Abstract
Background
To systematically evaluate the prevalence of post-sequelae and chronic obstructive pulmonary disease assessment test (CAT) scoring one year after hospital discharge among older COVID-19 patients, as well as potential risk factors.
Methods
A multi-center prospective cohort study involving 1,233 eligible older COVID-19 patients was conducted. All patients were followed-up between Mar 1, 2021 and Mar 20, 2021. CAT scoring was adopted to measure symptom burden in COVID-19 patients.
Results
Of the 1233 eligible cases, 630 (51.1%) reported at least one sequelae. The top six post-sequelae included fatigue (32.4%), sweating (20.0%), chest tightness (15.8%), anxiety (11.4%), myalgia (9.0%), and cough (5.8%). Severe patients had significantly higher percentage of fatigue, sweating, chest tightness, myalgia, and cough (P<0.05), while anxiety was universal in all subjects. Sweating, anxiety, palpitation, edema of lower limbs, smell reduction, and taste change were emerging sequelae. Disease severity during hospitalization (OR: 1.46, 95% CI: 1.15–1.84, P = 0.002), and follow-up time (OR: 0.71, 95% CI: 0.50–0.99, P = 0.043) were independently associated with risk of post-sequelae, while disease severity during hospitalization was significantly associated with increased risk of emerging sequelae (OR: 1.33, 95% CI: 1.03–1.71, P = 0.029). The median of CAT score was 2 (0–5) in all patients, and a total of 120 patients (9.7%) had CAT scores ≥10. Disease severity during hospitalization (OR: 1.81, 95% CI: 1.23–2.67, P = 0.003) and age (OR: 1.07, 95% CI: 1.04–1.09, P<0.001) were significantly associated with increased risk of CAT scores ≥10.
Conclusions
While the dramatic decline in the prevalence rate of persistent symptoms is reassuring, new sequelae among older COVID-19 patients cannot be ignored. Disease severity during hospitalization, age, and follow-up time contributed to the risk of post-sequelae and CAT scoring one year after hospital discharge among older COVID-19 patients. Our study provides valuable clues for long-term post-sequelae of the older COVID-19 patients, as well as their risk factors.
Keywords: COVID-19, Sequelae, Older people, Wuhan, SARS-CoV-2
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues to pose a global threat.1 As of August 2021, it has caused more than 109 million confirmed cases with more than 4.4 million deaths worldwide.2 The coronavirus (including severe acute respiratory syndrome [SARS] and Middle East respiratory syndrome [MERS]) pandemic has caused long-term pulmonary, cardiovascular, neuropsychiatric and systemic sequelae in the affected patients.3 , 4 Continued attention to global proliferation needs to be accompanied by systematic research on the long-term sequelae of COVID-19 recovery to establish an evidence-based system of prognostic assessment and health promotion.5
Both the occurrence and prognosis of COVID-19 were closely related to older age.6, 7, 8 To date, series of studies have reported the potential short- to long-term sequelae of COVID-19 recovery, ranging from two months to one year.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 However, the older patients have not attracted enough attention and no specific studies quantified the temporal trends and associated risk factors for sequelae of COVID-19 in older patients, especially long-term sequelae. In addition, although there were currently no agreed measures to assess the pulmonary burden of COVID-19, chronic obstructive pulmonary disease (COPD) assessment test (CAT), an eight-item questionnaire designed to quantify health status impairment in COPD patients, was adopted to measure pulmonary burden in COVID-19 patients and recognized by the scientific community.26
Here we systematically evaluated the post-sequelae one year after hospital discharge among older COVID-19 patients in a multi-center prospective cohort study, we also explored the risk factors of post-sequelae and CAT scoring one year after hospital discharge among older COVID-19 patients.
Materials and methods
Study design and patients
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline was implemented to enhance the reporting quality of this manuscript. Totally included in this multi-center prospective cohort study were real-time reverse transcription polymerase chain reaction (RT-PCR)-confirmed older COVID-19 patients (age ≥ 60) who were discharged from Huoshenshan Hospital and Taikang-Tongji Hospital (both in Wuhan, China) between Feb 12 and Apr 10, 2020.8 All discharged patients met the uniform discharge criteria of the World Health Organization interim guidance.27 The follow-up was conducted between Mar 1, 2021 and Mar 20, 2021. Exclusion criteria included (1) participants who refused, (2) those who could not be contacted, and (3) those who died before the follow-up visit. This study was approved by the Daping Hospital of Army Medical University (Ethics number 202,153). Verbal informed consent was obtained from all patients or their legal guardians prior to the survey due to the telephone follow-up.
