Abstract
Rationale: Although clinical trials have found that pulmonary rehabilitation (PR) can reduce the risk of readmissions after hospitalization for a chronic obstructive pulmonary disease (COPD) exacerbation, less is known about PR’s impact in routine clinical practice.
Objectives: To evaluate the association between initiation of PR within 90 days of discharge and rehospitalization(s).
Methods: We analyzed a retrospective cohort of Medicare beneficiaries (66 years of age or older) hospitalized for COPD in 2014 who survived at least 30 days after discharge.
Measurements and Main Results: We used propensity score matching and estimated the risk of recurrent all-cause rehospitalizations at 1 year using a multistate model to account for the competing risk of death. Of 197,376 total patients hospitalized in 4,446 hospitals, 2,721 patients (1.5%) initiated PR within 90 days of discharge. Overall, 1,534 (56.4%) patients who initiated PR and 125,720 (64.6%) who did not were rehospitalized one or more times within 1 year of discharge. In the propensity-score–matched analysis, PR initiation was associated with a lower risk of readmission in the year after PR initiation (hazard ratio, 0.83; 95% confidence interval, 0.77–0.90). The mean cumulative number of rehospitalizations at 1 year was 0.95 for those who initiated PR within 90 days and 1.15 for those who did not (P < 0.001).
Conclusions: After hospitalization for COPD, Medicare beneficiaries who initiated PR within 90 days of discharge experienced fewer rehospitalizations over 1 year. These results support findings from randomized controlled clinical trials and highlight the need to identify effective strategies to increase PR participation.
Keywords: physical exercise, chronic lung disease, readmissions
At a Glance Commentary
Scientific Knowledge on the Subject
Meta-analyses of randomized controlled trials have found that initiating pulmonary rehabilitation (PR) after an exacerbation can reduce the risk of readmission and improve survival. However, the number of patients studied in trials remains low, PR interventions vary among studies, most trials have been performed in Europe, and results have shown significant heterogeneity. Moreover, the results of randomized trials are not always fully generalizable to routine clinical settings, where patient populations are more diverse and where care is less protocolized.
What This Study Adds to the Field
In this observational cohort study of nearly 200,000 patients 66 years of age or older hospitalized with a chronic obstructive pulmonary disease exacerbation, only 1.5% of the patients initiated PR within 90 days of discharge. Patients who initiated PR within this time frame were less likely to have a rehospitalization within 1 year of discharge, and they spent more days at home. Because these findings reflect the experience of diverse patients receiving care in routine clinical settings, our results support and strengthen current guideline recommendations surrounding the role of PR after an exacerbation.
Chronic obstructive pulmonary disease (COPD) is one of the most common chronic conditions worldwide. COPD exacerbations—which result in more than 1.5 million emergency department visits and 700,000 hospitalizations annually in the United States—account for about half of annual healthcare expenditures for COPD (1). Among Medicare beneficiaries 65 years or older admitted to the hospital with a COPD exacerbation, approximately 20% are readmitted within 30 days, and 64% are readmitted within 1 year of discharge (2, 3). Several factors, including previous disease severity, comorbidities, previous hospital admission, poor health status, and not being involved in routine physical activity, were found to be associated with a higher risk of rehospitalization after an exacerbation of COPD (4, 5). In addition to increased healthcare costs, hospitalizations are associated with severe and prolonged deterioration in health status and an increased mortality risk (2, 6).
Pulmonary rehabilitation (PR) is a structured program of exercise, self-management education, and support that aims to improve the physical and psychological condition of people with COPD and promote health-enhancing behaviors (7). Several small randomized controlled trials (RCTs) and two meta-analyses have suggested that early initiation of PR can reduce the risk of rehospitalization (pooled odds ratio from meta-analysis, 0.44; 95% confidence interval [CI], 0.21–0.91) and death (8–10). On the basis of these data, guidelines for the treatment of patients with COPD from the American Thoracic Society, the European Respiratory Society, and the American College of Chest Physicians recommend initiation of PR within 3 weeks of an exacerbation (11, 12). However, little is known about the effectiveness of PR in preventing readmissions in routine clinical practice settings, and results from prior studies are conflicting. One large retrospective study from the United Kingdom compared rates of hospital admissions and visits to primary care 1 year before and 1 year after initiation of PR and showed that participation in PR was not associated with fewer exacerbations (13). In contrast, the latest UK COPD PR National Audit found that completion of PR was associated with a lower risk of hospitalization and time spent in the hospital (14). Therefore, we sought to determine the association between initiation of PR within 90 days of discharge from a hospitalization for COPD exacerbation and 1-year all-cause rehospitalization in Medicare patients. Some of the results of these studies have been previously reported in the form of an abstract and a publication (15, 16).
Methods
Design, Setting, and Subjects
We conducted a retrospective cohort study of fee-for-service Medicare beneficiaries 66 years of age or older who were hospitalized for a COPD exacerbation in 2014. Hospitalizations for COPD were defined by the International Classification of Diseases, 9th Revision, Clinical Modification codes used by the Center for Medicare and Medicaid Services to calculate readmissions and mortality measures (see Table E1 in the online supplement). To create an inception cohort, we excluded patients who had participated in PR in the year before the index admission. For individuals with multiple COPD hospitalizations in 2014, we considered the first hospitalization to be the index admission. To allow for the assessment of comorbidities and prior healthcare use, we limited the analysis to individuals with a full year of Medicare coverage before the index admission. To emulate inclusion/exclusion criteria for a clinical trial, we excluded patients with a low probability of referral or initiation of PR after discharge, including the following: patients who died within 30 days of discharge, those who would qualify for cardiac rehabilitation on the basis of an acute cardiac event or coronary surgery, patients with other diagnoses that might interfere with participation (e.g., dementia, metastatic cancer, or paralysis), those admitted from or discharged to hospice, and individuals discharged to a nursing home who remained in the facility for more than 30 days.
