Abstract
Psoriasis is a chronic inflammatory autoimmune condition manifested by the hyperproliferation of keratinocytes with buildup of inflammatory red patches and scales on skin surfaces. The available treatment options for the management of psoriasis have various drawbacks, and the clinical need for effective therapeutics for this disease remain unmet; therefore, the approaches of drug repurposing or drug repositioning could potentially be used for treating indications of psoriasis. The undiscovered potential of drug repurposing or repositioning compensates for the limitations and hurdles in drug discovery and drug development processes. Drugs initially approved for other indications, including anticancer, antidiabetic, antihypertensive, and anti-arthritic activities, are being investigated for their potential in psoriasis management as a new therapeutic indication by using repurposing strategies. This article envisages the potential of various therapeutics for the management of psoriasis.
Keywords: Drug repurposing, Psoriasis, Inflammation, Clinical development, Regulatory consideration
Graphical abstract
Highlights
-
•
Psoriasis is an autoimmune inflammatory skin disorder with complex physiology.
-
•
Conventional treatments for psoriasis cause severe adverse effects; therefore an unmet need remains for safer and more effective therapies for psoriasis.
-
•
Various drugs that effectively decrease the inflammation and proliferation of skin cells can be repurposed for the management of psoriasis.
-
•
Repurposed drugs provide various incentives to the pharmaceutical industry.
Abbreviations
- DC
Dendritic Cells
- UV
Ultra violet
- EU
European Union
- US
United States
- NDA
New Drug Application
- LPS
Lipo-polysaccharides
- PGA
Physician's Global Assessment
- PASI
Psoriasis Area and Severity Index
- IMQ
Imiquimod
- TNF
Tumor Necrosis Factor
- IL
Interleukins
- NF-kB
Nuclear Factor kappa B
- STAT
Signal Transducer and Activator of Transcription
- MAPK
Mitogen-activated Protein Kinase
- BTK
Bruton's Tyrosine Kinase
- JAK
Janus Kinase
- cAMP
Cyclic Adenosine Monophosphate
1. Introduction
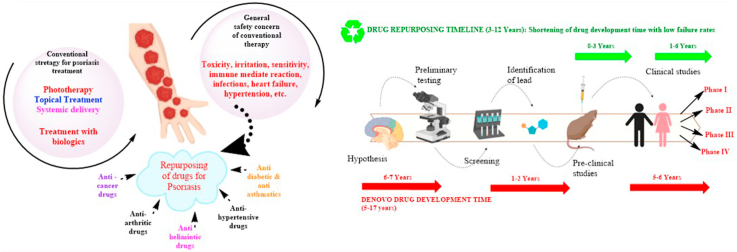
Psoriasis is a severe autoimmune disorder that is attributed to the hyperproliferation of skin cells and is usually characterized by red spots; thick, and scaly skin; and lesions resulting from hyperkeratosis, dilation of dermal capillaries, and parakeratosis (Harden et al., 2015). The innate and adaptive immunity that shields the body from invading pathogens can mistake its own cells for foreign elements, thus resulting in the eruption of a massive immunogenic response (Haahtela, 2019). In psoriatic conditions, the body produces antimicrobial peptides, which act as antigens presented to T cells by antigen-presenting cells called dendritic cells (DCs). T-lymphocytes (T cells) tend to attack normal skin cells and increase the amounts of inflammatory mediators in the blood and the rate of skin cell proliferation (Brembilla et al., 2018). T-cell mediated increases in the concentrations of inflammatory and pro-inflammatory cytokines such as interleukin (IL)-23, IL-17, IL-12, tumor necrosis factor alpha (TNF-α), and interferon gamma (INF-γ) in the body are the key hallmark of psoriasis (Arican et al., 2005). The released inflammatory cytokines are responsible for potentiating and continuing the cycle of inflammation in the body and thus, worsening the psoriatic condition (Lowes et al., 2014) (Albanesi, 2019). The contribution of these inflammatory cytokines to the pathogenesis of psoriasis has been demonstrated by the utilization of anticytokine antibodies, which are highly efficacious in treating psoriasis. Major contributions to Psoriasis are made by IL-23 and IL-17A. IL-23 is responsible for activation of IL-17 producing T cells (Hawkes et al., 2018). IL-17, specifically IL-17A, increases the proliferation and erratic differentiation of keratinocytes, and enhances the release of antimicrobial peptides and the inflammatory response (Deng et al., 2016). These newly produced skin cells begin to grow upon the older cells, thus leading to the formation of scaly skin (Anthony, 2006). The potential causative agents in the evolution of psoriasis may include genetic triggers, sunburn, infections, injury, severe stress, vitamin D deficiency, and certain medications. Other possible causes adversely affecting psoriasis conditions include cigarette smoking, alcohol consumption, obesity, and stress (AL-MUTAIRI et al., 2010; Zeng et al., 2017; Neimann et al., 2006). The common sites for the occurrence of spots and scaly skin include the scalp, knees, elbows, and legs. Drying of the skin can cause soreness, itching, burning, and sometimes bleeding (Kuchekar et al., 2011). To date, options for psoriasis treatment are lacking, although existing therapeutics are being used clinically to reduce inflammation and slow epidermal proliferation (Thorneloe et al., 2017). More than 120 million people are affected by psoriasis worldwide (Yip, 1984; Parisi et al., 2013).
The treatment regimens currently used for psoriasis primarily include phototherapy, psoralen with UVA (PUVA), and topical and systemic therapies (Greaves and Weinstein, 1995). The use of these conventional modalities is often associated with systemic toxicity, a prominent safety concern; moreover, their long-term use is associated with adverse effects (AEs) (Boehncke and Brembilla, 2018). Conventional treatment options leave clinical needs unmet in terms of bioavailability, skin permeation, and challenges concerning dosing frequency. Consequently, an efficacious, affordable, and safe therapeutic option for psoriasis is needed (Boehncke and Brembilla, 2018; Feldman et al., 2016). Drug repurposing is a potential, realistic and pragmatic tool to address the unmet need of available treatment options for psoriasis, with an emphasis on minimizing the impediments to drug development (Pushpakom et al., 2019; Oprea and Mestres, 2012).
Current therapeutic trends for treating psoriatic disorders include drugs already approved and used for other diseases that are now being investigated for the management of psoriasis, so that their applications may be repurposed or extended (Oprea and Mestres, 2012). Drug repurposing, or using a combination of two or more drugs, for psoriatic indications is an attractive approach to explore and reutilize the potential of drugs that are currently approved and used for other indications, and to investigate their unrealized prospects in treating new disease conditions (Baker et al., 2018). This strategy for extending the usage of drugs is also termed to as drug re-profiling, drug reformulation, drug repositioning, and drug combination (Ashburn and Thor, 2004). Drug repurposing is beneficial in the management of rare diseases, such as some genetic disorders and autoimmune diseases, because the traditional approaches are highly expensive and time-consuming. Therefore, drug repurposing is a potential approach to overcome obstacles in the development of new drugs and can also aid in exploring and reinvestigating the available marketed products for their therapeutic efficacy in new indications (Sekhon, 2013). Drug repurposing mainly requires identification of existing and already approved drug molecules for novel therapeutic reuse or of newer targets for already approved drugs. The regulatory benefits include potential bypassing of phase I and phase II clinical trials, particularly when similar or lower doses to those in previous validation data are used, and safety profiles from preclinical and clinical studies are available (Breckenridge and Jacob, 2019). Some factors that must be considered in repurposing any drug include the tolerability of the approved drug for new therapeutics, site-specificity, and the relevance of the drug's use for novel indications (Hernandez et al., 2017).
The present review focuses on discussing the redevelopment of old drugs for repurposing as psoriasis treatments, including different classes of drugs, their clinical data analysis, and challenges associated with drug repositioning. A brief overview of the regulatory pathway for drug approval for repurposed indications is also provided.
2. Repurposing of approved therapeutics for psoriasis: current need
Psoriasis is a condition with a complex pathophysiology and an unmet need for novel effective therapeutics. There is a lack of well-structured treatment strategies for treating psoriasis (Boehncke and Brembilla, 2018). The available treatment options for psoriasis include topical therapy, phototherapy, and systemic therapy. If the psoriasis is mild to moderate, then topical therapy is used. Moderate to severe psoriasis is characterized by the development of patches and scaly skin over a large surface area of the body, thus requiring systemic therapy. Topical treatments for mild to moderate psoriatic conditions include vitamin analogues, tars, corticosteroids, dithranol, and retinoids (Samarasekera et al., 2013). Topical therapy includes corticosteroids, calcipotriene, or tazarotene, and has the advantages of rapidly acting, localized effects, efficacy, an absence of severe AEs, and the ability to stop therapy immediately (Samarasekera et al., 2013). However, several drawbacks exist, such as tachyphylaxis, atrophy of the skin, adrenal suppression, and skin irritation. Generally, a combination of two drugs is preferred for topical treatment. If psoriasis spreads to more than 20% of the body, then topical treatment becomes cumbersome (Mason et al., 2013). For moderate to severe psoriasis phototherapy or systemic therapy is preferred. UVB or PUVA therapy is used (Archier et al., 2012). Phototherapy is confined to a specific region of the body and is very effective but can lead to the generation of squamous cell carcinoma, particularly in White people (Stern, 2007). Systemic agents are generally used, including methotrexate (whose drawbacks include bone marrow suppression, hepatotoxicity, liver cirrhosis, and contraindications in pregnant women) (Kozub and Simaljakova, 2011), cyclosporine (whose drawbacks include nephrotoxicity, hypertension, hypertrichosis, electrolyte imbalance, and gastrointesinal disturbances), and retinoids (whose drawbacks include lower efficacy as a monotherapy and the consequent need for combination therapies with topical agents and phototherapy; skin xerosis; conjunctivitis sicca cheilitis; and teratogenicity (Lebwohl et al., 2004; Warren and Griffiths, 2008). Phototherapy is an excellent alternative in conditions in which systemic therapy fails. However, AEs, such as antiproliferative and hepatotoxicity effects at non-target sites, are associated with systemic therapy. Systemic therapy and phototherapy are long-term therapies, and their use is limited because of frequent AEs and safety issues (Koo, 1999).
Methotrexate is a competitive folic acid antagonist used in systemic therapy against psoriasis. It requires many safety considerations, such as hepatotoxicity, pulmonary fibrosis, and various skin irritabilities. Severe AEs, such as fetal death and abnormalities, including myelosuppression, render it unsafe for use in pregnancy and with other conditions (Kozub and Simaljakova, 2011). However, cyclosporine causes nephrotoxicity, increases the levels of triglycerides and the risk of infections because of its immunosuppressive actions, leads to hypertension, and also causes malignancy (Paul et al., 2003). The combination therapy of PUVA causes photo-damage to the skin and eyes, and also increases the incidence of skin cancer. A risk of fetal death or fetal abnormalities has been reported with the administration of oral retinoids, such as acitretin, during pregnancy (Gollnick, 1996). Administration of oral retinoids can also lead to hepatotoxicity, dose-related alopecia, bone-related disorders, and increased levels of triglycerides. Use of high amounts of fumaric acid in psoriasis treatment can cause renal damage (Hoefnagel et al., 2003). The AEs outweigh the benefits provided by these treatment regimens for psoriasis, and thus the continued use of therapy through multiple routes of administration is difficult to justify. Moreover the probability of disease recurrence cannot be overlooked (Naldi and Griffiths, 2005). Some of the safety issues concerned with systemic therapy are listed in Table 1.
Table 1.
