Abstract
Background:
Conventionally fractionated radiotherapy is a common treatment for men with localized prostate cancer. A growing consensus suggests that stereotactic body radiation therapy (SBRT) is similarly effective but less costly and more convenient for patients. The SpaceOAR hydrogel rectal spacer placed between the prostate and rectum reduces radiation-induced rectal injury in patients receiving conventionally fractionated radiotherapy, but spacer efficacy with SBRT is unclear. The purpose of this research was to assess the clinical utility of the hydrogel rectal spacer in men receiving SBRT for prostate cancer.
Methods:
We performed systematic searches of Medline, Embase, and the Cochrane Central Register of Controlled Trials for studies in men who received the SpaceOAR hydrogel spacer prior to SBRT (≥5.0 Gy fractions) for treatment of localized prostate center. Rectal irradiation results were compared to controls without spacer implant; all other outcomes were reported descriptively owing to lack of comparative data incuding perirectal separation distance, rectal irradiation on a dosimetric curve, gastrointestinal (GI) toxicity, and freedom from biochemical failure. GI toxicity was reported as the risk of a grade 2 or 3+ bowel complication in early (≤3 months) and late (>3 months) follow-up.
Results:
In 11 studies with 780 patients, SBRT protocols ranged from 7 to 10 Gy per fraction with total dose ranging from 19 to 45 Gy. Perirectal distance achieved with the rectal spacer ranged from 9.6 to 14.5 mm (median 10.8 mm). Compared to controls receiving no spacer, SpaceOAR placement reduced the radiation delivered to the rectum by 29% to 56% across a dosimetric profile curve. In early follow-up, grade 2 GI complications were reported in 7.0% of patients and no early grade 3+ GI complications were reported. In late follow-up, the corresponding rates were 2.3% for grade 2 and 0.3% for grade 3 GI toxicity. Over 16 months median follow-up, freedom from biochemical failure ranged from 96.4% to 100% (pooled mean 97.4%).
Conclusions:
SpaceOAR hydrogel spacer placed between the prostate and rectum prior to SBRT is a promising preventative strategy that increases the distance between the prostate and rectum, reduces rectal radiation exposure, and may lower the risk of clinically important GI complications.
Keywords: hydrogel spacer, prostate cancer, radiotherapy, SpaceOAR, stereotactic body radiation therapy
1. Introduction
Prostate cancer is the most common malignancy in men with an annual incidence of 192,000 cases in the United States[1] and 365,000 cases in Europe.[2] Prostate cancer treatments include watchful waiting, active surveillance, radiotherapy, or radical prostatectomy, with the choice dependent on patient preferences, pathological tumor characteristics, risk of progression, and existing comorbidities. External beam radiotherapy is a common treatment for men with localized prostate cancer[3] and is better tolerated than surgery by older patients and those with existing comorbidities.[4] Conventionally fractionated radiotherapy (CfRT) utilizing 74 to 80 Gy radiation applied in 1.8 to 2.0 Gy fractions has been a mainstay of treatment across the localized disease spectrum.[5] However, in recent years there has been a shift to moderate hypofractionation (2.5–4 Gy per fraction), which has similar late toxicity outcomes as CfRT but slightly higher acute toxicity.[6] While CfRT and moderate hypofractionation are effective in maintaining biochemical disease control, they involve a prolonged treatment course involving approximately 36 to 40 treatments, which may deter patients from choosing these options.
Stereotactic body radiation therapy (SBRT) using ≥5.0 Gy fractions is an emerging treatment that provides prostate cancer control outcomes and rates of late gastrointestinal (GI) toxicity similar to those of CfRT and moderate hypofractionation, but applied in fewer treatment sessions which is more convenient and less costly to patients, and more efficient for providers since it involves less hospital capacity and utilizes fewer resources.[7] Yet concerns remain around toxicity rates for SBRT in the short- and long-term.[8] While utilization of SBRT in men with localized prostate cancer has recently increased, strategies to mitigate potential risk of radiation-induced GI toxicities should be explored. Rectal irradiation owing to overlap of the rectum with the planning target volume and associated rectal complications are known risks with radiotherapy due to the anatomic proximity of the rectum to the prostate. A key consideration with SBRT is maintaining a balance between delivering higher therapeutic dosages to the prostate while minimizing rectal complications.
