Abstract
Brain oxidative signaling pathways have been identified as important targets for alleviating food deprivation-induced changes in metabolic gate-ways. Previous studies have shown that prenatal and early postnatal malnutrition alters leptin and ghrelin signaling via oxidative pathways. Thus, it has been hypothesized that agents with antioxidant properties might be beneficial for the mitigation of prenatal and early postnatal food scarcity-induced oxidative damage. Quercetin and kaempferol are natural bioflavonoids with proven antioxidant properties. In this study, we evaluated their effects on prenatal maternal food consumption, maternal and pup weights, biomarkers of orexigenic and anorexigenic hormones and oxidative stress in rats. Rats were allotted into different treatment groups (n = 6) in three different experiments (prenatal, postnatal food-deprivations or both). Prenatal-food restriction (PrNFR) was induced by 50% of ad libitum accessible diet during pregnancy till parturition and postnatal-food restriction (PsNFR) was simulated by litter-enlargement to 16 pups per mother from postnatal day (PND) 2. Rats in each experiment were concurrently treated with vehicle (10 mL/kg), quercetin (50, 100 and 200 mg/kg, p.o.) or kaempferol (50, 100 and 200 mg/kg, p.o.) respectively. A third experimental group consisted of both protocols. Quercetin and kaempferol dose-dependently increased the body weights of pups exposed to PrNFR, PsNFR and PrNFR-PsNFR at PNDs 1–22 respectively. Both compounds increased maternal body weights but attenuated maternal food-intake at prenatal days 7 and 14 due by PrNFR. Quercetin and kaempferol reduced brain malondialdehyde concentrations and increased glutathione levels in PrNFR, PsNFR and PrNFR-PsNFR-exposed offspring of rats. Importantly, quercetin and kaempferol significantly (p < 0.05) prevented PrNFR-, PsNFR- or PrNFR-PsNFR-induced alterations in leptin and ghrelin levels. Cumulatively, quercetin and kaempferol increased pup and maternal weights and attenuated maternal food-intake of rats submitted to PrNFR, PsNFR and PrNFR-PsNFR respectively, likely via nutrigenomic modulations of orexigenic/anorexigenic hormones and inhibition of brain oxidative stress.
Keywords: Food restriction, Leptin, Ghrelin, Oxidative stress, Quercetin, Kaempferol
Abbreviations: PrNFR, Prenatal-food restriction; PsNFR, postnatal-food restriction; PND, postnatal day; QCET, Quercetin; KFAM, Kaempferol; DMSO, dimethyl sulfoxide; VO, vaginal opening; BPS, balano-preputal separation; ANOVA, one-way analysis of variance; BMI, body mass index
Highlights
-
•
PrNFR and PsNFR reduce maternal and fetal weights.
-
•
Quercetine and kaempferol increase PrNFR and PsNFR-induced weight loss.
-
•
Quercetine and kaempferol attenuate PrNFR and PsNFR-induced ghrelin alteration.
-
•
Quercetine and kaempferol attenuate PrNFR and PsNFR-induced leptin alteration.
-
•
Quercetine and kaempferol reduced PrNFR and PsNFR-induced brain oxidative stress.
Graphical abstract
1. Introduction
Nutritional status during pregnancy and early childhood has a significant impact on body composition, antioxidant status and orexigenic/anorexigenic hormone signaling, and can explain at least about 25% of the variation in maturation and other physiological measures of child development (Gualillo et al., 2012; Soliman et al., 2014). During pregnancy, the mother must preserve standard health conditions and metabolism in order to maintain availability of nutrients for the fetus (Engelbregt et al., 2000). Consequently, early malnutrition is known to change the original programming of organs, especially during developmental phases, which can result in long term metabolic changes in weight and growth patterns, as well as feeding behavior (Engelbregt et al., 2000; Engelbregt et al., 2001). It is well-known that metabolic adaptations to under-nutrition are linked to changes in endocrine microenvironments as well as orexigenic and anorexigenic hormones (Gualillo et al., 2012; Bouret et al., 2004). Ghrelin, which is an orexigenic hormone primarily expressed in the stomach and hypothalamus, is one of the most researched gut-derived molecules and is an endogenous ligand for growth hormone secretagogues and food intake (Gualillo et al., 2012; Rojas et al., 2011). However, the relationship between metabolic pathways with regards to body fat stores and weight within the neuroendocrine reproductive system was unclear until the discovery of leptin, which is the long-sought metabolic key to fat store and puberty (Engelbregt et al., 2000; Engelbregt et al., 2001; Nazian & Cameron, 1999).
Mechanistically, peripheral ghrelin and leptin are known to enter into certain areas of the brain through the blood brain barrier to modulate cascade of events including signal transduction and activator of transcription-3 (STAT3) and MAPK pathways, from where they are known the regulate various “hunger and satiety centres” in the lateral hypothalamic area, arcuate and paraventricular nuclei (Rojas et al., 2011; Hu et al., 2003; Sucajtys-Szulc et al., 2010). However, several studies have shown opposite correlations between human and animal ghrelin and leptin levels, feeding behavior, body mass and pubertal onset (Gualillo et al., 2012; Bouret et al., 2004; Quennell et al., 2011). For a long time, it was hypothesized that ghrelin and leptin played important roles in the central regulation of body weight and thus, it has been reported that fasting or under-nutrition has significant implication in the pathophysiology of several metabolic diseases like growth hormone deficiencies, anorexia nerovosa, cachexia, gastrointestinal motility disorders and obesity (Gualillo et al., 2012; Quennell et al., 2011; Cordido et al., 2009). Previous studies have shown that chronic prenatal and postnatal food restrictions markedly increased circulating levels of ghrelin and gastric ghrelin mRNA expression (Gualillo et al., 2012), but decreased leptin signaling via pleiotropic metabolic mechanisms involving increased generation of free radicals (Luo et al., 2015). Preclinical investigations in rodent and cell lines studies support the role of redox balancing in modulating the expression reproductive genes (Turpaev, 2002), metabolic functions (Pi et al., 2010) and growth hormones (Luo et al., 2015). Indeed, oxidative stress, which is a state of increased free radical generation and reduced protective antioxidant enzymes, is thought to be central to the pathogenesis of different feeding behaviors and mental disorders (Gualillo et al., 2012; Quennell et al., 2011; Cordido et al., 2009; Schiavone et al., 2013). For example, pre and postnatal food restrictions have been repeatedly linked with several perinatal adverse conditions including diabetes, preeclampsia, preterm birth, stillbirths, low birth weight and poor feeding behavior that are predictive of heightened risk of mental and metabolic diseases in postnatal life (Schiavone et al., 2013; Agarwal et al., 2005). Since mitochondria are major hub of metabolic activity, investigations have shown strong correlations between prenatal food deprivation-induced glucose insufficiency, placental insufficiency, dysregulated mitochondrial homeostasis in placental samples and altered redox catalytic activity (Swolin-Eide et al., 2002; Turpin et al., 2015), which can lead to increased vulnerability factor for the onset of neurodevelopmental disorders (Turpin et al., 2015). Together, oxidative signaling has been established as a common pathway involved in the metabolic gate-way and brain development (Luo et al., 2006). Previous studies have shown that supplementations with flavonoids exhibit strong nutrigenomic potentials and possibly mitigate various malnutrition-induced neonatal anomalies and neuroendocrine developmental diseases by modulation of orexigenic and anorexigenic hormones via antioxidant pathways (Agarwal et al., 2005; Duhig et al., 2016).
Quercetin (2-(3,4-hihydroxyphenyl)3,5,7-trihydroxy-4H-chromen-4-one)) and kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) [Fig. 1] are polyphenol antioxidants commonly distributed in different plants such as Curcuma domestica valeton, Cuscuta reflexa, Daucus carota, and Emblica officinalis (Wijdan & Marra, 2019). They are also found in large quantities in fruits and vegetables including apple, grape, orange, wild garlic, onion, red wine, black tea, broccoli, endive and wild leeks (Wijdan & Marra, 2019; Makris et al., 2006). Increasing bodies of evidence have shown that quercetin and kaempferol have gained international recognitions as safe essential components of our daily dietary life based on their antioxidant activities (Wijdan & Marra, 2019; Makris et al., 2006). Also, comprehensive toxicological studies have shown that quercetin (Wijdan & Marra, 2019) and kaempferol (Makris et al., 2006) supplementation for 12 weeks via different routes of administration were associated with marked beneficial effects without observable adverse effects on rodents and humans. Accordingly, various reports have shown diverse pharmacological activities of quercetin and kaempferol to include but not limited to anti-inflammatory (Devi et al., 2015), antioxidant (Ozgen et al., 2016), anticancer (Kashyap et al., 2017; Hashemzaei et al., 2017), antihypertensive (Wijdan & Marra, 2019; Marcin et al., 2018), antidiabetic (Khalid et al., 2015; Prabhu et al., 2018) and neuroprotective (Doğan et al., 2015; Costa et al., 2016) effects. Also, it has been reported that quercetin and kaempferol enhance reproductive functionality (Trivedi et al., 2009; Jamalan et al., 2016). Importantly, quercetin and kaempferol improve bone mineralization, bone microstructure and osteoblast function in estrogen deficiency-induced bone loss and ovariectomized rats (Trivedi et al., 2009). Different studies on whole animal and cultured human endothelia cells have also reported that quercetin and kaempferol attenuate some metabolic syndromes associated with prenatal and early postnatal stress via mechanisms linked to inhibition of oxidative stress and inflammatory processes (Wijdan & Marra, 2019; Crespo et al., 2008). Clinical studies on hypertensive and diabetic conditions conducted showed that quercetin and kaempferol exhibit profound beneficial activities (Wijdan & Marra, 2019; Dower et al., 2015; Brüll et al., 2017). Additionally, quercetin and kaempferol prevented pregnancy-induced cardiovascular dysfunctions in humans and experimental animals (Wijdan & Marra, 2019; Adam-Vizi & Seregi, 1982) as well as protect against the adverse effects of chemotherapeutic treatments during pregnancy (Doğan et al., 2015). Hence, these findings further suggest their potential benefits in conditions associated with prenatal and early postnatal under-nutrition, whose pathological abnormality is strongly associated with oxidative stress. As part of our on-going studies on the effects of quercetin and kaempferol on prenatal and postnatal food deprivation, this present study was further aimed at investigating the protective effects of quercetin and kaempferol on prenatal and early postnatal food deprivation-induced alterations in maternal food consumption, maternal and fetal weights, estimation of leptin and ghrelin hormonal levels, as well as measurement of oxidative stress in the brains of rats.
