Abstract
A 23- year-man post female to male (FTM) gender transition was found to have bilateral papilloedema at a routine optician visit. The patient was referred on for formal ophthalmological and neurological assessments. Optical coherence tomography (OCT) confirmed the presence of bilateral papilloedema. The patient was entirely asymptomatic and had no medical history. He took testosterone intramuscularly once per month. Neurological examination was otherwise normal. Investigations including routine blood panels, CT brain, MRI brain and cerebral MR venogram were all normal. Lumbar puncture yielded cerebrospinal fluid (CSF) normal in appearance but demonstrated raised intracranial pressure. In the absence of other causative aetiologies a diagnosis of idiopathic intracranial hypertension (IIH) was made. Treatment was commenced with acetazolamide and the patient was discharged with outpatient ophthalmological and neurological follow-up.
Keywords: neuroopthalmology, sexual and gender disorders
Background
A number of case reports have been published describing the emergence of idiopathic intracranial hypertension (IIH) in gender transition, particularly female to male (FTM) transition. This appears to occur in close relation to the commencement of testosterone, though no definitive mechanism has been described. This case is important as it highlights this potential association between patients undergoing FTM transition and IIH. As gender transition becomes more common worldwide it is important that we are aware of possible side effects of the associated treatments.
Case presentation
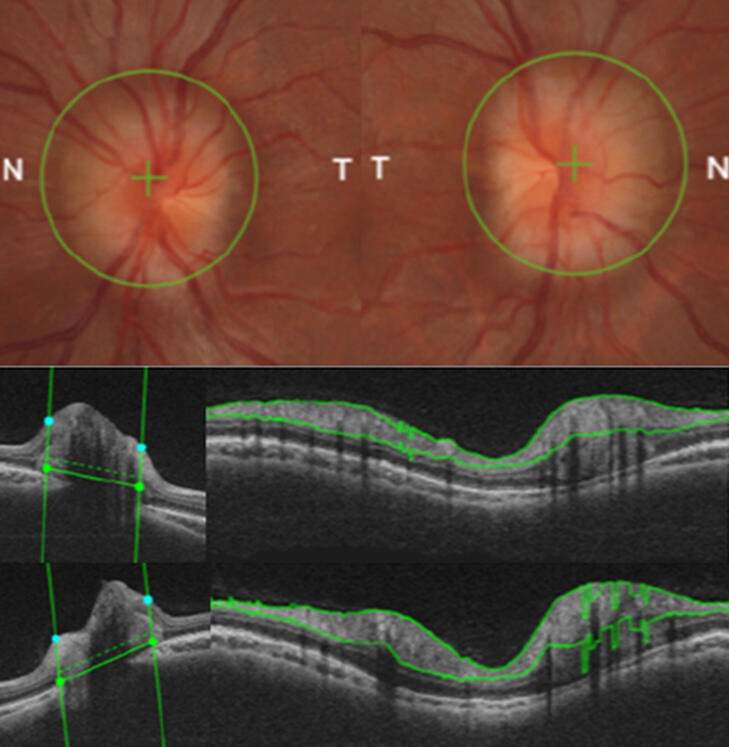
In July 2020, a 23-year-old man 4 years post FTM gender transition was found to have bilateral papilloedema at an optician visit for routine screening prior to applying for a driver’s licence. The patient was referred on for formal ophthalmological and neurological assessments. This demonstrated normal acuity of 6/6 bilaterally and normal intraocular pressures. Bilateral papilloedema was confirmed on optical coherence tomography (OCT) (figure 1). There was no constriction of the visual fields.
Figure 1.
Ophthalmological investigation; papilloedema was demonstrated on direct visualisation of the optic disc (above) and thereafter confirmed on optical coherence tomography (OCT). Horizontal and circular tomograms show marked swelling of the optic disc (middle—left eye; below—right eye).
The patient had undergone bilateral mastectomy as part of gender transition in 2017 and was taking depot testosterone intramuscularly monthly but no other regular medications. He had no other medical history; specifically no history of deep venous thrombosis or pulmonary embolism. He was entirely asymptomatic in terms of headaches or visual symptoms and a comprehensive neurological examination revealed no other abnormalities, with no restriction of eye movements. Vital signs were stable—RR 19, Sats 100%, BP 120/60, HR 75 regular, temp 36.1°C. The patient’s BMI was raised at 29.8 kg/m2.