Rectal spacers placed between the prostate and rectum have emerged as an effective tool to increase the distance between these anatomical structures in order to limit radiation-induced rectal injury.[9–11] A variety of rectal spacers are available including hydrogel spacer, hyaluronic acid, and rectal balloons, each intended to provide prostate-rectum separation but via slightly different mechanisms of action. In a systematic review with meta-analysis of men receiving CfRT for localized prostate cancer, the addition of an absorbable polyethylene glycol hydrogel spacer (SpaceOAR, Boston Scientific, Marlborough, MA, USA) was shown to be safe, reduced rectal irradiation, lowered the risk of rectal complications, and improved quality of life compared to CfRT without spacer placement.[12] To the authors’ knowledge, no systematic reviews have focused on SpaceOAR hydrogel spacer placement in conjunction with SBRT. Therefore, we performed a systematic review with the objective of determining the clinical utility of the SpaceOAR hydrogel spacer in men receiving SBRT for prostate cancer.
2. Methods
The review methods adhered to the guidance provided by the preferred reporting items for systematic reviews and meta-analyses (PRISMA)[13] and the protocol was prospectively registered at the Research Registry (reviewregistry973). Ethical approval and patient consent were not required because this is a systematic review and meta-analysis of previously published studies. The authors agree to make the raw data from this analysis available upon reasonable request. Systematic searches of Medline, Embase, and the Cochrane Central Register of Controlled Trials identified potentially eligible studies using keyword and MeSH term combinations including anatomic (prostat∗), disease (cancer, carcinoma), and treatment (hydrogel, perirectal spacer, polyethylene glycol, rectal spacer, SpaceOAR) terms. Manual keyword-driven searches were additionally performed using the Directory of Open Access Journals, Google Scholar, and the reference lists of included papers and relevant review articles.
Eligible studies were controlled trials or observational studies that enrolled men who received the SpaceOAR hydrogel spacer prior to receiving SBRT (≥5.0 Gy fractions) for treatment of localized or locally advanced prostate center.[5] We excluded review articles, case reports with less than 5 patients, studies that did not report an outcome specified in this review, and studies of other rectal spacers such as hyaluronic acid and rectal balloons. In order to maximize available evidence and reduce the risk of publication bias, we included unpublished or grey literature study data with no publication date or language restrictions. The final search was performed in August 2020.
Two experienced systematic reviewers (LM, DF) independently reviewed titles and abstracts of potentially eligible studies. We retrieved the references from each electronic database and merged them within a consolidated reference manager file for study screening and classification. Following exclusion of clearly ineligible studies, we reviewed the full-text of remaining articles. A medical translator assisted with non-English language translations. Among eligible studies, data were extracted by the same 2 reviewers and discrepancies were resolved by discussion. When multiple papers were published using a common patient population, we preferentially extracted outcome data from the paper with the largest sample size for early outcomes and from the paper with the longest follow-up duration for late outcomes.
From each study, we recorded manuscript metadata, study characteristics, patient characteristics, and main outcomes. Outcomes of this review were perirectal separation distance, rectal irradiation, GI toxicity, and freedom from biochemical failure (FFBF). Rectal irradiation data were extracted from dose-response curves and reported as a percentage of the rectal volume receiving 50% (range 41%–60%), 70% (range 61%–80%), and 90% (range 81%–100%) of the maximum prescribed radiation dose. GI toxicity was reported as the risk of a grade 2 or 3+ bowel complication in early (≤3 months) and late (>3 months) follow-up.
