Abstract
Neurodegenerative diseases (ND) are of vast origin which are characterized by gradual progressive loss of neurons in the brain region. ND can be classified according to the clinical symptoms present (e.g. Cognitive decline, hyperkinetic, and hypokinetic movements disorder) or by the pathological protein deposited (e.g., Amyloid, tau, Alpha-synuclein, TDP-43). Alzheimer’s disease preceded by Parkinson’s is the most prevalent form of ND world-wide. Multiple factors like aging, genetic mutations, environmental factors, gut microbiota, blood-brain barrier microvascular complication, etc. may increase the predisposition towards ND. Genetic mutation is a major contributor in increasing the susceptibility towards ND, the concept of one disease-one gene is obsolete and now multiple genes are considered to be involved in causing one particular disease. Also, the involvement of multiple pathological mechanisms like oxidative stress, neuroinflammation, mitochondrial dysfunction, etc. contributes to the complexity and makes them difficult to be treated by traditional mono-targeted ligands. In this aspect, the Poly-pharmacological drug approach which targets multiple pathological pathways at the same time provides the best way to treat such complex networked CNS diseases. In this review, we have provided an overview of ND and their pathological origin, along with a brief description of various genes associated with multiple diseases like Alzheimer’s, Parkinson’s, Multiple sclerosis (MS), Amyotrophic Lateral Sclerosis (ALS), Huntington’s and a comprehensive detail about the Poly-pharmacology approach (MTDLs and Fixed-dose combinations) along with their merits over the traditional single-targeted drug is provided. This review also provides insights into current repurposing strategies along with its regulatory considerations.
Keywords: Neurodegenerative diseases, Alzheimer’s disease, Parkinson’s disease, Drug repurposing, Gene therapy
Abbreviations
- ND
Neurodegenerative disease
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- FTD
Frontotemporal dementia
- MCI
Mild Cognitive Impairment
- APP
Amyloid Precursor Protein
- NFT
Neurofibrillary tangles
- UPR
Unfolded protein response
- ALS
Amyotrophic lateral sclerosis
- BMAA
β-N-methylamino-L-alanine
- BBB
Blood brain barrier
- GWAS
Genome-wide association studies
- EOAD
Early onset Alzheimer’s disease
- LOAD
Late onset Alzheimer’s disease
- SNP
Single nucleotide polymorphism
- TLR
Toll like receptor
- HD
Huntington’s disease
- MTDL
Multi-target directed ligands
- MAO
Monoamine oxidase
- GSH
Glutathione
- LD
L-Dopa
- API
Active Pharmaceutical ingredient
- FDC
Fixed dose combination
- COMT
Catechol-O-methyl transferase
- COM
Composition of matter
- MOU
Method of use
- NDA
New drug application
- SNpc
Substantia Nigra pars compacta
- MPTP
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- TH
Tyrosine Hydroxylase
- AMPK
AMP-kinase
- PPARγ
Peroxisome proliferator activated receptor gamma
- DOPAC
Dihydroxyphenylacetic acid
- HVA
Homovanillic acid
- Mac-1
Macrophage antigen-1
- iNOS
Inducible nitric oxide synthase
- MAO-B
Monoamine oxidase-B
- MPP+
1-methyl-4-pyridinium
- 6-OHDA
6-hydroxydopamine
- HY
Hoehn and Yahr
- UPDRS
Unified Parkinson Disease Scale- Motor score
- GLP-1
Glucagon like peptide-1
- GLP-1R
GLP-1 receptor
- GPCR
G-protein coupled receptor
- DPP-IV
Dipeptidyl peptidase IV
- LPS
Lipopolysaccharide
- MDS-UPDRS
Movement Disorders Society Unified Parkinson’s Disease Rating Scale
- MRI
Magnetic resonance imaging
- Aβ
Amyloid beta
- ARB
Angiotensin receptor blockers
- CCB
Calcium channel blockers
- AT1R
Angiotensin II type 1 receptor
- APP-CTF
Amyloid β protein precursor C-terminal fragment
- DHP
Dihydropyridines
- STZ
Strptozotocin
- SSRIs
Selective serotonin reuptake inhibitors
- MCI
Mild cognitive impairment
- TGF-β1
transforming growth factor-β1
1. Introduction
Neurons are one of the most special types of cells present in the human body. Their physiological role, as well as their cellular structure consisting of axon and dendrites, is very unique as compared to other cells in the human body. They are present throughout the body but most of them come together to form a complex network of millions of synapses and cell-bodies in the brain. The human brain though only small in size and weighing in the range of 1300–1400 g consumes a lot of energy and oxygen as compared to other organ systems (Gallagher et al., 1998; Heymsfield et al., 1985). Our brain accounts for almost 20% energy consumption at rest (Gallagher et al., 1998). Most portion of this energy is spent by neurons in maintaining the function at synapses such as neurotransmitter release, recycling of vesicles, reuptake of neurotransmitters and maintaining, and restoring membrane potentials (Watts et al., 2018). Moreover, healthy neurons do not store glucose in the form of glycogen hence are entirely dependent on the cerebral blood flow for the requirements of glucose (Rai et al., 2018). Due to such high energy requirements, even a slight mitochondrial dysfunction drastically increases the oxidative stress and makes them susceptible to damage. Although few regions in the brain are more susceptible to neuronal degeneration as compared to others, the reason behind this is still not clear. Also, due to a lack of regenerative capacity, the damage is permanent. Environmental pollution is one of the major risk factors in the development of NDs (Calderon-Garciduenas et al., 2016). Considering the current modern lifestyle and increasing environmental pollution the cases of ND are only going to increase in the near future. Currently, Alzheimer’s disease (AD) is the most common ND followed by Parkinson’s disease (PD). Even if such diseases have been present since many decades their exact pharmacotherapy is still unavailable. As a result, in the current scenario, the novel innovations in the field of polypharmacology seem like a ray of hope in combating such multi-mechanistic ND. Apart from polypharmacology, drug repurposing is also an emerging area in the management of various types of ND.
2. Types of ND
Broadly, ND can be classified based on the clinical symptoms observed or the pathological protein present (Kovacs, 2017; Dugger and Dickson, 2017).
2.1. Classification based on clinical symptoms
The focal region/regions at which neuronal damage occurs determines the clinical symptoms associated with the disease.
2.1.1. Cognitive diseases
Diseases that cause significant cognitive decline are classified under this group. Example- AD and Frontotemporal dementia (FTD). The cognitive decline observed in AD is due to lesions/neuronal death in specific neuroanatomical locations such as Hippocampus, Entorhinal complex, and associated cortical regions while in FTD neuronal degeneration is primarily observed in the frontal and temporal lobes. The advancement in neuroimaging systems and the development of automated segmentation techniques have shed light on the global and subfield details of the Hippocampus. A metanalysis of MRI studies shows that on average 23–24% bilateral hippocampal volume reduction is observed in AD patients as compared to normal ageing controls (Shi et al., 2009; de Flores et al., 2015). A study conducted in 2015, indicates that a metric system combining hippocampal volume (HV) and hippocampal atrophy rate can give a better understanding of the disease progression from mild cognitive impairment (MCI) to AD (McRae-McKee et al., 2019).
2.1.2. Hypokinetic movement disorder
Such disorders are marked by symptoms such as difficulty in movement, bradykinesia, and rigidity. The most classical example is PD. The loss of dopaminergic neurons in Substantia Nigra creates deficiency of dopamine in the nigrostriatal pathway which leads to the observed hypokinetic motor symptoms. The clinical symptoms associated with PD can be seen at any time, whenever the neuronal degeneration in Substantia Nigra reaches 30–70% of its total population (Cheng et al., 2010).
2.1.3. Hyperkinetic movement disorder
Hyperkinetic disorders are exemplified by non-volitational movements that occur spontaneously or are superimposed on voluntary movements. Example- Huntington’s disease (HD).
This is a traditional simplistic way of classification, in current scenario ND may encompass all three type of clinical features.
2.2. Classification based on pathological protein present
Almost all NDs are characterized by abnormal protein aggregation. These insoluble protein aggregates deposit in various anatomical locations and is considered as one of the many reasons leading to neuronal death. Amyloid beta, Tau, TDP-43, alpha synuclein, Huntingtin are the major pathological proteins involved.
2.2.1. Amyloid based classification
Neurodegenerative conditions under this category are primarily characterized by the deposition of the Amyloid-β protein. According to the Amyloid hypothesis theory, Aβ is the major pathological proteinaceous deposition in the senile plaques present in AD (Dugger and Dickson, 2017). Aβ is generated by improper cleavage of a transmembrane protein called the “Amyloid precursor protein” (APP). APP has diverse physiological functions in the human body some of which include neuronal development, Homeostasis, and intraneuronal transport of lipids like cholesterol (Muller and Zheng, 2012). The processing of APP occurs by 2 pathways (Kojro and Fahrenholz, 2005; Chen et al., 2017).
2.2.1.1. Non-amyloidogenic pathway
In this pathway, the amyloid precursor protein is cleaved by α-secretase followed by γ-secretase to produce peptide3 (p3) and APPs α. This is a protective pathway since the APPs-alfa released has a neuroprotective and neurotrophic activity. This pathway precludes the formation of Amyloid beta.
2.2.1.2. Amyloidogenic pathway
Herein, the APP is initially cleaved by β-secretase followed by γ-secretase which results in the production of Aβ 42 and Aβ 40 fragment. Aβ 40 is freely soluble and hence resists aggregation while the Aβ 42 fragment is highly insoluble and tends to aggregate into oligomers. Such oligomers when accumulated in synapses hamper neurotransmission and subsequently cause neurodegeneration. The Aβ (1–42) processing of APP protein in AD can be attributed to genetic mutations in APP, PSEN1, PSEN2, and APOE. The details regarding the genetic mutations will be discussed ahead in the review.
2.2.2. Tau based classification
Tau is a microtubule stabilizing protein present in the axons of neurons. Abnormal tau protein aggregates are indicated in various diseases like AD, frontal-temporal dementia and PD (Pirscoveanu et al., 2017). Previously, in AD, tau protein aggregation was considered secondary to the Aβ deposition. However, recent literature related to AD shows that Tau pathology resembles more closely to neuronal degeneration associated with regions like Hippocampus and Entorhinal cortex as compared to Aβ plaques. In short, it can be concluded that the ancient perspective indicating Aβ as the only pathological protein in AD is no longer valid and both Aβ and Tau are responsible for the complex pathophysiology of AD (Musiek and Holtzman, 2015).
Inside a cell, Tau has important physiological functions such as maintaining the structure of the microtubule and regulating axonal transport. For performing such functions an optimum tau phosphorylation is important. However, in various ND hyperphosphorylation of tau is observed. In AD deposition of Aβ is thought to exacerbate tau phosphorylation and its downstream pathological processes. Such hyperphosphorylation weakens the microtubule-tau interaction and leads to the intraneuronal cytotoxic protein aggregates in the form of Neurofibrillary tangles (NFT).
2.2.3. Alpha synuclein
α-Synuclein is the major constituent of the Lewy body (Stefanis, 2012). The ND classified under this type shows characteristic deposition of protein aggregate in the form of Lewy bodies. α-synuclein is considered as the primary protein pathology in PD but, its role in the formation of senile plaque in AD is also well documented. Microscopic analysis of the brain associated with PD and multiple system atrophy (MSA) shows Lewy body inclusions in the neuron and oligodendrocytes respectively (Dickson, 2012). Such Lewy bodies are neurotoxic and contribute to the neurodegeneration associated with PD through multiple mechanisms such as oxidative stress and unfolded protein response (UPR). Regulation of glucose, modulation of calmodulin and regulation of vesicle trafficking are a few of the major roles performed by α-synuclein (Emamzadeh, 2016). The genetic mutation (duplication, triplication and point mutation) in the SNCA gene results in the formation of pathological misfolded α-synuclein and PD.
2.2.4. TDP-43
ALS is the major disease associated‘ with pathological neuronal inclusions of TDP-43 protein. TARDBP is the gene encoding for TDP-43. A missense mutation in the TARDBP gene is thought to be responsible for the pathological cytoplasmic accumulation of TDP-43. In normal conditions, TDP-43 is localized in the nucleus and binds to ss-DNA, ss-RNA, proteins and is thought to regulate neuronal plasticity (Prasad et al., 2019). TDP-43 is also thought to have a major pathological role in frontotemporal lobar dementia.
2.2.5. Trinucleotide repeat sequences (Huntingtin/Ataxin)
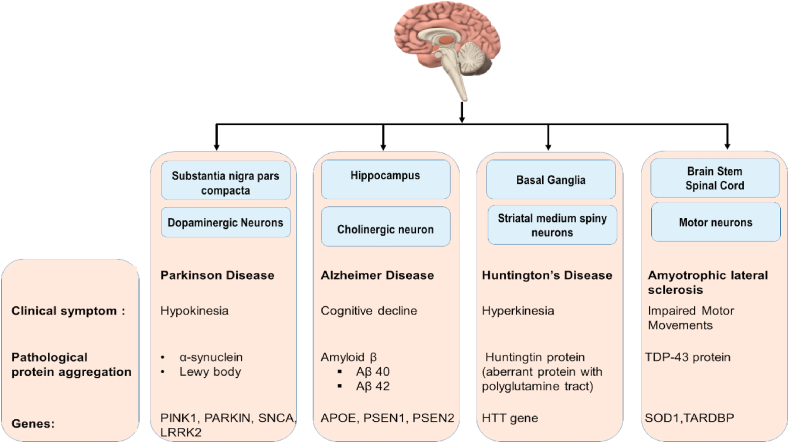
In such diseases, there is an abnormal expansion of a gene with a trinucleotide. The most common trinucleotide expansion being of CAG. Abnormal CAG expansion is observed in HD, Spinal, Bulbar Muscular Atrophy, and Spino-Cerebellar Ataxias. This repeat causes transcription of aberrantly long proteins having poly-glutamine tracts. Such proteins lose their normal cellular function and instead form cytotoxic protein aggregates which eventually leads to neurodegeneration (Paulson, 2018). An overview of the classification system is shown in Fig. 1.
Fig. 1.
Depictive classification of ND.
3. Multifactorial pathological origin
3.1. Ageing
Ageing is the major risk factor associated with ND. It is thought that an interplay between ageing, genetic predisposition and environmental factors dictate the type of neurodegeneration that may occur (Wyss-Coray, 2016). The most common forms of sporadic ND like Parkinson’s and Alzheimer’s are late onset and usually start to show symptoms at around 65 years of age. Ageing has been thought to contribute to neurodegeneration by various mechanisms such as Genetic instability, Impaired DNA repair mechanism, Low-grade chronic neuroinflammation, Increased Endoplasmic Reticulum stress, microvascular breakdown, decreased antioxidant enzymes and decreased autophagy.
Genetic instability (GI) refers to the mutations, base mispairing, and strand breaks observed in DNA. Such events are normally repaired by the DNA repair pathways. However, in aged individuals such damage may become permanent due to impaired DNA repair mechanisms. This leads to a cascade of events resulting in increased oxidative stress, mitochondrial dysfunction and cell senescence which cumulatively leads to neurodegeneration. (Hou et al., 2019).
Autopsy studies of aged brain with no prior neurological complications also shows the presence of protein aggregates such as Amyloidβ, Lewy bodies and TDP-43. However, such proteins are present in a concentration that is not pathological (Wyss-Coray, 2016; Hou et al., 2019). Such misfolded protein aggregates are cleared by a process called Autophagy. Autophagy can be defined as a process in which misfolded proteins and other cellular debris are subjected to lysosomal degradation via the formation of an autophagosome. Impairment in micro-autophagy with age may result in the build-up of such misfolded proteins which in turn can promote chronic-low grade neuro-inflammation, and subsequent neurodegeneration (Metaxakis et al., 2018; Walker, 2018). Such impairment in autophagy related processes can be attributed to hyperactivation of the mTOR pathway and instability of LAMP2A protein present on the lysosomal membrane. Also, increased aggregated protein burden in the aged brain leads to activation of unfolded protein response (UPR). In normal homeostatic conditions, UPR tends to increase cell survival by activating the autophagy process, decreasing protein translation and upregulating chaperones associated with the endoplasmic reticulum. However, sustained activation of UPR in aged individuals may cause activation of pro-apoptotic events leading to cell death and neurodegeneration (Cirone, 2020).
3.2. Genetic predisposition
Genetics is one of the main factors which determine the onset of ND. Familial forms of ND are due to the genetic mutations and accounts for 5–10% of the ND. The genetic predisposition of an individual can be classified into two types (Pihlstrom et al., 2017).
3.2.1. Mendelian based inheritance
Here, a mutation in gene is propagated through generations via autosomal dominant form. The classic example of such mendelian based inheritance of genetic mutation is found in AD in which a genetic mutation in either of the three genes APP, PSEN1 and PSEN2 may result in early onset familial AD. Similarly, in PD genetic mutations in SNCA, LRRK1, PARKIN, and PINK1 are related to early onset familial PD (Pihlstrom et al., 2017).
3.2.2. Genes that confer high susceptibility to ND
Mutations in such genes increases the probability of developing ND. A complex interplay between the other etiological factors like ageing and environmental factor determines the onset of disease. Example- APOE gene confers high susceptibility to AD based on age and dietary factors. APOE is one of the major causes for Late onset AD (Pihlstrom et al., 2017).
3.3. Environmental factors
Environmental risk factors are one of the leading causes of ND. Most often, an early age exposure to environmental/occupational toxins culminates into neurodegeneration in the later stages of an individual’s life. Some of the metals which cause neurotoxicity are lead, mercury, arsenic, aluminium, cadmium as well as some nanoparticles and pesticides. They are involved in dysregulating the synthesis and degradation of APP, Aβ and tau protein through different mechanisms like oxidative stress, mutations in protein related to autophagy and lipid peroxidation (Chin-Chan et al., 2015). Similarly, the destruction of dopaminergic neurons in substantia nigra region can be associated with exposure to environmental toxicants like MPTP, pesticides like paraquat and insecticide like rotenone (Klingelhoefer and Reichmann, 2015). Also, inhalation of volatile chemical solvents such as trichloroethylene (TCE) and SiO2 is well proven causative factor for mitochondrial dysfunction.
3.4. Lifestyle/diet
Lifestyle factors in midlife have an important influence on the risk of developing a ND during later life (Schulz and Deuschl, 2015). A Sedentary lifestyle increases the risk of ND. The common factors contributing to a sedentary lifestyle are socio-economic status, age and gender, advancement in technologies, and long working hours. Low concentrations of the endogenous antioxidant component like glutathione, and high proportion of polyunsaturated fatty acids (PUFAs) makes brain an ideal target for oxidative damage (Abdel Moneim, 2015). Lipid peroxidation in brain creates reactive oxygen species (ROS) which could react with macromolecules and may cause impairment in the function of membrane proteins e.g., inhibition of glutamate transporters, inhibition of Na+/K+ ATPases, inhibition of antioxidant enzymes like SOD1 and dysregulation in calcium homeostasis. Further, disruption of Ca2+ homeostasis can lead to a cascade of intracellular events and excitotoxicity. Intake of antioxidants like flavonoids, blueberries and strawberries (anthocyanins), is thought to slow the rate of cognitive decline (Feng and Wang, 2012). Intake of nuts specifically almond, hazelnut, and walnut, which are rich natural sources of monounsaturated and polyunsaturated fatty acids is associated with anti-inflammatory, anti-oxidant, anti-hyperlipidemic, and neuroprotectant property against AD (Grosso and Estruch, 2016; Gorji et al., 2018). Also, physical activity is associated with better cognitive function in aged individuals (Weuve et al., 2004).
3.5. Gut microbiota
Living inside everyone are trillions of microorganisms, microscopic organisms, infections, parasites, and other life shapes that are aggregately known as the microbiome. Different organs have microbial occupants, however, the gathering that has pulled the most consideration in biomedical exploration is the one in the gut. A few examinations have indicated that the intestinal microbiota sends and gets a variety of signals to and from the brain, it has become progressively clear that microorganism related bidirectional gut-brain flagging assumes a part in human cognitive capacities, brain health and brain pathophysiology (Collins and Bercik, 2009). A few examinations have featured that an expansion in intestinal porousness can permit the movement from the gut to the systemic flow of either microorganisms or their components and metabolites (e.g., β-N-methylamino-L-alanine-BMAA, a neurotoxin produced by Cyanobacteria and Lipopolysaccharide) (Kohler et al., 2016; de la Fuente-Nunez et al., 2018). Bacterial components like LPS after reaching the bloodstream can cross the compromised BBB in ND patients and activate toll-like receptor 4 which could further aggravate the neuroinflammation and neurodegeneration. Similarly, BMAA has been found in significant concentrations in the brains of AD, PD and ALS patients (Brenner, 2013). BMAA in the bloodstream can cross BBB by active transport mechanisms (Xie et al., 2011). Once inside the brain, BMAA binds and activates the excitatory glutamate receptors causing excitotoxicity and oxidative stress mediated neuronal death (Caller et al., 2018; Chiu et al., 2012). Such BMAA-associated toxicity is just one possible causal link in a field which is still under exploration i.e., microbiota-gut-brain axis and neurodegeneration.
3.6. Brain microvascular breakdown
Endothelial brokenness has been ensnared as a pivotal occasion in the advancement of a few CNS issues, such as AD, PD, ALS, multiple sclerosis (MS), human immunodeficiency virus (HIV)-1-associated neurocognitive disorder and traumatic brain injury (Grammas et al., 2011). Capillaries are the smallest cerebral blood vessels; they represent around 85% of cerebral vessel length and are a significant site of the blood-brain barrier (BBB) (Zlokovic, 2008). Keeping up BBB integrity is pivotal. Since, breakdown of BBB can open the entry to inflammatory leukocytes and bacterial endotoxins into the cerebrum, which could start a chain of immune response and neuro-inflammatory events culminating into ND. BBB breakdown in the hippocampus occurs prior to hippocampal atrophy, as seen in aged individuals and patients suffering from AD (Montagne et al., 2015, 2016). A slight alteration in BBB integrity may result in the formation of microbleeds in the brain, such microhemorrhages can be seen frequently in patients suffering from AD (Brundel et al., 2012). Many studies show that brain capillary damage and cerebral perfusion abnormalities might be an early biomarker of intellectual debilitation and neurodegeneration (Shams and Wahlund, 2016; Melzer et al., 2011; Nation et al., 2019).
3.7. Metabolic disorders
As everyone continuously ages, the pervasiveness of obesity, metabolic diseases, and neurodegenerative problems keep on ascending, as they are carefully interconnected. Metabolic diseases and neurodegeneration are firmly related. Numerous investigations demonstrated that hyperglycemia may be connected to AD pathophysiology. At the point when mice are controlled at high measures of glucose, there is an increase in tau cleavage and apoptosis bringing about the neuronal demise in mice cerebrum (Kim et al., 2009). Similarly, impaired insulin signaling, and Insulin resistance (IR) has also been found in the CNS of AD patients. Interestingly, glucose transport mediated in most of the brain parts is not insulin dependent. However, few regions of the brain like the hippocampus (involved in memory) express GLUT4 channels which are insulin dependent glucose transporters. Insulin resistance in the brain could disrupt the energy homeostasis/metabolism at the GLUT4 rich Hippocampal neurons, to bring about HC neuronal degeneration as seen in AD pathology. Moreover, recently, a clinical study carried out by De La Monte et al. reports derangement in CSF and peripheral systemic insulin levels in MCI and AD patients. The study further confirms that a decreased level of Insulin is observed in the CSF of patients suffering from MCI/AD as compared to the normal controls. Such a decreased insulin level suggests that AD is not a monomolecular protein pathology while a multi-molecular protein pathology that is related to insulin linked metabolic irregularities (de la Monte et al., 2019). The role of Insulin resistance in a cognitive disease like Alzheimer’s is further discussed in a more detailed manner by Rhea et al. in their review (Rhea et al., 2020).
