Abstract
The ubiquitous Fer protein-tyrosine kinase has been proposed to regulate diverse processes such as cell growth, cell adhesion, and neurite outgrowth. To gain insight into the biological function of Fer, we have targeted the fer locus with a kinase-inactivating missense mutation (ferD743R). Mice homozygous for this mutation develop normally, have no overt phenotypic differences from wild-type mice, and are fertile. Since these mice lack both Fer and the testis-specific FerT kinase activities, these proteins are clearly not essential for development and survival. No differences were observed in overall cellularity of bone marrow, spleen, or thymus in the absence of Fer activity. While most platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation was unchanged in ferD743R homozygous embryonic fibroblasts, cortactin phosphorylation was reduced. However, Fer kinase activity was not required for PDGF-induced Stat3, p120ctn, or epidermal growth factor (EGF)-induced β-catenin phosphorylation. Also, no defects were observed in changes to the actin cytoskeleton, adherens junctions, or focal adhesions in PDGF- or EGF-stimulated ferD743R homozygous embryonic fibroblasts. Therefore, Fer likely serves a redundant role in regulating cell growth, cell adhesion, retinal development, and spermatogenesis but is required for efficient phosphorylation of cortactin.
The nonreceptor protein-tyrosine kinase (PTK) Fer (15, 29, 36) and its closely related counterpart Fps/Fes (1, 40, 44) make up the only known members of a distinct subclass of PTKs. They share a similar domain structure including an N-terminal half containing three predicted coiled-coil (CC) motifs, a central Src homology 2 (SH2) domain, and a C-terminal catalytic domain. Both Fer and Fps/Fes (hereafter referred to as Fps) form trimers mediated by their CC motifs (5, 7, 24, 39). However, heterotypic interactions were not detected between Fer and Fps (7). Insertion of a proline residue to disrupt either the first or second CC motif in Fer abolished trimerization but not kinase activity (7). In contrast, a deletion within the first CC motif in Fps results in enhanced kinase activity, suggesting an autoregulatory function (5). This effect on activity is not observed for Fer (7) and may reflect differences in the regulation of Fps and Fer. It is noteworthy that when expressed at similar levels, Fps is not as highly phosphorylated as Fer (7). Also, in cultured bone marrow-derived macrophages, Fer is much more highly phosphorylated than Fps (A. Craig and Y. Senis, unpublished data). Alternatively, the deletions made in Fps (5) may affect other domains, including the catalytic domain. The SH2 domains of Fps and Fer function in mediating phosphotyrosine-dependent interactions with putative substrates (21, 25). The SH2 domain in Fps has also been implicated in mediating intramolecular interactions (17, 28).
Fer is ubiquitously expressed, while Fps expression is highest in myeloid, endothelial, epithelial, and neuronal cells (4, 8, 9, 11, 32). A unique feature of fer is the existence of a testis-specific isoform, called FerT, that is driven by a testis-specific promoter and arises by alternative splicing (10). FerT lacks the N-terminal CC domains of Fer but shares the same exons encoding the SH2 and kinase domains of Fer. ferT mRNA accumulates in primary spermatocytes during the pachytene stage of meiotic prophase and is thought to play a role in spermatogenesis (23).
Fer is activated downstream of the epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) receptors in fibroblasts (24, 25). In mast cells, Fer is activated downstream of the FcɛRI, a high-affinity receptor for immunoglobulin E (IgE) (37). The role of Fer in these signaling pathways is not known, although interactions have been observed between Fer and β-catenin and p120ctn (formerly called p120CAS) (24, 41) and between Fer and cortactin (25). All of these proteins become tyrosine phosphorylated upon stimulation of cells with either EGF or PDGF, and Fer is postulated to mediate this phosphorylation, either partly or in total. Overexpression of Fer in a tetracycline-regulatable cell system results in elevated p120ctn phosphorylation and reduced cell adhesion due to dissolution of adherens junctions (41). Recently, a role for Fer in regulating cross-talk between cadherin-catenin complexes and focal adhesions has been proposed (31). Studies on the regulation of N-cadherin by neurocan and its effects on neurite extension in chick retinal cells, suggest that Fer may be shuttling from adherens junctions to focal adhesions. Treatment of cells with a membrane-permeable peptide corresponding to the juxtamembrane sequence of N-cadherin, which mimics the cellular response to neurocan, resulted in a loss of Fer, p120ctn, and β-catenin from cadherin complexes, followed by the association of Fer with focal adhesions (2, 30). These studies together with those described above suggest that Fer regulates cell adhesion and possibly retinal development. However, the precise role of Fer in either cadherin-catenin complexes or focal adhesions is not known. Fer may also regulate cell growth, since overexpression of Fer from Drosophila melanogaster caused malignant transformation of rodent fibroblasts (35).
Fps is activated in hematopoietic cells in response to numerous cytokines including interleukin-3 (IL-3) (13), IL-4 (20), IL-6 (33), granulocyte-macrophage colony-stimulating factor (GM-CSF) (13), and erythropoietin (14). To help address the role of Fps in these signaling pathways, a transgenic mouse line was generated in which the fps locus was targeted with a kinase-inactivating mutation (42). Surprisingly, mice lacking Fps activity have no major defects in myeloid differentiation and display only subtle differences in activation of signal transducers and activators of transcription (Stat3 and Stat5) in response to GM-CSF. Since many cell types express both Fps and Fer, there is a possibility that more pronounced defects could be masked by functional redundancy between these highly homologous kinases.
We generated transgenic mice that harbor a kinase-inactivating mutation in the fer locus to address the question of redundancy between Fps and Fer and to determine the biological function of Fer. We show that while Fer and FerT kinase activity is completely abolished in homozygous animals, no overt defects in viability or fertility are observed. The major EGF and PDGF signaling pathways appear to be intact in embryonic fibroblasts in the absence of Fer kinase activity. Although cortactin phosphorylation is reduced in the absence of Fer activity, no defects are apparent with regard to subcellular localization and cell migration. Also, phosphorylation of p120ctn and β-catenin in response to PDGF or EGF does not require Fer activity. Formation of adherens junctions and focal adhesions are also indistinguishable in the absence or presence of Fer activity.
MATERIALS AND METHODS
Construction of the fer targeting vector.
Genomic fer sequences were cloned from a murine 129/SVJ library in λDASH2 (Stratagene), which was kindly provided by Janet Rossant (University of Toronto). A 12-kbp genomic clone (called 3B) was obtained that contained the final two exons of fer, which were numbered 19 and 20 based on our genomic clones and available human and mouse genomic sequences (unpublished data). We chose to target the fer allele with a kinase-inactivating mutation of aspartate 743 to arginine (D743R), which is predicted to disrupt the catalytic loop (6). The targeting construct was produced in the context of pPNT-NHS14 (42), a modified version of pPNT (43). The EcoRI site in pPNT-NHS14 was eliminated by EcoRI digestion, blunting with T4 DNA polymerase (New England Biolabs [NEB]), and religation with T4 DNA ligase (NEB), resulting in the clone pPNT-NHS14ΔRI. A 6-kbp XbaI fragment from clone 3B (containing exons 19 and 20) was then cloned into the NheI site of pPNT-NHS14ΔRI. The D743R mutation, which also generates an NruI site, was introduced within the 896-bp EcoRI fragment that spans exon 19 and was swapped for the EcoRI fragment bearing the wild-type fer sequence within the long arm of homology of the targeting vector (Fig. 1A). A 0.9-kbp XbaI-NheI fragment from clone 3B was then cloned into the XbaI site located between the PGK-neo-pA and PGK-tk-pA cassettes, thus providing a short arm of homology.
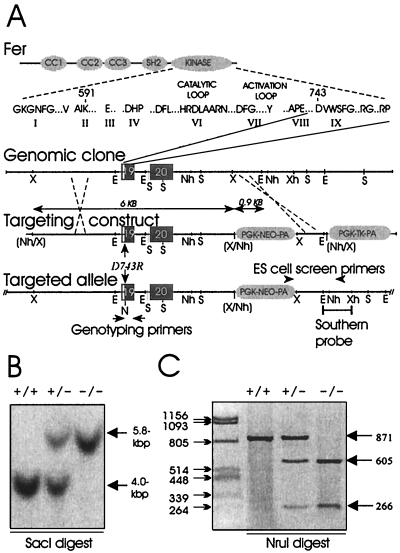
FIG. 1.
