Abstract
Background
Midwives are primary providers of care for childbearing women around the world. However, there is a lack of synthesised information to establish whether there are differences in morbidity and mortality, effectiveness and psychosocial outcomes between midwife‐led continuity models and other models of care.
Objectives
To compare midwife‐led continuity models of care with other models of care for childbearing women and their infants.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (25 January 2016) and reference lists of retrieved studies.
Selection criteria
All published and unpublished trials in which pregnant women are randomly allocated to midwife‐led continuity models of care or other models of care during pregnancy and birth.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. The quality of the evidence was assessed using the GRADE approach.
Main results
We included 15 trials involving 17,674 women. We assessed the quality of the trial evidence for all primary outcomes (i.e. regional analgesia (epidural/spinal), caesarean birth, instrumental vaginal birth (forceps/vacuum), spontaneous vaginal birth, intact perineum, preterm birth (less than 37 weeks) and all fetal loss before and after 24 weeks plus neonatal death using the GRADE methodology: all primary outcomes were graded as of high quality.
For the primary outcomes, women who had midwife‐led continuity models of care were less likely to experience regional analgesia (average risk ratio (RR) 0.85, 95% confidence interval (CI) 0.78 to 0.92; participants = 17,674; studies = 14; high quality), instrumental vaginal birth (average RR 0.90, 95% CI 0.83 to 0.97; participants = 17,501; studies = 13; high quality), preterm birth less than 37 weeks (average RR 0.76, 95% CI 0.64 to 0.91; participants = 13,238; studies = eight; high quality) and less all fetal loss before and after 24 weeks plus neonatal death (average RR 0.84, 95% CI 0.71 to 0.99; participants = 17,561; studies = 13; high quality evidence). Women who had midwife‐led continuity models of care were more likely to experience spontaneous vaginal birth (average RR 1.05, 95% CI 1.03 to 1.07; participants = 16,687; studies = 12; high quality). There were no differences between groups for caesarean births or intact perineum.
For the secondary outcomes, women who had midwife‐led continuity models of care were less likely to experience amniotomy (average RR 0.80, 95% CI 0.66 to 0.98; participants = 3253; studies = four), episiotomy (average RR 0.84, 95% CI 0.77 to 0.92; participants = 17,674; studies = 14) and fetal loss less than 24 weeks and neonatal death (average RR 0.81, 95% CI 0.67 to 0.98; participants = 15,645; studies = 11). Women who had midwife‐led continuity models of care were more likely to experience no intrapartum analgesia/anaesthesia (average RR 1.21, 95% CI 1.06 to 1.37; participants = 10,499; studies = seven), have a longer mean length of labour (hours) (mean difference (MD) 0.50, 95% CI 0.27 to 0.74; participants = 3328; studies = three) and more likely to be attended at birth by a known midwife (average RR 7.04, 95% CI 4.48 to 11.08; participants = 6917; studies = seven). There were no differences between groups for fetal loss equal to/after 24 weeks and neonatal death, induction of labour, antenatal hospitalisation, antepartum haemorrhage, augmentation/artificial oxytocin during labour, opiate analgesia, perineal laceration requiring suturing, postpartum haemorrhage, breastfeeding initiation, low birthweight infant, five‐minute Apgar score less than or equal to seven, neonatal convulsions, admission of infant to special care or neonatal intensive care unit(s) or in mean length of neonatal hospital stay (days).
Due to a lack of consistency in measuring women's satisfaction and assessing the cost of various maternity models, these outcomes were reported narratively. The majority of included studies reported a higher rate of maternal satisfaction in midwife‐led continuity models of care. Similarly, there was a trend towards a cost‐saving effect for midwife‐led continuity care compared to other care models.
Authors' conclusions
This review suggests that women who received midwife‐led continuity models of care were less likely to experience intervention and more likely to be satisfied with their care with at least comparable adverse outcomes for women or their infants than women who received other models of care.
Further research is needed to explore findings of fewer preterm births and fewer fetal deaths less than 24 weeks, and all fetal loss/neonatal death associated with midwife‐led continuity models of care.
Plain language summary
Midwife‐led continuity models of care compared with other models of care for women during pregnancy, birth and early parenting
What is the issue?
There are several ways to look after the health and well‐being of women and babies during pregnancy, birth and afterwards – these ways are called ‘models of care’. Sometimes, an obstetrician or another doctor is the lead healthcare professional and at other times it is a midwife. Sometimes, the responsibility is shared between obstetricians and midwives. One of the models is called ‘the midwife‐led continuity model’. This is where the midwife is the lead professional starting from the initial booking appointment, up to and including the early days of parenting. We wanted to find out if women and babies do better with this midwife‐led continuity model, compared with other models.
Why is this important?
Midwife‐led continuity models provide care from the same midwife or team of midwives during the pregnancy, birth and the early parenting period, and many women value this. These midwives also involve other care‐providers if they are needed. Obstetrician‐led or family doctor‐led models are not usually able to provide the same midwife/wives throughout. We need to know if the midwife‐led continuity model is safe, and if it brings benefits to mothers and babies.
What evidence did we find?
We identified 15 studies involving 17,674 mothers and babies (search date 25 January 2016). We included women at low risk of complications as well as women at increased risk, but not currently experiencing problems. All the trials involved professionally‐qualified midwives and no trial included models of care that offered home birth. We used reliable methods to assess the quality of the evidence and looked at seven key outcomes: preterm birth (birth before 37 weeks of pregnancy); the risk of losing the baby in pregnancy or in the first month after birth; spontaneous vaginal birth (when labour was not induced and birth not assisted by forceps; caesarean birth; instrumental vaginal birth (births using forceps or ventouse); whether the perineum remained intact, and use of regional analgesia (such as epidural).
The main benefits were that women who received midwife‐led continuity of care were less likely to have an epidural. In addition, fewer women had episiotomies or instrumental births. Women’s chances of a spontaneous vaginal birth were also increased and there was no difference in the number of caesarean births. Women were less likely to experience preterm birth, and they were also at a lower risk of losing their babies. In addition, women were more likely to be cared for in labour by midwives they already knew. The review identified no adverse effects compared with other models.
The trials contributed enough high quality evidence for each key outcome to give us reliable results for each one. We can be reasonably confident that future trials would find similar results for these outcomes.
What does this mean?
Most women should be offered ‘midwife‐led continuity of care’. It provides benefits for women and babies and we have identified no adverse effects. However, we cannot assume the same applies to women with existing serious pregnancy or health complications, because these women were not included in the evidence assessed.
Summary of findings
Summary of findings for the main comparison. Midwife‐led compared with other models of care for childbearing women and their infants (all) for childbearing women.
| Midwife‐led compared with other models of care for childbearing women and their infants (all) for childbearing women | ||||||
| Patient or population: Pregnant women Settings: Australia, Canada, Ireleand, UK Intervention: Midwife‐led models of care Comparison: All other models of care for childbearing women and their infants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| other models of care for childbearing women and their infants (all) | Midwife‐led | |||||
| Preterm birth (less than 37 weeks) | Study population | RR 0.76 (0.64 to 0.91) | 13238 (8 RCTs) | ⊕⊕⊕⊕ HIGH | None of the included trials in this review had adequate blinding. We have not downgraded evidence (‐1) for risk of bias due to lack of blinding. | |
| 63 per 1000 | 48 per 1000 (41 to 58) | |||||
| Moderate | ||||||
| 59 per 1000 | 45 per 1000 (38 to 54) | |||||
| All fetal loss before and after 24 weeks plus neonatal death | Study population | RR 0.84 (0.71 to 0.99) | 17561 (13 RCTs) | ⊕⊕⊕⊕ High | ||
| 34 per 1000 | 29 per 1000 (24 to 34) | |||||
| Moderate | ||||||
| 20 per 1000 | 17 per 1000 (14 to 20) | |||||
| Spontaneous vaginal birth (as defined by trial authors) | Study population | RR 1.05 (1.03 to 1.07) | 16687 (12 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 658 per 1000 | 691 per 1000 (677 to 704) | |||||
| Moderate | ||||||
| 693 per 1000 | 727 per 1000 (713 to 741) | |||||
| Caesarean birth | Study population | RR 0.92 (0.84 to 1.00) | 17674 (14 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 155 per 1000 | 143 per 1000 (130 to 155) | |||||
| Moderate | ||||||
| 156 per 1000 | 144 per 1000 (131 to 156) | |||||
| Instrumental vaginal birth (forceps/vacuum) | Study population | RR 0.90 (0.83 to 0.97) | 17501 (13 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 143 per 1000 | 129 per 1000 (119 to 139) | |||||
| Moderate | ||||||
| 179 per 1000 | 161 per 1000 (149 to 174) | |||||
| Intact perineum | Study population | RR 1.04 (0.95 to 1.13) | 13186 (10 RCTs) | ⊕⊕⊕⊕ HIGH 1 | ||
| 269 per 1000 | 279 per 1000 (255 to 304) | |||||
| Moderate | ||||||
| 333 per 1000 | 346 per 1000 (316 to 376) | |||||
| Regional analgesia (epidural/spinal) | Study population | RR 0.85 (0.78 to 0.92) | 17674 (14 RCTs) | ⊕⊕⊕⊕ HIGH 2 | ||
| 270 per 1000 | 229 per 1000 (211 to 248) | |||||
| Moderate | ||||||
| 287 per 1000 | 244 per 1000 (224 to 264) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Statistical heterogeneity, I² = 54%. We did not downgrade the evidence for heterogeneity with I2 < 60%.
2Statistical heterogeneity, I² = 57%.
Background
Description of the condition
In many parts of the world, midwives are the primary providers of care for childbearing women (ten Hoope‐Bender 2014). There are, however, considerable variations in the organisation of midwifery services and in the education and role of midwives (UNFPA 2014). Furthermore, in some countries, e.g. in North America, medical doctors are the primary care providers for the vast majority of childbearing women, while in other countries, e.g. Australia, New Zealand, The Netherlands, the United Kingdom and Ireland, various combinations of midwife‐led continuity, medical‐led, and shared models of care are available. Childbearing women are often faced with different opinions as to which option might be best for them (De Vries 2001). There is much debate about the clinical and cost effectiveness of the different models of maternity care (Ryan 2013) and hence continuing debate on the optimal model of care for routine ante‐, intra‐ and postnatal care for healthy pregnant women (Sutcliffe 2012; Walsh 2012). This review complements other work on models of maternity care and attributes thereof, specifically, the work of Hodnett (Hodnett 2012) and Olsen (Olsen 2012), in which the relationships between the various birth settings and pregnancy outcomes were evaluated systematically. This review also subsumes the Cochrane review, 'Continuity of caregivers during pregnancy, childbirth, and the postpartum period' (Hodnett 2000).
Description of the intervention
Whilst it is difficult to categorise maternity models of care exclusively due to the influence of generic policies and guidelines, it is assumed that the underpinning philosophy of a midwife‐led model of care is normality and the natural ability of women to experience birth without routine intervention. The midwife‐led continuity model of care is based on the premise that pregnancy and birth are normal life events. The midwife‐led continuity model of care includes: continuity of care; monitoring the physical, psychological, spiritual and social well being of the woman and family throughout the childbearing cycle; providing the woman with individualised education, counselling and antenatal care; attendance during labour, birth and the immediate postpartum period by a known midwife; ongoing support during the postnatal period; minimising unnecessary technological interventions; and identifying, referring and co‐ordinating care for women who require obstetric or other specialist attention. Differences between midwife‐led continuity and other models of care often include variations in philosophy, relationship between the care provider and the pregnant woman, use of interventions during labour, care setting (home, home‐from‐home or acute setting) and in the goals and objectives of care (Rooks 1999).
Midwife‐led continuity models of care
Midwife‐led continuity of care has been defined as care where "the midwife is the lead professional in the planning, organisation and delivery of care given to a woman from initial booking to the postnatal period" (RCOG 2001). Some antenatal and/or intrapartum and/or postpartum care may be provided in consultation with medical staff as appropriate. Within these models, midwives are, however, in partnership with the woman, the lead professional with responsibility for assessment of her needs, planning her care, referral to other professionals as appropriate, and for ensuring provision of maternity services. Thus, midwife‐led continuity models of care aim to provide care in either community or hospital settings, normally to healthy women with uncomplicated or 'low‐risk' pregnancies. In some models, midwives provide continuity of midwifery care to all women from a defined geographical location, acting as lead professional for women whose pregnancy and birth is uncomplicated, and continuing to provide midwifery care to women who experience medical and obstetric complications in partnership with other professionals.
Some models of midwife‐led continuity of care provide continuity of care to a defined group of women through a team of midwives sharing a caseload, often called 'team' midwifery. Thus, a woman will receive her care from a number of midwives in the team, the size of which can vary. Other models, often termed 'caseload midwifery', aim to offer greater relationship continuity, by ensuring that childbearing women receive their ante‐, intra‐ and postnatal care from one midwife or her/his practice partner (McCourt 2006). There is continuing debate about the risks, benefits, and costs of team and caseload models of midwife‐led continuity of care (Ashcroft 2003; Benjamin 2001; Green 2000; Johnson 2005; Waldenstrom 1998).
Other models of care
Other models of care include the following (a) Obstetrician‐provided care. This is common in North America, where obstetricians are the primary providers of antenatal care for most childbearing women. An obstetrician (not necessarily the one who provides antenatal care) is present for the birth, and nurses provide intrapartum and postnatal care. (b) Family doctor‐provided care, with referral to specialist obstetric care as needed. Obstetric nurses or midwives provide intrapartum and immediate postnatal care but not at a decision‐making level, and a medical doctor is present for the birth. (c) Shared models of care, where responsibility for the organisation and delivery of care, throughout initial booking to the postnatal period, is shared between different health professionals.
At various points during pregnancy, childbirth, and the postnatal period, responsibility for care can shift to a different provider or group of providers. Care is often shared by family doctors and midwives, by obstetricians and midwives, or by providers from all three groups. In some countries (e.g. Canada and The Netherlands), the midwifery scope of practice is limited to the care of women experiencing uncomplicated pregnancies, while in other countries (e.g. United Kingdom, France, Australia and New Zealand), midwives provide care to women who experience medical and obstetric complications in collaboration with medical colleagues. In addition, maternity care in some countries (e.g. Republic of Ireland, Iran and Lebanon), is predominantly provided by a midwife but is obstetrician‐led, in that the midwife might provide the actual care, but the obstetrician assumes overall responsibility for the care provided to the woman throughout her ante‐, intra‐ and postpartum periods.
How the intervention might work
Continuity of care is a means of delivering care in a way which acknowledges that a woman’s health needs are not isolated events, and should be managed over time (Reid 2002). This longitudinal aspect allows a relationship to develop between patients and their providers of care, and contributes to the patients' perception of having a provider who has knowledge of their medical history, and similarly an expectation that a known provider will care for them in the future (Haggerty 2003). Continuity refers to a ‘coordinated and smooth progression of care from the patient’s point of view’ (Freeman 2007) and therefore woman‐centredness is an important aspect in the delivery of continuity of care.
The general literature on continuity notes that a lack of clarity in definition and measurement of different types of continuity has been one of the limitations in research in this field (Haggerty 2003). Continuity has been defined by Freeman 2007 as having three major types ‐ management, informational and relationship. Management continuity involves the communication of both facts and judgements across team, institutional and professional boundaries, and between professionals and patients. Informational continuity concerns the timely availability of relevant information. Relationship continuity means a therapeutic relationship of the service user with one or more health professionals over time. Relationship/personal continuity over time has been found to have a greater effect on user experience and outcome (Saultz 2003; Saultz 2004; Saultz 2005). It has been argued that neither management nor informational continuity can compensate for lack of an ongoing relationship (Guthrie 2008). Midwife‐led continuity models of care have generally aimed to improve continuity of care over a period of time. Some models of midwife‐led care offer continuity with a group of midwives, and others offer personal or relational continuity, and thus the models of care that are the foci of this review are defined as follows.
Why it is important to do this review
There has been a lack of a single source of synthesised evidence on the effectiveness of midwife‐led continuity models of care when compared with other models of care. This review attempts to provide this evidence.
Objectives
The primary objective of this review is to compare the effects of midwife‐led continuity models of care with other models of care for childbearing women and their infants. We also explore whether the effects of midwife‐led continuity of care are influenced by: 1) models of midwife‐led care that provide differing levels of relationship continuity; 2) varying levels of obstetrical risk.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials including trials using individual‐ or cluster‐randomisation methods. We also included quasi‐randomised trials, where allocation may not have been truly random (e.g. where allocation was alternate or not clear).
Types of participants
Pregnant women.
Types of interventions
Models of care are classified as midwife‐led continuity of care, and other or shared care on the basis of the lead professional in the antepartum and intrapartum periods. In midwife‐led continuity models of care, the midwife is the woman's lead professional, but one or more consultations with medical staff are often part of routine practice. Other models of care include: a) where the physician/obstetrician is the lead professional, and midwives and/or nurses provide intrapartum care and in‐hospital postpartum care under medical supervision; b) shared care, where the lead professional changes depending on whether the woman is pregnant, in labour or has given birth, and on whether the care is given in the hospital, birth centre (free standing or integrated) or in community setting(s); and c) where the majority of care is provided by physicians or obstetricians.
Types of outcome measures
Primary outcomes
Birth and immediate postpartum
Regional analgesia (epidural/spinal)
Caesarean birth
Instrumental vaginal birth (forceps/vacuum)
Spontaneous vaginal birth (as defined by trial authors)
Intact perineum
Neonatal
Preterm birth (less than 37 weeks)
All fetal loss before and after 24 weeks plus neonatal death
Secondary outcomes
Antenatal hospitalisation
Antepartum haemorrhage
Induction of labour
Amniotomy
Augmentation/artificial oxytocin during labour
No intrapartum analgesia/anaesthesia
Opiate analgesia
Attendance at birth by known midwife
Episiotomy
Perineal laceration requiring suturing
Mean labour length (hours)
Postpartum haemorrhage
Breastfeeding initiation
Duration of postnatal hospital stay (days)
Low birthweight (less than 2500 g)
Five‐minute Apgar score less than or equal to seven
Neonatal convulsions
Admission to special care nursery/neonatal intensive care unit
Mean length of neonatal hospital stay (days)
Fetal loss less than 24 weeks and neonatal death
Fetal loss equal to/after 24 weeks and neonatal death
Perceptions of control during labour and childbirth
Mean number of antenatal visits
Maternal death
Cord blood acidosis
Postpartum depression
Any breastfeeding at three months
Prolonged perineal pain
Pain during sexual intercourse
Urinary incontinence
Faecal incontinence
Prolonged backache
Breastfeeding on hospital discharge (not pre‐specified)
Maternal satisfaction (not pre‐specified)
Cost (not pre‐specified)
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (25 January 2016).
The Register is a database containing over 20,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the PCG Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Trials Search Co‐ordinator searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
For search methods used in an earlier update of this review (Hatem 2008), seeAppendix 1.
Searching other resources
We searched for further studies in the reference list of the studies identified.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see (Sandall 2015).
For this update, the following methods were used for assessing the three reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
One of the review authors (D Devane) is a co‐author of one of the included studies (Begley 2011), so was not involved in data extraction or in the 'Risk of bias' assessment for this study.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update we assessed the quality of the evidence using the GRADE approach as outlined in the GRADE Handbook. In order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons of midwife‐led versus all other models of care for childbearing women and their infants.
Preterm birth (less than 37 weeks)
All fetal loss before and after 24 weeks plus neonatal death
Spontaneous vaginal birth (as defined by trial authors)
Caesarean birth
Instrumental vaginal birth (forceps/vacuum)
Intact perineum
Regional analgesia (epidural/spinal)
We used GRADEpro Guideline Development Tool to import data from Review Manager (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. We have not downgraded evidence for heterogeneity with an I² < 60%. We have not downgraded for risk of bias due to lack of blinding.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. In future updates, as appropriate, we will use the standardised mean difference to combine trials that measure the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We included a cluster‐randomised trial in the analyses along with individually‐randomised trials (North Stafford 2000). This trial found a negative ICC so no adjustment was made for clustering. We considered it reasonable to combine the results from cluster‐randomised trials and individually‐randomised trials if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
Other unit of analysis issues
Multiple pregnancies were included and both infants included in the denominator.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analysis we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014).
As there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or where substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect had not been clinically meaningful, we would not have combined trials. The results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses.
Caseload versus team models of midwifery care
Low‐risk versus mixed‐risk status
The following outcomes were used in subgroup analyses.
Regional analgesia (epidural/spinal)
Caesarean birth
Instrumental vaginal birth (forceps/vacuum)
Spontaneous vaginal birth (as defined by trial authors)
Intact perineum
Preterm birth (< 37 weeks)
All fetal loss before and after 24 weeks plus neonatal death
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
Our search strategy identified 88 citations relating to 38 studies in total. The updated search in May 2015 identified 11 new reports. Four were additional reports of an already included study McLachlan 2012; three new reports were included as Tracy 2013; two reports were excluded (Famuyide 2014; Gu 2013); and one was an additional reports of an excluded study (Walker 2012). A final report, Allen 2013, was eligible for the review and included, though this trial was a feasibility study and presents no usable data.
The updated search in January 2016 identified three new reports relating to three already included studies in the review (Begley 2011; McLachlan 2012; Tracy 2013). Additional data were extracted from these new reports on the following outcomes: cost (economic cost of care analysis) Begley 2011; and maternal satisfaction (maternal experiences of childbirth) McLachlan 2012. These data were reported narratively. No additional data were extracted from the additional report of Tracy 2013 which reports on the number of midwives and health professionals seen by a subset of publicly funded pregnant women.
Included studies
We included 15 trials involving 17,674 randomised women in total (Allen 2013; Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; MacVicar 1993; McLachlan 2012; North Stafford 2000; Rowley 1995; Tracy 2013; Turnbull 1996; Waldenstrom 2001). SeeCharacteristics of included studies table.
Included studies were conducted in the public health systems in Australia, Canada, Ireland and the United Kingdom with variations in model of care, risk status of participating women and practice settings. The Zelen method was used in three trials (Flint 1989; Homer 2001; MacVicar 1993), and one trial used cluster‐randomisation (North Stafford 2000).
Four studies offered a caseload model of care (McLachlan 2012; North Stafford 2000; Tracy 2013; Turnbull 1996) and 10 studies provided a team model of care: (Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; MacVicar 1993; Rowley 1995; Waldenstrom 2001). The composition and modus operandi of the teams varied among trials. Levels of continuity (measured by the percentage of women who were attended during birth by a known carer varied between 63% to 98% for midwife‐led continuity models of care to 0.3% to 21% in other models of care).
Eight studies compared a midwife‐led continuity model of care with a shared model of care (Begley 2011; Biro 2000; Flint 1989; Hicks 2003; Homer 2001; Kenny 1994; North Stafford 2000; Rowley 1995), three studies compared a midwife‐led continuity model of care with medical‐led models of care (Harvey 1996; MacVicar 1993; Turnbull 1996), and three studies compared midwife‐led continuity of care with various options of standard care including shared, medical‐led and shared care (McLachlan 2012; Tracy 2013; Waldenstrom 2001).
Participating women received ante‐, intra‐ and postpartum care in 13 studies (Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; McLachlan 2012; North Stafford 2000; Rowley 1995; Tracy 2013; Turnbull 1996; Waldenstrom 2001), and antenatal and intrapartum care only in one study (MacVicar 1993).