Exposure: PR Participation within 90 Days of Discharge
Receipt of PR was defined on the basis of one or more charges for PR (Healthcare Common Procedure Coding System codes G0424 [COPD-specific] and G0237, G0238, and G0239 [nonspecific]). Charges were obtained from the Medicare outpatient file, which contains claims data from institutional outpatient providers (i.e., hospital outpatient-based facilities), and the Medicare carrier file, which contains claims from noninstitutional providers (i.e., physicians’ offices). We computed the number of days between hospital discharge and the first PR session and recorded instances in which the first session occurred within 90 days of discharge.
Outcomes
Our primary outcome was rehospitalization due to any cause, including observation and inpatient status encounters, within 1 year of discharge from the index hospitalization. We excluded elective readmissions using the 2015 U.S. Centers for Medicare and Medicaid Services planned readmission algorithm methodology (17). A secondary outcome was readmission due to a COPD exacerbation defined as a principal diagnosis of COPD or a principal diagnosis of acute respiratory failure paired with a secondary diagnosis of COPD with exacerbation. To account for differences in follow-up period due to mortality, and because they are considered patient-centered outcomes, we also evaluated the number of person-days per year spent in the hospital or in the emergency department out of the total number of days of follow-up and person-days per year spent in a skilled nursing facility.
Patient, Hospital, and Community Factors
We recorded patient demographics, including age, sex, race/ethnicity, Medicaid dual eligibility, current tobacco use, and whether the patient was admitted from home or a skilled nursing facility. We assessed individual comorbidities using Agency for Healthcare Research and Quality comorbidity software and computed a longitudinal Charlson comorbidity score (18). We calculated each patient’s risk of frailty and considered a patient to be frail if the probability of frailty was >20% (19). As proxies for COPD severity, we recorded the following factors: use of home oxygen in the 90 days before admission, receipt of mechanical ventilation during the index hospitalization, and a count of all-cause hospitalizations in the 12 months before the index admission. In addition, we assessed emergency department visits and readmissions that occurred within the first 90 days after discharge from the index hospitalization but before the initiation of PR. We noted the date of PR initiation among those starting more than 90 days after discharge and with a date of death occurring less than a year after discharge. We determined geographic accessibility of PR by calculating the distance from the age-65-and-over population-weighted centroid of each individual’s zip code of residence to the nearest PR provider (20). For each index admission, we recorded hospital characteristics that included the number of beds, geographic region, teaching status, and whether the hospital served a rural or urban community. In addition, we used patient zip code to characterize several community-level factors that have previously been linked to hospital readmission rates. These include socioeconomics, demographics, and access to care factors (21).
Statistical Analysis
We compared the characteristics of patients who began PR within 90 days and those who did not, including patients who never participated in PR and those who began PR more than 90 days after discharge. Outcomes among patients starting PR more than 90 days after discharge were censored at PR start day. Given the large sample size, we used absolute standardized differences instead of P values; we considered a value >10% to reflect a clinically meaningful difference between groups (22). Outcomes were compared via chi-square tests.
We calculated a propensity score for initiation of PR within 90 days of discharge using a nonparsimonious generalized estimating equation logistic regression model that accounted for the clustering of patients within hospitals. The model included all aforementioned patient sociodemographic characteristics, tobacco use, comorbidities, frailty, markers of COPD severity, hospital characteristics, and selected interaction terms. In propensity model development, we excluded patients discharged from hospitals where PR was not provided, because such patients had no possibility of receiving it. We then applied model coefficients to estimate a propensity for initiation of PR to all patients included in the study.
Our primary goal was to evaluate the association of PR within 90 days of discharge and risk of readmissions within 1 year of discharge while accounting for the following: 1) the time-varying exposure to PR (patients begin PR at different times after discharge), 2) recurrent rehospitalizations, and 3) the competing risk of death. We applied a multistate model, an analytical approach that accounts for the risk of death (23). In the multistate model, after discharge from the index admission, a patient can transition to rehospitalization (T1), death without rehospitalization (T2), or survival to year’s end without readmission. After a rehospitalization, a patient can transition to additional rehospitalization (T1) one or more times (not distinguished by count in this model), death after rehospitalization (T3), or survival to the year’s end without either event (Figure 1A). These transitions were explicitly modeled by defining the current risk-set of transitions for each patient for each event (readmission, death), conditional on the event history of the patient. We chose to model all recurrent readmissions with a single risk estimate rather than modeling risk of a first readmission separately from a second, given that the first had occurred, because the index admission was not the first-ever admission for many patients. Cox regression models were then used to assess the association between PR and recurrent readmissions over 1 year.
Figure 1.
