Abstract
Background and objective
An increasing trend of asthma prevalence was observed in Asia; however, contributions of environmental and host-related risk factors to the development of this disease remain uncertain. This study aimed to perform a systematic review and meta-analysis for asthma-associated risk factors reported in Asia.
Methods
We systematically searched three public databases (Web of Science, PubMed, and Scopus) in Feb 2021. We only included articles that reported environmental and host-related risk factors associated with asthma in the Asian population. Random-effect meta-analyses were conducted for frequently reported asthma-associated risk factors to provide an overall risk estimate of asthma development.
Results
Of 4030 records obtained from public databases, 289 articles were selected for review. The most frequently reported asthma-associated risk factor was the family history of allergy-related conditions. The random-effect asthma risk estimates (pooled odds ratio, OR) were 4.66 (95% confidence interval (CI): 3.73–5.82) for the family history of asthma, 3.50 (95% CI: 2.62–4.67) for the family history of atopy, 3.57 (95% CI: 3.03–4.22) for the family history of any allergic diseases, 1.96 (95% CI: 1.47–2.61) for the family history of allergic rhinitis, and 2.75 (95% CI: 1.12–6.76) for the family history of atopic dermatitis. For housing-related factors, including the presence of mold, mold spots, mold odor, cockroach, water damage, and incense burning, the random-effect pooled OR ranged from 1.43 to 1.73. Other risk factors with significant pooled OR for asthma development included male gender (1.30, 95% CI: 1.23–1.38), cigarette smoke exposure (1.44, 95% CI: 1.30–1.60), cigarette smoking (1.66, 95% CI: 1.44–1.90), body mass index (BMI)–related parameters (pooled OR ranged from 1.06 to 2.02), various types of air pollution (NO2, PM10, and O3; pooled OR ranged from 1.03 to 1.22), and pre- and perinatal factors (low birth weight, preterm birth, and cesarean section; pooled OR ranged from 1.14 to 1.32).
Conclusions
The family history of asthma was the most frequently reported risk factor for asthma development in Asia with the highest risk estimate for asthma development. This suggests a major role of the genetic component in asthma pathogenesis. Further study on asthma genetics is required to improve the current understanding of asthma etiology.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40101-021-00273-x.
Keywords: Asthma, Review, Risk, Meta-analysis
Background
Asthma is one of the most common respiratory syndromes affecting more than 300 million individuals worldwide [1, 2]. Based on the findings from the International Study of Asthma and Allergies in Childhood (ISAAC) reported in 1998, the prevalence of asthma in the Asia-Pacific region was lower as compared with the western European and Oceania regions [3]. However, the ISAAC phase III (2007) has reported a reduction in the 12-month prevalence of asthma-related symptoms in western European and Oceania regions, whereas the same prevalence was increased in the Asia-Pacific region. Given the increasing trend of asthma prevalence in the Asia-Pacific region, further understanding of the disease-associated risk factors specific to this region may provide opportunities to develop better prevention and prognostic and therapeutic approaches for asthma disease management.
To date, numerous studies have been conducted to investigate the asthma-associated risk factor. The family history of asthma was frequently identified in disease-affected individuals, suggesting the high heritability nature of asthma development [4, 5]. Environmental and host-related factors such as obesity [6], air pollutant exposures [7, 8], and tobacco smoke exposures [9] have also been found to significantly correlate with asthma susceptibility. Meta-analysis studies were performed to collectively analyze and summarize the overall risk effects of these asthma-associated risk factors [10–12]. However, risk factors summarized in these meta-analyses, including the overall effect sizes estimated, may not be entirely generalizable to the Asian population due to global differences in cultural, lifestyle, socioeconomic, and ethnic backgrounds. Here, we provide an up-to-date review of studies that reported asthma-associated risk factors in the Asian population. The meta-analysis will be performed to evaluate the overall risk estimate for asthma and to provide a better understanding of asthma manifestation in Asia.
Methods
Search strategy
The current systematic review study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13, 14]. The PRISMA checklist was included in Table S1. We searched Web of Science, PubMed, and Scopus databases in February 2021, to retrieve all publications related to asthma-associated risk factors. Search terms were listed in Table S2, which included “asthma”, “epidemiology”, “risk”, and the names of 51 Asian countries, dependencies, or other territories.
Selection criteria
After the process of deduplication and exclusion of irrelevant articles based on titles and abstracts, we retrieved the full text of the remaining articles and screened against the inclusion and exclusion criteria. We included studies that fulfilled both of the criteria: (1) aimed to identify asthma-associated risk factors or asthma comorbidities and (2) have provided an estimation of the effect size of studied risk factors, such as the odds ratio (OR) with corresponding 95% confidence intervals (CIs). Also, we excluded studies that (1) only investigated non-human subjects, (2) only investigated risk factors associated with asthma severity, (3) only examined subjects from non-Asian countries, (4) have unclear study design, and (5) were review or meta-analysis studies. The quality of included studies was further assessed using JBI Critical Appraisal Tool Checklist containing eight criteria [15]. At each of the reviewing stages, the screening of papers and extraction of data was performed by the first author (Sio YY) independently, followed by further discussion with advice from the corresponding author (Chew FT).
Data retrieval
The following data were extracted from selected articles: names of authors, year of publication, country or region of study, sample size and basic characteristics of the study cohort, study design, disease definition, risk factors, and their corresponding effect sizes (odds ratio), confidence intervals, and p values of asthma association.
Statistical analysis
To perform the random-effect meta-analysis, we extracted the OR and 95% CI reported from each study of interest. These study findings were combined using the random-effect model with the pooled OR and 95% CI also computed. We used a chi-square-based test to examine any heterogeneity presented in the pooled risk estimate, with the inconsistency index (I2) also computed. The funnel plot was drawn based on the standard errors of the reported effect estimates of the risk factors, followed by visual inspection to examine any publication bias. The STATA version 13.0 statistical software was used for all statistical analyses reported in the current study.
Results
Study characteristics
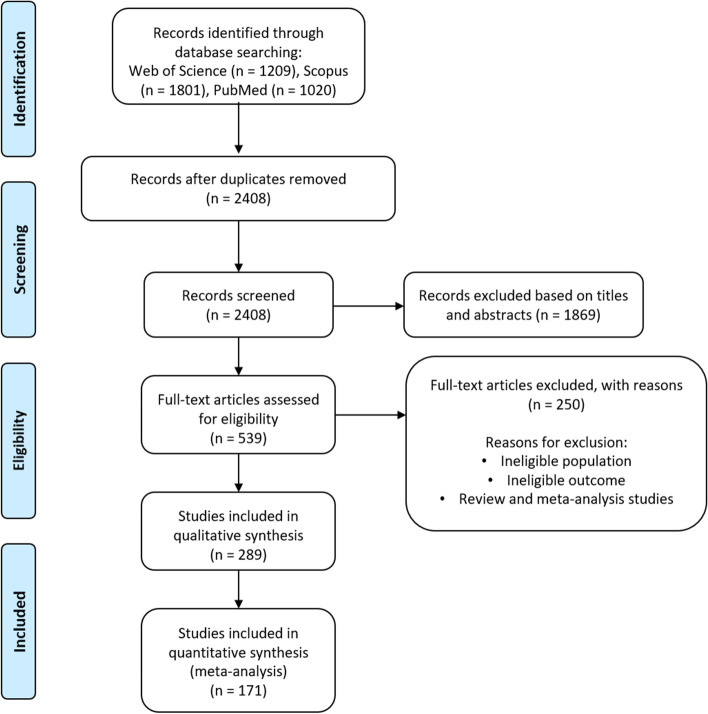
Figure 1 (PRISMA diagram) illustrates the overall search and review process of the current study. The initial literature search using Web of Science, Scopus, and PubMed databases has shortlisted 4030 articles that are potentially relevant to the scope of the current review. After removal of duplicates and screening of titles and abstracts of these search records, 539 articles were selected for full-text review. Finally, 289 papers were included in the systematic review process, and their study characteristics and reported asthma-associated risk factors were summarized in Table S3. Of these, 23 were cohort-based or longitudinal studies, 35 were case–control studies, and 231 were cross-sectional studies (Table S3). For the region of study, 73 out of these 289 reported studies were performed in mainland China, whereas Taiwan and India each contributed 38 and 28 publications, respectively (Table S4). The remaining studies were conducted in 25 other countries or regions in Asia, as summarized in Table S4. Other characteristics of these reviewed studies were mostly heterogeneous, including the definitions of asthma and risk factors, study size, study population, and statistical analysis approach (Table S3).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart illustrating the study selection procedure for systematic review and meta-analysis on risk factors of asthma in the Asian population
Results overview
We identified 31 major categories of asthma-associated risk factors that were reported in at least 3 studies (Table S5). Of these, 15 major categories of asthma risk factors were reported in at least 20 studies, which include family medical history, housing (condition, environment, size, type, etc.), age, gender, cigarette smoke exposure, cigarette smoking, body mass index (BMI)–related factors, pet exposure, educational level, urbanization, air pollution, breastfeeding, dietary habits, cooking fume exposure, and socioeconomic status (Table S5). Further, we also identified 9 common asthma comorbidities that were reported in at least 3 studies. These include atopy (26 studies) [16–41], allergic rhinitis (AR, 21 studies) [19, 26, 35, 42–59], respiratory infections (20 studies) [27, 40, 44, 49, 57, 59–73], eczema/atopic dermatitis (AD, 18 studies) [40, 44, 45, 47, 53–59, 62, 70, 72, 74–79], gastroesophageal reflux disease (5 studies) [19, 44, 47, 67, 80], chronic rhinosinusitis (5 studies) [19, 57, 76, 81, 82], food allergy (4 studies) [26, 62, 76, 82], otitis (3 studies) [67, 76, 82], and bronchitis (3 studies) [44, 57, 83] (Table S6).
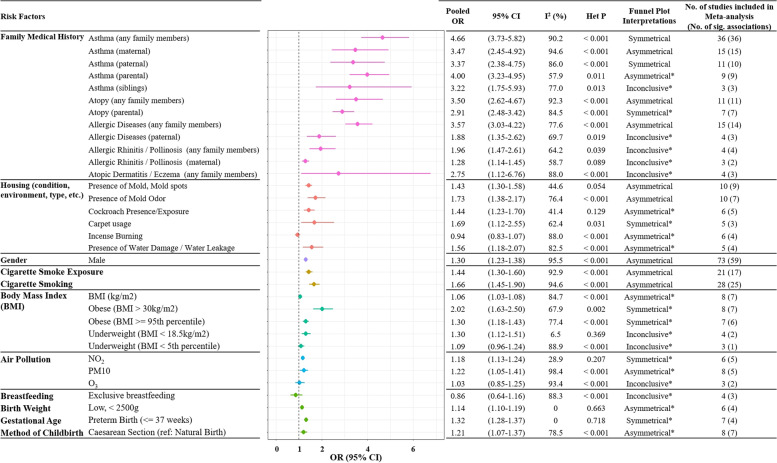
Results from the random-effect meta-analyses for risk factors including family medical history, housing-related factors, gender, cigarette smoke exposure, cigarette smoking, body mass index (BMI), air pollution, and pre- and perinatal factors are shown in Figures S1, S2, S3, S4, S5, S6, S7, S8, S9, S10, S11, S12, S13, S14, S15, S16, S17, S18, S19, S20, S21, S22, S23, S24, S25, S26, S27, S28, S29, S30, S31, S32, S33 and summarized in Fig. 2. These results were also discussed further in the subsequent sections. Besides, meta-analysis was not performed for other risk factors that were also frequently reported, given most studies were heterogeneous on their assessment and analytical approaches for these risk factors.
Fig. 2.
Meta-analyses of risk factors associated with asthma in Asia. The pooled odds ratios (ORs) for each asthma-associated risk factor were computed using the random-effect meta-analysis, with 95% confidence intervals (95% CIs) also included. Results from the heterogeneity test, including the I2 value and the heterogeneity p value (Het P) were also included in the figure. Publication biases were assessed based on the symmetry of funnel plots for each meta-analysis. The asterisk (*) indicates an inconclusive interpretation of the funnel plot because of the small number of studies included in the meta-analysis (n < 10)
Family medical history
Overall, 91 studies in Asia investigated the associations between the family history of various allergy-related diseases and the risk of asthma development [20, 22, 23, 25–27, 31, 32, 34–36, 42, 44–46, 51, 52, 57, 59, 62, 65–68, 70–73, 76–79, 82–140]. Among these, the family medical history of asthma (any family members) was most frequently studied and significantly associated with an increased risk of asthma (36 studies) [20, 25–27, 32, 34, 35, 44–46, 51, 57, 59, 76, 77, 79, 82, 83, 86, 95, 101, 102, 104–106, 108–110, 112, 116, 123, 128, 129, 133, 137, 140, 141]. In the random-effect meta-analysis performed for the family history of asthma (any family members) based on these 36 studies, the combined risk estimate for asthma development was increased significantly (pooled OR = 4.66, 95% CI: 3.73–5.82, I2 = 90.2%, heterogeneity p value < 0.001; Fig. 2 and Fig. S1). Further, we also performed meta-analyses for the family medical history of asthma in specific family groups separately, including paternal asthma (11 studies) [26, 27, 62, 68, 92, 113, 114, 117, 119, 120, 138], maternal asthma (15 studies) [26, 27, 62, 68, 73, 84, 89, 92, 113, 117, 119, 120, 123, 134, 138], parental asthma (9 studies) [26, 66, 72, 78, 88, 93, 118, 121, 122], and sibling’s asthma (4 studies) [26, 65, 123, 138]. The combined risk estimates for asthma were also significantly increased in these four meta-analyses (pooled OR ranged between 3.22 and 4; Fig. 2 and Figs. S2, S3, S4, S5). Significant heterogeneities were observed in all random-effect meta-analyses performed for the family medical history of asthma (Fig. 2 and Figs. S1, S2, S3, S4, S5), indicating that these included findings had different study outcomes across each other.
The family history of atopy was frequently associated with an increased risk of asthma in the Asian population (10 studies, Table S5) [36, 71, 94, 96–98, 103, 107, 124, 139]. Using these findings, in the random-effect meta-analysis for the family history of atopy, the combined risk estimate for asthma was significantly increased (pooled OR = 3.50, 95% CI: 2.62–4.67, I2 = 92.3%, heterogeneity p value < 0.001; Fig. 2 and Fig. S6). Seven studies have further evaluated the risk of asthma development in subjects with parental atopy [23, 85, 111, 119, 127, 135, 136]. In the random-effect meta-analysis using these findings, the combined risk estimate for asthma was also significantly increased (pooled OR = 2.91, 95% CI: 2.48–3.42, I2 = 84.5, heterogeneity p value < 0.001; Fig. 2 and Fig. S7).
The family medical history of other allergic diseases, such as AR or AD, was also reported as an asthma risk factor in Asia. Four studies have significantly associated the family history of AR with an increase in asthma risk [44, 46, 57, 109]. The pooled OR, calculated from the random-effect meta-analysis, also showed an overall increase in asthma risk (1.96, 95% CI: 1.47–2.61, I2 = 64.2%, heterogeneity p value = 0.039; Fig. 2 and Fig. S8). Additionally, in the random-effect meta-analysis for the family history of maternal AR (3 studies) [62, 84, 138], the combined risk estimate for asthma was significantly increased (pooled OR = 1.28, 95% CI: 1.14–1.45, I2 = 58.7%, heterogeneity p value = 0.089; Fig. 2 and Fig. S9). Four studies have investigated the association between the family history of AD and asthma risk [32, 44, 46, 57]. Of these, three studies have shown a significant association between this risk factor and an increased asthma risk [32, 46, 57]. In the random-effect meta-analysis using findings from these four studies, the pooled OR was 2.75 (95% CI: 1.12–6.76, I2 = 88%, heterogeneity p value < 0.001; Fig. 2 and Fig. S10).
