Abstract
Background
Following an outbreak of coronavirus disease 2019 (COVID-19) on the cruise ship Diamond Princess, passengers and crew were followed-up to determine prognosis. This study examined the epidemiological determinants of COVID-19 natural history using these follow-up data.
Methods
Infection status, diagnosis, clinical symptoms and prognosis were analysed for all passengers and crew members on the Diamond Princess. In addition, the risk of infection associated with exposure within cabin rooms, as well as the risks of various clinical manifestations of disease, along with their epidemiological determinants, were analysed.
Results
The adjusted odds ratio (aOR) of infection for individuals tested by polymerase chain reaction on or after 12 February 2020 compared with individuals tested before this date was 0.53 [95% confidence interval (CI) 0.39–0.72], reflecting decreased transmission during onboard quarantine. Among infected individuals, older age was associated with elevated odds of symptomatic illness (aOR 1.01, 95% CI 1.00–1.02), severe disease (aOR 1.08, 95% CI 1.05–1.12) and death (aOR 1.12, 95% CI 1.05–1.21).
Conclusions
Severe COVID-19 disease, death and symptomatic illness were more frequent among older individuals on the Diamond Princess. Older elderly cases (age ≥80 years) had the highest risks of severe disease and death. Inter-room transmission was prevented successfully by the onboard quarantine.
Keywords: Coronavirus disease 2019 (COVID-19), Natural history, Secondary transmission, Secondary attack rate, Asymptomatic ratio, Case fatality ratio
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has engulfed all regions of the world very swiftly, and has yet to be brought under control in many nations (World Health Organization, 2020). Difficulties in controlling the pandemic may be related to the natural history of infection: unlike severe acute respiratory syndrome coronavirus, infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) can be asymptomatic (Ali et al., 2020, Arons et al., 2020, Buitrago-Garcia et al., 2020, Kronbichler et al., 2020, Mizumoto et al., 2020, Nishiura et al., 2020a, Rothe et al., 2020, Sakurai et al., 2020). This point was initially raised by a case report from Germany (Rothe et al., 2020), and at a similar time by an early analysis of publicly available data on the Diamond Princess (Mizumoto et al., 2020), followed by an analysis of traveller data from evacuation flights from Wuhan to Tokyo (Nishiura et al., 2020a). Even in the absence of symptoms, secondary transmission can take place (Du et al., 2020; Nishiura et al., 2020b). Heterogeneity of disease manifestations plays a key role in transmission: the symptoms of younger individuals are often very mild, and their close contact in a confined space often leads to transmission (Nishiura et al., 2020c).
Although several epidemiological studies of the natural history of COVID-19 have been published, risk factors for some of the key variables in the disease course have yet to be identified. For instance, while the asymptomatic ratio has been estimated to be approximately 20–30% of infections (Ali et al., 2020, Arons et al., 2020, Buitrago-Garcia et al., 2020, Kronbichler et al., 2020, Mizumoto et al., 2020, Nishiura et al., 2020a, Rothe et al., 2020, Sakurai et al., 2020), the clinical determinants of asymptomatic infection have yet to be explored. Although risk factors for COVID-19-associated mortality, including age and underlying chronic diseases, have been identified (Ruan, 2020; Verity et al., 2020; Zhou et al., 2020), the predictors of severe disease manifestations are not yet fully understood. Moreover, epidemiological studies aiming to better characterize heterogeneous COVID-19 outcomes have not yet quantified the unconditional risk of transmission (e.g. secondary attack rate measured in confined spaces, such as households). These elements of disease heterogeneity can be clarified only by observing transmission and disease course over time in a substantial number of exposed and infected individuals of different ages.
The Japanese experience of an outbreak on the cruise ship Diamond Princess, starting on 3 February 2020, offers an interesting opportunity to study the natural history of COVID-19 (Field Briefing: Diamond Princess COVID-19 Cases, 20 Feb Update, 2020; Kakimoto et al., 2020). During the outbreak, transmission chains occurred in a confined space, with all passengers forced to remain in their cabins as part of an onboard quarantine. The presence of symptoms was explored consistently for all 3711 persons onboard, including 2666 passengers and 1045 crew members, and all underwent polymerase chain reaction (PCR) testing when symptoms were observed. Moreover, transmission events in cabin rooms shared with infected persons were recorded over time.
