 18 | 6 | 2 | 21 | 7 | 2 | 1.462 | 0.2266 |
| Lwin [49] 2006 | 116/219 | 39 | 58 | 19 | 64 | 117 | 38 | 1.537 | 0.2151 |
| Hu [50] 2007 | 110/115 | 55 | 39 | 16 | 61 | 42 | 12 | 1.330 | 0.2488 |
| Markan [52] 2007 | 153/133 | 105 | 40 | 8 | 105 | 28 | 0 | 1.841 | 0.1749 |
| Tang [54] 2007 | 252/195 | 139 | 93 | 20 | 138 | 51 | 6 | 0.232 | 0.6298 |
| Xing [55] 2007 | 686/509 | 202 | 184 | 300 | 182 | 105 | 222 | 174.111 | 0 |
| Hui [56] 2007 | 261/271 | 83 | 129 | 49 | 104 | 123 | 44 | 0.560 | 0.4542 |
| Deng [57] 2007 | 151/138 | 108 | 35 | 8 | 91 | 40 | 7 | 0.863 | 0.3529 |
| Fridman [59] 2008 | 40/86 | 15 | 21 | 4 | 39 | 38 | 9 | 0.003 | 0.9545 |
| Ilhan [60] 2008 | 78/100 | 36 | 32 | 10 | 72 | 26 | 2 | 0.038 | 0.8445 |
| Lin [61] 2008 | 50/123 | 19 | 27 | 4 | 73 | 44 | 6 | 0.037 | 0.8479 |
| Luo [62] 2008 | 442/195 | 260 | 151 | 31 | 138 | 51 | 6 | 0.232 | 0.6298 |
| Soares [63] 2008 | 12/16 | 3 | 9 | 0 | 9 | 5 | 2 | 0.825 | 0.3638 |
| Deshmukh [65] 2009 | 42/118 | 22 | 16 | 4 | 52 | 48 | 18 | 1.501 | 0.2205 |
| Fakhrzadeh [66] 2009 | 160/76 | 99 | 44 | 17 | 36 | 31 | 9 | 0.335 | 0.5628 |
| Ng [67] 2009 | 38/80 | 14 | 14 | 10 | 40 | 32 | 8 | 0.181 | 0.6702 |
| Liu [69] 2011 | 155/140 | 58 | 70 | 27 | 74 | 47 | 19 | 5.943 | 0.0148 |
| Liu H [70] 2011 | 146/112 | 54 | 59 | 33 | 61 | 39 | 12 | 2.155 | 0.1421 |
| Jin [72] 2011 | 405/400 | 215 | 140 | 50 | 204 | 144 | 52 | 10.047 | 0.0015 |
| Ma [73] 2011 | 122/45 | 6 | 115 | 1 | 0 | 44 | 1 | 41.172 | 0 |
| Cao [75] 2012 | 112/147 | 33 | 53 | 26 | 49 | 68 | 30 | 0.514 | 0.4736 |
| Fowdar [76] 2012 | 377/393 | 170 | 174 | 33 | 175 | 183 | 35 | 1.746 | 0.1863 |
| Yin [77] 2012 | 670/682 | 244 | 358 | 68 | 322 | 309 | 51 | 3.946 | 0.0470 |
| Zhang [78] 2012 | 189/165 | 128 | 53 | 8 | 117 | 41 | 7 | 1.835 | 0.1755 |
| Alghasham [79] 2012 | 26/250 | 18 | 8 | 185 | 65 | – | – | ||
| Bayramoglu [80] 2013 | 125/99 | 65 | 38 | 22 | 56 | 38 | 5 | 0.200 | 0.6543 |
| Fridman [81] 2013 | 75/150 | 29 | 40 | 6 | 71 | 64 | 15 | 0.011 | 0.9174 |
| Yao [82] 2013 | 150/150 | 32 | 69 | 49 | 61 | 67 | 22 | 0.263 | 0.6078 |
| Yang [83] 2013 | 200/200 | 39 | 99 | 62 | 61 | 89 | 50 | 2.303 | 0.1292 |
| Cai [84] 2014 | 200/200 | 39 | 99 | 62 | 61 | 89 | 50 | 2.303 | 0.1292 |
| Bayramoglu [87] 2015 | 125/99 | 65 | 38 | 22 | 56 | 38 | 5 | 0.200 | 0.6543 |
| Nassereddine [88] 2015 | 101/102 | 47 | 40 | 14 | 54 | 45 | 3 | 3.176 | 0.0747 |
| Wei [90] 2015 | 246/348 | 143 | 82 | 21 | 260 | 78 | 10 | 1.888 | 0.1695 |
| Wen [91] 2015 | 174/634 | 45 | 53 | 76 | 258 | 291 | 85 | 0.042 | 0.8370 |
| Pérez-Razo [92] 2015 | 372/391 | 112 | 174 | 87 | 90 | 200 | 101 | 0.222 | 0.6375 |
| 209/209 | 34 | 98 | 67 | 35 | 108 | 56 | 1.898 | 0.1683 | |
| Amrani-Midoun [93] 2016 | 82/72 | 37 | 36 | 9 | 44 | 25 | 3 | 0.055 | 0.8142 |
| Fan [94] 2016 | 214/494 | 37 | 102 | 75 | 119 | 234 | 141 | 1.272 | 0.2593 |
| Dwivedi [95] 2017 | 100/223 | 71 | 24 | 5 | 184 | 34 | 5 | 4.541 | 0.0331 |
| Wu [98] 2016 | 123/120 | 73 | 39 | 11 | 70 | 40 | 10 | 1.481 | 0.2235 |
| Ghogomu [99] 2016 | 41/50 | 3 | 24 | 14 | 45 | 5 | 0 | 0.139 | 0.7098 |
| Zhang [100] 2017 | 220/128 | 45 | 122 | 53 | 52 | 56 | 20 | 0.569 | 0.4507 |
| Zhao [101] 2017 | 200/200 | 54 | 99 | 47 | 80 | 91 | 29 | 0.143 | 0.7056 |
| Liu [102] 2019 | 934/1075 | 200 | 439 | 295 | 214 | 505 | 356 | 2.060 | 0.1512 |
| Nong [103] 2019 | 122/110 | 15 | 58 | 49 | 35 | 59 | 16 | 1.229 | 0.2675 |
| Wu [104] 2019 | 250/200 | 91 | 103 | 56 | 88 | 88 | 24 | 0.077 | 0.7816 |
| Zhao [105] 2019 | 120/120 | 81 | 34 | 5 | 75 | 38 | 7 | 0.540 | 0.4623 |
| Candrasatria [106] 2020 | 213/202 | 134 | 73 | 6 | 157 | 42 | 3 | 0.010 | 0.9205 |
HWE, Hardy–Weinberg equilibrium.
TABLE 4.
The genotype distribution and HWE of MTHFR A1298C polymorphism in this meta-analysis. The bold value is C/T T/T A/C C/C genotype data is incomplete, and HWE evaluation cannot be performed.
| First author/Year | Number of samples | Genotype of cases | Genotype of controls | HWE | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cases/controls | A/A | A/C | C/C | A/A | A/C | C/C | Chi | p | |
| Kahleova [34] 2002 | 164/173 | 79 | 62 | 23 | 77 | 75 | 21 | 0.171 | 0.679 |
| Tylicki [44] 2005 | 90/90 | 38 | 43 | 9 | 36 | 45 | 9 | 0.880 | 0.3481 |
| Markan [52] 2007 | 153/133 | 99 | 43 | 11 | 112 | 17 | 4 | 8.277 | 0.004 |
| Ng [67] 2009 | 79/39 | 37 | 35 | 7 | 22 | 14 | 3 | 0.134 | 0.7143 |
| Wang [107] 2010 | 195/213 | 132 | 56 | 7 | 134 | 68 | 11 | 0.377 | 0.5393 |
| Wang [108] 2010 | 203/225 | 138 | 57 | 8 | 139 | 75 | 11 | 0.046 | 0.8297 |
| Demirel [109] 2011 | 50/50 | 25 | 19 | 6 | 14 | 33 | 3 | 7.494 | 0.0062 |
| Fowdar [76] 2012 | 368/386 | 165 | 151 | 52 | 162 | 173 | 51 | 0.201 | 0.6539 |
| Alghasham [79] 2012 | 26/250 | 15 | 11 | 144 | 106 | – | – | ||
| Wei [90] 2015 | 246/348 | 157 | 78 | 11 | 213 | 121 | 12 | 1.073 | 0.3003 |
| Liu [102] 2019 | 930/1074 | 679 | 229 | 22 | 801 | 250 | 23 | 0.448 | 0.5034 |
HWE, Hardy–Weinberg equilibrium.
Meta-analysis Results
MTHFR C677T Polymorphism
In Table 5, we summarized the association between MTHFR C677T and EH. In the overall population, EH was found to be at a high risk across all genetic models (TT vs. CC: OR = 1.71, 95% CI: 1.44–2.02; TC vs. CC: OR = 1.26, 95% CI: 1.15–1.39; TT + TC vs. CC: OR = 1.37, 95% CI: 1.24–1.52; TT vs. TC + CC: OR = 1.51, 95% CI: 1.31–1.74; T vs. C: OR = 1.33, 95% CI: 1.22–1.45).
TABLE 5.
Pooled results and sensitivity analysis of the association between MTHFR C677T polymorphism and essential hypertension. The meaning of bold is in different subgroups, there are statistically significant gene models. In other words, it is the genetic model associated with EH.
