Abstract
Background:
To find out, based on the available recent randomized controlled trials (RCTs), if the nonsurgical interventions commonly used for knee osteoarthritis patients are valid and quantify their efficiency.
Methods:
The database of MEDLINE and EMBASE were searched for RCTs evaluating nonsurgical treatment strategies on patients with mild to moderate knee osteoarthritis. A Bayesian random-effects network meta-analysis was performed. The primary outcome was the mean change from baseline in the Western Ontario and McMaster university (WOMAC) total score at 12 months. Raw mean differences with 95% credibility intervals were calculated. Treatments were ranked by probabilities of each treatment to be the best.
Results:
Thirteen trials assessed 7 strategies with WOMAC at 12 months: injection of platelet rich plasma (PRP), corticosteroids, mesenchymal stem cells (MSCs), hyaluronic acid, ozone, administration of nonsteroidal anti-inflammatory drugs with or without the association of physiotherapy. For treatment-specific effect size, a greater association with WOMAC decrease was found significantly for MSCs (mean difference, −28.0 [95% CrI, −32.9 to −22.4]) and PRP (mean difference, −19.9 [95% CrI, −24.1 to −15.8]). Rank probabilities among the treatments indicated that MSCs had a much higher probability (P = .91) of being the best treatment compared with other treatments, while PRP ranked as the second-best treatment (P = .89).
Conclusion:
In this systematic review and network meta-analysis, the outcomes of treatments using MSCs and PRP for the management of knee osteoarthritis were associated with long-term improvements in pain and function. More high quality RCTs would be needed to confirm the efficiency of MSCs and PRP for the treatment of patients with knee osteoarthritis.
Keywords: knee, orthopedics, osteoarthritis, rheumatology, therapeutics
1. Introduction
Knee osteoarthritis (OA) is a common chronic degenerative disease due to bone and cartilage degeneration affecting up to 19% of adults aged 45 and older,[1] and is a major contributor to functional and social impairment, disability, reduced independence, and poorer quality-of-life.[2,3] Its clinical features mainly include cartilage degenerative lesions, with clinical manifestations such as limited range of motion in the knee, joint swelling, pain, stiffness and deformity (Figure S1, Supplemental Digital Content). Radiographic evidence of knee OA according to the Kellgren and Lawrence classification is present in approximately 30% of adults over the age of 65.[4] Worldwide estimates report that 9.6% of men and 18.0% of women over the age of 60 years have symptomatic OA[5] and that the number of people affected by OA will increase by about 50% over the next 20 years.[6,7] Therefore, there is an increasing need for urgent attention to this disease.
Current knee OA treatment strategies use surgical and non-surgical interventions.[6,8,9] Total knee replacement also known as total knee arthroplasty is considered an effective procedure for treating end-stage knee OA. However, not all individuals with knee OA can or even wish to proceed with surgery due to various comorbidities and/or age or health-related restrictions. Additionally, access to surgical intervention may be limited or delayed in many countries due to budgetary restrictions and limited resources, such as operating time or surgeon availability. Moreover, perioperative complications such as loosening,[10] infection,[11] instability,[12] fractures,[13] pain or discomfort may occur during and after total knee replacement.[14,15] Furthermore, the augmentation in the number of young patients undergoing knee surgery also increases the lifetime risk of requiring revision surgery.[16] For all these reasons, 15% to 30% of patients have reported dissatisfaction after total knee arthroplasty.[17] Therefore, as the majority of non-surgical interventions are safer, have a lower cost, and mobilize a less technical platform, they are required in the first step of the knee OA management before the need for surgery.[6,8] Indeed, these non-surgical interventions are meant to reduce or eliminate pain and improve joint function through cartilage repair which can delay or avoid the need for arthroplasty.
Although there are several guidelines for knee OA management, there has been no consensus reached concerning the efficacy of many available non-surgical treatment strategies. Due to the large number of publications evaluating multiple types of osteoarthritis treatments, recent meta-analyses seem to be an essential way especially with the appearance of new innovative treatments. The aim of our study was to evaluate the long-term efficiency of these treatments published recently by using a Bayesian approach. This method allows comparison of all available non-surgical knee OA strategies.
2. Methods
2.1. Literature search strategy
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement.[18]
The literature was screened and extracted by the authors using the electronic database of MEDLINE (PubMed) and EMBASE. We chose to evaluate a consequent amount of recent studies, namely between January 1, 2017 and March 1, 2020. This led to the evaluation of 864 clinical trials. Titles and abstracts were screened in order to determine if the identified articles met the inclusion and exclusion criteria. The full-text of selected articles was then further evaluated.
Eligible trials included placebo-controlled RCTs and those comparing any active treatment alone or in combination with another intervention. The inclusion and exclusion criteria are described below:
2.2. Inclusion criteria
Placebo and active-controlled randomized controlled trials (RCTs).
Patients with early knee OA (Kellgren–Lawrence grades 1–3).
At least 6 months of follow up.
Full version in English.
Studies that perform a patient global assessment using WOMAC total score and/or visual analogue scale (VAS) for pain.
2.3. Exclusion criteria
Additionally, trials will be excluded if they are:
Animal studies.
Studies which evaluated clinical postoperative outcomes after total knee arthroplasty.
Studies published prior to 2017, or after March 1, 2020.
Surgical interventions or perioperative treatments.
Books, reviews, meta-analyses, study protocols, case reports, expert opinions commentary, conference papers, unpublished results.
Studies evaluating patients with severe OA (Kellgren–Lawrence grade 4).
Studies focusing only on specific categories of patients (e.g., obese patients).
Studies with only graphs without available or accessible data.
