Abstract
The DNA damage response (DDR) is now known to play an important role in both cancer development and its treatment. Targeting proteins such as ATR (Ataxia telangiectasia mutated and Rad3-related) kinase, a major regulator of DDR, has demonstrated significant therapeutic potential in cancer treatment, with ATR inhibitors having shown anti-tumour activity not just as monotherapies, but also in potentiating the effects of conventional chemotherapy, radiotherapy, and immunotherapy. This review focuses on the biology of ATR, its functional role in cancer development and treatment, and the rationale behind inhibition of this target as a therapeutic approach, including evaluation of the progress and current status of development of potent and specific ATR inhibitors that have emerged in recent decades. The current applications of these inhibitors both in preclinical and clinical studies either as single agents or in combinations with chemotherapy, radiotherapy and immunotherapy are also extensively discussed. This review concludes with some insights into the various concerns raised or observed with ATR inhibition in both the preclinical and clinical settings, with some suggested solutions.
Keywords: DDR, ATR, ATM, ATR inhibitors, Chemo- and radiosensitisers
Graphical abstract
Highlights
-
•
The DNA Damage Response (DDR) is of significant interest in cancer research.
-
•
ATR kinase is a promising target for cancer therapeutics and drug discovery.
-
•
ATR kinase inhibitors have recently begun evaluation in cancer clinical trials.
-
•
First comprehensive review of the history and current status of ATR inhibitor development.
1. Introduction: An overview of DNA damage response (DDR) machinery in cancer
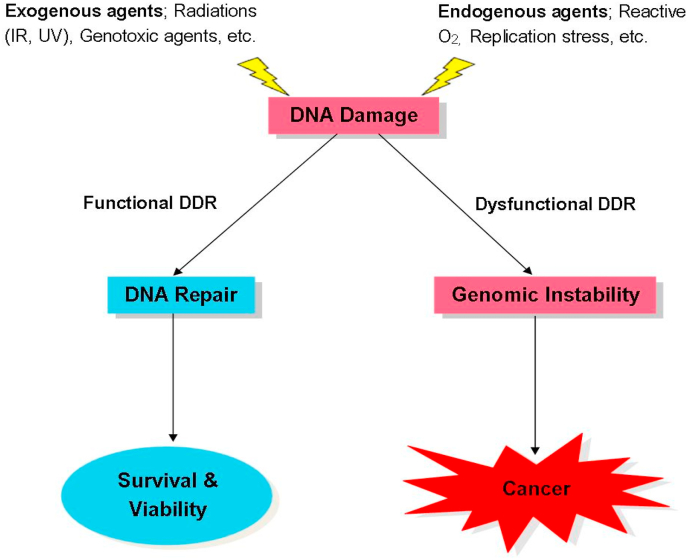
The integrity of human DNA is constantly subject to damage either by exogenous exposure to radiation or genotoxic agents, or by endogenous reactive and oxidative by-products of normal cellular metabolisms. This damage, if not repaired or incorrectly repaired, becomes lethal to the cell or organism (De Bont and van Larebeke, 2004; Jackson and Bartek, 2009). Constant and efficient repair of this DNA damage is therefore essential and biologically significant in preventing cellular death and many human diseases including cancer (Jackson and Bartek, 2009; Roos and Kaina, 2006). Thus, considering the genomic threat posed by DNA damage, cells respond to this DNA damage by activating a complex but distinct network of signalling pathways collectively termed the DNA Damage Response (DDR) that repair damage to constantly maintain the integrity of the genome and prevent the development of diseases such as cancer (Fig. 1).
Fig. 1.
DDR and cancer development. The presence of DNA damage either by exogenous or endogenous agents triggers the functional mechanisms of DDR leading to the rapid and efficient repair of DNA damage through cell cycle arrest and delays, and in some cases, apoptosis of cells when DNA damages accumulate beyond repair. This maintains genomic integrity which is critical for cell survival and viability. In contrast, DDR dysfunctions, which may be due to mutations and/or dysregulation of DDR mechanisms, can lead to inefficient or unrepaired DNA damage that in turn destabilise the genome of these cells. Genomic instability induces various aberrant cellular behaviours leading to the development of cancers.
The DDR comprises processes and mechanisms through which DNA damage is detected and repaired to maintain genomic stability and integrity, and this is significant to the survival and viability of a cell or organism (De Bont and van Larebeke, 2004). DDR pathways are crucial to both the development of cancers and their treatments, as cancer cells with defective DDR mechanisms exhibit high sensitivity to certain therapeutics, notably DNA damaging agents (Karnitz and Zou, 2015; Fong et al., 2009) and also many of these defects are known to drive cancer formation (Jackson and Bartek, 2009; Kandoth et al., 2013).
Genomic instability is a widely known hallmark of cancer, which may occur as a result of dysfunctional and/or dysregulation of DDR mechanisms (Hanahan and Weinberg, 2000, 2011; Negrini et al., 2010). For example, in hereditary cancers such as breast cancer, genomic instability, which drives these cancers, is known to result from mutations in DNA repair genes such as BRCA 1/2 (Bartkova et al., 2005). Defects in DDR mechanisms are generally known as major drivers of development in most cancers (Bartkova et al., 2005, 2006; Gorgoulis et al., 2005), such that functional loss (mutation) and/or dysregulation of key DDR genes and proteins are the primary molecular features of many cancers (Kandoth et al., 2013; Lavin, 2008). Such deficiencies in DDR either confer a growth advantage on tumours, thereby breaking the proliferation barrier posed by DDR and allowing the aggressive transformation of pre-cancerous cells to malignant tumours (Gorgoulis et al., 2005; Bartkova et al., 2006), or they increase the risk of cancer development. For example, women with mutated BRCA genes are more predisposed to developing breast and ovarian cancers than those with non-mutated genes (Pal et al., 2005; Levy-Lahad and Friedman, 2007). It is also known, however, and has become more apparent recently that the impairment of DDR mechanisms may significantly impact on the success (or otherwise) of cancer treatments, especially DNA damaging therapies such as cisplatin, irinotecan, gemcitabine, and ionizing radiation (IR) (Woods and Turchi, 2013). These DNA-damaging therapies function by inducing DNA damage, which is cytotoxic to highly proliferating cells (Johnson and Westcott, 2016). The response of cancer cells to such DNA damage therefore critically determines the success of these treatments. The inability of the cancer cells to efficiently and rapidly repair sufficiently high levels of DNA damage due to their DDR impairments will ultimately lead to cell death and hence account for the increased treatment efficacy of these therapies.
This positive therapeutic impact of DDR impairment of cancer cells on cancer treatment is mostly overcome by the inherent ability of tumours to activate (or re-activate) DDR mechanisms as a strategic response to escape the potentially lethal therapeutic effects of these anti-cancer therapies, however (Woods and Turchi, 2013; Karnitz et al., 2005). This perhaps explains the poor response and tumour resistance often observed in most solid tumours with these agents in the clinic. For example, tumour cells that have been shown to emerge as resistant to the DNA-damaging agents (cisplatin and gemcitabine) after prolonged treatments, are also accompanied by extremely high expression of DDR genes and proteins (Bao et al., 2006; Oliver et al., 2010). These findings clearly underscore the crucial role of DDR pathways in both the development and treatment of cancers. DDR and its regulators have therefore become attractive and promising strategic targets for novel cancer therapy: the exploitation of these pathways can provide a platform to develop novel anti-cancer drugs that can act as chemo- and/or radiosensitisers to enhance the therapeutic response of current conventional DNA-damaging anti-cancer therapies (Fong et al., 2009; Garrett and Collins, 2011; Bornstein and Jimeno, 2016). Moreover, the interplay between major DDR regulators in response to DNA damage creates a ‘synthetic lethality’ like dependency, where functional loss of DDR components as observed in most tumour cells leads to a greater reliance on the residual DDR factors to maintain viability and survival following DNA damage (Kaelin, 2005; Reaper et al., 2011). Tumour cells are known to exhibit severe and excessive DNA damage during tumourigenesis due to oncogenic-induced replication stress and genomic instability, which may trigger apoptosis or senescence of these cells if left unrepaired (Hanahan and Weinberg, 2011; Bartkova et al., 2006). Thus, with most DDR key regulators, including ATM and P53, being mutated or dysregulated in the majority of tumours (Karnitz et al., 2005), tumour cells are more likely to rely on residual pathways such as the ATR pathway in order to repair and survive this self-inflicted excessive DNA damage, and its consequential cell death (Nghiem et al., 2001; Weber and Ryan, 2015; Foote et al., 2015).
Targeting these residual DDR pathways may therefore be selectively toxic to cancer cells with mutations in certain DDR genes. An example of this ‘synthetic lethality’ concept is the inhibition of poly ADP-ribose polymerase (PARP), a DNA damage repair enzyme, which has been shown to be selectively lethal to cancer cells harbouring mutations in some DDR genes, including BRCA1/2 (Fong et al., 2009; Bryant et al., 2005; Farmer et al., 2005). This observation has led to the development of PARP inhibitors olaparib, rucaparib and niraparib which are all currently approved by the Food and Drug Administration (FDA) and European Medicine Agency (EMA) for the treatment of ovarian cancers (Bitler et al., 2017; Jiang et al., 2019). Olaparib and rucaparib have also been approved by the FDA for the treatment of prostate cancer (BRCA 1/2 and ATM gene mutated) (National Cancer Institute Website, 2020), with various other candidates including veliparib, talazoparib and fluzoparib at various stages of clinical development (Bitler et al., 2017; Jiang et al., 2019; Wang et al., 2019).
These DDR response signalling pathways have in recent times gained significant attention in cancer therapy, with various DDR proteins including ATR, ATM, DNA-PK, CHK1, CHK2, Wee1, and PARP now all considered promising targets for anti-cancer drug development (Jackson and Bartek, 2009; Carrassa and Damia, 2017; Brown et al., 2017). With the exception of PARP inhibitors (which have been successfully approved for clinical use) inhibitors of other DDR proteins have yet to realise their clinical potential (Brown et al., 2017). The ATR-CHK1 pathway, a major pathway of the DDR machinery, is one of most (if not the most) researched DDR pathways in cancer (Jackson and Bartek, 2009). Despite over two decades of enormous efforts, an ATR inhibitor for cancer treatment is yet to be clinically successful. In this review, we comprehensively discuss the functional role of ATR in DDR, particularly in relation to cancer development and treatment, and focus on the preclinical studies describing potential ATR inhibition in cancer therapy; hence the rationale behind the development of ATR inhibitors for cancer treatment. The progress and current status of all published ATR inhibitors (13 at the time of writing) since the report of the first (Schisandrin B), including the new generation of ATR inhibitors that has entered clinical development, are also discussed. We conclude by offering some insights into concerns that have been raised and reportedly observed following use of ATR inhibitors in the clinic, and consider how these shortcomings may be addressed so that this promising class of agent fulfils its potential.
1.1. Structure of ATR
ATR (Ataxia telangiectasia and Rad3 related protein) is a member of the phosphatidylinositol 3-kinase-related kinase (PIKK) family of serine/threonine protein kinases, which also includes ATM (Ataxia telangiectasia mutated protein), DNA-dependent protein kinase (DNA-PK), mammalian target of rapamycin (mTOR), suppressor of morphogenesis in genitalia (SMG1) and transformation/transcription domain-associated protein (TRRAP) (Cimprich and Cortez, 2008; Lempiainen and Halazonetis, 2009). Despite the lack of a kinase domain and activity of TRRAP (McMahon et al., 1998), it is still considered a member of this family since it shows high sequence similarities with other PIKK members (Lempiainen and Halazonetis, 2009). The cellular functions of this PIKK family are diverse, ranging from crucial roles in DDR (ATM, ATR and DNA-PK), control of cell growth, proliferation, metabolism (mTOR and ATM), nonsense-mediated mRNA decay (SMG1) and epigenetic regulation of transcription (TRRAP) (Weber and Ryan, 2015; Lempiainen and Halazonetis, 2009; Shiloh and Kastan, 2001), with their dysfunctions implicated in range of diseases, including immunodeficiency, neurological disorder and notably cancer (Jackson and Bartek, 2009).
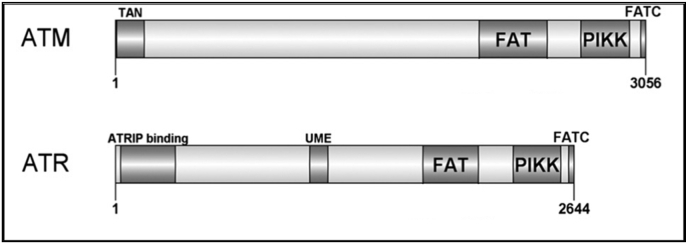
ATR is one of the two apical regulators of the DDR pathway that initiate and coordinate cellular responses to DNA damage and stress in cooperation with other DDR proteins (Jackson and Bartek, 2009; Cimprich and Cortez, 2008). These responses include activation of cell cycle checkpoints, DNA repair, transcriptional response and induction of apoptosis if necessary (Sancar et al., 2004). ATR is a relatively large protein at 300 kDa, and consisting of 2644 amino acids (Cimprich and Cortez, 2008; Gately et al., 1998). Structurally, ATR kinase shares significant similarities in its distinct domain architecture and sequence homology with ATM (another apical regulator of the DDR pathways) (Fig. 2), (Weber and Ryan, 2015; Lempiainen and Halazonetis, 2009; Flynn and Zou, 2011; Bosotti et al., 2000) which suggests similarities in the regulation mechanisms of these kinases (Cimprich and Cortez, 2008). For example, both kinases are known to preferentially phosphorylate serine or threonine residues followed by glutamine (SQ/TQ motif) (Matsuoka et al., 2007). However, despite exhibiting a large degree of similarity in most of their domains, the N-terminal domains of the two kinases ATM and ATR, are observed to share low sequence homology, and since members of the PIKK family are known to interact with distinct regulatory partners (Flynn and Zou, 2011), it is believed that the uniqueness of this N-terminal region is essential for differential association with their distinct and relative adapter proteins and downstream substrates (Cortez et al., 2001; Fernandes et al., 2005). For example, the N-terminus of both ATM and ATR contain either a distinct protein interacting domain or binding sites for Tel1 (TAN) (Seidel et al., 2008), p53 and BRCA1 (Fernandes et al., 2005), and ATRIP (Cortez et al., 2001), which are essential for the signalling of these kinases upon various types of DNA damage.