| Variable | n (cases/controls) | TT vs. CC | TC vs. CC | TT + TC vs. CC | TT vs. TC + CC | T vs. C | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P h / I 2 (%) | OR (95%CI) | P h / I 2 (%) | OR (95%CI) | P h / I 2 (%) | OR (95%CI) | P h / I 2 (%) | OR (95%CI) | P h / I 2 (%) | ||
| Overall | 63 (11,556/12,523) | 1.71 (1.44–2.02) a | <0.001/69.2 | 1.26 (1.15–1.39) a | <0.001/57.0 | 1.37 (1.24–1.52) a | <0.001/66.5 | 1.51 (1.31–1.74) a | <0.001/66.0 | 1.33 (1.22–1.45) a | <0.001/76.6 |
| Ethnicity | |||||||||||
| Asian | 42 (8,636/9,206) | 1.74 (1.43–2.12) a | <0.001/71.1 | 1.34 (1.21–1.48) a | 0.003/42.0 | 1.44 (1.28–1.61) a | <0.001/63.6 | 1.49 (1.26–1.77) a | <0.001/70.3 | 1.35 (1.24–1.46) a | <0.001/76.0 |
| Caucasian | 17 (2,255/2,575) | 1.73 (1.27–2.36) a | 0.012/50.1 | 1.06 (0.93–1.20) | 0.108/31.8 | 1.17 (1.04–1.32) | 0.048/39.5 | 1.62 (1.34–1.96) | 0.026/45.1 | 1.62 (1.34–1.96) a | 0.003/56.6 |
| Mixed | 3 (624/692) | 0.84 (0.61–1.16) | 0.422/0.0 | 1.06 (0.62–1.79) a | 0.057/60.2 | 1.03 (0.64–1.66) a | 0.078/56.0 | 1.00 (0.78–1.30) | 0.406/0.0 | 0.99 (0.79–1.25) | 0.205/34.6 |
| African | 1 (41/50) | 377.00 (18.37–7737.29) | – | 72.00 (15.83–327.45) | – | 114.00 (25.56–508.40) | – | 53.26 (3.06–927.31) | – | 32.93 (12.05–90.00) | – |
| Geographic region | |||||||||||
| East Asia | 38 (7,924/8,300) | 1.72 (1.40–2.12) a | <0.001/72.0 | 1.31 (1.19–1.45) a | 0.014/36.5 | 1.41 (1.26–1.58) a | <0.001/58.1 | 1.47 (1.23–1.76) a | <0.001/71.7 | 1.33 (1.20–1.47) a | <0.001/75.3 |
| South Asia | 2 (253/356) | 4.21 (0.84–21.07) | 0.239/27.8 | 1.60 (1.07–2.40) | 0.549/0.0 | 1.82 (1.23–2.67) | 0.767/0.0 | 3.82 (0.73–20.11) | 0.230/30.7 | 1.89 (1.34–2.66) | 0.961/0.0 |
| Western Asia | 5 (514/624) | 2.87 (0.95–8.67) a | 0.007/75.4 | 0.97 (0.53–1.79) a | 0.006/75.7 | 1.24 (0.72–2.11) a | <0.001/74.7 | 2.90 (1.14–7.35) a | 0.028/67.0 | 1.44 (0.83–2.50) a | <0.001/84.0 |
| Southeast Asia | 2 (459/550) | 3.40 (1.72–6.73) | 0.552/0.0 | 1.96 (1.48–2.61) | 0.830/0.0 | 2.10 (1.60–2.76) | 0.905/0.0 | 2.81 (1.43–5.53) | 0.544/0.0 | 1.98 (1.56–2.50) | 0.667/0.0 |
| Europe | 5 (727/777) | 1.76 (1.27–2.44) | 0.575/0.0 | 1.03 (0.82–1.28) | 0.440/0.0 | 1.17 (0.95–1.44) | 0.439/0.0 | 1.76 (1.30–2.38) | 0.779/0.0 | 1.25 (1.08–1.45) | 0.479/0.0 |
| Oceania | 3 (714/732) | 1.23 (0.86–1.76) | 0.380/0.0 | 1.08 (0.87–1.34) | 0.574/0.0 | 1.10 (0.89–1.36) | 0.404/0.0 | 1.17 (0.83–1.65) | 0.549/0.0 | 1.09 (0.93–1.29) | 0.330/9.9 |
| Africa | 5 (339/460) | 3.79 (1.02–14.01) a | 0.002/75.8 | 2.42 (1.03–5.69) a | 0.000/85.0 | 2.78 (1.13–6.83) a | <0.001/87.5 | 2.47 (0.84–7.31) a | 0.015/67.5 | 2.32 (1.11–4.84) a | <0.001/89.6 |
| South America | 1 (12/16) | 0.54 (0.02–14.35) a | – | 5.40 (0.98–29.67) a | – | 3.86 (0.75–19.84) a | – | 0.23 (0.01–5.30) a | – | 1.53 (0.50–4.75) a | – |
| North America | 2 (614/708) | 0.819 (0.53–1.27) | 0.219/34.1 | 0.76 (0.58–1.00) | 0.676/0.0 | 0.77 (0.60–0.99) | 0.436/0.0 | 0.99 (0.70–1.39) | 0.235/30.9 | 0.91 (0.71–1.16) | 0.146/48.1 |
| Sensitivity analysis of high-quality and HWE studies | |||||||||||
| Overall | 20 (4,439/4,661) | 1.14 (1.00–1.30) | 0.032/39.8 | 1.08 (0.98–1.19) | 0.186/21.3 | 1.15 (1.01–1.29) a | 0.040/38.1 | 1.11 (0.99–1.23) | 0.159/23.7 | 1.14 (1.04–1.25) a | 0.005/50.2 |
| Ethnicity | |||||||||||
| Asian | 15 (3,067/3,271) | 1.17 (0.99–1.36) | 0.180/24.8 | 1.14 (1.01–1.28) | 0.265/16.8 | 1.17 (1.04–1.30) | 0.105/32.9 | 1.09 (0.96–1.24) | 0.333/10.8 | 1.10 (1.02–1.19) | 0.018/48.6 |
| Caucasian | 4 (800/800) | 1.60 (0.87–2.92) a | 0.067/58.2 | 1.08 (0.88–1.33) | 0.704/0.0 | 1.14 (0.93–1.39) | 0.451/0.0 | 1.49 (0.86–2.60) a | 0.083/55.0 | 1.18 (0.96–1.45) | 0.168/40.6 |
| Mixed | 1 (572/590) | 0.83 (0.60–1.15) a | 0.113/60.2 | 0.76 (0.57–1.02) | 0.378/0.0 | 0.78 (0.59–1.02) | 0.204/38.0 | 1.01 (0.78–1.31) a | 0.151/51.4 | 0.95 (0.70–1.29) a | 0.075/68.4 |
| African | – | – | – | – | – | – | – | – | – | – | – |
| Geographic region | |||||||||||
| East Asia | 13 (2,701/2,936) | 1.13 (0.97–1.32) | 0.292/15.1 | 1.08 (0.95–1.22) | 0.721/0.0 | 1.10 (0.98–1.23) | 0.512/0.0 | 1.07 (0.94–1.22) | 0.474/0.0 | 1.07 (0.99–1.15) | 0.261/18.1 |
| South Asia | 1 (153/133) | 17.00 (0.97–298.31) | – | 1.43 (0.82–2.49) | – | 1.71 (1.00–2.94) | – | 15.60 (0.89–272.86) | – | 1.90 (1.17–3.10) | – |
| Southeast Asia | 1 (213/202) | 2.34 (0.58–9.55) | – | 2.04 (1.31–3.18) | – | 2.06 (1.34–3.17) | – | 1.92 (0.47–7.79) | – | 1.85 (1.26–2.71) | – |
| Europe | 1 (232/215) | 1.95 (1.04–3.64) | – | 1.32 (0.88–1.96) | – | 1.42 (0.97–2.08) | – | 1.67 (0.93–3.01) | – | 1.35 (1.03–1.78) | – |
| Oceania | 2 (467/483) | 1.01 (0.64–1.60) | 0.755/0.0 | 0.99 (0.76–1.30) | 0.784/0.0 | 0.99 (0.77–1.29) | 0.735/0.0 | 1.01 (0.65–1.56) | 0.811/0.0 | 1.01 (0.82–1.21) | 0.713/0.0 |
| Africa | 1 (101/102) | 5.36 (1.45–19.81) | – | 1.02 (0.57–1.82) | – | 1.29 (0.75–2.24) | – | 5.31 (1.48–19.10) | – | 1.52 (0.99–2.34) | – |
| South America | – | – | – | – | – | – | – | – | – | – | – |
| North America | 1 (572/590) | 0.83 (0.60–1.15) a | 0.113/60.2 | 0.76 (0.57–1.02) | 0.378/0.0 | 0.78 (0.60–1.02) | 0.204/38.0 | 1.01 (0.78–1.31) a | 0.151/51.4 | 0.95 (0.70–1.29) a | 0.075/68.4 |
A random-effects model was used.
HWE, Hardy–Weinberg equilibrium.
Next, a subgroup analysis was performed by ethnicity and geographic region. An increased risk of hypertension could be found in Asian (TT vs. CC: OR = 1.74, 95% CI: 1.43–2.12; TC vs. CC: OR = 1.34, 95% CI: 1.21–1.48; TT + TC vs. CC: OR = 1.44, 95% CI: 1.28–1.61; TT vs. TC + CC: OR = 1.49, 95% CI: 1.26–1.77; T vs. C: OR = 1.35, 95% CI: 1.24–1.46), Caucasian (TT vs. CC: OR = 1.73, 95% CI: 1.27–2.36; TT + TC vs. CC: OR = 1.17, 95% CI: 1.04–1.32; TT vs. TC + CC: OR = 1.62, 95% CI: 1.34–1.96; T vs. C: OR = 1.62, 95% CI: 1.34–1.96), and African (TT vs. CC: OR = 377.00, 95% CI: 18.37–7737.29; TC vs. CC: OR = 72.00, 95% CI: 15.83–327.45; TT + TC vs. CC: OR = 114.00, 95% CI: 25.56–508.40; TT vs. TC + CC: OR = 53.26, 95% CI: 3.06–927.31; T vs. C: OR = 32.93, 95% CI: 12.05–90.00). The same happened in the East Asia region (TT vs. CC: OR = 1.72, 95% CI: 1.40–2.12; TC vs. CC: OR = 1.31, 95% CI: 1.19–1.45; TT + TC vs. CC: OR = 1.41, 95% CI: 1.26–1.58; TT vs. TC + CC: OR = 1.47, 95% CI: 1.23–1.76; T vs. C: OR = 1.33, 95% CI: 1.20–1.47), South Asia region (TC vs. CC: OR = 1.60, 95% CI: 1.07–2.40; TT vs. TC + CC: OR = 1.82, 95% CI: 1.23–2.67; T vs. C: OR = 1.89, 95% CI: 1.34–2.66), Southeast Asia region (TT vs. CC: OR = 3.40, 95% CI: 1.72–6.73; TC vs. CC: OR = 1.96, 95% CI: 1.48–2.61; TT + TC vs. CC: OR = 2.10, 95% CI: 1.60–2.76; TT vs. TC + CC: OR = 2.81, 95% CI: 1.43–5.53; T vs. C: OR = 1.98, 95% CI: 1.56–2.50), Europe region (TT vs. CC: OR = 1.76, 95% CI: 1.27–2.44; TT vs. TC + CC: OR = 1.76, 95% CI: 1.30–2.28; T vs. C: OR = 1.25, 95% CI: 1.08–1.45), and Africa region (TT vs. CC: OR = 3.79, 95% CI: 1.02–14.01; TC vs. CC: OR = 2.42, 95% CI: 1.03–5.69; TT + TC vs. CC: OR = 2.78, 95% CI: 1.13–6.83; T vs. C: OR = 2.32, 95% CI: 1.11–4.84).
MTHFR A1298C Polymorphism
The association between MTHFR A1298C and EH is shown in Table 6. No significant association was found in both the overall population and the subgroup analyzed by ethnicity. However, according to the subgroup analyzed by geographical origin, a clear risk correlation between the two was found in South Asia region (AC vs. AA: OR = 2.86, 95% CI: 1.53–5.34; CC + AC vs. AA: OR = 2.91, 95% CI: 1.64–5.15; C vs. A: OR = 2.60, 95% CI: 1.59–4.26). A conservation correlation has been found in the West Asia region (CC + AC vs. AA: OR = 0.39, 95% CI: 0.17–0.89).
TABLE 6.
Pooled results and sensitivity analysis of the association between MTHFR A1298C polymorphism and essential hypertension. The meaning of bold is in different subgroups, there are statistically significant gene models. In other words, it is the genetic model associated with EH.
