Abstract
Objective.
To determine the cost effectiveness of pembrolizumab/lenvatinib (P/L) versus standard-of-care carboplatin/paclitaxel (C/T) as first-line systemic therapy for patients with advanced/recurrent endometrial cancer.
Methods.
We designed a Markov model to simulate treatment outcomes for advanced/recurrent endometrial cancer patients whose tumors are either microsatellite stable (MSS) or have high microsatellite instability (MSI-high). We adopted a healthcare sector perspective for the analysis. Model inputs for costs, health utility, and clinical estimates were obtained from the literature including data from GOG0209 and KEYNOTE-146. Primary outcomes included costs of care, quality-adjusted life years (QALYs), and the incremental cost-effectiveness ratio (ICER). The time-horizon was three years and the discount rate was 3% annually.
Results.
In a MSS cohort, compared to C/T, first-line treatment with P/L increased treatment costs by $212,670 and decreased QALYs by 0.28 per patient. In a MSI-high cohort, compared to C/T, P/L increased costs by $313,487 and increased QALYs by 0.11 per patient, representing an ICER of $2,849,882 per QALY. Sensitivity analyses found that the price of the new drugs was the most important determinant of the ICER and that the price of the new drugs would need to decrease by 85% to $2817 per cycle to reach a $150,000/QALY threshold.
Conclusion.
In the MSS model, we found that first-line therapy for advanced or recurrent endometrial cancer with P/L increased costs and worsened outcomes compared to C/T. In the MSI-high model, P/L improved survival and QALYs compared to C/T but was not cost-effective at the current cost of the drugs.
Keywords: Advanced and recurrent endometrial cancer, Cost-effectiveness, Immunotherapy
1. Introduction
Outcomes for women with advanced or recurrent endometrial cancer are poor with a five year survival of approximately 20% (1). Additionally, about 10–15% of endometrial cancer patients will have recurrent disease within three years of initial diagnosis. Another subset of patients will present with metastatic disease, which is very difficult to treat and palliate (2). Standard first-line treatment for patients with advanced or recurrent endometrial cancer is treatment with carboplatin and paclitaxel (3). However, for those patients whose tumors do not respond to or who do not tolerate this therapy, newer immunotherapy and molecular targeted agents have expanded treatment options for this group. National Comprehensive Cancer Network (NCCN) guidelines now include the use of everolimus and letrozole, bevacizumab, and pembrolizumab as non-cytotoxic options for second-line treatment in certain settings (4–6).
Immunotherapy has transformed the landscape for the treatment of advanced or recurrent endometrial cancer. KEYNOTE-158 and a study by Le et al. were phase II trials that examined the use of single-agent pembrolizumab, a monoclonal antibody that targets programmed death-ligand receptor 1 (PD-L1), in patients who were previously treated for advanced or recurrent cancers and whose tumors had high microsatellite instability (MSI-high). These studies led to the Food and Drug Administration’s (FDA) approval of single-agent pembrolizumab for patients whose tumors are MSI-high irrespective of cancer type (6,7). In these trials, patients with endometrial cancer were found to have impressive antitumor activity with objective response rates (ORRs) of 57.1% and 53.0% in KEYNOTE-158 and Le et al., respectively (6,7). However, only 15–30% of endometrial tumors are MSI-high, leaving most patients whose tumors are microsatellite stable (MSS) without this treatment option, particularly those with non-endometrioid tumor histologies, as these tumors are rarely MSI-high (8,9).
KEYNOTE-146 was a phase II single-arm trial that examined the use of pembrolizumab combined with lenvatinib, a multi-kinase inhibitor, in patients with previously- treated advanced or recurrent endometrial cancer (10). The authors of this study report an ORR of 38.9% and deemed this combination to have a manageable toxicity profile. This compared favorably to historical second-line cytotoxic and hormonal treatment regimens whose ORRs range from 10 to 30% (5,11–13). This study led to an accelerated FDA approval for pembrolizumab/lenvatinib (P/L) for second-line treatment for patients with MSS tumors but not MSI-high tumors (10). Although limited by the small sample size of patients with MSI-high tumors, response rates were distinctly different for the MSI-high (n = 11) and MSS subgroups (n = 94); patients with MSI-high tumors had an ORR of 63.6% compared to 37.2% in those with MSS tumors (10). Given the success of this drug combination in this phase II setting, phase III trials to investigate the use of pembrolizumab and lenvatinib for the treatment of patients with advanced or recurrent endometrial cancer with both MSS and MSI-high tumors are underway (14,15). As we await the results of ongoing trials, there is considerable uncertainty about how best to utilize these newer, significantly more expensive agents (16,17).
Markov modeling offers an approach to carry out virtual trials, using diverse data sources including single-arm trials, to assess the potential clinical and economic effects of different treatment regimens. The purpose of this study was to use Markov modeling to evaluate the cost-effectiveness of P/L versus C/T as first-line treatment in advanced or recurrent endometrial cancer. We hypothesize that treatment with P/L versus C/T would improve survival and quality of life in those patients with MSI-high tumors but would increase cost and would not be considered cost-effective.
2. Methods
2.1. Model overview and Markov states
TreeAge Pro Software (2020 version; Williamstown, MA) was used to develop a Markov model to evaluate the cost-utility of P/L compared to C/T in the treatment of advanced or recurrent endometrial cancer patients. There were three Markov health states used in our model: Respond, Progress, and Die (Fig. 1). We made several assumptions based upon clinical experience and the published literature. First, only subjects entering the respond health state received cancer treatment. Therefore, adverse drug events only occurred among these subjects. Second, the most common grade 3 and 4 adverse events reported in primary clinical trials for each treatment were used for each treatment arm’s transitional “adverse event”. Third, subjects who died due to progressive disease were assumed to receive palliative care at the end-of-life (18). Lastly, chemotherapy cycles were rounded up from treatment 21-day intervals to one month to represent a month in a Markov cycle and to mirror a lenvatinib 28-day cycle.