3.8. Complexity of ND
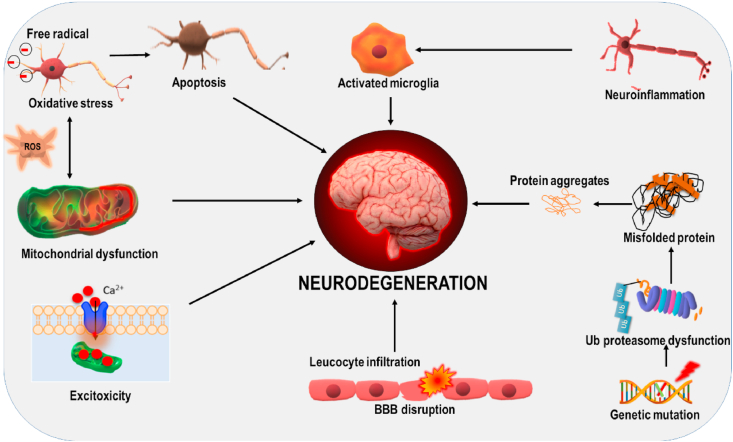
Different Pathological mechanism contribute to neurodegeneration, all of these mechanisms are interlinked to each other and contribute to the complexity observed in ND. Fig. 2 shows varied pathological mechanisms involved in ND.
Fig. 2.
Different pathological mechanisms contribute to neurodegeneration; all of these mechanisms are interconnected. Activation of one pathway may cause stimulation of other pathway leading to further aggravation of the disease condition.
4. One disease multiple gene
There are number of ND which are distinguished from their clinical or anatomic distribution (Dugger and Dickson, 2017). Many advances have been made in the past few decades in understanding the pathological pathways contributing to the progression of neurodegeneration diseases, from which genetics is one of the major contributors. The genetic study has been revolutionized from the initial sequencing of the human genome (Lander et al., 2001), followed by the international HapMap project and 1000 genome projects. The methodology like High throughput screening, genotyping array have made advances in generating genetic data. Genome-wide association study (GWAS) has become principle study design from 2005 for the study of complex genetics, where thousands and millions of single-nucleotide polymorphisms (SNPs) can be analyzed for understanding of one disease (Pihlstrom et al., 2017). There are innumerous genes that are associated with the progression of ND, which are discussed below:
4.1. Alzheimer’s disease
Alzheimer’s is a fatal form of dementia which is the sixth major leading cause of death in the United State (Taylor et al., 2017). One of the major risk factors for the development of AD is inheritance or mutation in genes. Based on the onset of the disease it is divided into Early onset of Alzheimer disease (EOAD) which mostly occurs before the age of 65 years and accounts for approximately 10% in total AD cases and the latter is Late onset of Alzheimer disease (LOAD) which is more common and mostly occurs after the age of 65years (Giri et al., 2016). The risk factor for EOAD mainly involves three rare mutations in APP, PSEN1 & PSEN2. APP is localized and cloned on chromosome 21. Down syndrome patients with partial or full duplication of chromosome 21 which results in duplication of APP gene are at greater risk for developing early onset of the disease (Hunter and Brayne, 2018). Later, it was observed that not all the families with EOAD showed linkage with chromosome 21. Subsequent studies found the linkage with chromosome 14 associated with mutation in PSEN1 and chromosome 1 which involved the homology of PSEN1, which was PSEN2 is also responsible for early onset of AD (Naj and Schellenberg et al., 2017). The apolipoprotein E (ApoE) located on chromosome 19q13.2 is the only and major genetic factor associated with LOAD found until now, of which ApoE4 is found to be the main cause of AD and ApoE3 to a lesser extent. Genome-wide association study (GWAS) has reformed the genetic study and the multi-stage multi analysis associated with SNP has found out almost 10 genes related with AD: ABCA7, BIN1, CD33, CLU, CR1, CD2AP, EPHA1, MS4A and PICALM (Misra et al., 2018).
According to the cellular functions carried out by the translated protein they can be categorized into: i) Lipid metabolism: ABCA7 and CLU (Jun et al., 2010; Hollingworth et al., 2011), ii) Immune response and inflammation: CR1, EPHA1, CD33, TREM (Jun et al., 2010; Hollingworth et al., 2011; Jin et al., 2015) and iii) Endocytosis and synaptic function: BIN1, CD2AP, EPHA1, PICALM (Jin et al., 2015; Naj et al., 2011).
4.1.1. Genes associated in lipid metabolism
ABCA7 (ATP binding cassette transporter A7) gene located on chromosome 19p13.3 is highly expressed in hippocampus CA1 neuron and microglial cells and encodes for ABCA7 for lipoprotein transport in the cell. In GWAS the G allele rs3764650 SNP was found to be the risk factor for AD as it is associated with hippocampal and cortical atrophy and cognitive deficits in patients (Almeida et al., 2018).
4.1.2. Gene associated in immune response and inflammation
Neuroinflammation is one of the essential hallmarks of AD which is the response of the immune system.CR1 is located on chromosome 1q32 which is expressed widely on blood cells. CR1 codes for complementary regulatory protein. A study found rs6656401, an SNP variant that is strongly associated with LOAD (Lambert et al., 2009).
EPHA1 is located on chromosome 7q34, is a member of the tyrosine kinase family. This gene is implicated in immune function, chronic inflammation, synaptic plasticity and cellular membrane processes. The SNP, rs11767557 was associated with a decreased risk of LOAD (Hollingworth et al., 2011).
TREM2 (Triggering receptor expressed on myeloid cell 2) gene is located on chromosome 6q21.1, highly expressed on the cell surface of microglia and throughout the central nervous system and encodes for single-pass type 1 membrane (Giri et al., 2016). Replogle et al. reported variant G rs6910730 to be associated with cognitive decline. A rare TREM2 missense variant T rs75932628 was strongly associated with AD pathology (Replogle et al., 2015).
4.1.3. Genes associated with endocytosis and synaptic function
CD2AP is located on chromosome 6p12, expressed in the brain and in AD patients, encodes for CD2-associated protein which is a scaffolding molecule and regulates actin cytoskeleton. It is functionally involved in receptor mediated endocytosis, apoptosis, cell adhesion, intracellular trafficking. SNP rs9296559 and rs9349407 in CD2AP is suspected to be at risk of LOAD. SNP rs9349407 has been found to be associated with neuritic plaque (Shulman et al., 2013). The CD2AP gene is strongly associated with LOAD risk and plays an important role in receptor mediated endocytosis, which is believed to be disrupted during the early stages of AD (Dunstan et al., 2016).
PICLAM (Phosphatidylinositol binding Clathrin assembly protein) is located on chromosome 11q14, expressed in all tissues and prominently in neurons. PICLAM is involved in clathrin-mediated endocytosis and also appears to be involved in the trafficking of VAMP (Harold et al., 2009). SNP rs3851179 is associated with thickening of entorhinal cortex and hippocampal degeneration and rs3851179 & APOE ε4 are strongly associated with brain atrophy and cognitive decline (Biffi et al., 2010).
4.2. Parkinson’s disease
PD is the second most prevalent irreversible, progressive, complex ND after Alzheimer’s, which affects almost 2–3% of the total population above the age of 65yrs. The neuropathological hallmark includes dopaminergic neuronal loss in substantia nigra and aggregates of α-synuclein (Poewe et al., 2017). From 1990 to 2016 the cases of Parkinson’s have almost increased by 2.4 times (Collaborators and G.B.D.P.s.D, 2018). PD was first considered to be a non-genetic disorder and only of ‘sporadic’ origin, but in the last few decades, it was found to be strongly associated with genetic background. Along with the environmental factors, hereditary factor is equally responsible for the risk of PD. The multiple genetic loci and genes which are associated with PD are as follows:
PARK1 & PARK4 locus both are mapped on chromosome 4q21 associated with SNCA, gene that encodes for α-synuclein. Three rare missense mutation: A53T, A30P and E46K occur in SNCA, from which A53T was found most frequently. Duplication and triplication of PARK4 are considered to be toxic. Duplication of gene resembles idiopathic PD while triplicate is responsible for early onset and rapid disease advancement (Lesage and Brice, 2009). Mutations (duplication/triplication) are pathologically responsible for cognitive decline, autonomic dysfunction and neuronal loss in the nigral and hippocampal region (Farrer, 2006).
PARK2 loci are plotted on chromosome 6q25.2 and is associated with parkin gene, mutations may lead to autosomal recessive form of PD. Parkin is an ubiquitin e3 ligase that works along with PINK1 in regulating mitophagy. A study reported that compound heterozygotic mutations in PARK2 with single point mutation of G403C and deletion mutation of exon 6 might contribute to the development of EOPD (Fang et al., 2019). The loci have been found to be associated with Lewy body and tau pathology and also to neuronal loss in the nigral region.
PARK5: The UCHL1 (Ubiquitin carboxyl-terminal esterase L1) gene on loci PARK5 is located on chromosome 4p14, encodes for ubiquitin thiolesterase. The UCHL1 protein is amply present in neurons throughout the brain. This protein is associated with ubiquitin proteasome system, which helps in removing abnormal and misfolded proteins. A Missense mutation in this gene occurs by replacing amino acid leucine with methionine at 93 position (I93M), which results in a disruption in the normal functioning of the ubiquitin proteasome system. This mutation is associated with autosomal dominant PD (Selvaraj and Piramanayagam, 2019).
PARK6: PARK6 locus is located on chromosome 1p 36 associated with PINK1 (PTEN-induced kinase 1) gene, a mitochondrial serine/threonine protein kinase and is associated with autosomal recessive early onset of PD. PINK1 function is to identify the damaged mitochondria to prevent their accumulation inside the cell. PINK1 p.I3689 mutation is found to be involved in PD which affects kinase activity and PARKIN activation (Ando et al., 2017).
PARK7: PARK7 loci associated with DJ-1gene located on chromosome 1p36.23, is also responsible for autosomal recessive PD. It is known to be involved in the protection of the brain from oxidative stress. Takahashi-niki K et al., found 4 mutants associated with DJ-1 out of which two were homozygous mutations (L166P, M26I0) and two heterozygous mutations (R98Q, D1498) (Takahashi-Niki et al., 2004). After the mutation, the normal functionality like its antioxidant and neuroprotective effects of the protein is lost.
PARK8: PARK8 locus is identified on chromosome 12q12, associated with LRRK2 (leucine rich repeat kinase 2). It helps in vesicular transport, protein-protein interaction, and also in autophagy (Selvaraj and Piramanayagam, 2019). A number of mutations are involved in LRRK2 which can be held responsible for phosphorylation of α-synuclein and tau, a key factor associated with aggregation and accumulation of unfolded protein in many ND (Zimprich et al., 2004).
PARK9: Located on chromosome 1p36 and is associated with ATP13A2 (ATPase 13A2) gene. Several missense mutations have been known to cause PD pathogenicity but the exact mechanism is still unknown (Klein and Westenberger, 2012). Mutation in ATP13A2 is not only associated with PD but also in other ND like Kufor-Rabef syndrome and neuronal ceroid lipofuscinoses. Similarly, PARK10 identified on chromosome 1p32 still remains to be associated with a gene. In addition to all this causative PD gene, there are several PARK loci that are identified by GWAS for causing pathogenicity in PD patients. In a recent meta-analysis, more than 800 GWAS have found multiple loci associated with PD like: CCD62/H1P1R, ACMSD/THEMI63, DGKQ/GAK, HLA, MCCC1/LAMP3, ITGA8, STK39, and SYT11/RAB25 (Zhang et al., 2018a).
Other ND which involves multiple gene mutations in their pathology are:
4.3. Multiple sclerosis
MS is a chronic, inflammatory, autoimmune disorder affecting the CNS. Th1 and Th17 are the major pathogenetic factor as they produce pro-inflammatory cytokines and chemokines like IL-6, IL-9, IL-12, IL-17, IL-21, IL-22, IL-23, IL-26, TNF-α, TNF-ꞵ, and INF-γ (Jadidi-Niaragh and Mirshafiey, 2011). The IL-6 functional gene is located on chromosome 7p21; it is responsible for activation of microglia and astrocytes, induction of cerebrovascular adhesion molecules and transportation of B & T lymphocytes across BBB into the CNS. A SNP in rs1800975 has been found as a risk factor for MS. Another important proinflammatory factor IL-7gene is found to be located on chromosome 5p13.2, which encodes for CD127 protein. It plays a crucial role in the modulation of T lymphocyte. A causal variation in gene SNP rs6897932 in exon6 was also observed to be impacting in MS. Similarly, IL7RA rs3194051, rs6897932, rs987107 and rs11567686 variants may be involved in contributing genetic susceptibility to MS (Benesova et al., 2018). Also, HLA (Human leukocyte antigen) DRB1∗1501 is one of the major gene thought to be involved in MS (Alcina et al., 2012).
4.4. Amyotrophic lateral sclerosis
ALS is a fatal, progressive ND. There are a plethora of genes that are associated with ALS: SOD1gene is located on chromosome 21q22.1, and it is documented to have more than 150 mutations which are seen to be spread all over the translated protein. Such aberrant protein aggregates when accumulated, may result in the death of motor neurons leading to the development of ALS phenotype. Out of all the genes, a genetic mutation in SOD1 alone accounts for up to 20% of familial type ALS. The second important gene associated with ALS is TARDBP located on chromosome 1 and codes for TDP-43 protein which plays an important role in regulating RNA splicing and transport. In dysregulated/pathological neurons, TDP-43 is found to be ubiquitinated in cytoplasm forming aggregates (Jeon et al., 2019). In a study, a single base pair change at position 1028 (A1028G) in TDP-43 was found to be affected in familial ALS (Yokoseki et al., 2008). Other mutated genes involved in the progression of ALS are ANG, FUS, VCP, OPTN, C9Oorf72, UBQLN2, SQSTM1, MATR3, TBK1 (Taylor et al., 2016).
4.5. Huntington’s disease
HD is a fatal, single gene disorder that involves mutation in CAG triplicate repeat at the start point of the exon in the HTT gene on chromosome 6. Almost 10–35 abnormal repeats have been linked to HD (Ghosh and Tabrizi, 2018). Such a gene when translated forms a cytotoxic protein (in this case Huntingtin) with long polyglutamine tracts.
In short, it can be concluded that all neurogenerative diseases, in one or the other way, are associated with multiple genes and are of multifactorial origin. In fact, the gene mutation in one disease may be the risk factor for the other disease. As a result, still more research is needed to be done in the field of genetics to understand the pathological role of genes related to ND in a better way. Genes mentioned above are briefly summarized in Table 1.
Table 1.
Genes which are related to various NDs are listed in the following table.
| Disease | Gene | Location | Role | Reference |
|---|---|---|---|---|
| AD | APP | Chromosome-21 | APP is responsible for regulating physiological processes such as neural proliferation, migration, differentiation, plasticity, and synaptogenesis. | Hunter and Brayne (2018) |
| PSEN1 | Chromosome-14 | PSEN1 is core gene in the γ-secretase complex responsible for generation of Aβ from APP. | Naj and Schellenberg et al., 2017 | |
| PSEN2 | Chromosome-1 | Regulate APP processing through γ-secretase to generate Aβ. | Naj and Schellenberg et al., 2017 | |
| ApoE | Chromosome-19q13.2 | Involved in metabolism of fats, cholesterol homeostasis | (Jun et al., 2010; Hollingworth et al., 2011) | |
| ABCA7 | Chromosome 19p13.3 | Highly expressed in hippocampus CA1 neuron and microglial cells and encodes for ABCA7 for lipoprotein transport in cell. | (Jun et al., 2010; Hollingworth et al., 2011) | |
| CLU | Chromosome 8p21.1 | Show protective effect in AD by encoding for apolipoprotein J (APOJ) which transport cholesterol to brain and is responsible Aꞵ clearance. | (Jun et al., 2010; Hollingworth et al., 2011; Jin et al., 2015) | |
| CR1 | Chromosome 1q32 | Expressed widely on blood cells and also found in dissolved blood plasma. CR1 codes for a complementary regulatory protein |
(Jun et al., 2010; Jin et al., 2015) | |
| EPHA1 | Chromosome 7q34 | This gene is implicated in immune function, chronic inflammation, synaptic plasticity and cellular membrane processes. | (Jun et al., 2010; Jin et al., 2015) | |
| TREM2 | Chromosome 6q21.1 | Highly expressed on cell surface of microglia and throughout the CNS and encodes for single-pass type 1 membrane. | (Jun et al., 2010; Hollingworth et al., 2011; Jin et al., 2015) | |
| BIN1 | Chromosome 2q14.3 | Role in synaptic vesicle endocytosis, inflammation, intracellular, APP trafficking, Clathrin mediated endocytosis and apoptosis. | (Hollingworth et al., 2011; Naj et al., 2011) | |
| CD2AP | Chromosome 6p12 | Encodes for CD2-associated protein which is a scaffolding molecule, regulates the actin cytoskeleton. | (Hollingworth et al., 2011; Naj et al., 2011) | |
| PICLAM | Chromosome 11q14 | Involved in clathrin-mediated endocytosis and trafficking of VAMP | (Hollingworth et al., 2011; Naj et al., 2011; Harold et al., 2009) | |
| PD | PARK1 and PARK4 | Chromosome 4q21 | They are pathologically responsible for cognitive decline, autonomic dysfunction and neuronal loss in nigral and hippocampal region. | (Lesage and Brice, 2009; Farrer, 2006; Fang et al., 2019) |
| PARK2 | Chromosome 6q25.2 | The loci are associated with PARKIN, which regulates Mitophagy | Fang et al. (2019) | |
| PARK3 | Chromosome 2p13 | Encodes tetra-hydro biopterin, which is a cofactor for Tyrosine Hydroxylase (converts tyrosine to L-DOPA) | Yamagishi (2017) | |
| PARK5 | Chromosome 4p14 | Encodes for ubiquitin thiolesterase (UCHL1) protein which is amply present in neurons throughout the brain and is associated with ubiquitin proteasome system to remove abnormal and misfolded protein. | Selvaraj and Piramanayagam (2019) | |
| PARK6 | Chromosome 1p35-p36 | Associated with PINK1 gene whose function is to identify the damaged mitochondria and target it to prevent it from accumulation. | Ando et al. (2017) | |
| PARK7 | Chromosome 1p36.23 | Involved in protection of brain from oxidative stress. | Takahashi-Niki et al. (2004) | |
| PARK8 | Chromosome 12q12 | It helps in vesicular transport, protein-protein interaction and also, in Autophagy. | Selvaraj and Piramanayagam (2019) | |
| PARK9 | Chromosome 1p36 | Exact role not known | Klein and Westenberger (2012) | |
| PARK10 | Chromosome 1p32 | Not identified | ||
| MS | IL-6 | Chromosome 7p21 | Responsible for activation of microglia and astrocytes, induction of cerebrovascular adhesion molecules and transportation of B and T lymphocytes along with activated microglial cells by breakdown of BBB into the CNS. | Benesova et al. (2018) |
| IL-7 | Chromosome 5p13.2 | It plays a crucial role in modulation of T lymphocyte effector function, lymphocyte development and acetylation. | Benesova et al. (2018) | |
| ALS | SOD1 | Chromosome 21q22.1 | Encodes antioxidant enzyme, SOD. Mutation is Responsible for 20% of familial type of ALS. | Jeon et al. (2019) |
| TARDBP | Chromosome 1 | Plays an important role in regulating RNA splicing and transport. | (Jeon et al., 2019; Yokoseki et al., 2008) | |
| HD | HTT | Chromosome 6 | Mutation in HTT causes HD | Ghosh and Tabrizi (2018) |
5. Polypharmacology
Every drug molecule available in the pharmaceutical market is basically a chemical compound made up of numerous heterocyclic scaffolds substituted with different functional groups. The functional groups along with the stereochemistry determine the target affinity of the drug molecule. Specific drug-target interaction produces a therapeutic effect while at the same time unintended drug-target interaction may lead to some serious side effects. Polypharmacology is a broad term that refers to either a single drug acting on multiple targets or a combination of drugs that can modulate multiple targets simultaneously to resolve the pathogenic symptoms of a particular disease (Reddy and Zhang, 2013; Jalencas and Mestres, 2013; Albertini et al., 2020). The drug discovery program in previous years had a major focus on “one drug-one target strategy”. However, the failure of any single monofunctional targeted drug to show considerable therapeutic benefits in complex neurodegenerative conditions like Alzheimer’s and Parkinson’s is leading towards a gradual shift towards polypharmacological drug discovery. Also, the multifactorial nature of ND and the curiosity to discover possible off-targets for current therapeutic drugs (drug repurposing) serves as an additional motivating factor to move towards a polypharmacological approach for the treatment of ND.
5.1. One drug-multiple target polypharmacological approach
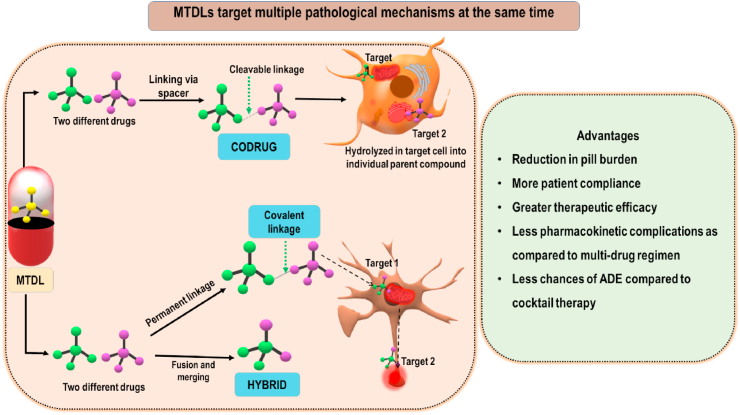
Drugs classified under this group are also called multi-target directed ligands (MTDL) or simply multi-target ligands/drugs (Albertini et al., 2020). MTDLs when compared to the already existing single monofunctional targeted drugs can target multiple signaling pathways at the same time and as a result, MTDLs show greater therapeutic efficacy credited because of their synergistic or additive mechanisms. Also, the chances of drug resistance by other biological compensating mechanisms and by gene mutations is reduced (Van der Schyf, 2011). MTDLs is a broad term and can be further classified into 1) Co-ligands and 2) Hybrid ligands. A technical comparison between both classes of ligands is shown in Fig. 3. A few of MTDLs which are not yet approved but might show therapeutic benefit in various ND conditions are discussed in the following section.
Fig. 3.
Depictive comparison between codrugs and hybrids. Also, advantages of MTDLs over combinational and single-target ligands is shown.
5.1.1. MTDLs in AD
The current FDA approved drug regime for AD consists of either acetylcholinesterase inhibitors or NMDA antagonists. Although AD is very well known since many decades, the drug discovery cycle remains risky and hence the current therapy is only limited and effective to a certain extent. The MTDLs formed from already established and approved drugs is the next strategy adopted by many research firms in order to search for an effective treatment.
5.1.1.1. Ibuprofen-lipoic acid codrug
NSAID’s and their chronic use have long been associated to be used in AD for their anti-inflammatory potential and ability to cross BBB (Imbimbo et al., 2010; Van Dam et al., 2010). Similarly, alpha-lipoic acid which is available in the market as a daily supplement is super anti-oxidant with the capability to permeate BBB (Packer et al., 1995).
Alkyldiamine group when used as a linker can successfully link the two compounds via the formation of a metabolically cleavable amide group. Codrug of this kind (Ibuprofen-alfa lipoic acid) showed a better in vitro antioxidant profile along with enhanced in vivo anti-amyloid aggregation property than the parent compounds alone. Also, the compound should have a greater BBB permeation due to the masking of the polar carboxylic acid groups (Sozio et al., 2010).
