Abstract
Importance
COVID-19 altered lifestyles and disrupted routine health care. Whether blood pressure (BP) control worsened during COVID-19 is unknown.
Objective
To understand whether home BP control worsened during COVID-19 across the United States (US) .
Design, Setting, and Participants
A population-based analysis of home BP data from 72,706 participants enrolled in a digital health hypertension control program. Data was compared before (January 2019 to March 2020) and during (April 2020 to August 2020) COVID-19.
Main Outcomes and Measures
Monthly mean home BP readings, systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) were quantified before and during the pandemic. Multivariable adjustments were made for age, sex, race, region, and months enrolled. Home BP readings were also classified based on monthly averages and highest home BP readings into risk groups: Stage 2 HTN: BP> = 135 or DBP> = 85; Uncontrolled HTN: SBP> = 145 or DBP> = 95; or Severely uncontrolled HTN: SBP> = 160 or DBP> = 100).
Results
Overall, 72,706 participants were enrolled in a digital health hypertension program between 1/1/2019 and 8/31/2020. Compared with participants pre-COVID-19 (n = 33,440), those during COVID-19 (n = 39,266) were of similar age (mean 53.0 ± 10.7 years vs 53.3 ± 10.8 years); sex (46% vs 50.6% female) and race (29.1% vs 34.2% non-white). Relative to pre-Covid (Apr-Aug 2019) the mean monthly number of home BP readings rose during COVID-19 (Apr-Aug, 2020), from 7.3 to 9.3 per month (P < .001). During COVID-19, participants had higher monthly adjusted mean SBP (131.6 mmHg vs. 127.5 mmHg, P < .001); DBP (80.2 mmHg vs. 79.2 mmHg, P < .001); and MAP (97.4 mmHg vs. 95.3 mmHg; P < .001). Relative to the pre-pandemic period, during COVID-19 the proportion of participants with a mean monthly BP classified as uncontrolled or severely uncontrolled hypertension also rose, 15% vs 19% and 4% vs 5%, respectively
Conclusions and Relevance
Based on home BP readings, mean monthly BP rose modestly after COVID-19, despite increased utilization of home monitoring. Further studies are needed to examine the longitudinal effects of the pandemic on cardiovascular disease risk factors, the impact of these on long-term population health.
The COVID-19 pandemic has affected millions worldwide. In the United States 30 million have contracted the virus, and of these, more than 500,000 have died.1 , 2 COVID-19 has also had multiple direct and indirect negative impacts on individuals’ health and well-being. Many are out-of-work, socially isolated, sedentary, and depressed. Furthermore, fears of viral exposure have disrupted routine in-person health care visits, vital for the treatment of chronic conditions.3
Hypertension is a highly prevalent chronic condition and a strong modifiable risk factor for cardiovascular disease (CVD). The risk of CVD in individuals rises sharply with increasing blood-pressure (BP)4., 5., 6., 7., 8. and even a few mmHg rise in BP at the population level can mean significant increased rates of heart attack, stroke, heart failure and chronic kidney disease.5 More recently, individuals with hypertension have been found to have higher risk for death among those contracting COVID-19.9 Prior to the pandemic hypertension control rates remained suboptimal; nearly a quarter of adults with hypertension are not at treatment goals.10 How this may have changed during the pandemic remains largely unknown. To date, though there is very limited information on population level blood control rates during the COVID-19 pandemic, there are reports from death certificate data11 that deaths from ‘hypertension’ have risen substantially in 2020.
Using data from a large national digital health BP disease management program run by Livongo, we examined community-based trends in home BP measurement and control. Specifically, we examined three questions: (1) Relative to similar periods before COVID-19, did individuals monitor their home BP more or less during COVID-19?; (2) Did population mean BP rise during COVID-19?; and (3) Did rates of severely uncontrolled BP also rise with COVID-19?
Statistical methods
Program characteristics
The Livongo Hypertension program is a digital chronic condition management program that combines: (1) a Food and Drug Administration cleared, cellular-connected, BP monitor and upper arm cuff; (2) unlimited scheduled access to clinicians (e.g., nutritionists, dieticians, exercise physiologists) for goal setting, and lifestyle education based on guideline recommendations; and (3) mobile phone application on iOS and Android platforms. The BP monitor technology used by Livongo has been clinically validated to be within +/− 3 mmHg of actual BP. The technology platform automatically uploads all BP readings taken. It also gives participants reminders for BP checking, and allows participants to send Health Summary Reports of BP readings to care providers, family members, and friends via email. The program provides evidence-based lifestyle and health advice, however, it does not directly give any specific medication treatment recommendations. The participants are not paid to participate in the program. In contrast, the program had a cost to participate which was typically born by the participant's employer and/or their health insurance provider.