Definitions and data acquisition
The disease severity during hospitalization was defined by World Health Organization (WHO) guideline for COVID-19.28 The severe refers to fever or suspected respiratory infection, plus one of the following: respiratory rate > 30 breaths/min; severe respiratory distress; or SpO2 ≤ 93% on room air.28 Fever was defined as an axillary temperature of 37.3 °C or higher. The baseline characteristics, including demographic characteristics, coexisting disorders, and the clinical symptoms, were extracted from the electronic medical records using a uniformed questionnaire by two trained physicians, and validated by a telephone-interview. The one-year follow up was conducted via telephone interview by trained physicians using a uniformed questionnaire including self-reported symptoms, and CAT score items, of which ≥ 10 (the threshold for maintenance treatment in COPD) and >2 (the median value) were treated as categorical outcomes. Patients were asked to report any sustained, intermittent, and emerging symptoms, respectively. The patient's current symptoms were carefully documented and evaluated by specialists to distinguish from their pre-disease status or other underlying diseases that were not associated with COVID-19. All survey data was double entered and validated using EpiData (version 3.1, EpiData Association, Odense, Denmark) software, and disputes were arbitrated by the expert committees composed of experts of respiratory and critical care medicine, and epidemiology.
Statistical analyses
Categorical variables were described using frequency rates and percentages, while continuous variables were described using the median/interquartile range (IQR) values. Categorical variables were compared using the chi-squared test or fisher's exact test as appropriate, while continuous variables were compared using Manne-Whitney U test. The missing values of all potential predictors (missing rate of less than 5.0%) were imputed by expectation-maximization (EM) method. To test for the risk of bias due to the patients who were lost to follow-up, 1:1 propensity score-matching (PSM) between the lost to follow-up subpopulation and the enrolled subpopulation was carried out. Univariate logistic regression analysis was used to screen the potential risk factors which reached a P value of less than 0.1, and calculate their odds ratios (ORs) and corresponding confidence intervals (CIs). Then, the independent risk factors were derived from a stepdown selection process in multivariate logistic regression model. Multivariable adjusted logistic regression analysis was then used for exploring independent risk factors associated with any post-sequelae, CAT scores ≥10 or >2 (the median value). All statistical analyses were conducted using the R software version 4.0.0 (Institute for Statistics and Mathematics, Vienna, Austria). The reported statistical significance levels were all 2-sided, and a level of < 0.05 was considered statistically significant.
Results
Baseline characteristics of the older COVID-19 patients
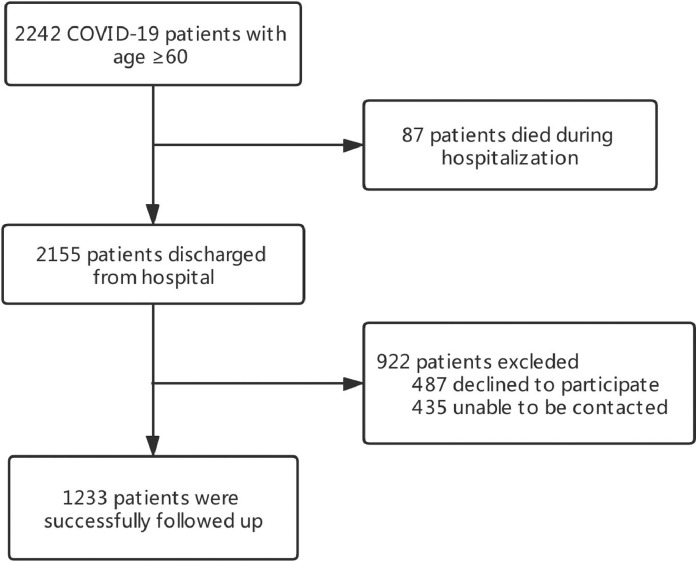
Fig. 1 presented the flowchart of the inclusion procedure of the older COVID-19 patients. Totally 2242 COVID-19 patients with age ≥ 60 were admitted to these two hospitals, and 87 (3.88%) patients died during hospitalization. Of the remaining 2155 discharged patients, 1233 (57.2%) were available for one year follow-up (487 declined to participate and 435 were unable to be contacted). Baseline demographic and clinical characteristics were summarized in Table 1 . In brief, the median age of the eligible patients was 68.0 (IQR: 64.0–73.0) years old, with 591 (47.9%) were male. A total of 438 (35.5%) patients were categorized as severe. The median duration of hospital stay was 15.0 (10.0–22.0) days and the median time from discharge to follow-up was 363.0 (357.0–371.0) days. During hospitalization, the most common symptoms were fever (77.3%), cough (68.9%), fatigue (58.5%), anorexia (53.8%), and short of breath (45.2%). Compared with non-severe cases, the severe were elder, more likely to be male, had more coexisting disorders, clinical symptoms, and receive more treatments.
Fig. 1.
flowchart of the inclusion of the older COVID-19 patients.
Table 1.