Domain structure of Fer and targeting strategy of fer locus. (A) Fer consists of three CC motifs, a central SH2 domain, and a kinase domain. The catalytic subdomains are shown, with subdomain IX encoded by exon 19 contained within genomic clone 3B. The targeting vector contains a 6-kbp long arm of homology, followed by the PGK-neo cassette, a 0.9-kbp short arm of homology, and a PGK-tk cassette. The D743R mutation was generated within exon 19, which also introduces an NruI (N) site. A schematic of the targeted allele following homologous recombination is shown, with positions of PCR primers and Southern blot probe indicated. Positions of XbaI (X), XhoI (Xh), NheI (Nh), EcoRI (E), and SacI (S) sites are shown. Sites in parentheses were destroyed. (B) Southern blot analysis of SacI-digested genomic DNA from animals that were wild type (+/+), heterozygous (+/−), or homozygous (−/−) for ferD743R. Hybridization with a probe located 3′ to the sequence used for the short arm of homology reveals bands of 4.0 kbp for the wild-type allele and 5.8 kbp for the ferD743R allele. (C) Genotyping PCR analysis of genomic DNA from +/+, +/−, or −/− animals. The resulting 871-bp fragment is resistant to NruI digestion in wild-type samples, yields additional 605- and 266-bp bands in heterozygous samples, and yields only 605- and 266-bp fragments in homozygous samples.
ES cell culture and chimeric mouse production.
Mouse embryonic stem (ES) cells (R1; passage 8) were kindly provided by Andras Nagy (34). Propagation, electroporation, and selection of recombinant clones were carried out as described previously (42). Geneticin (G418; GibcoBRL)-resistant cells were plated on gelatin-coated 24-well plates and screened by PCR using a sense primer (p2/neo21; 5′-CCGCTTCCTCGTGCTTTACGG-3′), corresponding to sequences in the 3′ region of the PGK-neo cassette, and an antisense primer (oligo1573; 5′-CGTAGCACAGTGCTGTGGTGAC-3′), located just 3′ to sequences used for the short arm of homology in the targeting construct (Fig. 1A). Individual positive clones (FerD-R 20 and FerD-R 153) were expanded on embryonic fibroblasts, and chimeric mice were produced using the darning needle aggregation method (34). Chimeric males were bred with 129/SVJ females, resulting in germ line transmission and establishment of stable lines from both clones.
Genotyping by Southern blotting and PCR.
Routine genotyping was performed with total DNA from tail biopsy samples from weaning age pups as templates for PCR using genotyping primers (Intron 18 forward [5′-TGGGGAAGGGAAGACATTTTGTAGC-3′] and Intron 19 reverse [5′-GGAAACTAGAAGCATTTTCACTTGG-3′]) which span the D743 codon in exon 19. The PCR-generated 871-bp fragment was subsequently digested with NruI (NEB), yielding bands of 605 and 266 bp in the presence of the D743R mutation. In addition, Southern blot analysis of SacI-digested tail DNA was done using a 1.8-kbp EcoRI-XhoI fragment from clone 3B as a probe (Fig. 1A). The wild-type allele yielded a 4-kb band, while the targeted allele produced a 5.8-kb band, due to the insertion of the PGK-neo-pA cassette.
Immune complex kinase assays.
Livers and testes were isolated from male 5-month-old littermates that were wild type, heterozygous, or homozygous for the D743R mutation. Lysates were generated by homogenization in KLB (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1% [vol/vol] Nonidet P-40, 0.5% [wt/vol] sodium deoxycholic acid, 10 μg of aprotinin and 10 μg of leupeptin per ml, 100 μM sodium orthovanadate, 100 μM phenylmethylsulfonyl fluoride), using an Ultra-Turrax T25 homogenizer (Janke & Kunkel, IKA-Labortechnik). Lysates were clarified by centrifugation at 12,000 × g for 10 min at 4°C. Protein concentrations were determined (Bio-Rad), and 1 mg of extract was precleared with 25 μl of GammaBind-Sepharose (Amersham Pharmacia Biotech), then subjected to immunoprecipitation with either 5 μl of anti-FerLA serum or anti-Fps/Fer serum (also called anti-FpsQE [7, 12]; raised against the SH2 domain of Fps but also cross-reactive with Fer and FerT). After incubation for 1.5 h, 25 μl of GammaBind-Sepharose was added for an additional 30 min at 4°C. Precipitates were washed three times in KLB and once in KRB (20 mM Tris-HCl [pH 7.5], 10 mM MnCl2, 100 μM sodium orthovanadate). Kinase reactions were performed by resuspending the immune complex in 30 μl of KRB supplemented with 5 μCi of [γ-32P]ATP and incubation for 20 min at 30°C. Reactions were terminated by addition of 30 μl of 2× sodium dodecyl sulfate (SDS) sample buffer, heated for 5 min at 100°C, and resolved by SDS-polyacrylamide gel electrophoresis (PAGE). Duplicate gels were either transferred to polyvinylidene difluoride membranes (Immobilon) using a semidry apparatus (Bio-Rad) or incubated at 50°C in 1 M KOH for 2 h and then fixed and dried on a gel dryer (Bio-Rad). Autoradiography was performed on both membranes and dried gels, then membranes were probed with anti-FerLA (1:500) or anti-Fps/Fer (1:500) antibodies, and proteins were visualized by enhanced chemiluminescence (NEN Life Science Products) using peroxidase-conjugated anti-rabbit secondary antibody (1:10,000; Boehringer Mannheim Biochemica).
Isolation of mouse embryonic fibroblasts.
Males and females heterozygous for the D743R mutation were bred, and 10 to 11 days following the detection of a vaginal plug, females were sacrificed by cervical dislocation. Embryos were subsequently isolated and placed in phosphate-buffered saline (PBS; calcium and magnesium free). The head and internal organs were removed, and carcasses were incubated in PBS supplemented with 20% fetal bovine serum (FBS; HyClone) and 0.25% collagenase (GibcoBRL) for 30 min at 37°C. After pipetting up and down, large clumps were allowed to settle out, and single-cell suspensions were transferred to fresh tubes. Following centrifugation at 300 × g for 5 min, cells were washed once with Dulbecco modified Eagle medium (DMEM; GibcoBRL)–10% FBS (HyClone)–2 mM glutamine (GibcoBRL)–2 mM antimicrobial/antimycotic (GibcoBRL), resuspended in 10 ml of the same media, plated on 100-mm-diameter tissue culture plates (Sarstedt), and grown at 37°C in 5% CO2 in a humidified incubator. Plates were usually confluent the following day. Cells were trypsinized and either split 1:3 or frozen in liquid nitrogen in DMEM–20% FBS–10% dimethyl sulfoxide. PCR genotyping was performed on a fraction of the cells as described above.
Growth factor stimulation, immunoprecipitation, and immunoblotting.
Embryonic fibroblasts were cultured to ≈80% confluency on 100-mm-diameter plates and starved in DMEM for 2 days; then fresh DMEM–10 mM HEPES-KOH (pH 7.5) was added with or without recombinant human PDGF β chain (PDGF-BB; 20 ng/ml; R&D). In some experiments, cells were stimulated with recombinant human EGF (100 ng/ml; Intergen). Cells were then washed with cold PBS–100 μM sodium orthovanadate, lysed in 1.5 ml of KLB, and scraped from the plates using a rubber policeman. After clarification of the lysates, 100-μl aliquots were retained for soluble cell lysates, while the remainder was divided equally between several tubes for immunoprecipitation with different antisera, including anti-FerLA rabbit polyclonal antibody (4 μl/sample), anti-p120ctn mouse monoclonal antibody (1 μg/sample; Transduction Labs), anticortactin mouse monoclonal antibody (4 μg/sample; Upstate Biotechnology Inc.), and anti-β-catenin mouse monoclonal antibody (1 μg/sample; Santa Cruz). Antiphosphotyrosine blotting of soluble cell lysates and immunoprecipitations were performed with monoclonal antibody PY99 (1:1,000; Santa Cruz). Antibodies used for Western blotting were anti-FerLA (1:500), anti-p120ctn (1:1,000), anti-cortactin (1:1,000), anti-β-catenin (1:1,000), anti-phospho-Akt (1:2,000; NEB), anti-Akt (1:1,000; NEB), anti-phospho-Stat3 (Y704; 1:2,000; Upstate Biotechnology), anti-Stat3 (1:1,000; NEB), anti-phospho-Erk1/2 (1:500; Santa Cruz), and anti-Erk (1:500; Santa Cruz). Proteins present on Western blots were revealed by enhanced chemiluminescence after incubation with either horseradish peroxidase-conjugated anti-rabbit (Boehringer Mannheim Biochemica), or horseradish peroxidase-conjugated anti-mouse IgG (Amersham Pharmacia Biotech).
