Abstract
Background
Immune dysregulation and inflammation in patients with SARS-CoV-2 is associated with a poor clinical outcome. We investigated the value of the inflammatory markers tryptophan and kynurenine in predicting the survival outcome of patients with SARS-CoV-2.
Methods
The study included 252 inpatients with a SARS-CoV-2 infection hospitalized between August 2020 and April 2021. Two groups were generated based on disease survival (survival group: n = 199; deceased group: n = 53). Plasma concentrations of tryptophan, kynurenine and interleukin-6 (IL-6) were measured on admission. In a subset of patients (n = 105; 81 survivors and 24 deceased) concentrations of tryptophan and kynurenine were checked 7 days after admission. The kynurenine/tryptophan ratio (TRP/KYN ratio) was calculated.
Results
On admission, the deceased group showed significantly higher concentrations of kynurenine and a significantly higher KYN/TRP ratio compared to the survival group (p-values < 0.001). Kynurenine and the KYN/TRP ratio significantly correlated with IL-6 (ρ = 0.441 and 0.448, p-values < 0.001). In the survival group, kynurenine and the KYN/TRPratio were significantly lower after seven days (p-values < 0.001). In the deceased group, no significant differences were found between the measurements.
Conclusion
Kynurenine and the KYN/TRP ratio are potentially useful parameters in predicting the survival outcome in SARS-CoV-2 positive patients.
Keywords: Tryptophan, Kynurenine, SARS-CoV-2
1. Introduction
The clinical presentation of patients infected with SARS-CoV-2 is highly variable. There is a broad range from asymptomatic infections to severe cases leading to death [1]. The immune system plays a crucial role in the pathogenesis of SARS-CoV-2 infections [1]. Immune dysregulation is a major factor associated with a poor outcome in SARS-CoV-2 infected subjects [1]. Hence, biomarkers reflecting the shift of the immune system towards imbalance and inflammation can contribute to the prognostic evaluation of SARS-CoV-2 infected patients and eventually influence clinical decision-making.
The main degradation pathway of the essential amino acid tryptophan leads to the production of kynurenins, molecules involved in immune reactions and inflammation [2]. The central catabolic reaction in the kynurenine pathway is the oxidation of tryptophan by the tryptophan 2, 3-dioxygenase (TDO) and the subsequent formation of kynurenine in the liver. Under inflammatory conditions this reaction is enhanced by the induction of the indolamine-pyrrole 2,3-dioxygenase (IDO) [3]. Thus, metabolites of the kynurenine pathway are potential markers of inflammation. In the context of an infection with SARS-CoV-2 they might have the potential of prognostic markers.
Therefore, in this study we aimed to investigate the clinical use of tryptophan and kynurenine in predicting the survival outcome of patients with SARS-CoV-2.
2. Methods
2.1. Study design and population
In this retrospective study, data of 252 inpatients with verified SARS-CoV-2 infection, which were hospitalized during the period of August 2020 to April 2021 at the General Hospital Hochsteiermark (Leoben, Austria), were evaluated. All patients provided their written informed consent. A whole panel of laboratory analyses, among them markers of tryptophan metabolism (tryptophan, kynurenine, KYN/TRP ratio), inflammatory markers (e.g. C-reactive protein (CRP), procalcitonin, interleukin-6 (IL-6)), D-dimer and hematological parameters (e.g. leukocytes, lymphocytes, hemoglobin, platelets), which were performed from blood samples collected on admission and after 7 days, was investigated. Additionally, data about survival and death during hospitalization were evaluated.
This study was approved by the Ethical Committee of the Medical University of Graz (Graz, Austria) (33–634 ex 20/21) and carried out in accordance with the current version of the Declaration of Helsinki.