Fifteen studies have collectively analyzed the family history of any allergic disease as a risk factor for asthma [31, 52, 70, 77, 86, 91, 100, 113, 115, 117, 126, 130–132, 140]. Of these, we removed one study [132] from the subsequent meta-analysis due to a different risk factor definition as compared with the other studies. In the random-effect meta-analysis for the family history of any allergic disease (14 studies) [31, 52, 70, 77, 86, 91, 100, 113, 115, 117, 126, 130, 131, 140], the combined odds ratio showed a significant increase in asthma risk (combined OR = 3.57, 95% CI: 3.03–4.22, I2 = 77.6%, heterogeneity p value < 0.001; Fig. 2 and Fig. S11). Four studies have investigated the risk of asthma development in subjects with paternal allergic diseases [87, 90, 113, 117]. In the random-effect meta-analysis using these findings, the asthma risk estimate was also increased significantly (combined OR = 1.88, 95% CI: 1.35–2.62, I2 = 69.7%, heterogeneity p value = 0.019; Fig. 2 and Fig. S12).
Housing-related factors
A total of 76 studies have investigated housing-related factors and their association with asthma [16–18, 25, 26, 38, 52, 53, 58–60, 67, 72, 73, 79, 82, 85, 88, 91, 94, 95, 97, 98, 105, 111, 118–120, 125, 127, 129, 130, 135, 136, 138, 142–182]. In these studies, frequently investigated housing-related risk factors of asthma included household dampness (18 studies) [17, 67, 72, 82, 85, 88, 125, 130, 142, 143, 146, 147, 150, 157, 166, 171, 174, 180], traffic pollution (14 studies) [16, 18, 53, 58, 127, 129, 130, 135, 148, 149, 172, 173, 176, 181], the presence of mold or mold spots (11 studies) [111, 119, 120, 129, 136, 142, 143, 150, 151, 160, 174], the presence of mold odor (10 studies) [85, 142, 143, 146, 147, 150, 151, 160, 174, 183], cockroach exposures (9 studies) [98, 111, 120, 130, 136, 160, 163, 165, 172], housing type (7 studies) [26, 135, 155, 162, 167, 178, 179], size of housing (5 studies) [26, 105, 127, 142, 155], and carpet usage (5 studies) [125, 130, 135, 149, 160]. Household dampness was associated with an increased asthma risk in 16 studies significantly (Table S5) [17, 67, 72, 82, 85, 88, 130, 142, 143, 146, 147, 150, 157, 166, 171, 180]; however, two other studies reported a mixed or insignificant result for this risk factor [125, 174]. Further, in these reviewed studies, the dampness of the housing environment was assessed by measuring the presence of damp stains [143, 150, 157]; dampness of clothes [142, 143], bed [142, 143], floor [17], or wall [67]; or general household dampness [82, 88, 142, 166, 171]. Meta-analysis was not performed for this risk factor due to the heterogeneity in assessment approaches of household dampness. Nevertheless, meta-analysis was performed for the presence of water damage or leakage in the household environment (5 studies) [111, 136, 142, 154, 174], and the combined random-effect risk estimate for asthma was significantly increased (pooled OR = 1.56, 95% CI: 1.18–2.07, I2 = 82.5%, heterogeneity p value < 0.001; Fig. 2 and Fig. S13).
The presence of mold, mold spots, or mold odor in the household environment was reported to be significantly associated with a greater risk of developing asthma in 12 studies [111, 119, 120, 129, 136, 142, 143, 146, 147, 150, 151, 160]. By contrast, four other studies have shown a mixed or insignificant association with asthma for this risk factor [85, 174, 183]. Further, 11 out of 12 studies that showed significant findings for this risk factor were all conducted in mainland China [142, 143, 146, 147, 150, 151] and Taiwan [111, 119, 120, 136, 160]. This may suggest an ethnic- or region-specific association of this risk factor with asthma. In the random-effect meta-analysis for the presence of mold or mold spot in the house based on 10 studies [111, 119, 120, 129, 136, 143, 150, 151, 160, 174], the combined asthma risk estimate was increased (pooled OR = 1.43, 95% CI: 1.30–1.58, I2 = 44.6%, heterogeneity p value = 0.054; Fig. 2 and Fig. S14). In the random-effect meta-analysis for the presence of mold odor based on 10 studies [85, 142, 143, 146, 147, 150, 151, 160, 174, 183], a similar trend of increasing combined asthma risk estimate was also observed (pooled OR = 1.73, 95% CI: 1.38–2.17, I2 = 76.4%, heterogeneity p value < 0.001; Fig. 2 and Fig. S15).
Six studies have examined the overall presence of cockroaches in the household [98, 111, 130, 160, 165, 172]; five of these studies [98, 111, 160, 165, 172] showed a significantly higher risk of developing asthma in the presence of this risk factor. In the random-effect meta-analysis for the presence of cockroaches in the household environment (6 studies) [98, 111, 130, 160, 165, 172], the combined risk estimate for asthma was increased (pooled OR = 1.44, 95% CI: 1.23–1.70, I2 = 41.4%, heterogeneity p value = 0.129; Fig. 2 and Fig. S16). Further, four studies have compared different frequencies of cockroach exposure in the household, and all have shown significant associations with asthma for increased exposure frequencies [120, 136, 160, 163].
The usage of carpet in the housing environment was significantly associated with an increased risk of asthma as reported in three studies [130, 149, 160], while two studies [125, 135] reported mixed or insignificant associations for this risk factor. In the random-effect meta-analysis for the usage of carpet in the household environment (5 studies) [125, 130, 135, 149, 160], the combined risk estimate for asthma was increased (pooled OR = 1.69, 95% CI: 1.12–2.55, I2 = 62.4%, heterogeneity p value = 0.031; Fig. 2 and Fig. S17). Incense burning was also frequently studied as a household risk factor contributing to asthma; three studies significantly associated incense burning with decreased asthma risk [38, 120, 170], while one study has associated this factor with increased asthma risk [172] separately. Further, two other studies have reported a mixed or insignificant association between incense burning and asthma [125, 182]. In the meta-analysis for incense burning (6 studies) [38, 120, 125, 170, 172, 182], the combined risk estimates for asthma is not significant (pooled OR = 0.94, 95% CI: 0.83–1.07, I2 = 88%, heterogeneity p value < 0.001; Fig. 2 and Fig. S18).
Lastly, meta-analysis was not performed for the presence of traffic pollution or traffic exposure near the housing environment, given multiple studies have used different assessment approaches for this risk factor. However, all ten studies that reported significant findings have consistently associated traffic pollution or exposure with an increased risk of asthma development [16, 18, 53, 58, 127, 129, 130, 148, 149, 172]. Also, meta-analysis was not performed for other frequently studied housing-related asthma risk factors, including the type and size of housing, due to the same reason of heterogeneity in assessment approaches.
Gender
The association between gender and asthma was reported in 75 studies [16, 18, 19, 21, 22, 33, 35, 37, 38, 40, 42, 43, 47, 49, 56, 58, 62, 67, 70, 71, 73, 74, 79, 82–87, 92, 93, 95, 97, 99, 104, 105, 107, 110, 116, 118, 120, 122, 124–127, 130, 131, 133, 135–137, 139, 142, 159, 170, 179, 184–201]. Of these, 58 studies have observed male subjects having a higher asthma susceptibility as compared with that of the female subjects significantly [16, 18, 22, 37, 38, 40, 47, 56, 58, 62, 67, 70, 71, 73, 74, 79, 82–84, 86, 87, 92, 95, 97, 99, 104, 105, 107, 116, 118, 120, 122, 124–127, 130, 131, 135, 136, 139, 142, 159, 170, 179, 184–194, 197–200]. By contrast, 15 studies showed females having higher asthma risk than males significantly [19, 21, 33, 35, 42, 49, 85, 93, 110, 133, 137, 195, 196, 201]. Of these 75 studies, we removed 2 studies [125, 126] from the subsequent meta-analysis due to their missing information in risk factor definition and inconsistency in disease definition. In the random-effect meta-analysis for gender (73 studies), the combined risk estimate for the male developing asthma was increased (pooled OR = 1.30, 95% CI: 1.23–1.38, I2 = 95.5%, heterogeneity p value < 0.001; Fig. 2 and Fig. S19).
Cigarette smoke exposure and cigarette smoking
The effect of passive cigarette smoke exposure on the risk of asthma was frequently studied in Asia (63 studies) [19, 27, 36, 38, 40, 43, 52–54, 58, 60, 62, 66, 67, 70, 73, 77–79, 87, 90, 92, 93, 100, 101, 104, 105, 107, 111, 114, 116, 119, 120, 124, 125, 135, 136, 139, 142, 145, 147, 148, 160, 162, 168, 170, 172, 179, 182, 183, 198–200, 202–210]. These studies have used different analytical methods to assess its influence on the susceptibility to asthma, including the number of cigarettes exposed per day [27, 54, 100, 120, 160, 208], the number of persons smoking in the house [53, 105, 114, 207], smoking in the presence of the subject [168], the duration of exposure [93, 160, 168, 207], the exposure during mother’s pregnancy [52, 60, 104, 111, 160, 168], the avoidance of cigarette smoke exposure [119], or the presence of father, mother, or any family member who is a smoker [67, 116, 124, 147, 148, 206]. We included findings from 21 studies in the random-effect meta-analysis for the overall associations of passive cigarette smoke exposure with asthma [19, 43, 62, 66, 77, 90, 92, 101, 107, 111, 127, 136, 142, 162, 170, 179, 183, 202–205]. In this meta-analysis, the combined risk estimate for asthma was significantly increased (pooled OR = 1.44, 95% CI: 1.30–1.60, I2 = 92.9%, heterogeneity p value < 0.001; Fig. 2 and Fig. S20).
A total of 36 studies have investigated the association between cigarette smoking and asthma [26, 35, 38, 42–44, 49, 51, 54, 56, 61, 90, 100, 101, 112, 125, 128, 139, 142, 162, 167, 170, 179, 185, 196, 198, 200, 203, 209–216]. These studies have used different approaches in the definition of active cigarette smoking, including ever actively smoking [162, 167, 216], former smokers [101, 128, 185, 214], or current smokers [26, 42–44, 49, 51, 54, 56, 61, 90, 100, 101, 112, 125, 128, 142, 170, 196, 203, 211–214]. We included findings from 21 studies in the random-effect meta-analysis for the overall associations of cigarette smoking with asthma [26, 38, 42–44, 49, 51, 54, 56, 61, 90, 100, 101, 112, 128, 139, 142, 170, 179, 196, 198, 200, 203, 211–215]. In this meta-analysis, the combined risk estimate for asthma was increased (pooled OR = 1.66, 95% CI: 1.45–1.90, I2 = 94.6%, heterogeneity p value < 0.001; Fig. 2 and Fig. S21).
Collectively, our analysis indicated that exposure to cigarette smoke, either via passive exposure or cigarette smoking, was both associated with an overall increase in asthma risk within the Asian population.
Body mass index (BMI)
A total of 37 studies in Asia have investigated the association between BMI and asthma [19, 38, 42–45, 47, 49, 61, 125, 135, 153, 162, 167, 185, 186, 196, 200, 201, 204, 213, 217–232]. These studies have used different BMI cut-offs for defining overweight, obesity, and underweight status, while other studies have also analyzed BMI as a continuous variable (Table S5). In the random-effect meta-analysis for BMI as a continuous variable (8 studies) [38, 43, 49, 135, 153, 201, 204, 230], the overall risk estimate for asthma was increased (pooled OR = 1.06, 95% CI: 1.03–1.08, I2 = 84.7%, heterogeneity p value < 0.001; Fig. 2 and Fig. S22). This suggests an increase in BMI was associated with an increase in asthma risk. Eight studies have also investigated the association between obese (BMI > 30 kg/m2) and asthma development [162, 167, 185, 196, 213, 217, 222, 225]. In the random-effect meta-analysis using these findings, the asthma risk estimate was increased for this risk factor (pooled OR = 2.02, 95% CI: 1.63–2.50, I2 = 67.9%, heterogeneity p value = [77, 233] 0.002; Fig. 2 and Fig. S23). The association between obesity (BMI ≥ 95th percentile) and asthma was also frequently studied (7 studies) [45, 219, 221, 223, 226, 231, 232]. In the random-effect meta-analysis for this factor, the asthma risk estimate was increased (pooled OR = 1.30, 95% CI: 1.18–1.43, I2 = 77.4%, heterogeneity p value < 0.001; Fig. 2 and Fig. S24).
Four studies have investigated the association between underweight (BMI < 18.5 kg/m2) and asthma [42, 167, 185, 217]. In the random-effect meta-analysis for this factor, the overall asthma risk estimate was increased (pooled OR = 1.30, 95% CI: 1.12–1.51, I2 = 6.5%, heterogeneity p value = 0.369; Fig. 2 and Fig. S25). However, in the random-effect meta-analysis for 3 others studies that used BMI < 5th percentile as the definition of underweight [223, 231, 232], the overall risk estimate for asthma was not significantly changed (pooled OR = 1.09, 95% CI: 0.96–1.24, I2 = 88.9%, heterogeneity p value < 0.001; Fig. 2 and Fig. S26).
Air pollution
There were 24 studies that investigated the associations between different types of air pollution and the risk of developing asthma. These air pollution-related parameters included the levels of NO2 (9 studies) [127, 135, 172, 182, 234–238], particulate matter less than 10 μm (PM10, 11 studies) [47, 54, 127, 135, 136, 172, 182, 187, 235, 236, 239], PM2.5 (3 studies) [42, 234, 240], O3 (4 studies) [54, 135, 136, 234], CO (3 studies) [54, 136, 234], nitrogen oxides (3 studies) [136, 241, 242], and SO2 (5 studies) [135, 136, 234, 236, 238]. In the random-effect meta-analysis for NO2 pollution (6 studies) [127, 135, 172, 182, 234, 235], the overall asthma risk estimate was increased (pooled OR = 1.18, 95% CI: 1.13–1.24, I2 = 88.9%, heterogeneity p value < 0.001; Fig. 2 and Fig. S27). In the random-effect meta-analysis for PM10 pollution (8 studies) [47, 54, 127, 135, 172, 182, 187, 235], the overall asthma risk estimate was increased (pooled OR = 1.22, 95% CI: 1.05–1.41, I2 = 98.4%, heterogeneity p value < 0.001; Fig. 2 and Fig. S28). In the random-effect meta-analysis for O3 pollution (3 studies) [54, 135, 234], the overall asthma risk estimate was not significantly changed (pooled OR = 1.03, 95% CI: 0.85–1.25, I2 = 93.4%, heterogeneity p value < 0.001; Fig. 2 and Fig. S29). Meta-analysis was not performed for other types of air pollution as most studies were heterogeneous on their assessment approaches of air pollution level.
Pre- and perinatal factors
Multiple pre- and perinatal factors were also frequently studied as a risk factor contributing to asthma, including breastfeeding (26 studies) [27, 36, 40, 44, 64, 65, 69, 70, 77, 85, 86, 89, 111, 122, 126, 127, 131, 133, 135, 138, 153, 199, 243–246], birth weight (17 studies) [66, 69, 84, 85, 87, 126, 127, 130, 132, 133, 183, 199, 228, 247, 248], gestational age (11 studies) [36, 69, 77, 84, 85, 126, 133, 140, 233, 247, 248], and the method of childbirth (10 studies) [36, 47, 64, 84, 126, 131, 133, 249–251]. Given most studies were heterogeneous on their assessment approaches and analytical methods for these risk factors, meta-analyses were only performed for exclusive breastfeeding, low birth weight (< 2500 g), preterm birth (≤ 37 weeks), and childbirth by caesarean section (reference category: natural birth). In the random-effect meta-analysis for exclusive breastfeeding (4 studies) [64, 77, 122, 199], the overall asthma risk estimate was not significantly changed (pooled OR = 0.86, 95% CI: 0.64–1.16, I2 = 88.3%, heterogeneity p value < 0.001; Fig. 2 and Fig. S30). In the random-effect meta-analysis for low birth weight (< 2500 g, 6 studies) [69, 84, 87, 127, 199, 247], the overall asthma risk estimate was increased (pooled OR = 1.14, 95% CI: 1.10–1.19, I2 = 0%, heterogeneity p value = 0.663; Fig. 2 and Fig. S31). In the random-effect meta-analysis for preterm birth (≤ 37 weeks, 6 studies) [77, 84, 85, 140, 233, 247], the overall asthma risk estimate was increased (pooled OR = 1.32, 95% CI: 1.28–1.37, I2 = 0%, heterogeneity p value = 0.718; Fig. 2 and Fig. S32). In the random-effect meta-analysis for childbirth by caesarean section (8 studies) [36, 47, 84, 126, 131, 133, 249, 250], the overall asthma risk estimate was increased (pooled OR = 1.21, 95% CI: 1.07–1.37, I2 = 78.5%, heterogeneity p value < 0.001; Fig. 2 and Fig. S33).