Flow cytometry.
Single-cell suspensions from bone marrow of 6- to 12-week-old mice were prepared and processed for flow cytometry as described previously (42). In addition, single-cell suspensions were obtained from spleen and thymus by mincing the tissues, followed by passage through a 21-gauge needle. Cells were resuspended at a density of 20 × 106/ml and processed the same as bone marrow cells (42). Bone marrow cells were incubated with the following antibody pairs: (i) phycoerythrin (PE)-conjugated rat anti-mouse Ly-6G and fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD11b, (ii) PE-conjugated rat anti-mouse TER-119 and FITC-conjugated rat anti-mouse CD44, and (iii) PE-conjugated rat anti-mouse CD45R/B220 and FITC-conjugated rat anti-mouse IgM. Splenocytes were incubated with PE-conjugated rat anti-mouse CD45R/B220 and FITC-conjugated rat anti-mouse IgM. Thymocytes were analyzed with PE-conjugated rat anti-mouse CD8 and FITC-conjugated rat anti-mouse CD4 (all conjugated antibodies were from PharMingen Canada except for rat anti-mouse IgM, which was purchased from Serotec).
Immunofluorescence.
Wild-type and ferD743R homozygous embryonic fibroblasts were plated on gelatinized coverslips (18 by 18 mm). Cells were starved overnight in DMEM and stimulated with either EGF (100 ng/ml, 2 h) or PDGF (20 ng/ml, 4 h). Coverslips were rinsed with PBS, fixed in 2% paraformaldehyde–0.1% Triton X-100 for 30 min at 4°C, and blocked in PBS–20% filtered goat serum for 30 min at room temperature, followed by PBS–2% bovine serum albumin (BSA) for 2 h. Primary mouse monoclonal antibodies were diluted in PBS–2% BSA and were applied to the cells at the following dilutions: anti-cortactin, 1:200; antivinculin (Sigma clone hVIN-1), 1:400; anti-p120ctn, 1:50; anti-β-catenin, (Transduction Labs), 1:100; anti-α-catenin (Transduction Labs), 1:50; and antipaxillin (Transduction Labs), 1:100. Cells were incubated overnight at 4°C. Coverslips were washed three times in PBS and incubated with a mixture of Alexa Fluor 568 goat anti-mouse IgG (1:400; Molecular Probes) and FITC-conjugated phalloidin (1:400; stock, 100 μg/ml; Sigma) in PBS–2% BSA for 1 h in the dark at room temperature. Coverslips were washed three times with PBS and then mounted on microscope slides using FluoroGuard antifade (Bio-Rad). Coverslips were analyzed by confocal microscopy (Insight Plus [Meridian] microscope, air-cooled argon laser) using a 60× oil immersion objective (NA1.2). Alexa Fluor 568 signals were obtained by excitation at 514 nm and captured using a 620 ± 20-nm band-pass filter. FITC signals were obtained by excitation at 488 nm and captured using a 530 ± 15-nm band-pass filter. Images were pseudo-colored and overlaid using MaximDL software.
RESULTS
Targeting of the murine fer gene.
To investigate the biological function of the PTK Fer, we chose to target the fer allele with a kinase-inactivating mutation. Initially we were not successful in cloning fer genomic sequences encoding the conserved lysine in subdomain II, which we previously used for targeting the fps proto-oncogene (42). However, we did obtain a large clone that contained the final two exons of fer (Fig. 1A). These exons were numbered 19 and 20 based on our cloning and available mouse and human genomic sequences (unpublished data). Exon 19 sequences encoded kinase subdomain IX, which includes aspartate 743. This residue contributes to the conformational stability of the catalytic loop by forming hydrogen bonds with the backbone amide groups of the catalytic loop residues (18). We reasoned that mutation of aspartate 743 to arginine (D743R) might destabilize the catalytic loop and result in inactivation of the Fer kinase domain. In addition, since the testis-specific isoform FerT (10) shares the SH2 and kinase domains of Fer, the D743R mutation should also inactivate FerT. We have tested this hypothesis by generating the D743R mutation in the fer cDNA, and we observed a loss of kinase activity when the mutation was expressed in bacteria or in mammalian cells (6). For targeting the fer allele with the D743R mutation, we generated a targeting vector that consisted of (i) a 6-kbp long arm of homology containing the final two exons of fer, (ii) the Neo-positive selectable marker, (iii) a 0.9-kbp short arm of homology corresponding to 3′ flanking sequences to the fer locus, and (iv) the negative selection marker human herpes simplex virus thymidine kinase, located 3′ to the short arm of homology (Fig. 1A). The D743R mutation was generated within the long arm of homology, resulting in the introduction of an NruI restriction site which was used for genotyping analysis.
The linearized targeting vector was electroporated into the R1 murine ES cell line (34), and combined G418-ganciclovir selection was applied. A total of 700 clones were screened by PCR across the short arm of homology (Fig. 1A). We identified five positive clones, which corresponded to a targeting frequency of approximately 0.7%. However, because homologous recombination could have occurred either 5′ or 3′ from the D743R mutation, we also used a secondary PCR strategy which amplified a 871-bp fragment from genomic DNA that spanned the D743R mutation. Digestion of this PCR product with NruI resulted in production of 605- and 266-bp fragments if the D743R mutation was present (Fig. 1C). Two targeted lines, one which contained the D743R mutation (Fer 153) and one which retained the wild-type coding sequence line (Fer 20) were subsequently used to produce chimeric mice by the darning needle aggregation method (34), and germ line transmission was achieved for both lines. Heterozygous offspring were identified by PCR from tail biopsy DNA with the same primers as used in the ES cell screen (Fig. 1A). Heterozygous cross heterozygous breeding pairs were established for both lines, and the resulting offspring were genotyped by both Southern blotting and PCR. The Fer 20 line, which contained the Neo cassette immediately downstream of the fer locus but retained wild-type coding sequence, was bred to homozygosity. Western blot analysis showed that Fer expression was unchanged and that insertion of the Neo cassette did not alter transcription or stability of fer mRNA (data not shown); as a result, this line was not studied further. However, the Fer 153 line produced viable offspring that were wild type, heterozygous, or homozygous by Southern blotting of SacI-digested tail DNA (Fig. 1B) and by PCR genotyping (Fig. 1C). Therefore, we successfully targeted the fer locus with the kinase-inactivating D743R mutation, but mice homozygous for this mutation looked and behaved similarly to their wild-type and heterozygous littermates (data not shown).
Mice homozygous for the ferD743R allele express inactive Fer.
To verify that the D743R mutation disrupted Fer activity when expressed from the fer allele, we performed immune complex kinase assays on tissue homogenates from wild-type, heterozygous, and homozygous mice. Liver homogenates were subjected to immunoprecipitation with Fer antisera, followed by in vitro kinase reactions (Fig. 2A). Western blotting of soluble cell lysates identified the 94-kDa Fer protein for all genotypes; however, the amount of Fer was greatly reduced in homozygous lysates (top panel). This has been observed consistently in all tissues examined and in all primary cell cultures derived from ferD743R mice. This is likely due to ubiquitination and degradation, since the FerD743R protein, but not wild-type Fer, was recognized by an antiubiquitin antibody (data not shown). Fer kinase activity was observed in both wild-type and heterozygous but not homozygous homogenates (middle panel). Fer was detected in liver homogenates from all three genotypes by immunoprecipitation followed by Western blotting (bottom panel), thus verifying that the FerD743R protein is present at reduced steady state levels and is catalytically inactive.