Fig. 1.
Chemical structures of quercetin and kaempferol (Wijdan et al., 2019).
2. Materials and methods
2.1. Experimental animals
Male and female Wistar rats (170–200 g; 12–16 weeks old) were obtained from the Animal House Facility of the Faculty of Basic Medical Sciences, Delta State University, Abraka, Nigeria. Animals were accommodated in plastic cage (27 × 30 × 42 cm) in a 25 ± 1 °C environment with light/dark cycle of 12:12. Animals were fed with standard animal pellets and water at free will throughout the experiments. Animals were acclimatized for a period of 7 days prior to the start of experiments. Afterward, each animal was randomly distributed to various treatment groups. Six (6) animals or pups were approved based on ethical considerations. Guide from National institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85–23, revised 1985) in accordance with the animal rights laws of the Delta State University (REC/FBMS/DELSU/19/078) were strictly followed.
2.2. Drugs and chemicals
Quercetin – QCET (50, 100 and 200 mg/kg), kaempferol – KFAM (50, 100 and 200 mg/kg) were procured from Sigma–Aldrich, St. Louis, USA. They were dissolved in dimethyl sulfoxide (DMSO) (0.1 M) and administered orally (p.o.) by gavage. The DMSO (10 mL/kg, p.o.) (0.1 M) solution was also administered orally in a volume of 10 mL/kg per body weight, as a control. The doses of quercetin (Prabhu et al., 2018) and kaempferol (Khalid et al., 2015) used in this study were based on results obtained from preliminary and previous investigations.
2.3. Experimental design
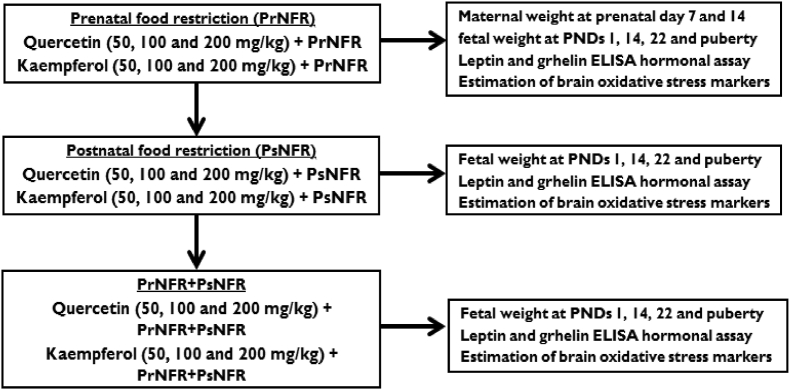
2.3.1. Experiment 1: Prenatal food deprivation
Constant patterns of vaginal cyclic period of female Wistar rats were established during the last 10 days baseline period. Rats were accommodated individually in a standard rat cage with wood shavings as bedcovers with free access to water. Programmed mating was carried out by monitoring the oestrus cycle of the rats before the introduction of the male Wistar rats. Day 1 of pregnancy was confirmed by the presence of spermatozoa after a vaginal smear. Thereafter, the animals were randomly distributed into various treatment groups (n = 6). Group 1, which served as control was fed ad libitum with a standard rodent diet for 21 days; however, rats in group 2 were food deprived for 21 days and served as negative control. Groups 3–5 rats were food deprived and treated with quercetin (50, 100 and 200 mg/kg, p.o.). Animals in groups 6–8 were food deprived and but received kaempferol (50, 100 and 200 mg/kg, p.o.) consecutively till date of delivery (days 21–22). Prenatal food restriction (PrNFR) was simulated as previously described but with minor modifications (Gualillo et al., 2012), including 50% reduction in the quality and quantity of rodent pellets ad libitum. This was estimated by the quantity of food consumed by the control group on the preceding days from day 1 of gestation onward to delivery date (days 21–22). Nevertheless, the control group was fed ad libitum with a standard rodent diet for 21 days without any form of prenatal starvation. Maternal food consumption was measured by weighing the left-over of the original quantity of food served across all treatment groups at prenatal days 7 and 14 (Engelbregt et al., 2000). Also, maternal/dam weights were measured across all treatment groups at prenatal days 7 and 14. Pups (number of stillbirths, 6–10 litters, litter size (4–8 cm), sex ratio per litter etc) were delivered naturally between days 21–22 (Anachuna et al., 2020). Litters were housed three males or three females per cage with dam, and dams were fed with standard diet. Mean litter weights were measured at postnatal days (PNDs) 1, 7, 14, 22 and at puberty (Scheme 1). The litters (brothers and sisters) were housed together with the mothers in standard rat cages enriched with wood shavings as bedcovering with food and water ad libitum with standard temperature and 12 h light and dark cycle.
Scheme 1.
Experimental protocols.
2.3.2. Experiment 2: Postnatal food deprivation
Postnatal food restriction (PsNFR) was induced by cross fostering and increasing the litters to 16 pups per mother from PND 2 till weaning (day 24), for a total period of 22 days of postnatal under nutrition (Anachuna et al., 2020). Postnatal food deprived rats were collected into various experimental groups, each holding 6 rats (3 males and 3 females) per group. Animals in group 1 were fed through one mother (i.e., 6 pups per mother) ad libitum until weaning (22 days) which served as the normal control. Group 2 was postnatally food deprived (among 16 pups per maternal mother) and served as the negative experimental control. Groups 3–5 were food deprived postnatally (among 16 pups per maternal mother) but received quercetin (50, 100 and 200 mg/kg, p.o.), while groups 6–8 were food deprived postnatally (among 16 pups per maternal mother) and received kaempferol (50, 100 and 200 mg/kg, p.o.) consecutively until weaning (Scheme 1).
2.3.3. Experiment 3: Prenatal and postnatal food deprivations
Scheduled mattings were carried out in the Wistar rats by monitoring the oestrus cycle of the rats before the introduction of the male Wistar rats as described in experiment 1. Day 1 after confirmation of pregnancy by the presence of spermatozoa, prenatal food deprivation (50% reduction in the quality and quantity of rodent pellets ad libitum) was initiated according to previously described model but with minor modifications (Engelbregt et al., 2000). This was based on the amount of food consumed by the control group on the previous days, from day 1 of gestation onward to the end of gestation (days 21–22). At day 21–22, pups were delivered spontaneously. Immediately, postnatal food deprivation was initiated by expanding the numbers of litters to 16 pups per mother from PND 2 until weaning (day 24), as previously described above. The prenatal and postnatal food deprived (PrNFR-PsNFR) rats (male and female) were grouped into different treatment groups (n = 6; 3 males and 3 females). Litters were housed three males and three females per cage. The PrNFR-PsNFR animals in group 1 were fed by one mother (consisting of 6 pups per mother) ad libitum until weaning (22 days) and served as the normal control. PrNFR-PsNFR group 2 was given 10 mL/kg of vehicle and was used as the negative control. PrNFR-PsNFR groups 3–5 received quercetin (50, 100 and 200 mg/kg, p.o.), whereas PrNFR-PsNFR groups 6–8 were treated with kaempferol (50, 100 and 200 mg/kg, p.o.). Mean litter weights were measured at postnatal days (PNDs) 1, 7, 14, 22 and at puberty (Scheme 1). The litters (brothers and sisters) were housed together with the mothers in standard rat cages enriched with wood shavings as bedcoverings, with food and water ad libitum (Engelbregt et al., 2000; Anachuna et al., 2020).
2.4. Animal measurements, euthanization and tissue collection
Prenatal maternal weights were measured on weekly basis. Following natural birth, litter sizes were measured at PND 1 across all treatment groups. Onward, the litter sizes were measured (in both the males and females) at PNDs 7, 14, 22 and at puberty with a digital weighing balance (DIGI 520, Japan) (Anachuna et al., 2020). Thereafter, male and female animals were decapitated and blood where collected through intracardiac puncture. Blood serum were collected and stored at −20 °C for the determination of serum leptin and ghrelin concentrations. Also, the whole brain was immediately dissected on a flat ice tray, weighed, homogenized with 10% w/v sodium phosphate buffer (0.1 M, pH 7.4) and centrifuged 3000 rpm for 10 min for biochemical assays of brain glutathione (GSH) and malondialdehyde (MDA) levels and were expressed as nmol/g tissue.
2.5. Estimation of serum leptin and ghrelin concentrations
Serum concentrations of leptin (Sigma Aldrich) and ghrelin (Sigma Aldrich) of offspring were measured using enzyme-linked immunosorbent assays according to manufacturer's protocols. Thereafter, the concentrations of leptin and ghrelin were expressed in ng/mL.
2.6. Measurements of brain oxidative stress markers of offspring
Brain lipid peroxidation (MDA) of offspring was assayed using previous protocol with thiobarbituric acid (TBA) and trichloroacetic acid (TCA) (Adam-Vizi & Seregi, 1982). Briefly, 0.1 mL of the brain supernatants of rats were diluted 20 times using 0.15 M Tris-KCl buffer and mixed with TCA (0.5 mL, 30%) and thiobarbituric acid (0.5 mL, 0.75%). The mixture was heated for 45 min in a water bath at 80 °C. The reaction mixture was cooled, centrifuged for 5 min at 4000 rpm and the absorbance of the supernatant was read at 532 nm with spectrophotometer. MDA concentrations were calculated from the molar extinction coefficient (1.56 × 105/M/cm) and the absorbance index, and expressed as nmol MDA/g tissue.