The present study aimed to examine the epidemiological determinants of the heterogeneous natural history of COVID-19 using data collected from the passengers and crew of the Diamond Princess. Differential risks of in-room secondary transmission, symptom development, severe manifestations and death were explored. The frequency of in-room secondary transmission frequency was used to assess the effectiveness of the onboard quarantine.
Methods
Diamond Princess data
As described elsewhere (Expert Taskforce for the COVID-19 Cruise Ship Outbreak, 2020; Field Briefing: Diamond Princess COVID-19 Cases, 20 Feb Update, 2020; Kakimoto et al., 2020), the outbreak on Diamond Princess was recognized at sea by 3 February 2020. All passengers were strictly required to remain in their cabins for a period of 14 days starting on 5 February 2020. Disembarkation of passengers occurred from 19 February to 23 February 2020 and crew members disembarked by 1 March 2020. During the onboard quarantine, daily health check-ups were conducted and any individuals with symptoms (including fever or cough) were tested by PCR. Individuals whose cabinmates tested positive for SARS-CoV-2 were tested irrespective of symptoms. All crew members underwent PCR testing during disembarkation irrespective of symptoms. When a diagnosis was confirmed, cases were isolated in inland hospitals near Yokohama or in distant prefectures including Aichi and Fukushima. PCR-negative symptomatic passengers were tested repeatedly during the precautionary isolation period. In addition to onboard recording of data, hospitalized cases were followed-up to record prognosis. When individuals recovered or died, follow-up was completed.
Infection status, diagnosis, symptoms and prognosis were analysed for all passengers and crew on the Diamond Princess, including a follow-up survey of prognosis until 8 June 2020. The original data collected by the Ministry of Health, Labour and Welfare included age, sex, nationality, PCR test results, symptoms, disease severity and prognosis. Cabin information (e.g. presence of windows and ventilation) was also collected. Comorbidity data were only partially available via self-reported medication information in cabin health records. These data were disregarded in this study. Nationality was categorized as Asian or other because Asians dominated both the passengers and crew.
Outcomes and explanatory variables
Exploiting the unique features of the Diamond Princess outbreak (i.e. complete observations on passengers and crew in whom infection and symptoms were identified explicitly), two different types of risk were examined: (i) the risk of infection from in-cabin exposure; and (ii) the risk of disease among PCR-positive infected individuals. The latter category involved multiple risks, including risk of symptomatic infection, risk of severe disease and risk of death. These risks were quantitated, and potential factors associated with outcomes were explored, as discussed elsewhere (Gandhi et al., 2020).
Risk of infection in exposed individuals
Among the 2666 passengers housed in 1344 cabins during the quarantine, this study focused on cabins containing at least one guest with PCR-confirmed infection, designated as the index case in that cabin. The secondary attack rate was calculated for cabinmates. Single rooms and rooms without confirmed cases were excluded from the analysis. Four passengers without PCR results were also excluded from the analysis. When there were two people (Persons 1 and 2) infected in the same cabin, there were three possible interpretations: (i) Person 1 was the index case and transmitted the virus to Person 2; (ii) Persons 1 and 2 acquired infection outside the cabin simultaneously (co-primary cases); or (iii) Persons 1 and 2 acquired infection outside the cabin independently (Bailey, 1956; Klinkenberg and Nishiura, 2011). Considering the COVID-19 serial interval distribution (Ali et al., 2020; Prete et al., 2020), PCR-confirmed infections among cabinmates that occurred ≥4 days apart were considered to reflect Interpretation (i). All PCR-negative passengers whose cabinmates were positive were considered as controls.
Risks of symptomatic illness, severe disease and death among infected individuals
When considering the risks of symptomatic illness, severe disease and death among infected individuals, this study focused on all PCR-positive passengers and crew. Of 721 cases who tested positive onboard, 686 cases were fully followed-up. The remaining 35 cases were evacuated to Japan immediately and were lost to follow-up. Among these 686 cases, nine cases whose symptoms were not fully recorded were excluded. Of the remaining 677 cases, 300 remained asymptomatic. Of the remaining 377 cases, 48 experienced severe disease and 13 died.