Demographic and clinical characteristics of the COVID-19 patients aged ≥60 years by disease severity during hospitalization.
| Variables | Total patients (N = 1233) | Severe (N = 438) | Non-severe (N = 795) | P-value (Severe vs. Non-severe) |
|---|---|---|---|---|
| Gender, male | 591(47.9%) | 227(51.8%) | 364(45.8%) | 0.042 |
| Age (Years), median (IQR) | 68(64–73) | 69.5(65–75) | 67(64–72) | <0.001 |
| 60–69 | 716(58.1%) | 219(50.0%) | 497(62.5%) | <0.001 |
| 70–79 | 388(31.5%) | 157(35.8) | 231(29.1%) | |
| ≥80 | 129(10.5%) | 62(14.2%) | 67(8.4%) | |
| Smoking | ||||
| Never | 784(88.6%) | 303(90.4%) | 481(87.4%) | 0.370 |
| Former | 18(2.0%) | 5(1.5%) | 13(2.4%) | |
| Active | 83(9.4%) | 27(8.1%) | 56(10.2%) | |
| Coexisting disorders-no.(%) | ||||
| 0 | 523(42.4%) | 160(36.5%) | 363(45.7%) | <0.001 |
| 1 | 340(27.6%) | 118(27.0%) | 222(27.9%) | |
| ≥2 | 370(30.0%) | 160(36.5%) | 210(26.4%) | |
| Hypertension | 516(41.8%) | 212(48.4%) | 304(38.2%) | 0.001 |
| Diabetes | 234(19.0%) | 96(21.9%) | 138(17.4%) | 0.051 |
| Cardiovascular disease | 169(13.7%) | 78(17.8%) | 91(11.4%) | 0.002 |
| Coronary heart disease | 128(10.4%) | 60(13.7%) | 68(8.6%) | 0.005 |
| Cerebrovascular disease | 72(5.8%) | 32(7.3%) | 40(5.0%) | 0.103 |
| Chronic liver disease | 53(4.3%) | 18(4.1%) | 35(4.4%) | 0.808 |
| Chronic kidney disease | 31(2.5%) | 10(2.3%) | 21(2.6%) | 0.700 |
| COPD | 19(1.5%) | 10(2.3%) | 9(1.1%) | 0.116 |
| Tumor | 32(2.6%) | 15(3.4%) | 17(2.1%) | 0.174 |
| Symptoms-no.(%) | ||||
| 0 | 19(2.1%) | 4(1.2%) | 15(2.7%) | 0.026 |
| 1–2 | 171(18.9%) | 52(15.3%) | 119(21.1%) | |
| >2 | 713(79.0%) | 283(83.5%) | 430(76.2%) | |
| Cough | 849(68.9%) | 326(74.6%) | 523(65.8%) | 0.001 |
| Fever | 699(77.3%) | 279(82.3%) | 420(74.3%) | 0.006 |
| Anorexia | 663(53.8%) | 243(55.5%) | 420(52.9%) | 0.384 |
| Fatigue | 721(58.5%) | 270(61.8%) | 451(56.7%) | 0.085 |
| Short breath | 556(45.2%) | 228(52.2%) | 328(41.3%) | <0.001 |
| Chest tightness | 398(32.3%) | 151(34.6%) | 247(31.1%) | 0.211 |
| Myalgia | 323(26.3%) | 111(25.4%) | 212(26.7%) | 0.611 |
| Expectoration | 228(18.5%) | 101(23.1%) | 127(16.0%) | 0.002 |
| Dyspnea | 111(9.0%) | 69(15.8%) | 42(5.3%) | <0.001 |
| Diarrhea | 78(6.3%) | 22(5.0%) | 56(7.1%) | 0.164 |
| Sore throat | 71(5.8%) | 21(4.8%) | 50(6.3%) | 0.285 |
| Nausea | 28(2.3%) | 11(2.5%) | 17(2.1%) | 0.670 |
| Vomiting | 34(2.8%) | 15(3.4%) | 19(2.4%) | 0.285 |
| Dizziness | 30(2.4%) | 11(2.5%) | 19(2.4%) | 0.892 |
| Chill | 25(2.0%) | 18(4.1%) | 7(0.9%) | <0.001 |
| Headache | 27(2.2%) | 8(1.8%) | 19(2.4%) | 0.519 |
| Nasal congestion | 9(0.7%) | 6(1.4%) | 3(0.4%) | 0.076 |
| Hemoptysis | 7(0.6%) | 4(0.9%) | 3(0.4%) | 0.253 |
| Treatment approach | ||||
| ICU admission | 40(3.2%) | 35(8.0%) | 5(0.6%) | <0.001 |
| Mechanical Ventilation | 17(1.4%) | 15(3.4%) | 2(0.3%) | <0.001 |
| Corticosteroid-related therapy | 103(8.4%) | 63(14.4%) | 40(5.0%) | <0.001 |
| Length of hospital stay, days | 15(10–22) | 15.5(10–24) | 15(10–21) | 0.007 |
| Time from discharge to follow-up, days | 363(357–371) | 360(356–368) | 365(357–371) | <0.001 |
Post-sequelae and CAT scoring one year after hospital discharge among older COVID-19 patients
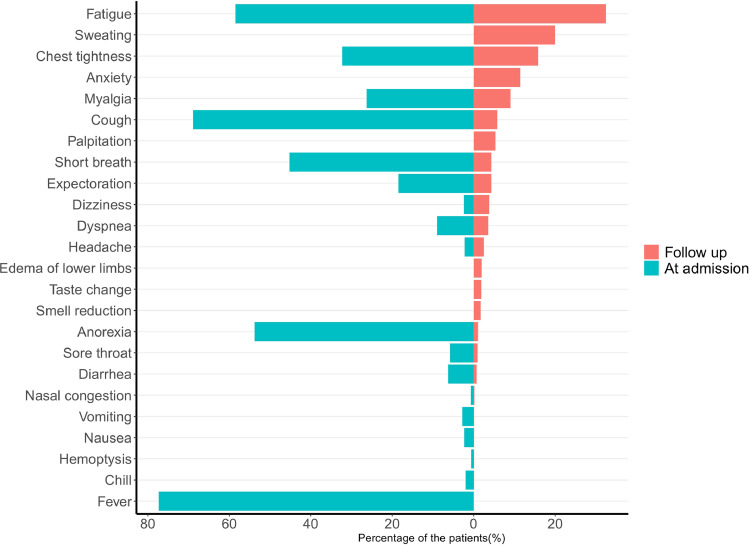
