Supplemental Digital Content is available in the text.
Key Words: health care access, SARS-CoV-2, COVID-19, health disparities, health care seeking
Abstract
Objectives:
Equitable access to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing is important for reducing disparities. We sought to examine differences in the health care setting choice for SARS-CoV-2 testing by race/ethnicity and insurance. Options included traditional health care settings and mobile testing units (MTUs) targeting communities experiencing disproportionately high coronavirus disease 2019 (COVID-19) rates.
Methods:
We conducted a retrospective, observational study among patients in a large health system in the Southeastern US. Descriptive statistics and multinomial logistic regression analyses were employed to evaluate associations between patient characteristics and health care setting choice for SARS-CoV-2 testing, defined as: (1) outpatient (OP) care; (2) emergency department (ED); (3) urgent care (UC); and (4) MTUs. Patient characteristics included race/ethnicity, insurance, and the existence of an established relationship with the health care system.
Results:
Our analytic sample included 105,386 adult patients tested for SARS-CoV-2. Overall, 55% of patients sought care at OP, 24% at ED, 12% at UC, and 9% at MTU. The sample was 58% White, 24% Black, 11% Hispanic, and 8% other race/ethnicity. Black patients had a higher likelihood of getting tested through the ED compared with White patients. Hispanic patients had the highest likelihood of testing at MTUs. Patients without a primary care provider had a higher relative risk of being tested through the ED and MTUs versus OP.
Conclusions:
Disparities by race/ethnicity were present in health care setting choice for SARS-CoV-2 testing. Health care systems may consider implementing mobile care delivery models to reach vulnerable populations. Our findings support the need for systemic change to increase primary care and health care access beyond short-term pandemic solutions.
The novel coronavirus disease 2019 (COVID-19) pandemic has impacted over 195,000,000 persons worldwide as of July 2021.1 In the United States, the burden of COVID-19 has been felt more among minority and low-income populations, who have faced a higher prevalence of disease and mortality than White and high-income populations.2,3 Racial and economic disparities in COVID-19 are multifactorial, influenced by social determinants of health and the cumulative impact of structural racism.4–6 Early testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is critical to protect against the viral spread by facilitating early isolation and allows for earlier intervention to improve COVID-19 outcomes.7,8 Early data indicated disparate access to SARS-CoV-2 testing, with Black patients being more likely to be tested in an emergency department (ED) or inpatient setting compared with an ambulatory setting and less likely to receive any testing compared with White patients.2,9,10 Disparities in testing access may reflect barriers to health care unequally impacting racial/ethnic minority populations, such as less health insurance coverage, transportation difficulties, or living in neighborhoods lacking medical facilities.6,11,12 However, there has been an increased positivity rate among racial/ethnic minority patients who are tested,13 emphasizing the need for more evidence to understand where patients seek testing for SARS-CoV-2. This is particularly valuable for the Southern region of the US, where there are higher proportions of minority and uninsured residents and lower health care quality compared with other US regions.14,15 Considering the historical context, the South continues to feel the impact of structural racism on disparate health care outcomes, making it an important geographical area of study.16
In response to SARS-CoV-2 testing inequities, health care systems implemented new care delivery models, including mobile testing units (MTUs) and community-based drive-through sites, to reach underserved patients in highly impacted communities.17,18 Mobile clinic models are an effective platform for reaching underserved populations that can be adapted to specific care needs.19,20 Atrium Health, a large integrated health care system in the Southeast, implemented MTUs that deployed to areas identified as “hotspots” with the highest SARS-CoV-2 positivity rates beginning in mid-April 2020.21 In partnership with community stakeholders, Atrium Health expanded health care access options by providing testing at local churches and other trusted community settings. At the time of deployment, increasing access to testing among the local Hispanic community was a priority, given the disproportionately high positivity rates among that population.
Our study fills a gap in the existing literature by examining differences in health care setting choice for SARS-CoV-2 testing within a health care system offering various test setting options. Our primary aim was to examine racial/ethnic differences in health care setting for SARS-CoV-2 testing, and the secondary aim was to explore the role of a patient’s socioeconomic status (SES), measured by insurance status,22 as well as neighborhood deprivation, and having a primary care provider (PCP) relationship. Options for testing included MTUs, outpatient (OP) care, ED, and urgent care (UC) settings.
STUDY DATA AND METHODS
Study Setting
Atrium Health is a large, integrated health care system in the Southeast US with 42 hospitals and >1500 care locations in North Carolina, South Carolina, and Georgia. The study was approved by the Institutional Review Board at Atrium Health.
Data
Electronic medical records and billing data were obtained from Atrium Health’s Enterprise Data Warehouse. Data were extracted for patients aged 18 or older tested for SARS-CoV-2 between April 12, 2020, and September 30, 2020 (n=176,471). There was only one observation (which was the first test) per patient. Patients were excluded if they received a SARS-CoV-2 antibody test to focus on acute infection. Patients were excluded if test circumstances suggested that they had limited autonomy in choice of test setting including: (1) institutionalized patients (eg, skilled nursing or correctional facilities); (2) those tested for workplace exposure, where the choice of testing location may have been made by their employer, or (3) tested for preprocedural reasons or planned hospital admission. Patients tested through the ED and then admitted to the hospital remained in the sample. The sample was limited to patients residing in North Carolina or South Carolina (due to lack of MTU access in Georgia) and to patients with a documented insurance status. The final analytic sample comprised 105,386 patients, which is diagramed in Figure 1.
FIGURE 1.
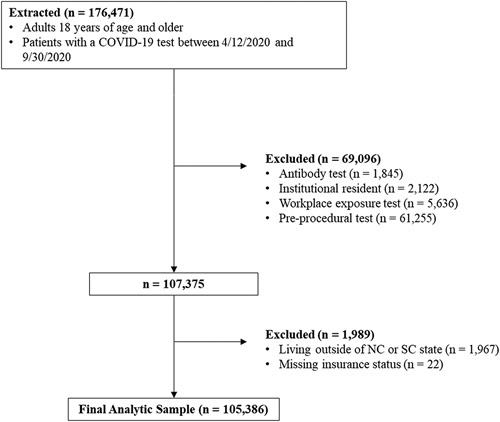
Flow diagram of the final analytic sample with inclusion and exclusion criteria. COVID-19 indicates coronavirus disease 2019; NC, North Carolina; SC, South Carolina.
The outcome variable of interest was the health care setting for SARS-CoV-2 testing, defined as the location of a patient’s first test order with 4 categories: (1) OP care; (2) UC; (3) ED; or (4) MTU. Patients who chose the OP setting called their provider’s office or a call center and were either tested at the OP office or at an external testing site for testing ordered by their provider. Patients who chose UC could either walk into an UC facility or make a same-day appointment and be tested onsite. Patients could also walk into an ED facility and be tested onsite. Last, starting in mid-April 2020, patients could get tested without an appointment by going to an MTU.