Statistical analysis
All risks were derived from binomial distributions, and their 95% confidence intervals (CIs) were calculated from Agresti's score interval. In multi-variable logistic regression analyses, explanatory variables for secondary attack rate included age, sex, date of PCR (before or on/after 12 February 2020) and the presence of windows in the cabin. Date of PCR was dichotomized because the onboard quarantine started on 5 February 2020, and the mean delay from infection to diagnosis was estimated as 7 days (i.e. the mean incubation period of 5 days plus a 2-day lag from symptom onset to diagnosis). With regard to risks associated with the clinical spectrum of disease, explanatory variables included age, sex and nationality (Asian or other). Nationality was included because the epidemic in the Western Pacific region has been less intensive compared with Western countries (Kayano and Nishiura, 2020; Kenyon, 2020; Yamamoto and Bauer, 2020).
Results
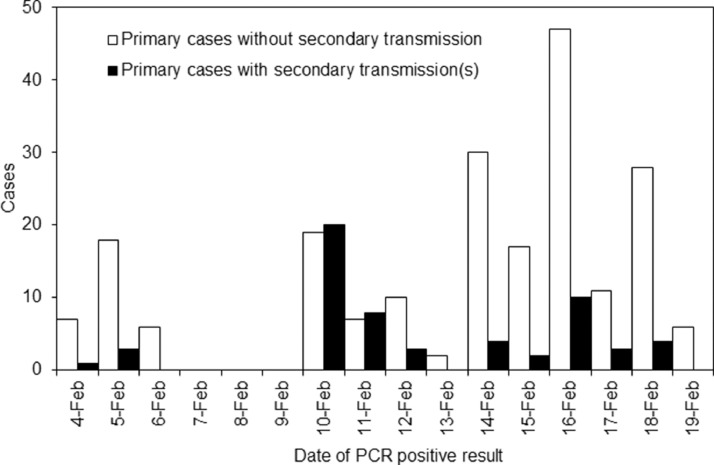
In total, 266 passengers were exposed to SARS-CoV-2 within their cabins, of whom 58 (21.8%) were infected (PCR positive). Eighty-nine and 177 exposed passengers were tested by PCR before and on/after 12 February 2020, respectively (Figure 1 ), of whom 32 (36.0%) and 26 (14.7%), respectively, were infected. The average age of passenger cases was 64.7 years, with lower and upper quartile boundaries of 62 and 74 years, respectively. Men accounted for 40.2% of passenger cases, and sex was not associated with in-room transmission (P=0.79). The univariate odds ratio (OR) of secondary transmission on or after 12 February 2020 was 0.41 (95% CI 0.26–0.64) compared with before this date. Multi-variable analysis also identified PCR testing on or after 12 February 2020 as an explanatory variable with an adjusted OR (aOR) of 0.53 (95% CI 0.39–0.72) (Table 1 ). Older age of exposed individuals was marginally associated with increased odds of infection (aOR 1.02, 95% CI 1.00–1.05; P=0.08).
Figure 1.
Date of confirmation of primary cases among cabinmates and secondary transmission on Diamond Princess. Numbers of individuals with infected cabinmates are shown according to the date of the first positive polymerase chain reaction (PCR) result for the primary case (n=266); primary cases were classified as with or without secondary transmission events. Following the onboard quarantine starting on 5 February 2020, and assuming a mean incubation period of 5 days and a lag from illness onset to diagnosis of 2 days, the curve indicates that the proportion of exposures within cabins resulting in secondary transmission was greatly reduced by the quarantine.
Table 1.
Multi-variable logistic regression model describing the risk of infection among exposed individuals with infected cabinmates (n=266).
| Adjusted OR (95% CI) | P-value | |
|---|---|---|
| Age (years) | 1.02 (1.00–1.05) | 0.08 |
| Male sex | 1.04 (0.76–1.42) | 0.79 |
| Primary case tested PCR positive on or after 12 February 2020 | 0.53 (0.39–0.72) | <0.01a |
| Windows in cabins | 1.25 (0.88–1.84) | 0.22 |
OR, odds ratio; CI, confidence interval; PCR, polymerase chain reaction; AIC, Akaike's information criterion.aSignificant association. The effect of age was modelled per 1-year increase. R2U=0.072, AICc=269.