Some midwife‐led continuity models included routine visits to the obstetrician or family physicians (GPs), or both. The frequency of such visits varied. Such visits were dependent on women's risk status during pregnancy (Biro 2000); routine for all women (one to three visits) (Flint 1989; Harvey 1996; Kenny 1994; MacVicar 1993; McLachlan 2012; Rowley 1995; Waldenstrom 2001), or based on the development of complications (Hicks 2003; Tracy 2013; Turnbull 1996) or antenatal care from midwives and, if desired by the woman, from the woman's general practitioner (Begley 2011).
Women were classified as being at low risk of complications in eight studies (Begley 2011; Flint 1989; Harvey 1996; Hicks 2003; MacVicar 1993; McLachlan 2012; Turnbull 1996; Waldenstrom 2001) and as 'low and high' and 'high' in six studies (Biro 2000; Homer 2001; Kenny 1994; North Stafford 2000; Rowley 1995; Tracy 2013).
The midwifery models of care were hospital‐based in four studies (Biro 2000; MacVicar 1993; Rowley 1995; Waldenstrom 2001), or offered (i) antenatal care in an outreach community‐based clinic and intra‐ and postpartum care in hospital (Homer 2001); (ii) ante‐ and postpartum community‐based care with intrapartum hospital‐based care (Hicks 2003; North Stafford 2000; Tracy 2013; Turnbull 1996) (iii) antenatal and postnatal care in the hospital and community settings with intrapartum hospital‐based care or (iv) postnatal care in the community with hospital‐based ante‐ and intrapartum care (Flint 1989; Harvey 1996; Kenny 1994; McLachlan 2012). Four studies offered intrapartum care in home‐like settings, either to all women in the trial (Waldenstrom 2001), or to women receiving midwife‐led continuity of care only (Begley 2011; MacVicar 1993; Turnbull 1996).
Excluded studies
We excluded 22 studies (Berglund 1998; Berglund 2007; Bernitz 2011; Chambliss 1991; Chapman 1986; Famuyide 2014; Giles 1992; Gu 2013; Heins 1990; Hildingsson 2003; Hundley 1994; James 1988; Kelly 1986; Klein 1984; Law 1999; Marks 2003; Runnerstrom 1969; Slome 1976; Stevens 1988; Tucker 1996; Waldenstrom 1997; Walker 2012). SeeCharacteristics of excluded studies.
Risk of bias in included studies
SeeFigure 1; Figure 2 for summary of 'Risk of bias' assessments.
1.
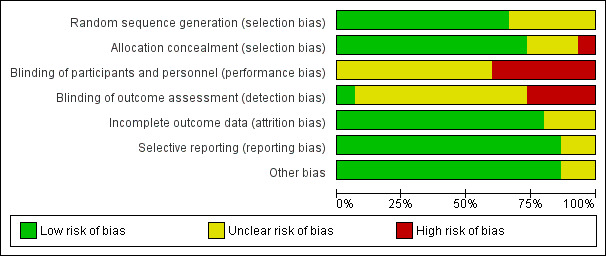
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
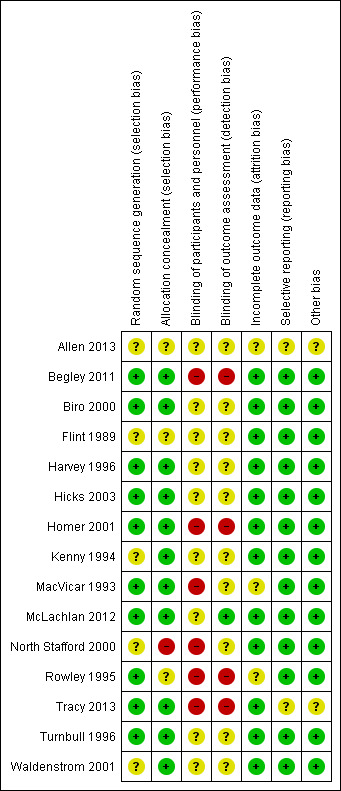
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Ten studies reported genuine random methods of generation of the randomisation sequence (Begley 2011; Biro 2000; Harvey 1996; Hicks 2003; Homer 2001; MacVicar 1993; McLachlan 2012; Rowley 1995; Tracy 2013; Turnbull 1996). Five gave no or insufficient information to form a clear judgement (Allen 2013; Flint 1989; Kenny 1994; North Stafford 2000; Waldenstrom 2001).
Allocation concealment was judged low risk of bias for 11 studies (Begley 2011; Biro 2000; Harvey 1996, Hicks 2003; Homer 2001; Kenny 1994; MacVicar 1993; McLachlan 2012; Tracy 2013; Turnbull 1996; Waldenstrom 2001). Three studies were judged unclear risk of bias: Rowley 1995 and Allen 2013 gave no information about the process of random allocation; and Flint 1989 used sealed opaque envelopes but did not specify any numbering. The North Stafford 2000 trial was a cluster‐randomised trial, whereby allocation concealment was not possible and it was judged high risk of bias for allocation concealment.
Blinding
Six of the included studies were judged as high risk in blinding of participants and personnel (Begley 2011; Homer 2001; MacVicar 1993; North Stafford 2000; Rowley 1995; Tracy 2013) and nine studies were of unclear risk of bias (Allen 2013; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Kenny 1994; McLachlan 2012; Turnbull 1996; Waldenstrom 2001).
One study was at low risk of bias for blinding of outcome assessment (McLachlan 2012), four were judged as high risk of bias (Begley 2011; Homer 2001; Rowley 1995; Tracy 2013), and 10 studies were at unclear risk of bias (Allen 2013; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Kenny 1994; MacVicar 1993; North Stafford 2000; Turnbull 1996; Waldenstrom 2001).
Incomplete outcome data
Twelve of the included studies were judged at low risk of bias for incomplete outcome data on the basis that attrition rate was less than 20% for all outcomes (other than satisfaction), or missing outcome data were balanced across groups (Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; McLachlan 2012; North Stafford 2000; Tracy 2013; Turnbull 1996; Waldenstrom 2001). Two of the studies (MacVicar 1993; Rowley 1995) did not provide sufficient information on loss to follow‐up and were judged as unclear. A feasibility study was also judged as unclear (Allen 2013).
Selective reporting
All outcomes stated in the methods section were adequately reported in the results in 13 studies (Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; MacVicar 1993; McLachlan 2012; North Stafford 2000; Rowley 1995; Turnbull 1996; Waldenstrom 2001). Two trials were judged to be of unclear risk of bias due to reporting: Allen 2013, a feasibility recruiting just one woman to the intervention and Tracy 2013, where we emailed the trial authors for clarification of data and additional data.
Other potential sources of bias
No other potential sources of bias were identified in most included studies. A feasibility study (Allen 2013) was considered of unclear risk, as was Tracy 2013, where a small proportion of women were crossed‐over from each arm.
Effects of interventions
See: Table 1
We used random‐effects for all analyses. Where we identified statistical heterogeneity (I² > 30%) we have reported the values of both Tau² and I². Because our subgroup analyses (reported below) did not generally explain heterogeneity found in specific primary outcomes, we discuss additional sources of heterogeneity below and in the discussion section of the review.
Comparison 1 (main comparison): midwife‐led continuity models of care versus other models of care for childbearing women and their infants ‐ all trials
Primary outcomes
Women randomised to midwife‐led continuity models of care were, on average, less likely to experience:
regional analgesia (epidural/spinal) (average risk ratio (RR) 0.85, 95% confidence interval (CI) 0.78 to 0.92; participants = 17,674; studies = 14; I² = 57%; high quality evidence) (Analysis 1.1);
instrumental vaginal birth (forceps/vacuum) (average RR 0.90, 95% CI 0.83 to 0.97; participants = 17,501; studies = 13; high quality evidence) (Analysis 1.3);
preterm birth < 37 weeks (average RR 0.76, 95% CI 0.64 to 0.91; participants = 13,238; studies = eight; I² = 33%; high quality evidence) (Analysis 1.6).
1.1. Analysis.
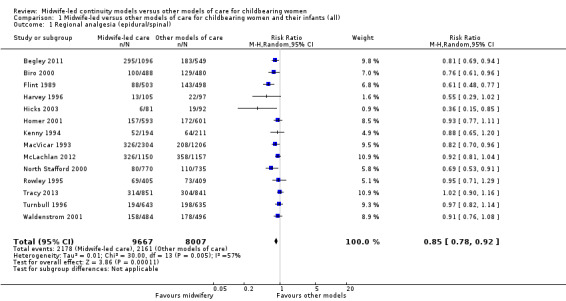
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 1 Regional analgesia (epidural/spinal).
1.3. Analysis.
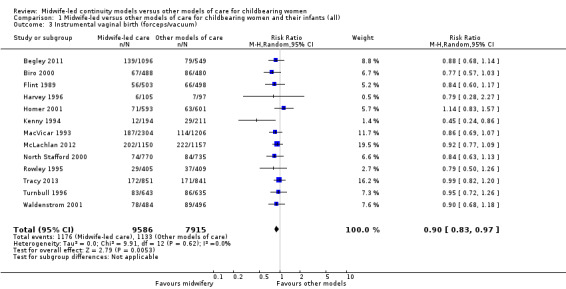
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 3 Instrumental vaginal birth (forceps/vacuum).
1.6. Analysis.
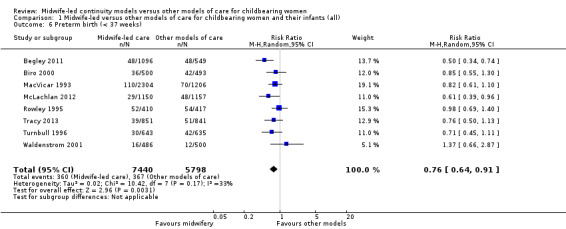
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 6 Preterm birth (< 37 weeks).
We conducted pre‐specified subgroup analysis to investigate heterogeneity in the above outcomes of regional analgesia and preterm birth. Assumed differences between caseload or team models of care versus other models of care could not explain the heterogeneity for these outcomes, and neither could potential differences between low‐risk and mixed‐risk groups of pregnant women (see analyses for regional analgesia Analysis 2.1 and Analysis 3.1 and for preterm birth Analysis 2.6 and Analysis 3.6).
2.1. Analysis.
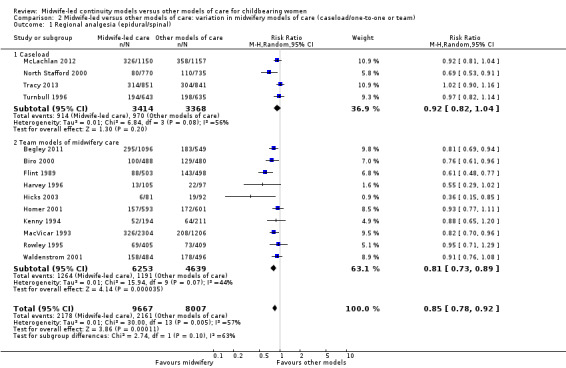
Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 1 Regional analgesia (epidural/spinal).
3.1. Analysis.
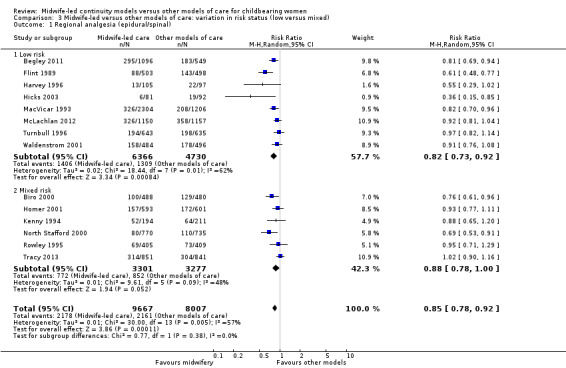
Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 1 Regional analgesia (epidural/spinal).
2.6. Analysis.
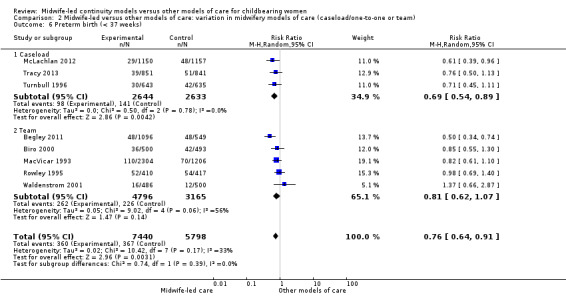
Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 6 Preterm birth (< 37 weeks).
3.6. Analysis.
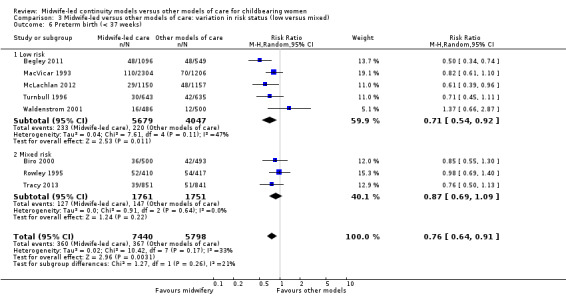
Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 6 Preterm birth (< 37 weeks).
Women randomised to midwife‐led continuity models of care were on average more likely to experience:
a spontaneous vaginal birth (average RR 1.05, 95% CI 1.03 to 1.07; participants = 16,687; studies = 12; high quality evidence) (Analysis 1.4).
1.4. Analysis.
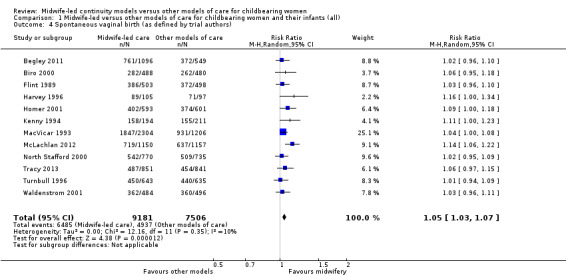
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 4 Spontaneous vaginal birth (as defined by trial authors).
There were no statistically significant differences between groups for the following outcomes:
caesarean birth (average RR 0.92, 95% CI 0.84 to 1.00; participants = 17,674; studies = 14; high quality evidence) (Analysis 1.2);
intact perineum (average RR 1.04, 95% CI 0.95 to 1.13; participants = 13,186; studies = 10; high quality evidence) (Analysis 1.5); there was moderate heterogeneity for this outcome (Heterogeneity: Tau² = 0.01; I² = 54%), and this could not be attributed to differences in the pre‐specified subgroups (see below and Analysis 2.5 and Analysis 3.5).
1.2. Analysis.
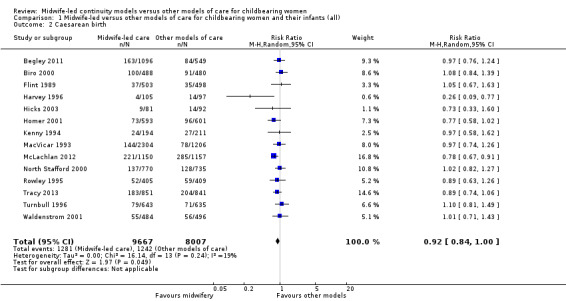
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 2 Caesarean birth.
1.5. Analysis.
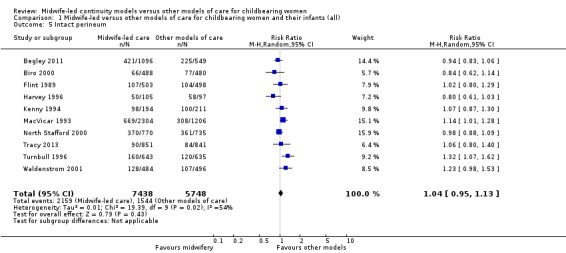
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 5 Intact perineum.
2.5. Analysis.
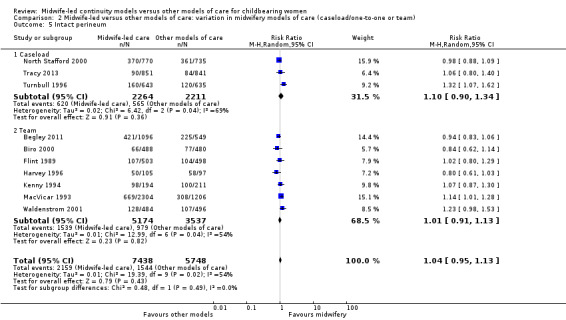
Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 5 Intact perineum.
3.5. Analysis.
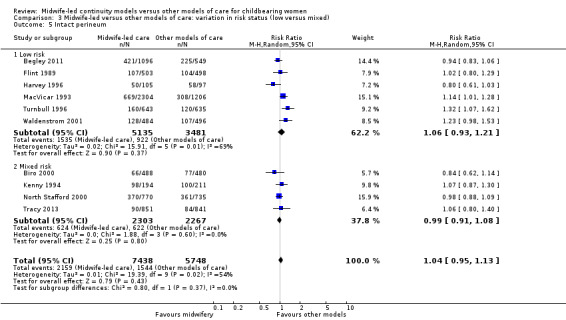
Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 5 Intact perineum.
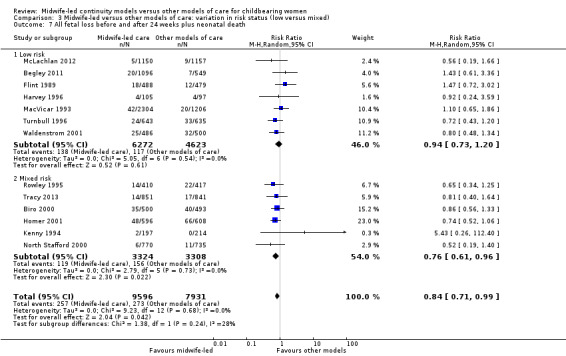
The difference in the average treatment effect in all fetal loss before and after 24 weeks plus neonatal death across included trials between women allocated to midwife‐led continuity models of care and women allocated to other models has an average RR of 0.84, with 95% CI 0.71 to 0.99. Given that (i) the 95% CI just reaches 0.99 and (ii) the absence of measurable heterogeneity in this outcome analysis, the probability is that midwife‐led continuity models of care are associated with a reduction in fetal loss and neonatal death by approximately 16%.
all fetal loss before and after 24 weeks plus neonatal death (average RR 0.84, 95% CI 0.71 to 0.99; participants = 17,561; studies = 13; high quality evidence) (Analysis 1.7).
1.7. Analysis.
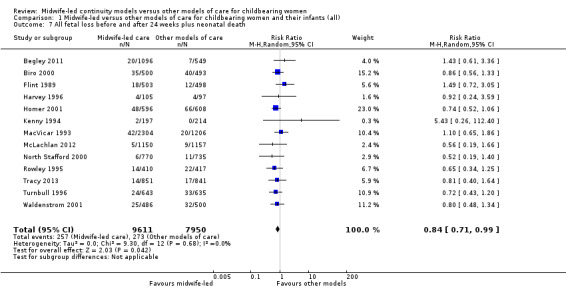
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 7 All fetal loss before and after 24 weeks plus neonatal death.
Secondary outcomes
Women randomised to midwife‐led continuity models of care were, on average, less likely to experience:
amniotomy (average RR 0.80, 95% CI 0.66 to 0.98; participants = 3253; studies = four; I² = 75%) (Analysis 1.11);
episiotomy (average RR 0.84, 95% CI 0.77 to 0.92; participants = 17,674; studies = 14; I² = 47%) (Analysis 1.16);
fetal loss less than 24 weeks and neonatal death (average RR 0.81, 95% CI 0.67 to 0.98; participants = 15,645; studies = 11) (Analysis 1.27).
1.11. Analysis.
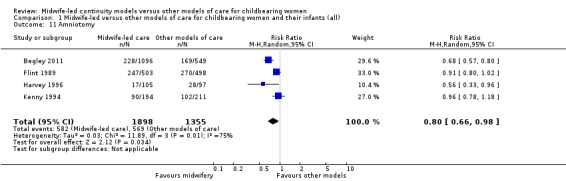
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 11 Amniotomy.
1.16. Analysis.
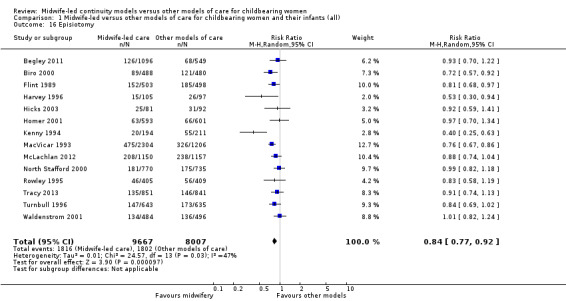
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 16 Episiotomy.
1.27. Analysis.
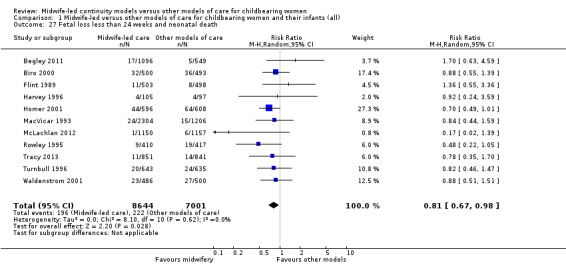
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 27 Fetal loss less than 24 weeks and neonatal death.
Women randomised to midwife‐led continuity models of care were on average more likely to experience:
no intrapartum analgesia/anaesthesia (RR 1.21, 95% CI 1.06 to 1.37; participants = 10,499; studies = seven; I² = 49%) (Analysis 1.13);
a longer mean length of labour (hours) (mean difference (MD) 0.50, 95% CI 0.27 to 0.74; participants = 3328; studies = three) (Analysis 1.18); however, there was evidence of skewness in the data from one of the trials in the analyses of length of labour (Turnbull 1996);
women allocated to midwife‐led continuity models of care were more likely to be attended at birth by a known midwife (RR 7.04, 95% CI 4.48 to 11.08; participants = 6917; studies = seven); however, the effect estimates for individual studies are highly variable, as reflected in substantial statistical heterogeneity (Tau² = 0.31; I² = 94%; Analysis 1.15).
1.13. Analysis.
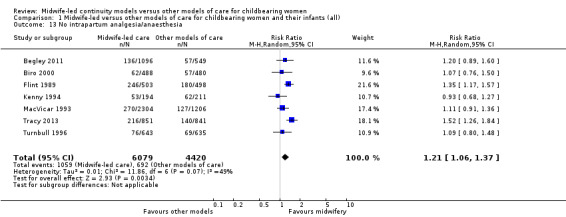
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 13 No intrapartum analgesia/anaesthesia.
1.18. Analysis.
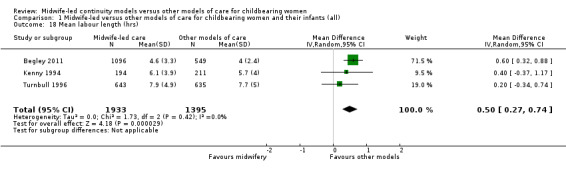
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 18 Mean labour length (hrs).