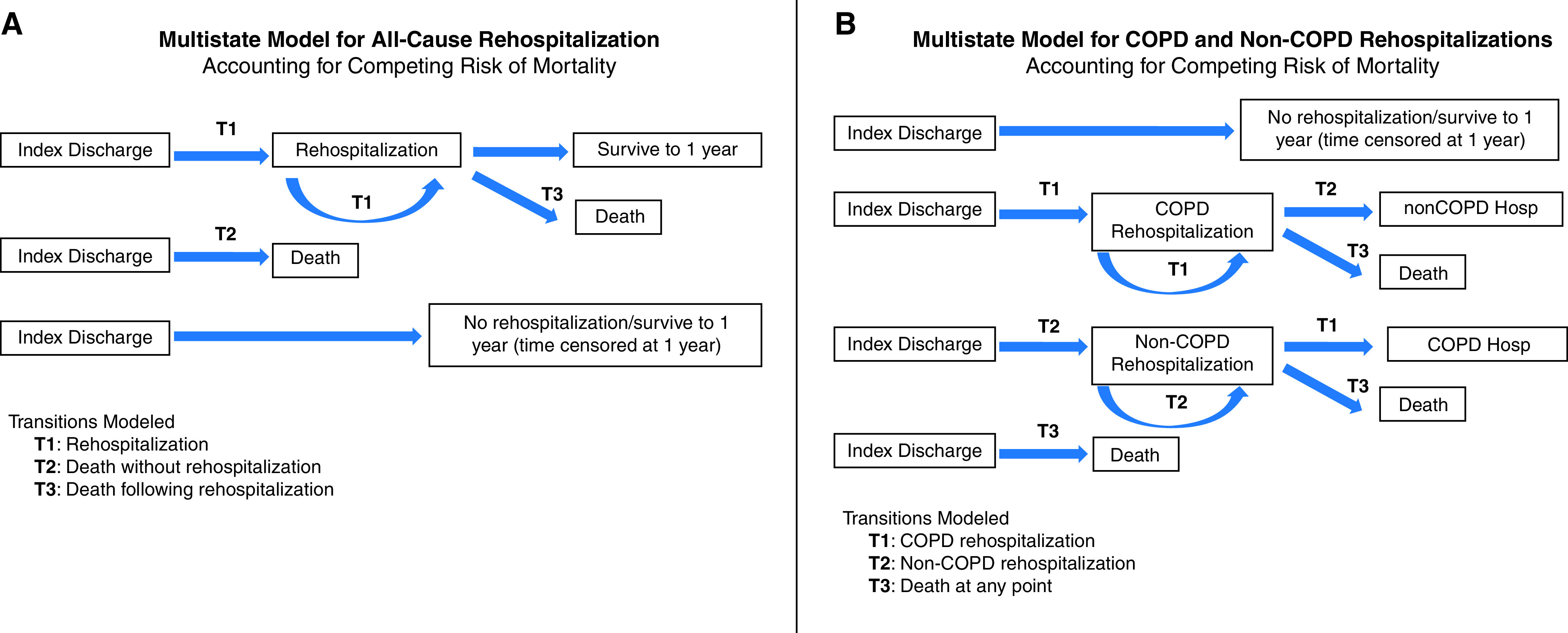
Multistate models for (A) all-cause rehospitalization and (B) chronic obstructive pulmonary disease (COPD) and non-COPD rehospitalizations. Hosp = hospitalization.
In a secondary analysis, we evaluated the association of PR within 90 days of discharge with COPD-specific readmissions. We again used a multistate model, allowing transition to COPD-specific readmissions (T1), any non-COPD readmissions (T2), or death (T3) at any point (Figure 1B). In these models, we assumed that the transition to COPD or other cause admission was independent of the prior admission type.
In our primary analysis, we matched each patient who began PR within 90 days of discharge to a patient who did not begin PR and had survived to the PR start date of the matched patient (to avoid immortal time bias). Using a multistate model, we modeled time from the matched PR start date and included readmissions and emergency department visits before the PR start day as additional covariates together with community factors. To assess heterogeneity of treatment effect, we evaluated interactions between PR initiation and age, frailty, comorbidity burden, and use of home oxygen before admission.
As a sensitivity analysis, we performed a landmark analysis, including only patients who survived at least 90 days after hospital discharge. The goal of the landmark method is to estimate, in an unbiased way, the time-to-event probabilities in each group conditional on the group membership of patients at a specific time point, the landmark time. The landmark analysis eliminates overlap between the treatment initiation period and outcome assessment (24). We used a multistate model to estimate the risk of readmission from 90 days to 1 year after discharge, and we accounted for the following patient factors: demographics, Medicaid dual eligibility, tobacco use, proxies for COPD severity, selected comorbidities, frailty risk, propensity for PR, readmissions within 90 days, and community factors.
All statistical testing was two-sided, using a 0.05 level of significance. All analyses were performed using SAS (version 9.4; SAS Institute, Inc.), and Figures were created using Stata (Release 16; StataCorp LLC). The project was approved by the Baystate Institutional Review Board.
Results
Full Cohort Characteristics and Unadjusted Outcomes
Drawn from 4,446 hospitals, the cohort included 197,376 patients of whom 2,721 (1.5%) initiated PR within 90 days of discharge (Figure E1). PR started within 3 weeks from index discharge among 496 (0.3%) patients. Compared with patients who did not initiate PR or started after 90 days, those who initiated PR within 90 days of discharge were younger, were more likely to be non-Hispanic White, and tended to live closer to a PR facility. PR patients were less likely to have been admitted to a hospital from a skilled nursing facility and less likely to have had an admission in the prior year. They had lower mean comorbidity scores and a lower risk of being frail. As we have previously reported, mortality within 1 year of discharge in those who participated in PR within 90 days of discharge was lower than in those who did not (15) (Table 1).
Table 1.