Quantitative meta-analysis was performed for rectal irradiation outcomes only since comparative data were unavailable for other outcomes. We performed random effects meta-analysis with inverse variance weighting where the statistic of interest was the weighted percentage mean difference in the percentage of rectal volume receiving distinct thresholds of the maximum prescribed radiation dose between groups treated with vs. without the SpaceOAR hydrogel spacer; a negative value favored SBRT with SpaceOAR and a positive value favored SBRT without SpaceOAR. Individual study results and pooled meta-analysis data were displayed with forest plots. We were unable to assess the potential for publication bias or to explore sources of heterogeneity with metaregression owing to the small number of available studies. All tests were two-sided and the threshold for statistical significance between groups was P < .05. Statistical analyses were conducted by a statistician using Review Manager v5.3 (Cochrane Collaboration, Copenhagen, Denmark).
3. Results
We identified 93 unique publications by keyword-driven systematic searches and 4 additional publications by manual searches. Ultimately, 11 studies reported in 14 papers[14–27] met eligibility criteria and were included in the systematic review. A PRISMA flow diagram depicting the study identification and selection results is shown in Figure 1. Owing to the lack of comparative outcome reporting, we extracted data only from the hydrogel spacer arm of each study for the systematic review. Among 11 studies (5 prospective) with 780 patients, SBRT protocols varied considerably using a total dose ranging from 19 to 45 Gy administered in 7 to 10 Gy per fraction. Among the studies in which patients were followed beyond the conclusion of SBRT, the median duration of follow-up was 20 months (range: 9–24 months) (Table 1). Patient characteristics in the included studies were reported inconsistently (Table 2). Mean patient age ranged from 69 to 73 years (median 70 years), prostate-specific antigen ranged from 6.3 to 9.8 ng/mL (median 8.2 ng/mL), androgen deprivation therapy usage was variable, and patients predominantly presented as intermediate risk.
Figure 1.
PRISMA study flow diagram. PRISMA = preferred reporting items for systematic reviews and meta-analyses, SBRT = stereotactic body radiation therapy.
Table 1.
Characteristics of studies utilizing stereotactic body radiation therapy and rectal hydrogel spacer for treatment of prostate cancer.
| Study | Design | N | Dose (Gy) | Fx | Gy/Fx | Follow-up∗ |
| Alongi, 2013[14] | P | 8 | 35 | 5 | 7 | [10 mo] |
| Chen, 2020[15] | R | 250 | 45 | 5 | 9 | 10 mo |
| Cuccia, 2020[16] | P | 10 | 35 | 5 | 7 | Treatment |
| Hwang, 2019[17]Hwang, 2018[18] | R | 50 | 36.25 | 5 | 7.25 | 20 mo |
| Jones, 2017[19]Folkert, 2017[20] | R | 36 | 45 | 5 | 9 | 9 mo |
| King, 2018[21] | P | 6 | 40 | 5 | 8 | 4 d |
| Ogita, 2019[22] | P | 40 | 36.25 | 5 | 7.25 | 3 mo |
| Pryor, 2019[23]Wilton, 2017[24] | P | 80 | 19–20† | 2 | 9.5–10 | 24 mo |
| Ruggieri, 2014[25] | R | 11 | 35 | 5 | 7 | Treatment |
| Saito, 2020[26] | R | 20 | 36.25 | 5 | 7.25 | Treatment |
| Zelefsky, 2019[27] | R | 269 | 37.5–40.0 | 5 | 7.5–8.0 | 24 mo |
Table 2.