Reduced glutathione concentration of brains of rats was assayed in the supernatants as established by Moron et al., (Moron et al., 1979). Brain supernatant was diluted (10x) and 20% of TCA was used to deproteinize the tissue. The end product was centrifuge at 4000 rpm for 10 min at 4 °C and the final supernatant was reacted in fresh test tubes with 51, 51-Dithiosnitrobenzoic acid (0.0006 M). The absorbance was read spectrophotometrically at 412 nm within 10 min, and the final concentrations of GSH was extracted from a standard curve of GSH and expressed as nmol GSH/g tissue.
2.7. Statistical analysis
Data were expressed as Mean ± S.E.M. (standard error of mean) after normality check. All data were analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc test for multiple comparisons where appropriate. Data were processed by Graph Pad Prism software version 5 (GraphPad Software, Inc. La Jolla, CA 92037 USA). A level of p < 0.05 was considered as statistically significant for all tests.
3. Results
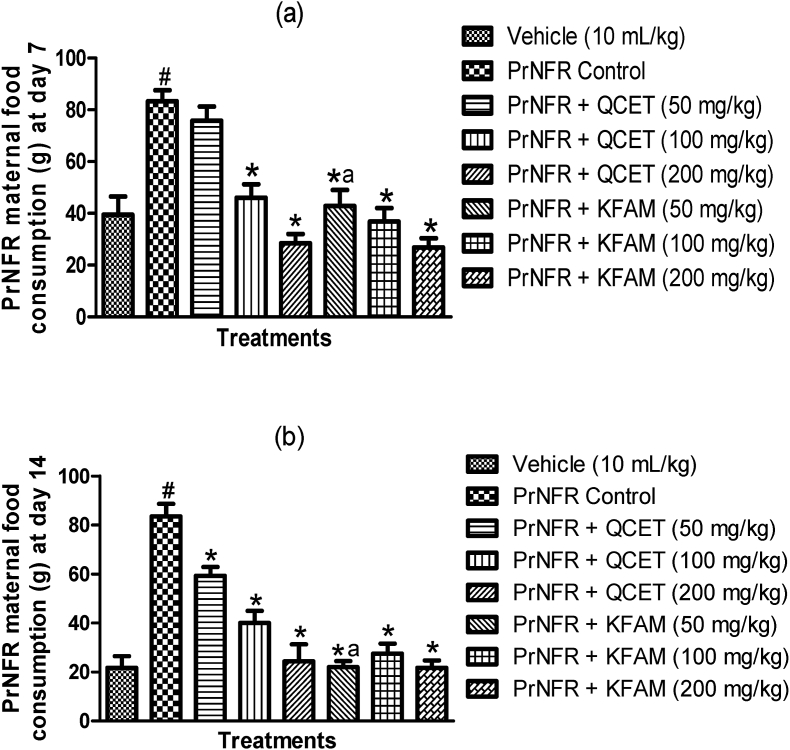
3.1. Effects of quercetin and kaempferol on maternal food consumption in rats exposed to prenatal food restriction
The effects of quercetin and kaempferol on maternal food consumption in rats exposed to prenatal food restriction at prenatal days 7 and 14 are shown in Fig. 2a and b. One-way ANOVA revealed significant differences in maternal food consumption in the various treatment groups at prenatal days 7 [F (7,40) = 16.26, p < 0.0001] (Fig. 2a) and 14 [F (7,40) = 24.88, p < 0.0001] (Fig. 2b). Accordingly, PrNFR caused a significant (p < 0.001) increase in maternal food consumption relative to vehicle control respectively at prenatal days 7 and 14. Quercetin (100 and 200 mg/kg, p.o.) and kaempferol (50, 100 and 200 mg/kg, p.o.) significantly (p < 0.001) decreased maternal food consumption at day 7 in a dose-dependent manner when compared with PrNFR control group (Fig. 2a). Similarly, quercetin (50, 100 and 200 mg/kg, p.o.) and kaempferol (50, 100 and 200 mg/kg, p.o.) significantly (p < 0.001) decreased maternal food consumption at prenatal day 14 when compared with PrNFR control group (Fig. 2b). Collectively, there were no significant difference between the effects of 100 and 200 mg/kg of quercetin and kaempferol treatment groups at prenatal days 7 and 14 respectively. However, kaempferol (50 mg/kg, p.o.) significantly decreased maternal food consumption at prenatal days 7 and 14 when compared with quercetin (50 mg/kg, p.o.) groups.
Fig. 2.
Effect of quercetin and kaempferol on prenatal food restriction-induced alteration in maternal food consumption at prenatal days 7 (a) and 14 (b). Bars represent the mean ± SEM of 6 animals per group. #P < 0.05 compared to vehicle group; ∗P < 0.05 compared to PrNFR control group; aP < 0.05 compared to Quercetin (50 mg/kg) group (One-way ANOVA followed by Bonferroni's post-hoc test). PrNFR = Prenatal food restriction, QCET = Quercetin, KFAM = Kaempferol.
3.2. Both quercetin and kaempferol increase maternal weights of rats exposed to prenatal food restrictions
One-way ANOVA revealed that PrNFR significantly reduced maternal weight (g) of rats at day 7 (p < 0.01) [F (7,40) = 5.587, p = 0.0002] (Fig. 3a) and day 14 (p < 0.001) [F (7,40) = 12.29, p < 0.0001] (Fig. 3b) when compared with control groups. Conversely, treatments with quercetin (200 mg/kg, p.o.) and kaempferol (50 and 200 mg/kg, p.o.) significantly (p < 0.05) increased maternal weight at prenatal days 7 and 14 when compared with PrNFR control groups respectively (Fig. 3a). Also, quercetin (50, 100 and 200 mg/kg, p.o.) and kaempferol (50, 100 and 200 mg/kg, p.o.) significantly (p < 0.05) increased maternal weight at day 14 relative to PrNFR-exposed group (Fig. 3b). There were no marked significant differences between the various dose levels of 50, 100 and 200 mg/kg of quercetin when compared with 50, 100 and 200 mg/kg of kaempferol treatments groups at prenatal days 7 and 14 respectively (Fig. 3a and b).
Fig. 3.
Effects of quercetin and kaempferol on prenatal food restriction-induced alteration in maternal weights (g) of rats at postnatal day 1 (a) and postnatal day 22 (b). Bars represent the mean ± SEM of 6 animals per group. #P < 0.05 compared to vehicle group; ∗P < 0.05 compared to PrNFR control group (One-way ANOVA followed by Bonferroni’s post-hoc test). PrNFR = Prenatal food restriction, QCET = Quercetin, KFAM = Kaempferol.
3.3. Effects of quercetin and kaempferol on the weights of pups of rats exposed to prenatal food restriction
The effects of quercetin and kaempferol on the weights of pups of rats exposed to prenatal food restriction are shown in Table 1. One-way ANOVA showed that PrNFR significantly (p < 0.05) decreased the weights of pups of rats at PND 1 [F (7,40) = 5.503, p = 0.0002], PND 14 [F (7,40) = 3.925, p = 0.0024] and PND 22 [F (7,40) = 3.896, p = 0.0024] when compared with vehicle control groups respectively. But no significant effect was observed in weight at puberty [F (7,40) = 1.454, p = 0.2115] when compared with vehicle control group. Quercetin (50, 100 and 200 mg/kg, p.o.) and kaempferol (50, 100 and 200 mg/kg, p.o.) significantly increased the weights of pups at PNDs 1 and 14 in comparison with PrNFR control groups (Table 1). Furthermore, quercetin (200 mg/kg, p.o.) and kaempferol (100 and 200 mg/kg, p.o.) also significantly increased the weights of pups at PND 22 when compared with PrNFR control group. Notably, no changes were produced by all doses of quercetin and kaempferol at pubertal period relative to PrNFR groups. Comparatively, no statistically significant effects were observed in the weights of pups exposed to PrNFR between the various dose levels of 50, 100 and 200 mg/kg of quercetin when compared with 50, 100 and 200 mg/kg of kaempferol treatments groups at PND 1, 14, 22 and at puberty respectively (Table 1).
Table 1.
Effects of quercetin and kaempferol on the weights of pups of rats exposed to prenatal food restriction at postnatal days (PNDs) 1, 14, 22 and at puberty.
| Treatment | Weight (g) at PND 1 | Weight (g) at PND 14 | Weight (g) at PND 22 | Pubertal weight (g) |
|---|---|---|---|---|
| Vehicle (10 mL/kg) | 5.60 ± 0.42 | 31.78 ± 2.41 | 37.73 ± 1.23 | 89.27 ± 1.54 |
| PrNFR Control | 3.30 ± 0.22# | 19.92 ± 1.50# | 28.35 ± 1.76# | 87.30 ± 1.94 |
| PrNFR + QCET (50 mg/kg) | 4.08 ± 0.23 | 28.60 ± 2.37 | 35.92 ± 2.12 | 90.53 ± 1.14 |
| PrNFR + QCET (100 mg/kg) | 5.08 ± 0.39∗ | 29.84 ± 1.65∗ | 36.58 ± 0.88 | 96.47 ± 4.14 |
| PrNFR + QCET (200 mg/kg) | 5.18 ± 0.32∗ | 30.98 ± 1.89∗ | 39.13 ± 2.91∗ | 97.45 ± 4.90 |
| PrNFR + KFAM (50 mg/kg) | 5.17 ± 0.33∗ | 33.20 ± 2.80∗ | 35.87 ± 1.11 | 89.02 ± 1.99 |
| PrNFR + KFAM (100 mg/kg) | 4.82 ± 0.31∗ | 30.25 ± 1.03∗ | 39.32 ± 1.89∗ | 90.55 ± 3.19 |
| PrNFR + KFAM (200 mg/kg) | 5.18 ± 0.27∗ | 30.23 ± 2.14∗ | 38.13 ± 1.45∗ | 92.91 ± 3.09 |
Values represent the mean ± SEM (n = 6). #P < 0.05 compared to vehicle group; ∗P < 0.05 compared to PrNFR control group (One-way ANOVA followed by Bonferroni's post-hoc test). PrNFR = Prenatal food restriction, QCET = Quercetin, KFAM = Kaempferol.