Database
This study included data from patients using the digital platform between January 1, 2019 and August 31, 2020. For this study, we defined the onset of the pandemic as April 1, 2020 to coincide with the shutdown imposed across many U.S. states at that time. Participant sex, race, and ethnicity were provided by the user at time of registration. Age, geographic region, account status, and enrollment status were based on the most recent information available at the time of the data cut (September 2020). Smoking status, height, weight, and body mass index (BMI) were self-reported and based on the most recent health status questionnaire. All available BP measurements collected in the digital platform from January 2019 to August 2020 were analyzed. These measures included systolic blood pressure (SBP), diastolic blood pressure (DBP), and frequency of BP readings. Mean arterial blood pressure (MAP) was calculated using the formula: MAP = (SBP + 2*DBP)/3.
For each user, the number of BP measurements per month were calculated. For the primary analysis, individuals who had no readings in a month (but did have readings in subsequent months) were assigned a zero value for that month. If the individual had no subsequent readings, they were conservatively ‘censored’ after a month of no readings and were considered to have self-discontinued the program. Based on all readings recorded, mean monthly SBP, DBP, and MAP for each participant were obtained. A sensitivity analysis was also performed limited to participants who had BP measurements in both the pre-pandemic and pandemic periods.
Participants were also classified monthly into major BP severity categories in two different ways: (1) based on their mean BP readings during the month or alternatively, (2) based on their highest single BP reading in each month (peak BP reading). The SBP and DBP measures were used to define hypertension classifications during each calendar month. Because home BP monitor values are typically lower then office-based BPs, we based our BP categories on current BP guidelines with refinements to the home thresholds as suggested by the current measurement guidelines.12., 13. Additionally, since we were specifically interested in whether there was an increase in higher risk, poorly controlled hypertension we classified mean monthly blood pressure into Normal/Stage 1 (defined as a home SBP of < 135 and DBP < 85); Stage 2 HTN (defined as home SBP of > = 135 or DBP> = 85); Uncontrolled HTN (defined as home SBP> = 145 or DBP > = 95), and Severely uncontrolled HTN (defined as home SBP> = 160 or DBP> = 100). These classifications of monthly BP was then repeated based on the highest single BP reading in a given month (peak BP reading).
Statistics
Summary statistics for these characteristics are presented for the overall cohort, and for users contributing data within each quarter of the study period. Continuous user characteristics are summarized using mean (SD), median (interquartile range), and range. Categorical characteristics are summarized using frequencies (percentages). Descriptive summaries were based on patients with available BP data. To assess whether changes in average monthly mean values of SBP, DBP, and MAP were associated with the advent of the COVID-19 pandemic, Generalized Estimating Equations (GEE) were used to fit linear models with repeated measures, assuming an independent working correlation structure for measurements within a patient. The model included factors for the associated month and year from which the mean values were determined, and included the interaction between year and month. Endpoints were analyzed in unadjusted models, as well as models adjusted for age, sex, race, region, and months-in-program (from first billing date in the program). In adjusted models, means were calculated using average covariate values for January, 2020.
The fitted model was used to estimate monthly means during the study period, and mean differences between 2020 vs. 2019 for calendar months January-August. To assess the “pandemic effect”, a “difference-in-differences” contrast was tested comparing the mean between-year difference in April-June (pandemic months in 2020, but not 2019) to the mean between-year difference in January-March (pre-pandemic months in both years). All model estimates are reported with a 95% confidence interval and p-value calculated using the robust covariance matrix estimate.
A sensitivity analysis was performed to compare the number of BP readings, SBP, DBP, and MAP during the first 3 months of 2020 (directly prior to COVID-19) to those seen from April to August 2020 (during COVID-19) to evaluate for additional pandemic related differences. Furthermore, an additional sensitivity analysis was performed on a subset of participants who had BP measurements in both the pre-pandemic and pandemic period. Additionally, to evaluate whether participants had higher peak BP during COVID-19, we repeated the classification analysis using participants’ highest single BP reading during each month.
A modeling strategy analogous to that described above was also employed to evaluate the frequency of BP monitoring. For the analysis of this count variable (monthly number of readings), GEE was used to fit a negative binomial log linear regression model with repeated measures, and relevant comparisons were quantified with rate ratios (in place of the differences used for this purpose in the linear models). For the multinomial hypertension endpoint, the odds of more severe vs less severe hypertension was modeled using a logit link, and cumulative odds ratios were used (in place of differences in linear models) to make the relevant comparisons.
