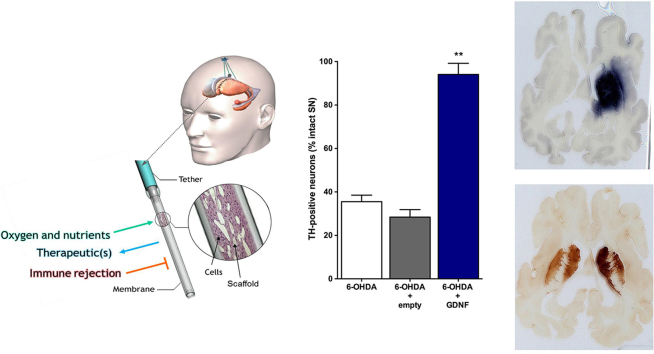
Abstract
Delivering glial cell line-derived neurotrophic factor (GDNF) to the brain is a potential treatment for Parkinson's Disease (PD). Here we use an implantable encapsulated cell technology that uses modified human clonal ARPE-19 cells to deliver of GDNF to the brain. In vivo studies demonstrated sustained delivery of GDNF to the rat striatum over 6 months. Anatomical benefits and behavioral efficacy were shown in 6-OHDA lesioned rats where nigral dopaminergic neurons were preserved in neuroprotection studies and dopaminergic fibers were restored in neurorecovery studies. When larger, clinical-sized devices were implanted for 3 months into the putamen of Göttingen minipigs, GDNF was widely distributed throughout the putamen and caudate producing a significant upregulation of tyrosine hydroxylase immunohistochemistry. These results are the first to provide clear evidence that implantation of encapsulated GDNF-secreting cells deliver efficacious and biologically relevant amounts of GDNF in a sustained and targeted manner that is scalable to treat the large putamen in patients with Parkinson's disease.
Keywords: Encapsulation, GDNF, Neuroprotection, Neuroregeneration
Graphical abstract
Highlights
-
•
GDNF is a potential therapy for Parkinson's Disease. To date, no efficacious means of delivering it have been effective.
-
•
Here we described an encapsulated cell technology to provide high levels of GDNF in parkinsonian rats and intact minipigs.
-
•We demonstrate:
-
a.Long-term efficacy by protection and recovery of the dopaminergic innervation and behavioral rescue in lesioned rats.
-
b.A widespread striatal delivery of GDNF that enhances dopaminergic function using rodent-sized and clinical-sized devices.
-
c.Both detailed pharmacodynamic and pharmacokinetic data are provided.
-
a.
-
•
The studies described here satisfy several pre-clinical requisites for continued clinical development of this approach.
1. Introduction
Parkinson's disease (PD) is a synucleinopathy characterized by progressive motor impairments secondary to the loss of striatal dopamine and loss of dopamine-secreting neurons in the substantia nigra. Treatment with glial cell line-derived neurotrophic factor (GDNF) has potential to slow or reverse the loss of dopaminergic function in PD patients. GDNF has consistently shown anatomical and behavioral benefits in 6-OHDA rats (Wang et al., 2002, Grondin et al., 2003, Dowd et al., 2005, Zheng et al., 2005), MPTP lesioned monkeys (Gash et al., 1996, Miyoshi et al., 1997, Kordower et al., 2000, Palfi et al., 2002) and aged monkeys (Kordower et al., 2000). GDNF has been tested in PD patients with encouraging but inconsistent results. The lack of robust clinical efficacy has been suggested to be a result of poor distribution of GDNF to the nigrostriatal system (Kordower et al, 19999, Lang et al., 2006, Nutt et al., 2003, Salvatore et al., 2006, Sherer et al., 2006).
Here we report on the continued development of a cell-based technology with the potential to deliver efficacious levels of GDNF, or other molecules, directly to the brain (Emerich et al., 1996, Kishima et al., 2004, Emerich et al., 2014, Lindner et al., 1995, Lindvall and Wahlberg, 2008). In this approach cells are engineered to secrete GDNF and are then enclosed in a semipermeable capsule, which is then implanted directly into the brain. The semipermeable nature of the membrane allows the cells to remain viable and distribute GDNF to the surrounding brain while also reducing immunological reactions to the encapsulated cells allowing cells from allo- and xenogeneic sources to be used. Building off previous studies using this system (Emerich et al., 2019, Eyjolfsdottir et al., 2016, Falcicchia et al., 2018, Fjord-Larsen et al., 2010, Fjord-Larsen et al., 2012a, Nanobashvili et al., 2019, Paolone et al., 2019, Simonato et al., 2017) we evaluated the long-term function and neurological benefits of an encapsulated clonal human cell line based on ARPE-19 when implanted into the brains of intact and parkinsonian rats and minipigs. Results indicated: (1) a widespread striatal delivery of GDNF and enhanced dopaminergic function in both rats and minipigs, (2) high and consistent (6 months) increases in striatal levels of GDNF, (3) that GDNF protected dopaminergic neurons when delivered prior to 6-OHDA lesions, and (4) that GDNF implants produced a significant improvement in neurological performance over a 62 week test period in rats with pre-existing 6-OHDA lesions (i.e. a neurorecovery effect). The demonstrations of long-term, controlled, and targeted delivery of GDNF provide ongoing support for continued development of this approach as a potential treatment for PD. In addition, this cell based technology allows for relatively rapid clinical evaluation as the regulatory framework, GMP manufacturing, and stereotactic instrumentation have already been addressed in the testing of devices secreting nerve growth factor (NGF) for the treatment of Alzheimer's patients (Wahlberg et al., 2012).
2. Methods and materials
2.1. Subjects
Adult male Sprague–Dawley rats (Harlan labs), ∼3 months old and weighing 225–250 g, were housed in groups of 4 in a temperature- and humidity-controlled colony room that was maintained on a 12 h light/dark cycle. Food and water were available ad libitum throughout the experiment. Female Göttingen Minipigs (Marshall Bioresources) approximately 3-6 months old and weighing approximately 10–15 kg were group housed. Animals were routinely fed Purina 5081 Mini Pig Pellets supplemented with treats including fresh fruit/produce. Food and water were available ad libitum throughout the experiment with the exception of food for 12 h prior to surgery. During the study, minipigs were routinely weighed once per week. Daily notes were taken by animal care facility staff regarding food consumption and health status. All animals were evaluated for behavior and health status by a board-certified veterinarian prior to study initiation and once monthly during the 3 months study period. All experimentation was conducted in accord with National Institutes of Health guidelines.
2.2. GDNF device production
ARPE-19 cells were cultured and modified to secrete GDNF as previously described (Fjord-Larsen et al., 2012a, Nanobashvili et al., 2019, Paolone et al., 2019). Cells were maintained using standard plating and passaging procedures in T-175 flasks with growth medium, DMEM + glutamax (1x) supplemented with 10% fetal bovine serum (Gibco). Cells were incubated at 37 °C, 90% humidity and 5% CO2. And passaged every 2–3 days.
Cells were encapsulated into hollow fiber membranes as previously described (Fjord-Larsen et al., 2012a, Nanobashvili et al., 2019, Paolone et al., 2019). For rodent implants, devices were manufactured from 7 mm segments of polyethersulfone membrane and loaded with cells at a density of 10,000 cells/μl using a custom manufactured automated cell-loading system. For minipig implants, 18 mm long devices were produced with a polyurethane tether attached via a titanium linker. Devices were kept in HE-SFM at 37 °C and 5% CO2 for 2–3 weeks prior to surgical implantation.
2.3. Surgical implantation of encapsulated cells
Rats were anesthetized with isoflurane (1–2%) and placed into a stereotaxic instrument (Stoelting, inc). A midline incision was made in the scalp, and a hole was drilled for the placement of a device into the striatum using a stainless-steel cannula mounted to the stereotaxic frame. The coordinates for implantation with respect to Bregma were: AP: 0.5, ML: ±3.0, and DV: −7.5.
Based on MRI-derived coordinates, minipigs were implanted with devices into the right putamen. The animal's head was fixed during surgery by a custom stereotactic device and continuously anesthetized via intubation and isoflurane (1–2%) administration. A craniotomy (1.5 cm × 1.5 cm) was made over the implant site and the dura was retracted exposing the cortical surface for placement of 2 cell-loaded polymer capsules centered in the putamen and separated by 5 mm. The capsules were stereotactically implanted using a cannula system attached to the stereotactic frame. At the conclusion of the surgical procedure, the tether was cut at the cortical surface and the dura, muscle, and skin were sutured using routine procedures.
2.4. 6-OHDA lesion
Two striatal sites (left striatum only) were injected with 10 μg 6-OHDA/site using a 28-gauge Hamilton syringe mounted to the stereotaxic frame at the following coordinates with respect to Bregma: (1) AP: 1.2, ML: ±2.5, DV: −5.0 and (2) AP: 0.2, ML: ±3.8, DV: −5.0. Two μl 6-OHDA per site was infused over 2 min. The cannula was left in place for an additional 2 min allowing the 6-OHDA to diffuse.