Fig. 2.
Schematic diagram of the domain structure of ATM and ATR. Known structural domains are shown for each protein; FRAP–ATM–TRRAP domain (FAT), FAT C-terminal domain (FATC), Phosphatidylinositol 3-kinase-related kinase domain (PIKK), Tel1/ATM N-terminal motif (TAN), ATR interacting protein (ATRIP), and UVSB PI3 kinase, MEI-41 and ESR1 domain (UME). ATM and ATR consist of 3656 and 2644 amino acids respectively. (Adapted from Weber and Ryan, 2015).
Besides ATM, ATR has also been shown to share some structural similarities and sequence homology with other DDR proteins, namely DNA-PK and mTOR, particularly within their catalytic loop with a conserved aspartate (D) residue that plays a key role in the kinase functions these proteins (Menolfi and Zha, 2020).
1.2. Signalling and functions of ATR in DDR
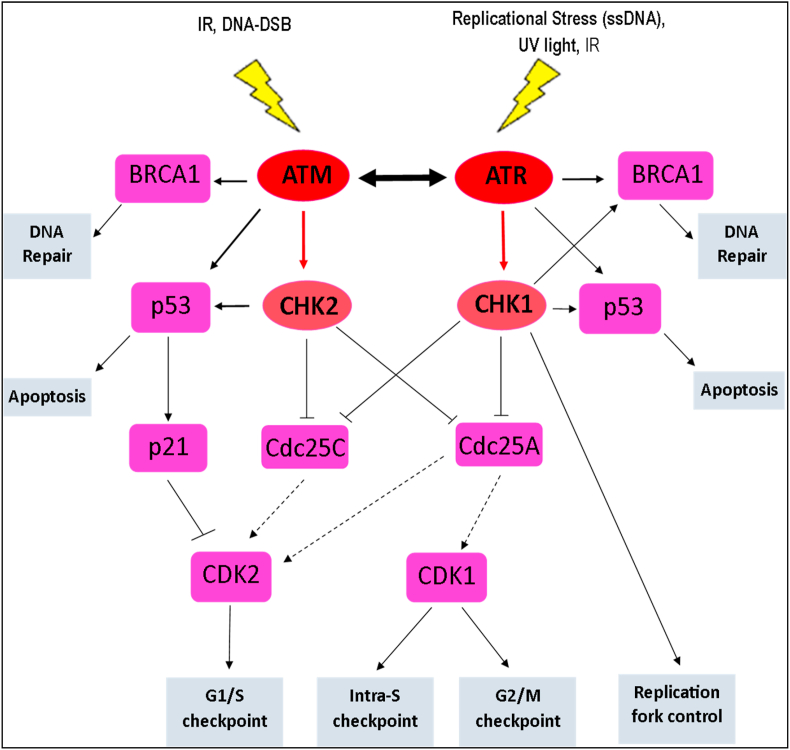
Although ATM and ATR are known to share multiple functional and structural similarities, as well as substrate phosphorylation specificity (serine or threonine residues followed by glutamine), they are primarily activated and respond to different types of DNA damage (Fig. 3). (Cimprich and Cortez, 2008) Whilst the activation of ATM kinase occurs in response to DNA double-strand breaks (DSB), which may occur as a result of either extrinsic IR exposure or intrinsic collapse of stalled replication forks, ATR kinase is activated by the extensive presence of single-stranded DNA structures (ssDNA) (Canman et al., 1998; van Gent et al., 2001; Bakkenist and Kastan, 2003).
Fig. 3.
The signalling cascades of ATM and ATR. ATM and ATR activate their distinct key mediator, CHK2 and CHK1 respectively, in response to respective DNA damage, and through various downstream substrates (p53, BRAC, Cdc25A, Cdc25c), which are commonly shared among these kinases, execute their respective functions to maintain the genomic integrity of cells. ATR and ATM may interconvert depending on the cellular content and the type of DNA damage to compensate for one another.
ATR is specifically activated in response to extensive single stranded DNA structures (ssDNA), which may primarily arise from a stalled DNA replication stress or other types of DNA damage, including resected DNA DSB, crosslinks, base adduct and inhibition of DNA polymerases (Cimprich and Cortez, 2008; Flynn and Zou, 2011). Also, in contrast to IR-induced activation of ATM, ATR is known to be activated by UV light rather than IR, and it is primarily, if not entirely, responsible for the initiation of the cellular response following UV-induced DNA damage (Abraham, 2001). However, despite reports describing ATR inactivation by IR, some studies have also suggested a delayed (compared to ATM signalling) but rapid indirect activation of ATR by IR in the presence ATM and Mre11 (Myers and Cortez, 2006). ATM and Mre11 are believed to be stimulated by IR-induced activation of ATR through the conversion of IR-induced DNA damage, normally DSB, into ssDNA which then activates ATR (Myers and Cortez, 2006).
The stability, localisation, activation and functions of ATR require a mutually dependent partner, namely ATR Interacting Protein (ATRIP) (Cortez et al., 2001). ATRIP is considered to be an obligatory subunit of the ATR kinase as no known phenotypic differences have been observed in organisms upon loss of ATR or ATRIP (Cortez et al., 2001). It is therefore suggested that ATR and ATRIP may exist and function as a complex (Flynn and Zou, 2011). While it was mentioned earlier that ssDNA activates the ATR pathway, it is actually the Replication Protein A (RPA)-coated ssDNA that triggers the activation of ATR, as most ssDNAs are rapidly coated by RPA once generated in cells (Flynn and Zou, 2011; Cimprich and Cortez, 2008). RPA binds to ssDNA through multiple oligonucleotide/oligosaccharide-binding folds (Marechal and Zou, 2015) and coordinates the recruitment and localised ATR-ATRIP complex to the sites of DNA damage and stress replication forks (Cortez et al., 2001). RPA-ssDNA is also crucial in the recruitment of topoisomerase (DNA) II binding protein 1 (TOPBP1), an activator of ATR in human cells (Kumagai et al., 2006).
Once ATR is activated, it executes its cellular functions through the phosphorylation and subsequent activation of many downstream substrates involved in a wide range of cellular processes of which many are not associated with DDR (Flynn and Zou, 2011). However, in response to DNA damage, ATR functions principally via the phosphorylation and activation of CHK1, the major downstream and best-studied target of ATR (Bartek and Lukas, 2003). This stabilises stalled replication forks, regulates origin firing, activates DNA damage repair and various checkpoints of the cell cycle, leading to the maintenance of earlier cycle arrest and induction of apoptosis or senescence when necessary (Cimprich and Cortez, 2008; Toledo et al., 2008). The crucial roles for ATR in DDR occur particularly at the S and G2 phases of the cell cycle (Sorensen et al., 2003). After activation by ATR, CHK1 subsequently phosphorylates and inhibits the activities of Cdc25A and Cdc25C to activate the intra S-checkpoint and the G2/M checkpoint of the cell cycle respectively (Sorensen et al., 2003; Sanchez et al., 1997; Xiao et al., 2003).
Cdc25 phosphatases are critical targets of the ATR signalling pathway via CHK1 due to their essential roles in the regulation and progression of the cell cycle (Flynn and Zou, 2011). CHK1 through the phosphorylation of Cdc25A mediates the degradation of Cdc25A leading to the subsequent inhibition of CDK1 and thus, inhibiting late replication origins during the intra-S checkpoint (Sorensen et al., 2003; Xiao et al., 2003). The inhibition of Cdc25C, through its phosphorylation by CHK1 also prevents the entry of the cell into the mitotic phase of the cell cycle, thus blocking the mitosis of cells with damaged DNA before DNA replication is completed (Sanchez et al., 1997).
In addition to this, ATR is also known to activate the G1/S cell cycle checkpoint through the phosphorylation and inhibition of Cdc25A by activated CHK1, in response to UV light-induced DNA damage (Bartek and Lukas, 2001). However, in conditions of continuous or excessive DNA damage, p53 is phosphorylated by both ATR and CHK1 for either continuous maintenance of the earlier cell cycle arrest or activation of apoptosis if necessary (Bartek and Lukas, 2001; Taylor and Stark, 2001). Unlike ATM, ATR has been shown to be an essential gene required for normal cell cycle progression even in the absence of genotoxic stress, and is important for cell viability and survival (Brown and Baltimore, 2000, 2003; Shechter et al., 2004). Total deletion of ATR is embryonically lethal in mice (Brown and Baltimore, 2000) and also results in defects in tissue homeostasis, depletion of progenitor cells in rapidly proliferating tissues and premature aging in adult mice (Brown and Baltimore, 2003; Ruzankina et al., 2007). Moreover, partial loss of ATR function in humans, though not lethal, results in a rare genetic disorder, Seckel syndrome. This is a disease characterized by dwarfism, intrauterine growth and mental retardation, severe microcephaly and a dysmorphic facial feature (O'Driscoll et al., 2003; Karnitz and Zou, 2015; Alderton et al., 2004). Cellular functions of ATM and ATR in response to DNA damage are known to partially overlap though are not necessary redundant, depending on the cellular context and type of DNA damage (Helt et al., 2005). For example, although CHK1 is known to be the specific key downstream target of ATR signalling, it can be also phosphorylated by ATM in response to IR (Sorensen et al., 2003). ATR is also known to phosphorylate, essentially, all downstream ATM-specific substrates such that in ATM-deficient cells, ATR activation is sufficient to activate the G1/S checkpoint - a principal function of ATM (Foote et al., 2015; Flynn and Zou, 2011).
ATR and ATM, as master controllers of DDR, may function closely together through their shared downstream targets, such that defects in one signalling pathway may significantly be compensated by the other, thus, ensuring efficient cellular response to various types of DNA damage in order to ultimately safeguard the integrity of the genome (Abraham, 2001; Cimprich and Cortez, 2008; Shiloh and Kastan, 2001).
1.3. ATR as a therapeutic target for cancer
Although ATR has been established as an essential protein whose functions are critical for both the viability and survival of cells (Brown and Baltimore, 2000, 2003; Schoppy et al., 2012), several studies have now demonstrated that the inhibition of functional kinase activity of ATR in tumour cells may either increase the sensitivity of tumour cells to genotoxic agents and/or cell death by apoptosis or cellular senescence, both of which have potential anti-tumour implications (Karnitz and Zou, 2015; Weber and Ryan, 2015).
The activity of ATR is crucial in all highly proliferative cells including tumour cells, particularly during the S-phase of the cell cycle, due to the replication stress associated with this phase of the cell cycle (Toledo et al., 2008; Brown and Baltimore, 2000; Ruzankina et al., 2007). However, the functions of ATR appear to be even more critical in many tumour cells than in normal cells, as tumour cells possess activated oncogenes such as ras, myc, and cyclin E, which are known to disrupt the normal cell cycle regulation, generating high replication stress as compared to normal cells (Hanahan and Weinberg, 2011; Toledo et al., 2008). In response to excessive replication stress, which ultimately generates high volumes of ssDNA, cells are more prone to cell death than survival if damage is not immediately repaired (Toledo et al., 2008). The ATR pathway is therefore critical for the survival of tumour cells, with several reports showing that inhibiting this DDR pathway is selectively toxic to tumour cells with high oncogene-driven replication damage (Helt et al., 2005; Gilad et al., 2010; Murga et al., 2011; Ferrao et al., 2012). For example, activation of the Ras oncogene with complete suppression of functional ATR has been shown to aggravate oncogene-induced replication stress leading to tumour growth arrest and substantial tumour cell death (Helt et al., 2005). Also, in HPV-related cancers such as cervical cancer, the expression and activities of oncoproteins such as E6 and E7 tends to increase the dependence of these cancer cells on the ATR pathway for survival as they imbalance the nucleotide pools for DNA synthesis, increasing the levels of replication stress (Bester et al., 2011; Wieringa et al., 2016).
Moreover, the loss of ATM functions, which is a common feature of most tumours, either through mutation of the ATM protein itself or its associated downstream targets, particularly p53, renders tumour cells defective at the G1 cell-cycle checkpoint as the ATM/p53 pathway is principally responsible for the activation of this checkpoint (Bolt et al., 2005; Jiang et al., 2009). ATM deficiency in tumour cells therefore renders cells more dependent on the intra-S-phase and G2/M checkpoints, of which ATR principally mediates, for survival following DNA damage (Cortez et al., 2001; Kastan et al., 1992). In recent times, ATR has been demonstrated as an essential regulator of immune checkpoint proteins such as PD-L1 in response to DNA damage, and with the post-translational regulation of PD-L1 now established to be mainly by ATR, the activation and inhibition of ATR clearly holds promising implications in immunotherapy. For example, as ATR plays a major role in PD-L1 regulation and with tumour cells evading T-cell mediated-killing by the up-regulation of DNA-damage induced PD-L1 on these cells (which is commonly observed with radiotherapy and DNA-damaging chemotherapy), the targeting of ATR is reported to potentiate chemo- and radiotherapy, in addition to promoting anti-tumour immune responses (Sun et al., 2018; Dillon et al., 2019).
In support of these findings, the ATR pathway has been reported to be activated in the course of most cancer chemotherapy treatments (Cimprich and Cortez, 2008), perhaps as a responsive measure to repair DNA damage induced by these agents, which consequently promotes tumour survival and drug resistance. Initially, due to the essentiality of ATR for cell viability and survival, its pharmacological inhibition was anticipated to potentially not be tolerated in vivo. However, hypomorphic mice with 10% of the usual functional ATR levels were reported to be remarkably normal, with preserved viability of highly proliferative tissues (Schoppy et al., 2012). Furthermore, this significantly low level of ATR was sufficient to induce synthetic lethality in oncogenic RAS-driven tumours, suggesting a favourable therapeutic index as complete inhibition of ATR may not be required to cause significant and selective toxicity in cancer cells (Schoppy et al., 2012). ATR has therefore been long considered and recognised as an attractive therapeutic target for anticancer therapy, with this discovery generating a growing interest in the development of ATR inhibitors.