Safety concerns associated with biologics and drugs used for the management of psoriasis.
| Drug/biologic | Safety concern |
|---|---|
| Methotrexate | Abnormalities, such as myelosuppression or fetal death Pulmonary fibrosis Hepatotoxicity, gastrointestinal irritation, and hypersensitivity Severe skin problems |
| Psoralen with UVA | Photosensitivity and photo damage Skin toxicity, skin cancer, and nausea Delayed sunburn, erythema, and ocular damage |
| Cyclosporin | Hypertension Hepatotoxicity, nephrotoxicity, and immunosuppression Increased triglyceride levels and cutaneous malignancy |
| Oral retinoids | Abnormalities, such as myelosuppression or fetal death Hypertension, hepatotoxicity, and nephrotoxicity Alopecia (dose related) and elevated triglyceride levels Ophthalmic irritation, such as drying Myalgia and worsened condition in long term therapy |
| Fumaric acid | Gastrointestinal irritation, transient renal damage, and flushing |
| Etanercept | Severe infectious conditions, such as tuberculosis and heart failure Pancytopenia and neurological events |
| Adalimumab | Infections, such as TB and respiratory tract infection Reaction at the site of injection or hypersensitivity reaction Lymphoma and neurological events |
| Efalizumab | Immune-mediated reaction |
| Alefacept | Malignancy and lymphopenia |
To mitigate these issues in conventional systemic therapy, new systemic agents with high target specificity and better immunological actions have been developed to treat moderate to severe psoriatic conditions. The new therapy regimen includes systemic delivery of some biologics, such as TNF-α inhibitors (etanercept and adalimumab) (Murdaca et al., 2009), T-cell growth inhibitors (Alefacept) (Krueger, 2002) and inhibitors of T-cell activation, and migration (Efalizumab) (Lebwohl et al., 2003). These biologics can lead to the development of various severe AEs, such as malignancy or lymphoma in the case of alefacept. In extreme cases, they can also cause immunosuppression or congestive heart failure. These systemic biologics may also lead to the development of bacterial infections or tuberculosis, and many other respiratory tract infections and complications (Egeberg et al., 2018). Safety aspects of biologics are further discussed in Table 1.
Evidence from numerous studies indicates a lacuna in the safe and effective treatment of psoriasis. Thus, new formulation strategies or repositioning/repurposing of existing drugs can be effective (Baker et al., 2018). Numerous drugs have been investigated with the aim of repurposing their roles for psoriasis treatment. The repurposing of drugs will not only decrease toxicity and enhance therapeutic efficacy, but also promisingly decrease the time required for development of novel formulations (Strittmatter, 2014).
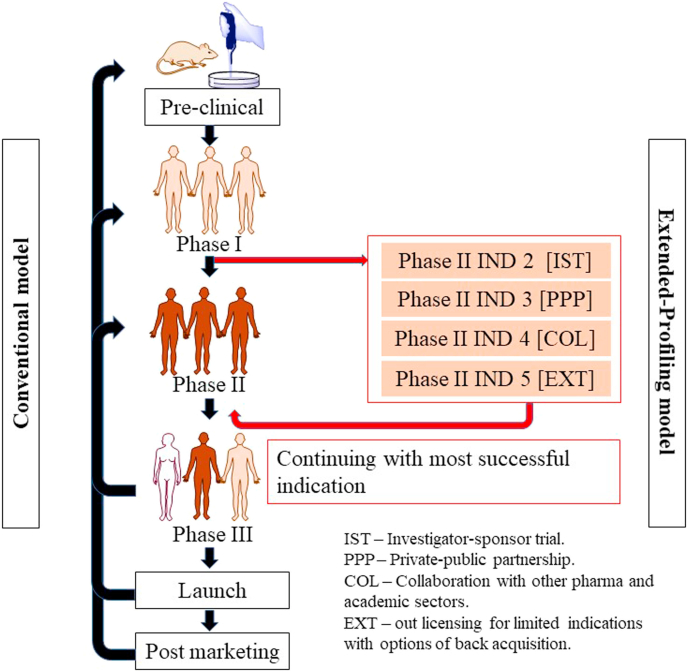
Conventionally, drug repositioning has followed the “recycling model,” wherein drugs, after failing at phase III, are shunted to drug repurposing. The major disadvantage with this model is the large investment in developing a molecule unable to provide satisfactory results in the original trial. Consequently, the risk to benefit ratio is high, thus discouraging pharmaceutical manufacturers and companies from investing in re-testing the molecule for different indications (Karaman and Sippl, 2019). The most promising approach uses the “extended-profiling model.” After the molecule completes proof-of-concept studies, it is further simultaneously investigated for all possible potential indications before proceeding to the next clinical trial phase. In this way, only drugs with studies supporting promising indications are taken forward. This pathway overcomes all lacunae in the conventional model and offers a wide range of benefits (Novac, 2013; Kiran et al., 2019).. (Fig. 1)
Fig. 1.
Diagrammatic representation of the two models of drug repositioning: conventional and extended profiling. The extended profiling model provides better outcomes with low failure rates and high efficacy along with time savings during the entire drug-development cycle.
Drug repositioning mainly requires a thorough understanding of the complex pathological events in psoriasis, which should help in developing targeted delivery systems (Naylor, 2015). The safety profile, immunological target, and efficacy of a drug candidate must be considered for its repurposing. Diseases that share common intercellular networks and have pathological features overlapping with those of psoriasis can form a basis for the selection criteria for repurposing candidates. Inhibition of the signaling pathways involved in the pathophysiology of psoriasis can provide a basis for assessing the antipsoriatic potential of drugs (Oprea et al., 2011a). Drugs used in cancer treatment, arthritis, and other inflammatory disorders are potential candidates for repurposing as psoriasis treatments (Xu and Zhang, 2017). These drug molecules can target specific immune-mediated factors (TNF-α) and other pro-inflammatory cytokines (interleukins) intricate in the pathogenesis of psoriasis, and thus may be repurposed for psoriasis (Ogawa and Okada, 2020).
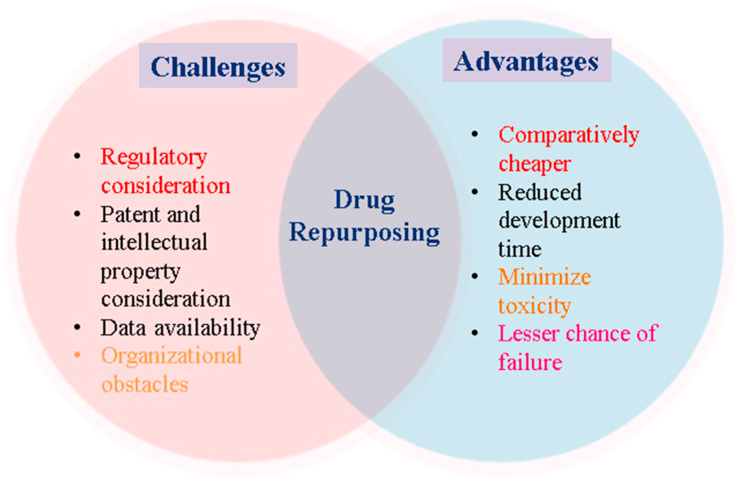
3. Advantages and challenges associated with the repurposing of drugs
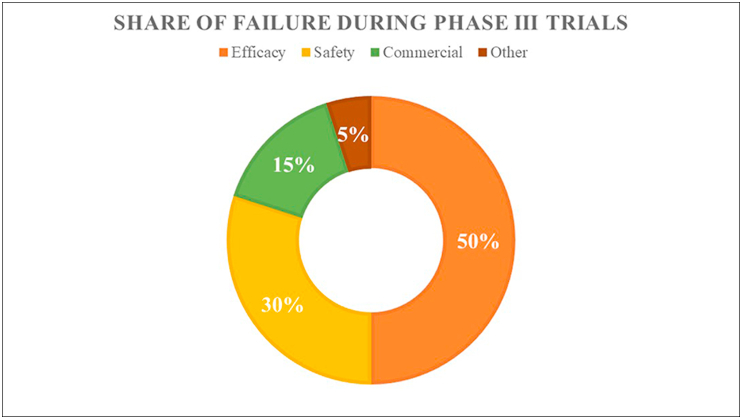
A famous saying quoted in the pharmaceutical industry is “fail early—fail fast.” Each year, thousands of molecules enter clinical trials, but almost 70% of drug molecules fail in phase II, and failure rates greater than 50% are associated with phase III clinical trials (DiMasi, 2001). The failure of drug molecules at later stages not only wastes company resources but also results in delays to develop and commercialize the appropriate pharmaceutical (DiMasi et al., 2003). The reasons for the failure of a drug are represented in Fig. 2. To address the high attrition rates faced by pharmaceutical companies, repositioning or drug repurposing provides a plethora of business opportunities with low-risk, highly cost-effective strategies that help in realizing the potential of drugs across wide area of usage (Xue et al., 2018). Drug discovery and development can be effectively bolstered by drug repurposing. The availability of prior knowledge on drug safety and bioavailability profiles strengthens the process and enables comparatively low failure rates and developmental costs, thus providing better therapeutic options for diseases with unmet clinical needs (Tobinick, 2009). Drug repositioning is often fruitful for rare diseases (orphan drugs), because of bonus of tax waivers, fast-tracked approval, and grants and monetary funding (Sardana et al., 2011). However, several challenges specific to drug repurposing include the following.
Fig. 2.
Percentages of failure in drug development and commercialization of drugs. Efficacy is a major cause of withdrawal from clinical trials. Drug repositioning may provide increased efficacy with comparatively low failure rates.
4. Patent and intellectual property consideration
Whenever, a molecule is granted a patent, much of the time span of the patent is used up in the initial period of the patent when the drug is in the developmental process. The repurposing of shelved molecules for new therapeutic areas will meet legal obstacles if the drugs are used in the same strength and dosage form (Smith, 2011). An efficient way of addressing this problem is using different strengths or new formulations that will not violate the existing intellectual property rights of “reference listed drugs.” Derivatization of a molecule is strongly not recommended, because doing so would produce a completely new molecule, thus moving away from repurposing. Another intellectual property rights consideration is the existence of literature describing different “off label” uses in clinical practice (Novac, 2013).
4.1. Regulatory consideration
Regulatory protection is another crucial hurdle associated with drug repurposing for new indications. Although a significant difference exists between the regulatory pathways of the European Union (EU) and the United States, most drugs are filed for repurposing well before the expiration of the patent of the original molecule. The EU operates in three major filing pathways: centralized, decentralized, and mutual recognition. When repurposed drugs are indicated for orphan indications (rare diseases), the EU grants 10 years of market exclusivity along with an additional 2 years of exclusivity if the pediatric investigational plan (PIP) under article 8(3) is complied with. However, applications for orphan drugs must be submitted via a rationalized system. Applications pertaining to new indications filed under article 10(5) are authorized with 1 year of exclusivity. The United States, in contrast, grants 3 years of market exclusivity. This short period of exclusivity will not provide substantial profits for companies, thus making the pharmaceutical industry reluctant to pursue repositioning (Murteira et al., 2014; Oprea and Overington, 2015).
4.2. Data availability
With increasing development of protocols in clinical studies, an increasing number of drugs are flooding the pipeline, regardless of their terminal failure at later stages. These studies provide the data collected in clinical trials, which are often not released into the public domain or have restricted access. Some data are less amenable to access and analysis to investigate drug repositioning (Pushpakom et al., 2019). Data integration has been demonstrated to provide important information such as the best indications, mechanisms of action, efficacy, and performance of molecules, thereby increasing the literature available for interpretation and the analytic power (Luo et al., 2017).