| Variable | n (cases/controls) | CC vs. AA | AC vs. AA | CC + AC vs. AA | CC vs. .AC + AA | C vs. A | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P h / I 2 (%) | OR (95%CI) | P h / I 2 (%) | OR (95%CI) | P h / I 2 (%) | OR (95%CI) | P h / I 2 (%) | OR (95%CI) | P h / I 2 (%) | ||
| Overall | 11 (2,504/2,979) | 1.07 (0.84–1.37) | 0.814/0.0 | 0.94 (0.77–1.17) a | 0.010/56.9 | 0.98 (0.87–1.10) a | 0.004/62.7 | 1.12 (0.89–1.43) | 0.889/0.0 | 1.01 (0.86–1.18) a | 0.015/56.0 |
| Ethnicity | |||||||||||
| Caucasian | 6 (777/988) | 1.03 (0.74–1.44) | 0.994/0.0 | 0.84 (0.64–1.10) | 0.230/27.3 | 0.87 (0.71–1.07) | 0.245/26.4 | 1.14 (0.83–1.55) | 0.929/0.0 | 0.96 (0.82–1.11) | 0.724/0.0 |
| Asian | 5 (1,727/1,991) | 1.11 (0.77–1.60) | 0.292/19.2 | 1.05 (0.77–1.44) a | 0.007/71.9 | 1.038 (0.90–1.20) a | 0.002/76.5 | 1.11 (0.77–1.60) | 0.488/0.0 | 1.07 (0.80–1.43) a | 0.001/77.5 |
| Geographic region | |||||||||||
| Europe | 2 (243/212) | 1.12 (0.61–2.05) | 0.748/0.0 | 0.99 (0.56–1.76) | 0.199/39.3 | 0.98 (0.67–1.42) | 0.238/28.3 | 1.18 (0.66–2.10) | 0.988/0.0 | 1.03 (0.77–1.36) | 0.385/0.0 |
| Oceania | 2 (458/476) | 0.99 (0.66–1.49) | 0.923/0.0 | 0.87 (0.66–1.14) | 0.876/0.0 | 0.89 (0.69–1.160) | 0.941/0.0 | 1.07 (0.73–1.57) | 0.885/0.0 | 0.96 (0.79–1.16) | 0.979/0.0 |
| South Asia | 1 (153/113) | 3.11 (0.96–10.08) | – | 2.86 (1.53–5.34) | – | 2.91 (1.64–5.15) | – | 2.50 (0.78–8.04) | – | 2.60 (1.59–4.26) | – |
| East Asia | 3 (1,328/1,512) | 0.91 (0.58–1.42) | 0.554/0.0 | 0.95 (0.76–1.18) | 0.254/27.0 | 0.98 (0.83–1.15) | 0.186/40.6 | 0.93 (0.60–1.44) | 0.662/0.0 | 0.93 (0.75–1.15) | 0.171/43.4 |
| Western Asia | 2 (76/300) | 1.12 (0.24–5.19) | – | 0.57 (0.19–1.73) | 0.063/71.1 | 0.39 (0.17–0.89) | – | 2.14 (0.50–9.07) | – | 0.70 (0.39–1.26) | – |
| Southeast Asia | 1 (246/346) | 1.24 (0.54–2.89) | – | 0.88 (0.62–1.24) a | – | 0.91 (0.65–1.27) a | – | 1.30 (0.57–3.00) | – | 0.96 (0.72–1.28) a | – |
| Sensitivity analysis of high-quality and HWE studies | |||||||||||
| Overall | 5 (1,786/1,988) | 0.95 (0.71–1.29) | 0.867/0.0 | 0.95 (0.82–1.09) | 0.506/0.0 | 0.95 (0.83–1.09) | 0.451/0.0 | 1.01 (0.75–1.34) | 0.900/0.0 | 0.97 (0.86–1.08) | 0.470/0.0 |
| Ethnicity | |||||||||||
| Caucasian | 2 (458/476) | 0.99 (0.66–1.49) | 0.923/0.0 | 0.87 (0.66–1.14) | 0.876/0.0 | 0.89 (0.69–1.16) | 0.941/0.0 | 1.07 (0.73–1.57) | 0.885/0.0 | 0.96 (0.79–1.16) | 0.979/0.0 |
| Asian | 3 (1,328/1,512) | 0.91 (0.58–1.42) | 0.554/0.0 | 0.98 (0.83–1.16) | 0.254/27.0 | 0.98 (0.83–1.15) | 0.186/40.6 | 0.928 (0.60–1.44) | 0.662/0.0 | 0.97 (0.84–1.12) | 0.171/43.4 |
| Geographic region | |||||||||||
| Europe | – | – | – | – | – | – | – | – | – | – | – |
| Oceania | 2 (458/476) | 0.99 (0.66–1.49) | 0.923/0.0 | 0.87 (0.66–1.14) | 0.876/0.0 | 0.89 (0.69–1.16) | 0.941/0.0 | 1.07 (0.73–1.57) | 0.885/0.0 | 0.96 (0.79–1.16) | 0.979/0.0 |
| East Asia | 3 (1,328/1,512) | 0.91 (0.58–1.42) | 0.554/0.0 | 0.98 (0.83–1.16) | 0.254/27.0 | 0.98 (0.83–1.15) | 0.186/40.6 | 0.93 (0.60–1.44) | 0.662/0.0 | 0.97 (0.84–1.12) | 0.171/43.4 |
A random-effects model was used.
HWE, Hardy–Weinberg equilibrium.
Heterogeneity and Sensitivity Analyses
In Table 5, we showed the very obvious heterogeneity. A meta-regression analysis was performed based on geographic origin, ethnicity, control group origin, gene quality control, matching, sample size, HWE, and literature quality assessment. Ethnicity was the source of heterogeneity in the final results (TC vs. CC: P = 0.035; TT + TC vs. CC: P = 0.042).
A sensitivity analysis was performed to rule out individual studies one by one, and the results were found to be stable. When we restricted the high-quality and HWE studies, there was no significant change in A1298C genotype. However, the results of the C677T genotype changed significantly in the overall population (TC vs. CC: OR = 1.08, 95% CI: 0.98–1.19, P h = 0.186, I 2 = 21.3%; TT vs. TC + CC: OR = 1.11, 95% CI: 0.99–1.23, P h = 0.159, I 2 = 23.7%), Asian population (TT vs. CC: OR = 1.17, 95% CI: 0.99–1.36, P h = 0.180, I 2 = 24.8%; TT vs. TC + CC: OR = 1.09, 95% CI: 0.96–1.24, P h = 0.333, I 2 = 10.8%), Caucasian population (TT vs. CC: OR = 1.60, 95% CI: 0.87–2.92, P h = 0.067, I 2 = 58.2%; TT + TC vs. CC: OR = 1.14, 95% CI: 0.93–1.39, P h = 0.451, I 2 = 0.0%; TT vs. TC + CC: OR = 1.49, 95% CI: 0.86–2.60, P h = 0.083, I 2 = 55.0%; T vs. C: OR = 1.18, 95% CI: 0.96–1.45, P h = 0.168, I 2 = 40.6%), and East Asia region (TT vs. CC: OR = 1.13, 95% CI: 0.97–1.32, Ph = 0.292, I 2 = 15.1%; TC vs. CC: OR = 1.08, 95% CI: 0.95–1.22, P h = 0.720, I 2 = 0.0%; TT + TC vs. CC: OR = 1.10, 95% CI: 0.98–1.23, P h = 0.512, I 2 = 0.0%; TT vs. TC + CC: OR = 1.07, 95% CI: 0.94–1.22, P h = 0.474, I 2 = 0.0%; T vs. C: OR = 1.07, 95% CI: 0.99–1.15, P h = 0.261, I 2 = 18.1%). All results are shown in Table 5 and Table 6.
Publication Bias
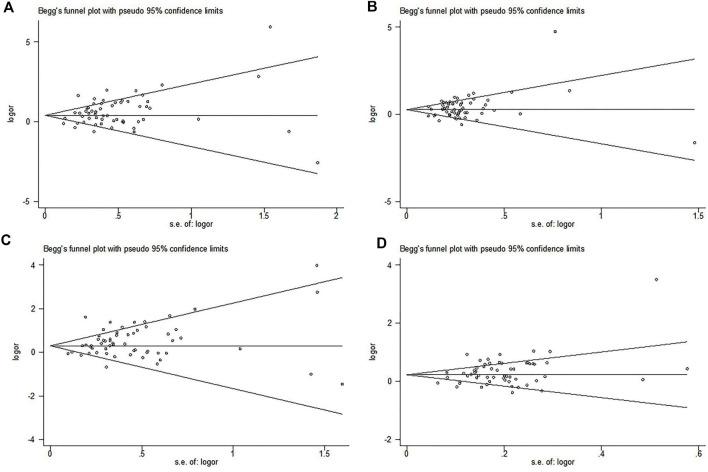
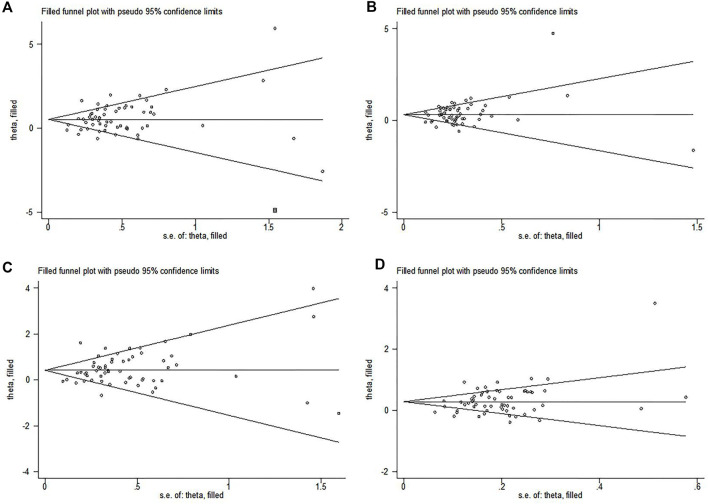
Begg’s funnel plot and Egger test were used to evaluate publication bias. For MTHFR C677T polymorphism, there was publication bias in four genetic models (TT vs. CC: P = 0.009; TT + TC vs. CC: P = 0.045; TT vs. TC + CC: P = 0.007; T vs. C: P = 0.005) (Figure 2). In order to further clarify the publication bias, we applied the trim-and-fill method, and the results of the overall population did not change (Figure 3). For MTHFR A1298C polymorphism, the shape of the funnel plot was uniform and symmetrical, indicating that there was no significant publication bias under all genetic models (P).
FIGURE 2.
Begg’s funnel plot to assess the publication bias of MTHFR C677T polymorphism in the overall population. (A) Allelic model, T vs. C; (B) dominant model, TT + TC vs. CC; (C) recessive model, TT vs. CC + TC; (D) homozygote genetic model, TT vs. CC.
FIGURE 3.
Trim-and-fill plots of the publication bias to assess MTHFR C677T polymorphism in the overall population. (A) Allelic model, T vs. C; (B) dominant model, TT + TC vs. CC; (C) recessive model, TT vs. CC + TC; (D) homozygote genetic model, TT vs. CC.
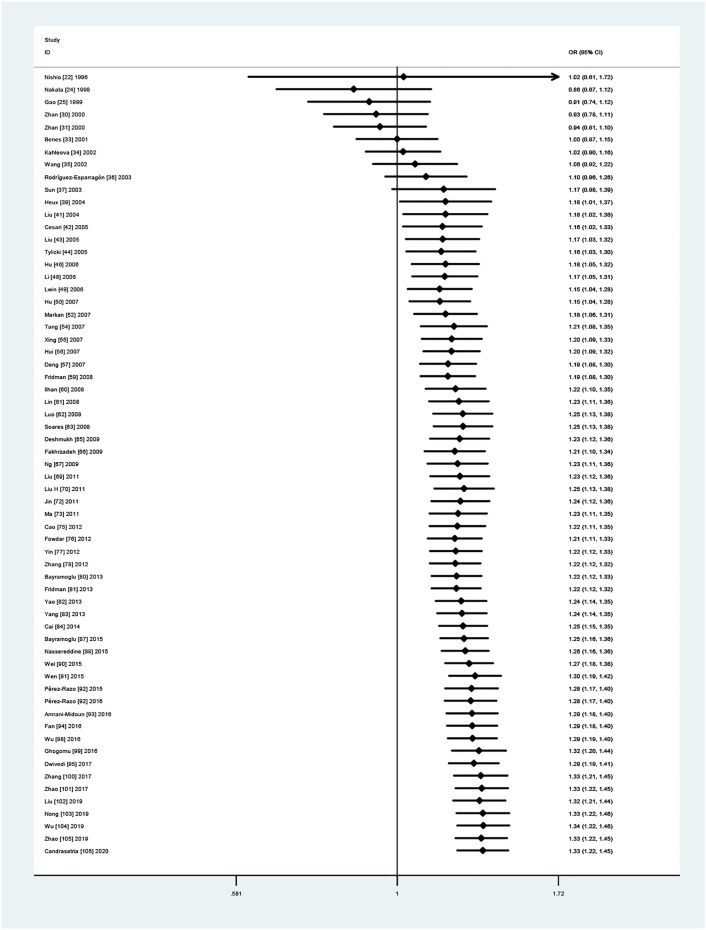
Cumulative Meta-analysis Results
According to year, the literature was included sequentially to evaluate the stability of cumulative effect size. The results showed that the correlation degree tended to be stable (Figure 4).
FIGURE 4.
A cumulative meta-analysis of forest plots was performed based on years (allelic model, T vs. C).
Credibility of the Positive Results
After FPRP, BFDP, and Venice criterion evaluation, results that met both FPRP <0.2, BFDP <0.8, and above moderate epidemiological evidence were only in the heterozygote (TC vs. CC) and recessive model (TT vs. CT + CC). Table 7 shows the details. This was different from the results of the sensitivity analyses that limited high-quality and HWE studies.
TABLE 7.