1.15. Analysis.
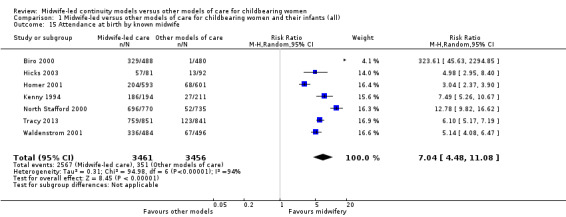
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 15 Attendance at birth by known midwife.
There were no statistically significant differences between groups for the following outcomes:
antenatal hospitalisation (average RR 0.95, 95% CI 0.85 to 1.05; participants = 7731; studies = seven; I² = 40%) (Analysis 1.8);
antepartum haemorrhage (average RR 0.89, 95% CI 0.57 to 1.40; participants = 3654; studies = four; I² = 31%) (Analysis 1.9);
induction of labour (average RR 0.93, 95% CI 0.86 to 1.01; participants = 17,501; studies = 13; I² = 47%) (Analysis 1.10);
augmentation/artificial oxytocin during labour (average RR 0.88, 95% CI 0.78 to 0.99; participants = 15,194; studies = 12; I² = 76%) (Analysis 1.12);
opiate analgesia (average RR 0.90, 95% CI 0.80 to 1.01; participants = 11,997; studies = 10; I² = 77%) (Analysis 1.14);
perineal laceration requiring suturing (average RR 1.02, 95% CI 0.96 to 1.10; participants = 15,104; studies = 10; I² = 53%) (Analysis 1.17);
postpartum haemorrhage (average RR 0.94, 95% CI 0.84 to 1.05; participants = 14,214; studies = 10) (Analysis 1.19);
breastfeeding initiation (average RR 1.12, 95% CI 0.81 to 1.53; participants = 2050; studies = two; I² = 81%) (Analysis 1.20);
mean length of postnatal hospital stay (days) (MD ‐0.10, 95% CI ‐0.29 to 0.09; participants = 3593; studies = three; Tau² = 0.02, I² = 58%) (Analysis 1.21);
low birthweight infant (RR 0.96, 95% CI 0.82 to 1.13; participants = 11,458; studies = seven) (Analysis 1.22);
five‐minute Apgar score less than or equal to seven (RR 0.98, 95% CI 0.73 to 1.32; participants = 12,546; studies = 11; I² = 32%) (Analysis 1.23);
neonatal convulsions (average RR 0.91, 95% CI 0.14 to 5.74; participants = 2923; studies = two) (Analysis 1.24);
admission of infant to special care or neonatal intensive care unit(s) (RR 0.90, 95% CI 0.78 to 1.04; participants = 17,561; studies = 13; I² = 43%) (Analysis 1.25);
mean length of neonatal hospital stay (days) (MD ‐3.63, 95% CI ‐7.57 to 0.30, participants = 1979; studies = two; Tau² = 6.69, I² = 80%) (Analysis 1.26);
fetal loss equal to/after 24 weeks and neonatal death (RR 1.00, 95% CI 0.67 to 1.49; participants = 17,359; studies = 12; I² = 0%) (Analysis 1.28).
1.8. Analysis.
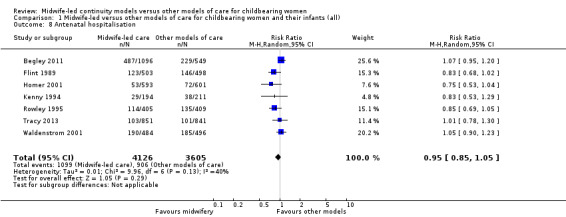
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 8 Antenatal hospitalisation.
1.9. Analysis.
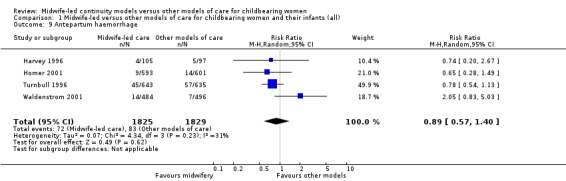
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 9 Antepartum haemorrhage.
1.10. Analysis.
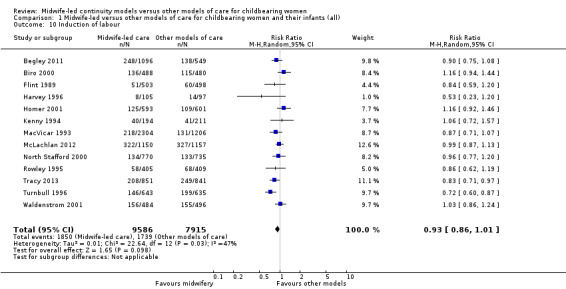
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 10 Induction of labour.
1.12. Analysis.
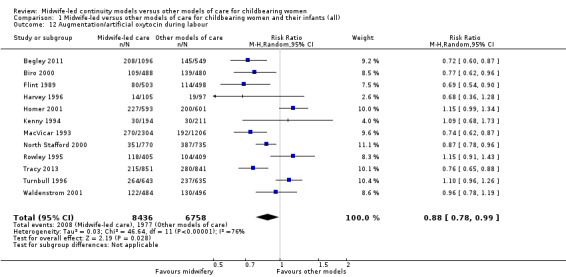
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 12 Augmentation/artificial oxytocin during labour.
1.14. Analysis.
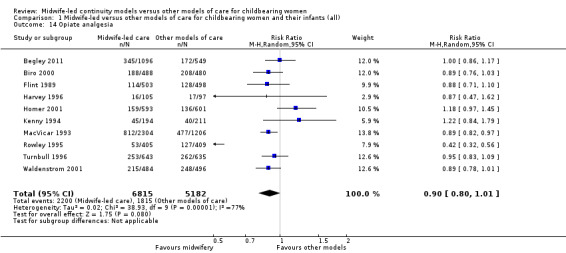
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 14 Opiate analgesia.
1.17. Analysis.
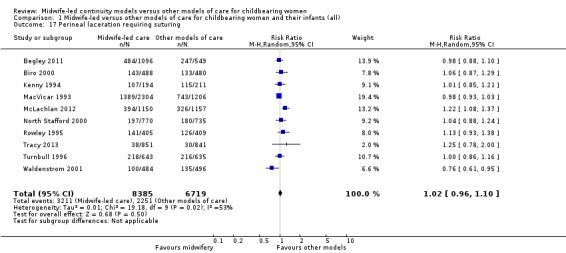
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 17 Perineal laceration requiring suturing.
1.19. Analysis.
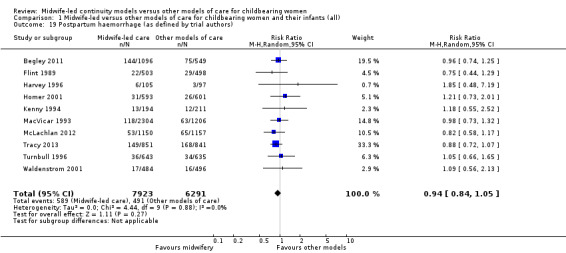
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 19 Postpartum haemorrhage (as defined by trial authors).
1.20. Analysis.
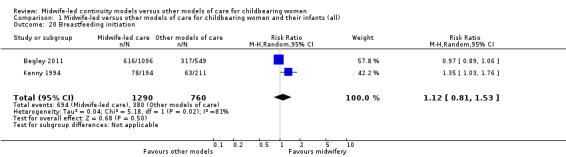
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 20 Breastfeeding initiation.
1.21. Analysis.
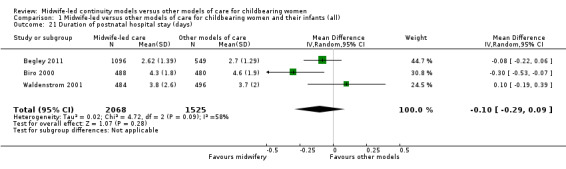
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 21 Duration of postnatal hospital stay (days).
1.22. Analysis.
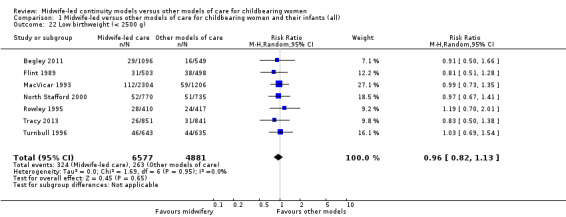
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 22 Low birthweight (< 2500 g).
1.23. Analysis.
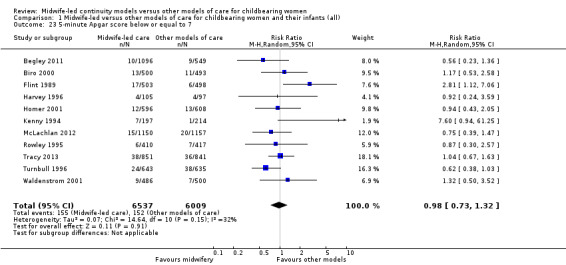
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 23 5‐minute Apgar score below or equal to 7.
1.24. Analysis.
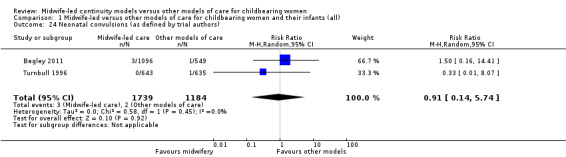
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 24 Neonatal convulsions (as defined by trial authors).
1.25. Analysis.
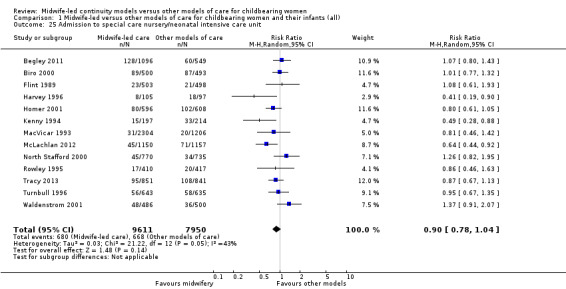
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 25 Admission to special care nursery/neonatal intensive care unit.
1.26. Analysis.
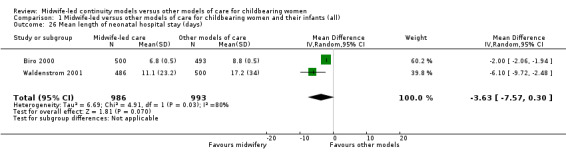
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 26 Mean length of neonatal hospital stay (days).
1.28. Analysis.
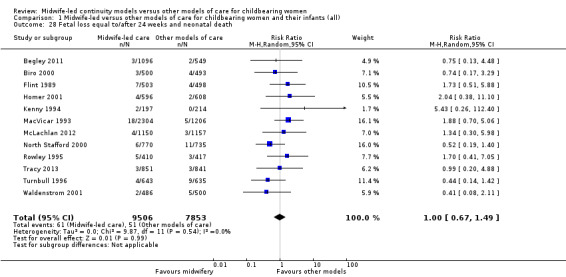
Comparison 1 Midwife‐led versus other models of care for childbearing women and their infants (all), Outcome 28 Fetal loss equal to/after 24 weeks and neonatal death.
There was substantial statistical heterogeneity in many of the analyses. The I² value was greater than 50% for 10 outcomes (antenatal hospitalisation, amniotomy, augmentation, opiate analgesia, attendance at birth by known carer, intact perineum, perineum requiring suturing, duration of postnatal hospital stay, duration of neonatal stay, breastfeeding initiation, and greater than 30% for a further six (antepartum haemorrhage, induction of labour, episiotomy, five‐minute Apgar score less than seven, preterm birth, admission to neonatal care). It is likely that heterogeneity could be due to the nature of the complexity of the intervention of a model of care, with variation in case mix and organisational setting.
Investigation of publication bias
Visual inspection of funnel plots for analyses where there were 10 or more studies (Analysis 1.1, Analysis 1.2, Analysis 1.3, Analysis 1.4, Analysis 1.5, Analysis 1.7, Analysis 1.10, Analysis 1.12, Analysis 1.14, Analysis 1.16, Analysis 1.17, Analysis 1.19, Analysis 1.23, Analysis 1.25, Analysis 1.27 and Analysis 1.28) suggested little evidence of asymmetry for most analyses. For three analyses (Analysis 1.1 regional analgesia, Analysis 1.2 caesarean delivery and Analysis 1.16 episiotomy), there was a some suggestion of asymmetry, though in all cases this was due to two small trials with large treatment effects in the same direction (Harvey 1996 and Hicks 2003, see Figure 3; Figure 4; Figure 5). There is therefore no strong evidence of reporting bias, though this is difficult to detect with the number of studies in this review, and whether it exists and the extent to which it affects the results may be clarified when more studies have been conducted.
3.
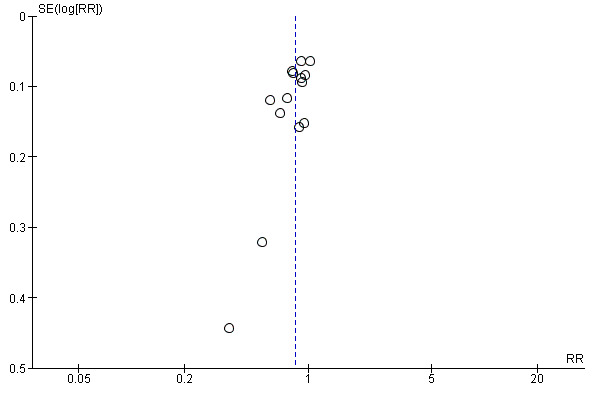
Funnel plot of comparison: 1 Midwife‐led versus other models of care for childbearing women and their infants (all), outcome: 1.1 Regional analgesia (epidural/spinal).
4.
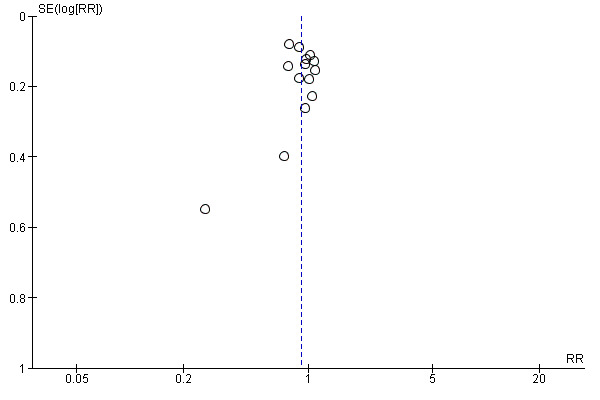
Funnel plot of comparison: 1 Midwife‐led versus other models of care for childbearing women and their infants (all), outcome: 1.2 Caesarean birth.
5.
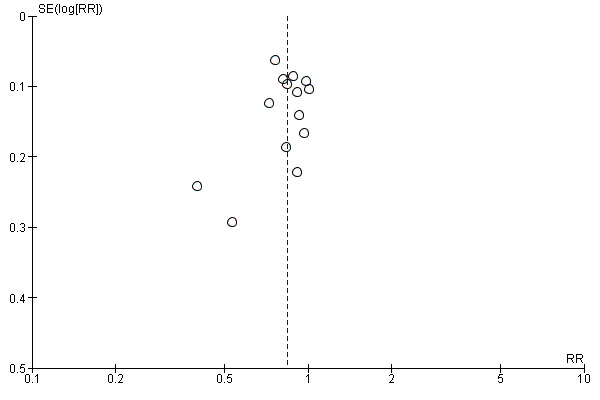
Funnel plot of comparison: 1 Midwife‐led versus other models of care for childbearing women and their infants (all), outcome: 1.16 Episiotomy.
Outcomes reported in single trials or not at all
It was not possible to analyse the following outcomes, either because data were not reported by any studies or they were reported in a way that did not allow extraction of the necessary data for meta‐analysis, or losses and exclusions were more than 20% of the randomised participants. No maternal deaths were reported. Only one trial reported the following outcomes: mean number of antenatal visits, perceptions of control, breastfeeding on discharge and postpartum depression and so results were not included in a meta‐analysis. No trials reported on longer‐term outcomes: any breastfeeding at three months; prolonged perineal pain; pain during sexual intercourse; urinary incontinence; faecal incontinence; and prolonged backache.
Subgroup analyses
Comparison 2: variation in midwifery models of care (caseload or one‐to‐one versus team)
Four trials randomised 6782 women to compare a caseload model of care (defined as one midwife carrying responsibility for a defined caseload of women in partnership with a midwife partner) with other models of care (McLachlan 2012; North Stafford 2000; Tracy 2013; Turnbull 1996). Caseload size was reported to be 45 women per midwife per year (McLachlan 2012), 35 to 40 women (North Stafford 2000), 40 women (Tracy 2013) and 32.4 women per midwife (Turnbull 1996). Ten trials randomised 11,183 women to compare team models of midwifery (defined as a group of midwives sharing responsibility for a caseload of women) with other models of care (Begley 2011; Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Homer 2001; Kenny 1994; MacVicar 1993; Rowley 1995; Waldenstrom 2001).
On the whole, there was no evidence of a difference between the caseload and team subgroups for any of the outcomes included in the subgroup analysis, which included caesarean section, instrumental vaginal birth, spontaneous vaginal birth, intact perineum, preterm birth < 37 weeks and all fetal loss before and after 24 weeks plus neonatal death.
There were borderline differences between subgroups for the outcome of regional analgesia (Test for subgroup differences: (P = 0.10), I² = 63.4%). Both caseload and team care (average RR 0.92, 95% CI 0.82 to 1.04; participants = 6782; studies = four; I² = 56%) and other models of care (average RR 0.81, 95% CI 0.73 to 0.89; participants = 10,892; studies = 10; I² = 44%) had substantial heterogeneity. Due to heterogeneity and to the small number of trials in each subgroup, we would advise caution when interpreting this result (Analysis 2.1).
Comparison 3: variation in risk status (low risk versus mixed)
Eight trials randomised 11,195 women to compare midwife‐led continuity models of care versus other models of care in women defined to be at low risk by trial authors (Begley 2011; Flint 1989; Harvey 1996; Hicks 2003; MacVicar 1993; McLachlan 2012; Turnbull 1996; Waldenstrom 2001). Six trials randomised over 6578 women to compare midwife‐led continuity models of care with other models of care in women defined to be at mixed risk of complications by trial authors (Biro 2000; Homer 2001; Kenny 1994; North Stafford 2000; Rowley 1995; Tracy 2013;). Of these, two trials excluded women who booked late ‐ after 24 weeks' gestation (Biro 2000; Homer 2001) and 16 weeks' gestation (Kenny 1994). Two trials excluded women with a substance misuse problem (Kenny 1994; Rowley 1995), and two trials excluded women with significant medical disease or previous history of a classical caesarean or more than two caesareans (Homer 2001), or women requiring admission to the maternal fetal medicine unit (Biro 2000).
There was no evidence of differences in treatment effect between the low risk and mixed risk subgroups for any of the outcomes included (see Analysis 3.1 to Analysis 3.7).
3.7. Analysis.
Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 7 All fetal loss before and after 24 weeks plus neonatal death.
Maternal satisfaction
Due to the lack of consistency in conceptualisation and measurement of women's experiences and satisfaction of care, a narrative synthesis of such data are presented. Ten studies reported maternal satisfaction with various components of the childbirth experiences (Biro 2000; Flint 1989; Harvey 1996; Hicks 2003; Kenny 1994; MacVicar 1993; McLachlan 2012; Rowley 1995; Turnbull 1996; Waldenstrom 2001).
Given the ambiguity surrounding the concept of satisfaction, it was not surprising to find inconsistency in the instruments, scales, timing of administration and outcomes used to 'measure' satisfaction across studies. Because of such heterogeneity and as might be expected, response rates of lower than 80% for most of these studies, meta‐analysis for the outcome of satisfaction was considered inappropriate and was not conducted.
Satisfaction outcomes reported in the included studies included maternal satisfaction with information, advice, explanation, venue of delivery, preparation for labour and birth, as well as giving choice for pain relief and behaviour of the carer. One study assessed perceptions of control in labour (Flint 1989), using a three‐point scale. For convenience and ease of understanding, tabulated results of the overall satisfaction or indicators which directly relate to staff attitude, or both, are presented in Table 2. In brief, the majority of the included studies, showed a higher level of satisfaction in various aspects of care in the midwife‐led continuity compared to the other models of care.
1. Women's experiences of care.
| Satisfaction | Intervention (n/N) | Control (n/N) | Relative rate | 95% CI | Statistical test | P value |
| Flint 1989* | ||||||
| Staff in labour (very caring) | 252/275 (92%) | 208/256 (81%) | 1.1 | 1.0‐1.2 | ||
| Experience of labour (wonderful/enjoyable) | 104/246 (42%) | 72/223 (32%) | 1.3 | 1.0‐1.8 | ||
| Satisfaction with pain relief (very satisfied) | 121/209 (58%) | 104/205 (51%) | 1.1 | 0.9‐1.4 | ||
| Very well prepared for labour | 144/275 (52%) | 102/254 (40%) | 1.3 | 1.0‐1.7 | ||
| MacVicar 1993 | N = 1663 | N = 826 | Difference | |||
| Very satisfied with antenatal care | 52% | 44% | 8.3% | 4.1‐12.5 | ||
| Very satisfied with care during labour | 73% | 60% | 12.9% | 9.1‐16.8 | ||
| Kenny 1994 | N = 213 | N = 233 | ||||
| Carer skill, attitude and communication (antenatal care) | 57.1/60 | 47.7/60 | t = 12.4 | 0.0001 | ||
| Convenience and waiting (antenatal care) | 14.8/20 | 10.9/20 | t = 10.1 | 0.0001 | ||
| Expectation of labour/birth (antenatal care) | 9.8/18 | 9.3/18 | t = 1.4 | 0.16 | ||
| Asking questions (antenatal care) | 8.5/12 | 6.9/12 | t = 6.6 | 0.0001 | ||
| Information/communication (labour and birth) | 28.3/30 | 24.8/30 | t = 7.48 | 0.0001 | ||
| Coping with labour (labour and birth) | 20.9/30 | 19.3/30 | t = 2.83 | 0.005 | ||
| Midwife skill/caring (labour and birth) | 22.7/24 | 21.3/24 | t = 3.44 | 0.0007 | ||
| Help and advice (postnatal care) | 21.0/24 | 19.7/24 | t = 1.88 | 0.06 | ||
| Midwife skill and communication (postnatal care) | 16.6/18 | 15.4/18 | t = 4.48 | 0.0001 | ||
| Managing baby (postnatal care) | 8.7/12 | 8.5/12 | t = 0.77 | 0.77 | ||
| Self‐rated health (postnatal care) | 7.5/12 | 7.1/12 | t = 1.67 | 0.10 | ||
| Rowley 1995 | OR | |||||
| Encouraged to ask questions | N/A | 4.22 | 2.72‐6.55 | |||
| Given answers they could understand | N/A | 3.03 | 1.33‐7.04 | |||
| Able to discuss anxieties | N/A | 3.60 | 2.28‐5.69 | |||
| Always had choices explained to them | N/A | 4.17 | 1.93‐9.18 | |||
| Participation in decision making | N/A | 2.95 | 1.22‐7.27 | |||
| Midwives interested in woman as a person | N/A | 7.50 | 4.42‐12.80 | |||
| Midwives always friendly | N/A | 3.48 | 1.92 ‐ 6.35 | |||
| Turnbull 1996 | n/N | n/N | Mean difference ‐ satisfaction score | |||
| Antenatal care | 534/648 | 487/651 | 0.48 | 0.55‐0.41 | ||
| Intrapartum care | 445/648 | 380/651 | 0.28 | 0.37‐0.18 | ||
| Hospital‐based postnatal care | 445/648 | 380/651 | 0.57 | 0.70‐0.45 | ||
| Home‐based postnatal care | 445/648 | 380/651 | 0.33 | 0.42‐0.25 | ||
| Waldenstrom 2001 | % | % | OR | |||
| Overall antenatal care was very good (strongly agree) | 58.2% | 39.7% | 2.22 | 1.66‐2.95 | < 0.001 | |
| Happy with the physical aspect of intrapartum care (strongly agree) | 58.6% | 42.5% | 1.94 | 1.46‐2.59 | < 0.001 | |
| Happy with the emotional aspect of intrapartum care (strongly agree) | 58.8% | 44.0% | 1.78 | 1.34‐2.38 | < 0.001 | |
| Overall postnatal care was very good (strongly agree) | 37.6% | 33.2% | 1.27 | 0.97‐1.67 | 0.08 | |
| Hicks 2003** | ||||||
| Care and sensitivity of staff (antenatal) | 1.32 | 1.77 | Mean difference? | 0.0000 | ||
| Care and sensitivity of staff (labour and delivery) | 1.26 | 1.58 | Mean difference? | 0.008 | ||
| Care and sensitivity of staff (postpartum at home) | 1.24 | 1.57 | Mean difference? | 0.0000 | ||
| Harvey 1996 | ||||||
| Labour and Delivery Satisfaction Index + | 211 | 185 | 26 | 18.8‐33.1 | 0.001 | |
| Biro 2000 | ||||||
| Satisfaction with antenatal care (very good) | 195/344 (57%) | 100/287 (35%) | 1.24 | 1.13‐1.36 | 0.001 | |
| Satisfaction with intrapartum care (very good) | 215/241 (63%) | 134/282 (47%) | 1.11 | 1.03‐1.20 | 0.01 | |
| Satisfaction with postpartum care in hospital (very good) | 141/344 (41%) | 102/284 (31%) | 0.92 | 0.82‐1.04 | 0.22 |
*: 99% Confidence interval (CI) for Flint study was reported N/A: not available **:Mean satisfaction scores are reported: lower scale indicates higher satisfaction. Satisfaction scores were calculated on a 5‐point ordinal scale in which 1 = very satisfied and 5 = very dissatisfied.