Fig. 1.
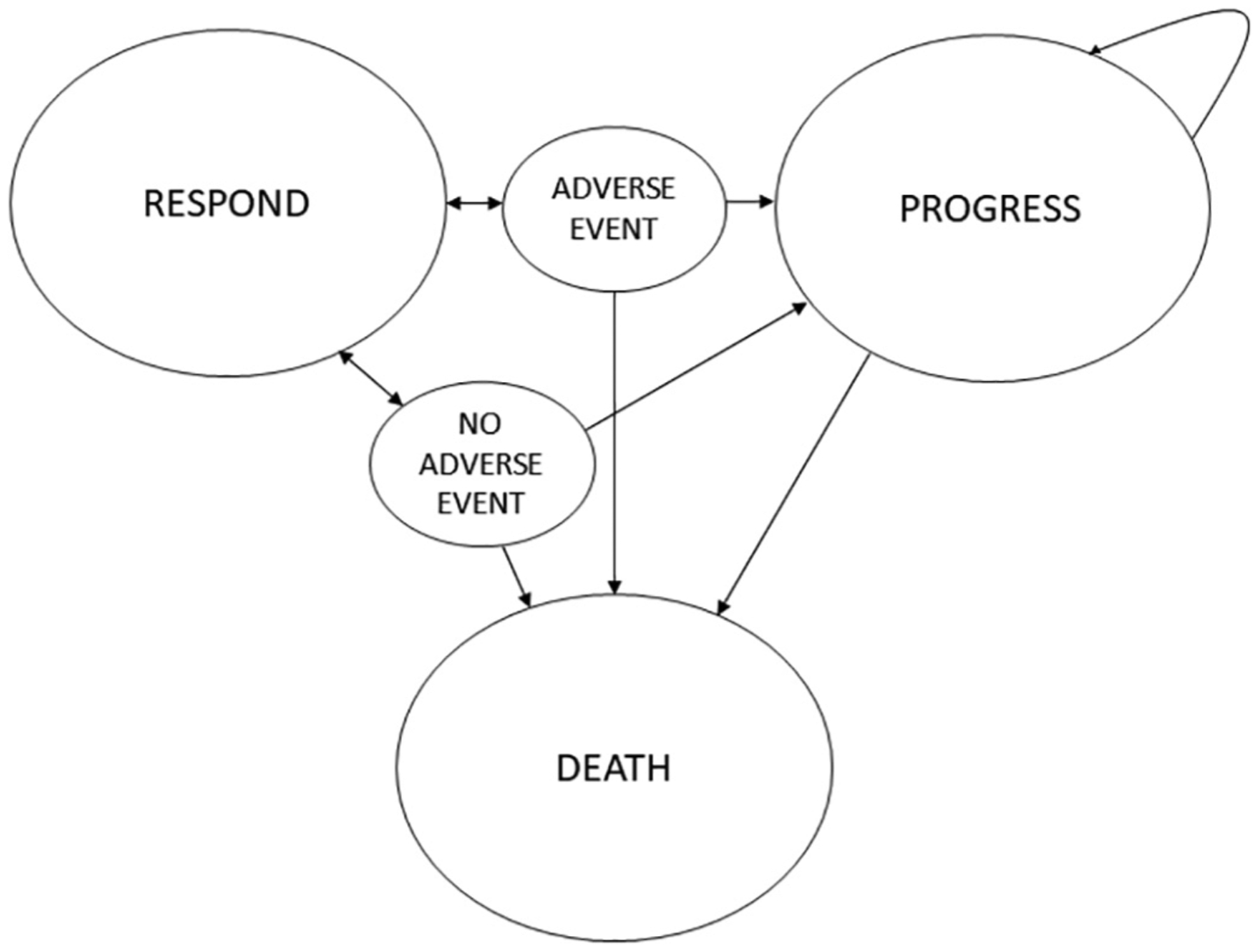
Markov state diagram.
A Markov decision tree was created from the Markov transition state diagram and is displayed in Supplementary Fig. 1. Two models were created to distinguish response rate by microsatellite instability status. The first model simulated first-line P/L versus C/T in tumors with MSS subtype and the second model simulated the same treatment in tumors with MSI-high subtype. A Markov cycle length of three months was chosen to represent the natural disease course and clinical assessment process. Patients undergoing treatment in clinical practice are not typically assessed for disease status at less than three month intervals, therefore we used a three month Markov cycle length with a risk of disease response, progression, and death assessed in each cycle. Half-cycle corrections were used. Time horizon was set to three years based on limited clinical trial data from KEYNOTE-146 available beyond 36 months.
Cost-effectiveness between treatments were expressed as the incremental cost effectiveness ratio (ICER) per quality-adjusted life year (QALY) gained. Willingness-to-pay thresholds in the United States was determined to be $150,000/QALY based on standard threshold levels for healthcare value-based assessments (19).
This economic evaluation model-based study was constructed using published clinical trial data with no identifiable patient data and was therefore determined to have exempt status from the University of Chicago Medicine Institutional Review Board.
2.2. Clinical estimates
Three-month transition probabilities were derived from progression free survival (PFS) and overall survival (OS) data from GOG0209 for C/T and KEYNOTE-146 for P/L (3,10). Three-month probabilities were abstracted from GOG0209 OS and PFS data by first digitizing the PFS and OS curves using Enguage Software, Inc. and directly abstracting the 3-month data (20). Transitional probabilities were then calculated with details of this process outlined in the methodology paper by del Campo et al. (21). Patients received an average of 18 treatment cycles for each treatment arm. The most common grade 3 or 4 adverse event described in each trial were used for the transition adverse event state for each respective treatment regimen. In KEYNOTE-146, the authors reported that 69.4% of treated patients experienced any grade 3 or 4 adverse events, of which 46.7% were reported as grade 3 or 4 hypertension (10). In GOG0209, patients receiving C/T experienced grade 3 or 4 myelosuppression-related events at the following incidence: leukopenia 50%, neutropenia 80%, anemia 17%, and thrombocytopenia 12% (3). Adverse-event related deaths and discontinuation rates reported in each study were used to determine transitional probabilities from adverse event states to other Markov states of respond, progress, or die.
