ABSTRACT
Background:
Endotheliopathy is a key element in COVID-19 pathophysiology, contributing to both morbidity and mortality. Biomarkers distinguishing different COVID-19 phenotypes from sepsis syndrome remain poorly understood.
Objective:
To characterize circulating biomarkers of endothelial damage in different COVID-19 clinical disease stages compared with sepsis syndrome and normal volunteers.
Methods:
Patients with COVID-19 pneumonia (n = 49) were classified into moderate, severe, or critical (life-threatening) disease. Plasma samples were collected within 48 to 72 h of hospitalization to analyze endothelial activation markers, including soluble Vascular Cell Adhesion Molecule-1 (sVCAM-1), von Willebrand Factor (VWF), A disintegrin-like and metalloprotease with thrombospondin type 1 motif no. 13 (ADAMTS-13) activity, thrombomodulin (TM), and soluble TNF receptor I (sTNFRI); heparan sulfate (HS) for endothelial glycocalyx degradation; C5b9 deposits on endothelial cells in culture and soluble C5b9 for complement activation; circulating dsDNA for neutrophil extracellular traps (NETs) presence, and α2-antiplasmin and PAI-1 as parameters of fibrinolysis. We compared the level of each biomarker in all three COVID-19 groups and healthy donors as controls (n = 45). Results in critically ill COVID-19 patients were compared with other intensive care unit (ICU) patients with septic shock (SS, n = 14), sepsis (S, n = 7), and noninfectious systemic inflammatory response syndrome (NI-SIRS, n = 7).
Results:
All analyzed biomarkers were increased in COVID-19 patients versus controls (P < 0.001), except for ADAMTS-13 activity that was normal in both groups. The increased expression of sVCAM-1, VWF, sTNFRI, and HS was related to COVID-19 disease severity (P < 0.05). Several differences in these parameters were found between ICU groups: SS patients showed significantly higher levels of VWF, TM, sTNFRI, and NETS compared with critical COVID-19 patients and ADAMTS-13 activity was significantly lover in SS, S, and NI-SIRS versus critical COVID-19 (P < 0.001). Furthermore, α2-antiplasmin activity was higher in critical COVID-19 versus NI-SIRS (P < 0.01) and SS (P < 0.001), whereas PAI-1 levels were significantly lower in COVID-19 patients compared with NI-SIRS, S, and SS patients (P < 0.01).
Conclusions:
COVID-19 patients present with increased circulating endothelial stress products, complement activation, and fibrinolytic dysregulation, associated with disease severity. COVID-19 endotheliopathy differs from SS, in which endothelial damage is also a critical feature of pathobiology. These biomarkers could help to stratify the severity of COVID-19 disease and may also provide information to guide specific therapeutic strategies to mitigate endotheliopathy progression.
Keywords: Coagulopathy, COVID-19, endothelium, SARS-CoV-2, sepsis, septic shock
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection of cells requires angiotensin-converting enzyme 2 (ACE2), with viral entry occurring subsequent to binding of primed viral spike proteins to ACE2 (1). ACE2 is expressed by type II pneumocytes in the alveolus, enterocytes, and also endothelial cells (1, 2). Infection with SARS-CoV-2 leads to the clinical disease COVID-19. Patients with laboratory-confirmed COVID-19 have a wide spectrum of clinical disease severity, with three stages of disease progression (3): stage I, an asymptomatic incubation period; stage II, presentation with mild to moderate symptoms, as a consequence of the immune response against the virus; and stage III, when the immune system has failed to contain the virus and a systemic hyperinflammatory response predominates (4). Most severe cases bear features of a cytokine release syndrome (CRS) associated with pulmonary infiltrates and a rapid worsening of respiratory failure, with marked elevation of acute phase reactants. Although most COVID-19 patients are asymptomatic or exhibit mild symptoms, those with advanced stages of disease present with clinical and analytical signs of severe endothelial injury (5), which may result from direct viral infection of endothelial cells (6) and excessive systemic immune cell activation induced by the virus (3).
Several pathways have been proposed to be responsible for endothelial pathobiology in COVID-19. Innate and adaptive immune activation, inflammation, loss of the protective barrier, and angiogenesis are among the identified mechanisms that lead to extensive thrombosis, decreased fibrinolysis, and end-organ damage (5, 6). Rapid response to viral infection activates innate immunity, in which the complement system plays a major role. In cases of severe COVID-19 infection, there is evidence of tissue damage consistent with microvascular injury due to complement activation (7, 8). Development of endothelial dysfunction provokes a shift from vascular equilibrium toward increased vasoconstriction, with endotheliitis marked by elevated cytokine production and release, which in turn induces a profound hypercoagulable state characterized by thrombosis and markedly impaired fibrinolysis (9, 10).
The endothelial glycocalyx plays a key role in the endothelial function (11). The pathological loss of this surface layer promotes a dysfunctional endothelial state and, consequently, tissue or organ dysfunction. The endothelial surface layer is enriched with glycosaminoglycans, of which heparan sulfate (HS) is the most common. There are many viral pathogens that utilize glycans for the initial interaction with host cells (11–14) including SARS-CoV-2. Presence of HS in the bloodstream likely serves as an indicator of endothelial barrier degradation (15). Inflammatory effects on these damaged endothelial cells propagate the release of acute phase reactants, such as von Willebrand Factor (VWF), and the shedding of cell-surface adhesion receptors into the circulation.
Endothelial dysfunction thus seems to be the underlying pathophysiological event for severe COVID-19 complications. The endothelium is also similarly profoundly injured in septic patients and in those with other infectious and inflammatory syndromes (16, 17). Features of the endotheliopathy recognized in COVID-19 have not yet been compared with those in other septic syndromes. Furthermore, markers of endothelial damage may constitute strong indicators of progression and severity in these different clinical contexts. The aim of the present study was to identify and validate biological markers of endothelial damage, complement and innate-immune system activation, and hemostatic alterations in COVID-19 patients with different clinical phenotypes, while comparing their respective profiles with those observed in other septic syndromes.
We hypothesized that circulating biomarkers of endothelial damage are increased in association with the severity of COVID-19 disease and that endothelial dysfunction observed in COVID-19 is different from the observed in sepsis and noninfectious inflammatory syndromes.
PATIENTS AND METHODS
Study population and sample collection
Adult patients hospitalized at a University Hospital with confirmed COVID-19 pneumonia (positive polymerase chain reaction (PCR) on nasopharyngeal swab samples and typical radiological chest imaging) were prospectively included between May 1 and May 31, 2020. Patients were classified into: moderate disease, with fever and respiratory symptoms with radiological findings of pneumonia; severe disease, with any of the following criteria: respiratory rate≥30 breaths/min, oxygen saturation ≤ 93% at room air or PaO2/FiO2≤300 mmHg; critical disease, with respiratory failure and requiring mechanical ventilation, shock or with other organ failure that requires intensive care unit (ICU) admission (18). The sample size was calculated based on previous studies of endothelial dysfunction in sepsis made by our group and that were enough to show differences (19).