Table 2 presented the post-sequelae and CAT scoring one year after hospital discharge, including systemic/general sequelae (34.6%), neurological sequelae (29.0%), cardiovascular sequelae (19.0%), respiratory sequelae (10.1%), and digestive system sequelae (1.9%). Of the 1233 eligible cases, 630 patients (51.1%) reported at least one sequelae at follow up, and the severe group had significantly higher percentage than the non-severe group (57.5% vs 47.5%, P = 0.001). The top six post-sequelae included fatigue (32.4%), sweating (20.0%), chest tightness (15.8%), anxiety (11.4%), myalgia (9.0%), and cough (5.8%). Severe patients had significantly higher percentage of fatigue, sweating, chest tightness, myalgia, and cough (P<0.05), while anxiety was universal in all subjects. Of them, fatigue, chest tightness, myalgia, and cough were sustained symptoms, although the prevalence rate dropped sharply (Fig. 2 ). Sweating, anxiety, palpitation, edema of lower limbs, smell reduction, and taste change were emerging sequelae (Fig. 2). The median of CAT score was 2 (0–5) in all patients, and the severe group had a significantly higher CAT score (3, IQR: 1–6) than the non-severe group (2, IQR: 0–5, p<0.001) (Table 2). A total of 120 patients (9.7%) had CAT scores ≥10, and 597 patients (48.4%) had CAT scores >2. The severe group had significantly higher proportion of patients with CAT score both ≥10 and >2 than the non-severe group (P<0.001).
Table 2.
Post-sequelae one year after hospital discharge among older COVID-19 patients by disease severity during hospitalization.
| Post-sequelae | Total patients (N = 1233) | Severe (N = 438) | Non-severe (N = 795) | P-value Severe vs. Non-severe |
|---|---|---|---|---|
| Any one of post-sequelae | 630(51.1%) | 252(57.5%) | 378(47.5%) | 0.001 |
| Systemic/general sequelae | 427(34.6%) | 178(40.6%) | 249(31.3%) | 0.001 |
| Fatigue | 400(32.4%) | 166(37.9%) | 234(29.4%) | 0.002 |
| Myalgia | 111(9.0%) | 52(11.9%) | 59(7.4%) | 0.009 |
| Chill | 1(0.1%) | – | 1(0.1%) | – |
| Respiratory sequelae | 124(10.1%) | 61(13.9%) | 63(7.9%) | 0.001 |
| Dyspnea | 44(3.6%) | 22(5.0%) | 22(2.8%) | 0.041 |
| Cough | 71(5.8%) | 34(7.8%) | 37(4.7%) | 0.025 |
| Expectoration | 53(4.3%) | 26(5.9%) | 27(3.4%) | 0.035 |
| Hemoptysis | 1(0.1%) | – | 1(0.1%) | – |
| Sore throat | 12(1.0%) | 7(1.6%) | 5(0.6%) | 0.129 |
| Nasal congestion | 2(0.2%) | 1(0.2%) | 1(0.1%) | – |
| Cardiovascular sequelae | 234(19.0%) | 111(25.3%) | 123(15.5%) | <0.001 |
| Edema of lower limbs | 24(1.9%) | 13(3.0%) | 11(1.4%) | 0.054 |
| Chest tightness | 195(15.8%) | 94(21.5%) | 101(12.7%) | <0.001 |
| Short breath | 53(4.3%) | 30(6.8%) | 23(2.9%) | 0.001 |
| Palpitation | 66(5.4%) | 29(0.6%) | 37(4.7%) | 0.142 |
| Neurological sequelae | 358(29.0%) | 142(32.4%) | 216(27.2%) | 0.052 |
| Dizziness | 47(0.8%) | 17(3.9%) | 30(3.8%) | 0.925 |
| Headache | 31(2.5%) | 16(3.7%) | 15(1.9%) | 0.058 |
| Anxiety | 141(11.4%) | 56(12.8%) | 85(10.7%) | 0.269 |
| Sweating | 246(20.0%) | 105(24.0%) | 141(17.7%) | 0.009 |
| Smell reduction | 21(1.7%) | 12(2.7%) | 9(1.1%) | 0.037 |
| Taste change | 23(1.9%) | 11(2.5%) | 12(1.5%) | 0.213 |
| Digestive system sequelae | 24(1.9%) | 9(2.1%) | 15(1.9%) | 0.838 |
| Diarrhea | 9(0.7%) | 3(0.7%) | 6(0.8%) | – |
| Nausea | 1(0.1%) | – | 1(0.1%) | – |
| Vomiting | 1(0.1%) | – | 1(0.1%) | – |
| Anorexia | 13(1.1%) | 6(1.4%) | 7(0.9%) | 0.561 |
| CAT scores | 2(0–5) | 3(1–6) | 2(0–5) | <0.001 |
| 0–10 | 1113(90.3%) | 376(85.8%) | 737(92.7%) | <0.001 |
| 10–20 | 100(8.1%) | 47(10.7%) | 53(6.7%) | |
| 22–30 | 13(1.1%) | 10(2.3%) | 3(0.4%) | |
| 30- | 7(0.6%) | 5(1.1%) | 2(0.3%) | |
| CAT scores ≥ 10 | 120(9.7%) | 62(14.2%) | 58(7.3%) | <0.001 |
| CAT scores > 2 | 597(48.4%) | 235(53.7%) | 362(45.5%) | 0.006 |
Fig. 2.