A second study (McLachlan 2012) assessed women's experience of childbirth in a postal survey. Women receiving caseload midwifery care were more likely to rate their experience of childbirth as very positive overall. These women reported a more positive experience of pain overall and more often reported feeling very proud of themselves. Women also felt more in control and more able to cope physically and emotionally; all of these outcome data are taken directly from the Machlachlan 2015 report and displayed in our Table 3 below.
2. McLachlan 2015 Women's experiences of birth.
| Outcome measure |
Caseload % scored 6 or 7*, with (n) |
Standard % scored 6 or 7*, with (n) |
Caseload RR** (ref = standard) |
|
Overall experience of childbirth (1 = very negative; 7 = very positive) |
71.2(697/979) | 62.6(516/824) | 1.47(1.21,1.80) |
|
Pain intensity (1 = no pain at all; 7= worst imaginable pain) |
57.8(565/978) | 58.0(478/825) | 0.99(0.82,1.20) |
|
Pain in relation to expectations (1 = much worse than expected; 7 = much better than expected) |
26.1(256/979)) | 22.7(186/820) | 1.21(0.97,1.50) |
|
Pain overall (1 = very negative; 7 = very positive) |
26.8(260/971) | 21.8(177/813) | 1.31(1.06,1.63) |
|
Anxiety during labour (1 = not at all anxious; 7 = very anxious) |
28.7(280/975) | 32.2(265/823) | 0.854(0.69,1.04) |
|
Experience of control (1 = completely out of control; 7 = in complete control) |
35.4(344/973) | 27.4(225/822) | 1.45(1.19,1.78) |
|
Coping physically (1 = much worse than expected; 7 = much better than expected) |
53.0(519/979) | 46.0(379/824) | 1.32(1.10–1.60) |
|
Coping emotionally (1 = much worse than expected; 7 = much better than expected) |
53.5(524/979) | 47.2(389/824) | 1.29(1.07–1.56) |
|
Feeling proud of self (1 = not all proud; 7 = very proud) |
80.5(786/976) | 72.2(594/823) | 1.59(1.28–1.99) |
|
Felt free to express feelings (1 = disagree strongly; 7 = agree strongly) |
80.7(788/977) | 71.6(586/819) | 1.66(1.33–2.06) |
|
Support by midwife (1 = no support at all; 7 = a lot of support) |
91.3(889/974) | 77.6(629/810) | 3.01(2.28–3.97) |
|
Support by doctor (if present) (1 = no support at all; 7 = a lot of support) |
53.0(334/630) | 54.1(329/608) | 0.96(0.77–1.20) |
|
Support by partner at all; 7 = a lot of support) |
91.5(892/975) | 89.7(733/817) | 1.23(0.89–1.69) |
*Scored ‘6’ or ‘7’ on seven‐point scale where ‘1’ was most negative response and ‘7’ most positive.
**Relative Risk (RR) with 95% confidence interval (CI) of rating statements as ‘6’ or ‘7’ on seven‐point scale, caseload/standard care.
Sensitivity analyses
We performed a sensitivity analysis excluding the cluster‐randomised North Staffordshire trial from all outcomes in the primary comparison (comparison 1) for which it had contributed data (North Stafford 2000). This did not alter the findings for any outcome, which remained consistent with overall findings with all trials included. Similarly, a sensitivity analysis for the primary outcomes including only the studies rated at low risk of bias (Begley 2011; Biro 2000; Harvey 1996; Hicks 2003; Homer 2001; McLachlan 2012; Turnbull 1996), found that there were only minor differences from the overall analyses. The main consequence was that confidence intervals were slightly wider, because of the smaller number of trials in the analysis. In no case were the conclusions of the analysis different. The primary outcome with the largest difference in this sensitivity analysis was preterm birth, where an analysis restricted to trials with lower risk of bias suggested a larger treatment effect: RR 0.64, (95% CI 0.51 to 0.81) compared with RR 0.77, (95% CI 0.62 to 0.94) in the overall analysis.
Economic analysis
Findings from economic analyses will vary depending on the structure of health care in a given country and what factors are included in the modelling. Due to the lack of consistency in measurement of economic evaluations, a narrative synthesis of such data are presented. Seven studies presented economic analysis in which various measures and items were included in the final cost estimation (Begley 2011; Flint 1987; Homer 2001; Kenny 1994; Rowley 1995; Tracy 2013; Young 1997).
Kenny 2015 reports an economic evaluation based on the Begley 2011 trial. Because the trial found no differences in the effect of type of care on any primary clinical outcome, the economic analysis compares only the costs of care rather than their cost‐effectiveness. Both midwifery‐led care and obstetric‐led care were shown to be equally safe and effective in the trial, but the costs of midwife‐led care were lower, contributing to a cost saving of EUR 182 per pregnant woman receiving midwife‐led care using an 'intention‐to‐treat' analysis.
Flint 1989 examined the costs for a subgroup of women (n = 49) and estimated costs for antenatal admission and antenatal care, and found antenatal care was 20% to 25% cheaper for women in the midwife‐led continuity of care group due to differences in staff costs. Women in the midwife‐led continuity of care group had fewer epidurals (GBP 19,360 versus GBP 31,460).
Kenny 1994 examined the costs of care in detail. The average cost/client in the antenatal period was AUD 158 midwife‐led continuity of care and AUD 167 control. For high‐risk women the average cost/client was AUD 390 midwife‐led continuity of care and AUD 437 control, and for low‐risk women AUD 119 midwife‐led continuity of care and AUD 123 control. The average cost per woman for intrapartum care was AUD 219 midwife‐led continuity of care and AUD 220 control and for postnatal care was AUD 745 midwife‐led continuity of care and AUD 833 control. The total cost/woman was AUD 1122 for midwife‐led continuity of care and AUD 1220 control.
Rowley 1995 used the Australian national cost weights for diagnostic‐related groups (AN‐DRGs) to estimate maternity care in each study group. The average cost per delivery was higher in the standard care group (AUD 3475) compared to the team‐midwifery group (AUD 3324). This method was limited to the acute inpatient and did not include antenatal or postnatal care cost estimations. An assessment of midwife salaries from the first antenatal visit up to and including labour and delivery care resulted in a cost of AUD 653 for each team care woman and $688 for each routine care woman. The amount of sick leave taken by team care midwives was half that taken by standard care midwives.
Tracy 2013 calculated cost outcomes per woman on the basis of activity‐based funding codes (Australian‐refined Diagnosis‐Related Group classification [DRG] codes). Expenditure data were obtained from the hospital financial systems, which provided detailed information about inpatient contacts for the mother and baby.The per‐woman cost of care calculated included both direct and indirect costs for each full episode of maternity care, taking account of the length of hospital stay for each woman. Direct and indirect costs were calculated for midwifery and obstetric clinical time; use of operating theatres, laboratory tests, imaging, wards, allied health, pharmacy; capital depreciation; and clinical overheads. Costs for each full episode of maternity care we recalculated from the sum of the services provided to the woman for the duration of her stay. Neonatal costs were not reported. Caseload midwifery care for unassisted vaginal birth cost significantly less than standard maternity care. This difference contributed to a significant difference in the overall median cost of birth per woman of AUD 566.74 (95% CI 106.17 to 1027.30) P = 0·02). However, the cost data showed several high‐cost outliers greater than $30,000, which were due to serious medical disorders, surgical complications, or accidental causes. The largest outlier, which cost more than $40,000, was due to a motor vehicle accident. The total cost of care per woman was AUD 566.74 (95% CI 106.17 to 1027.30); P = 0·02) less for caseload midwifery than for standard maternity care.
Young 1997 (cost analysis, Turnbull 1996) used the "individual patient‐based costing" approach, in which an assumption was made about the number of caseloads per midwife. When the assumption was based on a median caseload of 29 women per midwife, the cost of midwife managed care was not significantly different from the shared‐care group in the antenatal and intrapartum periods, but it was higher in the postpartum period. The authors also used an alternative assumption including a caseload of 39 women per midwife. A lower cost in the antenatal period for the midwife‐managed care was shown in comparison with the shared‐care group (mean: GPD 346 versus GPD 384, P = 0.05), but the postnatal care cost remained higher in the former group (GPD 444 versus GPD 397, respectively, P < 0.01). The authors did not recalculate the cost of intrapartum care for the second assumption, and used the same estimation as for the 29 caseload per midwife (since they indicated that the main effects were in the unit costs of clinic and home visits). They reported no significant differences between the midwifery and shared‐care group, in the cost of intrapartum care (GPD 280 versus GPD 276, P = 0.4).
Homer 2001 calculated the costs of all aspects of care from the healthcare provider's perspective, including salaries and wages; goods and services; and repair, maintenance and renewal (RMR). The associated costs for all stages of antenatal, intrapartum and postnatal care were calculated and presented as the mean cost per woman per group. The results showed a cost‐saving effect in the team midwifery group compared with the standard care arm of the study (mean cost per woman: AUD 2579 versus AUD 3483, respectively).
In summary, six studies presented cost data using different economic evaluation methods. All studies suggest a cost‐saving effect in intrapartum care. One study suggests a higher cost, and one study no differences in cost of postnatal care when midwife‐led continuity of care is compared with medical‐led maternity care. There is a lack of consistency in estimating maternity care cost among the available studies; however, there seems to be a trend towards the cost‐saving effect of midwife‐led continuity of care in comparison with medical‐led care.
Discussion
Summary of main results
This review summarises 15 trials involving 17,674 women that took place in four countries in a wide variety of settings and health systems. All trials involved midwife‐led continuity models of care that included either team or caseload midwifery, and women classified as at low or mixed risk. All trials included licensed midwives, and none included lay or traditional midwives. The review includes trials that compared midwife‐led continuity of care given both during the antepartum and the intrapartum period with other models of care, which included obstetricians or family physicians, or both, collaborating with nurses and midwives in a variety of organisational settings. No trial included models of care that offered out of hospital birth.
In the primary comparison, the results consistently show less use of some interventions for women who were randomised to receive midwife‐led continuity of care compared to women randomised to receive other models of care without detriment to outcomes. Specifically, women were on average less likely to experience regional analgesia, episiotomy, and instrumental birth. Women were on average more likely to experience spontaneous vaginal birth, a longer mean length of labour, and to be attended at birth by a known midwife, however, there were no differences in caesarean birth rates.
Stillbirth is not reported specifically due to differing gestational definitions, but is included within the outcome ‘Fetal loss equal to/after 24 weeks and neonatal death’. Women who were randomised to receive midwife‐led continuity of care compared to women randomised to receive other models of care were, on average, less likely to experience fetal loss less than 24 weeks and neonatal death and preterm birth before 37 weeks. The difference in the average treatment effect in all fetal loss before and after 24 weeks plus neonatal death across included trials between women allocated to midwife‐led continuity models of care and women allocated to other models has an average risk ratio (RR) of 0.84, with 95% confidence interval (CI) 0.71 to 0.99 (participants = 17561; studies = 13). Given that (i) the 95% CI just reaches 0.99 and (ii) the absence of measurable heterogeneity in this outcome analysis, the probability is that midwife‐led continuity models of care are associated with a reduction in fetal loss and neonatal death by approximately 16%.
We conducted pre‐specified subgroup analysis to investigate heterogeneity in the above outcomes of regional analgesia and preterm birth. The subgroup analyses of models of midwife‐led continuity of care and risk status did not find any significant subgroup interaction tests, indicating that there is no observable subgroup effect. It is possible that the complexity of the intervention in a range of settings and populations may influence the heterogeneity found.
Overall, we did not find any increased likelihood for any adverse outcome for women or their infants associated with having been randomised to a midwife‐led continuity model of care. These results were moderate in magnitude and generally consistent across all the trials.
It is possible that practice settings such as midwife‐led units can be a confounding influence on outcomes of midwife‐led continuity of care (Brocklehurst 2011), although home birth was not offered in any of the trials. Four trials offered care in midwife‐led units (Begley 2011; MacVicar 1993; Turnbull 1996; Waldenstrom 2001), which was available to women in both arms of one trial (Waldenstrom 2001), and only women in the midwife‐led group in three trials (Begley 2011; MacVicar 1993; Turnbull 1996). The increased likelihood of spontaneous vaginal birth in women randomised to midwife‐led continuity models of care may be a function of increased mobility due to less use of a range of analgesics, a much greater likelihood of attendance at birth by a known midwife, and the philosophy of care on offer. Midwife‐led continuity of care is a complex intervention, and it is impossible to unpick the relative importance of philosophy and continuity of care. However, in 10 trials, care was provided on the labour ward, suggesting a separate effect of birth setting. To what extent the observed benefits can be attributed to the model of midwifery care, midwifery philosophy, or to the quality and degree of relationship between the care provider and women was outside the scope of this review and requires an in‐depth exploration of the mechanisms through which midwife‐led care might work.
The possible effects on fetal loss and the substantive 24% reduction in preterm birth are important. Aetiology of both these events are complex but potentially influenced by models of care. Medical interventions to prevent fetal loss prior to 24 weeks do exist, as this is mostly due to spontaneous miscarriage, (and are dependent on quick access to care potentially influenced by continuity), such as cerclage and progesterone. These interventions are targeted to 'at risk' women, and may explain why mixed‐risk populations (with the improved access to care and appropriate referral) have the effect. Low‐risk women may not be referred or when referred the interventions not used due to lack of evidence in low‐risk women. There is insufficient detail in the trials to elucidate reasons for loss (e.g. intrauterine death or spontaneous miscarriage), and this would be important in future research.
Government and hospital policies affect how midwives are 'allowed' to practise, and/or the institutional structure within which midwives practise, and would thus affect practices and outcomes by limiting the potential of midwife‐led continuity of care in some settings. This is in contrast to models of health care which offer relationship continuity over time, which have been found to prevent clients falling through 'gaps in care' (Cook 2000). Women's experiences of care reported in the original studies include maternal satisfaction with information, advice, explanation, venue of delivery and preparation for labour and birth, as well as perceptions of choice for pain relief and evaluations of carers behaviour. In the majority of the included studies, satisfaction with various aspects of care appears to be higher in the midwife‐led continuity of care compared to the other models of care.
Overall completeness and applicability of evidence
Although there were limitations in the way that satisfaction‐related outcomes were assessed and reported, the majority of the included studies showed a higher level of satisfaction with various aspects of care in the midwife‐led continuity of care compared to the other models of care. Estimates of cost and resource use employed different economic evaluation methods. Results generally suggest a cost‐saving effect in intrapartum care; one study suggests a higher cost of postnatal care when midwife‐led continuity of care is compared with medical‐led care. However, there is a lack of consistency in estimating maternity care cost among the available studies, and there seems to be a trend towards a cost‐saving effect of midwife‐led continuity of care in comparison with medical‐led care.
Quality of the evidence
We assessed the quality of trial evidence for the following outcomes using the GRADE methodology: preterm birth < 37 weeks, all fetal loss before and after 24 weeks plus neonatal death, spontaneous vaginal birth (as defined by trialists), caesarean birth, instrumental vaginal birth, intact perineum and regional analgesia. All outcomes were graded as of high quality. Multiple trials of low risk of bias contributed to each outcome, and there were precise estimates with no heterogeneity greater than 60%. No trial included in this review had adequate blinding of participants, staff or outcomes assessors. We did not downgrade trial evidence for risk of bias due to lack of blinding. However, we understand that other authors might choose to do so. We would not expect blinding to affect the outcomes of preterm birth or fetal loss, but the argument could be made that blinding matters for mode of birth, intact perineum and use of analgesia.
Potential biases in the review process
We searched for further studies in the reference list of the studies identified, and did not apply any language or date restrictions. We made explicit judgements about whether studies were at high risk of bias using the GRADE approach. We carried out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result. No other potential sources of bias were identified in any of the included studies. There was no strong evidence of reporting bias, though this is difficult to detect with the number of studies in this review, and whether it exists and the extent to which it affects the results may be clarified when more studies have been conducted.
Agreements and disagreements with other studies or reviews
Studies of qualitative data can add understanding on why women experience fewer birth interventions within this model of care. One meta‐synthesis (Walsh 2012), suggests that lower rates of interventions could be linked to the "greater agency experienced by women and midwives within midwife‐led models", and that these effects are mediated, in part, by the smallness of scale in these settings. A review of reviews (Sutcliffe 2012), compared midwife‐led care during pregnancy and birth with physician‐led care resulted in similar findings to this review.
Authors' conclusions
Implications for practice.
Midwife‐led continuity of care confers important benefits and shows no adverse outcomes. However, due to the exclusion of women with significant maternal disease and substance abuse from some trials of women at mixed risk, caution should be exercised in applying the findings of this review to women with substantial medical or obstetric complications. Policy makers and healthcare providers should be aware that such benefits are conferred when midwives provide intrapartum care in hospital settings and also where midwives provide continuity through pregnancy and childbirth. Not all areas of the world have health systems where midwives are able to provide midwife‐led continuity models of care, and health system financing is a potential barrier to implementation. Policy makers who wish to achieve clinically important improvements in maternity care, particularly around normalising and humanising birth, and preventing preterm birth should consider midwife‐led continuity models of care and consider how financing of midwife‐led services can be reviewed to support this.
Implications for research.
Questions remain about the best way to organise midwife‐led continuity of care under varying conditions, and further comparisons of different models of midwife‐led continuity of care would be helpful. Further research should explore whether the observed benefits can be attributed to the model of continuity of midwifery care, philosophy, or to the quality and degree of relationship between the care provider and women. Further research is needed on more recently developed midwife‐led continuity models of care that include home birth and greater levels of relationship continuity in community settings to women classified at low and high risk of complications. One such model that should be evaluated is the community‐based caseload model of midwife‐led continuity of care. These models offer continuity of carer, with a named midwife working in partnership with associate midwives (usually two). They provide community‐based outreach and locally accessible services, in association with other care providers as necessary, with the option of intrapartum care provided at home, in a midwife‐led unit or in a hospital setting as appropriate. Others provide care to socially vulnerable women with promising results but further trials are required (Rayment‐Jones 2015).
Little is known about the interface between midwife‐led continuity models of care and the multi‐disciplinary network of support. Although continuity of care has been identified as a core component of a model of midwife‐led care, there is wide variation in the definition and measurement of continuity of care, which will require greater sophistication in future studies. Future research should also assess acceptability to midwives of different models of midwife‐led continuity of care that offer relational continuity.
Future trials in this area would benefit from drawing on a framework for trials of complex interventions, which explicitly requires theoretical modelling between processes and outcomes in the pre‐trial stage, and a process evaluation of the trial (Anderson 2008). All trials should provide greater description of intervention and standard models of care being assessed (Hoffman 2014) and include process evaluations of how they are being implemented (Moore 2014), using reporting guidelines for complex interventions. Future research in this area would benefit from exploring the theoretical underpinnings of these complex interventions and their associations with processes and outcomes and implementation reviews are helpful.
Questions remain about the mechanisms regarding why fetal loss is reduced, and why there are fewer preterm births in midwife‐led continuity models of care.
There remains relatively little information about the effects of midwife‐led continuity models of care on mothers' and babies' health and well being in the longer postpartum period. Future research should pay particular attention to outcomes that have been under‐researched, but are causes of significant morbidity, including postpartum depression, urinary and faecal incontinence, duration of caesarean incision pain, pain during intercourse, prolonged perineal pain and birth injury (to the baby). We will add these to the review outcomes when the review is updated as available, if not already specified in this review.
There were no trials in resource‐constrained countries and additional trials may be required in such settings.
Little is known about whether women feel they are part of the decision‐making process; sense of control; maternal self‐confidence; post‐traumatic stress disorder, coping after the birth. There is wide variation in the instruments used to measure women's views of and experiences of care. There is a need to develop meaningful, robust, valid and reliable methods to assess psychosocial outcomes and well being in pregnant and childbearing women. All trials should include an assessment of maternal and fetal well being. There is a lack of consistency in estimating maternity care cost, and further research using standard approaches of cost estimation is required which also includes cost to women and families. All trials should include economic analyses of the relative costs and benefits.
Given the heterogeneity in the choice of outcome measures routinely collected and reported in randomised evaluations of models of maternity care, a core (minimum) data set, such as that by Devane 2007, and a validated measure of maternal quality of life and well being would be useful not only within multi‐centre trials and for comparisons between trials, but might also be a significant step in facilitating useful meta‐analyses of similar studies. In addition, future trials should include measures of optimal outcomes for mothers and babies in addition to measures of morbidity.
Feedback
Bacon, May 2004
Summary
Are you planning to include intrapartum foetal death rates for women delivering in different types of unit, and with different levels of risk, as one of your outcome measures? We have been unable to find comparative data for a local review.
(Summary of comment from Sallie Bacon, May 2004)
Reply
We have not looked at intrapartum deaths specifically, but have addressed this issue in the 'Discussion'.