Results in all COVID-19 patients were compared with those obtained in healthy donors (n = 45), and critically ill COVID-19 patients were compared to other critically ill patients admitted to the ICU due to sepsis (S), septic shock (SS) (20) or with noninfectious systemic inflammatory response syndrome (NI-SIRS).
Furthermore, non-ICU patients were classified into two different groups based on clinical, radiological, and analytical early evolution (during the first 3 days of admission) after blood collection: worsening group (WG), with increased oxygen requirements, progression of radiological pulmonary infiltrates, and/or increased inflammation biomarkers (defined as an increase in C-reactive protein (CRP) and/or ferritin levels above twice the baseline levels) for which immunomodulatory treatment was administered; non-worsening group (NWG) with favorable outcome and response to standard therapy.
Patients’ characteristics, laboratory studies, and clinical outcomes were prospectively documented. Citrated blood samples were collected within the first 48 to 72 h of admission, centrifuged for plasma, aliquoted and stored at −40°C until used.
Study design
Endothelial damage was evaluated by measuring in plasma: sVCAM-1, VWF, ADAMTS-13 activity, thrombomodulin (TM), and soluble TNF receptor I (sTNFRI); HS levels, for endothelial glycocalyx degradation; C5b9 deposits on endothelial cells in culture, and soluble C5b9, for complement activation; presence of circulating dsDNA to measure neutrophil extracellular traps (NETs) presence; and α2-antiplasmin and PAI-1 to evaluate fibrinolysis (12, 21–24).
Markers of endothelial activation, glycocalyx degradation, and fibrinolysis
Plasma levels of VCAM-1 (R&D Systems, Minn), sTNFRI (Biomatik Corporation, Del), TM (Biomatik Corporation, Minn), HS (AttendBio Research, Spain), sC5b9 (Quidel, Calif), and PAI-1 antigen (Imubind, Toronto, Canada) were measured by ELISA. VWF antigen (VWF:Ag),VWF activity (VWF:GPIbM), and α2-Antiplasmin were evaluated in Atellica 360 (COAG, Siemens Healthineers, Germany). For ADAMTS-13 activity, fluorescence resonance energy transfer was applied (Fluoroskan Ascent FL; Thermolab Systems, Mass) (25). Deposits of C5b9 complex were measured as the area covered by C5b9 deposits expressed as average fold increases (mean ± standard error) of each condition versus control (23). Circulating dsDNA was quantified using Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Thermo Fisher, Mass).
Statistical analysis
Results are expressed as median and interquartile range for quantitative variables and as absolute count and percentages for qualitative variables. Statistical analysis was performed with nonparametric tests: comparison between two independent groups was performed using Mann–Whitney U test and Kruskal–Wallis one-way analysis was used for more than two independent samples, using SPSS statistical software (version 18.0.0; SPSS Inc, Chicago, III). Statistical significance considered when P < 0.05.
Study approval
Study approved by the local Ethics Committee (HCB/2020/0401) and conformed to the ethical guidelines of the Helsinki Declaration. Participants, or relatives, provided informed written consent before sample collection.
RESULTS
Patient characteristics
The descriptive characteristics of the patients with COVID-19 are summarized in Table 1. The clinical features were compared among the different groups of clinical severity. Hypertension was the most frequent comorbidity in all groups, especially in critically ill patients. Median days of symptoms before admission were similar among the different groups. Patients who needed oxygen supplementation (severe and critical disease) had increased inflammatory biomarkers such as CRP, ferritin, and D-dimer compared with those without oxygen requirements (moderate disease). Critical patients had also longer duration of hospital stay (LOS), but remarkably only one patient with COVID-19 who was admitted to the medical ICU died. Thirty-six (74%) patients were on prophylactic-dose anticoagulation; seven (14%) on intermediate-dose anticoagulation (enoxaparin 1 mg/kg once daily), and four (8%) on full-dose anticoagulation with two (4%) patients not anticoagulated in any way. All critical patients received immunomodulatory treatment with corticosteroids and other agents described in detail below: eight patients (80%) had already received therapy 24 to 72 h before blood sample collection and two patients (20%) the same day after blood sample collection.
Table 1.
Clinical characteristics of patients with COVID-19
| Overall n = 49 | Moderate disease n = 24 (49%) | Severe disease n = 15 (31%) | Critical disease n = 10 (20%) | |
| Male, n (%) | 26 (53%) | 10 (42%) | 9 (60%) | 7 (70%) |
| Age (years) | 61 (49–74) | 53 (43–71) | 68 (48–78) | 66 (52–75) |
| Comorbidities | ||||
| Hypertension | 20 (41%) | 6 (25%) | 7 (47%) | 7 (70%) |
| Diabetes | 12 (24.5%) | 6 (25%) | 4 (27%) | 2 (20%) |
| Pneumopathy | 5 (10%) | 0 | 2 (13%) | 3 (30%) |
| Heart disease | 9 (18%) | 4 (17%) | 2 (13%) | 3 (30%) |
| Active malignancy | 1 (2%) | 0 | 1 (7%) | 0 |
| Other immunosuppression | 3 (6%) | 0 | 1 (7%) | 2 (20%) |
| Chronic kidney disease | 4 (8%) | 1 (4%) | 1 (7%) | 2 (20%) |
| CRP (mg/dL) | 5 (3–9) | 4 (2–6) | 6 (4–9) | 14 (4–24)∗ |
| Ferritin (ng/mL) | 558 (268–1,026) | 278 (180–632) | 636 (422–1,230)∗ | 988 (562–1,436)∗ |
| D-dimer (ng/mL) | 600 (400–1,150) | 500 (400–775) | 800 (500–1,800)∗ | 1,050 (400–4,050)∗ |
| Acute Kidney Injury | 2 (4%) | 0 | 0 | 2 (20%) |
| Immunomodulatory therapy | 22 (45%) | 6 (25%) | 6 (40%) | 10 (100%)∗,† |
| Hospital length of stay (days) | 9 (4–15) | 6 (3–14) | 9 (4–11) | 20 (12–33)∗,† |
| Days of symptoms | 7 (5–10) | 6,5 (4–9) | 7 (5–13) | 6,5 (5–9) |
| 28-day mortality | 1 (2%) | 0 | 0 | 1 (10%) |
Data are expressed as median (interquartile range) or absolute count (percentage).
P < 0.05 compared with moderate disease group.
P < 0.05 compared with severe disease group.
CRP indicates C-reactive protein.