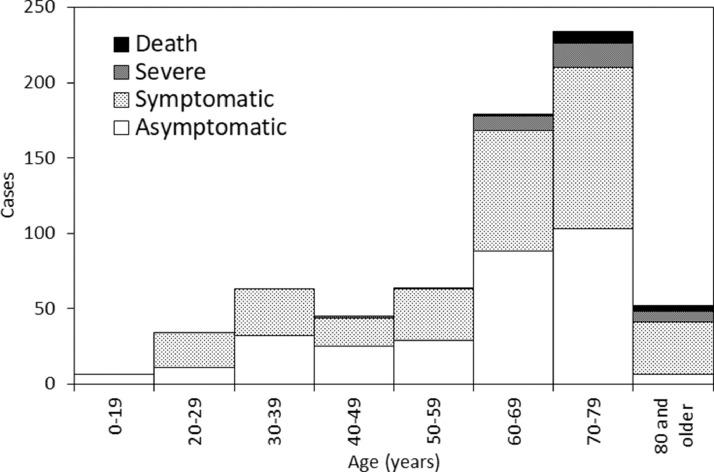
Figure 2 shows the age distribution of 677 PCR-positive individuals. Of these individuals, 377 (55.7%) were symptomatic, 48 (7.1%) had severe disease and 13 (1.9%) died. The proportion of symptomatic infected individuals was highest among cases aged ≥80 years (88.5%). Severe disease was observed most frequently among cases aged ≥80 years (21.1%), followed by cases in their 70s (10.3%) and 60s (6.1%). No cases aged <40 years experienced severe disease. Deaths were most common among cases aged ≥80 years (7.7%), followed by cases in their 70s (3.4%) and 60s (0.6%). No cases aged <60 years died. The infection fatality risk (IFR) among cases aged ≥70 years was estimated at 4.2% (95% CI 2.4–7.2).
Figure 2.
Age distribution of confirmed coronavirus disease 2019 (COVID-19) cases on the Diamond Princess. Of 721 confirmed cases, 677 were eligible and included in the analysis. Severity and presence of symptoms represented the worst state for each case. For instance, if an infected individual was diagnosed during the asymptomatic phase and later developed illness but recovered without complications, the case was classified as ‘symptomatic’. If a case was diagnosed as symptomatic and later developed severe illness but eventually recovered, the case was classified as ‘severe’.
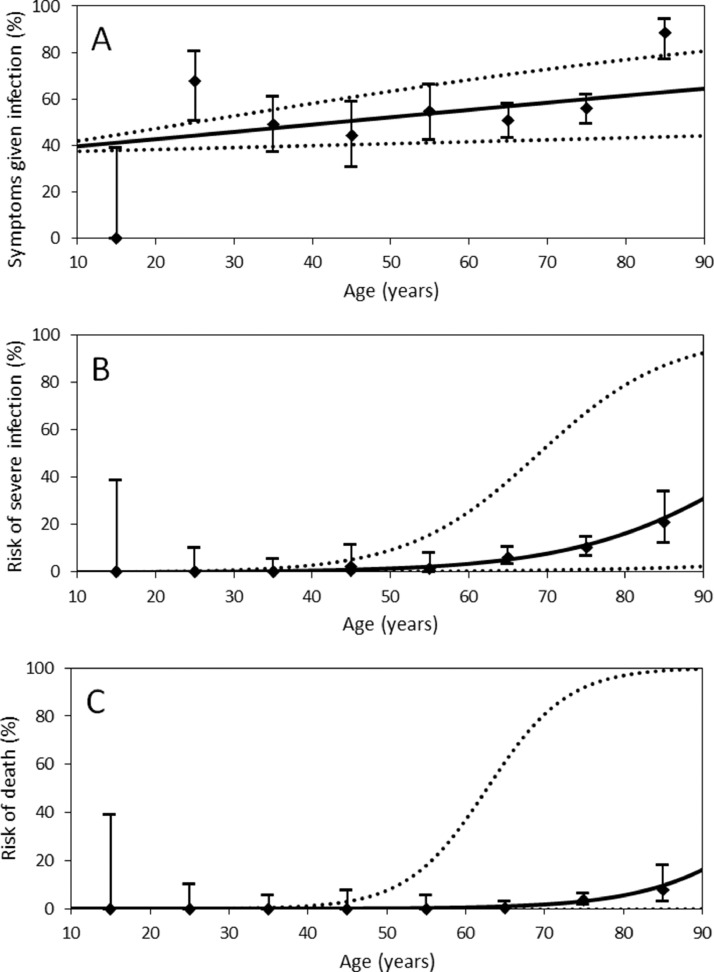
Figure 3 shows the risks of symptomatic infection, severe disease and death in each age group superimposed on the regression line. As summarized in Table 2 , multi-variable logistic regression showed that older age was significantly associated with symptomatic infection (aOR 1.01, 95% CI 1.00–1.02). Severe disease was more frequently observed in men (aOR 1.85, 95% CI 1.32–2.69) and older cases (aOR 1.08, 95% CI 1.05–1.12). Older age was significantly associated with risk of death (aOR 1.12, 95% CI 1.05–1.21).