Patient characteristics in studies of stereotactic body radiation therapy and rectal hydrogel spacer for treatment of prostate cancer.
| Risk category∗ | ||||||
| Study | Age (yr)∗ | PSA (ng/mL)∗ | ADT∗ | Low | Intermediate | High |
| Alongi, 2013[14] | [70] | [6.3] | [25%] | [65%] | [35%] | 0% |
| Chen, 2020[15] | — | — | — | 9% | 85% | 6% |
| Cuccia, 2020[16] | 70 | 9.3 | 30% | 30% | 70% | 0% |
| Hwang, 2019[17]Hwang, 2018[18] | 69 | 7.4 | 36% | 16% | 84% | 0% |
| Jones, 2017[19]Folkert, 2017[20] | — | — | — | 16% | 84% | 0% |
| King, 2018[21] | — | — | 100% | 0% | 100% | |
| Ogita, 2019[22] | 70 | — | 58% | 8% | 63% | 30% |
| Pryor, 2019[23]Wilton, 2017[24] | [70] | [8.9] | [54%] | 0% | [76%] | [24%] |
| Ruggieri, 2014[25] | 73 | — | — | 100% | 0% | |
| Saito, 2020[26] | 73 | 9.8 | — | 10% | 70% | 20% |
| Zelefsky, 2019[27] | [70] | [6.4] | [27%] | 10% | 90% | 0% |
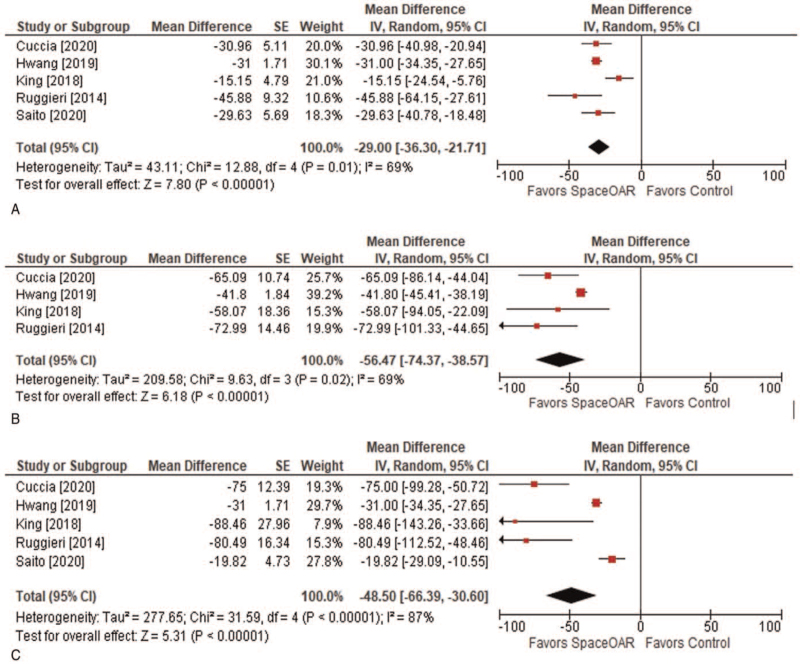
The perirectal distance achieved with SpaceOAR implant ranged from 9.6 to 14.5 mm (median 10.8 mm). Placement of the hydrogel spacer prior to SBRT effectively lowered the amount of radiation delivered to the rectum compared to SBRT with no spacer. Across the measured dosimetric profile curve represented as a percentage of the maximum prescribed radiation dose, rectal irradiation was 29% to 56% lower with vs. without hydrogel rectal spacer placement (Fig. 2). Grade ≥2 GI toxicity complications were uncommon. In early follow-up, grade 2 GI complications were reported in 7.0% of patients and no early grade 3+ complications were reported. In late follow-up, the corresponding pooled mean rates were 2.3% for grade 2 and 0.3% for grade 3 GI toxicity (Table 3). Over a median follow-up duration of 16 months (range 11–36 months), FFBF ranged from 96.4% to 100% (pooled mean 97.4%).
Figure 2.
Dosimetric profile of stereotactic body radiation therapy and rectal hydrogel spacer for treatment of prostate cancer. Values represent the percentage reduction with versus without SpaceOAR in the percentage of rectum receiving 50% (A), 70% (B), and (C) 90% of the maximum prescribed radiation dosage.
Table 3.