4.3. Organizational obstacles
A crucial drawback associated with drug repositioning is meager involvement of the pharmaceutical industry (Cragg et al., 2014). One of the reasons is the differences between the United States and the EU regulatory filing systems, with shorter spans of exclusivity rights. This short amount of time discourages most companies from pursuing drug repositioning, because few benefits can be attained (Smith, 2011). Another reason is the absence or scarcity of discovered therapeutic areas in the industry in which the new indication is being investigated, thus implying a lack of skilled personnel and funding resources. To eliminate this issue, external funding and outsourcing methods can be adopted by companies. Moreover, drug repurposing also requires upfront investment, and no such incentives are provided by the industry (Hernandez et al., 2017). Drug repurposing follows 505 (b) (2), also known as the hybrid New Drug Application (NDA) of the Food, Drug, and Cosmetic Act, which is discussed in later sections of this article (Halabi, 2018; Oprea et al., 2011b). Some advantages and challenges are summarized in Fig. 3.
Fig. 3.
Schematic representation of the advantages and limitations during repurposing or repositioning of drugs for new indications.
5. Effective repositioning of drugs for psoriasis
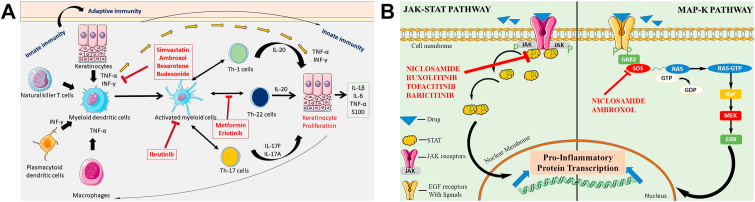
Various drugs have been investigated for repositioning in psoriasis. Psoriasis strongly affects quality of life, and may cause AEs including arthritis and heart conditions (Choi and Koo, 2003). Psoriasis is an inflammatory disorder (Meier and Sheth, 2009); thus the drug candidates with the potential to reduce the load of inflammatory mediators in the body or to inhibit inflammation could be investigated for their activity as antipsoriatic drugs. The psoriasis area and severity index (PASI) is a scale used to define psoriasis severity in the body, scoring the severity of the lesions and the area affected by the lesions. The severity of scale (scaliness), erythema (redness), and induration (thickness) are scored from 0 (no involvement) to 4 (severe involvement) (Kirby et al., 2001). The area of the body affected is scored on a scale of 0–6 based on the severity of the condition. A positive effect on the quality of life of a patient is also an indication of improvement in the PASI score, because it suggests a clinically meaningful benefit of the treatment (Gottlieb et al., 2003). A decrease in the PASI score to 75% is considered an effective measure of drug efficacy, but is clinically extremely stringent and has led to the failure of many drugs. PASI75 was therefore reduced to PASI50, reducing the accepted benchmark to a decrease of 50% to indicate a clinically significant improvement in psoriasis and aid in gaining drug regulatory approval (Carlin et al., 2004). At the stage of PASI50, a placebo can be differentiated from an effective medication. Potential candidate drugs which are been targeted to be repurposed as antipsoriatic drugs are also assessed with the physician global assessment (PGA) on a scale of 0–7 (clear to severe) along with the PASI score (Mease, 2011). Drugs repurposed for their therapeutic efficiency and safety in psoriasis are summarized in Table 2. The main mechanisms of action for these repurposed drugs in psoriasis are illustrated in Fig. 4 (a), (b).
Table 2.
Repurposed drugs for psoriasis.
| Drug | Therapeutic category | Mechanism of action/inhibitory pathway |
|||
|---|---|---|---|---|---|
| STAT | NF-κB | JAK | Immune cells | ||
| Niclosamide | Anthelminthic | ✓ | ✓ | ✓ | |
| Ibrutinib | Antineoplastic | ✓ | ✓ | ||
| Tofacitinib | Anti-arthritic | ✓ | ✓ | ||
| Ruxolitinib | Antineoplastic, immunomodulatory | ✓ | ✓ | ||
| Baricitinib | Anti-arthritic | ✓ | |||
| Paclitaxel | Antineoplastic | ✓ | ✓ | ||
| Bexarotene | Antineoplastic | ✓ | ✓ | ✓ | |
| Fenoldopam | Antihypertensive | ✓ | |||
| Theophylline | Bronchodilator | ✓ | ✓ | ||
| Metformin | Antidiabetic | ✓ | ✓ | ||
| Simvastatin | Antihyperlipidemic | ✓ | ✓ | ✓ | |
| Budesonide | Corticosteroid immunosuppressant | ✓ | |||
| Ambroxol | Mucolytic | ✓ | ✓ | ||
| Azathioprine | Anti-arthritic, immunomodulatory | ✓ | ✓ | ||
Fig. 4.
(a) Systematic illustration of the mechanisms of action of various drugs in psoriasis management and their cellular mechanisms. IL, interleukin; INF-γ, interferon gamma; Th cells, T-helper cells; TNF-α, tumor necrosis factor alpha.
Fig. 4 (b) Schematic illustration of the cellular mechanisms of drugs in psoriasis treatment, focusing on the JAK-STAT pathway. EGF, epidermal growth factor; GTP, guanosine triphosphate JAK, Janus kinase; STAT, signal transducer and activator of transcription.
5.1. Niclosamide
Niclosamide, an FDA approved anthelmintic drug, is a potential candidate used in the treatment of various solid tumors (Li et al., 2014). Niclosamide induces apoptosis, resulting in anticancer activity, and can also be used in fibrosis and rheumatoid arthritis because of its anti-inflammatory properties (Chen et al., 2018). Studies have reported several classical pathways in the context of psoriasis that are linked to inflammation and can be inhibited with niclosamide, including signal transduction and activator of transcription protein (STAT3), p65, nuclear factor kappa B (NF-κB) (Jin et al., 2010), Nuclear factor of activated T-cells (NFATc), and mitogen-activated protein kinase (MAPK) pathways, with minimal host toxicity (Li et al., 2014).
Thatikonda et al. have investigated the repurposing capability of niclosamide. Their results from in-vitro and in-vivo studies in mice suggest that this compound inhibits the hyperproliferation of keratinocytes through reactive oxygen species-mediated apoptosis. Furthermore, it abrogates the levels of lipopolysaccharide-induced cytokines in murine macrophages. These findings also supplement preclinical evidence indicating that niclosamide mitigates inflammation and hyperproliferation in skin cells by inhibiting STAT3, NF-κB, NFATc-1 (a transcription factor), and protein expression, thus reversing psoriatic conditions. The niclosamide treated group, in contrast to the imiquimod (IMQ) group, showed a decreased PASI index, with reduced skinfold and ear thickness, along with amelioration of spleen enlargement. The authors concluded that niclosamide may have a new role in antipsoriatic activity, thus possibly fulfilling the unmet need for psoriasis therapeutics (Thatikonda et al., 2020). Another study on the effect of niclosamide on DCs has concluded that the drug has the potential to exploit DC function. DCs link the environmental sensing of the innate immune system to the immune system's response. Synthesis and secretion of cytokines and chemokines, which modulate inflammatory responses and T-cell differentiation, and are responsible for the adaptive immune response, are key features of mature DCs (Bos et al., 2005). Moreover, DCs initiate the production of various pro-inflammatory markers, such as IL-6 and TNF-α, which are involved in the pathogenesis of various autoimmune and inflammatory disorders (Rashmi et al., 2009). Immature DCs lead to downregulation of the antigen-specific induction of T cells and INF-λ and decrease the proliferation of cells. Niclosamide strongly inhibits the function of IL-6 and TNF-α, thus providing novel insight into the role of niclosamide in various DC-mediated autoimmune inflammatory disorders, such as psoriasis. Consequently, the inhibition of DC activation and function with niclosamide treatment has a beneficial role in the management of psoriasis (Goodman et al., 2009).
5.2. KINASE inhibitors-role of tinib derivatives
5.2.1. Ibrutinib
Ibrutinib, a Bruton's tyrosine kinase (BTK) inhibitor, is a known anticancer therapeutic with potential and efficacy as an antipsoriatic agent (Sagiv-Barfi et al., 2015). Nadeem et al. have explored this BTK inhibitor in an IMQ-induced psoriasis model and identified a BTK-signaling function in innate immune cells, such as DCs and other γδ T cells in the skin; thus, BTK has a crucial function in psoriatic inflammation in mice, because DCs are involved in the amplification and secretion of various cytokines and other inflammatory mediators (Nadeem et al., 2020). This BTK signaling via the Toll like receptor downregulates transcription factors and protein kinases in DCs, thereby decreasing inflammatory mediators in CD11c + DCs and γδ + T cells. Thus, through its inhibition of dermal IL-17, IL-21, IL-23, IL-12, and TNF-α, ibrutinib may be a potential candidate to target BTK in psoriatic conditions. BTK inhibitors such as ibrutinib have been applied therapeutically and found to significantly decrease clinical and inflammatory factors associated with dermal inflammation (i.e., decreased ear thickness and weight, back skin thickness, myeloperoxidase activity, and oxidative stress in CD11c, DCs, and neutrophils), thus ameliorating dermal inflammation in an IMQ-induced model. Together, the data from various studies indicate that ibrutinib decreases dermal inflammation and improves PASI scores in IMQ mouse models (Al-Harbi et al., 2020).
5.2.2. Tofacitinib
Tofacitinib is a Janus kinase (JAK) inhibitor with significant immunosuppressive properties; it is mainly prescribed for treating rheumatoid arthritis, and it can also be repositioned for treating psoriatic conditions (Roskoski, 2016). Psoriasis develops through an immune-mediated process, and thus drugs that target these immune pathways can efficiently treat psoriasis. The JAK-STAT pathway is involved in the pathophysiology of inflammatory disorders, because generation of IL-23, along with IL-1β and IL-17, is directly linked to the JAK pathway (Chiricozzi et al., 2015). Moreover, the PASI score and duration of disease also depend on similar pathways. This drug modulates innate immune cells and thus can potentially decrease the expression of IL-23, IL-17, INF, and TNF-α (Berekmeri et al., 2018).
Studies predicting the clinical efficacy of tofacitinib in psoriasis have been completed in a phase IIb, placebo-controlled study in 59 patients at different doses (5, 10, 20, 30 and 50 mg/day) for 14 days. The study revealed a dose-dependent response with some AEs at higher doses. A remarkable decrease in keratin 16 and CD3+, CD8+, and CD25+ was observed. The psoriatic lesion severity sum score was used to measure erythema, induration, and scaling in index psoriasis lesions; a total score of 5–12 and an induration score of 2–4 were expected at baseline (Boy et al., 2009).
The efficacy of orally administered tofacitinib has been studied in patients with plaque-type psoriasis in a phase I study, with a dose of 5–50 mg twice daily or 60 mg once daily. At week 16, tofacitinib at 5 and 10 mg twice daily resulted in PGA score than the placebo. Improvements in psoriatic lesions were observed for all doses except the lowest dose, i.e., 5 mg twice daily, in contrast with the placebo. Two other large phase studies have indicated a 75% decrease in the PASI75 index and PGA scores for doses of 5 and 10 mg given twice daily for 16 weeks. The PASI75 was better with a 10 mg twice-daily dose compared with the 5 mg twice-daily dose, and was observed to be approximately 40% and 60%, respectively. The rates of severe AEs, and illnesses, malignancies, and discontinuations due to AEs, were all low and tended to be identical across groups. Nasopharyngitis was the most widespread AE among all classes (Papp et al., 2015).