Figure 3.
Age dependence in the natural history of coronavirus disease 2019 (COVID-19) infection (n=677). Age-dependent variation was examined with respect to (A) symptomatic illness, (B) risk of severe disease and (C) risk of death among infected individuals. Black diamonds show sample estimates plotted at the mid-point age of each age group (except for cases aged 0–19 years and for those aged ≥80 years, which were plotted at 15 and 85 years, respectively). Whiskers indicate the boundaries of the 95% confidence intervals, which were derived from Agresti's score interval. Solid curve lines show the risk of death based on univariate logistic regression taking age as a continuous explanatory variable. Dashed lines show the upper and lower 95% confidence intervals of the risks and were derived from the bootstrap method.
Table 2.
Multi-variable logistic regression models describing the risks of symptomatic illness, severe disease and death in infected individuals (n=677).
| Adjusted OR (95% CI) | P-value | |
|---|---|---|
| Symptomatic illness | ||
| Age (years) | 1.01 (1.00–1.02) | <0.01a |
| Male sex | 1.09 (0.94–1.27) | 0.27 |
| Nationality (Asian) | 1.10 (0.93–1.29) | 0.28 |
| Severe disease | ||
| Age (years) | 1.08 (1.05–1.12) | <0.01a |
| Male sex | 1.85 (1.32–2.69) | <0.01a |
| Nationality (Asian) | 0.86 (0.63–1.19) | 0.34 |
| Death | ||
| Age (years) | 1.12 (1.05–1.21) | <0.01a |
| Male sex | 1.74 (0.95–3.72) | 0.10 |
| Nationality (Asian) | 1.30 (0.70–2.78) | 0.44 |
OR, odds ratio; CI, confidence interval; AIC, Akaike's information criterion.
Significant association. The effect of age was modelled per 1-year increase. Male is compared with female. Model 1 (risk of symptomatic illness): R2U=0.011, AICc=928; Model 2 (risk of severe disease): R2U=0.146, AICc=304; Model 3 (risk of death): R2U=0.152, AICc=117.
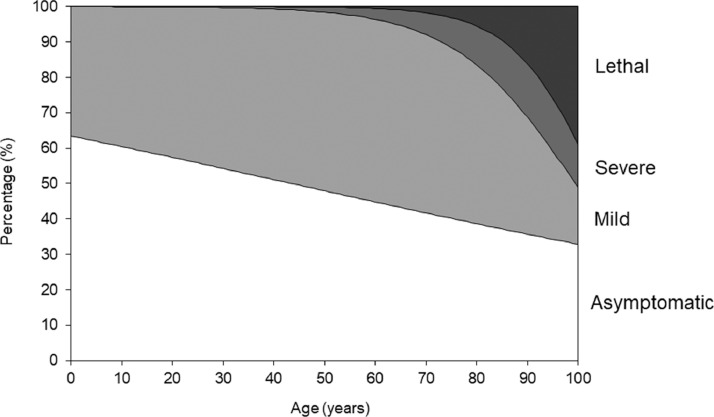
Using parameter estimates from logistic regression models, age-specific risks of symptomatic illness, severe disease and death were quantified to describe the natural course of infection (Figure 4 ). Young adults seldom experienced severe disease, and risk of symptomatic illness decreased in younger cases. The risks of severe disease, death and symptomatic illness increased with advancing age. Elderly individuals (age ≥80 years) had the highest risks of severe disease and death.