2. ATR inhibitors
Despite several earlier proof-of-principle studies (Nghiem et al., 2001; Sun et al., 2018; Dillon et al., 2019; Nishida et al., 2009; Wagner and Kaufmann, 2010) that have profoundly demonstrated functional loss of ATR as a potential sensitizer of cancer cells to conventional DNA damaging chemotherapies, IR and immunotherapy, the development of potent and specific small molecules targeting the ATR signalling pathway as an anti-cancer therapy was initially focused on the development of downstream CHK1 inhibitors rather than ATR kinase itself. The pharmaceutical development of specific ATR inhibitors was so delayed that several CHK1 inhibitors had already proceeded to clinical trials (although none had proven successful) when the first specific ATR inhibitor was reported (Sarkaria et al., 1999; Rundle et al., 2017). This delay was attributed to the earlier reported difficulties associated with obtaining the pure active form of the kinase protein for in vitro studies and its activating/interacting partners including ATIP, TopBP1 etc. which are required for developing assays and thus the screening of potential inhibitors (Rundle et al., 2017; Sarkaria et al., 1998). Additionally, the lack of structural information about the ATR kinase (the crystal structure of ATR still remains to be solved at the time of writing) is a hindrance to a rational drug design approach for selective and specific inhibitors.
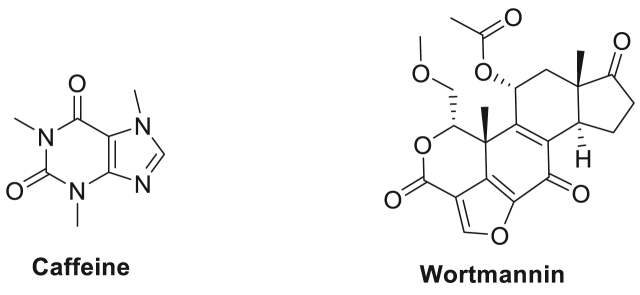
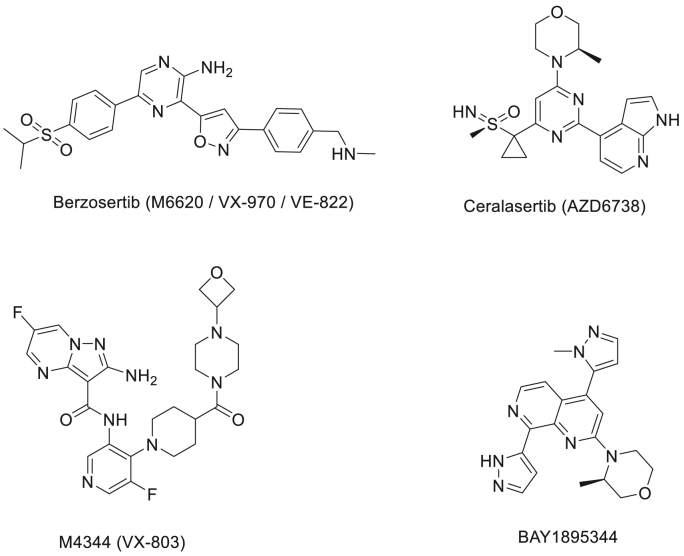
Caffeine and wortmannin (a fungal metabolite) (Fig. 4) are among the earliest agents used in the pioneering preclinical studies to demonstrate the sensitisation potential of ATR inhibition (IC50 = 1.1 mM and 1.8 μM respectively) to IR and other genotoxic agents on cancer cells, although these agents were also observed to inhibit multiple PIKKs (Nishida et al., 2009; Wagner and Kaufmann, 2010). Nevertheless, despite the weak and unspecific ATR inhibition demonstrated by these agents in vitro, and the high in vivo toxicity (Karve et al., 2012; Ihle et al., 2004), the observed chemo- and radio-sensitising effects of caffeine and wortmannin on various cancer cells greatly encouraged those engaged in the quest to develop more potent and specific ATR inhibitors for cancer treatment. Unfortunately, 20 years after these proof-of-principle studies, at the time of writing, only thirteen ATR inhibitors have been reported at various stages of drug development (Table 1), with none approved clinically. It is worth noting that four of these reported ATR inhibitors (Fig. 6), i.e. AZD6738, M6620 (VX-970), BAY1895344 and M4344 (VX-803) have progressed to phase I and II clinical trials in the last 8 years, however (see Table 2).
Fig. 4.
Chemical structures of caffeine and wortmannin, which are among the early agents used in ATR inhibition studies.
Table 1.
Summary of ATR inhibitors in preclinical and/or clinical development.
| Agent | Specificity [Primary Targets] | ATR Selectivity [Fold] |
|---|---|---|
| Preclinical development | ||
| Schisandrin B | ATR IC50 = 7.25 μM | Not reported |
| NU6027 | ATR; Ki = 0.1 μM CDK2; Ki = 1.3 μM CDK1; Ki = 2.5 μM |
ATM homologs > 1.5 |
| Dactolisib (NVP-BEZ235) | ATR; IC50 = 21 nM ATM; IC50 = 7 nM DNA-PK; IC50 = 5 nM mTOR; IC50 = 2 nM PI3K; IC50 = 2 nM |
No selectivity |
| EPT-46464 | ATR; IC50 = 14 nM mTOR; IC50 = 0.6 nM | ATM > 40, DNAPK > 2.5 PI3K > 12 |
| Torin 2 | ATR; IC50 < 10 nM mTOR; IC50 = 0.25 nM | No selectivity |
| VE-821 | ATR; IC50 = 26 nM | ATR homologs > 100 PI3K > 100 |
| AZ20 | ATR; IC50 = 5 nM | ATM, DNA-PK, PI3K > 600 mTOR ≤ 8 |
| Novartis's Tetrahydropyrazolo[1,5-a] pyrazines lead |
ATR; IC50 = 0.4 nM | ATM, DNA-PK, PI3K > 4000 mTOR > 100 |
| Novartis's Azabenzimidazole series lead |
ATR; IC50 = 0.5 nM | ATR homologs, PI3K > 30,000 |
| Clinical – Phase I | ||
| M4344 (VX-803) | ATR; IC50 < 0.2 nM | ATR homologs, PI3K > 100 |
| BAY1895344 | ATR; IC50 = 7 nM | ATM > 200 DNA-PK > 40 PI3K > 400 mTOR ≥ 6 mTORcellular > 60 |
| Clinical – Phase I & II | ||
| Berzosertib [M6620 (VX-970)] | ATR; IC50 = 0.2 nM | ATR homologs, PI3K > 100 |
| Ceralasertib [AZD6738] | ATR; IC50 = 4 nM | ATR homologs, PI3K > 300 |
Fig. 6.
The chemical structures of ATR inhibitors currently being evaluated in Phase I & II cancer clinical trials.
Table 2.
Major highlights of ATR inhibitors in clinical development.
| Agent | Major Highlights |
|---|---|
| Phase I | |
| M4344 (VX-803) |
|
| BAY1895344 |
|
| Phase I & II | |
| Berzosertib [M6620 (VX-970)] |
|
| Ceralasertib [AZD6738] |
|
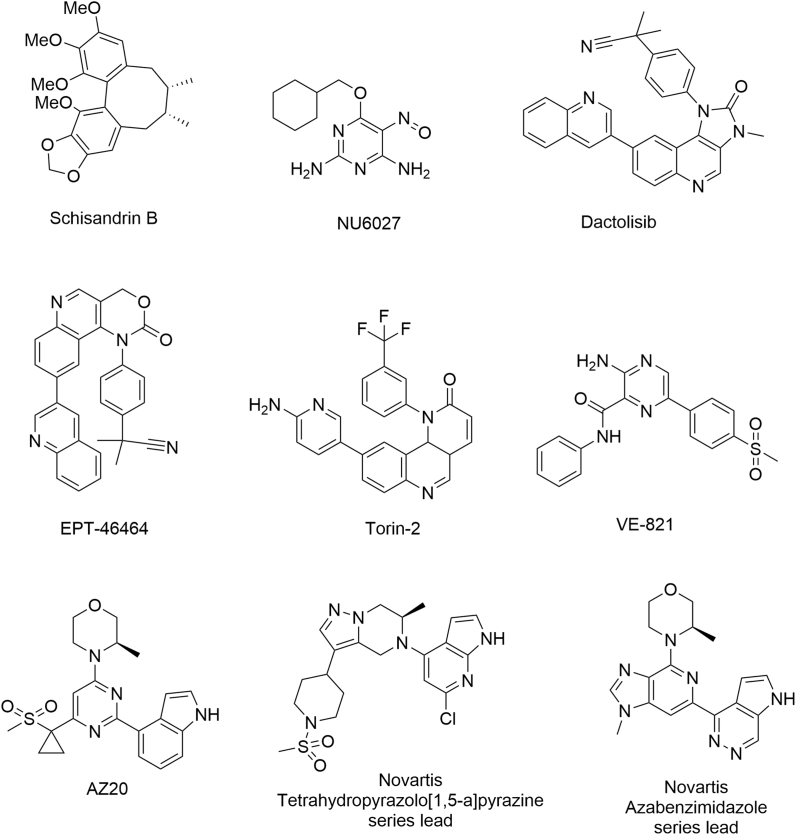
In this review, known ATR inhibitors have been grouped into 2 categories: those in preclinical development (Fig. 5), and those agents that have progressed to clinical evaluation (Fig. 6).
Fig. 5.
Chemical structures of reported ATR inhibitors in preclinical development.
2.1. ATR inhibitors in preclinical development
The first published ‘seemingly’ selective small molecule inhibitor of ATR was Schisandrin B (ATR IC50 = 7.25 μM) (Sarkaria et al., 1999). Schisandrin B is an active ingredient of Fructus Schisandrae, the fruit of the medicinal herb Schisandra chinensis (commonly known as the magnolia berry) which was identified as an inhibitor of ATR activity through a cell-based screen of herbal compounds. Schisandrin B was observed to inhibit UV-activated G2/M and S-phase checkpoints and also block the UV-induced ATR-dependent signalling pathway, which enhanced the sensitivity of cancer cells to UV treatment. However, despite the significant inhibition of ATR kinase activity by schisandrin B, the cellular ATR potency of this agent was considered weak as the observed sensitisation of UV-treated cancer cells occurred only at relatively high concentrations (≥30 μM), which may lead to off-target inhibition and systemic toxicities (Sarkaria et al., 1999). Additionally, schisandrin B was observed to inhibit not only the phosphorylation of ATR substrates at high concentrations but also substrates of ATM, thereby revealing a lack of selectivity. It is noteworthy that although schisandrin B was abandoned in its development as an ATR inhibitor, other studies have described it as an effective dual inhibitor of P-glycoprotein and multidrug resistance protein 1 (MRP1) (Sun et al., 2007), an effective cardio-protective agent against doxorubicin-induced cardiotoxicity (by enhancing glutathione redox cycling and inhibition of MAPK/p53 signalling) (Xu et al., 2011; Thandavarayan et al., 2015), a modulator of the NF-κB pathway in reducing cisplatin-induced toxicities (Giridharan et al., 2012), and as a blocker of epithelial-mesenchymal transition, hence reducing lung and bone metastasis (Liu et al., 2012). These effects further illustrate the lack of target selectivity, however.
In 2011, 12-years after schisandrin B was described as a potential ATR inhibitor, NU6027, a CDK2 inhibitor, was observed to be more potent against ATR kinase activity (ATR Ki = 0.1 μM) than its original intended target (CDK2 Ki = 1.3 μM) (Peasland et al., 2011; Arris et al., 2000). Unlike schisandrin B, NU6027 was identified as a low micromolar inhibitor of ATR activity in cancer cells (IC50 = 6.7 μM), with no observed inhibition of cellular ATM and DNA-PK even at high concentrations (≥10 μM). This cellular inhibition of ATR by NU6027 was observed to sensitise ovarian and breast cancer cells to various DNA-damaging anti-cancer therapeutics, although this was more profound with cisplatin (2-fold increase in potency) and hydroxyurea (3.2-fold increase).
Significantly, ATR inhibition by NU6027 also demonstrated for the first time a synthetic lethality with PARP inhibition (rucaparib), by inhibiting homologous recombination (Peasland et al., 2011). Despite its promise, NU6027 was ultimately not developed further due to a lack of ATR selectivity over the wider kinase family. That said, the reported observations with this agent, especially the observed synthetic lethality of ATR inhibition with PARP inhibition and XRCC1 defects, both provided a solid foundation on which to build, as well as generating further interest for the development of selective ATR inhibitors (Peasland et al., 2011). NU6027 remains a useful experimental tool in ATR research to this day.
With the tendency of small molecules cross-inhibiting ATR and its homologs ATM, DNA-PK and mTOR, and the fact that the kinase activity of ATR is restricted to the S/G2 phases of the cell cycle, cell-based screening for specific and potent ATR inhibitors was considered problematic due to the greater chance of false positives that could result from an indirect effect of the tested compound on the cell cycle (Rundle et al., 2017; Toledo et al., 2011). Crucially, a novel cell-screening platform was developed and reported in 2011 in which the measure of ATR activity was assessed by quantifying the pan-nuclear phosphorylation of H2AX (γH2AX), a process strictly dependent on ATR activity, thus overcoming the limitations of false positives with earlier screening assays (Toledo et al., 2011). Two compounds, NVP-BEZ235 and ETP-46464, each with potent (Nano molar) ATR inhibitory activity emerged from a pool of PI3K inhibitors using this screening approach (Toledo et al., 2011).
Interestingly, unlike ETP-46464 which was a novel compound, NVP-BEZ235, a dual PI3K/mTOR inhibitor (Maira et al., 2008), was already in phase I/II clinical trials for advanced solid tumours (NCT00620594; NCT01508104; NCT01658436: NCT01756118; NCT01658436) at the time of its identification as a potent ATR inhibitor. NVP-BEZ235 was reported to be highly active against ATR kinase itself (ATR IC50 = 21 nM) in cell-free assays, and also potent against ATR cellular activities (ATR IC50 = 100 nM). However, this compound was observed to also inhibit activities of other ATR homologs at relatively low nanomolar concentrations: ATM (IC50 = 7 nM) and DNA-PK (IC50 = 5 nM). NVP-BEZ235 was therefore referred by the authors as an inhibitor of DDR, rather than an inhibitor of ATR (Toledo et al., 2011). In view of this observation, and in addition to an earlier report which described NVP-BEZ235 as a potent radio-sensitizer for Ras-overexpressing tumours (Konstantinidou et al., 2009) (an observation which is more likely to be linked to the inhibition of ATR and its homologs rather than PI3K or mTOR inhibition) the anti-tumour activity of NVP-BEZ235 was suggested to be due to a potent effect on ATR activity rather its earlier reported PI3K/mTOR inhibition (Toledo et al., 2011). It is worth noting that the clinical development of NVP-BEZ235 as an anti-cancer agent has been halted due to an unexpected high degree of toxicity, and a lack of clinical efficacy (Wise-Draper et al., 2017; Carlo et al., 2016; Fazio et al., 2016) which may be due to a lack of knowledge of the potency of this compound on DDR and particularly ATR, which was not considered in the rational design of various clinical studies on this compound. NVP-BEZ235, now renamed as RTB101, is currently being evaluated in mild or moderate Parkinson's disease (resTORbio, 2019) although its clinical trials as a selective inhibitor of TORC1 on respiratory infections and their associated diseases (NCT04139915; NCT03373903) were halted due to the inability to meet the primary endpoint of the study (Kaeberlein, 2020).
