Abstract
Prostate cancer (PCa) is a major cause of morbidity and mortality in men worldwide. A geographic variation on the burden of the disease suggested that the environment, genetic makeup, lifestyle, and food habits modulate one's susceptibility to the disease. Although it has been generally thought to be an older age disease, and awareness and timely execution of screening programs have managed to contain the disease in the older population over the last decades, the incidence is still increasing in the population younger than 50. Existing treatment is efficient for PCa that is localized and responsive to androgen. However, the androgen resistant and metastatic PCa are challenging to treat. Conventional radiation and chemotherapies are associated with severe side effects in addition to being exorbitantly expensive. Many isolated phytochemicals and extracts of plants used in traditional medicine are known for their safety and diverse healing properties, including many with varying levels of anti-PCa activities. Many of the phytochemicals discussed here, as shown by many laboratories, inhibit tumor cell growth and proliferation by interfering with the components in the pathways responsible for the enhanced proliferation, metabolism, angiogenesis, invasion, and metastasis in the prostate cells while upregulating the mechanisms of cell death and cell cycle arrest. Notably, many of these agents simultaneously target multiple cellular pathways. We analyzed the available literature and provided an update on this issue in this review article.
Keywords: Prostate cancer, Chemotherapy, Radiation therapy, Natural products, Phytochemicals, Plant extract and compounds
Graphical abstract

Highlights
-
•
Prostate cancer in a major cause of death in older population worldwide.
-
•
Efficacies of current treatment options are limited in many cases.
-
•
Phytochemicals and extracts isolated from plants show anti-prostate cancer activity with unique mechanisms.
-
•
Certain phytochemicals alone or in combination with current chemotherapy show therapeutic promise.
Abbreviations
- 17/3βHSD
17/3β-hydroxysteroid dehydrogenase
- AP1
Activator protein 1
- ATM
ATM serine/threonine kinase
- ATP
Adenosine tri phosphate
- CCND1
Cyclin D1
- CD147
Cluster of differentiation 147
- CHEK2
Check point kinase 2
- ELAC2
Zinc phosphodiesterase ELAC protein 2
- ER-α
Estrogen receptor alpha
- ETV3
ETS variant transcription factor 3
- GSH
Reduced glutathione
- HMGCoA
3 hydroxy-3-methyl-glutaryl-coenzyme A reductase
- HOXB13
Homeobox B13
- IL1/6/8
Interleukine 1/6/8
- KMT2D
Histone-lysine N-methyl transferase 2D
- MAO-A
Monoamine oxidase-A
- MLH1
MutL homolog 1
- MMP2/9
Matrix metalloproteinase 2/9
- MSH2/6
Mut S homolog 2/6
- PALB2
Partner and localizer of BRCA2
- PDCD4
Programmed cell death protein 4
- PMS2
PMS1 homolog2, mismatch repair system component
- R1881
Metribolone
- RAD51D
Rad51 paralog D
- RNASEL
Ribonuclease 1
- S1P
Sphingosine -1-phospahate
- SCID
Severe combined immune deficient mice
- SMAD-3
Mothers against decapentaplegic homolog 3
- SPHK1
Sphingosine kinase 1
- StAR
Steroidogenic acute regulatory protein
- TGFβ-R1
Transforming growth factor β receptor type 1
- TIMP2/3
Tissue inhibitor of metalloproteinases 2
- TP53
Tumor protein 53
- ZFHX3
Zinc finger Homeobox 3
1. Introduction
Prostate cancer (PCa) is the second most common type of non-skin solid cancer next to lung cancer and the fifth leading cause of cancer related death in men. PCa mostly affects the population above 55 years. Men over 65 years are predisposed by 65% to the disease, which is 25% in men older than 75 (Fitzpatrick, 2008). Individuals with a family history are at a higher risk of the disease. Lifestyle and westernized diet rich in red or processed meat, sugar, fatty dairy products, refined grains, and fewer fruits and vegetables correlate well with their increased susceptibility to the disease (Torricelli et al., 2011; Attard et al., 2016). It is thought that the western diet does not itself cause the disease rather somewhat lacks the protective property of its counterpart of Southeast and East Asian countries where the PCa incidence is the least in the world (Torricelli et al., 2011; Adlercreutz, 2002; Chan et al., 1998a).
Current treatment strategies for PCa include active surveillance at the initial stages, followed by surgery and androgen deprivation therapy (ADT). Androgen that plays a vital role in the development and normal functioning of the prostate, has also been implicated in the initiation and growth of PCa about eight decades ago (Huggins and Hodges, 1941). PCa, although responds to ADT in the beginning, acquires androgen independence or castration resistance in subsequent 2 years, indicating a poor prognosis which is then treated with chemotherapy and other adjuvant therapies (Nurgali et al., 2018; de Gonzalez et al., 2013; Pearce, 2017). Despite significant progress with the existing PCa therapy, toxicity towards healthy normal cells, or non-specificity of these treatments remained a crucial constraint for treating PCa patients. Therefore, formulating an alternative therapy regimen to kill the PCa cells in a targeted manner requires an urgent attention. Herbal medicines have gained very high popularity since the early civilization because of their effectiveness against various ailments, including cancer. Research by various independent groups suggests the enormous potential of various plant extracts used in traditional and folk medicine and phytochemicals thereof with a novel mode of action as a potential remedy for deadly diseases like PCa (Cragg and Newman, 2013; Seca and Pinto, 2018).
1.1. Epidemiology
Globally, PCa is the second most common in incidence and the fifth-most leading cause of cancer-associated deaths in men. There were 1.276 million cases with 0.359 million deaths in 2018. It is the fourth most commonly diagnosed cancer after lung, breast, and colon in incidence, and the eight most trending causes of death in men and women combined (Bray et al., 2018). If the current trend continues, global PCa cases will rise to 2.2938 million in 2040. Although PCa is the most frequently diagnosed cancer in more than 50% (105 of 185) countries in the world, death due to PCa is the highest in Southern Africa (26.4%), followed by the Caribbean (25.4%) and middle Africa (22.4%). In both the UK and the USA, PCa is the most frequently occurring cancer in men (Bray et al., 2018; Ferlay et al., 2019).
1.2. Etiology
Men beyond the age of 50 are at a higher risk; six out of ten men older than 65 are affected by PCa. Race, ethnicity, and geographic variation can influence one's predisposition to PCa. African-American, African-Caribbean are more prone to develop PCa than Asian-American or Hispanic/Latino men. Developed countries like North America, North-Western Europe, Australia, and New Zealand carry a higher PCa burden. Autopsy results of PCa patients had revealed the highest incidence of PCa in African people (Gleason score 8+) with the lowest incidence encountered in the Asian people (Jalloh et al., 2013; Rebbeck and Haas, 2014; Rebbeck, 2017). Prostate-specific antigen levels (PSA) are significantly higher in African black men than white men (Vijayakumar et al., 1998). Genome-wide association study (GWAS), family-based study, and candidate gene association studies have identified multiple susceptibility markers with PCa. Chromosomes 8q24, 12q24, and 1q24-25 were shown to carry higher risk PCa loci (Cheng et al., 2008; Kote-Jarai et al., 2011; Gudmundsson et al., 2007; Yeager et al., 2007; Benafif and Eeles, 2016). Mutation in ZFHX3, TP53, and focal deletion in ETV3, and MYC amplification were associated with higher PCa death in African-American men. Truncation in KMT2D and amplification in CCND1 associate with primary and localized PCa in African-American men (Koga et al., 2020). Stress and consumption of an imbalanced diet act as critical risk factors for PCa (Chan and Giovannucci, 2001; Chan et al., 1998b; Giovannucci et al., 1998; Kolonel, 2001; Wolk, 2005).
Mounting evidence suggests a positive association between obesity and PCa (Calle et al., 2003; Allott et al., 2013). Altered levels of several hormones linked with obesity, such as higher estradiol levels, insulin, free insulin-like growth factor-1 (IGF-1), leptin, and a lower level of free testosterone and adiponectin add to an aggressive form of PCa (Chan et al., 1998b; Bosland, 2000; Price et al., 2012; Roberts et al., 2010; Buschemeyer and Freedland, 2007; Schnoeller et al., 2013). The meta-analysis revealed that 5–10% of the PCa had inherited genetic background (Watkins Bruner et al., 2003; Gallagher and Fleshner, 1998; Madersbacher et al., 2011). A person is at a higher risk of getting PCa if his father or brother has PCa. PCa is one of the several cancers having a high hereditary link (Lichtenstein et al., 2000). Mutations in breast cancer susceptibility gene 1 (BRCA1) and breast cancer susceptibility gene 2 (BRCA2) predispose one to a higher risk of developing PCa (Castro and Eeles, 2012; Nombela et al., 2019). DNA damage repair genes such as CHEK2, ATM, PALB2, and RAD51D can account for some of the hereditary PCa (Pritchard et al., 2016). Abnormality in DNA mismatch repair genes such as MSH2, MSH6, MLH1, and PMS2 (Albero-González et al., 2019; Nghiem et al., 2016) and tumor suppressor gene RNASEL (Xiang et al., 2003) are associated with heritable PCa (Malathi et al., 2007; Zhou et al., 1997; Rawla, 2019). Mutations in ELAC2 that support proliferation through the TGF-β signaling pathway, and HOXB13 an essential gene in prostate gland development, were implicated in hereditary PCa (Meyer et al., 2010; Noda et al., 2006). A small deletion mutation in the Xq26.3-q27.3 of the X chromosome carrying the androgen receptor (AR) gene has been linked with inherited PCa (Xu et al., 1998; Bergthorsson et al., 2000).
Alteration of AR gene expression due to amplification of AR gene, over production of androgen, and AR splice variants, and or its coregulator associate with AR therapy resistance/castration resistance prostate cancer (CRPC). Apart from AR, other cellular pathways such as the WNT signaling pathway play a critical role particularly in the late or advanced stage of PCa such as CRPC (Murillo-Garzón and Kypta, 2017). WNT pathway is deregulated in about a third of PCa (Chesire et al., 2000). Deregulation of both canonical and noncanonical WNT signaling pathways has been shown to be involved in PCa pathogenesis. Canonical WNT pathway (β-catenin dependent) functions through stabilization of β-catenin and subsequent activation of target genes involving ß-catenin-TCF/LEF1 transcription complexes (Komiya and Habas, 2008). The noncanonical WNT pathway (ß-catenin-independent) which is of two subtypes, the planer cell polarity and WNT/Ca2+ pathway were associated with the advanced stage of CRPC regulating genes involved in cell adhesion and migration, cell survival, and angiogenesis (Van Amerongen, 2012). In the canonical pathway, stabilization of β-catenin in the cytoplasm and nuclear translocation is controlled by tight regulation of function of the components of destruction complex composed of adenomatous polyposis coli (APC), glycogen synthase kinase 3 (GSK-3), casein kinase 1 (CK1), and Axin. Activating mutation of the β-catenin encoding gene CTNNB1 and inactivating mutation in APC and Axin gene associate with many CRPC (Beltran et al., 2013; Grasso et al., 2012). Upregulation of LEF1 gene, a target of ß-catenin-TCF-LEF1 transcription complex associates with CRPC. LEF1 correlated with malignant transformation and metastasis of PCa is also a target of ETS-regulated gene (ERG) which is upregulated in about half of PCa (Wu et al., 2013; Bauman et al., 2016). AR overexpression potentiated the expression of WNT- β-catenin target genes involved in PCa metastasis due to its ability to function as a partner of β-catenin-TCF-LEF1 transcription complex driving expression of genes involved in metastasis, although cooperation with β-catenin depends on the activation status of AR (Lee et al., 2015, 2016). Usually, WNT deregulated PCa cells become invasive when they acquire additional genomic changes (Francis, 2013). Evidence demonstrated WNT signaling pathway in immune evasion of PCa cells as well (Wu et al., 2013; Francis, 2013; Linch et al., 2017; Wang et al., 2018). Depending on the context, AR can also repress the expression of LEF1 and Axin2 genes (Lee et al., 2016).
Deregulation of upstream regulators i.e., the WNT ligands was also linked with PCa pathogenesis; WNT-1 is upregulated in primary and advanced PCa (Chen et al., 2004). Several WNT ligands associated with noncanonical WNT signaling pathways such as WNT-5A and WNT11 have been implicated in PCa invasiveness and resistance to ADT (Zhang et al., 2013; Volante et al., 2016; Rajan et al., 2014). Deregulation of secretory factors that normally antagonize functions of WNT ligands or WNT signaling components on cell membrane such as frizzled (FZD) have also been demonstrated to influence PCa with some linked with drug resistance (Murillo-Garzón and Kypta, 2017; Pashirzad et al., 2017). It is to be noted however that usually, WNT deregulation requires in combination with another genomic defect progression Downregulation of secreted frizzled-related proteins (sFRPs), Dickkopf-related proteins (DKKs), and WNT1 or increased expression of these inhibitors appeared to play a complex and context-dependent role, for example, a high level of Dickkopf-related proteins 1 (DKK1) was linked with poor treatment outcome (Rachner et al., 2014). Several studies demonstrated the critical roles of the tumor microenvironment (which secrete WNT ligands) in the growth of PCa including many other types of cancer (Hanahan and Weinberg, 2011). A few of the WNT family members secreted by the stromal cells were shown to be involved in tumor initiation, progression, and drug resistance of PCa (Murillo-Garzón and Kypta, 2017).
Like many other cancers and diseases, chronic inflammation is a driver of PCa (Stallone et al., 2014). In general, chronic inflammation is the underlying cause of more than 20% of cancers (De Marzo et al., 2007; Sfanos and De Marzo, 2012; Gurel et al., 2014; Mani et al., 2016; Kwon et al., 2014; Pal et al., 2014; Calcinotto et al., 2018), and close to 80% of prostate biopsy samples indicated the signatures of inflammation (Sfanos and De Marzo, 2012; Andriole et al., 2010; Banerjee et al., 2019). For example, imbalance in gut microbiome structure was linked with PCa (Liss et al., 2018; Sfanos et al., 2018; Golombos et al., 2018; Poutahidis, 2013). Chronic bacterial infection in the prostate results in the inactivation of the tumor suppressor, a homeobox protein NKX3.1 that plays an important regulatory role in DNA repair (Khalili et al., 2010; Bowen and Gelmann, 2010). Inflammatory cells secrete ROS with simultaneous incapacitation of DNA damage repair enzymes. For example, under chromic inflammation, AR signaling induces DNA damage through the recruitment of DNA topoisomerase 2β with simultaneous inactivation of DNA damage repair genes (de Bono et al., 2020).
2. Current treatment available for prostate cancer
General awareness and timely screening have significantly added to the survival of PCa. Localized PCa (Gleason score <6) in general has a good prognosis. PCa that has attained metastatic state, however, carries a poor prognosis with significantly reduced survival. In this condition, resection surgery, ADT, and radiation alone or in combination are standard treatment options; castration resistance, a more aggressive form of PCa (Gleason score >7), invariably develops resistance to these treatments within 2 years (Giacinti et al., 2014; Rice et al., 2019). These advanced treatments increase the median failure-free survival (FFS) to 11 months with 29% 2 years FFS, and median overall survival (OS) to 42 months with 2 years OS of 72% (James et al., 2015).
Several androgen inhibitors, such as enzalutamide, apalutamide, abiraterone, or orteronel, along with radiation or chemotherapy, are in use in the treatment regimens (Teply and Antonarakis, 2016). Radiotherapies such as intensity-modulated radiation therapy (IMRT), conformal radiation therapy (CRT), and proton beam radiation therapy help to treat PCa with high efficacy (Yu, 2016; Fischer-Valuck et al., 2017). Docetaxel and cabazitaxel administration improved survival (Oudard et al., 2017). Besides these, immunotherapy with cytotoxic T-cell activators sipuleucel-T, pembrolizumab, nivolumab, and atezolizumab lead to improved overall patients survival (De Velasco et al., 2017). PARP inhibitors such as olaparib, rucaparib, and niraparib are now being studied in detail for developing more efficient PCa drugs (Mateo et al., 2015; Abida et al., 2018; Subudhi, 2019). Despite notable advancements in therapeutic strategies, and recurrences of the disease are often observed (Nurgali et al., 2018; de Gonzalez et al., 2013; Carelle et al., 2002; Chow and Gandhi, 2017). Nevertheless, current treatments associate with toxic side effects on the healthy normal tissues underscoring an immediate need to find an alternate and more targeted therapy. In fact, many independent laboratories from different parts of the world have highlighted anticancer activities with unique mechanisms of action in phytochemicals isolated from different plants used in traditional and folk medicines.
3. Importance of natural products in prostate cancer therapy
PCa epidemiology indicated the geographical variation of the disease incidence and the critical role of diet and nutrients besides the role of genetic factors; there is a relatively lower incidence of the disease in Asia than in the western population. Asians adopting western lifestyles are at a higher risk of getting PCa suggesting the protective role of diets/nutrients of Asian countries on the disease (Torricelli et al., 2011). Notably, many phytochemicals and solvent extracts of plants used in the traditional medicine in India and China showed selective toxicity towards PCa tested in cell and animal-based experiments with minimal toxicity to normal cells, suggesting promising therapeutic potential against the disease (Harvey and Cree, 2010). We reviewed here briefly the activities of phytochemicals which are relatively well studied by listing their clinical trials in a table (Table 1, Fig. 1). Studies on the phytochemicals that were relatively less studied were summarized in tabular form (Table 2). There are solvent extracts prepared off various plants with noticeable anti-PCa cancer activities in the preclinical experimental models are also described here briefly (Table 3). Fig. 1 and Fig. 2 summarised the discussed activities and structures of the phytochemicals respectively. Only phytochemicals tested in both cell and preclinical animal models are considered in this review.
Table 1.