(Summary of response from Jane Sandall, November 2007)
Contributors
Sallie Bacon
Blake, 19 November 2013
Summary
The Society of Obstetricians and Gynaecologists of Canada (SOGC) is the longest established national organization for women’s reproductive care in North America, with membership made up of obstetricians, gynaecologists, nurses, midwives, family physicians and scientists. We have long supported a woman’s right to choose the care provider of her preference for obstetrical care, and we actively support and promote collaborative models of care. We were therefore very interested to read the review of midwifery‐led care that you published in August of this year. We were not surprised by the main findings cited in the abstract: less use of epidural or intra‐partum analgesia, fewer instrumental deliveries and, in consequence, fewer episiotomies, longer length of labour. These differences would be expected with the different model of care; for some women an unmediated delivery is a goal. However, for others, access to analgesia is a key consideration; we cannot conclude from this difference that the midwifery‐led model is better for all women. We were interested by the findings of fewer preterm births, fewer deaths <24weeks, findings which are unexplained, and for which it is unlikely that we could identify an explanation based on who was providing the care, given that there are few, if any, clinical interventions by any provider prior to 24 weeks which can affect these outcomes. Beyond these matters, however, we are primarily contacting you because the abstract failed to list the important outcomes which do not differ with provider: perineal trauma, induction of labour, oxytocin augmentation of labour, caesarean section, antenatal hospitalisation, post‐partum haemorrhage, length of hospital stay, initiation of breast feeding, neonatal Apgar score, admission to neonatal nursery, fetal loss or death >24 weeks. Our greatest concern is that, although the abstract failed to list or consider these fundamentally important clinical outcomes that were equivalent, the authors still asserted that “most women should be offered midwifery‐led continuity models of care and women should be encouraged to ask for this option…” We believe this conclusion received, and continues to receive, the bulk of media and lay attention. In fact, those who do not actually read the review but only the abstract will come away with an incorrect understanding that is not supported by the results, an outcome that appears to be self‐serving and misleading. We expect better from the Cochrane Collaboration. This was an opportunity to provide women with reassurance that they have healthful options for their pregnancy care, and that they can feel confident that, regardless of their choice, the outcomes will be similar with respect to a safe and healthy pregnancy and delivery. Instead, the way this issue has been positioned, and by the selective use of the data, the Cochrane appears to advocate for a particular model of care, a disservice to women and the many other health care professionals who care for them.
Comment received from Jennifer Blake, Society of Obstetricians and Gynaecologists of Canada, November 2013.
Reply
We are pleased to see the SOGC’s interest in our review and thank them for their comments.
We agree that findings of fewer preterm births and fewer deaths less than 24weeks are interesting. Midwife‐led continuity of care is a complex intervention, and it is impossible to unpick the relative importance of philosophy and continuity of care. We note in our review that questions remain about the mechanisms underlying these findings.
Our abstract is reported in original format in an effort to present information on multiple outcomes in as clear a manner as possible. Further to your comments, in the updated review, we have reformatted the presentation of outcomes in the abstract such that all primary outcomes are presented initially followed by all secondary outcomes. This will, we believe provide the reader with the totality of information on which to inform their health care decisions. Similarly, we have revised the conclusion to summarise the findings of the review and key areas for further research.
We trust this addresses your concerns.
Regards
Jane Sandall, August 2015
Contributors
Jane Sandall
What's new
| Date | Event | Description |
|---|---|---|
| 25 January 2016 | New citation required but conclusions have not changed | For this update the results and conclusions of this review remain unchanged. |
| 25 January 2016 | New search has been performed | Search updated. Three new trial reports identified relating to three studies already included in the review (Begley 2011; McLachlan 2012; Tracy 2013). Additional data have been added from two of the new reports on cost (Begley 2011) and maternal satisfaction (McLachlan 2012). The primary neonatal outcome "Overall fetal loss and neonatal death (fetal loss was assessed by gestation using 24 weeks as the cut‐off for viability in many countries)" was changed to "All fetal loss before and after 24 weeks plus neonatal death." The secondary neonatal outcomes, "Fetal loss and neonatal death less than 24 weeks" and "Fetal loss and neonatal death equal to/after 24 weeks" were changed to "Fetal loss less than 24 weeks and neonatal death" and "Fetal loss equal to/after 24 weeks and neonatal death". |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 23 September 2015 | Amended | Correction to abstract. Clarification of results for the outcomes "No intrapartum analgesia/anaesthesia" and "Attendance at birth by known midwife". |
| 31 May 2015 | New citation required but conclusions have not changed | Two new studies included (Allen 2013; Tracy 2013); two studies excluded (Famuyide 2014; Gu 2013). The conclusions remain the same. |
| 31 May 2015 | New search has been performed | Search updated. A 'Summary of findings' table has been incorporated. |
| 19 November 2013 | Feedback has been incorporated | Feedback 2 received from Jennifer Blake. |
| 2 May 2013 | New citation required and conclusions have changed | Two new studies included (Begley 2011; McLachlan 2012). In this update the evidence now suggests that women randomised to receive midwife‐led continuity models of care were less likely to experience preterm birth. There is now no evidence of a difference between different models of care in terms of antenatal hospitalisation and breastfeeding initiation. |
| 28 January 2013 | New search has been performed | Search updated. Methods updated. |
| 29 April 2009 | Amended | In response to feedback, we have clarified what is meant by midwife‐led care and have stressed the multi‐disciplinary network of care providers; have added information to the Abstract about the lack of effect on caesarean section; and revised the Abstract's conclusions from "All women" to "Most women should be offered midwife‐led models of care and women should be encouraged to ask for this option." |
| 9 November 2008 | Amended | Amended the graph labelling for control in childbirth (Analysis 1.32) and corrected a typographical error in the Results section. |
| 15 May 2008 | Amended | Converted to new review format. |
Acknowledgements
We are very grateful to the investigators who provided additional information: C Homer, H McLachlan, D Forster, P Brodie and S Tracy.
We thank Nancy Medley for her support in the production of the 'Summary of findings' table in the 2015 update. Nancy Medley's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Jane Sandall was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King's College Hospital NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Hora Soltani was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care Yorkshire and Humber at Sheffield Hallam University. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Appendices
Appendix 1. Search methods used in previous versions of this review
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (January 2008).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched the Cochrane Effective Practice and Organisation of Care Group's Trials Register (January 2008), Current Contents (1994 to January 2008), CINAHL (1982 to August 2006), Web of Science, BIOSIS Previews, ISI Proceedings, (1990 to 2008), and the WHO Reproductive Health Library (WHO‐RHL), No. 9. Through WHO‐RHL we obtained unpublished studies from the System for Information on Grey Literature In Europe (SIGLE). We used the search strategy detailed below, modifying it for each database as appropriate by checking each thesaurus for relevant subject headings and replacing them with text‐word search terms when a subject heading was not available.
We did not apply any language restrictions.
1 exp Pregnancy/ 2 exp Prenatal Care/ 3 exp Intrapartum Care/ 4 exp Obstetric Care/ 5 exp Postnatal Care/ 6 exp Midwifery/ 7 exp Midwifery Service/ 8 exp Obstetric Service/ 9 exp Home Childbirth/ 10 exp Alternative Birth Centers/ 11 or/1‐10 12 exp Continuity of Patient Care/ 13 exp Nursing Care Delivery Systems/ 14 (midwif$ adj2 team$).tw. 15 (midwif$ adj model$).tw. 16 (multidisciplinary adj team$).tw. 17 (share$ adj care).tw. 18 (midwif$ adj led).tw. 19 (midwif$ adj manag$).tw. 20 (medical$ adj led).tw. 21 (medical adj manag$).tw. 22 or/12‐21 23 exp Clinical Trials/ 24 11 and 22 and 23
Data and analyses
Comparison 1. Midwife‐led versus other models of care for childbearing women and their infants (all).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Regional analgesia (epidural/spinal) | 14 | 17674 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.78, 0.92] |
| 2 Caesarean birth | 14 | 17674 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.00] |
| 3 Instrumental vaginal birth (forceps/vacuum) | 13 | 17501 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.83, 0.97] |
| 4 Spontaneous vaginal birth (as defined by trial authors) | 12 | 16687 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.03, 1.07] |
| 5 Intact perineum | 10 | 13186 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.95, 1.13] |
| 6 Preterm birth (< 37 weeks) | 8 | 13238 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.64, 0.91] |
| 7 All fetal loss before and after 24 weeks plus neonatal death | 13 | 17561 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.71, 0.99] |
| 8 Antenatal hospitalisation | 7 | 7731 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.05] |
| 9 Antepartum haemorrhage | 4 | 3654 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.57, 1.40] |
| 10 Induction of labour | 13 | 17501 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 11 Amniotomy | 4 | 3253 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.66, 0.98] |
| 12 Augmentation/artificial oxytocin during labour | 12 | 15194 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 0.99] |
| 13 No intrapartum analgesia/anaesthesia | 7 | 10499 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.06, 1.37] |
| 14 Opiate analgesia | 10 | 11997 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.80, 1.01] |
| 15 Attendance at birth by known midwife | 7 | 6917 | Risk Ratio (M‐H, Random, 95% CI) | 7.04 [4.48, 11.08] |
| 16 Episiotomy | 14 | 17674 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.77, 0.92] |
| 17 Perineal laceration requiring suturing | 10 | 15104 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.96, 1.10] |
| 18 Mean labour length (hrs) | 3 | 3328 | Mean Difference (IV, Random, 95% CI) | 0.50 [0.27, 0.74] |
| 19 Postpartum haemorrhage (as defined by trial authors) | 10 | 14214 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.05] |
| 20 Breastfeeding initiation | 2 | 2050 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.81, 1.53] |
| 21 Duration of postnatal hospital stay (days) | 3 | 3593 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.29, 0.09] |
| 22 Low birthweight (< 2500 g) | 7 | 11458 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.82, 1.13] |
| 23 5‐minute Apgar score below or equal to 7 | 11 | 12546 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.73, 1.32] |
| 24 Neonatal convulsions (as defined by trial authors) | 2 | 2923 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.14, 5.74] |
| 25 Admission to special care nursery/neonatal intensive care unit | 13 | 17561 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.04] |
| 26 Mean length of neonatal hospital stay (days) | 2 | 1979 | Mean Difference (IV, Random, 95% CI) | ‐3.63 [‐7.57, 0.30] |
| 27 Fetal loss less than 24 weeks and neonatal death | 11 | 15645 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.98] |
| 28 Fetal loss equal to/after 24 weeks and neonatal death | 12 | 17359 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.67, 1.49] |
Comparison 2. Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Regional analgesia (epidural/spinal) | 14 | 17674 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.78, 0.92] |
| 1.1 Caseload | 4 | 6782 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.82, 1.04] |
| 1.2 Team models of midwifery care | 10 | 10892 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.73, 0.89] |
| 2 Caesarean birth | 14 | 17658 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.00] |
| 2.1 Caseload | 4 | 6782 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.05] |
| 2.2 Team models of midwifery care | 10 | 10876 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.05] |
| 3 Instrumental vaginal birth (forceps/vacuum) | 13 | 17965 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.97] |
| 3.1 Caseload | 4 | 6782 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.04] |
| 3.2 Team models of midwifery care | 9 | 11183 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.97] |
| 4 Spontaneous vaginal birth (as defined by trial authors) | 12 | 16687 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.03, 1.07] |
| 4.1 Caseload | 4 | 6782 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.00, 1.12] |
| 4.2 Team models of midwifery care | 8 | 9905 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.02, 1.07] |
| 5 Intact perineum | 10 | 13186 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.95, 1.13] |
| 5.1 Caseload | 3 | 4475 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.90, 1.34] |
| 5.2 Team | 7 | 8711 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.13] |
| 6 Preterm birth (< 37 weeks) | 8 | 13238 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.64, 0.91] |
| 6.1 Caseload | 3 | 5277 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.54, 0.89] |
| 6.2 Team | 5 | 7961 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.07] |
| 7 All fetal loss before and after 24 weeks plus neonatal death | 13 | 17527 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.71, 0.99] |
| 7.1 Caseload | 4 | 6782 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.48, 0.99] |
| 7.2 Team | 9 | 10745 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.73, 1.07] |
2.2. Analysis.
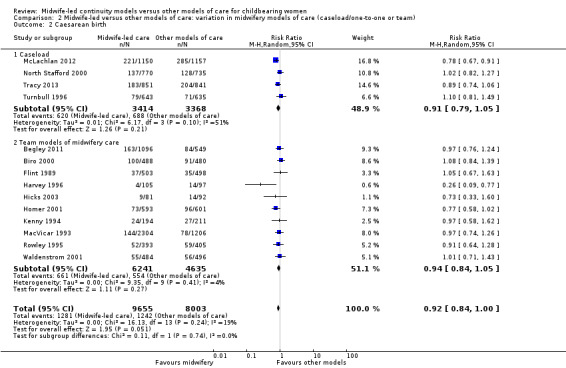
Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 2 Caesarean birth.
2.3. Analysis.
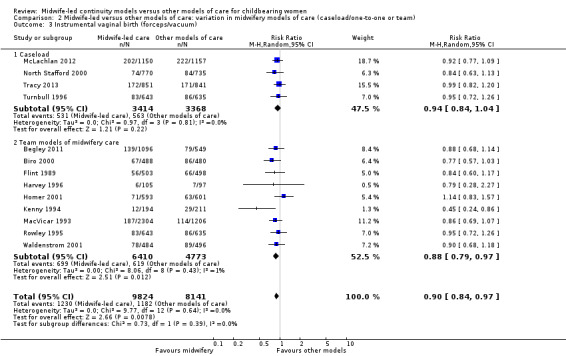
Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 3 Instrumental vaginal birth (forceps/vacuum).
2.4. Analysis.
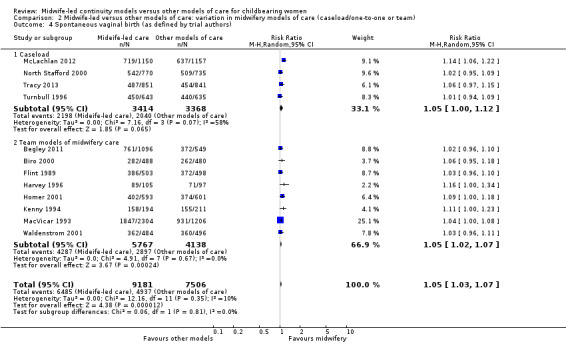
Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 4 Spontaneous vaginal birth (as defined by trial authors).
2.7. Analysis.
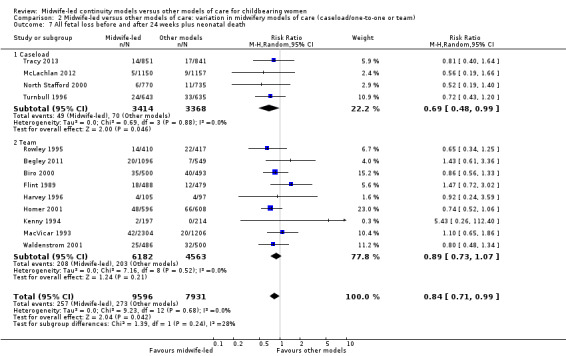
Comparison 2 Midwife‐led versus other models of care: variation in midwifery models of care (caseload/one‐to‐one or team), Outcome 7 All fetal loss before and after 24 weeks plus neonatal death.
Comparison 3. Midwife‐led versus other models of care: variation in risk status (low versus mixed).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Regional analgesia (epidural/spinal) | 14 | 17674 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.78, 0.92] |
| 1.1 Low risk | 8 | 11096 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 1.2 Mixed risk | 6 | 6578 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 1.00] |
| 2 Caesarean birth | 14 | 17674 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.00] |
| 2.1 Low risk | 8 | 11096 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.06] |
| 2.2 Mixed risk | 6 | 6578 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| 3 Instrumental vaginal birth (forceps/vacuum) | 13 | 17501 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.83, 0.97] |
| 3.1 Low risk | 7 | 10923 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.81, 0.99] |
| 3.2 Mixed risk | 6 | 6578 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.73, 1.04] |
| 4 Spontaneous vaginal birth (as defined by trial authors) | 12 | 16687 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.03, 1.07] |
| 4.1 Low risk | 7 | 10923 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.02, 1.08] |
| 4.2 Mixed risk | 5 | 5764 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.02, 1.10] |
| 5 Intact perineum | 10 | 13186 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.95, 1.13] |
| 5.1 Low risk | 6 | 8616 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.21] |
| 5.2 Mixed risk | 4 | 4570 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.91, 1.08] |
| 6 Preterm birth (< 37 weeks) | 8 | 13238 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.64, 0.91] |
| 6.1 Low risk | 5 | 9726 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.54, 0.92] |
| 6.2 Mixed risk | 3 | 3512 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.09] |
| 7 All fetal loss before and after 24 weeks plus neonatal death | 13 | 17527 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.71, 0.99] |
| 7.1 Low risk | 7 | 10895 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| 7.2 Mixed risk | 6 | 6632 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.61, 0.96] |
3.2. Analysis.
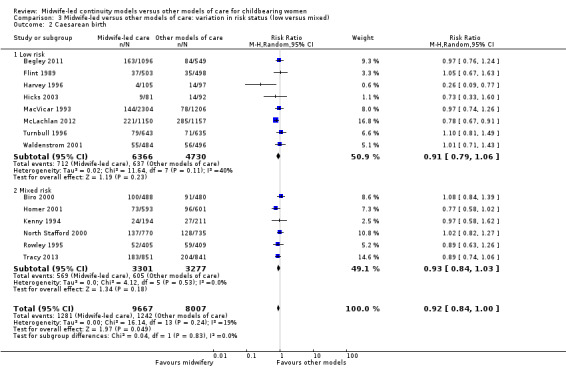
Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 2 Caesarean birth.
3.3. Analysis.
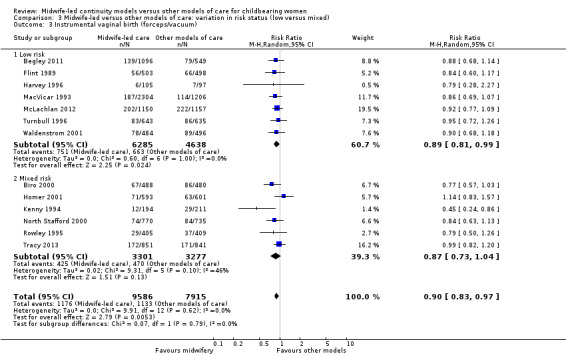
Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 3 Instrumental vaginal birth (forceps/vacuum).
3.4. Analysis.
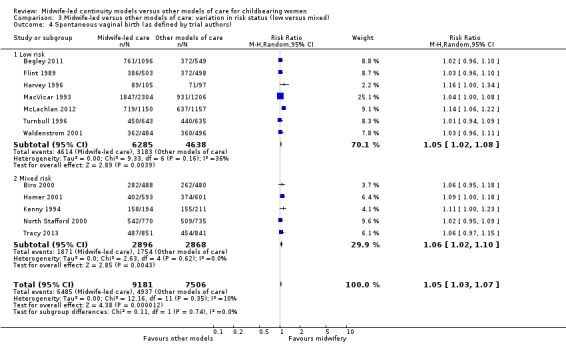
Comparison 3 Midwife‐led versus other models of care: variation in risk status (low versus mixed), Outcome 4 Spontaneous vaginal birth (as defined by trial authors).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 2013.
| Methods |
Study design: RCT. Duration of study: 2010‐2011. |
|
| Participants | Setting: inner city tertiary maternity hospital and associated community‐based clinic, Australia. Inclusion criteria: women were eligible for trial entry if they were: (a) aged between 13‐17 years of age (b) booked for public maternity care at the study hospital c) 23 weeks pregnant or less, d) single live fetus at time of recruitment. Exclusion criteria: maternal age 18 years or older, inability to provide consent (e.g. serious mental illness or lack of English fluency), residence outside of the hospital catchment area (because of the requirement for home visiting), 24 weeks gestation or greater, and multiple pregnancy. Participants randomised: 1 midwife‐led care, 0 to standard care. | |
| Interventions |
Experimental: women randomised to the intervention received antenatal, intrapartum and postnatal care from a known midwife. Control: women randomised to the control group were able to select any other available model of antenatal care including YWC, care with a GP, or a community‐ or hospital‐based antenatal clinic. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: Preterm birth Gestation Birthweight Mode of birth Apgar score less than 7 at 5 minutes Breastfeeding initiation Breastfeeding at hospital discharge Admission to a separate neonatal nursery Length of maternal and neonatal stay |
|
| Notes | This study was a feasibility study for a proposed randomised trial. Only 1 woman was recruited to receive the intervention, and the study was not continued. Authors concluded that an RCT with pregnant adolescents was not feasible according to specifications of the protocol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This study was a feasibility study. Only 1 woman received the intervention. This study contributed no data to the review. |
| Selective reporting (reporting bias) | Unclear risk | This study was a feasibility study. Only 1 woman received the intervention and no outcome data were reported. |
| Other bias | Unclear risk | This study was a feasibility study, and the study authors concluded that recruitment was not feasible according to the specifications outlined in the study protocol. |
Begley 2011.
| Methods |
Study design: RCT. Duration of study: 2004‐2007. |
|
| Participants | Setting: Health Service Executive, Dublin North‐East, Republic of Ireland. Inclusion criteria: women were eligible for trial entry if they were: (a) healthy with an absence of risk factors for complications for labour and delivery as identified in the ‘Midwifery‐led Unit (Integrated) Guidelines for Practitioners’ (at http://www.nehb.ie/midu/guidelines.htm); (b) aged between 16 and 40 years of age; and (c) within 24 completed weeks of pregnancy. Exclusion criteria: women with risk factors. Participants randomised: 1101 midwife‐led care, 552 to CLC. | |
| Interventions |
Experimental: women randomised to midwife‐led care (MLU) received antenatal care from midwives and, if desired, from their GPs for some visits. Where complications arose, women were transferred to CLU based on agreed criteria. Intrapartum care was provided by midwives in a MLU with transfer to CLU if necessary. Postnatal care was by midwives in the MLU for up to 2 days, with transfer of women or neonates to CLU if necessary (and back, as appropriate). On discharge, MLU midwives visited at home, and/or provided telephone support, up to the seventh postpartum day. Control: women randomised to consultant‐led care (CLU) received standard care: antenatal care provided by obstetricians supported by the midwifery and medical team; intrapartum and postpartum care (2 to 3 days in hospital) provided by midwives, overseen by consultants. Women were discharged into the care of Public Health Nurses. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: 5‐minute Apgar score below or equal to 7 Admission to special care nursery/NICU Amniotomy Antenatal hospitalisation Antepartum haemorrhage Augmentation/artificial oxytocin during labour Breastfeeding initiation Caesarean birth Duration of postnatal hospital stay (days) Episiotomy Fetal loss/neonatal death before 24 weeks Fetal loss/neonatal death equal to/after 24 weeks Induction of labour Instrumental vaginal birth (forceps/vacuum) Intact perineum Low birthweight (< 2500 g) Mean labour length Mean length of neonatal hospital stay (days) Neonatal convulsions (as defined by trial authors) No intrapartum analgesia/anaesthesia Opiate analgesia Fetal loss and neonatal death Perineal laceration requiring suturing Preterm birth (< 37 weeks) PPH (as defined by trial authors) Regional analgesia (epidural/spinal) Spontaneous vaginal birth (as defined by trial authors) Cost |
|
| Notes | Women were randomised to MLU or CLU in a 2:1 ratio. Kenny 2015 reports an economic analysis ‐ a comparison of the cost of care of the 2 types of services. We have described these results above ‐ data added 2016 update. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘Random integers were obtained using a random number generator…’ |
| Allocation concealment (selection bias) | Low risk | ‘…an independent telephone randomisation service.’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | '...lack of blinding of participants and carers...' |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 'Assessors for certain outcomes, such as laboratory tests, were blinded to study group.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up = 5 midwife‐led care, 3 CLC. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Low risk | No other bias identified. |
Biro 2000.