ETP-46464, on the other hand, was more specific and potent against ATR activity both in cell-free assays (ATR IC50 = 14 nM) and cellular assays (ATR IC50 = 25 nM), exhibiting some level of ATR selectivity over other ATR homologs; ATM (40-fold) and DNA-PK (2.5-fold) (Toledo et al., 2011). This observation led to ETP-46464 being recognised as the first highly potent and selective ATR inhibitor to be reported. Unfortunately, due to the poor pharmacological properties of ETP-46464 in mice, its further development as an anti-cancer therapy was abandoned (Toledo et al., 2011). Other potent, but unselective, ATR inhibitors have also been reported, for example Torin-2 (Liu, 2011, 2013). Torin-2, a highly potent mTOR inhibitor (IC50 = 0.25 nM) was reported to inhibit ATR and the activities of homologs in the low nanomolar range (IC50 < 10 nM) (Liu et al., 2013). The frequent observations of these highly potent mTOR inhibitors (NVP-BEZ235, ETP-46464 and Torin-2) exhibiting activities against other PIKK family members, particularly against those kinases involved in the DDR pathways (ATR and ATM) known to be essential for tumour growth (Gilad et al., 2010), seems to support the notion that the reported anti-tumour activity of these agents may be due to inhibition of these DDR kinases rather than mTOR inhibition (Weber and Ryan, 2015; Toledo et al., 2011).
With the discovery of a potent and selective ATR inhibitor still remaining elusive, Vertex Pharmaceuticals was reported to have identified a high throughput screen (HTS), which solved the earlier limitations of good, well-characterized chemical tools that hindered the development of potent and selective ATR inhibitors (Rundle et al., 2017; Charrier et al., 2011). The nature of this HTS providing a combination of structure-activity relationship (SAR) studies and homology modelling led to a better understanding of the interactions of potential inhibitors with the ATR active site (Charrier et al., 2011), knowledge which is crucial for rational drug design of potent, specific and selective inhibitors.
This HTS campaign led to the exciting discovery of the first series of potent and selective ATR inhibitors by Vertex Pharmaceuticals, of which VE-821 was identified as potent (ATR IC50 = 26 nM) in cell-free assays, but with more desirable selectivity (>100-fold) over ATR homologs ATM, DNA-PK and mTOR and other kinases (Reaper et al., 2011; Charrier et al., 2011). VE-821 was shown to strongly inhibit the phosphorylation of H2AX and CHK1, known markers of ATR activity, and also demonstrated a strong synergy with radiotherapy and with various genotoxic chemotherapeutics including gemcitabine, camptothecin, etoposide, cisplatin and carboplatin when evaluated in a panel of different cancer cells (Reaper et al., 2011; Prevo et al., 2012; Huntoon et al., 2013). This synergetic effect was most marked with cisplatin and carboplatin (DNA crosslinking agents), however. The apparent potency of cisplatin was observed to increase more than 10-fold with VE-821 in colon cancer cells with defective ATM signalling, for example (Reaper et al., 2011). This observation provided direct support to the earlier reported synthetic lethality interaction between ATR and the ATM-p53 pathway in cancer cells, such that cancer cells with defective ATM signalling are more reliant on ATR for survival from DNA damage (Reaper et al., 2011; Cortez et al., 2001; Kastan et al., 1992). Also, in ovarian cancer cells with depleted BRCA 1/2 or BRCA mutations (defective homologous recombination, HR), VE-821 was observed to further sensitise these cells not only to DNA damaging agents like cisplatin, but also to the PARP inhibitor, veliparib (Huntoon et al., 2013). In addition to these chemo-sensitisations, VE-821 was also shown to increase the sensitivity of various cancer cells to IR-induced cytotoxicity, regardless of the tumour type (Prevo et al., 2012; Pires et al., 2012). Interestingly, the observed radio-sensitisation effect of this agent was even more profound with hypoxic cancer cells (≥0.02–2.0% O2 concentration), as the presence of VE-821 was observed to sensitise radio-resistant hypoxic cancer cells to radiotherapy (Prevo et al., 2012; Pires et al., 2012). Hypoxia is a common feature of most solid tumours, inducing replication arrest even in the absence of DNA (Hammond et al., 2002). In response to replication stress, tumour cells activated their DDR pathways, which have been associated with the chemo- and radio-resistance of hypoxic cancer cells (Woods and Turchi, 2013; Sprong et al., 2006; Pires et al., 2010). Hypoxic cancer cells are more radio-resistant (2.5-3-fold), and hence the sensitisation of these cells by VE-821 through its ATR inhibition represented a major addition to the efficacy of radiotherapy. Even more importantly, VE-821 as monotherapy was shown to selectively induce cell death in cancer cells, but not normal cells. This again indicates the reliance of cancer cells on the ATR pathway for survival (Reaper et al., 2011; Pires et al., 2012). Unfortunately, despite this early promise, VE-821 was not progressed further, with no in vivo data published. However, the optimised analogue VE-822 (VX-970), with increased potency and selectivity against ATR (Fokas et al., 2012) was developed further and is currently in phase II clinical trials (see below, section 2.2). VE-821 remains a valid template for designing and evaluating novel inhibitors of ATR and also in understanding the significance of ATR signalling in various cancers (Gorecki et al., 2020; Middleton et al., 2018; Alsubhi et al., 2016; Al-Subhi et al., 2018).
In 2012, AstraZeneca discovered a hit through an HTS study of its previously described mTOR inhibitors series. The compound was over 10-fold more potent against ATR (IC50 = 30 nM) compared to the originally-intended target mTOR (IC50 = 330 nM), and about 100-fold selective over ATR homologs and PI3K kinases (Finlay et al., 2012; Foote et al., 2013). This hit compound was optimised into AZ20, a more potent ATR inhibitor (ATR IC50 = 5 nM) with improved selectivity (>600-fold) over ATR homologs DNA-PK and ATM, and PI3Kα kinases, although this agent was observed to have retained some residual inhibitory potency against the recombinant mTOR enzyme (≤8-fold-selectivity) (Foote et al., 2013). In vitro, AZ20 inhibited the activity of ATR in a concentration-dependent manner, including phosphorylation of its primary substrate CHK1, with prolonged exposure of AZ20 increasing γH2AX pan-nuclear staining (replication stress), which is indicative of ATR inhibition (Foote et al., 2013; Jacq et al., 2012). AZ20 also showed cytotoxic activity as a single agent, and profoundly so in combination with the selective ATM inhibitor, KU-60019, in various cancer cells, and at a tolerated dose (50 mg/kg once daily and 25 mg/kg twice daily). AZ20 was shown to induce significant tumour growth inhibition in LoVo (human colorectal cancer) xenografts (ATM-deficient) in vivo (Foote et al., 2013; Jacq et al., 2012). It is worth noting that AZ20 was the first ATR inhibitor to be reported with in vivo data. However, despite the promising preclinical data for this agent, the clinical development of AZ20 was halted due to poor solubility (10 μM aqueous solubility at pH 7.4 in phosphate buffer) and CYP inhibition (CYP3A4), which raised concerns of potential drug-drug interactions (the CYP3A family is the major metabolic enzyme for many drugs) (Foote et al., 2013). AZ20 was later optimised to AZD6738, which is currently being evaluated in phase II clinical trials (see below, section 2.2).
In 2014, a group at Novartis also reported the identification of an ATR inhibitor from a tetrahydropyrazolo[1,5-a]pyrazines series with potency in a cell free assay (ATR IC50 = 0.4 nM), and in a cellular assay (ATR IC50 = 0.037 μM) with 4000 to 10,000-fold selectivity over ATM, DNA-PK and PI3Kα kinases, and about 100-fold selectivity over mTOR (Barsanti et al., 2015a). This novel, potent and selective ATR inhibitor was discovered through an optimisation campaign of an HTS hit, a low nanomolar dual ATR and PI3Ka inhibitor (ATR IC50 = 26 nM; PI3Ka IC50 = 9 nM), using PI3Kα mutants as an ATR crystal structure surrogate in addition to ATR homology modelling. Unfortunately, despite this agent possessing acceptable potency, selectivity, aqueous solubility (37 μM aqueous solubility at pH 7.4 in phosphate buffer) and bioavailability (F = 64%), it demonstrated time-dependent inhibition of CYP3A4 activity (Kinact/Ki = 0.028 μl/min/pmol), which raised concerns about potential drug-drug interactions (DDIs) and safety, thereby halting its progression (Barsanti et al., 2015a). That said, this compound is arguably the most potent and selective ATR inhibitor to be reported (particularly over ATM, DNA-PK and PI3Kα kinases). The full extent of its preclinical data (both in vivo and most in vitro profiles) is unfortunately yet to be reported.
A year later, this same group from Novartis reported another highly potent (ATR IC50 = 0.5 nM), selective (>30,000-fold over ATR homologs) and soluble (>2400 μM aqueous solubility at pH 7.4 in phosphate buffer) azabenzimidazole derivative as an ATR inhibitor, this time devoid of any CYP3A4 and hERG liabilities (Barsanti et al., 2015b). This agent was reportedly discovered through modification of the core of a morpholino-imidazopyrimidine hit identified through a combined virtual screening and HTS campaign using a diverse subset of a Novartis compound collection. However, despite the potency of this morpholino-imidazopyrimidine hit against ATR (IC50 = 96 nM), and selectivity (>1000-fold) over ATR homologs, it harboured some pharmacokinetics and CYP3A4 concerns (Barsanti et al., 2015b). Hence, the modification of this hit into a potent and safer azabenzimidazole derivative ATR inhibitor. Unfortunately, this promising azabenzimidazole derivative ATR inhibitor demonstrated poor bioavailability (F = 7.5%) probably due to low membrane permeability and efflux, although a reasonable i. v. pharmacokinetics profile was observed. The progress of this compound is yet to be reported (Barsanti et al., 2015b).
2.2. ATR inhibitors that have progressed to cancer clinical trials
2.2.1. Berzosertib (M6620; VX-970; VE-822)
Berzosertib is the first ATR inhibitor to be evaluated in humans, with the first participant enrolled in a clinical study on December 10, 2012 (NCT02157792), about 13 years after the first selective ATR inhibitor was reported (Sarkaria et al., 1999). Initially discovered and developed by Vertex Pharmaceuticals as VE-822 (VX-970), an improved analogue of VE-821 (a potent and selective ATR inhibitor, which lacked the drug-like properties required for its progression as a clinical candidate), this agent has now been acquired by Merck KGaA, Germany, for further clinical development after exhibiting some preliminary clinical potential in a phase I trial (NCT02487095) (Thomas et al., 2017).
M6620 is a highly potent and selective ATR inhibitor, inhibiting ATR activity both in cell free assays (ATR IC50 = 0.2 nM) and in cellular assays (ATR IC50 = 19 nM) with over >100-fold selectivity over DNA-PK, ATM, and other PI3Kα kinases, in addition to its improved solubility (aqueous solubility of 52 μM) and pharmacokinetic properties compared to its precursor, VE-821 (Fokas et al., 2012; Knegtel et al., 2019).
Beginning with Fokas et al. (2012), the radiosensitising and chemosensitising potentials of M6620 (VX-970) have been widely studied in multiple preclinical models. For example, in PDAC pancreatic cancer cells and xenografts, VE-822 was shown to be a strong sensitizer for radiotherapy and chemotherapy both in vitro and in vivo, such that the efficacy of radiotherapy and chemo-radiotherapy in pancreatic tumour xenografts was profoundly enhanced in combination with VE-822 (Fokas et al., 2012). Interestingly, although VE-822 attenuated ATR signalling in normal cells, the observed VE-822-enhanced cytotoxicity of radiotherapy and chemotherapy was selective to cancer cells but not normal cells (Fokas et al., 2012). Also, in a panel of NSCLC cell lines, similar observations were reported (Hall et al., 2014). In this study, VX-970 was shown to strongly potentiate the cytotoxic effects of various DNA damaging drugs including cisplatin, oxaliplatin, gemcitabine, and etoposide selectively in lung cancer cell lines but not normal cells (Hall et al., 2014).
Consistent with previous reports on other ATR inhibitors, the synergistic effects of VX-970 were more profound with cisplatin as compared to other DNA damaging drugs, with the observed chemo-sensitivity more favourable in cells deficient in p53 (though no significant correlations were observed with p53 status) (Hall et al., 2014). Also, in a panel of 7 human patient-derived lung tumour xenografts, VX-970 was observed to significantly regress and delay tumour growth in six of these models in combination with cisplatin, even at VX-970 doses known to lack efficacy as a monotherapy, and in tumour models shown to be non-responsive to cisplatin monotherapy (Hall et al., 2014). Again in another study, VX-970 was demonstrated to be highly synergistic with cisplatin and etoposide in treatment-resistant SCLC models, such that VE-VX-790 in combination with cisplatin was more efficacious than a combination of cisplatin with etoposide both in vitro and in vivo (Nagel et al., 2019). Strikingly, the combination of VX-790 and cisplatin was reported to not only profoundly inhibit tumour growth in these SCLC mouse models, but also improved the survival of these mice (Nagel et al., 2019). These data were a strong pointer towards possible clinical success as a chemo-sensitizer of DNA damaging agents in lung cancer patients. Interestingly, further studies have since demonstrated the chemo- and radio sensitivity potential of VX-970 in a range of different cancers, including oral (Leszczynska et al., 2016; Shi et al., 2018), colon (Combes et al., 2019), brain (Chen et al., 2018) gastric (Yan et al., 2018), triple negative breast (Tu et al., 2018) and various paediatric solid tumours (Kurmasheva et al., 2018), in addition to lung cancers. For example, the cytotoxic activity of irinotecan (Jossé et al., 2014), and Pt-based chemotherapy (Combes et al., 2019) has been shown to be potentiated by VX-970 without additional toxicity in colon cancers, including in treatment-resistant models. Furthermore, the combination of VE-822 and oxaliplatin was observed to significantly promote antitumor T-cell responses, with increased survival in mouse models, just as reported with inhibitors of the ATR signalling (Combes et al., 2019). Also, in oesophageal tumours, M6620 was shown to significantly inhibit tumour growth in combination with cisplatin, carboplatin and radiation in vitro and in vivo (Leszczynska et al., 2016; Shi et al., 2018). It is noteworthy that although the chemosensitising ability of M6620 has been shown to be profound with conventional cytotoxic chemotherapy (particularly Pt-based chemotherapy), highly synergistic effects have also been reported in combination with other DDR-related pathway inhibitors, including CHK1 inhibitor V158411,134 WEE1 inhibitor AZD1775,135 and PARP inhibitor olaparib (Schmitt et al., 2017). The ability of VX-970 and other ATR inhibitors to potentiate the cytotoxicity of other DDR pathway inhibitors has been attributed to the observation that cancer cells activate alternative DDR pathways, in particular ATR, to counter the effects of this inhibition (Massey, 2016). Thus by inhibiting ATR kinase in addition to these other DDR pathways, the cytotoxic effects of these inhibitors are fully maximised. For example, in AML cells, the combination of AZD1775 and VX-970 has been shown to exhibit synergistic anti-leukemic activity, with AZD1775 increasing VE-822-induced DNA double-strand breaks, thereby enhancing VE-822-induced cell apoptosis and inducing DNA damage and cell cycle arrest (Qi et al., 2019).