Patient Characteristics in Full Cohort and among PR Initiators and Noninitiators or Late Initiators
| Patient Characteristics | Total | No PR/Late PR | PR within 90 Days of Discharge* | Absolute Standardized Differences (%) † |
|---|---|---|---|---|
| n (%) | 197,376 (100) | 194,655 (98.5) | 2,721 (1.5) | — |
| Age, yr | 76.9 ± 7.6 | 77.0 ± 7.6 | 74.5 ± 6.1 | 35.92 |
| Sex, F | 115,690 (58.6) | 114,263 (58.7) | 1,427 (52.4) | 12.62 |
| Race/ethnicity | 24.37 | |||
| Non-Hispanic White | 168,114 (85.2) | 165,594 (85.1) | 2,520 (92.6) | — |
| Black | 16,885 (8.6) | 16,759 (8.6) | 126 (4.6) | — |
| Hispanic | 8,084 (4.1) | 8,038 (4.1) | 46 (1.7) | — |
| Other | 4,293 (2.2) | 4,264 (2.2) | 29 (1.1) | — |
| Frailty index indicator cut-off > 20% | 71,860 (36.4) | 71,377 (36.7) | 483 (17.8) | 43.50 |
| Distance to nearest PR, miles | 9.8 ± 14.8 | 9.8 ± 14.8 | 5.8 ± 6.4 | 34.99 |
| Dual eligibility (Medicaid buy-in) | 52,284 (26.5) | 51,980 (26.7) | 304 (11.2) | 40.44 |
| Current tobacco user | 46,517 (23.6) | 45,922 (23.6) | 595 (21.9) | 4.12 |
| Weighted Charlson Comorbidity Index | 4.2 ± 3.2 | 4.2 ± 3.2 | 3.5 ± 2.9 | 25.14 |
| Prior year all-cause admissions | 22.46 | |||
| No admits | 103,676 (52.5) | 101,992 (52.4) | 1,684 (61.9) | — |
| One admit | 45,646 (23.1) | 45,062 (23.1) | 584 (21.5) | — |
| Two or more admits | 48,054 (24.4) | 47,601 (24.5) | 453 (16.7) | — |
| Home oxygen use | 62,834 (31.8) | 61,761 (31.7) | 1,073 (39.4) | 16.15 |
| Characteristics of index hospitalization | ||||
| Principal diagnosis | 2.93 | |||
| Acute respiratory failure | 31,892 (16.2) | 31,423 (16.1) | 469 (17.2) | — |
| COPD | 165,484 (83.8) | 163,232 (83.9) | 2,252 (82.8) | — |
| Pneumonia as secondary diagnosis | 40,191 (20.4) | 39,705 (20.4) | 486 (17.9) | 6.45 |
| Noninvasive ventilation | 15,175 (7.7) | 14,960 (7.7) | 215 (7.9) | 0.81 |
| Invasive ventilation | 6,332 (3.2) | 6,248 (3.2) | 84 (3.1) | 0.7 |
| Admitted from SNF | 24,482 (12.4) | 24,341 (12.5) | 141 (5.2) | 26.01 |
| Comorbidities | ||||
| Diabetes | 63,684 (32.3) | 62,999 (32.4) | 685 (25.2) | 15.93 |
| Congestive heart failure | 62,430 (31.6) | 61,874 (31.8) | 556 (20.4) | 26.07 |
| Deficiency anemias | 36,408 (18.4) | 36,071 (18.5) | 337 (12.4) | 17.06 |
| Psychoses/depression | 36,354 (18.4) | 35,893 (18.4) | 461 (16.9) | 3.92 |
| Renal failure | 35,719 (18.1) | 35,387 (18.2) | 332 (12.2) | 16.71 |
| Hypothyroidism | 35,156 (17.8) | 34,724 (17.8) | 432 (15.9) | 7.41 |
| Obesity | 29,430 (14.9) | 29,003 (14.9) | 427 (15.7) | 2.2 |
| Obstructive sleep apnea | 25,786 (13.1) | 25,320 (13) | 466 (17.1) | 11.53 |
| Neurological disorders | 14,389 (7.3) | 14,261 (7.3) | 128 (4.7) | 11.04 |
| Weight loss | 8,763 (4.4) | 8,665 (4.5) | 98 (3.6) | 4.32 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; PR = pulmonary rehabilitation; SNF = skilled nursing facility.
Data are presented as n (%) or mean ± SD unless otherwise indicated.
PR within 3 months of discharge.
Standardized differences of >10% are considered meaningful.
A total of 127,254 (64.5%) patients were rehospitalized at least once in the year after their index discharge, and 95,282 (48.3%) had at least one visit to the emergency department that did not result in hospitalization. Compared with patients who did not initiate PR or who began the program more than 90 days after discharge, initiation of PR within 90 days was associated with a lower all-cause readmission rate at 1 year (56.4% vs. 64.6%) and a lower mean number of rehospitalizations (1.2 vs. 1.5; P < 0.001). COPD-specific hospitalizations within 1 year were higher among those who started PR within 90 days (33.6% vs. 31.5%; P = 0.021). The number of days spent in the hospital per person-year (SD) was lower in those who initiated PR within 90 days of discharge (11.5 [19.1] d) than in those who did not (22.7 [34.3] d) (Table 2).
Table 2.
Unadjusted Outcomes in the Full Cohort and Propensity-Matched Cohort
| Full Cohort*
|
Propensity-Score–Matched Cohort
†
|
|||||
|---|---|---|---|---|---|---|
| No PR/ Late PR | PR within 90 Days of Discharge | P Value (Chi-square Test) | No PR/ Late PR | PR within 90 Days of Discharge | P Value (Conditional Regression) | |
| n (%) | 194,655 (98.5) | 2,721 (1.5) | — | 2,710 (50.0) | 2,710 (50.0) | — |
| Any unplanned all-cause rehospitalizations/SNF/ED visits in 1 year after discharge | 147,631 (75.8) | 1,901 (69.9) | <0.001 | 1,730 (63.8) | 1,732 (63.9) | 0.95 |
| Mortality 1 year after discharge | 38,104 (19.6) | 198 (7.3) | <0.001 | 382 (14.1) | 198 (7.3) | <0.001 |
| Number of days spent in the hospital per person-year | 22.7 ± 34.3 | 11.5 ± 19.1 | <0.001 ‡ | 11.7 ± 30.5 | 7.9 ± 18.9 | <0.001 |
| Number of days spent in nursing home per person-year | 4.9 ± 17.0 | 2.0 ± 8.7 | <0.001 ‡ | 2.9 ± 14.0 | 1.8 ± 7.8 | 0.0003 |
| Number of days spent in ED per person-year | 1.4 ± 2.9 | 1.1 ± 2.0 | 0.001 ‡ | 1.1 ± 2.3 | 1.0 ± 2.0 | 0.025 |
| Average number of unplanned rehospitalizations | 1.5 ± 1.9 | 1.2 ± 1.5 | <0.001 ‡ | 1.2 ± 1.7 | 1.1 ± 1.6 | 0.02 |
| Categories of number of unplanned rehospitalizations in 1 year after discharge | <0.001 | 0.091 | ||||
| No rehospitalizations during follow-up | 68,935 (35.4) | 1,187 (43.6) | — | 1,333 (49.2) | 1,368 (50.5) | — |
| One rehospitalization during follow-up | 55,626 (28.6) | 756 (27.8) | — | 576 (21.3) | 624 (23.0) | — |
| Two or more rehospitalizations during follow-up | 70,094 (36.0) | 778 (28.6) | — | 801 (29.6) | 718 (26.5) | — |
| Unplanned COPD rehospitalizations in 1 year after discharge | 61,344 (31.5) | 914 (33.6) | 0.021 | 735 (27.1) | 726 (26.8) | 0.78 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; ED = emergency department; PR = pulmonary rehabilitation; SNF = skilled nursing facility.