5.1.1.2. Tacrine based codrugs and hybrids
Tacrine was the first anti-cholinesterase drug approved by the FDA for AD (Crismon, 1994). However, due to the surfacing of adverse events related to hepatotoxicity, it was later withdrawn from the market. Recent research has shown that the modification of the free primary amine group in tacrine greatly reduces its hepatotoxic potential. As a result, all the tacrine based codrugs and hybrids use the primary amine group to attach a spacer to reduce hepatotoxicity. A vast majority of compounds showing anti-amyloidogenic, anti-BACE1, antioxidant, and MAO inhibition properties have been linked with Tacrine (Gonzalez et al., 2019; Wu et al., 2017). The resultant MTDLs showed an excellent in vitro data profile. The details of the mentioned codrugs are explained precisely by Wu et al. in their review (Wu et al., 2017).
5.1.1.3. MTDLs synthesized by merging of two or more drugs
Memoquine is an MTDL made by hybridization of a polyamine derived from proctamine and 1,4 benzoquinone from coQ10 (Dias and Viegas, 2014). In vitro and in vivo studies demonstrated excellent ache inhibitory and antioxidant potential of Memoquine. Moreover, it was also found to reduce Aβ42 burden along with anti-BACE1 activity (Capurro et al., 2013).
Recently, antagonism of the 5HT6 receptor has also emerged as an effective target in the treatment of AD. Antagonism of 5HT6 is considered to be pro-cholinergic, hence combining this property with an anticholinesterase inhibitor may prove to be synergetic in combating AD. Taking this into view Idalopirdine is developed, which is made by merging of tryptamine pharmacophore raised against 5HT6 receptor antagonist and benzylamine (ache inhibitor). The drug is still under investigation however, Clinical trial phase 3 conducted in 2017 for Idalopirdine failed to show any statistically significant result in alleviation of cognitive decline (Andrews et al., 2018; Atri et al., 2018).
5.1.2. MTDLs in PD
PD like AD is a complex multifactorial ND. Although, the list of FDA approved drugs for Parkinson’s is more as compared to AD, a single-target drug that could modify the disease outcome is still not found. The importance of polypharmacology in PD can be traced back to the 1970s when Amantadine (an antiviral agent) was approved by the FDA for the treatment of PD. Now, Amantadine is commonly used to alleviate the PD symptoms of tremors and rigidity, this classic example pinpoints the importance of polypharmacology in drug repurposing. Multi-drugs which are strategically developed and found to be effective in various in vitro and in vivo studies are discussed below.
5.1.2.1. L-dopa based MTDLs
L-dopa along with carbidopa in a fixed-dose combination is a gold standard for the treatment of PD. L-dopa increases the nigrostriatal dopaminergic levels but at the same time, the drug is a pro-oxidant and might increase the oxidative stress in the already vulnerable regions of the brain. As a result, there have been many attempts to synthesize MTDLs combining L-dopa along with other free radical scavenging or antioxidant agents like Caffeic acid, alfa-lipoic acid and Carnosine (Sozio et al., 2008; Di Stefano et al., 2006). Caffeic acid and carnosine conjugated L-dopa failed to show a significant antioxidant property but, a modest antioxidant property was found for the lipoic acid-L-dopa conjugated drug. Also, all the three agents mentioned showed good stability and increased LD and DA release in the brain. Recently, Franceschelli et al. showed the anti-oxidant and anti-apoptotic potential of LD-GSH codrug in an in vitro model (Franceschelli et al., 2019). The study showed that GSH-LD codrug increases the expression of anti-apoptotic proteins like bcl-2 and simultaneously reduces the pro-apoptotic proteins like bax and caspase-3 by triggering the PI3K/AKT pathway. The current literature available on the L-dopa based MTDLs shows themselves to be a potential therapeutic agent however, more in vivo studies should be conducted to validate the results.
5.1.2.2. MTDLs synthesized by merging of two or more drugs
M30 a novel MTDL is formed by strategically merging propylgylamine moiety present in the FDA approved Rasagiline into an antioxidant/iron-chelator moiety i.e. 8-hydroxy quinoline. The in vitro and in vivo studies of M30 shows strong free radical scavenger activity along with the inhibition of brain selective MAO-A and MAO-B. Moreover, it’s in vivo ability to restore dopaminergic neurons and to stabilize mitochondrial membrane potential in MPTP induced PD model makes m30 a potential therapeutic agent to be used in PD (Youdim and Oh, 2013; Youdim et al., 2014).
5.1.3. MTDLs in MS
MS is a chronic progressive auto-immune disorder characterized by demyelinating lesions and neuro-inflammation. The complications that occur in MS can be attributed to various inflammatory cytokines released by the lymphocytes infiltrated into the brain parenchyma. Also, the transformation of microglia and astrocytes to their reactive phenotype by a process called Microgliosis/Astrogliosis further adds up in the build-up of Pro-inflammatory and inflammatory mediators. The currently available treatment options in MS only target neuro-inflammation and overlooks other complications. As a result, there is a need for an alternative therapeutic approach that could target demyelination, polarization of microglias, neurodegeneration along with neuroinflammation. Targeting the mentioned pathomechanisms simultaneously would only be possible by polypharmacological approach and hence lately a lot of research has been carried out in this field (Ghasemi et al., 2017; Dobson and Giovannoni, 2019). Very recently, Rossi and colleagues developed a novel codrug for MS by connecting alpha-linolenic acid (ALA) to Valproic acid by an alkyldiamine linkage (Rossi et al., 2020). As previously discussed, such linkages strategically form amide groups by reacting with the free acid groups in the parent compound. The resultant codrug is stable and dissociates into the individual drug once inside the neurons/microglia/astrocyte with the help of hydrolysing enzymes. A detailed in vitro study performed on the ALA-VA codrug showed that the drug effectively blocked the polarization of microglia into M1 phenotype and was also shown to promote differentiation of oligodendrocyte precursor cell into oligodendrocytes. Additionally, neuroprotective property along with high BBB permeability of the co-drug makes it a promising agent for MS. Hopefully, in vivo studies might be performed in the future to validate the in vitro results.
5.2. Multiple drugs - multiple targets polypharmacological approach (polypharmacy)
The multiple pathogenic mechanisms associated with ND demands multiple targeted pharmacological therapies. MTDLs are definitely more efficacious pharmacokinetically and are patient compliant but, not all drugs can be made into MTDLs. So, more often a cocktail therapy or a fixed-dose combination of drugs is employed as a treatment strategy. Cocktail therapy is a combination of multiple dosage forms containing different APIs While, fixed dose combination is a single dosage form containing multiple APIs. From drug discovery point of view, the combination approach is less risky and more successful with respect to clinical transitions as compared to MTDLs. However, the major drawback of the “multiple drugs-multiple target” polypharmacological approach is the potential scope to cause drug-drug interactions and adverse side effects.
Few fixed dose combinations which might be useful in various neurodegenerative condition are discussed below.
5.2.1. Fixed dose combination in AD
Acetylcholinesterase inhibitors like galantamine and donepezil are the most widely used therapeutic drugs in AD. These agents reversibly inhibit acetylcholinesterase enzyme, thereby making more acetylcholine available in the brain region. Such an increase in acetylcholine in the brain synapses is considered to be responsible for alleviating the cognitive decline (Annicchiarico et al., 2007; Takeda et al., 2006; Wilkinson et al., 2004). Memantine, on the other hand, is an NMDA receptor antagonist. By blocking the NMDA receptors, it is thought to prevent the excitotoxicity associated neuronal death (Kutzing et al., 2012; Molinuevo et al., 2005). Preclinical studies conducted with Memantine demonstrated it to act on multiple targets like BACE1, Amyloid-B, BDNF, and NMDA receptor. Hence was considered to be a blockbuster in AD treatment however, clinical studies demonstrated it to be even less efficacious as compared to AChE inhibitors in alleviating the cognitive symptoms. However, further clinical trials conducted showed Memantine to be effective in reducing the cognitive deficits when combined with AChE inhibitor Donepezil (Deardorff and Grossberg, 2016). Both cholinergic and glutamatergic imbalance has long been associated as major pathophysiological mechanisms in AD. The FDC of Memantine and Donepezil effectively targets both the pathological target and was also found to show greater therapeutic benefits in moderate to severe AD than either drug when used alone. Another combination strategy is MAO-B inhibitors such as Rasagiline along with acetylcholinesterase inhibitors such as Rivastigmine. MAO-B is upregulated in ND which causes increased metabolism of neurotransmitters like dopamine, this leads to an increase in the generation of reactive oxygen species causing oxidative stress and neurodegeneration (Saura et al., 1994). Recently, MAO-B upregulation has also been suggested to increase amyloid beta levels (Schedin-Weiss et al., 2017). Such a combination of Rasagiline and Rivastigmine is very beneficial in such a scenario since it improves cognition and decreases oxidative stress. Based, on the mentioned benefits a hybrid compound called Ladostigil was developed (Weinstock et al., 2000). The drug has shown good neuroprotective and AChE inhibition activity in preclinical studies and currently it is still under clinical trials (Bar-Am et al., 2009).
5.2.2. Fixed dose combinations in PD
Various combination therapies have been taken up for the treatment of PD. Some drug combinations approved by FDA are levodopa+carbidopa, levodopa+benserazide and levodopa+carbidopa+entacapone. Monotherapy of levodopa is also known to be very effective (Poewe et al., 2010) but there are significant reported side effects and peripheral metabolism which limits the delivery to the brain. Given along with DOPA decarboxylase inhibitor, like carbidopa or benserazide, levodopa shows great advantage by significantly improving the levels of L-DOPA and subsequently dopamine in the brain. The DOPA decarboxylase inhibitors do not particularly act in such a way so as to treat Parkinson’s or even reduce symptoms of the disease as they do not cross the blood brain barrier (BBB), but they selectively stop the conversion of levodopa into dopamine peripherally, hence, reducing side effects. They also offer an advantage by being effective in reducing the chronic motor fluctuations as compared to monotherapy of levodopa (Celesia and Wanamaker, 1976; Ellis and Fell, 2017). Another combination of MAO-B inhibitors along with levodopa offers an advantage by allowing a reduction in the dose of levodopa that technically reduces off-targeting. Class of drugs called COMT and MAO inhibitors offers treatment in Parkinson’s by preserving dopamine levels produced endogenously (Salamon et al., 2020; Muller, 2009). These along with levodopa, when administered, prolongs the duration of action and also increases the t1/2 (half-life) of the drug. Such a combination is also seen to be advantageous in reducing motor symptoms associated with PD. Additionally, the combination also offers an advantage in wearing off cases of levodopa (Muller, 2009). Anti-cholinergic drugs and levodopa are also given in combination to overcome the dopaminergic and cholinergic imbalance associated with Parkinsonism. However, such combinations are not advised to be used in elderly patients as it may create confusion like state and impair cognitive abilities (Deardorff and Grossberg, 2016).
6. Repurposed drugs for NDs
6.1. Repurposing of approved therapeutics for ND
Although there has been a lot of advancement in the drug discovery field from past decades to the present there still exist certain challenges associated with the complete cure of many diseases. A new drug discovery program takes more than 10–12 years to bring the new drug into the market and this process involves a lot of money investment (Parvathaneni et al., 2019). The new drug also has to go through strict regulatory processes before its market approval. There are lot many molecules that enter the preclinical trials every year but only a few (1 in 10,000 molecules) of which can pave their path into the clinical studies (Basavaraj and Betageri, 2014). These limitations associated with the new drug discovery encourage the pharmaceutical companies as well as researchers to utilize the already approved drug molecules for the new indications. Investigational drug molecules which have failed to show the efficacy for their predetermined indications also have chances to their efficacy for new indication (Parvathaneni et al., 2019; Pushpakom et al., 2019). This strategy of utilizing already approved or investigational drug molecules for new indications is known as drug repurposing/repositioning/re-profiling (Martin and Bowden, 2019).
Unmet clinical needs have become one of the hurdles for the effective therapy of many diseases like cancer, ND, etc. Focusing only on ND including PD, AD, etc. is debilitating as their pathophysiology involves heterogeneous molecular mechanisms (Gitler et al., 2017). ND are caused due to progressive dysfunction and loss of neurons which lead to impairment in the motor and/or cognitive functions (Giorgini, 2013; Budd Haeberlein and Harris, 2015). Various therapeutics have been approved by different regulatory agencies for the treatment of various types of ND. But most of them are known to provide symptomatic relief along with a reduction in cognitive and motor functions (Hilbush et al., 2005). For the successful therapy of ND, the drug molecules must act on molecular mechanisms involved in the disease’s development and progression. Recent clinical trials have shown failures to newly developed diseases modifying therapeutics and these failures lead pharmaceutical companies and researchers to focus on drug repurposing in ND. The clinical trials study of these repurposed drugs has shown promising results for the treatment of ND.
6.2. Advantages and challenges associated with the repurposing of drugs
6.2.1. Advantages associated with the repurposing of drugs
One of the important advantages of drug repurposing is the regulatory approval process becomes easy as compared to new drug entities. This is because the already approved drugs possess the accepted safety and tolerability level in humans which leads to a reduced risk of failure (Jourdan et al., 2020). The already established safety will further avoid unanticipated obstruction in the trial phases (Strittmatter, 2014).
The time period required for the drug development will be condensed because of the availability of various data sets associated with preclinical analysis including safety and formulation development in certain cases (Pushpakom et al., 2019).
Another important advantage is the less investment in the drug development but it will be depending upon the developmental stage and process of the repositioned drug candidate.
These advantages offered by repurposing further increases the probabilities of the introduction of repurposed drug candidates into the market. But certain factors should be taken into consideration including formulation, dosage form, and route of administration of the repurposed drug as these factors may affect the drug’s safety profile. Hence in such cases re-evaluation of safety is required (Jourdan et al., 2020).
6.2.2. Challenges associated with the repurposing of drugs
Various drugs have been successfully repurposed for several disease conditions; however, it is not always fruitful for all the repurposed drugs. The drug repurposing poses certain challenges when it comes to the patent considerations and regulatory aspects along with organizational barriers (Pushpakom et al., 2019).
6.2.2.1. Patent considerations
When the repositioned drug does not possess market approval then it becomes an advantage for the repositioner to apply for patent protection for the repurposed drug for a new indication. But the drugs being used for the repositioning are not new, hence there may be the presence of prior art and which might lead to a repurposed drug unpatentable (Ashburn and Thor, 2004). For off-patent drugs, there exists another issue relating to the use of formulations and dosage forms used by generic drug manufacturers. This is because the generic drug manufacturer makes use of the ‘skinny labeling’ strategy where they legally label their generic drug product solitary for non-patented indications. This may lead to a reduction in profitability as there may be a difficulty on the stoppage of off-label usage for newly patented repurposed indications (Pushpakom et al., 2019).
6.2.2.2. Regulatory aspects
The regulatory aspects are the critical elements during the repositioning of drugs. Various regulatory pathways are available depending on the regulatory agencies for the application of repositioned drugs. Several regulatory agencies offer different exclusivity periods for the marketing of repurposed drugs but the offered timeline is not sufficient to make revenues out of it for the regaining of the invested money in the repurposing of drug candidates (Pritchard et al., 2017; Breckenridge and Jacob, 2019).
6.2.2.3. Organizational barriers in the pharmaceutical industry
Many pharmaceutical industries are working on drug repurposing in the area other than their primary disease area. For repurposing in such cases, these industries work in collaboration with academic organizations and smaller biotech companies (Pushpakom et al., 2019; Novac, 2013). The industries face certain organizational barriers when the area of focus of organization doesn’t match with that of the industries expectations and if the compound of interest has been discontinued from the organizational research and development (R&D) divisions which lead to the lack of support for the new indications (Ashburn and Thor, 2004). This lead to the lack of personnel who can work on the projects designed on the repurposing of a drug in the designated diseased area along with a lack of funding and resources for the headway of the idea within the industry. These barriers can be overcome by the utilization of external resources such as contract manufacturing organizations, pharmacovigilance, and regulatory support (Pushpakom et al., 2019).
6.3. Intellectual property consideration for repurposed therapeutics
The pharmaceutical companies working on drug repurposing seek patent protection for gaining exclusivity period after commercialization to get revenues in return for investments made for commercialization of repurposed drug molecule (Cavalla, 2013). As stated earlier, the patentability of a repurposed drug may be challenging as these drugs are not new to the researchers hence there may be a presence of prior art. The prior existence of such patents may become a hurdle for the commercialization of such repositioned drugs (Ashburn and Thor, 2004). But there are various ways to tackle this situation while a patent application for repurposed drugs. One of which is the patent protection by the composition of matter (COM) of the product. This type of patent is considered the strongest strategy for patent protection. It covers active pharmaceutical ingredients (API) contained in the product, novel formulations, and drug delivery mechanisms. Nonetheless, the application for the composition of matter for API and formulations occur early in the development phase of a new drug product which leads to the short patent life till the product reaches the market as compared to the time and money invested in bringing this product to the market (Smith, 2011).
This barrier can be overcome by the new COM protection with the help of the inclusion of a new patentable chemical entity in the repositioned drug product, delivery mechanisms which can be a patentable or else patentable combination of APIs. In some cases, the repositioned drug would be off-patent and hence generic (Smith, 2011). In these situations, the repositioner can file a Method of Use (MOU) or only ‘use’ patent especially when the drug has never been marketed (Ashburn and Thor, 2004).
6.4. Regulatory consideration for repurposed therapeutics
For the development of repurposed drugs, regulatory considerations are important elements. Considering two major regulatory markets; Europe and the United States (US), types of applications, pathways for regulatory submission and exclusivity benefits differ which are discussed in following section of the article.
In the US, new drug application is classified into different chemical types including new drug application (NDA) type 1 (new molecular entity), NDA type-2 (new active ingredient or new derivative), NDA type-3 (new dosage form), NDA type-4 (new drug combination), NDA type-5 (new formulation or new manufacturer), NDA type-6 (new indication). These applications can be filed under three possible regulatory pathways i.e. 505(b)(1), 505(b)(2), and 505(j) (Murteira et al., 2014). For minor changes such as labeling, new dosage forms, and strength in a product that is already approved as an NDA, a supplemental NDA has to be submitted by a company (Pushpakom et al., 2019).
In the European Union, applications for repurposed drugs can be filed under three possible routes such as centralized, decentralized, or national application. Article 6, 8(3), 10(3), 10a of Directive 2001/83/EC partially include different drug repurposing strategies (Murteira et al., 2014). The regulatory filing for a new chemical entity in the US will be given as five years as the initial period of exclusivity. But for previously approved activity which showed new clinical investigation such as new users will be given three years of additional exclusivity. In Europe, for new chemical entity filing will be given as eight years of grant for marketing authorization during this period no generic company can make use of investigators data while two years of a time period will be provided during which generic company can only rely on the data produced by investigator but cannot market the product. During the period of eight years of exclusivity, if the investigator identifies a new indication then 1 year of extra exclusivity period will be given for the investigator given that this new indication provides substantial value as compared to existing therapies (Breckenridge and Jacob, 2019).
6.5. Potential repurposed drugs for various ND
In the case of ND, the function of neuronal cells in the brain is majorly affected and thereby disturbing the quality of life. As said earlier there are various drugs already approved for the treatment of different ND but these are targeting only symptomatic relief but there is an absence of disease-modifying therapies due to a lack of complete understanding of the underlying mechanism of actions. Repurposing of already approved drugs opens a promising therapeutic route for the management of such debilitating ND. In the following section, we have provided potential repurposed drugs for the management of various ND.
6.5.1. Parkinson’s disease
PD is the most commonly observed movement disorder. It occurs due to the loss of dopaminergic neurons in the Substantia Nigra pars compacta (SNpc). The loss of dopaminergic neurons in SNpc leads to the deficiency of dopamine in SNpc which is responsible for the majority of PD symptoms (Dauer and Przedborski, 2003). The pathophysiology of PD is complex and but it is not fully understood. It has been observed that the accumulation of Lewy bodies represents the important marker in the neuropathology of PD (Reddy et al., 2014). The current therapy is dependent on dopaminergic drugs and there are no established disease-modifying therapeutic options available for the PD (Athauda and Foltynie, 2018). Also, there are side effects associated with the available therapies e.g. delivery of dopamine in the extra-striatal region, poor permeability across the blood-brain barrier, and variations in absorptions (Stoker and Barker, 2020). This shows the higher unmet clinical needs for the therapeutic management of PD and one of the options for overcoming such unmet clinical needs is the repurposing of already approved drugs.
6.5.1.1. Antidiabetic drugs
There is increasing evidence that suggests a linkage between type 2 diabetes mellitus and the development of PD. Both diseases are age-related and share similar pathological mechanisms. Insulin resistance is an underlying cause of the development of type 2 diabetes mellitus (Athauda and Foltynie, 2016). Insulin receptors are present in various parts of body cells including certain brain parts such as the hippocampus, basal ganglion, substantia nigra (Unger et al., 1991). Various studies have shown that insulin plays an important role in the regulation of neuronal survival and growth, dopaminergic transmission, and maintenance of synapses (Gerozissis, 2003). The various research studies observed a connection between the development of PD and loss of insulin signaling (Foltynie and Athauda, 2020). These clinical findings showing the role of insulin in the pathophysiology of PD suggests the use of antidiabetic drugs as a repurposed drug for the management of PD with the help of restoring the lost insulin signaling. Various evidence-based studies have been published in the repurposing of various antidiabetic drugs for the treatment of PD including metformin, thiazolidinediones, insulin, glucagon-like peptide-1 agonist, dipeptidyl peptidase 4 inhibitors, exenatide, etc.
Patil et al. studied the effect of metformin on the 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced PD mice model. They have administered metformin 500 mg/kg orally to mice for 21 days. The study results show a strong neuroprotective effect of metformin. This neuroprotective effect was evidenced by depletion in the oxidative stress along with maintenance of tyrosine hydroxylase (TH) positive dopaminergic neurons. After treatment with metformin, there was an increase in the locomotor and muscular activity in MPTP-induced parkinsonian mice. Metformin also showed a significant increase in brain-derived neurotrophic factor (BDNF) which is responsible for the neurotrophic effect of metformin (Patil et al., 2014). In another study with metformin by Lu et al. observed the neuroprotective effect of metformin on MPTP-induced parkinsonian mouse model by preventing dopaminergic neuronal cell death. In this study, metformin was administered to the mice in the dose of 5 mg/mL with drinking water for 3 weeks. It was observed that metformin improved motor impairment along with an increase in dopamine level in the striatum of MPTP-induced parkinsonian mice. Metformin showed significantly improved TH positive neurons in substantial nigra pars compact of MPTP induced parkinsonian mice. These results revealed the protective effect of metformin by preventing dopaminergic neuronal degeneration. Metformin also reduced (47.3%) the levels α-Synuclein positive neuronal cells which acts as a critical parameter for the development of PD (Lu et al., 2016). Currently, Paudel et al. published a detailed review on the role of metformin on PD. They have also provided various ongoing preclinical and clinical studies of metformin for the management of PD. It has been observed that metformin involves the reduction of phosphorylation and aggregation of α-synuclein, attenuation of oxidative stress, prevention of mitochondrial dysfunction, modulation of autophagy by activation of AMP-kinase (AMPK) along with a reduction of neurodegeneration and neuroinflammation (Foltynie and Athauda, 2020). The clinical studies were performed in the Taiwanese population for evaluation of the effect of metformin along with sulfonylureas on the PD risk in type-2 diabetes mellitus patients. The study results showed metformin may reduce the risk of PD in Taiwanese patients with type-2 diabetes as compared to treatment with sulfonylureas (Wahlqvist et al., 2012). These published studies show the potential of metformin as a neuroprotective agent in the treatment of PD.