2.3. Cost estimates
Costs for treatment drugs, supportive treatment drugs, diagnostic testing, and healthcare services were included in our model to represent the perspective of the payor. Details of cost inputs are included in Table 1 and an itemized listing of costs per treatment regimen are included in Supplementary Table 1. Drug prices were derived from the Centers for Medicare and Medicaid (CMS) Medicare Part B October 2020 Drug and Biological Average Sales Price Quarterly payment files and UpToDate average wholesale cost for acetaminophen and lenvatinib which were not included on the payment report (22,23). Routine chemotherapy-cycle laboratory test pricing was derived from the 2020 clinical laboratory schedule, and diagnostic testing and healthcare services were derived from the Medicare Physician Fee Schedule look-up tool (22,24,25). The cost of mismatch repair and PD-L1 immunohistochemistry testing was included as a baseline cost for both models and a pre-treatment echocardiogram was included for patients receiving P/L. The costs of grade 3 or 4 complications for the most common adverse event per treatment arm were derived from CMS and other accepted values in the literature (26). Treatment for grade 3 or 4 hypertension included the mean cost of treatment for monthly medication and monitoring and of inpatient or emergency treatment for hyper-tensive urgency and emergency. This cost amounted to a mean of $8420 per event (27,28). Treatment for grade 3 or 4 myelosuppression incorporated the cost of an injection of the granulocyte-colony stimulating factor analog pegfilgrastim, which was derived from the CMS Medicare Part B Drug and Biological Average Sales Price Quarterly payment files (22). Patients who moved from “progress” to “die” incurred the cost of palliative care, which has been calculated in previous studies (18). All costs were represented as 2020 United States Dollars (USD) and discounted annually at a rate of 3%.
Table 1.
Cost and utility model inputs.
| Input | Value | Source |
|---|---|---|
| Costs (per treatment cycle, $ USD) | ||
| PDL-1/MMRtesting (once) | 456 | (24) |
| Carboplatin/Paclitaxel | 1123 | (22,24,25) |
| Pembrolizumab/Lenvatinib | 19,986 | (22,24,25) |
| Palliative Care | 32,968 | (18) |
| Adverse Event: Hypertension, Grade 3 or 4 | 8402 | (27,28) |
| Adverse Event: Myelosuppression, Grade 3 or 4 | 3572 | (22) |
| Health Utility Values | ||
| Metastatic Endometrial Cancer | 0.63 | (31) |
| Progression | 0.36 | (30) |
| Respond | 0.71 | (30) |
| Disutility for Grade 3 or 4 Hypertensive event | −0.01 | (26,32) |
| Disutility for Grade 3 or 4 Myelosuppression | −0.10 | (30) |
| Disutility for transition to palliative care | −0.60 | (31) |
2.4. Utility estimates
Health utility values represent the present quality of life at that present state and are assigned a value from 1, (healthy) to 0 (death). Values for the health states utilized in the model are found in Table 1. A baseline advanced, metastatic endometrial cancer utility of 0.63 was derived from previous literature (29). There are no established health utility values for health states specific to endometrial cancer, therefore disutility values were adapted from metastatic breast cancer literature which utilized platinum-based treatment, resulting in a transitional “respond” utility of +0.075 and “progress” disutility of −0.272 (30). Disutility for progression to palliative care was ascertained from the ovarian cancer literature (31). Disutility for the most common grade 3 or 4 adverse events were ascertained from the literature specific to medication-related hypertension and myelosuppression health utilities (26,30,32). Utility values were discounted 3% annually.
2.5. Markov cohort population
A theoretical cohort was created for each model by estimating the proportion of patients with advanced or recurrent endometrial cancer who would be treated in the model. This was derived in the following manner. In the United States, 65,000 women are diagnosed with endometrial cancer annually with approximately 9–10% of newly diagnosed endometrial cancer patients diagnosed with advanced disease (1). Approximately 10–15% of the endometrial cancer population will recur within two to three years after their initial treatment (2). Using a conservative estimate for advanced (10%) and recurrent disease incidence (10%), we calculated a theoretical cohort of all patients with advanced or recurrent endometrial cancer of 13,000 patients. Literature estimates of MSI-high prevalence in advanced and recurrent endometrial cancer populations report that up to 30% of newly diagnosed endometrial cancers are MSI-high and up to 15% of recurrent tumors are MSI-high (8,9,33–35). This results in a cohort of 3000 patients for the MSI-high group and therefore, a cohort of 10,000 patients for the MSS group.
2.6. Sensitivity analysis
Extensive one-way sensitivity analysis was performed on key model inputs. Confidence intervals for cost estimates included a +/− 25% range for upper and lower bound and transition probabilities and utilities included a +/− 10% upper and lower bound. We then conducted a threshold analysis for the use of P/L to be cost-effective compared to C/T in the MSI-high model based on the willingness-to-pay threshold of $150,000 per QALY.
3. Results
Patient outcomes were estimated for a theoretical cohort of 10,000 women diagnosed with advanced or recurrent endometrial cancer with the MSS subtype over a three year period treated with either C/T or P/L. Results are displayed in Table 2. In the MSS cohort, treatment with C/T was superior to P/L at the three year time point, resulting in 399 less deaths and 520 more patients with response with a gain of +0.28 QALY per patient over the P/L strategy. First-line treatment with C/T resulted in a mean attributable cost of $48,848 and mean of 1.68 QALYs per patient. First-line treatment with P/L resulted in a mean attributable cost of $261,518 and mean of 1.40 QALYs per patient, representing a difference in costs of $212,670 and decrease in QALY by 0.28, with C/T considered a dominant strategy.
Table 2.
