Supplemental Digital Content is available in the text.
Keywords: apoptosis; athletes; cardiomegaly, exercise-induced; heart; heart injuries; necrosis
Abstract
Serological assessment of cardiac troponins (cTn) is the gold standard to assess myocardial injury in clinical practice. A greater magnitude of acutely or chronically elevated cTn concentrations is associated with lower event-free survival in patients and the general population. Exercise training is known to improve cardiovascular function and promote longevity, but exercise can produce an acute rise in cTn concentrations, which may exceed the upper reference limit in a substantial number of individuals. Whether exercise-induced cTn elevations are attributable to a physiological or pathological response and if they are clinically relevant has been debated for decades. Thus far, exercise-induced cTn elevations have been viewed as the only benign form of cTn elevations. However, recent studies report intriguing findings that shed new light on the underlying mechanisms and clinical relevance of exercise-induced cTn elevations. We will review the biochemical characteristics of cTn assays, key factors determining the magnitude of postexercise cTn concentrations, the release kinetics, underlying mechanisms causing and contributing to exercise-induced cTn release, and the clinical relevance of exercise-induced cTn elevations. We will also explain the association with cardiac function, correlates with (subclinical) cardiovascular diseases and exercise-induced cTn elevations predictive value for future cardiovascular events. Last, we will provide recommendations for interpretation of these findings and provide direction for future research in this field.
Cardiac troponins (cTn) are proteins that facilitate the contraction of cardiomyocytes after the influx of calcium into the cell. Because of their cardiac-specific isoforms, serological assessment of cTn is the gold standard to assess myocardial injury in clinical practice.1 A greater magnitude of chronically or acutely elevated cTn concentration is associated with lower event-free survival in patients2,3 and the general population.4
Exercise training improves cardiovascular function, lowers the risk for cardiovascular events, and promotes longevity. However, a bout of exercise can produce an acute rise in cTn concentrations,5 which may exceed the upper reference limit in a substantial number of individuals and meet the criteria for myocardial injury.1 Multiple studies over the past 3 decades have reported elevated cTn concentrations after exercise of different types, durations, and intensities and among subjects of different ages, sex, and health and training status.5–7 However, the clinical significance of such findings was not clear because of the descriptive nature, small sample size, and cross-sectional design of the studies, as well as a lack of long-term follow-up and mechanistic studies.
Recent studies shed new light on the underlying mechanisms and clinical relevance of exercise-induced cTn elevations. For example, endurance exercise can compromise cardiomyocyte sarcolemmal integrity,8 which may result in leakage of cTn fragments into the circulation.9 Furthermore, the magnitude of exercise-induced increases in cTn concentrations has been associated with occult obstructive coronary artery disease10 and an increased risk for mortality and major adverse cardiovascular events in middle-aged and older individuals.11
This narrative review will summarize recent insights into factors determining the magnitude of exercise-induced cTn release, the underlying mechanisms responsible for these elevations, and the clinical relevance and considerations for the interpretation of exercise-induced elevations in cTn concentrations.
Assessment of Cardiac Troponins
Molecular Basis
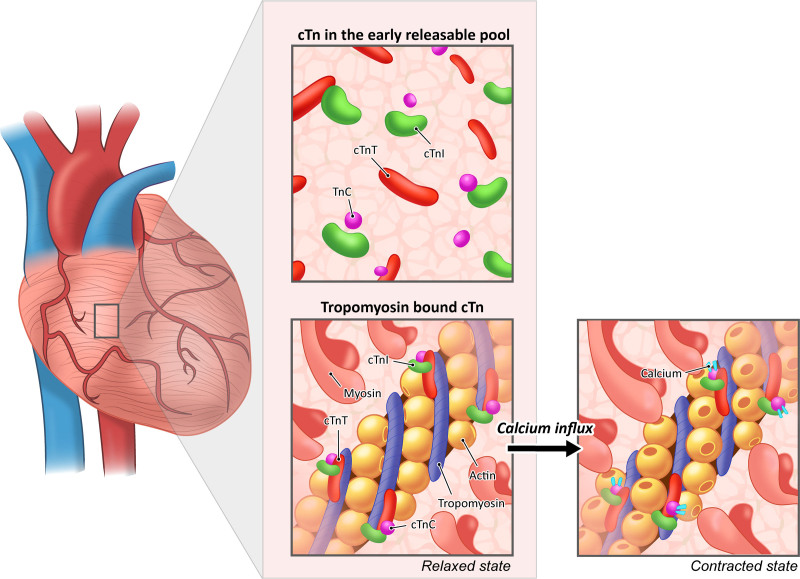
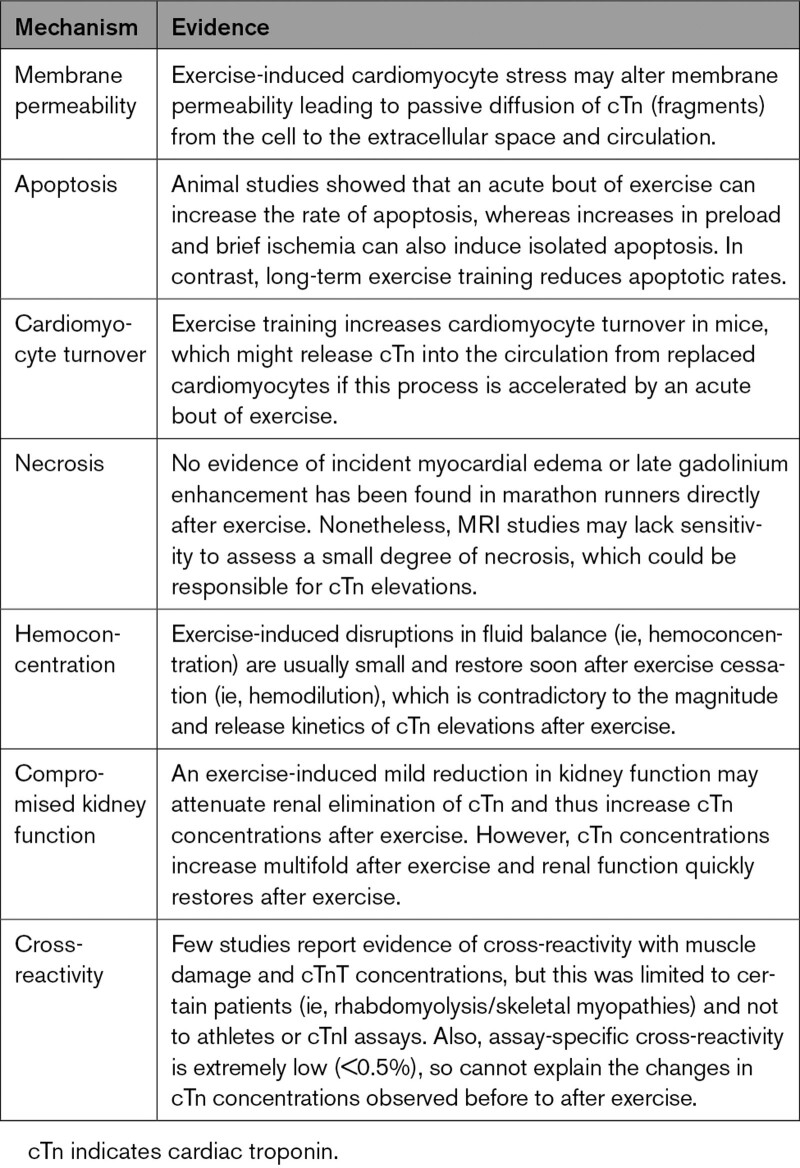
Troponin (Tn) is an intracellular protein complex that is part of the contractile apparatus of cardiac and skeletal muscle. Tn consists of 3 subunits (ie, I, T, and C) of which cardiac- and muscle-specific isoforms for I and T exist. Within cardiomyocytes, the cTn protein complex is attached to tropomyosin, a structural protein that is wrapped around the thin filament. With the influx of calcium, calcium ions bind to the TnC subunit, leading to a conformational change of the cTn complex, allowing the myosin head to bind to the actin filament, and leading to cardiomyocyte contraction (Figure 1). In addition to tropomyosin-bound cTn, cTn molecules are also present in an early releasable pool, and this fraction of cTn may be significantly larger than previously estimated (5%–10%).12
Figure 1.
The cTn complex plays an essential role in cardiomyocyte contraction. When an action potential reaches the cardiomyocyte, calcium enters the cell, leading to a conformational change after binding of calcium ions to the TnC subunit. Hence, the myosin binding sites are exposed, allowing the myosin head to bind to the actin filaments and facilitating cardiomyocyte contraction. Note that the majority of cTn complexes are bound to the actin filament but are also present in an early releasable pool. cTn indicates cardiac troponin.
Analytic Considerations
The first commercial cTn assays became available in 1996, but the rapid evolution of assay technology has tremendously improved the analytic sensitivity.13 High-sensitivity cTn assays are characterized by a low analytic coefficient of variation (coefficient of variation <10%) at the 99th percentile or upper reference limit (URL) established in apparently healthy individuals, and they have the ability to quantify cTn levels in >50% of those healthy individuals.13 Men typically have higher resting cTn levels,11,14 underlining the importance of sex-specific URLs.
More than 20 immunoassays are commercially available for cardiac troponin I (cTnI), ranging from contemporary, high-sensitivity to point-of-care assays, each using their own monoclonal antibodies specific to different epitopes of cTnI. Despite efforts by international workgroups, the standardization of cTnI measurements remains limited.15
Circulating cTn Forms
Different cTn forms and fragments have been identified in the circulation, caused either by intracellular or extracellular processes.16,17 The fragmentation process is not completely understood but may depend on degradation, phosphorylation, ubiquitination, complex formation, and binding to specific anti-cTn immunoglobulins. For patients with myocardial infarction, cardiac troponin T (cTnT) has predominantly been found intact (37 kDa) in the first hours after presentation, and as primary (29 kDa) and secondary fragments (15–20 kDa) thereafter.17 The primary fragment is cleaved at the N-terminal end of cTnT, whereas the secondary fragments are further cleaved at the C-terminal end.17,18 Patients with end-stage renal disease had only small cTnT fragments with molecular weights comparable to the secondary fragments as seen with myocardial infarction.19 It remains to be determined whether these secondary cTn forms are comparable between patients with end-stage renal disease and myocardial infarction or whether they represent different types of disease-specific fragments. It also remains a topic of discussion how cTn forms (either intact or fragments) are cleared from the blood circulation, but it is thought that smaller proteins pass through the glomerular membrane for clearance.20 After an acute myocardial infarction, however, extrarenal clearance turned out to dominate in studies on rats.21 Extrarenal clearance might be associated with scavenger receptor clearance; however, this topic has not been fully elucidated.
Exercise-Induced Cardiac Troponin Elevations
Brief Historical Perspective
The majority of studies examining the possibility of myocardial injury after exercise have used cTn as the marker of choice. However, initial evidence supporting the concept of exercise-induced cardiac injury was based on studies measuring serum CK-MB (creatine kinase myocardial band).22 Although CK-MB was widely adopted for the clinical diagnosis and management of acute coronary syndromes, it was subsequently shown to lack cardiac tissue specificity and sensitivity, especially among athletes in whom skeletal muscle CK-MB concentrations were higher (8.9±1.3% versus 3.3±0.7% in the gastrocnemius muscle of marathoners compared with untrained controls), and released in response to exercise-induced muscle injury.22 Accordingly, cTn replaced CK-MB as the gold-standard marker for myocardial injury following the Redefinition of Myocardial Infarction in 2000. Since the development of the first-generation cTn assays, >200 studies examining the effect of exercise on cTn release have been published.