2.2. Laboratory procedures
Tryptophan and kynurenine were measured in plasma samples by high-performance liquid chromatography (HPLC) with a simultaneous ultraviolet and fluorimetric detection system [4] . Separations were achieved on a Chromolith RP18e column (100 × 4.6 mm, 5 µm, Merck, Darmstadt, Germany) at 30 °C by isocratic elution with a mobile phase (pH 4.9) consisting of 50 mmol/L ammonium acetate, 250 mol/L zinc acetate and 3 % (v/v) acetonitrile, at a flow-rate of 0.8 mL/min. Kynurenine and tryptophan were detected on an Agilent 1200 VWD detector (Agilent, Palo Alto, CA, U.S.A.) at 235 nm. Acquisition and processing of the chromatograms were performed using an Agilent 1200 system equipped with a Chemstation software (Agilent, Palo Alto, CA, U.S.A.). The concentrations were determined as the peak-height measurement against external standards. All reagents were p.A. grade from Merck (Darmstadt, Germany). Intra- and inter-assay coefficients of variation (CVs) for different concentrations of tryptophan and kynurenine were in the range of 0.7% to 9.3%. The tryptophan break down index (=KYN/TRP) was calculated according to the literature [5].
CRP and creatinine (Jaffe) were performed on a cobas c503 and procalcitonin and IL-6 were measured on a cobas e801 (Roche Diagnostics, Rotkreuz, Switzerland) by standard laboratory procedures. D-Dimer was determined on a CS-5100 (Siemens Healthineers, Erlangen, Germany) and the hematological parameters (e.g. leukocytes, lymphocytes, hemoglobin, platelets) were measured on an XN-3000 (Sysmex Corporation, Kobe, Japan).
2.3. Statistical analysis
Normal distribution of the data was examined with the Kolmogorov-Smirnov test. Not normally distributed continuous variables were presented as medians with interquartile ranges (Q1-Q3). Categorical variables were expressed as percentages. The exact Mann-Whitney U test was performed for the comparison between two groups for not normally distributed continuous variables. For subgroup comparisons of categorical parameters Fisher’s exact test was used. Differences of tryptophan and kynurenine concentrations between hospital admission and after 7 days were calculated with the Wilcoxon test. Spearman’s rho (ρ) was performed to assess correlations between parameters of tryptophan metabolism and IL-6. A p-value < 0.05 was considered statistically significant. The analyses were performed using SPSS 26.0 statistical software (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Patients characteristics
A total of 252 inpatients with SARS-CoV-2 infection were included in this study. They all presented a positive SARS-CoV-2 PCR result. During hospitalization, 53/252 (21.0%) died, whereas 199/252 (79.0%) were discharged from our hospital after convalescence. The baseline characteristics of the study population are shown in Table 1 . The median age was 76 (range: 24–97) and 109 individuals were female (43.2%). In the deceased group, the median (Q1-Q3) age was significantly higher [82 (75–89) vs 74 (64–82)] compared to the survival group (p < 0.001). Deceased patients showed higher frequencies of intensive care unit (ICU) admittances (28.3 vs 16.6%, p = 0.054) and intubation (22.6 vs 5.5%, p < 0.001).
Table 1.
Basic characteristics and laboratory parameters of the study cohort.
|
SARS-CoV-2 positive Total (n = 252) |
SARS-CoV-2 positive Survived (n = 199) |