Figure 4.
Clinical manifestations and outcomes of coronavirus disease 2019 (COVID-19) as a function of age. Age dependence of clinical manifestations is summarized. Areas were drawn according to the best fit solutions of logistic regression models.
Discussion
This study explored the risks of secondary transmission and spectrum of COVID-19 manifestations and outcomes among passengers and crew on the Diamond Princess. The analysis showed that in-room secondary transmission occurred more frequently prior to the onboard quarantine, and older age of exposed persons was marginally associated with increased risk of infection (P=0.08). Follow-up of passengers and crew for 4 months after the end of quarantine clearly showed that age was the most important predictor of clinical course of infection. Older elderly cases tended to have greater risks of symptomatic illness, severe disease and death than cases of other ages, consistent with previous studies (Ruan, 2020; Verity et al., 2020). Due to the completeness of follow-up, it was concluded that the IFR among cases aged ≥70 years was 4.2%; a preliminary estimate based on the same publicly available data was 6.4% (Russell et al., 2020).
Two important take-home messages emerged from this study. First, the onboard quarantine restricting movement outside of cabins reduced the risk of infection by approximately 50% among passengers whose cabinmates had PCR-confirmed COVID-19. During the quarantine period, passengers were instructed to remain in their own cabins. All exposed individuals had been in contact with infected cabinmates by the time of PCR testing, and the results indicate that inter-room transmission was prevented successfully during the quarantine period. Second, it was possible to quantify the epidemiological determinants of clinical course of infection, especially as a function of age. As well as severe disease and death, symptomatic illness was found to be more common among older individuals. Older elderly individuals (age ≥80 years) had the highest risks of severe disease and death. Although the effect size (aOR) of elderly patients with regard to symptomatic illness, severe disease and death were 1.01, 1.08 and 1.12, respectively, these values represent the increment of risks with every single age, and the resulting increment for older elderly individuals tends to be very large.
Some of the negative results of this study are also informative. The presence of a window in cabins was not associated with risk of infection among exposed passengers with infected cabinmates. Thus, in-room airflow was not immediately evident as a major determinant of secondary transmission in situations of close contact. Nationality (Asian or other) was not associated with development of severe disease, despite the fact that cumulative COVID-19 incidence and deaths in Asia, especially in the Western Pacific and Southeast Asia, have been lower than in Europe and the USA (Kayano and Nishiura, 2020; Kenyon, 2020; Yamamoto and Bauer, 2020).
An important characteristic of COVID-19 is its heterogeneous transmissibility, especially in high-risk indoor settings such as pubs, bars and night clubs. This feature has been demonstrated to reduce cumulative incidence at the end of the epidemic (Britton et al., 2020; Diekmann et al., 2010; Gomes et al., 2020; Riou and Althaus, 2020). The present study adds to this body of knowledge by demonstrating that the risks of clinical manifestations are also highly heterogeneous, being highly dependent on age. This exercise yielded systematic age-dependent estimates of these risks, enabling calculation of the cumulative risk of death at the end of the epidemic.
Five important limitations of this study should be acknowledged. First, cases were diagnosed using PCR tests with limited sensitivity. Repeated PCR testing was performed among both symptomatic persons and close contacts of cases during the quarantine and disembarkation periods. Asymptomatic persons underwent PCR testing upon disembarkation alone. Unrecognized infections, especially prior to quarantine, and additional asymptomatic cases could have existed. Second, comorbidity data were incomplete and thus were not considered in this study. Metabolic comorbidities (e.g. obesity, diabetes, chronic obstructive lung disease and hypertension) are predictive of risk of COVID-19 death in hospitals (Abu-Raya, 2020; Zhou et al., 2020). Moreover, steroids and immunosuppressive drugs may reduce fatality rates in patients with severe COVID-19 (Brotherton et al., 2020; Guaraldi et al., 2020), and anti-hypertensive drugs (e.g. angiotensin-converting enzyme inhibitors) may increase the risk of severe outcomes (Cai, 2020). Assuming that elderly passengers on the Diamond Princess represent a wealthier fraction of the general population and were healthy in general when they boarded the ship, the age-dependent estimates could have underestimated the risks of symptomatic illness, severe disease and death. Third, racial differences among passengers and crew members were not considered, and nationality (Asian or other) alone was considered in this study. Fourth, health interviews during the quarantine were complicated by language barriers. However, the Diamond Princess crew were accustomed to resolving language barriers, especially for passengers who do not speak English. Finally, limited data were available on behaviours, compliance (e.g. mask wearing and hand hygiene) and relationships among cabinmates (e.g. spouses, relatives or friends).