Results of the Markov cohort model for patients with microsatellite stable endometrial cancer.
| N = 5000 each cohort | Pembrolizumab/Lenvatinib | Carboplatin/Paclitaxel |
|---|---|---|
| Respond (n) | 1106 | 1626 |
| Progress (n) | 742 | 622 |
| Death (n) | 3151 | 2752 |
| Cost ($) | 261,518 | 48,848 |
| Difference in costs | 212,670 | |
| Effectiveness (QALY) | 1.40 | 1.68 |
| Difference in QALYs | −0.28 |
A Markov cohort of 3000 MSI-high advanced or recurrent endometrial cancer patients were run through the MSI-high model with results in Table 3. In a MSI-high population, first-line P/L would result in 399 fewer deaths, representing approximately a 71.5% survival at three years compared to the C/T cohort with approximately 45% survival at three years. First-line treatment with C/T resulted in a mean attributable cost of $48,848 and mean of 1.68 QALYs per patient while P/L resulted in a mean attributable cost of $362,335 and mean survival of 1.79 QALYs per patient, with an ICER of $2,849,882 per QALY.
Table 3.
Results of the Markov cohort model for patients with high microsatellite instability endometrial cancer.
| N = 1500 each cohort | Pembrolizumab/Lenvatinib | Carboplatin/Paclitaxel |
|---|---|---|
| Respond (n) | 380 | 488 |
| Progress (n) | 692 | 187 |
| Death (n) | 427 | 826 |
| Cost ($) | 362,335 | 48,848 |
| Difference in costs | 313,487 | |
| Effectiveness (QALY) | 1.79 | 1.68 |
| Incremental effectiveness (QALY) | 0.11 | |
| ICER ($) | 2,849,882 |
A univariate sensitivity analysis was performed on key model inputs that included costs of regimens, response rates (reflected as transition probabilities), and health utility values which are displayed as a tornado diagram in Fig. 2. The most significant drivers of the model were found to be the costs of the pembrolizumab and lenvatinib. In a threshold analysis, as seen in Fig. 3, decreasing the pembrolizumab and lenvatinib drug costs by 85% to $2817 per cycle would result in an ICER less than $150,000, allowing the treatment regimen to be considered cost-effective.
Fig. 2.
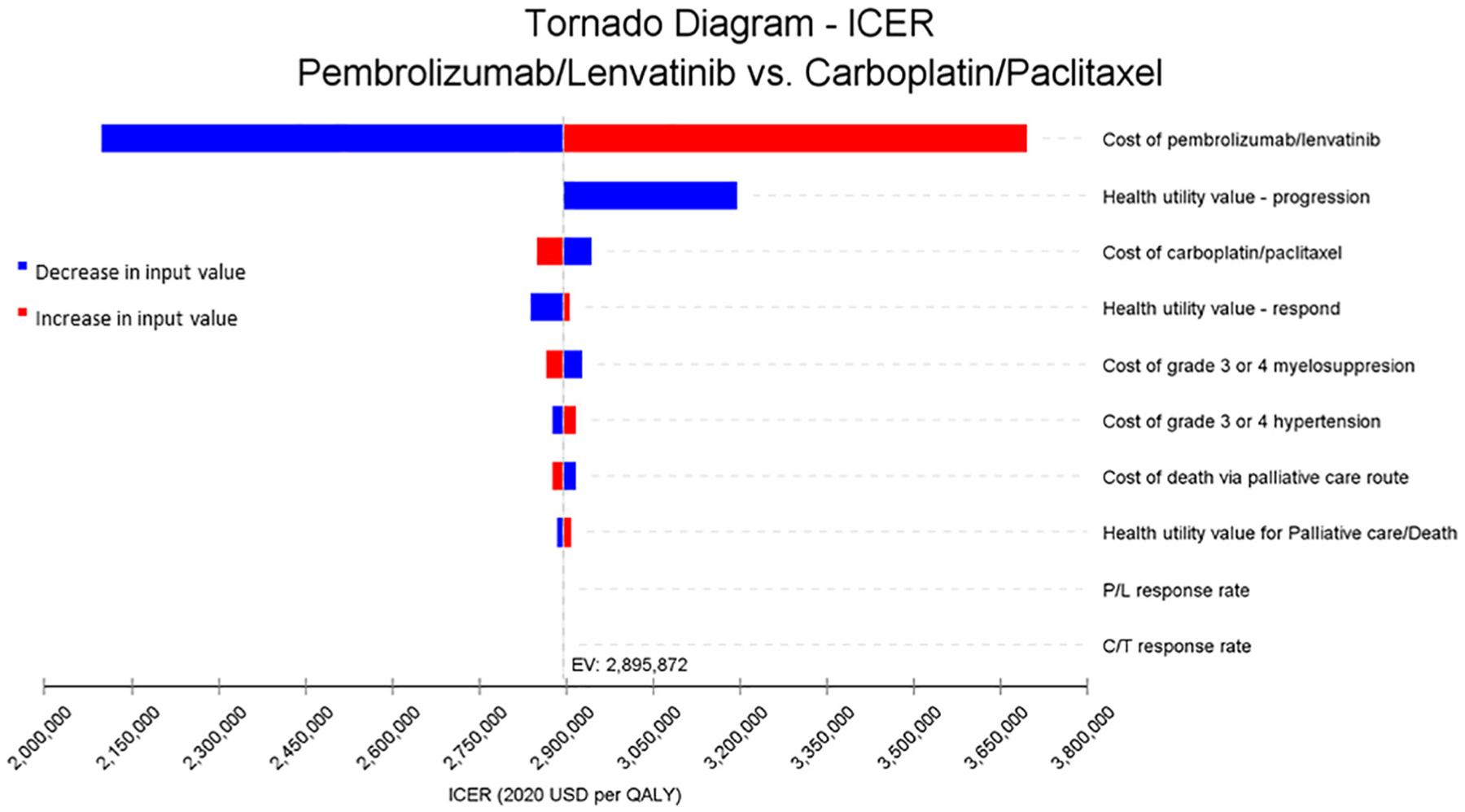
Tornado diagram based on one-way sensitivity analysis for MSI-high model for pembrolizumab/Lenvatinib versus carboplatin/paclitaxel. Abbreviations: C/T: carboplatin/paclitaxel, P/L: pembrolizumab/Lenvatinib.
