Abstract
Type 17 cytokines have been strongly implicated in mucosal immunity in part by regulating the production of antimicrobial peptides. Using a mouse model of C. rodentium infection which causes colitis, we found that intestinal IL-17RA and IL-17RC was partially required for control of infection in the colon and IL-17 regulates the production of luminal hydrogen peroxide as well as expression of Tnsf13. Reduced Tnfsf13 expression was associated with a profound defect in generating C. rodentium specific IgA+ antibody secreting cells. Taken together, intestinal IL-17R signaling plays key roles in controlling invading pathogens in part by regulating luminal hydrogen peroxide as well as regulating the generation of pathogen specific IgA+ antibody secreting cells.
Introduction
IL-17 signaling is thought to play critical roles in immunity in barrier organs such as the skin, lung and gastrointestinal tract (1, 2). IL-17 producing cells such as Th17 and group 3 ILC3s are abundant in the intestine in part due to the development of the gut microbiota including species of bacteria that can adhere to epithelial cells such as segmented filamentous bacteria (SFB) (3) (4, 5). We have recently reported that epithelial IL-17R controls SFB growth in the terminal ileum and regulates the release of H2O2, expression of the polymeric immunoglobulin receptor (PIGR) and secretion of IgA in the small intestine (6). Endogenous hydrogen peroxide (H2O2) is generated from the apical site of intestinal epithelial cells via NADPH oxidases such as the Nox family and is toxic to nearly all aerobic organisms (7) (8). On the other hand, IgA directly binds to bacteria and can trap antigens in the mucus layer and prevent them from binding to cell surface receptors (9, 10).
In addition to SFB colonization, attaching and effacing pathogens such as C. rodentium induce IL-17 producing cells (5, 11). C. rodentium can attach in the cecum and the colon and inoculation has long been used as a model of attaching and effacing organisms in the GI tract similar to strains of E. coli that cause human colitis (11). The induction of IL-17 producing cells has been assumed to contribute to pathogenic bacteria clearance by production of antimicrobial peptides and regulating the recruitment of myeloid cells. During C. rodentium infection, IL-17A expression is increased in colon as well as IL-22 produced from Th17 cells and ILC3s. IL-22 has an indispensable role in protection from this infection (12) (11, 13). IL-22 signals to gut epithelial cells and regulates the expression of several key antimicrobial proteins including S100A8/A9, Lipocalin-2, and RegIIIβ and RegIIIγ (12) (11, 13). Il22−/− mice develop a lethal infection with C. rodentium and this can be partially rescued by gavage administration of recombinant RegIIIγ (12). Moreover, IL-17A or IL-17F deficient mice show enhanced susceptibility to C. rodentium, which is associated with impaired β-defensin expression (2). Genetic deficiency of IL-17RE, the receptor from IL-17C also develop lethal C. rodentium infection and IL-17C can synergize with IL-22 in the induction of S100A8/A9 as well as lipocalin-2 (14). IL-17RE can be expressed in colonic epithelium as well as specific T cells and hematopoietic progenitors (2) (15). Moreover, IL-17C uses IL-17RA as a co-receptor. What is unclear, is the role of epithelial IL-17RA and IL-17RC in mucosal immunity against C. rodentium infection.
In this study, we infected villin-cre Il17rafl/fl mice with C. rodentium and assessed bacterial growth and dissemination at day 14 as well as gene expression in the colon. Compared to Cre- littermate animals, villin-cre Il17rafl/fl mice showed higher bacterial dissemination to the liver. There was significant induction of several NADPH oxidases in the colons of Cre- control mice including Nox1, Duox2, and Duoxa2 that were substantially reduced in Cre+ mice. Moreover, these genes were induced in vitro by IL-17 and were associated with increased generation of H2O2 in organoid cultures. In vivo, we also observed that intestinal IL-17RA or IL-17RC controlled the expression in the colon, of both Pigr and Tumor Necrosis Factor Ligand Superfamily Member 13 (Tnfsf13) after C. rodentium infection. This resulted in reduced C. rodentium specific IgA producing cells in spleen, bone marrow and lamina propria of colon in villin-cre Il17rafl/fl mice. Moreover, Tnfsf13 overexpression was able to protect against C. rodentium infection in these mice along with an increase in C. rodentium IgA producing cells. Our findings indicate that IL-17R signaling in epithelial cells regulates H2O2 generation as well as IgA secreting cells by regulation of Tnfsf13. These data reveal new regulation of intestinal mucosal immunity by IL-17 receptors.
Materials and Methods
Mice
All mice were on a C57BL/6 background and were housed in pathogen-free conditions at DCM Tulane University. Nox1 KO mice were purchased from The Jackson Laboratory. Il17rafl/fl and Il17rcfl/fl have been described (6). Il17rafl/fl X villin cre, and Il17rcfl/fl X villin cre mice on C57BL/6 background were generated and maintained at Department of Comparative Medicine (DCM) Tulane University. All of the animal studies were conducted with the approval of the Tulane University Institutional Animal Care and Use Committee.
Human Colonoids
The human colonoids were provided by the Washington University in St. Louis Digestive Diseases Research Cores Center Precision Animal Models and Organoids Core to Dr. Good’s laboratory and cultured in L-WRN media as described before (16). The patient in which the colonoids were derived from was a 55 year old black female with normal endoscopic evaluations and no clinical findings.
Citrobacter infection
Bacteria were prepared by shaking at 37 °C overnight in LB broth. C57BL/6, Il17rafl/fl villin cre, Il17rcfl/fl villin cre and Nox1 KO mice were inoculated by gavage with 1 × 109 CFU of C. rodentium (ATCC 51459).
Adenovirus transduction
Il17rafl/fl X villin cre mice were intraperitoneally injected with Ad-Tnfsf13 or Ad-zsGreen (5 × 109 PFU/mouse) at day 3 post infection. Ad-Tnfsf13 is a E1-E3-human AD5 vector that expressed murine TNFSF13 off the CMV promoter. The adenovirus vectors were provided by Vector Biolabs.
Tissue collection, histology and colony-forming unit counts
Colon tissue was dissected from the mice and we used the terminal 0.5cm piece for histologic analysis and the next 0.5cm piece for CFU analysis. Histology segments were fixed in 10% neutral buffered formalin and paraffin-embedded tissue sections were stained with H&E. Spleen, liver and colon were weighed and homogenized. Samples were serially diluted and plated in triplicate in MacConkey agar (Remel). Colonies were counted after 24 h of incubation at 37 °C.
RNAscope
Paraffin-embedded colon sections from Il17rafl/fl X villin cre mice underwent in situ hybridization according to the manufacturer’s instructions (RNAscope 2.5 HD Assay – RED, Advanced Cell Diagnostics (ACD) - a Bio-Techne brand, CA, USA). Briefly, after hydrogen peroxide treatment, we performed target retrieval, created a hydrophobic barrier and applied Tnfsf13 probe (RNAscope® Probe Mm-Tnfsf13-O1, ACD) and Nox1 probe (RNAscope® Probe Mm-Nox1-C2, ACD) for hybridization. After hybridization, tissues were stained with 50% Hematoxylin.
Lamina propria cell isolation.
Colons were harvested from the mice and incubated in PBS containing 10 mM EDTA with shaking for 45 min at room temperature. After vortexing for 10 s, the tissue pieces were washed with PBS, minced into 1–2 mm pieces, and incubated in Iscove’s Modified Dulbecco’s Medium liquid (IMDM) containing Liberase TL (Roche) with shaking for 45 min at 37 °C. After digestion, the cell suspensions were filtered using a 70 μM cell strainer.
Flow cytometry
Spleen, bone marrow and lamina propria cells were stained with PE-conjugated anti-IgA (mA-6E1, eBioscience), FITC-conjugated anti-CD3e (17A2, Biolegend), APC-conjugated anti- CD138 (281–2, BD Pharmingen), and Alexa Fluor 700-conjugated anti- B220 (RA3–6B2, BD Pharmingen). Stained cells were analyzed on a Cytek Aurora (CYTEK) with FlowJo software (TreeStar).
ELISA
hTNFSF13 ELISA was performed using human colonoid culture supernatant. ELISA was performed as per manufacturer’s instructions (LEGEND MAX Human APRIL/TNFSF13 ELISA Kit, BioLegend). Colonoids were incubated with hIL-17A (100 ng/ml) or hTNFα (100 ng/ml) for 24 or 48 hr.