Based on this highly promising preclinical data, M6620 has entered at least 19 registered phase I and II clinical trials since the start of its first trial in 2012 (www.clinicaltrial.gov). The majority (17) of these trials are committed to evaluating M6620 in combinations: with radiotherapy (NCT02589522; NCT04052555), with chemotherapy including DNA-damaging agents; topotecan, cisplatin, carboplatin, gemcitabine, irinotecan, paclitaxel, doxorubicin, docetaxel and capecitabine (NCT02157792; NCT03896503; NCT03641313; NCT03517969; NCT02627443; NCT02595931; NCT02595892; NCT02567409; NCT02487095), with PARP inhibitor veliparib (NCT02723864), with angiogenesis inhibitor bevacizumab (NCT03704467) chemo-radiotherapy (NCT03641547; NCT02567422), and even with immunotherapy avelumab (NCT04216316) in various metastatic and refractory solid tumours. Two clinical trials (NCT03309150; NCT03718091) are assessing M6620 as a monotherapy in advanced and selected solid tumours.
The majority of these trials are still active and recruiting, with full data of the two completed trials (NCT02157792; NCT03704467) yet to be reported, therefore the full clinical potential of M6620 is not known. However, the phase I arm of a single phase I/II trial (NCT02487095) has been fully reported (Thomas et al., 2018). In this trial, 21 patients with advanced solid tumours (with defective ATM-p53 signalling) were enrolled to assess the tolerability, safety, and antitumor activity of M6620 in combination with topotecan. This combination was generally well-tolerated, even at the highest planned dose level (topotecan 1.25 mg/m2, days 1–5; M6620 210 mg/m2, days 2 and 5). Unfortunately, the trial was reported to be associated with various grade 3/4 haematological toxicities, including anaemia, lymphopenia, leukopenia, neutropenia, thrombocytopenia and nausea. The observed toxicity profile of this combination was dose-limiting and largely mirrored that of topotecan monotherapy. The trial also provided some promising clinical activity, with 2 partial responses (≥18 months, ≥ 7 months), 7 stable disease responses (ranging from 3 to 12 months; median, 9 months) and more interestingly, 3 out of 5 patients with platinum-refractory SCLC also exhibiting a partial response (≥7 months) or stable disease responses (10 and ≥6 months) (Thomas et al., 2018). Based on this clinical activity in platinum-refractory SCLC, a new randomised phase II trial has been initiated for this combination in SCLC cancer and small cell cancers outside of the lungs (NCT03896503).
In addition to this report, preliminary data from 5 different arms of a just-completed single trial (NCT02157792) have also been separately presented, albeit in the form of conference abstracts (Yap et al., 2015; Shapiro et al., 2016; O'Carrigan et al., 2016; Plummer et al., 2016; Plummer et al., 2018). In all these reports, M6620 as a monotherapy or in combination with carboplatin (Yap et al., 2015; O'Carrigan et al., 2016), or with cisplatin (Shapiro et al., 2016), or with gemcitabine (Plummer et al., 2016, 2018), in various advanced solid tumours was well-tolerated, although the maximum tolerated dose in the case of the combination with cisplatin or gemcitabine was not reached due to dose-limiting haematological toxicities, including grade 3 drug hypersensitivity, and treatment-emergent adverse events (Shapiro et al., 2016; Plummer et al., 2016, 2018). Common non-dose-limiting toxicities included fatigue, nausea, cytopenias, hypotension and myelosuppression. M6620, however, was best tolerated as a monotherapy even at 480 mg/m2, with no dose-limiting toxicities or drug-related G3-4 adverse effects observed (Yap et al., 2015). The median half-life of M6620 also was determined as 16 h, with no accumulation (Yap et al., 2015; Shapiro et al., 2016; Plummer et al., 2016), with evidence of target modulation and anti-tumour responses with either M6620 monotherapy, or in combination with other cytotoxic agents. For example, a RECIST complete response (19+ months) was observed with M6620 as monotherapy (60 mg/m2) in an advanced colorectal cancer patient with a tumour with confirmed loss of ATM (Yap et al., 2015; O'Carrigan et al., 2016). In addition to this, stable disease was also observed in 4 other patients with M6620 alone (11–17.4 weeks), in 8 patients with M6620 + carboplatin (5+ to 20+ weeks) (Yap et al., 2015), and in 18/33 patients with M6620 + gemcitabine (Plummer et al., 2018). The combination of M6620 with cisplatin also led to partial response in 4/28 patients including 3 patients with platinum-resistant/refractory tumours (mesothelioma, ovarian and TNBC), and a neuroendocrine prostate cancer patient (Shapiro et al., 2016). Fortunately, after 8 years of recruiting, the full trial (NCT02157792) has now been completed (March 11, 2020) with 200 participants, and the publication of this data is currently eagerly awaited. Hopefully this data will reveal the true clinical potential of VX-970 either as a monotherapy or in combination with chemotherapy and radiotherapy in these advanced solid tumours.
2.2.2. Ceralasertib (AZD6738)
Ceralasertib (developed by AstraZeneca), an improved sulfoximine morpholinopyrimidine analogue of AZ20, is highly potent against ATR activity both in cell free assays (ATR IC50 = 4 nM) and in cellular assays (ATR IC50 = 74 nM) with improved selectivity (300-fold) over ATR homologs DNA-PK, ATM, mTOR and other PI3Kα kinases (Jones et al., 2013; Guichard et al., 2013; Foote et al., 2018). More importantly, this orally-administered ATR inhibitor exhibits improved aqueous solubility (661 μM at pH 7.4 in phosphate buffer) and bioavailability, and is devoid of the CYP3A4 time-dependent inhibition observed with AZ20 (Foote et al., 2018). Based on very promising pre-clinical data, AZD6738 was first admitted to a clinical trial in 2013 (NCT01955668), and is currently being assessed in various phase I and II clinical trials.
Preclinically, AZD6738 has been extensively studied, and is still being studied, in various tumour models both as a single agent, and in combination with DNA damaging agents (including cisplatin, cyclophosphamide etc), PARP inhibitors (olaparib), antimetabolites (gemcitabine), radiotherapy, and even immunotherapy. (Jones et al., 2013; Guichard et al., 2013; Foote et al., 2018; Kim et al., 2017; Vendetti et al., 2015, 2018). As a single agent, AZD6738 was demonstrated to cause significant tumour growth inhibition of ATM- and p53-deficient cells, both in vitro and in xenograft models in vivo. AZD6738 was observed to increase the accumulation of high volumes of unrepaired DNA damage in these ATM-deficient cells, leading to cell death due to mitotic catastrophe (Kwok et al., 2015). Also, AZD6738 has been demonstrated to significantly inhibit cancer cell growth, with profound sensitivity in cells with ATM-pathway defects when used as a single agent across different panels of cancer cell lines in vitro (Guichard et al., 2013). In a LoVo xenograft model (ATM-deficient) in vivo, AZD6738 achieved near complete tumour growth inhibition at well-tolerated doses, although this observation was absent in an HT29 xenograft model (ATM-proficient) despite the sensitivity of these cells to the drug in vitro (Guichard et al., 2013; Foote et al., 2018). AZD6738 was additionally shown to induce cell death and senescence in a panel of non-small cell lung cancer (NSCLC) cells, and other cancer cell lines in vitro, regardless of p53/ATM status (Vendetti et al., 2015; Dillon et al., 2017). Similar observations have been reported in a panel of human breast cancer cell lines (Kim et al., 2017). Moreover, in gastric cancer cells with ATM deficiency, AZD6738 was shown to induce synthetic lethality in this model both in vitro and in vivo (Min et al., 2017). Also, in another study, the analysis of pancreatic ductal adenocarcinoma (PDAC) samples revealed at least one genetic alteration in approx. 93% of these cases, which was responsible for the sensitivity of these cells to this agent. The phenomenon of synthetic lethality may account for this observation (Wallez et al., 2018).
Despite the promising data associated with use of AZD6738 as a monotherapy, particularly in ATM-deficient cancer cells as summarised above, the biggest clinical potential for this agent across a variety of tumour types is perhaps in combination with other therapies. It has demonstrated strong synergy with various classes of chemotherapy and radiotherapy. (Vendetti et al., 2015, 2018; Dillon et al., 2017; Wallez et al., 2018; Bukhari et al., 2019; Jin et al., 2018). For example, in NSCLC cell lines, AZD6738 has been shown to potentiate the cytotoxicity of cisplatin and gemcitabine regardless of their ATM kinase signalling status. However, in the ATM-deficient subset of these NSCLC cell lines, AZD6738 was seen to potently synergize with cisplatin, inducing rapid cell death (Vendetti et al., 2015). For example, the synergistic effect of AZD6738 (1 μM) on cisplatin sensitivity was more profound (10-17-fold increase) in ATM-deficient cells (shRNA ATM knockdown H460 and A549 cells), as compared to the corresponding ATM-proficient cells (H460 and A549 wild type cells), with an approx. 2-fold increase (Vendetti et al., 2015). Also, in both ATM-proficient (H460) and ATM–deficient (H23) lung cancer xenografts in vivo, the combination of AZD6738 and cisplatin was well-tolerated and strongly inhibited tumour growth; by approx. 76% in ATM-proficient and 85% in ATM-deficient xenografts, with some mice strikingly demonstrating complete tumour resolution despite the fact that these models had shown no response to cisplatin alone (Vendetti et al., 2015). In another study, AZD6738 in combination with carboplatin or cyclophosphamide was also well-tolerated, exhibiting anti-tumour growth activity and regression (Guichard et al., 2013). Similar synergistic observations have also been reported with AZD6738 in combination with gemcitabine in pancreatic ductal adenocarcinoma (PDAC) both in vitro and in vivo (Wallez et al., 2018). In a panel of both mouse and human PDAC cell lines, this combination was shown to synergistically inhibit cell growth, even with cells which were shown to be highly resistant to both drugs alone, in vitro. The combination was also shown to induce tumour regression in PDAC tumour models in vivo, with a significant improvement in survival observed at well-tolerated doses (Wallez et al., 2018). Similarly to the reported chemo-sensitisation potential of AZD6738, radio-sensitisation has also been extensively demonstrated both in vitro and in vivo (Dillon et al., 2017, 2019; Vendetti et al., 2018). For instance, in multiple panels of cancer cell lines AZD6738 was shown to uniformly potentiate the cytotoxic activity of radiation, even at clinically relevant doses, regardless of the p53 and/or BRCA status, both in vitro and in vivo models (Dillon et al., 2017). In these models, AZD6738 was observed to inhibit HR, abrogate radiation-induced G2 cell-cycle checkpoint arrest and promote the generation of acentric dysfunctional micronuclei due to aberrant mitosis and mitotic catastrophe (Dillon et al., 2017). Remarkably, the synergistic effects of AZD6738 have recently been shown not to be limited to cytotoxic agents or radiotherapy, but also to other molecularly targeted small molecule agents, such as AZD1775 (Wee1 inhibitor), olaparib (PARP inhibitor), acalabrutinib (Bruton's tyrosine kinase inhibitor) (Guichard et al., 2013; Bukhari et al., 2019; Jin et al., 2018; Clack et al., 2015). For example, in triple-negative breast cancer cells, the growth inhibition activity of AZD1775 was observed to be strongly potentiated by AZD6738, both in vitro and in vivo. The combination of AZD1175 and AZD6738 was observed to strongly induce mitotic catastrophe, ultimately leading to cell death and also inactivated RAD51-mediated homologous recombination, which enhanced the sensitivity of these cells to cisplatin and a PARP inhibitor, independent of BRCA status (Jin et al., 2018). Also, on a panel of breast cancer cell lines and cancer stem cells, this combination has been shown to exhibit highly synergistic cytotoxicity (Bukhari et al., 2019). Lastly, the influence of AZD6738 on tumour immunology and its implication in immunotherapy has also been reported. In a colorectal cancer model, AZD6738 was reported to strongly impact CD8+ T cell–dependent immune responses following radiation, and to potentiate CD8+ T cell–dependent anti-tumour responses in these tumour models, with immunologic memory generated in complete responder mice (Vendetti et al., 2018). Interestingly, AZD6738 was also shown to attenuate the up-regulation of radiation-induced PD-L1 expression on tumour cells, and strikingly decreases tumour-infiltrating regulatory T cells, which perhaps contributes to the highly synergetic effect observed with radiation and AZD6738 in combination (Vendetti et al., 2018). In another recent study, administration of AZD6738 following radiation in HPV-driven malignancies was also observed to boost the immunogenic effects of radiation, with a marked increase in antigen presentation, infiltration of immune cells including CD8+, CD3+, NK cells, and production of radiation-induced tumour-derived cytokine production, such as CCL2, CCL5, CXCL10, and CXCL9 (Dillon et al., 2019).