We chose the WOMAC total score and/or visual analogue scale (VAS) for pain because these instruments are the most used in the literature for their high level of validity and reliability[19,20] in patients with knee osteoarthritis. Furthermore, knee pain and function are likely to be the factors that matter the most to patients, physicians, and caregivers.
The WOMAC is a disease-specific and self-administered questionnaire used in the evaluation of hip and knee OA. It consists of 24 questions, grouped into 3 subscales including pain (5 questions), stiffness (2 questions), and physical function (17 questions) for the activity of daily living during the past 48 hours.[19] In the Likert scale version, each answer is scored on a scale from 0 to 4: 0 represents “none” and 4 represents “extreme.” In the VAS version, each answer is scored on a 100-mm VAS: 0 represents “none” and 100 mm represents “extreme,” thus, higher scores on the WOMAC indicate worse pain, stiffness, and functional limitations.
When pain severity was assessed on a 100-mm or 10-cm VAS, higher score indicates greater pain intensity.
2.4. Outcomes and data extraction
The primary outcome was mean change from baseline to 12 months (long-term) with the WOMAC total score. Secondary outcomes were changes from baseline to 3 months (short-term), to 6 months (middle-term) with WOMAC and from baseline to 3 months, to 6 months, and to 12 months with VAS.
For each outcome, the change from baseline was extracted at each time point if reported; otherwise, numerical data for the outcome were extracted at baseline and at each time point. Other extracted data included baseline demographic characteristics (age, sex, body mass index), clinical characteristics (Kellgren–Lawrence grade), and the dose of each treatment.
Treatments administered at different doses have been considered as a single intervention. If the same trial compared different treatment doses, the trial has been split in more pairwise comparisons against hyaluronic acid (HA) or placebo. This methodological choice has been taken assuming that no correlation structure is evident among different dose effects in the same randomized controlled trial.
Only trials with extractable data were included. No additional information was requested from authors.
2.5. Quality and risk of bias assessment
Quality was assessed independently by the authors. The Quality of the included trials was assessed using the Cochrane Collaboration tool for assessing risk of bias in RCTs.[21] Each study was evaluated as low, high, or unclear risk of bias according to the randomization, allocation concealment, blinding, completeness of outcome data, and selective outcome reporting. The GRADE methodology was used to assess the quality of evidence (GRADEpro, McMaster University, 2020).
2.6. Data synthesis and analysis
The imputation of the correlation method was used when standard deviations were available for absolute baseline and follow-up values, but not for the mean change values[22] by using the correlation value r = 0.5. This was selected as a plausible value based on other studies.[23]
When studies did not report mean change, these values were calculated as the arithmetic difference between baseline and follow-up. VAS and WOMAC scale scores were all normalized to a scale from 0 to 100 to ensure comparability between all the studies for each outcome measure.
A Bayesian multiple treatment network meta-analysis[24] with random effects and uninformative priors was performed and considered both placebo- and active-controlled trials. The analysis was performed on the raw mean difference with 95% credibility intervals for the treatment-specific and relative effect sizes for all eligible trials using the WOMAC or the VAS.
The reference treatment group to be compared against is “Placebo.” In the case of the absence of placebo “Hyaluronic acid” is used as the reference treatment because HA is widely used by physicians and is also the most commonly used treatment in the trials. The minimum clinical important difference for the total WOMAC score is 10.[25]
The between-study standard deviation was modeled using a uniform distribution of the 0 to 10 interval.[26] A random effects model was computed using Markov chain Monte Carlo methods with Gibbs sampling based on simulations of 200,000 iterations in each of 4 chains.
The number of iterations is considered sufficiently large to produce accurate posterior estimates.
Homogeneity and consistency assumptions were evaluated using node splitting method.
A rankogram plot is used to graph the probabilities of each treatment having each of the different possible ranks among the treatments. The treatment rank probabilities are based on the marginal effect measures. A higher event probability implies a better treatment.
The analyses were conducted using the R-evolution version 4.0.3 and the pcnetmeta package version 2.7 that interfaces with Just Another Gibbs Sample version 4.3.0 for computing a Markov chain Monte Carlo simulation.
The sensitivity analyses were conducted to evaluate the robustness of the model. The post hoc sensitivity analyses were performed using alternative statistical methods to those described above.
A sensitivity analysis based on a Fixed effect model instead of the Random effect model has been conducted for each outcome. Furthermore, a sensitivity analysis has been conducted for each outcome based on an empirical informative prior on heterogeneity distribution: inverse-gamma distribution instead of the uninformative prior.
2.7. Ethical approval
Ethical approval was not necessary due to the study design.