Summary of key outcomes in studies of stereotactic body radiation therapy and rectal hydrogel spacer for treatment of prostate cancer.
| Gastrointestinal toxicity | FFBF | ||||||
| Study | Perirectal distance (mm) | Early grade 2 | Early grade 3 | Late grade 2∗ | Late grade 3∗ | Rate | Follow-up (mo)∗ |
| Alongi, 2013[14] | — | — | 0/8 | 0/[5] | 0/[5] | 8/8 | [11] |
| Chen, 2020[15] | — | 18/250 | 0/250 | 10/250 | 1/250 | 241/250 | 36 |
| Cuccia, 2020[16] | 9.9 | 0/10 | 0/10 | — | — | ||
| Hwang, 2019[17]Hwang, 2018[18] | 9.6 | 2/50 | 0/50 | 0/50 | 0/50 | 50/50 | 20 |
| Jones, 2017[19]Folkert, 2017[20] | 11.7 | 1/44 | 0/44 | — | 0/44 | 44/44 | 12 |
| King, 2018[21] | — | 0/6 | 0/6 | — | — | — | — |
| Ogita, 2019[22] | — | 7/40 | 0/40 | — | — | — | — |
| Pryor, 2019[23]Wilton, 2017[24] | — | — | 0/80 | — | — | — | — |
| Ruggieri, 2014[25] | 14.5 | — | — | — | — | — | — |
| Saito, 2020[26] | — | — | — | — | — | — | — |
| Zelefsky, 2019[27] | — | — | — | 3/269 | — | — | — |
4. Discussion
A growing body of evidence supports the use of SBRT in the treatment of localized prostate cancer owing to its comparable efficacy, lower cost, and improved patient convenience relative to CfRT and moderate hypofractionation.[7] Prostate tumors have a low alpha/beta ratio of 1.5 to 2 versus 3 in the rectum, which suggests that prostate cancer cells have a high sensitivity to dose per fraction and therefore are perhaps more responsive to SBRT.[28] The most recent American Society for Radiation Oncology and National Comprehensive Cancer Network guidelines state that SBRT should be conditionally recommended for low-risk patients and moderate-risk patients under clinical trial surveillance or multi-institutional registry.[5,29,30] Using the evidence available, we demonstrated that the SpaceOAR hydrogel spacer placed between the prostate and rectum prior to SBRT increases the distance between the prostate and rectum, lowers rectal radiation exposure, and may lower the risk of important GI complications. Prospective comparative trials are needed to bolster the current body of evidence for SpaceOAR in the setting of SBRT.
An encouraging finding of this systematic review was the low risk (<3%) of late grade ≥2 GI toxicity in men treated with SpaceOAR. Of the patients included, 561 of 780 underwent dose-escalated SBRT regimens (37.5–45 Gy in 5 fractions), demonstrating low toxicity rates when using a rectal spacer, even with high-dose radiotherapy regimens. These results compare favorably to a recent meta-analysis that reported late grade ≥2 GI toxicity rates of 10.0% for SBRT.[31] It is likely that the magnitude of prostate-rectum separation that was achieved following spacer placement, along with the resulting decrease in rectal irradiation, was responsible for sparing patients from radiation-induced GI complications. It is also plausible that the hydrogel spacer may additionally be helpful during treatment since hypofractionated RT is associated with higher rates of early GI complications relative to CfRT.[6] These hypotheses are supported by a lower risk of early and late GI toxicity observed in men treated with CfRT with vs. without the SpaceOAR rectal spacer.[12] The totality of this evidence suggests that in men receiving radiation therapy, regardless of fractionation, insertion of SpaceOAR is responsible for significant reductions in rectal radiation dose and is associated with a favorably low rate of GI side effects.