Investigations
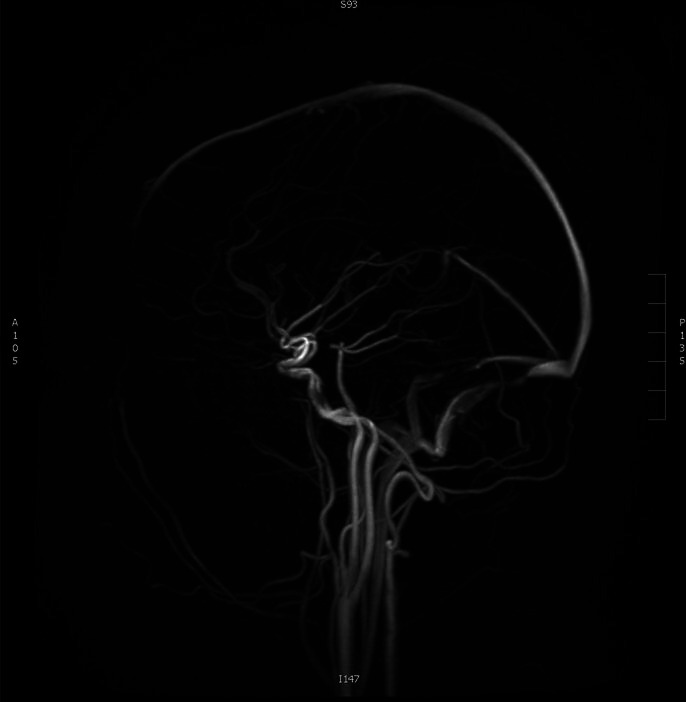
MRI brain and MR venogram were reported normal with no evidence of cerebral venous thrombosis or space occupying lesion (figure 2).
Figure 2.
MR venogram; no evidence of cerebral venous sinus thrombosis.
Routine blood work including full blood count, renal profile, liver function testing, coagulation screen and C reactive protein were all within normal parameters.
Lumbar puncture was performed with the patient lying in the left decubitus position. This yielded colourless cerebrospinal fluid (CSF). The opening pressure was raised at 35 cm of H2O (normal range 5–20 cm H2O). The closing pressure was 15 cm H2O. CSF protein was 149 mg/L (200–400). CSF glucose was 3.7 mmol/L with concurrent serum glucose of 5.2 mmol/L and a ratio of 0.7. CSF cell count was one white cell/cm3. CSF culture was negative. Cytology revealed no abnormal cells. Serum testosterone was normal at 20.34 nmol/L (8.33–30.19). Nasal swab PCR for SARS-CoV-2 infection was negative.
In the absence of a structural abnormality to cause raised intracranial pressure, the patient was diagnosed with IIH. Treatment with acetazolamide 250 mg two times per day was commenced with monitoring of his papilloedema as a gauge of treatment response given the asymptomatic nature of his presentation.
Outcome and follow-up
On follow-up in clinic, the patient is doing well and reports no side effects of his treatment. Specifically when questioned he reports no abdominal pain to indicate nephrolithiasis and no paraesthesia. Follow-up ophthalmological review had shown resolution of the papilloedema after six months of treatment with acetazolamide.
Discussion
A number of cases of IIH have been reported in the setting of gender transition.1–5 Though no specific mechanism has been confirmed, a number of potential causes have been hypothesised. One case by Hornby et al suggests dysregulation of androgens as a potentially causative.2 Interestingly, this case references three further similar case reports all of which involve FTM transition, as in our case.3 4 6 These cases describe a correlation between the commencement of testosterone and symptoms. This theory is further backed by three similar case reports and also by a report of recurring IIH with reinstatement of testosterone treatment by Qureshi et al.5 It would be a difficult decision for a person going through a transition to consider stopping transgender hormone therapy if it was concluded to be an aetiological factor in the IIH. We did not recommend this course of action in this case. Although there is no current evidence-based consensus for the management of hormone therapy in transgender patients with IIH, almost all the patients in referenced case reports achieved remission with standard treatment using acetazolamide and weight management and did not require cessation of androgen treatment.