Another study on 12 human volunteers with plaque psoriasis for 12 weeks randomized the patients and administered 10 mg of tofacitinib or placebo twice per day for 12 weeks. The authors concluded that pSTAT1 and pSTAT3 staining in lesional skin keratinocytes was higher at baseline but decreased after 1 day of tofacitinib treatment (baseline, 1290 pSTAT1+ cells/m2; day 1, 332 pSTAT1+ cells/m2; and nonlesional, 155 pSTAT1+ cells/m2). After days 1 and 3 of tofacitinib treatment, epidermal thickness and KRT16 mRNA expression were significantly and gradually reduced. After 1 and 2 weeks, decreases in DC and T-cell numbers were observed, respectively. Major decreases in the IL-23/TH17 pathways were identified at week 4 and continued through week 12. Changes in the expression of psoriasis-related genes and a decrease in the expression of the IL-17 gene were closely associated with improvements in clinical and histologic features (Krueger et al., 2016).
Tofacitinib is also being investigated as a topical preparation. In a vehicle-controlled clinical trial, the efficacy of two tofacitinib topical ointments was evaluated. In the study, 71 human patients with mild to moderate psoriasis were randomized 2:1:2:1–2% tofacitinib ointment 1, vehicle 1, 2% tofacitinib ointment 2, and vehicle 2, each administered twice daily for 14 days to a specified single area of 300 cm2. The study demonstrated improvements in the psoriatic condition. Tofacitinib ointment 1 was well tolerated and effective in the treatment of plaque psoriasis, in contrast to vehicle, and showed a remarkable improvement in the psoriasis severity, PASI index, and PGA. Topical tofacitinib for the prevention of psoriasis should be studied further. Thus, tofacitinib can be repurposed as a therapeutic regimen for psoriasis (Ports et al., 2013).
5.2.3. Ruxolitinib
Ruxolitinib is a JAK inhibitor with potential immunomodulatory and antineoplastic properties. It selectively binds JAK-1 and JAK-2, and inhibits these protein tyrosine kinases (Alves de Medeiros et al., 2016). Inhibition of the JAK signaling pathway is also responsible for the decrease in cellular proliferation and inflammation by blocking of the production of proinflammatory cytokines, such as TNF-α and interleukins (IL-2, IL-4, IL-15, IL-21, IL-12, and IL-23), driven by T cells. Therefore, ruxolitinib's activity as a JAK inhibitor endows it with immunomodulatory actions, and it may potentially be used in immune-mediated disorders such as psoriasis (Alves de Medeiros et al., 2016) (Mesa, 2010). It is being explored for the management of mild to severe psoriatic conditions, and has been suggested to locally, rather than systemically, inhibit various factors that enhance the severity of psoriasis. Ruxolitinib has gained much interest, owing to the feasibility of its topical application in psoriasis (Hosking et al., 2018).
An open clinical trial has been performed with 28 participants divided into five groups treated for 28 days with three different concentrations (0.5%, 1%, and 1.5%) of ruxolitinib cream, compared with betamethasone (0.05%) and calcipotriene (0.005%). In contrast to the placebo, both the 1% and 1.5% creams decreased lesion thickness, erythema, and scaling, and decreased the lesion area. The efficacious doses of ruxolitinib decreased the composite lesion rate by more than 50%, as compared with just 32% for the vehicle. Topical application of the cream was well tolerated, and decreases in erythema, lesion thickness, and scaling, in addition to overall improvement in the PASI score and PGA were observed. Ruxolitinib thus can be used as a topical therapy for psoriasis, and is safe, well tolerated, and clinically active (Punwani et al., 2012).
In another study, in five consecutive cohorts of five patients 18–65 years of age, topical ruxolitinib phosphate 1% or 1.5% cream was administered once daily or twice daily for 4 weeks to 2–20% body surface area. Erythema, scaling, and thickness of the target lesions were graded on a scale of 0–4. Improvements in lesion scores were seen after treatment with Ruxolitinib phosphate cream at 1.0% once daily or 1.5% twice daily. Topical INCB018424 decreased transcriptional markers of immune cell lineage/activity in lesional skin, with clinical progress and reductions in markers of T-helper (Th) 17 lymphocyte activation, DC activation, and epidermal hyperplasia (Punwani et al., 2015).
5.2.4. Baricitinib
Baricitinib has been validated for the management of rheumatoid arthritis, because it functions through TNF-α inhibition. It is a selective inhibitor of JAK (subtype JAK-1and JAK-2) that is involved in the Th-17 signaling pathway (Genovese et al., 2016). The Th-17 signaling pathway is important, because inflammation is mediated by T cells and is responsible for the production of interleukins and TNF (Kuwabara et al., 2017). Blockage of JAK-mediated intercellular signaling pathways can inhibit the gene transcription of various proinflammatory cytokines participating in the pathogenesis of inflammatory conditions, such as psoriasis (Banerjee et al., 2017). A study conducted by Eli Lilly has tested a dose range in a phase IIb trial to determine the safety and efficacy of oral baricitinib in 271 North American participants with moderate to severe psoriasis. In the study, patients were randomized and administered oral doses (2, 4, 8. or 10 mg once per day) for 12 weeks, on the basis of improvements in the PASI score by a certain percentage. At 12 weeks, the main endpoint was PASI75. At week 12, the 8 mg (43%) and 10 mg (54%) baricitinib groups reached PASI75 more often than the placebo group. At 12 weeks, all baricitinib-treated groups had larger mean increases in their PASI scores and had higher rates of PASI50 than the placebo group, except for the 2 mg group; statistically significant PASI90 responses were obtained in the baricitinib-treated groups. Significant changes in the PASI index on the basis of total dermatology life quality index were observed for baricitinib compared with the placebo. The study clearly described the efficacy of baricitinib for psoriasis, because it improved the PASI index, and no signs of infection were observed (Papp et al., 2016).
5.3. Paclitaxel
Paclitaxel, a taxol derivative used in chemotherapy for cancer to regulate fast-proliferating cells, has also been found to have anti-angiogenic and anti-inflammatory properties (Long, 1994). The ability of paclitaxel to regulate cell growth has been used to control the hyperproliferation of skin cells during psoriasis (Rahman et al., 2015). Kilfoyle et al. have formulated TyroSpheres for topical administration of paclitaxel for the management of psoriasis. TyroSpheres increase the cytotoxicity of loaded paclitaxel against human keratinocytes (the IC50 of paclitaxel-TyroSpheres is approximately 45% lower than that of free paclitaxel) (Kilfoyle et al., 2012). Yin et al. evaluated the efficacy of paclitaxel in an IMQ-induced psoriatic model. The group treated with 0.3% paclitaxel showed better outcome than the other categories by a large margin. Furthermore, in terms of the PASI, the 0.3% paclitaxel group had a greater effect on skin thickness reduction than was observed in the 0.1% paclitaxel group. The findings suggested that paclitaxel is well tolerated on mouse skin when used topically as an ointment. The results showed decreased skin and ear thickness in mice, as compared with that of psoriatic skin (Yin et al., 2019).
Paclitaxel has shown clinical efficacy in a phase II pilot study in 12 patients with moderate to severe psoriasis. Paclitaxel was found to have anti-inflammatory and anti-angiogenic properties. Dose-dependent effects were observed in the PASI index; a higher dose (75 mg/m2 for six doses at 4-week intervals) of drug produced more significant effects than a lower dose (37.5 mg/m2 for six doses at 4-week intervals). All five patients showed a change in PASI (mean PASI decrease by 59.7%, median PASI decrease by 59.6%, range: 40.3–79.2%) at stage I. Four of the seven patients finished stage II, and all showed a change in PASI (decrease by 45.9% on average, median: 45.0%, range: 14.6–79.1%). Most patients tolerated micellar paclitaxel well. The overall results showed improvements in the PASI score and severity of disease. Therefore, paclitaxel is a noteworthy option for the management of psoriatic conditions at various stages of severity (Ehrlich et al., 2004).
5.4. Bexarotene
Bexarotene, a retinoid X receptor used for its anticancer properties, has been repurposed for its antipsoriatic activity (Farol and Hymes, 2004). Saka et al. developed a liposomal system for bexarotene by formulating a gel and evaluated its antipsoriatic efficacy. They performed pharmacodynamic studies in mice with IMQ-induced psoriasis (BALB/c model) and found that bexarotene effectively downregulates interleukins (IL-22, IL-17, and IL-6) and TNF-α. The results indicated improvements in the PASI score and plaque elevation. The cytokine levels in the negative control group were considerably higher than those in the other groups. Similarly, as opposed to negative control, the therapy groups demonstrated substantial decreases in different cytokine levels. As compared with plain drug group, both the positive control and liposomal gel groups were more effective in lowering cytokine levels (Saka et al., 2020).
In another phase II multicenter clinical trial study performed by Smit et al. the role of bexarotene was explored in human participants for safety, tolerability, and efficacy in psoriasis. From this trial, the authors concluded that no severe adverse outcomes were observed, and bexarotene was well tolerated in most participants. Notable amelioration of the psoriasis condition was observed after the administration of bexarotene, as indicated by improved PASI, PGA, and plaque elevation. The overall response rates for PASI showed a ≥50% improvement, thus indicating the antipsoriatic effect of bexarotene. Hence, these findings demonstrate that bexarotene is a potential clinical candidate for psoriasis management (Smit et al., 2004).
5.5. Fenoldopam mesylate
Fenoldopam mesylate, a selective dopamine receptor agonist, is used to treat hypertension. Reports have suggested that it also possesses antiproliferative properties and thus can be used as an antipsoriatic drug (Tumlin et al., 2000). The antiproliferative activity of the drug is regulated by the dopamine receptors (D1, D4, and D5 receptors) on T cells. Under normal conditions, T-cell activation produces many types of dopamine receptors (DRs) in normal ranges, whereas psoriatic skin contains approximately 20 times the number of DRs found in normal healthy skin. Depolarization of DRs may potentially be useful in managing psoriatic conditions, because it downregulates the production of proteins and proinflammatory mediators such as CD28, CD69, and IL-2. Depolarization of dopamine receptors can be achieved by inhibition of activated T cells (Levite, 2016). The use of this drug is limited because of its physicochemical properties (pH sensitivity and oxidation). Doppalapudi et al. have attempted to prepare a stable topical formulation of fenoldopam and evaluated it in-vitro and in-vivo for its antipsoriatic efficacy in an IMQ-induced mouse model. Compared with the FD solution, FD formulations elicited a greater decrease in interleukin levels (p < 0.0001 for IL-17 and IL-23; p < 0.01 for TNF-α). A 2–5-fold decrease in the TNF-α concentration was observed in the formulation treated group compared with the IMQ control group. The results indicated a significant decrease in the inflammatory cytokines, and the authors concluded that the stable formulation with fenoldopam may be a useful approach for the treatment of psoriasis (Doppalapudi et al., 2020).