Publication bias
Publication bias was assessed using a funnel plot for each of the 33 meta-analyses performed in this current study (Fig. 2 and Figs. S1, S2, S3, S4, S5, S6, S7, S8, S9, S10, S11, S12, S13, S14, S15, S16, S17, S18, S19, S20, S21, S22, S23, S24, S25, S26, S27, S28, S29, S30, S31, S32, S33). Of these, 23 meta-analyses have insufficient studies (n < 10) to be comprehensively analyzed for publication bias. Of the remaining 10 meta-analyses, symmetrical funnel plots were observed for the analyses for the overall family history of asthma and paternal asthma (Fig. 2, Figs. S1, and S3). However, for the analyses for 8 other risk factors (maternal asthma, overall family history of atopy, overall family history of allergic diseases, household presence of mold, household presence of mold odor, male gender, cigarette smoke exposure, and cigarette smoking), these funnel plots were asymmetrical, suggesting publication biases (Fig. 2, Figs. S2, S6, S8, S13, S14, S19, S20, S21).
Other factors not included in meta-analysis
Meta-analysis was not performed for multiple asthma risk factors that were frequently reported, including parental or participant’s educational level, pet exposure, urbanization, and dietary habits, given these factors were assessed differently among reviewed studies. Overall, seven studies reported that a higher educational level of the participant was significantly associated with a lower risk of asthma [42, 49, 162, 167, 196, 213, 216], while 11 studies have provided mixed or insignificant findings [26, 125, 178, 179, 197, 252] (Table S5) [42, 49, 162, 167, 196, 213, 216]. Besides, five studies reported that a higher parental educational level is significantly associated with a lower risk of asthma [31, 58, 86, 93, 100], while eight studies reported that a lower parental educational level is significantly associated with a lower risk of asthma [38, 47, 54, 104, 120, 126, 138, 170]. Five studies reported mixed or insignificant associations between parental educational level and asthma risk.
The associations between pet exposures and asthma risk were investigated in 33 studies [16, 18, 27, 36, 44, 58, 66, 67, 71, 77, 79, 90, 97, 101, 107, 113, 117, 119, 125, 130, 132, 135, 139, 160, 165, 169, 172, 178, 199, 204, 253–255]. Of these findings, an increased risk of asthma was significantly associated with the exposure to cats (7 studies) [27, 58, 90, 113, 117, 172, 253] or dogs (7 studies) [16, 44, 71, 90, 113, 117, 160]. A study had also associated the exposure to both cats and dogs with increasing asthma susceptibility [18]. Multiple studies have also associated asthma with exposure to a specific group of animals, such as farm animals [113, 117, 169], furred pets [101, 204, 254], or overall pet animals [44, 66, 67, 97, 107, 117, 119]. Stratification of the study cohort based on the duration or frequency of exposure [27, 160], number of pets owned [113, 165], or exposure to animals at a specific stage of life [27, 113, 165, 253] was also reported to be significantly associated with asthma development. Overall, in most studies, exposure to animals was shown to associate with an increased risk of asthma, whereas only five studies have shown the asthma-protective effect from pet or farm animal exposures [66, 119, 132, 169, 254].
Further, 28 studies have investigated and compared the effect of living in urban, suburban, or rural areas on the risk of developing asthma [31, 42, 43, 49, 54, 62, 78, 84, 87, 100, 105, 107, 121, 127, 129, 142, 157, 172, 179, 189–192, 197, 199, 213, 235, 256]. Increasing urbanization level was shown to be significantly associated with increased asthma risk in 17 studies [31, 54, 84, 100, 107, 121, 127, 129, 142, 157, 172, 190–192, 213, 235, 256], whereas an opposite trend of decreasing asthma risk due to this increase was shown in four studies [43, 49, 105, 197].
The associations between dietary habits and asthma were studied and reported in 23 articles [16, 18, 23, 31, 47, 65, 71, 77, 86, 91, 96, 153, 166, 191, 195, 196, 200, 256–261]. Of these, an increased risk of developing asthma was significantly associated with the consumption of meat (chicken, red meat, etc.) (6 studies) [71, 86, 153, 195, 196, 257] or junk foods (2 studies) [18, 257], whereas fruit consumption was correlated to reduced asthma risk (6 studies) [16, 96, 166, 196, 200, 257]. However, for the other types of food consumption, contradictory findings were often observed from the literature. For instance, dairy product consumption was associated with either an increase [23, 86] or a decrease [16, 31, 71, 195, 196] in asthma risk. Similarly, contradictory findings were also reported on the effects of consumption of seafood (including fish) [16, 256, 259, 260] and vegetables [16, 31, 196] on the risk of developing asthma.
The association between asthma and cooking fume exposure was investigated and reported in 21 studies [16, 26, 42, 101, 116, 118, 130, 155, 166–168, 172, 175, 178, 179, 181, 182, 212, 213, 215, 262]. Increasing risk of developing asthma was reported for various routes of exposure to cooking fumes, including the exposure to direct oil fumes [101], cooking without a chimney or a fan [168, 213], eating in the kitchen [116], and cooking in the house without a separate kitchen [167, 179, 212]. Five studies have further shown the usage of wood [16, 155], coal [101, 215, 262], gas [118, 130, 166], high-pollution fuels [167], fuel mix [179], and biomass/solid fuels [179, 212, 213] as cooking fuels for household cooking was associated with increased asthma risk, as compared with the usage of low-pollution fuels such as electricity. Seven studies reported a mixed or insignificant association between cooking fume exposure and asthma [26, 42, 172, 175, 178, 181, 182].
Lastly, the association between socioeconomic status and asthma was studied and reported in 21 articles [33, 40, 47, 62, 68, 70, 78, 88, 105, 115, 125, 133, 162, 178, 179, 197–199, 212, 232, 252]. This risk factor was assessed differently as income [33, 40, 47, 62, 68, 70, 78, 105, 115, 125, 133, 178, 198, 199], standard of living index [162, 179, 252], socioeconomic status [232], financial standing [88], wealth index [212], or wealth category [197] across multiple studies. Increasing socioeconomic status was shown to be significantly associated with increased asthma risk in four studies [33, 40, 47, 232], whereas an opposite trend of decreasing asthma risk due to this increase was shown in nine studies [68, 88, 105, 115, 133, 162, 179, 199, 212]. Also, eight studies reported a mixed or insignificant association between socioeconomic factor and asthma [62, 70, 78, 125, 178, 197, 198, 252].
Discussion
The current systematic review and meta-analysis study aimed to summarize and estimate the overall risk estimates of frequently reported asthma risk factors in the Asian population. We included 289 studies that were published from the year 1993 to 2021. In these studies, 15 major categories of asthma risk factors were reported in at least 20 studies, including family medical history, housing (condition, environment, size, type, etc.), age, gender, cigarette smoke exposure, cigarette smoking, BMI-related factors, pet exposure, educational level, urbanization, air pollution, breastfeeding, dietary habits, cooking fume exposure, and socioeconomic status. For most of these risk factors, we conducted random-effect meta-analyses and demonstrated overall significant associations between these factors and asthma in the Asian population. To our knowledge, this is the most up-to-date systematic review and meta-analysis of asthma-associated risk factors in Asia. The current study identified major factors that are frequently and significantly associated with the manifestation of asthma in this region. Further, these asthma risk factors can be divided into modifiable and non-modifiable factors to be used as an effective target of asthma preventive medicine. Modifiable factors include housing (condition, environment, size, type, etc.), cigarette smoke exposure, cigarette smoking, BMI-related factors, pet exposure, educational level, urbanization, air pollution, breastfeeding, dietary habits, cooking fume exposure, and socioeconomic status. These factors can be targeted in primary asthma preventive measures that focus on the prevention of disease development. Non-modifiable asthma risk factors, including family medical history, age, and gender, can be used as a target in secondary and tertiary asthma preventive measures that focus on early disease detection and reduction of disease severity.
Overall, the family medical history of various allergy-related conditions was most frequently studied and reported to be significantly associated with the risk of asthma development. Of these family medical conditions, frequently reported was the family history of asthma, which was found to significantly associate with asthma development in 37 studies performed in Asia (Table S5) [20, 25–27, 32, 34, 35, 44–46, 51, 57, 59, 76, 77, 79, 82, 83, 86, 95, 101, 102, 104–106, 108–110, 112, 116, 123, 125, 128, 129, 133, 137, 140, 141]. Our findings are in concordance with the meta-analysis result performed previously using 6 independent studies, which showed an overall increase in asthma risk for preschool children with a family history of asthma (pooled OR = 2.20, 95% CI: 1.54–3.14) [12]. This suggests a high heritability of asthma and the genetic component may underlie the disease pathogenesis process. Multiple asthma candidate genes have been discovered to date, with the heritability of this disease estimated to range from 35 to 95% [263–266]. Nevertheless, the genetic pathway leading to asthma development is not well understood and should be explored further to improve the current understanding of asthma pathogenesis.
In this meta-analysis study, we also observed overall significant associations between asthma and multiple housing-related risk factors, including housing dampness, presence of water damage, carpet usage, and exposures to mold and cockroaches. Indoor dampness and the presence of mold in the household were shown to associate with increased asthma risk in a previously conducted meta-analysis study [267]. This indicates allergenic sensitizations towards fungal spores and conidia might associate with asthma development, which is in concordance with previous epidemiological and immunological evidence [268–270]. Similarly, the usage of carpet in the household environment might also increase an individual’s sensitization to house dust mite allergens, which was consistently shown to increase the risk of developing asthma in the tropical region of Asia [32, 271–273]. Lastly, sensitization to cockroaches was also frequently reported as an important risk factor for asthma (reviewed in [274]). Given these consistent associations reported in several studies, action should be taken to reduce the respective allergen load in the household environment to decrease the risk of asthma development.
This current meta-analysis focused on studies conducted in Asia. By comparing our results to meta-analyses that were focused on the general global outcomes, multiple region-specific risk factors were observed. For instance, an overall increase in asthma risk was associated with black carbon pollution in a previous meta-analysis [11], while this current meta-analysis did not observe any study reporting this association. The protective effect of exclusive breastfeeding against asthma was shown to be significant using meta-analysis [275]; however, this current study did not show a significant association (potentially due to the combined sample size). Besides, the overall asthma risk was increased for household water damage in our current meta-analysis; however, a previous meta-analysis [267] did not show a significant overall association for this risk factor, suggesting differential environmental factors may be more predominant in Asia. Exposure to cats was shown to significantly reduce the risk of asthma in a previous meta-analysis [276]. Although we did not perform a meta-analysis on this risk factor due to the heterogeneity across studies, those that evaluated this factor have shown an increased risk for asthma associated with exposure to cats. Collectively, these observations suggest findings from previous global meta-analyses may not entirely be generalizable to the Asian population, and slight variations may occur.
Our study has several limitations that should be addressed. First, in the meta-analyses for most of the risk factors, the number of studies included was too small (n < 10) for the comprehensive assessment of publication bias using the funnel plot [277]. Besides, we also detected a significant level of heterogeneity in most of the meta-analyses performed. This may be due to the differences in cultural, lifestyle, geographical, and ethnic background that may influence the associations between most factors and asthma. Additional study is therefore required to further validate these identified factors that were associated with asthma in Asia.
Conclusion
In conclusion, the current review study has identified multiple environmental and host-related asthma risk factors in the Asian population. The risk factors identified in our meta-analysis can improve the current understanding of asthma etiology and develop better preventive, therapeutic, and prognostic approaches for asthma.
Supplementary Information
Additional file 1: Figures S1–S33. Forest plots and funnel plots for random-effect meta-analysis of the different asthma risk factors.
Additional file 2: Table S1. PRISMA 2009 Checklist.
Additional file 3: Table S2. Keywords used to perform literature search in three publication databases (Web of Science, Scopus, and Pubmed) to retrieve articles reporting asthma-associated risk factors in Asia.
Additional file 4: Table S3. Study characteristics and reported asthma-associated risk factors of 289 studies included in the systematic review process.
Additional file 5: Table S4. Reported publications on asthma-associated risk factors from countries, dependencies, or other territories within Asia (1993-2021).
Additional file 6: Table S5. Summary of frequently reported asthma-associated risk factors in the Asian population (1993-2021).
Additional file 7: Table S6. Summary of frequently reported asthma comorbidities in the Asian population (1993-2021).
Acknowledgments
We would like to thank all authors who contributed to the studies that we have reviewed and all participants involved in these studies.
Abbreviations
- AR
Allergic rhinitis
- AD
Atopic dermatitis
- BMI
Body mass index
- CI
Confidence interval
- ISAAC
International Study of Asthma and Allergies in Childhood
- OR
Odds ratio
- PM10
Particulate matter less than 10 μm
- PM2.5
Particulate matter less than 2.5 μm
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Authors’ contributions
FTC conceived and supervised the current review study. YYS conducted the literature review process and analyzed the data. YYS wrote the manuscript. All authors reviewed and approved the manuscript.
Funding
F.T.C. has received research support from the Singapore Ministry of Education Academic Research Fund, Singapore Immunology Network (SIgN), National Medical Research Council (NMRC) (Singapore), Biomedical Research Council (BMRC) (Singapore), and the Agency for Science Technology and Research (A*STAR) (Singapore); Grant Numbers are N-154-000-038-001, R-154-000-191-112, R-154-000-404-112, R-154-000-553-112, R-154-000-565-112, R-154-000-630-112, R-154-000-A08-592, R-154-000-A27-597, R-154-000-A91-592, R-154-000-A95-592, R-154-000-B99-114, BMRC/01/1/21/18/077, BMRC/04/1/21/19/315, BMRC/APG2013/108, SIgN-06-006, SIgN-08-020, NMRC/1150/2008, and H17/01/a0/008. F.T.C. has received consulting fees from Sime Darby Technology Centre, First Resources Ltd, Genting Plantation, and Olam International, outside the submitted work. Y.Y.S. has received research support from the NUS Resilience & Growth Postdoctoral Fellowships with grant number R-141-000-036-281. All funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data used and included in this study are available from the corresponding author (Chew Fook Tim).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The Global Asthma Report 2014. http://www.globalasthmareport.org/resources/Global_Asthma_Report_2014.pdf.