SARS-CoV-2 positive Died (n = 53) |
p-value | |
|---|---|---|---|---|
| Basic characteristics | ||||
| Age, years | 76 (65–83) | 74 (64–82) | 82 (75–89) | < 0.001 |
| Sex, female/male | 109/143 | 81/118 | 28/25 | 0.113 |
| ICU | 48 (19.0%) | 33 (16.6%) | 15 (28.3%) | 0.054 |
| Intubation | 23 (9.1%) | 11 (5.5%) | 12 (22.6%) | < 0.001 |
| Biochemical parameters | ||||
| Tryptophan, µmol/L on admission | 45.9 (39.5–53.0) (n = 249) | 46.9 (39.5–53.6) (n = 196) | 44.4 (39.8–52.4) | 0.436 |
| Tryptophan, µmol/L after 7 days | 46.5 (38.7–60.4) (n = 106) | 46.9 (38.9–60.9) (n = 82) | 46.0 (38.7–60.0) (n = 24) | 0.991 |
| Kynurenine, µmol/L on admission | 4.95 (3.65–6.58) | 4.57 (3.54–5.90) | 5.91 (5.12–7.85) | < 0.001 |
| Kynurenine, µmol/L after 7 days | 4.46 (3.39–6.08) (n = 106) | 4.31 (3.36–5.48) (n = 82) | 6.04 (4.34–9.11) (n = 24) | 0.003 |
| KYN/TRP ratio on admission | 10.4 (7.8–15.2) (n = 249) | 9.7 (7.3–14.5) (n = 196) | 13.9 (10.3–20.5) | < 0.001 |
| KYN/TRP ratio after 7 days | 10.7 (6.7–15.3) (n = 106) | 9.4 (6.2–13.5) (n = 82) | 13.5 (9.3–17.9) (n = 24) | 0.003 |
| CRP, mg/L | 59.0 (25.5–104.0) (n = 250) | 52.4 (21.0–96.4) (n = 198) | 86.8 (39.8–137.5) (n = 52) | 0.001 |
| Procalcitonin, ng/mL | 0.12 (0.06–0.24) (n = 183) | 0.1 (0.05–0.17) (n = 141) | 0.23 (0.14–0.55) (n = 42) | < 0,001 |
| Interleukin-6, ng/mL | 49.7 (24.3–109.0) (n = 171) | 42.2 (19.4–81.6) (n = 134) | 110.0 (56.0–171.0) (n = 37) | < 0,001 |
| Creatinine, µmol/L | 90.2 (74.3–116.7) (n = 251) | 89.2 (72.5–107.9) | 98.1 (79.6–148.5) (n = 52) | 0.011 |
| D-Dimer, mg/L | 0.94 (0.66–1.98) (n = 160) | 0.86 (0.57–1.82) (n = 126) | 1.73 (0.77–3.54) (n = 34) | 0.002 |
| Hematological parameters | ||||
| Leukocytes, x109/L | 6.9 (4.8–9.2) | 6.6 (4.7–8.8) | 7.9 (5.6–11.5) | 0.016 |
| Lymphocytes, x109/L | 0.93 (0.63–1.26) (n = 194) | 0.95 (0.68–1.28) (n = 157) | 0.74 (0.49–1.20) (n = 37) | 0.044 |
| Hemoglobin, g/L | 133 (119–147) | 135 (120–148) | 130 (109–141) | 0.032 |
| Platelets, x109/L | 197 (144–249) | 195 (144–247) | 205 (143–268) | 0.475 |
Data are presented as medians (interquartile range: Q1-Q3) or n (%). For subgroup comparisons of non-normally distributed metric or categorical variables the exact Mann-Whitney U test or the Fisher‘s exact test were used. A p-value < 0.05 was considered statistically significant. ICU, intensive care unit; KYN, kynurenine; TRP, tryptophan; CRP, C-reactive protein.
As shown in Table 1, the 53 patients, who died during hospitalization, had significantly higher plasma CRP, PCT and IL-6 levels compared to the 199 survived individuals (all p-values < 0.05). Creatinine, D-dimer, leukocytes, lymphocytes, and hemoglobin also differed significantly between these two groups (all p-values < 0.05).
3.2. Tryptophan metabolism
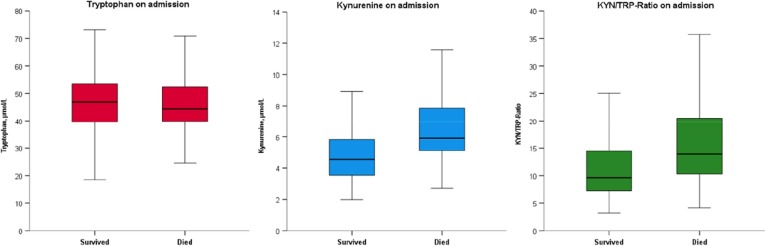
As presented in Table 1 and Fig. 1 A, no statistically significant difference of serum tryptophan levels could be found between the deceased and survival groups on admission. However, the kynurenine concentrations (5.91 (5.12–7.85) vs 4.57 (3.54–5.90), p < 0.001) and the KYN/TRP ratios (13.94 (10.29–20.47) vs 9.65 (7.26–14.48), p < 0.001) were significantly higher in the deceased group compared to the survival group (Table 1, Fig. 1B and 1C). As shown in Fig. 2 B and 2C, kynurenine and the KYN/TRP ratio showed a positive correlation with IL-6 (ρ = 0.441 and ρ = 0.448, p-values < 0.001).
