Abstract
Objectives:
Sleep comprises one-third of one’s life, yet little is known about sleep in developing countries. Furthermore, many studies in industrialized countries have reported that sleep duration and quality decline with aging, but whether this association persists globally is unknown. This study’s objectives were to characterize sleep in a community without electricity in Haiti and to examine associations between measures of sleep and age.
Methods:
Fifty-eight Haiti residents (50% women) in four age groups, 18–30, 31–50, 51–64, and ≥65 years participated. Three days of wrist actigraphy were used to estimate sleep patterns.
Results:
Mean (standard deviation) values of sleep measures were: 20:57 (0:40) for sleep onset, 4:54 (0:43) for sleep end, 9.3 (1.2) h for time in bed, 7.0 (1.0) h for sleep duration, 54 (24) min awake after sleep onset, and 88.7 (5.4)% for sleep maintenance (percentage of sleep period actually spent sleeping). There were no significant differences in the sleep measures between men and women. Regression analyses adjusting for sex, household size, and number of people sleeping in the same room indicated that only sleep fragmentation differed by age group. Specifically, mean fragmentation was higher in the youngest age group than all other age groups, which did not differ from one another.
Conclusions:
Average time in bed in this Haitian sample was greater than previously reported for industrialized countries like the United States (9.3 versus. 7–8 h);, however, actual sleep duration averaged only 7 h. No age-related decline in sleep duration or quality was observed in Haiti.
The majority of sleep research has been conducted in industrialized areas, particularly the United States, Europe, and Japan. Very little is known about the sleep patterns of people living in less industrialized or developing areas. Sleep patterns may vary among industrialized and nonindustrialized areas due to sociocultural differences. Indeed, Worthman and Melby (2002) identified several factors that vary among cultures and could impact sleep patterns. These factors include bedding type and sleeping location, number of individuals sharing a sleeping space, proximity of animals (pets, livestock or wild), and nighttime rituals or travel (Worthman and Melby, 2002). It has also been proposed that the use of electricity for light artificially shortens human exposure to nocturnal darkness, extends the active period, and leads to a consolidated bedtime and shorter sleep duration than would be observed in a natural light-dark cycle (Wehr, 1999). The use of electricity can disrupt circadian rhythms, and this could lead to increased sleep deficiency (Czeisler, 2013). Thus, individuals living in areas with limited or no access to electricity may exhibit different sleep-wake patterns than have been observed in industrialized countries. In order to obtain a more comprehensive understanding of human sleep, sleep needs to be examined in a variety of cultures and environments. In addition, understanding variation in sleep patterns has important implications for health since sleep loss has been associated with impairments in a variety of health domains including neurobehavioral performance, mental health, immune function, appetite regulation, and glucose metabolism (Benca and Quintas, 1997; Bonnet, 2000; Ford and Cooper-Patrick, 2001; Spiegel et al., 2005).
A few studies have examined sleep in communities outside of the United States, Europe, and Japan. These have included a study among an agricultural population in Brazil, the Native Terena (Reimão et al., 2000), a study of men in Abidjan, a large city in the Ivory Coast (Bogui et al., 2002), a study among college students in Mexico City, among college students in Ethiopia (Lemma et al., 2012) and among college students in Nigeria (Oluwole, 2010). The primary limitation of all of these studies is that they relied on self-reported sleep duration and timing. One study among women in Senegal did use hip accelerometry to estimate time in bed (Ndiaye and Benefice, 2007), but this is not validated to estimate sleep duration or quality. Thus, objective estimates of sleep patterns in developing countries are lacking.
Previous research that used polysomnography to examine sleep and aging has suggested that objectively measured sleep duration and quality deteriorates with advancing age and, of course, these studies were conducted in industrialized countries. These studies reported that sleep in older adults is more shallow and characterized by more frequent awakenings, reduced sleep efficiency (percentage of time in bed actually spent sleeping), less slow-wave sleep (the deepest state of nonrapid eye movement sleep), and shorter total sleep time (Hornung et al., 2005; Ohayon et al., 2004; Unruh et al., 2008; Van Cauter et al., 2000). In a large cohort study in the USA, brief arousals from sleep significantly but modestly increased with age (Redline et al., 2004). Another commonly reported hallmark of aging is the progressive advancement of the circadian timing of sleep such that sleep onset and wake time occur at earlier clock times (Carrier et al., 1997). Finally, a study that compared sleep of younger (18–30 years) and older (60–75 years) adults using wrist actigraphy found that older adults went to bed and woke earlier, had shorter total sleep time, lower sleep efficiency, and greater wake after sleep onset (WASO) (Yoon et al., 2003). In the industrialized world, consolidated short sleep may involve a chronic disturbance of normal sleep regulation, including some degree of sleep deprivation and a misalignment of sleep relative to the natural dark period, throughout the lifetime. Associations between aging and sleep may therefore differ in regions without electricity if residents maintain sleep patterns that follow the light-dark cycle more closely and are not exposed to chronic sleep deprivation.