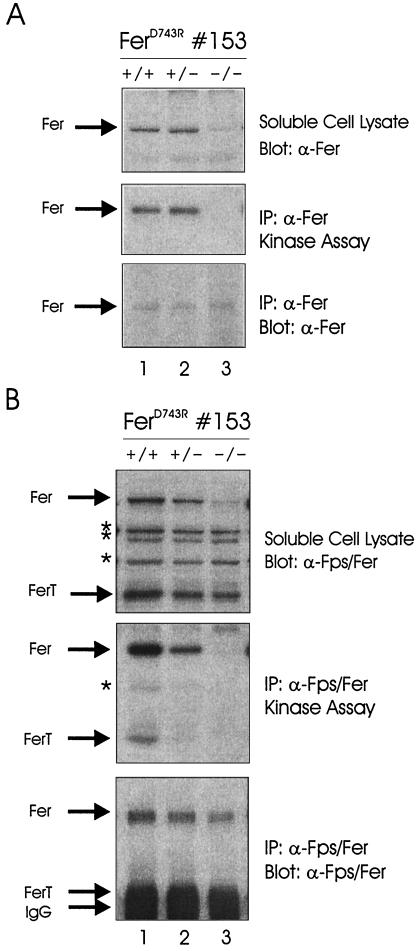
FIG. 2.
Analysis of Fer expression and kinase activity in ferD743R mice. (A) Liver homogenates from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) ferD743R mice (strain 153) were subjected to immunoprecipitation (IP) with Fer antisera, followed by in vitro kinase reactions. The position of Fer is indicated on the left. (B) Testis homogenates from +/+, +/−, or −/− ferD743R male mice were subjected to immunoprecipitation with Fps/Fer antisera (raised against the SH2 domain of Fps but cross-reactive with Fer and FerT), followed by in vitro kinase reactions. The positions of Fer, FerT, and the IgG heavy chain are indicated on the left. Cross-reacting bands in the soluble cell lysate, and a putative partial degradation product in the kinase assay, are indicated with asterisks.
To assess Fer and FerT activity in testis, homogenates were prepared and subjected to immunoprecipitation with anti-Fps/Fer antiserum (which reacts with Fps, Fer, and FerT), followed by in vitro kinase reactions (Fig. 2B). Western blotting of soluble cell lysates identified the 94-kDa Fer and the 51-kDa FerT proteins for all three genotypes (top panel). Only trace amounts of the 92-kDa Fps protein were found in testis samples, along with several cross-reacting proteins (top panel). The 51-kDa band that we have indicated as FerT was not detected with this antiserum in other tissues such as spleen and liver, and it was not recognized by our Fer antiserum, which was raised against N-terminal sequences not found in FerT (data not shown). Therefore, it seems that FerT is less destabilized than Fer by the D743R mutation (compare lanes 1 and 3). Kinase activities of Fer and FerT are clearly reduced in heterozygous homogenates and completely disrupted in homozygous homogenates (middle panel). Western blotting of the immunoprecipitations revealed reduced but detectable levels of Fer (bottom panel). Unfortunately, we have been unable to resolve the FerT band from the IgG heavy chain in Western blotting analysis. Overall, these results indicate that homozygous ferD743R mice lack both Fer and FerT kinase activities.
Genotypic analysis of ferD743R offspring.
Genotypic analysis of over 300 pups from 50 litters derived from heterozygous cross heterozygous breeding pairs indicated a genetic distribution of 61 (20%) wild type, 180 (59%) heterozygous, and 65 (21%) homozygous for the ferD743R allele (Table 1). Similar percentages were obtained when male and female offspring were analyzed separately. This clearly demonstrates that Fer and FerT kinase activities are not required for embryonic development and maturation. To test whether Fer and FerT were required for fertility, homozygous males were set up with females of all three genotypes. Normal-sized litters were obtained from each of these breedings, and the offspring were of the expected genotypes (data not shown). These results suggest that Fer and FerT kinase activities are not required for male or female fertility. Litter sizes were also compared between wild type × wild type, heterozygous × heterozygous, and homozygous × homozygous breeding pairs (Table 2). Average litter sizes from these breeding pairs were 6 ± 2, 6 ± 2, and 7 ± 2, respectively. Pups from homozygous × homozygous parents were normal in size and appearance compared to those from other breeding pairs. Although FerT has been proposed to play a role in spermatogenesis (10), our results indicate that FerT kinase activity is dispensible for male fertility.
TABLE 1.
Genotypic analysis of offspring from heterozygous × heterozygous breeding pairs
| Gender | No. (%) of indicated genotypea
|
||
|---|---|---|---|
| Wild type | Heterozygous | Homozygous | |
| Male | 25 (17) | 95 (64) | 28 (19) |
| Female | 36 (23) | 85 (54) | 37 (23) |
| Combined | 61 (20) | 180 (59) | 65 (21) |
Cumulative data from 7 separate heterozygous × heterozygous 129/SVJ breeding pairs active over continuous periods of 9 months.
TABLE 2.
Litter sizes
| Genotypea | No. of breeding pairs | Total no. of litters born | No. of pups born/litter (mean ± SD) |
|---|---|---|---|
| Wild type × wild type | 2 | 5 | 6 ± 2 |
| Heterozygous × heterozygous | 7 | 50 | 6 ± 2 |
| Homozygous × homozygous | 3 | 6 | 7 ± 2 |
All breeding pairs were inbred 129/SVJ mice.
Cellularity of tissues from ferD743R mice.
Flow cytometry was used to examine whether the levels of hematopoietic precursors in bone marrow, or mature lymphocytes in spleen and thymus, were altered in ferD743R homozygous mice (data not shown). No significant differences were observed in bone marrow from mice of the different genotypes in the levels of Ly-6G+ CD11b+ myeloid precursors (wild type, 41%; heterozygous, 41%; homozygous, 40%), TER-119+ CD44lo erythroid precursors (wild type, 9%; heterozygous, 9%; homozygous, 7%), or B220+ IgM+ immature B cells (wild type, 12%; heterozygous, 9%; homozygous, 12%). The levels of B220+ IgM+ B cells in spleen were also unaffected by loss of Fer kinase activity (wild type, 46%; heterozygous, 61%; homozygous, 52%). Also, T-cell populations in thymus were similar in animals of each genotype: CD4+ CD8+ (wild type, 82%; heterozygous, 85%; homozygous, 77%), CD4+ CD8− (wild type, 11%; heterozygous, 8%; homozygous, 12%), and CD4− CD8+ (wild type, 6%; heterozygous, 5%; homozygous, 8%). Therefore, Fer kinase activity is not required for myelopoiesis, erythropoiesis, or lymphopoiesis.
PDGF signaling in ferD743R embryonic fibroblasts.
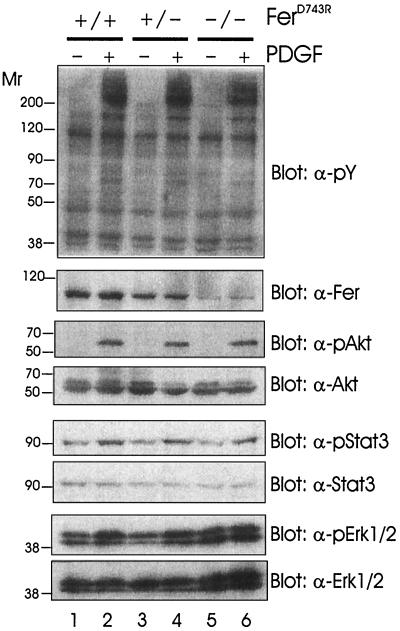
Since Fer is activated downstream of EGF and PDGF receptors (24, 25), we wanted to assess whether either of these signaling pathways was impaired in the absence of Fer activity. Embryonic fibroblasts were established from day 10.5 embryos, and their genotypes were determined by PCR (as described in Materials and Methods). Early-passage cells were grown until they approached confluency; then they were starved for 2 days without serum and subsequently stimulated with PDGF-BB for 5 min. No obvious differences were observed between overall profiles of tyrosine-phosphorylated proteins in PDGF-stimulated wild-type, heterozygous, or homozygous ferD743R cell lysates (Fig. 3, top panel). As described above for tissue samples, the amount of Fer was lower in heterozygous and homozygous cell lysates (second panel). Using a panel of phosphospecific and control antibodies, we analyzed some of the major downstream signaling targets. Akt (PKB) activation was observed in lysates from cells of all three genotypes (third panel), indicating that activation of the phosphatidylinositol 3′-kinase pathway does not require Fer activity. Although recent studies suggest that Fer can associate with and phosphorylate Stat3 (38), our data indicate that Fer is not the physiological kinase of Stat3 since Stat3 phosphorylation upon PDGF stimulation was the same in the absence and presence of Fer activity (fifth panel). Phosphorylation of p44 Erk1 and p42 Erk2 was elevated in response to PDGF in lysates from cells of each genotype (seventh panel). Basal levels of Erk1/2 phosphorylation were slightly elevated in homozygous ferD743R cells (compare lanes 1, 3, and 5). However, blotting with the control Erk antibody indicated slightly higher amounts of Erk1/2 in the homozygous ferD743R cell lysates (bottom panel, compare lanes 1 to 4 with lanes 5 and 6). In time courses of activation up to 40 min, we have not observed any reproducible differences in activation of these signaling proteins (data not shown).