Data are presented as n (%) or mean ± SD unless otherwise indicated.
Outcomes assessed from discharge until 1 year or PR start after 90 days or death.
Outcomes assessed from matched PR start day until 1 year after discharge or PR start after 90 days or death.
Kruskal-Wallis test.
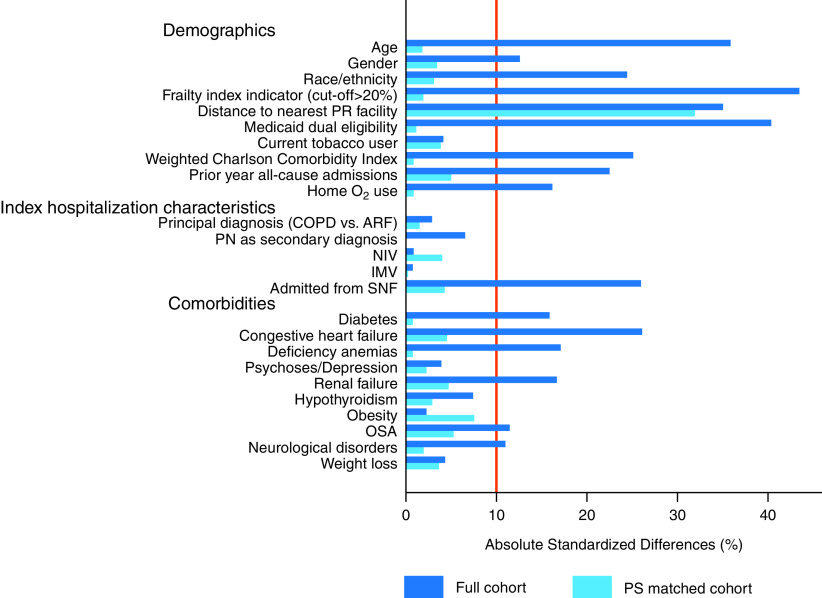
Propensity-Matched Analysis
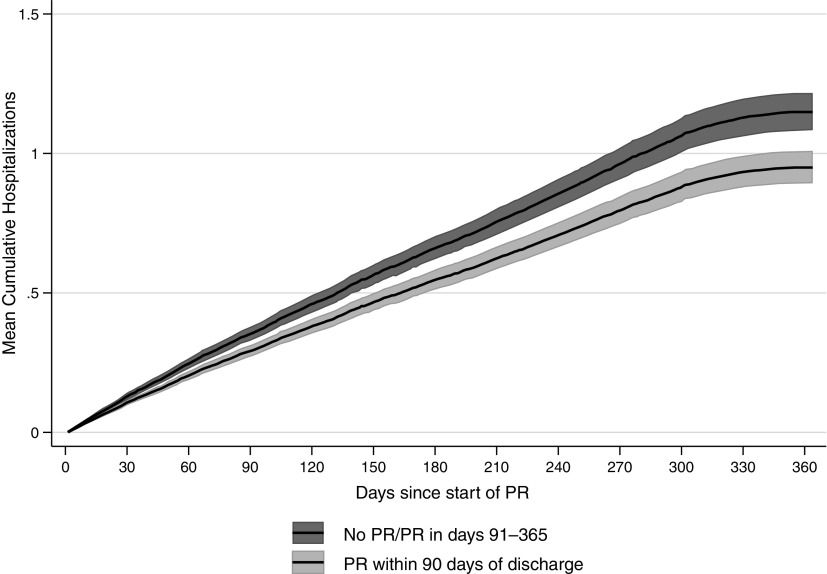
Overall, 99.6% of PR initiators were matched on their propensity for PR, and their characteristics were well balanced other than the distance to PR facility, which was greater in the non-PR group (Figure 2). The risk of readmission after PR initiation was lower among those who initiated PR within 90 days of discharge than in those who did not: hazard ratio (HR), 0.83 (95% CI, 0.77–0.90). Figure 3 shows the multivariable-adjusted risk of readmission over time, by PR participation. The readmission curves start to diverge early, with a mean cumulative number of readmissions of 0.95 readmissions at 1 year among patients who began PR within 90 days and 1.15 readmissions among patients who did not. There were no significant interactions between PR and the patient characteristics of age, frailty, comorbidity burden, or use of home oxygen.
Figure 2.
Absolute standardized differences for patient characteristics in the full cohort and propensity analysis. ARF = acute respiratory failure; COPD = chronic obstructive pulmonary disease; IMV = invasive mechanical ventilation; NIV = noninvasive ventilation; OSA = obstructive sleep apnea; PN = pneumonia; PR = pulmonary rehabilitation; PS = propensity score; SNF = skilled nursing facility.
Figure 3.
Multistate analysis using Cox proportional regression in the propensity-matched analysis. PR = pulmonary rehabilitation.