3.4. Effects of quercetin and kaempferol on the weights of pups of rats exposed to postnatal food restriction
Table 2 shows the effects of quercetin and kaempferol on the weights of pups of rats exposed to postnatal food restriction. Analysis with one-way ANOVA showed that there were significant differences between various treatment groups in the weights of pups at PND 14 [F (7,40) = 4.600, p = 0.0008], PND 22 [F (7,40) = 6.800, p < 0.0001] and at puberty [F (7,40) = 4.573, p = 0.0008], but not at PND 1 [F (7,40) = 0.3115, p = 0.9445]. Chronic postnatal food restriction (PsNFR) significantly (p < 0.05) decreased the weights of pups of rats at PNDs 14, 22 and at puberty but not at PND 1, when compared with vehicle control groups respectively. At PND 1, no significant effects were observed following treatments with quercetin (50–200 mg/kg, p.o.) and kaempferol (50–200 mg/kg, p.o.) when compared with PsNFR control group. Meanwhile, high doses of quercetin (200 mg/kg, p.o.) and kaempferol (200 mg/kg, p.o.) significantly (p < 0.001) increased the weights of pups of rats when compared with PsNFR control groups at PND 14 (Table 2). Moreover, at PND 22 and at puberty, quercetin (100 and 200 mg/kg, p.o.) and kaempferol (100 and 200 mg/kg, p.o.) significantly (p < 0.001) raised the weights of pups of rats subjected to PsNFR relative to PsNFR control groups respectively. There were no significant differences in the weights of pups exposed to PsNFR between the various dose levels of quercetin (50, 100 and 200 mg/kg, p.o.) when compared with kaempferol (50, 100 and 200 mg/kg, p.o.) treatments groups at PND 1, 14, 22 and at puberty respectively (Table 2).
Table 2.
Effects of quercetin and kaempferol on the weights of pups of rats exposed to postnatal food restriction at postnatal days (PNDs) 1, 14, 22 and at puberty.
| Treatment | Weight (g) at PND 1 | Weight (g) at PND 14 | Weight (g) at PND 22 | Pubertal weight (g) |
|---|---|---|---|---|
| Vehicle (10 mL/kg) | 4.85 ± 0.25 | 31.83 ± 2.20 | 37.17 ± 1.07 | 98.48 ± 2.50 |
| PsNFR Control | 4.78 ± 0.13 | 15.86 ± 1.41# | 19.13 ± 0.55# | 64.98 ± 4.33# |
| PsNFR + QCET (50 mg/kg) | 4.78 ± 0.22 | 23.43 ± 2.68 | 26.29 ± 2.47 | 84.87 ± 2.37 |
| PsNFR + QCET (100 mg/kg) | 4.88 ± 0.13 | 24.52 ± 2.55 | 32.77 ± 3.10∗ | 99.58 ± 7.19∗ |
| PsNFR + QCET (200 mg/kg) | 4.75 ± 0.20 | 27.98 ± 2.07∗ | 32.10 ± 2.86∗ | 98.97 ± 5.43∗ |
| PsNFR + KFAM (50 mg/kg) | 5.02 ± 0.08 | 23.15 ± 1.74 | 23.52 ± 0.73 | 83.53 ± 4.12 |
| PsNFR + KFAM (100 mg/kg) | 4.68 ± 0.25 | 20.18 ± 2.35 | 34.21 ± 2.55∗ | 87.01 ± 8.02 |
| PsNFR + KFAM (200 mg/kg) | 4.87 ± 0.10 | 27.77 ± 3.10 ∗ | 34.83 ± 3.79∗ | 94.62 ± 5.06∗ |
Values represent the mean ± SEM of 6 animals per group. #P < 0.05 compared to vehicle group; ∗P < 0.05 compared to PsNFR control group (One-way ANOVA followed by Bonferroni's post-hoc test). PsNFR = Postnatal food restriction, QCET = Quercetin, KFAM = Kaempferol.
3.5. Effects of quercetin and kaempferol on the weights of pups of rats exposed to both prenatal and postnatal food restrictions
Analysis with one-way ANOVA markedly showed that there were significant differences between various treatment groups in the weights of pups at PND 1 [F (7,40) = 12.63, p < 0.0001], PND 14 [F (7,40) = 10.84, p < 0.0001], PND 22 [F (7,40) = 11.45, p < 0.0001] and at puberty [F (7,40) = 15.62, p < 0.0001]. PrNFR-PsNFR significantly (p < 0.001) reduced the weights of pups of rats at PNDs 1, 14, 22 and at puberty, when compared with vehicle control groups respectively. However, at PND 1, Bonferroni post-hoc test showed that quercetin (50, 100 and 200 mg/kg, p.o.) and kaempferol (50, 100 and 200 mg/kg, p.o.) significantly increased the weights of pups of rats exposed to both PrNFR-PsNFR (Table 3). Also, quercetin (200 mg/kg, p.o.) and kaempferol (100 and 200 mg/kg, p.o.) significantly (p < 0.001) increased the weights of pups at PNDs 14 and 22 when compared with PrNFR-PsNFR control group (Table 3). Additionally, Bonferroni post-hoc test showed only the highest doses of quercetin (200 mg/kg, p.o.) and kaempferol (200 mg/kg, p.o.) significantly (p < 0.001) elevated the pubertal weights of pups of rats submitted to both PrNFR-PsNFR when compared with PrNFR-PsNFR control group. Furthermore, there were no significant differences in the weights of pups exposed to both PrNFR-PsNFR between the various dose levels of 50, 100 and 200 mg/kg of quercetin when compared with kaempferol (50, 100 and 200 mg/kg, p.o.) treatments groups at PND 1, 14, 22 and at puberty respectively (Table 3).
Table 3.
Effects of quercetin and kaempferol on the weights of pups of rats exposed to both prenatal and postnatal food restrictions at postnatal days (PNDs) 1, 14, 22 and at puberty.
| Treatment | Weight (g) at PND 1 | Weight (g) at PND 14 | Weight (g) at PND 22 | Pubertal weight (g) |
|---|---|---|---|---|
| Vehicle (10 mL/kg) | 4.92 ± 0.11 | 29.89 ± 1.53 | 37.37 ± 2.85 | 91.12 ± 0.83 |
| PrNFR + PsNFR Control | 3.18 ± 0.20# | 12.01 ± 1.52# | 17.30 ± 1.19# | 55.25 ± 4.45# |
| PrNFR + PsNFR + QCET (50 mg/kg) | 4.85 ± 0.11∗ | 10.92 ± 0.95 | 20.31 ± 1.27 | 59.24 ± 2.71 |
| PrNFR + PsNFR + QCET (100 mg/kg) | 4.95 ± 0.07∗ | 19.64 ± 1.49 | 24.15 ± 2.20 | 64.38 ± 2.04 |
| PrNFR + PsNFR + QCET (200 mg/kg) | 4.63 ± 0.18∗ | 25.28 ± 2.77∗ | 29.58 ± 3.04∗ | 84.16 ± 7.04∗ |
| PrNFR + PsNFR + KFAM (50 mg/kg) | 4.67 ± 0.23∗ | 15.95 ± 3.04 | 19.88 ± 0.48 | 59.68 ± 0.94 |
| PrNFR + PsNFR + KFAM (100 mg/kg) | 4.70 ± 0.23∗ | 24.80 ± 2.04∗ | 34.52 ± 2.10∗ | 73.66 ± 6.55 |
| PrNFR + PsNFR + KFAM (200 mg/kg) | 4.93 ± 0.10∗ | 26.10 ± 2.77∗ | 33.89 ± 3.35∗ | 102.0 ± 4.96∗ |
Values represent the mean ± SEM (n = 6). #P < 0.05 compared to vehicle group; ∗P < 0.05 compared to PrNFR-PsNFR control group (One-way ANOVA followed by Bonferroni's post-hoc test). PrNFR = Prenatal food restriction, PsNFR = Postnatal food restriction, QCET = Quercetin, KFAM = Kaempferol.
3.6. Quercetin and kaempferol attenuate prenatal food restriction-induced alterations on leptin and ghrelin concentrations in rats
Bonferroni post-hoc analysis showed that PrNFR significantly (p < 0.05) decreased the levels of leptin [F (7,40) = 8.913, p < 0.0001] (Fig. 4a) but not ghrelin [F (7,40) = 8.518, p < 0.0001] (Fig. 4b) when compared with vehicle control groups. Quercetin (200 mg/kg, p.o.) and kaempferol (100 and 200 mg/kg, p.o.) induced a significant (p < 0.05) increase in leptin levels of rats when compared with PrNFR control group (Fig. 4a). Furthermore, quercetin (50–200 mg/kg, p.o.) and kaempferol (50 and 100 mg/kg, p.o.) did not produce any significant (p > 0.05) changes in the ghrelin levels when compared with PrNFR control, although high dose of kaempferol (200 mg/kg, p.o.) significantly (p < 0.05) decreased the levels of ghrelin when compared with PrNFR control (Fig. 4b). By comparison, there were no significant differences in leptin levels between 50, 100 and 200 mg/kg of quercetin in comparison with 50, 100 and 200 mg/kg of kaempferol treatment groups (Fig. 4a). Although there were no significant differences in leptin concentrations between 50 and 100 mg/kg dose levels of quercetin and kaempferol, 200 mg/kg of kaempferol significantly (p < 0.05) reduced leptin levels when compared with quercetin (200 mg/kg) (Fig. 4b).