C. rodentium antigen preparation
C. rodentium was cultured in 200ml LB and incubated at 37 °C, shaken at 200rpm overnight and then harvested. The pellet was re-suspended in 5ml of lysis buffer (50mM Tris pH 8.0, 10% glycerol, 0.1% Triton X-100, 100ug/ml lysozyme (Thermo Scientific), 1mM EDTA, 1mM PMSF and proteases inhibitor cocktail (Thermo Scientific). After incubation on ice for 30min, the lysate was sonicated for 20 s (5% Duty cycle and 50% Power output) on and off for 8–10 times till sample was no longer viscous and then centrifuged at 10,000rpm for 30min at 4 °C. The supernatant protein concentration was measured by BCA protein assay (Pierce).
ELISPOT
C. rodentium-specific IgA ASCs from the spleen, BM and lamina propria were enumerated using ELISPOT. Briefly, 96-well ELISPOT plates were coated with C. rodentium antigen, and then blocked with media containing 10% FBS. Cells from spleen, BM and lamina propria were seeded at an initial concentration of 1 × 106, 5 × 105 and 5 × 104 cells/well respectively. After 16 hours, plates were washed and probed with biotinylated anti-mouse IgA (Mabtech) for 2 hours. After the treatment, the plates were incubated with alkaline phosphatase (AP) -conjugated streptavidin. Spots were visualized and enumerated using a CTL-Immunospot S5 MicroAnalyzer. No spots were detected in cultures using cells from uninfected mice.
Western blotting
Fecal pellets (1–2 pellets) were collected from individual mice and dissolved in PBS (50 mg/ml) containing protease inhibitors (Thermo Scientific) and 1 mM PMSF. BCA assay was performed to quantify protein and 7.5 μg protein was used for western blotting. Western blotting were performed in non-reducing condition using 4–20% gradient SDS-PAGE gels and reducing condition using 10% SDS-PAGE gels (Bio-Rad system) and transferred to nitrocellulose membranes. The blot was probed with goat anti-mouse IgA-HRP (Southern Biotech). After incubation with IgA-HRP-conjugated anti-mouse antibody, membranes were washed and incubated with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). Signal was detected using Bio-Rad ChemiDoc MP imaging system. IgA (Catalog No. 553476, BD Pharmingen) was used as positive control.
Mouse formation of monolayers
Whole mouse colon was resected and rinsed with cold PBS to remove debris. The tissue was then transferred to Hank’s Balanced Salt Solution (HBSS) containing 10 mM EDTA and incubated for 40 minutes with gentle shaking at room temperature. After incubation, the tissue was placed into cold PBS containing with 0.1% BSA and pipetting over 10 times and then filtered through a 70μm nylon filter (Thermo Scientific). The filtered crypts were re-suspended in mouse IntestiCult Organoid Growth Medium (STEMCELL TECHNOLOGIES) containing trans-4-[(1R)-1-Aminoethyl]-N-4-pyridinylcyclohexanecarboxamide dihydrochloride (Y-27632) and 6-[[2-[[4-(2,4-Dichlorophenyl)-5-(5-methyl-1H-imidazol-2-yl)-2-pyrimidinyl]amino]ethyl]amino]-3-pyridinecarbonitrile (CHIR99021) (Tocris) and then plated in 24 well plate after coating collagen type IV α1 (SIGMA).
Amplex Red assay
To measure hydrogen peroxide production from colonic organoid cultures, 50ul of cell supernatants from organoid monolayer cultures after stimulation with IL-17 (R&D SYSTEMS) ot TNFα (R&D SYSTEMS) or vehicle were subjected to an Amplex Red assay following the manufacturer’s instructions (Life Technologies). Briefly, primary mouse colonic epithelial cells were incubated with IL-17, TNFα, or control condition media for 24 or 48 hrs. The condition media was removed and replaced with 50ul HBSS with calcium and magnesium (Thermo Scientific) before incubation with Phorbol-12-myristate-13-acetate (PMA) (50 ng/ml) and ionomycin (750 ng/ml) for 1hr. Supernatants (50 uL) were incubated with Amplex Red regent and HRP for 30 min and fluorescence was measured (excitation 530/emission 590) using a microplate reader (BioTek). To calculate the quantity of H2O2, a standard curve of known H2O2 concentrations was developed using the Amplex Red assay.
Hydrogen peroxide with C. rodentium assays
To evaluate bacteriostatic activity, 100ul of hydrogen peroxide (0–100μM) (Fisher Chemical) in LB broth and 100ul of C rodentium 1 ×103 bacterial cells(also in LB broth) were incubated in 96 well plates for 9 hours at 37 °C and then OD600 absorbance was measured on a microplate reader (BioTek). To evaluate bactericidal activity, 100ul of hydrogen peroxide (0–10 mM) in LB broth and 100ul of C rodentium 1 ×103 cells in LB broth were incubated in 96 well plates for 1 hour at 37 °C and then plated on LB plate overnight at 37 °C and CFU were counted.
ELISA for C. rodentium antibodies.
Serum and fecal samples were harvested on day 14 after infection. ELISA plates were coated with C. rodentium antigen. Coated plates were washed with washing buffer (0.05% Tween 20 in PBS), blocked for 2 h with blocking buffer (1% BSA and 0.1% Tween 20 in PBS), and washed before the addition of fecal samples adjusting for total IgA (125ng/ml). After a 2 hour incubation at room temperature, the plates were washed and the Ig was detected with goat anti-mouse IgA conjugated with horseradish peroxidase (Southern Biotech), diluted 1/5,000 in assay diluent (0.5% BSA and 0.05% Tween 20 in PBS), and incubated for 2 h at room temperature. After washing, TMB peroxidase substrate (Southern Biotech) was added to each well. Absorbance was read at 450 nm on a microplate reader (BioTek).
RNA sequencing
Total RNA from colon (1–4 μg) of Il17ra fl/fl X villin cre+ and littermate control cre- mice were used as starting material for deep sequencing using Illumina TrueSeq RNA Sample Preparation v2 Guide. Briefly, mRNA was purified with oligo-dT beads, fragmented with magnesium and heat-catalyzed hydrolysis, and used as a template for first- and second-strand cDNA synthesis with random primers. The cDNA 3’ ends were adenylated, followed by adaptor ligation and a 15-cycle PCR to enrich DNA fragments. Quantification of cDNA libraries were performed by using Kapa Biosystems primer premix kit with Illumina-compatible DNA primers. The cDNA libraries were pooled at a final concentration 1.8 pM. Single-read sequencing was performed on a NextSeq 550. RNAseq data have been deposited in GEO accession number: https://www.ncbi.nlm.nih.gov/geo/ GSE160157.
RT-PCR
Tissues were homogenized and RNA was isolated using Trizol RNA isolation techniques as per manufacturer’s instruction. Isolated RNA were reverse transcribed into cDNA using iScript kit (Bio-rad). TaqMan Gene Expression Master Mix (Applied Biosystems) were used for qPCR. Primer-probes were purchased from Applied Biosystems. The product codes are Pigr (Mm00465049_m1), Nox1 (Mm00549170_m1), Duox2 (Mm01326247_m1), Duoxa2 (Mm00470560_m1), Tnfsf13 (Mm03809849_s1), Tnfsf13b (Mm00446345_m1) TNFSF13 (Hs00601664_g1), HPRT (Hs02800695_m1) and Hprt (Mm00446968_m1). Gene expression were quantified and normalized to Hprt. RT-PCR was also performed using Biorad Sso advanced supermix (Bio-Rad) for C. rodentium gene expression (Forward atgccgcagatgagacagttg and Reverse cgtcagcagccttttcagcta). These primers were described as in previous report (17).
Statistical Analysis
Data were analyzed in GraphPad Prism (GraphPad Software, La Jolla, CA). For multiple group comparisons we used a one-way analysis of variance (ANOVA) with a Tukey’s multiple comparison post-test to calculate P values. Two group comparisons were done with student’s t test or Mann-Whitney U test. P values of AUC were analyzed by student’s t test using total area and SE of the AUC values. P values of <0.05 will be considered significant.
Results
Epithelial IL-17 receptor signaling is partially required for protection from C. rodentium infection.