Our main explanatory variable of interest was self-reported race/ethnicity, which was categorized as Hispanic, White, Black, or Other (all non-Hispanic). Our secondary explanatory variable of interest, insurance status at the time of the patient’s first SARS-CoV-2 test, was categorized as commercial, Medicaid, uninsured, Medicare, dual Medicare-Medicaid, or Other (payers that did not align with major insurance categories). Neighborhood disadvantage was measured using the Area Deprivation Index (ADI), a validated index used to study disparities in care and outcomes, constructed from publicly available data using 17 indicators of poverty, educational attainment, and housing quality to measure community characteristics.23–25 We calculated a continuous ADI score by census tract using the American Community Survey 5-year 2014–2018 estimates,26 with each state serving as a reference area for localized rankings. The continuous score was grouped into quintiles (Q1–Q5), with Q1 representing affluent communities with the lowest level of social deprivation. Patient addresses were geo-coded to a census tract level and merged with ADI quintiles.27 Patients with missing addresses, or those with P.O. Box listed as an address, were flagged as missing and not excluded from the sample.
Additional characteristics included sex, age, comorbidity burden measured by the Charlson Comorbidity Index (CCI) indicator for CCI ≥1 (3-y look back period from the date of the test; CCI not age-adjusted),28 asymptomatic status, and history of ED visits and hospitalizations in 6 months before March 1, 2020. Having an established primary care relationship was measured by having an attributed PCP at Atrium Health at the time of the testing encounter. To capture patients living in the public health priority regions prioritized for MTU site locations,29 we categorized patient residence as within an MTU priority location or not.
Statistical Analysis
Descriptive statistics both by race/ethnicity and the health care settings for SARS-CoV-2 testing were reported. Multinomial logistic regression analysis was used to assess associations between health care setting for COVID-19 testing and race/ethnicity. Adjusted models included insurance, ADI quintiles, missing ADI, residence in the MTU priority areas, sex, age, CCI, asymptomatic status, PCP attribution, and prior ED and hospital utilization, with and without interactions between race/ethnicity and insurance. Given the use of insurance status as a measure for SES, we chose to stratify the sample by insurance to better examine differences in a health care setting for testing by race/ethnicity within each insurance group and by proxy between those of similar SES. The adjusted model was estimated separately for each insurance group stratum. In all models, OP was the reference health care setting outcome alternative. SEs were adjusted for clustering at the census tract level.
Estimates were exponentiated and reported as relative risk ratios (RRRs). An example follows: the RRR reporting the relative risk of choosing ED versus OP for Black patients compared with White patients is computed as the ratio of the relative risk of seeking care at ED versus OP if Black (numerator) over the relative risk of seeking care at ED versus OP if White (denominator, reference category). The relative risk is the ratio of probabilities of seeking care at ED and OP. RRR>1 implies a higher relative risk of choosing ED versus OP for Black compared with White patients, and RRR<1 implies a smaller relative risk. Estimates from the stratified models were used to predict probabilities of each choice for each combination of race/ethnicity and insurance group while keeping the remaining variables at their observed values; average probabilities in each insurance group were presented graphically. Analyses were conducted using Stata 15 and R, version 4.0.2.30 The ADI was estimated using the R “sociome” package.31
STUDY RESULTS
Descriptive Statistics
The study population was 58% White, 24% Black, and 11% Hispanic patients. The sample was 58% female, and 19% were aged 65 years or older. The majority (47%) had commercial insurance, 21% were uninsured, 10% had Medicaid, 17% had Medicare, and 4% had dual Medicare-Medicaid. A higher percentage of Black and Hispanic patients than White patients were uninsured, did not have a PCP, and lived in a community with high area deprivation (Supplemental Digital Content—Table S1, http://links.lww.com/MLR/C344).
Most patients accessed SARS-CoV-2 testing through OP (55%) or ED (24%), followed by UC (12%) and MTU (9%) (Table 1). Among patients who chose OP, 64% were White, 20% were Black, and 7% were Hispanic. Among patients who chose the ED, 53% were White, 35% were Black, and 10% were Hispanic. The MTU patients were predominantly Hispanic (46%), followed by White (25%) and Black (22%) patients. Of UC patients, 67% were White, 21% were Black, and 9% were Hispanic. Patients living in the most disadvantaged census tract (Q5 ADI) were disproportionately represented at MTUs and EDs, comprising 23% of patients tested at MTUs and 22% tested at ED. Medicaid patients and those with prior ED visits and hospitalizations were disproportionately represented in the ED setting compared with OP setting, while uninsured patients and those without a PCP were disproportionately represented at MTU. All differences were statistically significant.
TABLE 1.
Patient Characteristics by Health Care Setting for SARS-CoV-2 Testing (N=105,386)
| Setting [n (%)] | ||||||
|---|---|---|---|---|---|---|
| Characteristics | OP | ED | UC | MTU | Total [n (%)] | P |
| Total | 58,183 (55.2) | 24,912 (23.6) | 12,567 (11.9) | 9724 (9.2) | 105,386 | |
| Race/ethnicity | ||||||
| White | 37,220 (64.0) | 13,116 (52.6) | 8353 (66.5) | 2438 (25.1) | 61,127 (58.0) | <0.001 |