List of phytochemicals undergone clinical trials for PCa therapy.
| Kind of supplement/Compound | Type of study | No. of patients and time period | Outcome | Reference |
|---|---|---|---|---|
| CURCUMIN | ||||
| Curcumin | A randomized, double-blind, placebo-controlled trial in patients who received intermittent androgen deprivation (IAD). | n = 97, oral Curcumin (1440 mg/day) or placebo for six months | Curcumin intake did not significantly change the overall off-treatment duration of IAD but PSA enhancement was suppressed. Curcumin was safe and well tolerated by patients. | Choi et al. (2019) |
| Curcumin | A double-blinded, randomized, placebo-controlled study in patients during radiotherapy | n = 40, Curcumin (total 3 g/day) or placebo during external-beam radiation therapy of up to 74 Gy for 3 months | Curcumin intake significantly increased total antioxidant capacity, with a reduction in SOD activity. PSA level was lowered in both the groups but nonsignificant differences in treatment outcomes between the groups. | Hejazi et al. 2016) |
| Curcumin | A pilot clinical trial in patients during radiotherapy | n = 40, 3 g/d Curcumin (6 × 500 mg capsules, n = 20), or placebo group (n = 20) during external-beam radiation therapy for 20 weeks | Curcumin intake provided radioprotection by reducing the severity of radiotherapy-related urinary symptoms. But did not reduce the intensity of bowel and other treatment related symptoms. | Hejazi et al. (2013) |
| LYCOPENE | ||||
| Fruit juice containing lycopene | In vivo double-blind placebo-controlled matched study | n = 60, a daily supplement for 28 days | Serum antioxidant, folate and, lLycopene level were increased while oxidative stress markers and homocysteine levels were decreased | Kawashima et al. (2007) |
| Lycopene tablet | A phase II randomized clinical trial before radical prostatectomy | n = 26, 15 mg Lycopene tablet twice a day for 3 weeks | Decreased PSA, IGF-1, and connexin-43 level with a reduction in chance and growth of PCa | Kucuk et al. (2001) |
| Lycopene with green tea catechins | A phase II randomized placebo-controlled trial | n = 133, daily green tea drink (3 cups, unblinded) or capsules [blinded, 600 mg flavan-3-ol ()-Epigallocatechin-3-gallate (EGCG) or placebo] and Lycopene-rich foods (unblinded) or capsules (blinded, 15 mg Lycopene or placebo) for 6 months | Reduced PCa risk. Lycopene and EGCG concentration was increased in men having an increased risk of PCa. | Lane et al. (2018) |
| Lycopene | A human intervention trial | n = 23, 40 mg Lycopene for 2 weeks | Showed cancer-preventive potential through the reduction in oxidative and other DNA damages. | Pool-Zobel et al. (1997) |
| Lycopene | An unblinded, randomized, Phase I clinical trial | n = 61, 30 mg Lycopene with a multivitamin | Increased serum level of Lycopene with reduced PSA level. | Bunker et al. (2007) |
| Lycopene | A phase II randomized trial among men with high-grade prostatic intraepithelial neoplasia (HGPIN) | n = 58, 30 mg Lycopene for 6 months | No significant change in serum PSA, IGF-1/3, MCM-2, and p27 levels. People in the Lycopene group had more extensive atrophy and less extensive HGPIN. | Gann et al. (2015) |
| Lycopene and green tea | The Pro-diet randomized controlled trial | n = 133, daily Lycopene (n = 44 assigned 15 mg capsules/day; 44 assigned a Lycopene-rich diet; 45 assigned placebo) and green tea (n = 45 assigned 600 mg/day epigallocatechin gallate; 45 assigned green tea drink; 43 assigned placebo) for 6 months | Serum Lycopene and EGCG level increased with a little reduction in serum IGF-1/2, IGFBP-2/3 level. | Biernacka et al. (2019) |
| Lycopene with tomato sauce | A randomized placebo-controlled study with a nonrandomized 5th arm study | n = 32, daily 30 mg Lycopene containing tomato sauce for 3 weeks before radical prostatectomy. | Serum and prostate Lycopene levels were increased with a concomitant decrease in PSA and Leukocyte DNA S–OH deoxyguanosine/deoxyguanosine (SOHdG) level. Reduced DNA damage with high apoptotic index in hyperplastic and neoplastic cells. | Bowen et al. (2002) |
| Lycopene and soy isoflavone | A phase II clinical trial | n = 71, 15 mg capsule of Lycopene alone or a capsule of Lycopene in combination with 40 mg of soy isoflavone twice daily for 6 months | Lycopene and combinatorial treatment with soy isoflavone did not reduce PSA level instead stabilized its level in the patient's serum. Both can delay the progression of hormone-refractory and hormone-sensitive PCabut did not have an additive effect. | Vaishampayan et al. (2007) |
| Lycopene | Phase I-II trial relapsed PCa patients | n = 36, Lycopene 15, 30, 45, 60, 90, and 120 mg/day for 1 year | Decreased serum PSA level with an increase in serum Lycopene level | Clark et al. (2006) |
| POLYPHENOLS FROM TEA | ||||
| Green tea, black tea | Randomized Phase II clinical trial | n = 113, 6 cups/day for 3–8 weeks before radical prostatectomy | Modulation of NF-κB in radical prostatectomy tissue, urinary 8- OHdG, and serum PSA levels were significantly decreased mainly in green tea, but not in the black tea group. | Henning et al. (2015) |
| Green tea | In men with clinically localized PCa | n = 17, 6 cups/day for 3–8 weeks | (−)-Epigallocatechin and (−)-Epicatechin were present in methylated form within prostatectomy tissue and this methylated EGCG may efficiently modulate its preventive effect on PCa. | Wang et al. (2010) |
| Polyphenon E (PolyE) | A placebo-controlled, randomized clinical trial in men with high-grade prostatic intraepithelial neoplasia (HGPIN) and/or atypical small acinar proliferation (ASAP) | n = 97, 400 mg EGCG for 1 year | Serum PSA level was decreased followed by accumulation of EGCG in plasma and was well tolerated in patients | Kumar et al. (2015) |
| Tea polyphenols polyphenon E (PolyE) | Short term supplementation study in PCa patients | n = 26, 800 mg EGCG, and lesser amounts of (−)-Epicatechin, (−)-Epigallocatechin, and (−)-Epicatechin-3-gallate (a total of 1.3 g of tea polyphenols) daily until radical prostatectomy | Decrease in serum PSA level, hepatocyte growth factor, and VEGF in PCa patients was observed with no increase in liver enzymes. | McLarty et al. (2009) |
| Polyphenon E | A randomized, double-blind, placebo-controlled trial in patients scheduled for radical prostatectomy. | n = 50, 800 mg EGCG or placebo daily for 3–6 weeks | Low accumulation of EGCG in the prostate tissue, favorable though insignificant changes in PSA, IGF, and oxidative DNA damage in blood leukocytes. | Nguyen et al. (2012) |
| POMEGRANATE JUICE | ||||
| Pomegranate juice | A phase II, Simon two-stage clinical trial for men with rising PSA after surgery or radiotherapy | n = 46, daily 8 ounces of pomegranate 570 mg total polyphenol gallic acid equivalents for | Elongation of PSA doubling time was observed in patients without any adversity along with a reduction in cell proliferation, increase in apoptosis, serum nitric oxide and reductions in oxidative state and sensitivity to oxidation of serum lipids in vitro LNCaP cells | Pantuck et al. (2006) |
| Pomegranate juice (POMx) | A randomized, multi-center, double-blind phase II study | n = 104, daily 1 or 3 g of POMx for 18 months | Prolongation of PSA doubling time (⩾6) months without adverse side effects. | Paller et al. (2013) |
| RESVERATROL AND GRAPES | ||||
| Resveratrol | A randomized placebo-controlled clinical study | n = 66, two doses of Resveratrol 150 mg or 1000 mg Resveratrol daily for 4 months. | Decreased (at higher dose) serum levels of the androgen precursors like androstenedione, DHEA, and DHEAS, while prostate volume and circulating levels of PSA, testosterone, free testosterone, and dihydrotestosterone were unchanged. | Kjær et al. (2015) |
| (MPX) Muscadine grape skin extract (Vitis rotundifolia) | A phase I/II Study in patients with recurrent PCa | n = 14, 500–4000 mg for 6.2–29.7 months | A higher dose of MPX is safe but serum PSA level were not reduced from baseline. | Paller et al. (2015) |
| MuscadinePlus (MPX), a commercial preparation of pulverized muscadine grape skin | A multicenter, placebo-controlled, two-dose, double-blinded trial in men with biochemically recurrent PCa | n = 125, 500 mg MPX (low), or 4000 mg MPX (high) daily or placebo for 12 months | No significant change in the PSA doubling time in the treated (two concentrations) versus the control group. MPX intake did not cause any adverse toxicity to the patients | Paller et al. (2018) |
| SOY ISOFLAVONES | ||||
| Genistein | A phase II placebo-controlled, randomized, double-blind clinical trial with patients before prostatectomy | n = 47, 30 mg Genistein or placebo capsules daily for 3–6 weeks. | A notable reduction in the mRNA level of androgen-related biomarker KLK4, but non-significant reduction in other PCa attributes like androgen and cell cycle. It changed the expression of several biomarkers related to PCa prediction and progression. | Lazarevic et al. (2012) |
| Soy isoflavone | A double-blinded, randomized, placebo-controlled trial | n = 86, soy isoflavone capsules (80 mg/d of total isoflavones, 51 mg/d aglucon units) for up to six weeks before scheduled prostatectomy | A short-term intervention did not change serum hormone levels, total cholesterol, or PSA but some genes related to cell cycle control and apoptosis were downregulated in the treated tumor tissue. | Hamilton-Reeves et al. (2013) |
| Isoflavone | A phase II, randomized, double-blind, placebo-controlled trial in men with rising PSA | n = 158, oral isoflavone (60 mg⁄ day) for 12 months | PSA levels did not change significantly but among all 53 patients aged ≥65 years, showed significantly less PCa incidence in the isoflavone group. | Miyanaga et al. (2012) |
| SULFORAPHANE & BROCCOLI | ||||
| Sulforaphane | A double-blinded, randomized, placebo-controlled multicenter trial in patients with increasing PSA after radical prostatectomy. | n = 78, daily oral administration of 60 mg of a stabilized free Sulforaphane for 6 months followed by 2 months without treatment (M6–M8). | Caused a significant reduction in log PSA slope and also prolonged the PSA doubling time by 86% compared to the placebo group. Sulforaphane was well-tolerated among patients. | Cipolla et al. (2015) |
| Sulforaphane-rich broccoli sprout extracts | A phase II study in patients with recurrent PCa | n = 20, 200 μmoles/day of Sulforaphane-rich extracts for a maximum period of 20 weeks | Appeared safe in the patient, prolonged on-treatment PSA doubling time but did not lead to ≥50% PSA declines in the majority of patients. | Alumkal et al. (2015) |
| Sulforaphane | A phase II single-arm study in patients with recurrent PCa | n = 20, 200 μmol of Sulforaphane extracts for up to 20 weeks per day | Lowered PSA level altogether, with an increase in PSA doubling time (6 months pre-study vs. 9.4 months on-study) and without any adverse side effects. It blocked HDAC function. | Alumkal et al. (2013) |
| Broccoli rich diet | A randomized open-label trial in patients with high-grade prostate intraepithelial neoplasia (HGPIN) | n = 22, 400 gm of broccoli/week and 400 gm peas/week for 1 year | Interacted with glutathione S-transferase mu 1 (GSTM1) and modulated signaling pathways such as TGF-β, Insulin signaling, EGF receptor associated with inflammation and carcinogenesis in the prostate. | Traka et al. (2008) |
Abbreviations: PSA, prostate-specific antigen; IGF1/3, insulin-like growth factor 1/3; EGCG, epi galloyl catechin 3 gallate; SOD, superoxide dismutase; CM-2, minichromosome maintenance protein complex; IGFBP-2/3, insulin-like growth factor binding proteins; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone-sulphate; KLK4, kallikrein 4; HDAC, histone deacetylases.
Fig. 1.
Mechanism of action of indicated phytochemicals on different cancer-promoting pathways. Abbreviations: AR, androgen recetor; PSA, prostate specific antigen; CYP17, cytochrome P450 17α-hydroxylase/17,20-lyase; GSK3β, glycogen synthase kinase 3 beta; Akt, protein kinase B; FOXO-3A, forkhead box O-3A; NKX3.1, NK3 homeobox 1; EGCG, epigalloyl catechin 3-gallate; Wnt, wingless-related integration site; SHH, sonic hedgehog; TGFβ, transforming growth factor beta; SMAD-4, mothers against decapentaplegic homolog- 4; PI3K, Phosphoinositide-3-kinase; mTOR, mammalian target of rapamycin; EGFR, epidermal growth factor receptor; IGF-1, insulin like growth factor-1; FGFR, fibroblast growth factor receptor; MYC, myelogenous cytoma; miRNA, micro ribonucleic acid; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; COX-2, cyclooxygenase-2; IL1/6/8/10, interleukin 1/6/8/10; TNF-α, tumor necrosis factor-α; Rb, retinoblastoma protein; MDM2, mouse double minute 2 homolog; CDK2/4/6, cyclin dependent kinase 2/4/6; MALAT1, metastasis associated lung adenocarcinoma transcript 1; HIF-1α, hypoxia-inducible factor 1-alpha; MMP2/9, matrix metalloproteinase-2/9; VEGF, vascular endothelial growth factor; SPOCK1, (SPARC (Osteonectin), Cwcv And Kazal Like Domains Proteoglycan 1); TIMP-3, tissue inhibitor of metalloproteinases-3; EGR-1, early growth response protein-1; E-cadh, E-cadherin, N-cadh, N-cadherin; ZO-1, zonula occludens protein 1; TWIST-1, TWIST related protein-1; ZEB-1, zinc finger E-box binding homeobox 1; MAO-A, monoamine oxidase-A; AMPK, AMP-activated protein kinase; PARP 1, Poly [ADP-ribose] polymerase 1; PDCD4, programmed cell death protein 4; PTEN, phosphatase and tensin homolog; Bcl2, B cell lymphoma-2; Bax, Bcl-2 associated X-protein; LC3B, microtubule-associated protein 1A/1B-light chain 3; RIPK3, Receptor-interacting serine/threonine-protein kinase 3; MAPK, mitogen-activated protein kinases; p-JNK, phospho-c-Jun N-terminal kinase; p-ERK1/2, extracellular signal-regulated kinase ½; ER stress, endoplasmic reticulam stress; ROS, reactive oxygen species.
Table 2.
List of phytochemicals showing therapeutic promise against PCa in preclinical model.