3. Results
3.1. Study selection
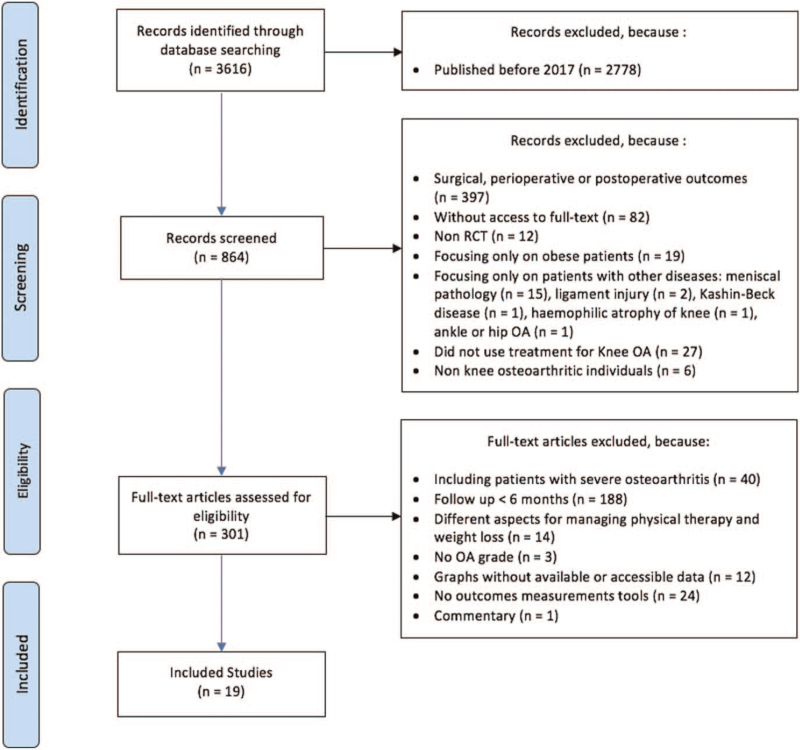
A total of 838 studies were identified through database searching. After analysis in accordance with the inclusion and exclusion criteria, 19 RCTs (N = 2488 patients) met the eligibility criteria and were included in this meta-analysis. The diagram in Fig. 1 summarizes the selection process.
Figure 1.
Trial selection process.
The mean age of the included patients is 57.65 ± 3.82 and a higher proportion (around 65%) of women than men with a mean body mass index of 28.17 ± 2.04. Ten of the 19 trials included >100 participants in all groups.
Disease severity was defined based on Kellgren–Lawrence radiological grading classification. Among the 19 studies, 5 have included patients with Kellgren–Lawrence grade 1 and 2; 9 studies with Kellgren–Lawrence 2 and 3; 4 studies with Kellgren–Lawrence grade 1–3 and 1 study with Kellgren–Lawrence grade 3.
3.2. Study characteristics
A total of 16 different interventions were studied in these RCTs. The included studies comprised physical and pharmacologic approaches but not any psychosocial or mind-body studies. Injection of platelet rich plasma (PRP) was assessed in 8 trials27–33,40, HA in 12 trials28–30,33,34,36–38,40–42,44, mesenchymal stem cells (MSCs) in 2 trials34,35, corticosteroids (CS) in 2 trials28,31, nonsteroidal anti-inflammatory drugs (NSAID) in 1 trial29,45, physical therapy associated with NSAID in 1 trial35, prolotherapy (dextrose) in 1 trial27, dexamethasone (Dex) in 1 trial37, combination of dexamethasone and hyaluronic acid (Dex + HA) in 1 trial37, ozone injection in 1 trial33, stromal vascular fraction (SVF) in 1 trial41, bone marrow aspirate concentrate in 1 trial43, amniotic suspension allograft in 1 trial42, administration of Q-Actin in 1 trial39, glucosamine chondroitin in 1 trial39, and Chondroitin sulfate in 1 trial45.
Out of the 19 RCTs included, 4 were placebo-controlled and 15 were comparing ≥2 interventions. Included trials are presented by outcome measures: WOMAC in Table S1, Supplemental Digital Content VAS in Table S2, Supplemental Digital Content.
The methodology quality and risk of bias for the included studies are displayed in Figure S2, Supplemental Digital Content, Supplemental Digital Content. Overall, all patients were randomized to receive an OA treatment. Seven studies maintained allocation concealment but the other studies failed to describe it clearly. The risk of performance bias was high in 13 studies and unclear in 1 study. It might be important for the physicians to be aware of treatments for patient safety. Detection bias was high in 3 studies and unclear in 4 studies. The attrition bias was high in 8 studies and unclear in 1 study. Unlike performance and attrition biases, reporting bias was low in >75% of the evaluated studies.
The quality of evidence for the primary outcome studies according to the GRADE system is presented in Table S5, Supplemental Digital Content.
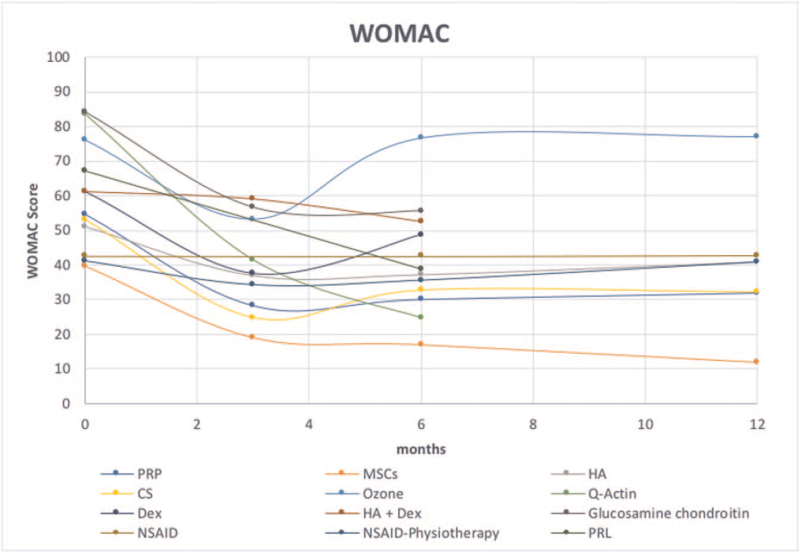
The treatments that have been compared together are described in Table S4, Supplemental Digital Content by the time point, the outcome measure, number of trials, and number of patients. The total score variation from baseline to the last follow-up are presented for each treatment in Fig. 2 for WOMAC and in Figure S3, Supplemental Digital Content for VAS.
Figure 2.
Curves showed the total scores variation of knee OA strategies from baseline to the last follow-up visit according to Western Ontario and McMaster university (WOMAC). OA = osteoarthritis.
