Abstract
Background
Despite the growing burden of chronic kidney disease (CKD), disease knowledge and understanding are still lacking, especially in Bangladesh.
Objective
The aim of this study was to evaluate the outcome of a health education intervention in order to enhance knowledge, health-related quality of life (QOL), and motivation regarding healthy lifestyles among rural and periurban adults suffering from CKD.
Methods
A parallel-group (1:1) randomized controlled trial is ongoing in the Mirzapur subdistrict, Bangladesh, where two groups of patients with CKD are being compared. Patients aged 18 years and over with CKD (stages 1-3) were enrolled in November 2020. Patients were randomly allocated into either the intervention group (n=63) or the control group (n=63). The control group received usual treatment, while the intervention group received health education through a CKD campaign facilitated by a nephrologist and via mHealth (ie, periodic mobile phone calls) from community health workers. Both groups were followed up for a period of 6 months. The primary endpoint is patients’ increased knowledge measured using the Chronic Kidney Disease Knowledge Questionnaire. The secondary endpoints are improved QOL measured using the standardized EuroQol 5-Dimension 5-Level (EQ-5D-5L) questionnaire as well as improvements in the levels of blood pressure, BMI, serum creatinine, fasting blood sugar, hemoglobin, cholesterol, high-density lipoprotein cholesterol, triglyceride, serum uric acid, blood urea nitrogen, and albumin to creatinine ratio.
Results
Enrollment of participants began in November 2020; the intervention and follow-up were completed in May 2021. We enrolled 126 patients in the study. Patients’ mean ages were 57.97 (SD 15.03) years in the control group and 57.32 (SD 14.37) years in the intervention group. There were 45 out of 63 (71%) females in the control group and 38 out of 63 (60%) females in the intervention group. In addition, there were 38 out of 63 (60%) literate patients in the control group and 33 out of 63 (52%) literate patients in the intervention group.
Conclusions
It is expected that a combined approach, incorporating both a CKD campaign and mHealth, for health education may be an effective tool for increasing knowledge and improving QOL among patients with CKD.
Trial Registration
ClinicalTrials.gov NCT04094831; https://clinicaltrials.gov/ct2/show/NCT04094831
International Registered Report Identifier (IRRID)
DERR1-10.2196/30191
Keywords: chronic kidney disease, campaign, mHealth, knowledge, Bangladesh
Introduction
Background
Chronic kidney disease (CKD) is a public health concern worldwide [1] and directly affects the global burden of morbidity and mortality [2]. CKD is associated with substantial financial costs for both patients and health care systems, and low- and middle-income countries (LMIC) have a much higher economic burden of CKD than developed countries [3]. If the stage of CKD advances in patients, the cost of medical treatment increases [4]. In LMIC, most people with kidney failure have insufficient access to lifesaving renal replacement therapy [5]. Diabetes, hypertension, obesity, and aging are the leading associated factors for CKD throughout the world [6]. These comorbidities make patients with CKD more vulnerable to end-stage renal disease (ESRD) [7].
Knowledge and self-management behaviors must be learned and practiced in order to slow the progression of CKD [8]. Studies have documented that knowledge is essential for slowing kidney function advancement through behavior adjustments, such as physical exercise, dietary changes, patient monitoring (eg, blood pressure [BP] and blood glucose), and adherence to medication [9]. Patients with CKD may benefit from knowledge to change their behavior, improve their outcomes, and lower their mortality rates [10]. Considering the increasing prevalence of CKD, only 6% of the general population and 10% of those at high risk are aware of their CKD status in LMIC [11]. This low health literacy and unawareness in the early stage of the disease are ultimately responsible for CKD disease progression [12]. It has been noted, however, that proper CKD knowledge and management as well as mitigation of CKD-related risks may delay the progression to ESRD and other interrelated health consequences [10,13]. However, most of the patient education research regarding CKD has focused on patients with ESRD. A few studies on CKD have been conducted in Bangladesh, where most were prevalence studies based on a hospitalized urban population; however, there has been no such study in rural Bangladesh describing the knowledge of CKD and health-related quality of life (QOL).
