Abstract
Background and aims
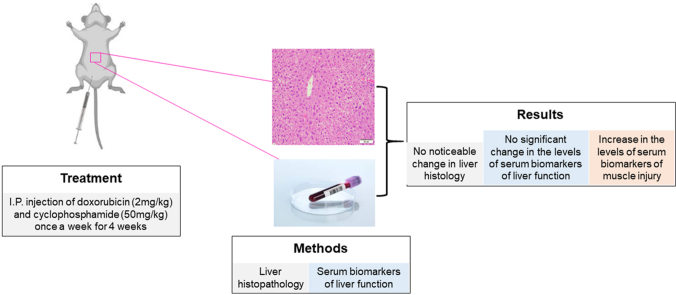
Chronic exposure to chemotherapeutics can lead to severe adverse events including hepatotoxicity. A combination chemotherapy regimen of doxorubicin (DOX) and cyclophosphamide (CPS) is employed in treatment of several cancers such as leukemia, lymphoma, and breast cancer. It is not well understood whether a combination therapy with DOX and CPS can induce hepatotoxicity. We therefore sought to determine whether co-administration of DOX and CPS at their clinically relevant doses and frequency results in hepatotoxicity.
Methods
Male C57BL/6J mice received one intraperitoneal injection of saline or DOX-2 mg/kg and CPS-50 mg/kg once a week for 4 weeks. After the treatment period, liver histology and various serum biomarkers of hepatotoxicity were assessed.
Results
Co-treatment with DOX and CPS did not alter the serum levels of alanine aminotransferase (ALT), alkaline phosphatase (ALP), bilirubin, albumin, globulin, or total protein. Similarly, co-administration of DOX and CPS did not result in a noticeable change in liver histology. However, it was notable that the concomitant treatment with DOX and CPS resulted in a significant increase in serum levels of aspartate aminotransferase (AST). Elevated serum AST levels were also associated with increased serum creatinine kinase (CK) levels, suggesting that the elevated serum AST levels are likely due to muscle injury following the co-administration of DOX and CPS.
Conclusions
Taken together, our results, for the first time, suggest that co-administration of DOX and CPS, at their clinically relevant doses and frequency does not induce a significant hepatotoxicity in the mice.
Keywords: Cyclophosphamide (CPS), Doxorubicin (DOX), Hepatotoxicity, Liver injury, Muscle injury
Graphical abstract
1. Introduction
Cancer patients are often administered with a combination of anticancer drugs to improve therapeutic efficacy. Adverse drug reactions are very common while taking chemotherapeutics, particularly in patients taking multiple anticancer drugs. For example, the combination treatment with doxorubicin (DOX) and cyclophosphamide (CPS), used in the treatment of several cancers such as leukemia, lymphoma, and breast cancer, has been shown to exhibit adverse effects such as cardiac and hematological toxicity.1 However, it is not well understood whether combination treatment with DOX and CPS results in hepatotoxicity. While serum biomarker analysis is the mainstay to detect hepatotoxicity in humans, both serum biomarkers and histopathological analyses are employed to detect hepatotoxicity in pre-clinical animal studies. Serum biomarkers of hepatic dysfunction include altered levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin and albumin.2,3
Studies report conflicting evidence for the hepatotoxic potential of monotherapy of DOX or CPS in humans. While some studies suggested that DOX or CPS alone can cause hepatic injury in cancer patients, other studies claimed that monotherapy of DOX or CPS at clinically relevant dosages has a low potential to induce hepatotoxic effects.4, 5, 6 It has been reported that DOX or CPS, when administered within the maximum tolerated dose, can cause hepatotoxicity in rats.7, 8, 9 However, it is important to note that the dosages of these drugs used in the rat studies were beyond the clinically relevant doses. Together, the above published studies convey confounding results whether or not monotherapy of DOX or CPS alone can cause hepatotoxicity in humans or rodents.
To date, one study reported the potential hepatotoxic effect of co-administration of DOX and CPS in a small sample of human cancer patients.10 However, this study partially analyzes the typical serum biomarker profile of hepatotoxicity and falls short of studying liver histology to detect hepatotoxicity at the microscopic level. Notably, there are no published reports on whether a clinically relevant combination treatment with DOX and CPS has the potential to induce hepatotoxicity in rodents. Therefore, a study exploring the hepatotoxic effect of concomitant administration of DOX and CPS at a clinically relevant treatment regimen is needed.
In this study, we sought to determine whether the standard combination treatment with DOX and CPS has the potential to induce hepatotoxicity in C57BL/6J mice. Serum markers indicative of liver injury as well as liver histology were examined after co-administration of four cycles of DOX and CPS. For the first time, our results suggest that a clinically relevant combination treatment with DOX and CPS does not induce hepatotoxicity in a rodent model.