Effects estimates were heterogeneous and inconsistent among studies but the node-splitting analysis of inconsistency was statistically insignificant. Consequently, there were no significant differences between the direct and indirect comparisons in the main analysis (Figure S4, Supplemental Digital Content). A quantitative synthesis of the evidence through a network meta-analysis was appropriate.
3.3. Primary outcome
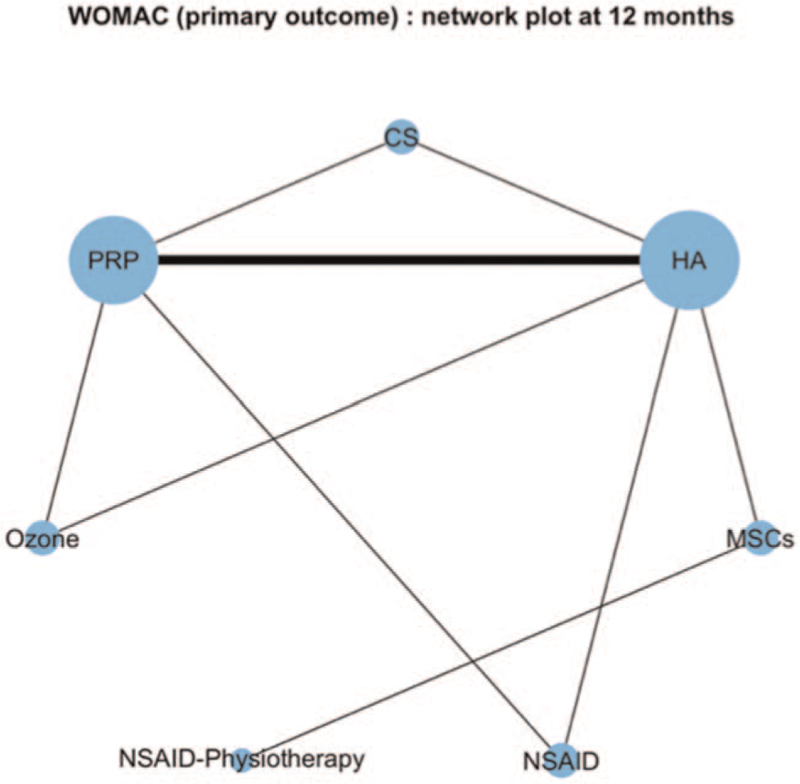
There were 15 trials assessing 7 strategies with WOMAC at 12 months: PRP, CS, MSCs, HA, Ozone, NSAID, and NSAID with association with physiotherapy (Table S4, Supplemental Digital Content). The network plot for the primary outcome appears in Fig. 3.
Figure 3.
Network plot for the primary outcome. The area of every circle is proportional to the number of randomly assigned patients and indicates the sample size. The width of the lines is proportional to the number of trials that directly compared the 2 strategies.
For treatment-specific effect size, a greater WOMAC decrease was significantly found for the MSCs (mean difference, −28.0 [95% CrI, −32.9 to −22.4]) and PRP (mean difference, −19.9 [95% CrI, −24.1 to −15.8]) associated to a clinically significant difference. Moreover, a significant mild improvement in the knee clinical status was found for HA (mean difference, –8.5 [95% CrI, −11.9 to −5.2]) but not for CS (mean difference, −9.5 [95% CrI, −15.0 to –0.7]), NSAID (mean difference, −5.4 [95% CrI, −13.9–0.7]), NSAID associated with physiotherapy (mean difference, –0.2 [95% CrI, −9.6–9.2]) and ozone injection (mean difference, 0.9 [95% CrI, −4.4–6.1]) (Figure S5, Supplemental Digital Content).
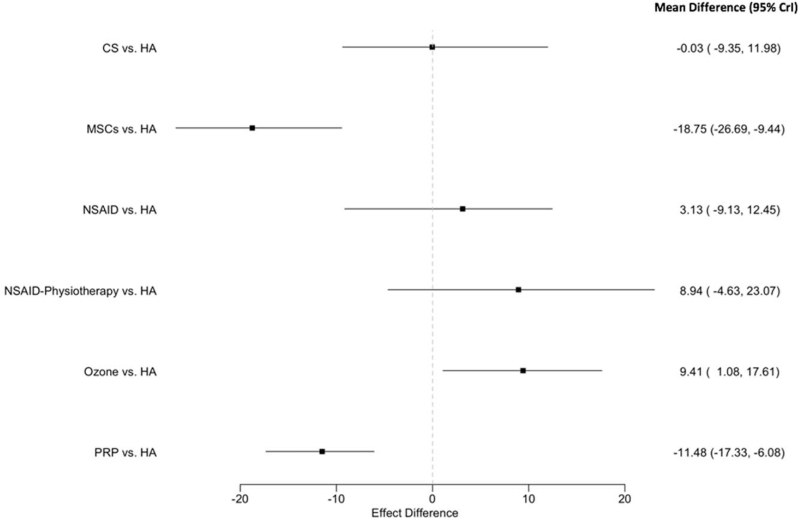
By comparing the treatments to hyaluronic acid “the reference treatment,” significant differences were observed between MSCs versus HA and PRP versus HA with an association with improvement in the WOMAC (decrease) for these 2 strategies but not for the 4 other strategies (Fig. 4).
Figure 4.
Forest plot for the strategies’ effects compared with the reference treatment for primary outcome (WOMAC score at 12 months). Estimates are expressed on a 0 to 100 scale. Point estimates refer to the posterior mean. The bars indicate 95% credibility intervals (CrIs). WOMAC = Western Ontario and McMaster university.