- 2.Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma P The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 3.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee: Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC). Eur Respir J. 1998;12(2):315–35. [DOI] [PubMed]
- 4.Burke W, Fesinmeyer M, Reed K, Hampson L, Carlsten C. Family history as a predictor of asthma risk. Am J Prev Med. 2003;24(2):160–169. doi: 10.1016/s0749-3797(02)00589-5. [DOI] [PubMed] [Google Scholar]
- 5.Liu T, Valdez R, Yoon PW, Crocker D, Moonesinghe R, Khoury MJ. The association between family history of asthma and the prevalence of asthma among US adults: National Health and Nutrition Examination Survey, 1999-2004. Genet Med. 2009;11(5):323–328. doi: 10.1097/GIM.0b013e31819d3015. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland ER. Linking obesity and asthma. Ann N Y Acad Sci. 2014;1311:31–41. doi: 10.1111/nyas.12357. [DOI] [PubMed] [Google Scholar]
- 7.Jung KH, Hsu SI, Yan B, Moors K, Chillrud SN, Ross J, Wang S, Perzanowski MS, Kinney PL, Whyatt RM, et al. Childhood exposure to fine particulate matter and black carbon and the development of new wheeze between ages 5 and 7 in an urban prospective cohort. Environ Int. 2012;45:44–50. doi: 10.1016/j.envint.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polosa R, Thomson NC. Smoking and asthma: dangerous liaisons. Eur Respir J. 2013;41(3):716–726. doi: 10.1183/09031936.00073312. [DOI] [PubMed] [Google Scholar]
- 10.Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS One. 2010;5(4):e10134. doi: 10.1371/journal.pone.0010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int. 2017;100:1–31. doi: 10.1016/j.envint.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Bao Y, Chen Z, Liu E, Xiang L, Zhao D, Hong J. Risk factors in preschool children for predicting asthma during the preschool age and the early school age: a systematic review and meta-analysis. Curr Allergy Asthma Rep. 2017;17(12):85. doi: 10.1007/s11882-017-0753-7. [DOI] [PubMed] [Google Scholar]
- 13.Khan KS, Kunz R, Kleijnen J, Antes G. Five steps to conducting a systematic review. J R Soc Med. 2003;96(3):118–121. doi: 10.1258/jrsm.96.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Lisy K, Qureshi R, Mattis P, et al. JBI Manual for Evidence Synthesis. In: Aromataris E, Munn Z, et al., editors. Systematic reviews of etiology and risk. 2020. [Google Scholar]
- 16.Alqahtani JM, Asaad AM, Awadalla NJ, Mahfouz AA. Environmental determinants of bronchial asthma among Saudi school children in Southwestern Saudi Arabia. Int J Environ Res Public Health. 2016;14(1):22. doi: 10.3390/ijerph14010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takaoka M, Suzuki K, Norback D. Current asthma, respiratory symptoms and airway infections among students in relation to the school and home environment in Japan. J Asthma. 2017;54(6):652–661. doi: 10.1080/02770903.2016.1255957. [DOI] [PubMed] [Google Scholar]
- 18.Alqahtani JM. Asthma and other allergic diseases among Saudi schoolchildren in Najran: the need for a comprehensive intervention program. Ann Saudi Med. 2016;36(6):379–385. doi: 10.5144/0256-4947.2016.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izuhara Y, Matsumoto H, Nagasaki T, Kanemitsu Y, Murase K, Ito I, Oguma T, Muro S, Asai K, Tabara Y, et al. Mouth breathing, another risk factor for asthma: the Nagahama study. Allergy. 2016;71(7):1031–1036. doi: 10.1111/all.12885. [DOI] [PubMed] [Google Scholar]
- 20.Wortong D, Chaiear N, Boonsawat W. Risk of asthma in relation to occupation: a hospital-based case-control study. Asian Pac J Allergy Immunol. 2015;33(2):152–160. doi: 10.12932/AP0487.33.1.2015. [DOI] [PubMed] [Google Scholar]
- 21.Lim FL, Hashim Z, Than LT, Md Said S, Hisham Hashim J, Norback D. Asthma, airway symptoms and rhinitis in office workers in Malaysia: associations with house dust mite (HDM) allergy, cat allergy and levels of house dust mite allergens in office dust. PLoS One. 2015;10(4):e0124905. doi: 10.1371/journal.pone.0124905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chae Y, Hahm MI, Ahn K, Kim J, Kim WK, Lee SY, Park YM, Han MY, Lee KJ, Kwon HJ. Indoor environmental factors associated with wheezing illness and asthma in South Korean children: phase III of the International Study of Asthma and Allergies in Childhood. J Asthma. 2014;51(9):943–949. doi: 10.3109/02770903.2014.930879. [DOI] [PubMed] [Google Scholar]
- 23.Feng M, Yang Z, Pan L, Lai X, Xian M, Huang X, Chen Y, Schroder PC, Roponen M, Schaub B, et al. Associations of early life exposures and environmental factors with asthma among children in rural and urban areas of Guangdong, China. Chest. 2016;149(4):1030–1041. doi: 10.1016/j.chest.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Bener A, Ehlayel MS, Bener HZ, Hamid Q. The impact of Vitamin D deficiency on asthma, allergic rhinitis and wheezing in children: an emerging public health problem. J Fam Community Med. 2014;21(3):154–161. doi: 10.4103/2230-8229.142967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Xiao W, Ma D, Zhang Y, Wang Q, Wang C, Ji X, He B, Wu X, Chen H, et al. Cross-sectional epidemiological survey of asthma in Jinan, China. Respirology. 2013;18(2):313–322. doi: 10.1111/resp.12005. [DOI] [PubMed] [Google Scholar]
- 26.Ding YP, Yao HX, Tang XL, He HW, Shi HF, Lin L, Li M, Chen S, Chen J, Wang HJ. An epidemiology study of bronchial asthma in the Li ethnic group in China. Asian Pac J Trop Med. 2012;5(2):157–161. doi: 10.1016/S1995-7645(12)60016-9. [DOI] [PubMed] [Google Scholar]
- 27.Al-Mousawi MS, Lovel H, Behbehani N, Arifhodzic N, Woodcock A, Custovic A. Asthma and sensitization in a community with low indoor allergen levels and low pet-keeping frequency. J Allergy Clin Immunol. 2004;114(6):1389–1394. doi: 10.1016/j.jaci.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Palmer LJ, Celedon JC, Weiss ST, Wang B, Fang Z, Xu X. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Respir Crit Care Med. 2002;165(11):1489–1493. doi: 10.1164/rccm.2107020. [DOI] [PubMed] [Google Scholar]
- 29.Leung TF, Lam CW, Chan IH, Li AM, Ha G, Tang NL, Fok TF. Inhalant allergens as risk factors for the development and severity of mild-to-moderate asthma in Hong Kong Chinese children. J Asthma. 2002;39(4):323–330. doi: 10.1081/jas-120002289. [DOI] [PubMed] [Google Scholar]
- 30.Celedon JC, Palmer LJ, Xu X, Wang B, Fang Z, Weiss ST. Sensitization to silk and childhood asthma in rural China. Pediatrics. 2001;107(5):E80. doi: 10.1542/peds.107.5.e80. [DOI] [PubMed] [Google Scholar]
- 31.Hijazi N, Abalkhail B, Seaton A. Diet and childhood asthma in a society in transition: a study in urban and rural Saudi Arabia. Thorax. 2000;55(9):775–779. doi: 10.1136/thorax.55.9.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung R, Ho P, Lam CW, Lai CK. Sensitization to inhaled allergens as a risk factor for asthma and allergic diseases in Chinese population. J Allergy Clin Immunol. 1997;99(5):594–599. doi: 10.1016/s0091-6749(97)70018-6. [DOI] [PubMed] [Google Scholar]
- 33.Goh DY, Chew FT, Quek SC, Lee BW. Prevalence and severity of asthma, rhinitis, and eczema in Singapore schoolchildren. Arch Dis Child. 1996;74(2):131–135. doi: 10.1136/adc.74.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung R, Ho P. Asthma, allergy, and atopy in three south-east Asian populations. Thorax. 1994;49(12):1205–1210. doi: 10.1136/thx.49.12.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oshikata C, Watanabe M, Ishida M, Kobayashi S, Kubosaki A, Yamazaki A, et al. Increase in asthma prevalence in adults in temporary housing after the Great East Japan earthquake. Int J Disaster Risk Reduction. 2020;50.
- 36.Boker F, Alzahrani A, Alsaeed A, Alzhrani M, Albar R. Cesarean section and development of childhood bronchial asthma: is there a risk? Open Access Maced J Med Sci. 2019;7(3):347–351. doi: 10.3889/oamjms.2019.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawlader MD, Ma E, Noguchi E, Itoh M, Arifeen SE, Persson LA, Moore SE, Raqib R, Wagatsuma Y. Ascaris lumbricoids infection as a risk factor for asthma and atopy in rural Bangladeshi children. Trop Med Health. 2014;42(2):77–85. doi: 10.2149/tmh.2013-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu YT, Chen WY, Wang TN, Tseng HI, Wu JR, Ko YC. Extreme BMI predicts higher asthma prevalence and is associated with lung function impairment in school-aged children. Pediatr Pulmonol. 2009;44(5):472–479. doi: 10.1002/ppul.21023. [DOI] [PubMed] [Google Scholar]
- 39.Sundaru H. House dust mite allergen level and allergen sensitization as risk factors for asthma among student in Central Jakarta. Med J Indonesia. 2006;15(1):5. [Google Scholar]
- 40.Lau YL, Karlberg J, Yeung CY. Prevalence of and factors associated with childhood asthma in Hong Kong. Acta Paediatr. 1995;84(7):820–822. doi: 10.1111/j.1651-2227.1995.tb13767.x. [DOI] [PubMed] [Google Scholar]
- 41.Celedon JC, Palmer LJ, Weiss ST, Wang B, Fang Z, Xu X. Asthma, rhinitis, and skin test reactivity to aeroallergens in families of asthmatic subjects in Anqing, China. Am J Respir Crit Care Med. 2001;163(5):1108–1112. doi: 10.1164/ajrccm.163.5.2005086. [DOI] [PubMed] [Google Scholar]
- 42.Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, Bai C, Kang J, Ran P, Shen H, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394(10196):407–418. doi: 10.1016/S0140-6736(19)31147-X. [DOI] [PubMed] [Google Scholar]
- 43.Masoompour SM, Mahdaviazad H, Ghayumi SMA. Asthma and its related socioeconomic factors: The Shiraz Adult Respiratory Disease Study 2015. Clin Respir J. 2018;12(6):2110–2116. doi: 10.1111/crj.12780. [DOI] [PubMed] [Google Scholar]
- 44.Lin J, Wang W, Chen P, Zhou X, Wan H, Yin K, Ma L, Wu C, Li J, Liu C, et al. Prevalence and risk factors of asthma in mainland China: the CARE study. Respir Med. 2018;137:48–54. doi: 10.1016/j.rmed.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Qureshi UA, Bilques S, Ul Haq I, Khan MS, Qurieshi MA, Qureshi UA. Epidemiology of bronchial asthma in school children (10-16 years) in Srinagar. Lung India. 2016;33(2):167–173. doi: 10.4103/0970-2113.177442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danansuriya MN, Rajapaksa LC, Weerasinghe A. Genetic, familial and environmental correlates of asthma among early adolescents in Sri Lanka: a case control study. World Allergy Organ J. 2015;8(1):19. doi: 10.1186/s40413-015-0068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li F, Zhou YC, Tong SL, Li SH, Jiang F, Jin XM, Yan CH, Tian Y, Deng SN, Shen XM. Environmental risk factor assessment: a multilevel analysis of childhood asthma in China. World J Pediatr. 2013;9(2):120–126. doi: 10.1007/s12519-013-0413-5. [DOI] [PubMed] [Google Scholar]
- 48.Higuchi O, Adachi Y, Itazawa T, Ito Y, Yoshida K, Ohya Y, Odajima H, Akasawa A, Miyawaki T. Rhinitis has an association with asthma in school children. Am J Rhinol Allergy. 2013;27(1):e22–e25. doi: 10.2500/ajra.2013.27.3846. [DOI] [PubMed] [Google Scholar]
- 49.Ekici A, Ekici M, Kocyigit P, Karlidag A. Prevalence of self-reported asthma in urban and rural areas of Turkey. J Asthma. 2012;49(5):522–526. doi: 10.3109/02770903.2012.677893. [DOI] [PubMed] [Google Scholar]
- 50.Al Ghobain MO, Al-Hajjaj MS, Al Moamary MS. Asthma prevalence among 16- to 18-year-old adolescents in Saudi Arabia using the ISAAC questionnaire. BMC Public Health. 2012;12:239. doi: 10.1186/1471-2458-12-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cakir E, Ersu R, Uyan ZS, Oktem S, Varol N, Karakoc F, Karadag B, Akyol M, Dagli E. The prevalence and risk factors of asthma and allergic diseases among working adolescents. Asian Pac J Allergy Immunol. 2010;28(2-3):122–129. [PubMed] [Google Scholar]
- 52.Uthaisangsook S. Risk factors for development of asthma in Thai adults in Phitsanulok: a university-based study. Asian Pac J Allergy Immunol. 2010;28(1):23–28. [PubMed] [Google Scholar]
- 53.Musharrafieh U, Al-Sahab B, Zaitoun F, El-Hajj MA, Ramadan F, Tamim H. Prevalence of asthma, allergic rhinitis and eczema among Lebanese adolescents. J Asthma. 2009;46(4):382–387. doi: 10.1080/02770900902777775. [DOI] [PubMed] [Google Scholar]
- 54.Ho WC, Hartley WR, Myers L, Lin MH, Lin YS, Lien CH, Lin RS. Air pollution, weather, and associated risk factors related to asthma prevalence and attack rate. Environ Res. 2007;104(3):402–409. doi: 10.1016/j.envres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Nga NN, Chai SK, Bihn TT, Redding G, Takaro T, Checkoway H, Son PH, Van DK, Keifer M, Trung le V, et al. ISAAC-based asthma and atopic symptoms among Ha Noi school children. Pediatr Allergy Immunol. 2003;14(4):272–279. doi: 10.1034/j.1399-3038.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 56.Leung R, Wong G, Lau J, Ho A, Chan JK, Choy D, Douglass C, Lai CK. Prevalence of asthma and allergy in Hong Kong schoolchildren: an ISAAC study. Eur Respir J. 1997;10(2):354–360. doi: 10.1183/09031936.97.10020354. [DOI] [PubMed] [Google Scholar]
- 57.Moussa MA, Skaik MB, Yaghy OY, Salwanes SB, Bin-Othman SA. Factors associated with asthma in school children. Eur J Epidemiol. 1996;12(6):583–588. doi: 10.1007/BF00499456. [DOI] [PubMed] [Google Scholar]
- 58.Rahimi Rad MH, Hejazi ME, Behrouzian R. Asthma and other allergic diseases in 13-14-year-old schoolchildren in Urmia: an ISAAC study. EMHJ. 2007;13:12. doi: 10.26719/2007.13.5.1005. [DOI] [PubMed] [Google Scholar]
- 59.Al-Mazam A, Mohamed AG. Risk factors of bronchial asthma in Bahrah, Saudi Arabia. J Family Community Med. 2001;8(1):33–39. [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Y, Hou J, Sheng Y, Kong X, Weschler LB, Sundell J. Modern life makes children allergic. A cross-sectional study: associations of home environment and lifestyles with asthma and allergy among children in Tianjin region, China. Int Arch Occup Environ Health. 2019;92(4):587–598. doi: 10.1007/s00420-018-1395-3. [DOI] [PubMed] [Google Scholar]
- 61.Nugmanova D, Sokolova L, Feshchenko Y, Iashyna L, Gyrina O, Malynovska K, Mustafayev I, Aliyeva G, Makarova J, Vasylyev A, et al. The prevalence, burden and risk factors associated with bronchial asthma in commonwealth of independent states countries (Ukraine, Kazakhstan and Azerbaijan): results of the CORE study. BMC Pulm Med. 2018;18(1):110. doi: 10.1186/s12890-018-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang CC, Chiang TL, Chen PC, Lin SJ, Wen HJ, Guo YL. Risk factors for asthma occurrence in children with early-onset atopic dermatitis: an 8-year follow-up study. Pediatr Allergy Immunol. 2018;29(2):159–165. doi: 10.1111/pai.12835. [DOI] [PubMed] [Google Scholar]