FIG. 3.
Analysis of major PDGF signaling pathways in ferD743R embryonic fibroblasts. Wild-type (+/+), heterozygous (+/−), and homozygous (−/−) ferD743R cells were starved and stimulated with PDGF-BB for 5 min. Soluble cell lysates were separated by SDS-PAGE and blotted for tyrosine-phosphorylated proteins (pY), Fer, phospho-Akt (pAkt), Akt, phospho-Stat3 (pStat3), Stat3, phospho-Erk1/2 (pErk1/2), and Erk1/2. Relative molecular weights are indicated in thousands on the left.
To verify that Fer is activated in response to PDGF in our cell system, the phosphorylation state of Fer was analyzed in PDGF-stimulated cell lysates. As described above, overall tyrosine phosphorylation profiles were similar in soluble cell lysates of the three genotypes (Fig. 4, top panel). Immunoprecipitation of Fer and blotting with antiphosphotyrosine revealed a marked increase in Fer phosphorylation in response to PDGF in both wild-type and heterozygous ferD743R cells (second panel, lanes 1 to 4). Interestingly, the inactive FerD743R protein in homozygous cells became tyrosine phosphorylated following treatment with PDGF (lanes 5 and 6). This suggests that an upstream kinase phosphorylates Fer and may regulate Fer activation. This is consistent with earlier findings showing elevated intrinsic kinase activity of Fer in growth factor-stimulated compared to starved cell lysates (24).
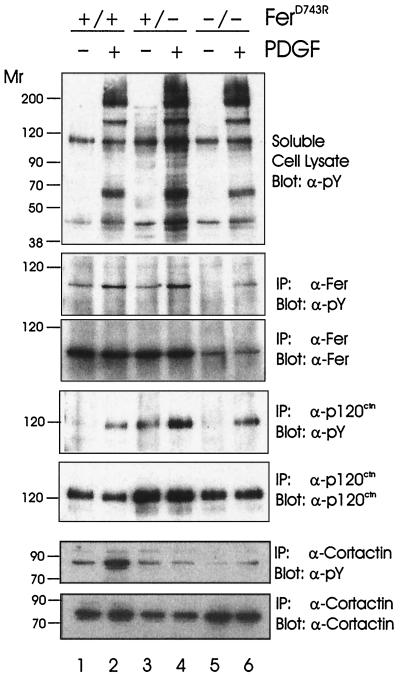
FIG. 4.
Impaired cortactin but not p120ctn phosphorylation in ferD743R embryonic fibroblasts. Wild-type (+/+), heterozygous (+/−), and homozygous (−/−) ferD743R cells were starved and stimulated with PDGF-BB for 5 min. Lysates were subjected to immunoprecipitation (IP) with Fer, p120ctn, and cortactin antibodies. Soluble cell lysates and immunoprecipitates were resolved by SDS-PAGE and blotted for tyrosine-phosphorylated proteins (pY), Fer, p120ctn, or cortactin. Relative molecular weight markers are indicated in thousands on the left.
We used a similar approach to assess the phosphorylation status of two putative substrates of Fer, p120ctn and cortactin (24, 25). PDGF stimulation caused an increase in p120ctn phosphorylation in the presence and absence of Fer kinase activity (Fig. 4, fourth panel). The overall amount of p120ctn phosphorylation was consistent with the amounts of p120ctn recovered in the immunoprecipitations (fifth panel). The higher levels of p120ctn in heterozygous cell lysates was due to slight overloading and was not observed in all experiments. Thus, p120ctn is not a substrate of Fer, at least under these conditions. However, the proposed interaction between p120ctn and Fer (24, 25, 41) may serve to bring Fer to the cadherin complex, where it may regulate other as yet unknown substrates.
Similar experiments were done to assess the phosphorylation status of cortactin (Fig. 4, bottom two panels). Although a substantial increase in cortactin phosphorylation was observed in wild-type cell lysates (lanes 1 and 2), this same increase in phosphorylation was not observed in heterozygous and homozygous ferD743R cells (lanes 3 to 6). Some of the reduced phosphorylation in heterozygous cells was likely due to reduced recovery of cortactin in these immunoprecipitates (bottom panel, lanes 3 and 4) and was not observed in other experiments. However, the recovery of cortactin from homozygous cells was similar to that in wild-type cells (compare lanes 1 and 2 with lanes 5 and 6), and yet the phosphorylation state of cortactin was greatly reduced in the absence of Fer activity (sixth panel, compare lanes 1 and 2 with lanes 5 and 6). These results are consistent with Fer playing a role in cortactin phosphorylation, but Fer is clearly not required for all tyrosine phosphorylation of cortactin. Other kinases implicated in cortactin phosphorylation include Src (45) and Fyn (3). We do not know if Fer phosphorylates cortactin directly, as we have been unable to observe a stable interaction between these proteins (data not shown).
EGF signaling in ferD743R embryonic fibroblasts.
In an analysis of EGF signaling similar to that shown for PDGF stimulation (Fig. 3), overall tyrosine phosphorylation profiles were similar in the absence and presence of Fer kinase activity (data not shown). Wild-type, heterozygous, and ferD743R homozygous cells also displayed similar EGF-induced activation of Akt (PKB), Stat3, and p44 Erk1/p42 Erk2. Therefore, Fer kinase activity is not required for efficient phosphorylation of Stat3 in response to either PDGF or EGF, suggesting that the effects observed by overexpression of Fer and Stat3 (38) do not reflect the physiological pathway leading to Stat3 activation. We have also observed normal levels of Stat3 activation in cultured bone marrow-derived macrophages stimulated with cytokines such as GM-CSF (data not shown).
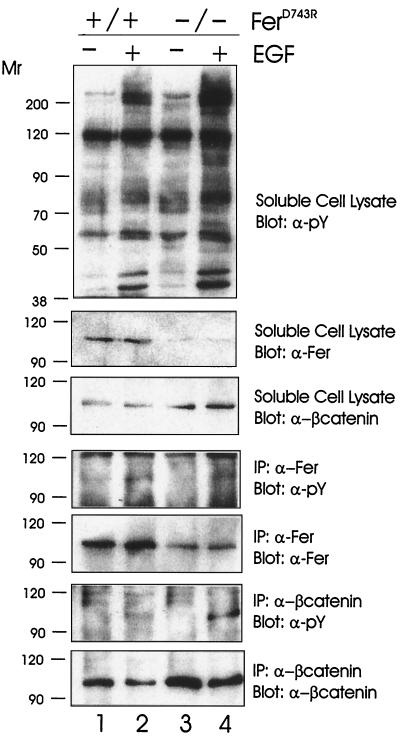
To confirm that Fer was indeed activated by EGF in this cell system, wild-type and ferD743R homozygous embryonic fibroblasts were stimulated with EGF, and soluble cell lysates were obtained. Western blotting with antiphosphotyrosine revealed similar profiles of phosphorylation in the absence and presence of Fer kinase activity (Fig. 5, top panel). The apparent higher levels of phosphorylation in the EGF-stimulated ferD743R homozygous lysate (lane 4) reflects slight overloading, as it was not observed in all experiments. The same blot was probed with anti-Fer, which revealed the characteristic reduction in FerD743R levels (second panel, compare lanes 1 and 2 with lanes 3 and 4). In contrast, the levels of β-catenin were higher in ferD743R homozygous lysates than in wild-type lysates (third panel), due to slight overloading. Anti-phosphotyrosine blotting of Fer immunoprecipitations revealed increased Fer phosphorylation in EGF-stimulated wild-type and ferD743R homozygous cell lysates (fourth panel). The observed phosphorylation of the inactive FerD743R protein (lane 4) indicates phosphorylation of Fer by an upstream kinase, as noted above for PDGF signaling.