| Methods |
Study design: RCT. Duration of study: 1996‐1998. |
|
| Participants | Setting: public tertiary hospital, Monash Medical Centre, Melbourne, Australia. Inclusion criteria: participants included women at low and high risk of complications. Exclusion criteria: women who requested shared obstetric care, needed care in the maternal‐fetal medicine unit, were > 24 weeks' gestation, did not speak English. Participants randomised: 502 team midwifery, 498 to standard care. | |
| Interventions | Experimental: team of 7 full‐time midwives who provided antenatal, intrapartum, and some postnatal care in hospital in consultation with medical staff. Doctors and team midwife jointly saw women at 12‐16, 28, 36, 41 weeks. Women at high risk of complications had individual care plans. Control: various options of care including shared care between GPs in the community and hospital obstetric staff, shared care between midwives in a community health centre and hospital obstetric staff, care by hospital obstetric staff only, and less commonly, care by hospital midwives in collaboration with obstetric staff. Women within these options experienced a variable level of continuity of care during their pregnancy, from seeing the same midwife or doctor at most visits to seeing several doctors and midwives. | |
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: 5‐minute Apgar score below or equal to 7 Admission to special care nursery/NICU Attendance at birth by known midwife Augmentation/artificial oxytocin during labour Duration of postnatal hospital stay (days) Episiotomy Fetal loss/neonatal death before 24 weeks Fetal loss/neonatal death equal to/after 24 weeks Induction of labour Intact perineum Instrumental vaginal birth (forceps/vacuum) Mean length of neonatal hospital stay (days) No intrapartum analgesia/anaesthesia Fetal loss and neonatal death Perineal laceration requiring suturing Preterm birth (< 37 weeks) Regional analgesia (epidural/spinal) Spontaneous vaginal birth (as defined by trial authors) |
|
| Notes | 2 groups similar at baseline. 80% of experimental group and 0.3% of standard group had previously met midwife attending labour. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Allocations were computer generated...' |
| Allocation concealment (selection bias) | Low risk | '...the research team member telephoned the medical records staff and asked them to select an envelope with the randomized treatment allocation.' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up = 14 team care, 18 standard care. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in results. |
| Other bias | Low risk | No other bias identified. |
Flint 1989.
| Methods |
Study design: RCT, Zelen design. Duration of study: 1983‐1985. |
|
| Participants | Setting: tertiary hospital and community settings, St George's Hospital, London, UK. Inclusion criteria: low risk of complications who booked at the study hospital and were likely to receive all their antenatal care at that hospital. Exclusion criteria: under 5 feet tall, serious medical problems, previous uterine surgery, past obstetric history of > 2 miscarriages/TOP/SB/NND, Rh antibodies. Participants randomised: 503 team‐midwifery, 498 to standard care (shared care). | |
| Interventions | Experimental: team of 4 midwives who provided antenatal, intrapartum and postnatal care in hospital, and postnatal care in the community for women in predefined geographic area. Obstetrician seen at 36 and 41 weeks as appropriate. Control: standard antenatal, intrapartum and postpartum care provided by assortment of midwives and obstetricians. | |
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: 5‐minute Apgar score below or equal to 7 Admission to special care nursery/NICU Amniotomy Antenatal hospitalisation Augmentation/artificial oxytocin during labour Caesarean birth Episiotomy Fetal loss/neonatal death before 24 weeks Fetal loss/neonatal death equal to/after 24 weeks High perceptions of control during labour and childbirth Induction of labour Intact perineum Instrumental vaginal birth (forceps/vacuum) Low birthweight (< 2500 g) No intrapartum analgesia/anaesthesia Opiate analgesia Fetal loss and neonatal death PPH (as defined by trial authors) Regional analgesia (epidural/spinal) Spontaneous vaginal birth (as defined by trial authors) |
|
| Notes | At baseline, more Asian women in control group (18% vs 10%) and more smokers in experimental group (30% vs 22%). Sub‐analysis of case notes found that 98% of experimental group and 20% of standard group had previously met midwife attending labour. Discrepancy in instrumental birth data. Date taken from report and not published paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | '...randomised into two groups by pinning sealed envelopes on their notes containing either the motto KNOW YOUR MIDWIFE or CONTROL GROUP' (Does not state if envelopes were number consecutively.). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up = 15 team care, 19 standard care. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in results. |
| Other bias | Low risk | No other bias identified. |
Harvey 1996.
| Methods |
Study design: RCT. Duration of study: 1992‐1994. |
|
| Participants | Setting: range of city hospitals and community settings in Alberta, Canada. Inclusion criteria: women at low risk of complications who requested and qualified for nurse‐midwife‐led care. Exclusion criteria: past history of caesarean section, primigravidas < 17 or > 37, > 24 weeks' gestation at time of entry to study. Participants randomised: 109 team‐midwife‐led care, 109 to standard care (Physician care). | |
| Interventions | Experimental: team of 7 nurse‐midwives who provided antenatal and intrapartum care in the hospital and postnatal care in the community. Obstetrician seen at booking and at 36 weeks. Control: physician care (family practice or obstetrician) which women chose from a range of city hospitals following usual process. | |
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: 5‐minute Apgar score below or equal to 7 Admission to special care nursery/NICU Amniotomy Antepartum haemorrhage Attendance at birth by known midwife Augmentation/artificial oxytocin during labour Caesarean birth Episiotomy Fetal loss/neonatal death before 24 weeks Induction of labour Instrumental vaginal birth (forceps/vacuum) Intact perineum Opiate analgesia Fetal loss and neonatal death PPH (as defined by trial authors) Regional analgesia (epidural/spinal) Spontaneous vaginal birth (as defined by trial author) |
|
| Notes | At baseline, more women in experimental group had longer period in education (16 years vs 15.23 years). Level of continuity not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...computer‐generated random allocation.' |
| Allocation concealment (selection bias) | Low risk | '...using a series of consecutively numbered, sealed, opaque envelopes...' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up = 4 team care and 12 standard care. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in results. |
| Other bias | Low risk | No other bias identified. |
Hicks 2003.
| Methods |
Study design: RCT. Duration of study: not stated. |
|
| Participants | Setting: tertiary hospital and community, city not stated but UK. Inclusion criteria: women at low risk of complications. Exclusion criteria: not stated. Participants randomised: 100 team‐midwife‐led care, 100 to standard care (shared care). | |
| Interventions | Experimental: team of 8 midwives who provided antenatal, intrapartum and postnatal care 24 hours a day, 7 days a week in both hospital and community. The team was attached to a GP practice. Referral to obstetrician as necessary. Control: shared care between community and hospital midwives and GPs and obstetricians when necessary. Women delivered by hospital midwife or community midwife if under domino scheme (1 midwife provides care for a woman throughout pregnancy, accompanies her into hospital for birth and returns home with her and baby a few hours after the birth, and care in postnatal period). | |
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: Induction of labour Instrumental vaginal birth (forceps/vacuum) Intact perineum Opiate analgesia Fetal loss and neonatal death PPH (as defined by trial authors) Regional analgesia (epidural/spinal) Spontaneous vaginal birth (as defined by trial authors) |
|
| Notes | 71% of experimental group and 14% of standard group had previously met midwife attending labour. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes '...had been shuffled previously by an individual not involved in the recruitment process, and then numbered consecutively'. |
| Allocation concealment (selection bias) | Low risk | 'Allocation was undertaken by giving each woman a sealed envelope containing one of the care options.' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up = 19 team care and 8 standard. Due to non‐response to questionnaires. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in results. |
| Other bias | Low risk | No other bias identified. |
Homer 2001.
| Methods |
Study design: RCT, Zelen method. Duration of study: 1997‐1998. |
|
| Participants | Setting: public tertiary hospital and community, Sydney, Australia. Inclusion criteria: women at low and high risk of complications. Exclusion criteria: women more than 24 weeks' gestation at their first visit to the hospital, women with an obstetric history of 2 previous caesareans or a previous classical caesarean and medical history of significant maternal disease. Participants randomised: 640 team‐midwife‐led care, 643 to standard care (shared care). | |
| Interventions | Experimental: 2 teams of 6 midwives sharing a caseload of 300 women a year/team. Antenatal care in outreach community‐based clinics. Intrapartum and postpartum hospital and community care. Obstetrician or obstetric registrar did not see women routinely, but acted as a consultant and reviewed women only as necessary. Women who developed complications during their pregnancy continued to receive care from the same group of carers. Control: standard care provided by hospital midwives and doctors in hospital‐based antenatal clinic, delivery suite and postnatal ward. Woman at high risk of complications were seen by obstetrician or registrar. Low‐risk women were seen by midwives and shared care with GPs in a shared model of care. | |
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: 5‐minute Apgar score below or equal to 7 Admission to special care nursery/NICU Antenatal hospitalisation Antepartum haemorrhage Attendance at birth by known midwife Augmentation/artificial oxytocin during labour Caesarean birth Episiotomy Fetal loss/neonatal death before 24 weeks Fetal loss/neonatal death equal to/after 24 weeks Induction of labour Instrumental vaginal birth (forceps/vacuum) Opiate analgesia Fetal loss and neonatal death PPH (as defined by trial authors) Regional analgesia (epidural/spinal) Spontaneous vaginal birth (as defined by trial authors) |
|
| Notes | 63% of experimental group and 21% of standard group had previously met midwife attending labour. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...computer‐generated random numbers...' |
| Allocation concealment (selection bias) | Low risk | '...group allocation was not revealed until the woman’s details were recorded by the administrative assistant.' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No (states 'unblinded'). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No (states 'unblinded'). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: team care 46, standard care 42. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in results. |
| Other bias | Low risk | No other bias identified. |
Kenny 1994.
| Methods |
Study design: RCT. Duration of study: 1992‐199. |
|
| Participants | Setting: Westmead public hospital, NSW, Australia. Inclusion criteria: women at low and high risk of complications. Exclusion criteria: women requiring use of the 'Drug use in pregnancy service' or booked after 16' weeks' gestation. Participants randomised: 213 team‐midwife‐led care, 233 to standard care (shared care). | |
| Interventions |
Experimental: team of 6.8 WTE midwives sharing a caseload. Provided antenatal and intrapartum care in hospital and postnatal care in hospital and community. Obstetrician saw all women at first visit and 32 weeks, and after 40 weeks, and as appropriate. Team midwife was on call for out‐of‐hours care. Control: low‐risk women seen in midwives' hospital antenatal clinics, and all other women seen by medical staff. Women received intrapartum care from delivery suite midwives, and postnatal care from midwives on postnatal ward and community postnatal care. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: 5‐minute Apgar score below or equal to 7 Admission to special care nursery/NICU Amniotomy Antenatal hospitalisation Attendance at birth by known midwife Augmentation/artificial oxytocin during labour Breastfeeding initiation Caesarean birth Episiotomy Fetal loss/neonatal death equal to/after 24 weeks Induction of labour Instrumental vaginal birth (forceps/vacuum) Intact perineum Mean labour length Mean number of antenatal visits No intrapartum analgesia/anaesthesia Opiate analgesia Fetal loss and neonatal death Perineal laceration requiring suturing PPH (as defined by trial authors) Regional analgesia (epidural/spinal) Spontaneous vaginal birth (as defined by trial authors) |
|
| Notes | 96% of experimental group and 13% of standard group had previously met midwife attending labour. Randomisation before consent to participate. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | '...allocated a numbered randomisation envelope (the number was recorded by the booking‐in midwife on a list of women booked in the session).' |
| Allocation concealment (selection bias) | Low risk | 'Allocated a numbered randomisation envelope (the number was recorded by the booking‐in midwife on a list of women booked in the session). When each woman returned for her first visit to the doctor at the antenatal clinic she was approached in the waiting room by a program midwife, reminded about the research and asked to sign a consent form. If the woman agreed to join the study, the randomisation envelope was opened and the woman informed of the type of care she was to receive and the appropriate future appointments made.' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up = 19 team care and 22 standard who either moved or had a miscarriage. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in results. |
| Other bias | Low risk | No other bias identified. |
MacVicar 1993.
| Methods |
Study design: RCT, Zelen method. Duration of study: 1989‐1991. |
|
| Participants | Setting: tertiary hospital and community in Leicester, UK. Inclusion criteria: women at low risk of complications. Exclusion criteria: women who had a previous caesarean section or difficult vaginal delivery, a complicating general medical condition, a previous stillbirth or neonatal death, or a previous small‐for‐gestational‐age baby, multiple pregnancy, Rhesus antibodies, and a raised level of serum alpha‐feto protein. Participants randomised: 2304 team midwifery, 1206 to standard care (shared care). | |
| Interventions | Experimental: team of 2 midwifery sisters assisted by 8 staff midwives provided hospital‐based antenatal, intrapartum (in hospital‐based 3 room home‐from‐home unit (no EFM or epidural) and hospital postnatal care only. All the staff were volunteers. Antenatal midwife‐led hospital clinic with scheduled visits at 26, 36 and 41 weeks' gestation. Intervening care shared with GPs and community midwives. Referral to obstetrician as appropriate. At 41 weeks mandatory referral to consultant. Postnatal care in community provided by community midwife and GP. Control group: shared antenatal care with GP and midwife. Intrapartum care provided by hospital staff. | |
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: Admission to special care nursery/NICU Augmentation/artificial oxytocin during labour Caesarean birth Episiotomy Fetal loss/neonatal death before 24 weeks Fetal loss/neonatal death equal to/after 24 weeks Induction of labour Intact perineum Instrumental vaginal birth (forceps/vacuum) Low birthweight (< 2500 g) No intrapartum analgesia/anaesthesia Opiate analgesia Fetal loss and neonatal death Perineal laceration requiring suturing PPH (as defined by trial authors) Preterm birth (< 37 weeks) Regional analgesia (epidural/spinal) Spontaneous vaginal birth (as defined by trial authors) |
|
| Notes | 2:1 randomisation ratio in favour of midwife‐led care. 189/2304 (8%) women opted out of team‐midwife care post‐randomisation. Analysis by intention‐to‐treat analysis. Level of continuity not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...by a random sequence...' |
| Allocation concealment (selection bias) | Low risk | ‘...sealed envelope...cards could not be read through the envelopes. Each envelope was numbered, and unused envelopes were not reallocated...’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated re participants but not possible to have achieved. Clinical staff were unaware whether a particular woman was in the control group or was not in the study. No information given re blinding of women in intervention arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information given on losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in results. |
| Other bias | Low risk | No other bias identified. |
McLachlan 2012.
| Methods |
Study design: RCT. Duration of study: 2007‐2010. |
|
| Participants | Setting: Royal Women’s Hospital (RWH), Melbourne, Australia. Inclusion criteria: low‐risk pregnant women; fewer than 24 completed weeks' gestation; a singleton pregnancy; and considered low obstetric risk at recruitment including an uncomplicated obstetric history. Exclusion criteria: previous caesarean section, history of stillbirth or neonatal death, 3 or more consecutive miscarriages, previous fetal death in utero, previous preterm birth (< 32 weeks), previous midtrimester loss/cervical incompetence/cone biopsy/known uterine anomaly, previous early onset of pre‐eclampsia (< 32 weeks' gestation), or rhesus iso‐immunisation; complications during the current pregnancy (such as multiple pregnancy or fetal abnormality); medical conditions (such as cardiac disease, essential hypertension, renal disease, pre‐existing diabetes, previous gestational diabetes, epilepsy, severe asthma, substance use, significant psychiatric disorders and obesity [BMI > 35] or significantly underweight [BMI < 17]). Participants randomised: 1156 caseload, 1158 standard care. | |
| Interventions |
Experimental: majority of care from a ‘primary’ caseload midwife at the hospital. The primary midwife collaborated with obstetricians and other health professionals and continued to provide caseload care if complications arose. Women saw an obstetrician at booking, at 36 weeks' gestation and postdates if required, and usually had 1 or 2 visits with a ‘back‐up’ midwife. Intrapartum care was provided in the hospital birthing suite. Where possible, primary midwife was on call for the woman’s labour and birth. The primary midwife (or a back‐up) attended the hospital on most days to provide some postnatal care and provided domiciliary care following discharge from hospital. Fulltime midwives had a caseload of 45 women per annum. During the trial there were 7.5 (at commencement) to 12 full‐time equivalent midwives employed in caseload care, equating to 10–14 midwives. Control: options included midwifery‐led care with varying levels of continuity, obstetric trainee care and community‐based care ‘shared’ between a general medical practitioner (GP) and the hospital, where the GP provided the majority of antenatal care. In the midwife and GP‐led models women saw an obstetrician at booking, 36 weeks' gestation and postdates if required, with other referral or consultation as necessary. In all standard‐care options, women were cared for by whichever midwives and doctors were rostered for duty when they came into the hospital for labour, birth and postnatal care. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: 5‐minute Apgar score below or equal to 7 Admission to special care nursery/NICU Caesarean birth Duration of postnatal hospital stay (days) Episiotomy Fetal loss/neonatal death before 24 weeks Fetal loss/neonatal death equal to/after 24 weeks Induction of labour Instrumental vaginal birth (forceps/vacuum) Low birthweight (< 2500 g) Fetal loss and neonatal death Preterm birth (< 37 weeks) PPH (as defined by trial authors) Regional analgesia (epidural/spinal) Spontaneous vaginal birth (as defined by trial authors) Maternal satisfaction |
|
| Notes | 'Around 90% of the women had a known carer in labour'. McLachlan 2015 reports the results of a postal survey of women's experiences of childbirth. Data for several relevant outcome domains are displayed in our additional Table 3 ‐ data added in 2016 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...using stratified permuted blocks of varying size.' |
| Allocation concealment (selection bias) | Low risk | 'Randomisation was undertaken using an interactive voice response system activated by telephone...' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Obstetric and medical outcome data (including type of birth) were obtained directly from the electronic obstetric database, blinded to treatment allocation. Data not available this way (e.g. continuity of carer) were manually abstracted (unblinded) from the medical record.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up = 6 caseload and 1 standard care. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported in results. |
| Other bias | Low risk | No other bias identified. |
North Stafford 2000.
| Methods |
Study design: RCT, cluster randomisation. Duration of study: not stated. |
|
| Participants | Setting: tertiary hospital and community, UK. Inclusion criteria: 'all‐risks’. Exclusion criteria: not stated. Participants randomised: 770 midwife‐led caseload care, 735 standard care (shared care). | |
| Interventions |
Experimental: caseload midwife‐led care. 3 geographic areas with 21 WTE midwives working in 3 practices offering a caseload model of care. Each midwife was attached to 2‐3 GP practices and cared for 35‐40 women. Midwives worked in pairs/threesomes. Caseload midwives were existing community midwives, plus new midwives recruited from community and hospital resulting in a mix of senior and junior staff. Monthly antenatal care in the community, intrapartum and postnatal care in hospital and postnatal care in the community provided. Control: shared care in the community between GPs, community midwives and obstetricians. Each community midwife cared for 100/150 women each. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: 5‐minute Apgar score below or equal to 7 Admission to special care nursery/NICU Attendance at birth by known midwife Augmentation/artificial oxytocin during labour Caesarean birth Episiotomy Fetal loss/neonatal death equal to/after 24 weeks Induction of labour Instrumental vaginal birth (forceps/vacuum) Intact perineum Low birthweight (< 2500 g) Fetal loss and neonatal death Perineal laceration requiring suturing Preterm birth (< 37 weeks) Regional analgesia (epidural/spinal) |
|
| Notes | 95% of experimental group and 7% of standard group had previously met midwife attending labour. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomisation was undertaken by one of the principal investigators...who had no prior knowledge of the area or medical and midwifery staff involved.... three pairs, one of each...randomised to receive caseload care and the other to traditional care.' |
| Allocation concealment (selection bias) | High risk | No information given about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 'It was not possible to mask allocation and both women and professionals were aware of the allocated type of midwifery care.' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: not reported but appears complete. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Low risk | No other bias identified. |
Rowley 1995.
| Methods |
Study design: RCT. Duration of study: 1991‐1992. |
|
| Participants | Setting: John Hunter hospital, Newcastle, NSW, Australia. Inclusion criteria: women booked for delivery at hospital of low and high risk. Exclusion criteria: women who had chosen shared antenatal care with their GP or had a substance abuse problem. Participants randomised: 405 team care, 409 standard care (shared care). | |
| Interventions | Experimental: team of 6 experienced and newly graduated midwives provided antenatal care, intrapartum care, and postnatal care in hospital. Women at low risk had scheduled consultations with an obstetrician at 12‐16, 36, 41 weeks and additional consultations as needed. Women at high risk had consultations with an obstetrician at a frequency determined according to their needs. Control: antenatal care from hospital physicians and intrapartum and postnatal care from midwives and doctors working in the delivery suite, and the postnatal ward. Women were usually seen by a doctor at each visit. Control‐group midwives were also a mix of experienced and newly qualified midwives. | |
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: 5‐minute Apgar score below or equal to 7 Admission to special care nursery/NICU Antenatal hospitalisation Augmentation/artificial oxytocin during labour Caesarean birth Episiotomy Fetal loss/neonatal death before 24 weeks Fetal loss/neonatal death equal to/after 24 weeks Induction of labour Instrumental vaginal birth (forceps/vacuum) Low birthweight (< 2500 g) Opiate analgesia Fetal loss and neonatal death Perineal laceration requiring suturing Preterm birth (< 37 weeks) Regional analgesia(epidural/spinal) Spontaneous vaginal birth (as defined by trial authors) |
|
| Notes | Degree of continuity not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Allocation to either team care or routine care was done by computer‐generated random assignments.' |
| Allocation concealment (selection bias) | Unclear risk | 'The women were allocated at random to team care or routine care....' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | '...the unblinded nature of the study could have led to differences in practice and measurement of outcomes...' |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | '...the unblinded nature of the study could have led to differences in practice and measurement of outcomes...' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not reported (appears minimal). |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported or explained in result. |
| Other bias | Low risk | No other bias identified. |
Tracy 2013.
| Methods | Study took place in 2 Australian centres (site 1: Royal Hospital for Women, Randwick; and site 2: Mater Mother’s Hospital, Brisbane). The randomised trial compared caseload midwifery with standard care. Women were recruited to the study from site 1 between December 2008 and May 2011, and from site 2 between June 2010 and May 2011. | |
| Participants | Women were included if they were less than 24 weeks pregnant at the booking visit, and aged 18 years and older. Women were excluded if they had planned to have an elective caesarean section, had a multiple pregnancy, or were planning to book with another care provider (e.g. a GP, caseload midwife, or private obstetrician). | |
| Interventions | Intervention: caseload midwifery care (receiving care through antenatal, intrapartum and postpartum, in hospital and in the community) from a named caseload midwife working in a small group of midwives known as a midwifery group practice (4 full‐time MWs). Each midwife provides care to 40 women a year as named midwife. The named midwife was on call for labour and birth. The caseload midwives were backed up when necessary by other caseload colleagues and by hospital staff during women’s stay in the postnatal ward. Community postnatal care was provided for up to 6 weeks. An obstetrician was allocated to each midwifery practice for consultation and referral using national guidelines. Total number randomised to intervention: 871. Comparison: standard care, which involved shared antenatal care from a GP and hospital midwives, labour and birth and postnatal hospital care from hospital midwives. It was unclear whether community postnatal care was provided in standard care. Total number randomised to standard care: 877. Data were collected at recruitment, at 36 weeks' gestation and at 6 weeks and 6 months postpartum. |
|
| Outcomes | Primary outcomes: Caesarean section (main PO), instrumental vaginal birth, unassisted vaginal birth, epidural analgesia, Apgar scores ≤ 7 at 5 minutes, admission to SCBU, preterm birth (GA < 37 weeks) Secondary outcomes: Antenatal admission to hospital; induction or augmentation of labour; perineal status after birth; blood loss after birth; GAs and birthweights of the infants; breastfeeding at hospital discharge, 6 weeks and 6 months postnatally; and perinatal and maternal mortality, hospital cost by mode of birth (cost of birth per woman) |
|
| Notes | Forti 2015, additional report of Tracy 2013 identified from 2016 update. This reports on a subset of publicly funded women randomised in the M@ngo trial (n = 420); women receiving caseload midwifery care saw fewer midwives and health professionals during their intrapartum care than did women in standard care. No additional data provided.