Findings from the initial descriptive studies using a simple preexercise, postexercise measurement design, coupled with the results of subsequent meta-analyses,6 suggest that circulating cTnI and cTnT concentrations are above the URL in >50% of athletes after endurance activities. In addition, evidence suggests that running events may be more likely to cause cTn elevation than cycling events.6 However, direct comparisons are not available. The mechanism is also unclear but may relate to the higher intensity of running versus cycling. Additional studies and further meta-analyses have replicated these initial findings, in general, and have also documented postexercise cTn elevations in a variety of populations including children, adolescents, postmenopausal women, and athletic animals.5 cTn elevation has also been associated with numerous exercise stimuli including endurance running, prolonged marching, basketball, high-intensity treadmill running, high-intensity cycling, and clinical exercise tests.23
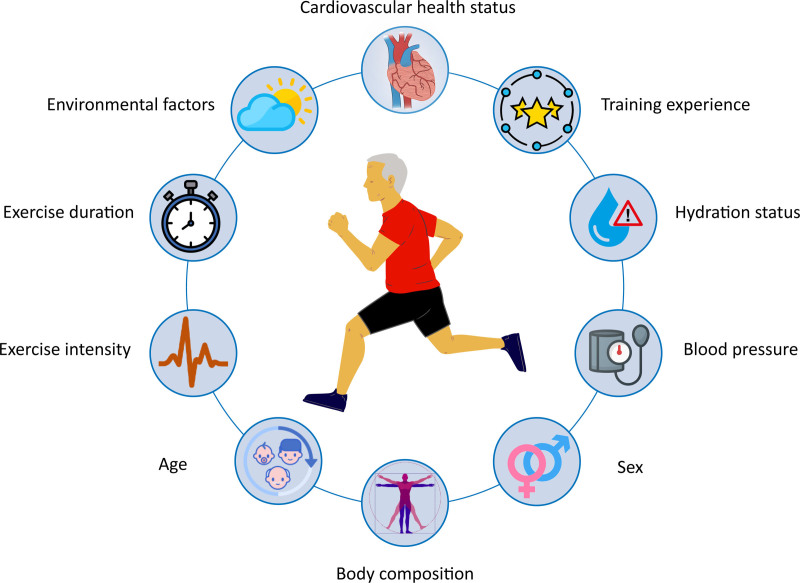
Predictors: Exercise Duration and Intensity
Numerous investigators have tried to examine which factors contribute to the release of cardiac-specific biomarkers. Age, training experience, blood pressure, environmental factors, exercise intensity, and exercise duration are among the predictors potentially associated with the magnitude of postexercise cTn concentrations14,24–26 (Figure 2). The variance explained by multivariate prediction models is low (r2<35%), however, and appears to be driven primarily by the intensity and duration of exercise. Early studies suggested that the magnitude of cTn release was positively related to the duration of exercise.24 However, a meta-analysis of 26 studies published in 2007 with exercise durations from 0.5 to 22 hours showed that postexercise cTn was inversely associated with exercise duration.6 Specifically, more athletes had a cTn concentration greater than the URL after marathon running than after substantially longer ultraendurance events. These data suggest that exercise intensity, rather than duration, may be the more potent stimulus for cTn release,27 because marathons are run at a higher intensity than ultraendurance events. In a recent study that documented a direct relationship among exercise heart rate, a surrogate for exercise intensity, and the prevalence of cTn after exercise,7 the importance of exercise intensity was also suggested.
Figure 2.
Factors driving the magnitude of exercise-induced troponin release. There is great variability in the effect of each factor on postexercise troponin concentrations across studies and all factors have limited predictive value (r2<35%). Exercise intensity and duration appear to have the largest impact on postexercise concentrations, likely reflecting overall cardiac workload.
To directly examine the effect of exercise duration and intensity, a recent study compared cTnI release following cycling at low (50%–60% lactate threshold for 60 minutes), moderate (60%–70% lactate threshold for 4 hours), and high intensities (80%–90% lactate threshold for 60 minutes).28 cTnI was elevated after both moderate- and high-intensity exercise but not after the low-intensity stimulus. Furthermore, cTn concentrations were significantly higher after the short-duration, high-intensity exercise than after the long-duration, moderate-intensity exercise.28 A similar study compared running at a moderate (60 minutes at 70% of peak heart rate) or high intensity (2 series of 12×30-second repeated sprints at 90% peak heart rate).29 cTnT was statistically higher 4 hours after the high-intensity than the moderate-intensity exercise. Also, a field study among 177 cyclists participating in a 91-km mountain bike race showed that the time spent performing high-intensity exercise (heart rate >150 bpm) was an independent predictor of postexercise cTnI and cTnT concentrations.30 In aggregate, these data suggest that postexercise cTn elevations are related to overall cardiac workload, the product of both duration and intensity.
Influence of Exercise Training
The heart remodels in response to exercise training, prompting several studies to examine the relationship of fitness or training status to postexercise cTn release.24,31 Event completion times or years of training were used as surrogates of fitness. More experienced marathon runners were less likely to have cTn elevations after the events than less experienced runners in several studies, but other studies have failed to confirm this relationship.26,32
Two recent studies have examined whether exercise training alters postexercise cTn release. One assessed cTnT concentrations at rest and after a 60-minute maximal run, before and after a 14-week training program in the intervention and control group.33 Before training, the 60-minute maximal run produced a heterogeneous cTnT response in both groups with 71% of subjects exceeding the URL. Baseline and postexercise cTnT were higher after training in the intervention group than in the control group.33 This may be because of a higher workload in the intervention group during the second maximal run as evidenced by a substantially higher speed (12.1±0.9 versus 10.7±0.9 km/h, P<0.05). The second study randomly assigned 48 young sedentary obese women to 12 weeks of high-intensity interval training, moderate-intensity continuous training, or no training and measured cTnT levels after the same absolute and relative (60% of Vo2max) exercise stimulus.34 Training significantly increased workload at 60% Vo2max. Before training, cTnT increased in all groups after exercise. After training, resting and postexercise cTnT concentrations at the same relative intensity were similar to pretraining values. However, cTnT did not increase after exercise at the same absolute intensity. These results suggest that exercise training reduces cTn increase after the same absolute, but not relative, intensity exercise. These studies collectively suggest that the magnitude of postexercise cTn release is affected by a combination of both training status and exercise intensity, because trained individuals require a greater absolute exercise stimulus to achieve the same relative stimulus.
Kinetics of cTn Concentrations
The kinetics of cTn concentrations after an acute myocardial infarction (AMI) are well described. Peak values of cTnI and cTnT occur ≈10 to 12 hours after an ST-segment–elevation AMI35 and remain elevated for 4 to 10 days, although the pattern and magnitude of cTn elevations is highly variable among patients because of both the size of the AMI and the rapidity of cTn washout influenced by reperfusion.
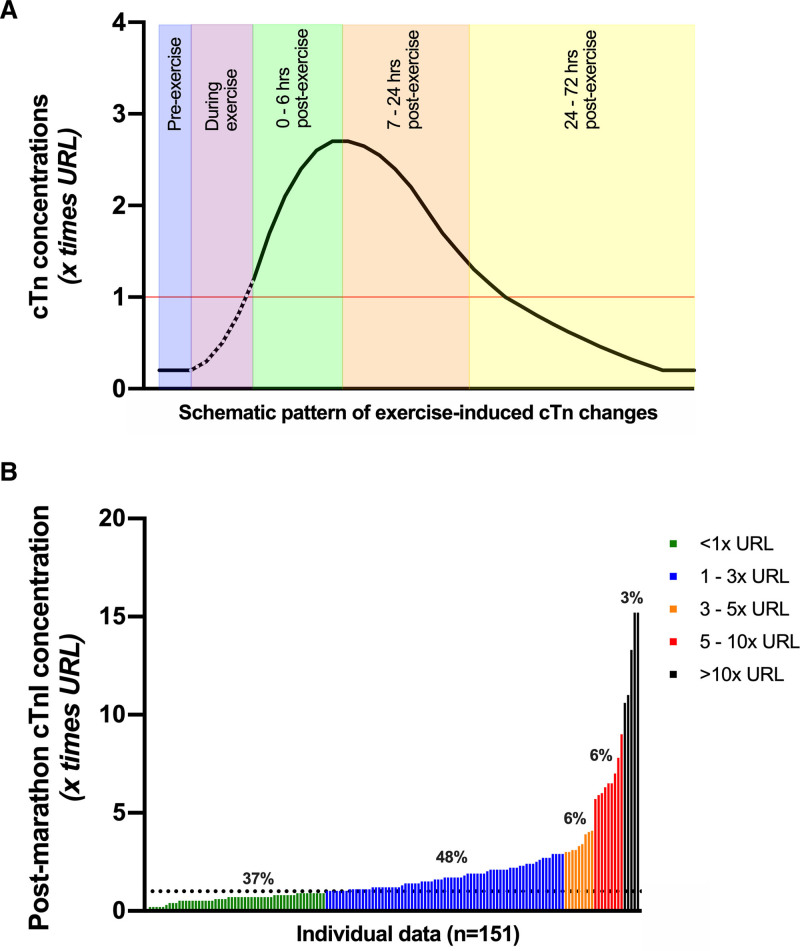
The kinetics of exercise-induced cTn concentrations are less clear. Middleton et al36 attempted to describe cTn kinetics during and after marathon exercise and reported a biphasic release pattern. Subsequently, several other studies assessed time-dependent changes in cTn concentrations up to 72 hours after exercise, of which the findings are summarized in Table S1 (cTnT) and Table S2 (cTnI). Differences in exercise duration, exercise intensity, and mode of exercise across studies exclude the possibility to perform a structured meta-analysis. Limited data are available to support or refute the potential of a biphasic release during exercise, but some important observations can nonetheless be made regarding postexercise concentrations. First, concentrations of cTn appear to progressively increase after exercise cessation with peak values typically occurring between 2 and 6 hours after exercise (Figure 3A). Second, the magnitude of cTn increase varies greatly among individuals,37 with some individuals demonstrating no or only very small changes in cTn concentrations, but others reporting values exceeding several times the URL Figure 3B. On average, peak postexercise cTn concentrations are ≈1 to 3 times the URL. The median change from baseline to postexercise concentrations was 10-fold (interquartile range: 5- to 19-fold) in marathon runners.37 Third, exercise-induced elevations in cTn concentrations are transient, with values returning to baseline after 48 to 72 hours postexercise. The early peak and smaller magnitude of exercise-related cTn elevations postexercise contrast with the greater magnitude and later peaking of cTn in AMI.
Figure 3.
Proposed pattern of exercise-induced elevations of cTn concentrations. A, Schematic illustration of the kinetics of cTn concentrations after a bout of endurance exercise. Changes of cTn concentrations during exercise are unclear (dashed line), but cumulative data show that cTn concentrations continue to rise after exercise cessation, with peak values reached between 2 and 6 hours after exercise. Complete normalization occurs within 24 to 72 hours after exercise. The 99th percentile or upper reference limit of normal (URL) is shown in red. B, Waterfall plot of individual (n=151) postmarathon cTnI concentrations, highlighting the large interindividual variation across athletes performing a similar endurance exercise bout. Data are pooled from participants of the Boston37 and Eindhoven26 marathons. Values in both plots are expressed as a multiple of the URL of the cTn assay. cTn indicates cardiac troponin.
Underlying Mechanisms
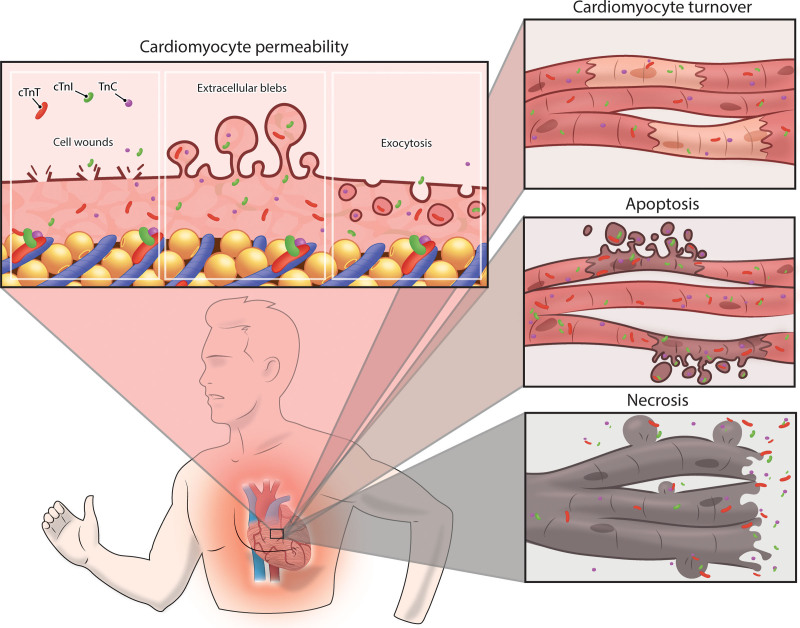
The mechanisms responsible for postexercise cTn increases remain controversial. cTn elevations were initially interpreted as irreversible damage, because the heart was considered a postmitotic organ whose cardiomyocytes could not be repaired or replaced. Hence, cTn release was considered pathognomonic of necrosis.38,39 There is increasing evidence, however, that cardiac mitosis does occur in adults at a rate of 0.5% to 1% of cardiomyocytes per year.40,41 This rate of mitosis may increase with exercise training.42,43 Transient increases in cTn occur not only after exercise, but also after atrial pacing44 and pharmacological stress testing,45 even in healthy individuals, highlighting the probability that not all cTn release is attributable to cardiomyocyte necrosis. The European Society of Cardiology’s Study Group on Biomarkers identified 3 possible causes for elevated cTn concentrations46: (1) reversible injury attributable to cell wounds, cytoplasmatic blebbing, or extracellular vesicle release; (2) injury attributable to apoptosis; and (3) irreversible injury attributable to myocardial necrosis (Figure 4). There are few direct data to support or reject a single release mechanism for exercise-induced elevations of cTn concentrations, but available evidence is presented in the following.
Figure 4.