Percentage of patients presenting with COVID-19-related sequelae during the acute phase of the disease (left) and at 1-year follow-up (right).
As the patients lost to follow-up before were a little older than those enrolled (Supplementary Table 1), PSM was conducted to evaluate the lost to follow-up bias in the sensitivity analysis. Totally 843 patients in the enrolled population were matched successfully with those lost to follow-up, and the baseline characteristics were comparable (Supplementary Table 1). We then compared the post-sequelae one year after hospital discharge between totally enrolled patients (n = 1233) and those selected by PSM (n = 843) (Supplementary Table 2). Most symptoms were similar to those of totally enrolled patients (Supplementary Table 2, all P >0.05). This indicates the lost to follow-up bias was negligible, and the enrolled patients were representative.
Risk factors of post-sequelae one year after hospital discharge among older COVID-19 patients
Risk factors for post-sequelae one year after hospital discharge were evaluated for all older COVID-19 patients (Table 3 -4 , and Supplementary Table 3–6). Disease severity during hospitalization (OR: 1.46, 95% CI: 1.15–1.84, P = 0.002), and follow-up time (OR: 0.71, 95% CI: 0.50–0.99, P = 0.043) were independently associated with risk of post-sequelae (Table 3). Table 4 presents the risk factors for emerging sequelae. Disease severity during hospitalization (OR: 1.36, 95% CI: 1.06–1.75, P = 0.016), and follow-up time (OR for per month: 0.68, 95% CI: 0.47–0.98, P = 0.038) were individually associated with either increased or decreased risk of emerging sequelae. In multivariable model, Disease severity during hospitalization was significantly associated with increased risk of emerging sequelae (OR: 1.33, 95% CI: 1.03–1.71, P = 0.029). We also explored risk factors for post-sequelae of each system, and disease severity during hospitalization, age, follow-up time, gender, and smoking were the main risk factors (Supplementary Table 3–6). Besides, our results revealed that corticosteroid-related therapy was associated with increased risk of both post-sequelae and emerging sequelae (P<0.001, Supplementary Table 7–8). To remove the potential confounding bias caused by disease severity during hospitalization, we also conducted stratified analyses of associations between any post-sequelae, emerging sequelae and corticosteroid-related therapy, and the results kept (Supplementary Table 7–8).
Table 3.
Logistic regression models to evaluate the risk factors for any post-sequelae.
| Variables | Univariate Logistic Analysis |
Multivariate Logistic Analysis |
||
|---|---|---|---|---|
| HR (95%CI) | P-value | OR (95%CI) | P-value | |
| Age, per year | 1.01(0.99–1.02) | 0.383 | ||
| Gender, male | 0.89(0.71–1.11) | 0.306 | ||
| Smoking | 0.94(0.75–1.17) | 0.560 | ||
| Severity during hospitalization | 1.50(1.18–1.89) | 0.001 | 1.46(1.15–1.84) | 0.002 |
| Coexisting disorders-no.(%) | ||||
| Hypertension | 0.95(0.76–1.79) | 0.673 | ||
| Diabetes | 1.01(0.76–1.34) | 0.949 | ||
| Cardiovascular disease | 0.91(0.66–1.26) | 0.579 | ||
| Coronary heart disease | 1.10(0.76–1.58) | 0.628 | ||
| Cerebrovascular disease | 1.14(0.71–1.84) | 0.591 | ||
| Chronic liver disease | 0.92(0.53–1.59) | 0.762 | ||
| Chronic kidney disease | 0.69(0.33–1.41) | 0.304 | ||
| COPD | 0.55(0.22–1.42) | 0.217 | ||
| Tumor | 0.74(0.36–1.50) | 0.401 | ||
| Time from discharge to follow-up, per month | 0.67(0.78–0.93) | 0.017 | 0.71(0.50–0.99) | 0.043 |
Table 4.