A P-value < .05 was considered statistically significant and no adjustment was made for multiple comparisons. Statistical analyses were performed by Duke Clinical Research Institute, (Durham, NC) using SAS software version 9.4 (SAS Institute, Cary, NC). The study was approved by the Institutional Review Board of the Duke Health System.
Results
The overall study population included 72,706 participants in the digital health hypertension platform between January 1, 2019 and August 31, 2020. Of the total population, 33,440 participants had their first BP reading on or before March 31, 2020 (pre-pandemic period), and 39, 266 participants had their first BP reading on or after April 1, 2020 (pandemic period). Table 1 describes characteristics of the overall study population. The overall mean age of the study population was 53.2 ± 10.8 years, with 8,297 (11%) of the cohort being ≥ 65 years of age. Overall, 48.5% of the cohort was female. The individuals came from a broad geographic distribution across United States Census Bureau (USCB) designated divisions with the most participants coming from the West South Central division (24.7%) followed by the South Atlantic (22%). Over half the participants came from the southern region. Of those individuals self-reporting race/ethnicity (N = 29,237), 68.6% were White, 17.7% were Black and 13.3% were Hispanic. Overall, 61.5% had a BMI >30 and 6.6% reported being a current smoker.
Table I.
Baseline characteristics of hypertension program participants.
| Overall (N = 72706) | Pre-Pandemic (N = 33440) | Pandemic (N = 39266) | |
|---|---|---|---|
| Age, yrs | 53.2 (10.8) | 53.0 (10.7) | 53.3 (10.8) |
| Gender (female) | 35242 / 72678 (48.5) | 15375 / 33422 (46.0) | 19867 / 39256 (50.6) |
| Race | |||
| White | 20046 / 29237 (68.6) | 11114 / 15665 (70.9) | 8932 / 13572 (65.8) |
| Black | 5172 / 29237 (17.7) | 2476 / 15665 (15.8) | 2696 / 13572 (19.9) |
| Asian | 1665 / 29237 (5.7) | 857 / 15665 (5.5) | 808 / 13572 (6.0) |
| Other | 2354 / 29237 (8.1) | 1218 / 15665 (7.8) | 1136 / 13572 (8.4) |
| Hispanic | 3912 / 29356 (13.3) | 1845 / 15734 (11.7) | 2067 / 13622 (15.2) |
| Smoker | 1807 / 27390 (6.6) | 925 / 14633 (6.3) | 882 / 12757 (6.9) |
| Height (inches) | 67.4 (4.1) | 67.5 (4.1) | 67.3 (4.1) |
| Weight (lbs) | 214.7 (54.0) | 218.2 (54.6) | 212.2 (53.4) |
| Body mass index | 33.1 (7.5) | 33.5 (7.6) | 32.8 (7.4) |
| BMI>=30 | 24281 / 39500 (61.5) | 10596 / 16635 (63.7) | 13685 / 22865 (59.9) |
| First Monthly Mean SBP | 134.7 (15.9) | 133.8 (16.2) | 135.5 (15.6) |
| First Monthly Mean DBP | 82.2 (10.2) | 82.1 (10.3) | 82.2 (10.0) |
In this table, user characteristics are categorized by the date of their first blood pressure reading in 2019-2020.
Summary Statistics: N, Mean (SD); n/N (%) for Discrete. Pre-Pandemic is first blood pressure reading on March 31, 2020 or before; Pandemic is 1 blood pressure reading on 4/1/2020 or after.
Comparing those who enrolled in the program prior to COVID-19 relative to after the onset of the pandemic, the mean age remained similar (53.0 ± 10.7 years vs 53.3 ± 10.8 years), however, the pandemic population had slightly more females (50.6% vs 46.0%), and more non-Whites 34.2% vs 29.1%. Approximately the same proportion of the participants enrolling pre-pandemic and during the pandemic were above the age of 65, 11.8% vs 11.1%. The mean BMI also remained similar, 33.5±7.6 vs. 32.8±7.4. The rest of the baseline characteristics were similar among patients enrolled in the pre vs during COVID-19 periods (Table I). Further breakdown of the population characteristics by quarter from January 2019 to August 2020 is provided in the supplement (Supplemental table 1).