2. Materials and methods
2.1. Materials
DOX and CPS were purchased from Sigma (St. Louis, MO, USA) and reconstituted in saline prior to administration in mice.
2.2. Animals and treatment
Eight weeks old male C57BL/6J mice weighing 25–35 g were housed in a temperature-controlled room with a 12-h day and night cycle with access to food and water ad libitum. The mice received humane care and in vivo experiments were carried after obtaining appropriate approvals from Auburn University's Institutional Animal Care and Use Committee (IACUC). To determine the potential hepatotoxic effect of DOX and CPS combination treatment, the mice received intraperitoneal (i.p.) injection of saline (n = 6) or chemotherapeutics (DOX-2 mg/kg and CPS-50 mg/kg) (n = 6), one injection per week for 4 weeks.
2.3. Tissue collection and processing
After 4 weeks of treatment, the mice from both control and chemotherapy groups were euthanized through CO2 inhalation. Trunk blood was collected through decapitation, and serum was separated for serum biomarker analysis. The livers were collected and processed for histological analysis.
2.4. Serum biomarker analysis
Serum ALT and AST concentrations were measured using a colorimetric enzymatic assay established and validated on the c 311 biochemistry analyzer (Roche, Indianapolis, IN, USA) at the Auburn University Clinical Pathology Laboratory. Serum was also used to test for other markers indicative of hepatotoxicity such as total protein, albumin, and globulin. Serum ALP and bilirubin levels were also assessed using the same method and instrument used for ALT and AST measurements.
2.5. Liver histology analysis
The representative liver sections were collected from various liver lobes, fixed in 10% neutral buffered formalin and paraffin embedded. Five μm thick liver sections were cut and stained with hematoxylin and eosin (H&E) for histological assessment.
2.6. Data and statistical analysis
Data are shown as the mean ± standard error of the mean (SEM). Analyses were performed using Prism-V software (La Jolla, CA, USA). The significance of the differences between groups was evaluated by Student's t-test. P < 0.05 was determined to be statistically significant.
3. Results
3.1. Effect of DOX and CPS combination treatment on liver histology
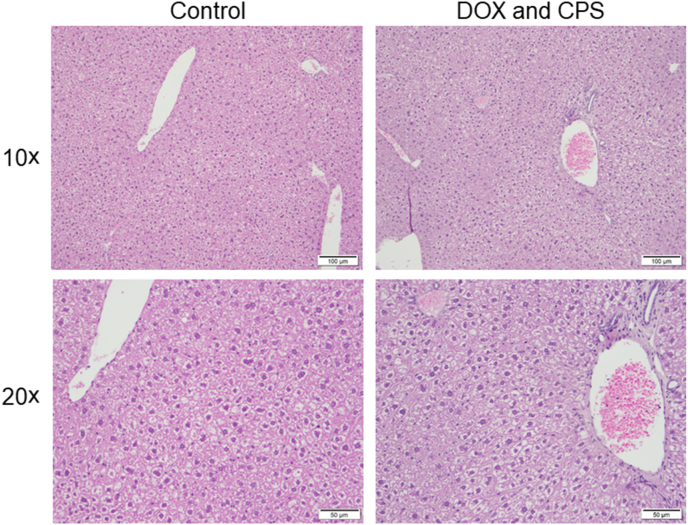
Co-administration of DOX and CPS did not result in a noticeable change in the liver histology at the end of the 4-cycle treatment as shown by H&E staining (Fig. 1).
Fig. 1.
Effect of DOX and CPS combination treatment on liver histology. Liver sections of control as well as DOX and CPS treated mice were stained with H&E for histological assessment. H&E staining of representative liver sections at 10 × and 20 × magnifications are shown. Abbreviations: CPS, cyclophosphamide; DOX, doxorubicin; H&E, hematoxylin and eosin.
3.2. Effect of DOX and CPS combination treatment on serum biomarkers of liver function
Co-administration of DOX and CPS did not significantly alter the serum levels of bilirubin, albumin, globulin, and total protein (Table 1) at the end of the 4-cycle treatment. However, co-administration of DOX and CPS significantly increased AST levels as well as CK levels.
Table 1.