| Black | 11,723 (20.1) | 8631 (34.6) | 2637 (21.0) | 2089 (21.5) | 25,080 (23.8) | |
| Hispanic | 3956 (6.8) | 2479 (10.0) | 1069 (8.5) | 4436 (45.6) | 11,940 (11.3) | |
| Other | 5284 (9.1) | 686 (2.8) | 508 (4.0) | 761 (7.8) | 7239 (6.9) | |
| Sex | ||||||
| Male | 22,988 (39.5) | 11,383 (45.7) | 5304 (42.2) | 4418 (45.4) | 44,093 (41.8) | <0.001 |
| Female | 35,195 (60.5) | 13,529 (54.3) | 7263 (57.8) | 5306 (54.6) | 61,293 (58.2) | |
| Age | ||||||
| 18–25 | 7590 (13.0) | 2881 (11.6) | 2680 (21.3) | 1628 (16.7) | 14,779 (14.0) | <0.001 |
| 26–34 | 9345 (16.1) | 3750 (15.1) | 2776 (22.1) | 2047 (21.1) | 17,918 (17.0) | |
| 35–44 | 9812 (16.9) | 3727 (15.0) | 2330 (18.5) | 2166 (22.3) | 18,035 (17.1) | |
| 45–54 | 11,033 (19.0) | 3919 (15.7) | 2160 (17.2) | 1789 (18.4) | 18,901 (17.9) | |
| 55–64 | 9670 (16.6) | 3858 (15.5) | 1452 (11.6) | 1173 (12.1) | 16,153 (15.3) | |
| 65–74 | 6922 (11.9) | 3319 (13.3) | 797 (6.3) | 643 (6.6) | 11,681 (11.1) | |
| 75–84 | 3016 (5.2) | 2341 (9.4) | 319 (2.5) | 252 (2.6) | 5928 (5.6) | |
| 85+ | 795 (1.4) | 1117 (4.5) | 53 (0.4) | 26 (0.3) | 1991 (1.9) | |
| Insurance | ||||||
| Commercial | 31,693 (54.5) | 6225 (25.0) | 8488 (67.5) | 3180 (32.7) | 49,586 (47.1) | <0.001 |
| Uninsured | 10,304 (17.7) | 5750 (23.1) | 1396 (11.1) | 4966 (51.1) | 22,416 (21.3) | |
| Medicaid | 4189 (7.2) | 4251 (17.1) | 1196 (9.5) | 626 (6.4) | 10,262 (9.7) | |
| Dual MM | 1571 (2.7) | 2193 (8.8) | 224 (1.8) | 113 (1.2) | 4101 (3.9) | |
| Medicare | 9741 (16.7) | 5972 (24.0) | 1073 (8.5) | 757 (7.8) | 17,543 (16.6) | |
| Other insurance | 685 (1.2) | 521 (2.1) | 190 (1.5) | 82 (0.8) | 1478 (1.4) | |
| Has PCP | 32,639 (56.1) | 9832 (39.5) | 6174 (49.1) | 2904 (29.9) | 51,549 (48.9) | <0.001 |
| Prior ED visits | 10,989 (18.9) | 11,388 (45.7) | 2270 (18.1) | 1445 (14.9) | 26,092 (24.8) | <0.001 |
| Prior hospitalization | 5312 (9.1) | 5902 (23.7) | 703 (5.6) | 503 (5.2) | 12,420 (11.8) | <0.001 |
| CCI≥1 | 19,751 (33.9) | 14,857 (59.6) | 2782 (22.1) | 1626 (16.7) | 39,016 (37.0) | <0.001 |
| Asymptomatic | 12,728 (21.9) | 1223 (4.9) | 1504 (12.0) | 2017 (20.7) | 17,472 (16.6) | <0.001 |
| ADI quintiles | ||||||
| Q1 | 17,449 (30.0) | 3495 (14.0) | 3504 (27.9) | 1830 (18.8) | 26,278 (24.9) | <0.001 |
| Q2 | 11,551 (19.9) | 4018 (16.1) | 2508 (20.0) | 2019 (20.8) | 20,096 (19.1) | |
| Q3 | 9003 (15.5) | 4195 (16.8) | 2215 (17.6) | 1389 (14.3) | 16,802 (15.9) | |
| Q4 | 9554 (16.4) | 5495 (22.1) | 1924 (15.3) | 1475 (15.2) | 18,448 (17.5) | |
| Q5 | 6520 (11.2) | 5499 (22.1) | 1594 (12.7) | 2246 (23.1) | 15,859 (15.0) | |
| Missing | 4106 (7.1) | 2210 (8.9) | 822 (6.5) | 765 (7.9) | 7903 (7.5) | |
| MTU priority area | 11,528 (19.8) | 6228 (25.0) | 2157 (17.2) | 4677 (48.1) | 24,590 (23.3) | <0.001 |
Prior ED visits and hospitalization were measured during 6 months before March 1, 2020.
ADI indicates Area Deprivation Index, with Q1 indicating lowest depravity; CCI≥1, Charlson Comorbidity Index score ≥1; ED, emergency department; MM, Medicaid and Medicare insurance; MTU, mobile testing unit; OP, outpatient; PCP, primary care physician; UC, urgent care.
P-values from χ2 tests.
Multinomial Logistic Regression Analyses
In the nonstratified models, the relative risk of seeking SARS-CoV-2 testing through the ED versus OP was higher if the patient was Black or Hispanic compared with White, and if the patient had any other payment source (Medicaid, Medicare, or uninsured) compared with commercial insurance (Supplemental Digital Content—Table S2, http://links.lww.com/MLR/C344). The relative risk of seeking testing through the MTU versus OP was higher if the patient was Black or Hispanic compared with White and if the patient was uninsured or had Medicare compared with commercial insurance.
Adjusted Models Stratified by Insurance Group
Average predicted probabilities of outcomes by race, computed from the adjusted model estimates, are displayed separately for each insurance group in Figure 2 and in Supplemental Digital Content—Table S3 (http://links.lww.com/MLR/C344). Commercially insured and Medicare populations were most likely to seek testing at OP regardless of race/ethnicity. In the Medicaid population, White and Hispanic patients had a higher predicted probability of getting tested at OP than ED, while Black patients were more likely to seek care at the ED (47%) than OP (37%). White and Black uninsured patients were most likely to seek care at OP (55% and 45%, respectively), while uninsured Hispanic patients were most likely to seek care at MTU (45%). Comparatively, uninsured White and Black patients had a lower predicted probability of using MTU. In all insurance groups, except for dual Medicare-Medicaid, the predicted probability of seeking care at OP and UC was highest if the patient was White. In all insurance categories, the predicted probability of seeking care at the ED was highest if the patient was Black. In all insurance categories, except for Medicare, the predicted probability of seeking care at MTU was highest if the patient was Hispanic.
FIGURE 2.
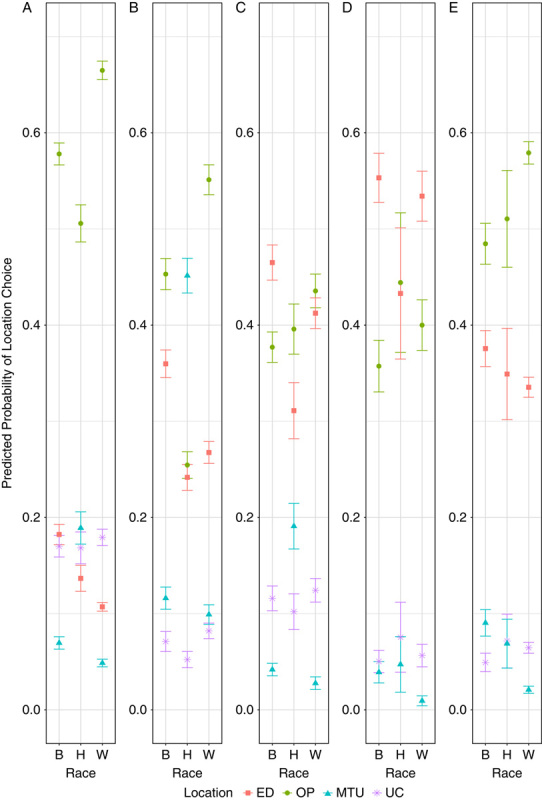
Predicted probability of health care setting for COVID-19 testing by race/ethnicity, stratified by insurance status: commercial (A); uninsured (B); Medicaid (C); dual Medicaid and Medicare (D); Medicare (E). The visualization depicts predicted probabilities and 95% confidence intervals. Estimates from the adjusted stratified models were used to predict probabilities of each health care setting for COVID-19 testing for combinations of race/ethnicity and insurance status while keeping the remaining variables at their observed values. COVID-19 indicates coronavirus disease 2019; ED, emergency department; MTU, mobile testing unit; OP, outpatient; UC, urgent care.
