Abstract
This study aimed to investigate the impact of hepatitis B virus (HBV) infection on the outcome of patients with advanced solid malignancies treated with programmed death receptor-1 (PD-1) inhibitors.
We retrospectively included patients treated with PD-1 inhibitors between August 2018 and April 2020. Propensity score matching (PSM) was performed to match the characteristics of the HBV and non-HBV groups. Objective response rate (ORR) and disease control rate (DCR) were compared between HBV and non-HBV groups using χ2 or Fisher exact tests. Kaplan-Meier and log-rank tests were used to analyze overall survival (OS) and progression-free survival (PFS).
A total of 120 patients, including 43 (35.8%) with HBV and 77 (64.2%) without HBV, were enrolled. Cases of HBV reactivation were not observed. In the entire study population, ORR and DCR did not significantly differ between both groups. After PSM, the study population comprised 39 patients, 15 with and 24 without HBV. The HBV group had an ORR of 55.6%, whereas the ORR in the non-HBV group was 36.8% (P = .35). Similarly, the DCR was 77.8% in the HBV group, as compared to 68.4% in the non-HBV group (P = .61). Additionally, HBV infection did not significantly affect OS (P = .54) and PFS (P = .64) in the unmatched cohort. Moreover, statistically significant differences regarding OS (P = .15) and PFS (P = .23) were also not detected after PSM.
In conclusion, the HBV infection status did not impact the therapy response or prognosis of patients treated with PD-1 inhibitors. Further prospective studies are needed to corroborate these findings.
Keywords: hepatitis B virus, prognosis, programmed death receptor-1 inhibitor, propensity score matching, response
1. Introduction
Cancer immunotherapy has been a breakthrough in the treatment of various malignancies in recent years. Immune checkpoint inhibitors (ICIs), especially programmed death receptor-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors, have demonstrated a survival benefit and/or durable disease control in advanced malignancies, including non-small cell lung cancer,[1] melanoma,[2] hepatocellular carcinoma,[3,4] renal cell carcinoma,[5] and microsatellite instability-high or mismatch repair-deficient solid tumors.[6] The indications for ICIs continue to expand at a fast rate.
More than 350 million people worldwide have chronic hepatitis B virus (HBV) infection, with about 75% located in Southeast Asia and the Western Pacific.[7] There is growing evidence showing that chronic HBV infection is strongly correlated with programmed death-ligand 1 (PD-L1) expression in tumor cells, suggesting a viral mediation of the systemic immune response.[8,9] The HBV X protein increases the expression of inflammatory cytokines, such as interleukin (IL)-6, IL-1β, and IL-18, thus promoting inflammatory damage.[10] In addition, the HBV X protein downregulates the level of eosinophil chemotactic protein-1,[11] affecting eosinophil function, including macrophage polarization and normalization of the tumor vasculature, which are known to promote tumor rejection.[12] Theoretically, HBV infection may affect the host immune status and alter the response to cancer immunotherapy.
In most clinical trials, particularly those testing immune-mediated therapy, patients with pre-existing hepatitis virus infection are excluded because of potentially insufficient treatment effects, increased toxicity, and fear of viral reactivation. However, hepatitis virus infection, especially HBV, is fairly prevalent among patients with cancer. A prospective multicenter study reported that 6.5% of 3000 newly diagnosed patients with cancer had a history of HBV infection, and 0.6% had concomitant HBV infection.[13] Unfortunately, data on the efficacy of ICIs in patients with HBV are limited and mostly found in case reports and case series.[14–17] To date, the effect of hepatitis viruses on the outcome of patients treated with ICIs remains unclear. Therefore, the influence of HBV on patients’ responses to ICIs needs to be further investigated.
This retrospective cohort study aimed to evaluate the impact of HBV infection on the outcome of cancer patients undergoing anti-PD-1 therapy. We divided the cohort into HBV and non-HBV groups to investigate the predictive and prognostic role of HBV infection in oncology clinical practice.