| Compound & chemical nature | Plant name and family | Mechanism of actions | In vitro | In vivo | Combination drug | References |
|---|---|---|---|---|---|---|
 Baicalein (Flavone) |
Scutellariabaicalensis (Lamiaceae) | Inhibited cell cycle regulatory proteins, G0/G1 arrest, CDK6, FOXM1, cyclin B, and Aurora B. Blocked cell proliferation through inducing apoptosis; reduced tumor overload in vivo. Blocked proliferation by inducing cell cycle arrest and apoptosis involving downregulating Ezrin expression. Downregulated cyclin D1, CDK4 while upregulating p53 and p21 levels. Decreased tumor overload in vivo by counteracting Ezrin function. It ameliorated benign prostate hyperplasia (BPH) through androgen-dependent apoptosis. Blocked prostate enlargement, production of DHT, and 5α-reductase activity type II in vivo. Increased Bax/Bcl-2 ratio, caspase-dependent apoptosis (Caspase 3, -8) and p-AMPK level, and reduced AR, PSA, PCNA, and Bcl-2 in vitro |
LNCaP, PC3 PC3 NA |
LNCaP xenograft PC3 xenograft Chemical (testosterone, DHT) induced BPH |
NA NA NA |
Yu et al. (2020) (Ma et al., 2020) (Jin and An, 2020) |
 Berberine (Isoquinoline alkaloid) Berberine (Isoquinoline alkaloid)
|
Berberis genus (Berberidaceae) |
Inhibits cell growth and induces apoptosis. It decreases transcriptional activity and signaling of AR induces AR protein degradation both in cells and tumor tissue. Metabolomics and network analyses suggested that berberine reversed the abnormality in metabolic biomarker expression by adjusting the perturbed metabolic pathways. Berberine inhibited p38 MAPK signaling to inhibit its downstream pro-oncogenic targets; inhibited tumor burden in vivo through inducing apoptosis. Inhibited benign prostatic hyperplasia induced by testosterone through inhibiting 5 alpha-reductase, AR, steroid coactivator-1 (SRC-1), and ERK signaling. Inhibited proliferation and growth in vitro and in vivo by inducing apoptosis, radio-sensitivity through inhibiting expression of VEGF and HIF1a. Acted in synergy with ionizing radiation. Inhibited tumor growth by facilitating p53 dependent and independent death and growth arrest at G0/G1 phase; enhanced p21 expression while decreasing cyclin D1, E and CDK 4, and -2 expression. |
LNCaP, 22Rv1, PC3, LAPC-4 C4–2B 22Rv1 RWPE-1 LNCaP, DU145 PC3, LNCaP, PWR1-E |
LNCaP grafted tumors 22Rv1 grafted nude mice Male Sprague–Dawley (SD) rats LNCaP grafted Balb/c nude mice PC3, LNCaP grafted Balb/c nude mice |
NA NA NA Ionizing radiation NA |
Li et al. (2011) (Li et al., 2017) (Youn et al., 2018) (Zhang et al., 2014) (Choi et al., 2009) |
 Betulinic Acid (BA) (Pentacyclic triterpene) |
Betula papyrifera (Betulaceae) |
Inhibited deubiquitinases to increase the levels of poly-ubiquitinated proteins and apoptosis in ex vivo and in vivo tumor model; lowered the levels of oncoproteins, cyclin D1 and AR protein. |
LNCaP, DU145, and PC3 |
TRAMP mice |
NA |
Reiner et al. (2013) |
 Capsaicin (Vanilloid type of compound) Capsaicin (Vanilloid type of compound)
|
Capsicum genus (Solanaceae) |
Oral administration increased the efficacy of radiotherapy and reduced the tumor burden; inhibited colony formation, NF-κB level, and DNA damage. Inhibited cell proliferation synergistically with docetaxel ex vivo and in vivo targeting PI3k/Akt/mTOR pathway. Overexpression of PTEN and inhibition of AMPK abrogated the functional synergy with docetaxel. Administration by oral route resulted in reduced metastatic potential, which was correlated with the reduction in p27/Kip1 expression and neuroendocrine differentiation in the tumor tissues; no toxicity to the liver or gastrointestinal tract was observed. Inhibited PC3 cell growth and proliferation both in vitro and in vivo. Antiproliferative effect on PCa cells was associated with increased p53, p21, and Bax level, and NFκB inhibition through suppression of IkBα degradation by blocking proteasome activity |
LNCaP, DU145, and PC3 LNCaP, PC3 PC3 PC3, DU145, LNCaP |
LNCaP grafted nu/nu athymic nude mice PC3 and LNCaP grafted athymic nude-Foxn1 (nu/nu) mice Transgenic adenocarcinoma of the mouse prostate (TRAMP) model. PC3 xenograft |
Increase radiosensitivity Synergizes docetaxel's activity in vitro and in vivo NA NA |
Venier et al. (2015a) (Sánchez et al., 2019) (Venier et al., 2015b) (Mori et al., 2006) |
 Delphinidin (Anthocyanin type of compound, specifically Anthocyanidin) Delphinidin (Anthocyanin type of compound, specifically Anthocyanidin)
|
Viola sp. (Violaceae)and Delphinium sp. (Ranunculaceae) |
Induced caspase-dependent apoptosis, G2/M arrest with reduction of p-IκB kinase γ; (NEMO), phosphorylation of NF-κB inhibitor y protein IκBα and phosphorylation of NF-κB/p65 at Ser536 and NF-κB/p50 at Ser529, and thus NF-κB/p65 nuclear translocation, and NF- κB DNA binding activity. Ameliorated tumor overload in vivo with a decrease in Bcl-2, Ki67, and PCNA levels. |
PC3, LNCaP, C4-2, 22Rv1 |
PC3 grafted nude mice |
NA |
Hafeez et al. (2008) |
 Fisetin (Flavonol) Fisetin (Flavonol)
|
Acacia greggii (Fabaceae) |
Reduced cell viability in vitro. Enhanced the ability of cabazitaxel to block cell proliferation through induction of apoptosis; reduced tumor growth, invasion, metastasis with reduction of PCNA, Ki67 levels while enhancing the level of Bax. Disrupted microtubule dynamics in PCa cells. Blocked PCa progression by inhibiting hyaluronan (HA) synthesis and degradation enzymes; increased the level of antiangiogenic high molecular mass hyaluronan (HMM-HA). Blocked tumor progression in TRAMP mice model and tumor xenograft studies. Blocked the expression and EGF-induced phosphorylation of Y-box binding protein-1 (YB-1) and markers of EMT (Vimentin, slug) while increasing E-cadherin to restrain EMT in vitro and in vivo. Prevented angiogenesis in vitro and in vivo by reducing VEGF endothelial NO synthase expression. Caused cell death by caspase-dependent apoptosis and increased Bax/Bcl-2 ratio and p53 level. Inhibited tube formation in HUVEC cells. Induced cell cycle arrest at G1 (strong) and G2/M (moderate) phases together with reducing cyclin D level. |
PrEC, 22Rv1, and C4-2, PC3M-luc-6, NCI/ADR-RES RWPE, NB11, NB26 RWPE-1, LNCaP, DU145, 22Rν1, PC-3, NB26 DU145, HUVEC |
22Rv1 and PC3 M-luc-6 grafted mice NB11 and NB26 grafted nude mice and TRAMP mice NB26 grafted nude mice Matrigel implanted Swiss albino mice |
Enhanced the efficacy of cabazitaxel NA NA NA |
Mukhtar et al. (2016) (Lall et al., 2016) (Khan et al., 2014) (Bhat et al., 2012) |
 Formononetin (Isoflavone derivative (O-methylated isoflavone)) Formononetin (Isoflavone derivative (O-methylated isoflavone))
|
Trifolium pretense (Fabaceae) | Inhibited cell proliferation by facilitating G1 arrest in PCa cells via downregulation of Akt/Cyclin D1/CDK4. Inhibited tumor overload in vivo. | PC3, DU145 | PC3 grafted nude mice | NA | Li et al. (2014) |
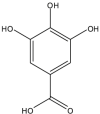 Gallic Acid (GA) (Polyphenolic compound) |
Vitis vinifera (Vitaceae) | Blocked PCa progression through inhibiting histone deacetylase 1, 2, and PCNA and upregulation of acetyl-p53. Enhanced intrinsic caspase-dependent apoptosis, DNA fragmentation with a decrease in mitochondrial membrane potential, and cyclin D1, -E1, and PCNA levels while upregulating p21/Waf1 level. Induced PCa cell death by apoptosis and reduced microvessel density and tumor overload in vivo. Inhibited tumor growth by apoptosis; reduced PCNA level, cell cycle markers cyclin B1, -E1, CDK2, -4, -6, and Cdc2 level. GA present in pomegranate peel extract reduced tumor burden in vivo accompanied by inhibition of TNF-α and VEGF expression. |
LNCaP, PC3 DU145, 22Rv1 NA PC3 |
PC3 grafted in BALB/c nu/nu male mice DU145, 22Rv1 grafted nude mice TRAMP mice PC3 xenograft |
NA NA NA NA |
Jang et al. (2020) (Kaur et al., 2009) (Raina et al., 2008) (Ma et al., 2015) |
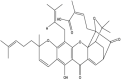 Gambogic Acid (Xanthone derivative) Gambogic Acid (Xanthone derivative)
|
Carciniahanburyi (Clusiaceae). |
Inhibited human umbilical vascular endothelial cell (HUVEC) proliferation, migration, invasion, tube formation, and microvessel growth at the nanomolar concentration in vitro. Reduced angiogenesis, VEGF-2, c-Src, focal adhesion kinase, and Akt level in vivo. |
PC3, HUVEC |
PC3 grafted SCID mice |
NA |
Yi et al. (2008) |
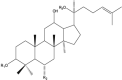 Ginsenosides (GN) (Triterpene saponin) |
Panax ginseng (Araliaceae). |
GN Rh2 blocked the growth of tumors developed with three different PCa cells by suppressing angiogenesis by inhibiting the expression of CNNM1, CD31, VEGF, and PDGF genes. GN-Rg3 showed synergistic activity with chemotherapeutics in killing PCa cells. Induced arrest in G0/G1 phase and apoptosis. Inhibited NF-κB, Bcl-2, IAP-1, XIAP, cyclin B, D1, E, and CDK 2, -4. Combination with docetaxel and cisplatin exhibited functional synergy in blocking PCa. GN-Rh2 blocked tumor growth and invasiveness. Activated TGF-β receptor signaling, regulators of cell cycle mediators, and MMPs. Downregulated cyclin D1, B1, MMP2/9 while increasing the level of pSMAD2 and p27. |
LNCaP, PC3, DU145 LNCaP, PC3, DU145 PC3 |
LNCaP, PC3, DU145 grafted nude mice NA PC3-luc xenograft |
NA Synergism with docetaxel and cisplatin NA |
Huang et al. (2019) (Kim et al., 2010) (Zhang et al., 2015) |
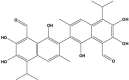 Gossypol (Polyphenolic) |
Gossypium hirsutum (Malvaceae) |
Blocked in vivo tumor proliferation by inducing DNA damage, p53, and apoptosis. Inhibited the expression of VEGF, Bcl-2, Bcl-xL, and tumor overload in vivo. Suppressed microvessel growth and sprouting in vitro and ex vivo. Inhibited p-Src (Tyr416), p-FAK (Tyr 397), p-Akt (S473), and p-ERK1/2 (Thr 202/Tyr 204) levels. Inhibited cell proliferation in vitro, and induced caspase-dependent apoptosis (Caspase3, -8), and blocks Bcl-2, PCNA, CD31 expression, and microvessel density in tumor tissue. Potentiated cell death in vitro and in vivo by apoptosis in combination with radiation; inhibited tumor angiogenesis. In combination, Sorafenib killed PCa cells more efficiently, involving apoptosis and autophagy in vitro and in vivo with reduced Bcl-2 level, Inhibited growth by apoptosis through mitochondrial pathway in PC3 cells and synergistically increased the anticancer activity of docetaxel both in vitro and in vivo. Blocked the expression of Bcl-xL while increasing the expression of Puma and Noxa. |
LAPC4, PC3, DU145 HUVEC, HMEC-1, PC3, DU145 PC3 PC3 PC3, DU145 PC3, DU145, LNCaP, WI38 |
DU145 xenograft in NOD/SCID mice PC3 xenograft and Sprague Dawley RAT PC3 xenograft PC3 grafted NCr-nu/nu nude mice PC3, DU145 xenograft PC3 grafted NCr-nu/nu nude |
NA NA NA Enhances the efficacy of radiotherapy Works synergistically with sorafenib Enhanced docetaxel efficacy in vitro and in vivo |
Volate et al. (2010) (Pang et al., 2011) (Zhang et al., 2010) (Xu et al., 2005) (Lian et al., 2012) (Meng et al., 2008) |
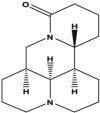 Matrine (Quinolizidine alkaloid) |
Sophora flavescens (Leguminosae) |
Blocked PCa cell growth, reversed EMT by activation of ER stress, and inhibition of unfolded protein response by inhibition of proteasomal chymotrypsin-like (CT-like) activity in the PCa cells in vitro and in vivo Blocked invasion through downregulating MMP2/9 and NF-κB signaling in vitro and in vivo |
DU145, PC3 DU145, PC3 |
DU145 xenograft DU145, PC3 xenografted nude mice |
NA NA |
Chang et al. (2018) (Huang et al., 2017) |
 Morelloflavone (Biflavonoid) |
Garcinia dulcis (Clusiaceae) | Prevented angiogenesis by reducing VEGF mediated microvessel formation and the RhoA and Rac1 GTPases. Blocked the phosphorylation and activation of Raf/MAPK/ERK pathway. Reduced tumor overload in vivo. | PC3, HUVEC | PC3 xenograft | NA | Pang et al. (2009) |
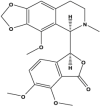 Noscapine (Benzylisoquinoline alkaloid) |
Papaver somniferum (Papaveraceae) |
Prophylactic feeding of Noscapine limited tumor growth and lymphatic metastasis. Enhanced the activity of paclitaxel and the Bax/Bcl-2 ratio. Reduced the mRNA levels of AR and PSA. |
PC3 LNCaP, PC3 |
PC3 xenografted athymic, Sim (NCr) nude mice NA |
NA Showed synergism with paclitaxel |
Barken et al. (2010) (Rabzia et al., 2017) |
 Silibinin (Flavolignan (Flavanoid unit linked to Phenyl Propane through an oxeran ring)) |
Silybum marianum (Asteraceae) | Hampered HIF-1α mediated signaling, angiogenesis, and lipogenesis in PCa cells. Reduced tumor load in vivo through inhibition of tumor vascularity; reduced the levels of HIF-1α, lipid, FASN, ACC, and NOX. . |
LNCaP, 22Rv1 | 22Rv1 xenograft | NA | (Deep et al., 2017) |
 Ursolic Acid (Pentacyclic triterpenoid) |
Cornus officinalis (Cornaceae) | Inhibited PI3K/Akt/mTOR pathway to induce apoptosis in vitro and in vivo | LNCaP, PC3 | LNCaP xenograft | NA | Meng et al. (2015) |
Abbreviations: Bax, Bcl2 associated protein X; Bcl2, B-cell leukemia/lymphoma 2; PI3K/Akt, phosphatidyl inositol-3-kinase/protein kinase B; EMT, epithelial to mesenchymal transition; ERK, extracellular signal regulatory protein kinase; NF-κB, nuclear factor kappa-light chain enhancer of activated B cells; CDKs, cyclin dependent kinases; AR, androgen receptor; PSA, prostate specific antigen; HIF-1α, hypoxia inducible factor-1 alpha; VEGF, vascular endothelial growth factor; STAT-3, signal transducer and activator of transcription 3; JNK, c-Jun-N-terminal kinase; PARP, poly (ADP-ribose) polymerase; AMPK, AMP-activated protein kinase; PTEN, phosphatase and tensin homolog; NKX3.1, NK3 Homeobox 1; PUMA, p53 upregulated modulator of apoptosis; NOXA, phorbol-12-myristate-13-acetate-induced protein 1; FOXO3a, forkhead box transcription factors 3a; TNF-α, tumor necrosis factor-α; MAPK, mitogen-activated protein kinase; Gn-Rh2, gonadotropin releasing hormone 2; CDKN1A, cyclin dependent kinase inhibitor 1A; Bak, Bcl-2 homologous antagonist/killer; Bad, Bcl-2 associated cell death; IκBα, I kappaB-alpha; PDK1, PIP3 dependent kinase 1; CRPC, castrate resistant prostate cancer; RhoA, ras homolog family member A; Rac1, ras-related C3 botulinum toxin substrate 1; PDEF, prostate epithelium-derived ETS transcription factor; Ki67, marker of proliferation Ki-67; PCNA, proliferating cell nuclear antigen; FOXM1, forkhead box M1; DHT, dihydrotestosterone; TRAMP, transgenic adenocarcinoma of mouse prostate; c-Src, phospho-proto-oncogene tyrosine-protein kinase c; phospho-focal adhesion kinase; Cdc2, cell division control 2; CD31, cluster of differentiation 31; FASN, fatty acid synthase; acetyl-CoA carboxylase; CNNM1, cyclin and CBS domain divalent metal cation transport mediator 1; IAP-1, inhibitor of apoptosis protein 1; XIAP, X-linked inhibitor of apoptosis protein; p-SMAD-2, phospho-mothers against decapentaplegic homolog 2; Raf, rapidly accelerated fibrosarcoma; VEGF-2, vascular endothelial growth factor-2.
Table 3.
Extracts of plants with anti-PCa activities.