The credibility results of the positive effects after FPRP, BFDP, and Venice criterion.
| Variables | Model | OR (95% CI) | I 2 (%) | Credibility | ||
|---|---|---|---|---|---|---|
| Prior probability of 0.001 | Venice criteria | |||||
| FPRP | BFDP | |||||
| MTHFR C677T | ||||||
| Overall | TT vs. CC | 1.71 (1.44–2.02) | 69.2 | <0.001 | <0.001 | ACC |
| Ethnicity | ||||||
| Asian | TT vs. CC | 1.74 (1.43–2.12) | 71.1 | 0.001 | 0.002 | ACB |
| Caucasian | TT vs. CC | 1.73 (1.27–2.36) | 50.1 | 0.746 | 0.935 | ACB |
| Geographic region | ||||||
| East Asia | TT vs. CC | 1.72 (1.40–2.12) | 72.0 | 0.004 | 0.020 | ACC |
| Europe | TT vs. CC | 1.76 (1.27–2.44) | 0.0 | 0.804 | 0.942 | AAB |
| Africa | TT vs. CC | 3.79 (1.02–14.01) | 75.8 | 0.998 | 0.998 | BCC |
| Sensitivity analysis (only studies with high quality and HWE) | ||||||
| Overall | TT vs. CC | 1.14 (1.00–1.30) | 39.8 | 0.981 | 0.999 | ABC |
| Overall | TC vs. CC | 1.26 (1.15–1.39) | 57.0 | 0.004 | 0.226 | ACB |
| Ethnicity | ||||||
| Asian | TC vs. CC | 1.34 (1.21–1.48) | 42.0 | <0.001 | 0.001 | ABB |
| Geographic region | ||||||
| East Asia | TC vs. CC | 1.31 (1.19–1.45) | 36.5 | <0.001 | 0.015 | ABC |
| Africa | TC vs. CC | 2.42 (1.03–5.69) | 85.0 | 0.997 | 0.998 | ACC |
| Sensitivity analysis (only studies with high quality and HWE) | ||||||
| Ethnicity | ||||||
| Asian | TC vs. CC | 1.14 (1.01–1.28) | 16.8 | 0.964 | 0.999 | AAB |
| Overall | TT + TC vs. CC | 1.37 (1.24–1.52) | 66.5 | <0.001 | <0.001 | ACC |
| Ethnicity | ||||||
| Asian | TT + TC vs. CC | 1.44 (1.28–1.61) | 63.6 | <0.001 | <0.001 | ACB |
| Caucasian | TT + TC vs. CC | 1.17 (1.04–1.32) | 39.5 | 0.915 | 0.997 | ABB |
| Geographic region | ||||||
| East Asia | TT + TC vs. CC | 1.41 (1.26–1.58) | 58.1 | <0.001 | <0.001 | ACC |
| Africa | TT + TC vs. CC | 2.78 (1.13–6.83) | 87.5 | 0.997 | 0.997 | ACC |
| Sensitivity analysis (only studies with high quality and HWE) | ||||||
| Overall | TT + TC vs. CC | 1.15 (1.01–1.29) | 38.1 | 0.945 | 0.998 | ABB |
| Ethnicity | ||||||
| Asian | TT + TC vs. CC | 1.17 (1.04–1.30) | 32.9 | 0.777 | 0.993 | ABC |
| Overall | TT vs. TC + CC | 1.51 (1.31–1.74) | 66.0 | <0.001 | 0.001 | ACC |
| Ethnicity | ||||||
| Asian | TT vs. TC + CC | 1.49 (1.26–1.77) | 70.3 | 0.011 | 0.217 | ACB |
| Caucasian | TT vs. TC + CC | 1.62 (1.34–1.96) | 45.1 | 0.003 | 0.036 | ABB |
| Geographic region | ||||||
| East Asia | TT vs. TC + CC | 1.47 (1.23–1.76) | 71.7 | 0.045 | 0.541 | ACB |
| Europe | TT vs. TC + CC | 1.76 (1.30–2.38) | 0.0 | 0.617 | 0.887 | AAB |
| Overall | T vs. C | 1.33 (1.22–1.45) | 76.6 | <0.001 | <0.001 | ACC |
| Ethnicity | ||||||
| Asian | T vs. C | 1.35 (1.24–1.46) | 76.0 | <0.001 | <0.001 | ACB |
| Caucasian | T vs. C | 1.62 (1.34–1.96) | 56.6 | 0.003 | 0.036 | ACB |
| Geographic region | ||||||
| East Asia | T vs. C | 1.33 (1.20–1.47) | 75.3 | <0.001 | 0.002 | ACC |
| Europe | T vs. C | 1.25 (1.08–1.45) | 0.0 | 0.764 | 0.991 | AAB |
| Africa | T vs. C | 2.32 (1.11–4.84) | 89.6 | 0.995 | 0.997 | BCC |
| Sensitivity analysis (only studies with high quality and HWE) | ||||||
| Overall | T vs. C | 1.14 (1.04–1.25) | 50.2 | 0.841 | 0.996 | ACC |
| Ethnicity | ||||||
| Asian | T vs. C | 1.10 (1.02–1.19) | 48.6 | 0.946 | 0.999 | ABC |
| MTHFR A1298C | ||||||
| Geographic region | ||||||
| Western Asia | CC + AC vs. AA | 0.39 (0.17–0.89) | – | 0.996 | 0.997 | B–– |
FPRP, false-positive report probability; BFDP, Bayesian false discovery probability; HWE, Hardy–Weinberg equilibrium.
Discussion
In 1996, a group of researchers first investigated the association between MTHFR C677T polymorphism and EH in Japan (Nishio et al., 1996). Then, another group of researchers studied MTHFR C677T and A1298C polymorphisms for the first time in the Czech Republic (Kahleová et al., 2002). Thereafter, many scholars worldwide partook in the research, but the results were inconsistent and controversial. Even though several meta-analyses (Qian et al., 2007; Niu et al., 2012; Yang B. et al., 2014; Yang K.-M. et al., 2014; Wu et al., 2014; Fu et al., 2019) appeared to investigate the association between the MTHFR SNPs, methodological problems, especially the inclusion of pregnancy-related hypertension and lack of quality assessment of the studies included, hampered the acceptance of their findings. Therefore, it is necessary to conduct a new, more reliable, and more comprehensive meta-analysis.
After data collection for this meta-analysis, HWE compliance checks of the control group, and literature quality score assignments, the effect sizes were combined using five genetic models. Ultimately, the C677T polymorphism was found to be a risk genotype in the overall population, East Asians, Southeast Asians, South Asians, Caucasians/Europeans (allelic model, homozygote model, dominant model, and recessive model), and Africans. It was possible that both were linked in the study population from African, South Asia region, and Southeast Asia region (Table 5). However, we are of the opinion that, based on a lack of studies on populations representative of Africa [one report (Ghogomu et al., 2016)], South Asia [two reports (Markan et al., 2007; Dwivedi and Sinha, 2017)], and South-East Asia [two reports (Wei et al., 2015; Candrasatria et al., 2020)], these results should be interpreted with caution until more studies on these underrepresented groups accumulate (Higgins, 2008). Then, we conducted a sensitivity analysis to choose high-quality studies and studies whose populations adhered to the assumptions of HWE. The results showed that C677T polymorphism was significantly associated with EH only in the overall population (allele, dominant, and homozygote model) and the Asian population (dominant, allele, and heterozygote model). When we used the FPRP, BFDP test, and the Venice criterion for evaluation, all significant associations were less-credible positive results, indicating that the association could not be noteworthy and could therefore be regarded as weak-level evidence. These tests are necessary to avoid random error and confounding bias, but sometimes they can alter the results of the epidemiological studies (Wakefield, 2007; Ioannidis et al., 2008; Zhao et al., 2018; Tian, 2019). At the same time, among the low-quality studies, there were four HWD studies, 28 unadjusted studies, 34 gene quality uncontrolled studies, and 34 no matching studies, which may be the main source of bias. In addition, we did not find evidence that the A1298C polymorphism was associated with EH. The South Asia [one report (Markan et al., 2007)] and Southeast Asia [one report (Wei et al., 2015)] results were also significantly less reliable because of the small number of studies (Tables 5–7). In conclusion, our findings are comparable to previous reports which observed that the MTHFR variant SNP at position 677 conveys a risk for hypertension; however, compared with the previous meta-analyses, the FPRP, BFDP, and Venice criterion indicated that this relationship is probably not causal. Some observational studies have shown that genetic changes are inseparable from the impact of environmental factors such as diet, exercise, smoking, and drinking. A method to explore the connection between genes and environmental factors is to study people in different regions and cultures (Anand, 2005). Hcy is an independent risk for EH (Zhong et al., 2017). Alam et al. found that the MTHFR 677 TT genotype was associated with hHcy in Indians (Alam et al., 2008). However, no association between MTHFR C677T polymorphism and hHcy was found in long-term resident Indians in the UK (Chambers et al., 2000). Further research suggested that the difference may be caused by different environmental factors such as diet and exercise.
We could find a significant heterogeneity between the articles included in the final results (Table 5 and Table 6). Heterogeneity exists naturally in meta-analysis, so sources of heterogeneity should be sought as far as possible. First, after excluding each study one by one, we believe that the included studies are within the confidence interval of the total effect size. We performed a sensitivity analysis which, in certain instances, can be useful to identify the source of heterogeneity. Therefore, we conducted a meta-regression analysis and found that ethnicity was the source of heterogeneity only in the dominant model and the heterozygote model. In addition, publication bias was also observed (Figure 2 suggests that small sample studies lead to publication bias). In order to identify and correct publication bias caused by the asymmetry of a funnel figure (Rothstein et al., 2005), we applied trim-and-fill method to remove the small sample study and (to) include missing studies. The results showed that there were no obvious changes before and after the cut to fill the consolidation effect (before the cut to fill the consolidation effect was statistically significant, while after the cut to fill the consolidation effect was statistically significant) (Figure 3); publication bias may not exist (Zhang and Zhong, 2009). In molecular epidemiological studies, small-sample research is very likely to have random errors and bias, resulting in unreliable final results (Ioannidis et al., 2008). Because the experimental design of small-sample research is not strict, its research quality is often low. Cumulative meta-analysis was carried out according to year. The results showed that, with the extension of year, the effect size did not change much, and the final results were gradually stable (Wang et al., 2009) (Figure 4). When we carried out a strict and high-quality design for small-sample research, the results were close to the real level.
Compared with previously published meta-analyses, this study has several advantages. Firstly, we have the largest sample size to date. A total of 66 case–control studies were included. For MTHFR C677T polymorphism, 63 studies were collected, including 11,556 case groups and 12,523 control groups. For MTHFR A1298C polymorphism, there were 11 studies with 2,504 case groups and 2,979 control groups. Secondly, we analyzed the sensitivity of the combined results by excluding individual studies one by one and limiting high-quality and HWE studies. Thirdly, meta-regression, cumulative meta-analysis, trim-and-fill method, and geographic origin subgroup analysis were used to verify the qualified combined effect size. Fourthly, we used the FPRP and BFDP tests that were first introduced to judge the credibility of the positive results. The Venice criterion was used to assess the cumulative level of epidemiological evidence for genetic association. The GWAS on Europeans was an association study for the detection of multiple SNPs. The method was regression analysis. An assumption of regression analysis is the independent distribution of data. However, the fact is that many individuals may have distant relationships, which will lead to false-positive results in an association analysis (O'Donnell and Nabel, 2008; Sul et al., 2018). However, our study conducted a credibility analysis of positive results obtained by traditional methods.
However, there are also shortcomings to our meta-analysis that should be kept in mind. Firstly, only Chinese and English studies were included in this study; studies in other languages were not included. There is a certain degree of heterogeneity. Secondly, this study did not include the data of family genetic aggregation, smoking, body weight, body mass index, gender, age, and medication history. Therefore, the results may be affected by confounding factors. Thirdly, the sources of the control group were not uniform, which may have classification bias. Fourthly, there were few studies on MTHFR C677T polymorphism in Africa, South Asia, Southeast Asia, and West Asia. Therefore, heterogeneity may exist. More research should be done in these areas. Fifthly, with regard to the MTHFR A1298C polymorphism, since there were only two studies in West Asia, it is still uncertain whether this SNP is associated with EH.