- 63.Jeng MJ, Lee YS, Tsao PC, Yang CF, Soong WJ. A longitudinal study on early hospitalized airway infections and subsequent childhood asthma. PLoS One. 2014;10(4):e0121906. doi: 10.1371/journal.pone.0121906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen YC, Tsai CH, Lee Y. Gestational medication use, birth conditions, and early postnatal exposures for childhood asthma. Clin Dev Immunol. 2012;2012:913426. doi: 10.1155/2012/913426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeh KW, Ou LS, Yao TC, Chen LC, Lee WI, Huang JL, Group PS Prevalence and risk factors for early presentation of asthma among preschool children in Taiwan. Asian Pac J Allergy Immunol. 2011;29(2):120–126. [PubMed] [Google Scholar]
- 66.Fernando D, Wickramasinghe P, Kapilananda G, Dewasurendra RL, Amarasooriya M, Dayaratne A. Toxocara seropositivity in Sri Lankan children with asthma. Pediatr Int. 2009;51(2):241–245. doi: 10.1111/j.1442-200X.2008.02687.x. [DOI] [PubMed] [Google Scholar]
- 67.Waked M, Salameh P. Risk factors for asthma and allergic diseases in school children across Lebanon. J Asthma Allergy. 2008;2:1–7. doi: 10.2147/jaa.s3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaman K, Takeuchi H, Md Y, El Arifeen S, Chowdhury HR, Baqui AH, Wakai S, Iwata T. Asthma in rural Bangladeshi children. Indian J Pediatr. 2007;74(6):539–543. doi: 10.1007/s12098-007-0104-0. [DOI] [PubMed] [Google Scholar]
- 69.S.A.L. AF, A.M. AD Risk factors for asthma among preschool children at Al-Najaf, Iraq: a case control study. Pakistan J Med Health Sci. 2020;14(3):5. [Google Scholar]
- 70.Tan TN, Shek LP, Goh DY, Chew FT, Lee BW. Prevalence of asthma and comorbid allergy symptoms in Singaporean preschoolers. Asian Pac J Allergy Immunol. 2006;24(4):175–182. [PubMed] [Google Scholar]
- 71.Demir AU, Karakaya G, Bozkurt B, Sekerel BE, Kalyoncu AF. Asthma and allergic diseases in schoolchildren: third cross-sectional survey in the same primary school in Ankara, Turkey. Pediatr Allergy Immunol. 2004;15(6):531–538. doi: 10.1111/j.1399-3038.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 72.Huang SL, Tsai PF, Yeh YF. Negative association of Enterobius infestation with asthma and rhinitis in primary school children in Taipei. Clin Exp Allergy. 2002;32(7):1029–1032. doi: 10.1046/j.1365-2745.2002.01424.x. [DOI] [PubMed] [Google Scholar]
- 73.Hallit S, Sacre H, Kheir N, Hobeika E, Hallit R, Waked M, Salameh P. Hygiene hypothesis: association between hygiene and asthma among preschool children in Lebanon. Allergol Immunopathol (Madr) 2021;49(1):135–145. doi: 10.15586/aei.v49i1.41. [DOI] [PubMed] [Google Scholar]
- 74.Shen CY, Lin MC, Lin HK, Lin CH, Fu LS, Fu YC. The natural course of eczema from birth to age 7 years and the association with asthma and allergic rhinitis: a population-based birth cohort study. Allergy Asthma Proc. 2013;34(1):78–83. doi: 10.2500/aap.2013.34.3625. [DOI] [PubMed] [Google Scholar]
- 75.Zhao T, Wang HJ, Chen Y, Xiao M, Duo L, Liu G, Lau Y, Karlberg J. Prevalence of childhood asthma, allergic rhinitis and eczema in Urumqi and Beijing. J Paediatr Child Health. 2000;36(2):128–133. doi: 10.1046/j.1440-1754.2000.00457.x. [DOI] [PubMed] [Google Scholar]
- 76.Ones U, Sapan N, Somer A, Disci R, Salman N, Guler N, Yalcin I. Prevalence of childhood asthma in Istanbul, Turkey. Allergy. 1997;52(5):570–575. doi: 10.1111/j.1398-9995.1997.tb02602.x. [DOI] [PubMed] [Google Scholar]
- 77.Dongol Singh S, Shrestha A. Risk factors associated with childhood asthma - a case control study. Kathmandu Univ Med J (KUMJ) 2018;16(64):290–295. [PubMed] [Google Scholar]
- 78.Jang Y, Shin A. Sex-based differences in asthma among preschool and school-aged children in Korea. PLoS One. 2015;10:e0140057. doi: 10.1371/journal.pone.0140057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawada T. Risk factors and prevalence of asthma or atopic dermatitis in young children by a questionnaire survey. J Nippon Med Sch. 2004;71(3):167–171. doi: 10.1272/jnms.71.167. [DOI] [PubMed] [Google Scholar]
- 80.Tsai MC, Lin HL, Lin CC, Lin HC, Chen YH, Pfeiffer S, Lin HC. Increased risk of concurrent asthma among patients with gastroesophageal reflux disease: a nationwide population-based study. Eur J Gastroenterol Hepatol. 2010;22(10):1169–1173. doi: 10.1097/MEG.0b013e32833fb68c. [DOI] [PubMed] [Google Scholar]
- 81.Ostovar A, Fokkens WJ, Pordel S, Movahed A, Ghasemi K, Marzban M, Farrokhi S. The prevalence of asthma in adult population of southwestern Iran and its association with chronic rhinosinusitis: a GA(2)LEN study. Clin Transl Allergy. 2019;9:43. doi: 10.1186/s13601-019-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ones U, Akcay A, Tamay Z, Guler N, Zencir M. Rising trend of asthma prevalence among Turkish schoolchildren (ISAAC phases I and III) Allergy. 2006;61(12):1448–1453. doi: 10.1111/j.1398-9995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 83.Gazala E, Ron-Feldman V, Alterman M, Kama S, Novack L. The association between birth season and future development of childhood asthma. Pediatr Pulmonol. 2006;41(12):1125–1128. doi: 10.1002/ppul.20442. [DOI] [PubMed] [Google Scholar]
- 84.Lin CH, Wang JL, Chen HH, Hsu JY, Chao WC. Shared prenatal impacts among childhood asthma, allergic rhinitis and atopic dermatitis: a population-based study. Allergy Asthma Clin Immunol. 2019;15(1):52. doi: 10.1186/s13223-019-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang S, Ou C, Liu R, Jiang H, Xie Z, Lam C, et al. Association between parental perceptions of odors and childhood asthma in subtropical South China with a hot humid climate. Building Environ. 2019;159.
- 86.Malaeb D, Hallit S, Sacre H, Malaeb B, Hallit R, Salameh P. Diet and asthma in Lebanese schoolchildren: a cross-sectional study. Pediatr Pulmonol. 2019;54(6):688–697. doi: 10.1002/ppul.24280. [DOI] [PubMed] [Google Scholar]
- 87.Toizumi M, Hashizume M, Nguyen HAT, Yasunami M, Kitamura N, Iwasaki C, Takegata M, Moriuchi H, Dang DA, Ariyoshi K, et al. Asthma, rhinoconjunctivitis, eczema, and the association with perinatal anthropometric factors in Vietnamese children. Sci Rep. 2019;9(1):2655. doi: 10.1038/s41598-019-39658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beridze V, Abuladze L, Partenadze N, Bakhtadze T, Lawson J, Zejda JE. Childhood asthma in Batumi, Georgia: prevalence and environmental correlates. J Asthma. 2018;55(1):43–49. doi: 10.1080/02770903.2016.1247169. [DOI] [PubMed] [Google Scholar]
- 89.Kashanian M, Mohtashami SS, Bemanian MH, Moosavi SAJ, Moradi Lakeh M. Evaluation of the associations between childhood asthma and prenatal and perinatal factors. Int J Gynaecol Obstet. 2017;137(3):290–294. doi: 10.1002/ijgo.12141. [DOI] [PubMed] [Google Scholar]
- 90.Ziyab AH. Prevalence and Risk Factors of asthma, rhinitis, and eczema and their multimorbidity among young adults in Kuwait: a cross-sectional study. Biomed Res Int. 2017;2017:2184193. doi: 10.1155/2017/2184193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang SP, Liu YL, Wang SB, Weng SF, Chen S, Zhang MJ, Dong L, Guo YH, Lin DR, Hua YH, et al. Trends in prevalence and risk factors of childhood asthma in Fuzhou, a city in Southeastern China. J Asthma. 2015;52(1):10–15. doi: 10.3109/02770903.2014.952434. [DOI] [PubMed] [Google Scholar]
- 92.Tavacol H, Rahimi Z, Cheraghi M, Ghatfan F, Baji Z, Rahmani H. A cross-sectional study of prevalence and risk factors for childhood asthma in Ahvaz city, Iran. Postepy Dermatol Alergol. 2015;32(4):268–273. doi: 10.5114/pdia.2015.53322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abidin E, Semple S, Rasdi I, Ismail S, Ayres J. The relationship between air pollution and asthma in Malaysian schoolchildren. Air Qual Atmosphere Health. 2014;7(4):12. [Google Scholar]
- 94.Becerir T, Akcay A, Duksal F, Ergin A, Becerir C, Guler N. Prevalence of asthma, local risk factors and agreement between written and video questionnaires among Turkish adolescents. Allergol Immunopathol (Madr) 2014;42(6):594–602. doi: 10.1016/j.aller.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 95.Khan AA, Tanzil S, Jamali T, Shahid A, Naeem S, Sahito A, Siddiqui FA, Nafees AA, Fatmi Z. Burden of asthma among children in a developing megacity: childhood asthma study, Pakistan. J Asthma. 2014;51(9):891–899. doi: 10.3109/02770903.2014.930882. [DOI] [PubMed] [Google Scholar]
- 96.Akcay A, Tamay Z, Hocaoglu AB, Ergin A, Guler N. Risk factors affecting asthma prevalence in adolescents living in Istanbul, Turkey. Allergol Immunopathol (Madr) 2014;42(5):449–458. doi: 10.1016/j.aller.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 97.Duksal F, Becerir T, Ergin A, Akcay A, Guler N. The prevalence of asthma diagnosis and symptoms is still increasing in early adolescents in Turkey. Allergol Int. 2014;63(2):189–197. doi: 10.2332/allergolint.13-OA-0612. [DOI] [PubMed] [Google Scholar]
- 98.Ugurlu E, Oncel SB, Evyapan F. Symptom prevalence and risk factors for asthma at the rural regions of Denizli, Turkey. J Thorac Dis. 2014;6(5):452–458. doi: 10.3978/j.issn.2072-1439.2014.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Norback D, Markowicz P, Cai GH, Hashim Z, Ali F, Zheng YW, Lai XX, Spangfort MD, Larsson L, Hashim JH. Endotoxin, ergosterol, fungal DNA and allergens in dust from schools in Johor Bahru, Malaysia- associations with asthma and respiratory infections in pupils. PLoS One. 2014;9(2):e88303. doi: 10.1371/journal.pone.0088303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lamnisos D, Moustaki M, Kolokotroni O, Koksoy H, Faiz M, Arifoglu K, Milton DK, Middleton N, Yiallouros PK. Prevalence of asthma and allergies in children from the Greek-Cypriot and Turkish-Cypriot communities in Cyprus: a bi-communal cross-sectional study. BMC Public Health. 2013;13:585. doi: 10.1186/1471-2458-13-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jie Y, Isa Z, Jie X, Ismail N. Asthma and asthma-related symptoms among adults of an acid rain plagued city in Southwest China: prevalence and risk factors. Polish J Environ Stud. 2013;22(3):10. [Google Scholar]
- 102.Oshnouei S, Salarilak S, Khalkhali A, Karamyar M, Rahimi Rad M, Delpishe A. Effects of acetaminophen consumption in asthmatic children. Iran Red Crescent Med J. 2012;14(10):641–646. [PMC free article] [PubMed] [Google Scholar]
- 103.Nathan AM, de Bruyne J, Khalid F, Arumugam K. Caesarean section and asthma in Malaysian children: a case-control study. Asian Pac J Allergy Immunol. 2012;30(3):204–208. [PubMed] [Google Scholar]
- 104.Lee SL, Lam TH, Leung TH, Wong WH, Schooling M, Leung GM, Lau YL. Foetal exposure to maternal passive smoking is associated with childhood asthma, allergic rhinitis, and eczema. Sci World J. 2012;2012:542983. doi: 10.1100/2012/542983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hwang GS, Choi JW, Yoo Y, Choung JT, Yoon CS. Residential environmental risk factors for childhood asthma prevalence in metropolitan and semirural cities in Korea. Asia Pac J Public Health. 2012;24(1):58–67. doi: 10.1177/1010539510373139. [DOI] [PubMed] [Google Scholar]
- 106.Mahdi B, Mahesh PA, Mysore RS, Kumar P, Jayaraj BS, Ramachandra NB. Inheritance patterns, consanguinity & risk for asthma. Indian J Med Res. 2010;132:48–55. [PubMed] [Google Scholar]
- 107.Selcuk ZT, Demir AU, Tabakoglu E, Caglar T. Prevalence of asthma and allergic diseases in primary school children in Edirne, Turkey, two surveys 10 years apart. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e711–e717. doi: 10.1111/j.1399-3038.2010.01008.x. [DOI] [PubMed] [Google Scholar]
- 108.Jain A, Vinod Bhat H, Acharya D. Prevalence of bronchial asthma in rural Indian children: a cross sectional study from South India. Indian J Pediatr. 2010;77(1):31–35. doi: 10.1007/s12098-009-0308-6. [DOI] [PubMed] [Google Scholar]
- 109.Alsowaidi S, Abdulle A, Bernsen R, Zuberbier T. Allergic rhinitis and asthma: a large cross-sectional study in the United Arab Emirates. Int Arch Allergy Immunol. 2010;153(3):274–279. doi: 10.1159/000314368. [DOI] [PubMed] [Google Scholar]
- 110.Alsowaidi S, Abdulle A, Bernsen R. Prevalence and risk factors of asthma among adolescents and their parents in Al-Ain (United Arab Emirates) Respiration. 2010;79(2):105–111. doi: 10.1159/000219248. [DOI] [PubMed] [Google Scholar]
- 111.Han YY, Lee YL, Guo YL. Indoor environmental risk factors and seasonal variation of childhood asthma. Pediatr Allergy Immunol. 2009;20(8):748–756. doi: 10.1111/j.1399-3038.2009.00871.x. [DOI] [PubMed] [Google Scholar]
- 112.Yoo S, Kim HB, Lee SY, Kim BS, Kim JH, Yu J, Kim BJ, Lee DH, Seong MW, Hong SJ. Effect of active smoking on asthma symptoms, pulmonary function, and BHR in adolescents. Pediatr Pulmonol. 2009;44(10):954–961. doi: 10.1002/ppul.21066. [DOI] [PubMed] [Google Scholar]
- 113.Dong GH, Ma YN, Ding HL, Jin J, Cao Y, Zhao YD, He QC. Pets keeping in home, parental atopy, asthma, and asthma-related symptoms in 12,910 elementary school children from northeast China. Indoor Air. 2009;19(2):166–173. doi: 10.1111/j.1600-0668.2008.00576.x. [DOI] [PubMed] [Google Scholar]
- 114.Joseph M, Zoubeidi T, Al-Dhaheri SM, Al-Dhaheri AA, Al-Dhaheri AA, Al-Kaabi FM, Al-Muhairi SJ, Joseph J. Paternal asthma is a predictor for childhood asthma in the consanguineous families from the United Arab Emirates. J Asthma. 2009;46(2):175–178. doi: 10.1080/02770900802604095. [DOI] [PubMed] [Google Scholar]
- 115.Talay F, Kurt B, Tug T, Yilmaz F, Goksugur N. Prevalence and risk factors of asthma and allergic diseases among schoolchildren in Bolu, Turkey. Acta Paediatr. 2008;97(4):459–462. doi: 10.1111/j.1651-2227.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 116.Pakhale S, Wooldrage K, Manfreda J, Anthonisen N. Prevalence of asthma symptoms in 7th- and 8th-grade school children in a rural region in India. J Asthma. 2008;45(2):117–122. doi: 10.1080/02770900701840220. [DOI] [PubMed] [Google Scholar]
- 117.Dong GH, Ding HL, Ma YN, Jin J, Cao Y, Zhao YD, He QC. Asthma and asthma-related symptoms in 16 789 Chinese children in relation to pet keeping and parental atopy. J Investig Allergol Clin Immunol. 2008;18(3):207–213. [PubMed] [Google Scholar]
- 118.Wong GW, Leung TF, Ma Y, Liu EK, Yung E, Lai CK. Symptoms of asthma and atopic disorders in preschool children: prevalence and risk factors. Clin Exp Allergy. 2007;37(2):174–179. doi: 10.1111/j.1365-2222.2007.02649.x. [DOI] [PubMed] [Google Scholar]