It has been observed from the various studies that thiazolidinediones (TZD) such as pioglitazone, rosiglitazone which are the activators of peroxisome proliferator-activated receptor-γ (PPAR- γ) showed neuroprotective effects in various NDs such as AD (Landreth et al., 2008), cerebral ischemia (White and Murphy, 2010), and PD (Wang et al., 2017). The TZD compounds primarily act on PPAR-γ receptors which are mainly found in adipose tissues and are involved in the regulation of glucose along with lipid metabolism (Hauner, 2002). Recent studies showed the expression of these receptors in astrocytes and neurons (Warden et al., 2016). These receptors also play an important role in the regulation of inflammatory response and anti-inflammatory related gene expression along with downregulation of inflammatory cytokines by acting on microglia/macrophages (Villapol, 2018).
Breidert et al. evaluated the role of pioglitazone on MPTP-induced Parkinson’s disease in a mouse model. MPTP leads to the loss of TH-positive neurons in disease mice but this loss has been prevented after treatment with pioglitazone in substantia nigra. MPTP intoxicated animals showed a significant reduction in dopamine and its metabolites including dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the striatum as compared to pioglitazone treated mice. MPTP administered mice showed increased activation of microglia which was supplemented by macrophage antigen-1 (Mac-1) and inducible nitric oxide synthase (iNOS) in substantia nigra. After treatment with pioglitazone, Mac-1 expression was significantly reduced (Breidert et al., 2002). In another study by Quinn et al. observed that pioglitazone has shown Monoamine oxidase-B (MAO-B) inhibitory activity in the MPTP mouse model of PD. After injection of MPTP in mice, there was a significant depletion of striatal dopamine, the simultaneous reduction in the TH-immunoreactivity along with neurotoxic metabolite of 1-methyl-4-pyridinium (MPP+). The increase in the concentration of neurotoxic metabolite, MPP+ was due to the overactivation of MAO-B in the striatum. After administration of pioglitazone in the dose of 20 mg/kg, twice a daily by oral route showed neuroprotection in MPTP intoxicated mice. The pioglitazone resulted in inhibition of the MAO-B enzyme responsible for the conversion of MPTP into its toxic metabolite MPP+ (Quinn et al., 2008). In contrast to these preclinical studies, phase-2 multicentre, double-blind, randomized clinical trials showed unfavorable outcomes for pioglitazone treated Parkinson’s patients at a dose of 15 mg/kg and 45 mg/kg (Neurol, 2015).
Various studies have documented the compromised signaling of insulin in PD patients (Morris et al., 2008). The main risk factor associated with the development of PD is age. Normal aging also shows the decrease in the peripheral insulin receptor signaling but in the case of PD, this decrease in insulin receptor signaling was found to be higher as compared to normal aging. Previous studies have observed a decrease in the mRNA levels of insulin receptors in the brain predominantly in the cortex, hypothalamus, and hippocampus. Fine et al. studied the effect of intranasal human insulin (Humulin) on the 6-hydroxydopamine (6-OHDA) induced PD in the rat model. They have found that intranasal insulin attenuated the motor dysfunction induced in the 6-OHDA induced rat model at a dose of 3 IU (Fine et al., 2020). The recent pilot, single-center, double-blinded, placebo-controlled clinical trial (NCT02064166) conducted on a small group of patients showed the efficacy of intranasal insulin for the management of PD. The subjects received 40 IU of intranasal human insulin, Novolin R (Novo Nordisk, Denmark) once daily for four weeks before the breakfast with help of a device Via Nase (Kurve technologies Seattle, WA). The administered dose of insulin via intranasal route was fund to be safe with no significant study-related adverse effects also no changes in the serum glucose levels and no hypoglycemic events. The patient receiving intranasal insulin showed improvement in verbal fluency in comparison with the baseline and placebo groups. There were improvements in the disability score on the Hoehn and Yahr (HY) score which is representative of the severity of PD in terms of motor functionality and performance. The Unified Parkinson Disease Scale- Motor score (UPDRS) was found to be decreased for intranasal insulin as compared to the baseline. Also, intranasal insulin was found to be well-tolerated and safe (Novak et al., 2019).
Glucagon like peptide-1 (GLP-1) is an incretin hormone secreted endogenously which is principally involved in glucose homeostasis and also involved in the activation of similar pathways as that of insulin (Drucker and Nauck, 2006). GLP-1 is primarily secreted in the L cells of the small intestine while a small amount of which is also secreted from the nerve endings of the having cell bodies in the nucleus of the solitary tract and caudal brainstem (Mortensen et al., 2003; Göke et al., 1995). GLP-1 exerts its action via GLP-1 receptor (GLP-1R) which is 7-transmembrane spanning G-protein coupled receptor (GPCR) (Reimann and Gribble, 2016). Pancreatic cells show wide expression of GLP-1R while neurons in the brain show selective expression of GLP-1R, mainly in the cerebellum, frontal cortex, hippocampus, hypothalamus, substantia nigra, and thalamus along with glial cells and astrocytes (Alvarez et al., 2005; Trapp and Cork, 2015).
The important function of GLP-1 in the brain is to reduce oxidative stress, stimulating neuronal growth as well as proliferation along with inhibition of apoptosis and modulation of inflammatory pathways (Hölscher, 2012). The downstream signaling of GLP-1 through its receptor is mainly responsible for the cellular survival along with inhibition of proapoptotic pathways which was found to be activated in the PD (Li et al., 2010a; Drucker, 2003). This endogenously secreted GLP-1 was found to be actively cleaved and degraded by a circulating enzyme known as dipeptidyl peptidase IV (DPP-IV) into an inactive metabolite that does not show any activity against GLP-1R (Deacon, 2004).
This degradation of GLP-1 lead to the discovery of an analog of GLP-1 which is a naturally occurring peptide called exendin-4 (Holz and Chepurny, 2003). This was recovered from the saliva of a venomous lizard known as the Gila monster (Heloderma suspectum) (Parkes et al., 2013). This natural analog of GLP-1 was found to be resistant to the circulating DDP-IV. Since then, the synthetic analogs based on the exendin-4 were developed namely, exenatide, dulaglutide, liraglutide, lixisenatide and which are licensed for the management of type-2 diabetes mellitus (Nielsen, 2005; Garber, 2012).
As discussed earlier one of the major unmet clinical needs of the PD is the unavailability of disease modifying therapeutic options. Disease modifying therapeutics are nothing but the therapeutic options having potential to reduce the neurodegeneration rate or are responsible for halting the disease process (Kalia et al., 2015). The GLP-1 analogs such as exenatide was found to be a potential disease modifying therapeutic for the management of PD (Lang and Espay, 2018).
Exenatide along with other GLP-1 agonists have shown promising therapeutic efficacy against various animal models of PD including 6-OHDA and lipopolysaccharide (LPS) (Li et al., 2009), 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine (MPTP) (Harkavyi et al., 2008), etc. These studies have revealed that after administration of neurotoxins, there was a steady loss of dopaminergic neurons that lead to the development of PD in the animals. This was further confirmed by behavioral, rotarod, pol test along with apomorphine challenge test, etc. the reversal of PD symptoms was observed significantly after the administration of GLP-1 agonists. The other analogs of GLP-1which were previously approved for type-2 diabetes mellitus have also been studied for the management of PD including liraglutide, lixisenatide (Liu et al., 2015), and semaglutide (Zhang et al., 2018b), etc.
These GLP-1 agonists are under clinical trials for the management of PD. Out of which exenatide has completed phase-2 of the randomized, double-blinded placebo-controlled clinical trial (NCT01971242). This study was conducted on 62 randomly assigned patients where 32 patients received exenatide (Bydureon) in the dose of 2 mg subcutaneously once a week while 30 patients were assigned a placebo. The primary outcome of this study was the difference in the Movement Disorders Society Unified PD Rating Scale (MDS-UPDRS) motor subscale (part 3). Exenatide was found to be well tolerated in PD patients. The off medication group of patients receiving exenatide showed improvement in the MDS-UPDRS score by 1.0 points while the placebo group showed worsening of MDS-UPDRS score by 2.1 points after the 60 weeks of treatment. Apart from the MDS-UPDRS score, there were no statistically significant differences in the other parameters were observed between exenatide treated and placebo group (Athauda et al., 2017).
6.5.1.2. Iron targeting agents
Various reports suggest that the iron leads to the induction of oxidation of dopamine which in turn causes the progression of PD due to the accumulation of dopamine-derived quinones along with reactive hydroxyl radicals (Jiang et al., 2013; El-Ayaan et al., 1997). Accumulation of iron in the SNpc may precede the beginning of the clinical symptoms of PD. This accumulation and deposition are mainly associated with aging (Mochizuki and Yasuda, 2012).
Treatments which are aiming to reduce the iron content are promising approaches for slowing disease progression in PD (Mounsey and Teismann, 2012). There is the number of chemical iron chelators including desferal (Jiang et al., 2006), deferiprone (Sun et al., 2018), apomorphine (Stacy and Silver, 2008), hydroxyquinolines (Mena et al., 2015) have already been approved for various disease conditions and also shown promising therapeutic options for the PD (Jiang et al., 2006; Kaur et al., 2003; Moreau et al., 2018). The randomized double-blinded, placebo-controlled clinical trial of deferiprone in PD patients evaluated the safety of the drug, changes of iron content in the brain with help of Magnetic resonance imaging (MRI), the clinical status of PD concerning UPDRS scores. The study results showed that the deferiprone was safe and might reduce the iron content in specific regions of the brain (Martin-Bastida et al., 2017). Iron chelation is associated with several unsolved issues including a dose of the drug, lack of specific target along a selection of disease stage for the enrolment of a patient. There also remains a question regarding the efficacy of iron chelation therapy on PD disease modification (Elkouzi et al., 2019).
6.5.1.3. Mitochondrial stress pathway targeting agents
Mitochondria represents the vital organelle of a cell playing important role in energy metabolism along with redox homeostasis (Yin et al., 2014). It has been reported that the involvement of excessive degradation of mitochondria in the pathophysiology of sporadic as well as familial PD (Twig and Shirihai, 2011). The drugs targeting mitochondrial stress pathways are promising therapeutic options in PD.
Inosine is a purine nucleoside that has shown a promising role as a neuroprotective agent. It acts as an anti-inflammatory agent and thereby providing protective action in neurons. Inosine metabolite, urate acts as an antioxidant and the individuals with elevated levels of urate in serum have shown a decreased risk of PD. In various animal models of PD such as MPTP (Nishikawa et al., 2017), rotenone (El-Shamarka et al., 2020), inosine has shown a promising anti-parkinsonian effect. These results have provoked the commencement of clinical studies of the inosine for the management of PD. In Sure-PD, a dose-ranging, randomized, double-blind, placebo-controlled trial (NCT00833690) of inosine, safety, tolerability, and urate elevating capacity of inosine was evaluated in an early stage of PD patients. A total of 164 patients were participated, out of which 75 patients met the eligibility criteria. The patients were randomized into 1:1:1 ratio groups based on placebo, inosine with a dose titrated to elevate mild levels of urate in the serum (up to 6.1–7.0 mg/dL), and inosine with a dose titrated to elevate the moderate levels of urate in the serum (up to 7.1–8.0 mg/dL) for 25 months. The inosine therapy was found to be safe and well-tolerated in PD patients, though three patients showed symptomatic nephrolithiasis. Urate levels in serum and cerebrospinal fluid were found to be increased with an increase in the dose of inosine (Schwarzschild et al., 2014). In another non-randomized, single-center, open-label clinical trial of inosine was conducted in Japan. In this trial, they have enrolled 10 Asian PD patients and the study was aimed at assessing the safety and efficacy of inosine. The dose of inosine was adjusted to maintain the serum urate levels in the range of 6.0–8.0 mg/dL. The inosine was found to be safe and well-tolerated in this study and the patients did not show any problematic adverse effects. In this study, the disease progression was not observed in all the participated patients. These study results were found to be satisfactory but the only limitation was found to be a small patient population targeting a specific region (Iwaki et al., 2017). Phase 3 (SURE-PD 3), multicenter, randomized, double-blind, placebo-controlled trial (NCT02642393) was conducted on 298 PD patients and completed recently. In this study, the capsules containing 500 mg of inosine was given orally to the treatment groups while lactose-containing capsules were given to the placebo group, three times a day for 24 months. The dose of inosine was further titrated to achieve serum urate levels in the range of 7.1–8.0 mg/dL. The primary outcome of the study was the estimation of the rate of change MDS-UPDRS I-III total score over 24 months after initiation of dopaminergic therapy.
6.5.2. Alzheimer’s disease
AD represents a devastating ND leading to irreversible, progressive impairment of cognitive function, loss of memory and independence, unusual behavior (Vargas et al., 2018). The molecular hallmarks of AD are extracellular deposition of amyloid-beta (Aβ) plaques and intracellular appearance of neurofibrillary tangles consists of hyperphosphorylated tau (Parihar and Hemnani, 2004; Kumar and Singh, 2015). The worldwide prevalence of AD is 4–8% and it has been estimated that the number of individuals affected by AD will reach up to 100 million by the year 2050 (Association, 2012). This shows the failure of available treatment options, the large unmet clinical needs, and thereby the requirement of disease-modifying agents for the cure of AD. The failure of attempts to develop superior drugs than the existing once along with failing clinical trials for AD prompted the utilization of drug repurposing strategy in AD.
These repurposed candidate drugs with high priority based on the higher level of supportive evidence can be divided into antihypertensives, antibiotics, antidiabetics, antidepressant’s (Corbett et al., 2012).
6.5.2.1. Antihypertensives
Various studies have reported the relationship between hypertension and increased risk of development of AD which shows the possibility for targeting therapeutics against hypertension and thereby reducing the risk of AD (Shih et al., 2018; Carnevale et al., 2016). Even though there exists a relationship between hypertension and AD but at the same time, it is also reported that this risk associated with hypertension does not appear to be significant in the later stages of life (Qiu et al., 2005). It has been proposed that antihypertensive shows some kind of independent mechanisms for neuroprotection against AD along with their direct action on blood pressure. These antihypertensives commonly, angiotensin receptor blockers (ARB) and calcium channel blockers (CCB) are mainly responsible for neuroprotective effects (Corbett et al., 2012).
ARB’s shows neuroprotection via directly acting on the brain or by showing peripheral effects. The central effects of ARB on the brain include blocking angiotensin II type 1 receptor (AT1R) inside the brain or blocking of AT1R outside the brain.
Various ARB’s such as losartan, telmisartan, irbesartan, olmesartan, valsartan, candesartan has been screened to study their neuroprotective effects in AD, and in a dose-dependent manner, they have shown attenuation of central effects of angiotensin II (Culman et al., 2002; Royea and Hamel, 2020).
Wang et al. screened 55 clinically approved antihypertensive drugs for their neuroprotective activity in AD with the help of primary neuronal culture generated from Tg2576 AD mouse. In vitro analysis showed valsartan as the only potential candidate for the attenuation of oligomerization of Aβ peptides into high molecular weight oligomeric peptides which are responsible for the deterioration of cognitive function. This attenuation of oligomerization of Aβ peptides was found to be at the 2-fold lower dose as compared to the dose used for hypertension therapy (Wang et al., 2007). The study was further escalated to the Tg2576 AD mice model where they have administered valsartan at the doses of 10 mg/kg/day to the 6 months old or 40 mg/kg/day to the 11.5 months old mice. The study results observed that potential benefit at the dose of 40 mg/kg/day (Wang et al., 2007).
The most remarkable outcome was observed in the study conducted by Danielyan et al., where they have observed the protective of valsartan in the APP/PS1 transgenic mouse model of AD after its intranasal administration. In this study, they have administered valsartan intranasally at the dose of 10 mg/kg/day to the APP/PS1 mice for 2 months which lead to the 3.7 fold reduction of Aβ plaques as compared to vehicle-treated mice. The valsartan-treated mice also showed a reduction in serum levels of various inflammatory cytokines and showed increased serum levels of IL-10 which is responsible for the suppression of inflammation. Additionally, losartan also increased the expression of tyrosine hydroxylase in the striatum as well as locus coeruleus (Danielyan et al., 2010).
The promising preclinical results of the ARBs as neuroprotective agents in AD have led to the investigation of some of these ARBs clinically. The compilation of completed, as well as ongoing clinical trials is summarized in Table 2.
Table 2.
Ongoing clinical trials conducted on ARBs for AD.
| Drug and dose | Study title | Patient population | NCT number | Outcome | Status | Reference |
|---|---|---|---|---|---|---|
| Telmisartan (80 mg) and ramipril | ONTARGET, a double-blind, double-dummy, randomized controlled trial with ramipril and telmisartan Parallel TRANSCEND trial with telmisartan alone |
25620 | NCT00153101 | In ONTARGET trial cognitive impairment was observed in 17% of allocated patients with telmisartan, ramipril, and a combination of telmisartan and ramipril groups. In TRANSCEND trial cognitive impairment was observed in 9% of 2694 patients with telmisartan treatment and 9% of 2689 patients with placebo. |
Completed | Anderson et al. (2011) |
| Candesartan (8–16 mg) once daily | Study on COgnition and Prognosis in Elderly (SCOPE) trial with a mean follow up of 3.5–3.7 years | 4937 | – | Candesartan showed a significant effect on blood pressure, mortality, and cerebrovascular events as compared to placebo. The mini-mental state examination (MMSE) scores were found to be stable in both groups. | Completed | Skoog et al. (2005) |
| Telmisartan (40 mg and 80 mg/day) and perindopril (2 mg, 4 mg, 8 mg/day) | Randomized, Open-Label, Proof of Concept Study in mild to moderate PD patients | 150 | NCT02085265 | Results are expected to be released in 2022 | Phase-2 Ongoing | – |
| Telmisartan (20 mg, 40 mg) | Health evaluation in African American patients (HEART) | 66 | NCT02471833 | Study results not posted | Phase-1 | – |
| Candesartan (8 mg orally once daily and further dose will be increased to 16 mg and 32 mg orally in 2 weeks) | Candesartan’s effects on AD and related biomarkers | 77 | NCT02646982 | Study results not posted | Phase-2 (Completed) | – |
In view with the various in vivo and in vitro in preclinical models showed promising therapeutic option of losartan and valsartan as potential candidates for neuroprotection in AD. But still, the clinical results of the various ARBs are conflicting when compared with its in vitro preclinical study results.
Calcium channel blockers (CCBs) are another class of antihypertensive which are mainly used to control blood pressure and angina (Eisenberg et al., 2004). These drugs are found to cross the blood-brain barrier easily, thereby inducing cerebral vasodilation and causing increased blood flow to the brain (Landmark et al., 1995; Hanyu et al., 2007; Forsman et al., 1990).
Anekonda et al. studied the effect of four L-type calcium channel blockers including diltiazem, isradipine, verapamil, and nimodipine for the investigation of its therapeutic effects in AD. This study was conducted on the human neuroblastoma/MC65 cell lines. All four compounds show protective effects on these cell lines against neurotoxicity induced by amyloid β protein precursor C-terminal fragment (APP-CTF). Isradipine was found to be more potent as compared to the other three compounds which showed protective effects against APP-CTF neurotoxicity in a nanomolar concentration (Anekonda et al., 2011). In another study by Paris et al. studied the effect of antihypertensives like dihydropyridines and non-dihydropyridines CCBs on Aβ production. This study was conducted on Chinese hamster ovary cells which were transfected with human APP751. The in vitro study results showed that out of all tested dihydropyridines (DHP), amlodipine, nitrendipine, and nilvadipine showed inhibition of Aβ production while others did not reduce the levels of Aβ nor they increased the levels. In vivo studies of nitrendipine and nilvadipine in a transgenic mouse model of AD (Tg PS1/APPsw) showed an acute reduction in brain Aβ levels along with improved clearance of Aβ across the blood-brain barrier. Amongst the other DHPs, nilvadipine was found to be the most effective drug which showed decreased load of Aβ in Tg APPsw (Tg2576) and Tg PS1/APPsw mice brains sideways improving learning abilities and spatial memory (Paris et al., 2011).
The promising in vitro and in vivo outcomes of some of CCBs encouraged the involvement of these CCBs in human AD patients. Table 3 shows summary of clinical trials of CCBs conducted on AD patients.
Table 3.
Summary of clinical trials of CCBs in AD.
| Drug and dose | Study title | Patient population | NCT number | Outcome | Status | Reference |
|---|---|---|---|---|---|---|
| Nitrendipine (10–40 mg/day) along with Enalapril maleate (5–20 mg/day) and hydrochlorothiazide (12.5–25 mg/day) | Double-blind, placebo-controlled Systolic hypotension in Europe (Syst-Eur) | 3228 | – | The incidence of dementia increased from 32 to 64 cases. As compared to controls, long term therapy of nitrendipine reduced the risk of dementia by 55% from 7.4 to 3.3 cases per 1,000 patient-years (43 versus 21 cases, P < 0.001). | Completed | Forette et al. (2002) |
| Nilvadipine | European, multicenter, double-blind, placebo-controlled trial in mild to moderate AD | 511 | NCT02017340 | Study results not posted | Phase-3, completed | – |
The various studies on CCBs against AD showed the strong potential of these candidates in lowering incident AD or dementia. There exists only one clinical trial of CCBs i.e. with nilvadipine was found to be effective against AD. The preclinical and clinical trial studies showed the effectiveness of nitrendipine, nilvadipine, and nimodipine at the doses prescribed in the clinic. These pieces of evidence show the promising potential of CCBs for the treatment as well as prevention of AD.
6.5.2.2. Antidiabetic drugs
One of the risk factors for the development of AD is type 2 diabetes mellitus. The relationship between type 2 diabetes mellitus is quite complex in nature and the crucial components are insulin resistance as well as inflammatory signaling pathways (Mittal and Katare, 2016). The available literature has revealed that various AD cases associated with type 2 diabetes mellitus showed hyperphosphorylation of tau proteins, increased concentration of cortical IL-6, abnormal regulation in the clearance of Aβ levels when compared with non-diabetic individuals (Kulstad et al., 2006; Freude et al., 2005). This growing evidence showing multiple links between type 2 diabetes mellitus and AD encouraged the use of antidiabetic drugs for the treatment of AD.
Various studies have reported the possible links between insulin signaling and AD development. This linking appears to be so crucial that AD is often referred to as neuroendocrine disorder or type 3 diabetes mellitus. The studies have revealed the impairment in insulin as well as insulin-like growth factor type I, II expression, and signaling in brains of AD patients (Steen et al., 2005). Insulin and insulin-like growth factors show neuroprotection and responsible for the regulation of phosphorylation of tau proteins which are the major components of neurofibrillary tangles that appear in AD (Carro and Torres-Aleman, 2004). An intraventricular administration of long-acting insulin (detemir) in streptozotocin (STZ) rat model of AD has shown neuroprotective effects in AD by improving cognitive behavior and learning ability (Shingo et al., 2013). Several preclinical studies of intranasal insulin in AD animal models have shown promising outcomes (Chapman et al., 2018). The success in the preclinical studies led to the testing of insulin clinical trials for AD management. There are currently 57 clinical trial studies on neuroprotective effects of various insulin analogs in AD patients, out of which 30 studies are completed and the remaining are ongoing (Department of Health).
The commonly prescribed antidiabetic drug, metformin which is a biguanide has shown an increase in insulin sensitivity. But the use of metformin in AD remains controversial due to preclinical studies that have revealed the attenuation of tau proteins by metformin (Kickstein et al., 2010; Li et al., 2012) while some of the clinical trials have observed a slight increase in the risk of AD after treatment with metformin (Imfeld et al., 2012; Moore et al., 2013).
Another class of synthetic antidiabetic drugs explored for the repurposing in AD is thiazolidinediones. The rationale for the use of these drugs in AD is due to the signs of increased expression of peroxisome proliferator-activated receptor-gamma (PPARγ) in AD patients (Kitamura et al., 1999). The preclinical studies with these agents have shown attenuation of some of the pathological mechanisms of AD, principally reducing inflammatory gene expression, and amyloid plaque burden (Jiang et al., 2008). These drugs not only act on the PPARγ receptor but also have several other mechanisms of action thereby ameliorating neurodegeneration (Pérez and Quintanilla, 2015). The outcome of clinical trials of rosiglitazone and pioglitazone revealed a promising outcome only for the pioglitazone in mild to moderate AD patients (Cheng et al., 2016).