Fig. 3.
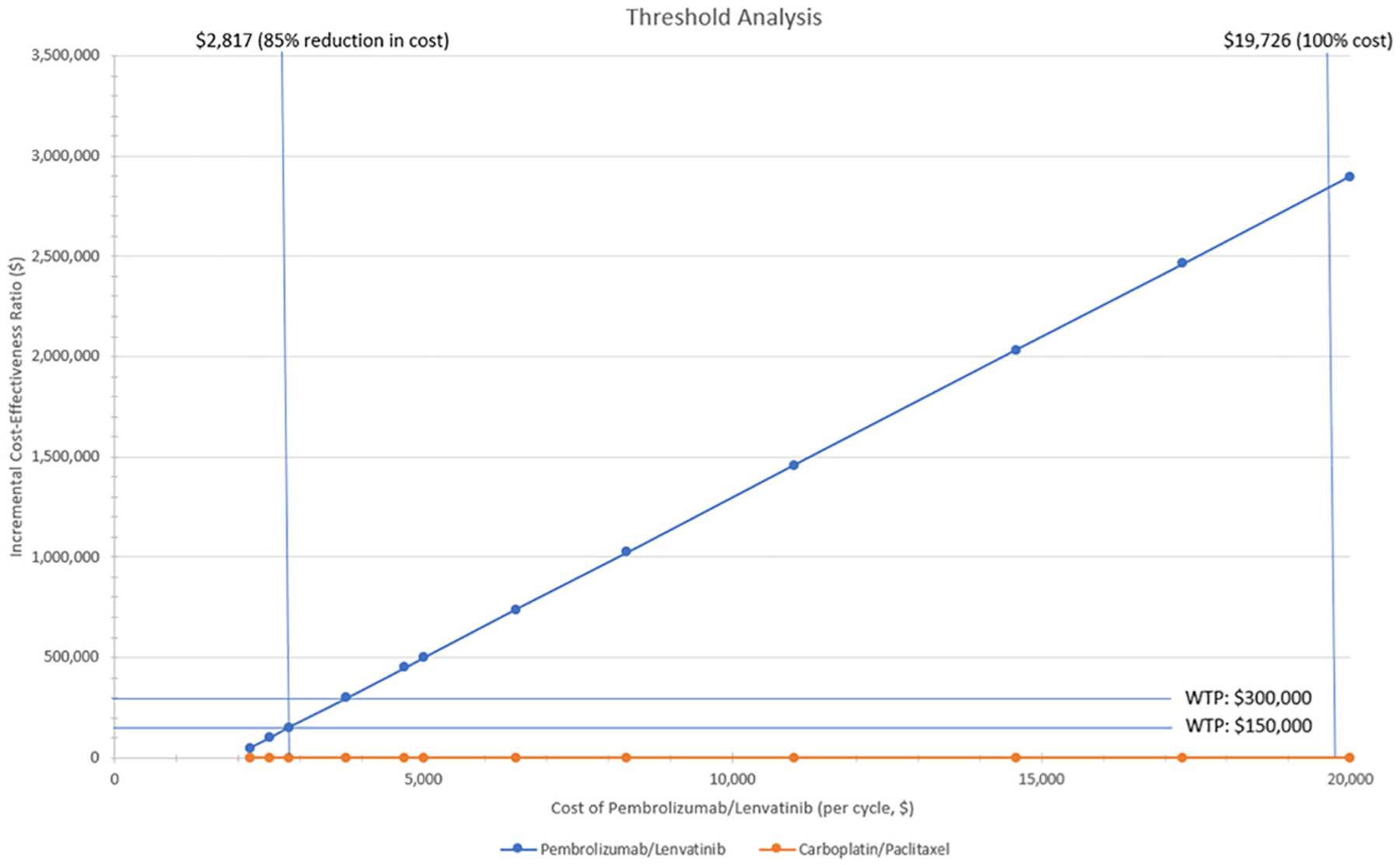
Threshold analysis for cost of Pembrolizumab/Lenvatinib.
4. Discussion
Using Markov models, we simulated first-line treatment options for advanced or recurrent endometrial cancer patient with pembrolizumab and lenvatinib versus carboplatin and paclitaxel for both the MSS and MSI-high molecular subtypes. We found that the P/L strategy for the MSS subtype reduced deaths and quality of life and increased costs compared to C/T, reducing support for P/L as a first-line therapy for patients with MSS endometrial cancer. In the MSI-high model, the P/L strategy reduced deaths and quality of life in three years compared to C/T but was not a cost-effective strategy with an ICER of $2,849,882 per QALY. In our threshold analysis, we found that decreasing the pembrolizumab and lenvatinib drug cost by 85% to $2817 per treatment cycle would result in an ICER less than $150,000. However, some prior studies have recommended using a higher willingness-to-pay in oncology patients given shortened lifespan, and authors have suggested that up to 200-$300,000/per QALY should be considered for these patients (36). Applied to our analysis, a decrease in the drug cost by 80% would achieve a cost-effective ratio with a willingness-to-pay of $300,000.
New treatment approaches including immunotherapy and molecularly targeted agents provide additional strategies to care for and improve patients’ survival and quality of life. These new drugs, however, are costly and contribute to the unsustainable growth of healthcare expenditures in the United States. A recent article examining the cost-effectiveness of pembrolizumab in non-biomarker selected hepatocellular carcinoma patients highlighted similar findings to our analysis: improvement in OS and QALYs, but the treatment was not cost-effective at its current price (16). Immunotherapy combinations in other economic analyses have been associated with steep costs of treatment, making comparisons to well-known, generic affordable medications (e.g. C/T) result in significantly higher ICERs (17,37).