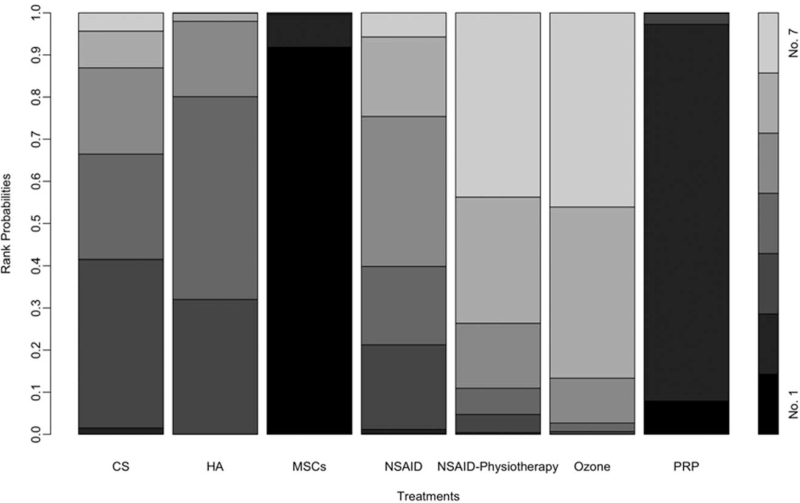
Rank probabilities indicate that MSCs have a much higher probability (P = .91) of being the best treatment among the treatments, however, PRP has a higher probability of being the second-best treatment (P = .89). CS and HA are ranked as the third and the fourth best treatments (P = .40 and P = .32, respectively). Figure 5 shows the plots of treatment rank probabilities.
Figure 5.
Plots of treatments rank probabilities for primary outcome. A darker area indicates the probability of being a higher rank, thus the black areas show the probabilities of being the best treatment.
Injection of ozone was significantly associated with increasing in WOMAC and worse knee status compared with the pretreatment. The injection of HA was significantly better than ozone injection for knee osteoarthritis.
3.4. Secondary outcomes
WOMAC at 3 and 6 months
The strategies were compared with hyaluronic acid (network plots appear in Figures S6a, Supplemental Digital Content and S6b, Supplemental Digital Content). A significant difference was only found between Q-Actin versus HA and associated with improvement in the WOMAC at 3 months (mean difference, −21.3 [95% CrI, −28.4 to −13.1]).
At 6 months, there is significant differences between CSE versus HA, MSCs versus HA, and PRP versus HA. Q-Actin showed a greater improvement in WOMAC (mean difference, −42.4 [95% CrI, −49.3 to −34.7]), compared with MSCs (mean difference, –9.2 [95% CrI, −15.6 to −2.9]) and PRP (mean difference, –8.8 [95% CrI, −13.4 to −4.8]). (Forest plots are presented in Figures S7a , Supplemental Digital Content, and S7b, Supplemental Digital Content).
VAS at 3, 6 and 12 months
Four of 16 RCTs with VAS have used placebo as the control group. The placebo group was defined as the reference treatment in order to make comparisons between the strategies. (Network plots appear in Figures S8a, Supplemental Digital Content, S8b, Supplemental Digital Content, and S8c, Supplemental Digital Content).
A significant difference was found between autologous adipose-derived stromal vascular fraction (SVF) and placebo associated with decreasing in pain at 3 months (mean difference, –12.7 [95% CrI, −24.1 to −3.3]) but not for other strategies.
At 6 months, SVF (mean difference, –19.9 [95% CrI, −31.8 to −10.7]), chondroitin sulfate (mean difference, –10.7 [95% CrI, −22.3 to 0.5]), and MSCs (mean difference, –10.0 [95% CrI, −20.3 to −0.2]) showed a significant difference associated with pain decrease compared with placebo.
A significant difference associated with decreasing in pain was found between SVF (mean difference, –23.2 [95% CrI, −32.0 to −15.7]), MSCs (mean difference, –20.6 [95% CrI, −28.1 to −12.6]), and PRP (mean difference, –15.0 [95% CrI, −20.7 to −11.4]) compared with hyaluronic acid (forest plots are presented in Figures S9a, Supplemental Digital Content, S9b, Supplemental Digital Content, and S9c, Supplemental Digital Content).
3.5. Sensitivity analyses
The sensitivity analyses were partially consistent with the results of the main analysis (Tables S6a–S6b, Supplemental Digital Content) when alternative statistical methods were used.
4. Discussion
In this systematic review and network meta-analysis, we chose to evaluate recent available randomized clinical trials in order to reflect contemporary practice. The interventions had different effects on the participants suffering from knee OA. In primary outcome (WOMAC at 12 months), MSCs and PRP were significantly better than the chosen control and associated with improvement in knee status. Otherwise, CS improved outcomes but did not perform better than the control.
Ozone injection is the only intervention for which knee pain and/or function got worse at the end of the study compared with the baseline. Ozone injection showed no improvement in pain and function at 12 months (+5.55% and +1.31%, respectively). In addition, the results of NSAID alone or with physical exercise (physiotherapy) were not associated with improvement in pain and function compared with the injection of HA. Otherwise, the combination of hyaluronic acid and dexamethasone was not associated with improvement in WOMAC at 3 and 6 months compared with the injection of hyaluronic acid alone.
Among all the interventions studied, the results of MSCs and PRP were the most consistent and associated with improvement in pain and articular function on the long-term. Moreover, Q-Actin (CSE) was associated with greater improvement in WOMAC at 3 and 6 months and the results of SVF were associated with greater improvement in pain found from the first evaluation at 3 months to the long-term evaluation. More studies with multiple outcomes should be carried out on the long term to confirm the results of these strategies.