Logistic regression models to evaluate the risk factors for emerging sequelae.
| Variables | Univariate Logistic Analysis |
Multivariate Logistic Analysis |
||
|---|---|---|---|---|
| HR (95%CI) | P-value | OR (95%CI) | P-value | |
| Age, per year | 1.01(1.00–1.03) | 0.141 | ||
| Gender, male | 0.85(0.66–1.08) | 0.188 | ||
| Smoking | 0.94(0.73–1.20) | 0.595 | ||
| Severity during hospitalization | 1.36(1.06–1.75) | 0.016 | 1.33(1.03–1.71) | 0.029 |
| Coexisting disorders-no.(%) | ||||
| Hypertension | 0.82(0.64–1.05) | 0.113 | ||
| Diabetes | 1.09(0.80–1.48) | 0.599 | ||
| Cardiovascular disease | 0.86(0.59–1.23) | 0.402 | ||
| Coronary heart disease | 0.90(0.59–1.35) | 0.597 | ||
| Cerebrovascular disease | 0.86(0.50–1.47) | 0.569 | ||
| Chronic liver disease | 0.95(0.52–1.75) | 0.863 | ||
| Chronic kidney disease | 0.98(0.50–2.16) | 0.968 | ||
| COPD | 0.64(0.21–1.93) | 0.427 | ||
| Tumor | 1.67(0.82–3.42) | 0.160 | ||
| Time from discharge to follow-up, per month | 0.68(0.47–0.98) | 0.038 | ||
Risk factors of CAT scoring one year after hospital discharge among older COVID-19 patients
Risk factors for CAT scoring one year after hospital discharge were also evaluated for all older COVID-19 patients (Table 5 -6 ). Disease severity during hospitalization (OR: 1.81, 95% CI: 1.23–2.67, P = 0.003) and age (OR: 1.07, 95% CI: 1.04–1.09, P<0.001) were significantly associated with increased risk of CAT scores ≥10 (Table 5). Age (OR: 1.08, 95% CI: 1.06–1.10, P<0.001) was significantly associated with increased risk of CAT scores >2 (the median), while follow-up time (OR for per month: 0.66, 95% CI: 0.47–0.93, P = 0.017) was inversely associated with increased risk of CAT scores >2 (Table 6).
Table 5.
Logistic regression models to evaluate the risk factors for CAT≥10.
| Variables | Univariate Logistic Analysis |
Multivariate Logistic Analysis |
||
|---|---|---|---|---|
| HR (95%CI) | P-value | OR (95%CI) | P-value | |
| Age, per year | 1.07(1.05–1.10) | <0.001 | 1.07(1.04–1.09) | <0.001 |
| Gender, male | 1.18(0.81–1.72) | 0.389 | ||
| Smoking | 0.99(0.69–1.41) | 0.946 | ||
| Severity during hospitalization | 2.10(1.43–3.06) | <0.001 | 1.81(1.23–2.67) | 0.003 |
| Coexisting disorders-no.(%) | ||||
| Hypertension | 1.20(0.82–1.74) | 0.352 | ||
| Diabetes | 0.90(0.55–1.47) | 0.664 | ||
| Cardiovascular disease | 1.48(0.90–2.42) | 0.123 | ||
| Coronary heart disease | 1.38(0.78–2.41) | 0.266 | ||
| Cerebrovascular disease | 2.40(1.30–4.45) | 0.005 | ||
| Chronic liver disease | 1.19(0.50–2.85) | 0.690 | ||
| Chronic kidney disease | 0.99(0.30–3.32) | 0.992 | ||
| COPD | 1.09(0.25–4.79) | 0.906 | ||
| Tumor | 0.29(0.04–2.17) | 0.229 | ||
| Time from discharge to follow-up, per month | 0.99(0.97–1.01) | 0.388 | ||
Table 6.