Many patient characteristics were associated with health care setting choice (Table 2). Having a PCP was associated with a lower relative risk of seeking care at ED versus OP and MTU versus OP in all insurance groups, and UC versus OP in the commercially insured and uninsured groups. The relative risk of seeking care at ED versus OP was higher if the patient lived in a more disadvantaged community, had comorbidities, had used the ED or was hospitalized in 6 months before the pandemic, and it was lower if the patient was asymptomatic. The relative risk of seeking care at UC versus OP was lower if the patient was hospitalized before the pandemic or was asymptomatic. The relative risk of seeking testing at MTU versus OP was lower if the patient had comorbidities (in all insurance groups but dual Medicaid-Medicare), had used the ED before the pandemic (among uninsured), or was asymptomatic (among uninsured).
TABLE 2.
Multinomial Logistic Regression Estimates of Health Care Setting for SARS-CoV-2 Testing, Stratified by Insurance Status
| Commercial | Uninsured | Medicaid | Dual MM | Medicare | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Explanatory Variables | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI | RRR | 95% CI |
| ED vs. OP setting | ||||||||||
| Race (reference=White) | ||||||||||
| Black | 2.09* | 1.92, 2.29 | 1.84* | 1.65, 2.05 | 1.36* | 1.21, 1.53 | 1.18* | 1.00, 1.38 | 1.39* | 1.24, 1.56 |
| Hispanic/Latino | 1.73* | 1.52, 1.97 | 1.95* | 1.72, 2.22 | 0.78* | 0.65, 0.92 | 0.70 | 0.50, 1.00 | 1.19 | 0.91, 1.56 |
| Other | 0.65* | 0.55, 0.76 | 0.22* | 0.18, 0.27 | 0.46* | 0.33, 0.64 | 0.88 | 0.61, 1.26 | 0.96 | 0.74, 1.24 |
| ADI quintiles (reference=Q1) | ||||||||||
| Q2 | 1.35* | 1.18, 1.53 | 1.46* | 1.21, 1.76 | 1.41* | 1.15, 1.75 | 1.18 | 0.88, 1.59 | 1.42* | 1.20, 1.68 |
| Q3 | 1.58* | 1.37, 1.82 | 2.12* | 1.78, 2.52 | 1.67* | 1.36, 2.05 | 1.04 | 0.77, 1.42 | 1.58* | 1.33, 1.88 |
| Q4 | 1.66* | 1.41, 1.94 | 2.37* | 2.00, 2.81 | 1.72* | 1.40, 2.11 | 1.00 | 0.72, 1.40 | 1.68* | 1.42, 1.99 |
| Q5 | 1.99* | 1.72, 2.30 | 2.95* | 2.49, 3.49 | 2.03* | 1.67, 2.48 | 1.28 | 0.94, 1.73 | 1.91* | 1.58, 2.31 |
| Missing | 1.74* | 1.48, 2.05 | 2.20* | 1.77, 2.74 | 2.23* | 1.75, 2.83 | 1.13 | 0.80, 1.60 | 1.69 | 1.40, 2.04 |
| MTU priority area | 0.92 | 0.83, 1.02 | 0.97 | 0.85, 1.10 | 1.15 | 1.00, 1.32 | 1.12 | 0.93, 1.35 | 0.96 | 0.83, 1.11 |
| Age (reference=18–25 or <75) | ||||||||||
| 26–34 | 0.81* | 0.73, 0.90 | 1.22* | 1.08, 1.39 | 0.93 | 0.82, 1.06 | ||||
| 35–44 | 0.79* | 0.72, 0.87 | 1.22* | 1.06, 1.39 | 1.03 | 0.90, 1.18 | ||||
| 45–54 | 0.79* | 0.71, 0.87 | 0.97 | 0.84, 1.11 | 0.96 | 0.80, 1.14 | ||||
| 55–64 | 0.78* | 0.70, 0.86 | 0.91 | 0.78, 1.06 | 0.85 | 0.72, 1.01 | ||||
| 65–74 | 0.79* | 0.65, 0.96 | 0.59* | 0.43, 0.81 | 1.12 | 0.75, 1.69 | ||||
| 75–84 | 0.81 | 0.45, 1.45 | 0.37* | 0.21, 0.64 | 0.57 | 0.25, 1.30 | 1.25 | 0.96, 1.64 | 1.50* | 1.38, 1.63 |
| 85+ | 1.83 | 0.53, 6.40 | 0.54 | 0.22, 1.32 | 0.73 | 0.22, 2.38 | 1.32 | 0.87, 2.00 | 2.94* | 2.38, 3.62 |
| Female | 0.69* | 0.65, 0.74 | 0.62* | 0.57, 0.66 | 0.74* | 0.66, 0.83 | 0.87 | 0.75, 1.01 | 0.82* | 0.77, 0.88 |
| Asymptomatic | 0.23* | 0.20, 0.27 | 0.17* | 0.14, 0.20 | 0.20* | 0.16, 0.25 | 0.22 | 0.16, 0.31 | 0.19* | 0.15, 0.23 |
| CCI≥1 | 2.00* | 1.86, 2.14 | 3.03* | 2.70, 3.39 | 1.67* | 1.50, 1.86 | 1.64* | 1.35, 2.00 | 3.26* | 0.93, 3.63 |
| Has PCP | 0.39* | 0.36, 0.42 | 0.35* | 0.31, 0.40 | 0.37* | 0.34, 0.42 | 0.53* | 0.44, 0.65 | 0.47* | 0.43, 0.52 |
| Prior ED use | 2.43* | 2.25, 2.62 | 3.24* | 2.94, 3.56 | 2.03* | 1.83, 2.25 | 1.75* | 1.52, 2.02 | 1.65* | 1.51, 1.79 |
| Prior hospitalization | 1.31* | 1.17, 1.45 | 1.42* | 1.20, 1.67 | 1.41* | 1.25, 1.60 | 1.49* | 1.28, 1.74 | 1.81* | 1.66, 1.98 |
| Constant | 0.22* | 0.19, 0.25 | 0.25* | 0.21, 0.29 | 0.69* | 0.56, 0.85 | 0.82 | 0.60, 1.13 | 0.18* | 0.16, 0.2 |
| UC vs. OP setting | ||||||||||
| Race (reference=White) | ||||||||||
| Black | 1.11* | 1.02, 1.20 | 1.08 | 0.90, 1.30 | 1.08 | 0.90, 1.30 | 1.00 | 0.71, 1.42 | 0.91 | 0.73, 1.14 |
| Hispanic/Latino | 1.26* | 1.11, 1.43 | 1.37* | 1.13, 1.66 | 0.89 | 0.70, 1.13 | 1.14 | 0.62, 2.09 | 1.26 | 0.80, 1.98 |