Among non-ICU COVID-19 patients, 12 patients were classified in the WG (worsening group) because they presented with increased oxygen requirements (58%); progression of radiological pulmonary infiltrates (58%), and/or increased biomarkers of inflammation (66%). These patients each received corticosteroids (67%), tocilizumab (42%), anakinra (17%), siltuximab (8%), or more than one of these treatments (24%) as part of their immunomodulatory therapy. The characteristics of these patients at the time of blood sample collection were compared with patients in the NWG (non-worsening group) (n = 27) (Table 2). Patients in the WG were older and presented with a greater hospital LOS, but there were no significant differences regarding sex, days of symptoms, comorbidities or inflammatory biomarkers at admission.
Table 2.
Comparison between worsening and not worsening non-ICU COVID-19 patients
| WG n = 12 (31%) | NWG n = 27 (69%) | P value | |
| Male, n (%) | 8 (67%) | 11 (41%) | P = 0.21 |
| Age (years) | 71 (60–79) | 52 (42–72) | P = 0.01 |
| Comorbidities | |||
| Hypertension | 6 (50%) | 7 (26%) | P = 0.24 |
| Diabetes | 5 (42%) | 5 (19%) | P = 0.26 |
| Pneumopathy | 0 | 2 (7%) | P = 0.73 |
| Heart disease | 4 (33%) | 2 (7%) | P = 0.21 |
| Active malignancy | 1 (8%) | 0 | P = 0.68 |
| Other immunosuppression | 0 | 1 (4%) | P = 0.87 |
| Chronic kidney disease | 1 (8%) | 1 (4%) | P = 0.82 |
| CRP (mg/dL) | 5.3 (4–8) | 4.5 (2–7,6) | P = 0.42 |
| Ferritin | 632 (292–1,451) | 431 (180–636) | P = 0.09 |
| D-dimer | 650 (400–1,025) | 500 (400–1,100) | P = 0.84 |
| Hospital length of stay (days) | 11 (8–22) | 6 (3–10) | P = 0.001 |
| Days of symptoms | 6 (4–10) | 8 (6–11) | P = 0.22 |
| Supplemental oxygen | 6 (50%) | 9 (33%) | P = 0.42 |
| Causes of worsening | |||
| Increased oxygen requirements | 7 (58%) | ||
| Progression of radiological pulmonary infiltrates | 7 (58%) | ||
| Increased inflammation biomarkers∗ | 8 (66%) | ||
| Immunomodulatory treatment | |||
| Corticosteroids | 8 (67%) | ||
| Tocilizumab | 5 (42%) | ||
| Anakinra | 2 (17%) | ||
| Siltuximab | 1 (8%) | ||
| More than 1 treatment | 3 (24%) | ||
Data are expressed as median (interquartile range) or absolute count (percentage).
Defined as increased CRP and/or ferritin levels above twice the baseline levels.
CRP indicates C-reactive protein; NWG, non-worsening group; WG, worsening group.
To compare the subset of critically ill COVID-19 patients with other critical inflammatory syndromes, we included patients with NI-SIRS, S and SS admitted to the ICU. Clinical characteristics of the different groups are shown in Table 3. There were no significant differences regarding sex or age. APACHE-II and SOFA II scores, as well as CRP levels were higher in patients with SS, compared with critical COVID-19 patients. ICU as well as hospital LOS were similar between patients with SS and critical COVID-19 disease but longer than in NI-SIRS and S patients. There were no deaths among patients with NI-SIRS and S, and similarly there were no differences between invasive mechanical ventilation and renal replacement therapy.
Table 3.
Clinical characteristics of patients with critical COVID-19 disease, NI-SIRS, sepsis, and septic shock
| Critical COVID-19 n = 10 | NI-SIRS n = 7 | Sepsis n = 7 | Septic shock n = 14 | |
| Male, n (%) | 7 (70%) | 4 (57%) | 5 (71%) | 8 (57%) |
| Age (years) | 66 (52–75) | 54 (46–59) | 68 (56–75) | 60 (48–72) |
| Comorbidities | ||||
| Hypertension | 7 (70%) | 2 (29%) | 4 (57%) | 6 (43%) |
| Diabetes | 2 (20%) | 1 (14%) | 2 (29%) | 3 (21%) |
| Pneumopathy | 3 (30%) | 0 | 0 | 0 |
| Heart disease | 3 (30%) | 1 (14%) | 1 (14%) | 1 (7%) |
| Active malignancy | 0 | 0 | 0 | 0 |
| Other immunosuppression | 2 (20%) | 0 | 1 (14%) | 0 |
| Chronic kidney disease | 2 (20%) | 0 | 0 | 0 |
| Primary diagnoses | ||||
| Infection source | 0 | |||
| Urinary | 2 (29%) | 3 (22%) | ||
| Pulmonary | 3 (43%) | 5 (71%) | 3 (22%) | |
| Soft tissues | 1 (14%) | 0 | 3 (22%) | |
| Endovascular | 2 (29%) | 0 | 2 (14%) | |
| Central Nervous System | 1 (14%) | 0 | 2 (14%) | |
| Abdominal | 0 | 1 (7%) | ||
| Cardiac surgery | 0 | 0 | ||
| Pulmonary surgery | 0 | 0 | ||
| Cardiovascular disease | 0 | 0 | ||
| Pulmonary Thromboembolism | 0 | 0 | ||
| APACHE II score | 14 (8–16) | 10 (9–20) | 15 (12–25) | 21 (14–25)∗ |
| SOFA II score | 4 (3–6) | 6 (5–12) | 7 (6–8)† | 11 (9–14)† |
| CRP (mg/dL) | 14 (4–24) | 6 (2,5–13) | 23 (3–30) | 28 (22–34)∗ |
| Platelet count (109/L) | 244 (187–302) | 132 (115–214) | 255 (171–280) | 153 (65–196)∗ |
| ICU length of stay (days) | 14 (6–34) | 4 (2–7)∗ | 3 (2–7)† | 10 (4–16) |
| Hospital length of stay (days) | 20 (12–33) | 11 (7–14)∗ | 10 (8–17)∗ | 18 (13–54) |
| 28-day mortality | 1 (10%) | 0 | 0 | 5 (36%) |
| Invasive Mechanical ventilation | 3 (30%) | 5 (57%) | 1 (14%) | 6 (43%) |
| Renal replacement therapy | 3 (30%) | 1 (14%) | 1 (14%) | 3 (21%) |
Data are expressed as median (interquartile range) or absolute count (percentage).
P < 0.05 compared with critical COVID-19 group.
P < 0.01 compared with critical COVID-19 group.
APACHE indicates Acute Physiology and Chronic Health Evaluation; CRP, C-reactive protein; ICU, intensive care unit; NI-SIRS, noninfectious systemic inflammatory response syndrome; SOFA, Sequential Organ Failure Assessment.