The primary aims of the present study were to (1) characterize sleep using an objective device, wrist actigraphy, in a community without electricity in rural Haiti, and (2) examine the associations between measures of sleep and age in this sample.
METHODS
Samples
A sample of Haitian adults was recruited from Fondwa, Haiti. Fondwa was a rural community in the Western Department of Haiti with a population of around 7,000 people. It was in a mountainous area of the country and was approximately a 3-h drive from Port Au Prince, the capital of Haiti. A key characteristic of the community at the time of this study was that there was no electricity available for the general population.
The study in Fondwa was conducted between January 30 and February 20, 2003. Day length at this time of year was between 11.03 and 11.75 h. Sunrise occurred between 6:09 and 6:25 and sunset occurred between 17:24 and 17:55. Four age groups of 15 subjects each were recruited in order to ensure a wide range of ages in the sample: (1) 18–35 years, (2) 36–50 years, (3) 51–64 years, and (4) 65 years and older. There were no other recruitment criteria. Women comprised 57, 64, 47, and 33% of each age group, respectively (Chi squared P = 0.37). Four local residents volunteered to assist with this study. All local assistants spoke French in addition to Creole. They informed other residents of the study through word of mouth. If interested, residents were instructed to come to the visitors’ center where investigators were staying. There, the consent form was read to them in Creole by one of the local assistants with an investigator present to help answer any questions the research volunteer had. After consent was obtained, a brief questionnaire was administered verbally in Creole by the investigator with the local assistant present to assist with questions.
The protocol was approved by the University of Chicago Institutional Review Board. Written signed consent was obtained after verbally explaining the study and reading the consent form to the subject. Consent was obtained in Creole with the assistance of local residents who were fluent in both Haitian Creole and French or English.
Wrist actigraphy
Subjects wore a wrist actigraphy monitor (Actiwatch-64, Mini Mitter, Bend OR) for 3 days continuously to estimate sleep characteristics. We had a limited number of monitors and time in Fondwa, and thus could not record more days. These Actiwatches were small, watch-like devices that have sensitive omnidirectional accelerometers that digitally record an integrated measure of gross motor activity, which is analyzed to identify sleep periods. Assessing sleep through actigraphy is therefore based on wrist movement, with the underlying premise that greater wrist movements are indicative of wake. Actigraphy has been validated against polysomnography, which is the gold standard for sleep measurement, demonstrating a correlation over 0.9 in healthy subjects (Jean-Louis et al., 1997). Unlike polysomnography, actigraphy does not appear to alter sleep behavior, as there is no “first night effect” (Ancoli-Israel et al., 2003). The devices recorded activity using 15-s epochs and the data were analyzed with the manufacturer’s software (Actiware 3.4) to calculate the sleep variables, which are described below.
In addition, participants were asked to write down the times they went to bed and woke up on a simple sleep diary form. A battery-operated digital clock was offered to those who did not own a watch or a clock, but only one subject used it (the remainder had access to wrist-watches). He was instructed to copy the numbers from the clock onto the form. The sleep log was used to identify bedtime and wake time for the actigraphy analysis, which is a common method for identification of time in bed for actigraphy analysis (Littner et al., 2003). Participants wore the actigraphy monitor either Monday through Wednesday nights or Thursday through Saturday nights. In this community, only Sunday was considered a non-work day.
Sleep characteristics
For each participant, we calculated the mean value from the 3 days of actigraphy for each of the following characteristics of sleep:
Time in bed. This is the interval between the time the participant said that he/she went to bed to sleep and the time he/she got out of bed.
Sleep duration. This is the total amount of sleep in hours obtained at night during time in bed.
Sleep onset time. This is the clock time of the initial sleep onset identified by the software.
Sleep end time. This is the clock time of the final morning awakening identified by the software.
Wake after sleep onset. This is the total amount of wake in hours between initial sleep onset and sleep end.
Sleep maintenance. This is the percentage of the period between sleep onset and sleep end (the “sleep period”) that is actually spent sleeping. It is called “sleep percentage” by the software but is described here as sleep “maintenance” because this value represents the ability to maintain sleep once one has fallen asleep initially. Higher values therefore indicate better sleep consolidation.
Sleep fragmentation. This is an index of restlessness during the sleep period. It is the sum of two percentages: (1) the percentage of the sleep period spent moving and (2) the percentage of the number of immobile phases (i.e., an epoch with no movement) that are only 1 min long or less. Fragmentation is an indicator of sleep disruption or disturbance and higher values indicate worse sleep quality.