The volume of rectum receiving radiation is highly correlated with the incidence of GI toxicities. With CfRT, the volume of rectum receiving ≥70 Gy radiation is a common metric used to measure rectal irradiation as CfRT protocols typically utilize total doses of 74 to 80 Gy. With SBRT in the current analysis, interpretation of rectal irradiation data is considerably more complex since the doses ranged from 19 to 45 Gy. For this reason, we reported rectal dosimetry as a percentage of the total radiation dose as opposed to using a fixed value. The clinical validity of this approach is uncertain since fixed toxicity thresholds have not been established for SBRT protocols. However, we did identify that regardless of total radiation dose, utilization of the hydrogel spacer lowered rectal radiation exposure by 29% to 56% across the measured dosimetric curve. In terms of oncologic outcome, follow-up in the included studies was too short to draw definitive conclusions, but the pooled FFBF rate of 97% at median 15 months follow-up is an encouraging result and suggests excellent midterm biochemical control.
This systematic review is novel in that the efficacy of the SpaceOAR hydrogel rectal spacer prior to SBRT was evaluated for the first time. Conclusions derived from this review were strengthened by using a pre-specified methodology that adhered to PRISMA guidelines and was prospectively registered. We additionally identified and adjusted for multiple papers reporting on common patients and included data from abstracts to minimize the risk of publication bias. On balance, there are several limitations of this review that must be acknowledged. First and most importantly is that there are no randomized trials comparing various rectal spacer strategies with SBRT. Any comparisons of rectal toxicity with vs. without a spacer prior to SBRT were necessarily descriptive in nature, prone to bias, and should be considered only as hypothesis-generating. Second, the SBRT protocols varied considerably among studies and patient characteristics were inconsistently reported. Third, late GI toxicity and FFBF data were available over mid-term follow-up only, and therefore, the long-term outcomes in patients treated with the hydrogel spacer prior to SBRT remain unknown. Fourth, this review did not investigate the effect of hydrogel spacer placement on toxicities in adjacent organs such as the bladder or penile bulb. Finally, none of the included studies reported bowel-related quality of life. Whether patients receiving SBRT realize an overall improvement in health utility following hydrogel spacer placement remains unclear. Overall, the evidence for hydrogel spacer placement prior to SBRT for prostate cancer treatment is promising, yet higher-quality comparative studies with longer term follow-up are needed to draw more definitive conclusions.
5. Conclusions
Utilization of the SpaceOAR hydrogel spacer placed between the prostate and rectum prior to SBRT is a promising preventative strategy that increases the distance between the prostate and rectum, lowers the amount of rectal radiation exposure, and may lower the risk of important GI complications.
Acknowledgments
The authors thank David Fay, PhD for assistance with literature review and data extraction. The authors had no writing assistance in the preparation of this manuscript.
Author contributions
Conceptualization: Heather A. Payne, Michael Pinkawa, Clive Peedell, Samir K. Bhattacharyya, Emily Woodward, Larry E. Miller.
Data curation: Larry E. Miller.
Formal analysis: Larry E. Miller.
Funding acquisition: Samir K. Bhattacharyya.
Investigation: Heather A. Payne, Michael Pinkawa, Clive Peedell, Samir K. Bhattacharyya, Emily Woodward, Larry E. Miller.
Methodology: Heather A. Payne, Michael Pinkawa, Clive Peedell, Emily Woodward, Larry E. Miller.
Project administration: Heather A. Payne, Michael Pinkawa, Clive Peedell, Samir K. Bhattacharyya, Emily Woodward, Larry E. Miller.
Resources: Samir K. Bhattacharyya.
Software: Larry E. Miller.
Supervision: Samir K. Bhattacharyya.
Validation: Heather A. Payne, Michael Pinkawa, Clive Peedell, Emily Woodward.
Writing – original draft: Larry E. Miller.
Writing – review & editing: Heather A. Payne, Michael Pinkawa, Clive Peedell, Samir K. Bhattacharyya, Emily Woodward.
Footnotes
Abbreviations: CfRT= conventionally fractionated radiotherapy, FFBF = freedom from biochemical failure, GI = gastrointestinal, PRISMA = preferred reporting items for systematic reviews and meta-analyses, SBRT = stereotactic body radiation therapy.
