Summary/Abstract
Diabetes mellitus (DM) is a systemic metabolic disease that affects 463 million adults worldwide and is a leading cause of cardiovascular disease, blindness, nephropathy, peripheral neuropathy, and amputation. Lipids have long been recognized as contributors to the pathogenesis and pathophysiology of DM and its complications, but recent discoveries have highlighted ceramides, a class of bioactive sphingolipids with cell signaling and second messenger capabilities, as particularly important contributors to insulin resistance and the underlying mechanisms of DM complications. Besides their association with insulin resistance and pathophysiology of type 2 diabetes, evidence is emerging that certain species of ceramides are mediators of cellular mechanisms involved in the initiation and progression of microvascular and macrovascular complications of DM. Advances in our understanding of these associations provides unique opportunities for exploring ceramide species as potential novel therapeutic targets and biomarkers. This review discusses the links between ceramides and the pathogenesis of DM and diabetic complications and identifies opportunities for novel discoveries and applications.
Introduction
Diabetes mellitus (DM), a global epidemic that affects 463 million adults worldwide, now ranks among the leading non-communicable public health challenges of the present era1–3. The number of people living with diabetes has increased by more than three-fold over the past two decades1–4. As the leading cause of blindness, amputation, and chronic kidney disease, and a major contributor to myocardial infarction (Ml), heart failure, stroke, and peripheral vascular disease (PVD), diabetes imposes a high toll on morbidity and mortality5–8. Type 2 diabetes (T2D) accounts for approximately 90-95% of the diabetes burden, whereas type 1 diabetes (T1D; typically seen in children) accounts for 5-10% of the disease burden. Insulin deficiency, usually triggered by autoimmune mechanisms in genetically susceptible persons, is the proximate cause of T1D. In contrast, the pathophysiology of T2D involves a complex interaction among genetic predisposition, insulin resistance, and β-cell dysfunction, among other contributory factors. Activation of pro-inflammatory and oxidative stress pathways is involved in the pathophysiology of both forms of diabetes as well as diabetes complications9. Approximately 7.3 million of the 34 million people affected with diabetes in the U.S. are undiagnosed and half of the 463 million people with diabetes worldwide also are undiagnosed1,10. Sadly, individuals with undiagnosed diabetes still are at risk of developing diabetes complications: in the United Kingdom Prospective Diabetes Study, nearly one-quarter of people with newly diagnosed T2D already had developed one or more complications9.
Sphingolipids are a group of specialized lipids containing an amino alcohol in their backbone and are present in all mammalian cells (Figure 1). They were originally assumed to only have structural roles, but certain types of bioactive sphingolipids have been found to be involved in cell signaling, growth/survival, differentiation, death, inflammation, and other processes11. Sphingolipids are structurally diverse and can be divided into different classes based on major variations in molecular structure12. Sphingolipid classes are further divided into different species by smaller differences in parameters such as chain length, allowing for the existence of tens of thousands of unique sphingolipid molecules in every cell13. Due to their ubiquity and widespread bioactivity, sphingolipid metabolic abnormalities and dysregulation have been implicated as potential driving forces in many pathologic conditions, including DM and DM-associated complications.
Figure 1: Diversity and Synthesis of Ceramides.
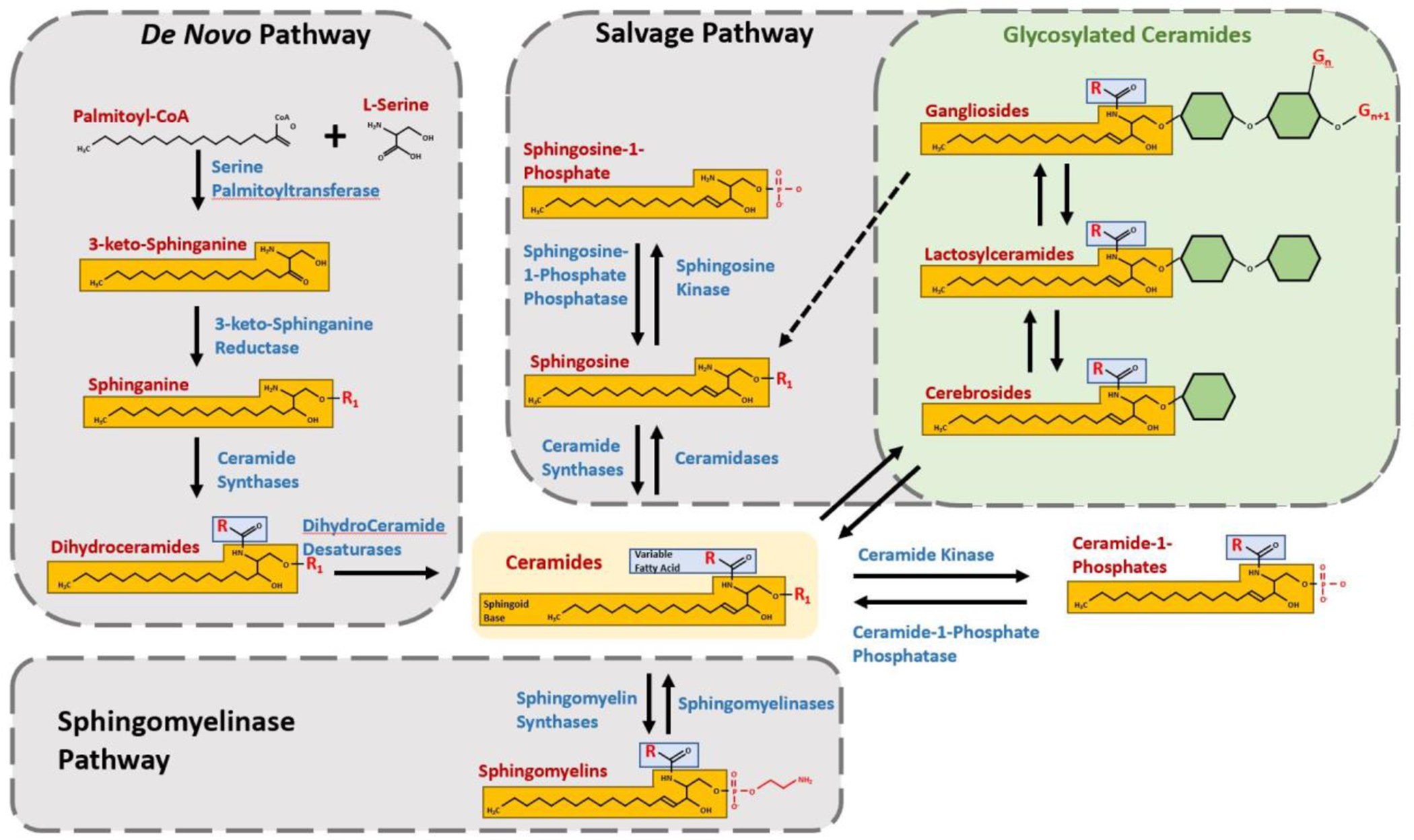
Synthesis of ceramide occurs via three main pathways, each of which utilizes enzymes with multiple isoforms, pathway-specific localization, and/or reactants with variable R-groups to yield a diverse array of Ceramide species with unique bioactivity. The De Novo Pathway occurs in the endoplasmic reticulum and begins with rate-limiting Serine Palmitoyltransferase-catalyzed condensation of Palmitoyl-CoA and L-Serine to form 3-keto-Sphinganine, which is then reduced to Sphinganine by 3-keto-Sphinganine Reductase. Ceramide Synthases 1-6 add a fatty acid chain (blue) of variable length (R) to form Dihydroceramide, which is then is converted to Ceramide by Dihydroceramide Desaturase and transported to the Golgi182. The Sphingomyelinase Pathway hydrolyzes Sphingomyelin to Ceramide via acid or neutral Sphingomyelinases 1-5, which are found in the plasma membrane, cytosol, mitochondria, endosomes, and lysosomes183, 184. The Salvage Pathway breaks down complex sphingolipids such as gangliosides in endo-lysosomal compartments to Sphingosine, which may then be transported to the ER and reacylated to Ceramide by Ceramide Synthases 1-5. Alternatively, Sphingosine (R1 = H) may be phosphorylated at R1 to Sphingosine-1-Phosphate by Sphingosine Kinase185–187. Ceramide may be phosphorylated at R1 by Ceramide Kinase to generate Ceramide-1-Phosphate, or it may be glycosylated to form Cerebrosides (Glucosylceramides and Galactosylceramides). Addition of a Galactose residue to a Glucosylceramide yields a Lactosylceramide, which may be further glycosylated or acquire sialic acid residues at various sites (Gn, Gn+1...) to form Gangliosides188.
At the center of the sphingolipid metabolic labyrinth is ceramide, which may be formed by three main pathways: de novo synthesis, hydrolysis of sphingomyelin (SM), and salvage of complex sphingolipids (Figure 1). The different routes of ceramide synthesis determine the initial localization and species of ceramide generated, and hence its bioactivity and downstream products13. Several decades of research have established the involvement of various forms of ceramide, and even systemic or plasma ratios of ceramide species, in important biological processes central to human diseases such as DM and its complications. While the involvement of ceramide in these diabetic complications is already evident and supported by literature, many new studies further implicate ceramide and help elaborate ceramide-mediated mechanisms underlying their pathophysiology. The emergence of ceramides as key mediators of the onset and progression of insulin resistance and diabetic complications provides unique opportunities to explore their potential utility as novel therapeutic targets and disease biomarkers. This review briefly discusses past research and recent findings regarding ceramide’s roles in insulin resistance and some of the major complications of diabetes. Currently, information on the role of ceramides in diabetes is disseminated in scores of publications in numerous biomedical journals. Some review articles published in the past decade have diligently assembled and discussed studies on the roles of ceramides in diabetes and its complications14–17. Some focus heavily on sphingolipid metabolism and signaling, while others emphasize mechanisms of pathogenesis. This review attempts to aggregate current mechanistic knowledge while also providing a comprehensive narrative regarding the roles of ceramides in various diabetic complications in a format that will be useful for clinicians and researchers.