2.5. GDNF ELISA
A commercially available GDNF ELISA kit (DuoSet® for human GDNF R&D Systems, Minneapolis, MN) was used to quantify GDNF device secretion prior to implantation and again following retrieval from the brain. Immediately following retrieval from the brain devices were incubated at 37 °C in HE-SFM. Media samples (4 h incubation) were collected to quantify GDNF. Supernatants from pulverized and centrifuged tissue samples were assessed for GDNF levels using the same ELISA.
2.6. Initial in vivo clonal screening/selection
The in vivo viability and continued GDNF secretion of 3 cell clones was evaluated to permit selection of a single clone for subsequent studies. Cells were encapsulated and implanted into intact rat striatum as described above. Devices were explanted 4 and 8 weeks later and GDNF secretion and cell viability were confirmed. Twenty four rats were randomly assigned to one of 6 treatment groups: 1–2) cell line clone-125 explanted after 4 or 8 weeks in vivo, 3–4) cell line clone-120 explanted after 4 or 8 weeks in vivo, 5–6) cell line clone-20 explanted after 4 or 8 weeks in vivo. Groups consisted of 4 animals each with bilateral implants providing a total of 8 explanted devices per group/time-point.
2.7. Long-term encapsulated cell function and tissue levels of GDNF in rats
Devices were implanted as described above. Devices (6–10/group) were then removed by placing the animal into the stereotactic frame, visualizing the proximal tip of the implant and gently removing it using microforceps. Devices were removed at 1 day, 3 days, 1 week, 2 weeks, and monthly from 1 to 6 months post implant and were immediately incubated at 37 °C in HE-SFM prior to processing for GDNF determinations. Immediately after device removal, the previously implanted striatum and overlying cortex were dissected free, placed in 1 ml of Tissue Protein Extraction Reagent (T-PER; Thermo Scientific, Rockford, Il) and flash frozen in liquid nitrogen. Supernatants from pulverized, and centrifuged tissue samples were assessed for GDNF levels.
2.8. Encapsulated cell function in intact versus 6-OHDA-lesioned striatum
A second study directly compared GDNF section from devices implanted into intact versus 6-OHDA-lesioned striatum. Animals received unilateral 6-OHDA lesions as described above. One month later, GDNF-secreting devices were bilaterally implanted into the striatum. Devices were retrieved at 2 (n = 6), 4 (n = 6), and 8 (n = 5) weeks later and GDNF levels were determined by ELISA.
2.9. Neuroprotection studies
Rats received unilateral striatal implants of cell-loaded devices followed 1 week later by infusion of 6-OHDA into the striatum adjacent to the implant. All animals were weighed and tested for neurological function prior to implantation and again at 2 and 4 weeks post lesion using the behavioral tests outlined below. At the conclusion of testing, animals were sacrificed and the brain processed for quantitative determinations of the ability of GDNF to protect lesioned dopaminergic neurons in the substantia nigra (see below for details). Sections throughout the striatum were processed for visualization of GDNF and tyrosine hydroxylase (TH) as described below. Three experimental groups were used: 1): 6-OHDA alone (n = 8); 2): 6-OHDA + empty device implants (n = 8); and 3): 6-OHDA + GDNF devices (n = 8).
2.10. Neurorecovery studies
Rats received unilateral intrastriatal injections of 6-OHDA followed, 1 month later, by implantation of encapsulated cells. Animals were tested for neurological function at 4, 8, 14, 20, 30, 40, 52, and 62 weeks following device implantation. Animals received: (1) 6-OHDA alone (n = 8); (2) 6-OHDA + empty device implants (n = 8); and (3) 6-OHDA + GDNF devices (n = 8).
2.11. Neurological evaluation
Using a previously published battery of tests (Tornøe et al., 2012, Schallert, 2006, Emerich et al., 2019, Nanobashvili et al., 2019), rats were evaluated to provide a behavioral measure of the extent of the lesion as well the magnitude of benefit provided by the GDNF implants. All tests were conducted 24 h prior to device implantation, 24 h prior to 6-OHDA injection, and again at the testing points outlined above for both the neuroprotection studies and the neurorecovery studies. All testing was performed in a dim light testing room and the individual tests included (in order of testing): the cylinder test of spontaneous forelimb use, the spontaneous forelimb placing use and the stepping test.
2.12. Histology and immunocytochemistry
Rats were deeply anesthetized and transcardially perfused with 200 mls of 0.9% ice-cold saline. Following saline perfusion, the rats were decapitated and the devices were removed. The brains were placed into Zamboni's fixative for 1 week and then transferred to 25% sucrose for 48 h. Brains were then treated overnight with 20% glycerol and 2% dimethylsulfoxide to prevent freeze-artifacts, and multiply embedded in a gelatin matrix using MultiBrain™ Technology (Neuroscience Associates, Knoxville, TN). The brains were cryo-sectioned coronally at 40 micron intervals. Every 6th section was processed for GDNF immunohistochemistry using a commercially-available antibody to human GDNF (1:1000; R&D systems). A separate series of adjacent sections was processed for the visualization of dopaminergic terminals within the striatum using TH immunocytochemistry (1:6000; Pel Freez). Additional TH-stained sections were taken throughout the substantia nigra to visualize and quantify the number of remaining dopaminergic neurons. A series of sections encompassing the rostral tip of the substantia nigra pars compacta to the caudal end of the substantia nigra pars reticulata was used for manually counting of TH-positive neurons on both the lesioned and control sides of the brain. Optical densitometry of TH-positive fibers in the striatum was performed on a series of 3 equally-spaced sections based on images captured using a Nikon E800 microscope coupled to a computer assisted morphometric system (iVision image analysis software; BioVision Technologies v 4.0.10, Exton, PA).
Pigs were anesthetized and the previous surgical site exposed to remove the implanted devices 12 weeks following implantation. Following device removal, the pigs were transcardially perfused with 2 L of 0.9% saline followed by 2 L of Zamboni's fixative. Brains were removed and immersion fixed for approximately 1 week in Zamboni's fixative prior to being transferred to 25% sucrose for 48 h. The brains were cryo-sectioned horizontally at 40 micron intervals. Every 6th section was saved and processed for GDNF immunohistochemistry using a commercially-available antibody to human GDNF (1:500; R&D systems). A separate series of adjacent sections was processed for the visualization of dopaminergic terminals within the striatum using TH immunocytochemistry (1:500; Pel Freez). Optical densitometry of TH-positive fibers in the striatum was performed on a series of 5 equally-spaced sections using NIH Image J software (v1.3 NIH).
2.13. Serum and CSF measurements
Samples of whole blood, serum, and CSF were taken from minipigs at implant and explant (3 months). These samples were sent to Idexx Laboratories (North Grafton, MA) to be analyzed for: complete blood count (CBC) with differential, liver and kidney profile, electrolytes and serum chemistries. The CSF was analyzed for total protein, cell count, glucose, phosphate, potassium, chloride and calcium. Samples were also analyzed for GDNF by ELISA.
2.14. Serum antibodies to human GDNF
The development of potential anti-GDNF antibodies was determined with a competitive ELISA specific for pig antibodies directed against human GDNF. Serum samples were incubated in 96 well plates coated with human GDNF. After washing, a biotinylated anti-human GDNF antibody was added and the amount of biotinylated antibody binding was measured after the addition of streptavidin-HRP conjugate and TMB substrate. The data was expressed as a ratio of sample optical density/pig serum optical density. A ratio of less than 0.70 was considered positive for specific human GDNF antibodies.
2.15. Statistical methods
Mixed-design ANOVAs were used to determine the effects of treatment, time, and any treatment × time interactions. Where applicable, post-hoc comparisons were conducted using t-test and Fisher's LSD test. Statistical analyses were performed using SPSS software. In cases of violation of sphericity assumption, Huyhn-Feldt correct F values are reported. Alpha was set at 0.05 and exact p values are reported for significant results (Greenwald et al., 1996). Mean values and standard errors of the mean (SEM) were calculated for each group and expressed as percentage of the intact hemisphere to facilitate graphical comparisons.