MSCs had the highest probability to be the best treatment with primary outcome and also associated with improvement in pain and function especially at mid and long term. Moreover, the greatest improvement of pain and function at 12 months compared with baseline were observed in MSCs intervention groups (–66.36% with WOMAC and –74.47% with VAS). However, MSCs injections were performed in trials in absence of a matrix, mimicking the natural cellular environment, and therefore cartilage regeneration could not be achieved. Indeed, stem cells need a support that provides a 3D environment for their proliferation, differentiation, and regeneration of cartilage.[46,47] Injection of PRP combined with MSCs and in the presence of a matrix implanted on bone and/or cartilage lesions could be an innovative strategy for treating knee osteoarthritis but larger RCTs are needed to confirm this hypothesis.
According to Kellgren–Lawrence and Outerbridge OA classifications, the articular cartilage fissures do not reach the subchondral bone for patients with grade II but only for grades III and IV. For this reason, it seems important to carry out studies evaluating OA treatments on groups of patients according to the severity of osteoarthritis, because this allows to have more relevant results and to select the most appropriate treatment for each patient.
The interventions using PRP or MSCs were not recommended by the American College of Rheumatology (ACR) guideline[48] for the management of knee osteoarthritis.
Actually, this guideline reviewed studies published until 2018 which could explain why the interventions of our included studies were not recommended. However, more recent studies published after 2018 show the efficacy and safety of some of these treatments such as PRP injections that are becoming more popular nowadays and recommended by the European League Against Rheumatism (EULAR) who considered in 2020 that intraarticular injections of PRP are an efficient treatment of early or moderate symptomatic knee osteoarthritis and may be useful in severe knee osteoarthritis.[49]
According to a 2013 article in American Association of Retired Persons, US hospitals charge $50,000, on average, for a total knee replacement. However, the mean price for a single unilateral knee PRP injection was $714 (95% CI: $691–737) and the cost of a single stem-cell treatment for osteoarthritis was estimated at $5156 (95% CI $4550–5762) based on data from 273 centers in the United States.[50] A medico-economic study focused on knee OA strategies appears crucial in order to provide information to public health decision-makers. Moreover, literature highlighted the important value of a medico-economic evaluation for knee OA treatment strategies.
Knee OA is considered a chronic disease and this study cannot confirm the efficiency of the evaluated interventions due to several limitations. First, the high statistical heterogeneity >75% for PRP strategy probably due to variation in protocols used in the included studies in important variables such as the volume of PRP, the frequency of the injections, and the control strategy.
Furthermore, overall quality of evidence, as qualified by GRADE was very low which means that further research is likely to show different results.
Second, the largest number of knee OA treatments studies published since 2017 evaluated different interventions on the short term. Only a small number of RCT studies evaluated interventions on the long-term (≥1 year of follow-up), although knee OA is considered a chronic disease. Furthermore, according to several studies, 50% of clinical trials go unreported, often because the results are negative,[51] which may also have introduced a bias.
Third, the small number of patients (<30 participants) included and evaluated for some strategies may introduce bias due to small study effects.
Fourth, the small number of publications using other outcome measures than VAS and WOMAC represents an important issue making it difficult to evaluate interventions according to other outcomes. Thus, the development of a universal outcome scale combining items as pain, function, and quality of life may be a solution to evaluate knee OA patients without the necessity to use many instruments. It would be beneficial to facilitate and strengthen the processing of future comparative studies.
Fifth, Freitag et al[35] showed better improvement in the MSCs groups compared with the control group represented by conservative treatments as exercise program prescribed by a physiotherapist or medical practitioner for at least 8 weeks, weight loss, analgesia, and biomechanical management. However, these interventions should be evaluated apart in a meta-analysis for a better understanding of the effectiveness of each one. In addition, many interventions have been excluded from our study (e.g., low-level laser therapy, transcutaneous electrical nerve stimulation, therapeutic ultrasound, Curcuma, etc) which may also have introduced bias.
Finally, the rank probabilities were used to compare the effectiveness between the different interventions, nevertheless, they show limitations, and the results should be interpreted with caution. For example, the safety of patients as well as the level of satisfaction and quality of life were not an outcome measures which is also considered a limitation to this study.
5. Conclusions
In this systematic review and network meta-analysis, the outcomes of treatments using MSCs and PRP for the management of knee osteoarthritis were associated with long-term improvements in pain and function. We suggest that more high quality randomized controlled trials would be needed to confirm the efficiency of MSCs and PRP for the treatment of patients with knee osteoarthritis.
Author contributions
Anne-Marie Musset, Damien Offner, and Moustafa Naja contributed in the conception and design of this article. Moustafa Naja and Damien Offner performed searches, analyses, and interpretations. Gabriel Fernandez De Grado provided statistical expertise. Moustafa Naja wrote the article. Damien Offner and Anne-Marie Musset contributed to the corrections. Nadia Benkirane-Jessel, Dominique Scipioni, and Henri Favreau made revision of the article for intellectual content. All authors have read and approved the final submitted manuscript.
Conceptualization: Moustafa Naja, Anne-Marie Musset, Damien Offner.
Formal analysis: Moustafa Naja, Gabriel Fernandez De Grado, Damien Offner.
Methodology: Moustafa Naja.
Supervision: Anne-Marie Musset, Damien Offner.
Writing – original draft: Moustafa Naja.