Barrington et al. previously looked at single-agent pembrolizumab versus other single-agent treatments in second-line strategies for patients with advanced or recurrent endometrial cancer based on the results of KEYNOTE-028 and Le et al. (7,35). They found that single-agent pembrolizumab was cost-effective only in patients with MSI-high tumors. Their ICER for pembrolizumab alone in this population was reported to be $147,249 and was cost-effective compared to pegylated liposomal doxorubicin (38). This finding aligns with the FDA-indication for the use of pembrolizumab in patients with MSI-high tumors.
A recent study by Batman et al. examined the cost-effectiveness of adding trastuzumab to C/T for patients with Her2/neu positive uterine serous cancers based on the phase II trial by Fader et al. (39,40). They found that for patients with Her2/neu tumors, the treatment strategy was cost-effective. Both the Batman et al. and the Barrington et al. studies, as well as our current analysis highlight the use of biomarker-driven treatment strategies to promote cost-effectiveness for newer agents. Unlike in patients with other cancers, such as lung and breast cancers, which utilize molecular classifications to guide treatment, these concepts are not as well established in endometrial cancer. Only recently have treatment guidelines started to incorporate molecular subtyping into decision-making strategies. Based on our results and the above studies, cost and quality of life data should be provided along with efficacy data when new drugs are evaluated; especially drugs that have variable response rates depending on biomarker status. These new treatments may offer improved quality of life and survival for outcomes for patients. However, identifying specific patient populations or altering various treatment costs may help improve the financial impact on our healthcare system, payors, and individual patients, who may ultimately shoulder this additional financial burden.
Our model allowed us to question how P/L could perform in the first-line setting in a short time horizon. We found that this treatment in patients with MSI-high endometrial cancer could result in improved overall survival with an improved quality of life but that it was very costly. With many factors affecting our cost-effectiveness still under investigation, we are optimistic that current phase III trials (NCT03517449, NCT03884101) may help to answer this question and further validate our model and findings (14,15).
Our model seeks to understand and explore options for patients with advanced or recurrent endometrial cancers that reflect real world decisions providers may be making regarding treatment sequences that include both standard cytotoxic options and newer therapeutics. The model is limited in that the publicly available trial data that were used as inputs had different inclusion criteria than the setting in which the treatments were studied here. KEYNOTE-146 included only patients who had received prior therapy, almost all of whom received a platinum-based doublet (10). While we used response rates for P/L based on these data, we do not know if response rates will be better or worse when this combination is used prior to a cytotoxic regimen such as in a first-line recurrent setting. In contrast, we used C/T from GOG0209 as the comparison group based on the NCCN guidelines and standard clinical practice. This trial enrolled patients who were chemo-naive and did not require them to have measurable disease. This difference in patients’ prior treatments may underestimate the survival benefit of P/L in our model. Toxicity in each arm of our model may have been over- or underestimated given the eligibility criteria used for both GOG0209 and KEYNOTE-146. For instance, chemo-naïve patients may tolerate chemotherapy better and without side effects compared to patients that may have pre-existing symptom burden or are more frail from previous treatment courses. Furthermore, because pembrolizumab cycles were modeled as once every 4 weeks in our study, cost of this medication may be underestimated in this model.
The ENGOT-en9/LEAP-001 (NCT 03884101) is a phase III trial investigating C/T versus P/L in advanced or recurrent endometrial cancer regardless of molecular tumor type (MSS/MSI-high) that began enrolling patients in 2019 with results expected by 2023 (14). We hypothesize that not only might the result from this trial continue to show a benefit to P/L treatment, but that the magnitude of effect in a chemotherapy-naive recurrent population may be more favorable and, therefore, could impact the cost-effectiveness of treatment. Additionally, KEYNOTE-775 (NCT03517449) is currently testing P/L versus single-agent paclitaxel or doxorubicin in the second-line setting and is expected to have a larger patient population of tumors of both MSS and MSI-high subtypes (15). With larger numbers and longer term PFS and OS data, these ongoing trials may shed light both on the ideal sequencing of treatments and on whether adding lenvatinib to pembrolizumab in MSI-high subtypes is beneficial.
One question that will not be answered by the ongoing studies is whether P/L would be more effective than pembrolizumab alone in the first-line treatment of patients with endometrial cancer with MSI-high tumors. KEYNOTE-158 and Le et al. reported an ORR of 53–57% with pembrolizumab alone in patients with endometrial cancer with MSI-high tumors (6,7). KEYNOTE-146 added lenvatinib to pembrolizumab treatment and reported an ORR of 63.6% in a MSI-high population. This difference in ORR between these trials may not be that different and would require a prospective clinical trial to determine, which may not be feasible. In addition, lenvatinib may not add benefit to pembroluzimab treatment when cost and side effect profile are included. Lenvatinib costs $9557 per cycle of treatment and has considerable side effects as a single agent and when combined with pembrolizumab (10). A phase 2 trial of second-line single-agent lenvatinib in advanced/recurrent endometrial cancer found that 87% of subjects had treatment-related side-effects, of which 59% were ≥ grade 3 and high rates of dose interruption (59%), reduction (30%) and treatment discontinuation (31%) (41). Evaluation of pembrolizumab in the first-line setting may warrant further study.
We acknowledge that our analysis was set to a three-year time horizon due to a small cohort and lack of meaningful follow-up past the 36-month time period in KEYNOTE-146. Future models will need to consider long-term data from KEYNOTE-146 to accurately model patient outcomes. The limitations in follow up in our model may also obscure the effect of patients who experience long term durable responses or complete responses with P/L. In KEYNOTE-146, three patients demonstrated a complete response. Although the absolute number is small, curative treatment in second-line endometrial cancer treatment is rare.