Ceramide and Insulin Resistance
Metabolic syndrome refers to a group of pathological disorders including obesity, hypertension, dyslipidemia, and hyperglycemia that acts as compounding risk factors for diabetes, cardiovascular disease, and other conditions18. Metabolic syndrome is closely related to insulin resistance, a condition characterized by impaired insulin-stimulated metabolic activities in certain tissues (skeletal muscle, adipocytes, liver, etc.)19. Insulin is produced by the pancreas to maintain glucose homeostasis, but in insulin resistance, insulin levels may be elevated due to lack of glucose uptake and feedback. Excess caloric intake causes expansion of adipose tissue, which increases adipocyte lipolysis and leads to ectopic deposition of lipids in non-adipose tissue20. Transport of sarcolemmal fatty acids21 and delivery of non-esterified fatty acids and triacylglycerols to peripheral tissues is also increased22. These fatty acids are esterified to diacylglycerol and triacylglycerol and stored in the liver, skeletal muscle, heart, and pancreas23, where they participate in ceramide biosynthesis and cause ceramide accumulation24, 25. Emerging evidence has implicated the accumulation of ceramide in metabolic tissues as contributing to impairment of insulin sensitivity and glucose homeostasis14, 26–29.
Obesity is a strong risk factor for diabetes30. Plasma analyses of obese individuals show significantly higher levels of ceramide31 and palmitate32, which, as the source of palmitoyl-CoA in the de novo pathway, is a precursor of ceramide biosynthesis (Figure 1). A multicohort study of diabetic patients showed that dysfunctional visceral adiposity and insulin resistance are associated with higher plasma ceramides33, suggesting that plasma ceramides could act as DM biomarkers34, 35. Elevated ceramides were also reported in the skeletal muscle, liver, and hypothalamus of obese humans and experimental obese rodents36. In another genetically obese animal model, the levels of hepatic and plasma C16:0 ceramide increased significantly. However, use of antisense oligonucleotides of the C16:0 ceramide biosynthetic enzyme ceramide synthase 6 (CerS6) in this model significantly reduced hepatic and plasma C16:0 ceramide while also increasing insulin sensitivity37. Similarly, in experimental high fat diet animals, increased levels of C18:0 ceramide were observed in skeletal muscle. Knocking out the CerS1 gene, which synthesizes C18:0 ceramide, leads to improved glucose homeostasis and insulin sensitivity38. High-fat diet in a Wistar rat model correlated with increased ceramide levels and decreased insulin sensitivity39. Other obese animal models showed increased levels of serine palmitoyl transferase (SPT) and dihydroceramide desaturase (DES), indicating increased activity of the de novo ceramide synthesis pathway (Figure 1)40. This is further supported by experiments showing that ablation of dihydroceramide desaturase 1 (DES1) in mice resolves hepatic steatosis and insulin resistance caused by obesogenic diet and leptin deficiency41. Leptin regulates lipid metabolism in the skeletal muscle and is responsible for dampening hyperlipidemia and lipotoxicity. Higher levels of ceramide detected in leptin-deficient mice suggests a relationship between ceramide synthesis and hyperlipidemia leading to decreased insulin action. Collectively, these results indicate that tissue-specific ceramide accumulation is dependent upon distinct ceramide species generated by the de novo and salvage pathways.
Ceramide-regulated insulin resistance occurs through multiple pathways (Figure 2). In one mechanism, ceramide activates PKCζ, which phosphorylates and inhibits the translocation of AKT/protein kinase B (PKB) to the plasma membrane42. Inhibiting translocation of AKT/PKB interferes with insulin-dependent GLUT glucose transporter proteins, blocking glucose uptake and impairing nutrient storage14. Ceramide also stimulates activation of cytosolic protein phosphatase 2A (PP2A), which is the primary phosphatase for AKT/PKB dephosphorylation43, 44. Increased accumulation of ceramide in the endoplasmic reticulum and/or mitochondria impairs insulin action and causes cellular stress 26,28.
Figure 2: Ceramides and Insulin Resistance.
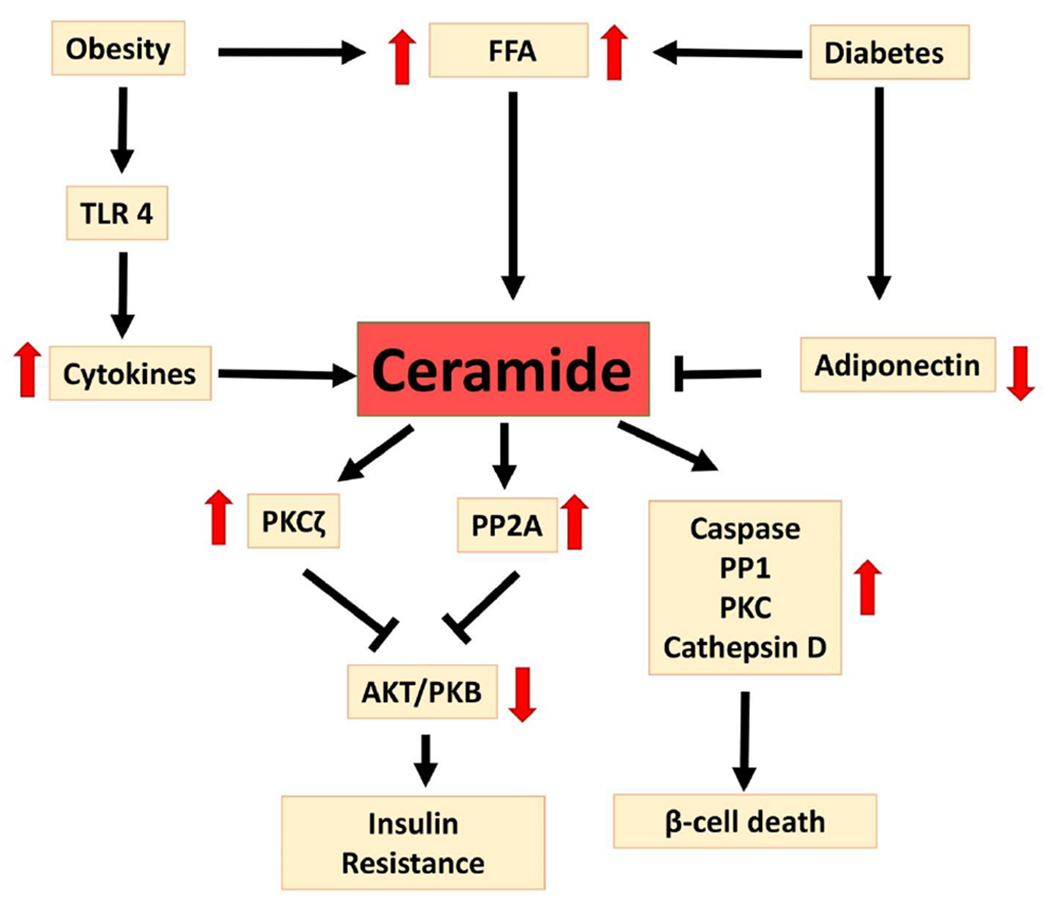
Ceramide plays a central role in insulin resistance and β-cell death. Obesity and hyperglycemia both increase circulating shorter-chain saturated free fatty acids (FFA) that serve as substrates for and induce de novo ceramide synthesis24, 25. Chronic inflammation in obesity is associated with TLR4 activation, which in turn increases cytokines and ceramide levels189. Diabetes is associated with decreased adiponectin, which is a known inhibitor of ceramide synthesis164. Ceramide activates PP2A and PKCζ, which inhibit AKT/PKB and decrease insulin sensitivity58, 59. Ceramide also activates pro-inflammatory and pro-apoptotic pathways via caspase, PP1, PKc, and cathepsin D15, 57, leading to pancreatic β-cell death53.
The chronic inflammation associated with obesity is due in part to accumulation of ceramides19. In blood samples of diabetic patients, increased levels of proinflammatory cytokines are correlated with dysregulated lipid metabolism and ceramide production. Obesity in leptin-deficient, genetically obese mice (ob/ob), is associated with elevated TNFα and hyperinsulinemia. The adipose tissue of these transgenic animals also show increased gene expression of the ceramide biosynthetic enzymes neutral sphingomyelinase (nSMase), acidic sphingomyelinase (aSMase), and SPT45. Ceramide elevation in adipose tissue increases NLRP3-dependent IL-1β46, and knock-out of NLRP3 in mouse models protects them from ceramide-mediated glucose intolerance, insulin resistance, and inflammation due to diet-induced obesity47. Similarly, silencing of proinflammatory genes (i.e., TNF, IL-1α, and stress-induced kinase JNK-1) reduces peripheral insulin resistance48, suggesting correlations between inflammation and insulin resistance. The ceramide-mediated inflammatory pathway is regulated by toll like receptors (TLRs). TLRs such as TLR4 can induce insulin resistance49 via IκKβ and NFκB signaling pathways50. As well as modulating insulin sensitivity, adiponectin plays an important role in controlling ceramide-mediated inflammation in metabolic disorders through AMPK, a serine threonine kinase51.
Lipotoxicity and hyperglycemia cause ceramide to accumulate in pancreatic β-cells52 and β-cell destruction can be prevented by blocking ceramide production53. Unlike T2D, in which cells do not respond properly to insulin, T1D is caused by inadequate production, secretion, and release of insulin due to loss of pancreatic β-cells. In Zucker diabetic fatty (ZDF) rats, inhibition of de novo ceramide biosynthesis by cycloserine attenuates Langerhans islet apoptosis and helps prevent hyperglycemia54. In ceramide-mediated β-cell dysfunction and cell death, the fatty acid transporter/cluster determinant 36 (FAT/CD36) plays a significant role. During obesity and hyperlipidemia, CD36 induces adipose tissue inflammation by enhancing macrophage activation and impairing insulin signaling55, and inhibition of CD36 is shown to prevent ceramide-induced thioredoxin-interacting protein activation by inhibiting NFκB and in turn protects β-cells from apoptosis56. In addition to interfering in signaling mechanisms, disrupting glucose transport and storage, and aggravating inflammation, ceramide enhances cell death by stimulating caspase, PKC, serine/threonine protein phosphatase 1 (PP1), and cathepsin D15, 57. In islets of Langerhans and pancreatic β-cell lines, it has been reported that ceramide dephosphorylates ERK1/2 by PP2A, which in turn inhibits insulin expression58,59. Ceramide-mediated activation of PKCζ not only inactivates AKT/PKB pathway but also phosphorylates and inactivates pancreatic and duodenal homeobox gene-1 (PDX-1), which is a transcription factor of insulin gene expression60. Hence, targeting ceramide synthesis as a therapeutic strategy to prevent insulin resistance could be an effective strategy, although global inhibition of ceramide production could be harmful due to its involvement in diverse physiologic mechanisms.