Patients who initiated PR within 90 days spent fewer days in the hospital or in a nursing home than those who did not or who started more than 90 days after discharge (number of days spent in the hospital per person-year: 7.9 d vs. 11.7 d; number of days spent in a nursing home per person-year: 1.8 d vs. 2.9 d).
Using the landmark approach initiation of PR within 90 days was associated with a similarly lower risk of readmission over the subsequent 9 months as in our primary analysis (HR, 0.87; 95% CI, 0.82–0.93).
COPD-Specific Readmissions
In the propensity-matched cohort, adjusting for unbalanced patient covariates as well as community characteristics, the initiation of PR within 90 days was associated with a lower risk of both COPD-specific rehospitalization (HR, 0.86; 95% CI, 0.76–0.97) and non-COPD rehospitalization (HR, 0.79; 95% CI, 0.71–0.87) in the year after PR initiation. Similar findings were observed using the landmark approach (see Table 3).
Table 3.
Covariate-Adjusted Outcomes in the Propensity-Matched Cohort and Landmark Cohort*
| Multistate Models | PS-Matched Cohort |
Landmark Cohort |
||||||
|---|---|---|---|---|---|---|---|---|
| HR | LL | UL | P Value | HR | LL | UL | P Value | |
| Unplanned all-cause rehospitalization | 0.83 | 0.77 | 0.9 | <0.001 | 0.87 | 0.82 | 0.93 | <0.001 |
| Unplanned COPD rehospitalization | 0.856 | 0.76 | 0.965 | 0.0108 | 0.927 | 0.862 | 0.998 | 0.0439 |
| Unplanned non-COPD rehospitalization | 0.788 | 0.712 | 0.872 | <0.001 | 0.856 | 0.802 | 0.915 | <0.001 |
D efinition of abbreviations: COPD = chronic obstructive pulmonary disease; HR = hazard ratio; LL = lower limit; PS = propensity score; UL = upper limit.
Covariate-adjusted models with robust SEs accounting for multiple rehospitalizations within patients and competing risk of mortality.
Discussion
In this large observational study of U.S. Medicare beneficiaries hospitalized for COPD in 2014, we found that participation in PR within 90 days of discharge was associated with a lower risk of all-cause and COPD-specific rehospitalization at 1 year than nonparticipation or participation after 90 days. Although the risk of readmission was high in both groups, it was significantly lower in those initiating PR within 90 days of discharge. In addition, the number of days that patients spent in the hospital per person-year among those who initiated PR within 90 days was lower than those who did not or who started more than 90 days after discharge. Our findings were robust to several analytic approaches that accounted for differences in the population of patients who received or did not receive PR, the time-varying nature of the PR exposure, recurrent readmissions, and the substantial competing risk of death. Furthermore, the results of the landmark analysis were comparable to the propensity-matched results, increasing confidence in the reliability of these effect estimates. These results complement existing evidence from randomized trials, adding further data suggesting that PR is associated with a lower risk of readmission among this highly vulnerable population.
A recent meta-analysis of 13 RCTs showed that PR initiated within 4 weeks of the COPD discharge decreased the number of days that patients spent in the hospital at the end of PR treatment by 4.3 days (1 trial, 180 patients; 95% CI, −6.8 to −1.7) and decreased hospital readmissions (6 trials, 319 patients; relative risk = 0.47; 95% CI, 0.29 to 0.75) (8). In addition, a 2016 Cochrane meta-analysis found that PR significantly reduced the mean number of hospital admissions per patient from 1.6 to 0.9 during the year after hospital admission for an exacerbation (8 studies; 810 participants) and reduced hospital readmissions (pooled odds ratio, 0.44); however, the results were heterogeneous (I2 = 77%) (9). Our study results are consistent with the findings from the randomized trials; however, the effect size that we estimated is somewhat attenuated from that seen in previous trials. This may be related to the more diverse population included in our study and to the less protocolized nature of PR in routine clinical settings.
Another key finding of our study is that patients who initiated PR within 90 days of discharge spent fewer days in the hospital and/or a nursing home during the year after discharge. “Home-time” (being alive and out of any healthcare institution) is an outcome prioritized by patients, especially the elderly, and could be a valuable outcome measure for future randomized clinical trials (25).
Readmissions after a hospitalization for COPD remain a challenging problem for patients and healthcare systems, and reducing readmissions has become a significant policy target in many countries. In the United States, COPD has been included as one of the conditions under the Medicare Hospital Readmissions Reduction Program, which imposes financial penalties on hospitals with high risk-adjusted readmission rates (26). The Hospital Readmissions Reduction Program has prompted many institutions to allocate resources to adopt strategies to reduce readmissions. However, there is little high-quality evidence to guide healthcare systems in these efforts. RCTs that have evaluated readmission reduction strategies for COPD have produced conflicting results, or have even increased the risk of readmission (27, 28). PR is one of the few interventions known to benefit exercise performance, functional status, and survival in patients with COPD. There are several mechanisms by which PR might reduce the risk of rehospitalization, including increasing exercise capacity and functional performance, promoting medication adherence, and enhancing integrated care through augmenting lines of communication among PR staff and other healthcare providers. In a secondary analysis, initiation of PR was associated with a lower risk of readmission due to COPD and a lower risk for other conditions as well. Our study was unable to explain why readmissions for conditions other than COPD might be affected by PR. It could be that exercise and the social components of PR lead to general improvements in health or that readmissions after a hospitalization for COPD are due to a wide range of other causes than COPD; however, it is also possible that this association reflects residual confounding due to unmeasured factors.
Although our results suggest that PR may be an effective strategy for mitigating the recurrent cycle of rehospitalizations in COPD, the low uptake of PR in the postdischarge setting is striking. The low participation in PR that we found has been reported in several prior studies (29, 30), and it is not unique to the United States (31, 32). Our results reinforce the urgent need for developing and testing new strategies to improve participation in PR.