The validity of wrist actigraphy has been compared to polysomnography, which is the gold standard. The overall agreement rate between actigraphy and polysomnography ranged from 72.1 to 96.5%, sensitivity ranged from 86.5 to 98.7% and specificity ranged from 27.7 to 67.1% (Van de Water et al., 2011). In general, actigraphic estimates of sleep duration, sleep percentage, and WASO are considered valid (Martin and Hakim, 2011; Van de Water et al., 2011). The sleep fragmentation index has not been validated against polysomnographic measures, but has been associated with health outcomes (Hinderliter et al., 2013; Knutson et al., 2011).
Other measures
Each participant also responded to a verbally administered questionnaire, which included age (years) and sex. We also asked the participants about household characteristics, including the total number of persons living in house and the total number of persons sleeping in same room as the participant. Finally, subjects were asked to rate their health using the responses “poor,” “fair,” “good,” “very good,” and “excellent.”
Statistical analysis
A total of 60 adults participated, however, two of these individuals did not have valid actigraphy data (one watch failed to record any data; the second person removed the watch after wearing it for only a few hours). Thus, a total of 58 residents of Fondwa were included in these analyses.
Descriptive statistics were estimated for each sleep variable as well as age and household characteristics. Analyses of variance were calculated comparing sleep characteristics between the four age groups and Bonferroni multiple comparison tests were used when the ANOVA indicated significant differences. Spearman’s rank correlation coefficients were calculated to compare sleep characteristics with the two household characteristics because they were right-skewed. We used independent student’s t-test to compare sleep characteristics between men and women. Linear regression models were then estimated to predict each sleep characteristic from the covariates, which included the four age groups (the oldest age group was the referent), sex (male is coded as 0 in the dummy variable), household size and number of people sleeping in the same room. All statistical analyses were conducted using StataSE 10 (College Station, TX). Of the 58 participants with valid actigraphy data, one person was missing household size and was therefore excluded from regression analyses including this variable. There were no other missing data. The estimated power to detect a 15-min change in sleep duration for every 10 years of age with a sample size of 58 people is 88% (Soper, 2013).
RESULTS
Ages in this sample ranged from 18 to 88 years and 50% were women. Table 1 presents a description of mean age, household characteristics, and sleep characteristics for the full sample and separately for men and women. Household sizes ranged from 2 to 12 people and the number of people sleeping in the same room as the participant ranged from one to nine people. No one slept in a room alone. There were no significant differences in mean sleep characteristics between men and women (all P > 0.05)
TABLE 1.
Study sample characteristics for full sample and by sex
| All | Men | Women | P | |
|---|---|---|---|---|
| n | 58 | 29 | 29 | |
| Age (years) | 48.6 (16.8) | 51.8 (15.8) | 45.5 (17.5) | 0.16a |
| Household size (n) | 5.5 (2.4) | 5.7 (2.5) | 5.4 (2.3) | 0.57a |
| Persons sleeping in same room (n) | 3.5 (2.0) | 3.4 (1.8) | 3.6 (2.2) | 0.75a |
| Subjective health; n (%) | 0.61b | |||
| Excellent | 1 (2%) | 1 (3.5%) | 0 | |
| Very good | 2 (3%) | 1 (3.5%) | 1 (3.5%) | |
| Good | 4 (7%) | 3 (10%) | 1 (3.5%) | |
| Fair | 45 (78%) | 22 (76%) | 23 (79%) | |
| Poor | 6 (10%) | 2 (7%) | 4 (14%) | |
| Sleep measures | ||||
| Sleep onset (hh:mm) | 20:57 (0:40) | 20:55 (0:35) | 20:59 (0:45) | 0.72a |
| Sleep end (hh:mm) | 4:54 (0:43) | 4:46 (0:40) | 5:01 (0:46) | 0.20a |
| Time in bed (h) | 9.3 (1.2) | 9.0 (1.1) | 9.5 (1.2) | 0.10a |
| Sleep duration (h) | 7.0 (1.0) | 7.0 (0.9) | 7.1 (1.1) | 0.84a |
| Wake after sleep onset (h) | 0.9 (0.4) | 0.8 (0.3) | 1.0 (0.5) | 0.24a |
| Sleep maintenance (%) | 88.7 (5.4) | 89.3 (4.3) | 88.0 (6.4) | 0.38a |
| Sleep fragmentation (%) | 17.9 (11.8) | 17.2 (10.3) | 18.5 (13.3) | 0.67a |
Data are presented as mean (standard deviation).
From Student’s t-test comparing sexes (unadjusted).
From a chi-squared test comparing sexes (unadjusted).