In the field of nephrology, both developed and developing countries are still implementing mobile health (mHealth) [14,15]. However, mobile phone call–based health education has great potential to provide CKD knowledge and improve QOL because it relies on basic mobile technology and requires limited literacy or numeracy skills [16]. In countries with minimal or no national health insurance, such as Bangladesh, CKD education in the early stages of the disease could be an integral part of patient management and the reduction of its related risk factors to slow down its progression; the need is greater in rural and periurban areas. Community health workers (CHWs) have the potential to make a significant difference in the community’s well-being as well as in the lives of people living with kidney disease [17]. A nephrologist-facilitated health campaign, on the other hand, has high potential for delivering CKD education. Thus, this study aimed to evaluate the outcome of a health education intervention in order to enhance knowledge, health-related QOL, and motivation about healthy lifestyle among rural and periurban adults suffering from CKD (stages 1-3).
Hypothesis
We hypothesize that the knowledge and health-related QOL of patients with CKD will be improved by health education provided by a CKD campaign and a mobile phone call–based intervention.
Methods
Design
This study is a community-based, single-centered, prospective, open-label, two-arm (1:1), randomized control trial involving patients with CKD and is being conducted in rural and periurban areas of Bangladesh. This study has been designed in accordance with the CONSORT (Consolidated Standards of Reporting Trials) [18] and SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guidelines [19]. The study flowchart is shown in Figure 1. The total study duration was planned to be 1 year; however, the intervention started in mid-November 2020 and the follow-up period was 6 months long. The last follow-up was completed in May 2021.
Figure 1.
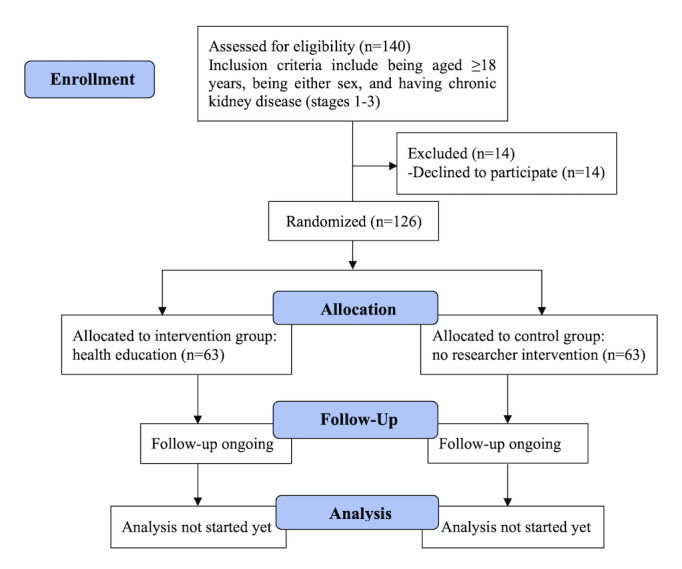
Flowchart of the study.
Study Site
This study was conducted in the demographic surveillance system (DSS) area of the Mirzapur subdistrict within the Tangail district, which is located about 60 km north of Dhaka, the capital city of Bangladesh. It has a mixed rural and periurban population [20]. The subdistrict has 13 unions—it is the smallest administrative unit—and 219 villages. Among the 10 unions in Mirzapur that comprise the DSS area, three unions—Mirzapur Sadar, Bhatgram, and Gorai—nearest to our sentinel health facility, which is popularly known as Kumudini Hospital, were selected purposively due to budgetary constraints and time limitations. The study participants were required to visit Kumudini Hospital Laboratory for their study-related investigations and had lower travel costs compared to other areas.
Study Population and Eligibility Criteria
We recruited patients for this study from among the residents of the DSS. Inclusion criteria for enrollment in the study were as follows: aged 18 years and older, either sex, diagnosed with CKD (stages 1-3: estimated glomerular filtration rate=30-59 mL/min/1.73 m2 and/or albumin to creatinine ratio [ACR]≥30 mg/g for more than 3 months) [21], had lived steadily in the locality for at least 5 years, had a personal cell phone or access to a shared phone, and had given written informed consent to participate. The following were excluded from the study: individuals who were hospitalized at the time of enrollment; had any known serious illness with questionable prognosis, for example, stage 4 or 5 CKD, malignancy, mental illness, congenital disease, and physical disability, if they had prescriptions; and did not agree to give consent.
Randomization
A permuted block-randomization technique was performed using a block size of 6 based on a computer-generated random number sequence. An experienced statistician, not involved in the study in any way, prepared the randomization table and placed the study IDs of the patients with CKD, along with their corresponding intervention allocations, into serially numbered sealed envelopes according to the randomization schedule; these corresponded to the serial numbers of the patients with CKD. These envelopes were kept in an office locker. Allocations to intervention and control groups were concealed in identical sealed envelopes that were only to be opened when the study patient was ready for enrollment under the supervision of the principal investigator. This took place after a patient with CKD was recruited to the study, after obtaining voluntary informed written consent and assignment of a study ID.