3. Results
3.1. GDNF secretion from selected cell clones
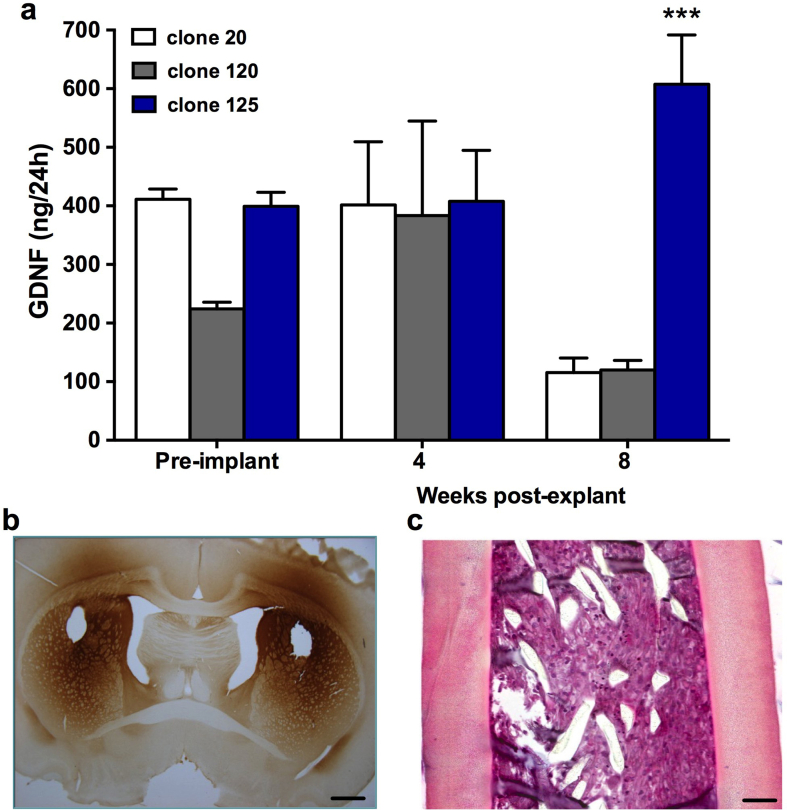
Prior to implantation, devices containing each of the 3 experimental clones were assessed for GDNF output. As shown in Fig. 1a, these levels ranged from approximately 200-400 ng/device/24 h (Clone-20: 411.13 ± 17.66; Clone-120: 224.19 ± 11.52; Clone-125: 399.17 ± 24.01) with GDNF-125 devices showing the highest consistency and overall output. After 4 weeks, GDNF levels in all clones were maintained with no appreciable decrease relative to pre-implant values (Clone-20: 401.46 ± 107.78; Clone-120: 383.34 ± 161.34; Clone-125: 407.64 ± 87.22). At the 8 week time point, both GDNF-20 and GDNF-120 showed a reduction in GDNF output by approximately 4-fold (Clone-20: 115.53 ± 24.74; Clone-120: 119.87 ± 16.56), while GDNF-125 devices increased in output by 50% (Clone-125: 607.53 ± 84.26). The results were confirmed by the statistical comparison (Clone-20 V Clone-125: t (7)=5.50, p = 0.0005; Clone-120 V Clone-125: t (7) = 5.38, p = 0.0001).
Fig. 1.
(a) ELISA analysis of encapsulated clonal ARPE-19 cell lines demonstrates robust continued secretion of GDNF from clone-125. While GDNF secretion from clones 20 and 120 decreased between 4 and 8 weeks, secretion from clone-125 remained stable and even rose during the same period in vivo. (b) Immunohistochemistry of tissue sections at 8 weeks post implant confirms robust diffusion of GDNF from encapsulated clone-125 cells throughput the implanted striatum and overlying cortex. Scale bar, 500 μm in panel b and 75 μm in panel c. H&E staining on longitudinal sections of devices confirms excellent viability of encapsulated clone −125 cells. Clone-125 was used for all subsequent studies. Data are expressed as mean ± SEM of 4 animals each with bilateral implants per group/time-point. ∗∗∗p<0.0001; Student's t test for unpaired data.
Based on the continued secretion of GDNF, clone-125 containing devices were analyzed to confirm cell viability. Explanted devices were fixed in formalin, embedded in Historesin, sectioned longitudinally and the viability, based on morphology, was confirmed on hematoxylin eosin (H&E)-stained sections. Histologically, GDNF-125 devices maintained high levels of cell density after 4 and 8 weeks of implantation (Fig. 1c). After 8 weeks in vivo, GDNF-125 devices showed no obvious signs of declining viability, with very few pyknotic nuclei, and with the majority of encapsulated cells maintaining a normal cytoplasmic volume and healthy overall appearance.
Immunohistochemical staining of GDNF secreted from clone-125 cells revealed robust distribution of the growth factor. Intense GDNF immunoreactivity was seen throughout the striatum and into the globus pallidus and ventral pallidum. Immunoreactivity was also observed in the corpus callosum and the overlying cortex adjacent to the implant site (Fig. 1b). Areas outside of the immediate site were completely devoid of GDNF immunoreactivity confirming the specificity of the staining. While not examined here, our previous studies have confirmed that these high levels of GDNF secretion are not associated with any alteration in reactive gliosis around the implant site (Falcicchia et al., 2018, Nanobashvili et al., 2019, Paolone et al., 2019).
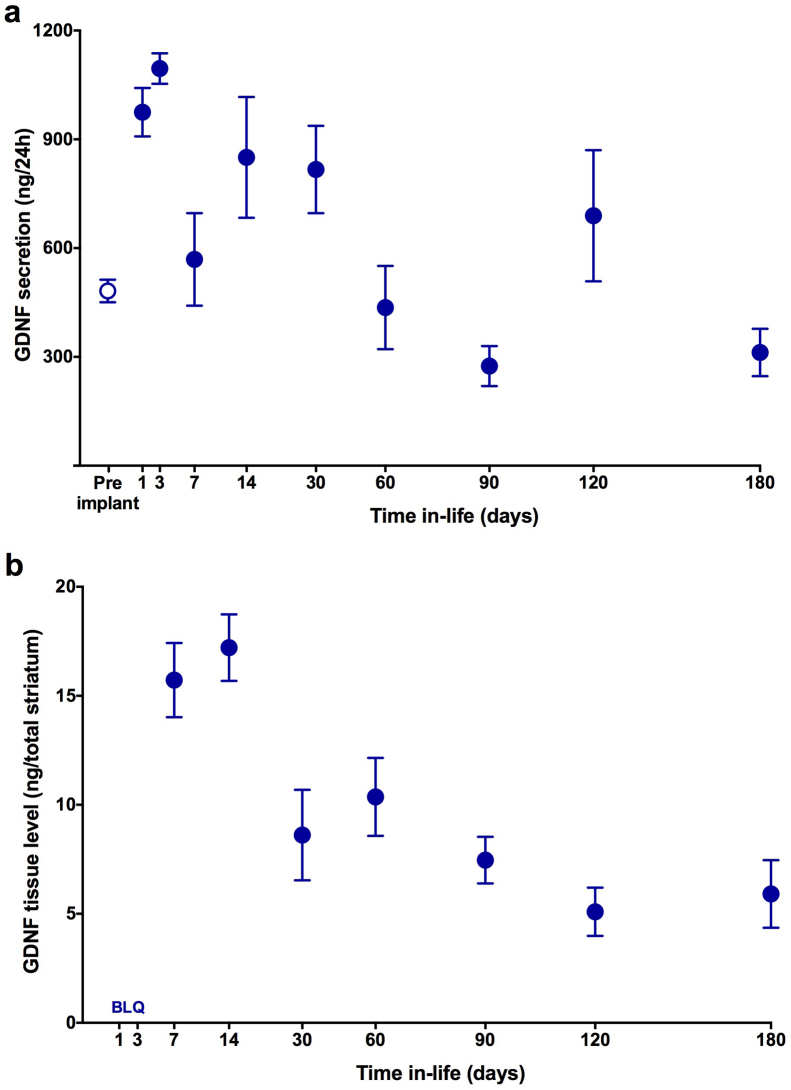
3.2. Long-term secretion and tissue distribution of GDNF
Quantitation of device output illustrated a continued and stable secretion of GDNF from the implanted devices. Prior to implantation, devices secreted approximately 480 ng/day (484.99 ± 31.29) of GDNF. Explanted devices showed an initial increase in secretion peaking at approximately 3–14 days (1095.15 ± 42.17) and then tapering to a sustained level that remained at pre-implant levels for the 6-month in vivo period (Fig. 2a; 312.31 ± 65.33). The continued delivery of GDNF to the striatum significantly elevated tissue concentrations of GDNF as determined by ELISA. Of note, as reported in previously published studies (Falcicchia et al., 2018, Emerich et al., 2019, Kishima et al., 2004, Paolone et al., 2019), we removed and analyzed the entire implanted striatum. This allowed us to gain insight into tissue concentrations of GDNF in the target striatum as opposed to using a protein-based average or highly variable, smaller tissue punches. Accordingly, the final data are expressed as ng of GDNF in the whole striatum (Hadaczek et al., 2010). Tissue concentrations of GDNF were elevated within 1 week following implantation, reaching peak levels at approximately 2 weeks (17.21 ± 1.53) and then plateauing thereafter to remain relatively constant for at least 6 months post implantation (Fig. 2b).
Fig. 2.
Long-term, sustained delivery of GDNF and resulting elevation tissue levels of GDNF within the implanted striatum. Fig. 2a demonstrates that GDNF device secretion peaks early post implantation but then stabilizes and remains consistent for at least 6 months in vivo. Fig. 2b confirms that GDNF delivery results in sustained tissue levels within the implanted striatum. When explanted from the striatum the output of the devices over time generally paralleled the enhanced tissue concentrations with an early peak in delivery followed by a longer, sustained output similar to that noted from the same devices immediately prior to implantation. All data are expressed as mean ± SEM of 4 animals each with bilateral implants per time-point. BLQ = below limit of quantitation.
3.3. Encapsulated cell function in intact versus 6-OHDA-lesioned striatum
Levels of GDNF secretion from explanted devices at 2–8 weeks post implantation are reported in Table 1. Prior lesioning of the dopaminergic innervation of the striatum with 6-OHDA, did not impact GDNF output from devices explanted at 2–8 weeks post implantation (Table 1).