| Plant name and plant part | Extract name | Mechanism of action | In vitro | In vivo | References |
|---|---|---|---|---|---|
| Leaves of Polyalthia longifolia | Methanolic extract (1) | Enhanced G1/S phase arrest, intrinsic apoptosis (Caspase3, -9), and ER stress (upregulated GRP78, ATF4, and IRE1α levels). XBP1, Calnexin, CDK4, -6, Cyclin A2, and Cyclin D1 were downregulated; reduced tumor overload in nude mice. | PC3, DU145, C4-2, PC3M-LUC-C6 | PC3M-LUC-C6 cell xenograft | Afolabi et al. (2019) |
| The root of Taraxacum officinale and Cymbopogon citratus | Aqueous extract of Taraxacum (Dandelion) (DRE) and Cymbopogon (lemongrass) ethanolic extract (LGE) (2) | Induced oxidative stress, and apoptosis via caspase cascade; reduced tumor overload alone and in synergy with taxol and mitoxantrone. | PC3, DU145 | DU145, PC3 xenograft | Nguyen (2019) |
| The seed extract of Litchi chinensis | n-butyl alcohol extract (3) | Induced mitochondrial-dependent apoptosis, G1/S phase arrest by blocking Akt signaling; Inhibited cell migration, invasion, and EMT through downregulation of AKT/GSK-3β signaling, Vimentin, and Snail while upregulating E-cadherin and β-catenin; ameliorated tumor overload in nude mice without toxicity to normal cells. | PC3, DU145, RM1, and C4–2B | PC3 xenograft | Guo et al. (2017) |
| Whole plant extract of Wedelia chinensis | Ethanolic extract (rich in flavonoids) (4) | Blocked the AR, HER2/3, and Akt signaling networks and increased the therapeutic efficacy of androgen ablation in PCa as well as in an in vivo adapted castration-resistant PCa (CRPC) model. | PC-3, DU145, 22Rv1, and LNCaP | LNCaP, 22Rv1 xenograft | Tsai et al. (2017b) |
| Leaves of Maytenus royleanus | Methanolic extract (5) | Potentiated caspase-mediated apoptosis, G2 arrest, and downregulation of cyclin/cdk networks; suppressed AR/PSA signaling ex vivo as well as in vivo; reduced tumor growth. | C4-2 and CWR22Rν1 | CWR22Rν1 xenograft | Shabbir et al. (2015) |
| Vegetative material of Sutherlandia frutescens | Methanolic extract (6) | Inhibited cellular growth via modulating Gli/Hh signaling; blocked the formation of poorly differentiated carcinoma in prostates of TRAMP mice. | PC3, LNCaP, TRAMP-C2 | Male B6FVB-F1 TRAMP mice | Lin et al. (2016) |
| Roots of Kalanchoe gastonis-bonnieri (BIRM) | Liquid extract (7) | Induced caspase 8 mediated apoptosis; degraded AR through the proteasome pathway; inhibited p-Akt and in vivo tumor growth. | DU145, LNCaP, PC-3ML | PC-3ML grafted in FOXN1 inbred athymic mice | Shamaladevi et al. (2016) |
| Acanthopanax trifoliatus | Extract rich in triterpenoids (8) | Potentiated cell death by apoptosis by suppressing NF-κB and STAT-3 in vitro and in vivo. | PC3 | PC3 xenograft | Li et al. (2015) |
| Leaves of Hibiscus sabdariffa | Polyphenol rich aqueous extract (9) | Induced cell death; blocked cell invasion via down-regulation of Akt/NF-κB/MMP-9 pathway in vitro and in vivo. | LNCaP | LNCaP xenograft | Chiu et al. (2015) |
| Roots of Eurycoma longifolia | Quassinoids rich containing methanolic extract (10) | Induced apoptosis; inhibited cell cycle arrest (G0/G1 and G2/M); modulated cyclin/cdk levels; blocked AR translocation to the nucleus and thereby PSA level; suppressed tumor growth in vivo. | LNCaP, PC-3, RWPE-1, WRL 68 | LNCaP xenograft | Tong et al. (2015) |
| Leaves and roots of Arum Palaestinum | Aqueous extract (11) | Inhibited PCa spheroid formation and tumor overload in vivo. | 22Rv1 | PC3-MM2 grafted nu/nu mice | Cole et al. (2015) |
| Knots of Pinus sylvestris | Lignan and stilbenoid rich ethanol-water extract (12) | Induced apoptotic death in vivo via enhancing TRAIL pathway at well-tolerated doses to the normal cells. | PC3M-luc2 | PC3M-luc2 grafted Foxn1nu nude mice | Yatkin et al. (2014) |
| Leaves of Azadirachta indica | Supercritical CO2 extract of leaves (13) | Inhibited dihydrotestosterone-induced androgen receptor and PSA levels; blocked Integrin b1, Calreticulin, and Focal adhesion kinase activation; ameliorated tumor growth and enhanced AKR1C2 level in vivo. | LNCaP-luc2, PC3 | LNCaP-luc2 xenograft | Wu et al. (2014) |
| Leaves of Piper betel | Methanolic extract (14) | Induced apoptosis in cells in vitro and in vivo tumor model. | PC3, DU145, 22Rv1,RWPE-1, PC3-luc cells | PC3-luc xenograft | Paranjpe et al. (2013) |
| Leaves of Ipomoea batatus | Polyphenol (Quinic acid (QA), caffeic acid, its ester clonogenic acid, and isochlorogenic acids, 4, 5-di-CQA, 3,5-di-CQA, and 3,4-di-CQA) rich methanolic extract (15) | Inhibited growth, proliferation of the cell, and progression of tumor xenograft. | PC3, PC3-luc cells | PC3-luc xenograft | Gundala et al. (2013) |
| Whole Zingiber officinale | Methanolic extract (16) | Induced cell cycle arrest and mitochondria-dependent apoptosis in vitro; reduced tumor burden and induced apoptosis in vivo. | PrEC, LNCaP, C4-2, C4–2B, DU145 and PC3 | PC3 xenograft | Karna et al. (2012) |
| Leaves of Hibiscus sabdariffa | Aqueous extract (17) | Induced apoptosis by intrinsic and extrinsic pathways in androgen-dependent PCa cells; reduced tumor burden in vivo. | Human CaP, LNCaP, PC3 and DU145 | LNCaP xenograft | Lin et al. (2012) |
| Leaves of Ipomoea batatus | Polyphenol rich methanolic extract (18) | Induced cell cycle arrests, and mitochondria-dependent apoptosis in vitro; reduced tumor burden via apoptotic death in tumor cells. | LNCaP, DU145, PC3, C4-2, C4–2B, PC3-luc | PC3-luc xenograft | Karna et al. (2011) |
| Leaves of Camellia ptilophylla | Aqueous extract (19) | Induced cell cycle arrest and caspase-mediated apoptosis; inhibited nuclear translocation and phosphorylation of NF-κB, and activation of IKKα, while inhibition of phosphorylation and degradation of IκBα and regressed tumor growth in vivo. | PC3 | PC3 xenograft | Peng et al. (2010) |
| Aerial parts of Saussurea involucrata | Different solvent extracts (20) | Ethyl acetate fraction blocked the proliferation of PCa cells, caused G1 arrest, modulated cell cycle markers, and induced mitochondria-dependent apoptosis; lowered the phosphorylation of EGFR, activation of Akt and STAT3 activity in vitro, and tumor growth in vivo. | PC3 and LNCaP | PC3 grafted BALB/c nude mice | Way et al. (2010) |
| Rhizome of Bergenia ligulata | Polyphenol rich ethyl acetate fraction (21) | Induced death of PC3 cells ex vivo and in vivo by oxidative stress-mediated by overactivation of MAO-A | PC3 | PC3 grafted SCID mice | Ghosh (2021) |
Abbreviations used in the table: ATF4, activating transcription factor; IRE1α, serine/threonine-protein kinase/endoribonuclease inositol-requiring enzyme 1 α; XBP1, X-Box binding protein 1; TRAIL, TNF-related apoptosis-inducing ligand; GRP78, glucose regulated protein 78; ER stress, endoplasmic reticulum stress; p-GSK3β, phospho-glycogen synthase kinase 3 beta; STAT3, signal transducer and activator of transcription 3; HER2/3, human epidermal growth factor receptor 2/3; AKR1C2, aldo-keto reductase family 1 member C2; IKKα, inhibitory κβ kinase α; IKBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; EGFR, epidermal growth factor receptor.
Fig. 2.
Chemical structures of indicated phytochemicals with demonstrated therapeutic promise against PCa in preclinical models.
3.1. Apigenin
Apigenin is a dietary flavonoid present in Anthemis sp. (Asteraceae) and many other plants with various medicinal properties such as antioxidant, anti-inflammatory, antiviral, neuroprotective, anticancer, and anti-metastatic properties with negligible toxicity to normal cells. It showed anticancer activity against different cancers, including PCa. It inhibited SPOCK1 (SPARC (Osteonectin), Cwcv And Kazal Like Domains Proteoglycan 1) expression and thus SPOCK1-induced expression of mesenchymal markers in different PCa cells, and there by PC3 induced xenograft tumor growth, proliferation, invasion, and metastasis (Chien et al., 2019). It inhibited p-Akt (S473), p-GSK3β, and p-BAD activities, expression, and autophosphorylation of IGFR and induced apoptotic cell death in vitro and in vivo (Kaur et al., 2008). Apigenin reduced PCa tumor growth and metastasis via targeting PI3K/Akt/FOXO signaling pathway. It was shown to induce cell cycle arrest at G0/G1 phase, to upregulate BIM and p27/Kip1 levels along with inhibition of cMyc expression and β-catenin signaling (Shukla et al., 2014; Shukla and Gupta, 2008). Apigenin inhibited PCa cell growth through modulating the cell cycle via increased expression of Waf1/p21, Kip1/p27, INK4a/p16, and INK4c/p18, and downregulation of cyclins D1, D2, E and Cdk2, Cdk4, and Cdk6, and Retinoblastoma protein phosphorylation (S780). It enhanced the binding of cyclin D1 to Waf1/p21 and Kip1/p27 by reducing the binding of cyclin E with Cdk2 in both 22Rv1, PC3 cell-induced xenograft tumor. Apigenin helped stabilize p53 activity by phosphorylation at serine 15 in 22Rv1 cell induced tumors (Shukla and Gupta, 2006), and ROS generation and p53-dependent apoptosis in vitro and in vivo, and potentiated p14ARF-mediated downregulation of MDM2 protein accompanied by inhibition of NF-κB/p65 function (Shukla and Gupta, 2008).
Apigenin blocked cell proliferation in vitro and in vivo, modulated IGF/IGF-IR signaling via upregulating IGFBP-3 and p27/Kip1, and inhibiting p-Akt, p-GSK3β, cyclin D1 (Shukla and Gupta, 2009). Apigenin blocked tumor growth by inducing cell cycle arrest and apoptosis through inhibiting class I HDACs (HDAC1, and -3) activities. It promoted acetylation of H3 and H4 and hyperacetylation of H3 on p21/Waf1 promoter; modulated Bax/Bcl-2 ratio, and increased p21/Waf1 expression in the tumor tissue (Pandey et al., 2012). Apigenin inhibited angiogenesis in tumor tissues by decreasing HIF1-α and VEGF expression (Fang et al., 2007).
3.2. Curcumin
Curcumin, a polyphenolic compound isolated from Curcuma longa, has been shown to exhibit many pharmacological properties such as anticancer, anti-inflammatory, antioxidant, neuroprotective, and radioprotective activities (Amalraj et al., 2017). Curcumin exerts its effect by targeting multiple signaling pathways such as AR signaling, NF-κB-, AP-1-, PI3k/Akt/mTOR-, and Wnt/β-catenin/TGF-β/MYC pathways (Abd Wahab et al., 2020; Katta et al., 2019). Because of the modulation of different signaling pathways simultaneously, it is not surprising that Curcumin acts as a potent inhibitor of PCa cells. This curcuminoid promotes apoptosis in LNCaP and PC3 cells by suppressing the epidermal growth factor receptor (EGFR) tyrosine kinase activity (Dorai et al., 2000). In DU145 cells, Curcumin could induce apoptosis by downregulating Bcl2 and upregulating caspase-3 and 8 via TNF activation and inhibition of NF-κB, protein kinase D (PKD), and β-catenin (Mukhopadhyay et al., 2001; Sundram, 2012). Curcumin was reported to induce NRF2 expression and the cellular antioxidant mechanism by restoring the hypomethylation status of CpG islands on the NRF2 promoter in TRAMP cell (Khor et al., 2011). It showed anti-PCa activity in an AR-dependent way by preventing the Wnt/β-catenin pathway along with inducing cell cycle arrest at the G2 phase and autophagy (Teiten et al., 2011). Curcumin kills PCa cells by inhibiting glyoxalases, increasing methylglyoxal levels, and decreasing cellular ATP and Glutathione (GSH) levels (Santel, 2008). Furthermore, in a PC3-xenograft model combination of Curcumin with α-tomatine inhibited tumor growth (Huang, 2015). Curcumin was found to inhibit cancer-associated fibroblast formation (CAFs), which mediates tumorigenesis, invasion, metastasis, and EMT in PCa. It ameliorates CAFs associated with ROS generation, IL-6, CXC chemokine receptor-4 (CXCR-4) receptor expression in PC3 cells through downregulating MAO-A/mTOR/HIF-1α signaling (Du et al., 2015). Analogs of Curcumin inhibited testosterone and DHT-induced AR activity (Schmidt and Figg, 2016).
In a recent study, Curcumin-loaded lipid nanoparticles have shown significant cytotoxicity in docetaxel-resistant PC3 and DU145 cells (Tanaudommongkon et al., 2020). Lipid polymer hybrid nanoparticles (aptamer-PLGA-PEG) of Curcumin and cabazitaxel have shown enhanced drug delivery, cytotoxicity towards PCa cells in vitro, and in vivo (Chen et al., 2020). Treatment PCa cells with a combination of Curcumin and metformin resulted in enhanced cytotoxicity and apoptosis (Eslami et al., 2020).
3.3. Epigallocatechin-3-gallate
Epigallocatechin-3-Gallate (EGCG) is a major polyphenolic compound present in green tea Camellia sinensis. EGCG carries various pharmacological properties, including anticancer, antioxidant, antidiabetic, cardioprotective, and neuroprotective activities (Chu et al., 2017). EGCG induces apoptosis in PC3 cells when treated alone and in combination with cisplatin, which works through activation of caspase 9 (Hagen et al., 2013). EGCG regresses PCa in TRAMP mice through suppressing AR, IGF-1, and its receptor insulin-like growth factor receptor-1 (IGF-R1) (Harper et al., 2007). From earlier reports, EGCG was shown to induce p53 independent apoptosis in both androgen-dependent (LNCaP) and androgen-independent (DU-145) cells (Gupta et al., 2003). It was reported that EGCG induced cell cycle arrest and apoptosis in LNCaP and DU-145 cells through modulating cyclin kinase inhibitor and cyclin-dependent kinase activities (Gupta et al., 2003). EGCG promoted apoptosis in human PCa cells through activation of p53 and inhibition of NF-κB by increasing the ratio of Bax/Bcl-2 (Hastak et al., 2003). Besides, EGCG inhibited R1881 induced nuclear localization of AR and its protein expression; it also decreased the androgen-regulated miRNA-21 and elevated the tumor suppressor miRNA-330 in the PCa xenograft model (Siddiqui et al., 2011). EGCG with doxorubicin reduced tumor growth significantly in CB17 SCID mice generated by metastatic PC-3M cells (Stearns et al., 2010). Green tea rich in EGCG combined with quercetin reduced tumor growth induced by androgen-sensitive LAPC-4 cells in the SCID mouse model (Wang et al., 2014a). EGCG reduced testosterone-induced benign prostate hyperplasia and fibrosis in rats through lowering oxidative stress, inflammation, collagen deposition, and angiogenetic growth factors. It also blocks the overexpression of HIF-1α, TGF-β1, TGF-βRI, AR, ER-α, and p-Smad3 while increasing the levels of ER-β and miR-133a/b (Zhou et al., 2018a). EGCG induced apoptosis in PCa cells and patient tissue samples through activating miR-520a-3p mediated Akt1 downregulation (Zhang et al., 2020a). Vasculogenic mimicry (VM), a process adapted by advanced PCa to forming vessels like networks needed for prostate tumorigenesis and aggressiveness, is restrained by EGCG treatment in PC3 cells through inhibition of vascular endothelial cadherin (VE-cadherin), N-cadherin, twist, vimentin, p-Akt, and Akt (Yeo, 2020). EGCG mediated dysregulation of cellular calcium homeostasis led to apoptotic death in PCa cells (Marchetti et al., 2020).
3.4. Ellagic acid
Ellagic acid is a polyphenolic compound found in different fruits and vegetables, like in Rubus sp. (Rosaceae), Vaccinium sp. (Ericaceae), known for its antioxidant and anticancer properties. It increased microtubule assembly but reduces tubulin polymerization by cabazitaxel. Ellagic acid inhibited drug efflux in castration-resistant PCa/22Rv1 cells but did not change the efficacy of docetaxel in vivo (Eskra et al., 2019). It blocked tumor progression by activating caspase 3 mediated apoptosis in a TRAM tumor model, modulated Bax/Bcl2 ratio, caspase 3 level. It decreased LNCaP cell growth, increased cell cycle-related proteins, p21Waf, P27Kip, cdk2, and cyclin E accompanied by downregulation of cyclin D1 and Cdk1expression levels in Sprague-Dawley (SD) rats (Naiki-Ito et al., 2015). Combined with luteolin and punicic acid, Ellagic acid inhibited tumor growth, metastasis, and angiogenesis in PCa cell induced xenograft and allograft tumors. Ellagic acid was shown to reduce CXCR-4, p-Akt/PI3K activities and block tube formation, IL-8, VEGF, and angiogenesis in HMVEC cells (Wang et al., 2014b).
3.5. Genistein
Genistein, an isoflavone, is isolated from leguminous plants (family Leguminosae) such as soybean (Glycine max), broad bean (Vicia faba), and chickpea (Cicer arietinum). The anti-inflammatory properties of Genistein are well known (Ghosh et al., 2016). Numerous studies revealed the cytotoxic potential of Genistein on multiple cancers, including PCa, which functions through modulating AR, PI3K/Akt, NF-κB, Hh, and Wnt signaling pathways. Genistein was shown to suppress different oncogenic miRNA expression, bone metastasis, and stemness in PCa (Fontana et al., 2020). It suppressed proliferation and metastasis in PC3 cells by inhibiting the expression of p38MAPK and MMP2 and inducing apoptosis through activating caspase 3 (Shafiee et al., 2020). A nanoliposome formulation of Genistein and anti-inflammatory drug Celecoxib induced ROS mediated apoptotic death in PC3 and LNCaP cells sparing the normal fibroblasts mainly through downregulation of cellular GSH and Prx-6 level along with inhibition of expression of COX-2 synthesis and Glut-1 transporters (Tian et al., 2019). A nanoliposome formulation of Genistein and Plumbagin was shown to induce apoptotic death in PC3 and LNCaP cells and in in vivo mouse model through inhibiting PI3K/Akt signaling and Glut-1 transporters (Song et al., 2020).
Genistein combined polysaccharide (GCP) impedes intracrine androgen synthesis by downregulating testosterone levels by ⁓3 fold and reducing the PSA level. It blocked the synthesis of 3βHSD, 17βHSD, StAR, SRB1, and CYP17A enzymes involved in the intracrine synthesis of AR while hindering cell growth, proliferation in LNCaP, and PC346C CaP cells through inducing apoptosis (Batra et al., 2020). Genistein elevates the effect of a shallow dose of irradiation (20 mGy/h) in DU145 cells to induce apoptotic cell death more efficiently (Altaf et al., 2019). A recent study with Genistein showed that it hindered the pathways involved in the transformation of a simple form of PCa to a highly lethal motility phenotype and long term use of soy product; Genistein can instigate compensatory changes in the response of biomarkers involved in cell motility (MEK4, MMP2) in human PCa cells (Zhang, 2019). In a clinical trial, Genistein pre-treatment showed differential DNA methylation and gene expression in PCa patients compared to placebo-treated ones. Overall, Genistein treatment resulted in reduced expression of MYC activity with enhanced PTEN function (Bilir et al., 2017).