To examine if the receptors for the Th17 cytokines, IL-17A and IL-17F, are required for intestinal immunity to C. rodentium, we infected mice with conditional deletion of Il17ra or Il17rc in the gastrointestinal tract. We orally inoculated Il17ra fl/fl villin cre+, Il17rc fl/fl villin cre+ and littermate cre- control mice with C. rodentium. Ten days post infection, colonic bacterial burdens and were found to be higher in both Il17ra fl/fl villin cre+ Il17rc fl/fl villin cre+ compared to cre- control mice (Fig. 1A) strongly implicating that these two receptors are critical for mucosal immunity to this infection. Moreover, bacterial burden as measured by C. rodentium RNA expression levels in the feces (Fig. 1B) as well as the liver, ileum, and colon, were higher in Il17rc fl/fl villin cre+ mice (Fig. 1C) demonstrating that the receptor chain for IL-17A and IL-17F plays a critical role. C. rodentium burden trended higher on day 7 and 14 post infection with higher C. rodentium RNA in the colon of Il17ra fl/fl villin cre mice at day 14 (Fig. S1 A and B). Histological analysis of the colon at day 14 showed massively disrupted epithelial architecture in the colons of Il17ra fl/fl villin cre+ as well as liver microabscesses (Fig. 1D arrows) and increased inflammatory cells (Fig. 1E) in the liver. C. rodentium was cleared in both littermate controls and Il17ra fl/fl villin cre+ Il17rc fl/fl villin cre+ mice by day 28. Interestingly, anti- C. rodentium specific IgG producing cells were increased in both IL-17 receptor deficient mice reflecting the higher bacteria burden (Figure S1C and D). As IgG plays a critical role in protection from C. rodentium infection (18), these systemic IgG responses likely contribute to clearance in this model.
Figure 1. Intestinal IL-17R is required for control of C. rodentium infection.
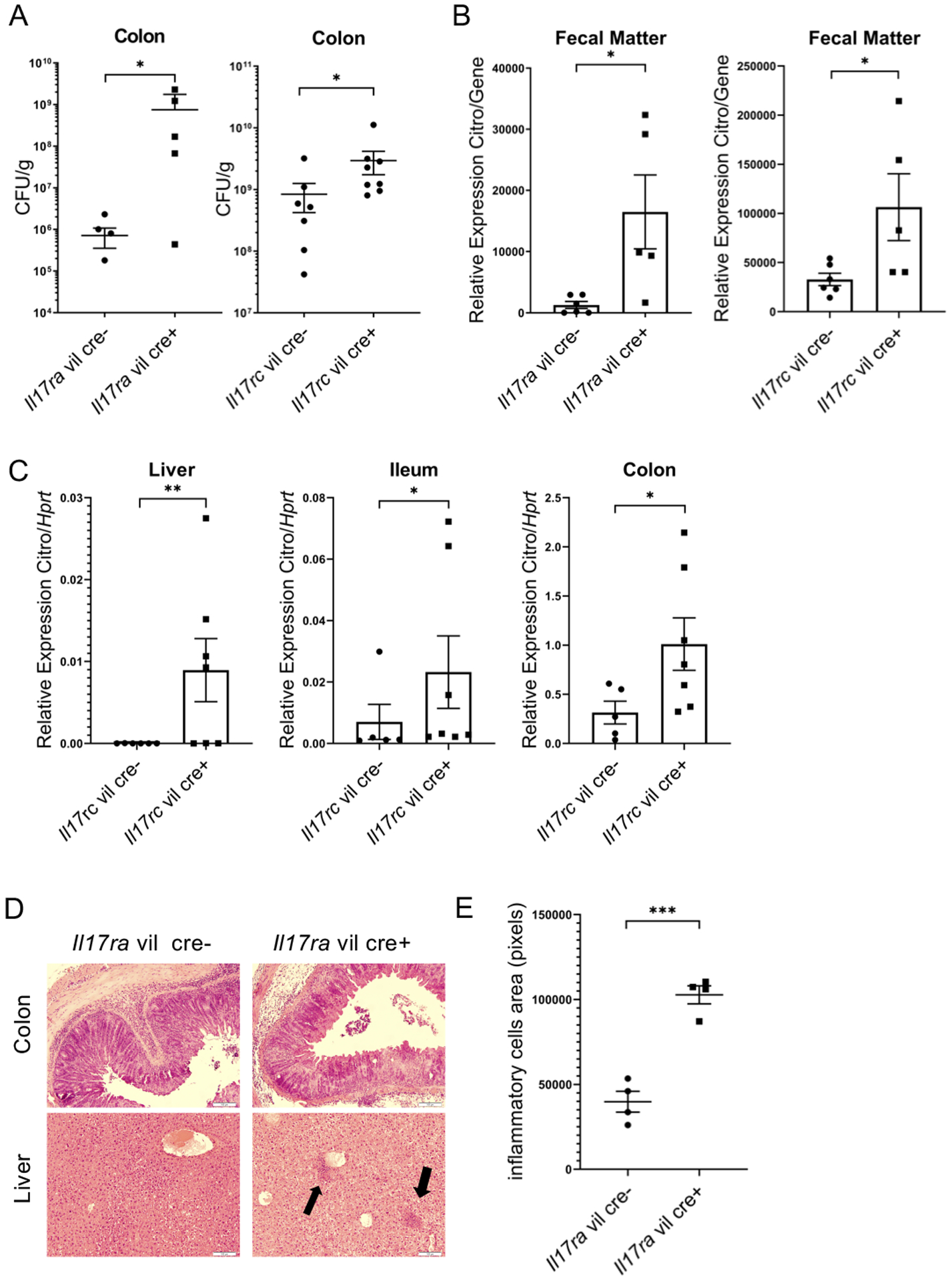
(A) Bacteria burden at day 10 in Il17ra fl/fl villin cre+ and Il17rc fl/fl villin cre+ and littermate cre- mice after C. rodentium gavage. (n=5–8 per group) (B) C. rodentium RNA expression in feces (measured by RT-PCR) at day 10 post C. rodentium infection in Il17ra fl/fl villin cre+, Il17rc fl/fl villin cre+ and littermate cre- mice (n=4 per group) (C) C. rodentium RNA expression (measured by RT-PCR) at day 10 in IL17rc fl/fl villin cre+ littermate cre- mice (n=5–8 per group) (D) H&E histology of liver and colon from Il17ra fl/fl villin cre+ and littermate cre- mice at day 14 p.i. The arrows indicate migrated inflammatory cells. (E) Inflammatory cell area in the liver. Significant differences are designated by 2-tailed Mann Whitney test (A and C) or 2-tailed Student’s t test (B and F). *, P < 0.05; **, P < 0.01. Values are means ± SEM.
Intestinal IL-17R signaling regulates apical NADPH oxidases and secretory IgA
To further understand how IL-17R signaling promotes protection from C. rodentium, we performed unbiased RNAseq analysis of the colon isolated at day 10 after C. rodentium infection. Nox1, Duox2, Duoxa2, Pigr and Tumor Necrosis Factor Ligand Superfamily Member 13 (Tnfsf13) were substantially reduced in the colons of Il17ra fl/fl villin cre+ mice (Fig. 2A) compared to cre- mice. Nox1 and Duox2 are members of NADPH oxidase and can generate apical hydrogen peroxide. Duoxa2 as a co-activator promotes Duox2 maturation to produce hydrogen peroxide. The expression of Nox1, Duox2 and Duoxa2 were substantially increased by C. rodentium infection but this increase was completely abrogated in the colons of both Il17ra fl/fl villin cre+ and Il17rc fl/fl villin cre+ mice when assayed by quantitative real time PCR (Fig. 2B and C).
Figure 2. Nox1, Duox2, Duoxa2, Pigr and Tnfsf13 are downregulated in IL-17 receptor deficient mice.