Writing – review & editing: Henri Favreau, Dominique Scipioni, Nadia Benkirane-Jessel, Anne-Marie Musset, Damien Offner.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AARP = American Association of Retired Persons, ASA = amniotic suspension allograft, BMI = Body mass index, BMAC = bone marrow aspirate concentrate, CrIs = credibility intervals, CS = corticosteroids, Dex = dexamethasone, GC = glucosamine chondroitin, GRADE = Grading of Recommendations Assessment, HA = hyaluronic acid, JAGS = Just Another Gibbs Sample, LLLT = Low-Level Laser Therapy (LLLT), MCMC = Markov chain Monte Carlo, MSCs = mesenchymal stem cells, NSAID = nonsteroidal anti-inflammatory, OA = osteoarthritis, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis, RCT = randomized controlled trial, SVF = stromal vascular fraction, TENS = Transcutaneous Electrical Nerve Stimulation, TKA = total knee arthroplasty, VAS = Visual Analogue Scale, WOMAC = Western Ontario and McMaster university.
How to cite this article: Naja M, Fernandez De Grado G, Favreau H, Scipioni D, Benkirane-Jessel N, Musset AM, Offner D. Comparative effectiveness of non-surgical interventions in the treatment of patients with knee osteoarthritis: a PRISMA-compliant systematic review and network meta-analysis. Medicine. 2021;100:49(e28067).
PROSPERO registration number: CRD42021226871.
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files];The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Hawker GA, Badley EM, Croxford R, et al. A population-based nested case-control study of the costs of hip and knee replacement surgery. Med Care 2009;47:732–41. [DOI] [PubMed] [Google Scholar]
- [2].Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013;21:1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Felson DT, Lawrence RC, Hochberg MC, et al. Osteoarthritis: new insights. Part 2: treatment approaches. Ann Intern Med 2000;133:726–37. [DOI] [PubMed] [Google Scholar]
- [4].Teitel AD, Zieve D. MedlinePlud Medical Encycolpedia. National Institutes of Health. “Osteoarthritis.” Last updated: Sept 26, 2011. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/000423.htm. Accessed June 29, 2021. [Google Scholar]
- [5].World Health Organization; 2016. Available at: http://www.who.int/chp/topics/rheumatic/en/. Accessed February 5, 2016. [Google Scholar]
- [6].Sakalauskiene G, Jauniskiene D. Osteoarthritis: etiology, epidemiology, impact on the individual and society and the main principles ofmanagement. Medicina (Kaunas) 2010;46:790–7. [PubMed] [Google Scholar]
- [7].Anderson AS, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol 2010;24:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Michael J, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int 2010;107:152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Glyn-Jones S, Palmer AJR, Agricola R, et al. Osteoarthritis. Lancet 2015;386:376–87. [DOI] [PubMed] [Google Scholar]
- [10].Han HS, Kang SB, Yoon KS. High incidence of loosening of the femoral component in legacy posterior stabilised-flex total knee replacement. J Bone Joint Surg Br 2007;89:1457–61. [DOI] [PubMed] [Google Scholar]
- [11].Springer BD, Cahue S, Etkin CD, Lewallen DG, McGrory BJ. Infection burden in total hip and knee arthroplasties: an international registry-based perspective. Arthroplast Today 2017;3:137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Petrie JR, Haidukewych GJ. Instability in total knee arthroplasty: assessment and solutions. Bone Joint J 2016;98-B: (1 suppl A): 116–9. [DOI] [PubMed] [Google Scholar]
- [13].Yoo JD, Kim NK. Periprosthetic fractures following total knee arthroplasty. Knee Surg Relat Res 2015;27:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shannak O, Palan J, Esler C. A regional registry study of 216 patients investigating if patient satisfaction after total knee arthroplasty changes over a time period of five to 20 years. Knee 2017;24:824–8. [DOI] [PubMed] [Google Scholar]
- [15].Martin G, Harris I. Complications of total knee arthroplasty. Literature review current through; 2020. This topic last updated: Mar 18, 2020. [Google Scholar]
- [16].Price AJ, Alvand A, Troelsen A, et al. Knee replacement. Lancet 2018;392:1672–82. [DOI] [PubMed] [Google Scholar]
- [17].Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res 2014;472:133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:03–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- [20].Alghadir AH, Anwer S, Iqbal A, Iqbal ZA. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res 2018;11:851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Higgins JPT, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abrams KR, Gillies CL, Lambert PC. Meta-analysis of heterogeneously reported trials assessing change from baseline. Stat Med 2005;24:3823–44. [DOI] [PubMed] [Google Scholar]
- [23].Galli G, Vadillo MA, Sirota M, Feurra M, Medvedeva A. A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) on episodic memory. Brain Stimul 2019;12:231–41. [DOI] [PubMed] [Google Scholar]
- [24].Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clement ND, Bardgett M, Weir D, Holland J, Gerrand C, Deehan DJ. What is the minimum clinically important difference for the WOMAC index after TKA? Clin Orthop Relat Res 2018;476:2005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health-Care Evaluation, Vol. 13. New York, NY: John Wiley & Sons; 2004. [Google Scholar]
- [27].Rahimzadeh P, Imani F, Faiz SHR, Entezary SR, Zamanabadi MN, Alebouyeh MR. The effects of injecting intra-articular platelet-rich plasma or prolotherapy on pain score and function in knee osteoarthritis. Clin Interv Aging 2018;13:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Huang Y, Liu X, Xu X, Liu J. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis: a prospective randomized controlled study. Intraartikuläre Injektionen mit plättchenreichem Plasma, Hyaluronsäure oder Kortikosteroiden bei Kniearthrose: Eine prospektive, randomisierte, kontrollierte Studie. Orthopade 2019;48:239–47. [DOI] [PubMed] [Google Scholar]