2. Materials and methods
2.1. Patients
In this retrospective study, patients who received PD-1 inhibitors at Ganzhou People's Hospital (The Affiliated Ganzhou Hospital of Nanchang University) between August 2018 and April 2020 were identified. Ethical approval for the study was granted by the Institutional Ethics Committee of Ganzhou People's Hospital, and the study complied with the principles of the Helsinki Declaration. The need for obtaining patient consent was waived due to the retrospective nature of our study. The inclusion criteria were clinical or histological diagnosis of advanced solid malignancies, at least one infusion of PD-1 inhibitors, and availability of baseline hepatitis B surface antigen (HBsAg) test results. Patients were excluded if they showed positivity for other viral markers, including antibodies against hepatitis A virus, hepatitis C virus (HCV), hepatitis D virus, hepatitis E virus, or human immunodeficiency virus (HIV). HBV infection was defined as a positive HBsAg test. Patients with HBV received regular antiviral therapy. Monitoring of serological markers, including HBsAg, anti-HBs antibody, anti-HBc antibody, HBeAg, anti-HBe antibody, and HBV DNA was performed every 1 to 3 months in patients with HBV.
2.2. Data collection
The baseline patient characteristics and laboratory values obtained 14 days preceding the initiation of PD-1 inhibitor treatment were assessed. Data on sex, age, HBV infection status, Eastern Cooperative Oncology Group (ECOG) score, primary tumor, PD-1 inhibitor substance, previous therapy, and treatment modality were extracted from medical records.
2.3. Response evaluation
Treatment response was evaluated by contrast-enhanced computed tomography or magnetic resonance imaging every 6 to 8 weeks after the first cycle of ICIs. Tumor efficacy was assessed as either complete (CR) or partial (PR) response, as well as stable (SD) or progressive (PD) disease, according to the Response Evaluation Criteria in Solid Tumors (RECIST) v.1.1. The objective response rate (ORR) was defined by the presence of either a CR or PR. The disease control rate (DCR) was defined as the sum of CR, PR, and SD.
2.4. Statistical analyses
Propensity score matching (PSM) was performed to match patients in the HBV and non-HBV groups according to tumor type. Statistical analyses were performed for the entire study cohort and separately for the PSM cohort.
Significance was evaluated using the chi-square or Fisher's exact tests to compare categorical variables between HBV and non-HBV groups. Overall survival (OS) was defined from the first cycle of PD-1 inhibitor administration to the date of cancer-related death or last contact. Progression-free survival (PFS) was defined as the time from the first treatment administration to the date of PD, cancer-related death, or last follow-up. The Kaplan-Meier and log-rank tests were used to analyze survival. A 2-tailed P value <.05 was considered significant. Statistical analyses were performed using SPSS Statistics v.26 (IBM) and R v.3.5 (R Core Team, R Foundation for Statistical Computing), and graphs were plotted using GraphPad Prism 8.0.2.
3. Results
3.1. Patient characteristics
Overall, 120 patients with advanced solid malignancies treated with PD-1 inhibitors were enrolled in the study. Using PSM, we minimized bias by adjusting for tumor type that may have an impact on treatment efficacy and survival. The baseline characteristics before and after PSM are depicted in Table 1. The unmatched cohort included 43 patients (35.8%) with HBV infection and 77 patients (64.2%) without HBV infection. The median age was 55 years, and patients in the HBV group were significantly older than the control group patients (P = .002). The main tumor types were liver cancer (n = 36, 30.0%), lung cancer (n = 27, 22.5%), esophageal cancer (n = 13, 10.8%), and melanoma (n = 12, 10.0%). Additionally, there were significant differences in tumor types between the two groups (P < .001). The histopathological findings of the patients are presented in Table S1, Supplemental Digital Content.
Table 1.
Patient characteristics.