Our study has several strengths. We studied a large sample of Medicare beneficiaries with complete use data for the year before and year after the index hospitalization and were able to ascertain readmissions and deaths with a high degree of accuracy, regardless of the hospital where the patients were admitted or where death occurred. To our knowledge, this is the first study of PR to apply a multistate model and consider the recurrent nature of readmissions, as well as the competing risk of death. This is critical given the high risk of death in patients with COPD and the difference in mortality rates among PR participants compared with nonparticipants. Multistate models represent an advance over prior other methods that do not account for competing risks. These results complement our previously published study, which found that PR is associated with a lower risk of death in the year after hospitalization (15).
Our study also has several limitations. Most importantly, this is an observational study, and treatment assignment was not random. Although we defined the cohort to emulate a target trial, adjusted for multiple patient characteristics (including surrogate markers of disease severity and frailty), and used several analytic techniques, it is possible that the association that we observed reflects residual unmeasured confounding. The Centers for Medicare and Medicaid Services claims lack granularity on social and environmental factors as well as on clinical characteristics such as markers of disease severity used in clinical trials (e.g., Global Initiative for Chronic Obstructive Lung Disease stages) and use of medications. However, we have also included county characteristics (socioeconomics, demographics, and access to care) that could affect participation in PR. Healthy user bias remains a possibility in any observational study involving voluntary participation in an exercise program, which could result in an overestimation of the association between PR and readmissions. Low exercise capacity could have contributed to this bias; however, our analysis controlled for frailty, sociodemographics, use of home oxygen, the Charlson index for comorbidity adjustment, and propensity for PR. In an attempt to minimize this bias, we excluded patients considered to have a low probability of participating in PR, such as those with dementia, metastatic cancer, or paralysis. Another limitation is related to the very small number of patients who initiated PR; they could represent a limited, self-selected subpopulation that is different from the larger population. This is why we matched the patients on the basis of their propensity for PR. We defined PR exposure as a binary variable (PR initiated vs. not within 90 days of discharge) and did not count the number of sessions completed; therefore, we could not establish any dose–response relationship. This decision was made because of the analytical difficulties associated with recurrent readmissions, variable time to accrue PR sessions, and overlap of outcomes with continued accrual of sessions. Our study sample was limited to patients aged 66 years or older; thus, our findings may not be generalizable to younger patients. Furthermore, the analyses were limited to patients enrolled in fee-for-service Medicare and did not include those in Medicare Advantage plans. Nevertheless, more than 66% of Medicare beneficiaries were still enrolled in fee-for-service programs in 2019 (33). Finally, we assessed participation in supervised PR programs, not PR conducted within the patient’s home. It is possible that some patients categorized in the nontreated group received PR at home or in a nursing home; however, this would have biased the results toward the null.
Conclusions
In routine clinical settings, among Medicare beneficiaries, the initiation of PR within 90 days of hospital discharge was associated with a lower risk of rehospitalization over the year after PR initiation. These results support findings from recent RCTs and, given the underuse of PR in this setting, highlight the need for effective strategies to increase participation in PR in this population.
Acknowledgments
Acknowledgment
Ethics committee approval: All procedures in this study were performed in accordance with the ethical standards of Baystate Health’s Institutional Review Board and in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Footnotes
Supported by the National Heart, Lung, and Blood Institute of the NIH under Award Number K24 HL132008-01 and 5R01HL133046-02. Q.R.P. is supported by the National Heart, Lung, and Blood Institute of the NIH under Award Number K23HL135440-01A1. The funders had no role in data collection, management, or analysis; study design or conduct; interpretation of study findings; or the preparation, review, or approval of the manuscript submitted for publication.
Author Contributions: M.S.S.: literature search, study design, data interpretation, and manuscript writing; P.S.P.: study design, data interpretation, data analysis, and manuscript writing; A.P.: data analysis, data interpretation, and manuscript review and editing; R.Z.: data interpretation and manuscript review and editing; K.A.S.: data interpretation and manuscript review and editing; T.C.L.: data interpretation and manuscript review and editing; Q.R.P.: data interpretation and manuscript review and editing; K.M.M.: data interpretation and manuscript review and editing; V.M.P.-P.: data interpretation and manuscript review and editing; and P.K.L.: literature search, study design, data interpretation, manuscript writing, and funding acquisition. A.P. and P.S.P. have accessed and verified the underlying data.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202012-4389OC on July 20, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res . 2013;5:235–245. doi: 10.2147/CEOR.S34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lindenauer PK, Dharmarajan K, Qin L, Lin Z, Gershon AS, Krumholz HM. Risk trajectories of readmission and death in the first year following hospitalization for COPD. Am J Respir Crit Care Med . 2018;197:1009–1017. doi: 10.1164/rccm.201709-1852OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goto T, Faridi MK, Gibo K, Toh S, Hanania NA, Camargo CA, Jr, et al. Trends in 30-day readmission rates after COPD hospitalization, 2006-2012. Respir Med . 2017;130:92–97. doi: 10.1016/j.rmed.2017.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bahadori K, FitzGerald JM. Risk factors of hospitalization and readmission of patients with COPD exacerbation--systematic review. Int J Chron Obstruct Pulmon Dis . 2007;2:241–251. [PMC free article] [PubMed] [Google Scholar]