- 119.Lee YL, Hsiue TR, Lee CH, Su HJ, Guo YL. Home exposures, parental atopy, and occurrence of asthma symptoms in adulthood in southern Taiwan. Chest. 2006;129(2):300–308. doi: 10.1378/chest.129.2.300. [DOI] [PubMed] [Google Scholar]
- 120.Lee YL, Lin YC, Hsiue TR, Hwang BF, Guo YL. Indoor and outdoor environmental exposures, parental atopy, and physician-diagnosed asthma in Taiwanese schoolchildren. Pediatrics. 2003;112(5):e389. doi: 10.1542/peds.112.5.e389. [DOI] [PubMed] [Google Scholar]
- 121.Shohat T, Green MS, Davidson Y, Livne I, Tamir R, Garty BZ. Differences in the prevalence of asthma and current wheeze between Jews and Arabs: results from a national survey of schoolchildren in Israel. Ann Allergy Asthma Immunol. 2002;89(4):386–392. doi: 10.1016/S1081-1206(10)62040-6. [DOI] [PubMed] [Google Scholar]
- 122.Takemura Y, Sakurai Y, Honjo S, Kusakari A, Hara T, Gibo M, Tokimatsu A, Kugai N. Relation between breastfeeding and the prevalence of asthma : the Tokorozawa Childhood Asthma and Pollinosis study. Am J Epidemiol. 2001;154(2):115–119. doi: 10.1093/aje/154.2.115. [DOI] [PubMed] [Google Scholar]
- 123.Wang TN, Chao YY, Wang TH, Chen CJ, Ko YC. Familial risk of asthma among adolescents and their relatives in Taiwan. J Asthma. 2001;38(6):485–494. doi: 10.1081/jas-100105869. [DOI] [PubMed] [Google Scholar]
- 124.Chhabra SK, Gupta CK, Chhabra P, Rajpal S. Risk factors for development of bronchial asthma in children in Delhi. Ann Allergy Asthma Immunol. 1999;83(5):385–390. doi: 10.1016/S1081-1206(10)62835-9. [DOI] [PubMed] [Google Scholar]
- 125.Razzaq S, Nafees AA, Rabbani U, Irfan M, Naeem S, Khan MA, Fatmi Z, Burney P. Epidemiology of asthma and associated factors in an urban Pakistani population: adult asthma study-Karachi. BMC Pulm Med. 2018;18(1):184. doi: 10.1186/s12890-018-0753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huo X, Chu S, Hua L, Bao Y, Du L, Xu J, Zhang J. The effect of breastfeeding on the risk of asthma in high-risk children: a case-control study in Shanghai, China. BMC Pregnancy Childbirth. 2018;18(1):341. doi: 10.1186/s12884-018-1936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Norback D, Lu C, Wang J, Zhang Y, Li B, Zhao Z, Huang C, Zhang X, Qian H, Sun Y, et al. Asthma and rhinitis among Chinese children - indoor and outdoor air pollution and indicators of socioeconomic status (SES) Environ Int. 2018;115:1–8. doi: 10.1016/j.envint.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 128.Lam HT, Ronmark E, Tu'o'ng NV, Ekerljung L, Chuc NT, Lundback B. Increase in asthma and a high prevalence of bronchitis: results from a population study among adults in urban and rural Vietnam. Respir Med. 2011;105(2):177–185. doi: 10.1016/j.rmed.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 129.Idani E, Raji H, Maraghi E, Aghababaeian H, Madadizadeh F, Maryam D. Risk factors associated with asthma among adults in Khuzestan, southwest Iran. Clin Epidemiol Glob Health. 2020;8(2):6. [Google Scholar]
- 130.Huang S, Garshick E, Weschler LB, Hong C, Li J, Li L, Qu F, Gao D, Zhou Y, Sundell J, et al. Home environmental and lifestyle factors associated with asthma, rhinitis and wheeze in children in Beijing, China. Environ Pollut. 2020;256:113426. doi: 10.1016/j.envpol.2019.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hu Y, Chen Y, Liu S, Jiang F, Wu M, Yan C, Tan J, Yu G, Hu Y, Yin Y, et al. Breastfeeding duration modified the effects of neonatal and familial risk factors on childhood asthma and allergy: a population-based study. Respir Res. 2021;22(1):41. doi: 10.1186/s12931-021-01644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arif AA, Veri SD. The association of prenatal risk factors with childhood asthma. J Asthma. 2019;56(10):1056–1061. doi: 10.1080/02770903.2018.1515224. [DOI] [PubMed] [Google Scholar]
- 133.Al Yassen AQ, Al-Asadi JN, Khalaf SK. The role of Caesarean section in childhood asthma. Malays Fam Physician. 2019;14(3):10–17. [PMC free article] [PubMed] [Google Scholar]
- 134.Spiegel E, Shoham-Vardi I, Goldbart A, Sergienko R, Sheiner E. Maternal asthma is an independent risk factor for long-term respiratory morbidity of the offspring. Am J Perinatol. 2018;35(11):1065–1070. doi: 10.1055/s-0038-1639507. [DOI] [PubMed] [Google Scholar]
- 135.Liu F, Zhao Y, Liu YQ, Liu Y, Sun J, Huang MM, Liu Y, Dong GH. Asthma and asthma related symptoms in 23,326 Chinese children in relation to indoor and outdoor environmental factors: the Seven Northeastern Cities (SNEC) Study. Sci Total Environ. 2014;497-498:10–17. doi: 10.1016/j.scitotenv.2014.07.096. [DOI] [PubMed] [Google Scholar]
- 136.Hwang BF, Lee YL, Lin YC, Jaakkola JJ, Guo YL. Traffic related air pollution as a determinant of asthma among Taiwanese school children. Thorax. 2005;60(6):467–473. doi: 10.1136/thx.2004.033977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang M, Wu T, Cheng L, Wang F, Wei Q, Tanguay RM. Plasma antibodies against heat shock protein 70 correlate with the incidence and severity of asthma in a Chinese population. Respir Res. 2005;6:18. doi: 10.1186/1465-9921-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Karunasekera KA, Jayasinghe JA, Alwis LW. Risk factors of childhood asthma: a Sri Lankan study. J Trop Pediatr. 2001;47(3):142–145. doi: 10.1093/tropej/47.3.142. [DOI] [PubMed] [Google Scholar]
- 139.Kalyoncu AF, Demir AU, Ozcakar B, Bozkurt B, Artvinli M. Asthma and allergy in Turkish university students: two cross-sectional surveys 5 years apart. Allergol Immunopathol (Madr) 2001;29(6):264–271. doi: 10.1016/s0301-0546(01)79068-4. [DOI] [PubMed] [Google Scholar]
- 140.Khalkhali HR, Oshnouei S, Salarilak S, Rahimi Rad M, Karamyar M, Khashabi J. Effects of antibiotic consumption on children 2-8 years of age developing asthma. Epidemiol Health. 2014;36:e2014006. doi: 10.4178/epih/e2014006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mathew A, Prince T, Remees R, Saravanapandian N, Ramalingam S, Srikanth K, Mathai J. Prevalence and risk factors of asthma in school going children in South India. Nepal J Epidemiol. 2012;2(1):8. [Google Scholar]
- 142.Wang J, Zhao Z, Zhang Y, Li B, Huang C, Zhang X, Deng Q, Lu C, Qian H, Yang X, et al. Asthma, allergic rhinitis and eczema among parents of preschool children in relation to climate, and dampness and mold in dwellings in China. Environ Int. 2019;130:104910. doi: 10.1016/j.envint.2019.104910. [DOI] [PubMed] [Google Scholar]
- 143.Cai J, Li B, Yu W, Wang H, Du C, Zhang Y, Huang C, Zhao Z, Deng Q, Yang X, et al. Household dampness-related exposures in relation to childhood asthma and rhinitis in China: a multicentre observational study. Environ Int. 2019;126:735–746. doi: 10.1016/j.envint.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 144.Zhang J, Sun C, Liu W, Zou Z, Zhang Y, Li B, Zhao Z, Deng Q, Yang X, Zhang X, et al. Associations of household renovation materials and periods with childhood asthma, in China: a retrospective cohort study. Environ Int. 2018;113:240–248. doi: 10.1016/j.envint.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 145.Hallit S, Raherison C, Waked M, Salameh P. Association between caregiver exposure to toxics during pregnancy and childhood-onset asthma: a case-control study. Iran J Allergy Asthma Immunol. 2017;16(6):488–500. [PubMed] [Google Scholar]
- 146.Bu Z, Wang L, Weschler L, Li B, Sundell J, Zhang Y. Associations between perceptions of odors and dryness and children's asthma and allergies: a cross-sectional study of home environment in Baotou. Build Environ. 2016;106:8. [Google Scholar]
- 147.Lin Z, Norback D, Wang T, Zhang X, Shi J, Kan H, Zhao Z. The first 2-year home environment in relation to the new onset and remission of asthmatic and allergic symptoms in 4246 preschool children. Sci Total Environ. 2016;553:204–210. doi: 10.1016/j.scitotenv.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 148.Singh S, Sharma BB, Sharma SK, Sabir M, Singh V, investigators Ic Prevalence and severity of asthma among Indian school children aged between 6 and 14 years: associations with parental smoking and traffic pollution. J Asthma. 2016;53(3):238–244. doi: 10.3109/02770903.2015.1087558. [DOI] [PubMed] [Google Scholar]
- 149.Idris IB, Ghazi HF, Zhie KH, Khairuman KA, Yahya SK, Abd Zaim FA, Nam CW, Abdul Rasid HZ, Isa ZM. Environmental air pollutants as risk factors for asthma among children seen in pediatric clinics in UKMMC, Kuala Lumpur. Ann Glob Health. 2016;82(1):202–208. doi: 10.1016/j.aogh.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 150.Lin Z, Zhao Z, Xu H, Zhang X, Wang T, Kan H, Norback D. Home dampness signs in association with asthma and allergic diseases in 4618 preschool children in Urumqi, China-the influence of ventilation/cleaning habits. PLoS One. 2015;10(7):e0134359. doi: 10.1371/journal.pone.0134359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hu Y, Liu W, Huang C, Zou ZJ, Zhao ZH, Shen L, Sundell J. Home dampness, childhood asthma, hay fever, and airway symptoms in Shanghai, China: associations, dose-response relationships, and lifestyle's influences. Indoor Air. 2014;24(5):450–463. doi: 10.1111/ina.12104. [DOI] [PubMed] [Google Scholar]
- 152.Dong GH, Qian ZM, Wang J, Trevathan E, Liu MM, Wang D, Ren WH, Chen W, Simckes M, Zelicoff A. Home renovation, family history of atopy, and respiratory symptoms and asthma among children living in China. Am J Public Health. 2014;104(10):1920–1927. doi: 10.2105/AJPH.2013.301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nahhas M, Bhopal R, Anandan C, Elton R, Sheikh A. Investigating the association between obesity and asthma in 6- to 8-year-old Saudi children: a matched case-control study. NPJ Prim Care Respir Med. 2014;24:14004. doi: 10.1038/npjpcrm.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang J, Li B, Yu W, Yang Q, Wang H, Huang D, Sundell J, Norback D. Rhinitis symptoms and asthma among parents of preschool children in relation to the home environment in Chongqing, China. PLoS One. 2014;9(4):e94731. doi: 10.1371/journal.pone.0094731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu W, Huang C, Hu Y, Zou Z, Zhao Z, Sundell J. Association of building characteristics, residential heating and ventilation with asthmatic symptoms of preschool children in Shanghai: A cross-sectional study. Indoor Built Environ. 2014;23(2):14. [Google Scholar]
- 156.Middleton N, Kolokotroni O, Lamnisos D, Koutrakis P, Yiallouros PK. Prevalence of asthma and respiratory symptoms in 15-17 year-old Greek-Cypriots by proximity of their community of residence to power plants: Cyprus 2006-07. Public Health. 2014;128(3):288–296. doi: 10.1016/j.puhe.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 157.Zhang M, Zhou E, Ye X, Sun Y, Sundell J, Yang X. Indoor environmental quality and the prevalence of childhood asthma and rhinitis in Wuhan area of China. Chin Sci Bull. 2013;58(34):7. [Google Scholar]
- 158.Wang H, Li B, Yang Q, Yu W, Wang J, Liu Y, Ou Y, Sundell J. Dampness in dwellings and its associations with asthma and allergies among children in Chongqing: a cross-sectional study. Chin Sci Bull. 2013;58(34):8. [Google Scholar]
- 159.Dhabadi BB, Athavale A, Meundi A, Rekha R, Suruliraman M, Shreeranga A, Gururaj S. Prevalence of asthma and associated factors among schoolchildren in rural South India. Int J Tuberc Lung Dis. 2012;16(1):120–125. doi: 10.5588/ijtld.11.0195. [DOI] [PubMed] [Google Scholar]
- 160.Chen YC, Tsai CH, Lee YL. Early-life indoor environmental exposures increase the risk of childhood asthma. Int J Hyg Environ Health. 2011;215(1):19–25. doi: 10.1016/j.ijheh.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 161.Zuraimi MS, Tham KW, Chew FT, Ooi PL, Koh D. Home air-conditioning, traffic exposure, and asthma and allergic symptoms among preschool children. Pediatr Allergy Immunol. 2011;22(1 Pt 2):e112–e118. doi: 10.1111/j.1399-3038.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 162.Subramanian SV, Ackerson LK, Subramanyam MA, Wright RJ. Domestic violence is associated with adult and childhood asthma prevalence in India. Int J Epidemiol. 2007;36(3):569–579. doi: 10.1093/ije/dym007. [DOI] [PubMed] [Google Scholar]
- 163.Tsai HJ, Tsai AC, Nriagu J, Ghosh D, Gong M, Sandretto A. Risk factors for respiratory symptoms and asthma in the residential environment of 5th grade schoolchildren in Taipei, Taiwan. J Asthma. 2006;43(5):355–361. doi: 10.1080/02770900600705326. [DOI] [PubMed] [Google Scholar]
- 164.Vedanthan PK, Mahesh PA, Vedanthan R, Holla AD, Liu AH. Effect of animal contact and microbial exposures on the prevalence of atopy and asthma in urban vs rural children in India. Ann Allergy Asthma Immunol. 2006;96(4):571–578. doi: 10.1016/S1081-1206(10)63552-1. [DOI] [PubMed] [Google Scholar]
- 165.Salo PM, Xia J, Johnson CA, Li Y, Avol EL, Gong J, London SJ. Indoor allergens, asthma, and asthma-related symptoms among adolescents in Wuhan, China. Ann Epidemiol. 2004;14(8):543–550. doi: 10.1016/j.annepidem.2003.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wong GW, Ko FW, Hui DS, Fok TF, Carr D, von Mutius E, Zhong NS, Chen YZ, Lai CK. Factors associated with difference in prevalence of asthma in children from three cities in China: multicentre epidemiological survey. BMJ. 2004;329(7464):486. doi: 10.1136/bmj.329.7464.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mishra V. Effect of obesity on asthma among adult Indian women. Int J Obes Relat Metab Disord. 2004;28(8):1048–1058. doi: 10.1038/sj.ijo.0802700. [DOI] [PubMed] [Google Scholar]
- 168.Zheng T, Niu S, Lu B, Fan X, Sun F, Wang J, Zhang Y, Zhang B, Owens P, Hao L, et al. Childhood asthma in Beijing, China: a population-based case-control study. Am J Epidemiol. 2002;156(10):977–983. doi: 10.1093/aje/kwf127. [DOI] [PubMed] [Google Scholar]
- 169.Melsom T, Brinch L, Hessen JO, Schei MA, Kolstrup N, Jacobsen BK, Svanes C, Pandey MR. Asthma and indoor environment in Nepal. Thorax. 2001;56(6):477–481. doi: 10.1136/thorax.56.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang TN, Ko YC, Chao YY, Huang CC, Lin RS. Association between indoor and outdoor air pollution and adolescent asthma from 1995 to 1996 in Taiwan. Environ Res. 1999;81(3):239–247. doi: 10.1006/enrs.1999.3985. [DOI] [PubMed] [Google Scholar]
- 171.Yang CY, Tien YC, Hsieh HJ, Kao WY, Lin MC. Indoor environmental risk factors and childhood asthma: a case-control study in a subtropical area. Pediatr Pulmonol. 1998;26(2):120–124. doi: 10.1002/(sici)1099-0496(199808)26:2<120::aid-ppul8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 172.Wang J, Zhang Y, Li B, Zhao Z, Huang C, Zhang X, Deng Q, Lu C, Qian H, Yang X, et al. Asthma and allergic rhinitis among young parents in China in relation to outdoor air pollution, climate and home environment. Sci Total Environ. 2021;751:141734. doi: 10.1016/j.scitotenv.2020.141734. [DOI] [PubMed] [Google Scholar]