FIG. 5.
EGF-stimulated β-catenin phosphorylation in the absence of Fer activity. Wild-type (+/+) and homozygous (−/−) ferD743R cells were starved and stimulated with EGF for 5 min. Lysates were subjected to immunoprecipitation (IP) with Fer and β-catenin-specific antibodies. Soluble cell lysates and immune complexes were resolved by SDS-PAGE and blotted for tyrosine-phosphorylated proteins (pY), Fer, and β-catenin. Relative molecular weight markers are indicated in thousands on the left.
Since Fer has been proposed to play a role in regulating several of the catenin family members, we analyzed β-catenin phosphorylation in wild-type and ferD743R homozygous cell lysates (Fig. 5, bottom two panels). We observed an EGF-dependent tyrosine phosphorylation of β-catenin in the absence and presence of Fer activity (sixth panel). The amount of phosphorylation correlated well with the amount of β-catenin recovered in the immunoprecipitations (bottom panel, compare lanes 2 and 4). Since β-catenin is also a 97-kDa tyrosine-phosphorylated protein, it was possible that the apparent phosphorylation of Fer in homozygous cell lysates could reflect coimmunoprecipitation of Fer and β-catenin. However, we did not detect β-catenin in our Fer immunoprecipitates by Western blotting (data not shown). Taken together, our results indicate that while EGF and PDGF signaling lead to activation of Fer, the catalytic activity of Fer is not required for phosphorylation of p120ctn or β-catenin. However, since the inactive FerD743R protein becomes phosphorylated upon EGF and PDGF stimulation, Fer could retain a scaffolding function whereby PTB or SH2 domain-containing proteins could still interact with Fer. But even kinase activity-independent roles of Fer would be impaired in ferD743R homozygous mice due to the reduced stability of FerD743R protein.
Localization of actin-, focal adhesion-, and cadherin-associated proteins in ferD743R homozygous cells.
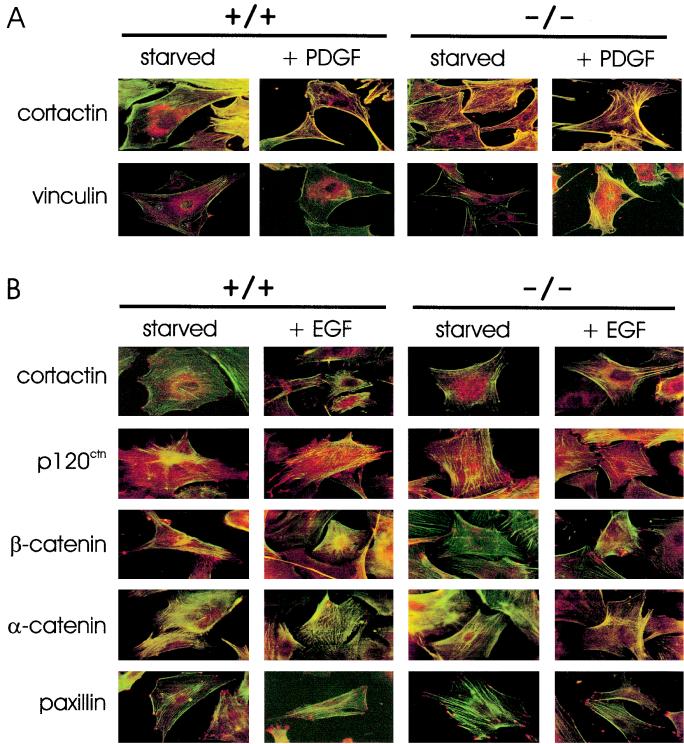
Fer has been proposed to regulate cross-talk between adherens junctions and focal adhesions (2), as well as the cortical actin-associated protein cortactin (25). This led us to test for differences in cellular localization of actin-, focal adhesion-, and cadherin-associated proteins in wild-type and ferD743R homozygous cells (Fig. 6). Wild-type and ferD743R homozygous embryonic fibroblasts were plated on gelatinized coverslips, starved of growth factors, and incubated with either PDGF for 4 h (Fig. 6A) or EGF for 2 h (Fig. 6B). We observed actin stress fibers that were anchored to cortical actin, located at the cell periphery in wild-type and ferD743R homozygous cells under both starvation and stimulation conditions (Fig. 6A). Overall, there were less cell-cell contacts for wild-type and ferD743R homozygous cells in the presence of PDGF compared to starved cells (compare columns 2 and 4 to columns 1 and 3). While cortactin was localized predominantly to the perinuclear region in starved cells, strong colocalization was observed with cortical actin in PDGF-treated wild-type and ferD743R homozygous cells. This correlated with cells that were extending lamellipodia. Vinculin, like cortactin, was mostly perinuclear in starved cells but showed strong colocalization with the ends of actin stress fibers and cortical actin in PDGF stimulated wild-type and ferD743R homozygous cells. Therefore, we conclude that changes to the actin cytoskeleton in response to PDGF, and the association with proteins such as cortactin and vinculin, remain intact in the absence of Fer activity. Although PDGF-induced phosphorylation of cortactin was reduced in the absence of Fer activity (Fig. 4), we have not observed a defect in cortactin localization, cortical actin, or in PDGF-induced cell motility (data not shown). Therefore, either the basal levels of cortactin phosphorylation in the absence of Fer are sufficient for regulation of cortactin, or other regulatory mechanisms are able to compensate for the lack of Fer activity in the control of cortactin localization.
FIG. 6.
Localization of the actin cytoskeleton and of actin-, cadherin-, or focal adhesion-associated proteins in ferD743R embryonic fibroblasts. Wild-type (+/+) and homozygous (−/−) ferD743R cells were plated on gelatinized coverslips, starved of growth factors, and stimulated with either PDGF (A) or EGF (B). The proteins denoted on the left were detected with monoclonal antibodies and Alexa Fluor 568 goat anti-mouse secondary (indicated by red color). Actin cytoskeleton was visualized by staining with FITC-phalloidin (indicated by green color). Yellow denotes colocalization of protein and actin. Representative images are shown following confocal microscopy.
We next examined the morphological changes of wild-type and ferD743R homozygous cells in response to EGF stimulation (Fig. 6B). As described above for PDGF treatment, cortactin showed predominantly perinuclear localization in starved cells, although some staining of membrane ruffles was observed. Upon stimulation of cells with EGF, colocalization was observed between cortactin and cortical actin structures in both wild-type and ferD743R homozygous cells. Thus, no defects in cortactin localization were evident in the absence of Fer activity for EGF or PDGF-stimulated cells.
Since Fer has been proposed to interact with, and possibly phosphorylate, several catenins (2, 24, 25, 41), we analyzed their localization in wild-type and ferD743R homozygous cells. While some diffuse staining was observed for p120ctn, we also observed punctate staining at positions of cell-cell contact, consistent with localization to adherens junctions. Many of these cell-cell contacts were maintained in EGF-stimulated cells. As expected, little colocalization was observed between p120ctn and the actin cytoskeleton. As expected, we did not see any defects in p120ctn localization in the absence of Fer activity, consistent with its normal phosphorylation state in ferD743R homozygous cells (Fig. 4). β-Catenin also localized in a punctate pattern at sites of cell-cell contact in starved cells but showed little staining in the periphery of EGF-stimulated wild-type cells. This is consistent with phosphorylated β-catenin being either targeted for degradation or performing its signaling role through interaction with the transcription factor Lef-1/TCF (26). While there was less cell-cell contact in ferD743R homozygous cells upon EGF stimulation, some punctate staining was observed at the periphery. Consistent with α-catenin being a vinculin-like protein that bridges β-catenin to actin filaments (16, 27), it displayed more colocalization with actin stress fibers as opposed to the punctate patterns observed for p120ctn and β-catenin. To visualize focal adhesions in wild-type and ferD743R homozygous cells, we examined paxillin localization under starvation and EGF stimulation conditions. As expected, paxillin localized to the ends of actin stress fibers. There were more focal adhesions in EGF-stimulated cells, indicating that the formation of focal adhesions does not require Fer kinase activity. Therefore, the appearance of Fer in focal adhesions and its proposed role in regulation of these complexes may be restricted to neurocan signaling in retinal cells (2, 30) and is likely not a general regulator of cell adhesion.