- [29].Buendía-López D, Medina-Quirós M, Fernández-Villacañas Marín MÁ. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: a randomized controlled trial. J Orthop Traumatol 2018;19:03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Su K, Bai Y, Wang J, Zhang H, Liu H, Ma S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol 2018;37:1341–50. [DOI] [PubMed] [Google Scholar]
- [31].Uslu Güvendi E, Aşkin A, Güvendi G, Koçyiğit H. Comparison of efficiency between corticosteroid and platelet rich plasma injection therapies in patients with knee osteoarthritis. Arch Rheumatol 2017;33:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Simental-Mendía M, Acosta-Olivo CA, Hernández-Rodríguez AN, et al. Intraarticular injection of platelet-rich plasma in knee osteoarthritis: single versus triple application approach. Pilot study. Acta Reumatol Port 2019;44:138–44. [PubMed] [Google Scholar]
- [33].Duymus TM, Mutlu S, Dernek B, Komur B, Aydogmus S, Kesiktas FN. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc 2017;25:485–92. [DOI] [PubMed] [Google Scholar]
- [34].Matas J, Orrego M, Amenabar D, et al. Umbilical cord-derived mesenchymal stromal cells (mscs) for knee osteoarthritis: repeated msc dosing is superior to a single msc dose and to hyaluronic acid in a controlled randomized phase i/ii trial. Stem Cells Transl Med 2019;8:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Freitag J, Bates D, Wickham J, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med 2019;14:213–30. [DOI] [PubMed] [Google Scholar]
- [36].Guo Y, Yang P, Liu L. Origin and efficacy of hyaluronan injections in knee osteoarthritis: randomized, double-blind trial. Med Sci Monit 2018;24:4728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maia PAV, Cossich VRA, Salles-Neto JI, Aguiar DP, de Sousa EB. Viscosupplementation improves pain, function and muscle strength, but not proprioception, in patients with knee osteoarthritis: a prospective randomized trial. Clinics (Sao Paulo) 2019;74:e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sun SF, Hsu CW, Lin HS, Liou IH, Chen YH, Hung CL. Comparison of single intra-articular injection of novel hyaluronan (HYA-JOINT Plus) with Synvisc-One for knee osteoarthritis: a randomized, controlled, double-blind trial of efficacy and safety. J Bone Joint Surg Am 2017;99:462–71. [DOI] [PubMed] [Google Scholar]
- [39].Nash RJ, Azantsa BKG, Sharp H, Shanmugham V. Effectiveness of Cucumis sativus extract versus glucosamine-chondroitin in the management of moderate osteoarthritis: a randomized controlled trial. Clin Interv Aging 2018;13:2119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ahmad HS, Farrag SE, Okasha AE, et al. Clinical outcomes are associated with changes in ultrasonographic structural appearance after platelet-rich plasma treatment for knee osteoarthritis. Int J Rheum Dis 2018;21:960–6. [DOI] [PubMed] [Google Scholar]
- [41].Hong Z, Chen J, Zhang S, et al. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop 2019;43:1123–34. [DOI] [PubMed] [Google Scholar]
- [42].Farr J, Gomoll AH, Yanke AB, Strauss EJ, Mowry KC. ASA Study Group. A randomized controlled single-blind study demonstrating superiority of amniotic suspension allograft injection over hyaluronic acid and saline control for modification of knee osteoarthritis symptoms [published correction appears in J Knee Surg. 2019; 32(11)e2]. J Knee Surg 2019;32:1143–54. [DOI] [PubMed] [Google Scholar]
- [43].Shapiro SA, Kazmerchak SE, Heckman MG, Zubair AC, O’Connor MI. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med 2017;45:82–90. [DOI] [PubMed] [Google Scholar]
- [44].Petterson SC, Plancher KD. Single intra-articular injection of lightly cross-linked hyaluronic acid reduces knee pain in symptomatic knee osteoarthritis: a multicenter, double-blind, randomized, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc 2019;27:1992–2002. [DOI] [PubMed] [Google Scholar]
- [45].Reginster J, Dudler J, Blicharski T, et al. Pharmaceutical-grade Chondroitin sulfate is as effective as celecoxib and superior to placebo in symptomatic knee osteoarthritis: the ChONdroitin versus CElecoxib versus Placebo Trial (CONCEPT). Ann Rheum Dis 2017;76:1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Keller L, Schwinté P, Gomez-Barrena E, Arruebo M, Benkirane-Jessel N. Smart Implants as a novel strategy to regenerate well-founded cartilage. Trends Biotechnol 2017;35:08–11. [DOI] [PubMed] [Google Scholar]
- [47].Keller L, Idoux-Gillet Y, Wagner Q, et al. Nano- engineered implant as a new platform for regenerative nanomedicine using 3D well-organized human cells spheroids. Int J Nanomed 2017;12:447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol 2020;72:220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Eymard F, Ornetti P, Maillet J, et al. GRIP (Groupe de Recherche sur les Injections de PRP, PRP Injection Research Group). Intra-articular injections of platelet-rich plasma in symptomatic knee osteoarthritis: a consensus statement from French-speaking experts. Knee Surg Sports Traumatol Arthrosc 2020;doi: 10.1007/s00167-020-06102-5. Epub ahead of print. Erratum in: Knee Surg Sports Traumatol Arthrosc. 2020 Oct 24;: PMID: 32583023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Piuzzi NS, Ng M, Chughtai M, et al. The stem-cell market for the treatment of knee osteoarthritis: a patient perspective. J Knee Surg 2018;31:551–6. [DOI] [PubMed] [Google Scholar]
- [51].World Health Organization; 2017. Available at: https://www.who.int/ictrp/results/en/. Accessed May 18, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.