Conclusion
In summary, this study provided a comprehensive analysis of MTHFR polymorphisms on the risk of EH in different populations worldwide. The results showed that MTHFR C677T gene polymorphism was associated with increased EH risks in overall population, East Asian, and Caucasian. However, after FPRP, BFDP, and Venice Criteria tests, the above-mentioned associations became less reliable. These results should be treated with caution because they could be false-positives. The final conclusion highlighted weak epidemiological credibility for a higher risk of EH of the MTHFR 677T allele. Therefore, more investigations are needed to provide researchers with conclusive evidence of whether MTHFR C677T is a genetic predictor of EH. Similarly, there was no significant association between the MTHFR A1298C polymorphism and EH. Therefore, MTHFR polymorphisms could not contribute to the development of hypertension. In the future, more studies are needed to repeatedly verify the current conclusions.
Acknowledgments
We thanked all the authors of the included original studies and references. Meanwhile, we would like to express our gratitude to the authors who participated in this meta-analysis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
In this study, HM conducted the research, collected data, analyzed data, and wrote papers. SH carried out the research design, data collection, statistical analysis, and revision of the paper. YY was responsible for sorting data and checking data. YX and LF designed the research and performed statistical analysis and revision of the paper. At the same time, YX also completed the generation of Figures 1–4 and Tables 5–7. HM and SH were responsible for making Tables 1–4 and Supplementary forms and filling in relevant information. XH was involved in designing the research, verifying data, and revising papers. All authors participated in the revision and uploading of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed nor endorsed by the publisher.
Supplementary Material
The supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.698590/full#supplementary-material
Abbreviations
BFDP, Bayesian false discovery probability; CI, confidence intervals; CNKI, China National Knowledge Infrastructure; EH, essential hypertension; FPRP, false-positive report probability; MTHFR, 5,10-methylenetetrahydrofolate reductase; OR, odds ratios; SNP, single-nucleotide polymorphism.
References
- Alam M. A., Husain S. A., Narang R., Chauhan S. S., Kabra M., Vasisht S. (2008). Association of Polymorphism in the Thermolabile 5, 10-methylene Tetrahydrofolate Reductase Gene and Hyperhomocysteinemia With Coronary Artery Disease. Mol. Cell Biochem. 310 (1-2), 111–117. 10.1007/s11010-007-9671-7 [DOI] [PubMed] [Google Scholar]
- Alghasham A., Settin A. A., Ali A., Dowaidar M., Ismail H. (2012). Association of MTHFR C677T and A1298C Gene Polymorphisms With Hypertension. Int. J. Health Sci. (Qassim). 6 (1), 3–11. 10.12816/0005968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrani-Midoun A., Kiando S. R., Treard C., Jeunemaitre X., Bouatia-Naji N. (2016). The Relationship Between MTHFR C677T Gene Polymorphism and Essential Hypertension in a Sample of an Algerian Population of Oran City. Int. J. Cardiol. 225, 408–411. 10.1016/j.ijcard.2016.10.027 [DOI] [PubMed] [Google Scholar]
- Anand S. S. (2005). The Value of Studying Gene-Environment Interactions in Culturally Diverse Populations. Can. J. Physiol. Pharmacol. 83 (1), 42–46. 10.1139/y05-004 [DOI] [PubMed] [Google Scholar]
- Arina C. A., Amir D., Siregar Y., Sembiring R. J. (2019). The Role of Polymorphism Gen Methylene Tetra Hydrofolate Reductase (MTHFR) C677T in Ischaemic Stroke Patients With and Without Hypertension. Open Access Maced J. Med. Sci. 7 (1), 29–32. 10.3889/oamjms.2019.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähr V., Oelkers W., Diederich S. (2003). Monogenic Hypertension. Med. Klin (Munich). 98 (4), 208–217. 10.1007/s00063-003-1245-1 [DOI] [PubMed] [Google Scholar]
- Bayramoglu A., Urhan Kucuk M., Guler H. I., Abaci O., Kucukkaya Y., Colak E. (2013). Is There Any Genetic Predisposition of MMP-9 Gene C1562T and MTHFR Gene C677T Polymorphisms With Essential Hypertension? Cytotechnology. 67 (1), 115–122. 10.1007/s10616-013-9665-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayramoglu A., Urhan Kucuk M., Guler H. I., Abaci O., Kucukkaya Y., Colak E. (2015). Is There Any Genetic Predisposition of MMP-9 Gene C1562T and MTHFR Gene C677T Polymorphisms With Essential Hypertension? Cytotechnology. 67 (1), 115–122. 10.1007/s10616-013-9665-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes P., Kanková K., Muzík J., Groch L., Benedík J., Elbl L., et al. (2001). Methylenetetrahydrofolate Reductase Polymorphism, Type II Diabetes Mellitus, Coronary Artery Disease, and Essential Hypertension in the Czech Population. Mol. Genet. Metab. 73 (2), 188–195. 10.1006/mgme.2001.3188 [DOI] [PubMed] [Google Scholar]
- Cai W., Sun Y., Peng K., Kwok H., Lei L., Wu S., et al. (2020). Physical-Activity-Related Injuries and Risk Factors Among Secondary School Students in Hong Kong. Int. J. Environ. Res. Public Health. 17, 747. 10.3390/ijerph17030747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., Yin L., Yang F., Zhang L., Cheng J. (2014). Association Between Hcy Levels and the CBS844ins68 and MTHFR C677T Polymorphisms With Essential Hypertension. Biomed. Rep. 2 (6), 861–868. 10.3892/br.2014.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y. M., Gong W. X. (2009). Linkage Study on Methylenetetra-Hydrofolate Reductase Single Nucleotide Polymorphisms and Hypertension in the Elderly With Rheumatoid Arthritis. Chin. J. Birth Health Hered. 17 (7), 14–17. 10.13404/j.cnki.cjbhh.2009.07.011.14 [DOI] [Google Scholar]
- Candrasatria R. M., Adiarto S., Sukmawan R. (2020). Methylenetetrahydrofolate Reductase C677T Gene Polymorphism as a Risk Factor for Hypertension in a Rural Population. Int. J. Hypertens. 2020, 4267246. 10.1155/2020/4267246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto P., Canto-Cetina T., Juárez-velázquez R., Rosas-Vargas H., Rangel-Villalobos H., Canizales-Quinteros S., et al. (2008). Methylenetetrahydrofolate Reductase C677T and Glutathione S-Transferase P1 A313G Are Associated With a Reduced Risk of Preeclampsia in Maya-Mestizo Women. Hypertens. Res. 31 (5), 1015–1019. 10.1291/hypres.31.1015 [DOI] [PubMed] [Google Scholar]
- Cao Z. Y. (2012). The Association between Methylenetetrahydrofolate Reductase Gene Polymorphism and Hypertensive Elderly Patient With Acute Myocardial Infarction. Chin. J. Gerontol. 32 (23), 5118–5120. 10.3969/j.issn.1005-9202.2012.23.007 [DOI] [Google Scholar]
- Cesari M., Zanchetta M., Burlina A., Pedon L., Maiolino G., Sticchi D., et al. (2005). Hyperhomocysteinemia Is Inversely Related With Left Ventricular Ejection Fraction and Predicts Cardiovascular Mortality in High-Risk Coronary Artery Disease Hypertensives. Arterioscler Thromb. Vasc. Biol. 25 (1), 115–121. 10.1161/01.atv.0000149674.62430.e7 [DOI] [PubMed] [Google Scholar]
- Chambers J. C., Ireland H., Thompson E., Reilly P., Obeid O. A., Refsum H., et al. (2000). Methylenetetrahydrofolate Reductase 677 C-->T Mutation and Coronary Heart Disease Risk in UK Indian Asians. Arterioscler Thromb. Vasc. Biol. 20 (11), 2448–2452. 10.1161/01.atv.20.11.2448 [DOI] [PubMed] [Google Scholar]
- Chen F., Wang Y., Xu Y., Zheng H., Song B., He Y., et al. (2009). Polymorphism of Methylene Tetrahydrofolate Reductase Gene in Henan Han Population. J. U Zhengzhou. 44 (2), 317–320. 10.3969/j.issn.1671-6825.2009.02.032 [DOI] [Google Scholar]
- Demir S. C., Evruke C., Ozgunen T., Kadayifci O., Altintas U., Kokangul S. (2006). The Relationship Between Pregnancy Induced Hypertension and Congenital Thrombophilia. Saudi Med. J. 27 (8), 1161–1166. 10.1016/j.revmed.2006.04.007 [DOI] [PubMed] [Google Scholar]
- Demirel Y., Dogan S., Uludag A., Silan C., Atik S., Silan F., et al. (2011). Combined Effect of Factor V Leiden, MTHFR, and Angiotensin-Converting Enzyme (Insertion/Deletion) Gene Mutations in Hypertensive Adult Individuals: a Population-Based Study from Sivas and Canakkale, Turkey. Genet. Test. Mol. Biomarkers. 15 (11), 785–791. 10.1089/gtmb.2011.0044 [DOI] [PubMed] [Google Scholar]
- Deng F. M. (2007). Polymorphism of eNOS and MTHFR Genes, Environmental Factors and Their Interaction Involved in the Pathogenesis of Essential Hypertension in Kazakh Ethnic of Xinjiang. [dissertation]. [Chengdu (Sichuan)]: Sichuan University. [Google Scholar]
- Deshmukh A., Rodrigue K. M., Kennedy K. M., Land S., Jacobs B. S., Raz N. (2009). Synergistic Effects of the MTHFR C677T Polymorphism and Hypertension on Spatial Navigation. Biol. Psychol. 80 (2), 240–245. 10.1016/j.biopsycho.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doris P. A. (2002). Hypertension Genetics, Single Nucleotide Polymorphisms, and the Common Disease:Common Variant Hypothesis. Hypertension. 39 (2 Pt 2), 323–331. 10.1161/hy0202.104087 [DOI] [PubMed] [Google Scholar]
- Dwivedi M. K., Sinha D. (2017). Role of MTHFR 677 C>T Polymorphism on Blood Homocysteine and Susceptibility to Hypertension. Int. J. Hum. Genet. 17 (3), 118–125. 10.1080/09723757.2017.1383619 [DOI] [Google Scholar]
- Fakhrzadeh H., Mirarefin M., Sharifi F. (2009). Association of Methylenetetrahydrofolate Reductase Gene Polymorphism (C677T) With Metabolic Syndrome in an Iranian Population. Tehran Homocysteine Survey. J. Diabetes Metab. Disord. 8, 37–46. [Google Scholar]
- Falaschetti E., Mindell J., Knott C., Poulter N. (2014). Hypertension Management in England: a Serial Cross-Sectional Study From 1994 to 2011. The Lancet. 383 (9932), 1912–1919. 10.1016/s0140-6736(14)60688-7 [DOI] [PubMed] [Google Scholar]