The GLP-1 peptides such as exenatide and liraglutide are the further class of antidiabetic drugs that improve the release of insulin. Various studies have reported the potential of these peptides as neuroprotective agents in AD (Cheng et al., 2016). Preclinical studies have shown the neuroprotective behavior of these peptides by reducing pathological markers of AD such as Aβ plaque load, reduction activation of microglia, and improvement in the memory behavior (McClean et al., 2011; Li et al., 2010b). The pilot study (NCT01255163) on AD patients has shown the safety and tolerability of exenatide in AD patients. But exenatide did not show any significant difference in comparison with placebo in clinical and cognitive measures, cortical volume, and thickness in MRI scans and serum, plasma, CSF, and plasma neuronal extracellular vesicles (EV) apart from the reduction in Aβ42 in EVs (Mullins et al., 2019). The clinical trial of liraglutide (NCT01843075) in a large AD patient population is currently ongoing at the Imperial College London.
6.5.2.3. Antibiotics
The recent evidence suggests a relationship between dysbiosis of microbes in the intestine and the development of AD (Jiang et al., 2017). Increasing proofs of linkage between gut microbes and the brain lead to the investigation of antibiotics in the management of AD. But only certain antibiotics have shown some hope in the reduction in neuroinflammation associated with dysbiosis can provide beneficial effects in AD. The antibiotics like rifampicin (Yulug et al., 2018), minocycline (Budni et al., 2016), and rapamycin (Wang et al., 2014) in the AD animal model have shown a reduction in the Aβ levels in the brain, inflammatory cytokines, and microglia activation. Although these antibiotics have shown anti-inflammatory effects and improvement in the cognitive functions in AD animal models but have shown controversial results in AD (Angelucci et al., 2019).
6.5.2.4. Antidepressants
Various studies have stated the depression as a risk factor for the development of AD especially when a depression is observed within two years of diagnosis of dementia (Ownby et al., 2006). It has been observed 30–50% as a prevalence rate of comorbidity of depression along with AD (Aboukhatwa et al., 2010). Several pathological mechanisms have been postulated which have provided the mechanistic relation between these two diseases. These include depletion of locus coeruleus neurons and central superior raphe nucleus (Aboukhatwa et al., 2010; Zweig et al., 1988). In case of depression, it leads to the release of high amounts of glucocorticoids which have adverse effects on hippocampus which causes the development of dementia symptoms (Sapolsky, 2000).
Depression and stress may responsible for the reduction in the neurogenesis (Warner-Schmidt and Duman, 2006). Reduction in the neurogenesis lead to the development of AD like symptoms such as acquiring and storage of information (Verret et al., 2007). Several studies have demonstrated the role of antidepressants for the neurogenesis in the brain particularly in the two regions of the dentate gyrus of hippocampus; subgranular zone and subventricular zone which play a vital role in learning and memory (Abrous et al., 2005; Pechnick et al., 2011; Duman et al., 2001; Malberg et al., 2000). Antidepressants have been divided into various classes such as monoamine oxidase inibitors, tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors. Various drugs from each class of antidepressants have shown neurogenesis in various animal models through different mechanism of actions (Kim et al., 2013).
SSRIs represents interesting class of antidepressants as compared to the other antidepressants due to the involvement of serotonergic system in the retention of memory as well as improvement in the learning ability. The drugs under the class of SSRIs have shown to delay the onset of AD (Mdawar et al., 2020).
MCI is called as impairment of cognitive performance and which possesses high risk of development of Alzheimer’s dementia. But an interference leading the delay of this progression by 5 years may reduce the chances of development of Alzheimer’s dementia by 57%. In the study conducted by Bartels et al. showed chronic administration SSRI delayed progression of MCI into Alzheimer’s dementia in patients with history of depression (Bartels et al., 2018).
Recently Torrisi et al. studied the neuroprotective effect of 2 s generation antidepressants i.e. SSRIs, fluoxetine and vortioxetine in the dose of 10 mg/kg intraperitoneally for 24 days. In this study, depressive like phenotype was induced in 2 months old C57BL/6 mice by intracerebroventricular injection of amyloid β (1–42) (Aβ1-42) oligomers. The fluoxetine (10 mg/kg) and vortioxetine (5 mg/kg, 10 mg/kg) was administered intraperitoneally before 7 days of Aβ injection. The administration of Aβ oligomer lead to significant memory deficits, shortage of transforming growth factor-β1 (TGF-β1), depressive like phenotype along with significant decrease in the synaptic proteins like synaptophysin and PSD95 in the hippocampus of mice brain. After the chronic administration of fluoxetine and vortioxetine maintained the levels of TGF-β1, synaptophysin and PSD95 in the hippocampus of brain of mice model. This study showed administration of fluoxetine and vortioxetine prevented cognitive defects and depressive like phenotype in AD mice model (Torrisi et al., 2019).
6.5.3. Multiple Sclerosis
MS is an autoimmune disorder of the central Nervous system that is mainly characterized by neural impairments such as neuroaxonal loss, demyelination, etc, and presents various phenotypes such as Primary Progressive Multiple Sclerosis (PPMS) (less common), Relapsing-Remitting Multiples Sclerosis (RRMS) that marches to Secondary Progressive Multiple Sclerosis (SPMS) over time (more common) (Ransohoff et al., 2015; Lublin et al., 2014). Precise etiology of MS is still under investigation, but based on various emerging technologies such as operational tools in neurobiology, neuroimaging, and neuroimmunology, the occurrence of MS is believed to be multifactorial that finally causes hyperactivation of neuronal axons triggering the inflammatory immune responses leading to irreversible neuronal disability. Probable promoters of neurodegeneration include oxidative injury, iron accumulation, remyelination failure, and mitochondrial damage that finally leads to localized inflammation (i.e., microglial activation or B-cell dysregulation) or augmented neurodegeneration (Mahad et al., 2015; Kawachi and Lassmann, 2017).
Achievement in the treatment can be achieved by blocking the entry of B and T lymphocytes from the peripheral blood to the CNS that invokes the inflammatory immune responses that subsequently leads to neuronal damage. Various anti-inflammatory drugs, immune modulators, neuroprotective agents, chemotherapeutic drugs are under investigation for repurposing in the treatment of the relapsing and progressive type of MS.
6.5.3.1. Drugs repurposed in the treatment of RRMS
RRMS is a phenotype of MS, where patients suffer from acute intermediate relapses along with stability periods in-between that gradually march to a worsen stage of progressive MS in which motor disability reflex increases independent of relapses (Lublin et al., 2014). Interferon-beta (IFN-β) was among the primary approved drugs for treating RRMS that mainly had significant immunomodulatory action on inflammatory cytokines (upregulation of anti-inflammatory cytokines and downregulation of pro-inflammatory cytokines) but did not prove to have potential effects on reducing disability advancement (Weinstock-Guttman et al., 1995; Kieseier, 2011). Several countries like Europe, North America had conducted randomized clinical trials to prove the effectiveness of IFN-β in treating disability but remained to be pointless (Kappos et al., 2001; Wolinsky et al., 2007). Other drugs such as glatiramer acetate and Mitoxantrone had only the slightest impact on treating MS and the investigation is terminated due to potential drawbacks (Wolinsky et al., 2007; Krapf et al., 2005).
Potential efficacy in the treatment of RRMS is achieved by repurposing drugs like rituximab and ocrelizumab that are IgG1 monoclonal antibody, primarily target CD20 B cells leading to depletion by apoptosis, cytotoxicity, and complement-mediated cytolysis and had shown a significant reduction in the number of relapses compared to placebo. Based on these successful results of ORATORIO trials in the treatment of progressive MS, ocrelizumab proved to have a significant rate in reducing disability (24%) and has been approved by USFDA in the treatment of progressive MS (Bhargava et al., 2018; Montalban et al., 2017).
BG-12 (dimethyl fumarate), initially approved for psoriasis has been repurposed as a new indication in treating RRMS due to its neuro and cytoprotective activities. The results confirmed that the annual relapse rate is reduced by 58% in the BG-12 group along with a decrease in MRI lesions compared to placebo at a span of 2 years (Gold et al., 2012).
Fingolimod phosphate (FTY720) is a natural referent of sphingosine and was the first approved oral therapy in treating RRMS. It mainly interferes in the lymphocyte trafficking, preventing the efflux of lymphocytes from lymphoid organs and also its infiltration into the CNS, inhibiting autoreactive interactions. It exerts immunomodulatory activity on binding to Sphingosine-1-Phosphate (S1P1) receptors (downregulating the receptors, inhibiting the synthesis of inflammatory mediators like cytokines, IL-6, 1β, TNF-α, and also upregulates the microglial production), thus enhancing neuroprotective activity (Hoffmann et al., 2015; Noda et al., 2013). Scientists have conducted various trials on adult male CD1 mice by collagenase VII-S (0.5 mL, 0.06 U) insertion at a dose of 0.5 μL to develop intra cerebral hemorrhage, followed by introducing fingolimod (0.5 mg/kg) after 30 min and once daily for 2 days. The results supported the neuroprotective behavior of fingolimod along with decreased inflammation (Watts et al., 2018). Recent preclinical evidences revealed the potential activity of (FTY720) in the reduction of brain atopy and loss volume but showed less effectiveness in reducing disability progression which was concluded from phase III INFORMS trial (Lublin et al., 2016).
6.5.3.2. Drugs repurposed for the treatment of progressive MS
Another category of drugs that are under investigation for treating progressive MS includes Sphingosine-1-Phosphate (SIP) Receptor Modulators such as Siponimod and fingolimod. Siponimod is a selective SIP-1,5 receptor modulator that can cross BBB and has direct neuroprotective effects on CNS and glial cells by decreasing recirculation and infiltration of potentially auto active lymphocytes. Patients treated with this drug showed a significant reduction in relapses (80%) and also had statistical benefit in T2 lesion volume and brain MRI lesions but suffered from some adverse effects like lymphopenia, macular edema, increased liver transaminases, varicella-zoster reactivation, bradycardia, and hypertension similar to fingolimod (Gentile et al., 2016).
Biotin is a B-complex, water-soluble vitamin essential as a co-factor for decarboxylase enzymes, has been evaluated for its activity in progressive MS (Shirani et al., 2016). The preliminary results of a phase III trial of (MD1003) high dose biotin in progressive MS (NCT02220933) have shown improvement in motor disability outcomes (13% of the exposed group vs 0% of the placebo group) comparatively at a dose of 100–300 mg/day). It was also studied that a high dose of biotin also had a positive impact on re-myelination, brain energy production, and also help to reduce virtual hypoxia in MS (Tourbah et al., 2015; Sedel et al., 2016). Ibudilast initially approved for ischemic stroke and asthma is investigated for the safety and efficacy in Secondary and Primary Progressive MS. The preclinical reports revealed its neuroprotective activity and also slowed down the progression pace but had a minor impact on reducing the MRI lesions (Mizuno et al., 2004).
Alpha-lipoic acid (ALA) is a natural antioxidant synthesized in the liver that mainly reduced levels of anti-inflammatory markers like IFN-gamma, TGF-beta, ICAM-1, and IL-4, in patients receiving ALA compared to placebo. The primary outcome was a significant reduction in percent change of brain volume (−0.21%) compared to placebo (−0.65%) and the brain atrophy reduced by 68% (Spain et al., 2017). Simvastatin, an anti-hyperlipidemic drug was investigated for its activity in RRMS and progressive MS, revealed its cell-protective and anti-inflammatory role but did not have much impact on lesion reduction or disease progression when used as a single drug or in combination with INF-β (Bhardwaj et al., 2012; Baldassari and Fox, 2018).
Several other drugs are currently under clinical trial investigation for its immunomodulatory, neuroprotective, and myelin repair efficacy in MS and some examples include MIS416 (NCT02228213), riluzole, amiloride and fluoxetine (NCT01910259), NeuroVax, a T-cell receptor peptide vaccine (NCT02057159), Sunphenon epigallocatechin-3-gallate, an antioxidant (NCT00799890), and Masitinib (NCT01433497).
Apart from these drugs, a continuous search for disease-modifying strategies that promote myelin repair is in progress that mainly helps to mitigate the root cause of this disease. Imidazole antifungals and Clemastine fumarate are two groups of compounds that are intensely screened to promote remyelination (Hubler et al., 2018; Green et al., 2017). Enhanced objectivity and sensitivity of disability metrics in MS will further improve the ability of clinical trials to yield more sensitive assessments of drug effectiveness.
6.5.4. Huntington’s disease
HD is an adult-onset ND that is mainly characterized by psychiatric disturbances, motor disabilities, and dementia (Martin and Gusella, 1986). The neuropathological changes are mainly associated with mutations encrypting the huntingtin (HTT) gene on chromosome 4 in humans (MacDonald et al., 1993). HTT gene mainly encodes various functional abilities such as translational, post-translational modifications, membrane-associated signaling, apoptotic prevention, vesicular, mitochondrial transport, and protein binding interactions (Rigamonti et al., 2000; Smith et al., 2009; Tian et al., 2014). Intense studies on the neurochemistry of this gene revealed that the HTT gene is a complex protein and it is divided into sub-domains featuring various binding capabilities. The N-terminal of the gene contains polyglutamine stretch encoding CAG trinucleotide sequences generally in a number 4 to 35 followed by three groups of HEAT repeats, which are essential for the protein binding and also contain sites for post-translational modifications (DiFiglia et al., 1995). Any sort of mutations in this expansion leads to abnormalities in the number of CAG nucleotides (>40) resulting in proteolysis causing accumulation of polyglutamine fragments in various cytoplasmic portions of the striatum, and other brain tissues that finally results in HD (DiFiglia et al., 1997).
Several in vivo animal studies have been conducted to explore the activity of the HTT gene. A model study of mice containing only 7 CAG repeats in the HTT gene, featured cognitive defects and motor impairment whereas, mice with overexpressed HTT gene lacking polyglutamine stretch, enhanced catabolic autophagy reducing mutations (Clabough and Zeitlin, 2006). Moreover, HTT was found to be a substrate for various proteases such as caspases and calcium-activated proteases such as calpains. Caspases are cysteine-aspartate proteases that mainly cleave HTT gene by promoting apoptotic pathway resulting in neural impairment (Orrenius et al., 2003; Wellington et al., 2002) whereas calpains get activated due to increased intracellular Ca+2 levels (mainly due to enhanced glutamate release or by membrane depolarisation) which further results in proteolytic cleavage of HTT gene resulting in cytotoxicity (Goll et al., 2003; Gafni and Ellerby, 2002).
Currently, several approaches are in use for the management of this disease but most of them are for symptomatic relief and are less approachable towards a complete cure. Extensive clinical studies are in line targeting various nuclear, transcriptional, translational protein factors to achieve disease-modifying therapies and some strategies are discussed below:
6.5.4.1. Strategies promoting mutant HTT degradation
The two approaches preferred in the treatment of HD is to promote the degradation of the mutant HTT gene either by macroautophagy or by regulation of proteostasis (Bano et al., 2011). Autophagy is a catabolic process considered as the primary destructive pathway in the pathogenesis of cancer as well as various NDs, which mainly involves the formation of autophagosome and transfers the unwanted and toxic contents to the lysosome for degradation that mainly facilitates cell stress survival, energy redemption and cell homeostasis (Levine and Kroemer, 2008; Noda et al., 2009; Longatti and Tooze, 2009). Promotion of this process helps in the destruction of various aggregated toxic proteins (polyglutamine aggregates) resulting in an enhanced recovery in the condition. The key regulator of autophagy is the ‘target of rapamycin’ (TOR) which on inhibition by rapamycin, activates the negative feedback mechanism of lysosomal autophagic degradation of aggregated proteins (Kroemer et al., 2010). Use of L-type Ca+2 channel blockers such as felodipine revealed the indirect activation of autophagy mainly by inactivating calpains and by enhancing the levels of Beclin-1- and Atg proteins that promote autophagy when administered via minipumps through subcutaneous route in mice (Williams et al., 2008; Russo et al., 2011; Yousefi et al., 2006). Even though this strategy (autophagy) seems to be approachable, but is not much effective because of its lack of protein recognition ability (p62 or polyubiquitin) and less sequestering capacity (Martinez-Vicente et al., 2010). Compounds known as Autophagosome-tethering compound (ATTEC) binds to both mutant HTT and autophagosome promoting autophagic pathway is quite amicable (Li et al., 2019).
Several studies have been conducted on the effect of proteostasis in neurogenerative diseases and one such study revealed that a decrease in the level of Insulin-like growth factor-1 (IGF-1) reduces proteotoxicity and increased the life span of the nematodes and mice (Cohen et al., 2006, 2009). Recent findings in C. elegans exposed the presence of protein MOAG-4/SERF1-2 which on the loss of its function, reduced the mutant protein aggregation of α-synuclein or β-amyloid and huntingtin (van Ham et al., 2010). By considering all these facts, autophagy and proteostasis have a profound role in treating HD.
6.5.4.2. Genetic approaches
Genetic approaches are considered as effective disease-modifying therapies that selectively act on mutant HTT (allele-specific therapies) or may act on both mutant HTT and wild HTT (non-allele-specific therapy).
Antisense oligonucleotides (ASO) are short, single-stranded DNA fragments containing 8–50 nucleotides and mainly bind to mRNA and arrests the translational progression of the mutant gene by promoting ribonuclease H degradation (Rossor et al., 2018). RG6042 (also known as HTTRX) is a non-allele-specific ASO that mainly facilitates RNA degradation on pairing with mRNA, by enhancing the levels of RNase H1, which further arrests the translational modifications of mutant and wild type of HTT gene by up to 63% when administered intrathecally followed by no dosing period of 4 months (Tabrizi et al., 2019; Kordasiewicz et al., 2012). The main drawback of this ASO is its non-specificity which becomes an important concern in the elimination of complete HTT gene during embryonic and post-natal stages. rs362307 and rs362331 are two allele-specific ASO that are under clinical trials which mainly target single nucleotide polymorphism (SNPs) associated with mutant HTT gene (Datson et al., 2017). Long-term use of ASO (non-specific) has shown adverse effects such as thrombocythemia, hepatic and renal effects, arthralgias, and so on (Liu and Zeitlin, 2017).
RNA interference (RNAi) is a defensive mechanism where the RNA shuts its expression to protect the cells from pathogens, producing double-stranded RNA (ds RNA) which now dice the RNA into 21 fragments that are loaded into a silencing complex, where the fragments either undergo degradation or may act as the template strand for DNA (Aguiar et al., 2017; Shannon, 2020). This RNAi may be selective or non-selective and do not cross BBB, thus cannot be administered intrathecally. Several non-coding RNA constructs are also in the study such as small interfering RNA (siRNA), microRNA (miRNA), and short hairpin RNA, which proved to be efficacious in reducing the mutation expression on HTT (Aguiar et al., 2017). AMT-130, AAV5, and VY-HTT01 are miRNA’s that are currently under investigation for their activity but the early results have profound efficacy in treating HD when administered via intraparenchymal route (Barker et al., 2020).
Monogenic disorders can be treated to a greater extent with genome editing (altering the gene sequence by inserting, deleting, or slicing of DNA sequence) strategies like zinc finger proteins or by transcription activators. Zinc finger proteins (ZFP) are specific amino acid sequences that mainly bind to the nucleases or transcription factors and modify the transcription process by cleavage of DNA. Engineered ZFP onto the CAG expands, successfully reduced the progression of mHTT (90%) and wHTT (15%) in stem cells derived neuron fibers and also in fibroblasts upholding its selective nature (Caron et al., 2018; Zeitler et al., 2019). On the other hand, transcription activator-like effectors are more specific binding proteins, which on binding to mHTT sequences causes gene silencing reducing the further mutagenesis (Fink et al., 2016).
In most cases, genetic approaches are considered as an effective way of treating HD than other strategies. Several repurposed drugs are under investigation for the management of HD that include Ceftriaxone (CFM), apomorphine, etc. Preclinical studies on male transgenic mice R6/2 revealed that, CFM mainly regulates the expression of GLT-1, that further increases the glutamate uptake, decreasing its deposition (Sari et al., 2010; Yimer et al., 2019). Subcutaneous administration of apomorphine (1 mg) had a significant recovery effect of motor dysfunction with 10–20 min and this effect is mainly due to its sedative and hypnotic effect which can be reversed by using haloperidol (2 mg) (Auffret et al., 2019). Copper (II) chelating drugs are under investigation for repurposing such as metformin, cyclodipeptides, and so on due to their neuroprotective effects on various ND (Lanza et al., 2018). Continuous research in genetic morphology is still in progress, which on success have a positive impact on the treatment of this rare autosomal ND shortly.
6.5.5. Batten disease
The neuronal ceroid lipofuscinoses (NCLs), also known as Batten disease is an inherited, autosomal neuropediatric disorder mainly characterized by seizures, dementia, visual and neural impairment, psychomotor disabilities finally resulting in premature death. The exact pathophysiology of this disease is still elusive, but due to the advent of advanced experimental technologies the cause for this disorder is found to be due to mutations occurring in one of the 13 genes (PPT1, TPP1, DNAJC5, CLN3, CLN5, CLN6, MFSD8, CLN8, CTSD, GRN, ATP13A2, CTSF, and KCDT7) and the other hallmark of this disease is the accumulation of autofluorescent (lipofuscin or ceroid), substances in the lysosomes which mainly differentiated this disease with other similar NDs (Zeman and Dyken, 1969).
The diagnosis of this disease can be done through disease onset, visual evaluations, brain MRI and EEG, or by examining the ultrastructure pathology of the deposits by genetic and biological tests (Kohan et al., 2009; Aldrich and Kielian, 2011). The most reliable approach opted in countries with advanced genetic technology is to go for enzyme activity and genetic testing whereas, in countries lacking these facilities, skin biopsy or blood examination can be an alternative approach to detect the lysosomal storage substances. In the case of enzyme assays, patient samples such as fibroblasts, leukocytes, chorionic villi, amniocytes, and dried blood spots are collected and mixed with specific substrates (hemoglobin-coupled synthetic substrates and Ala–Ala–Phe-coupled substrates for CTSD and TPP1) in which enzyme of interest cleaves the substrate for fluorophore formation and the absence of fluorophore confirms the presence of batten disease subtype respectively (Vines and Warburton, 1999; Sohar et al., 2000).
Promising therapeutic approaches include gene therapy, stem cell therapies, enzyme replacement therapies, and the use of several small molecular drugs that are repurposed for this purpose.
6.5.5.1. Gene therapy
NDs are best treated with gene therapy, as the change can be at the nuclear genetic level that mainly forbids the progression further and AAV mediated gene therapy is the most promising approach. In this process, the reintroduction of lysosomal enzymes occurs via viral proteins (AAV) into the CNS that help to up-regulate the enzyme activity, decreasing the accumulation of proteins. A case study in 2018 reported that patients with mucopolysaccharidosis type IIIA (a lysosomal storage disorder associated with mutations in the SGSH lysosomal enzyme), when administered with a single intravenous dose of self-complementary AAV9 (scAAV9) containing human SGSH (hSGSH), crossed BBB, increased the enzyme activity and reduced the accumulation of heparin sulfate in urine and CSF thus improving the cognitive abilities (Sawamoto et al., 2018). Successful AAV-mediated gene therapy to CNS is mediated by several factors including the route of administration, dose, tropism, immune responses to viral capsid or transgene. As AAV naturally affects humans, our immune system readily produces antibodies which may show a negative impact on the process of gene therapy thus, necessitating the continuous monitoring of the patient.
Several preclinical and clinical trials are under investigation to detect the activity of AAV-mediated enzyme delivery to combat various subtypes of Batten disease. Various AAV serotypes (AAV1, AAV2, AAV5, AAV6, AAV9) are employed for this purpose either as a single vector or in combination with other vectors (Johnson et al., 2019).