Fig. 4.
Effects of quercetin and kaempferol on leptin (a) and ghrelin (b) concentrations of rats exposed to prenatal food restriction. Bars represent the mean ± SEM of 6 animals per group. #P < 0.05 compared to vehicle group; ∗P < 0.05 compared to PrNFR control group; cP < 0.05 compared to Quercetin (200 mg/kg) group (One-way ANOVA followed by Bonferroni's post-hoc test). PrNFR = Prenatal food restriction, QCET = Quercetin, KFAM = Kaempferol.
3.7. Quercetin and kaempferol reverse the effects of postnatal food restriction on leptin and ghrelin concentrations of rats
The effects of quercetin and kaempferol on leptin and ghrelin concentrations of rats exposed to postnatal food restriction are shown Fig. 5a and b. PsNFR significantly (p < 0.05) decreased the levels of leptin concentration [F (7,40) = 3.786, p = 0.0031] (Fig. 5a) and increased ghrelin [F (7,40) = 3.757, p = 0.0032] (Fig. 5b) levels when compared with the PsNFR control group. Administration of quercetin (200 mg/kg, p.o.) and kaempferol (100 and 200 mg/kg, p.o.) induced a significant (p < 0.05) increase in the concentration of leptin of rats when compared with PsNFR control group (Fig. 5a). Similarly, quercetin (200 mg/kg, p.o.) and kaempferol (50, 100 and 200 mg/kg, p.o.) produced a significant (p < 0.05) decrease in the concentration ghrelin of rats when compared with PsNFR control group (Fig. 5b). Interestingly, treatment with lower doses of quercetin (50 and 100 mg/kg, p.o.) did not prevent the effect of PsNFR on ghrelin concentration when compared with PsNFR group (Fig. 5b). There were no significant differences in leptin and ghrelin concentrations between the dose levels of 50, 100 and 200 mg/kg of quercetin in comparison with 50, 100 and 200 mg/kg of kaempferol respectively (Fig. 5a and b).
Fig. 5.
Effects of quercetin and kaempferol on leptin (a) and ghrelin (b) concentrations of rats exposed to postnatal food restriction. Bars represent the mean ± SEM of 6 animals per group. #P < 0.05 compared to vehicle group; ∗P < 0.05 compared to PsNFR control group (One-way ANOVA followed by Bonferroni's post-hoc test). PsNFR = Postnatal food restriction, QCET = Quercetin, KFAM = Kaempferol.
3.8. Quercetin and kaempferol mitigate the effects of both prenatal and postnatal food restrictions on leptin and ghrelin concentrations of rats
Fig. 6a and b show the effects of quercetin and kaempferol on both prenatal and postnatal food restrictions-induced changes in leptin and ghrelin concentrations of rats. One-way ANOVA showed that the combination of PrNFR and PsNFR protocols significantly (p < 0.001) decreased the levels of leptin [F (7,40) = 6.854, p < 0.0001] (Fig. 6a) and increased ghrelin concentrations [F (7,40) = 4.373, p = 0.0011] (Fig. 6b) when compared with PrNFR-PsNFR control groups. But, Bonferroni post-hoc test indicated that treatment with the highest doses of both quercetin (200 mg/kg, p.o.) and kaempferol (200 mg/kg, p.o.) significantly (p < 0.05) increased the concentration of leptin of rats unlike the lower doses (50 and 100 mg/kg) when compared with PrNFR-PsNFR control groups (Fig. 6a). Also, quercetin (100 and 200 mg/kg, p.o.) and kaempferol (50, 100 and 200 mg/kg, p.o.) decreased the concentration of ghrelin of rats when compared with PrNFR-PsNFR control groups. Interestingly, there were no significant differences in leptin and ghrelin concentration between the dose levels of 50, 100 and 200 mg/kg of quercetin in comparison with 50, 100 and 200 mg/kg of kaempferol respectively (Fig. 6a and b).
Fig. 6.
Quercetin and kaempferol mitigate the effects of both prenatal and postnatal food restrictions on leptin (a) and ghrelin (b) levels in rats. Bars represent the mean ± SEM (n = 6). #P < 0.05 compared to vehicle group; ∗P < 0.05 compared to PrNFR-PsNFR control group (One-way ANOVA followed by Bonferroni's post-hoc test). PrNFR = Prenatal food restriction, PsNFR = Postnatal food restriction, QCET = Quercetin, KFAM = Kaempferol.
3.9. Effects of quercetin and kaempferol on prenatal food restriction-induced changes on brain oxidative stress of rats
Bonferroni post-hoc analysis showed that PrNFR significantly decreased the brain levels of GSH (p < 0.05) [F (7,40) = 4.325, p = 0.0012] (Fig. 7a) and increased MDA (p > 0.05) [F (7,40) = 5.972, p < 0.0001], although not significantly (Fig. 7b) when compared with vehicle control groups, suggesting increased oxidative stress. Quercetin (100 mg/kg, p.o.) and kaempferol (200 mg/kg, p.o.) significantly (p < 0.05) increased brain GSH concentrations of rats when compared with PrNFR control group (Fig. 7a). Also, quercetin (100 mg/kg, p.o.) and kaempferol (100 and 200 mg/kg, p.o.) significantly (p < 0.05) decreased the levels of MDA when compared with PrNFR control (Fig. 7b). By comparison, there were no significant differences in GSH and MDA concentrations between 50, 100 and 200 mg/kg of quercetin in comparison with 50, 100 and 200 mg/kg of kaempferol treatment groups, respectively (Fig. 7a and b).
Fig. 7.
Effects of quercetin and kaempferol on prenatal food restriction-induced alterations on brain oxidative stress: (a) glutathione, GSH and (b) malondialdehyde, MDA. Bars represent the mean ± SEM (n = 6). #P < 0.05 compared to vehicle group; ∗P < 0.05 compared to PrNFR control group (One-way ANOVA followed by Bonferroni's post-hoc test). PrNFR = Prenatal food restriction, QCET = Quercetin, KFAM = Kaempferol.
3.10. Quercetin and kaempferol attenuate postnatal food restriction-induced brain oxidative stress of rats
The effects of quercetin and kaempferol on brain GSH and MDA concentrations of rats exposed to postnatal food restriction are shown Fig. 8a and b. PsNFR significantly (p < 0.05) decreased brain levels of GSH concentration [F (7,40) = 4.706, p = 0.0006] (Fig. 8a) and increased MDA [F (7,40) = 9.692, p = p < 0.0001] (Fig. 8b) levels when compared with the PsNFR control group. No effect was observed with 50 mg/kg of quercetin in comparison with PsNFR control (Fig. 8a). Administration of quercetin (100 and 200 mg/kg, p.o.) and kaempferol (50, 100 and 200 mg/kg, p.o.) produced a significant (p < 0.05) increases in brain levels of GSH when compared with PsNFR control group (Fig. 8a). Similarly, quercetin (50, 100 and 200 mg/kg, p.o.) and kaempferol (50, 100 and 200 mg/kg, p.o.) elicited significant (p < 0.05) decreases in brain levels of MDA of rats when compared with PsNFR control group (Fig. 8b). There are no significant differences in GSH and MDA concentrations between the dose levels of 50, 100 and 200 mg/kg of quercetin in comparison with 50, 100 and 200 mg/kg of kaempferol respectively (Fig. 8a and b).
Fig. 8.
Quercetin and kaempferol attenuate postnatal food restriction-induced brain oxidative stress: (a) glutathione, GSH and (b) malondialdehyde, MDA. Bars represent the mean ± SEM (n = 6). #P < 0.05 compared to vehicle group; ∗P < 0.05 compared to PrNFR control group (One-way ANOVA followed by Bonferroni's post-hoc test). PsNFR = Postnatal food restriction, QCET = Quercetin, KFAM = Kaempferol.
3.11. Quercetin and kaempferol attenuate prenatal and postnatal food restriction-induced alteration on brain oxidative stress of rats
The effects of quercetin and kaempferol on both prenatal and postnatal food restrictions-induced brain oxidative stress is shown Fig. 9a and b. One-way ANOVA showed that the combination of PrNFR and PsNFR stress significantly (p < 0.001) decreased both brain levels of GSH [F (7,40) = 6.845, p < 0.0001] (Fig. 9a) and increased MDA concentrations [F (7,40) = 6.700, p = 0.0011] (Fig. 9b) when compared with PrNFR-PsNFR control groups. Bonferroni's post-hoc test showed that treatment with both quercetin (100 and 200 mg/kg, p.o.) and kaempferol (50, 100 and 200 mg/kg, p.o.) significantly (p < 0.05) increased the brain concentration of GSH of rats when compared with PrNFR-PsNFR control group (Fig. 9a). However, quercetin (50 mg/kg, p.o.) did not produce any significant effect. Furthermore, quercetin (100 and 200 mg/kg, p.o.) and kaempferol (100 and 200 mg/kg, p.o.) decreased the brain concentrations of MDA when compared with PrNFR-PsNFR control group (Fig. 9b). However, there were no significant differences in GSH and MDA levels between the various dose levels (50, 100 and 200 mg/kg) of quercetin and kaempferol respectively (Fig. 9a and b).
Fig. 9.