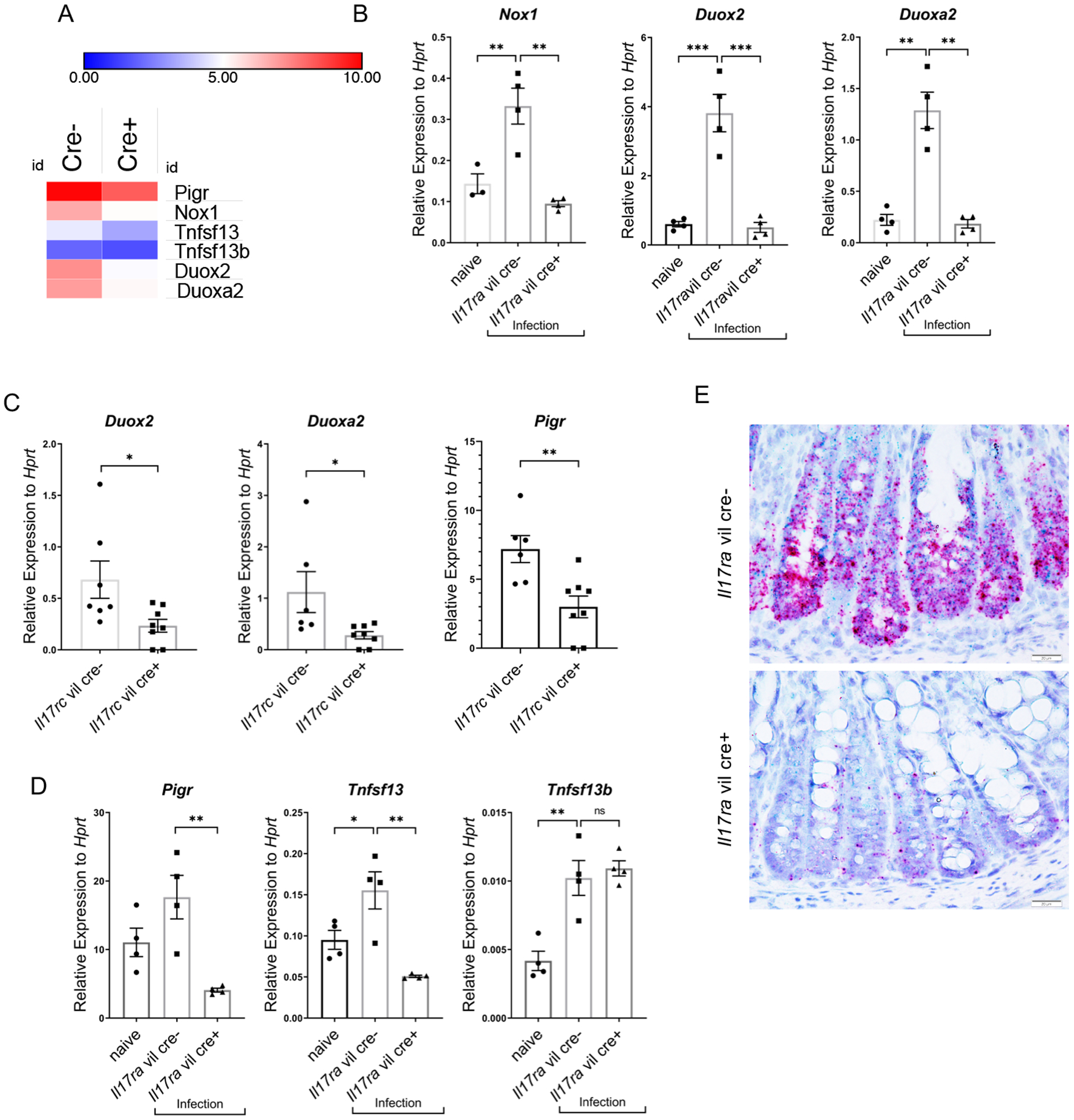
(A) Heat map of RNA-seq data in colon of differentially expressed in genes in colon tissue from Il17ra fl/fl villin Cre+ vs villin Cre- mice (n=3 each group) day 10 after C. rodentium infection. (B) Gene expression of Nox1, Duox2, and Duoxa2, by RT-PCR in colonic epithelial cells of Il17rafl/fl villin Cre+ vs villin Cre- mice day 14 post C. rodentium infection (n=3–4, two independent experiments). Naïve mice were uninfected Il17rafl/fl villin cre- mice. (C) Gene expression of Duox2, Duoxa2, and Pigr, as measured by RT-PCR from Il17rcfl/fl villin cre+ and littermate cre- mice, day 10 day post C. rodentium inoculation. (n=4). Significant differences are designated by 2-tailed Student’s t test. (D) Gene expression of Pigr, Tnfsf13 and Tnfsf13b in the colonic epithelial cells of Il17ra fl/fl villin Cre+ vs villin Cre- mice as measured by RT-PCR, day 14 post C. rodentium infection (n=3–4, two independent experiments). (E) RNAscope of Nox1 and Tnfsf13 mRNA expression in the colon of Il17rafl/fl villin cre+ (n = 4) and littermate control cre- (n = 4) mice 14 days after C. rodentium infection. Red deposits show Nox1 mRNA and green deposits show Tnfsf13 mRNA. Significant differences are designated by using ANOVA followed by Tukey’s multiple comparisons test (B and D). ns, non-significant difference; *, P < 0.05; **, P < 0.01; ***, P < 0.001. Values are means ± SEM.
In addition to observing a reduction of epithelial NADPH oxidase genes, there were also defects in genes important in mucosal humoral immunity. We have previously shown that epithelial deletion of IL-17RA results in reduced basal level of the polymeric immunoglobulin receptor (Pigr) in the small intestine (6) which is required for the transcytosis of secretory IgA (SIgA). Colonic expression of Pigr was induced by C. rodentium infection, but this was abrogated in both Il17rc fl/fl villin cre+ mice and Il17ra fl/fl villin cre+ (Fig. 2C and D). Infection also induced Tnfsf13 which was also dependent on intestinal Il17ra expression (Fig. 2D). In contrast, expression of Tnfsf13b was unaffected (Fig. 2D). Tnfsf13 is expressed in epithelial cells and myeloid cells such as dendritic cells. Using RNAscope, we investigated the cellular localization of Tnsf13 and Nox1 expression (Fig 2E). Tnfsf13 and Nox1 were highly expressed in epithelial cells in Il17ra fl/fl villin cre- whereas there was substantially reduced Tnfsf13 and Nox1 expression in Il17ra fl/fl villin cre+ mice (Fig. 2E).
IL-17 increases hydrogen peroxide in colonic epithelial cells inhibiting C. rodentium growth
Nox1, Duox2 and Duoxa2 expression are downstream of NF-κB and can regulate apical hydrogen peroxide. To investigate if these genes are directly regulated by IL-17 and generate hydrogen peroxide, we generated colonic epithelial cell monolayer cultures and stimulated these cells with IL-17A. IL-17A stimulation alone, substantially induced the expression of Nox1, Duox2 and Duoxa2 expression at both 24 and 48 hours whereas TNF-α alone did not induce these genes (Fig. 3A). Furthermore, IL-17A induced the production of hydrogen peroxide from organoid monolayer cultures (Fig. 3B). Hydrogen peroxide showed a dose-dependent ability to inhibit C. rodentium growth (Fig. 3C) as well as bactericidal activity at higher concentrations (Fig. 3D). To determine if Nox1 regulates C. rodentium growth, we infected Nox1 deficient mice or control mice with C. rodentium and monitored them for 7 and 14 days (Fig. 3E, Fig. S2A and B). Bacterial burdens in the liver and colon of Nox1 deficient mice were substantially higher at day 7 compared to control mice (Fig. 3E). At day 14 post-infection, bacterial CFU was not higher compared to controls but C. rodentium RNA was higher in the colon (Fig. S2A and B). Interestingly, Duox2 and Duoxa2 expression were also decreased in the colons of Nox1 deficient mice (Fig. 3F).
Figure 3. IL-17 induces Nox1, Duox2 and Duoxa2 expression and generation of hydrogen peroxide during C. rodentium infection.