6.5.5.2. Stem cell therapy
Cell therapies are at their early stage of progress which mainly aims to restore the activity of infected parts of cells by regeneration. Repurposing this approach in the effective treatment is complex, yet amendable to provide repair to some extent. A study reported that clonal neural stem cell (NSC) line, associated with lentivirus expressed a cytokine known as a ciliary neurotrophic factor (CNTF) had a positive effect on vision restoration when given intravitreally (Jankowiak et al., 2015). Human CNS derived stem cells (HuCNS- SCs) that secreted endogenous TPP1 and PTT1 were grafted into patients with Batten disease subtypes (CLN1 and CLN2) in phase I trial, were well tolerated with no adverse effects for a longer time, and helped to recover the condition (Selden et al., 2013). An ongoing phase 1 trial (NCT01586455) testing is in progress to test whether transplantation of human placental-derived stem cells aids patients with a range of diseases including NCLs.
6.5.5.3. Small molecule therapeutics/pharmacological approaches
Small molecule therapy is a combination of biological and pharmaceutical agents that mainly helps in monitoring the activities of the lysosome. Accumulation of proteins in lysosome is may be due to improper trafficking of enzymes by endoplasmic reticulum or by Golgi complex before entering the lysosome for degradation (Arvan et al., 2002). Pharmacological chaperones are one such molecule that binds to the target proteins and monitors the trafficking to the lysosome for degradation (Fan, 2008). Read through compounds are the entities which mainly prevent the premature termination codons that lead to nonsense mutations. Ataluren (also known as PTC124) is a small molecule administered orally to prevents premature termination by interacting with the ribosome and promoting the insertion of near-cognate tRNAs at the nonsense site (Roy et al., 2016) whereas, gentamicin, an aminoglycoside antibiotic binds to the aminoacyl-tRNA site of the 30S subunit, increased PPT1 and TPP1 transcript levels and enzyme activity in patients with CLN1 and CLN2 type (Sleat et al., 2001).
Studies have been conducted to unveil the efficacy of phosphodiesterase IV inhibitors (roflumilast, rolipram, and PF-06266047) on CLN3 juvenile batten subtype proved to be effective in improving cognitive and motor abilities along with lysosomal pathway in mice which is diagnosed by blood sample examination (Aldrich et al., 2016). Most of the pharmacological drugs (immunomodulators, antioxidants, neuroprotectants, NMDA, and AMPA receptor antagonists) have minimal effects on the actual disease pathophysiology and are mostly limited to symptomatic relief. Mycophenolate mofetil was initiated for the clinical trial in 2011 in mice for treating CLN3 subtype proved to have an improvement in motor activities but not had an effective clinical outcome on disease modification (Augustine et al., 2018). Teriflunomide, a pyrimidine antagonist reduced brain atrophy, neuronal loss, and retinal thinning by immunomodulation. Prednisolone reduced the levels of GAD65 autoantibodies and improved motor activities in older patients with CLN3 Batten disease but not seemed to be effective in younger patients (<13 years) (Åberg et al., 2008). Oxidative stress is the prominent feature in batten disease where mutated CLN3 reduces lysosomal arginine levels triggering various metabolic cycles, resulting in the production of Reactive Oxygen Species (ROS) that finally damage DNA. N-acetyl cysteine has shown positive results as an antioxidant in decreasing oxidative stress in these patients. Resveratrol had a dose-dependent impact on apoptotic markers and also on oxidative stress in patients with Batten disease (Kauss et al., 2020). The other repurposed drugs that provide symptomatic relief on Batten disease are flupirtine, an anti-apoptotic drug, and N-(tert- butyl) hydroxylamine, an antioxidant that showed an increase in motor and cognitive activities in Ppt1 mutant mice (Dhar et al., 2002; Sarkar et al., 2013).
6.5.5.4. Enzyme replacement therapy
This is the most effective strategy in treating lysosomal storage disorders, where the deficient lysosomal enzymes are replaced by purified recombinant enzymes administered via intrathecal, intracerebroventricular, or by an intravenous route which is now delivered to the receptor compartment by receptor-mediated uptake. CLN1, CLN2, CLN10, and CLN13 are the batten subtypes which are mainly caused due to lysosomal enzyme deficiencies like PPT1, TPP1, CTSD, and Cathepsin F (Mole and Cotman, 2015). Recombinant TPP1 is developed for the treatment of CLN2 type and administered via intracerebroventricularly at a dose of 300 mg. Results showed that in 78% of patients, the disease progression is much slower and some patients are monitored to study the long-term effects of this therapy concerning safety and efficacy (Schulz et al., 2018). Baseline clinical scores (including both the Hamburg CLN2 scale (NCL-2 rating scales) and Weill Cornell CNS scale, which quantify the loss of language, seizures, visual and motor abilities), vital signs, electrocardiography, EEG, and MRI findings were recorded before surgical implantation of the cannula and reservoir in the lateral ventricle of the right hemisphere. Blood and CSF samples are collected and monitored for immunogenicity, biopharmaceutical characteristics, and kinetic profile.
7. Conclusion
Neurodegeneration is a progressive, complex, and multifactorial etiological process associated with significant morbidity and mortality across the globe. In the current scenario, Alzheimer’s and Parkinson’s are the most common form of ND affecting the global population. The onset of such ND is erratic and difficult to predict since it is based on the cumulative effect of multiple factors like genetics, environment, diet, age, etc. As a result, the diagnosis of such ND is delayed and is only confirmed after the development of clinical symptoms. The current documented literature in the field of ND provides concrete evidence in the support of the involvement of multi-pathophysiological mechanisms in neurodegeneration. However, it can be hypothesized that during the initial stage of disease progression, only one pathophysiological mechanism is predominant over the other and hence, early diagnosis and early therapeutic intervention might be able to prolong the progression of neurodegeneration.
The present therapeutic regimen consists of various monotargeted ligands either administered alone or in a combination with others. Such mono-targeted drug therapy when initiated during the early stages can modify the disease outcome but fail to do so in later stages of the disease. As a result, there has been an increased focus on multi-targeted drug ligands which are more than capable of targeting multiple pathological mechanisms at the same time. Such drugs by acting on multiple targets might effectively delay the disease progression than a single-targeted agent used alone. Additionally, MTDLs show better patient compliance and can overcome the drug-resistance problem faced by the traditional drug regimen.
Drug repurposing represents emerging field to combat the mentioned NDs in order to provide disease modifying options.
Such newer therapeutic regimens are currently offering multiple options of drugs as well as affordable therapies. Aforementioned fields has brighter future also provides opportunities to explore these fields in wider way to obtain clinical success in the management of ND.
CRediT authorship contribution statement
Dharmendra Kumar Khatri: Conceptualization, Writing – review & editing, review & editing, Supervision. Amey Kadbhane: Writing – review & editing. Monica Patel: Writing – review & editing. Shweta Nene: Writing – review & editing. Srividya Atmakuri: Writing – review & editing. Saurabh Srivastava: Writing – review & editing, Review & editing, Supervision, Visualization. Shashi Bala Singh: Supervision, Visualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
All authors contributed equally.
Contributor Information
Dharmendra Kumar Khatri, Email: dharmendra.niperhyd@nic.in.
Shashi Bala Singh, Email: director@niperhyd.ac.in.
References
- Abdel Moneim A.E. The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab. Brain Dis. 2015;30(4):935–942. doi: 10.1007/s11011-015-9652-6. [DOI] [PubMed] [Google Scholar]
- Åberg L., et al. Intermittent prednisolone and autoantibodies to GAD65 in juvenile neuronal ceroid lipofuscinosis. Neurology. 2008;70(14):1218–1220. doi: 10.1212/01.wnl.0000307753.88839.29. [DOI] [PubMed] [Google Scholar]
- Aboukhatwa M., Dosanjh L., Luo Y. Antidepressants are a rational complementary therapy for the treatment of Alzheimer’s disease. Mol. Neurodegener. 2010;5(1):1–17. doi: 10.1186/1750-1326-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrous D.N., Koehl M., Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol. Rev. 2005;85(2):523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Aguiar S., van der Gaag B., Cortese F.A.B. RNAi mechanisms in Huntington’s disease therapy: siRNA versus shRNA. Transl. Neurodegener. 2017;6(1):1–10. doi: 10.1186/s40035-017-0101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini C., et al. From combinations to multitarget-directed ligands: a continuum in Alzheimer’s disease polypharmacology. Med. Res. Rev. 2020 doi: 10.1002/med.21699. [DOI] [PubMed] [Google Scholar]
- Alcina A., et al. Multiple sclerosis risk variant HLA-DRB1∗1501 associates with high expression of DRB1 gene in different human populations. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0029819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich A., Kielian T. Central nervous system fibrosis is associated with fibrocyte-like infiltrates. Am. J. Pathol. 2011;179(6):2952–2962. doi: 10.1016/j.ajpath.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich A., et al. Efficacy of phosphodiesterase-4 inhibitors in juvenile Batten disease (CLN3) Ann. Neurol. 2016;80(6):909–923. doi: 10.1002/ana.24815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J.F.F., et al. Updated meta-analysis of BIN1, CR1, MS4A6A, CLU, and ABCA7 variants in Alzheimer’s disease. J. Mol. Neurosci. 2018;64(3):471–477. doi: 10.1007/s12031-018-1045-y. [DOI] [PubMed] [Google Scholar]
- Alvarez E., et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem. 2005;92(4):798–806. doi: 10.1111/j.1471-4159.2004.02914.x. [DOI] [PubMed] [Google Scholar]
- Anderson C., et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10(1):43–53. doi: 10.1016/S1474-4422(10)70250-7. [DOI] [PubMed] [Google Scholar]
- Ando M., et al. The PINK1 p.I368N mutation affects protein stability and ubiquitin kinase activity. Mol. Neurodegener. 2017;12(1):32. doi: 10.1186/s13024-017-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews M., Tousi B., Sabbagh M.N. 5HT6 antagonists in the treatment of Alzheimer’s dementia: current progress. Neurol. Ther. 2018;7(1):51–58. doi: 10.1007/s40120-018-0095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anekonda T.S., et al. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer’s disease. Neurobiol. Dis. 2011;41(1):62–70. doi: 10.1016/j.nbd.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci F., et al. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflammation. 2019;16(1):1–10. doi: 10.1186/s12974-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annicchiarico R., et al. Rivastigmine in Alzheimer’s disease: cognitive function and quality of life. Therapeut. Clin. Risk Manag. 2007;3(6):1113–1123. [PMC free article] [PubMed] [Google Scholar]
- Arvan P., et al. Secretory pathway quality control operating in Golgi, plasmalemmal, and endosomal systems. Traffic. 2002;3(11):771–780. doi: 10.1034/j.1600-0854.2002.31102.x. [DOI] [PubMed] [Google Scholar]
- Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3(8):673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Association A.s. 2012 Alzheimer’s disease facts and figures. Alzheimer’s Dementia. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Athauda D., Foltynie T. Insulin resistance and Parkinson’s disease: a new target for disease modification? Prog. Neurobiol. 2016;145:98–120. doi: 10.1016/j.pneurobio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Athauda D., Foltynie T. Drug repurposing in Parkinson’s disease. CNS Drugs. 2018;32(8):747–761. doi: 10.1007/s40263-018-0548-y. [DOI] [PubMed] [Google Scholar]
- Athauda D., et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1664–1675. doi: 10.1016/S0140-6736(17)31585-4. 10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atri A., et al. Effect of idalopirdine as adjunct to cholinesterase inhibitors on change in cognition in patients with alzheimer disease: three randomized clinical trials. J. Am. Med. Assoc. 2018;319(2):130–142. doi: 10.1001/jama.2017.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffret M., Drapier S., Vérin M. New tricks for an old dog: a repurposing approach of apomorphine. Eur. J. Pharmacol. 2019;843:66–79. doi: 10.1016/j.ejphar.2018.10.052. [DOI] [PubMed] [Google Scholar]
- Augustine E.F., et al. vol. 43. Springer; 2018. Short-term administration of mycophenolate is well-tolerated in CLN3 disease (juvenile neuronal ceroid lipofuscinosis) pp. 117–124. (JIMD Reports). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassari L.E., Fox R.J. Therapeutic advances and challenges in the treatment of progressive multiple sclerosis. Drugs. 2018;78(15):1549–1566. doi: 10.1007/s40265-018-0984-5. [DOI] [PubMed] [Google Scholar]
- Bano D., et al. Neurodegenerative processes in Huntington’s disease. Cell Death Dis. 2011;2(11):e228. doi: 10.1038/cddis.2011.112. e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Am O., et al. The novel cholinesterase-monoamine oxidase inhibitor and antioxidant, ladostigil, confers neuroprotection in neuroblastoma cells and aged rats. J. Mol. Neurosci. 2009;37(2):135–145. doi: 10.1007/s12031-008-9139-6. [DOI] [PubMed] [Google Scholar]
- Barker R.A., et al. Huntingtin-lowering strategies for Huntington’s disease. Expet Opin. Invest. Drugs. 2020;29(10):1125–1132. doi: 10.1080/13543784.2020.1804552. [DOI] [PubMed] [Google Scholar]
- Bartels C., et al. Impact of SSRI therapy on risk of conversion from mild cognitive impairment to Alzheimer’s dementia in individuals with previous depression. Am. J. Psychiatr. 2018;175(3):232–241. doi: 10.1176/appi.ajp.2017.17040404. [DOI] [PubMed] [Google Scholar]
- Basavaraj S., Betageri G.V. Can formulation and drug delivery reduce attrition during drug discovery and development—review of feasibility, benefits and challenges. Acta Pharm. Sin. B. 2014;4(1):3–17. doi: 10.1016/j.apsb.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesova Y., Vasku A., Bienertova-Vasku J. Association of interleukin 6, interleukin 7 receptor alpha, and interleukin 12B gene polymorphisms with multiple sclerosis. Acta Neurol. Belg. 2018;118(3):493–501. doi: 10.1007/s13760-018-0994-9. [DOI] [PubMed] [Google Scholar]
- Bhardwaj S., Coleman C.I., Sobieraj D.M. Efficacy of statins in combination with interferon therapy in multiple sclerosis: a meta-analysis. Am. J. Health Syst. Pharm. 2012;69(17):1494–1499. doi: 10.2146/ajhp110675. [DOI] [PubMed] [Google Scholar]
- Bhargava P., et al. AAN Enterprises; 2018. Phase 1 Trial of Intrathecal Rituximab in Progressive MS Patients with Evidence of Leptomeningeal Contrast Enhancement (P3. 393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A., et al. Genetic variation and neuroimaging measures in Alzheimer disease. Arch. Neurol. 2010;67(6):677–685. doi: 10.1001/archneurol.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge A., Jacob R. Overcoming the legal and regulatory barriers to drug repurposing. Nat. Rev. Drug Discov. 2019;18(1):1–2. doi: 10.1038/nrd.2018.92. [DOI] [PubMed] [Google Scholar]
- Breidert T., et al. Protective action of the peroxisome proliferator-activated receptor-γ agonist pioglitazone in a mouse model of Parkinson’s disease. J. Neurochem. 2002;82(3):615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- Brenner S.R. Blue-green algae or cyanobacteria in the intestinal micro-flora may produce neurotoxins such as Beta-N-Methylamino-L-Alanine (BMAA) which may be related to development of amyotrophic lateral sclerosis, Alzheimer’s disease and Parkinson-Dementia-Complex in humans and Equine Motor Neuron Disease in horses. Med. Hypotheses. 2013;80(1):103. doi: 10.1016/j.mehy.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Brundel M., et al. High prevalence of cerebral microbleeds at 7Tesla MRI in patients with early Alzheimer’s disease. J. Alzheimers Dis. 2012;31(2):259–263. doi: 10.3233/JAD-2012-120364. [DOI] [PubMed] [Google Scholar]
- Budd Haeberlein S., Harris T. Promising targets for the treatment of neurodegenerative diseases. Clin. Pharmacol. Therapeut. 2015;98(5):492–501. doi: 10.1002/cpt.195. [DOI] [PubMed] [Google Scholar]
- Budni J., et al. The anti-inflammatory role of minocycline in Alzheimer s disease. Curr. Alzheimer Res. 2016;13(12):1319–1329. doi: 10.2174/1567205013666160819124206. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L., et al. Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: the clinical impact on children and beyond. Rev. Neurol. (Paris) 2016;172(1):69–80. doi: 10.1016/j.neurol.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Caller T., Henegan P., Stommel E. The potential role of BMAA in neurodegeneration. Neurotox. Res. 2018;33(1):222–226. doi: 10.1007/s12640-017-9752-7. [DOI] [PubMed] [Google Scholar]
- Capurro V., et al. Pharmacological characterization of memoquin, a multi-target compound for the treatment of Alzheimer’s disease. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale D., et al. Pathophysiological links among hypertension and Alzheimer’s disease. High Blood Pres. Cardiovasc. Prev. 2016;23(1):3–7. doi: 10.1007/s40292-015-0108-1. [DOI] [PubMed] [Google Scholar]
- Caron N.S., Dorsey E.R., Hayden M.R. Therapeutic approaches to Huntington disease: from the bench to the clinic. Nat. Rev. Drug Discov. 2018;17(10):729–750. doi: 10.1038/nrd.2018.133. [DOI] [PubMed] [Google Scholar]
- Carro E., Torres-Aleman I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur. J. Pharmacol. 2004;490(1–3):127–133. doi: 10.1016/j.ejphar.2004.02.050. [DOI] [PubMed] [Google Scholar]
- Cavalla D. Predictive methods in drug repurposing: gold mine or just a bigger haystack? Drug Discov. Today. 2013;18(11–12):523–532. doi: 10.1016/j.drudis.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Celesia G.G., Wanamaker W.M. L-dopa-carbidopa: combined therapy for the treatment of Parkinson’s disease. Dis. Nerv. Syst. 1976;37(3):123–125. [PubMed] [Google Scholar]
- Chapman C.D., et al. Intranasal insulin in Alzheimer’s disease: food for thought. Neuropharmacology. 2018;136:196–201. doi: 10.1016/j.neuropharm.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.F., et al. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017;38(9):1205–1235. doi: 10.1038/aps.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.C., Ulane C.M., Burke R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010;67(6):715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., et al. The peroxisome proliferators activated receptor-gamma agonists as therapeutics for the treatment of Alzheimer’s disease and mild-to-moderate Alzheimer’s disease: a meta-analysis. Int. J. Neurosci. 2016;126(4):299–307. doi: 10.3109/00207454.2015.1015722. [DOI] [PubMed] [Google Scholar]
- Chin-Chan M., Navarro-Yepes J., Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: alzheimer and Parkinson diseases. Front. Cell. Neurosci. 2015;9:124. doi: 10.3389/fncel.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu A.S., et al. Excitotoxic potential of the cyanotoxin beta-methyl-amino-L-alanine (BMAA) in primary human neurons. Toxicon. 2012;60(6):1159–1165. doi: 10.1016/j.toxicon.2012.07.169. [DOI] [PubMed] [Google Scholar]
- Cirone M. Perturbation of bulk and selective macroautophagy, abnormal UPR activation and their interplay pave the way to immune dysfunction, cancerogenesis and neurodegeneration in ageing. Ageing Res. Rev. 2020;58:101026. doi: 10.1016/j.arr.2020.101026. [DOI] [PubMed] [Google Scholar]
- Clabough E.B., Zeitlin S.O. Deletion of the triplet repeat encoding polyglutamine within the mouse Huntington’s disease gene results in subtle behavioral/motor phenotypes in vivo and elevated levels of ATP with cellular senescence in vitro. Hum. Mol. Genet. 2006;15(4):607–623. doi: 10.1093/hmg/ddi477. [DOI] [PubMed] [Google Scholar]
- Cohen E., et al. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313(5793):1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- Cohen E., et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139(6):1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators, G.B.D.P.s.D Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S.M., Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136(6):2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- Corbett A., et al. Drug repositioning for Alzheimer’s disease. Nat. Rev. Drug Discov. 2012;11(11):833–846. doi: 10.1038/nrd3869. [DOI] [PubMed] [Google Scholar]
- Crismon M.L. Tacrine: first drug approved for Alzheimer’s disease. Ann. Pharmacother. 1994;28(6):744–751. doi: 10.1177/106002809402800612. [DOI] [PubMed] [Google Scholar]
- Culman J., et al. The renin-angiotensin system in the brain: possible therapeutic implications for AT 1-receptor blockers. J. Hum. Hypertens. 2002;16(3):S64–S70. doi: 10.1038/sj.jhh.1001442. [DOI] [PubMed] [Google Scholar]
- Danielyan L., et al. Protective effects of intranasal losartan in the APP/PS1 transgenic mouse model of Alzheimer disease. Rejuvenation Res. 2010;13(2–3):195–201. doi: 10.1089/rej.2009.0944. [DOI] [PubMed] [Google Scholar]
- Datson N.A., et al. The expanded CAG repeat in the huntingtin gene as target for therapeutic RNA modulation throughout the HD mouse brain. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- de Flores R., La Joie R., Chetelat G. Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease. Neuroscience. 2015;309:29–50. doi: 10.1016/j.neuroscience.2015.08.033. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Nunez C., et al. Neuromicrobiology: how microbes influence the brain. ACS Chem. Neurosci. 2018;9(2):141–150. doi: 10.1021/acschemneuro.7b00373. [DOI] [PubMed] [Google Scholar]
- de la Monte S.M., et al. Early-stage Alzheimer’s disease is associated with simultaneous systemic and central nervous system dysregulation of insulin-linked metabolic pathways. J. Alzheim. Dis. 2019;68(2):657–668. doi: 10.3233/JAD-180906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon C.F. Circulation and degradation of GIP and GLP-1. Horm. Metab. Res. 2004;36(11/12):761–765. doi: 10.1055/s-2004-826160. [DOI] [PubMed] [Google Scholar]
- Deardorff W.J., Grossberg G.T. A fixed-dose combination of memantine extended-release and donepezil in the treatment of moderate-to-severe Alzheimer’s disease. Drug Des. Dev. Ther. 2016;10:3267–3279. doi: 10.2147/DDDT.S86463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. U. S. National Library of Medicine,. Available from: https://clinicaltrials.gov/ct2/results?cond=Alzheimer+Disease&term=insulin&cntry=&state=&city=&dist=&Search=Search.