Table 1.
GDNF secretion from encapsulated cells implanted into intact versus 6-OHDALesioned.
| Intact Striatum | 6-OHDA- Lesion | |
|---|---|---|
| Pre-Implant | 144.21 (8.14) | 147.69 (8.91) |
| 2 Weeks | 298.83 (129.15) | 323.86 (84.98) |
| 4 Weeks | 552.25 (142.91) | 564.27 (152.43) |
| 8 Weeks | 393.76 (56.82) | 378.33 (84.49) |
Data are expressed as mean ± SEM ng GDNF/24 h.
3.4. Neuroprotection study
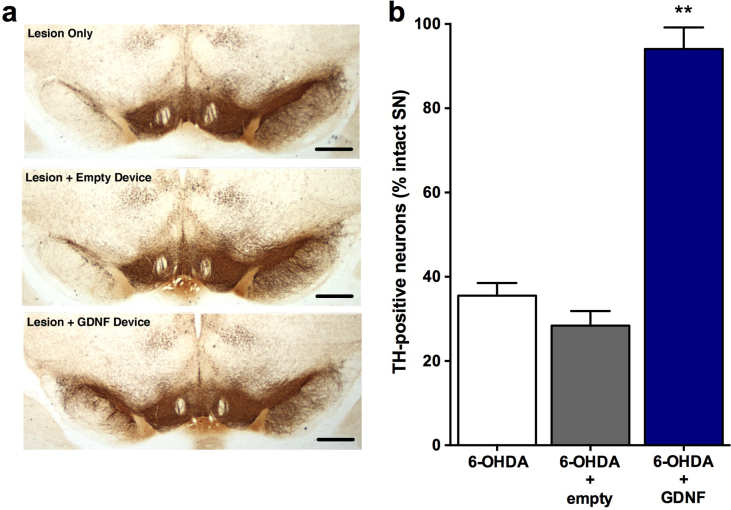
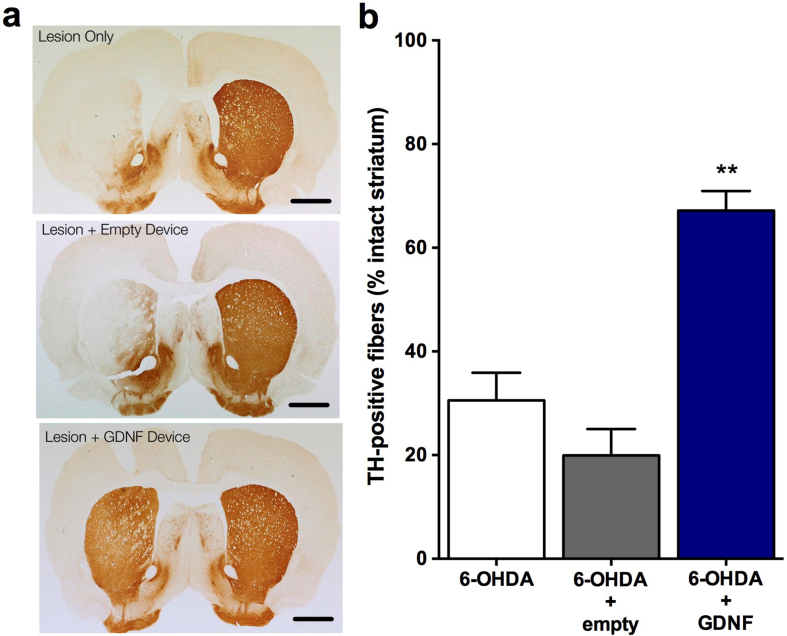
All animals recovered rapidly from surgery. Cage-side observations were performed daily and body weights were recorded periodically prior to surgery and during the 4 week testing period. No overt signs of behavioral toxicity were observed in any animal at any time. Likewise, no alterations in body weights were noted in treated animals relative to controls. Subsequent weight gain was rapid and comparable in all groups following 6-OHDA lesions. Before implantation and after retrieval, the encapsulated cells were assayed for GDNF secretion by ELISA. Before implantation, the encapsulated cells released 578.62 ± 28.95 ng GDNF/capsule/24 h. Post-explant values of GDNF from capsules explanted after 5 weeks in vivo averaged 507.59 ± 11.44 ng GDNF/capsule/24 h. GDNF was undetectable in media incubated with explanted empty capsules. Quantification of TH-positive neurons in the substantia nigra (Fig. 3a) revealed that the animals in the lesion only group had an extensive loss of TH-positive neurons (decreased 65% relative to the intact, contralateral side) at 4 weeks post lesion (Fig. 3b; 6-OHDA: 35.51 ± 3.01). A similar loss of TH-positive neurons (6-OHDA + empty: 28.41 ± 3.46; decreased 72%) was observed in lesioned animals receiving an empty device. In contrast, animals receiving GDNF implants exhibited a marked sparing of TH-positive neurons with only a 6% loss of neurons observed (6-OHDA + GDNF: 94.06 ± 5.12; F(2,21) = 82.36, p<0.001). Accompanying the loss of TH-positive neurons in the control groups was an extensive loss of TH-positive fibers in the striatum that was previously injected with 6-OHDA. The preservation of dopaminergic neurons in the GDNF-treated rats was associated with a notable preservation of TH-positive terminals in the striatum (Fig. 4a). Quantitative optical density measurements of TH fiber density confirmed these observations as the control animals (lesion only: 30.55 ± 5.35; and lesion + empty devices: 19.94 ± 5.10) displayed 70–80% reductions in TH fiber density while those animals receiving GDNF displayed a more modest 33% reduction of TH-positive fiber density (Fig. 4b; 6-OHDA + GDNF: 67.16 ± 3.77; (F(2,21) = 39.16; p<0.001).
Fig. 3.
Photomicrographs of substantia nigra stained for TH immunoreactivity illustrating loss of dopaminergic neurons following 6-OHDA and preservation of those neurons following GDNF treatment (a). Quantitation of dopaminergic neurons in the substantia nigra demonstrates virtually complete protection of lesioned neurons (b). Data are presented as mean ± SEM % of TH-positive neurons relative to the intact, non-lesioned hemisphere of 8 animals per group. Scale bars, 200 μm. Multiple comparisons were based on significant main effects of treatment resulting from the ANOVA described in Results: ∗∗p<0.01.
Fig. 4.
Photomicrographs of striatum stained for TH immunoreactivity (a) and quantitation of TH fiber density (b) following 6-OHDA with and without GDNF treatment illustrates a robust ability of GDNF to preserve dopaminergic fibers. Scale bars = 500 μm Data are presented as mean ± SEM % of TH-positive fibers relative to the intact, non-lesioned hemisphere of 8 animals per group. Multiple comparisons were based on significant main effects of treatment resulting from the ANOVA described in Results: ∗∗p<0.01.
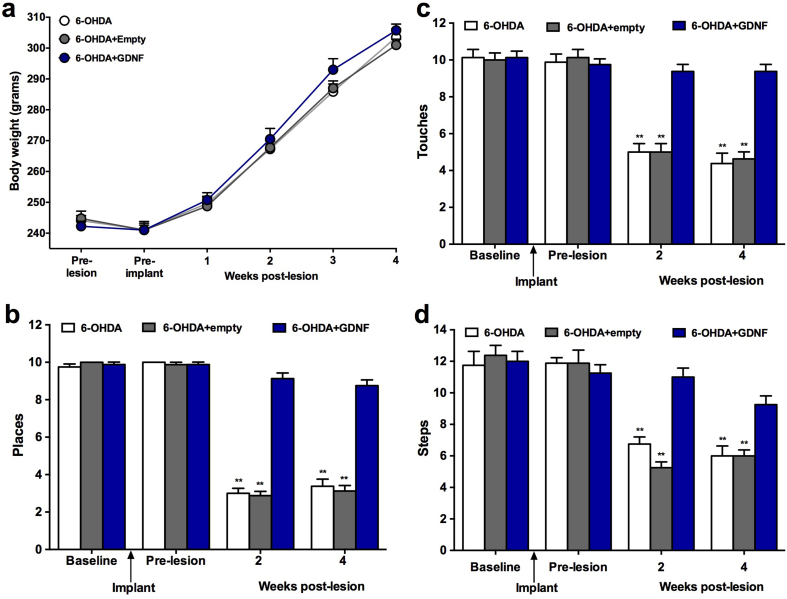
Tests of forelimb function in the cylinder, placing, and stepping tests confirmed that all groups of rats displayed normal forelimb use prior to the initiation of the study (pre-implant; Fig. 5). A modest decrease in body weight was observed following surgical implantation but this effect was not different from control animals that received sham surgery. However, no treatment related changes in body weights were noted over test period (Fig. 5a). Implantation of either empty devices or GDNF devices did not impact performance on any of the behavioral tasks. In contrast, the 6-OHDA lesion produced significant and comparable behavioral deficits in both the lesion only and lesion + empty device groups. This effect was consistent, did not vary when the animals were tested at 2 versus 4 weeks post-lesion and confirmed by the significant time × treatment interaction (Touches: F(6,63) = 13.68, p < 0.001; Places: F(6,63) = 78.97, p < .001; Steps: F(6,63) = 10.82, p < .001). Relative to the intact forelimb, performance on the contralateral and impaired forelimb was decreased 45–58%, 67%–72%, and 65%–70% in these control groups on the cylinder (Fig. 5b), placing (Fig. 5c), and stepping (Fig. 5d) tests, respectively. In contrast, the GDNF treated rats displayed virtually normal performance and the results were confirmed by a main effect of treatment (Touches: F(2,21) = 43.75, p < 0.001; Places: F(2,21) = 201.21, p < 0.001; Steps: F(2,21) = 7.26, p = 0.004). These neurological tests also confirmed the potent protection afforded by the GDNF implants. Use of the contralateral forelimb in the cylinder and placing tests was decreased 10–12% relative to the intact limb but this effect was not significant and did not differ from pre-implant performance. These benefits were also observed in the stepping test. Although a trend towards a deficit was seen in the GDNF-treated animals at 4 weeks post lesion, this effect did not reach statistical significance (Fig. 5d; p<0.10).