Quercetin and kaempferol reduce the combined effects of prenatal and postnatal food restrictions on brain glutathione (GSH) and malondialdehyde (MDA) concentrations of rats. Bars represent the mean ± SEM (n = 6). #P < 0.05 compared to vehicle group; ∗P < 0.05 compared to PrNFR control group (One-way ANOVA followed by Bonferroni's post-hoc test). PrNFR = Prenatal food restriction, PsNFR = Postnatal food restriction, QCET = Quercetin, KFAM = Kaempferol.
4. Discussion
In this study, we focused on evaluating the protective effects of quercetin and kaempferol on prenatal and early postnatal food deprivation-induced alterations in maternal and fetal weights, changes in orexigenic and anorexigenic hormones, as well as, brain oxidative status. Our data showed that the decreased body weights of pups at PNDs 1, 14, 22 and pubertal period due to prenatal food restriction (PrNFR)-, postnatal food restriction (PsNFR)- or the combination of PrNFR and PsNFR were significantly reversed by both quercetin and kaempferol in a dose-dependent manner. Also, both quercetin and kaempferol offered protection against prenatal food restriction-induced decrease in maternal weights, suggesting maternal protection. Importantly, we also showed that quercetin and kaempferol significantly prevented the suppression of leptin levels in rats submitted to PrNFR, PsNFR or both PrNFR-PsNFR stresses. Additionally, quercetin and kaempferol also reversed the negative effects of prenatal food restriction, postnatal food restriction or both PrNFR-PsNFR on ghrelin concentration of rats. Interestingly, quercetin and kaempferol decreased maternal food consumption at prenatal days 7 and 14, as evidenced by the remainder of food served. Furthermore, our study showed that quercetin and kaempferol mitigated PrNFR-, PsNFR- or both (PrNFR-PsNFR)-induced oxidative stress in the brains of rats, as evidenced by increased glutathione concentrations and decreased malondialdehyde levels.
Numerous investigations have shown that low birth weight is one of the prominent features that is known to be associated with prenatal food deprivation (Engelbregt et al., 2001). It has been hypothesized that weight plays an important role in the central regulation of puberty and it has therefore been suggested that in females, a critical weight is needed for menarche to occur (Engelbregt et al., 2000; Engelbregt et al., 2001). Consequently, previous studies have demonstrated that underfeeding during pregnancy resulted in growth retardation and a delayed onset of puberty (Engelbregt et al., 2000). Although earlier studies have proposed that more than weight, an appropriate body composition or a certain percentage of fat is needed to start puberty in rats (Bouret et al., 2004; Frisch & Revelle, 1971). Therefore, it is plausible to speculate that a low percentage of fat or low birth weight might delay the onset of certain developmental features including puberty and neurocognitive functions (Bouret et al., 2004; Frisch & Revelle, 1971). The relationship between metabolic pathway with regards body fat stores and weight and the neuroendocrine reproductive system was unclear until the discovery of leptin, which is a critical component of the metabolic gate-way to puberty (Engelbregt et al., 2001; Nazian & Cameron, 1999). Indeed, several studies have reported positive correlations between human and animal leptin levels, feeding behavior, body mass and pubertal onset (Engelbregt et al., 2001; Bouret et al., 2004; Quennell et al., 2011). A widely known theory suggests that continued reproductive function depends on maintaining a particular body fat composition or fat-to-lean ratio (Soliman et al., 2014; Engelbregt et al., 2000). This hypothesis is very much parallel to a set-point model postulated to explain the regulation of body weight. According to the set-point paradigm, rodents will defend a particular body weight by adjusting behavioral and physiological needs (Soliman et al., 2014; Frisch & Revelle, 1971). Of note, the level of consummatory behavior and metabolic processes are believed to be determined by a mechanism that compares the current level of body weight to a particular set point (Quennell et al., 2011; Sloboda et al., 2009). Thus, survival during extreme energetic challenges such as pre- and postnatal food deprivations require that mobilization and oxidation of free fatty acids from adipose tissue takes precedence over defense of a particular body fat content and weight (Sloboda et al., 2009).
In this study, PrNFR was found to cause markedly decreased body weight at PNDs 1, 14 and 22, except at pubertal period, which is in agreement with previous investigations (Imbesi & Castrogiovanni, 2008). The shift in body weight at puberty may represent a life course strategy intended to preserve fitness by reducing the risk of death before reproducing, or by allowing a greater number of successful reproductive episodes before death (Sloboda et al., 2009). Intriguingly, Engelbregt et al. (Engelbregt et al., 2000) showed that there was no significant difference in the BMI and body fat composition in rats exposed to intra-uterine growth retardation at puberty. Thus, it is possible to postulate that the well-reported phenomenon of metabolic programming as a result of poor prenatal nutrition might be a secondary outcome of a life history strategy to accelerate maturation. On the other hand, we showed that postnatal food deprivation decreased body weight at puberty as well as at PNDs 14 and 22, although no significant effect was observed at PND 1 as expected. Again, this observation led to the suggestion that the effect of food deprivation on body weight depends on the phase of development and growth. Accordingly, the combinational effects of both protocols, PrNFR-PsNFR was found to produce a more profound decrease in body weights. Interestingly, the repeated treatments with quercetin and kaempferol significantly reversed the adverse effects of prenatal, postnatal food restrictions or the combined effects on body weights. Fetal nutrition is determined by size and composition of the mother's body and by her diet. The nutritional need of the fetus depends on the intake of nutrients of the mother, and their distribution through maternal circulation and the placental transport mechanisms (Castrogiovanni & Imbesi, 2017). Indeed, malnourished mothers may be limited in their ability to adequately support the fetus. Clinically, there is mounting evidence showing that maternal nutritional status programs the risk of disease in the progeny as a result of low maternal weight, high levels of maternal oxidative stress and rapid cell aging (Curhan et al., 1996). In the present study, PrNFR induced a significant decrease in maternal weight at prenatal days 7 and 14 respectively. However, several studies have suggested that maternal antioxidant therapy reverses some of the deleterious effects suffered during prenatal food deprivation (Castrogiovanni & Imbesi, 2017). Thus, the ability of quercetin and kaempferol to increased maternal weight of rats exposed to food deprivation, suggests the beneficial effect of maternal antioxidant supplementation during food deprivation during pregnancy (Castrogiovanni & Imbesi, 2017).
Several studies have strongly implicated the role of leptin as a permissive regulatory factor for the onset of puberty and feeding behavior (Engelbregt et al., 2001; Castrogiovanni & Imbesi, 2017). Leptin, which is an anorexigenic peptide hormone synthesized by white adipose tissues, synchronizes growth and fertility based on food availability (Sloboda et al., 2009), via positive modulation of gonadotropin-releasing hormone including the kisspeptin (Iwasa et al., 2015). Overall, a reduction in fat volume or body weight triggers the fall in peripheral leptin concentration with corresponding increase in food intake (Engelbregt et al., 2001). On the other hand, an increase in fat volume or body weight causes an upsurge in peripheral leptin levels and reduced food intake (Quennell et al., 2011; Iwasa et al., 2015). Leptin is generally known as one of the upstream ligands for malonyl-CoA signaling pathway in the hypothalamus, and the concentration of hypothalamic malonyl CoA regulates the expressions of neuropeptides and food intake (Sucajtys-Szulc et al., 2010). Recently, it was reported that prolong fasting resulting from prenatal and postnatal food deprivations alters hypothalamic leptin levels and malonyl-CoA expression (Engelbregt et al., 2001; Iwasa et al., 2015). Also, studies have reported that prenatal under-nourishment causes drastic reduction of postnatal hypothalamic leptin surge, decrease malonyl-CoA expression (Delahaye et al., 2008) and retard pubertal programming and brain developments (Sucajtys-Szulc et al., 2010; Delahaye et al., 2008). Remarkably, previous investigation has shown that intravenous injection of leptin increased hypothalamic STAT3 phosphorylation and attenuated activation of AMPK and acetyl-CoA carboxylase, with associated increase in malonyl-CoA level and a decrease in feeding behavior (Sucajtys-Szulc et al., 2010). Accordingly, leptin, similarly to insulin, is destined to stop eating behavior and food intake once energy stores have been filled throught: activation of “satiety centre” including proopiomelanocortin/cocaine and amphetamine-regulated transcription neurons (POMC/CART) pathways and deactivation of the “hunger centre” in the lateral hypothalamic area, arcuate and paraventricular nuclei (Rojas et al., 2011; Sucajtys-Szulc et al., 2010). Thus, leptin-deficient rodents have been reported to demonstrate significantly reduction in weight, poor brain development as well as delayed pubertal onset and repression of fertility in adulthood through down-regulation of kisspeptin and antioxidant pathways (Quennell et al., 2011; Schiavone et al., 2013; Swolin-Eide et al., 2002).
Herein, we observed that prenatally and postnatally food deprived rats displayed lowered leptin levels, which is in agreement with previous findings (Engelbregt et al., 2001; Quennell et al., 2011; Iwasa et al., 2015). Moreover, the combinational effects of the two-hit factor of prenatal and postnatal food restriction caused a more significant drop in the leptin concentration. This finding further supports previous investigations, which showed that the combined effect of intrauterine growth retardation and postnatal food deprivation causes leptin depletion in rodents (Engelbregt et al., 2001). Remarkably our study showed that quercetin and kaempferol at different doses significantly increased serum leptin concentrations of rats exposed to prenatal, postnatal and/or prenatal-postnatal food restrictions. Studies have shown that supplementation with flavonoids enhance food consumption and inhibit anorexia via antioxidant activity (Schiavone et al., 2013; Swolin-Eide et al., 2002). This finding further suggests that the protective effects of quercetin and kaempferol during fasting may be related, in part, to leptin-mediated suppression of proteins that to play negative role in the pathology and progression of anorexic disorders. Therefore, this result further validates the earlier findings which showed that quercetin and kaempferol attenuate the negative effects of prenatal and postnatal food restrictions on gestational outcomes and the onset of puberty as reported elsewhere in our previous study (Anachuna et al., 2020). Correspondingly, prenatal food restriction was also found to induce a significant increase in prenatal maternal food consumption especially at days 7 and 14. This finding further supports other findings which showed that prenatal food deprivation causes increased maternal food consumption, decreased peripheral leptin concentration and body weight or fat volume (Engelbregt et al., 2000; Engelbregt et al., 2001; Nazian & Cameron, 1999). Thus, it is noteworthy to remark that the finding that quercetin and kaempferol decreased maternal food consumption during prenatal food deprivation, further confirms their beneficial effects in conditions associated with prenatal food scarcity in rodents.