- 173.Liu W, Cai J, Huang C, Chang J. Residence proximity to traffic-related facilities is associated with childhood asthma and rhinitis in Shandong, China. Environ Int. 2020;143:105930. doi: 10.1016/j.envint.2020.105930. [DOI] [PubMed] [Google Scholar]
- 174.Cai J, Li B, Yu W, Yao Y, Wang L, Li B, Wang Y, Du C, Xiong J. Associations of household dampness with asthma, allergies, and airway diseases among preschoolers in two cross-sectional studies in Chongqing, China: repeated surveys in 2010 and 2019. Environ Int. 2020;140:105752. doi: 10.1016/j.envint.2020.105752. [DOI] [PubMed] [Google Scholar]
- 175.Paudel U, Pant KP. Beyond smoking: environmental determinants of asthma prevalence in Western Nepal. J Health Pollut. 2020;10(25):200310. doi: 10.5696/2156-9614-10.25.200310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee JY, Leem JH, Kim HC, Lamichhane DK, Hwang SS, Kim JH, Park MS, Jung DY, Ko JK, Kwon HJ, et al. Effects of traffic-related air pollution on susceptibility to infantile bronchiolitis and childhood asthma: a cohort study in Korea. J Asthma. 2018;55(3):223–230. doi: 10.1080/02770903.2017.1313270. [DOI] [PubMed] [Google Scholar]
- 177.Takaoka M, Suzuki K, Norbäck D. The home environment of junior high school students in Hyogo, Japan – associations with asthma, respiratory health and reported allergies. Indoor Built Environ. 2016;25(1):13. [Google Scholar]
- 178.Al Ghamdi BR, Mahfouz AA, Abdel Moneim I, Khan MY, Daffallah AA. Altitude and bronchial asthma in south-western Saudi Arabia. EMHJ. 2008;14(1):7. [PubMed] [Google Scholar]
- 179.Mishra V. Effect of indoor air pollution from biomass combustion on prevalence of asthma in the elderly. Environ Health Perspect. 2003;111(1):71–78. doi: 10.1289/ehp.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yazicioglu M, Saltik A, Ones U, Sam A, Ekerbicer HC, Kircuval O. Home environment and asthma in school children from the Edirne region in Turkey. Allergol Immunopathol (Madr) 1998;26(1):5–8. [PubMed] [Google Scholar]
- 181.Zhang S, He Y, Liang H, Gao J, Li Y, Li Y, Wang L, Xie X, Sun M, Yuan C, et al. Higher environmental composite quality index score and risk of asthma and allergy in Northeast China. Allergy. 2021;76(6):1875–1879. doi: 10.1111/all.14672. [DOI] [PubMed] [Google Scholar]
- 182.Norback D, Lu C, Zhang Y, Li B, Zhao Z, Huang C, Zhang X, Qian H, Sun Y, Wang J, et al. Sources of indoor particulate matter (PM) and outdoor air pollution in China in relation to asthma, wheeze, rhinitis and eczema among pre-school children: synergistic effects between antibiotics use and PM10 and second hand smoke. Environ Int. 2019;125:252–260. doi: 10.1016/j.envint.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 183.Takaoka M, Suzuki K, Norbäck D. The home environment of junior high school students in Hyogo, Japan-associations with asthma, respiratory health and reported allergies. Indoor Built Environ. 2016;25(1):12. [Google Scholar]
- 184.Miyashita M, Kikuya M, Yamanaka C, Ishikuro M, Obara T, Sato Y, Metoki H, Nakaya N, Nagami F, Tomita H, et al. Eczema and asthma symptoms among schoolchildren in coastal and inland areas after the 2011 great East Japan earthquake: the ToMMo Child Health study. Tohoku J Exp Med. 2015;237(4):297–305. doi: 10.1620/tjem.237.297. [DOI] [PubMed] [Google Scholar]
- 185.Moradi-Lakeh M, El Bcheraoui C, Daoud F, Tuffaha M, Kravitz H, Al Saeedi M, Basulaiman M, Memish ZA, AlMazroa MA, Al Rabeeah AA, et al. Prevalence of asthma in Saudi adults: findings from a national household survey, 2013. BMC Pulm Med. 2015;15:77. doi: 10.1186/s12890-015-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yao J, Zhou Y, Wang J, Wu H, Liu H, Shi Y, Lei Q, Xia W, Ji C, Ye X, et al. Relationship between obesity and sex, and prevalence of asthma-like disease and current wheeze in Han children in Nanjing, China. J Int Med Res. 2015;43(1):139–146. doi: 10.1177/0300060514548289. [DOI] [PubMed] [Google Scholar]
- 187.Portnov BA, Reiser B, Karkabi K, Cohen-Kastel O, Dubnov J. High prevalence of childhood asthma in Northern Israel is linked to air pollution by particulate matter: evidence from GIS analysis and Bayesian model averaging. Int J Environ Health Res. 2012;22(3):249–269. doi: 10.1080/09603123.2011.634387. [DOI] [PubMed] [Google Scholar]
- 188.Sahebi L, Shabestary M. The prevalence of asthma, allergic rhinitis, and eczema among middle school students in Tabriz (northwestern Iran) Turkish J Med Sci. 2011;41(5):12. [Google Scholar]
- 189.El-Sharif NA, Nemery B, Barghuthy F, Mortaja S, Qasrawi R, Abdeen Z. Geographical variations of asthma and asthma symptoms among schoolchildren aged 5 to 8 years and 12 to 15 years in Palestine: the International Study of Asthma and Allergies in Childhood (ISAAC) Ann Allergy Asthma Immunol. 2003;90(1):63–71. doi: 10.1016/S1081-1206(10)63616-2. [DOI] [PubMed] [Google Scholar]
- 190.El-Sharif N, Abdeen Z, Qasrawi R, Moens G, Nemery B. Asthma prevalence in children living in villages, cities and refugee camps in Palestine. Eur Respir J. 2002;19(6):1026–1034. doi: 10.1183/09031936.02.01832001. [DOI] [PubMed] [Google Scholar]
- 191.Huang SL, Pan WH. Dietary fats and asthma in teenagers: analyses of the first Nutrition and Health Survey in Taiwan (NAHSIT) Clin Exp Allergy. 2001;31(12):1875–1880. doi: 10.1046/j.1365-2222.2001.01222.x. [DOI] [PubMed] [Google Scholar]
- 192.Hijazi N, Abalkhail B, Seaton A. Asthma and respiratory symptoms in urban and rural Saudi Arabia. Eur Respir J. 1998;12(1):41–44. doi: 10.1183/09031936.98.12010041. [DOI] [PubMed] [Google Scholar]
- 193.Lau YL, Karlberg J. Prevalence and risk factors of childhood asthma, rhinitis and eczema in Hong Kong. J Paediatr Child Health. 1998;34(1):47–52. doi: 10.1046/j.1440-1754.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 194.Laor A, Cohen L, Danon YL. Effects of time, sex, ethnic origin, and area of residence on prevalence of asthma in Israeli adolescents. BMJ. 1993;307(6908):841–844. doi: 10.1136/bmj.307.6908.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hallit S, Raherison C, Abou Abdallah R, Hallit R, Salameh P. Correlation of types of food and asthma diagnosis in childhood: a case-control study. J Asthma. 2018;55(9):966–974. doi: 10.1080/02770903.2017.1379535. [DOI] [PubMed] [Google Scholar]
- 196.Agrawal S, Pearce N, Ebrahim S. Prevalence and risk factors for self-reported asthma in an adult Indian population: a cross-sectional survey. Int J Tuberc Lung Dis. 2013;17(2):275–282. doi: 10.5588/ijtld.12.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Patel S, Ram U, Ram F, Patel SK. Socioeconomic and demographic predictors of high blood pressure, diabetes, asthma and heart disease among adults engaged in various occupations: evidence from India. J Biosoc Sci. 2020;52(5):629–649. doi: 10.1017/S0021932019000671. [DOI] [PubMed] [Google Scholar]
- 198.Booalayan H, Abdualrasool M, Al-Shanfari S, Boujarwa A, Al-Mukaimi A, Alkandery O, Akhtar S. Exposure to environmental tobacco smoke and prevalence of asthma among adolescents in a middle eastern country. BMC Public Health. 2020;20(1):1210. doi: 10.1186/s12889-020-09245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Furuhata M, Otsuka Y, Kaneita Y, Nakagome S, Jike M, Itani O, Ohida T. Factors associated with the development of childhood asthma in Japan: a nationwide longitudinal study. Matern Child Health J. 2020;24(7):911–922. doi: 10.1007/s10995-020-02944-0. [DOI] [PubMed] [Google Scholar]
- 200.Mansouri M, Sharifi F, Tabatabaee SS, Heidari E, Yaghubi H, Keshtkar A, Tabrizi YM, Arzaghi M, Varmaghani M. Prevalence of ever self-reported asthma and associated factors among university students in Iran: a population-based study. Int J Prev Med. 2020;11:54. doi: 10.4103/ijpvm.IJPVM_453_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lim JH, Lee DH, Lee SH, Kim JS, Jung HC, Cho SH. Asthma under control is inversely related with erosive esophagitis among healthy adults. PLoS One. 2019;14(1):e0210490. doi: 10.1371/journal.pone.0210490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fazlollahi MR, Najmi M, Fallahnezhad M, Sabetkish N, Kazemnejad A, Bidad K, Shokouhi Shoormasti R, Mahloujirad M, Pourpak Z, Moin M. Paediatric asthma prevalence: the first national population-based survey in Iran. Clin Respir J. 2019;13(1):14–22. doi: 10.1111/crj.12975. [DOI] [PubMed] [Google Scholar]
- 203.Awasthi S, Tripathi P, Prasad R. Environmental risk factors for asthma in Lucknow: a case-control study. Clin Epidemiol Glob Health. 2013;1(3):9. [Google Scholar]
- 204.Hong SJ, Lee MS, Sohn MH, Shim JY, Han YS, Park KS, Ahn YM, Son BK, Lee HB, Korean ISG. Self-reported prevalence and risk factors of asthma among Korean adolescents: 5-year follow-up study, 1995-2000. Clin Exp Allergy. 2004;34(10):1556–1562. doi: 10.1111/j.1365-2222.2004.02084.x. [DOI] [PubMed] [Google Scholar]
- 205.Gupta D, Aggarwal AN, Kumar R, Jindal SK. Prevalence of bronchial asthma and association with environmental tobacco smoke exposure in adolescent school children in Chandigarh, north India. J Asthma. 2001;38(6):501–507. doi: 10.1081/jas-100105871. [DOI] [PubMed] [Google Scholar]
- 206.Tabuchi T, Fujiwara T, Nakayama T, Miyashiro I, Tsukuma H, Ozaki K, Kondo N. Maternal and paternal indoor or outdoor smoking and the risk of asthma in their children: a nationwide prospective birth cohort study. Drug Alcohol Depend. 2015;147:103–108. doi: 10.1016/j.drugalcdep.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 207.Tsai CH, Huang JH, Hwang BF, Lee YL. Household environmental tobacco smoke and risks of asthma, wheeze and bronchitic symptoms among children in Taiwan. Respir Res. 2010;11:11. doi: 10.1186/1465-9921-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tanaka K, Miyake Y, Arakawa M, Sasaki S, Ohya Y. Prevalence of asthma and wheeze in relation to passive smoking in Japanese children. Ann Epidemiol. 2007;17(12):1004–1010. doi: 10.1016/j.annepidem.2007.07.108. [DOI] [PubMed] [Google Scholar]
- 209.Lee A, Lee SY, Lee KS. The use of heated tobacco products is associated with asthma, allergic rhinitis, and atopic dermatitis in Korean adolescents. Sci Rep. 2019;9(1):17699. doi: 10.1038/s41598-019-54102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kim SY, Sim S, Choi HG. Active, passive, and electronic cigarette smoking is associated with asthma in adolescents. Sci Rep. 2017;7:17789. doi: 10.1038/s41598-017-17958-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cho JH, Paik SY. Association between electronic cigarette use and asthma among high school students in South Korea. PLoS One. 2016;11(3):e0151022. doi: 10.1371/journal.pone.0151022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Agrawal S. Effect of indoor air pollution from biomass and solid fuel combustion on prevalence of self-reported asthma among adult men and women in India: findings from a nationwide large-scale cross-sectional survey. J Asthma. 2012;49(4):355–365. doi: 10.3109/02770903.2012.663030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Guddattu V, Swathi A, Nair NS. Household and environment factors associated with asthma among Indian women: a multilevel approach. J Asthma. 2010;47(4):407–411. doi: 10.3109/02770903.2010.481343. [DOI] [PubMed] [Google Scholar]
- 214.Xu X, Christiani DC. Occupational exposures and physician-diagnosed asthma. Chest. 1993;104(5):1364–1370. doi: 10.1378/chest.104.5.1364. [DOI] [PubMed] [Google Scholar]
- 215.Xu X, Niu T, Christiani DC, Weiss ST, Chen C, Zhou Y, Fang Z, Jiang Z, Liang W, Zhang F. Occupational and environmental risk factors for asthma in rural communities in China. Int J Occup Environ Health. 1996;2(3):172–176. doi: 10.1179/oeh.1996.2.3.172. [DOI] [PubMed] [Google Scholar]
- 216.Shahzad K, Akhtar S, Mahmud S. Prevalence and determinants of asthma in adult male leather tannery workers in Karachi, Pakistan: a cross sectional study. BMC Public Health. 2006;6:292. doi: 10.1186/1471-2458-6-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tomita Y, Fukutomi Y, Irie M, Azekawa K, Hayashi H, Kamide Y, Sekiya K, Nakamura Y, Okada C, Shimoda T, et al. Obesity, but not metabolic syndrome, as a risk factor for late-onset asthma in Japanese women. Allergol Int. 2019;68(2):240–246. doi: 10.1016/j.alit.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 218.Chen YC, Chih AH, Chen JR, Liou TH, Pan WH, Lee YL. Rapid adiposity growth increases risks of new-onset asthma and airway inflammation in children. Int J Obes (Lond) 2017;41(7):1035–1041. doi: 10.1038/ijo.2017.67. [DOI] [PubMed] [Google Scholar]
- 219.Wang D, Qian Z, Wang J, Yang M, Lee YL, Liu F, Liu MM, Zhao Y, Liu YQ, Huang MM, et al. Gender-specific differences in associations of overweight and obesity with asthma and asthma-related symptoms in 30 056 children: result from 25 districts of Northeastern China. J Asthma. 2014;51(5):508–514. doi: 10.3109/02770903.2014.892963. [DOI] [PubMed] [Google Scholar]
- 220.Chen YC, Tu YK, Huang KC, Chen PC, Chu DC, Lee YL. Pathway from central obesity to childhood asthma. Physical fitness and sedentary time are leading factors. Am J Respir Crit Care Med. 2014;189(10):1194–1203. doi: 10.1164/rccm.201401-0097OC. [DOI] [PubMed] [Google Scholar]
- 221.Okabe Y, Adachi Y, Itazawa T, Yoshida K, Ohya Y, Odajima H, Akasawa A, Miyawaki T. Association between obesity and asthma in Japanese preschool children. Pediatr Allergy Immunol. 2012;23(6):550–555. doi: 10.1111/j.1399-3038.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 222.Fukutomi Y, Taniguchi M, Nakamura H, Konno S, Nishimura M, Kawagishi Y, Okada C, Tanimoto Y, Takahashi K, Akasawa A, et al. Association between body mass index and asthma among Japanese adults: risk within the normal weight range. Int Arch Allergy Immunol. 2012;157(3):281–287. doi: 10.1159/000327555. [DOI] [PubMed] [Google Scholar]
- 223.Tanaka K, Miyake Y, Arakawa M, Sasaki S, Ohya Y. U-shaped association between body mass index and the prevalence of wheeze and asthma, but not eczema or rhinoconjunctivitis: the ryukyus child health study. J Asthma. 2011;48(8):804–810. doi: 10.3109/02770903.2011.611956. [DOI] [PubMed] [Google Scholar]
- 224.Okabe Y, Itazawa T, Adachi Y, Yoshida K, Ohya Y, Odajima H, Akasawa A, Miyawaki T. Association of overweight with asthma symptoms in Japanese school children. Pediatr Int. 2011;53(2):192–198. doi: 10.1111/j.1442-200X.2010.03197.x. [DOI] [PubMed] [Google Scholar]