Study Activities and Content
During home visits, the CHWs used a Portable Health Clinic (PHC) box with the essential diagnostic equipment for this research; details are described elsewhere [22,23]. At baseline, CHWs performed home visits to obtain written informed consent and to interview the study patients by administering field-tested standardized questionnaires. The questionnaires collected the following data: sociodemographic information, such as age, gender, marital status, religion, occupation, educational background, and income per month; patients’ current medical status, including medication use; patients’ medical history; and patients’ family history (3rd generation), including current and immediate-past medical status. The same information was collected at 3 months and at 6 months, in case of any changes from baseline. To evaluate the level of knowledge, awareness, and QOL on the part of the study patients, the CHWs also administered questionnaires at baseline to collect this data and administered the same questionnaire at 3 months and at 6 months.
CHWs also performed physical examinations at baseline and performed them again at 3 months and at 6 months to measure the following: BP, pulse, height, weight, waist and hip circumferences, and mid–upper arm circumference. However, during baseline measurements, the study patients were advised to visit Kumudini Hospital Laboratory for collection of blood and urine samples to estimate the condition of their kidneys and their related risk factors, such as serum creatinine, fasting blood sugar (FBS), hemoglobin, cholesterol, high-density lipoprotein cholesterol (HDL-c), triglyceride, serum uric acid, blood urea nitrogen (BUN), and ACR. The same laboratory investigations were done at the end of 6 months (Table 1).
Table 1.
Study activities.
| Schedule | Intervention group | Control group | |
| Baseline sessions | |||
|
|
Week 1 |
|
|
|
|
Weeks 1 and 2 |
|
|
|
|
Week 3 to month 3 |
|
|
| Intermediate sessions | |||
|
|
Week 1 of month 4 |
|
|
|
|
Months 4 to 6 |
|
|
| Final session | |||
|
|
End of month 6 |
|
|
aCKD: chronic kidney disease.
Training of CHWs
CHWs obtained written informed consent, performed physical examinations, interviewed the study participants after administering field-tested questionnaires, and provided health education. Training on the overall study procedure for CHWs was provided by the principal investigator. Training included the following: (1) general information about the study (ie, contact information, study overview, structure, and use of technology) and (2) role-specific information (ie, position description, recruitment, informed consent, data collection, use of an electronic database for data entry, proper use of backup paper copies, and self-evaluation form). Competency was assessed by practicing and role-playing in a private office. A nephrologist trained the CHWs, who then provided mHealth education to the study patients.
Intervention Group
The intervention group received health education through a CKD campaign using mHealth technology.
CKD Campaign
During the half-day CKD campaign, health education materials (ie, a leaflet as seen in Multimedia Appendix 1, a short textbook as seen in Multimedia Appendix 2, and a recording notebook) were provided to the study patients. A nephrologist facilitated the health campaign: the contents of the textbook and leaflet were discussed, and patients were taught how to measure salt using the spoon during preparation of their daily meals. The research team have diverse professional backgrounds and have established the content of the CKD textbook and leaflet in the patients’ native language (Bangla), based on the educational materials from the website of the National Kidney Foundation, New York, United States, after receiving permission. Important information related to CKD, such as basics of the kidney and kidney diseases, stages of disease, risk factors, and preventive measures, were used to develop the textbook and leaflet in the patients’ native language (Bangla).
mHealth Technology
Basic health education information about CKD was included in the content to be delivered through a mobile phone call to help patients gain knowledge and awareness and to improve their behaviors. Discussion about the basics of kidney diseases, risk factors, and preventive measures of CKD was performed by CHWs over a mobile phone call with the study patients. The patients had the liberty to discuss their health-related issues with the CHWs over a period of 10 minutes (Table 2). A nephrologist trained the CHWs in the provision of mHealth education.
Table 2.