Effect of DOX and CPS combination treatment on serum levels of total protein, albumin, globulin, ALP, ALT, AST, CK, and bilirubin.
| Serum parameters | Control | Combination treatment with DOX and CPS |
|---|---|---|
| Total protein (g/dL) | 5.81 ± 0.07 | 6.01 ± 0.09 |
| Albumin (A) (g/dL) | 3.47 ± 0.05 | 3.35 ± 0.03 |
| Globulin (G) (g/dL) | 2.33 ± 0.06 | 2.67 ± 0.08 |
| A/G Ratio | 1.47 ± 0.04 | 1.30 ± 0.04 |
| ALP (U/L) | 67.77 ± 3.64 | 62.93 ± 1.35 |
| ALT (U/L) | 137.67 ± 27.02 | 111.67 ± 3.66 |
| AST (U/L) | 536.67 ± 12.82 | 633.33 ± 21.39∗ |
| CK (U/L) | 29,997 ± 231 | 33,268 ± 141∗ |
| Bilirubin (mg/dL) | 0.08 ± 0.01 | 0.06 ± 0.01 |
Results are presented as the mean ± SEM (n = 6). The significance of the differences between groups was evaluated by Student's t-test. ∗P < 0.05 vs. the control group.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CPS, cyclophosphamide; DOX, doxorubicin; SEM, standard error of the mean.
4. Discussion
Co-administration of DOX and CPS is considered a standard chemotherapy in many cancer patients including breast cancer patients, and has been shown to improve disease outcome compared to monotherapy of DOX or CPS alone.11 However, the considerable side effects associated with these chemotherapeutic agents should also be considered during combination treatment. As most drugs undergo metabolism by the liver, damage to this organ often occurs during cytotoxic chemotherapy.12 However, the hepatotoxic effect of the combination therapy with these drugs is not well understood. Therefore, in this study, we set to determine whether the co-administration of DOX and CPS would induce hepatotoxicity in a healthy murine model at the end of a 4-cycle treatment.
We followed the clinically relevant treatment regimen of DOX-2 mg/kg and CPS-50 mg/kg once a week for 4 weeks in C57BL/6J mice.13 Additionally, we followed the published studies for the intraperitoneal route of administration of DOX and CPS.14, 15, 16 Following the treatment, livers were collected for gross and histological evaluation. Serum was also collected, and various serum markers of liver injury were measured. The co-administration of DOX and CPS, at their clinically relevant dosages, neither produced noticeable changes in liver histology nor significantly increased the serum levels of ALT, ALP, bilirubin, or total protein. These results suggest that a clinically relevant combination treatment with DOX and CPS does not induce hepatotoxicity at the end of a 4-cycle treatment. However, the concomitant treatment with DOX and CPS resulted in a significant increase in serum levels of AST and CK. The increase in AST and CK levels is most likely an indication of muscle injury after combination treatment. Indeed, previous studies have shown that DOX and CPS, depending on the dosage and frequency of dosing, can cause injury to and impair the function of cardiac/skeletal/smooth muscles.17, 18, 19, 20 Additionally, several studies have shown that the clinically relevant combination treatment with DOX and CPS can induce cardiac toxicity and cognitive deficits.21,22 In our study, we implemented the commonly used and clinically relevant therapeutic regimen of CPS and DOX that causes cardiac toxicity, and found that the combination treatment does not cause a noticeable hepatotoxicity.
The hepatic effect of DOX and CPS could be confounded by several factors such as age, gender, race, ethnicity, cancer pathogenesis, and other combinatorial drugs or supplements taken in addition to DOX and CPS. For example, in a retrospective study of human breast cancer patients administered with 4 cycles of DOX and CPS, only 8 Chinese patients, out of the 85 patients, 9.4% developed hepatitis (as indicated by raised ALT activity) during the combination chemotherapy.23 It is possible that other confounding factors could have also contributed to the development of acute toxicity. The authors pointed out that race or ethnicity on drug response should be considered, as there are documented differences between Chinese and Caucasians in the plasma binding and hepatic drug metabolism, and in this study, it was the Chinese participants that developed acute toxicity. Additionally, this observed chemotherapy-related toxicity could also be attributed to factors such as lower body mass index with higher percentage of body fat composition, and the popular practice of concurrent alternative medicine during chemotherapy in these Chinese patients.
As this study only explored the effects of DOX and CPS in healthy animals, there may exist some degree of variability if this chemotherapy protocol was administered to tumor-bearing animals. It is important to note that the immune status in healthy mice is different from tumor-bearing mice. As a result, it is likely that this differential immune status may alter the hepatic effect of DOX and CPS. Therefore, future studies are needed to explore the effects of DOX and CPS on cancer xenograft model of relevant cancer types. The present study does not take into account other drugs, such as antibiotics and analgesics, as well as other factors such as hepatic viruses that can play a role in making the patients more susceptible to the development or exacerbation of liver injury. Future studies are warranted to study this chemotherapy protocol in a healthy as well as a tumor-bearing animal or an animal receiving other drugs in addition to DOX and CPS.