- 225.Wang TN, Lin MC, Wu CC, Huang MS, Leung SY, Huang CC, Ho PS, Ko YC. Role of gender disparity of circulating high-sensitivity C-reactive protein concentrations and obesity on asthma in Taiwan. Clin Exp Allergy. 2011;41(1):72–77. doi: 10.1111/j.1365-2222.2010.03581.x. [DOI] [PubMed] [Google Scholar]
- 226.Tsai HJ, Tsai AC. The association of BMI and sedentary time with respiratory symptoms and asthma in 5th grade schoolchildren in Kaohsiung, Taiwan. J Asthma. 2009;46(1):9–15. doi: 10.1080/02770900802444229. [DOI] [PubMed] [Google Scholar]
- 227.Celedon JC, Palmer LJ, Litonjua AA, Weiss ST, Wang B, Fang Z, Xu X. Body mass index and asthma in adults in families of subjects with asthma in Anqing, China. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1835–1840. doi: 10.1164/ajrccm.164.10.2105033. [DOI] [PubMed] [Google Scholar]
- 228.Chen YC, Kuo HP, Hsia SM, Wu HT, Pan WH, Lee YL. Life course body mass index through childhood and young adulthood and risks of asthma and pulmonary function impairment. Pediatr Pulmonol. 2021;56(5):849–857. doi: 10.1002/ppul.25197. [DOI] [PubMed] [Google Scholar]
- 229.Lai L, Zhang T, Zeng X, Tan W, Cai L, Chen Y. Association between physician-diagnosed asthma and weight status among Chinese children: the roles of lifestyle factors. Int J Environ Res Public Health. 2020;17(5):1599. doi: 10.3390/ijerph17051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Myung J, Lee H, Kim TH, Han E. Relationships between self-reported asthma and pulmonary function and various measures of obesity. J Asthma. 2018;55(7):741–749. doi: 10.1080/02770903.2017.1362701. [DOI] [PubMed] [Google Scholar]
- 231.Lim MS, Lee CH, Sim S, Hong SK, Choi HG. Physical activity, sedentary habits, sleep, and obesity are associated with asthma, allergic rhinitis, and atopic dermatitis in Korean adolescents. Yonsei Med J. 2017;58(5):1040–1046. doi: 10.3349/ymj.2017.58.5.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gordon B, Hassid A, Bar-Shai A, Derazne E, Tzur D, Hershkovich O, Afek A. Association between asthma and body mass index and socioeconomic status: a cross-sectional study on 849,659 adolescents. Respirology. 2016;21(1):95–101. doi: 10.1111/resp.12645. [DOI] [PubMed] [Google Scholar]
- 233.Raheleh Z, Ahmad A, Abtin H, Roghaye Z, Sara H, Siavash R. The association between birth weight and gestational age and asthma in 6-7- and 13-14-year-old children. Scientifica (Cairo) 2016;2016:3987460. doi: 10.1155/2016/3987460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chen BY, Chen CH, Chuang YC, Wu YH, Pan SC, Guo YL. Changes in the relationship between childhood asthma and ambient air pollution in Taiwan: results from a nationwide survey repeated 5 years apart. Pediatr Allergy Immunol. 2019;30(2):188–194. doi: 10.1111/pai.12999. [DOI] [PubMed] [Google Scholar]
- 235.Son JY, Kim H, Bell ML. Does urban land-use increase risk of asthma symptoms? Environ Res. 2015;142:309–318. doi: 10.1016/j.envres.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 236.Deng Q, Deng L, Lu C, Li Y, Norback D. Parental stress and air pollution increase childhood asthma in China. Environ Res. 2018;165:23–31. doi: 10.1016/j.envres.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 237.Deng Q, Lu C, Li Y, Sundell J, Dan N. Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ Res. 2016;150:119–127. doi: 10.1016/j.envres.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 238.Deng Q, Lu C, Norback D, Bornehag CG, Zhang Y, Liu W, Yuan H, Sundell J. Early life exposure to ambient air pollution and childhood asthma in China. Environ Res. 2015;143(Pt A):83–92. doi: 10.1016/j.envres.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 239.Yang SI, Lee SY, Kim HB, Kim HC, Leem JH, Yang HJ, Kwon H, Seo JH, Cho HJ, Yoon J, et al. Prenatal particulate matter affects new asthma via airway hyperresponsiveness in schoolchildren. Allergy. 2019;74(4):675–684. doi: 10.1111/all.13649. [DOI] [PubMed] [Google Scholar]
- 240.Chen F, Lin Z, Chen R, Norback D, Liu C, Kan H, Deng Q, Huang C, Hu Y, Zou Z, et al. The effects of PM2.5 on asthmatic and allergic diseases or symptoms in preschool children of six Chinese cities, based on China, Children, Homes and Health (CCHH) project. Environ Pollut. 2018;232:329–337. doi: 10.1016/j.envpol.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 241.Zhao S, Liu S, Hou X, Beazley R, Sun Y, Dong S. Evidence of provincial variability in air pollutants-asthma relations in China. J Cleaner Prod. 2020;242. 10.1016/j.jclepro.2019.118553.
- 242.Hasunuma H, Sato T, Iwata T, Kohno Y, Nitta H, Odajima H, Ohara T, Omori T, Ono M, Yamazaki S, et al. Association between traffic-related air pollution and asthma in preschool children in a national Japanese nested case–control study. BMJ Open. 2016;6(2):e010410. doi: 10.1136/bmjopen-2015-010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Watanabe JI, Tanaka K, Nagata C, Furukawa S, Arakawa M, Miyake Y. Breastfeeding duration is inversely associated with asthma in Japanese children aged 3 years. J Asthma. 2018;55(5):511–516. doi: 10.1080/02770903.2017.1349793. [DOI] [PubMed] [Google Scholar]
- 244.Arif AA, Racine EF. Does longer duration of breastfeeding prevent childhood asthma in low-income families? J Asthma. 2017;54(6):600–605. doi: 10.1080/02770903.2016.1247167. [DOI] [PubMed] [Google Scholar]
- 245.Huang C, Liu W, Cai J, Weschler LB, Wang X, Hu Y, Zou Z, Shen L, Sundell J. Breastfeeding and timing of first dietary introduction in relation to childhood asthma, allergies, and airway diseases: A cross-sectional study. J Asthma. 2017;54(5):488–497. doi: 10.1080/02770903.2016.1231203. [DOI] [PubMed] [Google Scholar]
- 246.Miyake Y, Tanaka K, Sasaki S, Kiyohara C, Ohya Y, Fukushima W, Yokoyama T, Hirota Y, Osaka M. Child Health Study G: Breastfeeding and the risk of wheeze and asthma in Japanese infants: the Osaka Maternal and Child Health study. Pediatr Allergy Immunol. 2008;19(6):490–496. doi: 10.1111/j.1399-3038.2007.00701.x. [DOI] [PubMed] [Google Scholar]
- 247.Takata N, Tanaka K, Nagata C, Arakawa M, Miyake Y. Preterm birth is associated with higher prevalence of wheeze and asthma in a selected population of Japanese children aged three years. Allergol Immunopathol. 2019;47(5):6. doi: 10.1016/j.aller.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 248.Lu FL, Hsieh CJ, Caffrey JL, Lin MH, Lin YS, Lin CC, Tsai MS, Ho WC, Chen PC, Sung FC, et al. Body mass index may modify asthma prevalence among low-birth-weight children. Am J Epidemiol. 2012;176(1):32–42. doi: 10.1093/aje/kwr484. [DOI] [PubMed] [Google Scholar]
- 249.Chen G, Chiang WL, Shu BC, Guo YL, Chiou ST, Chiang TL. Associations of caesarean delivery and the occurrence of neurodevelopmental disorders, asthma or obesity in childhood based on Taiwan birth cohort study. BMJ Open. 2017;7(9):e017086. doi: 10.1136/bmjopen-2017-017086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lavin T, Franklin P, Preen DB. Association between caesarean delivery and childhood asthma in India and Vietnam. Paediatr Perinat Epidemiol. 2017;31(1):47–54. doi: 10.1111/ppe.12324. [DOI] [PubMed] [Google Scholar]
- 251.Chu S, Chen Q, Chen Y, Bao Y, Wu M, Zhang J. Cesarean section without medical indication and risk of childhood asthma, and attenuation by breastfeeding. PLoS One. 2017;12(9):e0184920. doi: 10.1371/journal.pone.0184920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kashyap GC, Sharma SK, Singh SK. Prevalence and predictors of asthma, tuberculosis and chronic bronchitis among male tannery workers: a study of Kanpur City, India. Clin Epidemiol Glob Health. 2021;9:7. [Google Scholar]
- 253.Luo S, Sun Y, Hou J, Kong X, Wang P, Zhang Q, Sundell J. Pet keeping in childhood and asthma and allergy among children in Tianjin area, China. PLoS One. 2018;13(5):e0197274. doi: 10.1371/journal.pone.0197274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Huang C, Hu Y, Liu W, Zou Z, Sundell J. Pet-keeping and its impact on asthma and allergies among preschool children in Shanghai, China. Chin Sci Bull. 2013;58(34):8. [Google Scholar]
- 255.Zhang HL, Wang BY, Luo Y, Li Y, Cai CS, Huang LL, et al. Association of pet-keeping in home with self-reported asthma and asthma-related symptoms in 11611 school children from China. J Asthma. 2020;58(12):1555–64. doi: 10.1080/02770903.2020.1818772. [DOI] [PubMed] [Google Scholar]
- 256.Norback D, Zhao ZH, Wang ZH, Wieslander G, Mi YH, Zhang Z. Asthma, eczema, and reports on pollen and cat allergy among pupils in Shanxi province, China. Int Arch Occup Environ Health. 2007;80(3):207–216. doi: 10.1007/s00420-006-0123-6. [DOI] [PubMed] [Google Scholar]
- 257.Takaoka M, Norback D. Diet among Japanese female university students and asthmatic symptoms, infections, pollen and furry pet allergy. Respir Med. 2008;102(7):1045–1054. doi: 10.1016/j.rmed.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 258.Lee SC, Yang YH, Chuang SY, Liu SC, Yang HC, Pan WH. Risk of asthma associated with energy-dense but nutrient-poor dietary pattern in Taiwanese children. Asia Pac J Clin Nutr. 2012;21(1):73–81. [PubMed] [Google Scholar]
- 259.Tsai HJ, Tsai AC. The association of diet with respiratory symptoms and asthma in schoolchildren in Taipei, Taiwan. J Asthma. 2007;44(8):599–603. doi: 10.1080/02770900701539509. [DOI] [PubMed] [Google Scholar]
- 260.Takemura Y, Sakurai Y, Honjo S, Tokimatsu A, Gibo M, Hara T, Kusakari A, Kugai N. The relationship between fish intake and the prevalence of asthma: the Tokorozawa childhood asthma and pollinosis study. Prev Med. 2002;34(2):221–225. doi: 10.1006/pmed.2001.0978. [DOI] [PubMed] [Google Scholar]
- 261.Ibrahim AA, Qamar B, Fituri S, Akbar ZA, Al-Abdi T, Shi Z. Association between soft drink consumption and asthma among Qatari adults. Nutrients. 2019;11(3):606. doi: 10.3390/nu11030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Fu QL, Du Y, Xu G, Zhang H, Cheng L, Wang YJ, et al. Prevalence and occupational and environmental risk factors of self-reported asthma: evidence from a cross-sectional survey in seven Chinese cities. Int J Environ Res Public Health. 2016;13(11):1084. doi: 10.3390/ijerph13111084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242(1):10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8(3):169–182. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 265.Weiss ST, Raby BA, Rogers A. Asthma genetics and genomics 2009. Curr Opin Genet Dev. 2009;19(3):279–282. doi: 10.1016/j.gde.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 266.Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7(2):95–100. doi: 10.1038/sj.gene.6364284. [DOI] [PubMed] [Google Scholar]
- 267.Quansah R, Jaakkola MS, Hugg TT, Heikkinen SA, Jaakkola JJ. Residential dampness and molds and the risk of developing asthma: a systematic review and meta-analysis. PLoS One. 2012;7(11):e47526. doi: 10.1371/journal.pone.0047526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sio YY, Pang SL, Say YH, Teh KF, Wong YR, Shah SMR, et al. Sensitization to airborne fungal allergens associates with asthma and allergic rhinitis presentation and severity in the Singaporean/Malaysian population. Mycopathologia. 2021;186(5):583–8. doi: 10.1007/s11046-021-00532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Goh KJ, Yii ACA, Lapperre TS, Chan AK, Chew FT, Chotirmall SH, Koh MS. Sensitization to Aspergillus species is associated with frequent exacerbations in severe asthma. J Asthma Allergy. 2017;10:131–140. doi: 10.2147/JAA.S130459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chew FT, Lim SH, Shang HS, Dahlia MD, Goh DY, Lee BW, Tan HT, Tan TK. Evaluation of the allergenicity of tropical pollen and airborne spores in Singapore. Allergy. 2000;55(4):340–347. doi: 10.1034/j.1398-9995.2000.00308.x. [DOI] [PubMed] [Google Scholar]
- 271.Andiappan AK, Puan KJ, Lee B, Nardin A, Poidinger M, Connolly J, Chew FT, Wang DY, Rotzschke O. Allergic airway diseases in a tropical urban environment are driven by dominant mono-specific sensitization against house dust mites. Allergy. 2014;69(4):501–509. doi: 10.1111/all.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Baratawidjaja IR, Baratawidjaja PP, Darwis A, Soo-Hwee L, Fook-Tim C, Bee-Wah L, Baratawidjaja KG. Prevalence of allergic sensitization to regional inhalants among allergic patients in Jakarta, Indonesia. Asian Pac J Allergy Immunol. 1999;17(1):9–12. [PubMed] [Google Scholar]
- 273.Daengsuwan T, Lee BW, Visitsuntorn N, Charoenratanakul S, Ruangrak S, Jirapongsananuruk O, Vichyanond P. Allergen sensitization to aeroallergens including Blomia tropicalis among adult and childhood asthmatics in Thailand. Asian Pac J Allergy Immunol. 2003;21(4):199–204. [PubMed] [Google Scholar]
- 274.Do DC, Zhao Y, Gao P. Cockroach allergen exposure and risk of asthma. Allergy. 2016;71(4):463–474. doi: 10.1111/all.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Gdalevich M, Mimouni D, Mimouni M. Breast-feeding and the risk of bronchial asthma in childhood: a systematic review with meta-analysis of prospective studies. J Pediatr. 2001;139(2):261–266. doi: 10.1067/mpd.2001.117006. [DOI] [PubMed] [Google Scholar]
- 276.Takkouche B, Gonzalez-Barcala FJ, Etminan M, Fitzgerald M. Exposure to furry pets and the risk of asthma and allergic rhinitis: a meta-analysis. Allergy. 2008;63(7):857–864. doi: 10.1111/j.1398-9995.2008.01732.x. [DOI] [PubMed] [Google Scholar]
- 277.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figures S1–S33. Forest plots and funnel plots for random-effect meta-analysis of the different asthma risk factors.
Additional file 2: Table S1. PRISMA 2009 Checklist.
Additional file 3: Table S2. Keywords used to perform literature search in three publication databases (Web of Science, Scopus, and Pubmed) to retrieve articles reporting asthma-associated risk factors in Asia.
Additional file 4: Table S3. Study characteristics and reported asthma-associated risk factors of 289 studies included in the systematic review process.
Additional file 5: Table S4. Reported publications on asthma-associated risk factors from countries, dependencies, or other territories within Asia (1993-2021).
Additional file 6: Table S5. Summary of frequently reported asthma-associated risk factors in the Asian population (1993-2021).
Additional file 7: Table S6. Summary of frequently reported asthma comorbidities in the Asian population (1993-2021).
Data Availability Statement
All data used and included in this study are available from the corresponding author (Chew Fook Tim).