Circulating biomarkers in COVID-19 patients: comparative analysis depending on disease severity and early evolution
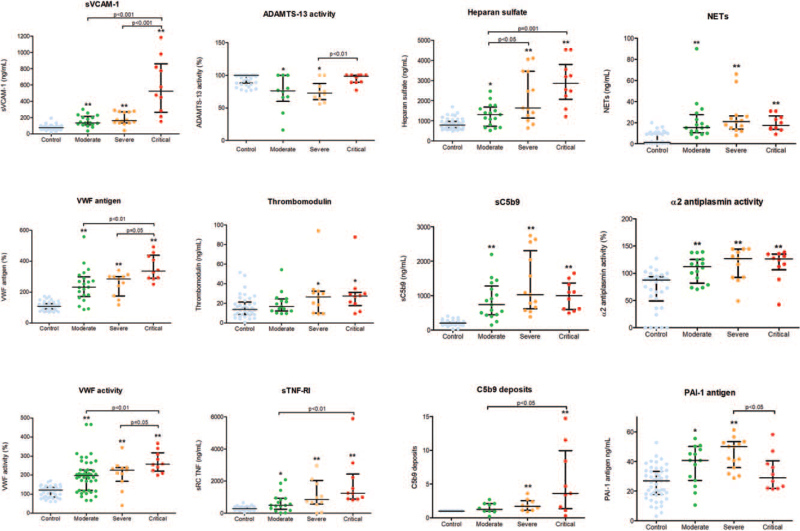
All circulating biomarkers of endothelial injury were significantly increased in all groups of patients with COVID-19 in comparison with healthy donors, with the unique exception of ADAMTS-13 activity that was in the normal range in all COVID-19 patients (median activity above 70%) (Fig. 1). Furthermore, a progressive increase in the levels of biomarkers of endothelial damage was observed in association with COVID-19 severity. There was a significant elevation of sVCAM-1 levels among de different groups (P < 0.01, for all). Also, plasma VWF antigen and VWF activity levels showed a strong association with COVID-19 clinical severity, with the differences among all groups proving statistically significant. Circulating HS levels were also associated with the severity of COVID-19, with significant differences between severe and moderate disease (P < 0.05), as well as between the critical and severe disease groups (P < 0.001).
Fig. 1.
Comparison of circulating endothelial biomarkers between different COVID-19 groups. Datapoints indicate individual measurements, whereas horizontal bars show median and interquartile ranges. ∗P < 0.05 compared with control group. ∗∗P < 0.001 compared with control group. C5b9 deposits are expressed as fold increase versus control. ADAMTS-13 indicates a disintegrin-like and metalloprotease with thrombospondin type 1 motif no. 13; NETs, neutrophil extracellular traps; PAI-1, plasminogen activator inhibitor-1; sC5b9, soluble C5b9; sTNF-RI, soluble tumor necrosis factor receptor I; sVCAM-1, soluble vascular cell adhesion molecule-1; VWF, Von Willebrand Factor.
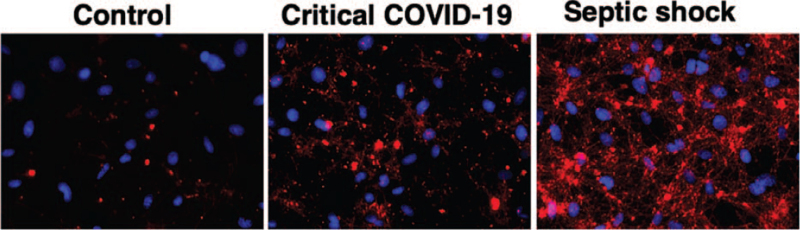
A progressive increase in C5b9 deposits on endothelial cells was also shown in association with COVID-19 severity, with significant differences between critical and moderate disease noted (P < 0.05) (Fig. 1). In Figure 2, micrographs show representative images of C5b9 deposits on endothelial cells in culture exposed to plasma samples from healthy donors (Control); a patient with critical COVID-19 and a patient with SS.
Fig. 2.
Complement C5b9 deposits on endothelial cells in culture. Representative micrographs showing deposits of C5b9 on human microvascular endothelial cells (HMEC-1) after exposure to plasma samples from healthy donors (Control), a critical COVID-19 patient and a patient with septic shock. C5b9 deposits were detected by using a specific primary antibody followed by a secondary antibody conjugated with Alexa594 (red). Cell nuclei were stained with DAPI (blue). Each field encloses 75,922 μm2 of cell culture preparation.
Interestingly, PAI-1 antigen levels were elevated but found to be lower in patients with critical disease compared to patients with moderate or severe disease (P < 0.05) (Figure 1). Conversely, plasma sC5b9, NETs, TM, and α2-antiplasmin activity did not differ significantly among the different severity groups (Figure 1).
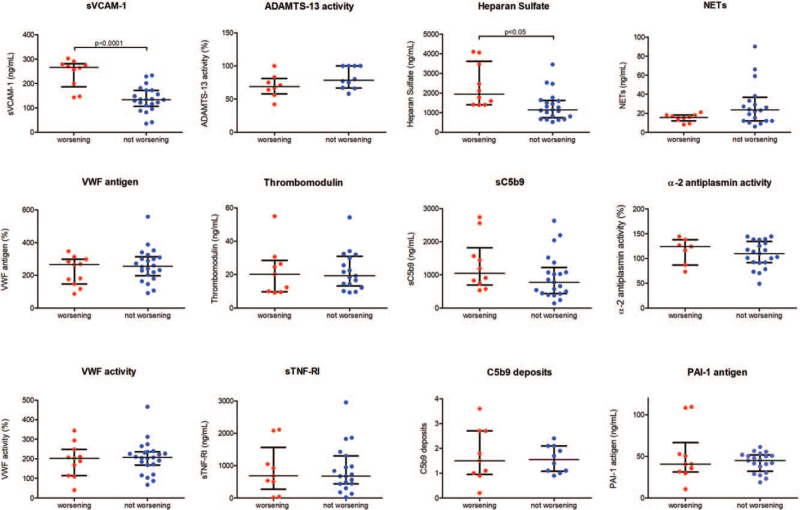
When comparing levels of biomarkers between COVID-19 patients in the early stages of the disease and as they evolved, we found statistically significant differences in HS and sVCAM-1 levels, which were significantly higher in the WG (Fig. 3).
Fig. 3.
Comparison of circulating endothelial biomarkers between worsening and not worsening non-ICU COVID-19 patients. Datapoints indicate individual measurements, whereas horizontal bars show median and interquartile ranges. C5b9 deposits are expressed as fold increase versus control. ADAMTS-13 indicates a disintegrin-like and metalloprotease with thrombospondin type 1 motif no. 13; NETs, neutrophil extracellular traps; PAI-1, plasminogen activator inhibitor-1; sC5b9: soluble C5b9; sTNF-RI, soluble tumor necrosis factor receptor I; sVCAM-1, soluble vascular cell adhesion molecule-1; VWF, Von Willebrand Factor.