A schematic overview of the potential underlying mechanisms of exercise-induced cTn release. An increased cardiomyocyte sarcolemmal permeability attributable to cell wounds, release of extracellular blebs, and increased exocytosis rates can be considered as reversible cardiac damage, resulting in a physiological increase of cardiac troponin concentrations. Similarly, an increased cardiomyocyte turnover may transiently increase cardiac troponin concentrations. A higher rate of apoptosis and especially necrosis should be classified as (micro)damage to the cardiomyocyte, representing a pathological response to exercise, which may have long-term health consequences. cTn indicates cardiac troponin.
Reversible Cardiac Injury
Macromolecules can exchange over the plasma membranes of viable cardiomyocytes and such release seems to occur through transient disruptions in the plasma membrane.47 Stressing the cardiomyocytes by contraction, β-adrenergic stimulation,47,48 stretching,49,50 or brief ischemia51,52 increases the rate of macromolecule release. These studies did not observe cardiomyocyte death on histological examination, but apoptosis could have occurred. In contrast, plasma membrane injury does not necessarily lead to cardiomyocyte death because (1) the cytoplasm is a macromolecular gel with restricted diffusion,53 (2) dystrophin complexes stabilize the membrane by forming links between the extracellular matrix and the contracting sarcomere,54 and (3) cell wound repair can restore small membrane holes after reoxygenation.48,54 Cardiomyocytes are therefore more resilient than previously thought.
The heart supplies most of the increased total body oxygen demand of exercise by increasing heart rate and stroke volume, which, in turn, increases myocardial oxygen demand, coronary blood flow, and cardiac preload and afterload. These responses increase cardiomyocyte stress and may alter membrane permeability, leading to passive diffusion of cTn from the cell to the extracellular space. This hypothesis has been examined in an explorative pilot study (n=11) using cardiac MRI of myocardial tissue water diffusivity (MD).8 MD is a quantitative measure of cardiomyocyte integrity and an increase in MD is indicative of increased cell membrane permeability. Marathon running increased cTnI concentrations and myocardial MD, thus demonstrating increased cell membrane permeability. Postmarathon cTnI values correlated directly with MD (r=0.66, P=0.03),8 suggesting that higher postexercise cTnI concentrations result, at least, in part, from greater cardiomyocyte membrane permeability. Both cTnI and MD returned to prerace values within 2 weeks after the marathon, indicating that these exercise-induced changes were transient.
The increase in membrane permeability after cardiomyocyte stress suggests that cTn molecules can leak from cardiomyocytes into the circulation, and this may be aided by degradation of cTn complexes. Ischemia is known to degrade cTn complexes. For example, ischemia reduces the size of cTnI and cTnT fragments in isolated rat hearts from 24 to 15 kDa and from 35 to 25 kDa, respectively,55 making them more readily able to pass through the membrane. Only small, degraded, cTnT fragments (<18 kDa) were found in postrace serum samples obtained from 10 marathon runners.9 These findings suggest that smaller fragments may leak into the circulation with the cardiac stress associated with exercise or ischemia, whereas larger fragments might only escape with destruction of the membrane after myocardial infarction.
Taken together, it is possible that exercise-induced cTn elevations are attributable, at least, in part, to reversible membrane damage of viable cardiomyocytes (Table 1). Whether this is the only mechanism responsible for exercise-induced cTn elevations, or occurs next to apoptosis or necrosis, is unknown. The magnitude of cTn release across individuals is extremely variable, even after the same exercise.37 It is possible that several mechanisms contribute to this variability and that the dominant mechanism differs between individuals with low and high magnitudes of postexercise cTn elevations (Figure 3B). Furthermore, it is unknown whether the putative changes in membrane permeability are entirely physiological or are an early marker of cardiac vulnerability and subsequent cardiac events.
Table 1.
Summary of Available Evidence for the Underlying Mechanisms of Exercise-Induced Elevations of cTn Concentrations
Apoptosis
Apoptosis or programmed cell death is part of normal cell turnover. Apoptotic processes can be activated through stress caused by oxidative overload, ischemia, and processes in other cells such as the detection of intracellular pathogens. Apoptosis should not produce cTn elevations, because intracellular content is not released when the apoptotic cell is fragmented and engulfed by other cells. However, cTn could be released during the destruction of apoptotic bodies or as cardiomyocyte apoptosis transitions to secondary necrosis.20
Few studies have explored the effects of exercise on apoptosis. Twelve weeks of exercise training reduced age-induced apoptosis in the left ventricle of rats, measured by less DNA fragmentation, terminal deoxynucleotidyl transferase dUTP nick end labeling–positive staining, and caspase-3 cleavage.56 A subsequent study demonstrated that exercise reduces the age-related increase in apoptotic signaling markers.57 A large study (n=64)58 of young (6-month-old) and middle-aged (12-month-old) mice randomly assigned the animals to cages with or without a functioning running wheel. Caspase-independent, Fas-dependent, and mitochondrial-dependent apoptotic pathways were reduced in both the young and middle-aged running mice.58 These findings agree with evidence in humans that exercise training is cardioprotective and helps preserve cardiac function during aging.
The acute effects of exercise on apoptosis are not well studied. A study (n=18) of young mice (2-month-old) assessed apoptosis at baseline or immediately after 8, 24, 48, and 72 hours of running at 60% to 70% of Vo2peak (n=3 animals per time point).59 Exercise produced a transient 150% increase in the rate of myocardial apoptosis at 24 hours after exercise, in part, because of catecholaminergic, but not oxidative, stress. These findings suggest that, in animals, exercise acutely increases the rate of apoptosis, whereas apoptotic rates are reduced with chronic exercise training.
The acute increase in left ventricular preload during exercise could contribute to increased apoptosis.60 Isolated rat hearts exposed to increased preload demonstrate intramyocardial cTnI proteolysis and cTnI release in the absence of ischemia.61 This cTnI degradation was blocked by antibodies that prevent the activation of endogenous calpains. Calpains are involved in cell signaling and cell cycle progression. Therefore, cellular calcium entry and proteolysis of cTnI may produce stretch-induced cardiomyocyte apoptosis.62 These findings have been confirmed in an in vivo swine model. Acute hemodynamic overload produced by phenylephrine infusion provoked transient left ventricular (LV) dysfunction, stretch-induced cardiomyocyte injury, elevated cTnI concentrations, and apoptosis in the absence of ischemia.63 Another study in swine evaluated changes in cTnI and apoptosis after 10 minutes of left anterior descending coronary artery occlusion with subsequent reperfusion for 24 hours.64 Brief ischemia produced a delayed cTnI release, with significant cTnI elevations starting 30 minutes after reperfusion. The cTnI elevations persisted for 24 hours. There was a concomitant, transient increase in apoptosis, with a 6-fold increase 1 hour after reperfusion, which normalized at 24 hours.64 In humans, 30, 60, and 90 seconds of balloon-induced coronary artery occlusion to induce ischemia increased cTn concentrations in patients without coronary artery disease (CAD), which continued to increase up to the end of sampling, 4 hours after ischemia.65 After 90 seconds of ischemia, patients had larger and quicker increases in cTn. cTnI and cTnT were >URL 3 hours after brief ischemia in 11% to 25% and 75% of patients, respectively.65 These findings indicate that isolated apoptosis can occur after increased preload or brief ischemia, conditions that may also occur during exercise.
An alternative explanation to increased cTn levels with exercise is that exercise increases cardiomyocyte turnover. C/EBPβ is a member of the bHLH gene family of DNA-binding transcription factors and decreases cardiomyocyte growth and proliferation.42 Exercise training decreased the expression of C/EBPβ in mice with swim training. Furthermore, the mice with reduced cardiac C/EBPβ levels were resistant to cardiac failure produced by pressure overload. These results indicate that exercise training decreases C/EBPβ, thereby decreasing its inhibition of cardiomyocyte turnover and increasing cardiac resilience to external stress. This hypothesis is supported by the observation that 8 weeks of running increased new cardiomyocytes 4.6-fold in adult mice, without evidence of systolic dysfunction or increased apoptosis.66 Both studies demonstrate that exercise activates the endogenous regenerative capacity of the mammalian heart, suggesting that replaced cardiomyocytes could release cTn into the circulation if this process is accelerated by an acute bout of exercise.
The association between exercise and apoptosis appears dependent on the time frame. Exercise training reduces apoptotic rates56–58 and increases cardiomyocyte growth and proliferation.42,66 In contrast, (supra)physiological challenges to untrained animals, such as forced running,59 volume overload,61,63 or ischemia,64 increase apoptosis with associated increases in cTn concentrations (Table 1). Whether these findings can be extrapolated to humans is not presently clear.
Cardiomyocyte Necrosis
Myocardial necrosis is the most frequent cause of cTn elevations unrelated to exercise. Cardiomyocyte metabolism shifts from aerobic to anaerobic pathways to produce ATP during myocardial ischemia. This shift to anaerobic metabolism eventually disrupts the sarcolemma. Ischemia >15 minutes irreversibly damages the cardiomyocyte,1 allowing intracellular proteins to enter the circulation. There is an old hypothesis67 that, after AMI, there is first an immediate and substantial release of cTn from an early releasable pool, followed by a smaller peak of cTn caused by the slower process of degrading myofibrils.1 Although this hypothesis has been disputed,12 the exact mechanism of cTn release remains to be unraveled. As discussed earlier, the cTn release after exercise is smaller, appears to peak sooner, and resolves faster than that observed with AMI. These differences make it unlikely that necrosis causes exercise-induced cTn elevations but does not exclude the possibility that a small degree of necrosis could produce elevated cTn concentrations in vulnerable individuals after exercise.
Acute cardiac necrosis cannot be definitely determined in vivo. Cardiac MRI studies of participants in the Manitoba,68 London,69 and Detroit70 marathons found no myocardial edema or late gadolinium enhancement despite increased cTn concentrations after exercise. The absence of myocardial edema or scar argue against cardiac necrosis, but cardiac MR is not sensitive enough to detect a small degree of necrosis. Only 40 mg of rat myocardial necrosis increases cTnT and cTnI >URL,71 but this would not be detected by cardiac MRI. It could be speculated that long-term exercise training could produce myocardial damage from repetitive single exercise sessions. This hypothesis is supported by the observation that lifelong endurance athletes have more late gadolinium enhancement (LGE) than their physically inactive peers and that the amount of LGE increases with the number of race completions and years of training.72 Thus, although no direct evidence exists of myocardial necrosis after exercise, it cannot be excluded as contributing to exercise-induced cTn increases in some cases (Table 1).
Noncardiac Explanations
Several alternative hypotheses have been suggested to contribute to exercise-induced elevations of cTn concentrations (Table 1). First, exercise-induced hemoconcentration may impact postexercise cTn concentrations, but the percentage change of fluid balance markers is (very) small relative to the increases in cTn concentrations. Also, any hemoconcentration is expected to be quickly restored with postexercise rehydration, which is contradictory to the progressive increase in cTn concentrations up to 2 to 6 hours after exercise. Evidence also suggests that hemodilution may occur after endurance exercise,73 so the role of hemoconcentration in elevated cTn concentrations after exercise is likely limited if not negligible.
Second, prolonged exercise and dehydration are associated with a compromised kidney function, but exercise-induced increases in cTn concentrations far exceed the modest reduction in renal function observed immediately after exertion. Cystatin C increased 21% to 25% immediately after a marathon run, indicating a similar relative decrease in renal function.74 This reduction in renal function may reduce renal cTn clearance and contribute to increased cTn concentrations, but cTn concentrations increase a median 1000% postmarathon,37 whereas renal function quickly recovers (<24 hours),74 demonstrating that the contribution of attenuated renal function to the magnitude of exercise-induced cTn elevations is limited.
Third, increases in cTn were hypothesized to be attributable to the cross-reactivity of the assay with skeletal Tn or skeletal muscle damage with cTn release. Cross-reactivity of cTn assays with skeletal Tn has been reported for cTnT75 and for certain assays of cTnI.76 For cTnT, it was estimated that cross-reactivity is limited to 0.003% (package insert Roche Diagnostics [2017-03, V9.0 English]) to 0.02%75 and for cTnI, 0.04% to 0.44%.76 Nevertheless, in a clinical setting of rhabdomyolysis, no association between CK and cTnT or cTnT was reported.77 In patients with neuromuscular diseases, end-stage renal disease, and even in healthy human skeletal muscle samples, cTnT but not cTnI was detected.78 Similarly, blood cTnT concentrations were often >URL in patients with skeletal myopathies, whereas cTnI was rarely elevated.79 Thus, skeletal muscle injury could conceivably contribute to exercise-induced cTnT increases but likely could not contribute to cTnI increases. We are also unaware of data showing increases in cTn, either T or I in muscle samples from exercise-trained subjects.