Number of blood pressure readings
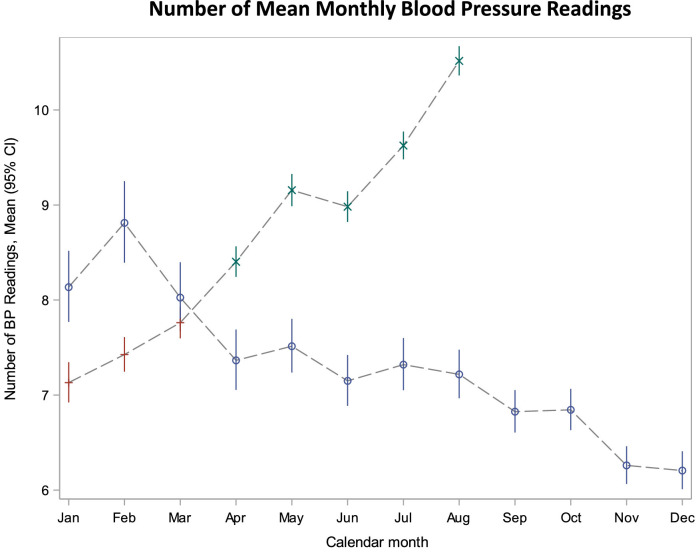
In 2019 (the pre-pandemic period) the number of BP readings using the digital health platform varied seasonally. The average number of readings per month in the pre-pandemic period (2019) was highest in February and then declined through November and December. However, after the onset of the pandemic (April 1, 2020) there was a significant rise in the mean number of BP readings per month compared with similar months in the pre-pandemic period (Figure 1 ). Comparing similar months in the pre- (April 1, 2019-August 31, 2019) and COVID-19 (April 1, 2020-August 31, 2019) periods, the average number of monthly BP readings rose from 7.3 readings registered per month to 9.3 per month (Figure 1). This relationship was constant after adjustment for participant characteristics (Supplemental Figure 1).
Figure 1.
Distribution of mean number of BP readings in 2019 and 2020 by month. The blue circles represent 2019; red plus represented Jan-Mar 2020; and green x represents the pandemic period of Apr-Aug 2020..
Blood pressure trends
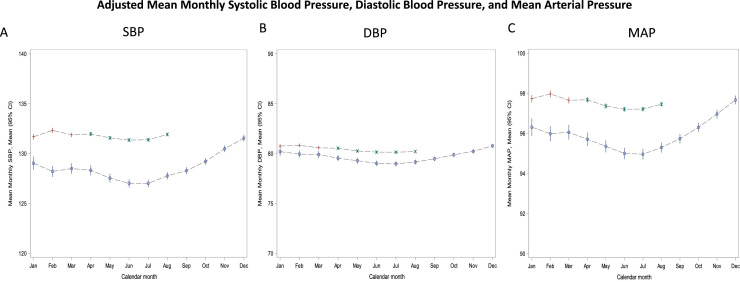
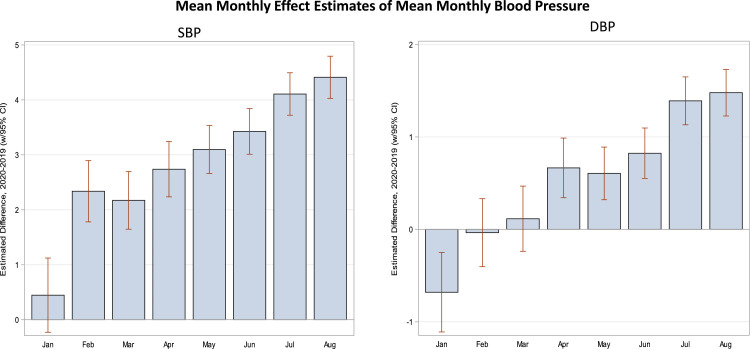
As illustrated in Figures 2 A-C, population mean monthly BP varied by season; with mean BPs being highest in the early winter and lowest in the summer months. Important to this study, when compared with corresponding monthly averages from 2019, mean monthly SBP, DBP, and MAP rose significantly during the months of COVID-19 (April 2020-August 2020). For example, the unadjusted mean SBP between April through August in 2020 vs 2019 were: 132.4 mmHg vs 128.8 mmHg P < .001) (See Supplemental Figure 2). Similarly, the adjusted mean SBP between April through August in 2020 vs 2019 were: 131.6 mmHg vs. 127.5 mmHg, respectively; P < .001 (Figure 2). Monthly mean DBP was also higher during COVID-19 months relative to their corresponding values prior to COVID-19, 80.4 mmHg vs 79.4 mmHg, P < .001 and adjusted mean monthly DBP 80.2 mmHg vs. 79.2 mmHg; P < .001. Monthly mean MAP after the onset of the pandemic was also higher, 97.8 mmHg vs. 95.9 mmHg, P <.001 and adjusted monthly mean MAP 97.4 mmHg vs. 95.3 mmHg, P < .001. Figure 3 provides the monthly change (delta) in population mean SBP and DBP comparing similar months in 2019 with those 2020. These data again demonstrate significantly higher differences in BP readings after COVID-19.