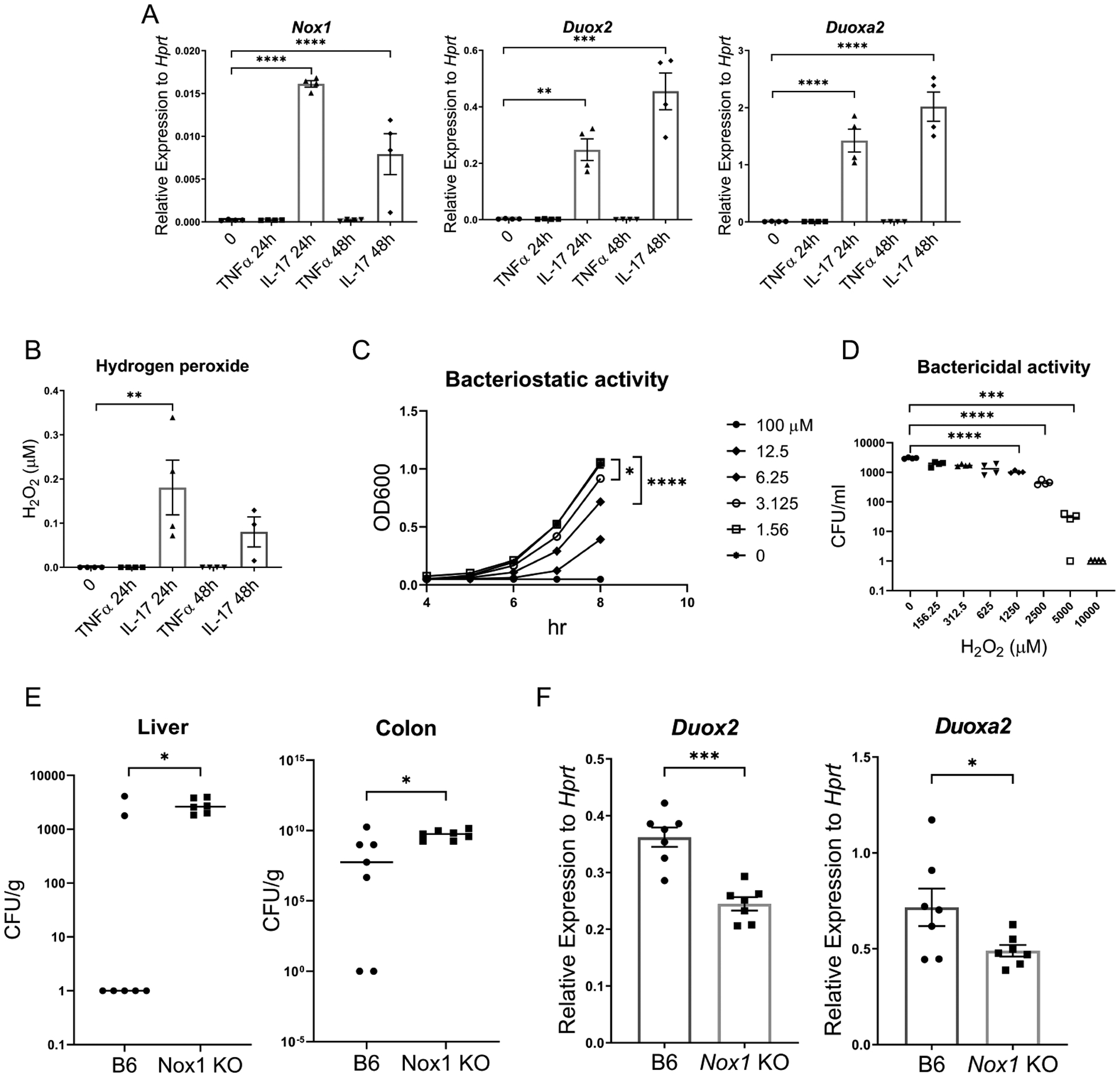
(A) Gene expression of Nox1, Duox2, and Duoxa2, in primary mouse colonic epithelial cell monolayers as measured by RT-PCR after stimulation IL-17A (100 ng/ml) and TNFα (100 ng/ml) for the indicated time. (n=4) (B) Apical hydrogen peroxide production from primary mouse epithelial cell monolayers as measured by the Amplex Red assay. The monolayer was stimulated by IL-17A (100 ng/ml) and TNFα (100 ng/ml) for the indicated time. (C) Bacteriostatic activity of hydrogen peroxide on C. rodentium growth. (D) Bactericidal activity of hydrogen peroxide on C. rodentium (E) C. rodentium burden at day 7 post-infection in Nox1 KO mice and B6 control mice (n=8 per group) (F) Gene expression of Duox2 and Duoxa2 expression in colon tissue measured by RT-PCR. Significant differences are designated by using ANOVA followed by Tukey’s multiple comparisons test (A-D). Significant differences are designated by 2-tailed Mann Whitney test (E) or 2-tailed Student’s t test (F). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Values are means ± SEM.
Intestinal IL-17R signaling is required for the generation of C. rodentium specific IgA.
As Tnfsf13 was regulated by IL-17R expression, we next determined if bacterial-specific dimeric IgA secretion was regulated by IL-17 receptor signaling in intestinal epithelial cells. Total fecal dimeric IgA was substantially reduced in the feces of Il17ra fl/fl villin cre+ mice compared to control mice whereas IgA H chains were intact (Fig. 4A). Furthermore, the kinetics of fecal C. rodentium specific IgA was substantially reduced in Il17ra fl/fl villin cre+ and Il17rc fl/fl villin cre+ mice over the course of infection (Fig. 4B). These results show that intestinal IL-17 signaling is essential for the generation of antigen specific dimeric IgA. To assess if IgA secreting cells (ASCs) were defective, we performed IgA ELISPOT assays. Total IgA positive cells collected from colonic lamina propria were not significantly different between Il17ra fl/fl villin cre+ mice and control mice pre-infection (Fig. 4C). However, there was a substantial decrease in C. rodentium specific IgA positive cells in Il17ra fl/fl villin cre+ at 14 days post infection in lamina propria (Fig. 4C). In contrast, Nox1 deficient mice mounted a C. rodentium specific IgA response in the lamina propria at day 14 (Fig.S2C) indicating that the IgA defect in Il17ra fl/fl villin cre+ defect is Nox1 independent. In Il17ra fl/fl villin cre+ we also observed that CD138+ IgA+ cells (plasma cells) were decreased in colonic lamina propria, spleen and bone marrow (BM) at 14 days post infection (Fig. 4D and S3).
Figure 4. IL-17 receptor signaling is critical for bacterial specific fecal IgA secretion.