Clinical Relevance
Exercise-induced increases in cTn have traditionally been interpreted as the only benign form of cTn release, because these elevations are mild, occur often, in apparently healthy individuals, and are not related to cardiac symptoms. However, cTn concentrations taken at rest in large populations and clinical studies predict mortality and cardiovascular morbidity,2,4 even within the normal range.3 The prognostic value of exercise-induced increases in cTn has rarely been studied80,81 but may have clinical relevance in some populations as discussed later on in this article.
Postexercise cTn and Cardiac Function
A number of studies have examined the association between postexercise reductions in cardiac function and cTn concentrations.7 Reductions in cardiac function after exercise are typically mild and transient and occur mostly after prolonged endurance events such as marathons, triathlons, and ultraraces. A meta-analysis using echocardiography found reductions in LV ejection fraction and diastolic function after such races.7 Postexercise cTn concentrations correlated directly with reductions in diastolic function measured as E/A ratio, but no association between cTn and change in LV ejection fraction was found, because only a few studies reported a significant association and not with the standard echocardiographic parameters. This is probably because of the limited number of studies (4/22) investigating the association between cTn and LV ejection fraction.7
Exercise appears to affect right ventricular (RV) more than LV function, possibly because the relative increase in RV wall stress with exercise is greater than in the LV.82 Eight studies measured RV function and exercise-induced cTn concentrations (Table S3). Only 4 of 8 studies reported a significant reduction in RV systolic function. Of those 4 studies, only 2 reported associations between RV systolic function and postexercise cTn concentrations, which found an association between exercise-induced cTn and the reduction in RV ejection fraction (r=0.49, P=0.00283), and RV basal (r=0.68), mid (r=0.70), and apical (r=0.72) strain (P<0.001 for all).25 The only 2 studies reporting postexercise reductions in RV function and associations with postexercise cTn found a direct correlation, but the absence of an association in the other studies may not have been reported. Potential explanations for discrepant outcomes between studies likely relate to the selection of endurance races, the timing of blood drawings, and the inclusion of less sensitive measures of systolic function (strain analyses were more likely to reveal reductions in cardiac function25 because both studies that used RV strain found reductions in RV function).
Overall, some evidence suggests that postexercise cTn concentrations are associated with decreased LV diastolic function and possibly with LV and RV systolic function, but this was observed in only a few studies, and the strength of the association was moderate. An important caveat of available evidence is that studies have only examined associations between cTn concentrations and cardiac function using a single postexercise assessment, most often acquired immediately after exercise cessation. Because cTn kinetics appear to show a delayed peak after exercise, a single postexercise cTn may obscure the true association between cTn and cardiac function.
Is Exercise-Induced cTn an Indicator of Subclinical Disease?
How and why exercise-induced cTn increases occur in ostensibly healthy people is unclear, but increases in cTn may reflect subclinical myocardial vulnerability. Epidemiological and clinical studies demonstrate that individuals with cardiovascular risk factors (CVRFs) and diseases (CVDs) have higher resting cTn concentrations11,84,85 than their healthy counterparts. Similarly, after short and prolonged exercise, individuals with CVD and CVRF showed larger cTn increases than their healthy peers.11,84 For example, patients with heart failure have higher baseline, exercise-induced cTnT concentrations than healthy controls after a short-graded bicycle exercise test.84 Among 725 long-distance walkers with an average age of 61 years, resting cTnI concentrations were higher (P<0.001), but the proportion of concentrations >URL was similar (P=0.86), in individuals with CVD (n=104, 7 [2–15] ng/L, 1.0% >URL), CVRF (n=186, 3 [0–9] ng/L, 1.7%), and healthy controls (n=435, 1 [0–5] ng/L, 1.2%). After 30 to 55 km of walking, cTnI concentrations increased in all groups (P<0.001), but patients with CVD more often had a postexercise cTn concentration >URL (16%) compared with individuals with CVRF (10%) and controls (6%; P=0.003).11
cTn elevations may indicate demand ischemia, so several studies have explored the association between the magnitude of exercise-induced cTn increases and significant CAD, but the results are inconsistent.86–88 Several studies have found no increase in cTn after a short-duration (<15 minutes) clinical exercise test in individuals with CAD.89 Other studies have reported significant cTn increases after clinical exercise or dobutamine stress tests. A recent meta-analysis including studies published between 2008 and 2016 found only minor increases in cTn concentrations after clinical exercise stress tests, with no difference in exercise-induced elevations of cTn concentrations between patients with inducible and noninducible ischemia (cTnT: 0.5 [0–0.9] ng/L versus 1.1 [0–2.2] ng/L, P=0.29; cTnI: 2.4 [0.2–4.7] ng/L versus 1.8 [0.6–3.0] ng/L, P=0.61).90 Similar findings were reported for pharmacological stress testing.90 Overall, these findings may be attributable to (1) exercise intensity or duration at the ischemia threshold being insufficient to produce cTn elevations sufficient to discriminate between those with and without severe CAD or myocardial ischemia; (2) cTn concentrations being measured too early after exercise and missing the cTn peak (Figure 3A); or (3) the lack of an association between myocardial ischemia and exercise-induced cTn elevations.
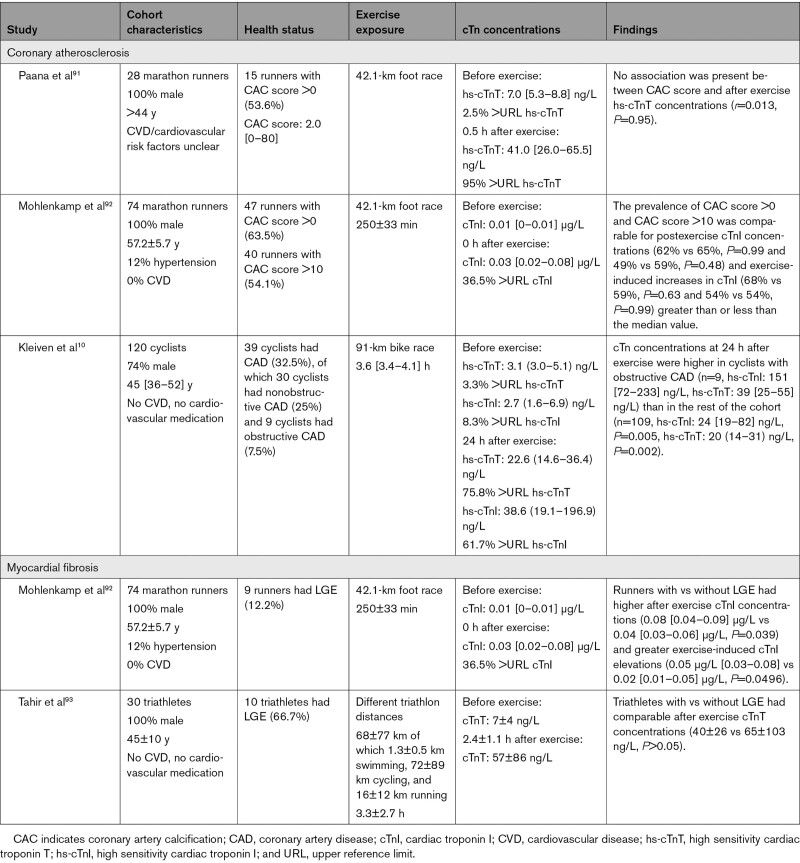
Observations among long-distance runners and cyclists largely confirm the findings from clinical studies with the majority of studies showing no association between cTn concentrations and CAD (Table 2). For example, no relation was found between postexercise cTnT concentrations and coronary artery calcification scores (r=–0.013, P=0.95) in 27 participants of the Paavo Nurmi marathon.91 Also, postexercise cTnI concentrations were not different between marathon runners with coronary artery calcification scores greater than or less than the median score (P>0.99).92 The North Sea Race Endurance Exercise Study (n=120) also found no relation between cTn concentrations 3 hours after a 91-km mountain bicycle race, although cyclists with occult obstructive CAD (n=9) had significantly higher cTnI and cTnT concentrations 24 hours after exercise than controls.10 The delayed cTn release in individuals with obstructive CAD may relate to impaired blood flow through the obstructed coronary arteries as is also seen with AMI. Future studies that evaluate whether postexercise cTn is a marker for occult CAD should include multiple time points of assessment.
Table 2.
Correlates Between Coronary Atherosclerosis, Myocardial Fibrosis, and Exercise-Induced cTn Release
Myocardial fibrosis has also been reported in ostensibly healthy endurance athletes. Only 2 studies to our knowledge have investigated the association between postexercise cTn concentrations and the presence of LGE (Table 2). German marathon runners with LGE (n=9) had higher cTnI concentrations immediately postmarathon than those without LGE (n=65).92 In contrast, triathletes with (n=10, 49±8 years) and without LGE (n=20, 42±10 years), showed no difference in postexercise cTnT concentrations collected at 2.4±1.1 hours posttriathlon (40±26 versus 65±103 ng/L).93 These 2 studies included only 19 individuals with LGE, so it is impossible to determine whether a relation exists.
In summary, CVRF and CVD are associated with higher resting and postexercise cTn concentrations. Most exercise studies have found no association between postexercise cTn elevations and CAD severity or myocardial fibrosis, but few studies have been performed and they used different exercise intensities, durations, and blood sampling protocols.
Prognostic Value in Patients
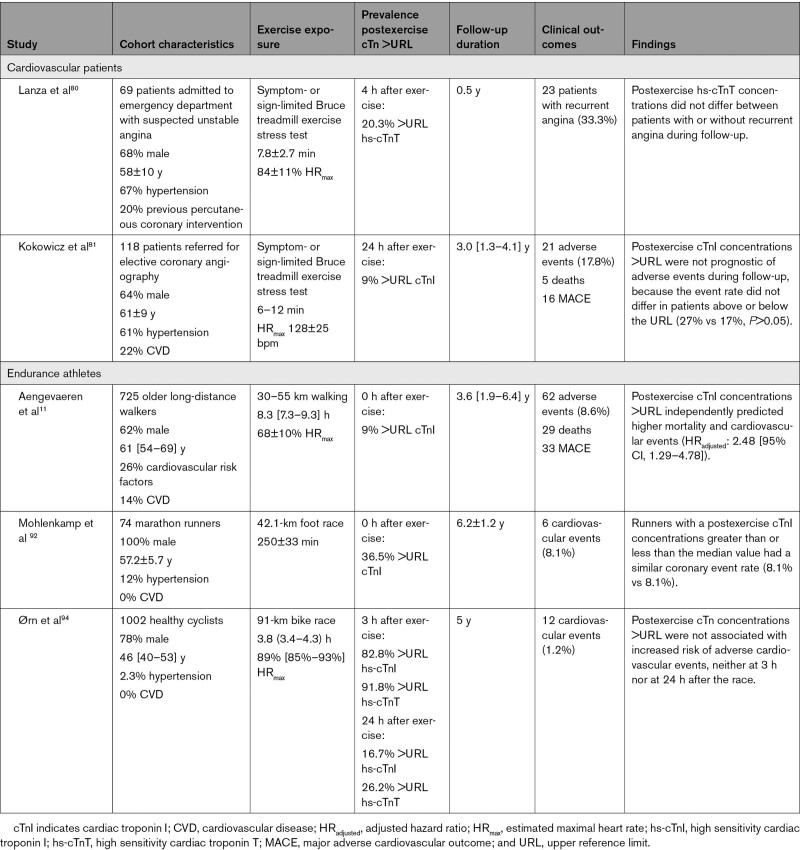
We are aware of only 2 exercise stress test studies evaluating the prognostic value of exercise-induced cTn concentrations80,81 (Table 3). Neither found a predictive relationship. There was no difference in 4 hours postexercise cTnT concentrations between patients with unstable angina who did (n=23) or did not (n=46) develop recurrent angina at 6-month follow-up.80 Another study reported no difference in the incidence of a composite end point (death, myocardial infarction, acute revascularization, hospitalization for unstable angina, or heart failure) in patients with CAD with 8 to 12 and 24-hour postexercise cTnI concentrations greater than versus less than the URL (27% versus 17%, P>0.05) during 36 (15–49) months of follow up.81 The absence of an association of exercise cTn with subsequent symptoms may be attributable to the short duration of exercise, sample size, sampling times, only a small number of clinical events, or the absence of a clinically important relationship.
Table 3.