Another possible aetiological factor was the patient’s raised BMI. An important factor in this patient’s workup was his karyotypic sex, female, which also confers a higher risk of IIH.7 8 Androgen excess, as a fundamental biological and diagnostic feature of polycystic ovarian syndrome (PCOS),9 should be considered in this case as PCOS can be associated with increased BMI, venous thrombo-embolism and resultant IIH.
Unfortunately, we cannot perfectly pinpoint the duration of IIH in our case given its asymptomatic nature. It may have been present and undetected from the initiation of treatment or may have developed subsequently. However, this presentation does raise the question of the prevalence of asymptomatic IIH among patients postgender transition and the mechanism by which it might occur. We recommend that IIH be considered as a differential diagnosis in people who develop new-onset headaches and/or visual symptoms postgender transition. In this case this was asymptomatic but we are not certain that an eye clinic review should be considered mandatory in all people undergoing this treatment.
Learning points.
Emerging patient considerations—gender transition is increasing in prevalence. Knowledge of the associated treatments and their side effects is important. The karyotypic sex of FTM patients means that they are at a higher risk for conditions such as IIH than their karyotypic male counterparts. Furthermore, complex aspects such as screening for conditions like breast cancer and incidence of prostatic cancer must be considered.
Community opticians have an evolving role in patient care. The patient’s papilloedema was detected on routine screening. This may have been otherwise undetected with resultant adverse effects.
Imaging of the brain with contrast enhanced CT or MR venogram is essential in patients with suspected IIH in order to exclude other causes for elevated CSF pressure.
Asymptomatic presentation—the patient was entirely asymptomatic at presentation. With that in mind, this may be more prevalent than we realise.
Footnotes
Contributors: BS and GPR directed the patient’s care and investigations along with writing the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Weinlander E, Derani T, Cornblath WT, et al. Intracranial hypertension in transgender patients. J Neuroophthalmol 2019;39:232–3. 10.1097/WNO.0000000000000736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hornby C, Mollan SP, Mitchell J, et al. What do transgender patients teach us about idiopathic intracranial hypertension? Neuroophthalmology 2017;41:326–9. 10.1080/01658107.2017.1316744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park S, Cheng CP, Lim LT, et al. Secondary intracranial hypertension from testosterone therapy in a transgender patient. Semin Ophthalmol 2014;29:156–8. 10.3109/08820538.2013.788678 [DOI] [PubMed] [Google Scholar]
- 4.Sigireddi RR, Lyons LJ, Lee AG. Idiopathic intracranial hypertension in a transgender female. Can J Ophthalmol 2019;54:e35–8. 10.1016/j.jcjo.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 5.Qureshi S, Qureshi K, Hassan A. Recurrent intracranial hypertension in a transgender female-to-male on testosterone therapy: a case report (5111). Neurology [Internet]. 2020 Apr 14 [cited 2021 Feb 12];94(15 Supplement). Available: https://n.neurology.org/content/94/15_Supplement/5111
- 6.Mowl AD, Grogg JA, Klein J. Secondary pseudotumour cerebri in a patient undergoing sexual reassignment therapy. Clin Exp Optom 2009;92:449–53. 10.1111/j.1444-0938.2009.00404.x [DOI] [PubMed] [Google Scholar]
- 7.Bruce BB, Kedar S, Van Stavern GP, et al. Idiopathic intracranial hypertension in men. Neurology 2009;72:304–9. 10.1212/01.wnl.0000333254.84120.f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels AB, Liu GT, Volpe NJ, et al. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri). Am J Ophthalmol 2007;143:635–41. 10.1016/j.ajo.2006.12.040 [DOI] [PubMed] [Google Scholar]
- 9.Azziz R, Carmina E, Dewailly D, et al. The androgen excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 2009;91:456–88. 10.1016/j.fertnstert.2008.06.035 [DOI] [PubMed] [Google Scholar]