Fig. 1.
(A) Comparison of plasma tryptophan concentrations (p = 0.436), (B) kynurenine concentrations (p < 0.001) and the (C) KYN/TRP ratios (p < 0.001) between survived (n = 199) and deceased (n = 53) patients with SARS-CoV-2 infection on admission.
Fig. 2.
Correlations between Interleukin-6 and tryptophan (ρ = −0.091, p = 0.241), kynurenine (ρ = 0.441, p < 0.001) and the KYN/TRP ratio (ρ = 0.448, p < 0.001).
From overall 105 patients (81 and 24 from the survival and deceased group), tryptophan and kynurenine levels were available on admission and 7 days after admission (Table 2 and Fig. 3 ). In the deceased group, no significant differences of tryptophan, kynurenine and KYN/TRP ratios were found between the two measurement points. In the survival group median kynurenine concentrations (4.3 (3.36–5.4) vs 5.39 (3.91–6.87), p < 0.001) and the KYN/TRP ratios (9.15 (6.20–13.16) vs 11.82 (8.54–17.11), p < 0.001) were significantly lower after 7 days compared to the measurements on admission. Tryptophan was observed significantly higher (46.78 (38.92–60.39) vs 44.47(37.30–51.81, p = 0.008) after 7 days.
Table 2.
Tryptophan, kynurenine and the KYN/TRP-ratios on admission and after 7 days.
| On admission | After 7 days | Mean Δ | p-value | ||
|---|---|---|---|---|---|
| Survived (n = 81) | |||||
| Tryptophan (µmol/L) | 44.5 (37.3–51.8) | 46.8 (38.9–60.4) | 4.58 (±17.08) | 0.008 | |
| Kynurenine (µmol/L) | 5.39 (3.91–6.87) | 4.30 (3.36–5.40) | −1.00 (±2.54) | < 0.001 | |
| KYN/TRP ratio | 11.8 (8.5–17.1) | 9.2 (6.2–13.2) | −3.73 (±10.90) | < 0.001 | |
| Died (n = 24) | |||||
| Tryptophan (µmol/L) | 46.5 (38.0–52.5) | 46.0 (38.7–60.0) | 0.45 (±34.80) | 0.864 | |
| Kynurenine (µmol/L) | 5.60 (4.21–6.95) | 6.04 (4.34–9.11) | 1.22 (±3.25) | 0.170 | |
| KYN/TRP ratio | 11.7 (8.7–15.5) | 13.5 (9.3–17.9) | 1.01 (±7.51) | 0.909 |
Data are presented as medians (IQR) or mean (±standard deviation). Differences of median concentrations between admission and after 7 days were calculated with the Wilcoxon test. A p-value < 0.05 was considered statistically significant.
Fig. 3.
Medians of tryptophan, kynurenine and the KYN/TRP ratio on admission and after 7 days separated by deceased patients (red line, n = 24) and survivors (blue line, n = 81).
4. Discussion
In the present study, we investigated the relationship between the clinical outcome (199 survived subjects vs. 53 deceased subjects) and the plasma concentrations of tryptophan and kynurenine in 252 SARS-CoV-2 positive inpatients. Patients, who died during hospitalization, were found with lower tryptophan but significantly higher kynurenine concentrations and significantly higher KYN/TRP ratios compared to individuals, who survived. Moreover, there was a significant difference in the course of the plasma concentrations of kynurenine over one week between the two groups.