Overview of Studies Assessing the Prognostic Value of Exercise-Induced cTn Release
Prognostic Value in Exercising Individuals
In contrast with a paucity of exercise/cTn studies in the clinical setting, a plethora of studies exist that follow endurance exercise events, but to our knowledge only 3 studies have assessed the prognostic value of these cTn elevations (Table 3). cTnI concentrations increased from baseline to postexercise in 74 male marathon runners (57±6 years), with 36.5% of the runners demonstrating postexercise cTnI concentrations >URL. During 6 years of follow-up, 6 CAD events occurred, evenly split between individuals with postmarathon cTnI concentrations greater than and less than the median value.92
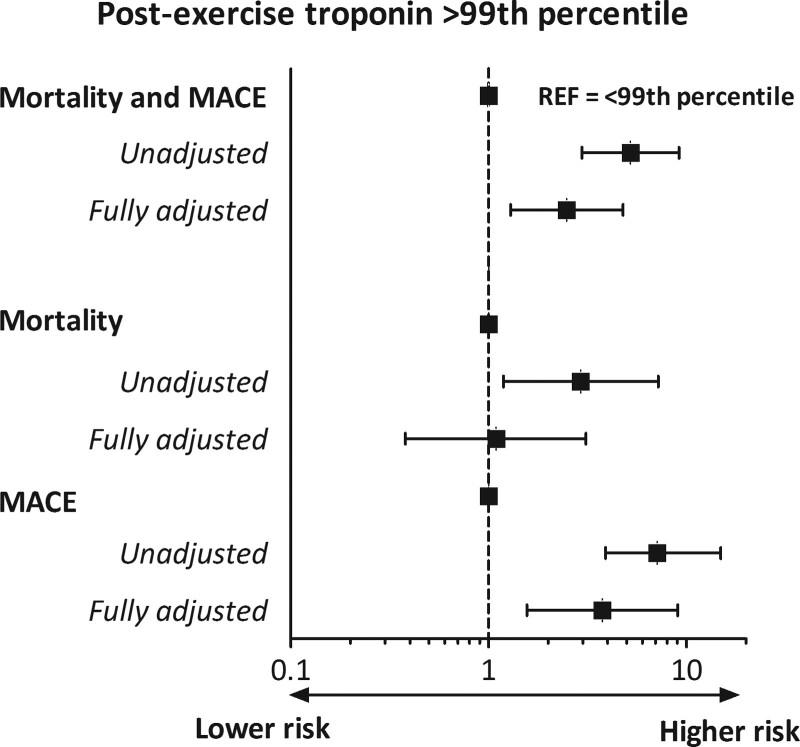
Our group previously followed 725 long-distance walkers (61.4 [54.4–69.1] years), 9% of whom had a cTnI concentration >URL ±10 minutes after walking 30 to 55 km.11 During a median follow-up of 43 (23–77) months, 62 participants experienced a composite end point of death (n=29, 47%), myocardial infarction (n=6, 10%), stroke (n=17, 27%), heart failure diagnosis (n=4, 6%), revascularization (n=5, 8%), or resuscitated sudden cardiac arrest (n=1, 2%). Of individuals with postexercise cTnI >URL, 27% experienced an end point compared with 7% of those with cTnI <URL (crude hazard ratio 5.21 [95% CI, 2.96–9.17]). After adjusting for age, sex, and the presence of CVD and CVRF, the hazard ratio was 3.21 (95% CI, 1.79–5.77). This decreased further to 2.48 (95% CI, 1.29–4.78) after adjusting for baseline cTnI. When separating mortality from other end points (Figure 5), mortality was not significantly associated with cTnI increases (fully adjusted hazard ratio, 1.09 [0.38–3.10]), whereas major adverse cardiovascular outcomes were strongly associated (fully adjusted hazard ratio, 3.75 [1.56–9.02]). This could be attributable to the lower statistical power of the independent outcomes or to examining all-cause and not cardiovascular mortality. The study cohort was not an athletic population but rather physically active individuals from the general population including older individuals with CVRF and CVD. Accordingly, these data are not applicable to younger cohorts of athletes with cTn values >URL, even when markedly elevated.
Figure 5.
Hazard ratios of postexercise troponin I > upper reference limit of normal for mortality and MACEs, and mortality and MACE separately, based on data from Aengevaeren et al.11 Troponin I was measured ≈10 minutes after 30 to 55 km of walking in 725 older long-distance walkers who experienced 62 events, 29 deaths, and 33 MACEs, during 43 [23–77] months follow-up. MACE indicates major adverse cardiovascular event.
Preliminary data examined postexercise cTn concentrations in 991 healthy participants (46 [40–53] years) in the North Sea Race 91-km bike race.94 Participants were followed for 5 years, and 12 (1.2%) experienced a cardiovascular event during follow-up. The prevalence of cTn >URL for cTnI and cTnT at 3 and 24 hours after exercise were 83% and 92%, and 17% and 27%, respectively. Postexercise cTn concentrations >URL were not associated with cardiovascular events at 3 (log-rank test, cTnI: P=0.11, cTnT: P=0.35) or 24 hours (cTnI: P=0.45, cTnT: P=0.06).94 The low event rate may have contributed to the absence of an association, but the near-significant result for cTnT at 24 hours is noteworthy.
Together, these 3 studies suggest that exaggerated postexercise cTn elevations may not be benign and may portend cardiovascular events in older individuals. It is unknown whether these findings can be extrapolated to younger subjects and to athletes performing vigorous activities.
Clinical Management and Considerations
Exercise-induced cTn elevations can lead to clinical confusion in the emergency department when individuals present postexercise with elevated cTn concentrations. Several clinical approaches have been suggested5 and remain appropriate. Clinicians should follow usual clinical protocols but be especially cognizant that exercise can increase cTn concentrations far above the URL and thus can explain cTn elevations after exercise. Patients presenting postexercise with any clinical concern for an acute coronary syndrome (with elevated cTn or not) should undergo the appropriate evaluation including 12-lead ECG, serial cTn testing, and some form of either noninvasive or invasive risk stratification as dictated by the overall clinical picture. However, assessment of CAD (eg, with coronary artery calcification scoring) is not indicated solely on the basis of lone postexercise cTn elevations.
Clinicians who oversee mass sporting events are not recommended to do on-site cTn testing outside of a clear research agenda unless future research supports its added value. If postexercise cTn elevations are found, the clinical significance is unclear, although some studies suggest that postexercise cTn elevations may portend future cardiac events in a small number of individuals.
Future Directions
Many studies have used 1 phlebotomy time point after exercise, and this time point often varies among studies, whereas only a few studies have investigated cTn release during exercise. Future studies should use similar time points for similar outcome measures. It appears that peak cTn values are achieved at 2 to 6 hours after a bout of endurance exercise. More research is needed to determine whether a specific postexercise time point may be predictive of future mortality and cardiovascular outcome.
There is evidence that both physiological and reversible, and pathological and irreversible myocardial injury, as well, might contribute to the exercise-induced cTn response, and this may be mediated by the population being studied. Mechanisms may also differ between populations because exercise-induced cTn release may be more likely related to reversible myocardial injury in healthy individuals, whereas irreversible myocardial injury might be more common in individuals with underlying CVD. Cellular and animal models, novel imaging techniques, and novel biomarker assays are needed to examine these possibilities. For example, a study using diffusion-weighted MRI could assess whether the larger exercise-induced elevations in cTn concentrations in individuals with CVRF are attributable to larger decreases in cardiomyocyte integrity. Assessing the types and sizes of cTn fragments and the appearance of apoptotic biomarkers would contribute to this analysis. Future work examining exercise-induced cTn release in healthy participants, and those with underlying cardiovascular disease, should examine the influence of coronary blood flow, because the differential response between these populations may be explained by the degree of coronary occlusion or vessel reactivity and the corollary impact on the washout of cTn stimulated by exercise.
Large prospective studies in clinical and recreational athletic populations with prolonged follow-up are needed to determine whether exercise-induced cTn predicts future cardiovascular events. Subsequent studies can then determine if altering the cTn response by exercise training or pharmacological treatment (eg, statins or aspirin) can alter cardiovascular outcomes.
Conclusions
Exercise of different types, durations, and intensities commonly increases cTn. cTn transiently increases after the performance of endurance exercise with peak values typically 2 to 6 hours after exercise. The underlying mechanisms are not clearly defined, but evidence supports the hypothesis that sarcolemmal permeability from reversible cardiac injury permits cTn fragments from an early releasable pool to leak from the cardiomyocyte. Evidence also suggests increased apoptosis or accelerated cardiomyocyte turnover attributable to myocardial stress or brief ischemia. Few studies have investigated the predictive value of exercise-induced cTn for cardiovascular events, but older long-distance walkers with a postexercise cTn concentration >URL experienced increased cardiovascular events. These findings need to be confirmed and the prognostic significance of cTn in younger athletic subjects needs to be determined.
Article Information
Sources of Funding
Drs Aengevaeren and Eijsvogels are financially supported by grants from the Dutch Heart Foundation (#2017T088 and #2017T051, respectively). Dr Mingels received funding from the Dutch government, Nederlandse organisatie voor gezondheidsonderzoek en zorginnovatie/Nederlandse Organisatie voor Wetenschappelijk Onderzoek (09150161810155).
Disclosures
Dr Mingels has received nonfinancial support from Abbott Diagnostics and Roche Diagnostics. The sponsors had no role in the design of the study, the analysis of the data, the preparation of the article, or the decision to submit the article for publication. The other authors reported no disclosures.
Supplemental Material
Tables S1–S3
References 95–127
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AMI
- acute myocardial infarction
- CAD
- coronary artery disease
- cTn
- cardiac troponin
- CVD
- cardiovascular disease
- CVRF
- cardiovascular risk factor
- LGE
- late gadolinium enhancement
- LV
- left ventricular
- MD
- mean diffusivity
- MRI
- magnetic resonance imaging
- RV
- right ventricular
- URL
- upper reference limit
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.121.056208.
For Sources of Funding and Disclosures, see page 1968.
Contributor Information
Vincent L. Aengevaeren, Email: vincent.aengevaeren@radboudumc.nl.
Aaron L. Baggish, Email: abaggish@partners.org.
Eugene H. Chung, Email: chungeug@umich.edu.
Keith George, Email: k.george@ljmu.ac.uk.
Øyunn Kleiven, Email: oyunn.kleiven@gmail.com.
Alma M.A. Mingels, Email: alma.mingels@mumc.nl.
Stein Ørn, Email: drsteinorn@hotmail.com.
Rob E. Shave, Email: rshave@mail.ubc.ca.
Paul D. Thompson, Email: paul.thompson@hhchealth.org.