- Fan S., Yang B., Zhi X., Wang Y., Wei J., Zheng Q., et al. (2016). Interactions of Methylenetetrahydrofolate Reductase C677T Polymorphism with Environmental Factors on Hypertension Susceptibility. Int. J. Environ. Res. Public Health. 13, 601. 10.3390/ijerph13060601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowdar J. Y., Lason M. V., Szvetko A. L., Lea R. A., Griffiths L. R. (2012). Investigation of Homocysteine-Pathway-Related Variants in Essential Hypertension. Int. J. Hypertens. 2012, 190923. 10.1155/2012/190923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen J., Juul K., Grande P., Jensen G. B., Schroeder T. V., Tybjærg-Hansen A., et al. (2004). Methylenetetrahydrofolate Reductase Polymorphism (C677T), Hyperhomocysteinemia, and Risk of Ischemic Cardiovascular Disease and Venous Thromboembolism: Prospective and Case-Control Studies From the Copenhagen City Heart Study. Blood. 104 (10), 3046–3051. 10.1182/blood-2004-03-0897 [DOI] [PubMed] [Google Scholar]
- Fridman O., Porcile R., Morales A. V., Gariglio L. O., Potenzoni M. A., Turk Noceto P. C. (2013). Association of Methylenetetrahydrofolate Reductase Gene 677C>T Polymorphism With Hypertension in Older Women in a Population of Buenos Aires City. Clin. Exp. Hypertens. 35 (3), 159–166. 10.3109/10641963.2012.690471 [DOI] [PubMed] [Google Scholar]
- Fridman O., Porcile R., Vanasco V., Junco M. N., Gariglio L., Potenzoni M. A., et al. (2008). Study on Homocysteine Levels and Methylenetetrahydrofolate Reductase Gene Variant (C677T) in a Population of Buenos Aires City. Clin. Exp. Hypertens. 30 (7), 574–584. 10.1080/10641960802251958 [DOI] [PubMed] [Google Scholar]
- Fu L., Li Y. n., Luo D., Deng S., Wu B., Hu Y. Q. (2019). Evidence on the Causal Link between Homocysteine and Hypertension from a Meta‐analysis of 40 173 Individuals Implementing Mendelian Randomization. J. Clin. Hypertens. 21 (12), 1879–1894. 10.1111/jch.13737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. Y., Zhan S. Y., Yin X. J., Hu Y. H., Li L. M. (1999). The Relationship Between Methylenetetrahydrofolate Reductase Polymorphism and Risk of Essential Hypertension. J. Beijing Med. Univ. 31 (4), 370–372. 10.3321/j.issn:1671-167X.1999.04.026 [DOI] [Google Scholar]
- Gheorghe A., Griffiths U., Murphy A., Legido-Quigley H., Lamptey P., Perel P. (2018). The Economic burden of Cardiovascular Disease and Hypertension in Low- and Middle-Income Countries: a Systematic Review. BMC Public Health. 18 (1), 975. 10.1186/s12889-018-5806-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghogomu S. M., Ngolle N. E., Mouliom R. N., Asa B. F. (2016). Association Between the MTHFR C677T Gene Polymorphism and Essential Hypertension in South West Cameroon. Genet. Mol. Res. 15, 28. 10.4238/gmr.15017462 [DOI] [PubMed] [Google Scholar]
- Heux S., Morin F., Lea R., Ovcaric M., Tajouri L., R. Griffiths L. (2004). The Methylentetrahydrofolate Reductase Gene Variant (C677T) as a Risk Factor for Essential Hypertension in Caucasians. Hypertens. Res. 27 (9), 663–667. 10.1291/hypres.27.663 [DOI] [PubMed] [Google Scholar]
- Higgins J. P. (2008). Cochrane Handbook For Systematic Reviews Of Interventions Version 5.1.0. The Cochrane Collaboration. Naunyn-Schmiedebergs Archiv Für Experimentelle Pathologie und Pharmakologie. 5 (2), S38. 10.1016/j.jhsa [DOI] [Google Scholar]
- Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring Inconsistency in Meta-Analyses. Bmj. 327 (7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R. L., Zhao S. Q., Niu G. M., Zhang C. Y., Hu R. L., Wang Z. G., et al. (2007). The Association Between Gene Polymorphisms of N5,10-Methylene Tetrahydrofolate Reductase (MTHFR) and Mongol Nation Patients with Primary Hypertension Disease and Hypertension Complicating Cerebrovascular Disease. Stroke Nerv Dis. 14, 13–15. [Google Scholar]
- Hu R., Niu G. M., Zhao S. G., Zhang C. Y., Hu R. L., Wang Z. G., et al. (2006). The Association Between Gene Polymorphisms of Methylene Tetrahydrofolate Reductase and Mongolian Patients With Drimary Hypertension. Chin. J. Hypertens. 14 (4), 274–276. 10.3969/j.issn.1673-7245.2006.04.010 [DOI] [Google Scholar]
- Hui P., Nakayama T., Morita A., Sato N., Hishiki M., Saito K., et al. (2007). Common Single Nucleotide Polymorphisms in Japanese Patients With Essential Hypertension: Aldehyde Dehydrogenase 2 Gene as a Risk Factor Independent of Alcohol Consumption. Hypertens. Res. 30 (7), 585–592. 10.1291/hypres.30.585 [DOI] [PubMed] [Google Scholar]
- Husemoen L. L. N., Skaaby T., Jørgensen T., Thuesen B. H., Fenger M., Grarup N., et al. (2014). MTHFR C677T Genotype and Cardiovascular Risk in a General Population Without Mandatory Folic Acid Fortification. Eur. J. Nutr. 53 (7), 1549–1559. 10.1007/s00394-014-0659-2 [DOI] [PubMed] [Google Scholar]
- Ilhan N., Kucuksu M., Kaman D., Ilhan N., Ozbay Y. (2008). The 677 C/T MTHFR Polymorphism Is Associated With Essential Hypertension, Coronary Artery Disease, and Higher Homocysteine Levels. Arch. Med. Res. 39 (1), 125–130. 10.1016/j.arcmed.2007.07.009 [DOI] [PubMed] [Google Scholar]
- Ioannidis J. P., Boffetta P., Little J., O'Brien T. R., Uitterlinden A. G., Vineis P., et al. (2008). Assessment of Cumulative Evidence on Genetic Associations: Interim Guidelines. Int. J. Epidemiol. 37 (1), 120–132. 10.1093/ije/dym159 [DOI] [PubMed] [Google Scholar]
- Jin Y., Zhao L. Y., Hou Y. T., Wang Z. F. (2011). Association Between MTHF Gene Polymorphism and Primary Hypertension Complicated With CHD. Chin. J. Geriatr. Heart Brain Vessel Dis. 13 (12), 1081–1083. 10.3969/j.issn.1009-0126.2011.12.008 [DOI] [Google Scholar]
- Kahleová R., Palyzová D., Zvára K., Zvárová J., Hrach K., Nováková I., et al. (2002). Essential Hypertension in Adolescents: Association With Insulin Resistance and With Metabolism of Homocysteine and Vitamins. Am. J. Hypertens. 15 (10 Pt 1), 857–864. 10.1016/s0895-7061(02)02984-9 [DOI] [PubMed] [Google Scholar]
- Kalita J., Srivastava R., Bansal V., Agarwal S., Misra U. K. (2006). Methylenetetrahydrofolate Reductase Gene Polymorphism in Indian Stroke Patients. Neurol. India. 54 (3), 260–263. 10.4103/0028-3886.27148 [DOI] [PubMed] [Google Scholar]
- Kearney P. M., Whelton M., Reynolds K., Muntner P., Whelton P. K., He J. (2005). Global Burden of Hypertension: Analysis of Worldwide Data. The Lancet. 365 (9455), 217–223. 10.1016/s0140-6736(05)17741-1 [DOI] [PubMed] [Google Scholar]
- Kobashi G., Yamada H., Asano T., Nagano S., Hata A., Kishi R., et al. (2000). Absence of Association between a Common Mutation in the Methylenetetrahydrofolate Reductase Gene and Preeclampsia in Japanese Women. Am. J. Med. Genet. 93 (2), 122–125. [DOI] [PubMed] [Google Scholar]
- Krzesinski J. M., Saint-Remy A. (2012). Essential Hypertension, a Complex Trait. Rev. Med. Liege. 67 (5-6), 279–285. [PubMed] [Google Scholar]
- Lau J., Antman E. M., Jimenez-Silva J., Kupelnick B., Mosteller F., Chalmers T. C. (1992). Cumulative Meta-Analysis of Therapeutic Trials for Myocardial Infarction. N. Engl. J. Med. 327 (4), 248–254. 10.1056/nejm199207233270406 [DOI] [PubMed] [Google Scholar]
- Li K., Sun Y., Chen L. (2000). Study on the Relationship Between Methylenetetrahydrofolate Reductase Gene Polymorphism and Plasma Homocysteine Level in Pregnancy Induced Hypertension Patients. Zhonghua Fu Chan Ke Za Zhi. 35 (4), 205–207. [PubMed] [Google Scholar]
- Li X., Huang W. (2006). The Analysis of MTHFR Gene Polymorphism in Patients with Renal Damage Caused by Hypertension and Patients With Renal Parenchymal Hypertension. J. Capital Univ. Med. Sci. 27 (4), 497–500. 10.3969/j.issn.1006-7795.2006.04.021 [DOI] [Google Scholar]
- Lin P. T., Cheng C. H., Wei J. C., Huang Y. C. (2008). Low Plasma Pyridoxal 5'-Phosphate Concentration and MTHFR 677C→T Genotypes Are Associated With Increased Risk of Hypertension. Int. J. Vitamin Nutr. Res. 78 (1), 33–40. 10.1024/0300-9831.78.1.33 [DOI] [PubMed] [Google Scholar]
- Liu H., Chen S., Ma P., Xu Q. (2011a). The Association Between Gene Polymorphisms of N5,10-Methylenetetrahydrofolate Reductase and Essential Hypertension in Patients of Ningxia Hui Nationality. Tianjin Med. J. 39 (12), 1095–1098. 10.3969/j.issn.0253-9896.2011.12.003 [DOI] [Google Scholar]
- Liu H. Y., Ma P., Xu Q. B. (2011b). The Correlation between Polymor-Phisms of N5,10 Methylenetetrahydrofolate Reductase and Essential Hypertension in Han Population in Ningxia. Guangdong Med. J. 32 (15), 1977–1980. 10.3969/j.issn.1001-9448.2011.15.014 [DOI] [Google Scholar]
- Liu J. W., Ye L., Liu J., Li X. Y. (2004). Methylenetetrahydrofolate Reductase Gene Polymorphism and Susceptibility to Peripheral Arterial Occlusive Disease in Hypertensive Patients. Chin. J. Geriatr. Brain Vessel Dis. 6 (1), 4–6. 10.3969/j.issn.1009-0126.2004.01.002 [DOI] [Google Scholar]
- Liu J., Ye L., Liu J., Li X. Y. (2005). Study on Homocysteine Metabolism Related Enzymes Gene Polymorphisms in Elderly Essential Hypertension Patients With Peripheral Arterial Occlusive Disease. Chin. J. Geriatr. 24 (3), 332–335. 10.3760/jissn:0254-9026.2005.05.003 [DOI] [Google Scholar]
- Liu S., Liu M., Li Q., Liu X., Wang Y., Mambiya M., et al. (2019). Association of Single Nucleotide Polymorphisms of MTHFR, TCN2, RNF213 With Susceptibility to Hypertension and Blood Pressure. Biosci. Rep. 39, BSR20191454. 10.1042/bsr20191454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. W., Tang Y., Chen H., Wu X. Y., Wu Y. A., Deng Y. L. (2008). Study on MTHFR C677T Polymorphism in Hypertensive Subjects With Blood Stasis Syndrome. J. Beijing Univ. Tradit Chin. Med. 31 (5), 351–354. 10.7666/dy1194718 [DOI] [Google Scholar]
- Lwin H., Yokoyama T., Yoshiike N., Saito K., Yamamoto A., Date C., et al. (2006). Polymorphism of Methylenetetrahydrofolate Reductase Gene (C677T MTHFR) Is Not a Confounding Factor of the Relationship Between Serum Uric Acid Level and the Prevalence of Hypertension in Japanese Men. Circ. J. 70 (1), 83–87. 10.1253/circj.70.83 [DOI] [PubMed] [Google Scholar]