Ceramide in Liver and Pancreatic Diseases
There is a strong connection between metabolic syndrome and dyslipidemia, insulin resistance, and dysglycemia. The liver and pancreas are two vital organs that regulate systemic lipid and glucose metabolism, but are also affected by their dysregulation. In fact, the pathophysiology of the most common metabolic disorder of the liver, non-alcoholic fatty liver disease (NAFLD), involves dyslipidemia, insulin resistance, hyperglycemia, inflammation, and their interplay61,62. Accumulation of cytotoxic lipids, especially ceramide, has been shown in metabolic NAFLD63,64, 65. Elevated levels of C16:0 and C18:0 dihydroceramide in human livers, indicating de novo ceramide biosynthesis elevation, were associated with NFALD and insulin resistance66. This finding was supported by animal studies wherein CerS6 deficient mice exhibited lower levels of C16:0 ceramide and are safeguarded from high fat-induced obesity, insulin resistance, and adipose tissue inflammation67. Further, Sprague-Dawley rats were protected from developing hepatic steatosis and fibrosis with NAFLD through inhibition of de novo ceramide biosynthesis by myriocin68. Involvement of acidic SMase and dihydroceramide desaturase has been reported in generation and accumulation of ceramide during NAFLD41, 69 (Figure 1). Like in NAFLD, blood samples from an experimental mouse model of non-alcoholic steatohepatitis (NASH) and liver biopsies from NASH patients show significant accumulation of C16:0 ceramide70. In addition to hepatic dihydroceramides, lactosylceramide levels were also found to be increased in insulin-resistant NASH patients64. Oxidative stress is one of the pathological hallmarks of NASH71, and increased levels of ceramides generate oxidative stress and inflammation in metabolic syndrome, which initiates fibrosis in NASH, leading to NAFLD72. In particular, C16:0 ceramide in the mitochondrial outer membrane is especially known to activate mitophagy and oxidative stress73.
Similar to the liver, the pancreas is another region where hyperglycemia and insulin resistance influence cytotoxic lipid deposition74. Pancreatitis susceptibility is linked to diabetes through metabolic changes which induce inflammation and generation of reactive oxygen species75, 76. Plasma sphingolipid metabolism is affected during acute pancreatitis, where significant increases in the ceramide levels and decreases in S1P have been reported in severe acute pancreatitis patients during early stage of the disease77. As discussed in the previous section, increased accumulation of ceramides influence secretion of insulin from pancreatic β-cells. The genetic deletion of sphingomyelin synthase 1 (SMS1) leads to accumulation of ceramide in islets of Langerhans, which affects mitochondrial function and subsequently inhibits insulin secretion78. Similar to inhibition of SMS1, siRNA knockdown of ceramide transporter protein (CERT) leads to accumulation of intracellular ceramide and pancreatic β-cell dysfunction during exposure with palmitic acid79. Palmitic acid induces de novo ceramide synthesis and this pathway appears to be involved in ceramide-induced pancreatic β-cell death and dysfunction (Figure 1). This information supports association of ceramide not only with insulin resistance and hyperglycemia, but also with liver and pancreatic diseases.
Ceramides in Diabetic Complications
The long-term complications of DM are classified into microvascular/neuropathic and macrovascular subgroups. The recognized microvascular and neuropathic complications of diabetes mellitus are diabetic retinopathy, diabetic nephropathy, and diabetic neuropathy. Diabetic retinopathy is the leading cause of blindness; diabetic nephropathy may progress to end-stage kidney disease (ESKD); and neuropathy is a major risk factor for lower extremity amputation. The development of microvascular complications of diabetes is a function of both the duration of the disease and the state of metabolic control. The pathogenesis of microvascular complications, though incompletely understood, involves mechanisms that include genetic predisposition; hyperglycemia-induced activation of the polyol, hexosamine, and PKC pathways; generation of advanced glycosylated end products; glomerular hyperfiltration; growth factor expression; oxidative stress; free radical damage; and abnormal endothelial, pericyte, and mesangial structure and/or function2, 3. Diabetic nephropathy and severe retinopathy also tend to cluster in families80.
The macrovascular complications of diabetes include cardiovascular disease (CVD), stroke, and PVD. Coronary artery disease, MI, and congestive heart failure are major components of CVD. Unlike microvascular complications, hyperglycemia is not the sole or major driver in the etiology of macrovascular complications. The risk factors for macrovascular disease include insulin resistance, hypertension, dyslipidemia, hyperglycemia, increased coagulation, impaired fibrinolysis, inflammation, oxidative stress, and lifestyle factors (cigarette smoking, obesity, physical deconditioning). These CVD risks frequently co-exist in people with DM. CVD is the leading cause of death in patients with diabetes and is 2-4 times more prevalent among people with diabetes compared to nondiabetic patients5, 8. Besides the traditional hyperglycemic and multifactorial landscape of diabetic complications, emerging evidence has implicated the involvement of ceramides in various mechanisms leading to diabetic complications in target tissues (heart, blood vessels, eyes, kidney, and peripheral nerves).
Ceramide in Diabetes-Induced Vascular Diseases
Cardiovascular disease can be a devastating complication of diabetes and obesity and continues to be a leading cause of death in developed countries81. Sphingolipids have been implicated in cardiovascular disease pathogenesis82, and even though it is unclear which lipid metabolites might directly cause cardiomyopathy, ceramide accumulation in multiple models correlates with these pathophysiological events and cardiac dysfunction83–85. Conventionally, cardiac dysfunction in T2D relates to generation of toxic lipid species by oxidation from excessive fatty acid uptake86. Studies of fenofibrate-induced lipid-lowering effects on cardiac function revealed that fenofibrate lowers plasma TG, cholesterol, and the ratio of ceramide C24:0/C16:0, and also minimally alters oxidative stress markers87, indicating that certain ceramide species may have independent or coordinated roles in cardiac dysfunction in diabetic patients. In recent years, several studies have found associations between circulating ceramide levels and adverse cardiovascular events, such as MI and stroke29, 88. These studies consistently showed that several long-chain ceramide species were associated with an increased risk of cardiac death and deleterious outcomes in patients with cardiovascular complications89. Studies have reported that elevated ceramide levels are associated with major adverse cardiovascular events90. In the same vein, several studies have also reported multiple ceramide species and their ratios as strong indicators of those events. C18:1 ceramide has been reported to be a key indicator of necrosis after coronary angiography procedures, as are C24:1 ceramide and sphingomyelin for cardiovascular mortality, while C22:0 and C24:0 ceramides strongly associate with reduced improvement in verbal memory in patients with coronary artery disease in response to exercise91.
One of the key features of heart failure is apoptotic cell loss92, and saturated free fatty acids (FFAs) have been shown to induce cardiomyocyte apoptosis and damage myofibrils93–97. Interestingly, inhibition of CerS was found to reverse this effect94. It has also been proposed that generation of ROS by ceramide could play a role in cardiomyocyte apoptosis97–99. In addition, mitochondrial fission stimulated by ceramide is also associated with early activation of cardiomyocyte apoptosis, further cementing the role of ceramide in this complication100. Ceramide has also been implicated in the process of atherosclerosis; degradation of sphingomyelin to ceramide by aSMase on low density lipoprotein (LDL) surfaces promotes their aggregation by ceramide-ceramide interactions, promoting atherosclerosis initiation101, 102. In addition, inhibition of DES1, the ultimate enzyme in the ceramide biosynthesis pathway that converts dihydroceramide to ceramide, has been shown to reverse pathogenesis of atherosclerosis and cardiomyopathy in rodent models103. Plasma ceramide levels are reported to be more precise than currently used lipid prediction markers, thus highlighting their potential use as biomarkers to identify patients in need of more aggressive treatment. Indeed, a plasma ceramide test kit for evaluation of cardiac risk was commercially released in 2016 by Mayo Medical Laboratories104. With ceramide being a strong biomarker for cardiac conditions like atherosclerosis, it may be interesting to further investigate it in fenofibrate-treated patients105.
In general, interventions such as inhibiting any of the de novo ceramide synthesis enzymes (namely SPT, CerS6 and DES1) in mouse models reportedly reduce cardiovascular complications such as atherosclerosis and lipotoxic cardiomyopathy17. Studies have shown that Adiponectin increases cellular ceramidase activity, thereby reducing ceramide levels, and exhibits cardioprotective properties14. Though the heart needs essential lipids, the heart is likely to have lipid accumulation resulting in severe complications in diabetes. Even though several studies using animal models have implicated ceramides in the pathogenesis of cardiovascular diseases, further research is necessary to delineate the exact nature of its toxicity based on structural variability.
Ceramide in Diabetic Nephropathy
Diabetes is the primary cause of chronic kidney disease (CKD) and ESKD worldwide106. Diabetic nephropathy is a highly complex disease which involves multiple pathogenic mechanisms including glomerular damage, hemodynamic dysfunction, inflammation, and fibrosis107. Ceramides are highly abundant in the kidney and several studies have shown that ceramide is involved in the pathogenesis of acute kidney injury caused by ischemic reperfusion, toxic insults, and oxidative stress14, 108. A recent study reported elevated levels of SPT, the rate-limiting enzyme in the de novo ceramide biosynthetic pathway, in renal tubular epithelial cells isolated from diabetic patients. The same study also reported prevention of tubular epithelial cell death by inhibition of ceramide synthesis109, implying a critical role of ceramide in diabetic nephropathy. Over the years, multiple studies have linked ceramides and elevated expression of SPT to apoptosis of mesangial, renal tubular epithelial, and microvascular endothelial cells in diabetic patients14.