3.6. Lycopene
Lycopene is a carotenoid pigment available abundantly in many fruits and vegetables such as, in tomato Lycopersicon esculentum or Solanum lycopersicum. Lycopene, through its antioxidant potentials, lowers the risk of cardiovascular diseases and skin damage (Chapter 11 - Lycopene: A, 2013). Lycopene was reported to carry chemopreventive and induce PCacell death by the mitochondrial apoptotic pathway (Gioti and Tenta, 2015; Konijeti et al., 2010; Goo et al., 2007). Lycopene was shown to inhibit different cancer cells, including PCa, by inhibiting HMG-CoA reductase and Ras function. The apoptotic cell death was also accompanied by induction of cell cycle arrest and inactivation of NF-κB and JNK and Akt (Palozza et al., 2010; Ivanov et al., 2007). Lycopene also inhibited the migration and invasion of PCa cells by inhibiting integrins (Gioti and Tenta, 2015). It was reported to induce apoptosis by decreasing Akt expression while increasing miR let-7f1 expression (microRNA Lethal-7) (Mirahmadi et al., 2020). Clinical trials revealed that Lycopene as a chemopreventive agent could reduce PCa malignancy from 24% to 36% by avoiding conversion of high-grade prostate intraepithelial neoplasia to PCa (Mohanty et al., 2005). Indonesian patient-derived PCa cells (Gleason score 6) were shown to be sensitized by Lycopene through reducing IGF-1 level (Tjahjodjati et al., 2020). Lycopene was reported to show anti-inflammatory activities by inhibiting IL1, -6, -8, and TNF-α in DU145, PC3, and LNCaP cells and tumor burden mice model (Jiang et al., 2019). It was reported to downregulate AR metabolism and signaling in in vitro and animal model of PCa (Applegate et al., 2019).
3.7. Piperine
Piperine is an alkaloid abundantly available in the Piperaceae family, specifically in species Piper nigrum commonly known as black pepper, used as a spice. Piperine shows many bioactivities like anxiolytic, antiaggregant, antioxidant, antispasmodic, antidepressant, antiasthmatic, and anticancer (Shityakov et al., 2019). Piperine induces cell death in a dose-dependent manner in PCa cells, PC-3 and LNCaP, through induction of apoptosis via obstructing voltage-gated K+ current (IK) and cell cycle arrest (Ba and Malhotra, 2018; George et al., 2019). It has also been reported that Piperine inhibited the migration and induced apoptotic cell death in DU145 cells through the downregulation of phospho-Akt/phospho-mTOR/MMP-9 (Zeng and Yang, 2018). Reports suggested that Piperine-mediated suppression of CYP3A4 activity helps enhance the docetaxel anticancer potency in the human CRPC xenograft mouse model when co-administered (Makhov et al., 2012). Piperine also induced apoptosis in LNCaP, PC3, and DU145 cells through enhancing the activation of caspase-3, PARP cleavage and inhibiting prostate PSA, STAT-3, and NF-κB (Samykutty, 2013). Piperine augmented docetaxel activity against taxane-resistant PCa tumors through the attenuation of cytochrome p450 (CYPs), especially CYP1B1, P-glycoprotein (P-gp) activity. CYPs are mainly involved in docetaxel's hepatic metabolism, and P-gp is a well-known multidrug-resistant protein involved in lowering docetaxel amount and effectivity inside the body (Li et al., 2018a).
3.8. Plumbagin
Plumbagin is a naphthoquinone isolated majorly from the roots of Plumbago rosea, an Indian medicinal plant (Anuf et al., 2014). Plumbagin shares many medicinal properties, such as antifertility, anticoagulant, antitumor, antimalarial, antimicrobial, and antidiabetic (Nayana et al. Mamta). Plumbagin was reported to exhibit selective inhibition of growth and invasion by promoting apoptosis via inhibiting Akt and NF-κB in hormone-refractory PCa DU145 but not in normal epithelial cells RWPE-1. Furthermore, it significantly slowed down DU145 induced xenograft in mice (Aziz et al., 2008). Plumbagin was also found to provoke apoptosis associated with ROS production and ER stress in DU145 and PC3 cells and the xenograft regression with minimal toxicity (Huang et al., 2018). Efficacy of ADT and subsequent survival of a mouse improved by the inclusion of Plumbagin in the treatment regimen (Abedinpour et al., 2017). Nanoemulsion formulation with Plumbagin showed potent antiproliferative activity towards PTEN-P2 PCa cells compared with Plumbagin alone (Chrastina et al., 2018). A recent report indicated that Plumbagin reduced the PTEN-P2 induced tumor growth only in castrated mice but failed in intact mice, and this revealed that dihydrotestosterone (DHT) was synthesized in testes mainly responsible for blocking prostate cell death (Rondeau et al., 2018).
3.9. Quercetin
Quercetin, a penta-hydroxylated flavonoid, is found in various fruits and vegetables, tea, berries, onions, apple, and tomatoes. This compound is endowed with multifarious medicinal properties such as anti-inflammatory, antioxidant, immunomodulatory, and anticancer properties. Quercetin impeded cell proliferation, invasion, migration, EMT, and in vivo tumor growth via downregulating IncRNA MALAT1, and inhibition of PI3K/Akt signaling (Lu et al., 2020a). It reversed docetaxel resistance through modulating AR and PI3K/Akt signaling in vitro and in vivo. Quercetin induced apoptosis and downregulated P-glycoprotein (P-gp), TWIST-1, and PSA level in vivo and enhanced docetaxel efficacy by reversing chemoresistance (Lu et al., 2020b).
With Resveratrol, Quercetin modulated promoter methylation, cell cycle, IGF1, Bcl-2, and PTEN signaling, and downregulated levels of EGFR, EGR3, and IL6, and upregulated IGFBP7 and NKX3.1 levels to reduce tumor overload by apoptosis (Singh et al., 2020a). Quercetin in combinatorial with metmorphin induced caspase-dependent apoptosis, and reduced invasion and Bcl-2, VEGF, and Akt/PI3K activities ex vivo and in vivo tumor model (Sun et al., 2018). It enhanced the therapeutic efficacy of paclitaxel, potentiated G2/M phase arrest, apoptosis, ER stress, produced ROS generation, and inhibited cell proliferation and migration in vitro and in vivo (Zhang et al., 2020b). Quercetin killed cells by inducing apoptosis by mitochondrial/ROS pathway accompanied by a reduction in the tyrosine-protein kinase-met (c-met) and PI3K/Akt pathway activities and could reverse doxorubicin resistance in vitro (Shu et al., 2018). Acted in synergy with next-generation anti-androgen enzalutamide in killing drug-resistant PCa cells by reducing expression of hnRNPA1, ARV7, AR, and AR–regulated genes PSA, NKX3.1, FKBP5, and UBE2C in vitro and in vivo (Tummala et al., 2017). Quercetin nanomicelles killed PCa cells in vitro and in vivo by apoptosis (Zhao et al., 2016).
Quercetin reduced tumor burden by inhibiting angiogenesis and metastasis through upregulating expression of anti-angiogenesis factor thrombospondin-1 (Yang et al., 2016). It inhibited the EGFR and PI3k/Akt pathway and the expression of PCNA, Vimentin, N-cadherin, cyclin D1, and mRNA level of snail, slug, and twist to ameliorate chemical (MNU + T) induced tumor overload in Sprague-Dawley male rats and inhibit (Firdous et al., 2014). Quercetin was also reported to block tumor growth by apoptosis through inhibition of proliferative activities of IGFIR, AR, and Akt (Sharmila et al., 2014).
3.10. Resveratrol
Resveratrol (RES) is a polyphenolic compound isolated from the dried stem of Vitis Vinifera (Tian and Liu, 2019; Park and Boo, 2013). The trans isomer of RES shows various biological activities such as antiinflammatory, antioxidant, antiaging, anticancer, immunomodulatory, neuroprotective, and cardioprotective activities. RES was shown to induce apoptotic death in LNCaP, DU145, and PC3 cells (Jasiński et al., 2013). RES induced increased apoptotic death in PCa cells in synergy with doxorubicin, taxol, methotrexate, cytarabine, actinomycin D via inhibiting survivin and promoting apoptosis (Gupta et al., 2011). It was shown to sensitize PC-3M-MM2 cells through the downregulation of Akt, ERK-1/2, estrogen, and IGF-1 and its receptor (IGF-1R) activities (Sheth, 2012). Furthermore, studies on Resveratrol reveal that it inhibits cyclooxygenase–2 (COX–2) and NF-κB in PCa cell lines and enhance apoptosis signals (Athar et al., 2009). Oral administration with Resveratrol reduced highly aggressive and androgen-receptor negative PCa cells PC-3M-MM2 and in SCID mouse through negative regulation of Akt and PCa associated microRNA such as miR21 and up-regulation of tumor suppressor PDCD4 levels (Sheth, 2012). RES inhibited PCa in vitro and in vivo model by inhibiting the antiapoptotic SphK1/S1P and prosurvival pathway, a downstream effector of the ERK1/2 pathway (Brizuela et al., 2010). RES tested clinically on benign prostate hyperplasia patients and found notably decreased androgens levels in serum without prostate tumor growth (Kjaer et al., 2015). RES, combined with Quercetin, reduced tumorigenesis in the TRAMP model by decreased oxidative stress and increasing apoptotic signals (Singh et al., 2020b). RES reduced PCa cell proliferation and apoptosis by inhibiting the expression of AR, AR-V7, and Akt pathway (Ye, 2020). RES blocked tumor invasion and migration through upregulating-eleven translocation-1 (TET1) along with TIMP2/3 levels and downregulating MMP2/9 and TNF-receptor associated factor 6 (TRAF6)/NF-κB/SLUG axis in PCa cells (Wang et al., 2020; Khusbu et al., 2020). Prostate fibrosis caused by inflammation was reduced by RES (Vicari et al., 2020). RES was shown to block hepatocyte growth factor (HGF) secretion by stromal cells, thus inhibiting invasion and EMT in PCa cells (Hsieh and Wu, 2020).
3.11. Sulforaphane
Sulforaphane, a natural isothiocyanate, is isolated from Brassica oleracea (family Brassicaceae). From ancient times numerous studies have revealed the efficacies of cruciferous vegetables in the amelioration of various ailments, including PCa. Sulforaphane (SFN) in these vegetables was shown to be majorly responsible for cytotoxicity towards PCa by inhibiting metastasis, invasion, and stemness via modulating AR, NF-κB, PI3k/Akt signaling pathway, energy metabolisms such as glycolysis, pentose phosphate pathway, and lipogenesis (Fontana et al., 2020). In a recent study, SFN treatment inhibited energy metabolism, i.e., enhanced glycolysis (Warburg effect) in PCa cells in-vitro and in vivo TRAMP and Hi-Myc mice model. SFN treatment blocked real-time extracellular acidification rate in LNCaP but not in PC3 cells, occluded the expression of hexokinase II (HKII), lactate dehydrogenase A (LDHA), and pyruvate kinase M2 (PKM2) involved in the glycolysis pathway in LNCaP and 22Rv1 cells (Singh et al., 2019). SFN treatment-induced lysosome-associated membrane protein-2 (LAMP-2) expression. RNAi of LAMP2 resulted in death in PC3 and 22Rv1 cells by autophagy (Hahm, 2020). A metabolite of SFN, Sulforaphane-cystein (SFN-Cys), and Sulforaphane-N-acetyl-cysteine (SFN-NAC) treatment caused apoptotic cell death via microtubule disruption and ERK1/2 activation in DU145 and PC3 cells with a longer half-life than SFN (Zhou et al., 2018b). SFN-Cys hindered invasion of DU145 and PC3 cells via downregulation of galectin-1 (helps in the invasion) mediated by ERK1/2 phosphorylation (Tian et al., 2016).
Inhibition of LNCaP and DU145 cells by SFN treatment was mediated through repression of activity of hTERT, the catalytic subunit of telomerase. This was mediated by changes in histone post-translational modifications linked with a higher risk of PCa recurrence, such as acetylation of histone H3 lysine 18 and di-methylation of histone H3 Lysine 4 in the regulatory elements within the hTERT promoter region. Moreover, SFN drives chromatin condensation through altered expression and recruitment of chromatin compactor MeCP2 resulting in hTERT repression (Abbas et al., 2016). SFN repressed the expression of ⁓100 long non-coding RNA, especially LINC0116 in PC3, LNCaP, and normal prostate epithelium (PREC) cells, which are known to mediate, cell cycle, signal transduction metabolism and are involved in PCa tumorigenesis (Beaver et al., 2017). A double-blind, randomized controlled clinical trial was conducted with 98 men scheduled for a biopsy. Patients who were given broccoli sprout extract (BSE, rich in SFN) or placebo showed a marked increase of SFN isothiocyanates and SFN level in plasma and urine along with the differential expression of 40 genes, including downregulation of AMACR and ARLNC1 involved in PCa development (Zhang et al., 2020c).
3.12. Triptolide
Triptolide, a diterpene, was isolated from Tripterygium wilfordii. Triptolide exhibits many potent pharmacological properties, such as an inhibitor of autoimmune, inflammatory, neurodegenerative, and cancer (Brinker et al., 2007). Triptolide induced apoptosis in LNCaP and PC-3 cells and inhibited tumor growth in the PC3 induced xenograft model in nude mice (Huang, 2012). It was shown to suppress invasion and migration of PCa cells through down-regulating the expression of caveolin-1, CD147, and MMPs (Yuan et al., 2016). Triptolide increases the sensitivity of metastatic castration-resistant PCa (mCRPC) cells and xenograft model towards the enzalutamide treatment via blocking AR binding and TFIIH and RNA polymerase II to its target promoter (Han et al., 2017).
Minnelide, a pro-drug of Triptolide pro-drug, reduced the tumor growth induced by CRPC 22Rv1 subcutaneously; the results revealed that minnelide had shown a significant tumor regression compared to Docetaxel and Enzalutamide via downregulating the expression of androgen receptor (Isharwal et al., 2017). Triptolide was shown to synergize doxorubicin activity in PC3 cells (Xu et al., 2018). Triptolide treatment caused AR protein degradation in LNCaP cells through intracellular Ca2+ accumulation and calpain activation (Li et al., 2018b). Benign prostate hyperplasia induced by testosterone propionate (TP) in the rat was regressed by Triptolide treatment (Yu-Rong et al., 2017).
3.13. Wedelolactone
Wedelolactone, a polyphenol, is isolated from Wedelia chinensis and Eclipta alba. Wedelolactone exhibited various pharmacological activities such as anticancer, antihypertensive, antihepatotoxic, anti-inflammatory, antiphospholipase, and antidote activity for snake poison (Xu et al., 2014). Wedelolactone showed a potent anticancer effect on PCa cells and tumor xenograft by inhibiting c-Myc at the transcription and translation level and promoting apoptosis. Moreover, Wedelolactone also showed functional synergy with enzalutamide in triggering apoptotic death in PCa cells (Sarveswaran et al., 2016). Wedelolactone treatment activated apoptosis in androgen-dependent and androgen-independent PCa cells by activating JNK and caspase 3 and suppressing PKCε but not Akt (Sarveswaran et al., 2012). Oral treatment of standardized herbal extract rich in Wedelolactone and other constituents like luteolin, and Apigenin inhibited tumor growth and metastasis in xenograft model of PC-3 and DU145. Also, it was shown that herbal extract in combination with docetaxel inhibited NF-κB activation with reduced general toxicity induced by docetaxel alone (Tsai et al., 2017a).
4. Conclusion
This review article with a brief overview of epidemiology and etiology of PCa gives a recent progress on anti-PCa activities of various phytochemicals and plant extracts reported from different laboratories. As it appeared significant insights had been achieved on the mode of actions of many of these agents/phytochemicals making them attractive for consideration for further development. Several of these agents target cancer hallmark pathways such as growth regulatory/metabolic (PI3/Akt/mTOR/cyclin-cdk, AR), angiogenesis (VEGF), pro-inflammatory (NF-kB), tumor suppressor (p53/Rb), and invasion and metastasis (WNT/β-catenin) pathways. In addition, some of these agents were shown to act in synergy with FDA-approved therapy regimens. Notably, many of the compounds were reported to simultaneously target multiple cellular pro-proliferative pathways making them, at least in principle, ideal candidates for consideration as alternate anticancer chemotherapeutic agents or for development in that direction given that a cancer cell is thrived by mutations/aberrations in multiple signaling pathways. Testing the immunomodulatory function of these agents is still lacking. Although, several compounds and extracts mentioned in the review were in different forms of human/clinical trials to carry forward the findings a step further for bedside application, progress in this direction is not much visible. Much needed progress will be achieved through active collaboration of basic scientist with practicing/clinical oncologists associated with the hospitals where a large number of eligible patients could be available for consideration for the trial. However, it should be noted that before taking up these steps, the candidate agents should be again undertaken for rigorous testing with healthy animals and PCa cell induced tumor xenograft. Anyway, many of the plant extracts described here are in many cases are promising sources for isolation of active principles to understand their potentials for further development.
CRediT authorship contribution statement
Suvranil Ghosh: Conceptualization, Data curation, Resources, Writing – original draft, Writing - review & editing. Joyita Hazra: Data curation, Resources, Writing – original draft. Koustav Pal: Data curation, Resources, Writing – original draft. Vinod K. Nelson: Data curation, Resources, Writing – original draft. Mahadeb Pal: Conceptualization, Project administration, Supervision, Validation, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work is supported by funding from SERB, DBT, and Bose Institute to MP. SG is a CSIR adhoc Senior research fellow. KP is an MBBS intern.