5.6. Theophylline
Theophylline, a xanthine derivative, is responsible for smooth muscle relaxation and is thus exploited for conditions such as bronchoconstriction (Barnes, 2013). It is broadly used in treating asthma and chronic obstructive pulmonary disorder as a bronchodilator. Another useful application of theophylline is psoriasis treatment. Studies have revealed that, at certain concentrations, it can significantly decrease the rate of proliferation of fibroblast cells in the skin (Papakostantinou et al., 2005). Touitou et al. have supported this finding by demonstrating an elevation in cyclic adenosine monophosphate (cAMP) levels. cAMP has a major role in autoimmune disorders such as psoriasis, because it monitors the activity of pro- and anti-inflammatory mediators. Drugs that downregulate the cAMP signaling pathway can significantly decrease the levels of anti-inflammatory factors within the immune system, and theophylline can perform this activity. Touitou et al. have performed in vitro evaluations in which a concentration of 1 mM theophylline significantly inhibited fibroblast proliferation. The authors attempted to enhance the penetration of theophylline through a topical route (Touitou et al., 1991). In another randomized controlled trial (double blind study) performed by Papakostantinou et al., a remarkable deterioration in the psoriatic area and level of inflammatory cytokines was observed. The effect of an ointment containing theophylline (1%), transcutol (20%), and oleic acid (10%) on the PASI score of the treated lesions was evaluated, and the ointment substantially increased the PASI scores of the treated lesions (two-tailed p = 0.002, Wilcoxon matched-pairs signed-rank test). An improvement of approximately >50% was achieved in the psoriasis area and severity index at 2 weeks, and no serious AEs were noted. Thus, theophylline is a potential candidate with good repurposing value in the management of psoriasis (Papakostantinou et al., 2005).
5.7. Metformin
Metformin, an antihyperglycemic agent, is usually prescribed to patients with type 2 diabetes for decreasing blood sugar levels (Rojas and Gomes, 2013). In psoriasis, the concentration of cytokines in the body steeply rise. These cytokines increase the frequency of metabolic syndrome. Metformin has been found to decrease the cardiovascular risks associated with metabolic syndrome (Ladeiras-Lopes et al., 2015). Thus, it can be given to patients with metabolic syndrome together with psoriasis. Metformin has antipsoriatic activity through activating the adenosine monophosphate-activated protein kinase (AMPK) enzyme. Activation of AMPK leads to the downregulation of cells including DCs, T cells, and macrophages. Metformin can thus be used as an antipsoriatic agent because it decreases the inflammatory mediators in the blood, which are a major concern during psoriasis (Chang and Choi, 2020). A study by Tsuji et al. has revealed that metformin blocks the upregulation of pro-IL-1 mRNA caused by TNF-α and IL-17A stimulation, on the basis of qRT-PCR analysis. The authors also performed ELISA studies indicating that metformin therapy blocks IL-1 secretion (mature IL-1) caused by TNF-α and IL-17A stimulation. These results suggest that metformin has various inhibitory effects on TNF-α- and IL-17A-induced pro-IL-1 synthesis and IL-1 secretion (mature IL-1) in normal human epidermal keratinocytes (NHEKs) by impairing NLRP3 inflammasome activation (Tsuji et al., 2020).
5.8. Simvastatin
Simvastatin belongs to the therapeutic class of β-hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase inhibitors (statins), which are used for the management of hypertension, because they lower the cholesterol levels in blood plasma (Wilkins et al., 2010). Clinical studies have demonstrated the immuno-modulatory and wound healing properties of statins. Owing to their significant immunomodulatory effects, they can modify the response to T cells and also inhibit MHC-II induction and the degranulation of mast cells, and can induce apoptosis (Zeiser, 2018). Therefore, simvastatin can downregulate inflammatory mediators such as interleukins (IL-6), TNF-α, and C-reactive protein; consequently, this drug may be useful in inflammatory disorders of the skin (Sameh et al., 2018). However, a potential role in the management of psoriasis remains controversial, because some studies have revealed that simvastatin improves the PASI index, whereas other studies have also reported deterioration of the skin lesions. A study by Adami et al. investigating the use of simvastatin in reducing hyperplasia has suggested that simvastatin can be used for the management of inflammatory disorders (Adami et al., 2012). Another pilot study investigating the potential of using statins in psoriatic conditions has identified improvements in the PASI score. In the study, patients with plaque psoriasis were administered simvastatin at 40 mg/kg for two weeks, and an improvement was observed in the dermatological life quality index and PASI index. These findings suggest that simvastatin at a dose of 40 mg/day is associated with clinical improvement in psoriasis and is well tolerated. Statins have several properties that may be relevant to psoriasis. Statins suppress the antigen leukocyte function antigen-1 (LFA-1), and selective LFA-1 blockade has been successfully used to treat psoriasis (Adami et al., 2012).
Combination therapy with simvastatin along with betamethasone administered topically has shown positive clinical outcomes in a double-blind clinical study in 30 patients with psoriasis. Patients were randomly divided into two groups: one receiving oral simvastatin at 40 mg/kg with topical betamethasone and another group receiving betamethasone alone for 8 weeks. The PASI scores were notably decreased with the combination treatment, as compared with betamethasone single therapy. Thus, simvastatin, owing to its anti-inflammatory and immunomodulatory actions, can be useful in treating psoriasis (Naseri et al., 2010).
5.9. Budesonide
Corticosteroids play a major role in the management of inflammatory dermatoses such as psoriasis. Corticosteroids have anti-inflammatory and immunosuppressive effects, and antiproliferative activity. De Jong et al. in a study on six volunteers, have elucidated the outcomes of budesonide in terms of hyperproliferation, and the levels of inflammatory cells and pro-inflammatory cytokines in psoriasis (De Jong et al., 1995). The patients were treated with budesonide ointment (0.025%) for 3 weeks, and biopsies (with histopathology) were conducted before and after the treatment. The data clearly showed a remarkable decrease in interleukins (IL-6 and IL-8), TNF-α, and monocytes; lower proliferation; and improvements in the PASI score. Their findings also suggest that, despite being clinically similar, psoriatic lesions vary in cytokine production among individuals (De Jong et al., 1995). Clinical scores and hyperproliferation increase after 1–3 weeks of corticosteroid therapy. In patients with elevated cytokine levels before therapy, steroid treatment decreases these levels.
Another randomized controlled trial has assessed the efficacy, safety, and tolerability of once-daily treatment with budesonide, compared with administration of a corticosteroid and a vitamin D3 analogue. In the study, 20 volunteers with plaque psoriasis were randomized to treatment with ointment containing calcipotriol 50 μg/g with betamethasone dipropionate 0.5 mg/g once daily, or cream containing budesonide 0.25 mg/g (in the morning) and tacalcitol 4 μg/g ointment (in the evening). Treatment with budesonide cream and tacalcitol ointment rapidly ameliorated lesions and provided relief from itching (Calzavara-Pinton et al., 2011).
5.10. Ambroxol
Ambroxol, a mucolytic agent used for the treatment of asthma, has been investigated for its protective action in psoriatic inflammation (Beeh et al., 2008). Sunkari et al. have demonstrated the inhibitory action of ambroxol on IMQ-induced inflammation, and have reported epidermal hyperplasia, a decrease in skin and ear thickness, and splenomegaly in Balb/C mice. They have investigated the role of ambroxol in decreasing inflammatory cytokines, and have observed a significant decrease in IL-1β, IL-6, IL-17, IL-22, IL-23, TGF-β, and TNF-α. Moreover, the expression of proteins in the pathogenesis pathway in psoriasis, such as iNOS, MAPKs, nitrotyrosine, and NF-κB, also decreased. Ambroxol caused a noticeable decrease in phenotypic changes and the PASI index in IMQ-induced psoriasis in the skin. The authors concluded that ambroxol has therapeutic efficacy against psoriasis (Sunkari et al., 2019).
5.11. Azathioprine
Azathioprine is an immunosuppressive drug prescribed for problems such as ulcerative colitis, rheumatoid arthritis, and Crohn's disease, and it also decreases the chance of kidney rejection after transplantation (Maltzman and Koretzky, 2003). Its use in inflammatory conditions such as psoriasis is attributed to its T-cell suppression. Azathioprine has been systematically explored by Gupta for psoriasis management. A study was performed in 10 patients given an intermediate higher dose of azathioprine (500 mg for 3 days, repeated every month) with continuous low-dose azathioprine (100 mg orally) daily in between the intermediate higher dose. After all care was discontinued, all 10 patients in phase IV remained in sustained remission for more than 5 years; as a result, azathioprine pulse treatment induces psoriasis remission for a period of five years (Gupta, 2015). The efficacy and safety of combination therapy with azathioprine in participants with moderate to severe psoriasis has been evaluated in retrospective cohort study in 23 patients. Of those 23, 17 patients received infliximab 3 mg/kg or 5 mg/kg with methotrexate, whereas 5 patients received infliximab 5 mg/kg with azathioprine. The PASI scores improved by 50% in most patients, and the treatment was well tolerated in patients. In addition, patient data was validated for a period of 4 weeks and as long as 5 years and 5 months. At the end of week 14, 91.3% of participants had a PASI of 50, 69.6% had a PASI of 75, and 39.1% had a PASI of 90 (Dalaker and Bonesrønning, 2009).
6. Clinical development of repurposed/repositioning of drugs for the management of psoriasis
The investigation of existed non-psoriatic drugs for antipsoriatic activity provides rapid possibilities to promote therapeutic approaches in clinical trials. The common molecular pathways and targets in the pathogenesis of different diseases motivate the discovery of therapeutics for psoriasis from non-psoriatic medicines (Cavalla, 2013). Common molecular origins of different diseases have been determined through proteomics, genomics, and informatics technologies. Analytical tools enabling research scientists to screen large numbers of available therapeutics against a specific disease target can also be developed (Hodos et al., 2016). Therefore, therapeutics initially identified as anti-arthritic, antidiabetic, immunomodulatory, antibiotic, antihypertensive, or anticancer drugs are being repurposed for psoriasis. The safety and efficacy of various non-psoriatic drugs for repurposing as potential antipsoriatic agents have been studied through various clinical trials. Data obtained from the clinical studies support the repurposing of the drugs in psoriasis. For a drug to obtain approval as a repurposed drug, its safety is of utmost importance (Oprea and Mestres, 2012; Novac, 2013). The fates of numerous drugs that were approved for different conditions and have now been repurposed as antipsoriatic drugs are described in Table 3.
Table 3.
Clinical development of various repurposed drugs for the management of psoriasis.
| Drug/API | Therapeutic indication | Status | Study details | ClinicalTrials.gov identifier |
|---|---|---|---|---|
| Paclitaxel | Cancer | Completed | Study to measure the safety and efficacy of paclitaxel in micellar form for severe psoriasis treatment | NCT00006276 |
| Paclitaxel | Cancer | Completed | Study to assess the potency of SOR007 as an ointment in psoriasis | NCT03004339 |
| Bexarotene | Cancer | Completed | Study on bexarotene with small-band UVB for psoriasis | NCT00151008 |
| Ruxolitinib | Cancer | Completed | Open-label study of topical drug to investigate the safety and efficacy in treating psoriasis | NCT00617994 |
| Ruxolitinib | Cancer | Completed | Study design for the application of ruxolitinib phosphate cream in psoriasis | NCT00820950 |
| Ruxolitinib | Cancer | Completed | Study (dose ranging) design for the application of ruxolitinib phosphate cream in psoriasis | NCT00778700 |
| Tofacitinib | Rheumatoid arthritis | Completed | Study of tofacitinib ointment for chronic psoriasis | NCT01831466 |
| Tofacitinib | Rheumatoid arthritis | Completed | Study measuring the safety and efficacy of CP-690,550 in Asian participants with moderate-severe psoriasis | NCT01815424 |
| Tofacitinib | Rheumatoid arthritis | Completed | Topical tofacitinib for chronic psoriasis | NCT00678561 |
| Tofacitinib | Rheumatoid arthritis | Completed | Determination of the effects of escalating multiple doses in psoriasis | NCT01736696 |
| Tofacitinib | Rheumatoid arthritis | Completed | Phase III randomized 2B, placebo-controlled trial for the evaluation of safety and efficacy of CP-690, 550 and etanercept in psoriasis | NCT01241591 |
| Tofacitinib | Rheumatoid arthritis | Completed | Study of the mechanism of action of CP-690, 550 in the skin of patients with moderate to severe psoriasis | NCT01710046 |
| Baricitinib | Rheumatoid arthritis | Completed | Double blind trial of baricitinib in participants with moderate to severe psoriasis | NCT01490632 |
| Metformin | Diabetes | Not recruited | Study designed to assess the safety and efficacy of metformin for the treatment of moderate psoriasis | NCT02644954 |
| Metformin | Diabetes | Not recruited | Treatment of psoriasis associated with metabolic disorders | NCT03629639 |
7. Regulatory challenges and pathways for repurposing of drugs
A critical aspect during the development of repurposed drugs is regulatory consideration. Specific guidelines and detailed regulations exist for drug-repurposing conditions.