| Unmatched | Matched | ||||||
| Characteristics | All (%) (n = 120) | HBV group (%) (n = 43) | Non-HBV group (%) (n = 77) | P | HBV group (%) (n = 15) | Non-HBV group (%) (n = 24) | P |
| Age, y | |||||||
| ≤55 | 61 (50.8) | 31 (40.3) | 30 (69.8) | 9 (60.0) | 10 (41.7) | ||
| >55 | 59 (49.2) | 46 (59.7) | 13 (30.2) | .002 | 6 (40.0) | 14 (58.3) | .265 |
| Median (range) | 55 (17–79) | ||||||
| Sex | |||||||
| Male | 90 (75.0) | 31 (72.1) | 59 (76.6) | 8 (53.3) | 19 (79.2) | ||
| Female | 30 (25.0) | 12 (27.9) | 18 (23.4) | .583 | 7 (46.7) | 5 (20.8) | .089 |
| ECOG score | |||||||
| 0–1 | 56 (47.1) | 16 (38.1) | 40 (51.9) | 8 (53.3) | 16 (66.7) | ||
| 2–3 | 63 (52.9) | 26 (61.9) | 37 (48.1) | .148 | 7 (46.7) | 8 (33.3) | .405 |
| Primary tumor | |||||||
| Liver cancer | 36 (30.0) | 32 (74.4) | 4 (5.2) | 4 (26.7) | 4 (16.7) | ||
| Lung cancer | 27 (22.5) | 2 (4.7) | 25 (32.5) | 2 (13.3) | 5 (20.8) | ||
| Esophgeal cancer | 13 (10.8) | 1 (2.3) | 12 (15.6) | 1 (6.7) | 1 (4.2) | ||
| Melanoma | 12 (10.0) | 4 (9.3) | 8 (10.4) | 4 (26.7) | 8 (33.3) | ||
| Others∗ | 32 (26.7) | 4 (9.3) | 28 (36.4) | <.001 | 4 (26.7) | 6 (25.0) | .978 |
| Treatment modality | |||||||
| PD-1inhibitor† monotherapy | 44 (36.7) | 13 (30.2) | 31 (40.3) | 7 (46.7) | 14 (58.3) | ||
| Combination therapy‡ | 76 (63.3) | 30 (69.8) | 46 (59.7) | .274 | 8 (53.3) | 10 (41.7) | .477 |
After PSM, the study population comprised a total of 39 patients (15 patients with HBV and 24 without HBV). For balance comparison, we evaluated standardized differences before and after PSM (Fig. S1, Supplemental Digital Content). After PSM, the HBV group included patients with liver cancer (n = 4), lung cancer (n = 2), esophageal cancer (n = 1), melanoma (n = 4), and others (n = 4), whereas the non-HBV group comprised patients with liver cancer (n = 4), lung cancer (n = 5), esophageal cancer (n = 1), melanoma (n = 8), and others (n = 6). No significant differences in tumor type, age, sex, ECOG score, and treatment modality were noted between the two groups (Table 1). Moreover, patients with HBV received regular antiviral therapy. None of these patients experienced HBV reactivation during or after anti-PD-1 therapy. HBV DNA and HBsAg levels before and after PD-1 inhibitor treatment are presented in Table S2, Supplemental Digital Content.
3.2. Association of HBV with response to PD-1 inhibitors before and after PSM
As shown in Table 2, 73 patients of the entire cohort were evaluated for treatment efficacy. Treatment responses were not assessed in 47 patients. Of those, 38 patients received one or two courses of immunotherapy and 9 additional patients were not evaluated by contrast-enhanced computed tomography or magnetic resonance imaging although they had been administered three to eight cycles of PD-1 inhibitors. Upon comparing Response Evaluation Criteria in Solid Tumors (RECIST)-based changes between patients in the HBV (5 PR, 9 SD, and 12 PD) and non-HBV (1 CR, 17 PR, 15 SD, and 14 PD) groups, no significant difference was found (P = .33).The ORR was 19.2% in the HBV group, while it was 38.3% in the non-HBV group (P = .09). Furthermore, patients with HBV had a DCR of 53.8% while that of the non-HBV group was 70.2% (P = .16).
Table 2.
Response evaluation according to HBV infection.
| Unmatched | Matched | |||||
| Cohort (n = 73) | HBV group | Non-HBV group | P | HBV group | Non-HBV group | P |
| RECIST change | ||||||
| CR | 0 | 1 | 0 | 1 | ||
| PR | 5 | 17 | 5 | 6 | ||
| SD | 9 | 15 | 2 | 6 | ||
| PD | 12 | 14 | .328 | 2 | 6 | .628 |
| Objective response | ||||||
| CR + PR | 5 | 18 | 5 | 7 | ||
| SD + PD | 21 | 29 | .093 | 4 | 12 | .350 |
| Disease control | 0 | 0 | ||||
| CR + PR + SD | 14 | 33 | 7 | 13 | ||
| PD | 12 | 14 | .162 | 2 | 6 | .609 |
After PSM, the ORR and DCR were 55.6% and 77.8% in the HBV group (5 PR, 2 SD, and 2 PD). Similarly, the non-HBV group (1 CR, 6 PR, 6 SD, and 6 PD) had an ORR of 36.8% and a DCR of 68.4%. No significant difference was detected between the 2 groups (Table 2).