High levels of glycosphingolipids in the kidney and urinary tract have been shown to contribute to the pathophysiological basis of several other renal diseases, including Fabry’s disease, hemolytic uremic syndrome, and bacterial adhesion in urinary tract infections110. The activation of biosynthetic pathways for glycosphingolipid production plays an important role in glomerulonephritis, acute kidney injury, kidney cancer, and diabetic nephropathy111. In addition to playing a role in renal hypertrophy in type 1 diabetic kidney disease112, glycosphingolipids have recently been shown to induce fibrosis in the early stages of type 2 diabetic kidney disease113. It has been hypothesized that excessive glucose increases glycosphingolipid production by increasing the availability of substrates namely ceramide and UDP-glucose113. It has been shown that reduced plasma levels of long- and very long-chain species of ceramides have predictive value for progression of macroalbuminuria (MA) in diabetic nephropathy114. This same study also showed that reduced levels of lactosylceramides and hexosylceramides (C18:1-H), are significantly associated with higher risk of progression to MA and CKD, respectively. Furthermore, it has been reported that elevated levels of advanced glycation end-products (AGE), usually found in diabetic conditions, are responsible for increased levels of several gangliosides in tissues, especially GM3. GM3 was shown to compromise renal pericyte and mesangial cell regeneration via inactivation of VEGF receptors and the receptor-associated AKT signaling pathway115. Mitochondrial damage in podocytes by reactive oxygen species (ROS) produced by ceramide accumulation was reported in a new study using Otsuka Long Evans Tokushima Fatty (OLETF) rats and mice on a high-fat diet (HFD)116. Their results also show albuminuria and histologic features characteristic of diabetic nephropathy and podocyte injury, which were reversed by treatment with myriocin, an inhibitor of SPT. An increase in ceramide accumulation and ROS generation in mitochondrial podocytes was also observed when cultured podocytes were exposed to high glucose, high free fatty acid, and angiotensin II in combination (GFA). However, pretreatment with myriocin protected them from GFA-induced damage of mitochondrial integrity. It has also been reported that in glomerular endothelial cells (GECs) and podocytes, lowering cellular ceramide levels by activation of acid ceramidase reduces palmitate-induced lipotoxicity, oxidative stress, and apoptosis117. In addition, other studies have shown that the expression of sphingomyelin phosphodiesterase acid-like 3B (SMPDL3b), a homologue of aSMase responsible for elevation of ceramide levels, is increased in diabetic kidney disease and targeting it could protect podocytes from damage118, 119.
In a study of bi-racial healthy adults with parental history of T2DM, adiponectin was shown to be a powerful risk marker of incident prediabetes120. Since it has been reported that adiponectin stimulates ceramidase activity via its receptors121, this study underscores the importance of ceramide as a biomarker for diabetes and its complications. It has also been reported that AdipoRon, a synthetic adiponectin agonist, can decrease ceramide-induced lipotoxicity in DKD by decreasing ceramide, oxidative stress, and apoptosis in glomerular endothelial cells and podocytes in db/db mouse model122. The study also showed that AdipoRon was able to reduce both albuminuria and lipid accumulation in the kidneys122. Recently, it was shown that administration of exogenous C1P was associated with protection from albuminuria and mesangial expansion in db/db mice in vivo123. Also, it was reported that the use of Fingolimod (FTY720), an FDA-approved drug for treatment of multiple sclerosis, renders nephroprotection in streptozotocin-induced diabetic nephropathy rat models124. As FTY720 inhibits ceramide synthase and reduces ceramide levels125, these studies highlight the potential of ceramide and ceramide-focused pharmacologic intervention in diabetic nephropathy.
The results of these experiments strongly imply that alteration of ceramide metabolic pathways is a factor in the pathophysiology of DKD. Considering the severity of renal dysfunction and failure as complications of diabetes, it is enormously important to further explore the role of ceramide in DKD pathogenesis.
Ceramide in Diabetic Retinopathy
Diabetic retinopathy (DR) is characterized by progressive retinal degeneration, neovascularization, and eventual blindness, and is the principal cause of blindness in people aged 20-65126. Although proper glycemic control is the mainstay preventative measure for DR, it has been shown that DR may develop and progress even in normoglycemic conditions127–129. Increased levels of sphingolipids, particularly ceramide, have been associated with various aspects of retinopathy. Analysis of sphingolipid composition of type 2 diabetic and non-diabetic post-mortem human tissue showed a relative increase in total ceramide, lactosylceramide (LacCer), and SM in diabetic vitreous samples. This study not only indicates that changes in sphingolipid composition in the vitreous as a result of T2D could be connected to pathologies of the retina, retinal vessels, vitreous, and surrounding tissues, but also underscores the importance of ceramide in complications resulting from DM130. In the pathogenesis of DR, apoptosis of pericytes (PCs) is an early event. This is generally thought to be a consequence of sustained hyperglycemia, which increases the concentration of the saturated free fatty acid palmitate. In an in-vitro study of cultured PCs, incubation with palmitate increased the cellular content of ceramide, leading to apoptosis. Inhibition of ceramide synthase and overexpression of N-acylsphingosine amidohydrolase 1 (ASAH1), an enzyme responsible for conversion of ceramide to sphingosine, reversed the proapoptotic effect of palmitate, underscoring the role of ceramide in the early pathogenesis of DR131. Animal models of streptozotocin-induced diabetes have shown significantly increased glucosylceramide levels in the retina. This study speculated that accumulation of glycosphingolipids leads to activation of the endoplasmic reticulum stress response, leading to insulin resistance and neuronal apoptosis and thereby contributing to the pathogenesis of DR132. In addition, several studies have reported an increase in ceramide due to activation of the sphingomyelinase pathway (Figure 1) to be critical to the pathogenesis of DR. aSMase is shown to be highly activated in diabetic retinas, particularly in the retinal endothelium. Increased ceramide levels due to conversion of sphingomyelin (SM) to ceramide by aSMase significantly contribute to retinal inflammation133. It has also been reported that the aSMase vascular isoform specifically increases in the retinas of diabetic animals during the vaso-degenerative stage133. Importantly, DHA, which is decreased in diabetic retinas134, downregulates the expression of aSMase in human retinal endothelial cells, leading to decrease in ceramide levels and protecting the retina from vaso-degeneration135–138. In addition, downregulation of aSMase with DHA prevents capillary formation and cytokine production135, 137–139. Supplementation with a DHA-rich diet has been shown to be protective against capillary loss in diabetic retinopathy in-vivo140. DHA supplementation was also observed to normalize impaired b-wave amplitude and latency time in diabetic rats140. Furthermore, increasing the retinal levels of n3-PUFAs, which were shown to be reduced in the retinas of human diabetic eyes141, reduced pathological retinal angiogenesis in a mouse model of oxygen-induced retinopathy140. These studies advocate for the beneficial role of lowering ceramide levels in DR pathology and also highlights the importance of ceramide in DR. In DR, mitochondrial damage leading to cell breakdown in the blood-retinal barrier (BRB) causes pathological abnormalities142. In-vivo studies of diabetic rats have demonstrated a relationship between ceramide and mitochondrial function143; aSMase-dependent accumulation of ceramide was observed in the mitochondria of STZ-induced diabetic rat retinas. Increases in ceramide were shown to be responsible for induction of proinflammatory cytokines like IL-1β and IL-6, and impaired mitochondrial function in-vitro. Increase in very long-chain (VLC) ceramides have been shown to stabilize tight junctions and prevent blood-retinal barrier dysregulation in in-vitro models. Elongation of very long-chain fatty acids protein 4 (ELOVL4) is responsible for production of VLC fatty acids that are incorporated in VLC ceramides. ELOVL4 is reduced to a great extent in STZ-induced diabetic rat retinas134, and overexpression of retinal ELOVL4 decreased basal, IL-1β-induced, and vascular endothelial growth factor (VEGF)-induced permeability in a Bovine retinal endothelial cell (BREC) model144.
Thus, increase in ceramide levels as a consequence of altered sphingolipid metabolism appear to be relevant to the pathogenesis of diabetic retinopathy. Given ceramide’s central role in sphingolipid metabolism, this also underscores the importance of modulating sphingolipid metabolism in general as an important aspect of treating this complication.
Ceramide in Diabetic Neuropathy
While hyperglycemia is a well-established contributor to nerve injury, multiple other factors play important roles in diabetic neuropathy (DN)145. Recent developments suggest modulation of sphingolipid metabolism significantly contributes to the development and progression of DN145 and multiple studies provide evidence that dysregulated lipid metabolism, both at cellular and macro levels, aggravates DN in diabetic animal models146. Abnormalities of axonal protein transport are thought to have an important role in the pathogenesis of DN, and earlier reports showing a positive effect of exogenous gangliosides on the axonal transport of structural proteins in the sensory fibers of streptozotocin-induced diabetic rats highlight the possible role of sphingolipids in DN147. Also, in-vitro studies of Schwann cell apoptosis by palmitate was reported to be significantly suppressed by ceramide synthase inhibitors, further supporting a role of ceramide148. Atypical deoxysphingolipids, which are toxic to neurons and pancreatic β-cells149,150, are formed when SPT metabolizes alanine or glycine instead of serine. Deoxysphingolipid levels are reported to be elevated in hereditary sensory and autonomic neuropathy type 1 and in type 2 DM17. It has also been reported that plasma levels of deoxysphingolipids are elevated in individuals with T1D, though not as much as in type 2 diabetes151–153. One study showed that 1-deoxydihydroceramide is a particularly important mediator for cytotoxicity towards insulin-producing pancreatic β-cells, and highlights the importance of targeting deoxysphingolipid biosynthesis with fenofibrate as a promising therapeutic approach for diabetic neuropathy 17. The fact that disproportionate increases in certain deoxysphingolipids are seen in diabetic individuals with neuropathy reinforces their importance in this complication. A recent pilot study of type I diabetic patients further suggests an important role of ceramide in neuropathy. The study was conducted in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) type 1 diabetes sub-cohort with the goal to assess the associations between multiple sphingolipid species, deoxysphingolipids, and free amino acids, and the presence of symptomatic neuropathy. Using mass spectroscopy, plasma levels of DSL and free amino acids were measured in participants with and without symptoms of neuropathy. The study showed significant associations between increased levels of ceramides, plasma long-chain deoxy-ceramides, and patients reporting neuropathy. This study also highlights the potential of plasma ceramide and deoxy-ceramide species as diagnostic and prognostic biomarkers in diabetic neuropathy152. Altered sphingolipid metabolism is a developing area of study in the context of neuropathy and further research might lead to new therapies targeting sphingolipid metabolism.