Figure 2.
Distribution of monthly mean systolic blood pressure (A), diastolic blood pressure (B), and mean arterial blood pressure (C) readings in 2019 and 2020 in adjusted models. The blue circles represent 2019; red plus represented Jan-Mar 2020; and green x represents the pandemic period of Apr-Aug 2020. Adjustments were made for age, sex, race, region, and months in program. BP is measured in mmHg.
Figure 3.
Mean differences between 2020 and 2019 by month for SBP and DBP. The average difference between 2019 and 2020 are displayed for systolic blood pressure and diastolic blood pressue.
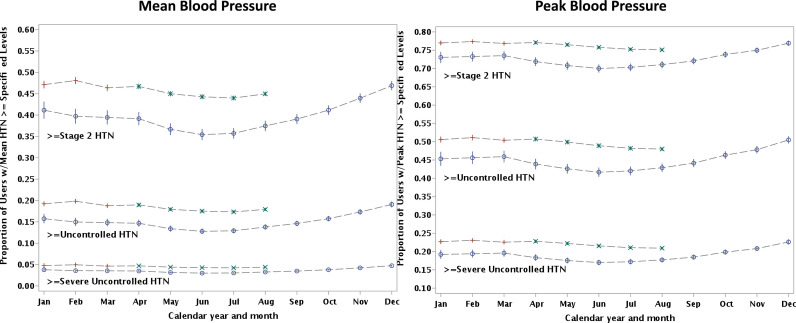
After the onset of the pandemic there was a modest increase in the percentage of participants classified as having high risk, uncontrolled or severely uncontrolled BP based on an average (mean) of all BP reading during a given month (Figure 4 ). Relative to pre-COVID-19, the adjusted proportion of participants after COVID-19 with a mean monthly adjusted BP reading classified as uncontrolled hypertension (home SBP> = 145 or DBP > = 95) rose from 13% to 18% after COVID-19. The proportion with adjusted mean SBP classified as severely uncontrolled BP (home SBP > = 160 or DBP > = 90) rose slightly from 3% to 4%.
Figure 4.
Distribution of the adjusted proportion of participants with Stage 2 or higher (>=135 or DBP>= 85 o), uncontrolled BP (>=145 or DBP>= 95) and severely uncontrolled (>=160 or DBP>=100) categories in 2019 and 2020 classified by either a) monthly mean BP or b) peak blood pressure. The blue circles represent 2019; red plus represented Jan-Mar 2020; and green x represents the COVID-19 period of Apr-Aug 2020. Adjustments were made for age, sex, race, region, and months in program.
In another sensitivity analysis, participant's BP control was classified based on the highest recorded value (peak) during a month as opposed to their mean monthly BP. This addressed whether there was evidence of high-risk elevations in BP during COVID-19. Relative to similar pre-pandemic months, during COVID-19, those with at least one BP reading that fell into the uncontrolled BP classification rose slightly (adjusted proportion 49% vs 43%). Similarly, those with at least one monthly reading classified as severely uncontrolled BP also rose slightly (adjusted rate 22% vs 18%).
As a second sensitivity analysis, we examined only the first 3 months of 2020 (directly prior to COVID-19) and compared these to results seen from April to August 2020 (during COVID-19). As can be seen in Table II , there still was a small but significant increase in monthly BPs seen in the first 3 months of 2020 relative to 2019. Thus, the temporal changes in BP correlate closely to the onset of COVID-19.
Table II.