References
- Abbas A., et al. Sulforaphane modulates telomerase activity via epigenetic regulation in prostate cancer cell lines. Biochem. Cell. Biol. 2016;94(1):71–81. doi: 10.1139/bcb-2015-0038. [DOI] [PubMed] [Google Scholar]
- Abd Wahab N.A., et al. Mechanism of anti-cancer activity of curcumin on androgen-dependent and androgen-independent prostate cancer. Nutrients. 2020;12(3):679. doi: 10.3390/nu12030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedinpour P., et al. Plumbagin improves the efficacy of androgen deprivation therapy in prostate cancer: a pre-clinical study. Prostate. 2017;77(16):1550–1562. doi: 10.1002/pros.23428. [DOI] [PubMed] [Google Scholar]
- Abida W., et al. TRITON2: an international, multicenter, open-label, phase II study of the PARP inhibitor rucaparib in patients with metastatic castration-resistant prostate cancer (mCRPC) associated with homologous recombination deficiency (HRD) J. Clin. Oncol. 2018;36:TPS388. [Google Scholar]
- Adlercreutz H. Phyto-oestrogens and cancer. Lancet Oncol. 2002;3(6):364–373. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- Afolabi S.O., et al. Polyalthia longifolia extract triggers ER stress in prostate cancer cells concomitant with induction of apoptosis: insights from in vitro and in vivo studies. Oxidative Medicine and Cellular Longevity. 2019 doi: 10.1155/2019/6726312. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albero-González R., et al. Immunohistochemical expression of mismatch repair proteins (MSH2, MSH6, MLH1, and PMS2) in prostate cancer: correlation with grade groups (WHO 2016) and ERG and PTEN status. Virchows Arch. 2019;475(2):223–231. doi: 10.1007/s00428-019-02591-z. [DOI] [PubMed] [Google Scholar]
- Allott E.H., Masko E.M., Freedland S.J. Obesity and prostate cancer: weighing the evidence. Eur. Urol. 2013;63(5):800–809. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altaf R., Kumi-Diaka J., Branly R. Irradiation augmentation of genistein-induced apoptosis in androgen-independent DU-145 prostate cancer cells in vitro. Journal of Cancer Prevention and Current Research. 2019;10(1):4–11. [Google Scholar]
- Alumkal J.J., et al. Sulforaphane treatment in men with recurrent prostate cancer. American Society of Clinical Oncology. 2013;31(15) 5017–5017. [Google Scholar]
- Alumkal J.J., et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Invest. N. Drugs. 2015;33(2):480–489. doi: 10.1007/s10637-014-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalraj A., et al. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives - a review. Jornal of Traditional and Complementary Medicine. 2017;7(2):205–233. doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriole G.L., et al. Effect of dutasteride on the risk of prostate cancer. N. Engl. J. Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- Anuf A.R., et al. Antiproliferative effects of Plumbago rosea and its purified constituent plumbagin on SK-MEL 28 melanoma cell lines. Pharmacogn. Res. 2014;6(4):312–319. doi: 10.4103/0974-8490.138280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate C.C., Rowles J.L., Erdman J.W. Can lycopene impact the androgen axis in prostate cancer?: a systematic review of cell culture and animal studies. Nutrients. 2019;11(3):633. doi: 10.3390/nu11030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athar M., et al. Multiple molecular targets of resveratrol: anti-carcinogenic mechanisms. Arch. Biochem. Biophys. 2009;486(2):95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard G., et al. Prostate cancer. Lancet. 2016;387(10013):70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- Aziz M.H., Dreckschmidt N.E., Verma A.K. Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Canc. Res. 2008;68(21):9024–9032. doi: 10.1158/0008-5472.CAN-08-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba Y., Malhotra A. Potential of piperine in modulation of voltage-gated K+ current and its influences on cell cycle arrest and apoptosis in human prostate cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2018;22(24):8999–9011. doi: 10.26355/eurrev_201812_16671. [DOI] [PubMed] [Google Scholar]
- Banerjee S., et al. Microbiome signatures in prostate cancer. Carcinogenesis. 2019;40(6):749–764. doi: 10.1093/carcin/bgz008. [DOI] [PubMed] [Google Scholar]
- Barken I., Geller J., Rogosnitzky M. Prophylactic noscapine therapy inhibits human prostate cancer progression and metastasis in a mouse model. Anticancer Res. 2010;30(2):399–401. [PubMed] [Google Scholar]
- Batra N., et al. Genistein Combined Polysaccharide (GCP) can inhibit intracrine androgen synthesis in prostate cancer cells. Biomedicines. 2020;8(8):282. doi: 10.3390/biomedicines8080282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman T.M., et al. Expression and colocalization of β-catenin and lymphoid enhancing factor-1 in prostate cancer progression. Hum. Pathol. 2016;51:124–133. doi: 10.1016/j.humpath.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver L.M., et al. Long noncoding RNAs and sulforaphane: a target for chemoprevention and suppression of prostate cancer. J. Nutr. Biochem. 2017;42:72–83. doi: 10.1016/j.jnutbio.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran H., et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur. Urol. 2013;63(5):920–926. doi: 10.1016/j.eururo.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benafif S., Eeles R. Genetic predisposition to prostate cancer. Br. Med. Bull. 2016;120:75–89. doi: 10.1093/bmb/ldw039. [DOI] [PubMed] [Google Scholar]
- Bergthorsson J.T., et al. Analysis of HPC1, HPCX, and PCaP in Icelandic hereditary prostate cancer. Hum. Genet. 2000;107(4):372–375. doi: 10.1007/s004390000384. [DOI] [PubMed] [Google Scholar]
- Bhat T.A., et al. Fisetin inhibits various attributes of angiogenesis in vitro and in vivo--implications for angioprevention. Carcinogenesis. 2012;33(2):385–393. doi: 10.1093/carcin/bgr282. [DOI] [PubMed] [Google Scholar]
- Biernacka K.M., et al. Effect of green tea and lycopene on the insulin-like growth factor system: the ProDiet randomized controlled trial. Eur. J. Canc. Prev. 2019;28(6):569. doi: 10.1097/CEJ.0000000000000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilir B., et al. Effects of genistein supplementation on genome-wide DNA methylation and gene expression in patients with localized prostate cancer. Int. J. Oncol. 2017;51(1):223–234. doi: 10.3892/ijo.2017.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosland M.C. Chapter 2: the role of steroid hormones in prostate carcinogenesis. JNCI Monographs. 2000;2000(27):39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]
- Bowen C., Gelmann E.P. NKX3. 1 activates cellular response to DNA damage. Canc. Res. 2010;70(8):3089–3097. doi: 10.1158/0008-5472.CAN-09-3138. [DOI] [PubMed] [Google Scholar]
- Bowen P., et al. Tomato sauce supplementation and prostate cancer: lycopene accumulation and modulation of biomarkers of carcinogenesis. Exp. Biol. Med. 2002;227(10):886–893. doi: 10.1177/153537020222701008. [DOI] [PubMed] [Google Scholar]
- Bray F., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Brinker A.M., et al. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae) Phytochemistry. 2007;68(6):732–766. doi: 10.1016/j.phytochem.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizuela L., et al. The sphingosine kinase-1 survival pathway is a molecular target for the tumor-suppressive tea and wine polyphenols in prostate cancer. FASEB (Fed. Am. Soc. Exp. Biol.) J. 2010;24(10):3882–3894. doi: 10.1096/fj.10-160838. [DOI] [PubMed] [Google Scholar]
- Bunker C.H., et al. A randomized trial of lycopene supplementation in Tobago men with high prostate cancer risk. Nutr. Canc. 2007;57(2):130–137. doi: 10.1080/01635580701274046. [DOI] [PubMed] [Google Scholar]
- Buschemeyer W.C., III, Freedland S.J. Obesity and prostate cancer: epidemiology and clinical implications. Eur. Urol. 2007;52(2):331–343. doi: 10.1016/j.eururo.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Calcinotto A., et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559(7714):363–369. doi: 10.1038/s41586-018-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle E.E., et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N. Engl. J. Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Carelle N., et al. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer. 2002;95(1):155–163. doi: 10.1002/cncr.10630. [DOI] [PubMed] [Google Scholar]
- Castro E., Eeles R. The role of BRCA1 and BRCA2 in prostate cancer. Asian J. Androl. 2012;14(3):409. doi: 10.1038/aja.2011.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.M., Giovannucci E.L. Dairy products, calcium, and vitamin D and risk of prostate cancer. Epidemiol. Rev. 2001;23(1):87–92. doi: 10.1093/oxfordjournals.epirev.a000800. [DOI] [PubMed] [Google Scholar]
- Chan J.M., Stampfer M.J., Giovannucci E.L. What causes prostate cancer? A brief summary of the epidemiology. Semin. Canc. Biol. 1998;8(4):263–273. doi: 10.1006/scbi.1998.0075. [DOI] [PubMed] [Google Scholar]
- Chan J.M., et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279(5350):563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- Chang J., et al. Matrine inhibits prostate cancer via activation of the unfolded protein response/endoplasmic reticulum stress signaling and reversal of epithelial to mesenchymal transition. Mol. Med. Rep. 2018;18(1):945–957. doi: 10.3892/mmr.2018.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapter 11 - lycopene: A review of chemical and biological activity related to beneficial health effects. Stud. Nat. Prod. Chem. 2013;40:383–426. [Google Scholar]
- Chen G., et al. Up-regulation of Wnt-1 and β-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2004;101(6):1345–1356. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- Chen Y., et al. Anti prostate cancer therapy: aptamer-functionalized, curcumin and cabazitaxel co-delivered, tumor targeted lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2020;127:110181. doi: 10.1016/j.biopha.2020.110181. [DOI] [PubMed] [Google Scholar]
- Cheng I., et al. 8q24 and prostate cancer: association with advanced disease and meta-analysis. Eur. J. Hum. Genet. 2008;16(4):496–505. doi: 10.1038/sj.ejhg.5201959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesire D.R., et al. Detection and analysis of β-catenin mutations in prostate cancer. Prostate. 2000;45(4):323–334. doi: 10.1002/1097-0045(20001201)45:4<323::aid-pros7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Chien M.-H., et al. Targeting the SPOCK1-snail/slug axis-mediated epithelial-to-mesenchymal transition by apigenin contributes to repression of prostate cancer metastasis. J. Exp. Clin. Canc. Res. 2019;38(1):1–17. doi: 10.1186/s13046-019-1247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C.-T., et al. Hibiscus sabdariffa leaf extract inhibits human prostate cancer cell invasion via down-regulation of Akt/NF-kB/MMP-9 pathway. Nutrients. 2015;7(7):5065–5087. doi: 10.3390/nu7075065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M.S., et al. Berberine inhibits p53-dependent cell growth through induction of apoptosis of prostate cancer cells. Int. J. Oncol. 2009;34(5):1221–1230. [PubMed] [Google Scholar]
- Choi Y.H., et al. A randomized, double-blind, placebo-controlled trial to evaluate the role of curcumin in prostate cancer patients with intermittent androgen deprivation. Prostate. 2019;79(6):614–621. doi: 10.1002/pros.23766. [DOI] [PubMed] [Google Scholar]
- Chow P.H., Gandhi M. Phase III multi-centre open-label randomized controlled trial of selective internal radiation therapy (SIRT) versus sorafenib in locally advanced hepatocellular carcinoma: the SIRveNIB study. American Society of Clinical Oncology: Asia-Paciifc Hepatocellular Carcinoma Trials Group. 2017;36(19):1913–1921. [Google Scholar]
- Chrastina A., et al. Plumbagin-loaded nanoemulsion drug delivery formulation and evaluation of antiproliferative effect on prostate cancer cells. BioMed Res. Int. 2018;2018:9035452. doi: 10.1155/2018/9035452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., et al. Green tea extracts epigallocatechin-3-gallate for different treatments. BioMed Res. Int. 2017;2017:5615647. doi: 10.1155/2017/5615647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla B.G., et al. Effect of sulforaphane in men with biochemical recurrence after radical prostatectomy. Canc. Prev. Res. 2015;8(8):712–719. doi: 10.1158/1940-6207.CAPR-14-0459. [DOI] [PubMed] [Google Scholar]
- Clark P.E., et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology. 2006;67(6):1257–1261. doi: 10.1016/j.urology.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Cole C., et al. Arum Palaestinum with isovanillin, linolenic acid and β-sitosterol inhibits prostate cancer spheroids and reduces the growth rate of prostate tumors in mice. BMC Compl. Alternative Med. 2015;15(1):1–8. doi: 10.1186/s12906-015-0774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta. 2013;1830(6):3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono J., et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020;382(22):2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- de Gonzalez A.B., et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int. J. Radiat. Oncol. Biol. Phys. 2013;86(2):224–233. doi: 10.1016/j.ijrobp.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marzo A.M., et al. Inflammation in prostate carcinogenesis. Nat. Rev. Canc. 2007;7(4):256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Velasco G., et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunology Research. 2017;5(4):312–318. doi: 10.1158/2326-6066.CIR-16-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deep G., et al. Silibinin inhibits hypoxia-induced HIF-1α-mediated signaling, angiogenesis and lipogenesis in prostate cancer cells: in vitro evidence and in vivo functional imaging and metabolomics. Mol. Carcinog. 2017;56(3):833–848. doi: 10.1002/mc.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorai T., Gehani N., Katz A. Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein. Mol. Urol. 2000;4(1):1–6. [PubMed] [Google Scholar]
- Du Y., et al. Curcumin inhibits cancer-associated fibroblast-driven prostate cancer invasion through MAOA/mTOR/HIF-1α signaling. Int. J. Oncol. 2015;47(6):2064–2072. doi: 10.3892/ijo.2015.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskra J.N., Schlicht M.J., Bosland M.C. Effects of black raspberries and their ellagic acid and anthocyanin constituents on taxane chemotherapy of castration-resistant prostate cancer cells. Sci. Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-39589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslami S.S., et al. Combination of curcumin and metformin inhibits cell growth and induces apoptosis without affecting the cell cycle in LNCaP prostate cancer cell line. Nutr. Canc. 2020:1–14. doi: 10.1080/01635581.2020.1783327. [DOI] [PubMed] [Google Scholar]
- Fang J., et al. Apigenin inhibits tumor angiogenesis through decreasing HIF-1α and VEGF expression. Carcinogenesis. 2007;28(4):858–864. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- Ferlay J., et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Canc. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- Firdous A., et al. Quercetin, a natural dietary flavonoid, acts as a chemopreventive agent against prostate cancer in an in vivo model by inhibiting the EGFR signaling pathway. Food & Function. 2014;5(10):2632–2645. doi: 10.1039/c4fo00255e. [DOI] [PubMed] [Google Scholar]
- Fischer-Valuck B.W., et al. Two-and-a-half-year clinical experience with the world's first magnetic resonance image guided radiation therapy system. Advances in Radiation Oncology. 2017;2(3):485–493. doi: 10.1016/j.adro.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick J.M. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(Suppl. 2):16–22. doi: 10.1111/j.1464-410X.2007.07487.x. [DOI] [PubMed] [Google Scholar]
- Fontana F., et al. Natural compounds in prostate cancer prevention and treatment: mechanisms of action and molecular targets. Cells. 2020;9(2):460. doi: 10.3390/cells9020460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis J.C., et al. β-catenin is required for prostate development and cooperates with PTEN loss to drive invasive carcinoma. PLoS Genet. 2013;9(1) doi: 10.1371/journal.pgen.1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R.P., Fleshner N. Prostate cancer: 3. Individual risk factors. CMAJ (Can. Med. Assoc. J.) : Canadian Medical Association journal = Journal de l'Association Medicale Canadienne. 1998;159(7):807–813. [PMC free article] [PubMed] [Google Scholar]
- Gann P.H., et al. A phase II randomized trial of lycopene-rich tomato extract among men with high-grade prostatic intraepithelial neoplasia. Nutr. Canc. 2015;67(7):1104–1112. doi: 10.1080/01635581.2015.1075560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George K., Thomas N.S., Malathi R. Piperine blocks voltage gated K+ current and inhibits proliferation in androgen sensitive and insensitive human prostate cancer cell lines. Arch. Biochem. Biophys. 2019;667:36–48. doi: 10.1016/j.abb.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Ghosh N., et al. Chronic inflammatory diseases: progress and prospect with herbal medicine. Curr. Pharmaceut. Des. 2016;22(2):247–264. doi: 10.2174/1381612822666151112151419. [DOI] [PubMed] [Google Scholar]
- Ghosh S., et al. Induction of monoamine oxidase A-mediated oxidative stress and impairment of NRF2-antioxidant defence response by polyphenol-rich fraction of Bergenia ligulata sensitizes prostate cancer cells in vitro and in vivo. Free Radical Biology and Medicine. 2021;172:136–151. doi: 10.1016/j.freeradbiomed.2021.05.037. [DOI] [PubMed] [Google Scholar]
- Giacinti S., et al. Resistance to abiraterone in castration-resistant prostate cancer: a review of the literature. Anticancer Res. 2014;34(11):6265–6269. [PubMed] [Google Scholar]
- Gioti K., Tenta R. Bioactive natural products against prostate cancer: mechanism of action and autophagic/apoptotic molecular pathways. Planta Med. 2015;81(7):543–562. doi: 10.1055/s-0035-1545845. [DOI] [PubMed] [Google Scholar]
- Giovannucci E., et al. Calcium and fructose intake in relation to risk of prostate cancer. Canc. Res. 1998;58(3):442–447. [PubMed] [Google Scholar]
- Golombos D.M., et al. The role of gut microbiome in the pathogenesis of prostate cancer: a prospective, pilot study. Jornal of Urology. 2018;111:122–128. doi: 10.1016/j.urology.2017.08.039. [DOI] [PubMed] [Google Scholar]
- Goo Y.A., et al. Systematic investigation of lycopene effects in LNCaP cells by use of novel large-scale proteomic analysis software. Proteonomics Clin. Appl. 2007;1(5):513–523. doi: 10.1002/prca.200600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso C.S., et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson J., et al. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat. Genet. 2007;39(5):631–637. doi: 10.1038/ng1999. [DOI] [PubMed] [Google Scholar]
- Gundala S.R., et al. Polar biophenolics in sweet potato greens extract synergize to inhibit prostate cancer cell proliferation and in vivo tumor growth. Carcinogenesis. 2013;34(9):2039–2049. doi: 10.1093/carcin/bgt141. [DOI] [PubMed] [Google Scholar]