Our model structure incorporated the most common side effects and their disutility into its QALY calculation. There are other treatment toxicities that would contribute to QALYs in both treatment strategies, such as the generalized fatigue experienced by P/L users and long-term neuropathy from the C/T group not captured in this model. In addition, many patients who will be treated with the P/L may have significant comorbidities (e.g. hypertension, renal disease) that may also alter treatment plans compared to trial populations. Understanding changes in cost due to dose reduction or discontinuation from adverse events or patient quality of life are challenging to model.
In summary, our model suggests that first-line use of P/L versus C/T may provide a clinical benefit in a MSI-high population, although it is not a cost-effective treatment option. However, this same clinical benefit may not be seen for P/L in a MSS population in the first-line setting. Based on our model, C/T should continue to be the first-line choice for patients with MSS tumors over P/L due to decreased efficacy and worsened quality of life, but P/L should continue to remain as a second-line treatment given limited options for this patient population. We eagerly await results of ENGOT-en9/LEAP-001 and KEYNOTE-775 and hope that they will determine what the most efficacious option is for patients with advanced or recurrent endometrial cancer (14,15). However, it is important to consider other treatment aspects, such as cost and quality of life, that impact both individual patients and our society as a whole. With the growing use of costly molecularly targeted and immunotherapies, we believe that economic analyses and predictive modeling approaches should be incorporated into decision processes for patients and physicians.
Supplementary Material
HIGHLIGHTS.
We modeled first-line pembrolizumab/lenvatinib(P/L) vs carboplatin/paclitaxel for advanced or recurrent endometrial cancer.
In our model, P/L improves overall survival and quality of life only in microsatellite instability-high tumors.
P/L is not cost-effective at its current price and cost would need to decrease by 85% to be cost-effective.
Acknowledgements
Authors thank Dr. Gini Fleming, MD for her critical review and mentorship related to the project topic and manuscript content.
This work was supported by Bears Care - the charitable beneficiary of the Chicago Bears Football Club (SAA).
Footnotes
Conflict of interest statement
SAA, EH, and NKL declare no conflict of interest. KCK served on an Advisory Board for LEAP Therapeutics through GOG Foundation, outside of the submitted work.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2021.05.038.
References
- [1].SEER, Surveillance Epidemiology, and End Results Program (SEER): Cancer Stat Facts: Uterine Cancer, National Institute of Health, 2020. [Google Scholar]
- [2].Fung-Kee-Fung M, Dodge J, Elit L, Lukka H, Chambers A, Oliver T, et al. , Follow-up after primary therapy for endometrial cancer: a systematic review, Gynecol. Oncol 101 (3) (2006) 520–529. [DOI] [PubMed] [Google Scholar]
- [3].Miller DS, Filiaci VL, Mannel RS, Cohn DE, Matsumoto T, Tewari KS, et al. , Carboplatin and Paclitaxel for Advanced endometrial cancer: final overall survival and adverse event analysis of a phase III trial (NRG oncology/GOG0209), J. Clin. Oncol 338 (33) (2020) 3841–3850 (JCO2001076). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Slomovitz BM, Jiang Y, Yates MS, Soliman PT, Johnston T, Nowakowski M, et al. , Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma, J. Clin. Oncol 33 (8) (2015) 930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aghajanian C, Sill MW, Darcy KM, Greer B, McMeekin DS, Rose PG, et al. , Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a gynecologic oncology group study, J. Clin. Oncol 29 (16) (2011) 2259–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. , Efficacy of Pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study, J. Clin. Oncol 38 (1) (2020) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. , Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade, Science. 357 (6349) (2017) 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Prendergast EN, Holman LL, Liu AY, Lai TS, Campos MP, Fahey JN, et al. , Comprehensive genomic profiling of recurrent endometrial cancer: implications for selection of systemic therapy, Gynecol. Oncol 154 (3) (2019) 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Basil JB, Goodfellow PJ, Rader JS, Mutch DG, Herzog TJ, Clinical significance of microsatellite instability in endometrial carcinoma, Cancer. 89 (8) (2000) 1758–1764. [DOI] [PubMed] [Google Scholar]
- [10].Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, et al. , Lenvatinib plus Pembrolizumab in patients with advanced endometrial cancer, J. Clin. Oncol 38 (26) (2020) 2981–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wadler S, Levy DE, Lincoln ST, Soori GS, Schink JC, Goldberg G, Topotecan is an active agent in the first-line treatment of metastatic or recurrent endometrial carcinoma: eastern cooperative oncology group study E3E93, J. Clin. Oncol 21 (11) (2003) 2110–2114. [DOI] [PubMed] [Google Scholar]
- [12].Lentz SS, Brady MF, Major FJ, Reid GC, Soper JT, High-dose megestrol acetate in advanced or recurrent endometrial carcinoma: a gynecologic oncology group study, J. Clin. Oncol 14 (2) (1996) 357–361. [DOI] [PubMed] [Google Scholar]
- [13].Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, et al. , Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study, J. Clin. Oncol 22 (19) (2004) 3902–3908. [DOI] [PubMed] [Google Scholar]
- [14].Marth Christian, Vulsteke Christof, Pérez Maria Jesus Rubio, Makker Vicky, Braicu Elena Ioana, McNeish Iain A., et al. ENGOT-en9/LEAP-001: A phase III study of first-line pembrolizumab plus lenvatinib versus chemotherapy in advanced or recurrent endometrial cancer, J. Clin. Oncol 38 (15) (2020). [Google Scholar]
- [15].Makker V, Herraez AC, Aghajanian C, Fujiwara K, Pignata S, Penson RT, et al. , A phase 3 trial evaluating efficacy and safety of lenvatinib in combination with pembrolizumab in patients with advanced endometrial cancer, J. Clin. Oncol 37 (15_suppl) (2019) (TPS5607-TPS). [Google Scholar]
- [16].Chiang CL, Chan SK, Lee SF, Wong IO, Choi HC, Cost-effectiveness of Pembrolizumab as a second-line therapy for hepatocellular carcinoma, JAMA Netw. Open 4 (1) (2021) e2033761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ding D, Hu H, Shi Y, She L, Yao L, Zhu Y, et al. , Cost-effectiveness of Pembrolizumab plus Axitinib versus Sunitinib as first-line therapy in advanced renal cell carcinoma in the U.S, Oncologist. 26 (2) (2021) (e290–e7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Smith S, Brick A, O’Hara S, Normand C, Evidence on the cost and cost-effectiveness of palliative care: a literature review, Palliat. Med 28 (2) (2014) 130–150. [DOI] [PubMed] [Google Scholar]
- [19].Neumann PJ, Cohen JT, Weinstein MC, Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold, N. Engl. J. Med 371 (9) (2014) 796–797. [DOI] [PubMed] [Google Scholar]
- [20].Mitchell M, Muftakhidinov B, Winchen T, Engauge Digitizer Software, [Survival curve digitizer]. Available from: http://markummitchell.github.io/engauge-digitizer 2020.