Comparing 2019 to 2020 changes in number of blood pressure readings SBP, DBP and, MAP seen in the first 3 months of 2020 before COVID-19 and those seen during covid-19
| Variable | Changes seen Jan-Mar, 2020 relative to Jan-Mar, 2019 (PreCOVID-19 Period) | Changes seen Apr-Aug 2020 relative to Apr-Aug 2019 (COVID-19 Period) | Pandemic Effect: Delta between changes in months Jan-Mar 2020 vs. 2019, and those during COVID months in 2020 vs. same months in 2019 | P value |
|---|---|---|---|---|
| # of Monthly Blood Pressure Readings1 | 0.89 (0.86-0.93) | 1.27 (1.24-1.41) | 1.42 (1.36-1.49) | < .001 |
| SBP | 1.65 (1.22-2.08) | 3.56 (3.27-3.84) | 1.90 (1.47-2.34) | < .001 |
| DBP | -0.20 (-0.48-0.09) | 0.9 (0.60-1.19) | 1.19 (0.91-1.48) | < .001 |
| MAP | 0.50 (0.2-0.79) | 1.89 (1.69-2.09) | 1.39 (1.09-1.69) | < .001 |
1 Statistics presented for the number of blood pressure readings represent unadjusted relative change in mean # of monthly blood pressure readings from Jan through March 2020 relative to the same months in 2019 (Column 1). These are compared relative to similar changes seen in blood pressure readings from April through August 2020 (COVID-19 period) relative to the same months in 2019 (column 2) and then Column 3 represents delta (ratio of ratios) between column 1 and 2 (reflecting any changes that may be more specific to COVID-19). Similar analyses were also done for SBP, DBP and MAP (where the measures represent mean differences between the time periods listed in mmHg).
As a final sensitivity analysis, we limited our patient population to those who had BP reading in both the pre-pandemic and post pandemic periods. This cohort consisted of a total of 7,025 participants. Baseline characteristics of this group (Supplemental table 2) were generally similar to those for the overall cohort with the exception that this group was slightly older (means ag 57.3 years vs 53.2 years. Supplement 3 provides adjusted mean monthly SBP, DBP, and, MAP for participants with BP readings in both Pre-Pandemic and Pandemic Periods Similar to the results in the overall analysis mean SBP (128.5 vs 126.5, P < .001), DBP (78.4 vs 77.9, P .006), and MAP (95.1 vs 94.1, P <.001) were higher in the post pandemic period relative to the pre-pandemic period (Supplemental Figure 3).
Discussion
Using data from over 72,000 persons using a home BP monitoring platform, this study provides a unique look into BP control during the COVID-19 pandemic. Compared with 2019 (prior to COVID-19), we found that individuals were actually more engaged with checking their home BP after COVID-19 relative to before. While population mean BP demonstrated significant seasonal variability throughout the study period, we did find that the population average mean monthly SBP, DBP, and MAP rose significantly during the pandemic compared with the comparable time periods in the prior year. These finding persisted after adjustment for age, sex, region of the country, and months enrolled in the digital health program. Finally, while there was a slight increase in severely elevated BP readings after COVID-19, these changes were modest compared with pre-COVID.
Our study demonstrated significant seasonal variability in BP results at the population level. SBP and DBP rose in the fall and peaked right after the winter holidays season. Then BP readings tended to fall and nadired in the summer months. These results are similar to prior studies that also demonstrate seasonal trends in BP.17., 18., 19. These seasonal changes have been described to changes in vasomotor tone from cooler temperatures, changes in blood viscosity, increased production of catecholamines, changes in exercise frequency, or changes in eating patterns and weight.20 , 21
Our major finding was that SBP, DBP and MAP rose significantly after the onset of COVID-19 relative to either similar months in the preceding year or compared with the first three months of 2020 (just before COVID-19). There are several potential explanations for the rise in population BP after COVID-19. Most importantly, stay-at-home orders, increases in working from home and sedentary behavior, isolation, pandemic-related stress, and large changes in personal lifestyle may have impacted hypertension control. Specifically, alcohol intake is known to increase BP, and multiple studies have shown increases in alcohol intake and binge drinking during the pandemic.22 , 23 Other studies have shown dramatic decreases in physical activity during the pandemic, with one study showing a 42% decrease in physical activity during lockdown.24
Furthermore, fears of viral exposure and closing of clinics disrupted routine in-person healthcare visits, vital for the treatment of chronic conditions.3 While many clinics moved to more telemedicine visits, the frequency of in-person visits to healthcare providers for chronic disease management declined markedly.25 , 26 Early in the pandemic, one in three US Adults surveyed reported being unable to get routine care due to the pandemic, while another 1 in 4 reported being unable to access prescription medications.26 Additionally, there were reports that certain commonly used BP lowering agents that inhibit the renin-angiotensin-aldosterone (RAAS) system may have adverse effects on a patient with COVID-19.27 , 28 While RAAS inhibitors were subsequently shown to be ‘safe’, it is possible that these negative news stories hurt patient compliance with anti-hypertensive medication in general, and RAAS inhibitors in particular29
While the changes in SBP and DBP observed in our study are modest at the individual level, these findings should be interpreted in the context of the population. Spread across thousands of individuals, even slight changes in population level BP can have measurable impact on long term cardiovascular risk.4., 5., 6. , 14 , 15 For example, difference in dietary salt studies has demonstrated that slight increases in SBP and DBP (as low as 2mmHg/1 mmHg at the population level) can predict higher cardiovascular events in population analyes.16
On a positive note, our study did find that patients appeared more willing to measure their home BP monitoring program during the pandemic than before, as evidenced by an increase in the number of BP readings after the onset of the pandemic compared with similar respective pre-pandemic months. Possible reasons for this include more time at home to record BP readings, a higher interest in health related issues with the pandemic, or a specific heightened awareness of hypertension as a risk factor for COVID 19.9 It is also possible that participants were more concerned about monitoring their own home BP as a result of potential postponements of their routine in-person provider visits after the onset of the pandemic. Despite this increased engagement, BP levels rose, providing an important reminder that monitoring alone was insufficient to counter the multifactorial reasons for pandemic-related elevations in BP.