DISCUSSION
To address the biological function of the Fer PTK, we have generated a transgenic mouse line devoid of Fer kinase activity (ferD743R). While Fer and FerT kinase activities are abolished in ferD743R homozygous animals, no overt defects were found in embryos or mature animals. Furthermore, ferD743R homozygous males and females were fully fertile, indicating that neither Fer nor FerT activity is required for gametogenesis or any other developmental function. Given the ubiquitous expression of Fer and its proposed roles in regulating cadherin-catenin complexes (24, 41), cortical actin (25), cell adhesion (41), cell growth (35), and neurite outgrowth (2, 30, 31), it is surprising that animals devoid of Fer activity appear to develop normally. This suggests that either the function of Fer in these pathways is not critical or another kinase may provide redundant functions. It is worth noting that all of the proposed roles of Fer are based predominantly on experiments performed on immortalized cell lines, and as such they may not reflect the physiological function of Fer. In the case of redundancy, it would seem unlikely that Fps could completely compensate for loss of Fer, since Fps displays a more restricted expression pattern. However, we now have the tools to address this question by generating compound transgenic mouse lines devoid of both Fps and Fer activities. These animals should allow us to determine if either the subtle defects described for mice devoid of Fps activity (42) or Fer activity are masked by functional redundancy of these PTKs in tissues with overlapping expression.
The recent publications involving Fer shuttling between N-cadherin complexes and focal adhesions in chick retinal cells (2, 30, 31) warrant investigation using the mouse model described in this study. We know that ferD743R homozygous animals are not blind, as they respond to visual cues (unpublished observations). However, a more detailed analysis of retinal development in mice devoid of Fer activity is likely needed.
One defect that was observed in embryonic fibroblasts devoid of Fer activity involved the cortical actin-associated protein cortactin (45, 46). Fer was shown previously to coimmunoprecipitate with cortactin and to contribute to its tyrosine phosphorylation (25). While we have not observed coimmunoprecipitation of Fer and cortactin (data not shown), the interaction was proposed to involve detergent-insoluble cytoskeleton structures. However, we did find that detergent-soluble cortactin was underphosphorylated in cells devoid of Fer activity (Fig. 4). This suggests that Fer contributes to cortactin phosphorylation, although whether this reflects a direct or indirect event is not known. Clearly, more study is required to delineate the molecular mechanism leading to cortactin phosphorylation and, more importantly, to determine what the precise function of cortactin is in cells. Another recent study implies a cell volume-regulated pathway involving Fer and cortactin (22). This report also suggests that the Src PTK family member Fyn functions upstream of Fer in this signaling pathway. Our observations of tyrosine phosphorylation of kinase inactive Fer (Fig. 4 and 5) also implies the action of an upstream PTK. The intrinsic activity of Fer is twofold higher in growth factor-stimulated lysates than in starved cell lysates (24; unpublished data). This would be consistent with phosphorylation by an upstream kinase either causing a conformational change in Fer or regulating interaction with other proteins that might affect its activity. Therefore, it will be important to identify both the upstream kinase and the site of phosphorylation in Fer using the cells devoid of Fer activity described in this study.
Since Fer is also activated in mast cells downstream of the FcɛRI, it will also be interesting to determine if inflammatory responses are impaired in mice devoid of Fer activity. Preliminary experiments suggest a hyperdegranulation response in ferD743R homozygous bone marrow-derived mast cells in response to calcium ionophore (unpublished data). This phenotype is similar to that described for mice devoid of the SH2-containing inositol phosphatase SHIP (19). Further experiments are under way to try to confirm this phenotype and establish whether Fer and SHIP function in the same signaling pathway.
ACKNOWLEDGMENTS
This work was supported by grant MT-11627 from the Medical Research Council of Canada (MRCC) and by the National Cancer Institute of Canada with funds from the Canadian Cancer Society. A.W.B.C. was supported by an MRCC postdoctoral fellowship.
We gratefully acknowledge Derek Schulze for help with confocal microscopy and for flow cytometry analysis, Yotis Senis and Waheed Sangrar for comments on the manuscript, and Kari Newcombe for technical assistance.
REFERENCES
- 1.Alcalay M, Antolini F, Van de Ven W J, Lanfrancone L, Grignani F, Pelicci P G. Characterization of human and mouse c-fes cDNA clones and identification of the 5′ end of the gene. Oncogene. 1990;5:267–275. [PubMed] [Google Scholar]
- 2.Arregui C, Pathre P, Lilien J, Balsamo J. The nonreceptor tyrosine kinase fer mediates cross-talk between N-cadherin and beta1-integrins. J Cell Biol. 2000;149:1263–1274. doi: 10.1083/jcb.149.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calautti E, Missero C, Stein P L, Ezzell R M, Dotto G P. Fyn tyrosine kinase is involved in keratinocyte differentiation control. Genes Dev. 1995;9:2279–2291. doi: 10.1101/gad.9.18.2279. [DOI] [PubMed] [Google Scholar]
- 4.Care A, Mattia G, Montesoro E, Parolini I, Russo G, Colombo M P, Peschle C. c-fes expression in ontogenetic development and hematopoietic differentiation. Oncogene. 1994;9:739–747. [PubMed] [Google Scholar]
- 5.Cheng H, Rogers J A, Dunham N A, Smithgall T E. Regulation of c-Fes tyrosine kinase and biological activities by N-terminal coiled-coil oligomerization domains. Mol Cell Biol. 1999;19:8335–8343. doi: 10.1128/mcb.19.12.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole L A, Zirngibl R, Craig A W, Jia Z, Greer P. Mutation of a highly conserved aspartate residue in subdomain IX abolishes Fer protein-tyrosine kinase activity. Protein Eng. 1999;12:155–162. doi: 10.1093/protein/12.2.155. [DOI] [PubMed] [Google Scholar]
- 7.Craig A W B, Zirngibl R, Greer P. Disruption of coiled-coil domains in Fer protein-tyrosine kinase abolishes trimerization but not kinase activation. J Biol Chem. 1999;274:19934–19942. doi: 10.1074/jbc.274.28.19934. [DOI] [PubMed] [Google Scholar]
- 8.Feldman R A, Gabrilove J L, Tam J P, Moore M A, Hanafusa H. Specific expression of the human cellular fps/fes-encoded protein NCP92 in normal and leukemic myeloid cells. Proc Natl Acad Sci USA. 1985;82:2379–2383. doi: 10.1073/pnas.82.8.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari S, Torelli U, Selleri L, Donelli A, Venturelli D, Moretti L, Torelli G. Expression of human c-fes onc-gene occurs at detectable levels in myeloid but not in lymphoid cell populations. Br J Haematol. 1985;59:21–25. doi: 10.1111/j.1365-2141.1985.tb02959.x. [DOI] [PubMed] [Google Scholar]
- 10.Fischman K, Edman J C, Shackleford G M, Turner J A, Rutter W J, Nir U. A murine fer testis-specific transcript (ferT) encodes a truncated Fer protein. Mol Cell Biol. 1990;10:146–153. doi: 10.1128/mcb.10.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greer P, Maltby V, Rossant J, Bernstein A, Pawson T. Myeloid expression of the human c-fps/fes proto-oncogene in transgenic mice. Mol Cell Biol. 1990;10:2521–2527. doi: 10.1128/mcb.10.6.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haigh J, McVeigh J, Greer P. The fps/fes tyrosine kinase is expressed in myeloid, vascular endothelial, epithelial, and neuronal cells and is localized in the trans-Golgi network. Cell Growth Differ. 1996;7:931–944. [PubMed] [Google Scholar]