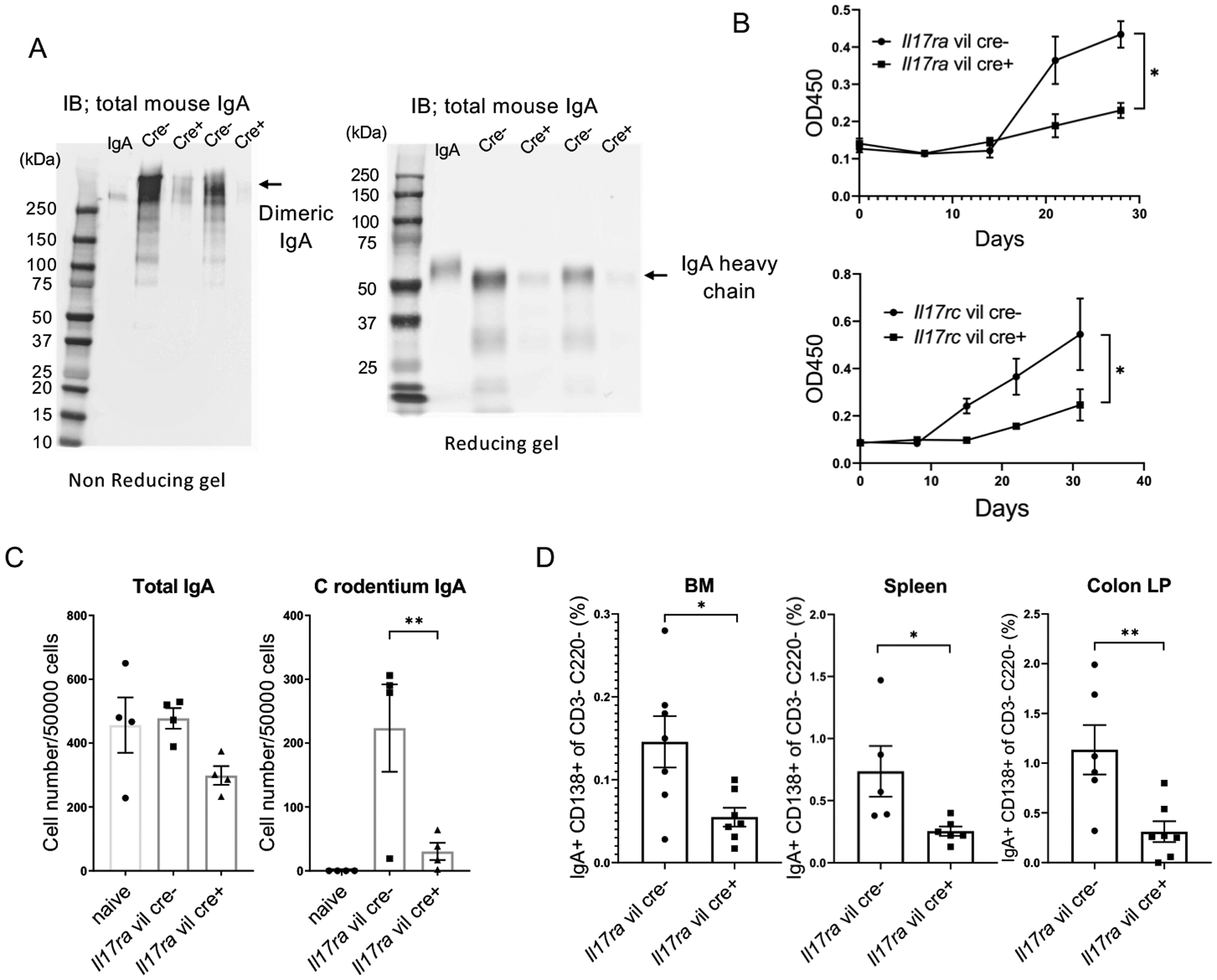
(A) Fecal IgA secretion was assayed by western blots after Il17rafl/fl villin cre+ and littermate cre- mice were infected orally with C. rodentium for 7 days using non reducing gel (left) and reducing gel (right) (n=5 per group). (B) Anti-C. rodentium specific fecal IgA (measured by ELISA using C. rodentium extract-coated plates). Feces were collected from C. rodentium infected Il17rafl/fl or Il17rcfl/fl villin cre+ and respective littermate cre- mice at serial time points post-infection. (n=4 per group). Significant differences in the area under the curve (AUC) are designated by 2-tailed Student’s t test. Values are means ± SEM. (C) C. rodentium specific IgA secreting cells in colonic lamina propria as measured by Elispot after 14 days post C. rodentium infection (n=4 per group). (D) CD138+ IgA+ cells in the B220- CD3- population collected from colonic LP, spleen and BM were measured by FACS on day 14 post C. rodentium infection in Il17ra fl/fl villin cre+ and littermate cre- mice. Significant differences are designated by using ANOVA followed by Tukey’s multiple comparisons test (C). Significant differences are designated by 2-tailed Student’s t test (D). *, P < 0.05; **, P < 0.01; ***, P < 0.001. Values are means ± SEM.
IL-17 regulates expression of Tnsf13 in human and mouse intestinal organoids.
We hypothesized that IL-17 signaling induces Tnfsf13 expression in epithelial cells and Tnfsf13 may enhance B cell activation and plasma cell proliferation. However, it is still unclear if IL-17A directly induces Tnfsf13 expression in gut epithelial cells. To address the question, we isolated organoids from mouse colon and generated monolayer cultures before incubation with TNFα or IL-17A for 24 or 48hr. In the murine monolayer culture, murine IL-17 induced Pigr and Tnsf13 but not Tnsf13b (Fig. 5A). In human colonoids, IL-17A added basolaterally resulted in increased expression of secreted TNFSF13 protein (Fig. 5B) as well as tissue TNFSF13 mRNA expression (Fig. 5B).
Figure 5. IL-17A induces TNFSF13 expression in human and mouse colonic organoid cultures and overexpression of Tnfsf13 increases IgA+ cells.
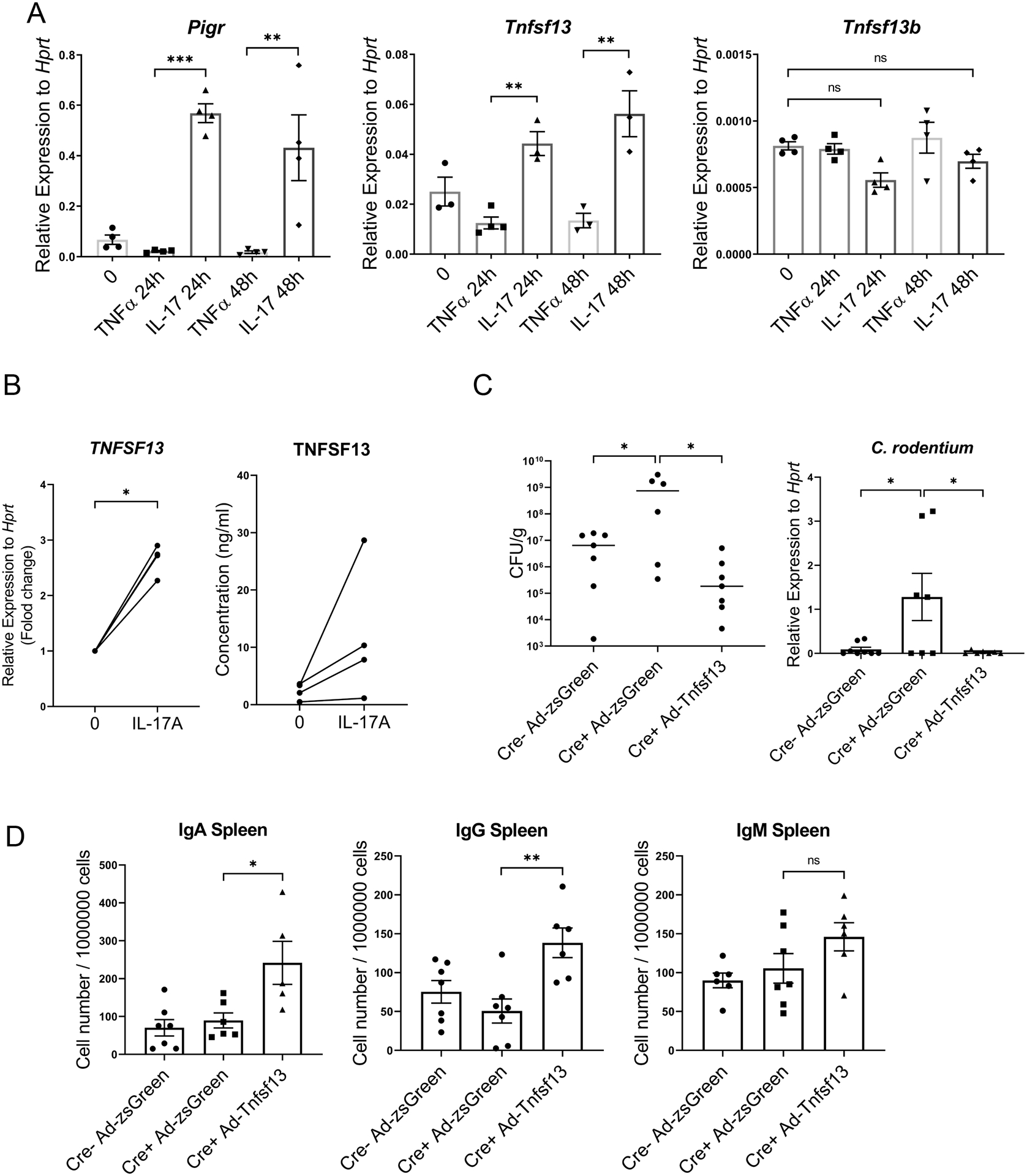
(A) Gene expression of Pigr, Tnfsf13 and Tnfsf13b in mouse colonic organoids as measured by RT-PCR. (n=4). (B) Left: Human TNFSF13 concentrations in human organoid culture medium was measured by ELISA. Right: TNSF13 mRNA expression in human colonic organoids measured by RT-PCR. (C-D) Effect of Tnfsf13 overexpression on Ig+ cells. Il17ra fl/fl villin cre+ and littermate cre- mice were intraperitoneally injected with Ad-Tnfsf13 or Ad-zsGreen at day3 post infection of C. rodentium and followed for 14 days. (C) Right: C. rodentium RNA expression in colon measured by RT-PCR, Left: bacteria burdens in colon (CFU). (D) C. rodentium specific IgA secreting cells in spleen were measured by Elispot. Significant differences are designated by 2-tailed Student’s t test (B). Significant differences are designated by using ANOVA followed by Tukey’s multiple comparisons test (A and D). *, P < 0.05; **, P < 0.01. Values are means ± SEM.