Underpinned by this large volume of promising pre-clinical data, AZD6738 is currently being assessed in no fewer than 25 phase I and II clinical trials (www.clinicaltrial.gov). In phase I clinical trials, AZD6738 is being evaluated as a monotherapy in haematological cancers (NCT01955668, NCT03770429) and in solid refractory tumours (NCT02223923, NCT03022409), but also in combination with radiation, chemotherapy and immunotherapy: radiation (NCT02223923), carboplatin (NCT02264678), paclitaxel (NCT02630199), gemcitabine (NCT03669601), olaparib (NCT02264678), acalabrutinib (NCT03328273, NCT03527147) and durvalumab (NCT02264678), in various advanced tumours including those with ATM-deficiency. In phase II clinical trials, AZD6738 is currently being assessed as a monotherapy in various solid tumours (NCT03682289), and in combination with olaparib in participants with various advanced solid malignancies (NCT02264678, NCT02576444, NCT03682289, NCT03463342, NCT03330847, NCT03334617, NCT03428607, and others). Also, in combination with immunotherapy, AZD6738 plus durvalumab (anti-PD-L1 inhibitor) is currently being assessed in advanced solid malignancies (NCT02264678), but also specifically in lung cancer (NCT03334617, NCT03833440), breast cancer (NCT03740893), refractory gastric adenocarcinoma (NCT03780608) and metastatic melanoma (NCT03780608). As most of these clinical trials are still on-going and recruiting, and the data from completed trials (NCT01955668) still not reported, the full clinical potential of the drug is yet to be revealed. That said, preliminary results for the phase I arm of a clinical trial (NCT02264678) have been reported in conference abstracts (Krebs et al., 2018; Yap et al., 2016). In this limited data set, AZD6738 was observed to be well-tolerated in patients in combination with carboplatin, olaparib and durvalumab, with the most commonly reported toxicities being thrombocytopenia, neutropenia and anaemia (for AZD6738 plus carboplatin or plus olaparib combination), plus immune toxicities, nausea/vomiting, musculoskeletal chest pain and dyspnoea (for the AZD6738 plus durvalumab combination) ≥ in 20% subjects (Krebs et al., 2018; Yap et al., 2016). Also the preliminary pharmacokinetics data for AZD6738 revealed rapid absorption of the drug, with peak plasma concentrations observed after 1.5 h, and an elimination half-life of 11 h. Interestingly, although this trial primarily focused on safety and tolerability, preliminary signals indicated that anti-tumour activity was observed in all the combinations evaluated in cancer patients with advanced solid tumours. For example, with AZD6738 plus carboplatin, 4 out of 33 patients with advanced cervical cancer and ovarian cancers achieved partial responses (Yap et al., 2016), and 7 of 39 patients with advanced breast, ovarian, prostate, pancreatic and ampullary cancer also achieved 1 complete response, 5 partial responses and 1 unconfirmed partial response with the AZD6738 plus olaparib combination. 4 out of 21 patients with either squamous cell carcinoma of the larynx or advanced NSCLC achieved 2 partial responses, and another patient with NSCLC achieved a confirmed complete response with the AZD6738 plus carboplatin combination (Krebs et al., 2018).
2.2.3. M4344 (VX-803)
M4344, formerly known as VX-803, is another orally bioactive ATR inhibitor which was originally developed by Vertex pharmaceuticals but has now been acquired by Merck KGaA, Germany (in addition to M6620) for further development. M4344 is by far the most potent ATR inhibitor reported, strongly inhibiting ATR activity both in cell free assays (ATR IC50 = 0.15 nM) and in cellular assays (ATR IC50 = 8 nM), with over 100-fold selectivity against a panel of 308 kinases, including ATR homologs and PI3K kinases (Zenke et al., 2019). Preclinical data for M4344 has not been extensively reported (Zenke et al., 2019), but M4334 was reported to exhibit synergy with several DNA-damaging chemotherapeutics, PARP and CHK1 inhibitors in a panel of 92 cancer cell lines, and has also demonstrated tumour regression in tumour models in vivo. While M4344 has entered two phase I clinical trials as a monotherapy or in combination with carboplatin and cisplatin for advanced solid tumours (NCT02278250), and in combination with niraparib (a PARP inhibitor) against PARP-resistant recurrent ovarian cancer (NCT04149145), at the time of writing, no clinical data has been reported.
2.2.4. BAY1895344
BAY1895344 is the most recently reported potent ATR inhibitor to have begun evaluation in the clinic (NCT03188965). It is a potent and selective orally-administered ATR inhibitor developed by Bayer AG (Germany) as an optimised version of BAY-937 (structure not disclosed). BAY-937 showed promising ATR inhibition both in cell-free (IC50 = 78 nM) and cellular (IC50 = 360 nM) assays, with anti-tumour activity demonstrated both in vitro and in vivo as either a single agent, or in combination with cisplatin (Luecking et al., 2017). Issues of low aqueous solubility, low bioavailability (rat) and more critically hERG inhibition observed with BAY-937 hindered further development, however. Hence the development of BAY1895344, an improved analogue with enhanced aqueous solubility, bioavailability across species, and with an absence of significant hERG liabilities (Luecking et al., 2017; Wengner et al., 2020). In vitro, BAY1895344 has proved to be a highly potent ATR inhibitor both in cell-free (IC50 = 7 nM) and cellular (IC50 = 36 nM) assays with selectivity over ATR homologs; ATM (>200-fold), DNA-PK (>40-fold), mTOR (≥6-fold) and PI3K (>400-fold) (Wengner et al., 2020). Interestingly, despite the mTOR inhibition observed at sub-micromolar concentrations (IC50 = 35 nM), it was observed to inhibit the PI3K/AKT/mTOR pathway with over 60-fold selectivity compared to its ATR inhibition. However, despite some level of ATR selectivity reported with BAY1895344, it is the least potent and least selective of the ATR inhibitors currently being evaluated in clinical trials (the other drugs being M6620, AZD6738, and M4334) (Fokas et al., 2012; Foote et al., 2018; Zenke et al., 2019).
In vitro, BAY1895344 was shown to exhibit strong antiproliferative activity across a panel of cancer cell lines with defective DDR pathways as a single agent, and also demonstrated profound synergetic effects in combination with DNA-damaging agents such as cisplatin, bleomycin, etc. and with DDR inhibitors targeting ATM, CHK1, DNA-PK, WEE1, and PARP (Wengner et al., 2020). Interestingly, defective ATM pathways were observed to greatly sensitise cancer cell lines to BAY1895344. Similar observations were also reported in various tumour models in vivo, with effective tumour growth inhibition or tumour growth delays observed with BAY1895344 as a monotherapy and in combination with olaparib, radiotherapy and with even hormone therapy in ovarian, prostate, colorectal and lymphoma models at well-tolerated doses (Wengner et al., 2020). Also, and surprisingly BAY1895344 demonstrated superior anti-tumour efficacy in human lymphoma xenografts compared to equivalent doses of M6620 and AZD6738, despite its relatively lower potency and selectivity against ATR. This unexpected observation was attributed to the superior pharmacokinetic properties of BAY1895344 compared to M6620 and AZD6738: the plasma exposure of BAY1895344 was observed to exceed the concentration levels required for antiproliferative activity (IC50) in tumour cells, thus accounting for this superior antitumor efficacy BAY1895344 as a monotherapy in vivo (Wengner et al., 2020).
With this promising pre-clinical data, BAY1895344 entered its first clinical investigation as a monotherapy in advanced solid tumours and lymphomas (NCT03188965) in 2017. More recently another trial evaluating the combination with pembrolizumab (PD-1 inhibitor) has been reported in advanced solid tumours (NCT04095273). Preliminary data (reported in conference abstract form) has revealed dose-limiting haematological toxicities associated with this agent in an on-going clinical trial for advanced solid tumours and lymphomas (NCT03188965). In addition, consistent with preclinical data, all patients that were observed with responses possessed tumours with ATM loss or ATM mutations (De Bono et al., 2019).
3. Conclusion
DDR pathways, particularly ATR-CHK1, have been demonstrated over recent decades to play critical roles in both the development of cancers, and their responses to classical cancer treatments, including cytotoxic chemotherapy, radiotherapy and even immunotherapy (Woods and Turchi, 2013; Sun et al., 2018). The search for a potent and selective ATR inhibitor for cancer treatment has accelerated in recent times, with several agents having entered various clinical investigations. Considering the promising preclinical data for these agents, there remains considerable optimism for successful clinical outcomes in these trials. Despite this optimism, there is also cause for caution. While the results of these clinical trials are eagerly awaited, preliminary data seem to validate some earlier concerns of toxicities raised with ATR inhibition as a cancer treatment (Karnitz and Zou, 2015). Despite some promising responses, including complete responses observed with some ATR inhibitors currently being investigated in the clinic (Krebs et al., 2018; Yap et al., 2016), the occurrence of dose-limiting haematological toxicities, and treatment-emergent adverse events seem to be a common observation with these agents, particularly when in combination with cytotoxic chemotherapy (perhaps the most likely clinical scenario), although these agents are well-tolerated as monotherapies, with no dose-limiting toxicities (Yap et al., 2015, 2016; De Bono et al., 2019). Interestingly, some of these toxicities had been earlier observed in various preclinical settings. For example, the dosing of carboplatin prior to administration of AZD6738 was not tolerated, leading to drastic weight loss in rats, while the reverse dosing sequence was tolerated (Clack et al., 2015). Also, several dose-limiting toxicities were reported to be observed with AZD6738 dosing, including changes in food consumption and body weight in dogs, rats and mice, in addition to bone marrow toxicity, hypocellularity in multiple lymphoid tissues and increase in alveolar macrophages, although recovery from these toxicities was observed after the termination of dosing (Vendetti et al., 2015). Again, the combination of BAY1895344 with carboplatin was observed to produce dose-dependent toxicity, which limited the potential therapeutic value of this combination, and potentially with other Pt-based chemotherapies (Wengner et al., 2020). It is therefore of no surprise that safety and tolerability of different dosing schedules are now the key emphasis for new entry ATR inhibitors (M4334 and BAY1895344) under clinical investigation, perhaps to avoid or minimise toxicities which may likely undermine the clinical potential of these promising ATR inhibitors.
ATR is an established essential protein whose functions are known to be critical for both the viability and survival of cells, although these functions are even more critical in many tumours for survival and growth (Toledo et al., 2008; Brown and Baltimore, 2000), hence the rational for ATR targeting as an anti-cancer therapy. However, considering the essentiality of the ATR kinase and its ATR-CHK1 pathway, the “non-selective” targeting of ATR may still hold the possibility to be lethal to normal cells, leading to serious toxicity, particularly in combination with other cytotoxic chemotherapeutics. It is worth stating that despite the selectivity, potency and promising pre-clinical data for this generation of ATR inhibitors, the clinical success of these agents particularly in combination with chemotherapy, radiotherapy and immunotherapy will rely on smart dosing strategies that limit the occurrence of haematological and other toxicities. Failing that, specific targeting of these agents (e.g. as tumour-targeted prodrugs (Gill et al., 2014; Barnieh et al., 2019)) may be necessary, and could provide a significant opportunity to circumvent the issues of systemic toxicity.
With the observed preliminary clinical responses reported in various ongoing clinical investigations, particularly in patients with ATM and HR defective tumours, the completion of these trials is likely to open multiple insights in the field of ATR and DDR in general.
Author Contributions
Conceived and designed review: All.
Supervisory team: PML, RAF.
Manuscript composition: FMB
Manuscript editing: FMB, RAF
Final proof-reading: All
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Prof Sherif El-Khamisy for reviewing the manuscript and for valuable feedback. Dr Goreti Ribeiro Morais is thanked for useful suggestions. The Ghana Educational Trust Fund is acknowledged for sponsoring FMB in his PhD studies.