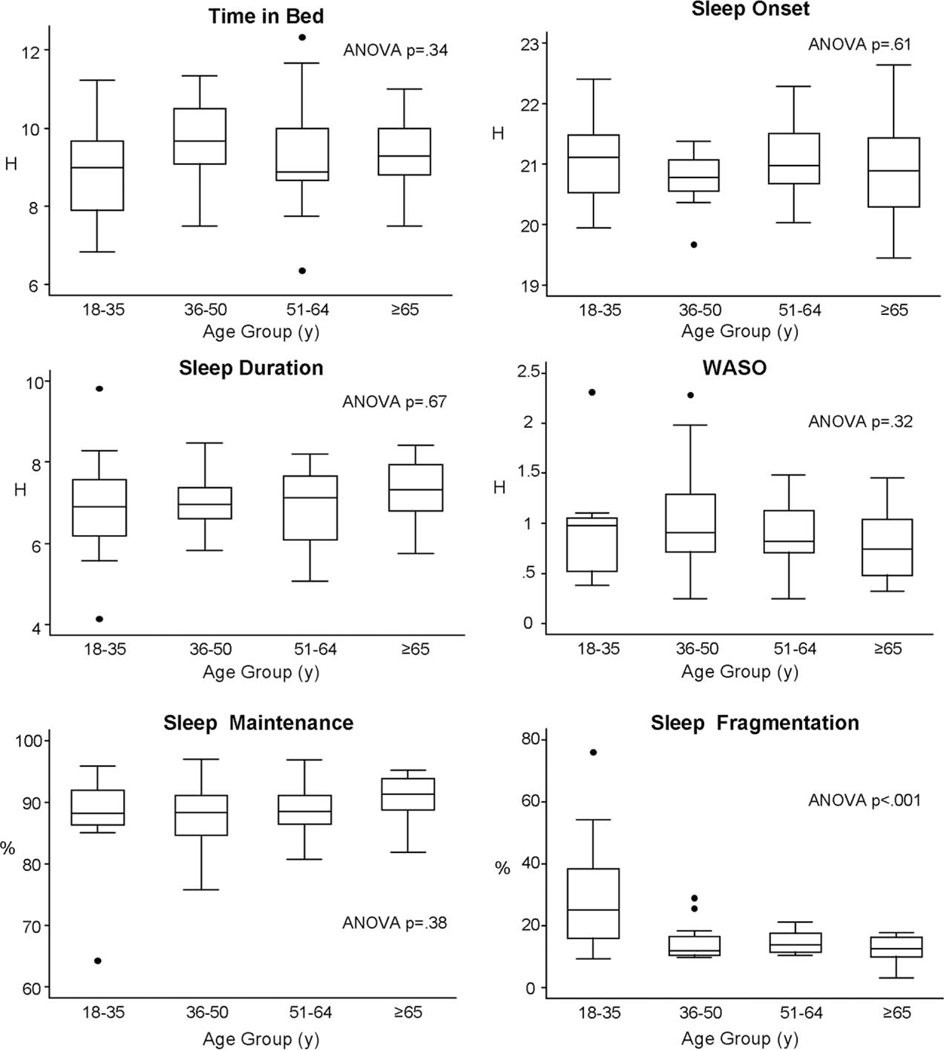
Figure 1 presents the unadjusted associations between age groups and each sleep characteristic in the Fondwa sample. Only sleep fragmentation was significantly different between the age groups in unadjusted analyses (ANOVA P < 0.001). Bonferroni tests indicated that the mean fragmentation among the youngest age group was significantly higher than the three older age groups but mean sleep fragmentation in these three older groups did not differ from one another. Household size was correlated with both sleep maintenance (ρ = −0.31, P = 0.02) and WASO (ρ = 0.38, P = 0.004), indicating reduced sleep consolidation and greater wake was associated with larger household size. Sleep end time was significantly positively correlated with number of people sleeping in the same room (ρ = 0.30, P = 0.04), indicating a later wake time was associated with a larger number of people sleeping in the same room. No other sleep characteristic was significantly correlated with either household size or number of people sleeping in the same room. Regression analyses (Table 2) adjusting for covariates indicated that only sleep fragmentation differed significantly between age groups. Specifically, sleep fragmentation was significantly higher in the youngest age group relative to the oldest age group (β = 17.8%, P < 0.001) but the other two age groups did not differ from the oldest group. In these regression analyses, sex was not significantly associated with any of the sleep characteristics (all P > 0.05). Larger household size was significantly associated with earlier sleep onset (6 min per person, P = 0.03), greater WASO (β = 4 min per person, P = 0.01), and lower sleep maintenance (β = −0.7% per person, P = 0.03). Number of people sleeping in the same room was associated with later sleep onset (β = 7 min per person, P = 0.03), and there was a trend for lower sleep maintenance (β = −0.7% per person, P = 0.06) and greater WASO (β = 4 min per person, P = 0.06).
Fig. 1.
Box plots showing the median and interquartile range of six sleep variables for the four age groups. The top of the box represents the 75th percentile; the bottom of the box, the 25th percentile; the line inside the box, the 50th percentile (median). Circles represent outliers. ANOVA P-values are also presented.
TABLE 2.