References
- 1.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Circulation. 2018; 138:e618–e651. doi: 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 2.Eggers KM, Jernberg T, Lindahl B. Cardiac troponin elevation in patients without a specific diagnosis. J Am Coll Cardiol. 2019; 73:1–9. doi: 10.1016/j.jacc.2018.09.082 [DOI] [PubMed] [Google Scholar]
- 3.Roos A, Bandstein N, Lundbäck M, Hammarsten O, Ljung R, Holzmann MJ. Stable high-sensitivity cardiac troponin T levels and outcomes in patients with chest pain. J Am Coll Cardiol. 2017; 70:2226–2236. doi: 10.1016/j.jacc.2017.08.064 [DOI] [PubMed] [Google Scholar]
- 4.Sze J, Mooney J, Barzi F, Hillis GS, Chow CK. Cardiac troponin and its relationship to cardiovascular outcomes in community populations: a systematic review and meta-analysis. Heart Lung Circ. 2016; 25:217–228. doi: 10.1016/j.hlc.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 5.Shave R, Baggish A, George K, Wood M, Scharhag J, Whyte G, Gaze D, Thompson PD. Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol. 2010; 56:169–176. doi: 10.1016/j.jacc.2010.03.037 [DOI] [PubMed] [Google Scholar]
- 6.Shave R, George KP, Atkinson G, Hart E, Middleton N, Whyte G, Gaze D, Collinson PO. Exercise-induced cardiac troponin T release: a meta-analysis. Med Sci Sports Exerc. 2007; 39:2099–2106. doi: 10.1249/mss.0b013e318153ff78 [DOI] [PubMed] [Google Scholar]
- 7.Donaldson JA, Wiles JD, Coleman DA, Papadakis M, Sharma R, O’Driscoll JM. Left ventricular function and cardiac biomarker release-the influence of exercise intensity, duration and mode: a systematic review and meta-analysis. Sports Med. 2019; 49:1275–1289. doi: 10.1007/s40279-019-01142-5 [DOI] [PubMed] [Google Scholar]
- 8.Aengevaeren VL, Froeling M, Hooijmans MT, Monte JR, van den Berg-Faay S, Hopman MTE, Strijkers GJ, Nederveen AJ, Bakermans AJ, Eijsvogels TMH. Myocardial injury and compromised cardiomyocyte integrity following a marathon run. JACC Cardiovasc Imaging. 2020; 13:1445–1447. doi: 10.1016/j.jcmg.2019.12.020 [DOI] [PubMed] [Google Scholar]
- 9.Vroemen WHM, Mezger STP, Masotti S, Clerico A, Bekers O, de Boer D, Mingels A. Cardiac troponin T: only small molecules in recreational runners after marathon completion. J Appl Lab Med. 2019; 3:909–911. doi: 10.1373/jalm.2018.027144 [DOI] [PubMed] [Google Scholar]
- 10.Kleiven Ø, Omland T, Skadberg Ø, Melberg TH, Bjørkavoll-Bergseth MF, Auestad B, Bergseth R, Greve OJ, Aakre KM, Ørn S. Occult obstructive coronary artery disease is associated with prolonged cardiac troponin elevation following strenuous exercise. Eur J Prev Cardiol. 2020; 27:1212–1221. doi: 10.1177/2047487319852808 [DOI] [PubMed] [Google Scholar]
- 11.Aengevaeren VL, Hopman MTE, Thompson PD, Bakker EA, George KP, Thijssen DHJ, Eijsvogels TMH. Exercise-induced cardiac troponin I increase and incident mortality and cardiovascular events. Circulation. 2019; 140:804–814. doi: 10.1161/CIRCULATIONAHA.119.041627 [DOI] [PubMed] [Google Scholar]
- 12.Starnberg K, Jeppsson A, Lindahl B, Hammarsten O. Revision of the troponin T release mechanism from damaged human myocardium. Clin Chem. 2014; 60:1098–1104. doi: 10.1373/clinchem.2013.217943 [DOI] [PubMed] [Google Scholar]
- 13.Apple FS, Collinson PO; IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem. 2012; 58:54–61. doi: 10.1373/clinchem.2011.165795 [DOI] [PubMed] [Google Scholar]
- 14.Kleiven Ø, Omland T, Skadberg Ø, Melberg TH, Bjørkavoll-Bergseth MF, Auestad B, Bergseth R, Greve OJ, Aakre KM, Ørn S. Race duration and blood pressure are major predictors of exercise-induced cardiac troponin elevation. Int J Cardiol. 2019; 283:1–8. doi: 10.1016/j.ijcard.2019.02.044 [DOI] [PubMed] [Google Scholar]
- 15.Apple FS, Sandoval Y, Jaffe AS, Ordonez-Llanos J; IFCC Task Force on Clinical Applications of Cardiac Bio-Markers. Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem. 2017; 63:73–81. doi: 10.1373/clinchem.2016.255109 [DOI] [PubMed] [Google Scholar]
- 16.Labugger R, Organ L, Collier C, Atar D, Van Eyk JE. Extensive troponin I and T modification detected in serum from patients with acute myocardial infarction. Circulation. 2000; 102:1221–1226. doi: 10.1161/01.cir.102.11.1221 [DOI] [PubMed] [Google Scholar]
- 17.Streng AS, de Boer D, van Doorn WP, Bouwman FG, Mariman EC, Bekers O, van Dieijen-Visser MP, Wodzig WK. Identification and characterization of cardiac troponin t fragments in serum of patients suffering from acute myocardial infarction. Clin Chem. 2017; 63:563–572. doi: 10.1373/clinchem.2016.261511 [DOI] [PubMed] [Google Scholar]
- 18.Cardinaels EP, Mingels AM, van Rooij T, Collinson PO, Prinzen FW, van Dieijen-Visser MP. Time-dependent degradation pattern of cardiac troponin T following myocardial infarction. Clin Chem. 2013; 59:1083–1090. doi: 10.1373/clinchem.2012.200543 [DOI] [PubMed] [Google Scholar]
- 19.Mingels AM, Cardinaels EP, Broers NJ, van Sleeuwen A, Streng AS, van Dieijen-Visser MP, Kooman JP, Bekers O. Cardiac troponin T: smaller molecules in patients with end-stage renal disease than after onset of acute myocardial infarction. Clin Chem. 2017; 63:683–690. doi: 10.1373/clinchem.2016.261644 [DOI] [PubMed] [Google Scholar]
- 20.Hammarsten O, Mair J, Möckel M, Lindahl B, Jaffe AS. Possible mechanisms behind cardiac troponin elevations. Biomarkers. 2018; 23:725–734. doi: 10.1080/1354750X.2018.1490969 [DOI] [PubMed] [Google Scholar]
- 21.Fridén V, Starnberg K, Muslimovic A, Ricksten SE, Bjurman C, Forsgard N, Wickman A, Hammarsten O. Clearance of cardiac troponin T with and without kidney function. Clin Biochem. 2017; 50:468–474. doi: 10.1016/j.clinbiochem.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 22.Siegel AJ, Silverman LM, Evans WJ. Elevated skeletal muscle creatine kinase MB isoenzyme levels in marathon runners. JAMA. 1983; 250:2835–2837 [PubMed] [Google Scholar]
- 23.Gresslien T, Agewall S. Troponin and exercise. Int J Cardiol. 2016; 221:609–621. doi: 10.1016/j.ijcard.2016.06.243 [DOI] [PubMed] [Google Scholar]
- 24.Fortescue EB, Shin AY, Greenes DS, Mannix RC, Agarwal S, Feldman BJ, Shah MI, Rifai N, Landzberg MJ, Newburger JW, et al. Cardiac troponin increases among runners in the Boston Marathon. Ann Emerg Med. 2007; 49:137–43, 143.e1. doi: 10.1016/j.annemergmed.2006.09.024 [DOI] [PubMed] [Google Scholar]
- 25.Neilan TG, Januzzi JL, Lee-Lewandrowski E, Ton-Nu TT, Yoerger DM, Jassal DS, Lewandrowski KB, Siegel AJ, Marshall JE, Douglas PS, et al. Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation. 2006; 114:2325–2333. doi: 10.1161/CIRCULATIONAHA.106.647461 [DOI] [PubMed] [Google Scholar]
- 26.Eijsvogels TM, Hoogerwerf MD, Maessen MF, Seeger JP, George KP, Hopman MT, Thijssen DH. Predictors of cardiac troponin release after a marathon. J Sci Med Sport. 2015; 18:88–92. doi: 10.1016/j.jsams.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 27.Eijsvogels TM, Hoogerwerf MD, Oudegeest-Sander MH, Hopman MT, Thijssen DH. The impact of exercise intensity on cardiac troponin I release. Int J Cardiol. 2014; 171:e3–e4. doi: 10.1016/j.ijcard.2013.11.050 [DOI] [PubMed] [Google Scholar]
- 28.Marshall L, Lee KK, Stewart SD, Wild A, Fujisawa T, Ferry AV, Stables CL, Lithgow H, Chapman AR, Anand A, et al. Effect of exercise intensity and duration on cardiac troponin release. Circulation. 2020; 141:83–85. doi: 10.1161/CIRCULATIONAHA.119.041874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weippert M, Divchev D, Schmidt P, Gettel H, Neugebauer A, Behrens K, Wolfarth B, Braumann KM, Nienaber CA. Cardiac troponin T and echocardiographic dimensions after repeated sprint vs. moderate intensity continuous exercise in healthy young males. Sci Rep. 2016; 6:24614. doi: 10.1038/srep24614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjørkavoll-Bergseth M, Kleiven Ø, Auestad B, Eftestøl T, Oskal K, Nygård M, Skadberg Ø, Aakre KM, Melberg T, Gjesdal K, et al. Duration of elevated heart rate is an important predictor of exercise-induced troponin elevation. J Am Heart Assoc. 2020; 9:e014408. doi: 10.1161/JAHA.119.014408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mingels A, Jacobs L, Michielsen E, Swaanenburg J, Wodzig W, van Dieijen-Visser M. Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin Chem. 2009; 55:101–108. doi: 10.1373/clinchem.2008.106427 [DOI] [PubMed] [Google Scholar]
- 32.Serrano-Ostáriz E, Terreros-Blanco JL, Legaz-Arrese A, George K, Shave R, Bocos-Terraz P, Izquierdo-Álvarez S, Bancalero JL, Echavarri JM, Quilez J, et al. The impact of exercise duration and intensity on the release of cardiac biomarkers. Scand J Med Sci Sports. 2011; 21:244–249. doi: 10.1111/j.1600-0838.2009.01042.x [DOI] [PubMed] [Google Scholar]
- 33.Legaz-Arrese A, López-Laval I, George K, Puente-Lanzarote JJ, Mayolas-Pi C, Serrano-Ostáriz E, Revilla-Martí P, Moliner-Urdiales D, Reverter-Masià J. Impact of an endurance training program on exercise-induced cardiac biomarker release. Am J Physiol Heart Circ Physiol. 2015; 308:H913–H920. doi: 10.1152/ajpheart.00914.2014 [DOI] [PubMed] [Google Scholar]
- 34.Nie J, Zhang H, He Y, Cao W, Liu Y, Kong Z, George K. The impact of high-intensity interval training on the cTnT response to acute exercise in sedentary obese young women. Scand J Med Sci Sports. 2019; 29:160–170. doi: 10.1111/sms.13344 [DOI] [PubMed] [Google Scholar]
- 35.Laugaudin G, Kuster N, Petiton A, Leclercq F, Gervasoni R, Macia JC, Cung TT, Dupuy AM, Solecki K, Lattuca B, et al. Kinetics of high-sensitivity cardiac troponin T and I differ in patients with ST-segment elevation myocardial infarction treated by primary coronary intervention. Eur Heart J Acute Cardiovasc Care. 2016; 5:354–363. doi: 10.1177/2048872615585518 [DOI] [PubMed] [Google Scholar]
- 36.Middleton N, George K, Whyte G, Gaze D, Collinson P, Shave R. Cardiac troponin T release is stimulated by endurance exercise in healthy humans. J Am Coll Cardiol. 2008; 52:1813–1814. doi: 10.1016/j.jacc.2008.03.069 [DOI] [PubMed] [Google Scholar]
- 37.Eijsvogels TM, Januzzi JL, Taylor BA, Isaacs SK, D’Hemecourt P, Zaleski A, Dyer S, Troyanos C, Weiner RB, Thompson PD, et al. Impact of statin use on exercise-induced cardiac troponin elevations. Am J Cardiol. 2014; 114:624–628. doi: 10.1016/j.amjcard.2014.05.047 [DOI] [PubMed] [Google Scholar]
- 38.Mair J. Tissue release of cardiac markers: from physiology to clinical applications. Clin Chem Lab Med. 1999; 37:1077–1084. doi: 10.1515/CCLM.1999.157 [DOI] [PubMed] [Google Scholar]
- 39.Takemura G, Kanoh M, Minatoguchi S, Fujiwara H. Cardiomyocyte apoptosis in the failing heart–a critical review from definition and classification of cell death. Int J Cardiol. 2013; 167:2373–2386. doi: 10.1016/j.ijcard.2013.01.163 [DOI] [PubMed] [Google Scholar]
- 40.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, et al. Dynamics of cell generation and turnover in the human heart. Cell. 2015; 161:1566–1575. doi: 10.1016/j.cell.2015.05.026 [DOI] [PubMed] [Google Scholar]
- 41.Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisén J, Giacca M, Hare JM, Houser S, Lee RT, et al. Cardiomyocyte regeneration: a consensus statement. Circulation. 2017; 136:680–686. doi: 10.1161/CIRCULATIONAHA.117.029343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010; 143:1072–1083. doi: 10.1016/j.cell.2010.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellison GM, Waring CD, Vicinanza C, Torella D. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart. 2012; 98:5–10. doi: 10.1136/heartjnl-2011-300639 [DOI] [PubMed] [Google Scholar]