Content of mobile health (mHealth) education that took place over a 10-minute mobile phone call.
| Topic | Content of mHealth education |
| Kidneys | Kidneys are bean shaped and positioned near the middle of your back on either side of your backbone. Your kidneys are part of the body’s urine system. Kidney functions include the following:
|
| Major risk factors for kidney disease | Some major risk factors include the following:
|
| Some ways to protect kidneys | Ways to protect kidneys include the following:
|
| Diabetes | Diabetes damages your kidneys. Managing blood sugar level slows kidney damage. Some advice includes the following:
|
| Hypertension | Getting your blood pressure back to normal can reduce kidney damage, and some blood pressure tablets actually protect your kidneys. Some advice includes the following:
|
Blood Pressure Check
The CHWs performed home visits where they checked patients’ BP once per week and continued over the study period.
Control Group
The control group received usual care and were followed up over the study period.
Sample Size
We assume that the average level of existing knowledge among patients with CKD (stages 1-3) is 40% [24], and that the average level of expected knowledge after the intervention will increase to 70% [25]. Therefore, considering 90% power and 20% loss to follow-up, the total sample size should be 126 (63 in each group). The sample size was estimated based on the following formula:
| N = ([p1 × q1 + p2q2] / [p2 – p1]2) × factor for α and β |
Here, p1=40%=0.40, the percentage of existing knowledge, and p2=70%=0.70, the percentage of expected knowledge after the intervention. In addition, q1 = 1 – p1 = 0.60; q2 = 1 – p2 = 0.30; power is equal to 90%; and loss to follow-up is equal to 20%.
Endpoints
The primary outcome is the evaluation of improved scores from the Chronic Kidney Disease Knowledge Questionnaire [24].
The secondary outcomes are as follows:
Improved QOL, as measured by the EuroQol 5-Dimension 5-Level (EQ-5D-5L) QOL questionnaire [26].
Improvements in the levels of BP, BMI, serum creatinine, FBS, hemoglobin, cholesterol, HDL-c, triglyceride, serum uric acid, BUN, and ACR.
Primary and secondary outcomes were measured at baseline, 3 months, and 6 months for both intervention and control groups, except for the laboratory investigation levels, which were measured at baseline and 6 months.
Measurements of Knowledge and QOL
Knowledge
Knowledge was measured using the Chronic Kidney Disease Knowledge Questionnaire, a 24-item scale with “true,” “false,” and “I don’t know” multiple-choice answer options, designed to assess CKD knowledge in patients with disease at stages 1 to 3. Knowledge scores were calculated by adding the number of correct answers divided by the total number of questions and multiplying by 100 to obtain a percentage score (Multimedia Appendix 3). For all questions, the answer “I don't know” was scored as incorrect.
The translation to Bangla was performed according to the state-of-the-art procedure of forward-backward translation. A physician and a university lecturer, both native speakers of Bangla and fluent in English, translated the questionnaire into Bangla first. The translated Bangla versions were compiled, and a single Bangla forward version was created. This forward version was then translated back into English by a professional translator with experience in medical translation and by one medical doctor who had not been involved in previous steps. The back-translated versions were then compiled and compared by the researcher, and all four versions were submitted to the expert committee that was formed for the validation study. The expert committee developed the questionnaire; pretesting was then conducted at the community level without any knowledge of diagnosis. The suggested changes were made accordingly based on the pretesting responses. Finally, the Bangla version of the CKD knowledge questionnaire was created.
Quality of Life
QOL was measured using the standardized EQ-5D-5L questionnaire (Multimedia Appendix 3). The EQ-5D-5L contains five dimensions: mobility, self-care, usual activities, pain or discomfort, and anxiety or depression [26,27]. Each dimension has five levels: no problems, slight problems, moderate problems, severe problems, and unable to or extreme problems. A higher score indicates better QOL [28].
Ethical Considerations
This study has been approved by the Research Review Board and the Ethical Review Board of icddr,b. The study was registered at ClinicalTrials.gov (NCT04094831). This study is being conducted in accordance with the Declaration of Helsinki [29]. The study objectives; the importance, risks, and benefits of the research; and the patients’ rights were explicitly communicated to all participants before recruitment. Participation was completely voluntary and written informed consent was obtained from all patients. Each study patient’s identity will remain anonymous.
Statistical Analysis
The intention-to-treat analysis will be performed to compare the outcomes of the intervention and control groups. All baseline indicators at the time of registration will be analyzed to ensure the comparability of the randomized samples. Categorical variables will be expressed as means and SDs and will be analyzed by chi-square tests, discrete variables will be expressed as frequencies and percentages, and continuous variables will be analyzed by t tests or Mann-Whitney U tests. Multiple comparisons will be performed by two-way analysis of variance tests for the evaluation of the outcome variables, such as CKD knowledge, physical measurements, and QOL at baseline, 3 months, and 6 months. However, outcome variables for laboratory findings were measured at baseline and 6 months. Data will be analyzed using SPSS (version 22.0; IBM Corp) and the significance level will be set at the level of P<.05.