Repurposed drugs follow the 505(b)(2) pathway of the Food, Drug, and Cosmetic Act. The 505(b)(2) pathway, also known as the hybrid NDA, is an amalgamation of the original NDA and Abbreviated New Drug Applications, which were enacted in 1984 under the Hatch-Waxman Amendments (Drug Price Competition and Patent Term Restoration Act) (Breckenridge and Jacob, 2019).
Section 505(b)(2) simplifies the clearance process for products that have already been approved, including some changes relative to the current version. Sponsors are often confronted with challenges in deciding the inquiries to be conducted to facilitate approval through the 505(b)(2) pathway. Drugs proposed through the 505(b)(2) pathway are accepted on the basis of evidence from previous trials not conducted by the sponsor, and/or findings obtained from preclinical studies and clinical outcomes from the available literature. (Murteira et al., 2014).
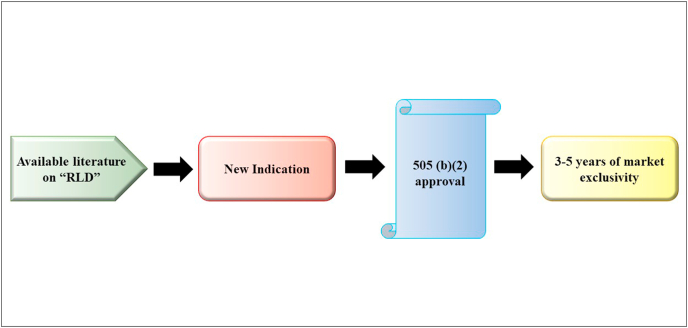
The reason for the enforcement of this act was to provide an added benefit to formulators and scientists to develop new pharmaceutical products by relying on the literature already available for “reference listed drugs” and developing novel formulations, new combinations, or novel routes of administration, or exploring novel indications. This approval pathway encouraged scientists to eliminate costly clinical trials and expedite the developmental process. Applicants are given exclusivity rights as part of the hybrid NDA approval pathway. Exclusivity, the most overused term in the pharmaceutical industry, refers to awarding exclusive rights to the claimant for a set period of time, prohibiting rivals from producing or formulating the substance by using the applicant's formula. This exclusivity period gives the applicant a hold over the market share for a specific time duration, during which the benefits can be reaped with a simultaneous reduction in market competition. The 505(b)(2) pathway provides 3–5 years of exclusivity to the applicant for new chemical entities. This exclusivity time period is longer than that provided through the Abbreviated New Drug Applications pathway (only 180 days). The basic features of the hybrid NDA are represented in Fig. 5. Under 505(b)(2), applicants must disclose the novel route of administration for which the drug is being repurposed, and comparison profiles with the primary route along with the indication must be provided (Witkowski, 2011).
Fig. 5.
Schematic representation depicting the regulatory approval pathway used in repurposing of drugs and market exclusivity, an attractive incentive for the pharmaceutical industry to pursue drug repositioning. RLD, reference listed drugs.
The European Medicines Agency (EMA) provides a parallel procedure for approval, as stated under article 10 of directive 2001/83/EC (specifically articles 6, 8(3), 10(3) and 10(5)). The procedure does not allow the use of non-proprietary studies containing safety data and high-quality reports as supportive data for application, in contrast to the hybrid NDA pathway. Article 10 does not offer any basis for the use of non-proprietary studies. Accordingly, any change in the definition or form of an already existing drug does not entitle it to be a new drug. EMA requires an extended period of 6 months for obtaining approval, in comparison to the FDA. Furthermore, all applications must contain a risk management plan in addition to all other described documents. Company filing 505(b)(2) application for drug repositioning in psoriasis offers various benefits involving low drug developmental cost, prior knowledge of the safety and bioavailability of the molecule, a low risk-benefit ratio, and the ability to generate high revenues during the time span of market exclusivity (Iglesias-Lopez et al., 2019; Giannuzzi et al., 2017).
8. Conclusion and future perspectives
The life cycle of a drug molecule begins with the phase of drug discovery and development, and finally enters the stage in which the drug is marketed and is made available to all patients. This transition requires a considerable amount of time and money, and thus is the major hurdle to bringing new drugs to the market. Drug repurposing provides an ambitious alternative pathway to exploit the capabilities of a single drug molecule to treat many disease conditions and to reuse its power for new therapeutic indications. This path is less expensive and time consuming than the conventional process. Therefore, drug repositioning is viewed as an attractive tool commercially, because preclinical and clinical developmental data for the drug (phase I and phase II trials) are already available. Psoriasis is a disease that shares many intercellular pathways and interconnected pathophysiology with multiple clinical conditions. Thus, repurposing drugs used to treat such conditions for psoriasis are expected to be beneficial. Drug repositioning will have a high-impact and long-lasting role in developing new therapeutics for psoriasis. It also proffers an opportunity to develop advanced understanding of drug design and to form new collaborations between academia and industry. The output of drug repurposing has been fruitful in treating autoimmune disorders and other rare diseases that are not focused on by industry because they provide low financial gain. Drugs that are repurposed for the new indication of psoriasis are thoroughly investigated for their safety and efficacy profiles. Furthermore, newer technologies must be applied in the development of novel formulations with different routes of administration and indications, to obtain more efficacious products. Drug repurposing or repositioning will accelerate drug discovery, increase productivity, and allow for the full potential of drug products to be harnessed.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Funding
This review received no external funding.
CRediT authorship contribution statement
Harsha Jain: Conceptualization, Writing – original draft, Methodology, Writing – review & editing. Aditi Rajan Bhat: Methodology, Writing – review & editing. Harshita Dalvi: Methodology, Writing – review & editing. Chandraiah Godugu: Supervision. Shashi Bala Singh: Project administration. Saurabh Srivastava: Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are thankful to the Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER), Hyderabad during the course of writing this review article.
References
- Adami M., et al. Simvastatin ointment, a new treatment for skin inflammatory conditions. J. Dermatol. Sci. 2012;66(2):127–135. doi: 10.1016/j.jdermsci.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Al-Harbi N.O., et al. Therapeutic treatment with Ibrutinib attenuates imiquimod-induced psoriasis-like inflammation in mice through downregulation of oxidative and inflammatory mediators in neutrophils and dendritic cells. Eur. J. Pharmacol. 2020;877:173088. doi: 10.1016/j.ejphar.2020.173088. [DOI] [PubMed] [Google Scholar]
- Albanesi C. Clinical Immunology. Elsevier; 2019. Immunology of psoriasis; pp. 871–878. e1. [DOI] [Google Scholar]
- Alves de Medeiros A.K., et al. JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PloS One. 2016;11(10) doi: 10.1371/journal.pone.0164080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-Mutairi N., et al. Comorbidities associated with psoriasis: an experience from the Middle East. J. Dermatol. 2010;37(2):146–155. doi: 10.1111/j.1346-8138.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- Anthony A. Innate and adaptive immunity and the pathophysiology of psoriasis. J. Am. Acad. Dermatol. 2006;54:867–880. doi: 10.1016/j.jaad.2005.10.057. [DOI] [PubMed] [Google Scholar]
- Archier E., et al. Efficacy of Psoralen UV-A therapy vs. Narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2012;26:11–21. doi: 10.1111/j.1468-3083.2012.04519.x. [DOI] [PubMed] [Google Scholar]
- Arican O., et al. Serum levels of TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediat. Inflamm. 2005;2005(5):273–279. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3(8):673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Baker N.C., et al. A bibliometric review of drug repurposing. Drug Discov. Today. 2018;23(3):661–672. doi: 10.1016/j.drudis.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., et al. JAK–STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77(5):521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P.J. Theophylline. American journal of respiratory and critical care medicine. 2013;188(8):901–906. doi: 10.1164/rccm.201302-0388PP. [DOI] [PubMed] [Google Scholar]
- Beeh K., et al. Antiinflammatory properties of ambroxol. Eur. J. Med. Res. 2008;13(12):557–562. [PubMed] [Google Scholar]
- Berekmeri A., et al. Tofacitinib for the treatment of psoriasis and psoriatic arthritis. Expet Rev. Clin. Immunol. 2018;14(9):719–730. doi: 10.1080/1744666X.2018.1512404. [DOI] [PubMed] [Google Scholar]
- Boehncke W.-H., Brembilla N.C. Unmet needs in the field of psoriasis: pathogenesis and treatment. Clin. Rev. Allergy Immunol. 2018;55(3):295–311. doi: 10.1007/s12016-017-8634-3. [DOI] [PubMed] [Google Scholar]
- Bos J., et al. Psoriasis: dysregulation of innate immunity. Br. J. Dermatol. 2005;152(6):1098–1107. doi: 10.1111/j.1365-2133.2005.06645.x. [DOI] [PubMed] [Google Scholar]
- Boy M.G., et al. Double-blind, placebo-controlled, dose-escalation study to evaluate the pharmacologic effect of CP-690,550 in patients with psoriasis. J. Invest. Dermatol. 2009;129(9):2299. doi: 10.1038/jid.2009.25. [DOI] [PubMed] [Google Scholar]
- Breckenridge A., Jacob R. Overcoming the legal and regulatory barriers to drug repurposing. Nat. Rev. Drug Discov. 2019;18(1):1–2. doi: 10.1038/nrd.2018.92. [DOI] [PubMed] [Google Scholar]
- Brembilla N.C., Senra L., Boehncke W.-H. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front. Immunol. 2018;9:1682. doi: 10.3389/fimmu.2018.01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzavara-Pinton P., et al. The separate daily application of tacalcitol 4 μg/g ointment and budesonide 0.25 mg/g cream is more effective than the single daily application of a two compound ointment containing calcipotriol 50 μg/g and betamethasone dipropionate 0.5 mg/g. Giornale italiano di dermatologia e venereologia: organo ufficiale. Societa italiana di dermatologia e sifilografia. 2011;146(4):295–299. [PubMed] [Google Scholar]
- Carlin C.S., et al. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J. Am. Acad. Dermatol. 2004;50(6):859–866. doi: 10.1016/j.jaad.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Cavalla D. Predictive methods in drug repurposing: gold mine or just a bigger haystack? Drug Discov. Today. 2013;18(11–12):523–532. doi: 10.1016/j.drudis.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Chang J.-E., Choi M.S. A molecular perspective on the potential benefits of metformin for the treatment of inflammatory skin disorders. Int. J. Mol. Sci. 2020;21(23):8960. doi: 10.3390/ijms21238960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., et al. Niclosamide: beyond an antihelminthic drug. Cell. Signal. 2018;41:89–96. doi: 10.1016/j.cellsig.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiricozzi A., et al. Tofacitinib for the treatment of moderate-to-severe psoriasis. Expet Rev. Clin. Immunol. 2015;11(4):443–455. doi: 10.1586/1744666X.2015.1013534. [DOI] [PubMed] [Google Scholar]