In addition, ghrelin, a 28-amino-acid peptide, is often called hunger hormone because of its orexigenic food-intake controlling ability through increase in gastric motility and acid secretion (Gualillo et al., 2012; Rojas et al., 2011; Inui, 2001). Evidence has shown that blood levels of ghrelin increases during intense hunger before meal, but decreases to baseline after a meal (Gualillo et al., 2012; Inui, 2001). However, ghrelin levels have been reported to be inversely proportion to BMI, and also studies have shown that there is a negative correlation between insulin secretion and ghrelin levels (Luo et al., 2015; Inui, 2001). Thus, ghrelin has emerged as an endocrine link between the stomach, hypothalamus and pituitary in maintaining adequate energy homeostasis and body weight (Gualillo et al., 2012; Luo et al., 2015). Indeed, exogenous ghrelin injection has been shown to induce positive energy equilibrium in rodents by reducing fat utilization without marked alteration in energy disbursement (Tschop et al., 2000; Gil-Campos et al., 2006). Also, ghrelin is known to enhance AMPK activity in the hypothalamus, reduce malonyl-CoA reactivity, stimulate palmitoyl transferase-1, induce mitochondria fatty acid metabolism, release neuropeptide-Y thereby leading to the activation of hunger centres (Rojas et al., 2011; Gil-Campos et al., 2006). Alterations of these pathways especially ghrelin, has been hypothesized as one of the basic patho-mechansims for several metabolic diseases involving deranged feeding behavior (Cordido et al., 2009). However, increasing body of evidence gleaned over the past years have shown that the levels of orexigenic and anorexigenic signals are mostly based on the nutritional status of the body (Gualillo et al., 2012; Cordido et al., 2009). Accordingly, although different preclinical and clinical studies have shown that no changes were observed in ghrelin levels in pregnant rats with free access to food and water (Gualillo et al., 2012), several reports have shown that chronic prenatal and postnatal food deprivations profoundly increased circulating levels of ghrelin and gastric ghrelin mRNA expression (Gualillo et al., 2012) via pleiotropic metabolic mechanisms including elevated levels of placental free radical generations due to hypermetabolism of the mitochondria (Luo et al., 2015).
Remarkably, oxidative stress again has been associated with several perinatal adverse conditions including diabetes, preeclampsia, preterm birth, low birth weight that are predictive of heightened risk of the metabolic syndromes and neurodevelopmental diseases in postnatal life (Schiavone et al., 2013; Agarwal et al., 2005). Hence, oxidative stress has long been established as a common pathway involved in the metabolic gate-way and brain development (Sucajtys-Szulc et al., 2010; Agarwal et al., 2005). Nevertheless, the result of this study further confirmed earlier reports that showed that chronic maternal nutritional restriction during pregnancy and early postnatal food deprivation increase serum ghrelin levels (Gualillo et al., 2012). It is possible that the prenatal and postnatal under-nutrition induced increased ghrelin levels as an adaptive mechanism to encourage pathologic adiposity due to food scarcity (Gualillo et al., 2012; Tschop et al., 2000). However, the findings that quercetin and kaempferol reduced ghrelin levels in prenatally and postnatally food deprived rats further supports the notion that compounds with antioxidant properties may be useful as adaptogens in providing resilience against the negative effects of prenatal and postnatal-induced stress. In addition, it is possible that the attenuating effects of quercetin and kaempferol on ghrelin levels could be to avoid abnormality in fetal programming, fetal growth, weight loss, metabolic, cardiovascular functions and brain damage via decrease in oxidative stress (Luo et al., 2015).
In this study, investigation of brain oxidative stress markers in rats exposed to prenatal food restriction, postnatal food restriction or both showed increased brain lipid peroxidation markers, MDA and decreased GSH levels, suggesting oxidative stress. Given the important role of GSH in house-keeping antioxidant activity, neuronal circuitry in brain development and neuroprotection (Aoyama et al., 2006), several reports have shown that neuronal GSH deficiency is associated with increased lipid peroxidation of brain regions enriched with polyunsaturated fatty acid tissues (Wood et al., 2009), changes in brain functions including learning and memory impairments and social deficits (Emokpae et al., 2020), increased susceptibility to neuropsychiatric diseases (Ben-Azu et al., 2018), and premature age-dependent neurodegeneration (Aoyama et al., 2006). Thus, the findings that repeated treatments with quercetin and kaempferol ameliorate prenatal and postnatal food restriction or combinational effects of both factors on brain oxidative stress further showed their beneficial effects in conditions associated gestational and early postnatal food starvation-mediated oxidative damages. The findings from this study reinforce the belief that compounds with antioxidant activity might be beneficial as adaptogens in providing protection against the negative effects of prenatal and postnatal food scarcity. Interestingly, our recent study showed that quercetin and kaempferol improved gestational outcomes and delayed pubertal onset in rats exposed to prenatal and postnatal food deprivation or/and their combined effects (Anachuna et al., 2020).
5. Conclusion
Cumulatively, our study identified possible connections between prenatal and early postnatal food restrictions, altered serum orexigenic/anorexigenic hormones and increased brain oxidative stress in Wistar rats, which can be reversed by antioxidant treatments. We showed that quercetin and kaempferol increased pup weights of rats submitted to prenatal, postnatal food deprivation or the combined effects via mechanisms related to modulation of leptin and ghrelin levels and antioxidant pathways. Therefore, quercetin and kaempferol supplementation could provide important nutritional interventions to combat against prenatal and early postnatal food scarcity-induced changes in metabolic and brain functions.
Author Contributions
Anachuna Kenneth Kelechi (AKK), Moke Goodies Emuesiri (MGE), Iyare Cordilia (IC), Katchy Nkiru (KN), Ben-Azu Benneth (BAB), Adeniyi Boluwatife (AB), Nwogueze Bartholomew Chukwuebuka (NBC), Iyare Eghosa (IE)
AKK, MGE, BAB and IE conceptualized the study; AKK, IC, KN, AB and NBC funded the study; AKK, IE, MGE, KN and AB supplied the drugs and reagents; IE and BAB supervised the study; KKA, MGE, IC, AB and KN carried out the study; KKA, BAB and AB did the biochemical analyses; BBA, KKA, MGE and IC did the data acquisition and analyses; KKA, BAB and IE did the literature search; KKA, BAB and IE wrote the first draft and did the review of the manuscript; and all authors approved the final draft of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are very thankful to the technical staff of the Department of Physiology, Delta State University, Abraka for their technical inputs in the study design and animal breeding. Also, we are grateful to the Department of Physiology, University of Nigeria for the technical assistance during the ELISA studies.
Contributor Information
Kenneth Kelechi Anachuna, Email: goldasaba20@gmail.com.
Benneth Ben-Azu, Email: pharmben4ever@yahoo.com, bbenazu@pums.edu.ng.