Despite these issues and limitations, this study offers critically relevant estimates of parameters underlying the age-dependent natural history of COVID-19. Compared with older individuals, younger cases have smaller risks of symptomatic illness and severe manifestation. Moreover, older elderly cases (age ≥80 years) had a higher risk of death than the younger elderly cases (65–79 years old), and yielded the highest risk of death.
Using the epidemiological data collected during an outbreak of COVID-19 on the cruise ship Diamond Princess, this study found that severe disease, death and symptomatic illness were more common among older individuals. Among older elderly cases, age ≥80 years was associated with the highest risks of severe disease and death. Another finding was that inter-room transmission was prevented successfully by the onboard quarantine.
Acknowledgments
Acknowledgments
The authors wish to thank Mr. Hiroaki Murayama for helpful discussions on data analysis, and Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Conflict of interest statement
None declared.
Funding
T.K. received funding from Japan Society for the Promotion of Science KAKENHI (21K10467) and Fujiwara Foundation. H.N. received funding from the Health and Labour Sciences Research Grant (19HA1003, 20CA2024, 20HA2007 and 21HB1002); Japan Agency for Medical Research and Development (AMED; JP20fk0108140 and JP20fk0108535); the Japan Society for the Promotion of Science KAKENHI (17H04701 and 21H03198); Environment Research and Technology Development Fund (JPMEERF20S11804) of the Environmental Restoration and Conservation Agency of Japan; the Inamori Foundation; GAP Fund Programme of Kyoto University; and the Japan Science and Technology Agency (JST) CREST programme (JPMJCR1413) and the SICORP programme (JPMJSC20U3 and JPMJSC2105). The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval
As described elsewhere (Expert Taskforce for the COVID-19 Cruise Ship Outbreak, 2020), this study aimed to provide additional epidemiological insights into the spread of COVID-19, an infectious disease and major public health threat. The use of simplified informed consent procedures was thus approved by the National Institute of Infectious Diseases. The study protocol was approved by the Ethics Review Committee of the Hokkaido University Graduate School of Medicine (ID: Med20-010) and Kyoto University Graduate School of Medicine (ID: R2673).
Data availability statement
Deidentified case data for the Diamond Princess outbreak are publicly available elsewhere (Expert Taskforce for the COVID-19 Cruise Ship Outbreak, 2020).
Author contributions
Tetsuro Kobayashi: conceptualization, investigation, formal analysis, Writing – original draft.
Keita Yoshii: conceptualization, formal analysis.
Natalie M. Linton: investigation.
Motoi Suzuki: investigation, resources, data curation.
Hiroshi Nishiura: conceptualization, investigation, formal analysis, writing – review and editing.
References
- Abu-Raya B. Predictors of refractory coronavirus disease (COVID-19) pneumonia. Clin Infect Dis. 2020;71:895–896. doi: 10.1093/cid/ciaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali ST, Wang L, Lau EHY, Xu XK, Du Z, Wu Y, et al. Serial interval of SARS-CoV-2 was shortened over time by nonpharmaceutical interventions. Science. 2020;369:1106–1109. doi: 10.1126/science.abc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey NTJ. On estimating the latent and infectious periods of measles: I. families with two susceptibles only. Biometrika. 1956;43:15. [Google Scholar]
- Britton T, Ball F, Trapman P. A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2. Science. 2020;369:846–849. doi: 10.1126/science.abc6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton H, Usuf E, Nadjm B, Forrest K, Bojang K, Samateh AL, et al. Dexamethasone for COVID-19: data needed from randomised clinical trials in Africa. Lancet Glob Health. 2020;8:e1125–e1126. doi: 10.1016/S2214-109X(20)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci AM, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8:e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann O, Heesterbeek JAP, Roberts MG. The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface. 2010;7:873–885. doi: 10.1098/rsif.2009.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Xu X, Wu Y, Wang L, Cowling BJ, Meyers LA. Serial interval of an outbreak of 2019 novel coronavirus diseases (COVID-19)-China, 2020. China CDC Weekly Res Lett. 2020;26:2019–2021. [Google Scholar]
- Expert Taskforce for the COVID-19 Cruise Ship Outbreak Epidemiology of COVID-19 outbreak on cruise ship quarantined at Yokohama, Japan, February 2020. Emerg Infect Dis. 2020;26:2591–2597. doi: 10.3201/eid2611.201165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field Briefing: Diamond Princess COVID-19 Cases, 20 Feb Update. National Institute of Infectious Diseases. 2020. Available at: https://www.niid.go.jp/niid/en/2019-ncov-e/9417-covid-dp-fe-02.html (Accessed 26 February 2020).