Fig. 5.
Normal weight gain and neurological performance is maintained in lesioned animals by implantation of GDNF devices. (a) Body weights of animals with and without GDNF treatment. (b–d) Performance in the cylinder, placing, and stepping tests are all significantly impaired at 2 and 4 weeks following intrastriatal injections of 6-OHDA. In contrast, performance on each of these tests is preserved by GNDF implants with treated animals performing comparably to pre-surgery levels (pre-implantation and pre-lesion). Data are presented as mean ± SEM of 8 animals per group. Multiple comparisons were based on significant main effects or interactions resulting from ANOVAs that are described in Results: ∗∗p<0.01.
3.5. Neurorecovery
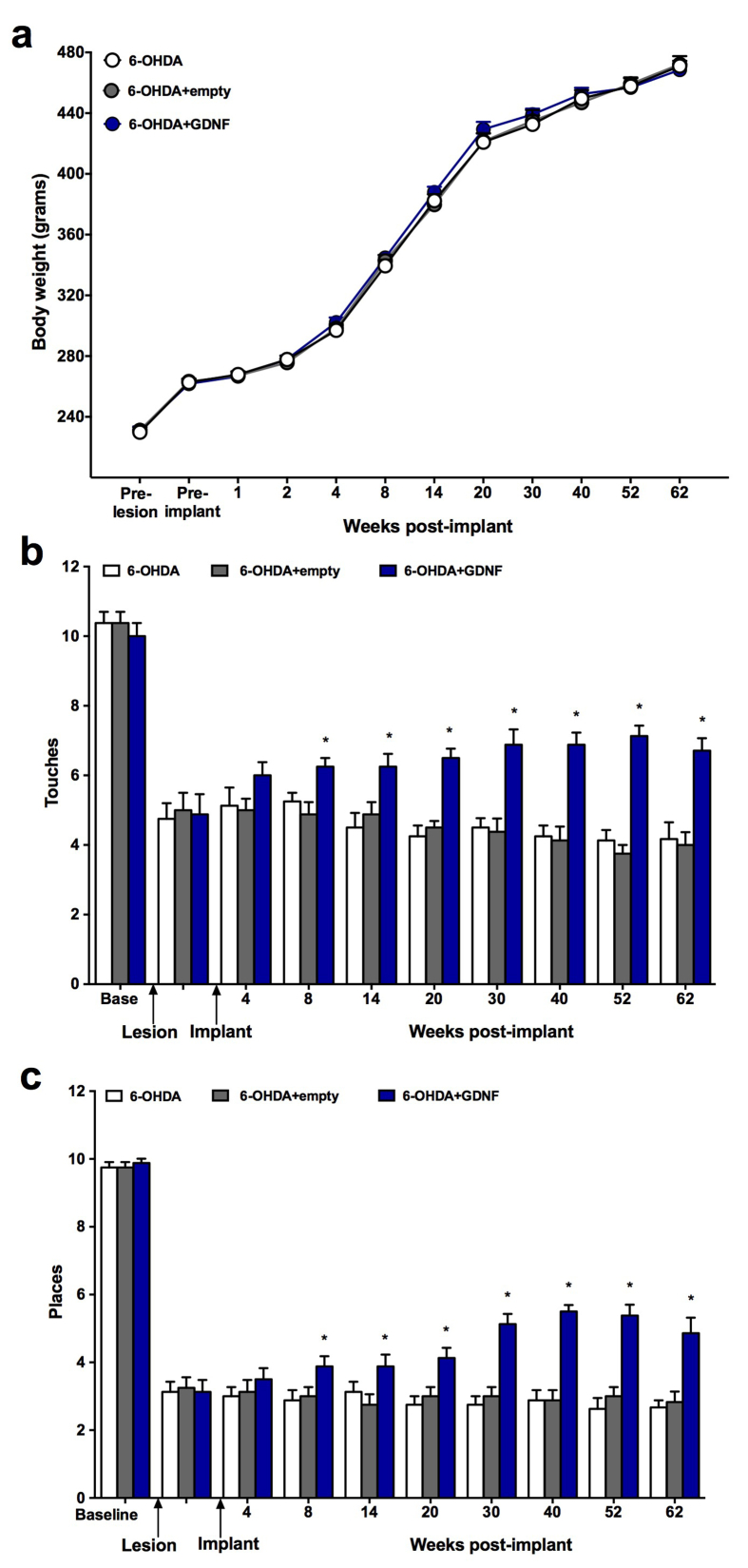
The studies evaluated the ability of cell-based GDNF, to promote neurological recovery following loss of dopaminergic neurons. Consistent with the neuroprotection studies, 6-OHDA produced clear and consistent deficits in performance on the cylinder, placing, and stepping tests (Fig. 6). Modest improvements in performance were noted on the cylinder and placing tests as early as 4 weeks post implantation although these effects did not achieve statistical significance. However, these small benefits continued to gradually grow in magnitude and confirmed by the significant time × treatment interaction (Touches: F(18,189) = 4.22, p < 0.001; Places: F(18,189) = 4.46) at 8 weeks (17–19% improvement) and peaking at 62 weeks post treatment (i.e, the latest time-point examined) when the improvement was >70% relative to controls. While these benefits were robust and consistent on the cylinder (main effect of treatment: F(2,21) = 37.17, p < 0.001) and placing tests (main effect of treatment: F(2,21) = 61.41, p < 0.001), no changes were observed on the stepping test (data not shown) in treated animals. No treatment related changes in body weights were noted over the 62 week test period (Fig. 6a).
Fig. 6.
Long-term, normal weight gain (a) and neurological performance in rats receiving GDNF implants 1 month post 6-OHDA. Then evaluated for 62 weeks. Testing on both the cylinder and placing tests (b-c) revealed a smooth and ever growing improvement in performance over the 62 week testing period. Data are presented as mean ± SEM of 8 animals per group. Multiple comparisons were based on significant main effects or interactions resulting from ANOVAs that are described in Results: ∗p<0.05.
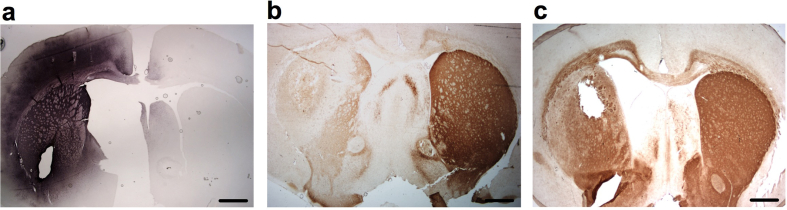
At the conclusion of the long-term study (62 weeks) the devices were retrieved, the brains processed and stained for GDNF and TH. All devices were easily retrieved intact without any evidence of adherent tissue. The GDNF output of the devices prior to implant was approximately 345 ng/day. At explant, GDNF output remained high at approximately 222 ng/day. The continued secretion of GDNF was paralleled by a robust and striatal-wide diffusion of GDNF (Fig. 7a). GDNF was visible throughout the implanted striatum as well as the overlying cortex which the implant extended through. Paralleling the robust neurological recovery and diffusion of GDNF throughout the striatum was a significant enhancement of striatal TH-positive fibers. While control animals exhibited a marked loss of dopaminergic fibers post 6-OHDA (Fig. 7b), animals treated with GDNF exhibited a dense TH fiber network surrounding the implant site and extending throughout the implanted striatum (Fig. 7c).
Fig. 7.
GDNF immunoreactivity in rat striatum 62 weeks following implantation of encapsulated GDNF-secreting cells (a) is associated with a pronounced preservation of TH-positive fibers that normally occurs post 6-OHDA (b–c). Scale bars = 500 μm.
3.6. GDNF distribution from clinical-sized devices in minipig brain
Following implantation, all animals recovered rapidly post-surgery and demonstrated normal activity and feeding behavior within several hours post-recovery. The formal monthly health status assessments confirmed the normal behavior of all animals at all times during the study. Food consumption was normal throughout the study and the pigs gained weight at a rate that would be anticipated by previously published guidelines provided by the vendor.