| Other | 0.50* | 0.44, 0.58 | 0.24 | 0.18, 0.31 | 0.75 | 0.47, 1.20 | 0.73 | 0.30, 1.76 | 0.72 | 0.45, 1.15 |
| ADI quintiles (reference=Q1) | ||||||||||
| Q2 | 1.08 | 0.95, 1.21 | 1.32* | 1.02, 1.70 | 1.15 | 0.89, 1.50 | 1.38 | 0.76, 2.52 | 1.28 | 0.99, 1.67 |
| Q3 | 1.18 | 0.97, 1.43 | 1.40* | 1.04, 1.88 | 1.35* | 1.01, 1.80 | 0.84 | 0.48, 1.47 | 1.38* | 1.05, 1.82 |
| Q4 | 0.99 | 0.83, 1.19 | 1.27 | 0.96, 1.68 | 1.02 | 0.76, 1.37 | 1.08 | 0.61, 1.91 | 1.14 | 0.86, 1.51 |
| Q5 | 1.28* | 1.03, 1.61 | 1.84* | 1.33, 2.56 | 1.19 | 0.88, 1.59 | 0.86 | 0.47, 1.54 | 1.18 | 0.87, 1.61 |
| Missing | 1.13 | 0.95, 1.34 | 1.12 | 0.82, 1.52 | 1.03 | 0.73, 1.47 | 0.89 | 0.44, 1.84 | 1.30 | 0.98, 1.73 |
| Crescent | 0.81* | 0.73, 0.90 | 0.63* | 0.51, 0.79 | 0.94 | 0.77, 1.16 | 0.83 | 0.54, 1.29 | 0.79 | 0.62, 1.01 |
| Age (reference=18–25 or <75) | ||||||||||
| 26–34 | 0.81* | 0.74, 0.88 | 1.06 | 0.91, 1.25 | 0.75* | 0.64, 0.88 | ||||
| 35–44 | 0.63* | 0.58, 0.69 | 0.96 | 0.81, 1.13 | 0.63* | 0.53, 0.75 | ||||
| 45–54 | 0.57* | 0.52, 0.61 | 0.63* | 0.52, 0.77 | 0.47* | 0.37, 0.61 | ||||
| 55–64 | 0.44* | 0.40, 0.49 | 0.54* | 0.42, 0.69 | 0.24* | 0.17, 0.33 | ||||
| 65–74 | 0.33* | 0.27, 0.42 | 0.63 | 0.39, 1.02 | 0.13* | 0.03, 0.54 | ||||
| 75–84 | 0.36* | 0.19, 0.67 | 0.08* | 0.01, 0.54 | 0.11* | 0.01, 0.81 | 0.76 | 0.46, 1.26 | 1.02 | 0.88, 1.19 |
| 85+ | 0.00* | 0.00, 0.00 | 0.00* | 0.00, 0.00 | 0.27 | 0.03, 2.33 | 0.40 | 0.14, 1.11 | 0.71 | 0.48, 1.06 |
| Female | 0.84* | 0.79, 0.88 | 0.65* | 0.58, 0.73 | 1.18 | 1.00, 1.41 | 1.63* | 1.15, 2.31 | 1.07 | 0.95, 1.22 |
| Asymptomatic | 0.49* | 0.44, 0.54 | 0.29* | 0.23, 0.36 | 0.28* | 0.22, 0.36 | 0.52* | 0.31, 0.85 | 0.57* | 0.45, 0.72 |
| CCI ≥1 | 0.73* | 0.68, 0.78 | 0.90 | 0.75, 1.08 | 0.80* | 0.68, 0.94 | 0.40* | 0.30, 0.54 | 0.62* | 0.54, 0.71 |
| Has PCP | 0.60* | 0.56, 0.64 | 1.42* | 1.24, 1.62 | 0.93 | 0.80, 1.07 | 1.27 | 0.92, 1.75 | 1.01 | 0.87, 1.17 |
| Prior ED use | 0.95 | 0.88, 1.02 | 1.24* | 1.06, 1.44 | 0.96 | 0.82, 1.11 | 1.19 | 0.87, 1.62 | 0.85 | 0.72, 1.01 |
| Prior hospitalization | 0.75* | 0.66, 0.84 | 0.69* | 0.52, 0.93 | 0.71* | 0.59, 0.85 | 0.63 | 0.44, 0.90 | 0.82 | 0.67, 1.01 |
| Constant | 0.74* | 0.67, 0.82 | 0.20* | 0.16, 0.26 | 0.46* | 0.35, 0.62 | 0.21* | 0.11, 0.39 | 0.14* | 0.12, 0.1 |
| MTU vs. OP setting | ||||||||||
| Race (reference=White) | ||||||||||
| Black | 1.65* | 1.47, 1.86 | 1.45* | 1.23, 1.71 | 1.76* | 1.32, 2.36 | 4.69* | 2.43, 9.05 | 5.48* | 4.32, 6.94 |
| Hispanic/Latino | 5.27* | 4.60, 6.03 | 10.75* | 9.33, 12.40 | 7.96* | 5.95, 10.63 | 4.57* | 1.85, 11.27 | 3.90* | 2.49, 6.09 |
| Other | 1.07 | 0.89, 1.29 | 0.86 | 0.69, 1.07 | 2.55* | 1.68, 3.87 | 1.72 | 0.42, 7.01 | 2.34* | 1.50, 3.64 |
| ADI quintiles (reference=Q1) | ||||||||||
| Q2 | 1.05 | 0.88, 1.26 | 0.97 | 0.78, 1.22 | 0.83 | 0.57, 1.22 | 1.47 | 0.44, 4.94 | 1.42 | 0.96, 2.10 |
| Q3 | 1.02 | 0.82, 1.28 | 1.09 | 0.87, 1.36 | 0.97 | 0.63, 1.49 | 1.74 | 0.54, 5.61 | 1.29 | 0.85, 1.98 |
| Q4 | 0.88 | 0.69, 1.13 | 0.85 | 0.68, 1.07 | 0.93 | 0.62, 1.38 | 1.49 | 0.52, 4.28 | 1.56* | 1.03, 2.37 |
| Q5 | 1.24* | 1.02, 1.50 | 1.12 | 0.90, 1.38 | 0.88 | 0.61, 1.29 | 1.98 | 0.71, 5.52 | 1.94* | 1.32, 2.86 |
| Missing | 1.04 | 0.82, 1.31 | 0.94 | 0.72, 1.23 | 1.03 | 0.65, 1.62 | 0.84 | 0.22, 3.21 | 1.32 | 0.81, 2.15 |
| Crescent | 2.03* | 1.76, 2.35 | 1.76* | 1.52, 2.03 | 2.42* | 1.93, 3.02 | 3.04* | 1.67, 5.54 | 3.60* | 2.74, 4.74 |
| Age (reference=18–25 or <75) | ||||||||||
| 26–34 | 0.92 | 0.80, 1.05 | 1.15* | 1.01, 1.31 | 0.83 | 0.65, 1.06 | ||||
| 35–44 | 0.89 | 0.78, 1.01 | 1.16* | 1.00, 1.34 | 0.95 | 0.73, 1.24 | ||||
| 45–54 | 0.94 | 0.82, 1.07 | 1.04 | 0.90, 1.20 | 1.12 | 0.83, 1.52 | ||||
| 55–64 | 0.95 | 0.81, 1.10 | 1.06 | 0.91, 1.25 | 1.30 | 0.93, 1.84 | ||||
| 65–74 | 0.95 | 0.71, 1.27 | 0.98 | 0.72, 1.34 | 0.61 | 0.24, 1.58 | ||||
| 75–84 | 0.80 | 0.29, 2.16 | 0.83 | 0.51, 1.35 | 0.00* | 0.00, 0.00 | 1.40 | 0.74, 2.65 | 1.33* | 1.09, 1.61 |
| 85+ | 0.00* | 0.00, 0.00 | 0.43 | 0.17, 1.07 | 0.00* | 0.00, 0.00 | 1.26 | 0.41, 3.88 | 0.50* | 0.29, 0.89 |