- Choi J., Koo J.Y. Quality of life issues in psoriasis. J. Am. Acad. Dermatol. 2003;49(2):57–61. doi: 10.1016/S0190-9622(03)01136-8. [DOI] [PubMed] [Google Scholar]
- Cragg G.M., Grothaus P.G., Newman D.J. New horizons for old drugs and drug leads. J. Nat. Prod. 2014;77(3):703–723. doi: 10.1021/np5000796. [DOI] [PubMed] [Google Scholar]
- Dalaker M., Bonesrønning J. Long-term maintenance treatment of moderate-to-severe plaque psoriasis with infliximab in combination with methotrexate or azathioprine in a retrospective cohort. J. Eur. Acad. Dermatol. Venereol. 2009;23(3):277–282. doi: 10.1111/j.1468-3083.2008.03039.x. [DOI] [PubMed] [Google Scholar]
- De Jong E., et al. Effects of topical treatment with budesonide on parameters for epidermal proliferation, keratinization and inflammation in psoriasis. J. Dermatol. Sci. 1995;9(3):185–194. doi: 10.1016/0923-1811(94)00376-p. [DOI] [PubMed] [Google Scholar]
- Deng Y., Chang C., Lu Q. The inflammatory response in psoriasis: a comprehensive review. Clin. Rev. Allergy Immunol. 2016;50(3):377–389. doi: 10.1007/s12016-016-8535-x. [DOI] [PubMed] [Google Scholar]
- DiMasi J.A. Risks in new drug development: approval success rates for investigational drugs. Clin. Pharmacol. Ther. 2001;69(5):297–307. doi: 10.1067/mcp.2001.115446. [DOI] [PubMed] [Google Scholar]
- DiMasi J.A., Hansen R.W., Grabowski H.G. The price of innovation: new estimates of drug development costs. J. Health Econ. 2003;22(2):151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- Doppalapudi S., et al. Fenoldopam mesylate for treating psoriasis: a new indication for an old drug. Int. J. Pharm. 2020;573:118726. doi: 10.1164/rccm.201302-0388PP. [DOI] [PubMed] [Google Scholar]
- Egeberg A., et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br. J. Dermatol. 2018;178(2):509–519. doi: 10.1111/bjd.16102. [DOI] [PubMed] [Google Scholar]
- Ehrlich A., et al. Micellar paclitaxel improves severe psoriasis in a prospective phase II pilot study. J. Am. Acad. Dermatol. 2004;50(4):533–540. doi: 10.1016/j.jaad.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Farol L.T., Hymes K.B. Bexarotene: a clinical review. Expet Rev. Anticancer Ther. 2004;4(2):180–188. doi: 10.1586/14737140.4.2.180. [DOI] [PubMed] [Google Scholar]
- Feldman S.R., et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. American health & drug benefits. 2016;9(9):504. [PMC free article] [PubMed] [Google Scholar]
- Genovese M.C., et al. Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 2016;374:1243–1252. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- Giannuzzi V., et al. Orphan medicinal products in Europe and United States to cover needs of patients with rare diseases: an increased common effort is to be foreseen. Orphanet J. Rare Dis. 2017;12(1):1–11. doi: 10.1186/s13023-017-0617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick H. Oral retinoids—efficacy and toxicity in psoriasis. Br. J. Dermatol. 1996;135:6–17. doi: 10.1111/j.1365-2133.1996.tb15661.x. [DOI] [PubMed] [Google Scholar]
- Goodman W.A., et al. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J. Immunol. 2009;183(5):3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb A.B., et al. The national psoriasis foundation psoriasis score (NPF-PS) system versus the psoriasis area severity index (PASI) and physician's global assessment (PGA): a comparison. J. Drugs Dermatol. JDD: J. Drugs Dermatol. JDD. 2003;2(3):260–266. [PubMed] [Google Scholar]
- Greaves M.W., Weinstein G.D. Treatment of psoriasis. N. Engl. J. Med. 1995;332(9):581–589. doi: 10.1056/NEJM199503023320907. [DOI] [PubMed] [Google Scholar]
- Gupta R. Prolonged remission of psoriasis with azathioprine pulse therapy. Indian J. Dermatol. 2015;60(4):360. doi: 10.4103/0019-5154.160480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahtela T. A biodiversity hypothesis. Allergy. 2019;74(8):1445–1456. doi: 10.1111/all.13763. [DOI] [PubMed] [Google Scholar]
- Halabi S.F. The drug repurposing ecosystem: intellectual property incentives, market exclusivity, and the future of new medicines. Yale JL & Tech. 2018;20:1. [Google Scholar]
- Harden J.L., Krueger J.G., Bowcock A.M. The immunogenetics of psoriasis: a comprehensive review. J. Autoimmun. 2015;64:66–73. doi: 10.1016/j.jaut.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes J.E., et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J. Immunol. 2018;201(6):1605–1613. doi: 10.4049/jimmunol.1800013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez J.J., et al. Giving drugs a second chance: overcoming regulatory and financial hurdles in repurposing approved drugs as cancer therapeutics. Frontiers in oncology. 2017;7:273. doi: 10.3389/fonc.2017.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos R.A., et al. In silico methods for drug repurposing and pharmacology. Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 2016;8(3):186–210. doi: 10.1002/wsbm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefnagel J., et al. Long-term safety aspects of systemic therapy with fumaric acid esters in severe psoriasis. Br. J. Dermatol. 2003;149(2):363–369. doi: 10.1046/j.1365-2133.2003.05433.x. [DOI] [PubMed] [Google Scholar]
- Hosking A.-M., Juhasz M., Mesinkovska N.A. Topical Janus kinase inhibitors: a review of applications in dermatology. J. Am. Acad. Dermatol. 2018;79(3):535–544. doi: 10.1016/j.jaad.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Iglesias-Lopez C., et al. Regulatory framework for advanced therapy medicinal products in Europe and United States. Front. Pharmacol. 2019;10:921. doi: 10.3389/fphar.2019.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., et al. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-κB pathway and generation of reactive oxygen species. Canc. Res. 2010;70(6):2516–2527. doi: 10.1158/0008-5472.CAN-09-3950. [DOI] [PubMed] [Google Scholar]
- Karaman B., Sippl W. Computational drug repurposing: current trends. Curr. Med. Chem. 2019;26(28):5389–5409. doi: 10.2174/0929867325666180530100332. [DOI] [PubMed] [Google Scholar]
- Kilfoyle B.E., et al. Development of paclitaxel-TyroSpheres for topical skin treatment. J. Contr. Release. 2012;163(1):18–24. doi: 10.1016/j.jconrel.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran T., et al. Repositioning drugs. Asian J. Pharmaceut. Res. 2019;9(4):268–272. doi: 10.5958/2231-5691.2019.00044.3. [DOI] [Google Scholar]
- Kirby B., et al. Physical and psychologic measures are necessary to assess overall psoriasis severity. J. Am. Acad. Dermatol. 2001;45(1):72–76. doi: 10.1067/mjd.2001.114592. [DOI] [PubMed] [Google Scholar]
- Koo J. Systemic sequential therapy of psoriasis: a new paradigm for improved therapeutic results. J. Am. Acad. Dermatol. 1999;41(3):S25–S28. doi: 10.1016/S0190-9622(99)70363-4. [DOI] [PubMed] [Google Scholar]
- Kozub P., Simaljakova M. Systemic therapy of psoriasis: methotrexate. Bratisl. Lek. Listy. 2011;112(7):390–394. [PubMed] [Google Scholar]
- Krueger G.G. Selective targeting of T cell subsets: focus on alefacept–a remittive therapy for psoriasis. Expet Opin. Biol. Ther. 2002;2(4):431–441. doi: 10.1517/14712598.2.4.431. [DOI] [PubMed] [Google Scholar]
- Krueger J., et al. Tofacitinib attenuates pathologic immune pathways in patients with psoriasis: a randomized phase 2 study. J. Allergy Clin. Immunol. 2016;137(4):1079–1090. doi: 10.1016/j.jaci.2015.12.1318. [DOI] [PubMed] [Google Scholar]
- Kuchekar A.B., et al. Psoriasis: a comprehensive review. International Journal of pharmacy & life sciences. 2011;2(6) [Google Scholar]
- Kuwabara T., et al. The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/3908061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladeiras-Lopes R., et al. Novel therapeutic targets of metformin: metabolic syndrome and cardiovascular disease. Expert Opin. Ther. Targets. 2015;19(7):869–877. doi: 10.1517/14728222.2015.1025051. [DOI] [PubMed] [Google Scholar]
- Lebwohl M., et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N. Engl. J. Med. 2003;349(21):2004–2013. doi: 10.1056/NEJMoa030002. [DOI] [PubMed] [Google Scholar]
- Lebwohl M., et al. Combination therapy to treat moderate to severe psoriasis. J. Am. Acad. Dermatol. 2004;50(3):416–430. doi: 10.1016/j.jaad.2002.12.002. [DOI] [PubMed] [Google Scholar]
- Levite M. Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol. 2016;216(1):42–89. doi: 10.1111/apha.12476. [DOI] [PubMed] [Google Scholar]
- Li Y., et al. Multi-targeted therapy of cancer by niclosamide: a new application for an old drug. Canc. Lett. 2014;349(1):8–14. doi: 10.1016/j.canlet.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H.J. Mayo Clinic Proceedings. Elsevier; 1994. Paclitaxel (Taxol): a novel anticancer chemotherapeutic drug. [DOI] [PubMed] [Google Scholar]
- Lowes M.A., Suarez-Farinas M., Krueger J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014;32:227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., et al. A network integration approach for drug-target interaction prediction and computational drug repositioning from heterogeneous information. Nat. Commun. 2017;8(1):1–13. doi: 10.1038/s41467-017-00680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltzman J.S., Koretzky G.A. Azathioprine: old drug, new actions. The Journal of clinical investigation. 2003;111(8):1122–1124. doi: 10.1172/JCI18384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A.R., et al. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst. Rev. 2013;(3) doi: 10.1111/bjd.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease P.J. Measures of psoriatic arthritis: tender and swollen joint assessment, psoriasis area and severity index (PASI), nail psoriasis severity index (NAPSI), modified nail psoriasis severity index (mNAPSI), mander/newcastle enthesitis index (MEI), leeds enthesitis index (LEI), spondyloarthritis research consortium of Canada (SPARCC), maastricht ankylosing spondylitis enthesis score (MASES), leeds dactylitis index (LDI), patient global for psoriatic arthritis, dermatology life quality index (DLQI), psoriatic arthritis quality of life (PsAQOL), functional assessment of chronic illness therapy–fatigue (facit-F), psoriatic arthritis response criteria (PsARC), psoriatic arthritis joint activity index (PsAJAI), disease activity in psoriatic arthritis (DAPSA), and composite psoriatic disease activity index (CPDAI) Arthritis Care Res. 2011;63(S11):S64–S85. doi: 10.1002/acr.20577. [DOI] [PubMed] [Google Scholar]
- Meier M., Sheth P.B. Clinical spectrum and severity of psoriasis. Management of psoriasis. 2009;38:1–20. doi: 10.1159/000232301. [DOI] [PubMed] [Google Scholar]
- Mesa R.A. Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis. Idrugs: the investigational drugs journal. 2010;13(6):394–403. [PubMed] [Google Scholar]
- Murdaca G., Colombo B., Puppo F. Anti-TNF-α inhibitors: a new therapeutic approach for inflammatory immune-mediated diseases: an update upon efficacy and adverse events. Int. J. Immunopathol. Pharmacol. 2009;22(3):557–565. doi: 10.1177/039463200902200301. [DOI] [PubMed] [Google Scholar]