- Guo H., et al. Litchi seed extracts diminish prostate cancer progression via induction of apoptosis and attenuation of EMT through Akt/GSK-3β signaling. Sci. Rep. 2017;7(1):1–13. doi: 10.1038/srep41656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Hussain T., Mukhtar H. Molecular pathway for (-)-epigallocatechin-3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch. Biochem. Biophys. 2003;410(1):177–185. doi: 10.1016/s0003-9861(02)00668-9. [DOI] [PubMed] [Google Scholar]
- Gupta S.C., et al. Chemosensitization of tumors by resveratrol. Ann. N. Y. Acad. Sci. 2011;1215:150–160. doi: 10.1111/j.1749-6632.2010.05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel B., et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiology & Prevention Biomarkers. 2014;23(5):847–856. doi: 10.1158/1055-9965.EPI-13-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeez B.B., et al. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-κB signaling. Canc. Res. 2008;68(20):8564–8572. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen R.M., et al. Epigallocatechin-3-gallate promotes apoptosis and expression of the caspase 9a splice variant in PC3 prostate cancer cells. Int. J. Oncol. 2013;43(1):194–200. doi: 10.3892/ijo.2013.1920. [DOI] [PubMed] [Google Scholar]
- Hahm E.-R., et al. The role of lysosome-associated membrane protein 2 in prostate cancer chemopreventive mechanisms of sulforaphane. Canc. Prev. Res. 2020;13(8):661–672. doi: 10.1158/1940-6207.CAPR-20-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Reeves J.M., et al. Short-term soy isoflavone intervention in patients with localized prostate cancer: a randomized, double-blind, placebo-controlled trial. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., et al. Triptolide inhibits the AR signaling pathway to suppress the proliferation of enzalutamide resistant prostate cancer cells. Theranostics. 2017;7(7):1914–1927. doi: 10.7150/thno.17852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Harper C.E., et al. Epigallocatechin-3-Gallate suppresses early stage, but not late stage prostate cancer in TRAMP mice: mechanisms of action. Prostate. 2007;67(14):1576–1589. doi: 10.1002/pros.20643. [DOI] [PubMed] [Google Scholar]
- Harvey A.L., Cree I.A.J.P.m. High-throughput screening of natural products for cancer therapy. Planta Med. 2010;76(11):1080–1086. doi: 10.1055/s-0030-1250162. [DOI] [PubMed] [Google Scholar]
- Hastak K., et al. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22(31):4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- Hejazi J., et al. A pilot clinical trial of radioprotective effects of curcumin supplementation in patients with prostate cancer. J. Canc. Sci. Ther. 2013;5(10):320–324. [Google Scholar]
- Hejazi J., et al. Effect of curcumin supplementation during radiotherapy on oxidative status of patients with prostate cancer: a double blinded, randomized, placebo-controlled study. Nutr. Canc. 2016;68(1):77–85. doi: 10.1080/01635581.2016.1115527. [DOI] [PubMed] [Google Scholar]
- Henning S.M., et al. Randomized clinical trial of brewed green and black tea in men with prostate cancer prior to prostatectomy. Prostate. 2015;75(5):550–559. doi: 10.1002/pros.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T.-c., Wu J.M. Resveratrol suppresses prostate cancer epithelial cell scatter/invasion by targeting inhibition of hepatocyte growth factor (HGF) secretion by prostate stromal cells and upregulation of E-cadherin by prostate cancer epithelial cells. Int. J. Mol. Sci. 2020;21(5):1760. doi: 10.3390/ijms21051760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., et al. PLoS Triptolide inhibits the proliferation of prostate cancer cells and down-regulates SUMO-specific protease 1 expression. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., et al. PLoS Combination of α-tomatine and curcumin inhibits growth and induces apoptosis in human prostate cancer cells. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0144293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., et al. Matrine suppresses invasion of castration-resistant prostate cancer cells by downregulating MMP-2/9 via NF-κB signaling pathway. Int. J. Oncol. 2017;50(2):640–648. doi: 10.3892/ijo.2016.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., et al. Plumbagin triggers ER stress-mediated apoptosis in prostate cancer cells via induction of ROS. Cell. Physiol. Biochem. 2018;45(1):267–280. doi: 10.1159/000486773. [DOI] [PubMed] [Google Scholar]
- Huang Y., et al. Ginsenoside Rh2 inhibits angiogenesis in prostate cancer by targeting CNNM1. J. Nanosci. Nanotechnol. 2019;19(4):1942–1950. doi: 10.1166/jnn.2019.16404. [DOI] [PubMed] [Google Scholar]
- Huggins C., Hodges C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Canc. Res. 1941;1(4):293. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- Isharwal S., et al. Minnelide inhibits androgen dependent, castration resistant prostate cancer growth by decreasing expression of androgen receptor full length and splice variants. Prostate. 2017;77(6):584–596. doi: 10.1002/pros.23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov N.I., et al. Lycopene differentially induces quiescence and apoptosis in androgen-responsive and-independent prostate cancer cell lines. Clin. Nutr. 2007;26(2):252–263. doi: 10.1016/j.clnu.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Jalloh M., et al. Evaluation of 4,672 routine prostate biopsies performed in six African countries. Journal Africain du Cancer/African Journal of Cancer. 2013;5(3):144–154. [Google Scholar]
- James N.D., et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019) Eur. Urol. 2015;67(6):1028–1038. doi: 10.1016/j.eururo.2014.09.032. [DOI] [PubMed] [Google Scholar]
- Jang Y.G., Ko E.B., Choi K.C. Gallic acid, a phenolic acid, hinders the progression of prostate cancer by inhibition of histone deacetylase 1 and 2 expression. JNB (J. Nutr. Biochem.) 2020;84:108444. doi: 10.1016/j.jnutbio.2020.108444. [DOI] [PubMed] [Google Scholar]
- Jasiński M., Jasińska L., Ogrodowczyk M. Resveratrol in prostate diseases - a short review. Central European Journal of Urology. 2013;66(2):144–149. doi: 10.5173/ceju.2013.02.art8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L.-N., Liu Y.-B., Li B.-H. Lycopene exerts anti-inflammatory effect to inhibit prostate cancer progression. Asian J. Androl. 2019;21(1):80. doi: 10.4103/aja.aja_70_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin B.R., An H.J. Baicalin alleviates benign prostate hyperplasia through androgen-dependent apoptosis. Aging (Albany NY) 2020;12(3):2142–2155. doi: 10.18632/aging.102731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karna P., et al. Polyphenol-rich sweet potato greens extract inhibits proliferation and induces apoptosis in prostate cancer cells in vitro and in vivo. Carcinogenesis. 2011;32(12):1872–1880. doi: 10.1093/carcin/bgr215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karna P., et al. Benefits of whole ginger extract in prostate cancer. Br. J. Nutr. 2012;107(4):473–484. doi: 10.1017/S0007114511003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katta S., et al. Curcumin-gene expression response in hormone dependent and independent metastatic prostate cancer cells. Int. J. Mol. Sci. 2019;20(19):4891. doi: 10.3390/ijms20194891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P., Shukla S., Gupta S. Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: an in vitro and in vivo study. Carcinogenesis. 2008;29(11):2210–2217. doi: 10.1093/carcin/bgn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M., et al. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharmacol. Res. 2009;26(9):2133–2140. doi: 10.1007/s11095-009-9926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima A., et al. Four week supplementation with mixed fruit and vegetable juice concentrates increased protective serum antioxidants and folate and decreased plasma homocysteine in Japanese subjects. Asia Pac. J. Clin. Nutr. 2007;16(3) [PubMed] [Google Scholar]
- Khalili M., et al. Loss of Nkx3. 1 expression in bacterial prostatitis: a potential link between inflammation and neoplasia. Am. J. Pathol. 2010;176(5):2259–2268. doi: 10.2353/ajpath.2010.080747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.I., et al. YB-1 expression promotes epithelial-to-mesenchymal transition in prostate cancer that is inhibited by a small molecule fisetin. Oncotarget. 2014;5(9):2462–2474. doi: 10.18632/oncotarget.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor T.O., et al. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011;82(9):1073–1078. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- Khusbu F.Y., et al. Resveratrol induces depletion of TRAF6 and suppresses prostate cancer cell proliferation and migration. Int. J. Biochem. Cell Biol. 2020;118:105644. doi: 10.1016/j.biocel.2019.105644. [DOI] [PubMed] [Google Scholar]
- Kim S.M., et al. Combination of ginsenoside Rg3 with docetaxel enhances the susceptibility of prostate cancer cells via inhibition of NF-kappaB. European Journal of Pharmacolgy. 2010;631(1–3):1–9. doi: 10.1016/j.ejphar.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Kjaer T.N., et al. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate. 2015;75(12):1255–1263. doi: 10.1002/pros.23006. [DOI] [PubMed] [Google Scholar]
- Kjær T.N., et al. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate. 2015;75(12):1255–1263. doi: 10.1002/pros.23006. [DOI] [PubMed] [Google Scholar]
- Koga Y., et al. Genomic profiling of prostate cancers from men with african and European ancestry. Clin. Canc. Res. 2020;26(17):4651–4660. doi: 10.1158/1078-0432.CCR-19-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonel L.N. Fat, meat, and prostate cancer. Epidemiol. Rev. 2001;23(1):72–81. doi: 10.1093/oxfordjournals.epirev.a000798. [DOI] [PubMed] [Google Scholar]
- Komiya Y., Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konijeti R., et al. Chemoprevention of prostate cancer with lycopene in the TRAMP model. Prostate. 2010;70(14):1547–1554. doi: 10.1002/pros.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kote-Jarai Z., et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat. Genet. 2011;43(8):785–791. doi: 10.1038/ng.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucuk O., et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiology & Prevention Biomarkers. 2001;10(8):861–868. [PubMed] [Google Scholar]
- Kumar N.B., et al. Randomized, placebo-controlled trial of green tea catechins for prostate cancer prevention. Canc. Prev. Res. 2015;8(10):879–887. doi: 10.1158/1940-6207.CAPR-14-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon O.-J., et al. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111(5):E592–E600. doi: 10.1073/pnas.1318157111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall R.K., et al. Dietary flavonoid fisetin increases abundance of high-molecular-mass hyaluronan conferring resistance to prostate oncogenesis. Carcinogenesis. 2016;37(9):918–928. doi: 10.1093/carcin/bgw071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J.A., et al. ProDiet: a phase II randomized placebo-controlled trial of green tea Catechins and lycopene in men at increased risk of prostate cancer. Canc. Prev. Res. 2018;11(11):687–696. doi: 10.1158/1940-6207.CAPR-18-0147. [DOI] [PubMed] [Google Scholar]
- Lazarevic B., et al. The effects of short-term genistein intervention on prostate biomarker expression in patients with localised prostate cancer before radical prostatectomy. Br. J. Nutr. 2012;108(12):2138–2147. doi: 10.1017/S0007114512000384. [DOI] [PubMed] [Google Scholar]
- Lee E., Ha S., Logan S.K., PLoS Divergent androgen receptor and beta-catenin signaling in prostate cancer cells. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0141589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., et al. Androgen signaling is a confounding factor for β-catenin-mediated prostate tumorigenesis. Oncogene. 2016;35(6):702–714. doi: 10.1038/onc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., et al. Berberine suppresses androgen receptor signaling in prostate cancer. Mol. Canc. Therapeut. 2011;10(8):1346–1356. doi: 10.1158/1535-7163.MCT-10-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., et al. Formononetin promotes cell cycle arrest via downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer cells. Cell. Physiol. Biochem. 2014;34(4):1351–1358. doi: 10.1159/000366342. [DOI] [PubMed] [Google Scholar]
- Li D., et al. Potent inhibitory effect of terpenoids from Acanthopanax trifoliatus on growth of PC-3 prostate cancer cells in vitro and in vivo is associated with suppression of NF-κB and STAT3 signalling. Journal of Functional Foods. 2015;15:274–283. [Google Scholar]
- Li X., et al. Metabolic characterization and pathway analysis of berberine protects against prostate cancer. Oncotarget. 2017;8(39):65022. doi: 10.18632/oncotarget.17531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., et al. Enhanced anti-tumor efficacy and mechanisms associated with docetaxel-piperine combination-in vitro and in vivo investigation using a taxane-resistant prostate cancer model. Oncotarget. 2018;9(3):3338. doi: 10.18632/oncotarget.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., et al. Alteration of androgen receptor protein stability by triptolide in LNCaP Cells. Medicina. 2018;54(3):39. doi: 10.3390/medicina54030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J., et al. Sorafenib sensitizes (-)-gossypol-induced growth suppression in androgen-independent prostate cancer cells via Mcl-1 inhibition and Bak activation. Mol. Canc. Therapeut. 2012;11(2):416–426. doi: 10.1158/1535-7163.MCT-11-0559. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P., et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000;343(2):78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- Lin H.-H., et al. Hibiscus sabdariffa leaf induces apoptosis of human prostate cancer cells in vitro and in vivo. Food Chem. 2012;132(2):880–891. [Google Scholar]
- Lin H., et al. Inhibition of Gli/hedgehog signaling in prostate cancer cells by “cancer bush” Sutherlandia frutescens extract. Cell Biol. Int. 2016;40(2):131–142. doi: 10.1002/cbin.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linch M., et al. Intratumoural evolutionary landscape of high-risk prostate cancer: the PROGENY study of genomic and immune parameters. Ann. Oncol. 2017;28(10):2472–2480. doi: 10.1093/annonc/mdx355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss M.A., et al. Metabolic biosynthesis pathways identified from fecal microbiome associated with prostate cancer. Eur. Urol. 2018;74(5):575–582. doi: 10.1016/j.eururo.2018.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., et al. Quercetin inhibits epithelial-to-mesenchymal transition (EMT) process and promotes apoptosis in prostate cancer via downregulating lncRNA MALAT1. Canc. Manag. Res. 2020;12:1741. doi: 10.2147/CMAR.S241093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., et al. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int. J. Biol. Sci. 2020;16(7):1121. doi: 10.7150/ijbs.41686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G.-Z., et al. Effect of pomegranate peel polyphenols on human prostate cancer PC-3 cells in vivo. Food Science and Biotechnology. 2015;24(5):1887–1892. [Google Scholar]
- Ma S.C., et al. Baicalein inhibits the proliferative activity of human prostate cancer cell line PC3 by downregulating Ezrin. J. Biol. Regul. Homeost. Agents. 2020;34(3):885–892. doi: 10.23812/20-145-A-44. [DOI] [PubMed] [Google Scholar]
- Madersbacher S., et al. The influence of family history on prostate cancer risk: implications for clinical management. BJU Int. 2011;107(5):716–721. doi: 10.1111/j.1464-410X.2010.10024.x. [DOI] [PubMed] [Google Scholar]
- Makhov P., et al. Co-administration of piperine and docetaxel results in improved anti-tumor efficacy via inhibition of CYP3A4 activity. Prostate. 2012;72(6):661–667. doi: 10.1002/pros.21469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi K., et al. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448(7155):816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani R.S., et al. Inflammation-induced oxidative stress mediates gene fusion formation in prostate cancer. Cell Rep. 2016;17(10):2620–2631. doi: 10.1016/j.celrep.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C., Gavazzo P., Burlando B. Epigallocatechin-3-gallate mobilizes intracellular Ca2+ in prostate cancer cells through combined Ca2+ entry and Ca2+-induced Ca2+ release. Life Sci. 2020;258:118232. doi: 10.1016/j.lfs.2020.118232. [DOI] [PubMed] [Google Scholar]
- Mateo J., et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 2015;373(18):1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarty J., et al. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Canc. Prev. Res. 2009;2(7):673–682. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- Meng Y., et al. Natural BH3 mimetic (-)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol. Canc. Therapeut. 2008;7(7):2192–2202. doi: 10.1158/1535-7163.MCT-08-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., et al. Ursolic acid induces apoptosis of prostate cancer cells via the PI3K/Akt/mTOR pathway. Am. J. Chin. Med. 2015;43(7):1471–1486. doi: 10.1142/S0192415X15500834. [DOI] [PubMed] [Google Scholar]
- Meyer M.S., et al. Genetic variation in RNASEL associated with prostate cancer risk and progression. Carcinogenesis. 2010;31(9):1597–1603. doi: 10.1093/carcin/bgq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirahmadi M., et al. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020;129:110459. doi: 10.1016/j.biopha.2020.110459. [DOI] [PubMed] [Google Scholar]
- Miyanaga N., et al. Prostate cancer chemoprevention study: an investigative randomized control study using purified isoflavones in men with rising prostate-specific antigen. Canc. Sci. 2012;103(1):125–130. doi: 10.1111/j.1349-7006.2011.02120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty N., et al. Lycopene as a chemopreventive agent in the treatment of high-grade prostate intraepithelial neoplasia. Urol. Oncol. 2005;23:383–385. doi: 10.1016/j.urolonc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Mori A., et al. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Canc. Res. 2006;66(6):3222–3229. doi: 10.1158/0008-5472.CAN-05-0087. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A., et al. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20(52):7597–7609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- Mukhtar E., et al. Fisetin enhances chemotherapeutic effect of cabazitaxel against human prostate cancer cells. Mol. Canc. Therapeut. 2016;15(12):2863–2874. doi: 10.1158/1535-7163.MCT-16-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo-Garzón V., Kypta R. WNT signalling in prostate cancer. Nat. Rev. Urol. 2017;14(11):683. doi: 10.1038/nrurol.2017.144. [DOI] [PubMed] [Google Scholar]
- Naiki-Ito A., et al. Ellagic acid, a component of pomegranate fruit juice, suppresses androgen-dependent prostate carcinogenesis via induction of apoptosis. Prostate. 2015;75(2):151–160. doi: 10.1002/pros.22900. [DOI] [PubMed] [Google Scholar]
- Nayana, S.K., A.I. Shalini, and B.S. Mamta, A simple method for isolation of plumbagin from roots of Plumbago rosea. Pharmaceut. Biol.. 43(6): p. 551-553.