| Female | 0.92* | 0.85, 0.99 | 0.88* | 0.81, 0.96 | 0.97 | 0.78, 1.19 | 0.76 | 0.52, 1.13 | 0.88 | 0.76, 1.02 |
| Asymptomatic | 0.96 | 0.87, 1.07 | 0.67* | 0.59, 0.76 | 0.98 | 0.76, 1.27 | 1.04 | 0.60, 1.78 | 0.94 | 0.73, 1.22 |
| CCI≥1 | 0.72* | 0.65, 0.80 | 0.64* | 0.56, 0.74 | 0.72* | 0.57, 0.89 | 0.73 | 0.45, 1.18 | 0.62* | 0.53, 0.74 |
| Has PCP | 0.63* | 0.58, 0.69 | 0.50* | 0.44, 0.57 | 0.60* | 0.50, 0.73 | 1.19 | 0.73, 1.94 | 0.68* | 0.56, 0.82 |
| Prior ED use | 0.93 | 0.84, 1.03 | 0.85* | 0.74, 0.97 | 0.95 | 0.78, 1.16 | 1.13 | 0.77, 1.65 | 0.95 | 0.78, 1.16 |
| Prior hospitalization | 0.82* | 0.68, 0.98 | 1.05 | 0.85, 1.29 | 0.95 | 0.72, 1.25 | 0.70 | 0.44, 1.09 | 0.83 | 0.66, 1.04 |
| Constant | 0.10* | 0.08, 0.12 | 0.18* | 0.15, 0.22 | 0.08* | 0.05, 0.12 | 0.01* | 0.00, 0.04 | 0.03* | 0.02, 0.05 |
ADI indicates Area Deprivation Index; CCI≥1, Charlson Comorbidity Index score ≥1; CI, confidence interval; ED, emergency department; MM, Medicaid and Medicare insurance; MTU, mobile testing unit; OP, outpatient; PCP, primary care physician; RRR, relative risk ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UC, urgent care.
P<0.05.
DISCUSSION
We sought to describe differences by race/ethnicity in the health care setting for SARS-CoV-2 testing while examining the role of insurance status and other patient characteristics. Disparities by race/ethnicity were present regardless of the patient’s insurance status or other characteristics. We found that Black patients were more likely than White patients to access testing through the ED in every insurance group, while Hispanic patients were more likely to access testing through the MTU than White and Black patients in all insurance groups, except for Medicare. Living in a more deprived neighborhood increased the relative risk of getting tested at ED versus OP. Finally, regardless of race/ethnicity and insurance group, having a PCP was associated with a lower relative risk of accessing testing through the ED, UC, or MTU than through the OP setting.
Our findings are consistent with literature documenting disparities in health care access by race/ethnicity. While evidence of disparities in SARS-CoV-2 testing locations is emerging, 2 prior studies found higher use of ED testing sites among Black patients than White patients.2,10 Research exploring health care utilization prepandemic found higher rates of ED use among Black compared with White patients.5,32,33 Structural racism and the accumulation of historical experiences with inequitable treatment,16,34 and poor communication can disincentivize Black persons from engaging with the health care system, including primary care.35,36 It is notable that the disparities persisted in a health care setting for testing despite the deliberate efforts of health systems to provide testing choices outside of the ED for at-risk communities through outreach campaigns.37
Differences in a health care setting for SARS-CoV-2 testing by race/ethnicity were present within every insurance group. Even among commercially insured patients, which generally represents patients with better access to care and higher SES than those with Medicaid or uninsured patients, Black and Hispanic patients were still more likely than White patients to access testing through the ED. Prior research has found that patients of lower SES tend to use more ED care than OP care.38,39 Our results may suggest that the commercially insured population in this study have a more heterogeneous economic status than would be suggested by insurance status alone. Compared with patients with other types of insurance, Black and White patients with Medicaid had a higher likelihood of accessing a SARS-CoV-2 test through the ED. Given that SARS-CoV-2 testing is free, this pattern may represent past familiarity with the health care system. Prior evidence shows that Medicaid patients face barriers to OP care, such as transportation,11 and lower acceptance rates by providers.40 Since Black patients are overrepresented in Medicaid in our sample, policies and interventions to improve access to OP care for patients with Medicaid may help mitigate disparities.