- Ma J., Yang P. (2011). The Correlation Between the Gene Polymorphisms of MTHFR and eNOS and Essential Hypertension of the Han Nationality in Yunnan Province. Chin. J. Cardio Res. 9 (12), 909–912. 10.3969/j.issn.1672-5301.2011.12.007 [DOI] [Google Scholar]
- Marinho C., Alho I., Arduíno D., Falcão L. M., Brás-Nogueira J., Bicho M. (2007). GST M1/T1 and MTHFR Polymorphisms as Risk Factors for Hypertension. Biochem. Biophysical Res. Commun. 353 (2), 344–350. 10.1016/j.bbrc.2006.12.019 [DOI] [PubMed] [Google Scholar]
- Markan S., Sachdeva M., Sehrawat B. S., Kumari S., Jain S., Khullar M. (2007). MTHFR 677 CT/MTHFR 1298 CC Genotypes Are Associated With Increased Risk of Hypertension in Indians. Mol. Cell Biochem. 302 (1-2), 125–131. 10.1007/s11010-007-9434-5 [DOI] [PubMed] [Google Scholar]
- Mendilcioglu I., Bilgen T., Arikan Y., Keser I., Simsek M., Timuragaoglu A. (2011). The Association Between Inherited Thrombophilias and Pregnancy-Related Hypertension Recurrence. Arch. Gynecol. Obstet. 284 (4), 837–841. 10.1007/s00404-010-1756-y [DOI] [PubMed] [Google Scholar]
- Mills K. T., Stefanescu A., He J. (2020). The Global Epidemiology of Hypertension. Nat. Rev. Nephrol. 16 (4), 223–237. 10.1038/s41581-019-0244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B., Hupuczi P., Papp Z. (2007). High Frequency of Methylenetetrahydrofolate Reductase 677TT Genotype in Hungarian HELLP Syndrome Patients Determined by Quantitative Real-Time PCR. J. Hum. Hypertens. 21 (2), 154–158. 10.1038/sj.jhh.1002122 [DOI] [PubMed] [Google Scholar]
- Nakata Y., Katsuya T., Takami S., Sato N., Fu Y., Ishikawa K., et al. (1998). Methylenetetrahydrofolate Reductase Gene Polymorphism: Relation to Blood Pressure and Cerebrovascular Disease. Am. J. Hypertens. 11 (8 Pt 1), 1019–1023. 10.1016/s0895-7061(98)00046-6 [DOI] [PubMed] [Google Scholar]
- Nassereddine S., Kassogue Y., Korchi F., Habbal R., Nadifi S. (2015). Association of Methylenetetrahydrofolate Reductase Gene (C677T) With the Risk of Hypertension in Morocco. BMC Res. Notes. 8, 775. 10.1186/s13104-015-1772-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton-Cheh C., Johnson T., Gateva V., Tobin M. D., Bochud M., Coin L., et al. (2009). Genome-wide Association Study Identifies Eight Loci Associated With Blood Pressure. Nat. Genet. 41 (6), 666–676. 10.1038/ng.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng X., Boyd L., Dufficy L., Naumo vs. ki N., Blades B., Travers C., et al. (2009). Folate Nutritional Genetics and Risk for Hypertension in an Elderly Population Sample. J. Nutrigenet Nutrigenomics. 2 (1), 1–8. 10.1159/000160079 [DOI] [PubMed] [Google Scholar]
- Nishio H., Lee M. J., Fujii M., Kario K., Kayaba K., Shimada K., et al. (1996). A Common Mutation in Methylenetetrahydrofolate Reductase Gene Among the Japanese Population. Jap J. Hum. Genet. 41 (2), 247–251. 10.1007/bf01875985 [DOI] [PubMed] [Google Scholar]
- Niu W.-Q., You Y.-G., Qi Y. (2012). Strong Association of Methylenetetrahydrofolate Reductase Gene C677T Polymorphism with Hypertension and Hypertension-In-Pregnancy in Chinese: a Meta-Analysis. J. Hum. Hypertens. 26 (4), 259–267. 10.1038/jhh.2011.11 [DOI] [PubMed] [Google Scholar]
- Nong Q., Lu W. Q., Pan X. S., Li T. Z., Wei B. M., Du H. L., et al. (2019). Blood Pressure and Homocysteine Levels in Patients With Hypertension: a Meta-Analysis. Chin. J. Pharm. Sci. 9 (19), 9–13. 10.3969/j.issn.2095-0616.2019.19.005 [DOI] [Google Scholar]
- Nursal A. F., Kaya S., Sezer O., Karakus N., Yigit S. (2018). MTHFR Gene C677T and A1298C Variants Are Associated With FMF Risk in a Turkish Cohort. J. Clin. Lab. Anal. 32, e22259. 10.1002/jcla.22259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell C. J., Lindpaintner K., Larson M. G., Rao V. S., Ordovas J. M., Schaefer E. J., et al. (1998). Evidence for Association and Genetic Linkage of the Angiotensin-Converting Enzyme Locus with Hypertension and Blood Pressure in Men but Not Women in the Framingham Heart Study. Circulation. 97 (18), 1766–1772. 10.1161/01.cir.97.18.1766 [DOI] [PubMed] [Google Scholar]
- O'Donnell C. J., Nabel E. G. (2008). Cardiovascular Genomics, Personalized Medicine, and the National Heart, Lung, and Blood Institute. Circ. Cardiovasc. Genet. 1 (1), 51–57. 10.1161/circgenetics.108.813337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Razo J. C., Cano-Martínez L. J., Vargas Alarcón G., Canizales-Quinteros S., Martínez-Rodríguez N., Canto P., et al. (2015). Functional Polymorphism Rs13306560 of the MTHFR Gene Is Associated With Essential Hypertension in a Mexican-Mestizo Population. Circ. Cardiovasc. Genet. 8 (4), 603–609. 10.1161/circgenetics.114.000942 [DOI] [PubMed] [Google Scholar]
- Powers R. W., Minish L. A., Lykins D. L., Ness R. B., Crombleholme W. R., Roberts J. M. (1999). Methylenetetrahydrofolate Reductase Polymorphism, Folate, and Susceptibility to Preeclampsia. J. Soc. Gynecol. Investig. 6 (2), 74–79. 10.1177/107155769900600205 [DOI] [PubMed] [Google Scholar]
- Qian X., Lu Z., Tan M., Liu H., Lu D. (2007). A Meta-Analysis of Association between C677T Polymorphism in the Methylenetetrahydrofolate Reductase Gene and Hypertension. Eur. J. Hum. Genet. 15 (12), 1239–1245. 10.1038/sj.ejhg.5201914 [DOI] [PubMed] [Google Scholar]
- Rafiq S., Anand S., Roberts R. (2010). Genome-Wide Association Studies of Hypertension: Have They Been Fruitful? J. Cardiovasc. Trans. Res. 3 (3), 189–196. 10.1007/s12265-010-9183-9 [DOI] [PubMed] [Google Scholar]
- Rajkovic A., Mahomed K., Rozen R., Malinow M. R., King I. B., Williams M. A. (2000). Methylenetetrahydrofolate Reductase 677 C--> T Polymorphism, Plasma Folate, Vitamin B(12) Concentrations, and Risk of Preeclampsia Among Black African Women From Zimbabwe. Mol. Genet. Metab. 69 (1), 33–39. 10.1006/mgme.1999.2952 [DOI] [PubMed] [Google Scholar]
- Ren Y., He Y. H., Cao M. J. (2018). Correlation Analysis of MTHFR and MTRR Gene Related Mutations With H Type Hypertension. J. Yan'an U. 16 (3), 73–76. 10.3969/j.issn.1672-2639.2018.03.021 [DOI] [Google Scholar]
- Rios D. R. A., Alpoim P. N., Godoi L. C., Mendes F. S., Lwaleed B., Sousa L. P., et al. (2017). Is There a Link Among Thrombophilia Factors and Preeclampsia? J. Thromb. Thrombolysis. 44 (4), 516–518. 10.1007/s11239-017-1556-3 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Esparragón F., Hernández-Perera O., Rodríguez-Pérez J. C., Anábitarte A., Díaz-Cremades J. M., Losada A., et al. (2003). The Effect of Methylenetetrahydrofolate Reductase C677T Common Variant on Hypertensive Risk Is Not Solely Explained by Increased Plasma Homocysteine Values. Clin. Exp. Hypertens. 25 (4), 209–220. 10.1081/ceh-120020391 [DOI] [PubMed] [Google Scholar]
- Rothstein E. C., Carroll S., Combs C. A., Jobsis P. D., Balaban R. S. (2005). Skeletal Muscle NAD(P)H Two-Photon Fluorescence Microscopy In Vivo: Topology and Optical Inner Filters. Biophysical J. 88 (3), 2165–2176. 10.1529/biophysj.104.053165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Singh A. K., Pandey P., Chandra S., Singh K. A., Gambhir I. S. (2016). Molecular Genetics of Essential Hypertension. Clin. Exp. Hypertens. 38 (3), 268–277. 10.3109/10641963.2015.1116543 [DOI] [PubMed] [Google Scholar]
- Soares A. L., Fernandes A. P., Cardoso J. E., Sousa M. O., Lasmar M. C., Novelli B. A., et al. (2008). Plasma Total Homocysteine Levels and Methylenetetrahydrofolate Reductase Gene Polymorphism in Patients With Type 2 Diabetes Mellitus. Pathophysiol Haemost. Thromb. 36 (5), 275–281. 10.1159/000252825 [DOI] [PubMed] [Google Scholar]
- Sohda S., Arinami T., Hamada H., Yamada N., Hamaguchi H., Kubo T. (1997). Methylenetetrahydrofolate Reductase Polymorphism and Pre-Eclampsia. J. Med. Genet. 34 (6), 525–526. 10.1136/jmg.34.6.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staessen J. A., Wang J., Bianchi G., Birkenhäger W. H. (2003). Essential Hypertension. The Lancet. 361 (9369), 1629–1641. 10.1016/s0140-6736(03)13302-8 [DOI] [PubMed] [Google Scholar]
- Su N. J., Li B., Fen J. H., Yu B. (2011). Study on the Relationship Between N 5,10-methtlenetetrahydrofolate Reductase Gene and Endothelial Nitric Oxide Synthase Gene Polymorphisms and Preeclampsia and Eclampsia in the Han Nationality Women of Guangdong. Prog. Obstet. Gynecol. 20 (3), 199–202. 10.13283/j.cnki.xdfckjz.2011.03.017 [DOI] [Google Scholar]
- Sul J. H., Martin L. S., Eskin E. (2018). Population Structure in Genetic Studies: Confounding Factors and Mixed Models. Plos Genet. 14 (12), e1007309. 10.1371/journal.pgen.1007309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. G., Li Y. M., Guo H. (2003). The Gene Polymorphisms of Homocysteine Metabolism-Related Enzymes and the Associated Factors in Isolated Systolic Hypertension. Chin. J. Cardiol. 31 (4), 269–273. 10.3760/jissn:0253-3758.2003.04.009 [DOI] [Google Scholar]
- Swartz M. K. (2011). The PRISMA Statement: a Guideline for Systematic Reviews and Meta-Analyses. J. Pediatr. Health Care. 25 (1), 1–2. 10.1016/j.pedhc.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Tang Y., Chen H., Wu X. Y., Luo J. W. (2007). The C677T Point Mutation of N5,10-Methylene Tetrahydrofolate Reductase (MTHFR) and Essential Hypertension. Mol. Cardiol. Chin. 7 (4), 205–207. 10.3969/j.issn.1671-6272.2007.04.004 [DOI] [Google Scholar]
- Thakkinstian A., McKay G. J., McEvoy M., Chakravarthy U., Chakrabarti S., Silvestri G., et al. (2011). Systematic Review and Meta-Analysis of the Association Between Complement Component 3 and Age-Related Macular Degeneration: A HuGE Review and Meta-Analysis. Am. J. Epidemiol. 173 (12), 1365–1379. 10.1093/aje/kwr025 [DOI] [PubMed] [Google Scholar]
- Tian J. (2019). The Association between Single Nucleotide Polymorphisms and the Incidence of Esophageal Cancer: Evaluation from Meta-Analysis and Genome-wide Association Studies. [dissertation]. [Chong qing (Sichuan)]: Chongqing Medical University. [Google Scholar]