Results from multivariate regression models predicting each sleep measure (bold text indicates p<.05)
| Outcome variable, β (p value) |
|||||||
|---|---|---|---|---|---|---|---|
| Independent variables | Time in bed (h) | Sleep onset (h) | Sleep end (h) | Sleep duration (h) | WASO (h) | Sleep maintenance (%) | Sleep fragmentation (%) |
| Age group (years) | |||||||
| 18–35 | −0.75(0.11) | 0.19 (0.45) | −0.22 (0.43) | −0.50 (0.21) | 0.09 (0.53) | −2.1 (0.28) | 17.8 (<0.001) |
| 36–50 | 0.05 (0.91) | −0.15 (0.55) | −0.33 (0.24) | −0.29 (0.47) | 0.11 (0.46) | −1.2(0.53) | 0.2 (0.95) |
| 51–64 | −0.19 (0.66) | 0.16 (0.50) | −0.19 (0.47) | −0.37 (0.33) | 0.02 (0.90) | −0.7(0.70) | 1.6 (0.66) |
| ≥65 | ref | ref | ref | ref | ref | ref | ref |
| Female sex | 0.60 (0.07) | −0.003 (0.99) | 0.27 (0.17) | 0.15 (0.59) | 0.13 (0.22) | −1.1 (0.40) | 0.7 (0.80) |
| Household size | 0.15 (0.06) | −0.10 (0.03) | 0.02 (0.60) | 0.06 (0.37) | 0.06 (0.01) | −0.7 (0.03) | 0.7 (0.28) |
| # Persons sleeping in same room | −0.06 (0.49) | 0.12 (0.03) | 0.10 (0.09) | −0.07 (0.35) | 0.06 (0.06) | −0.7(0.06) | 0.9 (0.24) |
| Intercept | 8.6 | 21.0 | 4.5 | 7.2 | 0.25 | 96.7 | 6.0 |
DISCUSSION
Sleep characteristics in Haiti included an average time in bed of 9.3 h, but an average sleep duration based on wrist actigraphy of only 7 h. Sleep onset occurred at approximately 21:00 on average and wake time was approximately 5:00 on average. No participant slept in a room alone and on average three to four people slept in the same room as the participant. A greater number of people sleeping in the same room had a modest association with sleep quality. For example, three additional people in the room would be associated with 3% lower sleep maintenance or 16 additional minutes spent awake after initially falling asleep on average. There were no differences in sleep characteristics between men and women. Finally, only sleep fragmentation was significantly associated with aging, which was actually lower at older ages suggesting better sleep quality at older ages.
The average time in bed and average sleep duration in Haiti were longer than has been reported in the US (see Table 3). For example, the 2010 Sleep in America Poll conducted by the National Sleep foundation found that Americans in general reported spending 7.3 h in bed on weekdays and 8.2 h on weekends and reported sleeping 6.6 h on weekdays and 7.3 h on weekends (National Sleep Foundation, 2010). For African Americans in this poll, average time in bed was 7.1 h on weekdays and 8.1 h on weekends while sleep duration was 6.2 h on weekdays and 6.9 h on weekends (National Sleep Foundation, 2010). In addition, average bedtime was 23:30 on weekdays and 0:10 on weekends for African Americans. These data suggest that Americans, particularly African Americans, spend less time in bed, sleep less, and go to bed later than residents of Fondwa. However, the poll’s estimates were all self-reported, which may overestimate sleep duration compared to actigraphy (Lauderdale et al., 2008). One study in Massachusetts compared total sleep duration based on one week of wrist actigraphy among different racial/ethnic groups, including African-Caribbean immigrants (Ertel et al., 2011). The majority of the African-Caribbean immigrant group was people born in Haiti (56%). The unadjusted mean (SD) sleep duration for the African-Caribbean immigrants was 6.3 (1.3) h, which is 40 min less than the mean observed in Fondwa (independent t-test, P<0.001). No information on sleep timing or quality was provided in this study. The immigrant sample in this US study included all full-time employees and the mean age was age 39.1 (SD 11.2), almost 10 years younger than the Fondwa sample. Thus, it is unclear if environmental or sociocultural differences between living in Massachusetts and living in Fondwa, Haiti contributed to these differences. The CARDIA sleep study, which also used wrist actigraphy, reported that mean (SD) time in bed was 7.1 (1.2) h for the whole sample combined and 7.6 (1.43) h for African American women and 7.1 (1.4) h for African American men. Sleep duration in this study averaged 6.1 h for the full sample and only 5.9 h for African American women and 5.1 h for African American men (Lauderdale et al., 2006), which is over an hour less than the Fondwa average. The CARDIA study did include a narrower range of ages, that is, 35–50 years, than the Haiti sample but the mean age in CARDIA was only 5 years younger than the Haitian sample (43.4 vs. 48.3 years). However, since there was no association between age and sleep duration in the Fondwa sample, the difference in ages between these two studies probably does not explain the 1-h difference in average sleep duration.
TABLE 3.