- [21].del Campo C, Bai J, Keller LR, Comparing Markov and non-Markov alternatives for cost-effectiveness analysis: insights from a cervical cancer case, Oper. Res. Health Care 21 (2019) 32–43. [Google Scholar]
- [22].2020 ASP Drug Pricing FIles [Internet]. Centers for Medicare & Medicaid Services, 2020, [cited 09.15.2020]. Available from https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2020-asp-drug-pricing-files.
- [23].Lenvatinib: Drug Information [Internet]. UpToDate2020 (cited 09.15.2020).
- [24].Clinical Laboratory Fee Schedule Files [Internet]. Centers for Medicare & Medicaid, 2020, [cited 10.15.2020]. Available from https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.
- [25].Physician Fee Schedule Search [Internet]. Centers for Medicare & Medicaid Services, 2020. [cited 12.20.2020]. Available from https://www.cms.gov/medicare/physician-fee-schedule/search?.
- [26].Tengs TO, Wallace A, One thousand health-related quality-of-life estimates, Med. Care 38 (6) (2000) 583–637. [DOI] [PubMed] [Google Scholar]
- [27].Minion LE, Bai J, Monk BJ, Robin Keller L, Ramez EN, Forde GK, et al. , A Markov model to evaluate cost-effectiveness of antiangiogenesis therapy using bevacizumab in advanced cervical cancer, Gynecol. Oncol 137 (3) (2015) 490–496. [DOI] [PubMed] [Google Scholar]
- [28].HCUPnet: Healthcare Cost and Utilization Project [Internet]. AHRQ, 2017, [cited 10/1/2020]. Available from http://www.hcup-us.ahrq.gov/.
- [29].Grann VR, Jacobson JS, Sundararajan V, Albert SM, Troxel AB, Neugut AI, The quality of life associated with prophylactic treatments for women with BRCA1/2 mutations, Cancer J. Sci. Am 5 (5) (1999) 283–292. [PubMed] [Google Scholar]
- [30].Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J, Health state utilities for metastatic breast cancer, Br. J. Cancer 95 (6) (2006) 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Havrilesky LJ, Broadwater G, Davis DM, Nolte KC, Barnett JC, Myers ER, et al. , Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment, Gynecol. Oncol 113 (2) (2009) 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stason WB, Weinstein MC, Public-health rounds at the Harvard School of Public Health. Allocation of resources to manage hypertension, N. Engl. J. Med 296 (13) (1977) 732–739. [DOI] [PubMed] [Google Scholar]
- [33].Hause RJ, Pritchard CC, Shendure J, Salipante SJ, Classification and characterization of microsatellite instability across 18 cancer types, Nat. Med 22 (11) (2016) 1342–1350. [DOI] [PubMed] [Google Scholar]
- [34].Lorenzi M, Amonkar M, Zhang J, Mehta S, Liaw K-L, Epidemiology of microsatellite instability high (MSI-H) and deficient mismatch repair (dMMR) in solid tumors: a structured literature review, J. Oncol 2020 (2020) 1807929. [Google Scholar]
- [35].Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. , Safety and antitumor activity of Pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study, J. Clin. Oncol 35 (22) (2017) 2535–2541. [DOI] [PubMed] [Google Scholar]
- [36].Nadler E, Eckert B, Neumann PJ, Do oncologists believe new cancer drugs offer good value? Oncologist. 11 (2) (2006) 90–95. [DOI] [PubMed] [Google Scholar]
- [37].Watson TR, Gao X, Reynolds KL, Kong CY, Cost-effectiveness of Pembrolizumab plus Axitinib Vs Nivolumab plus Ipilimumab as first-line treatment of advanced renal cell carcinoma in the US, JAMA Netw. Open 3 (10) (2020), e2016144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Barrington DA, Dilley SE, Smith HJ, Straughn JM Jr., Pembrolizumab in advanced recurrent endometrial cancer: a cost-effectiveness analysis, Gynecol. Oncol 153 (2) (2019) 381–384. [DOI] [PubMed] [Google Scholar]
- [39].Batman S, Bohn J, Weisenberger MW, Hersh A, Bruegl A, Caughey A, et al. , Trastuzumab with carboplatin/paclitaxel for treatment of advanced stage and recurrent uterine papillary serous carcinoma: a cost-effectiveness analysis, Gynecol. Oncol 160 (1) (2021) 214–218. [DOI] [PubMed] [Google Scholar]
- [40].Fader AN, Roque DM, Siegel E, Buza N, Hui P, Abdelghany O, et al. , Randomized phase II trial of carboplatin-paclitaxel compared with carboplatin-paclitaxel-Trastuzumab in advanced (stage III-IV) or recurrent uterine serous carcinomas that overexpress Her2/Neu (NCT01367002): updated overall survival analysis, Clin. Cancer Res 26 (15) (2020) 3928–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Vergote I, Powell MA, Teneriello MG, Miller DS, Garcia AA, Mikheeva ON, et al. , Second-line lenvatinib in patients with recurrent endometrial cancer, Gynecol. Oncol 156 (3) (2020) 575–582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