Comparative analysis of circulating biomarkers in COVID-19 and septic and inflammatory syndromes admitted to the ICU
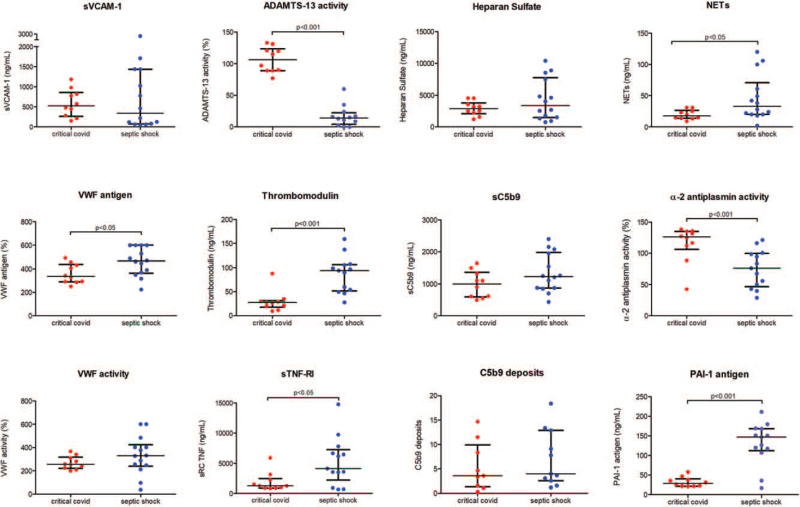
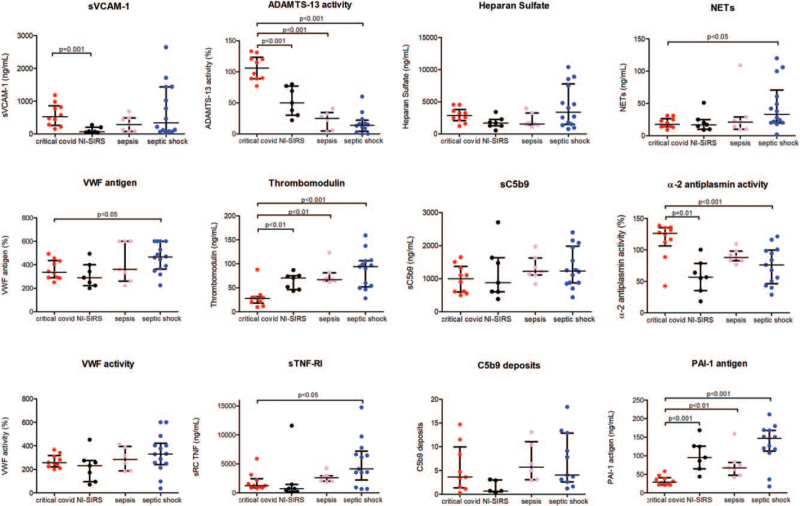
A distinctive pattern of biomarkers was observed between COVID-19 and SS patients (Fig. 4). Plasma VWF antigen, TM, and sTNFRI were increased in SS compared with patients with critical COVID-19 (P < 0.05, P < 0.001 and < 0.05 respectively). TM levels were higher in all groups with respect to critical COVID-19 (P < 0.01) (Fig. 5). In contrast with the normal values observed in COVID-19 patients, ADAMTS-13 activity was significantly lower in SS, S, and NI-SIRS (P < 0.001 in all) (Fig. 5).
Fig. 4.
Comparison of circulating endothelial biomarkers between critical COVID-19 and septic shock patients. Datapoints indicate individual measurements, whereas horizontal bars show median and interquartile ranges. C5b9 deposits are expressed as fold increase versus control. ADAMTS-13 indicates a disintegrin-like and metalloprotease with thrombospondin type 1 motif no. 13; NETs, neutrophil extracellular traps; PAI-1, plasminogen activator inhibitor-1; sC5b9: soluble C5b9; sTNF-RI, soluble tumor necrosis factor receptor I; sVCAM-1, soluble vascular cell adhesion molecule-1; VWF, Von Willebrand Factor.
Fig. 5.
Comparison of circulating endothelial biomarkers between critical COVID-19, NI-SIRS, sepsis, and septic shock patients. Datapoints indicate individual measurements, whereas horizontal bars show median and interquartile ranges. C5b9 deposits are expressed as fold increase versus control. ADAMTS-13 indicates a disintegrin-like and metalloprotease with thrombospondin type 1 motif no. 13; NETs, neutrophil extracellular traps; NI-SIRS, noninfectious systemic inflammatory response syndrome; PAI-1, plasminogen activator inhibitor-1; sC5b9, soluble C5b9; sTNF-RI, soluble tumor necrosis factor receptor I; sVCAM-1, soluble vascular cell adhesion molecule-1; VWF, Von Willebrand Factor.
There were no significant differences regarding VWF activity, sVCAM-1, and HS levels between critical COVID-19 and SS patients (Fig. 4).
In terms of complement activation, there were no differences between the respective ICU patients regarding sC5b9 levels. C5b9 deposits on endothelial cells showed a tendency to be increased when using SS samples versus COVID-19 patients (Fig. 2), though differences between both groups showed no statistical significance. NETs were significantly more elevated in SS patients than in critical COVID-19 (P < 0.05) (Fig. 4).
With regards to the fibrinolytic system, α2-antiplasmin activity was notably higher in patients with critical COVID-19 in comparison with SS and NI-SIRS patients (P < 0.001). Conversely, PAI-1 levels were elevated but significantly decreased in COVID-19 patients compared with the markedly higher levels seen in NI-SIRS, S, and SS patients (P < 0.001 in all).
Biomarkers levels from all patients are reported in the Supplementary table.
DISCUSSION
Biomarkers of three different biological processes associated with COVID-19 endotheliopathy, potentially involved in the extreme thrombotic morbidity and consequent mortality, have been evaluated in the present study: endothelial damage, complement activation, and fibrinolytic dysregulation. To our knowledge, this is the first study comparing biomarkers in COVID-19 with those obtained in other infectious or severe inflammatory syndromes. Our results demonstrate the presence of high levels of circulating endothelial stress products, glycocalyx degradation, marked activation of the complement system terminal pathway, and alteration of the molecules involved in the fibrinolysis process in COVID-19 patients. A biomarkers profile has been identified in COVID-19 patients with clear association with disease severity and exhibiting a distinctive pattern from the observed in septic syndromes.