Ceramides, Adiponectin, and FGF Interactions: Clinical Implications
Adiponectin, the most abundant product secreted from adipocytes, lowers hepatic and muscle triglycerides154 and is associated with improved insulin sensitivity, decreased cardiometabolic risk, and decreased risk of progression from prediabetes to J2D155–159. Indeed, a longitudinal cohort study showed that adiponectin levels could act as a biomarker for diabetes risk159. Furthermore, higher levels of adiponectin are associated with decreased risk of progression from normoglycemia to prediabetes in African American and European American offspring of parents with T2D160. Besides improvements in insulin sensitivity, other mechanisms of adiponectin’s favorable cardiometabolic profile include anti-inflammatory effects, decreased apoptosis and preservation of β-cell function, and reversal of lipotoxicity161–165. These numerous pro-cardiometabolic mechanisms appear to be unified by interactions among adiponectin, fibroblast growth factor 21 (FGF-21; a potent regulator of metabolism and energy utilization), and ceramides. Studies in rodent models show significant effects of FGF-21 in regulating blood glucose levels and increasing insulin sensitivity in mice, mediated by upregulation of adiponectin expression154, 164. FGF-21 accelerates the clearance of toxic ceramides in tissues. The observation that adiponectin-knockout mice are refractory to the ceramide-clearing effects of FGF-21 directly implicates adiponectin in the mechanism of ceramide clearance164. These interactions have some clinical relevance: the well-known insulin-sensitizing effect of PPAR-γ agonists used for diabetes treatment has been shown to be mediated, at least in part, by increased adiponectin expression166. Several other interventions that improve cardiometabolic risk (including exercise, weight loss, smoking cessation and treatment with PPAR-γ agonists, ACE inhibitors, and angiotensin II receptor blockers) are known to also increase adiponectin levels165,167–171, and, through that mechanism, could potentially decrease ceramide burden in metabolically active tissues. Adiponectin is believed to modulate ceramide levels via the AdipoRI and AdipoR2 receptors, which help regulate ceramidase activity in tissues172.
Conclusions and Future Directions: Ceramides as biomarkers and therapeutic targets
The advances in sphingolipid and diabetes research over the past few years provide additional support to establish ceramides as key actors in the pathophysiology of diabetes and associated complications. By further developing our understanding of the numerous roles of sphingolipids in mediating the processes underlying diabetes, foundations are laid for future breakthroughs and development of therapeutic treatments and disease biomarkers. Important advances in the field of sphingolipid dysregulation are by no means limited to ceramide or sphingolipids involved in diabetic complications. Indeed, important recent advances also include other sphingolipid classes involved in many other diseases but are beyond the scope of this review. Nevertheless, it should be emphasized that the ubiquity and widespread bioactivity of certain sphingolipids virtually ensures their involvement in the pathophysiology of a wide range of human diseases.
Targeted research into applications of sphingolipid screening are already beginning to yield useful insights which may be clinically translatable. For instance, a recent project analyzed ceramide species in participants of the Framingham Heart Study and the Study of Health in Pomerania, finding evidence that the ratio of C24:0/C16:0 ceramides isolated from peripheral blood may have utility as a biomarker for congestive heart failure173. Another study very recently published a significant association between incident T2D and elevated levels of saturated SM species in a cohort selected from participants in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL)174. Some experimental models have shown elevations in free fatty acids and triglycerides before anomalie s in plasma glucose become detectable17, 175, and the ratio of ceramide C18:0/016:0 has been shown to have predictive value for diabetes and prediabetes up to 10 years prior to onset176. The detection of sphingolipid perturbations in other diabetic tissues, such as the vitreous177, further supports their involvement in diabetic complications and suggests that unique biomarkers could be utilized for early detection or prognosis of specific diabetic complications. As the onset of these complications is generally quite insidious, effective biomarkers for screening could have a substantial impact on diabetes complication incidence and/or severity by widening the window for instituting early interventions.
Given what is known about the bioactive potential of ceramides, the changes in sphingolipid metabolism and regulation in diabetes as suggested by these studies may in fact contribute to diabetes pathology and could therefore eventually help identify pharmacologic targets. The validity of targeting sphingolipid metabolism in disease states is supported in various disease models, including retinal degeneration178, cancer179, atherosclerosis180, and inflammation181, to name a few. Identifying exploitable sphingolipid targets in diabetic complications could prove valuable in developing new therapeutic drugs. Currently, much of the data on the roles of ceramides in diabetes pathophysiology and complications have been derived from animal models and limited cross-sectional human studies. Longitudinal investigation of ceramide expression among people with prediabetes who progress to type 2 diabetes would provide valuable insights into the chronology and causal interactions between ceramides and incident diabetes. Similarly, exploring the role of ceramide in the initial transition from normoglycemia to prediabetes would extend such insight to an even more proximal stage of early dysglycemia. Longitudinal studies among people with diabetes that analyze repeated measurements of ceramide burden in relation to the development of microvascular and macrovascular complications over extended follow-up periods would be highly informative. Novel findings regarding ceramide involvement in diabetes are likely to yield additional clinically useful revelations as the relevant mechanisms of sphingolipid bioactivity are further unraveled. Building upon these findings will be critical to the long-term goals of eventually developing preventative and curative therapies for diabetic complications and other human diseases.
Highlights:
Our paper highlights classical concepts and recent developments regarding the role of ceramides, a class of bioactive sphingolipids with cell signaling and second messenger capabilities, in mechanisms involving insulin action, glucoregulatory, pathogenesis of diabetes and its complications. The emerging understanding of cross-talk among ceramides, adiponectin and FGF21 in mediating cardiometabolic risk and responses to interventions (thiazolidinediones, angiotensin inhibitors, etc.) is discussed and amplified in the sub-section of our review devoted to clinical translation.
Acknowledgements:
Dr. Mandal is supported by NIH grants EY022071 and EY031316, and grants from Research to Prevent Blindness Inc., USA. Dr. Dagogo-Jack is supported by NIH grants R01 DK067269, DK 48411, and DK62203.
Abbreviations
- DM
Diabetes mellitus
- MI
Myocardial infarction
- PVD
Peripheral vascular disease
- T2D
Type 2 diabetes
- T1D
Type 1 diabetes
- SM
Sphingomyelin
- CerS
Ceramide synthase
- SPT
Serine palmitoyl transferase
- DES
Dihydroceramide desaturase
- SMS1
Sphingomyelin synthase 1
- PKCζ
Protein kinase C zeta
- AKT/PKB
Protein kinase B
- PP2A
Protein phosphatase 2A
- Smase
Sphingomyelinase
- TNFα
Tumor necrosis factor alpha
- NLRP3
NOD-, LRR- and pyrin domain-containing protein 3
- IL-1β
Interleukin-1 Beta
- JNK-1
c-Jun N-terminal kinase-1
- TLR
Toll-like receptor
- IκKβ
Inhibitor of kappa B kinase beta
- NFκB
Nuclear factor kappa B
- AMPK
AMP-activated protein kinase
- ZDF
Zukar diabetic fatty rat
- FAT
Fatty acid transporter
- CD36
Cluster determinant 36
- PP1
Protein phosphatase 1
- ERK1/2
Extracellular-signal-regulated kinase 1/2
- PDX-1
Pancreatic and duodenal homeobox gene-1
- ESKD
End-stage kidney disease
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- CVD
Cardiovascular disease
- TG
Triglyceride
- FFA
Free fatty acid
- ROS
Reactive oxygen species
- LDL
Low density lipoprotein
- UDP
Uridine diphosphate
- CKD
Chronic kidney disease
- MA
Macroalbuminuria
- AGE
Advanced glycation end-products
- GM3
Monosialodihexosylganglioside
- VEGF
Vascular endothelial growth factor
- OLETF
Otsuka Long Evans Tokushima Fatty
- HFD
High-fat diet
- ROS
Reactive oxygen species
- GFA
Glial fibrillary acidic
- GECs
Glomerular endothelial cells
- SMPDL3b
Sphingomyelin phosphodiesterase acid-like 3B
- C1P
Cermaide-1-phosphate
- AdipoRon
Adiponectin receptor agonist
- FTY720
Fingolimod
- DKD
Diabetic Kidney Disease
- LacCer
Lactosylceramide
- PCs
Pericytes
- ASAH1
N-acylsphingosine amidohydrolase 1
- DHA
Docosahexaenoic acid
- n3-PUFAs
OMEGA-3-Polyunsaturated Fatty-Acids
- DR
Diabetic Retinopathy
- BRB
Blood-retinal barrier
- aSMase
Acid SphingoMyelinase
- VLC
Very Long Chain
- STZ
Streptozotocin
- ELOVL4
Elongation of very long-chain fatty acids protein 4
- BREC
Bovine Retinal Endothelial Cell
- DN
Diabetic Neuropathy
- EDIC
Epidemiology of Diabetes Interventions and Complications
- DCCT
Diabetes Control and Complications Trial
- DSL
Deoxy-sphingolipid
- FGF-21
Fibroblast growth factor 21
- ACE
Angiotensin converting enzyme
- PPAR-γ
Peroxisome proliferator-activated receptor gamma
- AdipoR
Adiponectin receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
References:
- 1.Sontag EM, Lotz GP, Yang G, et al. Detection of Mutant Huntingtin Aggregation Conformers and Modulation of SDS-Soluble Fibrillar Oligomers by Small Molecules. J Huntingtons Dis. 2012;1(1): 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladiwala AR, Lin JC, Bale SS, et al. Resveratrol selectively remodels soluble oligomers and fibrils of amyloid Abeta into off-pathway conformers. J Biol Chem. 2010;285(31): 24228–24237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1): 4–14. [DOI] [PubMed] [Google Scholar]
- 4.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA. 2014;312(12): 1218–1226. [DOI] [PubMed] [Google Scholar]
- 5.Lichtwitz O, Boissonnot M, Mercie M, Ingrand P, Leveziel N. Prevalence of macular complications associated with high myopia by multimodal imaging. J Fr Ophtalmol. 2016;39(4): 355–363. [DOI] [PubMed] [Google Scholar]
- 6.Egede LE, Dagogo-Jack S. Epidemiology of type 2 diabetes: focus on ethnic minorities. Med Clin North Am. 2005;89(5): 949–975, viii. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6): 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370(16): 1514–1523. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6): 1615–1625. [DOI] [PubMed] [Google Scholar]