Tnfsf13 overexpression induces IgA+ ASCs and protects from C. rodentium infection.
To determine if Tnfsf13 can rescue humoral immune responses in Il17ra fl/fl villin cre+ mice infected with C. rodentium, we generated a Tnfsf13 overexpression model using recombinant adenovirus (Ad). Il17ra fl/fl villin cre+ mice were intraperitoneally injected with Ad-Tnfsf13 or Ad-zsGreen as a control, at day 3 post infection. Both C. rodentium gene expression and bacterial burdens in the colon were lower in Il17ra fl/fl villin cre+ mice injected with Ad-Tnfsf13 compared with the Ad-zsGreen cohort (Fig. 5C). Additionally, Ad-Tnfsf13 significantly increased the number of C. rodentium specific IgA+ and IgG+ ASCs in the spleen (Fig. 5D). Ad-Tnfsf13 had no effect on IgM positive ASCs (Fig. 5D). In contrast, both IgA and IgG positive ASCs in LP were not increased in the Ad-Tnfsf13 or the Ad-zsGreen group (data not shown). This may be due to the inability of Tnfsf13 to access the lamina propria compartment as opposed to the systemic circulation. Hence, IL-17 induction of Tnfsf13 enhanced clearance of C. rodentium and increased C. rodentium specific IgA positive ASCs.
Discussion
Although type 17 immunity is essential for early immunity to C. rodentium (12), the peak of IL-17A expression has been reported to be approximately 12 days after C. rodentium infection (12). Moreover, ultimate clearance of C. rodentium requires class-switched antibody. Secreted IgA and IgM are thought to be insufficient but class-switched IgG is the critical isotype for organism clearance (18). However, IgA may be important in blocking re-infection. In support of a role for class-switched immunoglobulins, mice with defective T follicular helper function, that regulate B-cell proliferation and class-switching, have delayed clearance of C. rodentium (19, 20). IgA can potentially prevent epithelial invasion by pathogenic bacteria as well as neutralize bacterial virulence factors such as bacterial exotoxins (21) (22–24). Indeed administration of a monoclonal IgA against several species of pathologic bacteria prevents the development of dextran sodium sulfate (DSS)-induced colitis (9) and IgA coating can also identify pathologic bacteria in the setting of inflammatory bowel disease (10). These results suggest IgA plays critical roles in colitis development.
Here we elucidated the role of IL-17 receptors in intestinal epithelial cells in a model of infectious colitis and found that IL-17R regulates Pigr and lumenal SIgA in the colon as previously reported in the terminal ileum (6). Surprisingly, we also observed a defect in the generation of IgA+ antibody secreting cells specific to C. rodentium in the lamina propria of intestinal specific IL-17R−/− mice. This defect was associated with a failure to upregulate the expression of Tnfsf13 after C. rodentium infection. At steady state, we observed no differences in IgA+ antibody secreting cells in intestine suggesting that the reduced lumenal IgA prior to C. rodentium infection is likely due to reduced transport via reduced Pigr expression. However, after infection, there is a defect in both Pigr expression and a reduced number of pathogen specific antibody secreting cells. Tnfsf13 is produced by epithelial cells, dendritic cells and eosinophils and can induce IgA class switching and contribute to plasma cell survival (25) (26, 27) (28, 29).
It has been reported that Tnfsf13 deficient mice have impaired serum IgA levels at steady state as well as a reduction in antigen specific IgA production in the intestine after immunization with (4-Hydroxy-3-nitrophenylacetyl hapten is conjugated to Lipopolysaccharide) NP-LPS (30). This current study demonstrates that IL-17 is a critical regulator of Tnfsf13 in the intestinal epithelium and it is likely that this defect is causal in the reduced numbers of pathogen specific IgA+ antibody secreting cells. Tnfsf13 overexpression increased the pathogen specific IgA+ and IgG+ ASCs population in spleen. We did not observe differences in IgA+ ASC populations in lamina propria, which we interpret as lack of sufficient Tnfsf13 protein delivery to this compartment after adenovirus delivery. However, the increase IgA+ ASCs populations in spleen correlated with the previous study that showed that Tnfsf13 promotes IgA class-switching (30).
Furthermore, our findings indicate that IL-17R expression in colonic epithelial cells is a key regulator of induced H2O2 generation in the colon by regulating the expression of several NADPH oxidases including Nox1, Duox2 and Duoxa2 expression. Nox1 and Duox2 are highly expressed in the intestinal epithelial cells. These genes contribute to defense against pathogenic bacteria at the mucosal barrier (31) (32) (33). It has been reported that H2O2 released by Nox1 and Duox2 genes impair virulence of Campylobacter jejuni, which can cause diarrheal disease and colitis in (31). Duox2 deficient mice have impaired release of H2O2 and increased susceptibility to Helicobacter felis (34). In addition, Nox1 deficient mice allow segmented filamentous bacteria (SFB) overgrowth in the terminal ileum likely due to dysfunction of H2O2 release in intestine (6). Interestingly, in our study we also noted that Nox1 deficient mice also had a defect in Duox2 expression suggesting possible co-regulation of these oxidases as previously described (35). Recently it has been reported Nox1 and Duox2 can also be regulated by Toll-like receptor 4 signaling (36). This may explain the different kinetics of earlier bacterial growth in Nox1 deficient compared to IL-17 receptor deficient mice. An additional factor in this study is that Nox1 deficient mice may have different microbiome compared to IL-17 receptor deficient mice that were bred in house (6). It has also been reported that patients with intestinal disease such as irritable bowel syndrome and IBD and DSS-induced colitis models have a high level of oxidase expression in the intestine (37–40), however the regulation of these oxidases is poorly understood. Our study sheds light on the regulation of these oxidases in the colon by IL-17 receptor signaling.
Duox2 is also essential for synthesis of thyroid hormone in the thyroid gland (34). Some cases of congenital hypothyroidism (CH), are due to mutations in the DUOX2 gene (41). It has recently been reported that patients with increased risk for inflammatory bowel disease (IBD) (42) have an increase in Duox2 expression (38). Based on its role in thyroid hormone production, studies of Duox2 in the intestine will likely require conditional genetic approaches. The pathology and immune response in C. rodentium infection resembles certain forms of IBD as infectious colitis. Our data suggest that intestinal IL-17R signaling plays a beneficial role in infectious colitis by regulating ASC generation, transport of SIgA via Pigr regulation, as well as controlling luminal hydrogen peroxide concentrations.
Supplementary Material
Key points:
Intestinal IL-17R signaling plays a beneficial role in infectious colitis.
Intestinal IL-17R signaling regulates ASC generation and transport of SIgA
Expression of oxidases during infectious colitis require intestinal IL-17R
Acknowledgements
We would like to acknowledge the Washington University in St. Louis Digestive Diseases Research Cores Center Precision Animal Models and Organoids Core.
Grant support: The Digestive Diseases Research Cores Center Precision Animal Models and Organoids Core at Washington University was supported by grant P30DK052574. This work was also supported by the Louisiana Board of Regents Endowed Chairs for Eminent Scholars program, as well as by PHS grant R35HL139930 (JKK).