- 44.Turer AT, Addo TA, Martin JL, Sabatine MS, Lewis GD, Gerszten RE, Keeley EC, Cigarroa JE, Lange RA, Hillis LD, et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol. 2011; 57:2398–2405. doi: 10.1016/j.jacc.2010.11.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J. 2009; 30:162–169. doi: 10.1093/eurheartj/ehn504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mair J, Lindahl B, Hammarsten O, Müller C, Giannitsis E, Huber K, Möckel M, Plebani M, Thygesen K, Jaffe AS. How is cardiac troponin released from injured myocardium? Eur Heart J Acute Cardiovasc Care. 2018; 7:553–560. doi: 10.1177/2048872617748553 [DOI] [PubMed] [Google Scholar]
- 47.Clarke MS, Caldwell RW, Chiao H, Miyake K, McNeil PL. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ Res. 1995; 76:927–934. doi: 10.1161/01.res.76.6.927 [DOI] [PubMed] [Google Scholar]
- 48.Boutet M, Hüttner I, Rona G. Permeability alteration of sarcolemmal membrane in catecholamine-induced cardiac muscle cell injury. In vivo studies with fine structural diffusion tracer horse radish peroxidase. Lab Invest. 1976; 34:482–488 [PubMed] [Google Scholar]
- 49.Page E, Upshaw-Earley J, Goings G. Permeability of rat atrial endocardium, epicardium, and myocardium to large molecules. Stretch-dependent effects. Circ Res. 1992; 71:159–173. doi: 10.1161/01.res.71.1.159 [DOI] [PubMed] [Google Scholar]
- 50.Swildens J, de Vries AA, Li Z, Umar S, Atsma DE, Schalij MJ, van der Laarse A. Integrin stimulation favors uptake of macromolecules by cardiomyocytes in vitro. Cell Physiol Biochem. 2010; 26:999–1010. doi: 10.1159/000324013 [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Knapton A, Lipshultz SE, Weaver JL, Herman EH. Isoproterenol-induced cardiotoxicity in Sprague Dawley rats: correlation of reversible and irreversible myocardial injury with release of cardiac troponin T and roles of iNOS in myocardial injury. Toxicol Pathol. 2008; 36:277–278. doi: 10.1177/0192623307313010 [DOI] [PubMed] [Google Scholar]
- 52.Hickman PE, Potter JM, Aroney C, Koerbin G, Southcott E, Wu AH, Roberts MS. Cardiac troponin may be released by ischemia alone, without necrosis. Clin Chim Acta. 2010; 411:318–323. doi: 10.1016/j.cca.2009.12.009 [DOI] [PubMed] [Google Scholar]
- 53.Ellis RJ. Macromolecular crowding: obvious but underappreciated. Trends Biochem Sci. 2001; 26:597–604. doi: 10.1016/s0968-0004(01)01938-7 [DOI] [PubMed] [Google Scholar]
- 54.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005; 6:499–505. doi: 10.1038/nrm1665 [DOI] [PubMed] [Google Scholar]
- 55.McDonough JL, Arrell DK, Van Eyk JE. Troponin I degradation and covalent complex formation accompanies myocardial ischemia/reperfusion injury. Circ Res. 1999; 84:9–20. doi: 10.1161/01.res.84.1.9 [DOI] [PubMed] [Google Scholar]
- 56.Kwak HB, Song W, Lawler JM. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J. 2006; 20:791–793. doi: 10.1096/fj.05-5116fje [DOI] [PubMed] [Google Scholar]
- 57.No MH, Heo JW, Yoo SZ, Kim CJ, Park DH, Kang JH, Seo DY, Han J, Kwak HB. Effects of aging and exercise training on mitochondrial function and apoptosis in the rat heart. Pflugers Arch. 2020; 472:179–193. doi: 10.1007/s00424-020-02357-6 [DOI] [PubMed] [Google Scholar]
- 58.Cui JW, Hong Y, Kuo YM, Yu SH, Wu XB, Cui ZY, Lee SD. Voluntary exercise training attenuated the middle-aged maturity-induced cardiac apoptosis. Life Sci. 2020; 259:118187. doi: 10.1016/j.lfs.2020.118187 [DOI] [PubMed] [Google Scholar]
- 59.Arisi MF, Chirico EN, Sebeny R, Muthukumaran G, Mu A, De Jonghe BC, Margulies KB, Libonati JR. Myocardial apoptosis and mesenchymal stem cells with acute exercise. Physiol Rep. 2017; 5:e13297. doi: 10.14814/phy2.13297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.La Gerche A, Gewillig M. What limits cardiac performance during exercise in normal subjects and in healthy fontan patients? Int J Pediatr. 2010; 2010:791291. doi: 10.1155/2010/791291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng J, Schaus BJ, Fallavollita JA, Lee TC, Canty JM, Jr. Preload induces troponin I degradation independently of myocardial ischemia. Circulation. 2001; 103:2035–2037. doi: 10.1161/01.cir.103.16.2035 [DOI] [PubMed] [Google Scholar]
- 62.Cheng W, Li B, Kajstura J, Li P, Wolin MS, Sonnenblick EH, Hintze TH, Olivetti G, Anversa P. Stretch-induced programmed myocyte cell death. J Clin Invest. 1995; 96:2247–2259. doi: 10.1172/JCI118280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weil BR, Suzuki G, Young RF, Iyer V, Canty JM, Jr. Troponin release and reversible left ventricular dysfunction after transient pressure overload. J Am Coll Cardiol. 2018; 71:2906–2916. doi: 10.1016/j.jacc.2018.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weil BR, Young RF, Shen X, Suzuki G, Qu J, Malhotra S, Canty JM, Jr. Brief myocardial ischemia produces cardiac troponin I release and focal myocyte apoptosis in the absence of pathological infarction in swine. JACC Basic Transl Sci. 2017; 2:105–114. doi: 10.1016/j.jacbts.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Árnadóttir Á, Pedersen S, Hasselbalch RB, Goetze JP, Friis-Hansen LJ, Bloch-Münster AM, Skov Jensen J, Bundgaard H, Iversen K. Temporal release of high-sensitivity cardiac troponin T and I and copeptin after brief induced coronary artery balloon occlusion in humans. Circulation. 2021; 143:1095–1104. doi: 10.1161/CIRCULATIONAHA.120.046574 [DOI] [PubMed] [Google Scholar]
- 66.Vujic A, Lerchenmüller C, Wu TD, Guillermier C, Rabolli CP, Gonzalez E, Senyo SE, Liu X, Guerquin-Kern JL, Steinhauser ML, et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun. 2018; 9:1659. doi: 10.1038/s41467-018-04083-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katus HA, Remppis A, Scheffold T, Diederich KW, Kuebler W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol. 1991; 67:1360–1367. doi: 10.1016/0002-9149(91)90466-x [DOI] [PubMed] [Google Scholar]
- 68.Mousavi N, Czarnecki A, Kumar K, Fallah-Rad N, Lytwyn M, Han SY, Francis A, Walker JR, Kirkpatrick ID, Neilan TG, et al. Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am J Cardiol. 2009; 103:1467–1472. doi: 10.1016/j.amjcard.2009.01.294 [DOI] [PubMed] [Google Scholar]
- 69.O’Hanlon R, Wilson M, Wage R, Smith G, Alpendurada FD, Wong J, Dahl A, Oxborough D, Godfrey R, Sharma S, et al. Troponin release following endurance exercise: is inflammation the cause? a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2010; 12:38. doi: 10.1186/1532-429X-12-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trivax JE, Franklin BA, Goldstein JA, Chinnaiyan KM, Gallagher MJ, deJong AT, Colar JM, Haines DE, McCullough PA. Acute cardiac effects of marathon running. J Appl Physiol (1985). 2010; 108:1148–1153. doi: 10.1152/japplphysiol.01151.2009 [DOI] [PubMed] [Google Scholar]
- 71.Marjot J, Kaier TE, Martin ED, Reji SS, Copeland O, Iqbal M, Goodson B, Hamren S, Harding SE, Marber MS. Quantifying the release of biomarkers of myocardial necrosis from cardiac myocytes and intact myocardium. Clin Chem. 2017; 63:990–996. doi: 10.1373/clinchem.2016.264648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van de Schoor FR, Aengevaeren VL, Hopman MT, Oxborough DL, George KP, Thompson PD, Eijsvogels TM. Myocardial fibrosis in athletes. Mayo Clin Proc. 2016; 91:1617–1631. doi: 10.1016/j.mayocp.2016.07.012 [DOI] [PubMed] [Google Scholar]
- 73.Neumayr G, Pfister R, Hoertnagl H, Mitterbauer G, Prokop W, Joannidis M. Renal function and plasma volume following ultramarathon cycling. Int J Sports Med. 2005; 26:2–8. doi: 10.1055/s-2004-815717 [DOI] [PubMed] [Google Scholar]
- 74.Wołyniec W, Ratkowski W, Renke J, Renke M. Changes in novel AKI biomarkers after exercise. A systematic review. Int J Mol Sci. 2020; 21:E5673. doi: 10.3390/ijms21165673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vroemen WHM, de Boer D, Streng AS, Mingels AMA, Meex SJR. Elevated cardiac troponin T in skeletal myopathies: skeletal TnT cross-reactivity and/or cardiac TnT expression? J Am Coll Cardiol. 2018; 72:347–349. doi: 10.1016/j.jacc.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 76.Hyytiä H, Heikkilä T, Hedberg P, Puolakanaho T, Pettersson K. Skeletal troponin I cross-reactivity in different cardiac troponin I assay versions. Clin Biochem. 2015; 48:313–317. doi: 10.1016/j.clinbiochem.2014.12.028 [DOI] [PubMed] [Google Scholar]
- 77.du Fay de Lavallaz J, Zehntner T, Puelacher C, Walter J, Strebel I, Rentsch K, Boeddinghaus J, Nestelberger T, Twerenbold R, Mueller C. Rhabdomyolysis: a noncardiac source of increased circulating concentrations of cardiac troponin T? J Am Coll Cardiol. 2018; 72:2936–2937. doi: 10.1016/j.jacc.2018.09.050 [DOI] [PubMed] [Google Scholar]
- 78.Ricchiuti V, Apple FS. RNA expression of cardiac troponin T isoforms in diseased human skeletal muscle. Clin Chem. 1999; 45:2129–2135 [PubMed] [Google Scholar]
- 79.Schmid J, Liesinger L, Birner-Gruenberger R, Stojakovic T, Scharnagl H, Dieplinger B, Asslaber M, Radl R, Beer M, Polacin M, et al. Elevated cardiac troponin T in patients with skeletal myopathies. J Am Coll Cardiol. 2018; 71:1540–1549. doi: 10.1016/j.jacc.2018.01.070 [DOI] [PubMed] [Google Scholar]
- 80.Lanza GA, Mencarelli E, Melita V, Tota A, Gabrielli M, Sarullo F, Cordischi C, Potenza A, Cardone S, De Vita A, et al. Post-exercise high-sensitivity troponin T levels in patients with suspected unstable angina. PLoS One. 2019; 14:e0222230. doi: 10.1371/journal.pone.0222230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kokowicz P, Stec S, Flasińska K, Budaj A. [Troponin release following exercise test in patients with stable angina pectoris - risk factors and prognostic significance]. Kardiol Pol. 2010; 68:414–419. discussion 420 [PubMed] [Google Scholar]
- 82.La Gerche A, Heidbüchel H, Burns AT, Mooney DJ, Taylor AJ, Pfluger HB, Inder WJ, Macisaac AI, Prior DL. Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med Sci Sports Exerc. 2011; 43:974–981. doi: 10.1249/MSS.0b013e31820607a3 [DOI] [PubMed] [Google Scholar]
- 83.La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbüchel H, Prior DL. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012; 33:998–1006. doi: 10.1093/eurheartj/ehr397 [DOI] [PubMed] [Google Scholar]
- 84.Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P, Borlaug BA. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2018; 72:29–40. doi: 10.1016/j.jacc.2018.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Januzzi JL, Jr, Suchindran S, Coles A, Ferencik M, Patel MR, Hoffmann U, Ginsburg GS, Douglas PS; PROMISE Investigators. High-sensitivity troponin I and coronary computed tomography in symptomatic outpatients with suspected CAD: insights from the PROMISE Trial. JACC Cardiovasc Imaging. 2019; 12:1047–1055. doi: 10.1016/j.jcmg.2018.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eryol NK, Başar E, Ozdoğru I, Ciçek Y, Abaci A, Oğuzhan A, Topsakal R, Cetin S. Should troponin-T be assessed during exercise stress testing in patients with stable angina pectoris? Anadolu Kardiyol Derg. 2002; 2:132–137 [PubMed] [Google Scholar]
- 87.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, Obideen M, Abdelhadi N, Fang S, Ibeanu I, Pimple P, et al. Association between high-sensitivity cardiac troponin levels and myocardial ischemia during mental stress and conventional stress. JACC Cardiovasc Imaging. 2018; 11:603–611. doi: 10.1016/j.jcmg.2016.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samaha E, Brown J, Brown F, Martinez SC, Scott M, Jaffe AS, Davila-Roman VG, Nagele P. High-sensitivity cardiac troponin T increases after stress echocardiography. Clin Biochem. 2019; 63:18–23. doi: 10.1016/j.clinbiochem.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 89.Tveit SH, Cwikiel J, Myhre PL, Omland T, Berge E, Seljeflot I, Flaa A. Differential associations of cardiac troponin T and cardiac troponin I with coronary artery pathology and dynamics in response to short-duration exercise. Clin Biochem. 2021; 88:23–29. doi: 10.1016/j.clinbiochem.2020.11.005 [DOI] [PubMed] [Google Scholar]