How to cite this article: Payne HA, Pinkawa M, Peedell C, Bhattacharyya SK, Woodward E, Miller LE. SpaceOAR hydrogel spacer injection prior to stereotactic body radiation therapy for men with localized prostate cancer: a systematic review. Medicine. 2021;100:49(e28111).
Dr Payne reports receiving honoraria for advisory board attendance, travel expenses to medical meetings, and served as a consultant for AstraZeneca, Astellas, Janssen, Sanofi Aventis, Bayer, Ipsen, Ferring, Augmenix, and Novartis. Dr Pinkawa reported receiving grants from Augmenix Inc for studies, nonfinancial support from Augmenix Inc (material), and personal fees from Boston Scientific for presentations. Dr Peedell reports consulting and speaker fees from Boston Scientific; and speaker fees and travel expenses to medical meetings from Elekta and AstraZeneca. Ms Woodward and Dr Bhattacharyya report employment with Boston Scientific. Dr Miller reports consultancy with Boston Scientific.
This study was supported by Boston Scientific.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Fx = fraction, Gy = gray, P = prospective, R = retrospective.
Brackets denote estimated values.
Stereotactic body radiation therapy boost followed by radiation therapy using 46 Gy in 23 Fx or 36 Gy in 12 Fx.
ADT = androgen deprivation therapy, PSA = prostate-specific antigen.
Brackets denote estimated values.
FFBF = freedom from biochemical failure.
Brackets denote estimated values.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:07–30. [DOI] [PubMed] [Google Scholar]
- [2].Crocetti E. Epidemiology of Prostate Cancer in Europe. 2005. Available at: https://ec.europa.eu/jrc/en/publication/epidemiology-prostate-cancer-europe. Accessed August 25, 2020. [Google Scholar]
- [3].Chen J, Oromendia C, Halpern JA, et al. National trends in management of localized prostate cancer: a population based analysis 2004–2013. Prostate 2018;78:512–20. [DOI] [PubMed] [Google Scholar]
- [4].Trinh QD, Schmitges J, Sun M, et al. Open radical prostatectomy in the elderly: a case for concern? BJU Int 2012;109:1335–40. [DOI] [PubMed] [Google Scholar]
- [5].Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated radiation therapy for localized prostate cancer: an ASTRO, ASCO, and AUA evidence-based guideline. J Clin Oncol 2018;36:JCO1801097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Datta NR, Stutz E, Rogers S, et al. Conventional versus hypofractionated radiation therapy for localized or locally advanced prostate cancer: a systematic review and meta-analysis along with therapeutic implications. Int J Radiat Oncol Biol Phys 2017;99:573–89. [DOI] [PubMed] [Google Scholar]
- [7].Kishan AU, Dang A, Katz AJ, et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw Open 2019;2:e188006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].European Association of Urology. Prosate Cancer. 2020. Available at: https://uroweb.org/guideline/prostate-cancer/#6. Accessed December 8, 2020. [Google Scholar]
- [9].Mok G, Benz E, Vallee JP, et al. Optimization of radiation therapy techniques for prostate cancer with prostate-rectum spacers: a systematic review. Int J Radiat Oncol Biol Phys 2014;90:278–88. [DOI] [PubMed] [Google Scholar]
- [10].Tang Q, Zhao F, Yu X, et al. The role of radioprotective spacers in clinical practice: a review. Quant Imaging Med Surg 2018;8:514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Afkhami Ardekani M, Ghaffari H. Optimization of prostate brachytherapy techniques with polyethylene glycol-based hydrogel spacers: a systematic review. Brachytherapy 2019;19:13–23. [DOI] [PubMed] [Google Scholar]
- [12].Miller LE, Efstathiou JA, Bhattacharyya SK, et al. Association of the placement of a perirectal hydrogel spacer with the clinical outcomes of men receiving radiotherapy for prostate cancer: a systematic review and meta-analysis. JAMA Netw Open 2020;3:e208221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alongi F, Cozzi L, Arcangeli S, et al. Linac based SBRT for prostate cancer in 5 fractions with VMAT and flattening filter free beams: preliminary report of a phase II study. Radiat Oncol 2013;8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen L, Gao A, Gannavarapu BS, et al. Safety and outcome of stereotactic body radiation therapy (SBRT) with rectal hydrogel spacer for prostate cancer. J Clin Oncol 2020;38:76. [Google Scholar]