Means of age and sleep measures across the week in various samples (see text for references)
| n | Mean age (years) | Sleep measure | Bedtime/sleep onset (hh:mm) | Wake time/sleep end | Time in bed (h) | Sleep duration (h) | |
|---|---|---|---|---|---|---|---|
| Haiti | 58 | 49 | Wrist actigraphy | 20:57 | 4:54 | 9.3 | 7 |
| 2010 Sleep in America Poll, all race/ethnicities | 1,007 | 42 | Self-report | 23:49 | 6:31 | 7.5 | 6.8 |
| 2010 Sleep in America Poll, African Americans | 250 | 42 | Self-report | 23:59 | 6:21 | 7.4 | 6.4 |
| African-Caribbean immigrants | 118 | 39 | Wrist actigraphy | n/a | n/a | n/a | 6.3 |
| CARDIA Sleep Study, African Americans | 294 | 43 | Wrist actigraphy | n/a | n/a | 7.4 | 5.6 |
| Native Terena, Brazil | 65 | 37 | Self-report | 20:43 | 6:06 | 9 | n/a |
| College Students, Mexico City | 577 | 20 | Self-report | 23:28 | 6:56 | 7.5 | 7.2 |
| College Students, Nigeria | 276 | 25 | Self-report | n/a | n/a | n/a | 6.2 |
| College Students, Ethiopia | 2,551 | 21 | Self-report | n/a | n/a | n/a | 6.8 |
| Ivory Coast-African Descent | 12 | 26 | Self-report | n/a | n/a | 7 | 6.4 |
| Senegal women | 49 | 36 | Hip actigraphy | 22:09 | 6:06 | 8 | n/a |
A few other studies have examined sleep in non-Westernized or developing areas (see Table 3). For example, a study among the Native Terena, a rural agricultural group in Brazil, found that they spent an average of 9 h in bed, went to bed at 20:43 and woke at 6:06 on weekdays for all age groups combined (Reimão et al., 2000). Despite being based on self-report, average bedtime, and time in bed were similar to the averages in the Haiti sample; however, average wake time in the Native Terena was approximately 1 h later. Three separate studies administered questionnaires to college students. Among college students in Mexico City, mean self-reported sleep duration was 6.6 h on weekdays and 8.6 h on weekends (Valencia-Flores et al., 1998). Mean bedtime on weekdays was 23:17 and 23:53 on weekends, while mean wake time was 6:11 on weekdays and 8:50 on weekends. The large discrepancy between weekdays and weekends is likely to due to waking early for school during the week. Among Nigerian undergraduate students (n = 276) aged 19–35 years, mean sleep duration was 6.1 h (SD 1.0) on weekdays and 6.5 h (SD 1.3) on weekends (Oluwole, 2010). Finally, among undergraduate students in Ethiopia, mean duration of sleep per night was reported to be 6.79 ± 1.9 h (Lemma et al., 2012). Except for the weekend sleep duration among the students in Mexico City, mean self-reported sleep duration was shorter among these three samples of college students than the estimated sleep duration in the Haiti sample. The differences in sleep duration between these samples of college students and the Haiti sample could be due to the demands of attending college in addition to the younger age range. In a study among adults aged 19–29 years in Abidjan, a large city in the Ivory Coast, the sleep of people of African descent (n = 12) and of European descent (n = 12) was assessed via questionnaire (Bogui et al., 2002). Both groups spent 7 h in bed and obtained 6.4 h of sleep per night, both of which are shorter than in the Haiti sample. It is important to note that all of the subjects of European descent and two of those of African descent were from the military. The remaining subjects came from sports clubs. Thus, the sleep of these individuals may not be representative either of the city of Abidjan or of the Ivory Coast as a whole. An important limitation to all of these other studies is that they relied on self-reported sleep measures, which may not be entirely accurate (Lauderdale et al., 2008). One study among 49 adult women over 20 years of age in an agricultural region in Senegal used a more objective estimate of sleep, one night of hip accelerometry, to estimate time spent in bed (Ndiaye and Benefice, 2007). On average, these women went to bed at 22:09, woke at 6:06, therefore spending approximately 8 h in bed. Because accelerometers worn on the hip are not validated to estimate sleep duration or quality, these metrics are not available in this study. If these data from a single night are representative of habitual bedtimes, then this population of rural Senegalese women spent approximately 1 h less time in bed than the women in the sample from Haiti.