3.3. Effect of HBV infection on survival before and after PSM
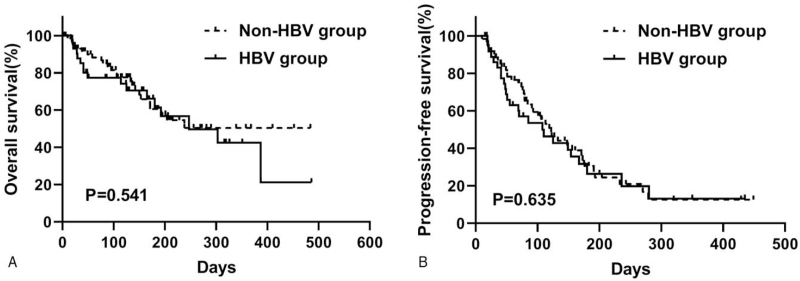
By the cutoff date in April 2020, 41 cancer-related deaths were observed among 120 patients (34.2%), and progression was found in 65 of 99 cases (65.7%). The median OS of the entire unmatched cohort was 303 days. Kaplan-Meier curves for OS and PFS, based on HBV infection, were used (Fig. 1). Patients with HBV had a median OS of 247 days (95% confidence interval [CI], 84–410), and the median OS was not reached in the non-HBV group (P = .54). The 6-month OS rate was 61.4% in the HBV group and 60.5% in the non-HBV group. In addition, the median PFS was 108 days (95% CI, 45–171) for HBV patients and 122 days (95% CI, 89–155) for non-HBV patients (P = .51).
Figure 1.
Kaplan-Meier curves of overall survival (A) and progression-free survival (B) based on HBV infection before propensity score matching. HBV = hepatitis B virus.
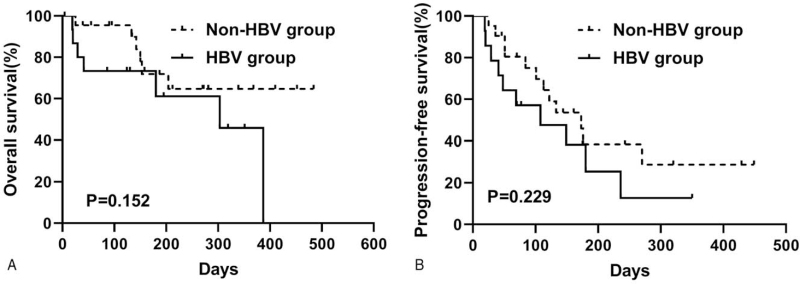
After PSM, the HBV group reached a median OS of 303 days (95% CI, 189–417), whereas the median OS was not reached in the non-HBV group (P = .15; Fig. 2A). The 6-month OS rate was 61.1% in the HBV group, as compared to 71.9% in the non-HBV group. The median PFS was 108 days (95% CI, 0–224) in the HBV group and 173 days (95% CI, 111–235) in the non-HBV group (P = .23; Fig. 2B).
Figure 2.
Kaplan-Meier curves of overall survival (A) and progression-free survival (B) based on HBV infection after propensity score matching. HBV = hepatitis B virus.
The predominant tumor type in the entire study cohort was liver cancer, comprising 35 hepatocellular carcinoma cases and one cholangiocellular carcinoma case. As shown in Fig. S2, Supplemental Digital Content, HBV infection did not significantly influence either OS (P = .92) or PFS (P = .72) in the liver cancer cohort. Patients with HBV had a median OS and PFS of 192 and 85 days, respectively. In the non-HBV group, the median OS and PFS were 153 and 84 days, respectively.