Deploying MTUs was a novel health care delivery approach in response to testing disparities.21 Our results suggest that the MTUs successfully connected the vulnerable and underserved to testing. The MTUs were the most likely health care setting for Hispanic and uninsured patients to access testing. This may be attributed to specific aspects of the outreach efforts and differences in care delivery through this model. Information and site schedules for the MTUs were available in both English and Spanish and distributed through Web sites, local radio, and stakeholder organizations in the Hispanic community. It was explicitly stated that a patient’s immigration status would never be reported and that insurance was not required. Noncitizens may be more likely to avoid seeking care in the ED due to concerns about their immigration status being revealed.41 Therefore, while the immigration status of the Hispanic population in this study is unknown, it is possible that, particularly for uninsured patients, this messaging may have impacted the decision to use the MTU for some patients. Also, the MTU location varied weekly based on what geographic areas had high COVID-19 prevalence. Subsequently, the increased test positivity rate among the Hispanic population in the study setting and the convenience of use for members of the essential workforce during that time may have also contributed to increased MTU usage.42 The success of the MTU in reaching populations that historically face barriers to care may have additional implications for other care delivery, including for COVID-19 vaccination, as racial/ethnic minority patients may experience reduced access to vaccines, and could also combat vaccine hesitancy by partnering within trusted community sites.43,44
Independent of race/ethnicity and insurance, we found that patients who did not have a PCP had a higher relative risk of seeking testing at the ED, UC, and MTU sites compared with OP care, consistent with prior literature that having a PCP is associated with decreased ED utilization.45,46 In the summer of 2020, Atrium Health was able to operationalize the benefit of having an established PCP to reach patients in community clinics and provided them information on how to use OP care for testing.37 However, similar to published literature, in our study, a lower percentage of Black and Hispanic patients had a PCP compared with White patients. Primary care clinics are often not located in predominately minority communities or in communities with lower median income levels.12 This may also explain our result showing the association between higher neighborhood deprivation and increased risk of testing through the ED. The Affordable Care Act’s Medicaid expansion to the lowest income group of uninsured patients is associated with decreased barriers to having a personal doctor and decreased ED use.47,48 Increasing access to a PCP, for example, through Medicaid expansion in North Carolina and South Carolina or by addressing the scarcity of primary care facilities in underserved areas, may help address disparities in testing and other care.
The study results may also have more generalizable implications related to factors impacting health care–seeking behaviors postpandemic. This study supports the assertion that the provision of insurance alone is important but may not be sufficient to bridge the care gap across racial/ethnic groups. Developing community-based interventions aimed at connecting patients to a PCP may be key to addressing barriers to care; however, patients may still face obstacles if other factors related to care access remain poorly described. More research is needed to better understand how patients make decisions about their health care, including the impact of travel time to a facility, wait time, convenient hours of operation, direct and indirect medical costs, and trust. The application of advanced analytical methods such as discrete choice analysis methods would also improve the evidence base. In addition, more qualitative research is needed to provide the lived experience context that is traditionally unmeasured in quantitative analyses.
Our study results should be interpreted in the context of its limitations. First, the use of the multinomial logistic regression model assumes the independence of irrelevant alternatives. It is possible that this assumption was violated in our model, however, because our study objective described the association between patient characteristics and choices among a fixed set of alternatives, we consider the model appropriate for this objective. Second, patient’s health was proxied through asymptomatic status, CCI score, and past ED and hospital utilization. However, there are unobserved components of health status not captured in this analysis, including illness severity at the time of testing, and thus unobserved heterogeneity bias may be present. The results should be generalized to other populations with caution because they are specific to the population of one regional health system. Finally, we excluded patients tested for workplace exposure given that employers may have chosen testing site location; for example, on occasion, MTUs were deployed to construction sites for employee testing.49 However, it is possible that in doing so, this exclusion may have omitted patients who had an individual choice for testing location, impacting study results. Our exclusion criteria, however, also is a strength of our study by limiting the study population to those patients who likely had autonomy in the testing location choice. In addition, while we refer to the location health care setting as a choice, the patient choice can be impacted by many factors, some of which including satisfaction with primary care or perceived convenience, were not able to be captured in this analysis.
In conclusion, the health care setting through which patients accessed SARS-CoV-2 testing varied by patient race/ethnicity and insurance status. Our results imply that the MTU model is a possible approach to addressing health care access disparities, particularly for the uninsured Hispanic population. Health care systems may consider implementing mobile delivery models for patient care, including COVID vaccination clinics, to reach patients who lack insurance or do not have a PCP. The consistency of our findings with previous prepandemic studies showing health care access disparities supports the need for systemic change to increase primary care and health care access for vulnerable communities beyond short-term pandemic solutions.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.lww-medicalcare.com.
ACKNOWLEDGMENTS
The authors acknowledge Amanda Aneralla for her work and time dedicated to coordination for the present study.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Alica Sparling, Email: alicasparling@gmail.com.
Morgan Walls, Email: morgan.walls@atriumhealth.org.
Carlene A. Mayfield, Email: carlene.mayfield@atriumhealth.org.
Jennifer S. Priem, Email: jennifer.priem@atriumhealth.org.
Jason Durham, Email: jason.durham@atriumhealth.org.
Timothy Hetherington, Email: timothy.hetherington@atriumhealth.org.
Yhenneko J. Taylor, Email: yhenneko.taylor@atriumhealth.org.
REFERENCES
- 1. World Health Organization (WHO). WHO coronavirus (COVID-19) dashboard. 2021. Available at: https://covid19.who.int . Accessed July 29, 2021.
- 2. Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood). 2020;39:1253–1262. [DOI] [PubMed] [Google Scholar]
- 3. Price-Haywood EG, Burton J, Fort D, et al. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382:2534–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J, Vargas-Bustamante A, Mortensen K, et al. Racial and ethnic disparities in health care access and utilization under the Affordable Care Act. Med Care. 2016;54:140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manuel JI. Racial/ethnic and gender disparities in health care use and access. Health Serv Res. 2018;53:1407–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopez L, Hart LH, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325:719. [DOI] [PubMed] [Google Scholar]
- 7. Shimizu K, Kondo K, Osugi Y, et al. Early COVID-19 testing is critical to end the pandemic. J Gen Fam Med. 2021;22:67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor PC, Adams AC, Hufford MM, et al. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21:382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lieberman-Cribbin W, Tuminello S, Flores RM, et al. Disparities in COVID-19 testing and positivity in New York City. Am J Prev Med. 2020;59:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weber E, Miller SJ, Astha V, et al. Characteristics of telehealth users in NYC for COVID-related CARE during the coronavirus pandemic. J Am Med Inform Assoc. 2020;27:1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolfe MK, McDonald NC, Holmes GM. Transportation barriers to health care in the united states: findings from the National Health Interview Survey, 1997–2017. Am J Public Health. 2020;110:815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsui J, Hirsch JA, Bayer FJ, et al. Patterns in geographic access to health care facilities across neighborhoods in the United States based on data from the national establishment time-series between 2000 and 2014. JAMA Netw Open. 2020;3:e205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egede LE, Walker RJ, Garacci E, et al. Racial/ethnic differences in COVID-19 screening, hospitalization, and mortality in Southeast Wisconsin. Health Aff (Millwood). 2020;39:1926–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Artiga S, Damico A. Health and health coverage in the south: a data update. Kaiser Family Foundation; 2016. [Google Scholar]
- 15. Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Inequalities in life expectancy among US counties, 1980 to 2014: temporal trends and key drivers. JAMA Intern Med. 2017;177:1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Churchwell K, Elkind MSV, Benjamin RM, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. [DOI] [PubMed] [Google Scholar]
- 17. Molling PE, Holst TT, Anderson BG, et al. Drive-through satellite testing: an efficient precautionary method of screening patients for SARS-CoV-2 in a rural healthcare setting. J Prim Care Community Health. 2020;11:2150132720947963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flynn EF, Kuhn E, Shaik M, et al. Drive-through COVID-19 testing during the 2020 pandemic: a safe, efficient, and scalable model for pediatric patients and health care workers. Acad Pediatr. 2020;20:753–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu SWY, Hill C, Ricks ML, et al. The scope and impact of mobile health clinics in the United States: a literature review. Int J Equity Health. 2017;16:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown-Connolly NE, Concha JB, English J. Mobile health is worth it! Economic benefit and impact on health of a population-based mobile screening program in New Mexico. Telemed J E-Health. 2014;20:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fiscus L, Towns R, Wood S, et al. Spotlight on the safety net: deploying mobile COVID-19 testing programs in North Carolina as an approach to improving health equity. N C Med J. 2021;82:80–82. [DOI] [PubMed] [Google Scholar]
- 22. Barnett JC, Berchick ER. Current population reports. Health insurance coverage in the United States: 2016. US Government Publishing Office; September 2017:P60–260.