References
- Abraham R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- Al-Subhi N., et al. Targeting ataxia telangiectasia-mutated- and Rad3-related kinase (ATR) in PTEN-deficient breast cancers for personalized therapy. Breast Canc. Res. Treat. 2018;169:277–286. doi: 10.1007/s10549-018-4683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderton G.K., et al. Seckel syndrome exhibits cellular features demonstrating defects in the ATR-signalling pathway. Hum. Mol. Genet. 2004;13:3127–3138. doi: 10.1093/hmg/ddh335. [DOI] [PubMed] [Google Scholar]
- Alsubhi N., et al. Chk1 phosphorylated at serine345 is a predictor of early local recurrence and radio-resistance in breast cancer. Mol. Oncol. 2016;10:213–223. doi: 10.1016/j.molonc.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arris C.E., et al. Identification of novel purine and pyrimidine cyclin-dependent kinase inhibitors with distinct molecular interactions and tumor cell growth inhibition profiles. J. Med. Chem. 2000;43:2797–2804. doi: 10.1021/jm990628o. [DOI] [PubMed] [Google Scholar]
- Bakkenist C.J., Kastan M.B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Bao S., et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Barnieh F.M., Race A.D., Shnyder S.D., Loadman P.M., Falconer R.A. Abstract 350: investigating the mechanisms of cellular uptake and metabolism of ICT2588, an MT-MMP-activated prodrug. Canc. Res. 2019;79:350. [Google Scholar]
- Barsanti P.A., et al. Structure-based drug design of novel potent and selective tetrahydropyrazolo[1,5-a]pyrazines as ATR inhibitors. ACS Med. Chem. Lett. 2015;6:37–41. doi: 10.1021/ml500353p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsanti P.A., et al. Structure-based drug design of novel, potent, and selective azabenzimidazoles (ABI) as ATR inhibitors. ACS Med. Chem. Lett. 2015;6:42–46. doi: 10.1021/ml500352s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartek J., Lukas J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol. 2001;13:738–747. doi: 10.1016/s0955-0674(00)00280-5. [DOI] [PubMed] [Google Scholar]
- Bartek J., Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Canc. Cell. 2003;3:421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Bartkova J., et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Bartkova J., et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- Bester A.C., et al. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell. 2011;145:435–446. doi: 10.1016/j.cell.2011.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitler B.G., Watson Z.L., Wheeler L.J., Behbakht K. PARP inhibitors: clinical utility and possibilities of overcoming resistance. Gynecol. Oncol. 2017;147:695–704. doi: 10.1016/j.ygyno.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt J., et al. The ATM/p53 pathway is commonly targeted for inactivation in squamous cell carcinoma of the head and neck (SCCHN) by multiple molecular mechanisms. Oral Oncol. 2005;41:1013–1020. doi: 10.1016/j.oraloncology.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bornstein E., Jimeno A. Olaparib for the treatment of ovarian cancer. Drugs Today. 2016;52:17–28. doi: 10.1358/dot.2016.52.1.2440714. [DOI] [PubMed] [Google Scholar]
- Bosotti R., Isacchi A., Sonnhammer E.L. FAT: a novel domain in PIK-related kinases. Trends Biochem. Sci. 2000;25:225–227. doi: 10.1016/s0968-0004(00)01563-2. [DOI] [PubMed] [Google Scholar]
- Brown E.J., Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- Brown E.J., Baltimore D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 2003;17:615–628. doi: 10.1101/gad.1067403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.S., O'Carrigan B., Jackson S.P., Yap T.A. Targeting DNA repair in cancer: beyond PARP inhibitors. Canc. Discov. 2017;7:20–37. doi: 10.1158/2159-8290.CD-16-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant H.E., et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- Bukhari A.B., et al. Inhibiting Wee1 and ATR kinases produces tumor-selective synthetic lethality and suppresses metastasis. J. Clin. Invest. 2019;129:1329–1344. doi: 10.1172/JCI122622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canman C.E., et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- Carlo M.I., et al. A phase ib study of BEZ235, a dual inhibitor of phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR), in patients with advanced renal cell carcinoma. Oncol. 2016;21:787–788. doi: 10.1634/theoncologist.2016-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrassa L., Damia G. DNA damage response inhibitors: mechanisms and potential applications in cancer therapy. Canc. Treat Rev. 2017;60:139–151. doi: 10.1016/j.ctrv.2017.08.013. [DOI] [PubMed] [Google Scholar]
- Charrier J.D., et al. Discovery of potent and selective inhibitors of ataxia telangiectasia mutated and Rad3 related (ATR) protein kinase as potential anticancer agents. J. Med. Chem. 2011;54:2320–2330. doi: 10.1021/jm101488z. [DOI] [PubMed] [Google Scholar]
- Chen E.M., et al. Biodegradable PEG-poly(ω-pentadecalactone-co-p-dioxanone) nanoparticles for enhanced and sustained drug delivery to treat brain tumors. Biomaterials. 2018;178:193–203. doi: 10.1016/j.biomaterials.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich K.A., Cortez D. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack G., Lau A., Pierce A., Smith S., Stephens C.J.A. Abstract O6.3; ATR inhibitor AZD6738. Ann. Oncol. 2015;26 [Google Scholar]
- Combes E., et al. Inhibition of ataxia-telangiectasia mutated and RAD3-related (ATR) overcomes oxaliplatin resistance and promotes antitumor immunity in colorectal cancer. Canc. Res. 2019;79:2933–2946. doi: 10.1158/0008-5472.CAN-18-2807. [DOI] [PubMed] [Google Scholar]
- Cortez D., Guntuku S., Qin J., Elledge S.J. ATR and ATRIP: partners in checkpoint signaling. Science. 2001;294:1713–1716. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- De Bono J.S., et al. First-in-human trial of the oral ataxia telangiectasia and Rad3-related (ATR) inhibitor BAY 1895344 in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2019;37 doi: 10.1158/2159-8290.CD-20-0868. 3007-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bont R., van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- Dillon M.T., et al. Radiosensitization by the ATR inhibitor AZD6738 through generation of acentric micronuclei. Mol. Canc. Therapeut. 2017;16:25–34. doi: 10.1158/1535-7163.MCT-16-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon M.T., et al. ATR inhibition potentiates the radiation-induced inflammatory tumor microenvironment. Clin. Canc. Res. 2019;25:3392. doi: 10.1158/1078-0432.CCR-18-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer H., et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- Fazio N., et al. A phase II study of BEZ235 in patients with everolimus-resistant, advanced pancreatic neuroendocrine tumours. Anticancer Res. 2016;36:713–719. [PMC free article] [PubMed] [Google Scholar]
- Fernandes N., et al. DNA damage-induced association of ATM with its target proteins requires a protein interaction domain in the N terminus of ATM. J. Biol. Chem. 2005;280:15158–15164. doi: 10.1074/jbc.M412065200. [DOI] [PubMed] [Google Scholar]
- Ferrao P.T., Bukczynska E.P., Johnstone R.W., McArthur G.A. Efficacy of CHK inhibitors as single agents in MYC-driven lymphoma cells. Oncogene. 2012;31:1661–1672. doi: 10.1038/onc.2011.358. [DOI] [PubMed] [Google Scholar]
- Finlay M.R., et al. Sulfonyl-morpholino-pyrimidines: SAR and development of a novel class of selective mTOR kinase inhibitor. Bioorg. Med. Chem. Lett. 2012;22:4163–4168. doi: 10.1016/j.bmcl.2012.04.036. [DOI] [PubMed] [Google Scholar]
- Flynn R.L., Zou L. ATR: a master conductor of cellular responses to DNA replication stress. Trends Biochem. Sci. 2011;36:133–140. doi: 10.1016/j.tibs.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokas E., et al. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis. 2012;3:e441. doi: 10.1038/cddis.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong P.C., et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- Foote K.M., Lau A., Nissink J.W. Drugging ATR: progress in the development of specific inhibitors for the treatment of cancer. Future Med. Chem. 2015;7:873–891. doi: 10.4155/fmc.15.33. [DOI] [PubMed] [Google Scholar]
- Foote K.M., et al. Discovery of 4-{4-[(3R)-3-Methylmorpholin-4-yl]-6-[1-(methylsulfonyl)cyclopropyl]pyrimidin-2-y l}-1H-indole (AZ20): a potent and selective inhibitor of ATR protein kinase with monotherapy in vivo antitumor activity. J. Med. Chem. 2013;56:2125–2138. doi: 10.1021/jm301859s. [DOI] [PubMed] [Google Scholar]
- Foote K.M., et al. Discovery and characterization of AZD6738, a potent inhibitor of ataxia telangiectasia mutated and Rad3 related (ATR) kinase with application as an anticancer agent. J. Med. Chem. 2018;61:9889–9907. doi: 10.1021/acs.jmedchem.8b01187. [DOI] [PubMed] [Google Scholar]
- Garrett M.D., Collins I. Anticancer therapy with checkpoint inhibitors: what, where and when? Trends Pharmacol. Sci. 2011;32:308–316. doi: 10.1016/j.tips.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Gately D.P., Hittle J.C., Chan G.K.T., Yen T.J. Characterization of ATM expression, localization, and associated DNA-dependent protein kinase activity. Mol. Biol. Cell. 1998;9:2361–2374. doi: 10.1091/mbc.9.9.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad O., et al. Combining ATR suppression with oncogenic ras synergistically increases genomic instability, causing synthetic lethality or tumorigenesis in a dosage-dependent manner. Canc. Res. 2010;70:9693–9702. doi: 10.1158/0008-5472.CAN-10-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J.H., et al. Tumor-targeted prodrug ICT2588 demonstrates therapeutic activity against solid tumors and reduced potential for cardiovascular toxicity. Mol. Pharm. 2014;11:1294–1300. doi: 10.1021/mp400760b. [DOI] [PubMed] [Google Scholar]
- Giridharan V.V., et al. Schisandrin B, attenuates cisplatin-induced oxidative stress, genotoxicity and neurotoxicity through modulating NF-kappaB pathway in mice. Free Radic. Res. 2012;46:50–60. doi: 10.3109/10715762.2011.638291. [DOI] [PubMed] [Google Scholar]
- Gorecki L., Andrs M., Rezacova M., Korabecny J. Discovery of ATR kinase inhibitor berzosertib (VX-970, M6620): clinical candidate for cancer therapy. Pharmacol. Ther. 2020;210:107518. doi: 10.1016/j.pharmthera.2020.107518. [DOI] [PubMed] [Google Scholar]
- Gorgoulis V.G., et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- Guichard S.M., et al. The pre-clinical in vitro and in vivo activity of AZD6738: a potent and selective inhibitor of ATR kinase. Canc. Res. 2013;73 [Google Scholar]
- Hall A.B., et al. Potentiation of tumor responses to DNA damaging therapy by the selective ATR inhibitor VX-970. Oncotarget. 2014;5:5674–5685. doi: 10.18632/oncotarget.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond E.M., Denko N.C., Dorie M.J., Abraham R.T., Giaccia A.J. Hypoxia links ATR and p53 through replication arrest. Mol. Cell Biol. 2002;22:1834–1843. doi: 10.1128/MCB.22.6.1834-1843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Helt C.E., Cliby W.A., Keng P.C., Bambara R.A., O'Reilly M.A. Ataxia telangiectasia mutated (ATM) and ATM and Rad3-related protein exhibit selective target specificities in response to different forms of DNA damage. J. Biol. Chem. 2005;280:1186–1192. doi: 10.1074/jbc.M410873200. [DOI] [PubMed] [Google Scholar]
- Huntoon C.J., et al. ATR inhibition broadly sensitizes ovarian cancer cells to chemotherapy independent of BRCA status. Canc. Res. 2013;73:3683–3691. doi: 10.1158/0008-5472.CAN-13-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle N.T., et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol. Canc. Therapeut. 2004;3:763–772. [PubMed] [Google Scholar]
- Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacq X., et al. Abstract 1823: AZ20, a novel potent and selective inhibitor of ATR kinase with in vivo antitumour activity. Canc. Res. 2012;72 1823-1823. [Google Scholar]
- Jiang H., et al. The combined status of ATM and p53 link tumor development with therapeutic response. Gene Dev. 2009;23:1895–1909. doi: 10.1101/gad.1815309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Li W., Li X., Bai H., Zhang Z. Current status and future prospects of PARP inhibitor clinical trials in ovarian cancer. Canc. Manag. Res. 2019;11:4371–4390. doi: 10.2147/CMAR.S200524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., et al. Combined inhibition of ATR and WEE1 as a novel therapeutic strategy in triple-negative breast cancer. Neoplasia. 2018;20:478–488. doi: 10.1016/j.neo.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., Westcott G. Olaparib recommendations for ovarian cancer patients. Future Oncol. 2016;12:149–151. doi: 10.2217/fon.15.257. [DOI] [PubMed] [Google Scholar]
- Jones C.D., Blades K., Foote K.M., Guichard S.M., et al. Discovery of AZD6738, a potent and selective inhibitor with the potential to test the clinical efficacy of ATR kinase inhibition in cancer patients. Canc. Res. 2013;73 [Google Scholar]
- Jossé R., et al. ATR inhibitors VE-821 and VX-970 sensitize cancer cells to topoisomerase I inhibitors by disabling DNA replication initiation and fork elongation responses. Canc. Res. 2014;74:6968. doi: 10.1158/0008-5472.CAN-13-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M. RTB101 and immune function in the elderly: interpreting an unsuccessful clinical trial. Transl. Med. Aging. 2020;4:32–34. [Google Scholar]
- Kaelin W.G. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Canc. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- Kandoth C., et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnitz L.M., Zou L. Molecular pathways: targeting ATR in cancer therapy. Clin. Canc. Res. 2015;21:4780–4785. doi: 10.1158/1078-0432.CCR-15-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnitz L.M., et al. Gemcitabine-induced activation of checkpoint signaling pathways that affect tumor cell survival. Mol. Pharmacol. 2005;68:1636–1644. doi: 10.1124/mol.105.012716. [DOI] [PubMed] [Google Scholar]
- Karve S., et al. Revival of the abandoned therapeutic wortmannin by nanoparticle drug delivery. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109:8230–8235. doi: 10.1073/pnas.1120508109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M.B., et al. A mammalian-cell cycle checkpoint pathway utilizing P53 and Gadd45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kim H.J., et al. Anti-tumor activity of the ATR inhibitor AZD6738 in HER2 positive breast cancer cells. Int. J. Canc. 2017;140:109–119. doi: 10.1002/ijc.30373. [DOI] [PubMed] [Google Scholar]
- Knegtel R., et al. Rational design of 5-(4-(Isopropylsulfonyl)phenyl)-3-(3-(4-((methylamino)methyl)phenyl)isoxazol-5-yl)pyrazin-2-amine (VX-970, M6620): optimization of intra- and intermolecular polar interactions of a new ataxia telangiectasia mutated and Rad3-related (ATR) kinase inhibitor. J. Med. Chem. 2019;62 doi: 10.1021/acs.jmedchem.9b00426. [DOI] [PubMed] [Google Scholar]
- Konstantinidou G., et al. Dual phosphoinositide 3-kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Canc. Res. 2009;69:7644–7652. doi: 10.1158/0008-5472.CAN-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M.G., et al. Abstract CT026: phase I study of AZD6738, an inhibitor of ataxia telangiectasia Rad3-related (ATR), in combination with olaparib or durvalumab in patients (pts) with advanced solid cancers. Canc. Res. 2018;78 [Google Scholar]
- Kumagai A., Lee J., Yoo H.Y., Dunphy W.G. TopBP1 activates the ATR-ATRIP complex. Cell. 2006;124 doi: 10.1016/j.cell.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Kurmasheva R.T., et al. Initial testing (stage 1) of M6620 (formerly VX-970), a novel ATR inhibitor, alone and combined with cisplatin and melphalan, by the Pediatric Preclinical Testing Program. Pediatr. Blood Canc. 2018;65 doi: 10.1002/pbc.26825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok M., et al. Synthetic lethality in chronic lymphocytic leukaemia with DNA damage response defects by targeting the ATR pathway. Lancet. 2015;385:S58. doi: 10.1016/S0140-6736(15)60373-7. [DOI] [PubMed] [Google Scholar]
- Lavin M.F. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat. Rev. Mol. Cell Biol. 2008;9 doi: 10.1038/nrm2514. 927-927. [DOI] [PubMed] [Google Scholar]
- Lempiainen H., Halazonetis T.D. Emerging common themes in regulation of PIKKs and PI3Ks. EMBO J. 2009;28:3067–3073. doi: 10.1038/emboj.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczynska K.B., et al. Preclinical testing of an Atr inhibitor demonstrates improved response to standard therapies for esophageal cancer. Radiother. Oncol. 2016;121:232–238. doi: 10.1016/j.radonc.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Lahad E., Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br. J. Canc. 2007;96:11–15. doi: 10.1038/sj.bjc.6603535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Zhang B., Liu K., Ding Z., Hu X. Schisandrin B attenuates cancer invasion and metastasis via inhibiting epithelial-mesenchymal transition. PloS One. 2012;7 doi: 10.1371/journal.pone.0040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., et al. Discovery of 9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phenyl) benzo[h][1,6]naphthyridin-2(1H)-one (Torin2) as a potent, selective, and orally available mammalian target of rapamycin (mTOR) inhibitor for treatment of cancer. J. Med. Chem. 2011;54:1473–1480. doi: 10.1021/jm101520v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., et al. Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR. Canc. Res. 2013;73:2574–2586. doi: 10.1158/0008-5472.CAN-12-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecking U.T., et al. Abstract 983: identification of potent, highly selective and orally available ATR inhibitor BAY 1895344 with favorable PK properties and promising efficacy in monotherapy and combination in preclinical tumor models. Canc. Res. 2017;77 doi: 10.1021/acs.jmedchem.0c00369. [DOI] [PubMed] [Google Scholar]
- Maira S.-M., et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Canc. Therapeut. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- Marechal A., Zou L. RPA-coated single-stranded DNA as a platform for post-translational modifications in the DNA damage response. Cell Res. 2015;25:9–23. doi: 10.1038/cr.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey A.J. Inhibition of ATR-dependent feedback activation of Chk1 sensitises cancer cells to Chk1 inhibitor monotherapy. Canc. Lett. 2016;383:41–52. doi: 10.1016/j.canlet.2016.09.024. [DOI] [PubMed] [Google Scholar]
- Matsuoka S., et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- McMahon S.B., Van Buskirk H.A., Dugan K.A., Copeland T.D., Cole M.D. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell. 1998;94:363–374. doi: 10.1016/s0092-8674(00)81479-8. [DOI] [PubMed] [Google Scholar]
- Menolfi D., Zha S. ATM, ATR and DNA-PKcs kinases—the lessons from the mouse models: inhibition ≠ deletion. Cell Biosci. 2020;10:8. doi: 10.1186/s13578-020-0376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton F.K., Pollard J.R., Curtin N.J. The impact of p53 dysfunction in ATR inhibitor cytotoxicity and chemo- and Radiosensitisation. Cancers. 2018;10:275. doi: 10.3390/cancers10080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min A., et al. AZD6738, A novel oral inhibitor of ATR, induces synthetic lethality with ATM deficiency in gastric cancer cells. Mol. Canc. Therapeut. 2017;16:566–577. doi: 10.1158/1535-7163.MCT-16-0378. [DOI] [PubMed] [Google Scholar]
- Murga M., et al. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors. Nat. Struct. Mol. Biol. 2011;18:1331–1338. doi: 10.1038/nsmb.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J.S., Cortez D. Rapid activation of ATR by ionizing radiation requires ATM and Mre11. J. Biol. Chem. 2006;281:9346–9350. doi: 10.1074/jbc.M513265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R., et al. Inhibition of the replication stress response is a synthetic vulnerability in SCLC that acts synergistically in combination with cisplatin. Mol. Canc. Therapeut. 2019;18:762. doi: 10.1158/1535-7163.MCT-18-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute Website https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-olaparib-rucaparib-prostate-cancer Accessed Date: July, 2020.