4. Discussion
Patients with cancer and HBV infection are not rare. Given the prevalence, it is inevitable that oncologists will manage patients with HBV infection. However, the efficacy of anti-PD-1 therapy in these patients is unclear because they are excluded from most oncology trials involving ICIs. To the best of our knowledge, this study is the first to demonstrate similar efficacy and survival for cancer patients with and without HBV undergoing PD-1 inhibitors in a clinical setting.
Data on the safety and efficacy of ICI therapy in patients with chronic viral infection and advanced-stage cancers are limited. Several case reports have described ICIs to be well-tolerated by patients with non-small cell lung cancer,[14,17,18] melanoma,[18,19] or hepatocellular carcinoma[15] and hepatitis virus infection. Shah et al[20] retrospectively evaluated treatment toxicity and response in 50 advanced-stage cancer patients who had HIV, HBV, or HCV infections and were treated with ICIs. In this case series, immunotherapy was shown to be safe, and responses to ICIs were prolonged among patients with chronic viral infection. Zhang et al[21] conducted a retrospective study on the utility of antiviral therapy in HBsAg-positive patients treated with ICIs in preventing HBV reactivation. Although HBV reactivation occurred in a small subset of patients who were seropositive for HbsAg, antiviral therapy and careful viral monitoring were allowed for safe administration of ICIs to HBV-infected cancer patients. However, the study did not provide details on the antitumor response and survival. In our study, which evaluated the impact of HBV infection in a large cohort of patients treated with anti-PD-1 therapy, patients with HBV received regular antiviral therapy. HBV reactivation was not observed in any patient during or after anti-PD-1 therapy.
In the entire unmatched study population, no significant correlation between HBV infection and RECIST change was found. Moreover, there were no significant differences in ORR and DCR regarding HBV infection before and after PSM. These efficacy results are consistent with those from clinical trials of anti-PD-1 therapy in patients with hepatocellular carcinoma. In the KEYNOTE-224 trial, ORRs were found in 5 (13%) of 39 hepatocellular carcinoma patients infected with HBV or HCV and 13 (20%) of 64 uninfected patients, with no difference among the groups.[4] In the CheckMate 040 trial, the ORR and DCR among HBV-infected hepatocellular carcinoma patients in the dose-expansion phase were 14% and 55%, respectively. However, these results were not matched for statistical comparisons with those of patients without HBV.[3]
In the present study, we also investigated the effects of HBV infection on the survival of patients treated with PD-1 inhibitors. In the unmatched cohort, there were no significant differences for OS and PFS between the two study groups. Moreover, statistically significant differences regarding OS and PFS were also not found after PSM, and in the liver cancer cohort, no differences in OS and PFS were observed between HBV and non-HBV patients. Similarly, a retrospective study on 32 metastatic non-small cell lung cancer patients with HBV suggested a lack of HBV impact on PFS associated with ICI therapy.[14] Another retrospective analysis reported that non-small cell lung cancer patients with previous HBV infection or pulmonary tuberculosis had better survival outcomes in terms of PFS and OS. However, this could be accounted for by the difference in baseline characteristics, owing to the small size of this study.[22] In the present study, we also observed substantial and prolonged responses in patients with HBV similar to those in non-HBV patients. Had these patients been excluded from ICI treatment because of their HBV infection, they would not have been able to experience the long-term benefits of ICI therapy. Considering the findings of our study, an HBV infection should not preclude cancer patients from receiving ICI treatment. Recently, published systematic reviews concluded that ICIs are safe and effective for advanced cancer patients with HBV.[23–25] Several ongoing trials are assessing the efficacy and toxicity of ICIs in patients with different malignancies and concurrent chronic viral infections.
The persistent existence of hepatitis virus infection results in antiviral CD8+ T cell exhaustion through negative co-stimulatory molecules, such as PD-1.[26] In a preclinical model, PD-1/PD-L1 blockade restored the function of exhausted virus-specific CD8+ T cells,[27] suggesting that PD-1 inhibitors might theoretically have a therapeutic application in chronic viral infection. Combination therapies incorporating immune checkpoint inhibitors and antiviral drugs can be explored to effectively treat chronic viral infection. Improving our understanding of the pathways involved in viral latency and tumor resistance to ICI therapy is critical to the rational development of immunotherapy in patients affected by cancer and chronic viral disease.