Logistic regression models to evaluate the risk factors for CAT > 2.
| Variables | Univariate Logistic Analysis |
Multivariate Logistic Analysis |
||
|---|---|---|---|---|
| HR (95%CI) | P-value | OR (95%CI) | P-value | |
| Age | 1.08(1.06–1.10) | <0.001 | 1.08 (1.06–1.10) | <0.001 |
| Gender, male | 0.96(0.77–1.20) | 0.719 | ||
| Smoking | 1.18(0.95–1.48) | 0.141 | ||
| Severity during hospitalization | 1.39(1.10–1.75) | 0.006 | ||
| Coexisting disorders-no.(%) | ||||
| Hypertension | 1.24(0.99–1.56) | 0.062 | ||
| Diabetes | 1.18(0.89–1.56) | 0.263 | ||
| Cardiovascular disease | 1.70(1.22–2.37) | 0.002 | ||
| Coronary heart disease | 1.70(1.17–2.47) | 0.005 | ||
| Cerebrovascular disease | 2.09(1.27–3.44) | 0.004 | ||
| Chronic liver disease | 1.11(0.64–1.93) | 0.707 | ||
| Chronic kidney disease | 1.30(0.64–2.67) | 0.470 | ||
| COPD | 0.96(0.39–2.38) | 0.926 | ||
| Tumor | 1.21(0.60–2.45) | 0.590 | ||
| Time from discharge to follow-up, per month | 0.61(0.44–0.85) | 0.004 | 0.66(0.47–0.93) | 0.017 |
Discussion
Age was an independent risk factor for the occurrence and prognosis of COVID-19, and older COVID-19 patients need more health monitoring and medical promotion. In the current study, we systematically evaluated the prevalence rate of post-sequelae and CAT scoring one year after hospital discharge among older COVID-19 patients, as well as the potential risk factors in a multi-center prospective cohort study. We revealed that more than half of the patients reported at least one sequelae one year after hospital discharge, and disease severity during hospitalization was independently associated with increased risk of post-sequelae and emerging sequelae. Totally 9.7% of patients had CAT scores ≥10, and 48.4% had CAT scores >2. Disease severity during hospitalization and age significantly associated with increased risk of CAT scores ≥10. Age was significantly associated with increased risk of CAT scores >2, while follow-up time was inversely associated with increased risk of CAT scores >2. To our knowledge, this should be the first study to focus on long-term post-sequelae of the older COVID-19 patients, as well as their risk factors.
Scientific and clinical evidence was evolving regarding the subacute and long-term effects of COVID-19, which may be caused by cellular damage, innate immune response and procoagulant state caused by SARS-CoV-2 infection.29 , 30 A systematic review reported that the median proportion of individuals experiencing at least one short-term persistent symptom was 72.5% (IQR: 55.0%−80.0%), which was higher than 51.1% in our study.31 However, we didn't find significant difference between several medium-term reports and our results. 9 , 12 , 13 , 15 , 32 Consistent with previous studies, fatigue, which was common after acute lung injury and associated with severe impairment of physical function and quality of life, was the most common symptom.33 Further, the emerging sequelae, including sweating, anxiety, palpitation, edema of lower limbs, smell reduction, and taste change, were all psychological responses and associated with posttraumatic stress disorder (PTSD).34, 35, 36 In our results, anxiety was universal in all subjects, no matter the severe or the non-severe. This indicated that the psychological comfort after hospital discharge of COVID-19 should not be neglected.
As SARS-CoV-2 is an emerging virus, no effective treatment has yet been developed. Corticosteroids, which may reduce inflammatory-induced lung injury, were used frequently for the treatment of viral infections, since high amount of cytokines can be induced by SARS-CoV,37 MERS-CoV38 and SARS-CoV-2 infections.39 However, clinical evidence reveals that corticosteroids cause decreased clearance of SARS-CoV and MERS-CoV and increased complications among survivors.40 In the recovery trial, the authors identified that dexamethasone reduced 28-day mortality among those receiving invasive mechanical ventilation or oxygen at randomization, but not among patients not receiving respiratory support.41 Except for dexamethasone helping benefit patients during hospitalization, however, the conclusion above also reveals that use of steroids should be more precise. Here, our results indicated that usage of corticosteroid-related therapy was associated with increased risk of both post-sequelae and emerging sequelae, although this might be biased by the disease severity during hospitalization, detailed dose and duration information, and self-reporting symptoms. Taking evidence above together, we next should establish more precise guidelines of corticosteroids use, and strike a balance between saving patients' lives during hospitalization and long-term sequelae.
Study strength and limitations
The strength of the current study includes the large sample size, detailed questionnaire on sequelae and use of CAT scoring system, long-term follow-up period, and focus on the older population. However, the interpretation and generalizability of the findings in the current study were also affected by several limitations. First, similar to other follow-up studies, high rate of lost follow-up possibly caused by individual willingness of patients not to be continuously concerned might lead to underestimation of the incidence of post-sequelae. However, the PSM suggests this bias might be limited. Second, telephone follow-up relied on patient self-reported symptoms may affect the accuracy of the post-sequelae survey and CAT scoring, although we performed rigorous quality control and repeat surveys of partial samples. Third, the absence of a non-COVID-19 control group and the absence of a pre-COVID-19 CAT assessment of the same patients limited the possibility of a comparative study. Fourth, currently the disease severity during hospitalization was defined by World Health Organization (WHO) interim guideline for COVID-19. Further more precise severity markers are warranted to classify the patients and guide precise treatment.