- Negrini S., Gorgoulis V.G., Halazonetis T.D. Genomic instability--an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- Nghiem P., Park P.K., Kim Y., Vaziri C., Schreiber S.L. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc. Natl. Acad. Sci. Unit. States Am. 2001;98:9092–9097. doi: 10.1073/pnas.161281798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H., et al. Inhibition of ATR protein kinase activity by schisandrin B in DNA damage response. Nucleic Acids Res. 2009;37:5678–5689. doi: 10.1093/nar/gkp593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carrigan B., et al. Phase I trial of a first-in-class ATR inhibitor VX-970 as monotherapy (mono) or in combination (combo) with carboplatin (CP) incorporating pharmacodynamics (PD) studies. J. Clin. Oncol. 2016;34 doi: 10.1200/JCO.19.02404. 2504-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll M., Ruiz-Perez V.L., Woods C.G., Jegg P.A., Goodship J.A. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat. Genet. 2003;33:497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- Oliver T.G., et al. Chronic cisplatin treatment promotes enhanced damage repair and tumor progression in a mouse model of lung cancer. Gene Dev. 2010;24:837–852. doi: 10.1101/gad.1897010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal T., et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- Peasland A., et al. Identification and evaluation of a potent novel ATR inhibitor, NU6027, in breast and ovarian cancer cell lines. Br. J. Canc. 2011;105:372–381. doi: 10.1038/bjc.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires I.M., et al. Effects of acute versus chronic Hypoxia on DNA damage responses and genomic instability. Canc. Res. 2010;70:925–935. doi: 10.1158/0008-5472.CAN-09-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires I.M., et al. Targeting radiation-resistant hypoxic tumour cells through ATR inhibition. Br. J. Canc. 2012;107:291–299. doi: 10.1038/bjc.2012.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer E.R., et al. Phase I trial of first-in-class ATR inhibitor VX-970 in combination with gemcitabine (Gem) in advanced solid tumors ( NCT02157792) J. Clin. Oncol. 2016;34 2513-2513. [Google Scholar]
- Plummer R., et al. Phase I dose expansion data for M6620 (formerly VX-970), a first-in-class ATR inhibitor, combined with gemcitabine (Gem) in patients (pts) with advanced non-small cell lung cancer (NSCLC) Ann. Oncol. 2018;29:viii519. [Google Scholar]
- Prevo R., et al. The novel ATR inhibitor VE-821 increases sensitivity of pancreatic cancer cells to radiation and chemotherapy. Canc. Biol. Ther. 2012;13:1072–1081. doi: 10.4161/cbt.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W., et al. Inhibition of Wee1 sensitizes AML cells to ATR inhibitor VE-822-induced DNA damage and apoptosis. Biochem. Pharmacol. 2019;164:273–282. doi: 10.1016/j.bcp.2019.04.022. [DOI] [PubMed] [Google Scholar]
- Reaper P.M., et al. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat. Chem. Biol. 2011;7:428–430. doi: 10.1038/nchembio.573. [DOI] [PubMed] [Google Scholar]
- resTORbio 2019. https://www.restorbio.com/clinical-trials/
- Roos W.P., Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Rundle S., Bradbury A., Drew Y., Curtin N.J. Targeting the ATR-CHK1 Axis in cancer therapy. Cancers. 2017;9:41. doi: 10.3390/cancers9050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y., et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell stem cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Lindsey-Boltz L.A., Unsal-Kacmaz K., Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu.l Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Sarkaria J.N., et al. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Canc. Res. 1998;58:4375–4382. [PubMed] [Google Scholar]
- Sarkaria J.N., et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent. Caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- Schmitt A., et al. ATM deficiency is associated with sensitivity to PARP1- and ATR inhibitors in lung adenocarcinoma. Canc. Res. 2017;77:3040–3056. doi: 10.1158/0008-5472.CAN-16-3398. [DOI] [PubMed] [Google Scholar]
- Schoppy D.W., et al. Oncogenic stress sensitizes murine cancers to hypomorphic suppression of ATR. J. Clin. Invest. 2012;122:241–252. doi: 10.1172/JCI58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel J.J., Anderson C.M., Blackburn E.H. A novel Tel1/ATM N-terminal motif, TAN, is essential for telomere length maintenance and a DNA damage response. Mol. Cell Biol. 2008;28:5736–5746. doi: 10.1128/MCB.00326-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro G., et al. Abstract CT012: phase 1 trial of first-in-class ATR inhibitor VX-970 in combination with cisplatin (Cis) in patients (pts) with advanced solid tumors ( NCT02157792) Canc. Res. 2016;76:CT012. [Google Scholar]
- Shechter D., Costanzo V., Gautier J. ATR and ATM regulate the timing of DNA replication origin firing. Nat. Cell Biol. 2004;6:648–655. doi: 10.1038/ncb1145. [DOI] [PubMed] [Google Scholar]
- Shi Q., et al. The identification of the ATR inhibitor VE-822 as a therapeutic strategy for enhancing cisplatin chemosensitivity in esophageal squamous cell carcinoma. Canc. Lett. 2018;432:56–68. doi: 10.1016/j.canlet.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., Kastan M.B. ATM: genome stability, neuronal development, and cancer cross paths. Adv. Canc. Res. 2001;83:209–254. doi: 10.1016/s0065-230x(01)83007-4. [DOI] [PubMed] [Google Scholar]
- Sorensen C.S., et al. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Canc. Cell. 2003;3:247–258. doi: 10.1016/s1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Sprong D., Janssen H.L., Vens C., Begg A.C. Resistance of hypoxic cells to ionizing radiation is influenced by homologous recombination status. Int. J. Radiat. Oncol. Biol. Phys. 2006;64:562–572. doi: 10.1016/j.ijrobp.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Sun M., Xu X., Lu Q., Pan Q., Hu X., Schisandrin B. A dual inhibitor of P-glycoprotein and multidrug resistance-associated protein 1. Canc. Lett. 2007;246:300–307. doi: 10.1016/j.canlet.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Sun L.-L., et al. Inhibition of ATR downregulates PD-L1 and sensitizes tumor cells to T cell-mediated killing. Am. J. Cancer Res. 2018;8:1307–1316. [PMC free article] [PubMed] [Google Scholar]
- Taylor W.R., Stark G.R. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- Thandavarayan R.A., et al. Schisandrin B prevents doxorubicin induced cardiac dysfunction by modulation of DNA damage, oxidative stress and inflammation through inhibition of MAPK/p53 signaling. PloS One. 2015;10 doi: 10.1371/journal.pone.0119214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A., et al. Phase I study of ATR inhibitor M6620 in combination with topotecan in patients with advanced solid tumors. J. Clin.l Oncol. 2017;36:1594–1602. doi: 10.1200/JCO.2017.76.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A., et al. Phase I study of ATR inhibitor M6620 in combination with topotecan in patients with advanced solid tumors. J. Clin. Oncol. 2018;36:1594–1602. doi: 10.1200/JCO.2017.76.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo L.I., Murga M., Gutierrez-Martinez P., Soria R., Fernandez-Capetillo O. ATR signaling can drive cells into senescence in the absence of DNA breaks. Gene Dev. 2008;22:297–302. doi: 10.1101/gad.452308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo L.I., et al. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nat. Struct. Mol. Biol. 2011;18:721–727. doi: 10.1038/nsmb.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu X., et al. ATR inhibition is a promising radiosensitizing strategy for triple-negative breast cancer. Mol. Canc. Therapeut. 2018;17:2462. doi: 10.1158/1535-7163.MCT-18-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gent D.C., Hoeijmakers J.H.J., Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat. Rev. Genet. 2001;2:196–206. doi: 10.1038/35056049. [DOI] [PubMed] [Google Scholar]
- Vendetti F.P., et al. The orally active and bioavailable ATR kinase inhibitor AZD6738 potentiates the anti-tumor effects of cisplatin to resolve ATM-deficient non-small cell lung cancer in vivo. Oncotarget. 2015;6:44289–44305. doi: 10.18632/oncotarget.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendetti F.P., et al. ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation. J. Clin. Invest. 2018;128:3926–3940. doi: 10.1172/JCI96519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J.M., Kaufmann S.H. Prospects for the use of ATR inhibitors to treat cancer. Pharmaceuticals. 2010;3:1311–1334. doi: 10.3390/ph3051311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallez Y., et al. The ATR inhibitor AZD6738 synergizes with gemcitabine in vitro and in vivo to induce pancreatic ductal adenocarcinoma regression. Mol. Canc. Therapeut. 2018;17:1670–1682. doi: 10.1158/1535-7163.MCT-18-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., et al. Pharmacologic characterization of fluzoparib, a novel poly (ADP-ribose) polymerase inhibitor undergoing clinical trials. Canc. Sci. 2019;110:1064–1075. doi: 10.1111/cas.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A.M., Ryan A.J. ATM and ATR as therapeutic targets in cancer. Pharmacol. Therapeut. 2015;149:124–138. doi: 10.1016/j.pharmthera.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Wengner A.M., et al. The novel ATR inhibitor BAY 1895344 is efficacious as monotherapy and combined with DNA damage–inducing or repair–compromising therapies in preclinical cancer models. Mol. Canc. Therapeut. 2020;19:26–38. doi: 10.1158/1535-7163.MCT-19-0019. [DOI] [PubMed] [Google Scholar]
- Wieringa H.W., van der Zee A.G.J., de Vries E.G.E., van Vugt M.A.T.M. Breaking the DNA damage response to improve cervical cancer treatment. Canc. Treat Rev. 2016;42:30–40. doi: 10.1016/j.ctrv.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Wise-Draper T.M., et al. A phase ib study of the dual PI3K/mTOR inhibitor dactolisib (BEZ235) combined with everolimus in patients with advanced solid malignancies. Targeted Oncol. 2017;12:323–332. doi: 10.1007/s11523-017-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D., Turchi J.J. Chemotherapy induced DNA damage response Convergence of drugs and pathways. Canc. Biol. Ther. 2013;14:379–389. doi: 10.4161/cbt.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z., et al. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J. Biol. Chem. 2003;278:21767–21773. doi: 10.1074/jbc.M300229200. [DOI] [PubMed] [Google Scholar]
- Xu Y., et al. Schisandrin B prevents doxorubicin-induced chronic cardiotoxicity and enhances its anticancer activity in vivo. PloS One. 2011;6 doi: 10.1371/journal.pone.0028335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H.H.N., et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell stem cell. 2018;23:882–897. doi: 10.1016/j.stem.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Yap T.A., et al. Abstract PR14: phase I trial of first-in-class ataxia telangiectasia-mutated and Rad3-related (ATR) inhibitor VX-970 as monotherapy (mono) or in combination with carboplatin (CP) in advanced cancer patients (pts) with preliminary evidence of target modulation and antitumor activity. Mol. Canc. Therapeut. 2015;14:PR14. doi: 10.1158/1535-7163.TARG-15-PR14. [DOI] [Google Scholar]
- Yap T.A., et al. 1LBA - phase I modular study of AZD6738, a novel oral, potent and selective ataxia telangiectasia Rad3-related (ATR) inhibitor in combination (combo) with carboplatin, olaparib or durvalumab in patients (pts) with advanced cancers. Eur. J. Canc. 2016;69 [Google Scholar]
- Zenke F.T., et al. Abstract 369: antitumor activity of M4344, a potent and selective ATR inhibitor, in monotherapy and combination therapy. Canc. Res. 2019;79 [Google Scholar]