In our study, the biomarkers of hepatotoxicity were measured at the end of the clinically relevant treatment period, which included 4 cycles of DOX and CPS administration. We did not measure the biomarkers of hepatotoxicity after each cycle of DOX and CPS administration. Although we did not notice a significant hepatotoxicity at the end of the 4 cycles of treatment period, it could not be ruled out that the mice may have developed acute hepatic toxicity during the treatment period, and that the mice may have recovered from the early liver injury by the time point when the biomarkers were studied. Although the dosage and frequency of administration of DOX and CPS used in our study is clinically relevant, DOX and CPS administration could run from 2 to 6 cycles at various doses.24 Future studies are needed to determine the dosage and frequency dependent hepatic effects of DOX and CPS. In the future, we are interested in conducting a comprehensive study in tumor-bearing mouse model with varying doses and cycles of DOX and CPS administration to study the hepatic effect of DOX and CPS co-administration.
Furthermore, our study included a sample size of 6 to test the hepatotoxic effect of DOX and CPS at a clinically relevant combination treatment regimen. Future studies are needed with an increased cohort size. Our study included only male mice. Therefore, to determine the sex as a biological variable, it will be interesting to examine whether the effect of DOX and CPS is sexually dimorphic.
5. Conclusion
For the first time, our results suggest that the co-administration of DOX and CPS, at their clinically relevant regimen, does not cause a significant hepatotoxicity in the healthy mouse model at the end of a 4-cycle treatment.
Authors’ contributions
Study concept and design: S. R Pondugula, M. Dhanasekaran. Drafting of the manuscript: S. R Pondugula, J. M Salamat, K. L Abbott, P. C Flannery, M. Govindarajulu, S. Ramesh, M. Sandey, C.-C. J Huang, M. Dhanasekaran. Data acquisition: J. M Salamat, K. L Abbott, M. Majrashi, M. Almaghrabi, M. Govindarajulu, S. Ramesh, K. Gill, N. Narayanan, E. McElroy, D. Desai, R. Nadar. Data analysis: J. M Salamat, K. L Abbott, M. Majrashi, M. Almaghrabi, M. Govindarajulu, S. Ramesh. Data interpretation: J. M Salamat, K. L Abbott, M. Govindarajulu, S. Ramesh. Methodology: M. Sandey. Critical revision of the manuscript for important intellectual content: S. K Onteru, C.-C. J Huang, Y. Iwaki, T. Moore.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
This project was supported by Animal Health and Disease Research Grant and Auburn University Research Initiative in Cancer Grant to S. R Pondugula. This work was also supported by the USA National Institutes of Health (NIH) R00 HD082686 Grant to C.-C. J Huang. The authors thank the Auburn University Clinical Pathology Lab for assistance with serum biomarker analysis.
Footnotes
Edited by Yuxia Jiang, Peiling Zhu and Genshu Wang.
Contributor Information
Satyanarayana R Pondugula, Email: srp0010@auburn.edu.
Muralikrishnan Dhanasekaran, Email: dhanamu@auburn.edu.
References
- 1.Jamali J, Dayo A, Adeel A, Qureshi Y, Khan T, Begum S. A survey on gastrointestinal adverse drug reactions of Doxorubicin and Cyclophosphamide combination therapy. J Pak Med Assoc. 2018;68:926–928. [PubMed] [Google Scholar]
- 2.Thapa BR, Walia A. Liver function tests and their interpretation. Indian J Pediatr. 2007;74:663–671. doi: 10.1007/s12098-007-0118-7. [DOI] [PubMed] [Google Scholar]
- 3.Gowda S, Desai PB, Hull VV, Math AA, Vernekar SN, Kulkarni SS. A review on laboratory liver function tests. Pan Afr Med J. 2009;3:17. [PMC free article] [PubMed] [Google Scholar]
- 4.Ricart AD. Drug-induced liver injury in Oncology. Ann Oncol. 2017;28:2013–2020. doi: 10.1093/annonc/mdx158. [DOI] [PubMed] [Google Scholar]
- 5.Damodar G, Smitha T, Gopinath S, Vijayakumar S, Rao Y. An evaluation of hepatotoxicity in breast cancer patients receiving injection Doxorubicin. Ann Med Health Sci Res. 2014;4:74–79. doi: 10.4103/2141-9248.126619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramadori G, Cameron S. Effects of systemic chemotherapy on the liver. Ann Hepatol. 2010;9:133–143. [PubMed] [Google Scholar]