- 13.Hanazono Y, Chiba S, Sasaki K, Mano H, Miyajima A, Arai K, Yazaki Y, Hirai H. c-fps/fes protein-tyrosine kinase is implicated in a signaling pathway triggered by granulocyte-macrophage colony-stimulating factor and interleukin-3. EMBO J. 1993;12:1641–1646. doi: 10.1002/j.1460-2075.1993.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanazono Y, Chiba S, Sasaki K, Mano H, Yazaki Y, Hirai H. Erythropoietin induces tyrosine phosphorylation and kinase activity of the c-fps/fes proto-oncogene product in human erythropoietin-responsive cells. Blood. 1993;81:3193–3196. [PubMed] [Google Scholar]
- 15.Hao Q L, Heisterkamp N, Groffen J. Isolation and sequence analysis of a novel human tyrosine kinase gene. Mol Cell Biol. 1989;9:1587–1593. doi: 10.1128/mcb.9.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R. The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc Natl Acad Sci USA. 1991;88:9156–9160. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hjermstad S J, Peters K L, Briggs S D, Glazer R I, Smithgall T E. Regulation of the human c-fes protein tyrosine kinase (p93c-fes) by its src homology 2 domain and major autophosphorylation site (Tyr-713) Oncogene. 1993;8:2283–2292. [PubMed] [Google Scholar]
- 18.Hubbard S R, Wei L, Ellis L, Hendrickson W A. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 1994;372:746–754. doi: 10.1038/372746a0. [DOI] [PubMed] [Google Scholar]
- 19.Huber M, Helgason C D, Damen J E, Liu L, Humphries R K, Krystal G. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc Natl Acad Sci USA. 1998;95:11330–11335. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izuhara K, Feldman R A, Greer P, Harada N. Interaction of the c-fes proto-oncogene product with the interleukin-4 receptor. J Biol Chem. 1994;269:18623–18629. [PubMed] [Google Scholar]
- 21.Jucker M, McKenna K, da Silva A J, Rudd C E, Feldman R A. The Fes protein-tyrosine kinase phosphorylates a subset of macrophage proteins that are involved in cell adhesion and cell-cell signaling. J Biol Chem. 1997;272:2104–2109. doi: 10.1074/jbc.272.4.2104. [DOI] [PubMed] [Google Scholar]
- 22.Kapus A, Di Ciano C, Sun J, Zhan X, Kim L, Wong T W, Rotstein O D. Cell volume-dependent phosphorylation of proteins of the cortical cytoskeleton and cell-cell contact sites: the role of Fyn and FER kinases. J Biol Chem. 2000;275:32289–32298. doi: 10.1074/jbc.M003172200. [DOI] [PubMed] [Google Scholar]
- 23.Keshet E, Itin A, Fischman K, Nir U. The testis-specific transcript (ferT) of the tyrosine kinase FER is expressed during spermatogenesis in a stage-specific manner. Mol Cell Biol. 1990;10:5021–5025. doi: 10.1128/mcb.10.9.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim L, Wong T W. The cytoplasmic tyrosine kinase FER is associated with the catenin-like substrate pp120 and is activated by growth factors. Mol Cell Biol. 1995;15:4553–4561. doi: 10.1128/mcb.15.8.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim L, Wong T W. Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase FER. J Biol Chem. 1998;273:23542–23548. doi: 10.1074/jbc.273.36.23542. [DOI] [PubMed] [Google Scholar]
- 26.Kirkpatrick C, Peifer M. Not just glue: cell-cell junctions as cellular signaling centers. Curr Opin Genet Dev. 1995;5:56–65. doi: 10.1016/s0959-437x(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 27.Knudsen K A, Soler A P, Johnson K R, Wheelock M J. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch C A, Moran M, Sadowski I, Pawson T. The common Src homology region 2 domain of cytoplasmic signaling proteins is a positive effector of v-Fps tyrosine kinase function. Mol Cell Biol. 1989;9:4131–4140. doi: 10.1128/mcb.9.10.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Letwin K, Yee S P, Pawson T. Novel protein-tyrosine kinase cDNAs related to fps/fes and eph cloned using anti-phosphotyrosine antibody. Oncogene. 1988;3:621–627. [PubMed] [Google Scholar]
- 30.Li H, Leung T C, Hoffman S, Balsamo J, Lilien J. Coordinate regulation of cadherin and integrin function by the chondroitin sulfate proteoglycan neurocan. J Cell Biol. 2000;149:1275–1288. doi: 10.1083/jcb.149.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lilien J, Arregui C, Li H, Balsamo J. The juxtamembrane domain of cadherin regulates integrin-mediated adhesion and neurite outgrowth. J Neurosci Res. 1999;58:727–734. doi: 10.1002/(sici)1097-4547(19991215)58:6<727::aid-jnr1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald I, Levy J, Pawson T. Expression of the mammalian c-Fes protein in hematopoietic cells and identification of a distinct Fes-related protein. Mol Cell Biol. 1985;5:2543–2551. doi: 10.1128/mcb.5.10.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda T, Fukada T, Takahashi-Tezuka M, Okuyama Y, Fujitani Y, Hanazono Y, Hirai H, Hirano T. Activation of Fes tyrosine kinase by gp130, an interleukin-6 family cytokine signal transducer, and their association. J Biol Chem. 1995;270:11037–11039. doi: 10.1074/jbc.270.19.11037. [DOI] [PubMed] [Google Scholar]
- 34.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulson R, Jackson J, Immergluck K, Bishop J M. The DFer gene of Drosophila melanogaster encodes two membrane-associated proteins that can both transform vertebrate cells. Oncogene. 1997;14:641–652. doi: 10.1038/sj.onc.1200875. [DOI] [PubMed] [Google Scholar]
- 36.Pawson T, Letwin K, Lee T, Hao Q L, Heisterkamp N, Groffen J. The FER gene is evolutionarily conserved and encodes a widely expressed member of the FPS/FES protein-tyrosine kinase family. Mol Cell Biol. 1989;9:5722–5725. doi: 10.1128/mcb.9.12.5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penhallow R C, Class K, Sonoda H, Bolen J B, Rowley R B. Temporal activation of nontransmembrane protein-tyrosine kinases following mast cell Fc epsilon RI engagement. J Biol Chem. 1995;270:23362–23365. doi: 10.1074/jbc.270.40.23362. [DOI] [PubMed] [Google Scholar]
- 38.Priel-Halachmi S, Ben-Dor I, Shpungin S, Tennenbaum T, Molavani H, Bachrach M, Salzberg S, Nir U. FER kinase activation of Stat3 is determined by the N-terminal sequence. J Biol Chem. 2000;275:28902–28910. doi: 10.1074/jbc.M003402200. [DOI] [PubMed] [Google Scholar]
- 39.Read R D, Lionberger J M, Smithgall T E. Oligomerization of the Fes tyrosine kinase. Evidence for a coiled-coil domain in the unique N-terminal region. J Biol Chem. 1997;272:18498–18503. doi: 10.1074/jbc.272.29.18498. [DOI] [PubMed] [Google Scholar]
- 40.Roebroek A J, Schalken J A, Verbeek J S, Van den Ouweland A M, Onnekink C, Bloemers H P, Van de Ven W J. The structure of the human c-fes/fps proto-oncogene. EMBO J. 1985;4:2897–2903. doi: 10.1002/j.1460-2075.1985.tb04020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosato R, Veltmaat J M, Groffen J, Heisterkamp N. Involvement of the tyrosine kinase Fer in cell adhesion. Mol Cell Biol. 1998;18:5762–5770. doi: 10.1128/mcb.18.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senis Y, Zirngibl R, McVeigh J, Haman A, Hoang T, Greer P A. Targeted disruption of the murine fps/fes proto-oncogene reveals that Fps/Fes kinase activity is dispensable for hematopoiesis. Mol Cell Biol. 1999;19:7436–7446. doi: 10.1128/mcb.19.11.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tybulewicz V L, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 44.Wilks A F, Kurban R R. Isolation and structural analysis of murine c-fes cDNA clones. Oncogene. 1988;3:289–294. [PubMed] [Google Scholar]
- 45.Wu H, Parsons J T. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H, Reynolds A B, Kanner S B, Vines R R, Parsons J T. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol. 1991;11:5113–5124. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]