- 10.Houck SA, Ren HY, Madden VJ, et al. Quality control autophagy degrades soluble ERAD-resistant conformers of the misfolded membrane protein GnRHR. Mol Cell. 2014;54(1): 166–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50 Suppl: S91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer zu Heringdorf D, van Koppen CJ, Jakobs KH. Molecular diversity of sphingolipid signalling. FEBS Lett. 1997;410(1): 34–38. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Liu Y, Sullards MC, Merrill AH, Jr. An introduction to sphingolipid metabolism and analysis by new technologies. Neuromolecular Med. 2010;12(4): 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaurasia B, Summers SA. Ceramides - Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol Metab. 2015;26(10): 538–550. [DOI] [PubMed] [Google Scholar]
- 15.Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis. 2013;12: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29(4): 381–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol. 2017;13(2): 79–91. [DOI] [PubMed] [Google Scholar]
- 18.Eckel RH, Alberti KG, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375(9710): 181–183. [DOI] [PubMed] [Google Scholar]
- 19.Sokolowska E, Blachnio-Zabielska A. The Role of Ceramides in Insulin Resistance. Front Endocrinol (Lausanne). 2019;10: 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horowitz JF, Coppack SW, Paramore D, Cryer PE, Zhao G, Klein S. Effect of short-term fasting on lipid kinetics in lean and obese women. Am J Physiol. 1999;276(2): E278–284. [DOI] [PubMed] [Google Scholar]
- 21.Bonen A, Parolin ML, Steinberg GR, et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004;18(10): 1144–1146. [DOI] [PubMed] [Google Scholar]
- 22.Bickerton AS, Roberts R, Fielding BA, et al. Adipose tissue fatty acid metabolism in insulin-resistant men. Diabetologia. 2008;51(8): 1466–1474. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207(6): 928–934. [DOI] [PubMed] [Google Scholar]
- 24.Boini KM, Xia M, Koka S, Gehr TW, Li PL. Sphingolipids in obesity and related complications. Front Biosci (Landmark Ed). 2017;22: 96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dekker MJ, Baker C, Naples M, et al. Inhibition of sphingolipid synthesis improves dyslipidemia in the diet-induced hamster model of insulin resistance: evidence for the role of sphingosine and sphinganine in hepatic VLDL-apoB100 overproduction. Atherosclerosis. 2013;228(1): 98–109. [DOI] [PubMed] [Google Scholar]
- 26.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15(5): 585–594. [DOI] [PubMed] [Google Scholar]
- 27.Ng ML, Wadham C, Sukocheva OA. The role of sphingolipid signalling in diabetesassociated pathologies (Review). Int J Mol Med. 2017;39(2): 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turpin-Nolan SM, Bruning JC. The role of ceramides in metabolic disorders: when size and localization matters. Nat Rev Endocrinol. 2020;16(4): 224–233. [DOI] [PubMed] [Google Scholar]
- 29.Summers SA. Could Ceramides Become the New Cholesterol? Cell Metab. 2018;27(2): 276–280. [DOI] [PubMed] [Google Scholar]
- 30.Blachnio-Zabielska A, Baranowski M, Zabielski P, Gorski J. Effect of high fat diet enriched with unsaturated and diet rich in saturated fatty acids on sphingolipid metabolism in rat skeletal muscle. J Cell Physiol. 2010;225(3): 786–791. [DOI] [PubMed] [Google Scholar]
- 31.Sanvicens N, Cotter TG. Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. J Neurochem. 2006;98(5): 1432–1444. [DOI] [PubMed] [Google Scholar]
- 32.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4): 311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neeland IJ, Singh S, McGuire DK, et al. Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: the Dallas Heart Study. Diabetologia. 2018;61(12): 2570–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borodzicz S, Czarzasta K, Kuch M, Cudnoch-Jedrzejewska A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015;14: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mucinski JM, Manrique-Acevedo C, Kasumov T, Garrett TJ, Gaballah A, Parks EJ. Relationships between Very Low-Density Lipoproteins-Ceramides, -Diacylglycerols, and -Triacylglycerols in Insulin-Resistant Men. Lipids. 2020. [DOI] [PubMed] [Google Scholar]
- 36.Choi S, Snider AJ. Sphingolipids in High Fat Diet and Obesity-Related Diseases. Mediators Inflamm. 2015;2015: 520618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raichur S, Brunner B, Bielohuby M, et al. The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach. Mol Metab. 2019;21: 36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turpin-Nolan SM, Hammerschmidt P, Chen W, et al. CerS1-Derived C18:0 Ceramide in Skeletal Muscle Promotes Obesity-Induced Insulin Resistance. Cell Rep. 2019;26(1): 1–10 e17. [DOI] [PubMed] [Google Scholar]
- 39.Zabielski P, Daniluk J, Hady HR, et al. The effect of high-fat diet and inhibition of ceramide production on insulin action in liver. J Cell Physiol. 2019;234(2): 1851–1861. [DOI] [PubMed] [Google Scholar]
- 40.Reali F, Morine MJ, Kahramanogullari O, et al. Mechanistic interplay between ceramide and insulin resistance. Sci Rep. 2017;7: 41231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaurasia B, Tippetts TS, Mayoral Monibas R, et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019;365(6451): 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stratford S, DeWald DB, Summers SA. Ceramide dissociates 3’-phosphoinositide production from pleckstrin homology domain translocation. Biochem J. 2001;354(Pt 2): 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chavez JA, Knotts TA, Wang LP, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278(12): 10297–10303. [DOI] [PubMed] [Google Scholar]
- 44.Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279(35): 36608–36615. [DOI] [PubMed] [Google Scholar]
- 45.Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006;55(9): 2579–2587. [DOI] [PubMed] [Google Scholar]
- 46.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2): 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22(13): 1111–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5): 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sims K, Haynes CA, Kelly S, et al. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J Biol Chem. 2010;285(49): 38568–38579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2): 98–107. [DOI] [PubMed] [Google Scholar]
- 51.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11): 1288–1295. [DOI] [PubMed] [Google Scholar]
- 52.El-Assaad W, Buteau J, Peyot ML, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144(9): 4154–4163. [DOI] [PubMed] [Google Scholar]
- 53.Teng W, Li Y, Du M, Lei X, Xie S, Ren F. Sulforaphane Prevents Hepatic Insulin Resistance by Blocking Serine Palmitoyltransferase 3-Mediated Ceramide Biosynthesis. Nutrients. 2019;11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273(49): 32487–32490. [DOI] [PubMed] [Google Scholar]
- 55.Kennedy DJ, Kuchibhotla S, Westfall KM, Silverstein RL, Morton RE, Febbraio M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc Res. 2011;89(3): 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karunakaran U, Moon JS, Lee HW, Won KC. CD36 initiated signaling mediates ceramide-induced TXNIP expression in pancreatic beta-cells. Biochim Biophys Acta. 2015;1852(11): 2414–2422. [DOI] [PubMed] [Google Scholar]
- 57.Ruvolo PP. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res. 2003;47(5): 383–392. [DOI] [PubMed] [Google Scholar]
- 58.Fontes G, Semache M, Hagman DK, et al. Involvement of Per-Arnt-Sim Kinase and extracellular-regulated kinases-1/2 in palmitate inhibition of insulin gene expression in pancreatic beta-cells. Diabetes. 2009;58(9): 2048–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo J, Qian Y, Xi X, Hu X, Zhu J, Han X. Blockage of ceramide metabolism exacerbates palmitate inhibition of pro-insulin gene expression in pancreatic beta-cells. Mol Cell Biochem. 2010;338(1-2): 283–290. [DOI] [PubMed] [Google Scholar]
- 60.Furukawa N, Shirotani T, Araki E, et al. Possible involvement of atypical protein kinase C (PKC) in glucose-sensitive expression of the human insulin gene: DNA-binding activity and transcriptional activity of pancreatic and duodenal homeobox gene-1 (PDX-1) are enhanced via calphostin C-sensitive but phorbol 12-myristate 13-acetate (PMA) and Go 6976-insensitive pathway. Endocr J. 1999;46(1): 43–58. [DOI] [PubMed] [Google Scholar]
- 61.Yki-Jarvinen H Ceramides: A Cause of Insulin Resistance in Nonalcoholic Fatty Liver Disease in Both Murine Models and Humans. Hepatology. 2020;71(4): 1499–1501. [DOI] [PubMed] [Google Scholar]
- 62.Samuel VT, Shulman GI. Nonalcoholic Fatty Liver Disease, Insulin Resistance, and Ceramides. N Engl J Med. 2019;381(19): 1866–1869. [DOI] [PubMed] [Google Scholar]
- 63.Pagadala M, Kasumov T, McCullough AJ, Zein NN, Kirwan JP. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol Metab. 2012;23(8): 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apostolopoulou M, Gordillo R, Koliaki C, et al. Specific Hepatic Sphingolipids Relate to Insulin Resistance, Oxidative Stress, and Inflammation in Nonalcoholic Steatohepatitis. Diabetes Care. 2018;41(6): 1235–1243. [DOI] [PubMed] [Google Scholar]
- 65.Wasilewska N, Bobrus-Chociej A, Harasim-Symbor E, et al. Increased serum concentration of ceramides in obese children with nonalcoholic fatty liver disease. Lipids Health Dis. 2018;17(1): 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luukkonen PK, Zhou Y, Sadevirta S, et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;64(5): 1167–1175. [DOI] [PubMed] [Google Scholar]
- 67.Turpin SM, Nicholls HT, Willmes DM, et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20(4): 678–686. [DOI] [PubMed] [Google Scholar]
- 68.Jiang M, Li C, Liu Q, Wang A, Lei M. Inhibiting Ceramide Synthesis Attenuates Hepatic Steatosis and Fibrosis in Rats With Non-alcoholic Fatty Liver Disease. Front Endocrinol (Lausanne). 2019;10: 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grammatikos G, Ferreiros N, Waidmann O, et al. Serum Sphingolipid Variations Associate with Hepatic Decompensation and Survival in Patients with Cirrhosis. PLoS One. 2015;10(9): e0138130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched proinflammatory extracellular vesicles in an IRE1alpha-dependent manner. J Lipid Res. 2016;57(2): 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masarone M, Rosato V, Dallio M, et al. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid Med Cell Longev. 2018;2018: 9547613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simon J, Ouro A, Ala-Ibanibo L, Presa N, Delgado TC, Martinez-Chantar ML. Sphingolipids in Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma: Ceramide Turnover. Int J Mol Sci. 2019;21(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hammerschmidt P, Ostkotte D, Nolte H, et al. CerS6-Derived Sphingolipids Interact with Mff and Promote Mitochondrial Fragmentation in Obesity. Cell. 2019;177(6): 1536–1552 e1523. [DOI] [PubMed] [Google Scholar]