References
- Adam-Vizi V., Seregi A. Receptor independent stimulatory effect of noradrenaline on Na+/K+-ATPase in rat brain homogenate, role of lipid peroxidation. Biochem Pharmacol. 1982;34:2231–2236. doi: 10.1016/0006-2952(82)90106-x. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Gupta S., Sharma R.K. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anachuna K.K., Ekhoye E.I., Iyare C., Katchy N., Ben-Azu B., Daubry T.M.E., et al. Altered gestational outcomes and delayed pubertal onset in prenatally and early postnatally food restricted male and female rats: mitigation by quercetin and kaempferol. Int J Physiol Pathophysiol Pharmacol. 2020;12:115–127. [PMC free article] [PubMed] [Google Scholar]
- Aoyama K., Suh S.W., Hamby A.M., Liu J., Chan W.Y., Chen Y., et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Ben-Azu B., Aderibigbe A.O., Omogbiya I.A., Ajayi A.M., Owoeye O., Olonode E.T., et al. Probable mechanisms involved in the antipsychotic-like activity of morin in mice. Biomed Pharmacother. 2018;105:1079–1090. doi: 10.1016/j.biopha.2018.06.057. [DOI] [PubMed] [Google Scholar]
- Bouret S.G., Draper S.J., Simerly R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Brüll V., Burak C., Stoffel-Wagner B., Siegfried W., Nickenig G., Müller C., et al. Acute intake of quercetin from onion skin extract does not influence postprandial blood pressure and endothelial function in overweight-to-obese adults with hypertension: a randomized, double-blind, placebo-controlled, crossover trial. Eur J Nutr. 2017;56:1347–1357. doi: 10.1007/s00394-016-1185-1. [DOI] [PubMed] [Google Scholar]
- Castrogiovanni P., Imbesi R. The role of malnutrition during pregnancy and its effects on brain and skeletal muscle postnatal development. J Funct Morphol Kinesiol. 2017;2:30. [Google Scholar]
- Cordido F., Isidro M.L., Nemina R., Sangiao S.,A. Ghrelin and growth hormone secretagogues, physiological and pharmacological aspect. Curr Drug Discov Technol. 2009;6:34–42. doi: 10.2174/157016309787581048. [DOI] [PubMed] [Google Scholar]
- Costa L.G., Jacqueline M.G., Roquè P.J., Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev. 2016;2016:1–9. doi: 10.1155/2016/2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo I., García-Mediavilla M.V., Gutiérrez B., Sánchez-Campos S., Tuñón M.J., González-Gallego J.A. Comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Brit J Nutr. 2008;100:968–976. doi: 10.1017/S0007114508966083. [DOI] [PubMed] [Google Scholar]
- Curhan G.C., Willet W.C., Rimm E.B., Spiegelman D., Ascherio A.L., Stampfer M.J. Birth weight and adult hypertension, diabetes mellitus and obesity in US men. Circulation. 1996;15:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- Delahaye F., Breton C., Risold P.Y., Enache M., Dutriez-Casteloot I., Laborie C., et al. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149:470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- Devi K.P., Malar D.S., Nabavi S.F., Sureda A., Xiao J., Nabavi S.M., et al. Kaempferol and inflammation: from chemistry to medicine. Pharmacol Res. 2015;99:1–10. doi: 10.1016/j.phrs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Doğan Z., Kocahan S., Erdemli E., Köse E., Yılmaz E.Z., Ekinci N., et al. Effect of chemotherapy exposure prior to pregnancy on fetal brain tissue and the potential protective role of quercetin. Cytotechnology. 2015;67:1031–1038. doi: 10.1007/s10616-014-9742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower J.I., Geleijnse J.M., Gijsbers L., Zock P.L., Kromhout P., Hollman P.C.H. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial. Am J Clin Nutr. 2015;101:914–921. doi: 10.3945/ajcn.114.098590. [DOI] [PubMed] [Google Scholar]
- Duhig K., Lucy C.C., Shennan A.H. vol. 9. 2016. pp. 113–116. (Oxidative stress in pregnancy and reproduction). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emokpae O., Ben-Azu B., Ajayi A.M., Umukoro S. D-ribose-Lcysteine attenuates lipopolysaccharide-induced memory deficits through inhibition of oxidative stress, release of proinflammatory cytokines, and nuclear factor-kappa B expression in mice. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:909–925. doi: 10.1007/s00210-019-01805-0. [DOI] [PubMed] [Google Scholar]
- Engelbregt M.I.A., Mieke J.T., Houdijk E.C.A.M., Popp-snijders C., Henriette A., Delemarre-van De Waal. The effects of intra-uterine growth retardation and postnatal undernutrition on onset of puberty in male and female rats. Pediatr Res. 2000;48:803–807. doi: 10.1203/00006450-200012000-00017. [DOI] [PubMed] [Google Scholar]
- Engelbregt M.J., van Weissenbruch M.M., Popp-Snijders C., Lips P., Delemarre-van de Waal A. Body mass index, body composition, and leptin at onset of puberty in male and female rats after intrauterine growth retardation and after early postnatal. Food Restriction Pediatric Research. 2001;50:474–478. doi: 10.1203/00006450-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Frisch E.R., Revelle R. Height and weight at menarche and a hypothesis of menarche. Arch Dis Child. 1971;46:695–701. doi: 10.1136/adc.46.249.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Campos M., Aguilera C.M., Canete R., Gil A. Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr. 2006;96:201–226. doi: 10.1079/bjn20061787. [DOI] [PubMed] [Google Scholar]
- Gualillo O., Jorge E.C., Nogueiras R., Seoane L.M., Arvat E., Ghigo E., et al. Effect of food restriction on ghrelin in normal-cycling female rats and in pregnancy. Obes Res. 2012;10:682–687. doi: 10.1038/oby.2002.92. [DOI] [PubMed] [Google Scholar]
- Hashemzaei M., Delarami Far A., Yari A., Heravi R.E., Tabrizian K., Taghdisi S.M., et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol Rep. 2017;38:819–828. doi: 10.3892/or.2017.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Cha S.H., Chohnan S., Lane M.D. Hypothalamic malonyl-CoA as a mediator of feeding behavior. Proc Natl Acad Sci USA. 2003;100:12624–12629. doi: 10.1073/pnas.1834402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbesi R., Castrogiovanni P. Embryonic and post natal development in experimental tryptophan deprived rats. A preliminary study. J Mol Histol. 2008;39:487–498. doi: 10.1007/s10735-008-9188-8. [DOI] [PubMed] [Google Scholar]
- Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci. 2001;2:551–560. doi: 10.1038/35086018. [DOI] [PubMed] [Google Scholar]
- Iwasa T., Matsuzaki T., Munkhzaya M., Tungalagsuvd A., Yamasaki M., Kuwahara A., et al. The effects of prenatal undernutrition and postnatal high-fat diet on hypothalamic Kiss 1 mRNA and serum leptin levels. Int J Dev Neurosci. 2015;42:76–79. doi: 10.1016/j.ijdevneu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Jamalan M., Ghaffari M.A., Hoseinzadeh P., Hashemitabar M., Zeinali M. Human sperm quality and metal toxicants: protective effects of some flavonoids on male reproductive function. Int J Fertil Steril. 2016;10:215. doi: 10.22074/ijfs.2016.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap D., Sharma A., Tuli H.S., Sak K., Punia S., Mukherjee T.K. Kaempferol–A dietary anticancer molecule with multiple mechanisms of action: recent trends and advancements. J Funct Food. 2017;30:203–219. doi: 10.1016/j.jff.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid S., Al-Numair G.C., Veeramani C., Alsaif M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015;20(5):198–209. doi: 10.1179/1351000214Y.0000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z.C., Fraser W.D., Julien P., et al. Tracing the origins of “fetal origins” of adult diseases: programming by oxidative stress? Med Hypotheses. 2006;66:38–44. doi: 10.1016/j.mehy.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Luo Z., Jean-François B., Nuyt A.M., Fraser W.M., Julien P., Audibert F., et al. Perinatal oxidative stress may affct fetal ghrelin levels in humans. Sci Rep. 2015;5:17881. doi: 10.1038/srep17881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris D.P., Kallithraka S., Kefalas P. Flavonols in grapes, grape products and wines: burden, profile and influential parameters. J Food Compos Anal. 2006;19:396–404. [Google Scholar]
- Marcin O., Przemysław Ł.M., Radosław K., Karolina W., Andrzej K., Hubert W., et al. Vivo, and clinical studies. Evidence-based complementary and alternative medicine. 2018. Pharmacological effect of quercetin in hypertension and its potential application in pregnancy-induced hypertension: review of in vitro; pp. 1–19. Article ID 7421489. [Google Scholar]
- Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Nazian S.J., Cameron D.F. Temporal relation between leptin and various indices of sexual maturation in the male rat. J Androl. 1999;20:487–491. [PubMed] [Google Scholar]
- Ozgen S., Kilinc O.K., Selamoglu Z. Antioxidant activity of quercetin: a mechanistic review. Turkish J Agri Food Sci Tech. 2016;4:1134–1138. [Google Scholar]
- Pi J., Zhang Q., Fu J., Woods C.G., Hou Y., Corkey B.E., et al. ROS signaling, oxidative stress and Nrf 2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu S., Vijayakumar S., Kothandaraman S., Palani M. Antidiabetic activity of quercetin extracted from Phyllanthus emblica L. Fruit: in silico and in vivo approaches. J Pharm Anal. 2018;8:109–118. doi: 10.1016/j.jpha.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quennell J.H., Howell C.S., Roa H.J., Augustine R.A., Grattan D.R., Anderson G.M. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152:1541. doi: 10.1210/en.2010-1100. 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas Joselyn, Arraiz Nailet, Aguirre Miguel, Velasco Manuel, Bermudez Valmore. AMPK as target for intervention in childhood and adolescent obesity. Journal of Obesity. 2011:19. doi: 10.1155/2011/252817. Article ID 252817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone S., Jaquet V., Trabace L., Krause K. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxidants Redox Signal. 2013;18:1475–1490. doi: 10.1089/ars.2012.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda D.M., Howie G.J., Pleasants A., Gluckman P.D., Vickers M.H. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PloS One. 2009;4 doi: 10.1371/journal.pone.0006744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman A., Sanctis V.D., Elalaily R. Nutrition and pubertal development. Indian J Endocrine Metabolism. 2014;18:39–47. doi: 10.4103/2230-8210.145073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucajtys-Szulc E., Jacek T., Elzbieta G., Justyna K., Ewa S., Ewa S., et al. Differential effect of prolonged food restriction and fasting on hypothalamic malonyl-CoA concentration and expression of orexigenic and anorexigenic neuropeptides genes in rats. Neuropeptides. 2010;44:17–23. doi: 10.1016/j.npep.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Swolin-Eide D., Dahlgren J., Nilsson C., Wikland K.A., Holmang A., Ohlsson C. Affected skeletal growth but normal bone mineralization in rat offspring after prenatal dexamethasone exposure. J Endocrinol. 2002;174:411–418. doi: 10.1677/joe.0.1740411. [DOI] [PubMed] [Google Scholar]
- Trivedi R., Kumar A., Gupta V., Kumar S., Nagar G.K., Romero J.R., et al. Effects of Egb 761 on bone mineral density, bone microstructure, and osteoblast function: possible roles of quercetin and kaempferol. Mol Cell Endocrinol. 2009;302:86–91. doi: 10.1016/j.mce.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Tschop M., Smiley D.L., Helman M.L. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Turpaev K.T. Reactive oxygen species and regulation of gene expression. Biochemistry. 2002;67:281–292. doi: 10.1023/a:1014819832003. [DOI] [PubMed] [Google Scholar]
- Turpin C.A., Sakyi S.A., Owiredu W.K. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth. 2015;15:189. doi: 10.1186/s12884-015-0624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijdan M.D., Marra M.V. Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients. 2019;11:2288. doi: 10.3390/nu11102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.J., Yucel M., Pantelis C., Berk M. Neurobiology of schizophrenia € spectrum disorders: the role of oxidative stress. Ann Acad Med Singapore. 2009;38:396. 396. [PubMed] [Google Scholar]