This study has several limitations, including its retrospective nature and small sample size. To our knowledge, however, this study involved the largest cohort of patients with HBV treated with ICIs. Moreover, the variation in the primary tumor type among patients may have been a confounder. The use of PSM improved the data quality but resulted in a smaller sample size. Given the correlation between HBV infection and PD-L1 expression, tumor mutation burden should be taken into account. To verify the effects of HBV infection on the efficacy and survival of cancer patients undergoing treatment with PD-1 inhibitors, large randomized trials are needed.
5. Conclusions
This is the first study to acknowledge that HBV infection does not significantly affect efficacy or survival in cancer patients receiving PD-1 inhibitors. Thus, HBV infection should not be a contraindication for ICI treatment. Regular monitoring of HBV DNA and antiviral prophylaxis is advised during or after ICI therapy. As more information on the tolerability of ICIs in patients with HBV is compiled, clinical trial eligibility criteria should be modified to allow more patients to benefit from investigational agents. This conclusion needs further confirmation by prospective studies.
Author contributions
WP and LZ: study design and manuscript writing and revision. PZ: data generation and statistical analysis. HL and ZL: data elaboration and manuscript revision. QN: data collection. All authors read and approved the final manuscript.
Conceptualization: Liting Zhong, Weiwei Peng.
Data curation: PinShun Zhong.
Investigation: Huafeng Liu, Qihong Nie.
Project administration: Huafeng Liu, Qihong Nie.
Software: Zelei Li.
Writing – original draft: Liting Zhong, PinShun Zhong.
Writing – review & editing: Weiwei Peng.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CR = complete response, CTLA-4 = cytotoxic T-lymphocyte-associated protein 4, DCR = disease control rate, ECOG = Eastern Cooperative Oncology Group, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCV = hepatitis C virus, HIV = human immunodeficiency virus, ICI = immune checkpoint inhibitor, IL = interleukin, ORR = objective response rate, OS = overall survival, PD = progressive disease, PD-1 = programmed death receptor-1, PD-L1 = programmed death-ligand 1, PFS = progression-free survival, PR = partial response, PSM = propensity score matching, RECIST = Response Evaluation Criteria in Solid Tumors, SD = stable disease.
How to cite this article: Zhong L, Zhong P, Liu H, Li Z, Nie Q, Peng W. Hepatitis B virus infection does not affect the clinical outcome of anti-programmed death receptor-1 therapy in advanced solid malignancies: Real-world evidence from a retrospective study using propensity score matching. Medicine. 2021;100:49(e28113).
LZ and PZ contributed equally to this work.
Funding: This work was supported by the Health Science and Technology Project of Jiangxi Province (202140765).
The authors report no conflicts of interest.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
Including Hodgkin lymphoma (n = 7), gastric adenocarcinoma (n = 5), colorectal adenocarcinoma (n = 3), soft tissue sarcoma (n = 3), cervical squamous cell carcinoma (n = 2), ovarian cancer (n = 2), ureteral urothelial carcinoma (n = 2), cutaneous squamous cell carcinoma (n = 2), squamous cell carcinoma (n = 1), small bowel adenocarcinoma (n = 1), nasopharyngeal carcinoma (n = 1), penile squamous cell carcinoma (n = 1), pancreatic cancer (n = 1), NK/T-cell lymphoma (n = 1).
Including pembrolizumab, nivolumab, toripalimab, camrelizumab, sintilimab.
Including PD-1 inhibitor plus chemotherapy (n = 41) and targeted agent (n = 35).
ECOG = Eastern Cooperative Oncology Group.
CR = complete response, PD = progressive disease, PR = partial response, SD = stable disease.