The lack of association between most characteristics of sleep and age in Fondwa differs from many studies in the United States that reported a decline in sleep duration and quality with aging based on both polysomnography (Hornung et al., 2005; Ohayon et al., 2004; Unruh et al., 2008; Van Cauter et al., 2000) and on actigraphy (Ohayon et al., 2004; Yoon et al., 2003). Some have challenged the notion that aging is invariably associated with deterioration in sleep. Poor sleep quality may simply be associated with existing illness and healthy older adults will not necessarily experience sleep problems (Vitiello, 2009; Vitiello et al., 2002). The Haiti data support the argument that sleep does not inevitably deteriorate with age. However, since this was a cross-sectional study, it is possible that there was a survival effect in our study. Specifically, individuals who had poor sleep quality or short sleep durations when younger may not have survived to older ages, and the older Fondwa residents in our study may represent the healthier individuals and better sleepers. In addition, there may be cultural or behavioral factors that differentially impact sleep at various ages and therefore mask any physiological age differences. For example, younger individuals may be responsible for the care of young children and infants (either their own children or a relative’s), but unfortunately we do not have information on the ages of the household members nor information on childcare practices and responsibilities for each participant. In addition to childcare, there may be other nocturnal household tasks that are the responsibility of the younger adult members (e.g., dealing with animals such as roosters) or social activities that occur at night. Finally, older individuals may have preferential access to the more comfortable sleeping locations (i.e., on the bed rather than on the floor). Ethnographic data about sleeping practices in Haiti and elsewhere are needed to understand potential cultural influences on sleep patterns by age.
In addition to age, the relationship between the sleep measures and two household characteristics were examined. Participants from a larger household had a small but significantly earlier sleep onset (by 6 min) and worse sleep quality based on a slightly greater WASO (4 min more) and lower sleep efficiency (0.5% lower). Sleeping in the same room with a greater number of people was associated with a slightly later sleep onset (6 min). The associations suggest that being from a larger household could impact sleep, but the effect size is small. Reasons why a larger household could impact sleep could include the noise and activities of others disturbing sleep by impacting the ability to fall or stay asleep, however, whether these small differences translate to any physiological or psychosocial effect is unclear.
There are some limitations to this study. First, this was a cross-sectional study, thus, how sleep changes within individuals over the course of their lifetime in Haiti cannot be determined. Second, we only have 3 days of wrist activity recordings and more days may have provided a more reliable estimate of habitual behavior. We also did not ask participants to include naps on the sleep log so we could not estimate napping behavior. Furthermore, we do not distinguish work days from free days and cannot assess how sleep changes across these types of days. We also did not have polysomnographic recordings of sleep which is considered the gold standard for assessing sleep and is required to identify the different sleep stages. Thus, we cannot determine whether age-related changes in sleep stages, particularly a decline in slow-wave sleep, or increases in microarousals, were present in this sample from Haiti. Gross body movements do decline in elderly adults (Gori et al., 2004) and therefore wrist actigraphy, which is based on movements, may overestimate sleep quality in older participants. However, other studies that used actigraphy did observe a decline in sleep quality with age (Yoon et al., 2003). Finally, we did not collect detailed information about the sleeping environment beyond the number of people sleeping in the same room as the participant. We do not know how many slept on a bed, on the floor or on another sleep surface. We also do not know if participants changed sleep location throughout the night. Future research should include an examination of these characteristics as they can impact sleep patterns.
This study provides novel information about variation in human sleep by examining sleep in a Haitian community without electricity. As sleep can affect many health domains, it is important to understand how people sleep around the world and these data provide one small piece of the picture. Additional similar studies would help to determine the degree of variability in sleep behavior among different cultures, which would inform debates about what, if anything, characterizes “normal” human sleep patterns, particularly with respect to aging.
ACKNOWLEDGMENTS
The author thanks Renate Schneider for her assistance conducting the study in Haiti, Eve Van Cauter for her input on the manuscript and study design, our local assistants in Fondwa and all the study volunteers.
Contract grant sponsor: National Institutes of Health National Institute on Aging; Contract grant number: PO1 AG011412.
Footnotes
CONFLICTS OF INTEREST: None.