- Gomes MGM, Corder RM, King JG, Langwig KE, Souto-Maior C, Carneiro J, et al. Individual variation in susceptibility or exposure to SARS-CoV-2 lowers the herd immunity threshold. medRxiv Prepr Serv Heal Sci. 2020 doi: 10.1101/2020.04.27.20081893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto K, Kamiya H, Yamagishi T, Matsui T, Suzuki M, Wakita T. Initial investigation of transmission of COVID-19 among crew members during quarantine of a cruise ship — Yokohama, Japan, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:312–313. doi: 10.15585/mmwr.mm6911e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano T, Nishiura H. A comparison of case fatality risk of COVID-19 between Singapore and Japan. J Clin Med. 2020;9:3326. doi: 10.3390/jcm9103326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. Why has COVID-19 spread more extensively in Europe than Asia? Preprints. 2020;19:1–37. [Google Scholar]
- Klinkenberg D, Nishiura H. The correlation between infectivity and incubation period of measles, estimated from households with two cases. J Theor Biol. 2011;284:52–60. doi: 10.1016/j.jtbi.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Kronbichler A, Kresse D, Yoon S, Lee KH, Effenberger M, Il Shin J. Asymptomatic patients as a source of COVID-19 infections: a systematic review and meta-analysis. Int J Infect Dis. 2020;98:180–186. doi: 10.1016/j.ijid.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:1–5. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H, Kobayashi T, Miyama T, Suzuki A, mok Jung S, Hayashi K, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H, Linton NM, Akhmetzhanov AR. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prete CA, Buss L, Dighe A, Porto VB, da Silva Candido D, Ghilardi F, et al. Serial interval distribution of SARS-CoV-2 infection in Brazil. J Travel Med. 2020;28:taa115. doi: 10.1093/jtm/taaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou J, Althaus CL. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25:1–5. doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCOV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan S. Likelihood of survival of coronavirus disease 2019. Lancet Infect Dis. 2020;20:630–631. doi: 10.1016/S1473-3099(20)30257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell TW, Hellewell J, Jarvis CI, Van Zandvoort K, Abbott S, Ratnayake R, et al. Estimating the infection and case fatality ratio for coronavirus disease (COVID-19) using age-adjusted data from the outbreak on the Diamond Princess cruise ship, February 2020. Euro Surveill. 2020;25:6–10. doi: 10.2807/1560-7917.ES.2020.25.12.2000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, Sasaki T, Kato S, Hayashi M, Tsuzuki SI, Ishihara T, et al. Natural history of asymptomatic SARS-CoV-2 infection. N Engl J Med. 2020;383:1–2. doi: 10.1056/NEJMc2013020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. COVID-19 Weekly epidemiological update.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200921-weekly-epi-update-6.pdf?sfvrsn=d9cf9496_6 Available at. [Google Scholar]
- Yamamoto N, Bauer G. Apparent difference in fatalities between Central Europe and East Asia due to SARS-COV-2 and COVID-19: four hypotheses for possible explanation. Med Hypoth. 2020;144 doi: 10.1016/j.mehy.2020.110160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H, Oshitani H, Kobayashi T, Saito T, Sunagawa T, Matsui T, et al., 2020c, Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19). medRxiv ;2020.02.28.20029272. http://medrxiv.org/content/early/2020/03/03/2020.02.28.20029272.abstract
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified case data for the Diamond Princess outbreak are publicly available elsewhere (Expert Taskforce for the COVID-19 Cruise Ship Outbreak, 2020).