The activation of the kynurenine pathway in SARS-CoV-2 positive patients was highlighted in recent articles. In the study of Lawler et al. indicators of the tryptophan metabolism (tryptophan, kynurenine, quinolinic acid, 3-hydroxykynurenine, kynurenic acid, xanthurenic acid, 3-hydroxyanthranilic acid) were evaluated in 10 SARS-CoV-2 positive subjects [6]. Compared to 49 SARS-CoV-2 negative subjects, tryptophan was significantly lower, whereas kynurenine, 3-hydroxykynurenine and quinolinic acid were significantly higher in the SARS-CoV-2-positive group [6]. In their metabolomic study, Thomas et al. illustrate the profound alteration of the kynurenine pathway in patients with SARS-CoV-2 infection [7]. The group of 33 SARS-CoV-2 positive subjects showed a significant decrease of tryptophan and increases of kynurenine, kynurenic acid and picolinic acid [7]. Similar findings were reported in other recent publications [8], [9], [10]. Lionetto et al. demonstrated the association between SARS-CoV-2 positivity and an elevated KYN/TRP ratio, including 89 SARS-CoV-2 positive patients, 305 SARS-CoV-2-negative hospitalized patients and 239 healthy controls [11]. The KYN/TRP ratio was significantly higher in the positive group compared to both other groups [11]. In addition, the SARS-CoV-2 positive patients were allocated in groups according to the disease severity [11]. Deceased patients and patients with a > 3-week stay in the ICU were observed with significantly higher KYN/TRP ratios compared to patients without the need of intensive care treatment [11].
These results are in line with our findings. In our cohort of SARS-CoV-2 positive patients, the KYN/TRP ratio of deceased patients was significantly higher compared to the survivors. This is indicative for a relationship between the survival-outcome of the disease and the plasma levels of tryptophan and kynurenine. Accordingly, a strong association between the disease severity and the altered kynurenine pathway indicators is corroborated by Marin-Corral et al. [12]. This study group found an increase of the KYN/TRP ratio with the severity of the COVID-19 disease among 49 SARS-CoV-2 positive patients [12]. Considering the consistent findings of our study and the current literature, a robust activation of the kynurenine pathway in SARS-CoV-2- positive patients is hypothesized. Furthermore, the association between the disease outcome and the plasma levels of kynurenine pathway metabolites demonstrates that indicators of tryptophan metabolism, especially kynurenine, may have the potential as prognostic biomarkers in individuals with SARS-CoV-2 infection.
Here, we found a positive correlation between kynurenine and the KYN/TRP ratio with IL-6. This is in accordance with a previous study from Thomas et al. [7], who found tryptophan to be negatively correlated with IL-6. IL-6 is a marker of inflammation and the main trigger of the indolamin-2,3-dioxygenase (IDO) expression. IDO is the key enzyme of the kynurenine pathway. It catalyzes the metabolization of tryptophan to N-formyl-kynurenine, which is further metabolized to kynurenine. Thus, the activation of IDO by IL-6 leads to high kynurenine levels and an enhancement of the KYN/TRP ratio.
In addition to tryptophan and kynurenine measurements at the time of admission, we assessed the course of these parameters and of the KYN/TRP ratio during one week. We included all patients from the total study cohort, who were measured for tryptophan and kynurenine on admission and after one week of hospitalization. On admission, no significant difference of the KYN/TRP ratio between the deceased (n = 24) and the survival (n = 81) group was observed. However, after one week they diverged, primarily due to a significant decrease of kynurenine in the survival group, indicating recovery from disease. In contrast, the deceased group showed a significant enhancement of the median kynurenine level, which may be a sign of worsening disease and poor outcome.
The major strength of this study is that for the first time longitudinal data of kynurenine and tryptophan measurements in hospitalized individuals with SARS-CoV-2 infection were evaluated.
Nevertheless, some limitations of this study must be considered. Due to the retrospective study design, only the routinely measured parameters were available for statistical analysis. One week after admission, not all study parameters (e.g. IL-6) were available for all patients. Furthermore, we had no information about how long the patients were symptomatic or if they already had been tested positive before admission. Therefore, it might be possible, that they were in slightly different stages of the COVID-19 disease. Another source of bias may be the parenteral substitution of amino acids in ICU patients, especially at the measurement one week after admission. We don’t have knowledge about the exact nutritional program of the included ICU patients. Hence, we cannot rule out an influence on the measurements of the tryptophan metabolites, although the effect might be supposedly weaker on the KYN/TRP ratio. In this study, we focused on the role of tryptophan and kynurenine in SARS-CoV-2 positive subjects. For a more comprehensive evaluation of the kynurenine pathway in this patient setting, the inclusion of other tryptophan metabolites (e.g. kynurenic acid, quinolinic acid, 3-hydroxykynurenine) should be considered in future study designs.