References
- [1].Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- [2].Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;18:01–6. [DOI] [PubMed] [Google Scholar]
- [5].Motzer RJ, Escudier B, George S, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 2020;126:4156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site–when a biomarker defines the indication. N Engl J Med 2017;1409–12. [DOI] [PubMed] [Google Scholar]
- [7].Dienstag JL. Hepatitis B virus infection. N Engl J Med 2008;359:1486–500. [DOI] [PubMed] [Google Scholar]
- [8].G. Lin WZ, Chen XH, Huang C, Lin XD, Huang YJ, Li C. Increase of programmed death ligand 1 in non-small-cell lung cancers with chronic hepatitis B. Ann Oncol 2017;516–7. [DOI] [PubMed] [Google Scholar]
- [9].Sallberg M, Pasetto A. Liver, tumor and viral hepatitis: key players in the complex balance between tolerance and immune activation. Front Immunol 2020;11:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ling LR, Zheng DH, Zhang ZY, et al. Effect of HBx on inflammation and mitochondrial oxidative stress in mouse hepatocytes. Oncol Lett 2020;19:2861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang DY, Zou LP, Liu XJ, Zhu HG, Zhu R. Chemokine expression profiles of human hepatoma cell lines mediated by hepatitis B virus X protein. Pathol Oncol Res 2016;22:393–9. [DOI] [PubMed] [Google Scholar]
- [12].Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol 2015;16:609–17. [DOI] [PubMed] [Google Scholar]
- [13].Ramsey SD, Unger JM, Baker LH, et al. Prevalence of hepatitis B virus, hepatitis C virus, and HIV infection among patients with newly diagnosed cancer from academic and community oncology practices. JAMA Oncol 2019;5:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Byeon SCJ, Jung HA. PD-1 inhibitors for non-small cell lung cancer patients with special issues: Real-world evidence. Cancer Med 2020;9:2352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pil Soo Sung HY, Nam Ik Han1, Jeong Won Jang, et al. Real-World Outcomes of Nivolumab in PatientsWith Unresectable Hepatocellular Carcinoma in an Endemic Area of Hepatitis B Virus Infection. Front Oncol 2020;10:1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Akar E, Baytekin HF, Deniz H, Tural D. Safe use of nivolumab in a patient with renal cell carcinoma and hepatitis B. J Oncol Pharm Pract 2019;26:1022–4. [DOI] [PubMed] [Google Scholar]
- [17].Pertejo-Fernandez A, Ricciuti P B, Hammond M SP, et al. Safety and efficacy of immune checkpoint inhibitors in patients with non-small cell lung cancer and hepatitis B or hepatitis C infection. Lung Cancer 2020;145:181–5. [DOI] [PubMed] [Google Scholar]
- [18].Kothapalli A, Khattak MA. Safety and efficacy of anti-PD-1 therapy for metastatic melanoma and non-small-cell lung cancer in patients with viral hepatitis: a case series. Melanoma Res 2018;28:155–8. [DOI] [PubMed] [Google Scholar]
- [19].Tio M, Rai R, Ezeoke OM, et al. Anti-PD-1/PD-L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. Eur J Cancer 2018;104:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shah NJ, Al-Shboor G, Blackburn M, et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J Immunother Cancer 2019;7:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang X, Zhou Y, Chen C, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer 2019;7:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gloria HJ, Chan YXG, Jia Li Low, Yiqing Huang, et al. Immune checkpoint inhibition for non-small cell lung cancer in patients with pulmonary tuberculosis or Hepatitis B: experience from a single Asian centre. Lung Cancer 2020;146:145–53. [DOI] [PubMed] [Google Scholar]
- [23].Tapia Rico G, Chan MM, Loo KF. The safety and efficacy of immune checkpoint inhibitors in patients with advanced cancers and pre-existing chronic viral infections (Hepatitis B/C, HIV): A review of the available evidence. Cancer Treat Rev 2020;86:102011. [DOI] [PubMed] [Google Scholar]
- [24].Dimitrios C, Ziogas FK, Evangelos Cholongitas, et al. Reconsidering the management of patients with cancer with viral hepatitis in the era of immunotherapy. Journal for immunotherapy of cancer 2020;8:e000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pu D, Yin L, Zhou Y, et al. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: a systematic review. Medicine 2020;99:e19013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nakamoto N, Cho H, Shaked A, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS pathogens 2009;5:e1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Balsitis S, Mason GV, ChaniewskiS PJ, Levine SM, Wichroski MJ. Safety and efficacy of anti-PD-L1 therapy in the woodchuck model of HBV infection. PLoS One 2018;13:e0190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.