Evaluation of potential markers of endothelial damage in COVID-19 patients showed that sVCAM-1, VWF, antigen and activity, TM and sTNFRI were elevated in COVID-19 patients versus healthy donors, progressively in association with disease severity. Higher levels of soluble VCAM-1, which participates in leukocyte recruitment to activated endothelial cells, and TM, with anticoagulant activity once thrombin-TM complexes form after coagulation activation, were consistently found in COVID-19 patients, as previously published. Of note, recent work postulates TM as a reliable predictor of mortality in COVID-19 patients (26, 27). From our results, both proteins emerge as potential prognostic tools to identify patients with worse early clinical evolution and provide direction for early therapeutic intervention. As for VWF, an acute phase reactant in conditions of systemic stress (28, 29), despite being elevated in all COVID-19 cases (27), and in contrast with recent publications (30), the activity of its metalloprotease ADAMTS-13 was consistently normal in our patients cohort.
The biomarkers of the COVID-19 endotheliopathy found here clearly follow a distinctive pattern with respect to other systemic septic and inflammatory syndromes. Patients with septic syndromes exhibit endothelial damage, glycocalyx degradation, and impaired fibrinolytic activity (31) together with strong inflammatory responses triggered by pathogens and the host immune system (16, 32). Comparative analysis between COVID-19 and septic syndromes indicated a different behavior. While sVCAM-1 levels were more elevated in critically ill COVID-19, TM and VWF levels were clearly higher in septic syndromes together with significantly reduced ADAMTS-13 activity (33, 34). Diminished presence of ADAMTS-13, as observed in SS, may result in insufficient cleavage of ultra-large VWF multimers, which are more reactive to platelets. Normal ADAMTS-13 activity is of particular interest in the characterization of COVID-19, as the associated thrombotic complications have distinct characteristics from disseminated intravascular coagulation (DIC), observed in sepsis (35, 36), and in other thrombotic microangiopathies (37). Regarding TM, lower levels in COVID-19 may confer reduced anticoagulant activity, likely contributing to the associated prothrombotic state.
Circulating HS was measured to evaluate endothelial glycocalyx degradation. In the MYSTIC study, severe alterations of the microcirculation and the endothelial glycocalyx in patients with COVID-19 were related to disease severity (38). HS seems crucial for the binding of SARS-CoV-2 to the cell membrane (39). Our present findings validate HS as a marker of COVID-19 severity and, similarly to sVCAM-1, HS may be a valuable prediction marker for worse clinical outcomes. Although HS levels were extremely high in some SS patients, differences with those in COVID-19 patients were not statistically significant. In relation to complement activation, responsible for endothelial damage in several microangiopathies (23) we found increased levels of sC5b9 in all COVID-19 patients with respect to healthy donors but did not differ from SS patients. Interestingly, C5b9 deposits on endothelial cells were higher when using samples from SS patients than COVID-19 patients.
Neutrophil extracellular traps (NETs) are elevated in septic and inflammatory syndromes in correlation with clinical severity and in association with thrombotic morbidity (40–42). In our study, we found higher levels of NETs in COVID-19 patients than in controls, but no significant differences among COVID-19 severity groups or between critically ill COVID-19 patients and other groups were observed.
Fibrinolysis alteration has been hypothesized as one of the causes of persistent thrombosis and vasculopathy in COVID-19 patients, with a hypofibrinolytic state predominating over coagulation hyperactivation (43, 44). Protein α2-antiplasmin inhibits plasmin proteolytic activity against fibrin through inactive plasmin–antiplasmin complexes. To our knowledge, α2-antiplasmin levels have been reported to be normal in COVID-19 patients (27). PAI-1 is a serpin secreted by endothelial cells that inhibits plasminogen activator. Elevations in PAI-1 levels have been observed in a large cohort of patients with non-COVID-19 acute respiratory distress syndrome with prognostic implications (44) as well as in SS patients (45). The evidence in COVID-19, however, is contradictory to date. Wang et al. (46) observed higher PAI-1 levels in patients with poorer clinical outcomes. Other groups have not found differences between critical and non-critical COVID-19 patients (27). In our study, both α2-antiplasmin and PAI-1 were higher in COVID-19 patients than in controls, without association with disease severity. Notably, α2-antiplasmin was significantly higher in COVID-19 patients than in NI-SIRS, sepsis, and even SS patients. Thus, evaluation of α2-antiplasmin levels together with ADAMTS-13 and sVCAM-1 may allow clear differentiation between the endotheliopathy occurring in COVID-19 and SS. While PAI-1 was relatively elevated in moderate and severe COVID-19, it was reduced in critically ill patients in comparison with levels in NI-SIRS and SS. It is worth noting the wide range of PAI-1 normal values (44, 45) in COVID-19 patients, which were higher on average than in controls but within a normal range in a high percentage of patients, limiting the strength of PAI-1 as a COVID-19-specific biomarker.
Several treatments have been proposed to reduce inflammatory and thrombotic complications in COVID-19 patients. Prophylactic doses of low molecular weight heparins have reduced mortality amongst high-risk patients (47, 48). Most of the patients included in our study were receiving heparin and, since heparin may protect the endothelium, levels of biomarkers could be even higher in its absence. Specifically, elevations in heparanase in COVID-19 may be partially neutralized by heparin. Nonetheless, the results reinforce the fact that endothelial damage and disordered fibrinolysis are crucial in the pathophysiology of COVID-19 severe complications and endothelial protection should be foreseen as a key strategy to prevent them (49). In this regard, there is currently a phase IIb randomized, double-blind, placebo-controlled clinical trial to evaluate the effect of defibrotide to treat the respiratory distress and CRS in COVID-19 patients (50). Defibrotide is a first-in-class oligonucleotide drug approved for the treatment of sinusoidal obstructive syndrome, a complication of hematopoietic stem cell transplantation underpinned by endothelial injury (51, 52), and also prevents endothelial damage in graft-versus-host disease (53), making it especially attractive as a therapeutic approach in this setting. Moreover, monoclonal antibodies to complement C5 protein, eculizumab, and ravulizumab are also being explored in COVID-19 patients (54–57). Also, another phase 2 study is currently evaluating a variety of fibrinolytic agents in these patients (58).
The biomarkers identified here for COVID-19 endotheliopathy clearly associate with clinical severity and exhibit a distinctive pattern from other septic and severe inflammatory syndromes. Specifically, sVCAM-1, α-2-antiplasmin, ADAMTS-13 activity, and PAI-1 behaved distinctly in critically ill COVID-19 patients as compared with other groups. The combination of these biomarkers could help to stratify the degree of endothelial dysfunction and coagulopathy in patients with critical COVID-19 disease and differentiate them from other severe inflammatory conditions (infectious and noninfectious) in the ICU. They could also provide information to guide specific therapeutic intervention. Elevations of sVCAM-1 and HS appear to be prognostic, since they were higher in patients with clinical worsening requiring immunosuppressive treatment. Further studies are warranted to validate these biomarkers as predictive tools and should be integrated into prospective studies.