Limitations
Our study has limitations that are important to note. First, our study defined the onset of the pandemic as April 1, 2020. This date was selected after major government shut-down across the United States. However, this date is somewhat arbitrary as some impacts of COVID-19 may have been felt prior to April in certain regions as other regions were relatively spared till later months.
Second, we also followed a population of individuals that were already enrolled in a home BP management program. By the nature of this, participants were typically employed, were younger and had the financial resources, and had higher interest in their health than the general population of patients with high BP. Second, as not all patients included in our study had BP readings continuously in the pre- and pandemic periods, we opted to study population averages. It is possible that there were changes in the population enrolling in the BP management program in the pandemic vs pre-pandemic period. While this is possible, the available baseline characteristics based on month of enrollment appeared similar and our results were not changed after adjusting for these baseline characteristics. We also performed a sensitivity analysis with a subgroup that did have readings during the pre-pandemic period and pandemic period that further validated our results. Furthermore, we did not have access to detailed clinical data such as medical co-morbidities or medication histories that would be needed for full adjustment. We also are unable to determine what drove the increase in BPs due to lack of data on medication adherence and dosing, healthcare utilization, or lifestyle and diet factors. Thus, while we established that BP rose modestly in community practice during the COVID-19 pandemic, future studies will need to further investigate detailed mechanisms for these changes Third, the Livongo Hypertension Program offered a number of support systems designed to control BP (See methods). Such interventions would be expected to lead to better BP control among participants over time. Thus, our results demonstrating an elevation in BP during Covid may represent an underestimation of the true impact of the COVID era in the overall community.
Finally, our results are limited to the first 5 months of the pandemic. It will be quite important to track whether changes seen in BP resolve after the pandemic ends, or if they persist longer-term. Further, longer-term analyses are indicated.
Conclusion
In our study of participants using a home BP monitoring platform we found that patients were more likely to measure their BP after the onset of the COVID-19 pandemic compared with before. Of concern though is that SBP, DBP and MAP rose modestly at the population level after COVID-19. There were also a modestly higher proportion of patients with stage 2 HTN, uncontrolled and severely uncontrolled BP readings during the pandemic. Finally, on a positive note, there was no evidence of a marked increase in the peak BP reading during any single month after COVID-19 compared with before. Future studies are needed to determine the underlying causes of this rise in population BP, to design strategies to mitigate their impact on future lockdowns, and whether these changes in BP initiated by COVID-19 are longstanding or transient.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ahj.2021.11.017.
Appendix. Supplementary materials
References
- 1.Worldometer COVID-19 Tracker. 2021. https://www.worldometers.info/coronavirus/.