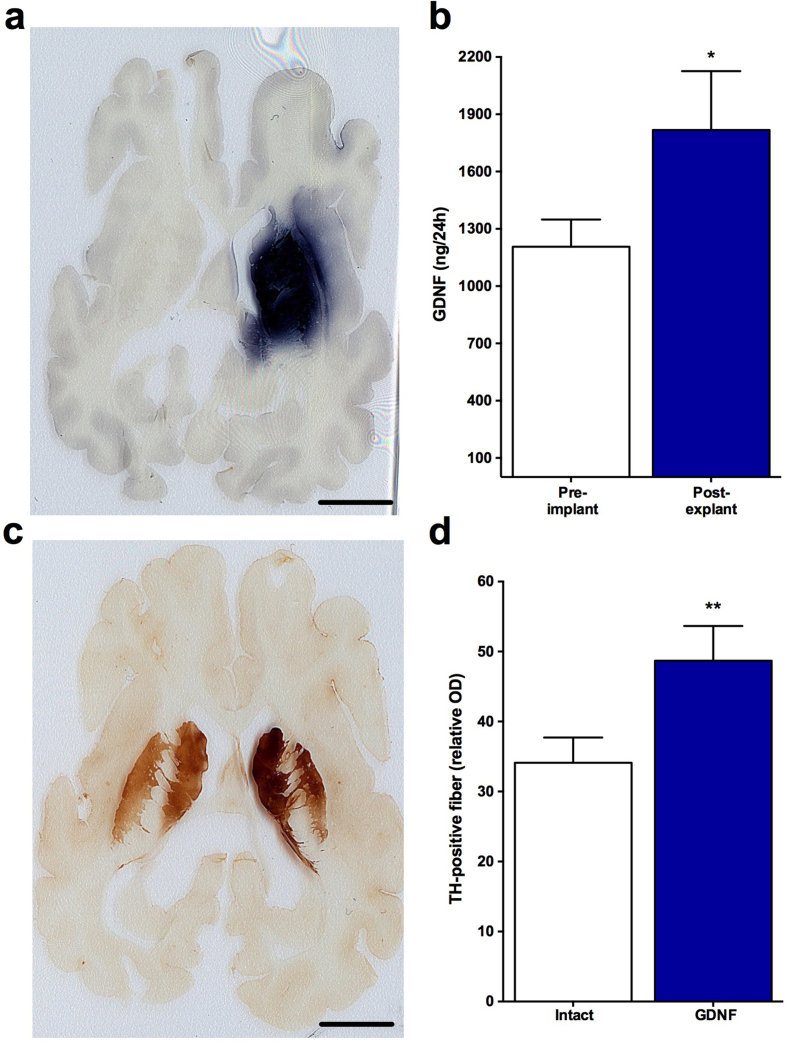
All devices were easily retrieved intact without any notable tissue adherence. Data from the 3 month test subjects demonstrated that all devices continued to secrete high levels of GDNF (Fig. 8b). Over the 3 month test period GDNF output increased by 51%. Immunohistochemistry confirmed that the secreted GDNF was widely distributed throughout the implanted striatum (Fig. 8a and b; pre-implant: 1206.33 ± 142.38; post-explant: 1817.42 ± 308.17; t (5) = 1.18, p = 0.021). As shown below in Fig. 8c and d, the diffusion of GDNF throughout the striatum from 2 devices robustly upregulated TH staining in the region of GDNF distribution. Quantitative determinations of the optical density of TH in the putamen and caudate confirmed the apparent upregulation of TH as the relative optical density of TH staining was increased by 48% in the treated striatum versus the non-implanted, control striatum (Intact: 34.11 ± 3.61; GDNF: 48.69 ± 4.97; t (5) = 5.13, p = 0.004).
Fig. 8.
Implantation of 2 clinical-sized devices into the right putamen of minipigs delivers GDNF for 12 weeks and produces widespread delivery of GDNF (a–b) throughout the implanted striatum. GDNF delivery is also associated with a profound biological upregulation on dopaminergic function as determined by enhanced TH-immunoreactivity and quantitative optical densitometry in the striatum (c–d). Scale bars = 5 mm. Data are presented as mean ± SEM of 6 animals. ∗p<0.05; ∗∗p<0.01, Student's t test.
3.7. Serum and CSF measurements
To assess the systemic safety and tolerability of GDNF, standard clinical chemistry and hematology was performed at baseline and again at explant 3 months later. In all pigs, the values for serum chemistry and blood counts at explant did not deviate from baseline ranges. Likewise, no changes were noted when the CSF was analyzed for total protein, cell count, glucose, phosphate, potassium, chloride and calcium (data not shown).
GDNF levels were quantified in serum and CSF using an ELISA specific for human GDNF that was validated for porcine serum and CSF matrices. GDNF was not detected in serum or CSF at baseline in any animal. In contrast, GDNF was detectable in serum, but not quantifiable, at 3 months in 4 out of 6 GDNF-treated animals (Table 2). CSF levels of GDNF were detectable and quantifiable in 5 out of 6 GDNF-treated pigs with levels ranging from 187 to 1001 pg/ml.
Table 2.
Serum and CSF measurements following GDNF implants in minipig Brain.
| Pig ID # |
Anti-GDNF Serum Antibodies |
CSF GDNF (pg/ml) |
Serum GDNF (pg/ml) |
|||
|---|---|---|---|---|---|---|
| IMPLANT | EXPLANT | IMPLANT | EXPLANT | IMPLANT | EXPLANT | |
| 1805 | NEG | NEG | BLQ | BLQ/DET | BLQ | BLQ |
| 1902 | NEG | NEG | BLQ | 187 | BLQ | BLQ/DET |
| 8936 | NEG | NEG | BLQ | 554 | BLQ | BLQ/DET |
| 1406 | NEG | NEG | BLQ | 249 | BLQ | BLQ |
| 1538 | NEG | NEG | BLQ | 1335 | BLQ | BLQ/DET |
| 2330 | NEG | NEG | BLQ | 1001 | BLQ | BLQ/DET |
BLQ: Below the Limit of Quantitation (94 pg/ml of serum and 64 pg/ml of CSF).
BLQ/DET: Below the Limit of Quantitation but Detectable.
3.8. Serum antibodies to human GDNF
Serum samples were collected at implant and at explant (3 months). Using a competitive ELISA to detect the presence of anti-GDNF antibodies, these analyses confirmed that the serum samples from all pigs were negative for anti-GDNF antibodies.
4. Discussion
While treating PD with pharmacologics such as levodopa and dopaminergic agonists provides temporary symptomatic relief of the motor symptoms, the progressive loss of dopaminergic neurons eventually renders these drugs ineffective and continued use results in side effects such as drug-induced dyskinesias (Fahn et al., 2004, Kucinski et al., 2013, Paolone et al., 2015). Trophic factors have been suggested to be a more optimal potential treatment to both restore the function of dopaminergic neurons while also protecting them from further damage and loss. In this regard, GDNF is a potent factor known to restore and augment nigrostriatal dopaminergic function in rodent and non-human primate models of PD (Dowd et al., 2005, Gash et al., 1996, Grondin et al., 2003, Kordower et al., 2000, Miyoshi et al., 1997, Palfi et al., 2002, Wang et al., 2002, Zheng et al., 2005).
Clinical evaluation of GDNF in PD patients has been promising but inconsistent, potentially because of insufficient distribution of GDNF throughout the nigrostriatal system. Several strategies are under development to optimize the diffusion and spread of trophic factors into brain tissue including direct brain infusion (Morrison, Lonser, & Oldfield, 2007), gene therapy approaches (Kordower et al., 2000, Palfi et al., 2002) and cell-based delivery using stem cells (Fjord-Larsen et al., 2012b, Gasmi et al., 2007, Shi et al., 2011). Here we describe the development of a delivery system based on implanting trophic factor-secreting cells that have been encapsulated inside of a polymer membrane. The pores of the membrane allow GDNF to cross the membrane into the surrounding brain tissue while protecting the encapsulated cells from host immune destruction. Previous iterations of this general technological approach were generally supportive of the notion but lacked the high-level section and long-term time duration shown here (Emerich et al., 1996, Kishima et al., 2004, Lindner et al., 1995). These novel data demonstrate, an implantable and removable encapsulated cellular device capable of providing high levels of human native and glycosylated GDNF suitable for dopaminergic neuroprotection and restoration (Hadaczek et al., 2010, Nanobashvili et al., 2019, Paolone et al., 2019). The long-term (at least 6 months) targeted delivery of GDNF over the majority of the nigrostriatal system without host-cell modification makes the system attractive for clinical translation in patients with Parkinson's disease. Even with significantly enhanced secretion of factors such as GDNF, multiple implant sites will still likely be required to deliver enough factor to cover the target region in a human brain. The present studies with 2 implants in minipig brain demonstrated robust delivery of GDNF throughput the entire striatum with diffusion distances >5 mm along the entire axis of each implant. Based on these data near complete coverage of the human putamen could be achieved with 3–4 implants identical in size and configuration to those used here. Multiple implants should not pose significant surgical or safety concerns as previous regulatory approved clinical trials have already incorporated the concurrent insertion of up to 4 implants into the brains of Alzheimer's patients with a good safety record (Wahlberg et al., 2012).