- Murteira S., et al. Drug reformulations and repositioning in the pharmaceutical industry and their impact on market access: regulatory implications. Journal of market access & health policy. 2014;2(1):22813. doi: 10.3402/jmahp.v2.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A., et al. Bruton's tyrosine kinase inhibitor suppresses imiquimod-induced psoriasis-like inflammation in mice through regulation of IL-23/IL-17A in innate immune cells. Int. Immunopharm. 2020;80:106215. doi: 10.1016/j.intimp.2020.106215. [DOI] [PubMed] [Google Scholar]
- Naldi L., Griffiths C. Traditional therapies in the management of moderate to severe chronic plaque psoriasis: an assessment of the benefits and risks. Br. J. Dermatol. 2005;152(4):597–615. doi: 10.1111/j.1365-2133.2005.06563.x. [DOI] [PubMed] [Google Scholar]
- Naseri M., et al. The remarkable beneficial effect of adding oral simvastatin to topical betamethasone for treatment of psoriasis: a double-blind, randomized, placebo-controlled study. Niger. J. Med.: journal of the National Association of Resident Doctors of Nigeria. 2010;19(1):58–61. doi: 10.4314/njm.v19i1.54216. [DOI] [PubMed] [Google Scholar]
- Naylor D.M. vol. 57. Drug Discovery; 2015. Therapeutic Drug Repurposing, Repositioning and Rescue. [Google Scholar]
- Neimann A.L., Porter S.B., Gelfand J.M. The epidemiology of psoriasis. Expet Rev. Dermatol. 2006;1(1):63–75. doi: 10.1586/17469872.1.1.63. [DOI] [Google Scholar]
- Novac N. Challenges and opportunities of drug repositioning. Trends Pharmacol. Sci. 2013;34(5):267–272. doi: 10.1016/j.tips.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Okada Y. The current landscape of psoriasis genetics in 2020. J. Dermatol. Sci. 2020 doi: 10.1016/j.jdermsci.2020.05.008. [DOI] [PubMed] [Google Scholar]
- Oprea T., Mestres J. Drug repurposing: far beyond new targets for old drugs. AAPS J. 2012;14(4):759–763. doi: 10.1208/s12248-012-9390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprea T.I., Overington J.P. Computational and practical aspects of drug repositioning. Assay Drug Dev. Technol. 2015;13(6):299–306. doi: 10.1089/adt.2015.29011.tiodrrr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprea T.I., et al. Drug repurposing from an academic perspective. Drug Discov. Today Ther. Strat. 2011;8(3–4):61–69. doi: 10.1016/j.ddstr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprea T.I., et al. Associating drugs, targets and clinical outcomes into an integrated network affords a new platform for computer-aided drug repurposing. Molecular informatics. 2011;30(2-3):100–111. doi: 10.1002/minf.201100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostantinou E., et al. Efficacy of 2 weeks' application of theophylline ointment in psoriasis vulgaris. J. Dermatol. Treat. 2005;16(3):169–170. doi: 10.1080/09546630510043202. [DOI] [PubMed] [Google Scholar]
- Papp K., et al. Tofacitinib, an oral J anus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br. J. Dermatol. 2015;173(4):949–961. doi: 10.1111/bjd.14018. [DOI] [PubMed] [Google Scholar]
- Papp K., et al. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br. J. Dermatol. 2016;174(6):1266–1276. doi: 10.1111/bjd.14403. [DOI] [PubMed] [Google Scholar]
- Parisi R., et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J. Invest. Dermatol. 2013;133(2):377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- Paul C.F., et al. Risk of malignancies in psoriasis patients treated with cyclosporine: a 5 y cohort study. J. Invest. Dermatol. 2003;120(2):211–216. doi: 10.1046/j.1523-1747.2003.12040.x. [DOI] [PubMed] [Google Scholar]
- Ports W., et al. A randomized phase 2a efficacy and safety trial of the topical J anus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br. J. Dermatol. 2013;169(1):137–145. doi: 10.1111/bjd.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punwani N., et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J. Am. Acad. Dermatol. 2012;67(4):658–664. doi: 10.1016/j.jaad.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Punwani N., et al. Downmodulation of key inflammatory cell markers with a topical Janus kinase 1/2 inhibitor. Br. J. Dermatol. 2015;173(4):989–997. doi: 10.1111/bjd.13994. [DOI] [PubMed] [Google Scholar]
- Pushpakom S., et al. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18(1):41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- Rahman M., et al. Nanomedicine-based drug targeting for psoriasis: potentials and emerging trends in nanoscale pharmacotherapy. Expet Opin. Drug Deliv. 2015;12(4):635–652. doi: 10.1517/17425247.2015.982088. [DOI] [PubMed] [Google Scholar]
- Rashmi R., Rao K., Basavaraj K. A comprehensive review of biomarkers in psoriasis. Clin. Exp. Dermatol.: Clin. Dermatol. 2009;34(6):658–663. doi: 10.1111/j.1365-2230.2009.03410.x. [DOI] [PubMed] [Google Scholar]
- Rojas L.B.A., Gomes M.B. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol. Metab. Syndrome. 2013;5(1):1–15. doi: 10.1186/1758-5996-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol. Res. 2016;111:784–803. doi: 10.1016/j.phrs.2016.07.038. [DOI] [PubMed] [Google Scholar]
- Sagiv-Barfi I., et al. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112(9):E966–E972. doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka R., et al. Enhanced penetration and improved therapeutic efficacy of bexarotene via topical liposomal gel in imiquimod induced psoriatic plaque model in BALB/c mice. J. Drug Deliv. Sci. Technol. 2020;58:101691. doi: 10.1016/j.jddst.2020.101691. [DOI] [Google Scholar]
- Samarasekera E., et al. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br. J. Dermatol. 2013;168(5):954–967. doi: 10.1111/bjd.12276. [DOI] [PubMed] [Google Scholar]
- Sameh N., et al. Prospective role of simvastatin on wound healing: review of the literature. J. Bioequivalence Bioavailab. 2018;10(2):36–42. doi: 10.4172/0975-0851.1000375. [DOI] [Google Scholar]
- Sardana D., et al. Drug repositioning for orphan diseases. Briefings Bioinf. 2011;12(4):346–356. doi: 10.1093/bib/bbr021. [DOI] [PubMed] [Google Scholar]
- Sekhon B.S. Repositioning drugs and biologics: retargeting old/existing drugs for potential new therapeutic applications. Journal of Pharmaceutical Education & Research. 2013;4(1) [Google Scholar]
- Smit J.V., et al. A phase II multicenter clinical trial of systemic bexarotene in psoriasis. J. Am. Acad. Dermatol. 2004;51(2):249–256. doi: 10.1016/j.jaad.2002.08.001. [DOI] [PubMed] [Google Scholar]
- Smith R.B. Repositioned drugs: integrating intellectual property and regulatory strategies. Drug Discov. Today Ther. Strat. 2011;8(3–4):131–137. doi: 10.1016/j.ddstr.2011.06.008. [DOI] [Google Scholar]
- Stern R.S. Psoralen and ultraviolet a light therapy for psoriasis. N. Engl. J. Med. 2007;357(7):682–690. doi: 10.1056/NEJMct072317. [DOI] [PubMed] [Google Scholar]
- Strittmatter S.M. Overcoming drug development bottlenecks with repurposing: old drugs learn new tricks. Nat. Med. 2014;20(6):590–591. doi: 10.1038/nm.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkari S., et al. Protective effects of ambroxol in psoriasis like skin inflammation: exploration of possible mechanisms. Int. Immunopharm. 2019;71:301–312. doi: 10.1016/j.intimp.2019.03.035. [DOI] [PubMed] [Google Scholar]
- Thatikonda S., Pooladanda V., Godugu C. Repurposing an old drug for new use: niclosamide in psoriasis-like skin inflammation. J. Cell. Physiol. 2020;235(6):5270–5283. doi: 10.1002/jcp.29413. [DOI] [PubMed] [Google Scholar]
- Thorneloe R., et al. Nonadherence to psoriasis medication as an outcome of limited coping resources and conflicting goals: findings from a qualitative interview study with people with psoriasis. Br. J. Dermatol. 2017;176(3):667–676. doi: 10.1111/bjd.15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobinick E.L. The value of drug repositioning in the current pharmaceutical market. Drug News Perspect. 2009;22(2):119–125. doi: 10.1358/dnp.2009.22.2.1303818. [DOI] [PubMed] [Google Scholar]
- Touitou E., et al. Enhanced permeation of theophylline through the skin and its effect on fibroblast proliferation. Int. J. Pharm. 1991;70(1–2):159–166. doi: 10.1016/0378-5173(91)90176-O. [DOI] [Google Scholar]
- Tsuji G., et al. Metformin inhibits IL-1β secretion via impairment of NLRP3 inflammasome in keratinocytes: implications for preventing the development of psoriasis. Cell death discovery. 2020;6(1):1–11. doi: 10.1038/s41420-020-0245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumlin J.A., et al. Fenoldopam, a dopamine agonist, for hypertensive emergency: a multicenter randomized trial. Acad. Emerg. Med. 2000;7(6):653–662. doi: 10.1111/j.1553-2712.2000.tb02039.x. [DOI] [PubMed] [Google Scholar]
- Warren R.B., Griffiths C.E. Systemic therapies for psoriasis: methotrexate, retinoids, and cyclosporine. Clin. Dermatol. 2008;26(5):438–447. doi: 10.1016/j.clindermatol.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Wilkins M.R., et al. Simvastatin as a treatment for pulmonary hypertension trial. Am. J. Respir. Crit. Care Med. 2010;181(10):1106–1113. doi: 10.1164/rccm.2009111-699OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski T.X. Intellectual property and other legal aspects of drug repurposing. Drug Discov. Today Ther. Strat. 2011;8(3–4):139–143. doi: 10.1016/j.ddstr.2011.06.007. [DOI] [Google Scholar]
- Xu X., Zhang H.-Y. The immunogenetics of psoriasis and implications for drug repositioning. Int. J. Mol. Sci. 2017;18(12):2650. doi: 10.3390/ijms18122650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H., et al. Review of drug repositioning approaches and resources. Int. J. Biol. Sci. 2018;14(10):1232. doi: 10.7150/ijbs.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., et al. A novel surfactant-free O/O paclitaxel ointment for the topical treatment of psoriasis. AAPS PharmSciTech. 2019;20(5):1–16. doi: 10.1208/s12249-019-1413-0. [DOI] [PubMed] [Google Scholar]
- Yip S.Y. The prevalence of psoriasis in the Mongoloid race. J. Am. Acad. Dermatol. 1984;10(6):965–968. doi: 10.1016/S0190-9622(84)80314-X. [DOI] [PubMed] [Google Scholar]
- Zeiser R. Immune modulatory effects of statins. Immunology. 2018;154(1):69–75. doi: 10.1111/imm.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., et al. Critical role of environmental factors in the pathogenesis of psoriasis. J. Dermatol. 2017;44(8):863–872. doi: 10.1111/1346-8138.13806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
With increasing development of protocols in clinical studies, an increasing number of drugs are flooding the pipeline, regardless of their terminal failure at later stages. These studies provide the data collected in clinical trials, which are often not released into the public domain or have restricted access. Some data are less amenable to access and analysis to investigate drug repositioning (Pushpakom et al., 2019). Data integration has been demonstrated to provide important information such as the best indications, mechanisms of action, efficacy, and performance of molecules, thereby increasing the literature available for interpretation and the analytic power (Luo et al., 2017).