The current study presents several limitations. A low number of patients has been included, especially for critically ill COVID-19 patients, sepsis, and NI-SIRS. In addition, endotheliopathy biomarkers have been measured only at a single time point in the first 72 h of admission and therefore the evolution of these biomarkers over time has not been evaluated. Further studies assessing serial sampling will help to understand mechanistic insights of the endotheliopathy in COVID-19. Moreover, most of the critically ill COVID-19 patients had already received an immunomodulatory treatment when blood samples were collected, mainly corticosteroids and/or tocilizumab. This happened due to the severity of the disease and, although time between the intervention and sample obtention was short (less than 36 h), we do not know the impact of these treatments on endothelial damage biomarkers measurements. Lastly, this is a single-center study, which may limit the generalizability of its results.
Supplementary Material
Acknowledgments
The authors thank Patricia Molina, Laura Bonastre, Estefanía Garcia, Pilar Gómez, and Marc Pino for their technical assistance. This study was supported by Fundació Clínic, Barcelona (HCB/2020/0401) and Jazz Pharmaceuticals (IST-16–10355). Results from the present study were presented in part at the ASH 62th Annual Meeting (2020).
Footnotes
Revised 12 May, 2021
SF and ABM-C contributed equally as first authors.
MD-R and PC contributed equally as last authors.
This study was supported by Fundació Clíinc, Barcelona (HCB/2020/0401), Jazz Pharmaceuticals (IST-16-10355), Fundació La Marató de TV3 (Project Code: 202026) and Instituto Carlos III, Spanish Government, FIS PI12/00832 cofinanciado con Fondo Europeo de Desarrollo Regional, Unión Europea, Una manera de hacer Europa.
ABM-C, SF, MP, PC, and MDR designed the study. SF, HV, AT, and SF contributed to sample collection. ABMC, MP, JMS, and STM performed the laboratory tests and analysis. SF and ABMC analyzed the clinical data and SF performed the statistical analysis. GE, EC, NJM, ER, DG-B, CCS, JMM, and PGR contributed with interpretation of data, correlative with outcomes, and hypothesis-generation for potential therapeutics. ABM-C, SF, PC, and MDR wrote the manuscript and all authors contributed in the editing and writing of the final manuscript. ABM-C and SF contributed equally as first authors and PC and MDR contributed equally as last authors.
ABM-C is an advisory board member for Siemens, outside of the submitted work. MDR and EC have been granted by and received honoraria from Jazz Pharmaceuticals. PC and SF have collaborated with Jansen, Gilead, Kite, MSD, Alexion, and Pfizer, outside of the submitted work. MP received speaker's fee from Jazz Pharmaceuticals. CCC has received research support from ADC Therapeutics and Rhizen Pharmaceuticals; has served as consultant or advisor for Servier, Novartis, Genenta Science srl, ADC Therapeutics, Roche, Sanofi, Karyopharm, and Jazz Pharmaceuticals; and has received honoraria for speaker engagements from Bristol-Myers Squibb, Merck Sharp & Dohme, Janssen Oncology, Astra-Zeneca. JMM reports grants and consulting honoraria from Jazz Pharmaceuticals, during the conduct of the study; consulting honoraria and travel expenses from Gilead, consulting honoraria from Novartis, Sandoz, and Takeda, outside the submitted work. PGR reports a consulting or advisory role for Karyopharm, Oncopeptides, Celgene, Janssen, Takeda, and Sanofi; and research funding from Oncopeptides, Celgene, Takeda, and Bristol-Myers Squibb . The rest of authors have no actual or potential conflict of interest to declare.
Supplemental digital content is available for this article.
REFERENCES
- 1.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203 (2):631–637, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan B, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111 (20):2605–2610, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ 27 (5):1451–1454, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Hear Lung Transplant 39 (5):405–407, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383 (2):120–128, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395 (10234):1417–1418, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int 98 (2):314–322, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 220:1–13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J 41 (32):3038–3044, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teuwen L-A, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol 20 (7):389–391, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cagno V, Tseligka ED, Jones ST, Tapparel C. Heparan sulfate proteoglycans and viral attachment: true receptors or adaptation bias? Viruses 11 (7):596, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oshima K, Haeger SM, Hippensteel JA, Herson PS, Schmidt EP. More than a biomarker: The systemic consequences of heparan sulfate fragments released during endothelial surface layer degradation (2017 Grover Conference Series). Pulm Circ 8 (1):2045893217745786, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koehler M, Delguste M, Sieben C, Gillet L, Alsteens D. Initial step of virus entry: virion binding to cell-surface glycans. Annu Rev Viro 7 (1):143–165, 2020. [DOI] [PubMed] [Google Scholar]
- 14.Stencel-Baerenwald JE, Reiss K, Reiter DM, Stehle T, Dermody TS. The sweet spot: defining virus-sialic acid interactions. Nat Rev Microbiol 12 (11):739–749, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care 23 (1):16, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101 (10):3765–3777, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Xing K, Murthy S, Liles WC, Singh JM. Clinical utility of biomarkers of endothelial activation in sepsis-a systematic review. Crit Care 16 (1):R7, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei P-F. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial version 7). Chin Med J (Engl) 133 (9):1087–1095, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández S, Palomo M, Molina P, Díaz-Ricart M, Escolar G, Téllez A, Seguí F, Ventosa H, Torramade-Moix S, Rovira M, et al. Progressive endothelial cell damage in correlation with sepsis severity. Defibrotide as a contender. J Thromb Haemost 19 (8):1948–1958, 2021. [DOI] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315 (8):801–810, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mir E, Palomo M, Rovira M, Pereira A, Escolar G, Penack O, Holler E, Carreras E, Díaz-Ricart M. Endothelial damage is aggravated in acute GvHD and could predict its development. Bone Marrow Transplant 52 (9):1317–1325, 2017. [DOI] [PubMed] [Google Scholar]