Immunohistochemistry confirmed that GDNF was distributed throughout the striatum and that this widespread distribution resulted in a virtually complete protection of dopaminergic neurons in the 6-OHDA-lesioned substantia nigra and preservation of TH-positive fibers in the striatum. These same animals demonstrated normal motor performance when evaluated on 3 separate neurological tests (cylinder, placing, and stepping tests). While modest residual deficits were noted on the cylinder and stepping tests in treated animals these changes were very small (<5% change from baseline) and did not reach statistical significance. While the neuroprotection paradigm described in this report required relatively short-term GDNF delivery, subsequent data provided substantial support for the ability of encapsulated cells to promote long-term, controlled, and functional delivery of GDNF to the nigrostriatal system. First, studies in rats quantified the 6-month continued delivery of GDNF to the striatum. Compared to pre-implant levels, GDNF secretion from explanted devices initially increased for the first couple of weeks and then stabilized at approximately pre-implant levels for the remaining 6 months. Second, the release of GDNF from the encapsulated cells resulted in robust elevations of GDNF in the tissue surrounding the implant site. Again, GDNF levels in tissue initially peaked early but then settled into a long (6 months) and stable elevation within the implanted striatum. Third, GDNF delivery from encapsulated cells promoted neurological recovery in the already lesioned nigrostriatal system (i.e; a neurorecovery/restorative model). When rats received implants post 6-OHDA a slow, steady improvement was noted in both the cylinder and placing tests. This improvement started within 1–2 months and continued to gain momentum over the 62 week test period. The observation of long-term (at least 62 weeks) GDNF immunoreactivity throughout the rodent striatum together with a marked recovery in TH-positive fiber density in the previously denervated striatum suggest that at least some of the motor recovery is accounted for by sprouting or regeneration of post-lesion, residual dopaminergic terminals. Follow up studies will be required to precisely correlate the timing of GDNF delivery to neurochemical and/or anatomical changes that contribute to neurological recovery. Nonetheless, the combined neuroprotective and neurorestorative actions described here suggest the potential of encapsulated cell-based GDNF delivery to provide a potent first in class disease modifying therapy.
5. Conclusion
These results describe the use of polymer-encapsulated GDNF-secreting cells for direct and local delivery of GDNF to the brain, in hopes of developing a treatment for PD patients. These studies demonstrated long-term and stable bioactive/efficacious effects on the nigrostriatal dopaminergic system. As such, this delivery system represents a novel version of previous encapsulated cell-based systems to deliver trophic factors to the brain and may be developed into a disease modifying and effective treatment for PD and other diseases (Saito et al., 2017, Tornøe et al., 2012, Falcicchia et al., 2018, Emerich et al., 2019, Nanobashvili et al., 2019, Orive et al., 2019, Paolone et al., 2019).
Author disclosure
LUW and DFE are employees of Gloriana Therapeutics a for profit biotechnology company which is developing the encapsulated cell technology to treat CNS diseases. WJB and TF were employed at Gloriana Therapeutics.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Author contributions
Conceptualization, Methodology: LUW, DFE.
Data curation, Writing- Original draft preparation: LUW, DFE, JHK, WJB, TF, GP.
Visualization, Investigation: LUW, DFE.
Supervision: LUW, DFE.
Writing- Reviewing and Editing: LUW, DFE, JHK, GP.
Declaration of competing interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgements
This research was supported by the Michael J. Fox Foundation for Parkinson's Research to LUW. Title of the grant: Pre-clinical Development of Encapsulated Cell Therapy for Parkinson's Disease.
Contributor Information
Lars U. Wahlberg, Email: LUW@GlorianaTx.com.
Giovanna Paolone, Email: giovanna.paolone@univr.it.
References
- Dowd E., Monville C., Torres E.M., Wong L.F., Azzouz M., Mazarakis N.D., et al. Lentivector-mediated delivery of GDNF protects complex motor functions relevant to human Parkinsonism in a rat lesion model. Eur. J. Neurosci. 2005;22:2587–2595. doi: 10.1111/j.1460-9568.2005.04414.x. http://doi:10.1111/j.1460-9568.2005.04414.x [DOI] [PubMed] [Google Scholar]
- Emerich D.F., Kordower J.H., Chu Y., Thanos C., Bintz B., Paolone G., et al. Widespread striatal delivery of GDNF from encapsulated cells prevents the anatomical and functional consequences of excitotoxicity. Neural. Plast. 2019;11:2019–2027. doi: 10.1155/2019/6286197. http://doi:10.1155/2019/6286197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich D.F., Orive G., Thanos C., Tornoe J., Wahlberg L.U. Encapsulated cell therapy for neurodegenerative diseases: From promise to product. Adv. Drug Deliv. Rev. 2014;67:131–141. doi: 10.1016/j.addr.2013.07.008. http://doi:10.1016/j.addr.2013.07.008 [DOI] [PubMed] [Google Scholar]
- Emerich D.F., Plone M., Francis J., Frydel B.R., Winn S.R., Lindner M.D. Alleviation of behavioral deficits in aged rodents following implantation of encapsulated GDNF-producing fibroblasts. Brain. Res. 1996;736:99–110. http://doi:10.1016/0006-8993(96)00683-X [PubMed] [Google Scholar]
- Eyjolfsdottir H., Eriksdotter M., Linderoth B., Lind G., Juliusson B., Kusk P., et al. Targeted delivery of nerve growth factor to the cholinergic basal forebrain of alzheimer's disease patients: Application of a second-generation encapsulated cell biodelivery device. Alzheimers Res. Ther. 2016;8:30–39. doi: 10.1186/s13195-016-0195-9. http://doi:10.1186/s13195-016-0195-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S., Oakes D., Shoulson I., Kieburtz K., Rudolph A., Lang A., et al. Parkinson Study Group. Levodopa and the progression of Parkinson's disease. N Engl. J. Med. 2004;12:183–189. doi: 10.1056/NEJMoa033447. http://doi:10.1056/NEJMoa033447 [DOI] [PubMed] [Google Scholar]
- Falcicchia C., Paolone G., Emerich D.F., Lovisari F., Bell W., Fradet T., et al. Seizure-suppressant and neuroprotective effects of encapsulated BDNF-producing cells in a rat model of temporal lobe epilepsy. Mol. Ther. Methods Clin. Dev. 2018;9:211–224. doi: 10.1016/j.omtm.2018.03.001. http://doi:10.1016/j.omtm.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjord-Larsen L., Kusk P., Emerich D.F., Thanos C., Torp M., Bintz B., et al. Increased encapsulated cell biodelivery of nerve growth factor in the brain by transposon-mediated gene transfer. Gene. Ther. 2012;19:1010–1017. doi: 10.1038/gt.2011.178. http://doi:10.1038/gt.2011.178 [DOI] [PubMed] [Google Scholar]
- Fjord-Larsen L., Kusk P., Tornøe J., Juliusson B., Torp M., Bjarkam C.R., et al. Long-term delivery of nerve growth factor by encapsulated cell biodelivery in the Göttingen minipig basal forebrain. Mol. Ther. 2010;18:2164–2172. doi: 10.1038/mt.2010.154. http://doi:10.1038/mt.2010.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjord-Larsen L., Kusk P., Torp M., Sørensen J.C.H., Ettrup K., Bjarkam C.R., et al. Encapsulated cell biodelivery of transposon-mediated high-dose NGF to the göttingen Mini pig basal forebrain. Open Tiss. Engin. Regen. Med. Journal. 2012;5:35–42. http://doi:10.2174/1875043501205010035 [Google Scholar]
- Gash D.M., Zhang Z., Ovadia A., Cass W.A., Yi A., Simmerman L., et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. http://doi:10.1038/380252a0 [DOI] [PubMed] [Google Scholar]
- Gasmi M., Brandon E.P., Herzog C.D., Wilson A., Bishop K.M., Hofer E.K., et al. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: Long-term efficacy and tolerability of CERE-120 for Parkinson's disease. Neurobiol. Dis. 2007;27:66–76. doi: 10.1016/j.nbd.2007.04.003. http://doi:10.1016/j.nbd.2007.04.003 [DOI] [PubMed] [Google Scholar]
- Greenwald A.G., Gonzalez R., Harris R.J., Guthrie D. Effect sizes and p values: What should be reportedand what should be replicated? Psychophysiology. 1996;33:175–183. doi: 10.1111/j.1469-8986.1996.tb02121.x. http://doi:10.1111/j.1469-8986.1996.tb02121.x [DOI] [PubMed] [Google Scholar]