- 90.Samaha E, Avila A, Helwani MA, Ben Abdallah A, Jaffe AS, Scott MG, Nagele P. High-sensitivity cardiac troponin after cardiac stress test: a systematic review and meta-analysis. J Am Heart Assoc. 2019; 8:e008626. doi: 10.1161/JAHA.118.008626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paana T, Jaakkola S, Bamberg K, Saraste A, Tuunainen E, Wittfooth S, Kallio P, Heinonen OJ, Knuuti J, Pettersson K, et al. Cardiac troponin elevations in marathon runners. Role of coronary atherosclerosis and skeletal muscle injury. The MaraCat Study. Int J Cardiol. 2019; 295:25–28. doi: 10.1016/j.ijcard.2019.08.019 [DOI] [PubMed] [Google Scholar]
- 92.Möhlenkamp S, Leineweber K, Lehmann N, Braun S, Roggenbuck U, Perrey M, Broecker-Preuss M, Budde T, Halle M, Mann K, et al. Coronary atherosclerosis burden, but not transient troponin elevation, predicts long-term outcome in recreational marathon runners. Basic Res Cardiol. 2014; 109:391. doi: 10.1007/s00395-013-0391-8 [DOI] [PubMed] [Google Scholar]
- 93.Tahir E, Scherz B, Starekova J, Muellerleile K, Fischer R, Schoennagel B, Warncke M, Stehning C, Cavus E, Bohnen S, et al. Acute impact of an endurance race on cardiac function and biomarkers of myocardial injury in triathletes with and without myocardial fibrosis. Eur J Prev Cardiol. 20192047487319859975. doi: 10.1177/2047487319859975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orn S, Melberg T, Omland T, Skadberg O, Bjorkavoll-Bergseth M, Erevik CB, Hansen MW, Auestad B, Bergseth R, Aakre KM, et al. Is cardiac troponin elevation following strenuous exercise clinically relevant in healthy subjects? Eur Heart J. 2020; 41suppl 2ehaa946–312. doi: 10.1093/ehjci/ehaa946.3121 [Google Scholar]
- 95.Tian Y, Nie J, Tong TK, Cao J, Gao Q, Man J, Shi Q, Liu W. Changes in serum cardiac troponins following a 21-km run in junior male runners. J Sports Med Phys Fitness. 2006; 46:481–488 [PubMed] [Google Scholar]
- 96.Middleton N, Shave R, George K, Whyte G, Hart E, Atkinson G. Left ventricular function immediately following prolonged exercise: a meta-analysis. Med Sci Sports Exerc. 2006; 38:681–687. doi: 10.1249/01.mss.0000210203.10200.12 [DOI] [PubMed] [Google Scholar]
- 97.Frassl W, Kowoll R, Katz N, Speth M, Stangl A, Brechtel L, Joscht B, Boldt LH, Meier-Buttermilch R, Schlemmer M, et al. Cardiac markers (BNP, NT-pro-BNP, troponin I, troponin T, in female amateur runners before and up until three days after a marathon. Clin Lab. 2008; 54:81–87 [PubMed] [Google Scholar]
- 98.Nie J, Tong TK, Shi Q, Lin H, Zhao J, Tian Y. Serum cardiac troponin response in adolescents playing basketball. Int J Sports Med. 2008; 29:449–452. doi: 10.1055/s-2007-989236 [DOI] [PubMed] [Google Scholar]
- 99.Lippi G, Impellizzeri F, Salvagno GL, Mion M, Zaninotto M, Cervellin G, Guidi GC, Schena F, Plebani M. Kinetics of highly sensitive troponin I and T after eccentric exercise. Clin Chem Lab Med. 2010; 48:1677–1679. doi: 10.1515/CCLM.2010.308 [DOI] [PubMed] [Google Scholar]
- 100.Scherr J, Braun S, Schuster T, Hartmann C, Moehlenkamp S, Wolfarth B, Pressler A, Halle M. 72-h kinetics of high-sensitive troponin T and inflammatory markers after marathon. Med Sci Sports Exerc. 2011; 43:1819–1827. doi: 10.1249/MSS.0b013e31821b12eb [DOI] [PubMed] [Google Scholar]
- 101.Nie J, Tong TK, George K, Fu FH, Lin H, Shi Q. Resting and post-exercise serum biomarkers of cardiac and skeletal muscle damage in adolescent runners. Scand J Med Sci Sports. 2011; 21:625–629. doi: 10.1111/j.1600-0838.2010.01096.x [DOI] [PubMed] [Google Scholar]
- 102.Tian Y, Nie J, Huang C, George KP. The kinetics of highly sensitive cardiac troponin T release after prolonged treadmill exercise in adolescent and adult athletes. J Appl Physiol (1985). 2012; 113:418–425. doi: 10.1152/japplphysiol.00247.2012 [DOI] [PubMed] [Google Scholar]
- 103.Ma G, Liu Y, Liu K. Influence of repeated bouts of table tennis training on cardiac biomarkers in children. Pediatr Cardiol. 2014; 35:711–718. doi: 10.1007/s00246-013-0842-x [DOI] [PubMed] [Google Scholar]
- 104.Li F, Hu Y, Nie J, Fu FH. Effects of acute, intermittent exercise in hypoxic environments on the release of cardiac troponin. Scand J Med Sci Sports. 2016; 26:397–403. doi: 10.1111/sms.12463 [DOI] [PubMed] [Google Scholar]
- 105.Li F, Nie J, Lu Y, Tong TK, Yi L, Yan H, Fu FH, Ma S. The impact of intermittent exercise in a hypoxic environment on redox status and cardiac troponin release in the serum of well-trained marathon runners. Eur J Appl Physiol. 2016; 116:2045–2051. doi: 10.1007/s00421-016-3460-5 [DOI] [PubMed] [Google Scholar]
- 106.Klinkenberg LJ, Luyten P, van der Linden N, Urgel K, Snijders DP, Knackstedt C, Dennert R, Kietselaer BL, Mingels AM, Cardinaels EP, et al. Cardiac troponin T and I release after a 30-km run. Am J Cardiol. 2016; 118:281–287. doi: 10.1016/j.amjcard.2016.04.030 [DOI] [PubMed] [Google Scholar]
- 107.Legaz-Arrese A, Carranza-García LE, Navarro-Orocio R, Valadez-Lira A, Mayolas-Pi C, Munguía-Izquierdo D, Reverter-Masía J, George K. Cardiac biomarker release after endurance exercise in male and female adults and adolescents. J Pediatr. 2017; 191:96–102. doi: 10.1016/j.jpeds.2017.08.061 [DOI] [PubMed] [Google Scholar]
- 108.Li F, Yi L, Yan H, Wang X, Nie J, Zhang H, Fu FHK, Zang Y, Yang S, Lu Y. High-sensitivity cardiac troponin T release after a single bout of high-intensity interval exercise in experienced marathon runners. J Exerc Sci Fit. 2017; 15:49–54. doi: 10.1016/j.jesf.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vassalle C, Masotti S, Lubrano V, Basta G, Prontera C, Di Cecco P, Del Turco S, Sabatino L, Pingitore A. Traditional and new candidate cardiac biomarkers assessed before, early, and late after half marathon in trained subjects. Eur J Appl Physiol. 2018; 118:411–417. doi: 10.1007/s00421-017-3783-x [DOI] [PubMed] [Google Scholar]
- 110.Broz P, Rajdl D, Novak J, Hromadka M, Racek J, Trefil L, Zeman V. High-sensitivity troponins after a standardized 2-hour treadmill run. J Med Biochem. 2018; 37:364–372. doi: 10.1515/jomb-2017-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Skadberg O, Kleiven O, Orn S, Bjorkavoll-Bergseth MF, Melberg TH, Omland T, Aakre KM. The cardiac troponin response following physical exercise in relation to biomarker criteria for acute myocardial infarction; the North Sea Race Endurance Exercise Study (NEEDED) 2013. Clin Chim Acta. 2018; 479:155–159. doi: 10.1016/j.cca.2018.01.033 [DOI] [PubMed] [Google Scholar]
- 112.Martínez-Navarro I, Sánchez-Gómez J, Sanmiguel D, Collado E, Hernando B, Panizo N, Hernando C. Immediate and 24-h post-marathon cardiac troponin T is associated with relative exercise intensity. Eur J Appl Physiol. 2020; 120:1723–1731. doi: 10.1007/s00421-020-04403-8 [DOI] [PubMed] [Google Scholar]
- 113.Li F, Nie J, Zhang H, Fu F, Yi L, Hopkins W, Liu Y, Lu Y. Effects of matched intermittent and continuous exercise on changes of cardiac biomarkers in endurance runners. Front Physiol. 2020; 11:30. doi: 10.3389/fphys.2020.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nie J, Zhang H, Kong Z, Wang C, Liu Y, Shi Q, George K. The impact of exercise modality and menstrual cycle phase on circulating cardiac troponin T. J Sci Med Sport. 2020; 23:309–314. doi: 10.1016/j.jsams.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 115.Huang C, Kong Z, Nie J, Pan M, Zhang H, Shi Q, George K. Impact of high-intensity interval and moderate-intensity continuous exercise on heart rate variability and cardiac troponin. J Sports Med Phys Fitness. 2021; 61:1301–1308. doi: 10.23736/S0022-4707.20.11657-8 [DOI] [PubMed] [Google Scholar]
- 116.Bernat-Adell MD, Collado-Boira EJ, Moles-Julio P, Panizo-González N, Martínez-Navarro I, Hernando-Fuster B, Hernando-Domingo C. Recovery of inflammation, cardiac, and muscle damage biomarkers after running a marathon. J Strength Cond Res. 2021; 35:626–632. doi: 10.1519/JSC.0000000000003167 [DOI] [PubMed] [Google Scholar]
- 117.Aengevaeren VL, Van Kimmenade RR, Ordonez-Llanos J, Garcia-Osuna A, Kaier T, Marber M, Froeling M, van den Berg-Faay S, Hooijmans MT, Monte JR, et al. Cardiac biomarker kinetics and their association with MR measures of cardiomyocyte integrity following a marathon run: implications for post-exercise biomarker testing. 2021; 10:e020039. doi: 10.1161/JAHA.120.020039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lippi G, Schena F, Montagnana M, Salvagno GL, Guidi GC. Influence of acute physical exercise on emerging muscular biomarkers. Clin Chem Lab Med. 2008; 46:1313–1318. doi: 10.1515/CCLM.2008.250 [DOI] [PubMed] [Google Scholar]
- 119.Carranza-García LE, George K, Serrano-Ostáriz E, Casado-Arroyo R, Caballero-Navarro AL, Legaz-Arrese A. Cardiac biomarker response to intermittent exercise bouts. Int J Sports Med. 2011; 32:327–331. doi: 10.1055/s-0030-1263138 [DOI] [PubMed] [Google Scholar]
- 120.Lippi G, Schena F, Dipalo M, Montagnana M, Salvagno GL, Aloe R, Guidi GC. Troponin I measured with a high sensitivity immunoassay is significantly increased after a half marathon run. Scand J Clin Lab Invest. 2012; 72:467–470. doi: 10.3109/00365513.2012.697575 [DOI] [PubMed] [Google Scholar]
- 121.Carmona G, Roca E, Guerrero M, Cussó R, Irurtia A, Nescolarde L, Brotons D, Bedini JL, Cadefau JA. Sarcomere disruptions of slow fiber resulting from mountain ultramarathon. Int J Sports Physiol Perform. 2015; 10:1041–1047. doi: 10.1123/ijspp.2014-0267 [DOI] [PubMed] [Google Scholar]
- 122.López-Laval I, Legaz-Arrese A, George K, Serveto-Galindo O, González-Rave JM, Reverter-Masia J, Munguía-Izquierdo D. Cardiac troponin I release after a basketball match in elite, amateur and junior players. Clin Chem Lab Med. 2016; 54:333–338. doi: 10.1515/cclm-2015-0304 [DOI] [PubMed] [Google Scholar]
- 123.Skadberg O, Kleiven O, Bjorkavoll-Bergseth M, Melberg T, Bergseth R, Selvag J, Auestad B, Greve OJ, Dickstein K, Aarsland T, et al. Highly increased Troponin I levels following high-intensity endurance cycling may detect subclinical coronary artery disease in presumably healthy leisure sport cyclists: The North Sea Race Endurance Exercise Study (NEEDED) 2013. Eur J Prev Cardiol. 2017; 24:885–894. doi: 10.1177/2047487317693130 [DOI] [PubMed] [Google Scholar]
- 124.Park MH, Shin KA, Kim CH, Lee YH, Park Y, Ahn J, Kim YJ. Effects of long-distance running on cardiac markers and biomarkers in exercise-induced hypertension runners: an observational study. Ann Rehabil Med. 2018; 42:575–583. doi: 10.5535/arm.2018.42.4.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rubio-Arias JÁ, Ávila-Gandía V, López-Román FJ, Soto-Méndez F, Alcaraz PE, Ramos-Campo DJ. Muscle damage and inflammation biomarkers after two ultra-endurance mountain races of different distances: 54 km vs 111 km. Physiol Behav. 2019; 205:51–57. doi: 10.1016/j.physbeh.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 126.Sierra APR, Lima GHO, da Silva ED, Maciel JFS, Benetti MP, de Oliveira RA, Martins PFO, Kiss MAP, Ghorayeb N, Newsholme P, et al. Angiotensin-converting enzyme related-polymorphisms on inflammation, muscle and myocardial damage after a marathon race. Front Genet. 2019; 10:984. doi: 10.3389/fgene.2019.00984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gaudreault V, Tizon-Marcos H, Poirier P, Pibarot P, Gilbert P, Amyot M, Rodés-Cabau J, Després JP, Bertrand O, Larose E. Transient myocardial tissue and function changes during a marathon in less fit marathon runners. Can J Cardiol. 2013; 29:1269–1276. doi: 10.1016/j.cjca.2013.04.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.