- Tylicki L., Födinger M., Puttinger H., Rutkowski P., Strozecki P., Tyszko S., et al. (2005). Methylenetetrahydrofolate Reductase Gene Polymorphisms in Essential HypertensionRelation With the Development of Hypertensive End-Stage Renal Disease. Am. J. Hypertens. 18 (11), 1442–1448. 10.1016/j.amjhyper.2005.05.012 [DOI] [PubMed] [Google Scholar]
- Vazquez-Alaniz F., Lumbreras-Márquez M. I., Sandoval-Carrillo A. A., Aguilar-Durán M., Méndez-Hernández E. M., Barraza-Salas M., et al. (2014). Association of COMT G675A and MTHFR C677T Polymorphisms with Hypertensive Disorders of Pregnancy in Mexican Mestizo Population. Pregnancy Hypertens. Int. J. Women's Cardiovasc. Health. 4 (1), 59–64. 10.1016/j.preghy.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Wakefield J. (2007). A Bayesian Measure of the Probability of False Discovery in Genetic Epidemiology Studies. Am. J. Hum. Genet. 81 (2), 208–227. 10.1086/519024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Su A. J., Wu G. Z., Zhang Y., Chen Y. L. (2010a). Relationship of Homocysteine, Methylenetetrahydrofolate Reductase (MTHFR) A1298C Polymorphism and Essential Hypertension in Xinjiang Kazakhs. Chin. J. Misdiagn. 10 (16), 3787–3790. [Google Scholar]
- Wang H., Wu G. Z., Zhang Y., Zhang X. Y., Chen Y. L., Ai S. K. (2010b). Association of Homocysteine and its Metabolic Enzyme Genes Polymorphisms With Essential Hypertension in Xinjiang Kazakhs. J. Clin. Rehab Tissue Eng. Res. 14 (33), 6247–6252. 10.3969/j.issn.1673-8225.2010.33.041 [DOI] [Google Scholar]
- Wang L., Guo H., Li Y. M. (2002). MTHFR Gene C677T Polymorphisms and Variation of Plasma Homocysteine Levels. Tianjin Med. 30 (10), 579–582. 10.3969/j.issn.0253-9896.2002.10.001 [DOI] [Google Scholar]
- Wang Y., Xu X., Huo Y., Liu D., Cui Y., Liu Z., et al. (2015). Predicting Hyperhomocysteinemia by Methylenetetrahydrofolate Reductase C677T Polymorphism in Chinese Patients With Hypertension. Clin. Appl. Thromb. Hemost. 21 (7), 661–666. 10.1177/1076029613519849 [DOI] [PubMed] [Google Scholar]
- Wang Z. G., Wang R., Zhang Y., Zhi F., Yang Y. L. (2009). 2-[6,8-Dibromo-3-(4-hydroxy-cyclo-hexyl)-1,2,3,4-tetra-hydro-quinazolin-2-yl]-6-methoxy-phenol. Acta Crystallogr. Sect E Struct. Rep. Online. 65 (Pt 3), o550. 10.1107/s1600536809005182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L. K., Menon S., Griffiths L. R., Gan S. H. (2015). Signaling Pathway Genes for Blood Pressure, Folate and Cholesterol Levels Among Hypertensives: an Epistasis Analysis. J. Hum. Hypertens. 29 (2), 99–104. 10.1038/jhh.2014.53 [DOI] [PubMed] [Google Scholar]
- Weisberg I., Jacques P. F., Selhub J., Bostom A. G., Chen Z., Curtis Ellison R., et al. (2001). The 1298A→C Polymorphism in Methylenetetrahydrofolate Reductase (MTHFR): In Vitro Expression and Association With Homocysteine. Atherosclerosis. 156 (2), 409–415. 10.1016/s0021-9150(00)00671-7 [DOI] [PubMed] [Google Scholar]
- Wen C., Lv J.-F., Wang L., Zhu W.-F., Wan F.-S., Wang X.-Z. (2015). Association of a Methylene Tetrahydrofolate Reductase C677T Polymorphism With Several Blood Chemical Levels in a Chinese Population. Genet. Test. Mol. Biomarkers. 19 (1), 24–29. 10.1089/gtmb.2014.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.-L., Hu C.-Y., Lu S.-S., Gong F.-F., Feng F., Qian Z.-Z., et al. (2014). Association Between Methylenetetrahydrofolate Reductase (MTHFR) C677T/A1298C Polymorphisms and Essential Hypertension: a Systematic Review and Meta-Analysis. Metabolism. 63 (12), 1503–1511. 10.1016/j.metabol.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Wu Z. Q., Xu Y. J. (2016). Analysis on Gene Polymorphism of MTHFR C677T in Han Population of south China Area. Int. J. Lab. Med. 37 (13), 1791–1795. 10.3969/j.issn.1673-4130.2016.13.017 [DOI] [Google Scholar]
- Wu Z. Y., Li X. M., Guo T. L., Lin Y. (2019). Study on the Relationship Between Methylenetrahydrofolate Reductase C677T Gene Polymorphism and Serum Homocysteine Level in Patients With Essential Hypertension in Fujian Han People. J. Clin. Ration Drug Use. 12 (29), 10–15. 10.15887/j.cnki.13-1389/r.2019.29.005 [DOI] [Google Scholar]
- Xing X. R., Hua Q. (2007). Relationships Between the Polymorphism of Methylenetetrahydrofolate Reductase Gene C677T and Hypertension, Cardiac Structure and Function. Med. J. Chin. PLA. 32 (7), 741–744. 10.3321/j.issn:0577-7402.2007.07.028 [DOI] [Google Scholar]
- Xue W.-Q., He Y.-Q., Zhu J.-H., Ma J.-Q., He J., Jia W.-H. (2014). Association of BRCA2 N372H Polymorphism with Cancer Susceptibility: a Comprehensive Review and Meta-Analysis. Sci. Rep. 4, 6791. 10.1038/srep06791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Fan S., Zhi X., Li Y., Liu Y., Wang D., et al. (2014a). Associations of MTHFR Gene Polymorphisms With Hypertension and Hypertension in Pregnancy: a Meta-Analysis From 114 Studies With 15411 Cases and 21970 Controls. PLoS One. 9 (2), e87497. 10.1371/journal.pone.0087497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K.-M., Jia J., Mao L.-N., Men C., Tang K.-T., Li Y.-Y., et al. (2014b). Methylenetetrahydrofolate Reductase C677T Gene Polymorphism and Essential Hypertension: A Meta-Analysis of 10,415 Subjects. Biomed. Rep. 2 (5), 699–708. 10.3892/br.2014.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Chen Z.-Y., Lin Y. (2013). Advancement of Targeted Ultrasound Contrast Agents and Their Applications in Molecular Imaging and Targeted Therapy. Curr. Pharm. Des. 19 (8), 1516–1527. 10.2174/1381612811319080019 [DOI] [PubMed] [Google Scholar]
- Yang X. L., Gu D. F. (2016). Distribution and Status of Salt Sensitive Genes Associated with Hypertension [Conference Presentation]. The 14th Chinese congress of Cardio-Brain Medicine. Beijing: China. [Google Scholar]
- Yao R. Y., Zhang H., Zhang J., Li D., Fan Y. (2013). Detection of C677T Polymorphism of MTHFR Gene in Patients With Primary Hypertension of Han Nationality in Henan Province. Chin. J. Gerontol. 33 (05), 1001–1003. 10.3969/j.issn.1005-9202.2013.05.005 [DOI] [Google Scholar]
- Yilmaz H., Unlüçerçi Y., Gürdöl F., Isbilen E., Isbir T. (2004). Association of Pre-Eclampsia With Hyperhomocysteinaemia and Methylenetetrahydrofolate Reductase Gene C677T Polymorphism in a Turkish Population. Aust. New Zealand J. Obstet. Gynaecol. 44 (5), 423–427. 10.1111/j.1479-828x.2004.00283.x [DOI] [PubMed] [Google Scholar]
- Yin R.-X., Wu J.-Z., Liu W.-Y., Wu D.-F., Cao X.-L., Miao L., et al. (2012). Association of Several Lipid-Related Gene Polymorphisms and Blood Pressure Variation in the Bai Ku Yao Population. Am. J. Hypertens. 25 (8), 927–936. 10.1038/ajh.2012.55 [DOI] [PubMed] [Google Scholar]
- Yu W. (2010). Relationship Between Polymorphism of Methylenetetrahydrofolate Reductase Gene and Pregnancy Induced Hypertension Syndrome. J. Henan Med. Col Staff Workers. 22 (6), 648–651. 10.3969/j.issn.1008-9276.2010.06.005 [DOI] [Google Scholar]
- Zhan S., Gao Y., Yin X., Huang Y., Hu Y., Li L. (2000a). A Case-Control Study on the Relationship Between Abnormal Homocysteine Metabolism and Essential Hypertension. Zhonghua Liu Xing Bing Xue Za Zhi. 21 (3), 194–197. [PubMed] [Google Scholar]
- Zhan S., Gao Y. Y., Yin X. J., Hu Y. H., Li L. M., Vivian N., et al. (2000b). Elevated Serum Homocystein, MTHFR Gene Mutation and Essential Hypertension in Chinese. Chin. J. Hypertens. 8 (1), 21–25. 10.3969/j.issn.1673-7245.2000.01.008 [DOI] [Google Scholar]
- Zhang J., Wang Y. X., Sun Q., Zhang X. Y., Sun M., Zhang J. X., et al. (2018). Analysis of the Relationship Between Methylene Tetrahydrofolate Reductase Gene C667T Polymorphism and EH. Chin. Med. Equipment. 15 (8), 126–133. 10.3969/J.ISSN.1672-8270.2018.08.036 [DOI] [Google Scholar]
- Zhang T. S., Zhong W. Z. (2009). Performance of the Nonparametric Trim and Fill Method in Stata. J. Evid-based Med. 9 (4), 240–242. 10.3969/j.issn.1671-5144.2009.04.026 [DOI] [Google Scholar]
- Zhang Y., Wang H., Zhang X. Y., Wang L., Wu G. Z. (2012). Relationship Between Homocysteine, Methylene Tetrahydrofolate Reductase C677T Polymorphisms and Essential Hypertension in Kazak Nationality in Xinjiang. J. Clin. Cardiol. (China). 28 (8), 570–573. 10.13201/jissn.1001-1439.2012.08.004 [DOI] [Google Scholar]
- Zhang Y. Y., Liang R., Ma S. L. (2017). Guo, X.kStudy on the Association Between 5, 10-methylenetrahydrofolate Reductase C677T Gene Polymorphism and Senile Simple Systolic Hypertension. Chin J. Geriatr. Cardio-Cerebrovascular Dis. 19 (8), 821–824. 10.3969/jissn.1009-0126.2017.08.01 [DOI] [Google Scholar]
- Zhao C. L., Zhang X. W., Wei Y. Q., Wu J. H., Chen X. P. (2019). Polymorphism of Homocysteine and its Metabolic Enzyme MTHFR C677T in Hypertensive Population in Guangxi. J. Guangxi Med. U. 36 (4), 570–573. 10.16190/j.cnki.45-1211/r.2019.04.018 [DOI] [Google Scholar]
- Zhao X., Qiu C. F., Shi Z. H., Deng Z. W., Weng H., Yang Y. H., et al. (2018). Evaluation of the Credibility of Evidence in Meta-Analysis of Genetic Association. Chin. J. Evid-based Med. 18 (8), 883–887. 10.7507/1672-2531.201804142 [DOI] [Google Scholar]
- Zhao Y. M., Li P., Xu W. Y., Xie W. C., Lin Z. H., Huang C. J., et al. (2017). The Relationship Between Plasma Homocysteine and Methylene Tetrahydrofolate Reductase Gene Polymorphism and Essential Hypertension in Han People in Yulin Area, Southwest China. Chin. J. Cardio Res. 15 (6), 528–532. 10.3969/jissn.1672-5301.2017.06.013 [DOI] [Google Scholar]
- Zhong F., Zhuang L., Wang Y., Ma Y. (2017). Homocysteine Levels and Risk of Essential Hypertension: A Meta-Analysis of Published Epidemiological Studies. Clin. Exp. Hypertens. 39 (2), 160–167. 10.1080/10641963.2016.1226888 [DOI] [PubMed] [Google Scholar]
- Zusterzeel P. L. M., Visser W., Bolm H. J., Peters W. H. M., Heil S. G., Steegers E. A. P., et al. (2000). Methylenetetrahydrofolate Reductase Polymorphisms in Preeclampsia and the HELLP Syndrome. Hypertens. Pregnancy. 19 (3), 299–307. 10.1081/prg-100101991 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.