- Grondin R., Zhang Z., Ai Y., Gash D.M., Gerhardt G.A. Intracranial delivery of proteins and peptides as a therapy for neurodegenerative diseases. Prog. Drug Res. 2003;61:101–123. doi: 10.1007/978-3-0348-8049-7_4. http://doi:10.1007/978-3-0348-8049-7_4 [DOI] [PubMed] [Google Scholar]
- Hadaczek P., Johnston L., Forsayeth J., Bankiewicz K.S. Pharmacokinetics and bioactivity of glial cell line-derived factor (GDNF) and neurturin (NTN) infused into the rat brain. Neuropharmacology. 2010;58:1114–1121. doi: 10.1016/j.neuropharm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishima H., Poyot T., Bloch J., Dauguet J., Condé F., Dollé F., et al. Encapsulated GDNF-producing C2C12 cells for Parkinson's disease: A pre-clinical study in chronic MPTP-treated baboons. Neurobiol. Dis. 2004;16:428–439. doi: 10.1016/j.nbd.2004.03.012. http://doi:10.1016/j.nbd.2004.03.012 [DOI] [PubMed] [Google Scholar]
- Kordower J.H., Emborg M.E., Bloch J., Ma S.Y., Chu Y., Leventhal L., et al. Neurodegeneration prevented by lentiviral delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. http://doi:10.1126/science.290.5492.767 [DOI] [PubMed] [Google Scholar]
- Kordower J.H., Palfi S., Chen E.Y., Ma S.Y., Sendera T., Cochran E.J., et al. Clinicopathological findings following intraventricular glial-derived neurotrohpic factor in a patient with Parkinson's disease. Ann. Neurol. 1999;46:419–424. doi: 10.1002/1531-8249(199909)46:3. <419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Kucinski A., Paolone G., Bradshaw M., Albin R.L., Sarter M. Modeling fall propensity in Parkinson's disease: Deficits in the attentional control of complex movements in rats with cortical-cholinergic and striatal-dopaminergic deafferentation. J Neurosci. 2013;33:16522–16539. doi: 10.1523/JNEUROSCI.2545-13.2013. http://doi:10.1523/JNEUROSCI.2545-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A.E., Gill S., Patel N.K., Lozano A., Nutt J.G., Penn R., et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrohpic factor infusion in Parkinson's disease. Ann. Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. http://doi:10.1002/ana.20737 [DOI] [PubMed] [Google Scholar]
- Lindner M.D., Winn S.R., Baetge E.E., Hammang J.P., Gentile F.T., Doherty E., et al. Implantation of encapsulated catecholamine and GDNF-producing cells in rats with unilateral dopamine depletions and parkinsonian symptoms. Exp. Neurol. 1995;131:62–76. doi: 10.1016/0014-4886(95)90059-4. http://doi:10.1016/0014-4886(95)90059-4 [DOI] [PubMed] [Google Scholar]
- Lindvall O., Wahlberg L.U. Encapsulated cell biodelivery of GDNF: A novel clinical strategy for neuroprotection in Parkinson's disease? Exp. Neurol. 2008;209:82–88. doi: 10.1016/j.expneurol.2007.08.019. http://doi:10.1016/j.expneurol.2007.08.019 [DOI] [PubMed] [Google Scholar]
- Miyoshi Y., Zhang Z., Ovadia A., Lapchak P.A., Collins F., Hilt D., et al. Glial cell line-derived neurotrophic factor-levodopa interactions and reduction of side effects in parkinsonian monkeys. Ann. Neurol. 1997;42:208–214. doi: 10.1002/ana.410420212. http://doi:10.1002/ana.410420212 [DOI] [PubMed] [Google Scholar]
- Morrison P.F., Lonser R.R., Oldfield E.H. Convective delivery of glial cell line-derived neurotrophic factor in the human putamen. J. Neurosurg. 2007;107:74–83. doi: 10.3171/JNS-07/07/0074. http://doi:10.3171/JNS-07/07/0074 [DOI] [PubMed] [Google Scholar]
- Nanobashvili A., Melin E., Emerich D., Tornøe J., Simonato M., Wahlberg L.U., et al. Unilateral ex vivo gene therapy by GDNF in epileptic rats. Gene. Ther. 2019;26:65–74. doi: 10.1038/s41434-018-0050-7. http://doi:10.1038/s41434-018-0050-7 [DOI] [PubMed] [Google Scholar]
- Nutt J.G., Burchiel K.J., Comella C.L., Jankovic J., Lang A.E., Laws E.R., Jr., et al. ICV GDNF Study Group, Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;14:69–73. doi: 10.1212/wnl.60.1.69. http://doi:10.1212/wnl.60.1.69 [DOI] [PubMed] [Google Scholar]
- Orive G., Echave M.C., Dolatshahi-Pirouz A., Paolone G., Emerich D.F. Advances in cell-laden hydrogels for delivering therapeutics. Expert. Opin. Biol. Ther. 2019;19:1219–1222. doi: 10.1080/14712598.2019.1654452. http://doi:10.1080/14712598.2019.1654452 [DOI] [PubMed] [Google Scholar]
- Palfi S., Leventhal L., Chu Y., Ma S.Y., Emborg M., Bakay R., et al. Lentivirally delivered glial cell line-derived neurotrophic factor increases the number of striatal dopaminergic neurons in primate models of nigrostriatal degeneration. J. Neurosci. 2002;22:4942–4954. doi: 10.1523/JNEUROSCI.22-12-04942.2002. http://doi:10.1523/JNEUROSCI.22-12-04942.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G., Brugnoli A., Arcuri A., Mercatelli D., Morari M. Eltoprazine prevents levodopa-induced dyskinesias by reducing striatal glutamate and direct pathway activity. Mov. Disord. 2015;30:1728–1738. doi: 10.1002/mds.26326. http://doi:10.1002/mds.26326 [DOI] [PubMed] [Google Scholar]
- Paolone G., Falcicchia C., Lovisari F., Kokaia M., Bell W., Fradet T., et al. Long-term, targeted delivery of GDNF from encapsulated cells is neuroprotective and reduces seizures in the pilocarpine model of epilepsy. J Neurosci. 2019;39:2244–2256. doi: 10.1523/JNEUROSCI.0435-18.2018. http://doi:10.1523/JNEUROSCI.0435-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N., Washio A., Miyashita K., Wahlberg L.U., Emerich D.F., Morotomi T., et al. Effects of polymer encapsulated glial cell line-derived neurotrophic factor secreting cells on odontoblast-like cell survival. J. Regen. Med. 2017;6:3–11. http://doi:10.4172/2325-9620.1000140 [Google Scholar]
- Salvatore M.F., Ai Y., Fischer B., Zhang A.M., Grondin R.C., Zhang Z., et al. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp. Neurol. 2006;202:497–505. doi: 10.1016/j.expneurol.2006.07.015. http://doi:10.1016/j.expneurol.2006.07.015 [DOI] [PubMed] [Google Scholar]
- Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. http://doi:10.1016/j.nurx.2006.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer T.B., Fiske B.K., Svendsen C.N., Lang A.E., Langston J.W. Crossroads in GDNF therapy for Parkinson's disease. Mov. Disord. 2006;21:136–141. doi: 10.1002/mds.20861. http://doi:10.1002/mds.20861 [DOI] [PubMed] [Google Scholar]
- Shi D., Chen G., Lv L., Li L., Wei D., Gu P., et al. The effect of lentivirus-mediated TH and GDNF genetic engineering mesenchymal stem cells on Parkinson's disease rat model. Neurol. Sci. 2011;32:41–51. doi: 10.1007/s10072-010-0385-3. http://doi:10.1007/s10072-010-0385-3 [DOI] [PubMed] [Google Scholar]
- Simonato M., Falcicchia C., Paolone G. In: Cell Therapy. Emerich D.F., Orive G., editors. Humana Press; 2017. Cell therapy for epilepsy; pp. 85–97. pp.85-98. [DOI] [Google Scholar]
- Tornøe J., Torp M., Jørgensen J.R., Emerich D.F., Thanos C., Bintz B., et al. Encapsulated cell-based delivery of Meteorin is neuroprotective in the quinolinic acid rat model of neurodegenerative disease. Restor. Neurol. Neurosci. 2012;30:25–36. doi: 10.3233/RNN-2012-110199. http://doi:10.3233/RNN-2012-110199 [DOI] [PubMed] [Google Scholar]
- Wahlberg L.U., Lind G., Almqvist P.M., Kusk P., Tornøe J., Juliusson B., et al. Targeted delivery of nerve growth factor via encapsulated cell biodelivery in alzheimer disease: A technology platform for restorative neurosurgery. J. Neurosurg. 2012;117:340–347. doi: 10.3171/2012.2.JNS11714. http://doi:10.3171/2012.2.JNS11714 [DOI] [PubMed] [Google Scholar]
- Wang L., Muramatsu S., Lu Y., Ikeguchi K., Fujimoto K., Okada T., et al. Delayed delivery of AAV-GDNF prevents nigral degeneration and promotes functional recovery in a rat model of Parkinson's disease. Gene. Ther. 2002;9:381–389. doi: 10.1038/sj.gt.3301682. http://doi:10.1038/sj.gt.3301682 [DOI] [PubMed] [Google Scholar]
- Zheng J.S., Tang L.L., Zheng S.S., Zhan R.Y., Zhou Y.Q., Goudreau J., et al. Delayed gene therapy of glial cell line-derived neurotrophic factor is efficacious in a rat model of Parkinson's disease. Brain Res. Mol. Brain Res. 2005;134:155–161. doi: 10.1016/j.molbrainres.2004.06.029. http://doi:10.1016/j.molbrainres.2004.06.029 [DOI] [PubMed] [Google Scholar]