- Dhar S., et al. Flupirtine blocks apoptosis in batten patient lymphoblasts and in human postmitotic CLN3-and CLN2-deficient neurons. Ann. Neurol.: Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2002;51(4):448–466. doi: 10.1002/ana.10143. [DOI] [PubMed] [Google Scholar]
- Di Stefano A., et al. L-dopa- and dopamine-(R)-alpha-lipoic acid conjugates as multifunctional codrugs with antioxidant properties. J. Med. Chem. 2006;49(4):1486–1493. doi: 10.1021/jm051145p. [DOI] [PubMed] [Google Scholar]
- Dias K.S., Viegas C., Jr. Multi-target directed drugs: a modern approach for design of new drugs for the treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2014;12(3):239–255. doi: 10.2174/1570159X1203140511153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D.W. Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb. Perspect. Med. 2012;2(8) doi: 10.1101/cshperspect.a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M., et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 1995;14(5):1075–1081. doi: 10.1016/0896-6273(95)90346-1. [DOI] [PubMed] [Google Scholar]
- DiFiglia M., et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277(5334):1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Dobson R., Giovannoni G. Multiple sclerosis - a review. Eur. J. Neurol. 2019;26(1):27–40. doi: 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- Drucker D.J. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol. Endocrinol. 2003;17(2):161–171. doi: 10.1210/me.2002-0306. [DOI] [PubMed] [Google Scholar]
- Drucker D.J., Nauck M.A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- Dugger B.N., Dickson D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017;9(7) doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Nakagawa S., Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology. 2001;25(6):836–844. doi: 10.1016/S0893-133X(01)00358-X. [DOI] [PubMed] [Google Scholar]
- Dunstan M.L., et al. vol. 12. 2016. P1-145: the Role of CD2AP in APP Processing; pp. P458–P459. [Google Scholar]
- Eisenberg M.J., Brox A., Bestawros A.N. Calcium channel blockers: an update. Am. J. Med. 2004;116(1):35–43. doi: 10.1016/j.amjmed.2003.08.027. [DOI] [PubMed] [Google Scholar]
- El-Ayaan U., et al. Anaerobic oxidation of dopamine by iron (III) J. Chem. Soc., Dalton Trans. 1997;(16):2813–2818. [Google Scholar]
- El-Shamarka M.E., Kozman M.R., Messiha B.A. The protective effect of inosine against rotenone-induced Parkinson’s disease in mice; role of oxido-nitrosative stress, ERK phosphorylation, and A2AR expression. N. Schmied. Arch. Pharmacol. 2020:1–13. doi: 10.1007/s00210-019-01804-1. [DOI] [PubMed] [Google Scholar]
- Elkouzi A., et al. Emerging therapies in Parkinson disease—repurposed drugs and new approaches. Nat. Rev. Neurol. 2019;15(4):204–223. doi: 10.1038/s41582-019-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J.M., Fell M.J. Current approaches to the treatment of Parkinson’s Disease. Bioorg. Med. Chem. Lett. 2017;27(18):4247–4255. doi: 10.1016/j.bmcl.2017.07.075. [DOI] [PubMed] [Google Scholar]
- Emamzadeh F.N. Alpha-synuclein structure, functions, and interactions. J. Res. Med. Sci. 2016;21:29. doi: 10.4103/1735-1995.181989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J.-Q. A counterintuitive approach to treat enzyme deficiencies: use of enzyme inhibitors for restoring mutant enzyme activity. Biol. Chem. 2008;389(1):1–11. doi: 10.1515/BC.2008.009. [DOI] [PubMed] [Google Scholar]
- Fang Y.Q., et al. Compound heterozygous mutations in PARK2 causing early-onset Parkinson disease: a case report. Medicine (Baltim.) 2019;98(5):e14228. doi: 10.1097/MD.0000000000014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer M.J. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat. Rev. Genet. 2006;7(4):306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- Feng Y., Wang X. Antioxidant therapies for Alzheimer’s disease. Oxid. Med. Cell. Longev. 2012;2012:472932. doi: 10.1155/2012/472932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J.M., et al. Intranasal delivery of low-dose insulin ameliorates motor dysfunction and dopaminergic cell death in a 6-OHDA rat model of Parkinson’s Disease. Neurosci. Lett. 2020;714:134567. doi: 10.1016/j.neulet.2019.134567. [DOI] [PubMed] [Google Scholar]
- Fink K.D., et al. Allele-specific reduction of the mutant huntingtin allele using transcription activator-like effectors in human huntington’s disease fibroblasts. Cell Transplant. 2016;25(4):677–686. doi: 10.3727/096368916X690863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltynie T., Athauda D. Progress in Brain Research. Elsevier; 2020. Repurposing anti-diabetic drugs for the treatment of Parkinson’s disease: rationale and clinical experience; pp. 493–523. [DOI] [PubMed] [Google Scholar]
- Forette F., et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch. Intern. Med. 2002;162(18):2046–2052. doi: 10.1001/archinte.162.18.2046. [DOI] [PubMed] [Google Scholar]
- Forsman M., et al. Effects of nimodipine on cerebral blood flow and neuropsychological outcome after cardiac surgery. Br. J. Addiction: Br. J. Anaesth. 1990;65(4):514–520. doi: 10.1093/bja/65.4.514. [DOI] [PubMed] [Google Scholar]
- Franceschelli S., et al. Modulation of apoptotic cell death and neuroprotective effects of glutathione-L-dopa codrug against H2O2-induced cellular toxicity. Antioxidants. 2019;8(8) doi: 10.3390/antiox8080319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freude S., et al. Peripheral hyperinsulinemia promotes tau phosphorylation in vivo. Diabetes. 2005;54(12):3343–3348. doi: 10.2337/diabetes.54.12.3343. [DOI] [PubMed] [Google Scholar]
- Gafni J., Ellerby L.M. Calpain activation in Huntington’s disease. J. Neurosci. 2002;22(12):4842–4849. doi: 10.1523/JNEUROSCI.22-12-04842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D., et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am. J. Physiol. 1998;275(2):E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- Garber A.J. Novel GLP-1 receptor agonists for diabetes. Expet Opin. Invest. Drugs. 2012;21(1):45–57. doi: 10.1517/13543784.2012.638282. [DOI] [PubMed] [Google Scholar]
- Gentile A., et al. Siponimod (BAF312) prevents synaptic neurodegeneration in experimental multiple sclerosis. J. Neuroinflammation. 2016;13(1):1–13. doi: 10.1186/s12974-016-0686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerozissis K. Brain insulin: regulation, mechanisms of action and functions. Cell. Mol. Neurobiol. 2003;23(1):1–25. doi: 10.1023/A:1022598900246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi N., Razavi S., Nikzad E. Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J. 2017;19(1):1–10. doi: 10.22074/cellj.2016.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R., Tabrizi S.J. Clinical features of huntington’s disease. Adv. Exp. Med. Biol. 2018;1049:1–28. doi: 10.1007/978-3-319-71779-1_1. [DOI] [PubMed] [Google Scholar]
- Giorgini F. Springer; 2013. Understanding Neuronal Dysfunction and Loss in Neurodegenerative Disease. [DOI] [PubMed] [Google Scholar]
- Giri M., Zhang M., Lu Y. Genes associated with Alzheimer’s disease: an overview and current status. Clin. Interv. Aging. 2016;11:665–681. doi: 10.2147/CIA.S105769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler A.D., Dhillon P., Shorter J. The Company of Biologists Ltd; 2017. Neurodegenerative Disease: Models, Mechanisms, and a New Hope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göke R., et al. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur. J. Neurosci. 1995;7(11):2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Gold R., et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012;367(12):1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- Goll D.E., et al. The calpain system. Physiol. Rev. 2003 doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Gonzalez J.F., et al. Developments with multi-target drugs for Alzheimer’s disease: an overview of the current discovery approaches. Expet Opin. Drug Discov. 2019;14(9):879–891. doi: 10.1080/17460441.2019.1623201. [DOI] [PubMed] [Google Scholar]
- Gorji N., Moeini R., Memariani Z. Almond, hazelnut and walnut, three nuts for neuroprotection in Alzheimer’s disease: a neuropharmacological review of their bioactive constituents. Pharmacol. Res. 2018;129:115–127. doi: 10.1016/j.phrs.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Grammas P., Martinez J., Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expet Rev. Mol. Med. 2011;13:e19. doi: 10.1017/S1462399411001918. [DOI] [PubMed] [Google Scholar]
- Green A.J., et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390:2481–2489. doi: 10.1016/S0140-6736(17)32346-2. 10111. [DOI] [PubMed] [Google Scholar]
- Grosso G., Estruch R. Nut consumption and age-related disease. Maturitas. 2016;84:11–16. doi: 10.1016/j.maturitas.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Hanyu H., et al. Favourable effects of nilvadipine on cognitive function and regional cerebral blood flow on SPECT in hypertensive patients with mild cognitive impairment. Nucl. Med. Commun. 2007;28(4):281–287. doi: 10.1097/MNM.0b013e32804c58aa. [DOI] [PubMed] [Google Scholar]
- Harkavyi A., et al. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J. Neuroinflammation. 2008;5(1):19. doi: 10.1186/1742-2094-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold D., et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner H. The mode of action of thiazolidinediones. Diabetes/Metabol. Res. Rev. 2002;18(S2):S10–S15. doi: 10.1002/dmrr.249. [DOI] [PubMed] [Google Scholar]
- Heymsfield S.B., et al. Differences between brain mass and body weight scaling to height: potential mechanism of reduced mass-specific resting energy expenditure of taller adults. J. Appl. Physiol. 1985;106(1):40–48. doi: 10.1152/japplphysiol.91123.2008. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbush B.S., et al. New prospects and strategies for drug target discovery in neurodegenerative disorders. NeuroRx. 2005;2(4):627–637. doi: 10.1602/neurorx.2.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F.S., et al. Fingolimod induces neuroprotective factors in human astrocytes. J. Neuroinflammation. 2015;12(1):184. doi: 10.1186/s12974-015-0393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P., et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher C. Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs. 2012;26(10):871–882. doi: 10.2165/11635890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Holz G.G., Chepurny O.G. Glucagon-like peptide-1 synthetic analogs: new therapeutic agents for use in the treatment of diabetes mellitus. Curr. Med. Chem. 2003;10(22):2471–2483. doi: 10.2174/0929867033456648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019;15(10):565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- Hubler Z., et al. Accumulation of 8, 9-unsaturated sterols drives oligodendrocyte formation and remyelination. Nature. 2018;560(7718):372–376. doi: 10.1038/s41586-018-0360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S., Brayne C. Understanding the roles of mutations in the amyloid precursor protein in Alzheimer disease. Mol. Psychiatr. 2018;23(1):81–93. doi: 10.1038/mp.2017.218. [DOI] [PubMed] [Google Scholar]
- Imbimbo B.P., Solfrizzi V., Panza F. Are NSAIDs useful to treat Alzheimer’s disease or mild cognitive impairment? Front. Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imfeld P., et al. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case–control study. J. Am. Geriatr. Soc. 2012;60(5):916–921. doi: 10.1111/j.1532-5415.2012.03916.x. [DOI] [PubMed] [Google Scholar]
- Iwaki H., et al. One year safety and efficacy of inosine to increase the serum urate level for patients with Parkinson’s disease in Japan. J. Neurol. Sci. 2017;383:75–78. doi: 10.1016/j.jns.2017.10.030. [DOI] [PubMed] [Google Scholar]
- Jadidi-Niaragh F., Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand. J. Immunol. 2011;74(1):1–13. doi: 10.1111/j.1365-3083.2011.02536.x. [DOI] [PubMed] [Google Scholar]
- Jalencas X., Mestres J.J.M. Vol. 4. 2013. On the Origins of Drug Polypharmacology; pp. 80–87. 1. [Google Scholar]
- Jankowiak W., et al. Sustained neural stem cell-based intraocular delivery of CNTF attenuates photoreceptor loss in the nclf mouse model of neuronal ceroid lipofuscinosis. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0127204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon G.S., et al. Pathological modification of TDP-43 in amyotrophic lateral sclerosis with SOD1 mutations. Mol. Neurobiol. 2019;56(3):2007–2021. doi: 10.1007/s12035-018-1218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., et al. Neuroprotective effects of iron chelator Desferal on dopaminergic neurons in the substantia nigra of rats with iron-overload. Neurochem. Int. 2006;49(6):605–609. doi: 10.1016/j.neuint.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Heneka M., Landreth G.E. The role of peroxisome proliferator-activated receptor-γ PPARγ) in Alzheimer’s disease. CNS Drugs. 2008;22(1):1–14. doi: 10.2165/00023210-200822010-00001. [DOI] [PubMed] [Google Scholar]
- Jiang D., et al. Inhibition of the Fe (III)-catalyzed dopamine oxidation by ATP and its relevance to oxidative stress in Parkinson’s disease. ACS Chem. Neurosci. 2013;4(9):1305–1313. doi: 10.1021/cn400105d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., et al. The gut microbiota and Alzheimer’s disease. J. Alzheim. Dis. 2017;58(1):1–15. doi: 10.3233/JAD-161141. [DOI] [PubMed] [Google Scholar]
- Jin S.C., et al. TREM2 is associated with increased risk for Alzheimer’s disease in African Americans. Mol. Neurodegener. 2015;10:19. doi: 10.1186/s13024-015-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.B., et al. Therapeutic landscape for Batten disease: current treatments and future prospects. Nat. Rev. Neurol. 2019;15(3):161–178. doi: 10.1038/s41582-019-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan J.P., et al. Drug repositioning: a brief overview. J. Pharm. Pharmacol. 2020 doi: 10.1111/jphp.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun G., et al. Meta-analysis confirms CR1, CLU, and PICALM as alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch. Neurol. 2010;67(12):1473–1484. doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia L.V., Kalia S.K., Lang A.E. Disease-modifying strategies for Parkinson’s disease. Mov. Disord. 2015;30(11):1442–1450. doi: 10.1002/mds.26354. [DOI] [PubMed] [Google Scholar]
- Kappos L., et al. Final analysis of the European multicenter trial on IFNβ-1b in secondary-progressive MS. Neurology. 2001;57(11):1969–1975. doi: 10.1212/wnl.57.11.1969. [DOI] [PubMed] [Google Scholar]
- Kaur D., et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease. Neuron. 2003;37(6):899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Kauss V., Dambrova M., Medina D.L. Pharmacological approaches to tackle NCLs. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2020;1866(9):165553. doi: 10.1016/j.bbadis.2019.165553. [DOI] [PubMed] [Google Scholar]
- Kawachi I., Lassmann H. Neurodegeneration in multiple sclerosis and neuromyelitis optica. J. Neurol. Neurosurg. Psychiatr. 2017;88(2):137–145. doi: 10.1136/jnnp-2016-313300. [DOI] [PubMed] [Google Scholar]
- Kickstein E., et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc. Natl. Acad. Sci. Unit. States Am. 2010;107(50):21830–21835. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieseier B.C. The mechanism of action of interferon-β in relapsing multiple sclerosis. CNS Drugs. 2011;25(6):491–502. doi: 10.2165/11591110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kim B., et al. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150(12):5294–5301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-J., Kim W., Kong S.-Y. Antidepressants for neuro-regeneration: from depression to Alzheimer’s disease. Arch Pharm. Res. (Seoul) 2013;36(11):1279–1290. doi: 10.1007/s12272-013-0238-8. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., et al. Increased expression of cyclooxygenases and peroxisome proliferator-activated receptor-γ in Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 1999;254(3):582–586. doi: 10.1006/bbrc.1998.9981. [DOI] [PubMed] [Google Scholar]
- Klein C., Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2(1):a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingelhoefer L., Reichmann H. Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors. Nat. Rev. Neurol. 2015;11(11):625–636. doi: 10.1038/nrneurol.2015.197. [DOI] [PubMed] [Google Scholar]
- Kohan R., et al. An integrated strategy for the diagnosis of neuronal ceroid lipofuscinosis types 1 (CLN1) and 2 (CLN2) in eleven Latin American patients. Clin. Genet. 2009;76(4):372–382. doi: 10.1111/j.1399-0004.2009.01214.x. [DOI] [PubMed] [Google Scholar]
- Kohler C.A., et al. The gut-brain Axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in Alzheimer’s disease. Curr. Pharmaceut. Des. 2016;22(40):6152–6166. doi: 10.2174/1381612822666160907093807. [DOI] [PubMed] [Google Scholar]
- Kojro E., Fahrenholz F. The non-amyloidogenic pathway: structure and function of alpha-secretases. Subcell. Biochem. 2005;38:105–127. doi: 10.1007/0-387-23226-5_5. [DOI] [PubMed] [Google Scholar]
- Kordasiewicz H.B., et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron. 2012;74(6):1031–1044. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G.G. Concepts and classification of neurodegenerative diseases. Handb. Clin. Neurol. 2017;145:301–307. doi: 10.1016/B978-0-12-802395-2.00021-3. [DOI] [PubMed] [Google Scholar]
- Krapf H., et al. Effect of mitoxantrone on MRI in progressive MS: results of the MIMS trial. Neurology. 2005;65(5):690–695. doi: 10.1212/01.wnl.0000174439.70369.7a. [DOI] [PubMed] [Google Scholar]
- Kroemer G., Mariño G., Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulstad J., et al. Differential modulation of plasma β-amyloid by insulin in patients with Alzheimer disease. Neurology. 2006;66(10):1506–1510. doi: 10.1212/01.wnl.0000216274.58185.09. [DOI] [PubMed] [Google Scholar]
- Kumar A., Singh A. A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol. Rep. 2015;67(2):195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Kutzing M.K., Luo V., Firestein B.L. Protection from glutamate-induced excitotoxicity by memantine. Ann. Biomed. Eng. 2012;40(5):1170–1181. doi: 10.1007/s10439-011-0494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J.C., et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 2009;41(10):1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lander E.S., et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Landmark K., et al. Nitrendipine and mefruside in elderly hypertensives: effects on blood pressure, cardiac output, cerebral blood flow and metabolic parameters. J. Hum. Hypertens. 1995;9(4):281. [PubMed] [Google Scholar]
- Landreth G., et al. PPARγ agonists as therapeutics for the treatment of Alzheimer’s disease. Neurotherapeutics. 2008;5(3):481–489. doi: 10.1016/j.nurt.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A.E., Espay A.J. Disease modification in Parkinson’s disease: current approaches, challenges, and future considerations. Mov. Disord. 2018;33(5):660–677. doi: 10.1002/mds.27360. [DOI] [PubMed] [Google Scholar]
- Lanza V., et al. Repurposing of copper (II)-chelating drugs for the treatment of neurodegenerative diseases. Curr. Med. Chem. 2018;25(4):525–539. doi: 10.2174/0929867324666170518094404. [DOI] [PubMed] [Google Scholar]
- Lesage S., Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 2009;18(R1):R48–59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106(4):1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., et al. Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J. Neurochem. 2010;113(6):1621–1631. doi: 10.1111/j.1471-4159.2010.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., et al. GLP-1 receptor stimulation reduces amyloid-β peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J. Alzheim. Dis. 2010;19(4):1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., et al. Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol., Biochem. Behav. 2012;101(4):564–574. doi: 10.1016/j.pbb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., et al. Allele-selective lowering of mutant HTT protein by HTT–LC3 linker compounds. Nature. 2019;575(7781):203–209. doi: 10.1038/s41586-019-1722-1. [DOI] [PubMed] [Google Scholar]
- Liu J.-P., Zeitlin S.O. Is huntingtin dispensable in the adult brain? J. Huntingt. Dis. 2017;6(1):1–17. doi: 10.3233/JHD-170235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., et al. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience. 2015;303:42–50. doi: 10.1016/j.neuroscience.2015.06.054. [DOI] [PubMed] [Google Scholar]
- Longatti A., Tooze S. Vesicular trafficking and autophagosome formation. Cell Death Differ. 2009;16(7):956–965. doi: 10.1038/cdd.2009.39. [DOI] [PubMed] [Google Scholar]
- Lu M., et al. Metformin prevents dopaminergic neuron death in MPTP/P-induced mouse model of Parkinson’s disease via autophagy and mitochondrial ROS clearance. Int. J. Neuropsychopharmacol. 2016;19(9):pyw047. doi: 10.1093/ijnp/pyw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin F.D., et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin F., et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:1075–1084. doi: 10.1016/S0140-6736(15)01314-8. 10023. [DOI] [PubMed] [Google Scholar]
- MacDonald M.E., et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72(6):971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Mahad D.H., Trapp B.D., Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- Malberg J.E., et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J.H., Bowden N.A. Drug repurposing—overcoming the translational hurdles to clinical use. Pharmacol. Res. Perspect. 2019;7(6) doi: 10.1002/prp2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J.B., Gusella J.F. Huntingtons disease. N. Engl. J. Med. 1986;315(20):1267–1276. doi: 10.1056/NEJM198611133152006. [DOI] [PubMed] [Google Scholar]
- Martin-Bastida A., et al. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci. Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M., et al. Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat. Neurosci. 2010;13(5):567. doi: 10.1038/nn.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean P.L., et al. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J. Neurosci. 2011;31(17):6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-McKee K., et al. Combining hippocampal volume metrics to better understand Alzheimer’s disease progression in at-risk individuals. Sci. Rep. 2019;9(1):7499. doi: 10.1038/s41598-019-42632-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdawar B., Ghossoub E., Khoury R. Selective serotonin reuptake inhibitors and Alzheimer’s disease. Neural Regen. Res. 2020;15(1):41. doi: 10.4103/1673-5374.264445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer T.R., et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson’s disease. Brain. 2011;134(Pt 3):845–855. doi: 10.1093/brain/awq377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena N.P., et al. The novel mitochondrial iron chelator 5-((methylamino) methyl)-8-hydroxyquinoline protects against mitochondrial-induced oxidative damage and neuronal death. Biochem. Biophys. Res. Commun. 2015;463(4):787–792. doi: 10.1016/j.bbrc.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Metaxakis A., Ploumi C., Tavernarakis N. Autophagy in age-associated neurodegeneration. Cells. 2018;7(5) doi: 10.3390/cells7050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A., Chakrabarti S.S., Gambhir I.S. New genetic players in late-onset Alzheimer’s disease: findings of genome-wide association studies. Indian J. Med. Res. 2018;148(2):135–144. doi: 10.4103/ijmr.IJMR_473_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K., Katare D.P. Shared links between type 2 diabetes mellitus and Alzheimer’s disease: a review. Diabetes Metabol. Syndr.: Clin. Res. Rev. 2016;10(2):S144–S149. doi: 10.1016/j.dsx.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Mizuno T., et al. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46(3):404–411. doi: 10.1016/j.neuropharm.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Yasuda T. Iron accumulation in Parkinson’s disease. J. Neural. Transm. 2012;119(12):1511–1514. doi: 10.1007/s00702-012-0905-9. [DOI] [PubMed] [Google Scholar]
- Mole S.E., Cotman S.L. Genetics of the neuronal ceroid lipofuscinoses (Batten disease) Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2015;1852(10):2237–2241. doi: 10.1016/j.bbadis.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinuevo J.L., Llado A., Rami L. Memantine: targeting glutamate excitotoxicity in Alzheimer’s disease and other dementias. Am. J. Alzheimers Dis. Other Demen. 2005;20(2):77–85. doi: 10.1177/153331750502000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A., et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A., et al. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol. 2016;131(5):687–707. doi: 10.1007/s00401-016-1570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalban X., et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 2017;376(3):209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- Moore E.M., et al. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013;36(10):2981–2987. doi: 10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C., et al. Iron as a therapeutic target for Parkinson’s disease. Mov. Disord. 2018;33(4):568–574. doi: 10.1002/mds.27275. [DOI] [PubMed] [Google Scholar]
- Morris J.K., et al. Measures of striatal insulin resistance in a 6-hydroxydopamine model of Parkinson’s disease. Brain Res. 2008;1240:185–195. doi: 10.1016/j.brainres.2008.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen K., et al. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul. Pept. 2003;114(2–3):189–196. doi: 10.1016/s0167-0115(03)00125-3. [DOI] [PubMed] [Google Scholar]