5. Conclusion
This study demonstrated an association between indicators of tryptophan metabolism, especially kynurenine and the KYN/TRP ratio, and the survival-outcome of hospitalized patients with a SARS-CoV-2 infection. In deceased SARS-CoV-2 positive subjects, the concentrations of kynurenine and the KYN/TRP ratio showed a different course during one week compared to patients, who survived the disease. Our findings suggest that kynurenine and the KYN/TRP ratio have the potential to predict the clinical outcome of SARS-CoV-2 patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.García L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front. Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cervenka I., Agudelo L.Z., Kynurenines R.JL. Tryptophan's metabolites in exercise, inflammation, and mental health. Science. 2017 doi: 10.1126/science.aaf9794. 357(6349):eaaf9794. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q., Liu D., Song P., Zou M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. (Landmark Ed). 2015;20:1116–1143. doi: 10.2741/4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hervé C., Beyne P., Jamault H., Delacoux E. Determination of tryptophan and its kynurenine pathway metabolites in human serum by high-performance liquid chromatography with simultaneous ultraviolet and fluorimetric detection. J. Chromatogr. B Biomed. Appl. 1996;675(1):157–161. doi: 10.1016/0378-4347(95)00341-X. [DOI] [PubMed] [Google Scholar]
- 5.Myint A.M., Kim Y.K., Verkerk R., Park S.H., Scharpé S., Steinbusch H.W., et al. Tryptophan breakdown pathway in bipolar mania. J. Affect. Disord. 2007;102(1–3):65–72. doi: 10.1016/j.jad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Lawler N.G., Gray N., Kimhofer T., Boughton B., Gay M., Yang R., et al. Systemic perturbations in amine and kynurenine metabolism associated with acute SARS-CoV-2 infection and inflammatory cytokine responses. J. Proteome Res. 2021;20(5):2796–2811. doi: 10.1021/acs.jproteome.1c00052. [DOI] [PubMed] [Google Scholar]
- 7.Thomas T., Stefanoni D., Reisz J.A., Nemkov T., Bertolone L., Francis R.O., et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5(14) doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimhofer T., Lodge S., Whiley L., Gray N., Loo R.L., Lawler N.G., et al. Integrative modeling of quantitative plasma lipoprotein, metabolic, and amino acid data reveals a multiorgan pathological signature of SARS-CoV-2 infection. J. Proteome Res. 2020;19(11):4442–4454. doi: 10.1021/acs.jproteome.0c00519. [DOI] [PubMed] [Google Scholar]
- 9.López-Hernández Y., Monárrez-Espino J., Oostdam A.H., Delgado J.E.C., Zhang L., Zheng J., et al. Targeted metabolomics identifies high performing diagnostic and prognostic biomarkers for COVID-19. Sci. Rep. 2021;11(1):14732. doi: 10.1038/s41598-021-94171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danlos F.X., Grajeda-Iglesias C., Durand S., Sauvat A., Roumier M., Cantin D., et al. Metabolomic analyses of COVID-19 patients unravel stage-dependent and prognostic biomarkers. Cell Death Dis. 2021;12(3):258. doi: 10.1038/s41419-021-03540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lionetto L., Ulivieri M., Capi M., De Bernardini D., Fazio F., Petrucca A., et al. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: An observational cohort study. Biochim. Biophys. Acta, Mol. Basis Dis. 2021;1867(3) doi: 10.1016/j.bbadis.2020.166042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marín-Corral J., Rodríguez-Morató J., Gomez-Gomez A., Pascual-Guardia S., Muñoz-Bermúdez R., Salazar-Degracia A., et al. Metabolic signatures associated with severity in hospitalized COVID-19 patients. Int. J. Mol. Sci. 2021;22(9):4794. doi: 10.3390/ijms22094794. [DOI] [PMC free article] [PubMed] [Google Scholar]