- 74.Biden TJ, Robinson D, Cordery D, Hughes WE, Busch AK. Chronic effects of fatty acids on pancreatic beta-cell function: new insights from functional genomics. Diabetes. 2004;53 Suppl 1: S159–165. [DOI] [PubMed] [Google Scholar]
- 75.Solanki NS, Barreto SG, Saccone GT. Acute pancreatitis due to diabetes: the role of hyperglycaemia and insulin resistance. Pancreatology. 2012;12(3): 234–239. [DOI] [PubMed] [Google Scholar]
- 76.Kumar K, Manrai M, Sood AK, Sharma R. A clinical study of insulin resistance in patients with chronic pancreatitis. Diabetes Metab Syndr. 2017; 11 Suppl 1: S283–S286. [DOI] [PubMed] [Google Scholar]
- 77.Kononczuk T, Lukaszuk B, Zendzian-Piotrowska M, et al. Plasma Sphingolipids in Acute Pancreatitis. Int J Mol Sci. 2017;18(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yano M, Watanabe K, Yamamoto T, et al. Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J Biol Chem. 2011;286(5): 3992–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo J, Zhu JX, Deng XH, et al. Palmitate-induced inhibition of insulin gene expression in rat islet beta-cells involves the ceramide transport protein. Cell Physiol Biochem. 2010;26(4-5): 717–728. [DOI] [PubMed] [Google Scholar]
- 80.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328(23): 1676–1685. [DOI] [PubMed] [Google Scholar]
- 81.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859): 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang XC, Paultre F, Pearson TA, et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20(12): 2614–2618. [DOI] [PubMed] [Google Scholar]
- 83.Harmancey R, Wilson CR, Taegtmeyer H. Adaptation and maladaptation of the heart in obesity. Hypertension. 2008;52(2): 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Son NH, Park TS, Yamashita H, et al. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117(10): 2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tao R, Zhang J, Vessey DA, Honbo N, Karliner JS. Deletion of the sphingosine kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc Res. 2007;74(1): 56–63. [DOI] [PubMed] [Google Scholar]
- 86.Wende AR, Symons JD, Abel ED. Mechanisms of lipotoxicity in the cardiovascular system. Curr Hypertens Rep. 2012;14(6): 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peterson LR, Jiang X, Chen L, et al. Alterations in plasma triglycerides and ceramides: links with cardiac function in humans with type 2 diabetes. J Lipid Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tippetts TS, Holland WL, Summers SA. The ceramide ratio: a predictor of cardiometabolic risk. Journal of Lipid Research. 2018;59(9): 1549–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laaksonen R, Ekroos K, Sysi-Aho M, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. European Heart Journal. 2016;37(25): 1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meeusen JW, Donato LJ, Bryant SC, Baudhuin LM, Berger PB, Jaffe AS. Plasma Ceramides: a novel predictor of major adverse cardiovascular events after coronary angiography. Arterioscler Thromb Vasc Biol. 2018;38(8): 1933–1939. [DOI] [PubMed] [Google Scholar]
- 91.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nature reviews Molecular cell biology. 2018;19(3): 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen QM, Tu VC. Apoptosis and heart failure: mechanisms and therapeutic implications. Am J Cardiovasc Drugs. 2002;2(1): 43–57. [DOI] [PubMed] [Google Scholar]
- 93.de Vries JE, Vork MM, Roemen TH, et al. Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res. 1997;38(7): 1384–1394. [PubMed] [Google Scholar]
- 94.Dyntar D, Eppenberger-Eberhardt M, Maedler K, et al. Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes. 2001;50(9): 2105–2113. [DOI] [PubMed] [Google Scholar]
- 95.Hickson-Bick DL, Sparagna GC, Buja LM, McMillin JB. Palmitate-induced apoptosis in neonatal cardiomyocytes is not dependent on the generation of ROS. Am J Physiol Heart Circ Physiol. 2002;282(2): H656–664. [DOI] [PubMed] [Google Scholar]
- 96.Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. J Biol Chem. 1997;272(6): 3324–3329. [DOI] [PubMed] [Google Scholar]
- 97.Sparagna GC, Hickson-Bick DL, Buja LM, McMillin JB. A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2000;279(5): H2124–2132. [DOI] [PubMed] [Google Scholar]
- 98.Pchejetski D, Kunduzova O, Dayon A, et al. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res. 2007;100(1): 41–49. [DOI] [PubMed] [Google Scholar]
- 99.Suematsu N, Tsutsui H, Wen J, et al. Oxidative stress mediates tumor necrosis factoralpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107(10): 1418–1423. [DOI] [PubMed] [Google Scholar]
- 100.Parra V, Eisner V, Chiong M, et al. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. 2008;77(2): 387–397. [DOI] [PubMed] [Google Scholar]
- 101.Devlin CM, Leventhal AR, Kuriakose G, Schuchman EH, Williams KJ, Tabas I. Acid Sphingomyelinase Promotes Lipoprotein Retention Within Early Atheromata and Accelerates Lesion Progression. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(10): 1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kinnunen PKJ, Holopainen JM. Sphingomyelinase Activity of LDL: A Link between Atherosclerosis, Ceramide, and Apoptosis? Trends in Cardiovascular Medicine. 2002;12(1): 37–42. [DOI] [PubMed] [Google Scholar]
- 103.Blitzer JT, Wang L, Summers SA. DES1: A Key Driver of Lipotoxicity in Metabolic Disease. DNA and cell biology. 2020;39(5): 733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nicholls M Plasma ceramides and cardiac risk. European Heart Journal. 2017;38(18): 1359–1360. [Google Scholar]
- 105.Croyal M, Kaabia Z, Leon L, et al. Fenofibrate decreases plasma ceramide in type 2 diabetes patients: A novel marker of CVD? Diabetes & metabolism. 2018;44(2): 143–149. [DOI] [PubMed] [Google Scholar]
- 106.Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The Global Epidemiology of Diabetes and Kidney Disease. Adv Chronic Kidney Dis. 2018;25(2): 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vallon V, Komers R. Pathophysiology of the diabetic kidney. Compr Physiol. 2011; 1(3): 1175–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zager RA, Conrad S, Lochhead K, Sweeney EA, Igarashi Y, Burkhart KM. Altered sphingomyelinase and ceramide expression in the setting of ischemic and nephrotoxic acute renal failure. Kidney International. 1998;53(3): 573–582. [DOI] [PubMed] [Google Scholar]
- 109.Liu G, Han F, Yang Y, et al. Evaluation of sphingolipid metabolism in renal cortex of rats with streptozotocin-induced diabetes and the effects of rapamycin. Nephrology Dialysis Transplantation. 2010;26(5): 1493–1502. [DOI] [PubMed] [Google Scholar]
- 110.Shayman JA, Radin NS. Structure and function of renal glycosphingolipids. Am J Physiol. 1991;260(3 Pt 2): F291–302. [DOI] [PubMed] [Google Scholar]
- 111.Mather AR, Siskind LJ. Glycosphingolipids and kidney disease. Adv Exp Med Biol. 2011;721: 121–138. [DOI] [PubMed] [Google Scholar]
- 112.Zador IZ, Deshmukh GD, Kunkel R, Johnson K, Radin NS, Shayman JA. A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J Clin Invest. 1993;91(3): 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Subathra M, Korrapati M, Howell LA, et al. Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells. Am J Physiol Renal Physiol. 2015;309(3): F204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lopes-Virella MF, Baker NL, Hunt KJ, et al. Glycosylated sphingolipids and progression to kidney dysfunction in type 1 diabetes. Journal of clinical lipidology. 2019; 13(3): 481–491.e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vukovic I, Bozic J, Markotic A, Ljubicic S, Ticinovic Kurir T. The missing link - likely pathogenetic role of GM3 and other gangliosides in the development of diabetic nephropathy. Kidney Blood Press Res. 2015;40(3): 306–314. [DOI] [PubMed] [Google Scholar]
- 116.Woo CY, Baek JY, Kim AR, et al. Inhibition of Ceramide Accumulation in Podocytes by Myriocin Prevents Diabetic Nephropathy. Diabetes & metabolism journal. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Choi SR, Lim JH, Kim MY, et al. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism: clinical and experimental. 2018;85: 348–360. [DOI] [PubMed] [Google Scholar]
- 118.Fornoni A, Sageshima J, Wei C, et al. Rituximab Targets Podocytes in Recurrent Focal Segmental Glomerulosclerosis. Science Translational Medicine. 2011;3(85): 85ra46–85ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yoo T- H, Pedigo CE, Guzman J, et al. Sphingomyelinase-Like Phosphodiesterase 3b Expression Levels Determine Podocyte Injury Phenotypes in Glomerular Disease. Journal of the American Society of Nephrology. 2015;26(1): 133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jiang Y, Owei I, Wan J, Ebenibo S, Dagogo-Jack S. Adiponectin levels predict prediabetes risk: the Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study. BMJ Open Diabetes Research & Care. 2016;4(1): e000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nature Medicine. 2011; 17(1): 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Choi SR, Lim JH, Kim MY, et al. Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism: clinical and experimental. 2018;85: 348–360. [DOI] [PubMed] [Google Scholar]
- 123.Mitrofanova A, Mallela SK, Ducasa GM, et al. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nature Communications. 2019;10(1): 2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Awad AS, Rouse MD, Khutsishvili K, et al. Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney International. 2011;79(10): 1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen H, Tran JT, Eckerd A, et al. Inhibition of de novo ceramide biosynthesis by FTY720 protects rat retina from light-induced degeneration. J Lipid Res. 2013;54(6): 1616–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2012;12(4): 346–354. [DOI] [PubMed] [Google Scholar]
- 127.Beck-Nielsen H, Olesen T, Mogensen CE, et al. Effect of near normoglycemia for 5 years on progression of early diabetic retinopathy and renal involvement. Diabetes Res. 1990;15(4): 185–190. [PubMed] [Google Scholar]
- 128.Zhang L, Krzentowski G, Albert A, Lefebvre PJ. Risk of developing retinopathy in Diabetes Control and Complications Trial type 1 diabetic patients with good or poor metabolic control. Diabetes Care. 2001;24(7): 1275–1279. [DOI] [PubMed] [Google Scholar]
- 129.Su EN, Alder VA, Yu DY, Yu PK, Cringle SJ, Yogesan K. Continued progression of retinopathy despite spontaneous recovery to normoglycemia in a long-term study of streptozotocin-induced diabetes in rats. Graefes Arch Clin Exp Ophthalmol. 2000;238(2): 163–173. [DOI] [PubMed] [Google Scholar]