References
- 1.Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, Alcorn JF, Weaver CT, and Kolls JK. 2011. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity. 35: 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, and Iwakura Y. 2009. Differential roles of IL-17A and IL-17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. [DOI] [PubMed] [Google Scholar]
- 3.Ivanov II, Frutos RL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, and Littman DR. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host.Microbe 4: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, Sczesnak A, Liao JJ, Torres VJ, Jenkins MK, Lafaille JJ, and Littman DR. 2014. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature 510: 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, Ishikawa E, Shima T, Hara T, Kado S, Jinnohara T, Ohno H, Kondo T, Toyooka K, Watanabe E, Yokoyama S, Tokoro S, Mori H, Noguchi Y, Morita H, Ivanov II, Sugiyama T, Nunez G, Camp JG, Hattori M, Umesaki Y, and Honda K. 2015. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell 163: 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar P, Monin L, Castillo P, Elsegeiny W, Horne W, Eddens T, Vikram A, Good M, Schoenborn AA, Bibby K, Montelaro RC, Metzger DW, Gulati AS, and Kolls JK. 2016. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity 44: 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravindra Kumar S, and Imlay JA. 2013. How Escherichia coli tolerates profuse hydrogen peroxide formation by a catabolic pathway. J Bacteriol 195: 4569–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geiszt M, and Leto TL. 2004. The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem 279: 51715–51718. [DOI] [PubMed] [Google Scholar]
- 9.Okai S, Usui F, Yokota S, Hori IY, Hasegawa M, Nakamura T, Kurosawa M, Okada S, Yamamoto K, Nishiyama E, Mori H, Yamada T, Kurokawa K, Matsumoto S, Nanno M, Naito T, Watanabe Y, Kato T, Miyauchi E, Ohno H, and Shinkura R. 2016. High-affinity monoclonal IgA regulates gut microbiota and prevents colitis in mice. Nat Microbiol 1: 16103. [DOI] [PubMed] [Google Scholar]
- 10.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, Ruggiero E, Cho JH, Goodman AL, and Flavell RA. 2014. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158: 1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silberger DJ, Zindl CL, and Weaver CT. 2017. Citrobacter rodentium: a model enteropathogen for understanding the interplay of innate and adaptive components of type 3 immunity. Mucosal immunology 10: 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, De Sauvage FJ, and Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature medicine 14: 282–289. [DOI] [PubMed] [Google Scholar]
- 13.Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, and Weaver CT. 2012. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 37: 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin SD, and Qian Y. 2011. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat.Immunol 12: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, Modrusan Z, Sai T, Lee W, Xu M, Caplazi P, Diehl L, de VJ, Balazs M, Gonzalez L Jr., Singh H, Ouyang W, and Pappu R. 2011. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat.Immunol 12: 1159–1166. [DOI] [PubMed] [Google Scholar]
- 16.VanDussen KL, Sonnek NM, and Stappenbeck TS. 2019. L-WRN conditioned medium for gastrointestinal epithelial stem cell culture shows replicable batch-to-batch activity levels across multiple research teams. Stem Cell Res 37: 101430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sagaidak S, Taibi A, Wen B, and Comelli EM. 2016. Development of a real-time PCR assay for quantification of Citrobacter rodentium. J Microbiol Methods 126: 76–77. [DOI] [PubMed] [Google Scholar]
- 18.Maaser C, Housley MP, Iimura M, Smith JR, Vallance BA, Finlay BB, Schreiber JR, Varki NM, Kagnoff MF, and Eckmann L. 2004. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infection and immunity 72: 3315–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho H, Jaime H, de Oliveira RP, Kang B, Spolski R, Vaziri T, Myers TG, Thovarai V, Shen Z, Fox JG, Leonard WJ, and Kelsall BL. 2019. Defective IgA response to atypical intestinal commensals in IL-21 receptor deficiency reshapes immune cell homeostasis and mucosal immunity. Mucosal immunology 12: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai X, Chi X, Qiao Q, Xie S, Wan S, Ni L, Wang P, Jin W, and Dong C. 2020. T Follicular Helper Cells Regulate Humoral Response for Host Protection against Intestinal Citrobacter rodentium Infection. J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandtzaeg P 2013. Secretory IgA: Designed for Anti-Microbial Defense. Front Immunol 4: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pabst O, and Slack E. 2020. IgA and the intestinal microbiota: the importance of being specific. Mucosal immunology 13: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunker JJ, Flynn TM, Koval JC, Shaw DG, Meisel M, McDonald BD, Ishizuka IE, Dent AL, Wilson PC, Jabri B, Antonopoulos DA, and Bendelac A. 2015. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity 43: 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fransen F, Zagato E, Mazzini E, Fosso B, Manzari C, El Aidy S, Chiavelli A, D’Erchia AM, Sethi MK, Pabst O, Marzano M, Moretti S, Romani L, Penna G, Pesole G, and Rescigno M. 2015. BALB/c and C57BL/6 Mice Differ in Polyreactive IgA Abundance, which Impacts the Generation of Antigen-Specific IgA and Microbiota Diversity. Immunity 43: 527–540. [DOI] [PubMed] [Google Scholar]
- 25.Berek C 2016. Eosinophils: important players in humoral immunity. Clin Exp Immunol 183: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, and Cerutti A. 2007. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26: 812–826. [DOI] [PubMed] [Google Scholar]
- 27.Shang L, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, Maussang D, Berin C, Unkeless JC, Mayer L, Abreu MT, and Lira SA. 2008. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology 135: 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerutti A, Chen K, and Chorny A. 2011. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol 29: 273–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen DC, Garimalla S, Xiao H, Kyu S, Albizua I, Galipeau J, Chiang KY, Waller EK, Wu R, Gibson G, Roberson J, Lund FE, Randall TD, Sanz I, and Lee FE. 2018. Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion. Nat Commun 9: 3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, Mizoguchi E, and Geha RS. 2004. Impaired IgA class switching in APRIL-deficient mice. Proceedings of the National Academy of Sciences of the United States of America 101: 3903–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corcionivoschi N, Alvarez LA, Sharp TH, Strengert M, Alemka A, Mantell J, Verkade P, Knaus UG, and Bourke B. 2012. Mucosal reactive oxygen species decrease virulence by disrupting Campylobacter jejuni phosphotyrosine signaling. Cell host & microbe 12: 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rada B, and Leto TL. 2008. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol 15: 164–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer H 2009. Mechanisms and function of DUOX in epithelia of the lung. Antioxid Redox Signal 11: 2453–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grasberger H, El-Zaatari M, Dang DT, and Merchant JL. 2013. Dual oxidases control release of hydrogen peroxide by the gastric epithelium to prevent Helicobacter felis infection and inflammation in mice. Gastroenterology 145: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pircalabioru G, Aviello G, Kubica M, Zhdanov A, Paclet MH, Brennan L, Hertzberger R, Papkovsky D, Bourke B, and Knaus UG. 2016. Defensive Mutualism Rescues NADPH Oxidase Inactivation in Gut Infection. Cell host & microbe 19: 651–663. [DOI] [PubMed] [Google Scholar]
- 36.Burgueno JF, Fritsch J, Gonzalez EE, Landau KS, Santander AM, Fernandez I, Hazime H, Davies JM, Santaolalla R, Phillips MC, Diaz S, Dheer R, Brito N, Pignac-Kobinger J, Fernandez E, Conner GE, and Abreu MT. 2020. Epithelial TLR4 signaling activates DUOX2 to induce microbiota-driven tumorigenesis. Gastroenterology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aerssens J, Camilleri M, Talloen W, Thielemans L, Gohlmann HW, Van Den Wyngaert I, Thielemans T, De Hoogt R, Andrews CN, Bharucha AE, Carlson PJ, Busciglio I, Burton DD, Smyrk T, Urrutia R, and Coulie B. 2008. Alterations in mucosal immunity identified in the colon of patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 6: 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haberman Y, Tickle TL, Dexheimer PJ, Kim MO, Tang D, Karns R, Baldassano RN, Noe JD, Rosh J, Markowitz J, Heyman MB, Griffiths AM, Crandall WV, Mack DR, Baker SS, Huttenhower C, Keljo DJ, Hyams JS, Kugathasan S, Walters TD, Aronow B, Xavier RJ, Gevers D, and Denson LA. 2014. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest 124: 3617–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sommer F, and Backhed F. 2015. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal immunology 8: 372–379. [DOI] [PubMed] [Google Scholar]
- 40.Kato M, Marumo M, Nakayama J, Matsumoto M, Yabe-Nishimura C, and Kamata T. 2016. The ROS-generating oxidase Nox1 is required for epithelial restitution following colitis. Exp Anim 65: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan M, Huang Y, Jiang X, Li P, Tang C, Jia X, Chen Q, Chen W, Sheng H, Feng Y, Wu D, and Liu L. 2016. The Prevalence, Clinical, and Molecular Characteristics of Congenital Hypothyroidism Caused by DUOX2 Mutations: A Population-Based Cohort Study in Guangzhou. Horm Metab Res 48: 581–588. [DOI] [PubMed] [Google Scholar]
- 42.Grasberger H, Noureldin M, Kao TD, Adler J, Lee JM, Bishu S, El-Zaatari M, Kao JY, and Waljee AK. 2018. Increased risk for inflammatory bowel disease in congenital hypothyroidism supports the existence of a shared susceptibility factor. Sci Rep 8: 10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