LITERATURE CITED
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. 2003. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26:342–392. [DOI] [PubMed] [Google Scholar]
- Benca RM, Quintas J. 1997. Sleep and host defenses: a review. Sleep 20: 1027–1037. [PubMed] [Google Scholar]
- Bogui P, Keita M, Dah C, Fidier N, Buguet-Brown ML, Buguet A. 2002. Le sommeil d’Africains et d’Européens en Côte d’Ivoire: étude par questionnaires. Santé 12:263–270. [PubMed] [Google Scholar]
- Bonnet M. 2000. Sleep deprivation. In: Kryger M, Roth T, Dement WC, editors. Principles and practices of sleep medicine, 3rd ed. Philadelphia, PA: WB Saunders. p 53–71. [Google Scholar]
- Carrier J, Monk TH, Buysse DJ, Kupfer DJ. 1997. Sleep and morning-eveningness in the ‘middle’ years of life (20–59 y). J Sleep Res 6:230–237. [DOI] [PubMed] [Google Scholar]
- Czeisler CA. 2013. Perspective: casting light on sleep deficiency. Nature 497:S13–S13. [DOI] [PubMed] [Google Scholar]
- Ertel KA, Berkman LF, Buxton OM. 2011. Socioeconomic status, occupational characteristics, and sleep duration in African/Caribbean immigrants and US White health care workers. Sleep 34:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D, Cooper-Patrick L. 2001. Sleep disturbances and mood disorders: an epidemiologic perspective. Depress Anxiety 14:3–6. [DOI] [PubMed] [Google Scholar]
- Gori S, Ficca G, Giganti F, Di Nasso I, Murri L, Salzarulo P. 2004. Body movements during night sleep in healthy elderly subjects and their relationships with sleep stages. Brain Res Bull 63:393–397. [DOI] [PubMed] [Google Scholar]
- Hinderliter AL, Routledge FS, Blumenthal JA, Koch G, Hussey MA, Wohlgemuth WK, Sherwood A. 2013. Reproducibility of blood pressure dipping: relation to day-to-day variability in sleep quality. J Am Soc Hypertens. doi:pii: S1933-1711(13)00097-1. 10.1016/j.jash.2013.06.001. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Hornung OP, Danker-Hopfe H, Heuser I. 2005. Age-related changes in sleep and memory: commonalities and interrelationships. Exp Gerontol 40:279–285. [DOI] [PubMed] [Google Scholar]
- Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H. 1997. The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Percept Motor Skills 85:207–216. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. 2011. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care 34:1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. 2008. Self-reported and measured sleep duration: how similar are they? Epidemiology 19:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, Liu K. 2006. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol 164:5–16. [DOI] [PubMed] [Google Scholar]
- Lemma S, Gelaye B, Berhane Y, Worku A, Williams MA. 2012. Sleep quality and its psychological correlates among university students in Ethiopia: a cross-sectional study. BMC Psychiatry 12:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, Hirshkowitz M, Kapen S, Kramer M, Loube D, Wise M, Johnson SF. 2003. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep 26:337–341. [DOI] [PubMed] [Google Scholar]
- Martin JL, Hakim AD. 2011. Wrist actigraphy. Chest 139:1514–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation. 2010. 2010 Sleep in America Poll. Washington, DC: National Sleep Foundation. [Google Scholar]
- Ndiaye GM, Benefice E. 2007. Patterns of daily activity and time spent in bed of adult women and adolescent and preadolescent girls from a rural community in Senegal, West Africa. Ann Hum Biol 34:454–469. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. 2004. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 27:1255–1273. [DOI] [PubMed] [Google Scholar]
- Oluwole OS. 2010. Sleep habits in Nigerian undergraduates. Acta Neurol Scand 121:1–6. [DOI] [PubMed] [Google Scholar]
- Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. 2004. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med 164:406–418. [DOI] [PubMed] [Google Scholar]
- Reimão R, Souza J, Gaudioso C, Guerra H, Alves A, Oliveira J, Gnobie N, Silvério D. 2000. Nocturnal sleep pattern in native Brazilian Terena adults. Arq Neuropsiquiatr 58:233–238. [DOI] [PubMed] [Google Scholar]
- Soper D. 2013. A-priori sample size calculator for multiple regression (Online Software).
- Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. 2005. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol 99:2008–2019. [DOI] [PubMed] [Google Scholar]
- Unruh ML, Redline S, An MW, Buysse DJ, Nieto FJ, Yeh JL, Newman AB. 2008. Subjective and objective sleep quality and aging in the sleep heart health study. J Am Geriatr Soc 56:1218–1227. [DOI] [PubMed] [Google Scholar]
- Valencia-Flores M, Castaño VA, Campos RM, Rosenthal L, Resendiz M, Vergara P, Aguilar-Roblero R, Ramos GG, Bliwise DL. 1998. The siesta culture concept is not supported by the sleep habits of urban Mexican students. J Sleep Res 7:21–29. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Plat L. 2000. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 284:861–868. [DOI] [PubMed] [Google Scholar]
- Van de Water AT, Holmes A, Hurley DA. 2011. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography—a systematic review. J Sleep Res 20(1 Pt 2):183–200. [DOI] [PubMed] [Google Scholar]
- Vitiello MV. 2009. Recent advances in understanding sleep and sleep disturbances in older adults: growing older does not mean sleeping poorly. Curr Dir Psychol Sci 18:316–320. [Google Scholar]
- Vitiello MV, Moe KE, Prinz PN. 2002. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res 53:555–559. [DOI] [PubMed] [Google Scholar]
- Wehr TA. 1999. The impact of changes in Nightlength (Scotoperiod) on human sleep. In: Turek FW, Zee PC, editors. Regulation of sleep and circadian rhythms. New York: Marcel Dekker, pp. 263–285. [Google Scholar]
- Worthman C, Melby M. 2002. Toward a comparative developmental ecology of human sleep. In: Carskadon M, editor. Adolescent sleep patterns: biological, social, and psychological influences. New York: Cambridge University Press. p 69–117. [Google Scholar]
- Yoon IY, Kripke DF, Youngstedt SD, Elliott JA. 2003. Actigraphy suggests age-related differences in napping and nocturnal sleep. J Sleep Res 12: 87–93. [DOI] [PubMed] [Google Scholar]