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomly assigned by a telephone‐based computer randomisation service provided by ANHMRC clinical trials randomisation centre to each group. |
| Allocation concealment (selection bias) | Low risk | As above, centralised allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the study it is not possible to blind participants or clinicians. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Due to the nature of the study it is not possible to blind participants or clinicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawls and losses outlined in a trial profile in Tracy 2013. 20/871 lost or withdrew from caseload care; 36 lost or withdrew from standard care. Pregnancies lost before 20 weeks and terminations of pregnancy have been added back in (see Notes above). |
| Selective reporting (reporting bias) | Unclear risk | Authors were emailed for length of neonatal stay and antepartum haemorrhage; these were mentioned in the protocol and were not included in publications. Answer expected 9.3.15. Authors emailed for GA of the 2 terminations of pregnancy for lethal abnormalities. Authors asked to clarify if length of stay outcome is for infants or women. |
| Other bias | Unclear risk | 19 (2%) women crossed over from caseload to standard care and 65 (7%) crossed over from standard to caseload care. |
Turnbull 1996.
| Methods |
Study design: RCT. Duration of study: 1993‐1994. |
|
| Participants | Setting: Glasgow Royal Maternity Hospital, Scotland, United Kingdom. Inclusion criteria: women at low risk of complications. Exclusion criteria: women booking after 16 weeks of pregnancy, not living in catchment area or with medical/obstetric complications. Participants randomised: 648 caseload, 651 standard care (shared care). | |
| Interventions | Experimental: caseload midwifery provided by 20 midwives who volunteered to join the MDU. Each pregnant woman had a named midwife whom she met at her first booking visit who aimed to provide the majority of care. When the named midwife was not available, care was provided by up to 3 associate midwives. Women were not seen by medical staff at booking. Antenatal care was provided at home, community‐based clinics or hospital clinics. Intrapartum care was in hospital (MDU ‐ 3 rooms with fewer monitors and homely surroundings) or main labour suite. Postnatal care was provided in designated 8‐bed MDU ward and community. A medical visit was scheduled where there was a deviation from normal. Control: all women seen by medical staff at booking. Shared antenatal care with from midwives, hospital doctors and GPs/family doctors. Intrapartum care from labour ward midwife on labour suite. Postnatal care on postnatal ward and community by community midwife. | |
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: 5‐minute Apgar score below or equal to 7 Admission to special care nursery/NICU Antepartum haemorrhage Augmentation/artificial oxytocin during labour Caesarean birth Episiotomy Fetal loss/neonatal death before 24 weeks Fetal loss/neonatal death equal to/after 24 weeks Induction of labour Instrumental vaginal birth (forceps/vacuum) Intact perineum Low birthweight (< 2500 g) Mean labour length Neonatal convulsions (as defined by trial authors) No intrapartum analgesia/anaesthesia Opiate analgesia Overall fetal loss and neonatal death Perineal laceration requiring suturing Postpartum depression PPH (as defined by trial authors) Preterm birth (< 37 weeks) Regional analgesia (epidural/spinal) Spontaneous vaginal birth (as defined by trial authors) |
|
| Notes | Women in the intervention group saw 7 fewer care providers across antenatal, labour and postnatal periods and 2 fewer providers during labour. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | '...random number tables...' |
| Allocation concealment (selection bias) | Low risk | 'The research team telephoned a clerical officer in a separate office for care allocation for each woman.' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Participants: not stated. Personnel: clinical staff were unaware whether a particular woman was in the control group or was not in the study. No information given for women in intervention arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 'Clinical data were gathered through a retrospective review of records by the research team who were not involved in providing care.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 5 team care and 16 shared care. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported or explained in result. |
| Other bias | Low risk | No other bias identified. |
Waldenstrom 2001.
| Methods |
Study design: RCT. Duration of study: 1996‐1997. |
|
| Participants | Setting: Royal Women's Hospital, Melbourne, Australia. Inclusion criteria: women at low risk of complications. Exclusion criteria: non‐English speaking women, women > 25 weeks' gestation at booking, women with high‐risk criteria including previous obstetric complications, preterm delivery, IUGR, PET, previous fetal loss, significant medical disease, > 3 abortions, substance addiction, infertility > 5 years. Participants randomised: 495 team‐midwife care, 505 standard care (combination of different models of care). | |
| Interventions |
Experimental: team‐midwife care provided by team of 8 midwives who provided hospital‐based antenatal, intrapartum (delivery suite or family birth centre) and some postnatal care in collaboration with medical staff. Control: standard care included different options of care being provided mostly by doctors, care mainly by midwives in collaboration with doctors (midwives clinics), birth centres and shared care between GPs and hospital doctors. |
|
| Outcomes | Outcomes considered in the review and reported in or extracted from the study: 5‐minute Apgar score below or equal to 7 Admission to special care nursery/NICU Antenatal hospitalisation Antepartum haemorrhage Attendance at birth by known midwife Augmentation/artificial oxytocin during labour Caesarean birth Duration of postnatal hospital stay(days) Episiotomy Fetal loss/neonatal death before 24 weeks Fetal loss/neonatal death equal to/after 24 weeks Induction of labour Instrumental vaginal birth (forceps/vacuum) Intact perineum Mean length of neonatal hospital stay (days) Opiate analgesia Overall fetal loss and neonatal death Perineal laceration requiring suturing PPH (as defined by trial authors) Preterm birth (< 37 weeks) Regional analgesia (epidural/spinal) Spontaneous vaginal birth (as defined by trial authors) |
|
| Notes | 65% and 9% of experimental (team) and control (standard) group participants had previously met midwife attending labour. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given. |
| Allocation concealment (selection bias) | Low risk | 'The research midwife rang a clerk at the hospital's information desk who opened an opaque, numbered envelope that contained information about the allocated group.' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated but unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: 11 team care and 9 standard‐care group. |
| Selective reporting (reporting bias) | Low risk | Outcome reporting: all outcomes stated in the methods section were adequately reported or explained in result. |
| Other bias | Low risk | No other bias identified. |
BMI: body mass index CLC: consultant‐led care CLU: consultant‐led unit EFM: electronic fetal monitoring GA: gestational age GP: general practitioner IUGR: intrauterine growth restriction MDU: midwifery development unit MLU: midwife‐led unit NICU: neonatal intensive care unit PET: positron emissions tomography PPH: postpartum haemorrhage RCT: randomised controlled trial SCBU: special care baby unit vs: versus WTE: whole time equivalent
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berglund 1998 | This study was a retrospective study comparing outcomes for 2 groups of women who gave birth in 1990 and 1992. |
| Berglund 2007 | This study compared risk assessment by physicians with midwives reporting new mothers to the doctor. It does not compare midwife‐led with other models of care. |
| Bernitz 2011 | This study compared women giving birth in 3 different birth units: the special unit for high‐risk women; the normal unit; and the midwife‐led unit. It does not compare midwife‐led with other models of care throughout pregnancy and birth. |
| Chambliss 1991 | Women admitted in labour were assigned to either midwife‐led or a resident physician and antenatal care was not part of the intervention. |
| Chapman 1986 | This study compares similar models of care occurring in 2 different birth environments rather than comparing 2 different models of care. The same group of community midwives cared for the women in both groups. Method of randomisation is not stated. |
| Famuyide 2014 | This study did not provide continuity of care from antenatal through to intrapartum period. |
| Giles 1992 | The study compares 2 models of antenatal care, i.e. antenatal care by midwives and obstetricians or antenatal care by midwives only. Intrapartum and postpartum care are not part of the intervention. |
| Gu 2013 | This study did not provide continuity of care from antenatal through to intrapartum period. |
| Heins 1990 | The study presents a randomised trial of nurse‐midwifery prenatal care to reduce low birthweight: intrapartum and postpartum care are not part of the intervention. |
| Hildingsson 2003 | The aim of the study was to determine women's interest in home birth and in‐hospital birth centre care in Sweden and to describe the characteristics of these women. It did not compare the models of care in these 2 settings. |
| Hundley 1994 | The main objective was to compare care and delivery of low‐risk women in a midwife‐managed delivery unit with care and delivery in the consultant‐led labour ward. It is not indicated if women in the birth centre group had antenatal midwifery‐led care. |
| James 1988 | This study compared a schematic approach to antenatal care only and conventional shared care. There are no data available. |
| Kelly 1986 | Study protocol only, search strategy did not reveal any evidence that the trial was conducted and completed. |
| Klein 1984 | The intervention involved the comparison of 2 birthing environments. |
| Law 1999 | In this study, the randomisation took place on the admission to labour ward, thus the study compared intrapartum care only. |
| Marks 2003 | This study aimed to compare continuity of midwifery care with standard midwifery care in reducing postnatal depression in women with a past history of depression. Thus midwife‐led care is not being compared to another model of care. |
| Runnerstrom 1969 | The primary reason for exclusion is the fact that the study did not compare a midwifery model of care to another model. The purpose of the investigation was to study the effectiveness or non‐effectiveness of nurse‐midwives in a supervised hospital environment. The population of the study comprised student nurse‐midwives and compared their services to those of MD residents in the same unit. Moreover, there are not enough comparable data. |
| Slome 1976 | Large loss to follow‐up after randomisation. A total of 66.5% in the treatment group and 63.5% in the control group were excluded or lost to the study. |
| Stevens 1988 | The care was not midwifery‐led. Both groups received shared care. 1 group received most of their care at a satellite clinic in their neighbourhood, which was an inner‐city, socio‐economically deprived area. The other group received care at the hospital clinic. Women receiving satellite clinic care also had additional social support from link workers during pregnancy. It was a comparison of the same model of care at different settings. |
| Tucker 1996 | The study compares a shared care model vs a medical‐led model. The primary analyses are not included. |
| Waldenstrom 1997 | This study compared birth centre care ‐ characterised by comprehensive antenatal, intrapartum and postpartum care, on the same premises with a home‐like environment and the same team of midwives ‐ to the standard obstetric care divided into antenatal care at neighbourhood antenatal clinics, intrapartum care in hospital delivery wards, and postpartum care in hospital postpartum wards. In the standard obstetric care, a woman usually meets with the same midwife, at the antenatal clinic, throughout pregnancy. In the delivery ward she meets a new staff team, and in the hospital postpartum ward, yet another staff team. Thus, the study compares continuous midwifery‐led caseload model of care to team midwifery‐led care. |
| Walker 2012 | This study compared care provided by general physicians, obstetric nurses and professional midwives in a cluster‐RCT in Mexico. It does not compare midwife‐led with other models of care throughout pregnancy and birth. Abstract only available. |
RCT: randomised controlled trial vs: versus
Characteristics of ongoing studies [ordered by study ID]
Nagle 2011.
| Trial name or title | Continuity of midwifery care and gestational weight gain in obese women: a randomised controlled trial. |
| Methods | A 2‐arm unblinded randomised controlled trial. |
| Participants | Primigravid women with a BMI ≥ 30 who are less than 17 weeks' gestation, recruited from maternity services in Victoria, Australia. |
| Interventions | Women allocated to the intervention arm will be cared for in a midwifery continuity of care model and receive an informational leaflet on managing weight gain in pregnancy. Women allocated to the control group will receive routine care in addition to the same informational leaflet. |
| Outcomes | The primary outcome is the proportion of women with a gestational weight gain within IOM guidelines. Secondary outcomes: provision of care in line with the standards within the UK guidelines, women's satisfaction with care. |
| Starting date | Unclear. |
| Contact information | cate.nagle@deakin.edu.au, School of Nursing and Midwifery, Deakin University, Geelong Waterfront campus, 1 Gheringhap St, Geelong Victoria, 3217, Australia. |
| Notes | Trial Registration: Australian New Zealand Clinical Trials Registry ACTRN12610001078044. |
BMI: body mass index IOM: Institute of Medicine
Differences between protocol and review
Breastfeeding on hospital discharge, maternal satisfaction were added as outcomes for the 2015 update.
In the 2016 update, some of the primary and secondary outcomes were clarified:
The primary neonatal outcome "Overall fetal loss and neonatal death (fetal loss was assessed by gestation using 24 weeks as the cut‐off for viability in many countries)" was changed to "All fetal loss before and after 24 weeks plus neonatal death."
The secondary neonatal outcomes, "Fetal loss and neonatal death less than 24 weeks" and "Fetal loss and neonatal death equal to/after 24 weeks" were changed to "Fetal loss less than 24 weeks and neonatal death" and "Fetal loss equal to/after 24 weeks and neonatal death".
Contributions of authors
Declan Devane (DD)
DD contributed to the protocol by contributing to the design and writing.
DD contributed to the review by contributing to the design of the review, appraising the quality of and extracting data from selected papers, contributing to the interpretation of data, writing the review and providing a methodological and clinical perspective.
Simon Gates (SG) SG provided methodological and statistical expertise in the development of the review, and assisted with analysis of data and interpretation of results.
Jane Sandall (JS) JS contributed to the protocol by contributing to the design and writing. JS contributed to the design, screened retrieved papers against inclusion criteria and appraised quality of papers.
JS has been the contact author for the review since July 2006 and is first author of the review. Since 2006, she has co‐ordinated the review process, written to authors for additional information, managed data for the review, re‐extracted data from papers, re‐entered data into Review Manager, re‐entered data for the included studies section, analysed and interpreted data, and provided a clinical and policy perspective. She has rewritten the Plain Language Summary, Abstract, Background, Methods, Description of studies, Methodological quality, Results, Analysis, Discussion and wrote the final draft of the review.
JS revised the review in response to feedback from referees and the editor. When making the revisions, JS updated the search and identified four new reports, and contacted authors for additional data, which were assessed by JS and DD, and which she included in the revised version.
JS in the guarantor for the review.
Andrew Shennan (AS)
AS provided specialist obstetric expertise, and assisted with interpretation of results.
Hora Soltani (HS) HS contributed to the design and commented on the first draft of the protocol.
HS contributed to the development of the protocol and review by contributing to the design, evaluation of the quality of the articles against the inclusion/exclusion criteria, data extraction, writing to authors for clarification of original article information, data interpretation, commenting on as well as writing the review.
Sources of support
Internal sources
Women's Health Academic Centre, King's Health Partners, King's College, London, UK.
Sheffield Hallam University, Sheffield, UK.
Health Services Executive, Dublin North East, Ireland.
Trinity College, Dublin, Ireland.
External sources
-
National Institute for Health Research, UK.
2013 update. NIHR Programme of centrally‐managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS: 10/4001/02
2015 update. UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
The research was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) South London at King’s College Hospital NHS Foundation Trust and CLAHRC Yorkshire and Humber at Sheffield Hallam University. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health, UK.
Declarations of interest
Declan Devane is a co‐author in one of the included trials in this review (Begley 2011). Jane Sandall was and is principal investigator for two studies evaluating models of midwife‐led continuity of care (Sandall 2001), and co‐investigator on the 'Birthplace in England Research Programme', an integrated programme of research designed to compare outcomes of births for women planned at home, in different types of midwifery units, and in hospital units with obstetric services. Declan and Jane were not involved in assessing or data extraction for these studies.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Allen 2013 {published data only}
- Allen J, Stapleton H, Tracy S, Kildea S. Is a randomised controlled trial of a maternity care intervention for pregnant adolescents possible? An Australian feasibility study. BMC Medical Research Methodology 2013;13:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begley 2011 {published and unpublished data}
- Begley C, Devane D, Clarke M, McCann C, Hughes P, Reilly M, et al. Comparison of Midwife‐led and Consultant‐led Care of Healthy Women at Low Risk of Childbirth Complications in the Republic of Ireland: a Randomised Trial [thesis]. Trinity College, University of Dublin, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley C, Devane D, Clarke M, McCann C, Hughes P, Reilly M, et al. Comparison of midwife‐led and consultant‐led care of healthy women at low risk of childbirth complications in the Republic of Ireland: a randomised trial. BMC Pregnancy and Childbirth 2011;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny C, Devane D, Normand C, Clarke M, Howard A, Begley C. A cost‐comparison of midwife‐led compared with consultant‐led maternity care in Ireland (the MidU study). Midwifery 2015;31(11):1032‐8. [DOI] [PubMed] [Google Scholar]
Biro 2000 {published data only}
- Biro MA, Waldenstrom U, Brown S, Pannifex JH. Satisfaction with team midwifery care for low‐ and high‐risk women: a randomized controlled trial. Birth 2003;30(1):1‐10. [DOI] [PubMed] [Google Scholar]
- Biro MA, Waldenstrom U, Pannifex JH. Team midwifery care in a tertiary level obstetric service: a randomized controlled trial. Birth 2000;27(3):168‐73. [DOI] [PubMed] [Google Scholar]
Flint 1989 {published data only}
- Flint C. Know your midwife. Nursing Times 1988;84:28‐32. [PubMed] [Google Scholar]
- Flint C, Poulengeris P, Grant AM. The 'Know your midwife' scheme ‐ a randomised trial of continuity of care by a team of midwives. Midwifery 1989;5:11‐6. [DOI] [PubMed] [Google Scholar]
- Flint C, Poulengeris, P. The 'Know your Midwife' Report. London: Heinemann, 1987. [Google Scholar]
Harvey 1996 {published data only}
- Harvey S, Jarrell J, Brant R, Stainton C, Rach D. A randomized, controlled trial of nurse‐midwifery care. Birth 1996;23:128‐35. [DOI] [PubMed] [Google Scholar]
- Harvey S, Rach D, Stainton MC, Jarrell J, Brant R. Evaluation of satisfaction with midwifery care. Midwifery 2002;18(4):260‐7. [DOI] [PubMed] [Google Scholar]
Hicks 2003 {published data only}
- Hicks C, Spurgeon P, Barwell F. Changing childbirth: a pilot project. Journal of Advanced Nursing 2003;42(6):617‐28. [DOI] [PubMed] [Google Scholar]
Homer 2001 {published data only}
- Homer C. Incorporating cultural diversity in randomised controlled trials in midwifery. Midwifery 2000;16:252‐9. [DOI] [PubMed] [Google Scholar]
- Homer C, Davis G, Brodie P, Sheehan A, Barclay L, Wills J, et al. Collaboration in maternity care: a randomised controlled trial comparing community‐based continuity of care with standard hospital care. BJOG: an international journal of obstetrics and gynaecology 2001;108:16‐22. [DOI] [PubMed] [Google Scholar]
- Homer CS, Davis GK, Brodie PM. What do women feel about community‐based antenatal care?. Australian & New Zealand Journal of Public Health 2000;24:590‐5. [DOI] [PubMed] [Google Scholar]
- Homer CS, Davis GK, Cooke M, Barclay LM. Women's experiences of continuity of midwifery care in a randomised controlled trial in Australia. Midwifery 2002;18(2):102‐12. [DOI] [PubMed] [Google Scholar]
- Homer CS, Matha DV, Jordan LG, Wills J, Davis GK. Community‐based continuity of midwifery care versus standard hospital care: a cost analysis. Australian Health Review 2001;24(1):85‐93. [DOI] [PubMed] [Google Scholar]
Kenny 1994 {published data only}
- Kenny P, Brodie P, Eckerman S, Hall J. Final Report. Westmead Hospital Team Midwifery Project Evaluation. Sydney: University of Sydney, 1994. [Google Scholar]
MacVicar 1993 {published data only}
- MacVicar J, Dobbie G, Owen‐Johnstone L, Jagger C, Hopkins M, Kennedy J. Simulated home delivery in hospital: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 1993;100:316‐23. [DOI] [PubMed] [Google Scholar]
McLachlan 2012 {published data only}
- Davey M, McLachlan H, Forster D. Timing of admission and selected aspects of intrapartum care: Relationship with caesarean section in the COSMOS (Caseload Midwifery) trial. Women & Birth 2013;26(Suppl 1):S3. [Google Scholar]
- Davey MA, McLachlan L, Forster D, Flood M. Influence of timing of admission in labour and management of labour on method of birth: results from a randomised controlled trial of caseload midwifery (COSMOS trial). Midwifery 2013;29(12):1297‐302. [DOI] [PubMed] [Google Scholar]
- Flood M, Forster DA, Davey MA, McLachlan HL. Serious adverse event monitoring in a RCT of caseload midwifery (COSMOS). Journal of Paediatrics and Child Health 2012;48(Suppl 1):113. [Google Scholar]
- McLachan H. A randomised trial comparing one‐to‐one midwifery care with standard hospital maternity care for women at low risk, in order to decrease operative birth and other interventions and increase the duration of breastfeeding and women's satisfaction with care, with no increase in costs of care. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 19 February 2008).
- McLachlan H, Forster D, Davey M‐A, Gold L, Biro M‐A, Flood M, et al. The effect of caseload midwifery on women's experience of labour and birth: Results from the COSMOS randomised controlled trial. Women & Birth 2013;26(Suppl 1):S13. [Google Scholar]
- McLachlan H, Forster D, Davey MA. The effect of caseload midwifery on women's experience of labour and birth: results from the COSMOS randomised controlled trial. International Confederation of Midwives 30th Triennial Congress. Midwives: Improving Women’s Health; 2014 June 1‐4; Prague, Czech Republic. 2014:C085.