- 130.Wilmott LA, Grambergs RC, Allegood JC, Lyons TJ, Mandal N. Analysis of sphingolipid composition in human vitreous from control and diabetic individuals. Journal of Diabetes and its Complications. 2019;33(3): 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-Induced Apoptosis in Cultured Bovine Retinal Pericytes. Diabetes. 2005;54(6): 1838–1845. [DOI] [PubMed] [Google Scholar]
- 132.Fox TE, Han X, Kelly S, et al. Diabetes Alters Sphingolipid Metabolism in the Retina. Diabetes. 2006;55(12): 3573–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Opreanu M, Tikhonenko M, Bozack S, et al. The Unconventional Role of Acid Sphingomyelinase in Regulation of Retinal Microangiopathy in Diabetic Human and Animal Models. Diabetes. 2011;60(9): 2370–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tikhonenko M, Lydic TA, Wang Y, et al. Remodeling of Retinal Fatty Acids in an Animal Model of Diabetes. Diabetes. 2010;59(1): 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Busik JV, Esselman WJ, Reid GE. Examining the role of lipid mediators in diabetic retinopathy. Clinical Lipidology. 2012;7(6): 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hammer SS, Busik JV. The role of dyslipidemia in diabetic retinopathy. Vision Research. 2017;139: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Opreanu M, Lydic TA, Reid GE, McSorley KM, Esselman WJ, Busik JV. Inhibition of Cytokine Signaling in Human Retinal Endothelial Cells through Downregulation of Sphingomyelinases by Docosahexaenoic Acid. Investigative Ophthalmology & Visual Science. 2010;51(6): 3253–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Simón MV, Prado Spalm FH, Vera MS, Rotstein NP. Sphingolipids as Emerging Mediators in Retina Degeneration. Frontiers in Cellular Neuroscience. 2019;13(246). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chakravarthy H, Navitskaya S, O'Reilly S, et al. Role of Acid Sphingomyelinase in Shifting the Balance Between Proinflammatory and Reparative Bone Marrow Cells in Diabetic Retinopathy. STEM CELLS. 2016;34(4): 972–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13(7): 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Futterman S, Kupfer C. The fatty acid composition of the retinal vasculature of normal and diabetic human eyes. Invest Ophthalmol. 1968;7(1): 105–108. [PubMed] [Google Scholar]
- 142.Levitsky Y, Hammer Ss, Fisher KP, et al. Mitochondrial Ceramide Effects on the Retinal Pigment Epithelium in Diabetes. 2020;21(11): 3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Colombini M Ceramide channels and mitochondrial outer membrane permeability. Journal of Bioenergetics and Biomembranes. 2017;49(1): 57–64. [DOI] [PubMed] [Google Scholar]
- 144.Kady NM, Liu X, Lydic TA, et al. ELOVL4-Mediated Production of Very Long-Chain Ceramides Stabilizes Tight Junctions and Prevents Diabetes-Induced Retinal Vascular Permeability. Diabetes. 2018;67(4): 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Callaghan BC, Gallagher G, Fridman V, Feldman EL. Diabetic neuropathy: what does the future hold? Diabetologia. 2020;63(5): 891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Perez-Matos MC, Morales-Alvarez MC, Mendivil CO. Lipids: A Suitable Therapeutic Target in Diabetic Neuropathy? Journal of diabetes research. 2017;2017: 6943851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Figliomeni B, Bacci B, Panozzo C, Fogarolo F, Triban C, Fiori MG. Experimental Diabetic Neuropathy: Effect of Ganglioside Treatment on Axonal Transport of Cytoskeletal Proteins. Diabetes. 1992;41(7): 866–871. [DOI] [PubMed] [Google Scholar]
- 148.Suzuki J, Akahane K, Nakamura J, et al. Palmitate induces apoptosis in Schwann cells via both ceramide-dependent and independent pathways. Neuroscience. 2011;176: 188–198. [DOI] [PubMed] [Google Scholar]
- 149.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274(34): 24202–24210. [DOI] [PubMed] [Google Scholar]
- 150.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45(1): 42–72. [DOI] [PubMed] [Google Scholar]
- 151.Dohrn MF, Othman A, Hirshman SK, et al. Elevation of plasma 1-deoxy-sphingolipids in type 2 diabetes mellitus: a susceptibility to neuropathy? European Journal of Neurology. 2015;22(5): 806–e855. [DOI] [PubMed] [Google Scholar]
- 152.Hammad SM, Baker NL, El Abiad JM, et al. Increased Plasma Levels of Select Deoxy-ceramide and Ceramide Species are Associated with Increased Odds of Diabetic Neuropathy in Type 1 Diabetes: A Pilot Study. NeuroMolecular Medicine. 2017;19(1): 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zuellig RA, Hornemann T, Othman A, et al. Deoxysphingolipids, Novel Biomarkers for Type 2 Diabetes, Are Cytotoxic for Insulin-Producing Cells. Diabetes. 2014;63(4): 1326–1339. [DOI] [PubMed] [Google Scholar]
- 154.Goetz R Metabolism: Adiponectin---a mediator of specific metabolic actions of FGF21. Nat Rev Endocrinol. 2013;9(9): 506–508. [DOI] [PubMed] [Google Scholar]
- 155.Duncan BB, Schmidt MI, Pankow JS, et al. Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2004;53(9): 2473–2478. [DOI] [PubMed] [Google Scholar]
- 156.Halperin F, Beckman JA, Patti ME, et al. The role of total and high-molecular-weight complex of adiponectin in vascular function in offspring whose parents both had type 2 diabetes. Diabetologia. 2005;48(10): 2147–2154. [DOI] [PubMed] [Google Scholar]
- 157.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7): 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360(9326): 57–58. [DOI] [PubMed] [Google Scholar]
- 159.Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian indians. Diabetes Care. 2003;26(12): 3226–3229. [DOI] [PubMed] [Google Scholar]
- 160.Jiang Y, Owei I, Wan J, Ebenibo S, Dagogo-Jack S. Adiponectin levels predict prediabetes risk: the Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study. BMJ Open Diabetes Res Care. 2016;4(1): e000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kusminski CM, Holland WL, Sun K, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18(10): 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Villarreal-Molina MT, Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie. 2012;94(10): 2143–2149. [DOI] [PubMed] [Google Scholar]
- 163.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8): 941–946. [DOI] [PubMed] [Google Scholar]
- 164.Holland WL, Adams AC, Brozinick JT, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17(5): 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kriketos AD, Gan SK, Poynten AM, Furler SM, Chisholm DJ, Campbell LV. Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care. 2004;27(2): 629–630. [DOI] [PubMed] [Google Scholar]
- 166.Kubota N, Terauchi Y, Kubota T, et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J Biol Chem. 2006;281(13): 8748–8755. [DOI] [PubMed] [Google Scholar]
- 167.Yang WS, Lee WJ, Funahashi T, et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86(8): 3815–3819. [DOI] [PubMed] [Google Scholar]
- 168.Yu JG, Javorschi S, Hevener AL, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51(10): 2968–2974. [DOI] [PubMed] [Google Scholar]
- 169.Sahebkar A Head-to-head comparison of fibrates versus statins for elevation of circulating adiponectin concentrations: a systematic review and meta-analysis. Metabolism: clinical and experimental. 2013;62(12): 1876–1885. [DOI] [PubMed] [Google Scholar]
- 170.Fontana V, de Faria AP, Oliveira-Paula GH, et al. Effects of angiotensin-converting enzyme inhibition on leptin and adiponectin levels in essential hypertension. Basic Clin Pharmacol Toxicol. 2014;114(6): 472–475. [DOI] [PubMed] [Google Scholar]
- 171.Hass A, Oz H, Mashavi M, Shargorodsky M. Role of RAAS and adipokines in cardiovascular protection: effect of different doses of angiotensin II receptor blocker on adipokines level in hypertensive patients. J Am Soc Hypertens. 2014;8(10): 709–714. [DOI] [PubMed] [Google Scholar]
- 172.Lopez X, Goldfine AB, Holland WL, Gordillo R, Scherer PE. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J Pediatr Endocrinol Metab. 2013;26(9–10): 995–998. [DOI] [PubMed] [Google Scholar]
- 173.Peterson LR, Xanthakis V, Duncan MS, et al. Ceramide Remodeling and Risk of Cardiovascular Events and Mortality. J Am Heart Assoc. 2018;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen GC, Chai JC, Yu B, et al. Serum sphingolipids and incident diabetes in a US population with high diabetes burden: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Am J Clin Nutr. 2020;112(1): 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007;65(6 Pt 2): S39–46. [DOI] [PubMed] [Google Scholar]
- 176.Hilvo M, Salonurmi T, Havulinna AS, et al. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia. 2018;61(6): 1424–1434. [DOI] [PubMed] [Google Scholar]
- 177.Wilmott LA, Grambergs RC, Allegood JC, Lyons TJ, Mandal N. Analysis of sphingolipid composition in human vitreous from control and diabetic individuals. J Diabetes Complications. 2019;33(3): 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stiles M, Qi H, Sun E, et al. Sphingolipid profile alters in retinal dystrophic P23H-1 rats and systemic FTY720 can delay retinal degeneration. J Lipid Res. 2016;57(5): 818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Das S, Chatterjee N, Bose D, Banerjee S, Jha T, Das Saha K. Antineoplastic impact of leishmanial sphingolipid in tumour growth with regulation of angiogenic event and inflammatory response. Apoptosis. 2015;20(6): 869–882. [DOI] [PubMed] [Google Scholar]
- 180.Jiang XC, Liu J. Sphingolipid metabolism and atherosclerosis. Handb Exp Pharmacol. 2013(216): 133–146. [DOI] [PubMed] [Google Scholar]
- 181.Dechecchi MC, Nicolis E, Mazzi P, et al. Modulators of sphingolipid metabolism reduce lung inflammation. Am J Respir Cell Mol Biol. 2011. ;45(4): 825–833. [DOI] [PubMed] [Google Scholar]
- 182.Mullen TD, Hannun YA, Obeid LM. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem J. 2012;441(3): 789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jenkins RW, Canals D, Hannun YA. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal. 2009;21(6): 836–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82(1): 27–44. [DOI] [PubMed] [Google Scholar]
- 185.Canals D, Salamone S, Hannun YA. Visualizing bioactive ceramides. Chem Phys Lipids. 2018;216: 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20(6): 1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Coant N, Sakamoto W, Mao C, Hannun YA. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv Biol Regul. 2017;63: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7(7): 537–551. [DOI] [PubMed] [Google Scholar]
- 189.Holland WL, Bikman BT, Wang LP, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121(5): 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]