- 23. Zhang Y, Ancker JS, Hall J, et al. Association between residential neighborhood social conditions and health care utilization and costs. Med Care. 2020;58:586–593. [DOI] [PubMed] [Google Scholar]
- 24. Kind AJ, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30 day rehospitalizations: an analysis of Medicare data. Ann Intern Med. 2014;161:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—The Neighborhood Atlas. N Engl J Med. 2018;378:2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The United States Census Bureau. American Community Survey 2014-2018 5-year estimates now available. 2019. Available at: www.census.gov/newsroom/press-releases/2019/acs-5-year.html . Accessed March 18, 2021.
- 27. Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93:1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tuty Kuswardhani RA, Henrina J, Pranata R, et al. Charlson Comorbidity Index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:2103–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cole AJ. Collaboration is critical: working together to optimize health in our communities. N C Med J. 2017;78:255–257. [DOI] [PubMed] [Google Scholar]
- 30. The R Foundation. R: The R Project for Statistical Computing. 2021. Available at: www.r-project.org/ . Accessed March 18, 2021.
- 31. Krieger N Dalton J Wang C, et al. Sociome: Operationalizing social determinants of health data for researchers. R package, version 1.4.2. 2020. Available at: https://rdrr.io/cran/sociome/ . Accessed March 18, 2021.
- 32. Taylor YJ, Spencer MD, Mahabaleshwarkar R, et al. Racial/ethnic differences in healthcare use among patients with uncontrolled and controlled diabetes. Ethn Health. 2019;24:245–256. [DOI] [PubMed] [Google Scholar]
- 33. Hanchate AD, Dyer KS, Paasche-Orlow MK, et al. Disparities in emergency department visits among collocated racial/ethnic Medicare enrollees. Ann Emerg Med. 2019;73:225–235. [DOI] [PubMed] [Google Scholar]
- 34. Yearby R. Structural racism and health disparities: reconfiguring the social determinants of health framework to include the root cause. J Law Med Ethics. 2020;48:518–526. [DOI] [PubMed] [Google Scholar]
- 35. Ben J, Cormack D, Harris R, et al. Racism and health service utilisation: a systematic review and meta-analysis. PLoS One. 2017;12:e0189900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arnett MJ, Thorpe RJ, Gaskin DJ, et al. Race, medical mistrust, and segregation in primary care as usual source of care: findings from the exploring health disparities in integrated communities study. J Urban Health. 2016;93:456–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mayfield CA, Sparling A, Hardeman G, et al. Development, implementation, and results from a COVID-19 messaging campaign to promote health care seeking behaviors among community clinic patients. J Community Health. 2020;46:728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kangovi S, Barg FK, Carter T, et al. Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood). 2013;32:1196–1203. [DOI] [PubMed] [Google Scholar]
- 39. Blanchard JC, Haywood YC, Scott C. Racial and ethnic disparities in health: an emergency medicine perspective. Acad Emerg Med. 2003;10:1289–1293. [DOI] [PubMed] [Google Scholar]
- 40. Hogash K, Heberlein M. Physician Acceptance of new medicaid patients: what matters and what doesn’t. Health Aff (Milwood). Health Affairs Blog. 2019. Available at: https://www.healthaffairs.org/do/10.1377/hblog20190401.678690/full/. Accessed March 20, 2021. [Google Scholar]
- 41. Maldonado CZ, Rodriguez RM, Torres JR, et al. Fear of discovery among Latino immigrants presenting to the emergency department. Acad Emerg Med. 2013;20:155–161. [DOI] [PubMed] [Google Scholar]
- 42. North Carolina Department of Health and Human Services (NCDHHS). NCDHHS selects organizations to address impact of COVID-19 on LatinX Community. 2020. Available at: www.ncdhhs.gov/news/press-releases/2020/06/26/ncdhhs-selects-organizations-address-impact-covid-19-latinx-community . Accessed August 1, 2021.
- 43. Burger AE, Reither EN, Mamelund S-E, et al. Black-White disparities in 2009 H1N1 vaccination among adults in the United States: a cautionary tale for the COVID-19 pandemic. Vaccine. 2021;39:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. ASPE. Disparities in COVID-19 vaccination rates across racial and ethnic minority groups in the United States. 2021. Available at: https://aspe.hhs.gov/reports/disparities-covid-19-vaccination-rates-across-racial-ethnic-minority-groups-united-states . Accessed August 1, 2021. [DOI] [PMC free article] [PubMed]
- 45. She Z, Gaglioti AH, Baltrus P, et al. Primary care comprehensiveness and care coordination in robust specialist networks results in lower emergency department utilization: a network analysis of medicaid physician networks. J Prim Care Community Health. 2020;11:2150132720924432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mortensen K. Access to primary and specialty care and emergency department utilization of medicaid enrollees needing specialty care. J Health Care Poor Underserved. 2014;25:801–813. [DOI] [PubMed] [Google Scholar]
- 47. Kino S, Kawachi I. The impact of ACA Medicaid expansion on socioeconomic inequality in health care services utilization. PLoS One. 2018;13:e0209935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sommers BD, Blendon RJ, Orav EJ, et al. Changes in utilization and health among low-income adults after Medicaid expansion or expanded private insurance. JAMA Intern Med. 2016;176:1501–1509. [DOI] [PubMed] [Google Scholar]
- 49. Becker’s Healthcare. “Not on our watch”: Atrium CEO tells Senate how his system is tackling health disparities. 2021. Available at: www.beckershospitalreview.com/hospital-management-administration/not-on-our-watch-atrium-ceo-tells-senate-how-his-system-is-tackling-health-disparities.html . Accessed August 1, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.lww-medicalcare.com.