- McLachlan H, Forster D, Davey MA, Farrell T, Gold L, Oats J, et al. A randomised controlled trial of caseload midwifery for women at low risk of medical complications (COSMOS) ‐ primary and secondary outcomes. Women and Birth 2011;24 Suppl 1:S13. [Google Scholar]
- McLachlan HL, Forster DA, Davey MA, Farrell T, Flood M, Shafiei T, et al. The effect of primary midwife‐led care on women's experience of childbirth: results from the COSMOS randomised controlled trial. BJOG: an International Journal of Obstetrics and Gynaecology 2015 [epub ahead of print]. [DOI] [PubMed]
- McLachlan HL, Forster DA, Davey MA, Farrell T, Gold L, Biro MA, et al. Effects of continuity of care by a primary midwife (caseload midwifery) on caesarean section rates in women of low obstetric risk: The COSMOS randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2012;119(12):1483‐92. [DOI] [PubMed] [Google Scholar]
- McLachlan HL, Forster DA, Davey MA, Farrell T, Gold L, Oats J, et al. A randomised controlled trial of caseload midwifery for women at low risk of medical complications (COSMOS): Maternal and infant outcomes. Journal of Paediatrics and Child Health 2011;47(Suppl 1):33. [Google Scholar]
- McLachlan HL, Forster DA, Davey MA, Farrell T, Gold L, Waldenstrom U, et al. A randomised controlled trial of caseload midwifery for women at low risk of medical complications (COSMOS): Women's satisfaction with care. Journal of Paediatrics and Child Health 2012;48(Suppl 1):41‐2. [Google Scholar]
- McLachlan HL, Forster DA, Davey MA, Lumley J, Farrell T, Oats J, et al. Cosmos: comparing standard maternity care with one‐to‐one midwifery support: a randomised controlled trial. BMC Pregnancy and Childbirth 2008;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
North Stafford 2000 {published data only}
- North Staffordshire Changing Childbirth Research Team. A randomised study of midwifery caseload care and traditional 'shared‐care'. Midwifery 2000;16:295‐302. [DOI] [PubMed] [Google Scholar]
Rowley 1995 {published data only}
- Rowley MJ, Hensley MJ, Brinsmead MW, Wlodarczyk JH. Continuity of care by a midwife team vs routine care during pregnancy and birth: a randomised trial. Medical Journal of Australia 1995;163:289‐93. [DOI] [PubMed] [Google Scholar]
Tracy 2013 {published data only}
- Forti A, Kildea S, Stapleton H. Intrapartum care for women: A sub‐study of the M@NGO RCT. Women and Birth 2015;28 Suppl:S14‐5. [Google Scholar]
- Hartz D, Hall B, Allen J, Lainchbury A, Forti A, Kildea S, et al. The M@NGO Trial: Does caseload midwifery reduce caesarean section operation rates?. Women & Birth 2013;26(Suppl 1):S8‐9. [Google Scholar]
- Hartz D, Tracy SK, Foureur M. Does caseload midwifery reduce casarean section rates. International Confederation of Midwives 30th Triennial Congress. Midwives: Improving Women’s Health; 2014 June 1‐4; Prague, Czech Republic. 2014:C086.
- Hartz DL, Tracy S, Kildea S, Tracy M, Foureur M, Hall B, et al. Does caseload midwifery reduce caesarean section operation rates: The m@ngo trial. Journal of Paediatrics and Child Health 2012;48(Suppl 1):27. [Google Scholar]
- Tracy K, Hartz L, Tracy B, Allen J, Forti A, Hall B, et al. Caseload midwifery care versus standard maternity care for women of any risk: M@NGO, a randomised controlled trial. Lancet 2013;382(9906):1723‐32. [DOI] [PubMed] [Google Scholar]
- Tracy SK. A randomised controlled trial of caseload midwifery care. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 31 July 2009) 2009.
- Tracy SK, Hartz D, Hall B, Allen J, Forti A, Lainchbury A, et al. A randomised controlled trial of caseload midwifery care: M@NGO (Midwives @ New Group practice Options). BMC Pregnancy and Childbirth 2011;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turnbull 1996 {published data only}
- Cheyne H, McGinley M, Turnbull D, Holmes A, Shields N, Greer I, et al. Midwife managed care: results of a randomised controlled trial of 1299 women. Prenatal and Neonatal Medicine 1996;1(Suppl 1):129. [Google Scholar]
- Holmes A, McGinley M, Turnbull D, Shields N, Hillan E. A consumer driven quality assurance model for midwifery. British Journal of Midwifery 1996;4(10):512‐8. [Google Scholar]
- McGinley M, Turnbull D, Fyvie H, Johnstone I, MacLennan B. Midwifery development unit at Glasgow Royal Maternity Hospital. British Journal of Midwifery 1995;3(7):362‐71. [Google Scholar]
- Shields N, Holmes A, Cheyne H. Knowing your midwife in labour. British Journal of Midwifery 1995;7(8):504‐10. [Google Scholar]
- Shields N, Reid M, Cheyne H, Holmes A, McGinley M, Turnbull D, et al. Impact of midwife‐managed care in the postnatal period: an exploration of psychosocial outcomes. Journal of Reproductive and Infant Psychology 1997;15:91‐108. [Google Scholar]
- Shields N, Turnbull D, Reid M, Holmes A, Cheyne H, McGinley M, et al. Satisfaction with midwife‐managed care in different time periods: a randomised controlled trial of 1299 women. Midwifery 1998;14:85‐93. [DOI] [PubMed] [Google Scholar]
- Shields N, Turnbull D, Reid M, Holmes A, Cheyne H, McGinley M, et al. Women's satisfaction and continuity of care with midwife managed care. Prenatal and Neonatal Medicine 1996;1(1):320. [Google Scholar]
- Turnbull D, Holmes A, Cheyne H, Shields N, McGinley M, McIlwaine G, et al. Does midwife‐led care work? The results of a randomised controlled trial of 1299 women. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:527.
- Turnbull D, Holmes A, Shields N, Cheyne H, Twaddle S, Harper Gilmour W, et al. Randomised, controlled trial of efficacy of midwife‐managed care. Lancet 1996;348:213‐8. [DOI] [PubMed] [Google Scholar]
- Turnbull D, McGinley M, Fyvie M, Johnstone IEA. Implementation and evaluation of a midwifery development unit. British Journal of Midwifery 1995;3(9):465‐8. [Google Scholar]
- Turnbull D, Reid M, McGinley M, Shields N. Changes in midwife attitudes to their professional role following implementation of the midwifery development unit. Midwifery 1995;11(3):110‐9. [DOI] [PubMed] [Google Scholar]
- Turnbull D, Shields N, McGinley M, Holmes A, Cheyne H, Reid M, et al. Professional issues: can midwife‐managed units improve continuity of care?. British Journal of Midwifery 1999;7(8):499‐503. [Google Scholar]
- Young D, Lees A, Twaddle S. The costs to the NHS of maternity care: midwife‐managed vs shared. British Journal of Midwifery 1997;5(8):465‐72. [Google Scholar]
- Young D, Shields N, Holmes A, Turnbull D, Twaddle S. Aspects of antenatal care. A new style of midwife‐managed antenatal care: costs and satisfaction. British Journal of Midwifery 1997;5:540‐5. [Google Scholar]
Waldenstrom 2001 {published data only}
- Waldenstrom U, Brown S, McLachlan H, Forster D, Brennecke S. Does team midwife care increase satisfaction with antenatal, intrapartum, and postpartum care? A randomized controlled trial. Birth 2000;27(3):156‐67. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, McLachlan H, Forster D, Brennecke S, Brown S. Team midwife care: maternal and infant outcomes. Australian and New Zealand Journal of Obstetrics and Gynaecology 2001;41(3):257‐64. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Berglund 1998 {published data only}
- Berglund A, Lindmark GC. Health services effects of a reduced routine programme for antenatal care. European Journal of Obstetrics & Gynecology and Reproductive Biology 1998;77(2):193‐9. [DOI] [PubMed] [Google Scholar]
Berglund 2007 {published data only}
- Berglund A, Lindberg M, Nystrom L, Lindmark G. Combining the perspectives of midwives and doctors improves risk assessment in early pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2007;86(2):177‐84. [DOI] [PubMed] [Google Scholar]
- Berglund A, Lindmark G. Midwife managed care ‐ impact on use of health services: and area‐based randomised controlled trial. XVI FIGO World Congress of Obstetrics & Gynecology (Book 4); 2000 Sept 3‐8; Washington DC, USA. 2000:116.
Bernitz 2011 {published data only}
- Bernitz S, Aas E, Oian P. Economic evaluation of birth care in low‐risk women. A comparison between a midwife‐led birth unit and a standard obstetric unit within the same hospital in Norway. A randomised controlled trial. Midwifery 2012;28(5):591‐9. [DOI] [PubMed] [Google Scholar]
- Bernitz S, Rolland R, Blix E, Jacobsen M, Sjoborg K, Oian P. Is the operative delivery rate in low‐risk women dependent on the level of birth care? A randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118(11):1357‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernitz S, Rolland R, Blix E, Jacobsen M, Sjoborg K, Oian PL. Is the operative delivery rate in low‐risk women dependent on birth care level? A randomised controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2012;91(Suppl 159):45‐6. [Google Scholar]
Chambliss 1991 {published data only}
- Chambliss L, Daly C, Medearis AL, Ames M, Turnquist R, Kayne M, et al. Significant differences in cesarean birth rates for resident physician and nurse midwife services are the result of selection criteria. American Journal of Obstetrics and Gynecology 1991;164:313. [Google Scholar]
- Chambliss LR, Daly C, Medearis AL, Ames M, Kayne M, Paul R. The role of selection bias in comparing cesarean birth rates between physician and midwifery management. Obstetrics & Gynecology 1992;80(2):161‐5. [PubMed] [Google Scholar]
Chapman 1986 {published data only}
- Chapman MG, Jones M, Spring JE, Swiet M, Chamberlain GVP. The use of a birthroom: a randomized controlled trial comparing delivery with that in the labour ward. British Journal of Obstetrics and Gynaecology 1986;93:182‐7. [DOI] [PubMed] [Google Scholar]
Famuyide 2014 {published data only}
- Famuyide A. OB Nest; redefining continuity of care for expectant mothers. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 24 March 2014] 2014.
Giles 1992 {published data only}
- Giles W, Collins J, Ong F, MacDonald R. Antenatal care of low risk obstetric patients by midwives. A randomized controlled trial. Medical Journal of Australia 1992;157:158‐61. [PubMed] [Google Scholar]
Gu 2013 {published data only}
- Gu C, Wu X, Ding Y, Zhu X, Zhang Z. The effectiveness of a Chinese midwives' antenatal clinic service on childbirth outcomes for primipare: A randomised controlled trial. International Journal of Nursing Studies 2013;50(12):1689‐97. [DOI] [PubMed] [Google Scholar]
Heins 1990 {published data only}
- Heins HC, Nance NW, McCarthy BJ, Efird CM. A randomized trial of nurse‐midwifery prenatal care to reduce low birth weight. Obstetrics & Gynecology 1990;75:341‐5. [PubMed] [Google Scholar]
Hildingsson 2003 {published data only}
- Hildingsson I, Waldenstrom U, Radestad I. Swedish women's interest in home birth and in‐hospital birth center care. Birth 3003;30(1):11‐22. [DOI] [PubMed] [Google Scholar]
Hundley 1994 {published data only}
- Hundley VA, Cruickshank FM, Lang GD, Glazener CMA, Milne JM, Turner M, et al. Midwife managed delivery unit: a randomised controlled comparison with consultant led care. BMJ 1994;309:1400‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley VA, Cruickshank FM, Milne JM, Glazener CMA, Lang GD, Turner M, et al. Satisfaction and continuity of care: staff views of care in a midwife‐managed delivery unit. Midwifery 1995;11:163‐73. [DOI] [PubMed] [Google Scholar]
- Hundley VA, Donaldson C, Lang GD, Cruickshank FM, Glazener CMA, Milne JM, et al. Costs of intrapartum care in a midwife managed delivery unit and a consultant led labour ward. Midwifery 1995;11:103‐9. [DOI] [PubMed] [Google Scholar]
James 1988 {unpublished data only}
- James DK. A comparison of a schematic approach to antenatal care and conventional shared care. Personal communication 1988.
Kelly 1986 {unpublished data only}
- Kelly J. Comparison of two different methods of delivering antenatal care, one with components provided by an obstetrician, the other by a midwife. Personal communication 1986.
Klein 1984 {published data only}
- Klein M, Papageorgiou AN, Westreich R, Spector‐Dunsky L, Elkins V, Kramer MS, et al. Care in a birth room vs a conventional setting: a controlled trial. Canadian Medical Association Journal 1984;131:1461‐6. [PMC free article] [PubMed] [Google Scholar]
Law 1999 {published data only}
- Law YY, Lam KY. A randomized controlled trial comparing midwife‐managed care and obstetrician‐managed care for women assessed to be at low risk in the initial intrapartum period. Journal of Obstetrics & Gynaecology Research 1999;25:107‐12. [DOI] [PubMed] [Google Scholar]
Marks 2003 {published data only}
- Marks MN, Siddle K, Warwick C. Can we prevent postnatal depression? A randomized controlled trial to assess the effect of continuity of midwifery care on rates of postnatal depression in high‐risk women. Journal of Maternal‐Fetal and Neonatal Medicine 2003;13:119‐27. [DOI] [PubMed] [Google Scholar]
Runnerstrom 1969 {published data only}
- Runnerstrom L. The effectiveness of nurse‐midwifery in a supervised hospital environment. Bulletin of the American College of Midwives 1969;14:40‐52. [DOI] [PubMed] [Google Scholar]
Slome 1976 {published data only}
- Slome C, Wetherbee H, Daly M, Christensen K, Meglen M, Thiede H. Effectiveness of certified nurse‐midwives. A prospective evaluation study. American Journal of Obstetrics and Gynecology 1976;124:177‐82. [DOI] [PubMed] [Google Scholar]
Stevens 1988 {unpublished data only}
- Stevens A. A randomised controlled trial of community antenatal care in central Birmingham. Personal communication 1988.
Tucker 1996 {published data only}
- Ratcliffe J, Ryan M, Tucker J. The costs of alternative types of routine antenatal care for low‐risk women: shared care vs care by general practitioners and community midwives. Journal of Health Services & Research Policy 1996;1:135‐40. [DOI] [PubMed] [Google Scholar]
- Tucker JS, Hall MH, Howie PW, Reid ME, Barbour RS, Florey CD, et al. Should obstetricians see women with normal pregnancies? A multicentre randomised controlled trial of routine antenatal care by general practitioners and midwives compared with shared care led by obstetricians. BMJ 1996;312:554‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waldenstrom 1997 {published data only}
- Waldenstrom U, Nilsson CA. A randomized controlled study of birth center care versus standard maternity care: effects on women's health. Birth 1997;24(1):17‐26. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Nilsson CA. Experience of childbirth in birth center care: a randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 1994;73:547‐54. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Nilsson CA. No effect of birth centre care on either duration or experience of breast feeding, but more complications: findings from a randomised controlled trial. Midwifery 1994;10:8‐17. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Nilsson CA. Women's satisfaction with birth center care: a randomized, controlled study. Birth 1993;20(1):3‐13. [DOI] [PubMed] [Google Scholar]
- Waldenstrom U, Nilsson CA, Winbladh B. The Stockholm birth centre trial: maternal and infant outcome. British Journal of Obstetrics and Gynaecology 1997;104(4):410‐8. [DOI] [PubMed] [Google Scholar]
Walker 2012 {published data only}
- Walker D, Demaria L, Gonzalez‐Hernandez D, Padron‐Salas A, Romero‐Alvarez M, Suarez L. Are all skilled birth attendants created equal? A cluster randomised controlled study of non‐physician based obstetric care in primary health care clinics in Mexico. Midwifery 2013;29(10):1199‐205. [DOI] [PubMed] [Google Scholar]
- Walker DM, DeMaria L, Suarez L, Gonzales D, Romero M, Padron A. Are all skilled birth attendants created equal? evidence from mexico. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S516‐S517. [Google Scholar]
References to ongoing studies
Nagle 2011 {published data only}
- Nagle C, Skouteris H, Hotchin A, Bruce L, Patterson D, Teale G. Continuity of midwifery care and gestational weight gain in obese women: a randomised controlled trial. BMC Public Health 2011;11:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Anderson 2008
- Anderson R. New MRC guidance on evaluating complex interventions. BMJ 2008;337:a1937. [DOI] [PubMed] [Google Scholar]
Ashcroft 2003
- Ashcroft B, Elstein M, Boreham N, Holm S. Prospective semistructured observational study to identify risk attributable to staff deployment, training, and updating opportunities for midwives. BMJ 2003;327(7415):584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2001
- Benjamin Y, Walsh D, Taub N. A comparison of partnership caseload midwifery care with conventional team midwifery care: labour and birth outcomes. Midwifery 2001;17(3):234‐40. [DOI] [PubMed] [Google Scholar]
Brocklehurst 2011
- Brocklehurst P, Hardy P, Hollowell J, Linsell L, Macfarlane A, McCourt C, et al. Perinatal and maternal outcomes by planned place of birth for healthy women with low risk pregnancies: the Birthplace in England national prospective cohort study. BMJ 2011;343:d7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 2000
- Cook RI, Render M, Woods DD. Gaps in the continuity of care and progress on patient safety. BMJ 2000;320:791‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Vries 2001
- Vries R, Benoit C, Teijlingen E, Wrede S. Birth by Design: Pregnancy, Maternity Care and Midwifery in North America and Northern Europe. New York: Routledge, 2001. [Google Scholar]
Devane 2007
- Devane D, Begley CM, Clarke M, Horey D, OBoyle C. Evaluating maternity care: a core set of outcome measures. Birth 2007;34(2):164‐72. [DOI] [PubMed] [Google Scholar]
Flint 1987
- Flint C, Poulengeris P. The 'Know your Midwife' Report. London: Heinemann, 1987. [Google Scholar]
Freeman 2007
- Freeman GK, Woloshynowych M, Baker R, Boulton M, Guthrie B, Car J, et al. Continuity of Care 2006: What Have We Learned Since 2000 and What are Policy Imperatives Now? Report for the National Co‐ordinating Centre for NHS Service Delivery and Organisation R & D (NCCSDO). London: NCCSDO, 2007. [Google Scholar]
Green 2000
- Green J, Renfrew M, Curtis PA. Continuity of carer: what matters to women? A review of the evidence. Midwifery 2000;16:186‐96. [DOI] [PubMed] [Google Scholar]
Guthrie 2008
- Guthrie B, Saultz JW, Freeman GK, Haggerty JL. Continuity of care matters.. BMJ 2008;337(a867):00. [DOI] [PubMed] [Google Scholar]
Haggerty 2003
- Haggerty JL, Reid RJ. Continuity of care: a multidisciplinary review. BMJ 2003;327(7425):1219‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodnett 2000
- Hodnett ED. Continuity of caregivers for care during pregnancy and childbirth. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD000062] [DOI] [PubMed] [Google Scholar]
Hodnett 2012
- Hodnett ED, Downe S, Walsh D. Alternative versus conventional institutional settings for birth. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD000012.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffman 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Johnson 2005
- Johnson M, Stewart H, Langdon R, Kelly P, Yong L. A comparison of the outcomes of partnership caseload midwifery and standard hospital care in low risk mothers. Australian Journal of Advanced Nursing 2005;22(3):21‐7. [PubMed] [Google Scholar]
McCourt 2006
- McCourt C, Stevens S, Sandall J, Brodie P. Working with women: developing continuity in practice. In: Page LA, McCandlish R editor(s). The New Midwifery. 2nd Edition. Churchill Livingstone, 2006:141‐66. [Google Scholar]
Moore 2014
- Moore G, Audrey S, Barker M, Bond L, Bonell C, Cooper C, et al. Process evaluation in complex public health intervention studies: the need for guidance. Journal of Epidemiology and Community Health 2014;68(2):101‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olsen 2012
- Olsen O, Clausen JA. Planned hospital birth versus planned home birth. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000352.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayment‐Jones 2015
- Rayment‐Jones H, Murrells T, Sandall J. An investigation of the relationship between the caseload model of midwifery for socially disadvantaged women and childbirth outcomes using routine data‐‐a retrospective, observational study. Midwifery 2015;31(4):409‐17. [DOI] [PubMed] [Google Scholar]
RCOG 2001
- Royal College of Obstetricians and Gynaecologists. The National Sentinel Caesarean Section Audit Report. London: RCOG Clinical Effectiveness Support Unit, 2001. [ISBN 1‐900364‐66‐2.] [Google Scholar]
Reid 2002
- Reid RJ, Haggerty JL, Mckendry R. Defusing the confusion: concepts and measures of continuity of health care. Report to the Canadian Health Services Research Foundation 2002.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rooks 1999
- Rooks JP. The midwifery model of care. Journal of Nurse‐Midwifery 1999;44(4):370‐4. [PubMed] [Google Scholar]
Ryan 2013
- Ryan P, Revill P, Devane D, Normand C. An assessment of the cost‐effectiveness of midwife‐led care in the United Kingdom. Midwifery 2013;29(4):368‐76. [DOI] [PubMed] [Google Scholar]
Sandall 2001
- Sandall J, Davies J, Warwick C. Evaluation of the Albany Midwifery Practice: Final Report. London: King's College, London, Florence Nightingale School of Nursing and Midwifery, 2001. [Google Scholar]
Saultz 2003
- Saultz JW. Defining and measuring interpersonal continuity of care. Annals of Family Medicine 2003;1(3):134‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saultz 2004
- Saultz JW, Albedaiwi W. Interpersonal continuity of care and patient satisfaction: a critical review. Annals of Family Medicine 2004;2(5):445‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saultz 2005
- Saultz JW, Lochner J. Interpersonal continuity of care and care outcomes: a critical review. Annals of Family Medicine 2005;3(2):159‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sutcliffe 2012
- Sutcliffe K, Caird J, Kavanagh J, Rees R, Oliver K, Dickson K, et al. Comparing midwife‐led and doctor‐led maternity care: a systematic review of reviews. Journal of Advanced Nursing 2012;68(11):2376‐86. [DOI] [PubMed] [Google Scholar]
ten Hoope‐Bender 2014
- Hoope‐Bender P, Bernis L, Campbell J, Downe S, Fauveau V, Fogstad H, et al. Improvement of maternal and newborn health through midwifery. Lancet Special Issue 2014;384(9949):1226‐35. [DOI] [PubMed] [Google Scholar]
UNFPA 2014
- UNFPA. The State of the World’s Midwifery 2014, A Universal Pathway, A Woman's Right to Health. UNFPA 2014.
Waldenstrom 1998
- Waldenstrom U, Turnbull D. A systematic review comparing continuity of midwifery care with standard maternity services. BJOG: an international journal of obstetrics and gynaecology 1998;105:1160‐70. [DOI] [PubMed] [Google Scholar]
Walsh 2012
- Walsh D, Devane D. A metasynthesis of midwife‐led care. Qualitative Health Research 2012;22(7):897‐910. [DOI] [PubMed] [Google Scholar]
Young 1997
- Young D, Lees A, Twaddle S. The costs to the NHS of maternity care: midwife‐managed vs shared. British Journal of Midwifery 1997;5(8):465‐72. [Google Scholar]
References to other published versions of this review
Hatem 2008
- Hatem M, Sandall J, Devane D, Soltani H, Gates S. Midwife‐led versus other models of care for childbearing women. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004667.pub2] [DOI] [PubMed] [Google Scholar]
Sandall 2013
- Sandall J, Soltani H, Gates S, Shennan A, Devane D. Midwife‐led continuity models versus other models of care for childbearing women. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD004667.pub3] [DOI] [PubMed] [Google Scholar]
Sandall 2015
- Sandall J, Soltani H, Gates S, Shennan A, Devane D. Midwife‐led continuity models versus other models of care for childbearing women. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD004667.pub4] [DOI] [PubMed] [Google Scholar]