- 22.Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Martine C, Ghita G, Escolar G, Carreas E. Endothelial dysfunction after hematopoietic stem cell transplantation: role of the conditioning regimen and the type of transplantation. Biol Blood Marrow Transplant 16 (7):985–993, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Palomo M, Blasco M, Molina P, Lozano M, Praga M, Torramade-Moix S, Martinez-Sanchez J, Cid J, Escolar G, Carreas E, et al. Complement activation and thrombotic microangiopathies. Clin J Am Soc Nephrol 14 (12):1719–1732, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gloude NJ, Khandelwal P, Luebbering N, Lounder DT, Jodele S, Alder MN, Lane A, Wilkey A, Lake KE, Litts B, et al. Circulating dsDNA, endothelial injury, and complement activation in thrombotic microangiopathy and GVHD. Blood 130 (10):1259–1266, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kremer Hovinga JA, Mottini M, Lämmle B. Measurement of ADAMTS-13 activity in plasma by the FRETS-VWF73 assay: comparison with other assay methods. J Thromb Haemost 4 (5):1146–1148, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Tong M, Jiang Y, Xia D, Xiong Y, Zheng Q, Chen F, Zou L, Xiao W, Zhu Y. Elevated expression of serum endothelial cell adhesion molecules in COVID-19 patients. J Infect Dis 222 (6):894–898, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a singlecentre, cross-sectional study. Lancet Haematol 7 (8):e575–e582, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hovinga JAK, Zeerleder S, Kessler P, Raomani de Wit T, van Mourik JA, Hack CE, ten Cate H, Reitsma PH, Wuillemin WA, Lämmle B. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J Thromb Haemost 5 (11):2284–2290, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Kawecki C, Lenting PJ, Denis CV. von Willebrand factor and inflammation. J Thromb Haemost 15 (7):1285–1294, 2017. [DOI] [PubMed] [Google Scholar]
- 30.Martinelli N, Montagnana M, Pizzolo F, Friso S, Salvago GL, Forni GL, Gianesin B, Morandi M, Lunardi C, Lippi G, et al. A relative ADAMTS13 deficiency supports the presence of a secondary microangiopathy in COVID 19. Thromb Res 193:170–172, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gando S. Role of fibrinolysis in sepsis. Semin Thromb Hemost 39 (4):392–399, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascón GA, Hernandez G, Murray P, De Backer D. ADQI XIV Workgroup. The Endothelium in Sepsis. Shock 45 (3):259–270, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwameis M, Schörgenhofer C, Assinger A, Steiner MM, Jilma B. VWF excess and ADAMTS13 deficiency: a unifying pathomechanism linking inflammation to thrombosis in DIC, malaria, and TTP. Thromb Haemost 113 (4):708–718, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aibar J, Castro P, Espinosa G, Fernández S, Hernández C, Rinaudo M, Butjosa M, Tàssies D, Reverter JC, Nicolás JM. ADAMTS-13 in critically ill patients with septic syndromes and noninfectious systemic inflammatory response syndrome. Shock 43 (6):556–562, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Fogarty H, Townsend L, Ni Cheallaigh C, Bergin C, Martin-Loeches I, Browne P, Bacon CL, Gaule R, Gillett A, Byrne M. COVID19 coagulopathy in Caucasian patients. Br J Haematol 189 (6):1044–1049, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, Falco M, Albano G, Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 18 (7):1747–1751, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincent JL, Castro P, Hunt BJ, Jörres A, Praga M, Rojas-Suarez J, Watanabe E. Thrombocytopenia in the ICU: disseminated intravascular coagulation and thrombotic microangiopathies—what intensivists need to know. Crit Care 22 (1):158, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse PR, Fobker M, Kühn J, Braune S, Göbel U, Thölking G, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis 24 (1):145–157, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Haan CAM, Li Z, te Lintelo E, Bosch BJ, Haijema BJ, Rottier PJM. Murine coronavirus with an extended host range uses heparan sulfate as an entry receptor. J Virol 79 (22):14451–14456, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar S, Gupta E, Kaushik S, Srivastava VK, Saxena J, Mehta S, Jyoti A. Quantification of NETs formation in neutrophil and its correlation with the severity of sepsis and organ dysfunction. Clin Chim Acta 495:606–610, 2019. [DOI] [PubMed] [Google Scholar]
- 41.Czaikoski PG, Mota JMSC, Nascimento DC, Sônego F, Vargar F, Castanheira S, Melo PH, Scortegagna GT, Silva RL, Barroso-Sousa R, et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS One 11 (2):e0148142–e1148142, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang S, Qi H, Kan K, Chen J, Xie H, Guo X, Zhang L. Neutrophil extracellular traps promote hypercoagulability in patients with sepsis. Shock 47 (2):132–139, 2017. [DOI] [PubMed] [Google Scholar]
- 43.Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer M, Urban S, Nydam TL, Moore PK, McIntyre RC. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg 231 (2):193–203e1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Esiner MD. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med 35 (8):1821–1828, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García-Segarra G, Espinosa G, Tassies D, Oriola J, Aibar J, Bové A, Castro P, Reverter JC, Nicolás JM. Increased mortality in septic shock with the 4G/4G genotype of plasminogen activator inhibitor 1 in patients of white descent. Intensive Care Med 33 (8):1354–1362, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, Yaffe MB, Morre HB, Barrett CD. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost 18 (7):1752–1755, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thachil J, Tang N, Gando S, Falanga A, CAttaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 18 (5):1023–1026, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 18 (5):1094–1099, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richardson E, Carlo-Stella C, Jara R, Vlodavsky I, Iacobelli M, Fareed J, Mo C, O’Gorman P, Yanik G, Palomo M, et al.Response to Maccioetal et al. “Multifactorial pathogenesis of COVID-19-related coagulopathy: Can defibrotide have a role in the early phases of coagulation disorders?”. J Thromb Haemost 18 (11):3111–3113. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Defibrotide as Prevention and Treatment of Respiratory Distress and Cytokine Release Syndrome of Covid 19. Available at: https://clinicaltrials.gov/ct2/show/NCT04348383 Accessed March 10, 2021. [Google Scholar]
- 51.Richardson PG, Carreras E, Iacobelli M, Nejadnik B. The use of defibrotide in blood and marrow transplantation. Blood Adv 2 (12):1495–1509, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson PG, Riches ML, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM, Arai S, Grupp SA, Guinan EV, Martin PL, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood 127 (13):1656–1665, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez-Sanchez J, Hamelmann H, Palomo M, Mir E, Moreno-Castaño AB, Torramade S, Rovira M, Escolar G, Cordes S, Kalupa M, et al. Acute graft-vs.-host disease-associated endothelial activation in vitro is prevented by defibrotide. Front Immuno 10:2339, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eculizumab (Soliris) in Covid-19 Infected Patients. Available at: https://clinicaltrials.gov/ct2/show/NCT04288713 Accessed March 10, 2021. [Google Scholar]
- 55. CORIMUNO19-ECU: trial Evaluating Efficacy and Safety of Eculizumab (Soliris) in Patients With COVID-19 Infection, Nested in the CORIMUNO-19 Cohort. Available at: https://clinicaltrials.gov/show/NCT04346797. [Epub ahead of print]. [Google Scholar]
- 56.Diurno F, Numis FG, Porta G, Cirillo F, Maddaluno S, Ragazzino A, De Negri P, Di Gennaro C, Pagano A, Allegorico E, et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Revr Medl Pharmacoll Sci 24 (7):4040–4047, 2020. [DOI] [PubMed] [Google Scholar]
- 57. Efficacy and Safety Study of IV Ravulizumab in Patients With COVID-19 Severe Pneumonia. Available at: https://clinicaltrials.gov/ct2/show/NCT04369469 Accessed March 10, 2021. [Google Scholar]
- 58. Fibrinolytic Therapy to Treat ARDS in the Setting of COVID-19 Infection. Available at: https://clinicaltrials.gov/ct2/show/NCT04357730 Accessed March 4, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.