- Mounsey R.B., Teismann P. Chelators in the treatment of iron accumulation in Parkinson’s disease. Int. J. Cell Biol. 2012;2012 doi: 10.1155/2012/983245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T. Levodopa/carbidopa and entacapone in the treatment of Parkinson’s disease: efficacy, safety and patient preference. Patient Prefer. Adherence. 2009;3:51–59. doi: 10.2147/ppa.s4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U.C., Zheng H. Physiological functions of APP family proteins. Cold Spring Harb. Perspect. Med. 2012;2(2):a006288. doi: 10.1101/cshperspect.a006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins R.J., et al. A pilot study of exenatide actions in Alzheimer’s disease. Curr. Alzheimer Res. 2019;16(8):741–752. doi: 10.2174/1567205016666190913155950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murteira S., et al. Drug reformulations and repositioning in the pharmaceutical industry and their impact on market access: regulatory implications. J. Market Access Health Pol. 2014;2(1):22813. doi: 10.3402/jmahp.v2.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek E.S., Holtzman D.M. Three dimensions of the amyloid hypothesis: time, space and’wingmen. Nat. Neurosci. 2015;18(6):800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj A.C., Schellenberg G.D., Alzheimer’s C. Disease Genetics Genomic variants, genes, and pathways of Alzheimer’s disease: an overview. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017;174(1):5–26. doi: 10.1002/ajmg.b.32499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj A.C., et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat. Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation D.A., et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019;25(2):270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurol L. Pioglitazone in early Parkinson’s disease: a phase 2, multicentre, double-blind, randomised trial. Lancet Neurol. 2015;14(8):795–803. doi: 10.1016/S1474-4422(15)00144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L.L. Incretin mimetics and DPP-IV inhibitors for the treatment of type 2 diabetes. Drug Discov. Today. 2005;10(10):703–710. doi: 10.1016/S1359-6446(05)03460-4. [DOI] [PubMed] [Google Scholar]
- Nishikawa N., et al. Effect of inosine on MPTP-induced Parkonson’s disease mice: high performance liquid chromatography and fluorescent activated cell sorting studies. J. Neurol. Sci. 2017;381:590–591. [Google Scholar]
- Noda T., Fujita N., Yoshimori T. The late stages of autophagy: how does the end begin? Cell Death Differ. 2009;16(7):984–990. doi: 10.1038/cdd.2009.54. [DOI] [PubMed] [Google Scholar]
- Noda H., et al. Fingolimod phosphate promotes the neuroprotective effects of microglia. J. Neuroimmunol. 2013;256(1–2):13–18. doi: 10.1016/j.jneuroim.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Novac N. Challenges and opportunities of drug repositioning. Trends Pharmacol. Sci. 2013;34(5):267–272. doi: 10.1016/j.tips.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Novak P., Pimentel Maldonado D.A., Novak V. Safety and preliminary efficacy of intranasal insulin for cognitive impairment in Parkinson disease and multiple system atrophy: a double-blinded placebo-controlled pilot study. PloS One. 2019;14(4) doi: 10.1371/journal.pone.0214364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S., Zhivotovsky B., Nicotera P. Regulation of cell death: the calcium–apoptosis link. Nat. Rev. Mol. Cell Biol. 2003;4(7):552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Ownby R.L., et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatr. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L., Witt E.H., Tritschler H.J. alpha-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995;19(2):227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- Parihar M., Hemnani T. Alzheimer’s disease pathogenesis and therapeutic interventions. J. Clin. Neurosci. 2004;11(5):456–467. doi: 10.1016/j.jocn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Paris D., et al. Selective antihypertensive dihydropyridines lower Aβ accumulation by targeting both the production and the clearance of Aβ across the blood-brain barrier. Mol. Med. 2011;17(3–4):149–162. doi: 10.2119/molmed.2010.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes D.G., Mace K.F., Trautmann M.E. Discovery and development of exenatide: the first antidiabetic agent to leverage the multiple benefits of the incretin hormone, GLP-1. Expet Opin. Drug Discov. 2013;8(2):219–244. doi: 10.1517/17460441.2013.741580. [DOI] [PubMed] [Google Scholar]
- Parvathaneni V., et al. Drug repurposing: a promising tool to accelerate the drug discovery process. Drug Discov. Today. 2019;24(10):2076–2085. doi: 10.1016/j.drudis.2019.06.014. [DOI] [PubMed] [Google Scholar]
- Patil S., et al. Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience. 2014;277:747–754. doi: 10.1016/j.neuroscience.2014.07.046. [DOI] [PubMed] [Google Scholar]
- Paulson H. Repeat expansion diseases. Handb. Clin. Neurol. 2018;147:105–123. doi: 10.1016/B978-0-444-63233-3.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechnick R.N., et al. Antidepressants stimulate hippocampal neurogenesis by inhibiting p21 expression in the subgranular zone of the hipppocampus. PloS One. 2011;6(11) doi: 10.1371/journal.pone.0027290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez M.J., Quintanilla R.A. Therapeutic actions of the thiazolidinediones in Alzheimer’s disease. PPAR Res. 2015;2015 doi: 10.1155/2015/957248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlstrom L., Wiethoff S., Houlden H. Genetics of neurodegenerative diseases: an overview. Handb. Clin. Neurol. 2017;145:309–323. doi: 10.1016/B978-0-12-802395-2.00022-5. [DOI] [PubMed] [Google Scholar]
- Pirscoveanu D.F.V., et al. Tau protein in neurodegenerative diseases - a review. Rom. J. Morphol. Embryol. 2017;58(4):1141–1150. [PubMed] [Google Scholar]
- Poewe W., et al. Levodopa in the treatment of Parkinson’s disease: an old drug still going strong. Clin. Interv. Aging. 2010;5:229–238. doi: 10.2147/cia.s6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W., et al. Parkinson disease. Nat. Rev. Dis. Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- Prasad A., et al. Molecular mechanisms of TDP-43 misfolding and pathology in amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2019;12:25. doi: 10.3389/fnmol.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard J.-L.E., O’Mara T.A., Glubb D.M. Enhancing the promise of drug repositioning through genetics. Front. Pharmacol. 2017;8:896. doi: 10.3389/fphar.2017.00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushpakom S., et al. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18(1):41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- Qiu C., Winblad B., Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Quinn L., et al. The PPARγ agonist pioglitazone is effective in the MPTP mouse model of Parkinson’s disease through inhibition of monoamine oxidase B. Br. J. Pharmacol. 2008;154(1):226–233. doi: 10.1038/bjp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai A., et al. Glycogen synthase protects neurons from cytotoxicity of mutant huntingtin by enhancing the autophagy flux. Cell Death Dis. 2018;9(2):201. doi: 10.1038/s41419-017-0190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff R.M., Hafler D.A., Lucchinetti C.F. Multiple sclerosis—a quiet revolution. Nat. Rev. Neurol. 2015;11(3):134. doi: 10.1038/nrneurol.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A.S., Zhang S. Polypharmacology: drug discovery for the future. Expet Rev. Clin. Pharmacol. 2013;6(1):41–47. doi: 10.1586/ecp.12.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D.H., Misra S., Medhi B. Advances in drug development for Parkinson’s disease: present status. Pharmacology. 2014;93(5–6):260–271. doi: 10.1159/000362419. [DOI] [PubMed] [Google Scholar]
- Reimann F., Gribble F.M. G protein-coupled receptors as new therapeutic targets for type 2 diabetes. Diabetologia. 2016;59(2):229–233. doi: 10.1007/s00125-015-3825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Replogle J.M., et al. A TREM1 variant alters the accumulation of Alzheimer-related amyloid pathology. Ann. Neurol. 2015;77(3):469–477. doi: 10.1002/ana.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea E.M., Raber J., Banks W.A. ApoE and cerebral insulin: trafficking, receptors, and resistance. Neurobiol. Dis. 2020;137:104755. doi: 10.1016/j.nbd.2020.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigamonti D., et al. Wild-type huntingtin protects from apoptosis upstream of caspase-3. J. Neurosci. 2000;20(10):3705–3713. doi: 10.1523/JNEUROSCI.20-10-03705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M., et al. α-Linolenic acid–valproic acid conjugates: toward single-molecule polypharmacology for multiple sclerosis. ACS Med. Chem. Lett. 2020:2280–2287. doi: 10.1021/acsmedchemlett.0c00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossor A.M., Reilly M.M., Sleigh J.N. BMJ Publishing Group Ltd; 2018. Antisense Oligonucleotides and Other Genetic Therapies Made Simple. [DOI] [PubMed] [Google Scholar]
- Roy B., et al. Ataluren stimulates ribosomal selection of near-cognate tRNAs to promote nonsense suppression. Proc. Natl. Acad. Sci. Unit. States Am. 2016;113(44):12508–12513. doi: 10.1073/pnas.1605336113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royea J., Hamel E. GeroScience; 2020. Brain Angiotensin II and Angiotensin IV Receptors as Potential Alzheimer’s Disease Therapeutic Targets; pp. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R., et al. Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis. 2011;2(4) doi: 10.1038/cddis.2011.29. e144-e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon A., et al. Fixed-dose combination therapy for Parkinson’s disease with a spotlight on entacapone in the past 20 years: a reduced pill burden and a simplified dosing regime. Expet Opin. Pharmacother. 2020:1–14. doi: 10.1080/14656566.2020.1806237. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatr. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sari Y., et al. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington’s disease. J. Biomed. Sci. 2010;17(1):1–5. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar C., et al. Neuroprotection and lifespan extension in Ppt1−/− mice by NtBuHA: therapeutic implications for INCL. Nat. Neurosci. 2013;16(11):1608–1617. doi: 10.1038/nn.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J., et al. Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience. 1994;62(1):15–30. doi: 10.1016/0306-4522(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Sawamoto K., et al. Gene therapy for mucopolysaccharidoses. Mol. Genet. Metabol. 2018;123(2):59–68. doi: 10.1016/j.ymgme.2017.12.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedin-Weiss S., et al. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. 2017;9(1):1–19. doi: 10.1186/s13195-017-0279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J.B., Deuschl G. [Influence of lifestyle on neurodegenerative diseases] Nervenarzt. 2015;86(8):954–959. doi: 10.1007/s00115-014-4252-y. [DOI] [PubMed] [Google Scholar]
- Schulz A., et al. Study of intraventricular cerliponase alfa for CLN2 disease. N. Engl. J. Med. 2018;378(20):1898–1907. doi: 10.1056/NEJMoa1712649. [DOI] [PubMed] [Google Scholar]
- Schwarzschild M.A., et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 2014;71(2):141–150. doi: 10.1001/jamaneurol.2013.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedel F., et al. Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis. Neuropharmacology. 2016;110:644–653. doi: 10.1016/j.neuropharm.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Selden N.R., et al. Central nervous system stem cell transplantation for children with neuronal ceroid lipofuscinosis. J. Neurosurg. Pediatr. 2013;11(6):643–652. doi: 10.3171/2013.3.PEDS12397. [DOI] [PubMed] [Google Scholar]
- Selvaraj S., Piramanayagam S. Impact of gene mutation in the development of Parkinson’s disease. Genes Dis. 2019;6(2):120–128. doi: 10.1016/j.gendis.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams S., Wahlund L.O. Cerebral microbleeds as a biomarker in Alzheimer’s disease? A review in the field. Biomarkers Med. 2016;10(1):9–18. doi: 10.2217/bmm.15.101. [DOI] [PubMed] [Google Scholar]
- Shannon K.M. Recent advances in the treatment of Huntington’s disease: targeting DNA and RNA. CNS Drugs. 2020;34(3):219–228. doi: 10.1007/s40263-019-00695-3. [DOI] [PubMed] [Google Scholar]
- Shi F., et al. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: meta-analyses of MRI studies. Hippocampus. 2009;19(11):1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- Shih Y.-H., et al. Hypertension accelerates Alzheimer’s disease-related pathologies in pigs and 3xTg mice. Front. Aging Neurosci. 2018;10:73. doi: 10.3389/fnagi.2018.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo A.S., et al. Intracerebroventricular administration of an insulin analogue recovers STZ-induced cognitive decline in rats. Behav. Brain Res. 2013;241:105–111. doi: 10.1016/j.bbr.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Shirani A., Okuda D.T., Stüve O. Therapeutic advances and future prospects in progressive forms of multiple sclerosis. Neurotherapeutics. 2016;13(1):58–69. doi: 10.1007/s13311-015-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman J.M., et al. Genetic susceptibility for Alzheimer disease neuritic plaque pathology. JAMA Neurol. 2013;70(9):1150–1157. doi: 10.1001/jamaneurol.2013.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog I., et al. Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: study on COgnition and Prognosis in the Elderly (SCOPE) Am. J. Hypertens. 2005;18(8):1052–1059. doi: 10.1016/j.amjhyper.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Sleat D.E., et al. Aminoglycoside-mediated suppression of nonsensemutations in late infantile neuronal ceroid lipofuscinosis. Eur. J. Paediatr. Neurol. 2001;5:57–62. doi: 10.1053/ejpn.2000.0436. [DOI] [PubMed] [Google Scholar]
- Smith R.B. Repositioned drugs: integrating intellectual property and regulatory strategies. Drug Discov. Today Ther. Strat. 2011;8(3–4):131–137. [Google Scholar]
- Smith R., et al. Mutant huntingtin interacts with β-tubulin and disrupts vesicular transport and insulin secretion. Hum. Mol. Genet. 2009;18(20):3942–3954. doi: 10.1093/hmg/ddp336. [DOI] [PubMed] [Google Scholar]
- Sohar I., Lin L., Lobel P. Enzyme-based diagnosis of classical late infantile neuronal ceroid lipofuscinosis: comparison of tripeptidyl peptidase I and pepstatin-insensitive protease assays. Clin. Chem. 2000;46(7):1005–1008. [PubMed] [Google Scholar]
- Sozio P., et al. New L-dopa codrugs as potential antiparkinson agents. Arch. Pharm. (Weinheim) 2008;341(7):412–417. doi: 10.1002/ardp.200700228. [DOI] [PubMed] [Google Scholar]
- Sozio P., et al. Ibuprofen and lipoic acid diamides as potential codrugs with neuroprotective activity. Arch. Pharm. (Weinheim) 2010;343(3):133–142. doi: 10.1002/ardp.200900152. [DOI] [PubMed] [Google Scholar]
- Spain R., et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol.-Neuroimmunol. Neuroinflammation. 2017;4(5) doi: 10.1212/NXI.0000000000000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy M., Silver D. Apomorphine for the acute treatment of “off” episodes in Parkinson’s disease. Park. Relat. Disord. 2008;14(2):85–92. doi: 10.1016/j.parkreldis.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Steen E., et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J. Alzheim. Dis. 2005;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Stefanis L. alpha-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2(2):a009399. doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker T.B., Barker R.A. vol. 9. 2020. (Recent Developments in the Treatment of Parkinson’s Disease). F1000Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter S.M. Overcoming drug development bottlenecks with repurposing: old drugs learn new tricks. Nat. Med. 2014;20(6):590–591. doi: 10.1038/nm.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Pham A.N., Waite T.D. Mechanism underlying the effectiveness of deferiprone in alleviating Parkinson’s disease symptoms. ACS Chem. Neurosci. 2018;9(5):1118–1127. doi: 10.1021/acschemneuro.7b00478. [DOI] [PubMed] [Google Scholar]
- Tabrizi S.J., et al. Targeting huntingtin expression in patients with Huntington’s disease. N. Engl. J. Med. 2019;380(24):2307–2316. doi: 10.1056/NEJMoa1900907. [DOI] [PubMed] [Google Scholar]
- Takahashi-Niki K., et al. Reduced anti-oxidative stress activities of DJ-1 mutants found in Parkinson’s disease patients. Biochem. Biophys. Res. Commun. 2004;320(2):389–397. doi: 10.1016/j.bbrc.2004.05.187. [DOI] [PubMed] [Google Scholar]
- Takeda A., et al. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer’s disease. Int. J. Geriatr. Psychiatr. 2006;21(1):17–28. doi: 10.1002/gps.1402. [DOI] [PubMed] [Google Scholar]
- Taylor J.P., Brown R.H., Jr., Cleveland D.W. Decoding ALS: from genes to mechanism. Nature. 2016;539(7628):197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.A., et al. Deaths from Alzheimer’s disease - United States, 1999-2014. MMWR Morb. Mortal. Wkly. Rep. 2017;66(20):521–526. doi: 10.15585/mmwr.mm6620a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., et al. Soluble N-terminal fragment of mutant Huntingtin protein impairs mitochondrial axonal transport in cultured hippocampal neurons. Neurosci. Bull. 2014;30(1):74–80. doi: 10.1007/s12264-013-1393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S.A., et al. Fluoxetine and vortioxetine reverse depressive-like phenotype and memory deficits induced by Aβ1-42 oligomers in mice: a key role of transforming growth factor-β1. Front. Pharmacol. 2019;10:693. doi: 10.3389/fphar.2019.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourbah A., et al. AAN Enterprises; 2015. Effect of MD1003 (High Doses of Biotin) in Progressive Multiple Sclerosis: Results of a Pivotal Phase III Randomized Double Blind Placebo Controlled Study (PL2. 002) [Google Scholar]
- Trapp S., Cork S.C. PPG neurons of the lower brain stem and their role in brain GLP-1 receptor activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309(8):R795–R804. doi: 10.1152/ajpregu.00333.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G., Shirihai O.S. The interplay between mitochondrial dynamics and mitophagy. Antioxidants Redox Signal. 2011;14(10):1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger J.W., Livingston J.N., Moss A.M. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog. Neurobiol. 1991;36(5):343–362. doi: 10.1016/0301-0082(91)90015-s. [DOI] [PubMed] [Google Scholar]
- Van Dam D., Coen K., De Deyn P.P. Ibuprofen modifies cognitive disease progression in an Alzheimer’s mouse model. J. Psychopharmacol. 2010;24(3):383–388. doi: 10.1177/0269881108097630. [DOI] [PubMed] [Google Scholar]
- Van der Schyf C.J. The use of multi-target drugs in the treatment of neurodegenerative diseases. Expet Rev. Clin. Pharmacol. 2011;4(3):293–298. doi: 10.1586/ecp.11.13. [DOI] [PubMed] [Google Scholar]
- van Ham T.J., et al. Identification of MOAG-4/SERF as a regulator of age-related proteotoxicity. Cell. 2010;142(4):601–612. doi: 10.1016/j.cell.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Vargas D.M.d., et al. Alzheimer’s disease master regulators analysis: search for potential molecular targets and drug repositioning candidates. Alzheimer’s Res. Ther. 2018;10(1):59. doi: 10.1186/s13195-018-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L., et al. Alzheimer’s-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. J. Neurosci. 2007;27(25):6771–6780. doi: 10.1523/JNEUROSCI.5564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S. Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cell. Mol. Neurobiol. 2018;38(1):121–132. doi: 10.1007/s10571-017-0554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines D.J., Warburton M.J. Classical late infantile neuronal ceroid lipofuscinosis fibroblasts are deficient in lysosomal tripeptidyl peptidase I. FEBS Lett. 1999;443(2):131–135. doi: 10.1016/s0014-5793(98)01683-4. [DOI] [PubMed] [Google Scholar]
- Wahlqvist M.L., et al. Metformin-inclusive sulfonylurea therapy reduces the risk of Parkinson’s disease occurring with Type 2 diabetes in a Taiwanese population cohort. Park. Relat. Disord. 2012;18(6):753–758. doi: 10.1016/j.parkreldis.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Walker K.A. Inflammation and neurodegeneration: chronicity matters. Aging. 2018;11(1):3–4. doi: 10.18632/aging.101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., et al. Valsartan lowers brain β-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J. Clin. Invest. 2007;117(11):3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., et al. Targeting the mTOR signaling network for Alzheimer’s disease therapy. Mol. Neurobiol. 2014;49(1):120–135. doi: 10.1007/s12035-013-8505-8. [DOI] [PubMed] [Google Scholar]
- Wang Y., et al. Neuroprotective effect and mechanism of thiazolidinedione on dopaminergic neurons in vivo and in vitro in Parkinson’s disease. PPAR Res. 2017;2017 doi: 10.1155/2017/4089214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden A., et al. Localization of PPAR isotypes in the adult mouse and human brain. Sci. Rep. 2016;6:27618. doi: 10.1038/srep27618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt J.L., Duman R.S. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16(3):239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Watts M.E., Pocock R., Claudianos C. Brain energy and oxygen metabolism: emerging role in normal function and disease. Front. Mol. Neurosci. 2018;11:216. doi: 10.3389/fnmol.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M., et al. TV3326, a novel neuroprotective drug with cholinesterase and monoamine oxidase inhibitory activities for the treatment of Alzheimer’s disease. J. Neural. Transm. Suppl. 2000;(60):157–169. doi: 10.1007/978-3-7091-6301-6_10. [DOI] [PubMed] [Google Scholar]
- Weinstock-Guttman B., et al. The interferons: biological effects, mechanisms of action, and use in multiple sclerosis. Ann. Neurol.: Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1995;37(1):7–15. doi: 10.1002/ana.410370105. [DOI] [PubMed] [Google Scholar]
- Wellington C.L., et al. Caspase cleavage of mutant huntingtin precedes neurodegeneration in Huntington’s disease. J. Neurosci. 2002;22(18):7862–7872. doi: 10.1523/JNEUROSCI.22-18-07862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J., et al. Physical activity, including walking, and cognitive function in older women. J. Am. Med. Assoc. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- White A.T., Murphy A.N. Administration of thiazolidinediones for neuroprotection in ischemic stroke: a pre-clinical systematic review. J. Neurochem. 2010;115(4):845–853. doi: 10.1111/j.1471-4159.2010.06999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D.G., et al. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: the relationship between pharmacological effects and clinical efficacy. Drugs Aging. 2004;21(7):453–478. doi: 10.2165/00002512-200421070-00004. [DOI] [PubMed] [Google Scholar]
- Williams A., et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 2008;4(5):295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky J.S., et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann. Neurol. 2007;61(1):14–24. doi: 10.1002/ana.21079. [DOI] [PubMed] [Google Scholar]
- Wu W.Y., et al. Novel multitarget-directed tacrine derivatives as potential candidates for the treatment of Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2017;32(1):572–587. doi: 10.1080/14756366.2016.1210139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539(7628):180–186. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., et al. 22nd Annual Symposium on ALS/MND, Sydney, Australia. 2011. Tracking brain uptake and protein incorporation of cyanobacterial toxin BMAA. [Google Scholar]
- Yamagishi S.J.E.N. The unsolved mystery of PARK3 locus of Parkinson’s disease. 2017;5:192–195. [Google Scholar]
- Yimer E.M., Hishe H.Z., Tuem K.B. Repurposing of the β-lactam antibiotic, ceftriaxone for neurological disorders: a review. Front. Neurosci. 2019;13:236. doi: 10.3389/fnins.2019.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F., Boveris A., Cadenas E. Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxidants Redox Signal. 2014;20(2):353–371. doi: 10.1089/ars.2012.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoseki A., et al. TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann. Neurol. 2008;63(4):538–542. doi: 10.1002/ana.21392. [DOI] [PubMed] [Google Scholar]
- Youdim M.B., Oh Y.J. Promise of neurorestoration and mitochondrial biogenesis in Parkinson’s disease with multi target drugs: an alternative to stem cell therapy. Exp. Neurobiol. 2013;22(3):167–172. doi: 10.5607/en.2013.22.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim M.B., et al. Promises of novel multi-target neuroprotective and neurorestorative drugs for Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(Suppl. 1):S132–S136. doi: 10.1016/S1353-8020(13)70032-4. [DOI] [PubMed] [Google Scholar]
- Yousefi S., et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 2006;8(10):1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- Yulug B., et al. Therapeutic role of rifampicin in Alzheimer’s disease. Psychiatr. Clin. Neurosci. 2018;72(3):152–159. doi: 10.1111/pcn.12637. [DOI] [PubMed] [Google Scholar]
- Zeitler B., et al. Allele-selective transcriptional repression of mutant HTT for the treatment of Huntington’s disease. Nat. Med. 2019;25(7):1131–1142. doi: 10.1038/s41591-019-0478-3. [DOI] [PubMed] [Google Scholar]
- Zeman W., Dyken P. Neuronal ceroid-lipofuscinosis (Batten’s disease): relationship to amaurotic family idiocy? Pediatrics. 1969;44(4):570–583. [PubMed] [Google Scholar]
- Zhang P.L., et al. Genetics of Parkinson’s disease and related disorders. J. Med. Genet. 2018;55(2):73–80. doi: 10.1136/jmedgenet-2017-105047. [DOI] [PubMed] [Google Scholar]
- Zhang L., et al. Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson’s disease mouse model. Neuropeptides. 2018;71:70–80. doi: 10.1016/j.npep.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Zimprich A., et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zweig R.M., et al. The neuropathology of aminergic nuclei in Alzheimer’s disease. Ann. Neurol.: Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1988;24(2):233–242. doi: 10.1002/ana.410240210. [DOI] [PubMed] [Google Scholar]