- 5. Müllerova H, Maselli DJ, Locantore N, Vestbo J, Hurst JR, Wedzicha JA, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest . 2015;147:999–1007. doi: 10.1378/chest.14-0655. [DOI] [PubMed] [Google Scholar]
- 6. Seidel D, Cheung A, Suh ES, Raste Y, Atakhorrami M, Spruit MA. Physical inactivity and risk of hospitalisation for chronic obstructive pulmonary disease. Int J Tuberc Lung Dis . 2012;16:1015–1019. doi: 10.5588/ijtld.12.0050. [DOI] [PubMed] [Google Scholar]
- 7. Nici L, Raskin J, Rochester CL, Bourbeau JC, Carlin BW, Casaburi R, et al. Pulmonary rehabilitation: WHAT WE KNOW AND WHAT WE NEED TO KNOW. J Cardiopulm Rehabil Prev . 2009;29:141–151. doi: 10.1097/HCR.0b013e3181a85cda. [DOI] [PubMed] [Google Scholar]
- 8. Ryrsø CK, Godtfredsen NS, Kofod LM, Lavesen M, Mogensen L, Tobberup R, et al. Lower mortality after early supervised pulmonary rehabilitation following COPD-exacerbations: a systematic review and meta-analysis. BMC Pulm Med . 2018;18:154. doi: 10.1186/s12890-018-0718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Puhan MA, Gimeno-Santos E, Cates CJ, Troosters T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2016;12:CD005305. doi: 10.1002/14651858.CD005305.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greening NJ, Williams JEA, Hussain SF, Harvey-Dunstan TC, Bankart MJ, Chaplin EJ, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ . 2014;349:g4315. doi: 10.1136/bmj.g4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wedzicha JA, Miravitlles M, Hurst JR, Calverley PM, Albert RK, Anzueto A, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J . 2017;49:1600791. doi: 10.1183/13993003.00791-2016. [DOI] [PubMed] [Google Scholar]
- 12. Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med . 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 13. Moore E, Newson R, Joshi M, Palmer T, Rothnie KJ, Singh S, et al. Effects of pulmonary rehabilitation on exacerbation number and severity in people with COPD: an historical cohort study using electronic health records. Chest . 2017;152:1188–1202. doi: 10.1016/j.chest.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 14. Steiner M, McMillan V, Lowe D, et al. London: Royal College of Physicians; 2017. https://www.rcplondon.ac.uk/projects/outputs/pulmonary-rehabilitation-beyond-breathing-better. [Google Scholar]
- 15. Lindenauer PK, Stefan MS, Pekow PS, Mazor KM, Priya A, Spitzer KA, et al. Association between intiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among Medicare beneficiaries. JAMA . 2020;323:1813–1823. doi: 10.1001/jama.2020.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stefan MS, Priya A, Pekow PS, Pinto-Plata VM, Mazor KM, Spitzer KA, et al. The association between pulmonary rehabilitation and rehospitalizations in patients with COPD [abstract] Am J Respir Crit Care Med . 2020;201:A2503. doi: 10.1164/rccm.202012-4389OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.2016 Measure information about the 30-day all-cause hospital readmission measure, calculated for the 2018 value-based payment modifier program. Washington, DC: U.S. Department of Health and Human Services; 2017. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/2016_acr_mif.pdf. [Google Scholar]
- 18. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care . 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 19. Segal JB, Chang H-Y, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care . 2017;55:716–722. doi: 10.1097/MLR.0000000000000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Missouri Census Data Center Columbia, MO: University of Missouri Center of Health Policy; https://mcdc.missouri.edu/applications/geocorr2014.html. [Google Scholar]
- 21.Social Explorer. https://www.socialexplorer.com/
- 22. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput . 2009;38:1228–1234. [Google Scholar]
- 23. Ieva F, Jackson CH, Sharples LD. Multi-state modelling of repeated hospitalisation and death in patients with heart failure: the use of large administrative databases in clinical epidemiology. Stat Methods Med Res . 2017;26:1350–1372. doi: 10.1177/0962280215578777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes . 2011;4:363–371. doi: 10.1161/CIRCOUTCOMES.110.957951. [DOI] [PubMed] [Google Scholar]
- 25. Lee H, Shi SM, Kim DH. Home time as a patient-centered outcome in administrative claims data. J Am Geriatr Soc . 2019;67:347–351. doi: 10.1111/jgs.15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hospital readmissions reduction program. Baltimore, MD: Centers for Medicare & Medicaid Services; 2021. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program. [Google Scholar]
- 27. Aboumatar H, Naqibuddin M, Chung S, Chaudhry H, Kim SW, Saunders J, et al. Effect of a hospital-initiated program combining transitional care and long-term self-management support on outcomes of patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA . 2019;322:1371–1380. doi: 10.1001/jama.2019.11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah T, Press VG, Huisingh-Scheetz M, White SR. COPD readmissions: addressing COPD in the era of value-based health care. Chest . 2016;150:916–926. doi: 10.1016/j.chest.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spitzer KA, Stefan MS, Priya A, Pack QR, Pekow PS, Lagu T, et al. Participation in pulmonary rehabilitation after hospitalization for chronic obstructive pulmonary disease among Medicare beneficiaries. Ann Am Thorac Soc . 2019;16:99–106. doi: 10.1513/AnnalsATS.201805-332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nishi SPE, Zhang W, Kuo Y-F, Sharma G. Pulmonary rehabilitation utilization in older adults with chronic obstructive pulmonary disease, 2003 to 2012. J Cardiopulm Rehabil Prev . 2016;36:375–382. doi: 10.1097/HCR.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yohannes AM, Connolly MJ. Pulmonary rehabilitation programmes in the UK: a national representative survey. Clin Rehabil . 2004;18:444–449. doi: 10.1191/0269215504cr736oa. [DOI] [PubMed] [Google Scholar]
- 32. Brooks D, Sottana R, Bell B, Hanna M, Laframboise L, Selvanayagarajah S, et al. Characterization of pulmonary rehabilitation programs in Canada in 2005. Can Respir J . 2007;14:87–92. doi: 10.1155/2007/951498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medicare Advantage. Kaiser Family Foundation; 2019. https://www.kff.org/medicare/fact-sheet/medicare-advantage/ [Google Scholar]