Results
The authors completed patient enrollment in November 2020, and the intervention and data collection were performed from November 2020 to May 2021. The results of the first analysis were made available in July 2021, as expected. We enrolled 126 patients (control group, n=63; intervention group, n=63) in the study. The mean age of the participants was 57.97 (SD 15.03) years for the control group and 57.32 (SD 14.37) years for the intervention group. A total of 71% (45/63) and 60% (38/63) of the patients were female in the control and intervention groups, respectively. A total of 67% (42/63) and 56% (35/63) of the patients were housewives in the control and intervention groups, respectively. A total of 79% (50/63) and 71% (45/63) of patients were married in the control and intervention groups, respectively. Furthermore, regarding the control and intervention groups, 60% (38/63) and 52% (33/63) of the patients were literate, 86% (54/63) and 78% (49/63) of the patients had a monthly income of US $100 per month or higher, 13% (8/63) and 16% (10/63) of the patients were current tobacco users, and 43% (27/63) and 30% (19/63) were current smokeless tobacco users, respectively (Table 3).
Table 3.
Demographic characteristics among the study participants.
| Characteristic | Control group participants (n=63) | Intervention group participants (n=63) | |
| Age in years, mean (SD) | 57.97 (15.03) | 57.32 (14.37) | |
| Gender, n (%) | |||
|
|
Female | 45 (71) | 38 (60) |
|
|
Male | 18 (29) | 25 (40) |
| Literacy, n (%) | |||
|
|
Illiterate | 25 (40) | 30 (48) |
|
|
Literate | 38 (60) | 33 (52) |
| Occupation, n (%) | |||
|
|
Housewife | 42 (67) | 35 (56) |
|
|
Farmer | 3 (5) | 4 (6) |
| Marital status, n (%) | |||
|
|
Married | 50 (79) | 45 (71) |
|
|
Widowed | 12 (19) | 18 (29) |
| Income (US $), n (%) | |||
|
|
<100/month | 9 (14) | 14 (22) |
|
|
≥100/month | 54 (86) | 49 (78) |
| Current tobacco smoker, n (%) | |||
|
|
Yes | 8 (13) | 10 (16) |
|
|
No | 55 (87) | 53 (84) |
| Current smokeless tobacco user, n (%) | |||
|
|
Yes | 27 (43) | 19 (30) |
|
|
No | 36 (57) | 44 (70) |
Discussion
Overview
This study’s overall effectiveness will be enhanced by the publication of this research protocol. A nephrologist-facilitated health campaign aims to increase knowledge and raise awareness about CKD-related health concerns by engaging people in discussions about important and influential health information. The aim of such events is to increase knowledge and understanding about CKD and its related risk factors, as well as to educate people on how they can avoid disease progression by living a healthy lifestyle. In addition, mHealth has been shown to enhance awareness, QOL, and behavior among low-income patients with chronic diseases, such as diabetes, and is currently being tested among low-income CKD patients [30]. While CKD management is a significant burden on health practitioners in low-capital settings, CHWs can be an important and underutilized resource for patient education. CHWs have been shown to be effective in assisting people in improving their health habits. A CHW intervention among patients with diabetes, for example, increased patients’ awareness and helped them control their blood glucose levels and BP [31]. To the best of our knowledge, this is the first research study in Bangladesh to evaluate the outcome of health education through a CKD campaign and mobile phone calls to reduce CKD-related burden. mHealth shows a lot of potential in terms of raising patient awareness and understanding of kidney disease, as well as improving kidney knowledge [16]. Furthermore, studies from both developed and developing countries have demonstrated the efficacy and user acceptance of mHealth technologies in the management of CKD among patients; however, the majority of them were limited to dialysis patients [14].
Strengths
A nephrologist facilitated the health campaign in this study, and CHWs provided health education through mobile phone calls in the patients’ native language (Bangla). Health education materials were developed using the same language for better understanding, even with minimum technical knowledge and skills. Furthermore, the study’s strengths include the unbiased systematic sampling approach used to recruit patients and the standard laboratory facility used to identify patients with CKD.