- 7.Aston WJ, Hope DE, Nowak AK, Robinson BW, Lake RA, Lesterhuis WJ. A systematic investigation of the maximum tolerated dose of cytotoxic chemotherapy with and without supportive care in mice. BMC Cancer. 2017;17:684. doi: 10.1186/s12885-017-3677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalender Y, Yel M, Kalender S. Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats. The effects of vitamin E and catechin. Toxicology. 2005;209:39–45. doi: 10.1016/j.tox.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud AM. Hesperidin protects against cyclophosphamide-induced hepatotoxicity by upregulation of PPARgamma and abrogation of oxidative stress and inflammation. Can J Physiol Pharmacol. 2014;92:717–724. doi: 10.1139/cjpp-2014-0204. [DOI] [PubMed] [Google Scholar]
- 10.Anber ZNH. Effect of doxorubicin and cyclophosphamide regimen versus taxane on liver enzymes in Iraqi women with breast cancer. Biomed Res. 2018;29 doi: 10.4066/biomedicalresearch.29-18-1140. [DOI] [Google Scholar]
- 11.Vriens BE, Aarts MJ, de Vries B, et al. Doxorubicin/cyclophosphamide with concurrent versus sequential docetaxel as neoadjuvant treatment in patients with breast cancer. Eur J Cancer. 2013;49:3102–3110. doi: 10.1016/j.ejca.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Grigorian A, O’Brien CB. Hepatotoxicity secondary to chemotherapy. J Clin Transl Hepatol. 2014;2:95–102. doi: 10.14218/JCTH.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alyamkina EA, Nikolin VP, Popova NA, et al. A strategy of tumor treatment in mice with doxorubicin-cyclophosphamide combination based on dendritic cell activation by human double-stranded DNA preparation. Genet Vaccines Ther. 2010;8:7. doi: 10.1186/1479-0556-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, Kim JS, Song MS, et al. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem. 2010;93:487–494. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Wagawade JD, Roy S, Hullatti KK, et al. Doxorubicin induced cognition impairment in rat model. Asian J Pharm Clin Res. 2015;8:301–304. [Google Scholar]
- 16.Kitamura Y, Hattori S, Yoneda S, et al. Doxorubicin and cyclophosphamide treatment produces anxiety-like behavior and spatial cognition impairment in rats: possible involvement of hippocampal neurogenesis via brain-derived neurotrophic factor and cyclin D1 regulation. Behav Brain Res. 2015;292:184–193. doi: 10.1016/j.bbr.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Fabris S, MacLean DA. Doxorubicin chemotherapy affects the intracellular and interstitial free amino acid pools in skeletal muscle. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Norren K, van Helvoort A, Argilés JM, et al. Direct effects of doxorubicin on skeletal muscle contribute to fatigue. Br J Cancer. 2009;100:311–314. doi: 10.1038/sj.bjc.6604858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayward R, Hydock D, Gibson N, Greufe S, Bredahl E, Parry T. Tissue retention of doxorubicin and its effects on cardiac, smooth, and skeletal muscle function. J Physiol Biochem. 2013;69:177–187. doi: 10.1007/s13105-012-0200-0. [DOI] [PubMed] [Google Scholar]
- 20.Crouch ML, Knowels G, Stuppard R, et al. Cyclophosphamide leads to persistent deficits in physical performance and in vivo mitochondria function in a mouse model of chemotherapy late effects. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salas-Ramirez KY, Bagnall C, Frias L, Abdali SA, Ahles TA, Hubbard K. Doxorubicin and cyclophosphamide induce cognitive dysfunction and activate the ERK and AKT signaling pathways. Behav Brain Res. 2015;292:133–141. doi: 10.1016/j.bbr.2015.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broder H, Gottlieb RA, Lepor NE. Chemotherapy and cardiotoxicity. Rev Cardiovasc Med. 2008;9:75–83. [PMC free article] [PubMed] [Google Scholar]
- 23.Ma B, Yeo W, Hui P, Ho WM, Johnson PJ. Acute toxicity of adjuvant doxorubicin and cyclophosphamide for early breast cancer -- a retrospective review of Chinese patients and comparison with an historic Western series. Radiother Oncol. 2002;62:185–189. doi: 10.1016/s0167-8140(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 24.Honkoop AH, van der Wall E, Feller N, et al. Multiple cycles of high-dose doxorubicin and cyclophosphamide with G-CSF mobilized peripheral blood progenitor cell support in patients with metastatic breast cancer. Ann Oncol. 1997;8:957–962. doi: 10.1023/a:1008259518263. [DOI] [PubMed] [Google Scholar]