- [16].Cuccia F, Mazzola R, Nicosia L, et al. Impact of hydrogel peri-rectal spacer insertion on prostate gland intra-fraction motion during 1.5 T MR-guided stereotactic body radiotherapy. Radiat Oncol 2020;15:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hwang ME, Mayeda M, Liz M, et al. Stereotactic body radiotherapy with periprostatic hydrogel spacer for localized prostate cancer: toxicity profile and early oncologic outcomes. Radiat Oncol 2019;14:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hwang ME, Black PJ, Elliston CD, et al. A novel model to correlate hydrogel spacer placement, perirectal space creation, and rectum dosimetry in prostate stereotactic body radiotherapy. Radiat Oncol 2018;13:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jones RT, Hassan Rezaeian N, Desai NB, et al. Dosimetric comparison of rectal-sparing capabilities of rectal balloon vs injectable spacer gel in stereotactic body radiation therapy for prostate cancer: lessons learned from prospective trials. Med Dosim 2017;42:341–7. [DOI] [PubMed] [Google Scholar]
- [20].Folkert MR, Zelefsky MJ, Hannan R, et al. Multi-institutional phase 2 trial of high-dose stereotactic body radiation therapy with temporary hydrogel spacer for low- and intermediate-risk prostate cancer. Int Jo Radiat Oncol 2017;99:1319. [Google Scholar]
- [21].King RB, Osman SO, Fairmichael C, et al. Efficacy of a rectal spacer with prostate SABR-first UK experience. Br J Radiol 2018;91:20170672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ogita M, Yamashita Y, Nozawa S, et al. A phase II study of stereotactic body radiotherapy with hydrogel spacer for prostate cancer; dosimetric comparison, acute toxicity and quality of life. Int J Radiat Oncol 2019;105:E298–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pryor D, Sidhom M, Arumugam S, et al. Phase 2 multicenter study of gantry-based stereotactic radiotherapy boost for intermediate and high risk prostate cancer (PROMETHEUS). Front Oncol 2019;9:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wilton L, Richardson M, Keats S, et al. Rectal protection in prostate stereotactic radiotherapy: a retrospective exploratory analysis of two rectal displacement devices. J Med Radiat Sci 2017;64:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ruggieri R, Naccarato S, Stavrev P, et al. Volumetric-modulated arc stereotactic body radiotherapy for prostate cancer: dosimetric impact of an increased near-maximum target dose and of a rectal spacer. Br J Radiol 2015;88:20140736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Saito M, Suzuki T, Sugama Y, et al. Comparison of rectal dose reduction by a hydrogel spacer among 3D conformal radiotherapy, volumetric-modulated arc therapy, helical tomotherapy, CyberKnife and proton therapy. J Radiat Res 2020;61:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zelefsky MJ, Pinitpatcharalert A, Kollmeier M, et al. Early tolerance and tumor control outcomes with high-dose ultrahypofractionated radiation therapy for prostate cancer. Eur Urol Oncol 2020;3:748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brenner DJ, Martinez AA, Edmundson GK, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002;52:06–13. [DOI] [PubMed] [Google Scholar]
- [29].Mohler JL, Antonarakis ES. NCCN guidelines updates: management of prostate cancer. J Natl Compr Canc Netw 2019;17:583–6. [DOI] [PubMed] [Google Scholar]
- [30].Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol 2019;20:1531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lehrer EJ, Kishan AU, Yu JB, et al. Ultrahypofractionated versus hypofractionated and conventionally fractionated radiation therapy for localized prostate cancer: a systematic review and meta-analysis of phase III randomized trials. Radiother Oncol 2020;148:235–42. [DOI] [PubMed] [Google Scholar]