Limitations
The patients in our study were randomly selected from the three unions of the Mirzapur subdistrict, and this does not represent the entire rural and periurban CKD population. In addition, data contamination from study patients and family members could be another study limitation. Therefore, the CHWs received verbal consent from patients not to disclose any study details to their neighbors. Finally, due to budgetary limitations and time constraints, our 6-month follow-up duration was relatively brief.
Conclusions
If our results show an enhancement of the study outcomes among patients with CKD (stages 1-3), we suggest integrating health education via a campaign and mHealth as effective tools at the national level. Further, we can improve patient knowledge and motivate patients with CKD regarding their health practices to improve their QOL.
Acknowledgments
Japan’s Grants-in-Aid for Scientific Research Program (KAKENHI) has provided funding for this study (grant 18H03113). We would like to express our gratitude to the study patients for their generous support. We are grateful to Dr Yuko Ito for her enormous support and Dr Ashir Ahmed (Kyushu University, Japan) for providing the Portable Health Clinic (PHC) box. The authors would like to express their heartfelt gratitude to the nephrologist, the community health workers (CHWs), and laboratory staff for their unconditional support. icddr,b is also grateful to the governments of Bangladesh, Canada, Sweden, and the United Kingdom for providing core and unrestricted support.
Abbreviations
- ACR
albumin to creatinine ratio
- BP
blood pressure
- BUN
blood urea nitrogen
- CHW
community health worker
- CKD
chronic kidney disease
- CONSORT
Consolidated Standards of Reporting Trials
- DSS
demographic surveillance system
- EQ-5D-5L
EuroQol 5-Dimension 5-Level
- ESRD
end-stage renal disease
- FBS
fasting blood sugar
- HDL-c
high-density lipoprotein cholesterol
- LMIC
low- and middle-income countries
- mHealth
mobile health
- PHC
Portable Health Clinic
- QOL
quality of life
- SPIRIT
Standard Protocol Items: Recommendations for Interventional Trials
Health education material: leaflet.
Health education material: short textbook.
Questionnaires.
Footnotes
Conflicts of Interest: None declared.
References
- 1.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020 Feb 29;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(20)30045-3 .S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lv J, Zhang L. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. doi: 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 3.George C, Mogueo A, Okpechi I, Echouffo-Tcheugui JB, Kengne AP. Chronic kidney disease in low-income to middle-income countries: The case for increased screening. BMJ Glob Health. 2017;2(2):e000256. doi: 10.1136/bmjgh-2016-000256. https://gh.bmj.com/lookup/pmidlookup?view=long&pmid=29081996 .bmjgh-2016-000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elshahat S, Cockwell P, Maxwell AP, Griffin M, O'Brien T, O'Neill C. The impact of chronic kidney disease on developed countries from a health economics perspective: A systematic scoping review. PLoS One. 2020;15(3):e0230512. doi: 10.1371/journal.pone.0230512. https://dx.plos.org/10.1371/journal.pone.0230512 .PONE-D-19-23506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White SL, Chadban SJ, Jan S, Chapman JR, Cass A. How can we achieve global equity in provision of renal replacement therapy? Bull World Health Organ. 2008 Mar;86(3):229–237. doi: 10.2471/blt.07.041715. http://europepmc.org/abstract/MED/18368211 .S0042-96862008000300017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan J, Wang C, Liu D, Qiao Y, Pan S, Jiang D, Zhao Z, Liang L, Tian F, Yu P, Zhang Y, Zhao H, Liu Z. Prevalence and risk factors of chronic kidney disease and diabetic kidney disease in Chinese rural residents: A cross-sectional survey. Sci Rep. 2019 Jul 18;9(1):10408. doi: 10.1038/s41598-019-46857-7. doi: 10.1038/s41598-019-46857-7.10.1038/s41598-019-46857-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng S, Shen F, Wen A, Wang L, Fan Y, Liu X, Liu H. Detecting lifestyle risk factors for chronic kidney disease with comorbidities: Association rule mining analysis of web-based survey data. J Med Internet Res. 2019 Dec 10;21(12):e14204. doi: 10.2196/14204. https://www.jmir.org/2019/12/e14204/ v21i12e14204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrauben SJ, Cavanaugh KL, Fagerlin A, Ikizler TA, Ricardo AC, Eneanya ND, Nunes JW. The relationship of disease-specific knowledge and health literacy with the uptake of self-care behaviors in CKD. Kidney Int Rep. 2020 Jan;5(1):48–57. doi: 10.1016/j.ekir.2019.10.004. https://linkinghub.elsevier.com/retrieve/pii/S2468-0249(19)31509-8 .S2468-0249(19)31509-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baay S, Hemmelgarn B, Tam-Tham H, Finlay J, Elliott MJ, Straus S, Beanlands H, Herrington G, Donald M. Understanding adults with chronic kidney disease and their caregivers' self-management experiences: A qualitative study using the theoretical domains framework. Can J Kidney Health Dis. 2019;6:1–12. doi: 10.1177/2054358119848126. https://journals.sagepub.com/doi/10.1177/2054358119848126?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed .10.1177_2054358119848126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai Y, Wang S, Tsai H, Chen T, Kung L, Hsiao P, Hsiao S, Hwang S, Chen H, Chiu Y. The interaction between self-care behavior and disease knowledge on the decline in renal function in chronic kidney disease. Sci Rep. 2021 Jan 11;11(1):401. doi: 10.1038/s41598-020-79873-z. doi: 10.1038/s41598-020-79873-z.10.1038/s41598-020-79873-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ene-Iordache B, Perico N, Bikbov B, Carminati S, Remuzzi A, Perna A, Islam N, Bravo RF, Aleckovic-Halilovic M, Zou H, Zhang L, Gouda Z, Tchokhonelidze I, Abraham G, Mahdavi-Mazdeh M, Gallieni M, Codreanu I, Togtokh A, Sharma SK, Koirala P, Uprety S, Ulasi I, Remuzzi G. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): A cross-sectional study. Lancet Glob Health. 2016 May;4(5):e307–e319. doi: 10.1016/S2214-109X(16)00071-1. https://linkinghub.elsevier.com/retrieve/pii/S2214-109X(16)00071-1 .S2214-109X(16)00071-1 [DOI] [PubMed] [Google Scholar]
- 12.Ricardo AC, Yang W, Lora CM, Gordon EJ, Diamantidis CJ, Ford V, Kusek JW, Lopez A, Lustigova E, Nessel L, Rosas SE, Steigerwalt S, Theurer J, Zhang X, Fischer MJ, Lash JP, CRIC Investigators Limited health literacy is associated with low glomerular filtration in the Chronic Renal Insufficiency Cohort (CRIC) study. Clin Nephrol. 2014 Jan;81(1):30–37. doi: 10.5414/CN108062. http://europepmc.org/abstract/MED/24219913 .11047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) Work Group Chapter 3: Management of progression and complications of CKD. Kidney Int Suppl (2011) 2013 Jan;3(1):73–90. doi: 10.1038/kisup.2012.66. https://linkinghub.elsevier.com/retrieve/pii/S2157-1716(15)31103-5 .S2157-1716(15)31103-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Chen H, Qazi H, Morita PP. Intervention and evaluation of mobile health technologies in management of patients undergoing chronic dialysis: Scoping review. JMIR Mhealth Uhealth. 2020 Apr 03;8(4):e15549. doi: 10.2196/15549. https://mhealth.jmir.org/2020/4/e15549/ v8i4e15549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: A systematic review. J Med Internet Res. 2015 Feb 24;17(2):e52. doi: 10.2196/jmir.3951. https://www.jmir.org/2015/2/e52/ v17i2e52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuot DS, Boulware LE. Telehealth applications to enhance CKD knowledge and awareness among patients and providers. Adv Chronic Kidney Dis. 2017 Jan;24(1):39–45. doi: 10.1053/j.ackd.2016.11.017. http://europepmc.org/abstract/MED/28224941 .S1548-5595(16)30145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narva AS, Norton JM, Boulware LE. Educating patients about CKD: The path to self-management and patient-centered care. Clin J Am Soc Nephrol. 2016 Apr 07;11(4):694–703. doi: 10.2215/CJN.07680715. https://cjasn.asnjournals.org/cgi/pmidlookup?view=long&pmid=26536899 .CJN.07680715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant S, Mayo-Wilson E, Montgomery P, Macdonald G, Michie S, Hopewell S, Moher D, on behalf of the CONSORT-SPI Group CONSORT-SPI 2018 Explanation and Elaboration: Guidance for reporting social and psychological intervention trials. Trials. 2018 Jul 31;19(1):406. doi: 10.1186/s13063-018-2735-z. https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-018-2735-z .10.1186/s13063-018-2735-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan A, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, Hróbjartsson A, Mann H, Dickersin K, Berlin JA, Doré CJ, Parulekar WR, Summerskill WSM, Groves T, Schulz KF, Sox HC, Rockhold FW, Rennie D, Moher D. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann Intern Med. 2013 Feb 05;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. https://www.acpjournals.org/doi/abs/10.7326/0003-4819-158-3-201302050-00583?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed .1556168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarker MHR, Moriyama M, Rashid HU, Chisti MJ, Rahman MM, Das SK, Uddin A, Saha SK, Arifeen SE, Ahmed T, Faruque A. Community-based screening to determine the prevalence, health and nutritional status of patients with CKD in rural and peri-urban Bangladesh. Ther Adv Chronic Dis. 2021;12:1–13. doi: 10.1177/20406223211035281. https://journals.sagepub.com/doi/10.1177/20406223211035281?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed .10.1177_20406223211035281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014 May;63(5):713–735. doi: 10.1053/j.ajkd.2014.01.416.S0272-6386(14)00491-0 [DOI] [PubMed] [Google Scholar]
- 22.Ahmed A, Kabir L, Kai E, Inoue S. GramHealth: A bottom-up approach to provide preventive healthcare services for unreached community. Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); July 3-7, 2013; Osaka, Japan. 2013. pp. 1668–1671. [DOI] [PubMed] [Google Scholar]
- 23.Islam R, Nohara Y, Rahman MJ, Sultana N, Ahmed A, Nakashima N. Portable Health Clinic: An advanced tele-healthcare system for unreached communities. Stud Health Technol Inform. 2019 Aug 21;264:616–619. doi: 10.3233/SHTI190296.SHTI190296 [DOI] [PubMed] [Google Scholar]
- 24.Gheewala PA, Peterson GM, Zaidi STR, Jose MD, Castelino RL. Public knowledge of chronic kidney disease evaluated using a validated questionnaire: A cross-sectional study. BMC Public Health. 2018 Mar 20;18(1):371. doi: 10.1186/s12889-018-5301-4. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-018-5301-4 .10.1186/s12889-018-5301-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang-Lindsey K, Yoon E. Measuring health-related outcomes after a peer-led educational intervention for African Americans with chronic kidney disease. J Nephrol Soc Work. 2019;43(1):33–44. https://www.kidney.org/sites/default/files/v43a_a3.pdf . [Google Scholar]
- 26.van Hout B, Janssen MF, Feng Y, Kohlmann T, Busschbach J, Golicki D, Lloyd A, Scalone L, Kind P, Pickard AS. Interim scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–715. doi: 10.1016/j.jval.2012.02.008. https://linkinghub.elsevier.com/retrieve/pii/S1098-3015(12)00058-7 .S1098-3015(12)00058-7 [DOI] [PubMed] [Google Scholar]
- 27.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011 Dec;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. http://europepmc.org/abstract/MED/21479777 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jahan Y, Rahman MM, Faruque ASG, Chisti MJ, Kazawa K, Matsuyama R, Moriyama M. Awareness development and usage of mobile health technology among individuals with hypertension in a rural community of Bangladesh: Randomized controlled trial. J Med Internet Res. 2020 Dec 07;22(12):e19137. doi: 10.2196/19137. https://www.jmir.org/2020/12/e19137/ v22i12e19137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shrestha B, Dunn L. The Declaration of Helsinki on medical research involving human subjects: A review of seventh revision. J Nepal Health Res Counc. 2020 Jan 21;17(4):548–552. doi: 10.33314/jnhrc.v17i4.1042. [DOI] [PubMed] [Google Scholar]
- 30.Krishna S, Boren SA. Diabetes self-management care via cell phone: A systematic review. J Diabetes Sci Technol. 2008 May;2(3):509–517. doi: 10.1177/193229680800200324. http://europepmc.org/abstract/MED/19885219 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Correia JC, Lachat S, Lagger G, Chappuis F, Golay A, Beran D, COHESION Project Interventions targeting hypertension and diabetes mellitus at community and primary healthcare level in low- and middle-income countries: A scoping review. BMC Public Health. 2019 Nov 21;19(1):1542. doi: 10.1186/s12889-019-7842-6. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-019-7842-6 .10.1186/s12889-019-7842-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Health education material: leaflet.
Health education material: short textbook.
Questionnaires.