Conclusions
Our study provides valuable clues for long-term post-sequelae of the older COVID-19 patients, as well as their risk factors. While the dramatic decline in the prevalence rate of persistent symptoms is reassuring, new sequelae cannot be ignored. Disease severity during hospitalization, age, and follow-up time contributed to the risk of long-term post-sequelae. Studies among different population and exploring relevant mechanisms are warranted to validate the results and popularize our findings.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Acknowledgments
The present study was funded by Outstanding Youth Science Foundation of Chongqing (cstc2020jcyj-jqX0014), Chongqing Talent Fund (CQYC202005003), and the Science Foundation for Outstanding Young People of the Army Medical University (grant to Pro Xiangyu Ma and Li). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributions
MX, LQ, CG and LL conceived and designed the study. MX, FX, MC, CY, and LQ drafted the paper and did the statistical analysis. FX, MC, CY, LH, ZK, YS, LL, CG collected the data. All authors approved the final draft of the manuscript for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.12.005.
Appendix. Supplementary materials
References
- 1.Moore J.P., Offit P.A. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA. 2021;325:821–822. doi: 10.1001/jama.2021.1114. [DOI] [PubMed] [Google Scholar]
- 2.WHO. WHO COVID-19 Dashboard. (2020).
- 3.Ahmed H., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehab Med. 2020;52 doi: 10.2340/16501977-2694. jrm00063. [DOI] [PubMed] [Google Scholar]
- 4.Honigsbaum M., Krishnan L. Taking pandemic sequelae seriously: from the Russian influenza to COVID-19 long-haulers. Lancet. 2020;396:1389–1391. doi: 10.1016/S0140-6736(20)32134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yelin D., et al. Long-term consequences of COVID-19: research needs. Lancet Infect Dis. 2020;20:1115–1117. doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koff W.C., Williams M.A. Covid-19 and immunity in aging populations - a new research agenda. N Engl J Med. 2020;383:804–805. doi: 10.1056/NEJMp2006761. [DOI] [PubMed] [Google Scholar]
- 7.Fang X., et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging. 2020;12:12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L., et al. Development and validation of a prognostic nomogram for predicting in-hospital mortality of COVID-19: a multicenter retrospective cohort study of 4086 cases in China. Aging. 2021;13:3176–3189. doi: 10.18632/aging.202605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Writing Committee for the, C.S.G. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Gassel R.J.J., et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am J Respir Crit Care Med. 2021;203:371–374. doi: 10.1164/rccm.202010-3823LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho-Schneider C., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosn J., et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peghin M., et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua-Huy T., et al. Persistent nasal inflammation 5 months after acute anosmia in patients with COVID-19. Am J Respir Crit Care Med. 2021;203:1319–1322. doi: 10.1164/rccm.202011-4258LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero-Duarte A., et al. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19:129. doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Borst B., et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labarca G., et al. Analysis of clinical symptoms, radiological changes and pulmonary function data 4 months after COVID-19. Clin Respir J. 2021 doi: 10.1111/crj.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y.M., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guler S.A., et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021;57 doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerum T.V., et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57 doi: 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skjorten I., et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J. 2021 doi: 10.1183/13993003.00996-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellan M., et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seessle J., et al. Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X., et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daynes E., Gerlis C., Briggs-Price S., Jones P., Singh S.J. COPD assessment test for the evaluation of COVID-19 symptoms. ThoraxThorax. 2021;76:185–187. doi: 10.1136/thoraxjnl-2020-215916. [DOI] [PubMed] [Google Scholar]
- 27.Organization, W.H. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. (January 28, 2020).
- 28.Organization, W.H. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance. (2020).
- 29.Montani D., et al. Multidisciplinary approach for post-acute COVID-19 syndrome: time to break down the walls. Eur Respir J. 2021;58 doi: 10.1183/13993003.01090-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nalbandian A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasserie T., Hittle M., Goodman S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shang Y.F., et al. Half-year follow-up of patients recovering from severe COVID-19: analysis of symptoms and their risk factors. J Intern Med. 2021 doi: 10.1111/joim.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan E., et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42:849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bracha H.S., et al. The STRS (shortness of breath, tremulousness, racing heart, and sweating): a brief checklist for acute distress with panic-like autonomic indicators; development and factor structure. Ann Gen Hosp Psychiatry. 2004;3:8. doi: 10.1186/1475-2832-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schafer L., et al. Nocturnal Olfactory Stimulation for Improvement of Sleep Quality in Patients With Posttraumatic Stress Disorder: a Randomized Exploratory Intervention Trial. J Trauma Stress. 2019;32:130–140. doi: 10.1002/jts.22359. [DOI] [PubMed] [Google Scholar]
- 36.Bedwell J.S., et al. Neurophysiological response to olfactory stimuli in combat veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2018;206:423–428. doi: 10.1097/NMD.0000000000000818. [DOI] [PubMed] [Google Scholar]
- 37.He L., et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falzarano D., et al. Treatment with interferon-alpha2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Group R.C., et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.