- 2.Monod M, Blenkinsop A, Xi X, et al. Age groups that sustain resurging COVID-19 epidemics in the United States (preprint) medRxiv. 2020 doi: 10.1101/2020.09.18.20197376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dejong C, Katz MH, Covinsky K. Deferral of care for serious non-COVID-19 conditions: a hidden harm of COVID-19. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.4016. [DOI] [PubMed] [Google Scholar]
- 4.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet. 2016;387 doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 5.Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality a systematic review and network meta-analysis. JAMA Cardiol. 2017;2 doi: 10.1001/jamacardio.2017.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360 doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 7.Navar AM, Peterson ED, Wojdyla D, et al. Temporal changes in the association between modifiable risk factors and coronary heart disease incidence. JAMA - J Am Med Assoc. 2016;316 doi: 10.1001/jama.2016.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark D, Colantonio LD, Min YI, et al. Population-attributable risk for cardiovascular disease associated with hypertension in black adults. JAMA Cardiol. 2019;4 doi: 10.1001/jamacardio.2019.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao C, Cai Y, Zhang K, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41:2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostchega Y, Zhang G, Hughes JP, Nwankwo T. Factors associated with hypertension control in US Adults Using 2017 ACC/AHA Guidelines: National Health and Nutrition Examination Survey 1999-2016. Am J Hypertens. 2018;31 doi: 10.1093/ajh/hpy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadhera RK, Shen C, Gondi S, et al. Cardiovascular deaths during the COVID-19 pandemic in the United States. J Am Coll Cardiol. 2021;77 doi: 10.1016/j.jacc.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American heart association. Hypertension. 2019;73 doi: 10.1161/HYP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Dov IZ, Ben-Arie L, Mekler J, Bursztyn M. Normal ambulatory blood pressure: a clinical practice-based comparison of two recently published definitions. J Hum Hypertens. 2005;19:565–567. doi: 10.1038/sj.jhh.1001866. [DOI] [PubMed] [Google Scholar]
- 14.Takashima N, Ohkubo T, Miura K, et al. Long-term risk of BP values above normal for cardiovascular mortality: A 24-year observation of Japanese aged 30 to 92 years. J Hypertens. 2012;30 doi: 10.1097/HJH.0b013e328359a9f7. [DOI] [PubMed] [Google Scholar]
- 15.Fujiyoshi A, Ohkubo T, Miura K, et al. Blood pressure categories and long-term risk of cardiovascular disease according to age group in Japanese men and women. Hypertens Res. 2012;35 doi: 10.1038/hr.2012.87. [DOI] [PubMed] [Google Scholar]
- 16.He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: A meta-analysis of randomized trials. Implications for public health. J Hum Hypertens. 2002;16 doi: 10.1038/sj.jhh.1001459. [DOI] [PubMed] [Google Scholar]
- 17.Sega R, Cesana G, Bombelli M, et al. Seasonal variations in home and ambulatory blood pressure in the PAMELA population. Pressione Arteriose Monitorate E Loro Associazioni. J Hypertens. 1998;16:1585–1592. doi: 10.1097/00004872-199816110-00004. [DOI] [PubMed] [Google Scholar]
- 18.Brennan PJ, Greenberg G, Miall WE, Thompson SG. Seasonal variation in arterial blood pressure. BMJ. 1982;285:919–923. doi: 10.1136/bmj.285.6346.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose G. Seasonal variation in blood pressure in man. Nature. 1961 doi: 10.1038/189235a0. [DOI] [PubMed] [Google Scholar]
- 20.Giaconi S, Ghione S, Palombo C, et al. Seasonal influences on blood pressure in high normal to mild hypertensive range. Hypertension. 1989;14:22–27. doi: 10.1161/01.HYP.14.1.22. [DOI] [PubMed] [Google Scholar]
- 21.James GD, Yee LS, Pickering TG. Winter-summer differences in the effects of emotion, posture and place of measurement on blood pressure. Soc Sci Med. 1990 doi: 10.1016/0277-9536(90)90126-D. [DOI] [PubMed] [Google Scholar]
- 22.Grossman ER, Benjamin-Neelon SE, Sonnenschein S. Alcohol consumption during the COVID-19 pandemic: a cross-sectional survey of US adults. Int J Environ Res Public Health. 2020;17:9189. doi: 10.3390/ijerph17249189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollard MS, Tucker JS, Green HD. Changes in Adult Alcohol Use and Consequences During the COVID-19 Pandemic in the US. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong JL, Lau T, Massar SAA, et al. COVID-19-related mobility reduction: heterogenous effects on sleep and physical activity rhythms. Sleep. 2021;44 doi: 10.1093/sleep/zsaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright A, Salazar A, Mirica M, et al. The invisible epidemic: neglected chronic disease management during COVID-19. J Gen Intern Med. 2020;35:2816–2817. doi: 10.1007/s11606-020-06025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirzinger A, Kearney A, Hamel L, Brodie M.KFF. Health tracking poll - early april 2020: the impact of coronavirus on life in America. Kaiser Fam Found. 2020 April. [Google Scholar]
- 27.Esler M, Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. 2020;38:781–782. doi: 10.1097/HJH.0000000000002450. [DOI] [PubMed] [Google Scholar]
- 28.Vaduganathan M, Vardeny O, Michel T, et al. Renin–Angiotensin–aldosterone system inhibitors in patients with covid-19. N Engl J Med. 2020 doi: 10.1056/nejmsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mancia G, Rea F, Ludergnani M, et al. Renin-Angiotensin-aldosterone system blockers and the risk of covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.