- Nghiem B., et al. Mismatch repair enzyme expression in primary and castrate resistant prostate cancer. Asian Journal of Neurology. 2016;3(4):223–228. doi: 10.1016/j.ajur.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M.M., et al. Randomized, double-blind, placebo-controlled trial of polyphenon E in prostate cancer patients before prostatectomy: evaluation of potential chemopreventive activities. Canc. Prev. Res. 2012;5(2):290–298. doi: 10.1158/1940-6207.CAPR-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C., et al. Dandelion root and lemongrass extracts induce apoptosis, enhance chemotherapeutic efficacy, and reduce tumour xenograft growth in vivo in prostate cancer. Evid. base Compl. Alternative Med. 2019 doi: 10.1155/2019/2951428. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda D., et al. ELAC2, a putative prostate cancer susceptibility gene product, potentiates TGF-β/Smad-induced growth arrest of prostate cells. Oncogene. 2006;25(41):5591–5600. doi: 10.1038/sj.onc.1209571. [DOI] [PubMed] [Google Scholar]
- Nombela P., et al. BRCA2 and other DDR genes in prostate cancer. Cancers. 2019;11(3):352. doi: 10.3390/cancers11030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurgali K., Jagoe R.T., Abalo R. Editorial: adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00245. 245-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudard S., et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial—FIRSTANA. J. Clin. Oncol. 2017;35(28):3189–3197. doi: 10.1200/JCO.2016.72.1068. [DOI] [PubMed] [Google Scholar]
- Pal S., et al. Chronic inflammation and cancer: potential chemoprevention through nuclear factor kappa B and p53 mutual antagonism. J. Inflamm. 2014;11(1):1–28. doi: 10.1186/1476-9255-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller C., et al. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis. 2013;16(1):50–55. doi: 10.1038/pcan.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller C.J., et al. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: safety, tolerability, and dose determination. Prostate. 2015;75(14):1518–1525. doi: 10.1002/pros.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller C.J., et al. Muscadine grape skin extract (MPX) in men with biochemically recurrent prostate cancer: a randomized, multicenter, placebo-controlled clinical trial. Clin. Canc. Res. 2018;24(2):306–315. doi: 10.1158/1078-0432.CCR-17-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palozza P., et al. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis. 2010;31(10):1813–1821. doi: 10.1093/carcin/bgq157. [DOI] [PubMed] [Google Scholar]
- Pandey M., et al. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: in vitro and in vivo study. Mol. Carcinog. 2012;51(12):952–962. doi: 10.1002/mc.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X., et al. Morelloflavone, a biflavonoid, inhibits tumor angiogenesis by targeting rho GTPases and extracellular signal-regulated kinase signaling pathways. Canc. Res. 2009;69(2):518–525. doi: 10.1158/0008-5472.CAN-08-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X., et al. (-)-Gossypol suppresses the growth of human prostate cancer xenografts via modulating VEGF signaling-mediated angiogenesis. Mol. Canc. Therapeut. 2011;10(5):795–805. doi: 10.1158/1535-7163.MCT-10-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantuck A.J., et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin. Canc. Res. 2006;12(13):4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- Paranjpe R., et al. Piper betel leaf extract: anticancer benefits and bio-guided fractionation to identify active principles for prostate cancer management. Carcinogenesis. 2013;34(7):1558–1566. doi: 10.1093/carcin/bgt066. [DOI] [PubMed] [Google Scholar]
- Park J., Boo Y.C. Isolation of resveratrol from vitis viniferae caulis and its potent inhibition of human tyrosinase. Evid. base Compl. Alternative Med. 2013:645257. doi: 10.1155/2013/645257. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashirzad M., et al. Role of Wnt5a in the pathogenesis of inflammatory diseases. J. Cell. Physiol. 2017;232(7):1611–1616. doi: 10.1002/jcp.25687. [DOI] [PubMed] [Google Scholar]
- Pearce A., et al. Incidence and severity of self-reported chemotherapy side effects in routine care: a prospective cohort study. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0184360. e0184360-e0184360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., et al. In vitro and in vivo effects of water extract of white cocoa tea (Camellia ptilophylla) against human prostate cancer. Pharmaceut. Res. 2010;27(6):1128–1137. doi: 10.1007/s11095-010-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool-Zobel B., et al. Consumption of vegetables reduces genetic damage in humans: first results of a human intervention trial with carotenoid-rich foods. Carcinogenesis. 1997;18(9):1847–1850. doi: 10.1093/carcin/18.9.1847. [DOI] [PubMed] [Google Scholar]
- Poutahidis T., et al. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0078898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A.J., et al. Insulin-like growth factor-I concentration and risk of prostate cancer: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiology and Prevention Biomarkers. 2012;21(9):1531–1541. doi: 10.1158/1055-9965.EPI-12-0481-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard C.C., et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 2016;375:443–453. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabzia A., et al. Synergistic anticancer effect of paclitaxel and noscapine on human prostate cancer cell lines. Iran. J. Pharm. Res. (IJPR) 2017;16(4):1432–1442. [PMC free article] [PubMed] [Google Scholar]
- Rachner T.D., et al. High serum levels of Dickkopf-1 are associated with a poor prognosis in prostate cancer patients. BMC Canc. 2014;14(1):1–6. doi: 10.1186/1471-2407-14-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina K., et al. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in TRAMP mice. Mol. Canc. Therapeut. 2008;7(5):1258–1267. doi: 10.1158/1535-7163.MCT-07-2220. [DOI] [PubMed] [Google Scholar]
- Rajan P., et al. Next-generation sequencing of advanced prostate cancer treated with androgen-deprivation therapy. Eur. Urol. 2014;66(1):32–39. doi: 10.1016/j.eururo.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawla P. Epidemiology of prostate cancer. World J. Oncol. 2019;10(2):63. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck T.R. Prostate cancer genetics: variation by race, ethnicity, and geography. Semin. Radiat. Oncol. 2017;27(1):3–10. doi: 10.1016/j.semradonc.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck T.R., Haas G.P. Temporal trends and racial disparities in global prostate cancer prevalence. Can. J. Urol. 2014;21(5):7496–7506. [PMC free article] [PubMed] [Google Scholar]
- Reiner T., et al. Betulinic acid selectively increases protein degradation and enhances prostate cancer-specific apoptosis: possible role for inhibition of deubiquitinase activity. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice M.A., Malhotra S.V., Stoyanova T. Second-generation antiandrogens in castration resistant prostate cancer. Frontiers in Oncology. 2019;9:801. doi: 10.3389/fonc.2019.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D.L., Dive C., Renehan A.G. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu. Rev. Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- Rondeau G., et al. Differential gene expression induced by anti-cancer agent plumbagin is mediated by androgen receptor in prostate cancer cells. Sci. Rep. 2018;8(1):2694. doi: 10.1038/s41598-018-20451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samykutty A., et al. Piperine, a bioactive component of pepper spice exerts therapeutic effects on androgen dependent and androgen independent prostate cancer cells. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez B.G., et al. Combination of the natural product capsaicin and docetaxel synergistically kills human prostate cancer cells through the metabolic regulator AMP-activated kinase. Canc. Cell Int. 2019;19(1):1–14. doi: 10.1186/s12935-019-0769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarveswaran S., Gautam S.C., Ghosh J. Wedelolactone, a medicinal plant-derived coumestan, induces caspase-dependent apoptosis in prostate cancer cells via downregulation of PKCε without inhibiting Akt. Int. J. Oncol. 2012;41(6):2191–2199. doi: 10.3892/ijo.2012.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel T., et al. Curcumin inhibits glyoxalase 1: a possible link to its anti-inflammatory and anti-tumor activity. PLoS One. 2008;3(10) doi: 10.1371/journal.pone.0003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarveswaran S., et al. Wedelolactone, an anti-inflammatory botanical, interrupts c-myc oncogenic signaling and synergizes with enzalutamide to induce apoptosis in prostate cancer cells. Mol. Canc. Therapeut. 2016;15(11):2791–2801. doi: 10.1158/1535-7163.MCT-15-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K.T., Figg W.D. The potential role of curcumin in prostate cancer: the importance of optimizing pharmacokinetics in clinical studies. Transl. Cancer Res. 2016;5(Suppl. 6):S1107. doi: 10.21037/tcr.2016.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoeller T., et al. Circulating free testosterone is an independent predictor of advanced disease in patients with clinically localized prostate cancer. World J. Urol. 2013;31(2):253–259. doi: 10.1007/s00345-012-0902-5. [DOI] [PubMed] [Google Scholar]
- Seca A.M.L., Pinto D.C.G.A. Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018;19(1):263. doi: 10.3390/ijms19010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfanos K.S., De Marzo A.M. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60(1):199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfanos K.S., et al. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 2018;15(1):11–24. doi: 10.1038/nrurol.2017.167. [DOI] [PubMed] [Google Scholar]
- Shabbir M., et al. Potent anti-proliferative, pro-apoptotic activity of the Maytenus royleanus extract against prostate cancer cells: evidence in in-vitro and in-vivo models. Oncotarget. 2015;10(3) doi: 10.1371/journal.pone.0119859. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shafiee G., et al. Beneficial effects of genistein in suppression of proliferation, inhibition of metastasis, and induction of apoptosis in PC3 prostate cancer cells. Arch. Physiol. Biochem. 2020:1–9. doi: 10.1080/13813455.2020.1717541. [DOI] [PubMed] [Google Scholar]
- Shamaladevi N., et al. The andean anticancer herbal product BIRM causes destabilization of androgen receptor and induces caspase-8 mediated-apoptosis in prostate cancer. Oncotarget. 2016;7(51):84201. doi: 10.18632/oncotarget.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmila G., et al. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014;33(4):718–726. doi: 10.1016/j.clnu.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Sheth S., et al. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shityakov S., et al. Phytochemical and pharmacological attributes of piperine: a bioactive ingredient of black pepper. Eur. J. Med. Chem. 2019;176:149–161. doi: 10.1016/j.ejmech.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Shu Y., et al. Quercetin reverses the doxorubicin resistance of prostate cancer cells by downregulating the expression of c-met. Oncology Letters. 2018;15(2):2252–2258. doi: 10.3892/ol.2017.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S., Gupta S. Molecular targets for apigenin-induced cell cycle arrest and apoptosis in prostate cancer cell xenograft. Mol. Canc. Therapeut. 2006;5(4):843–852. doi: 10.1158/1535-7163.MCT-05-0370. [DOI] [PubMed] [Google Scholar]
- Shukla S., Gupta S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radical Biol. Med. 2008;44(10):1833–1845. doi: 10.1016/j.freeradbiomed.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S., Gupta S. Apigenin suppresses insulin-like growth factor I receptor signaling in human prostate cancer: an in vitro and in vivo study. Mol. Carcinog. 2009;48(3):243–252. doi: 10.1002/mc.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S., et al. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis. 2014;35(2):452–460. doi: 10.1093/carcin/bgt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui I.A., et al. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB (Fed. Am. Soc. Exp. Biol.) J. 2011;25(4):1198–1207. doi: 10.1096/fj.10-167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.B., et al. Reversal of the Warburg phenomenon in chemoprevention of prostate cancer by sulforaphane. Carcinogenesis. 2019;40(12):1545–1556. doi: 10.1093/carcin/bgz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh C.K., et al. Quercetin–resveratrol combination for prostate cancer management in TRAMP mice. Cancers. 2020;12(8):2141. doi: 10.3390/cancers12082141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh C.K., et al. Quercetin-resveratrol combination for prostate cancer management in TRAMP mice. Cancers. 2020;12(8) doi: 10.3390/cancers12082141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.-y., et al. Improved drug delivery and anti-tumor efficacy of combinatorial liposomal formulation of genistein and plumbagin by targeting Glut1 and Akt3 proteins in mice bearing prostate tumor. Colloids Surf. B Biointerfaces. 2020:110966. doi: 10.1016/j.colsurfb.2020.110966. [DOI] [PubMed] [Google Scholar]
- Stallone G., et al. Pentraxin 3: a novel biomarker for predicting progression from prostatic inflammation to prostate cancer. Canc. Res. 2014;74(16):4230–4238. doi: 10.1158/0008-5472.CAN-14-0369. [DOI] [PubMed] [Google Scholar]
- Stearns M.E., et al. Combination therapy with epigallocatechin-3-gallate and doxorubicin in human prostate tumor modeling studies: inhibition of metastatic tumor growth in severe combined immunodeficiency mice. Am. J. Pathol. 2010;177(6):3169–3179. doi: 10.2353/ajpath.2010.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subudhi S.K., et al. A phase Ib/II study of niraparib combination therapies for the treatment of metastatic castration-resistant prostate cancer ( NCT03431350) American Society of Clinical Oncology. 2019;37(15) doi: 10.1200/JCO.2019.37.15_suppl.TPS5087. [DOI] [Google Scholar]
- Sun S., et al. Metformin combined with quercetin synergistically repressed prostate cancer cells via inhibition of VEGF/PI3K/Akt signaling pathway. Gene. 2018;664:50–57. doi: 10.1016/j.gene.2018.04.045. [DOI] [PubMed] [Google Scholar]
- Sundram V., et al. Curcumin attenuates β-catenin signaling in prostate cancer cells through activation of protein kinase D1. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaudommongkon I., et al. Curcumin nanoparticles and their cytotoxicity in docetaxel-resistant castration-resistant prostate cancer cells. Biomedicines. 2020;8(8):253. doi: 10.3390/biomedicines8080253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teiten M.H., et al. Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless signaling pathway. Int. J. Oncol. 2011;38(3):603–611. doi: 10.3892/ijo.2011.905. [DOI] [PubMed] [Google Scholar]
- Teply B.A., Antonarakis E.S. Novel mechanism-based therapeutics for androgen axis blockade in castration-resistant prostate cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2016;23(3):279. doi: 10.1097/MED.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Liu J. Resveratrol:A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2019:100. doi: 10.1002/jsfa.10152. [DOI] [PubMed] [Google Scholar]
- Tian H., et al. Sulforaphane-cysteine suppresses invasion via downregulation of galectin-1 in human prostate cancer DU145 and PC3 cells. Oncol. Rep. 2016;36(3):1361–1368. doi: 10.3892/or.2016.4942. [DOI] [PubMed] [Google Scholar]
- Tian J., et al. Nanoliposomal formulation encapsulating celecoxib and genistein inhibiting COX-2 pathway and Glut-1 receptors to prevent prostate cancer cell proliferation. Canc. Lett. 2019;448:1–10. doi: 10.1016/j.canlet.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Tjahjodjati S.S., Umbas R., Satari M. The protective effect of lycopene on prostate growth inhibitory efficacy by decreasing insulin growth factor-1 in Indonesian human prostate cancer cells. Res. Rep. Urol. 2020;12:137. doi: 10.2147/RRU.S232745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong K.L., et al. The in vitro and in vivo anti-cancer activities of a standardized quassinoids composition from Eurycoma longifolia on LNCaP human prostate cancer cells. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli P., et al. Synergic effect of α-tocopherol and naringenin in transglutaminase-induced differentiation of human prostate cancer cells. Amino Acids. 2011;41(5):1207–1214. doi: 10.1007/s00726-010-0788-8. [DOI] [PubMed] [Google Scholar]
- Traka M., et al. Broccoli consumption interacts with GSTM1 to perturb oncogenic signalling pathways in the prostate. PLoS One. 2008;3(7):e2568. doi: 10.1371/journal.pone.0002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.H., et al. A standardized herbal extract mitigates tumor inflammation and augments chemotherapy effect of docetaxel in prostate cancer. Sci. Rep. 2017;7(1):15624. doi: 10.1038/s41598-017-15934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.-H., et al. A standardized Wedelia chinensis extract overcomes the feedback activation of HER2/3 signaling upon androgen-ablation in prostate cancer. Front. Pharmacol. 2017;8:721. doi: 10.3389/fphar.2017.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummala R., et al. Quercetin targets hnRNPA1 to overcome enzalutamide resistance in prostate cancer cells. Mol. Canc. Therapeut. 2017;16(12):2770–2779. doi: 10.1158/1535-7163.MCT-17-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishampayan U., et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr. Canc. 2007;59(1):1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- Van Amerongen R. Alternative Wnt pathways and receptors. Cold Spring Harbor Perspectives in Biology. 2012;4(10) doi: 10.1101/cshperspect.a007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venier N.A., et al. Capsaicin: a novel radio-sensitizing agent for prostate cancer. Prostate. 2015;75(2):113–125. doi: 10.1002/pros.22896. [DOI] [PubMed] [Google Scholar]
- Venier N.A., et al. Capsaicin reduces the metastatic burden in the transgenic adenocarcinoma of the mouse prostate model. Prostate. 2015;75(12):1300–1311. doi: 10.1002/pros.23013. [DOI] [PubMed] [Google Scholar]
- Vicari E., et al. Resveratrol reduces inflammation-related prostate fibrosis. Int. J. Med. Sci. 2020;17(13):1864. doi: 10.7150/ijms.44443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar S., et al. Prostate-specific antigen levels are higher in African-American than in white patients in a multicenter registration study: results of RTOG 94-12. Int. J. Radiat. Oncol. Biol. Phys. 1998;40(1):17–25. doi: 10.1016/s0360-3016(97)00834-1. [DOI] [PubMed] [Google Scholar]
- Volante M., et al. Androgen deprivation modulates gene expression profile along prostate cancer progression. Hum. Pathol. 2016;56:81–88. doi: 10.1016/j.humpath.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Volate S.R., et al. Gossypol induces apoptosis by activating p53 in prostate cancer cells and prostate tumor-initiating cells. Mol. Canc. Therapeut. 2010;9(2):461–470. doi: 10.1158/1535-7163.MCT-09-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., et al. Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Canc. Prev. Res. 2010;3(8):985–993. doi: 10.1158/1940-6207.CAPR-09-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., et al. Enhanced inhibition of prostate cancer xenograft tumor growth by combining quercetin and green tea. JNB (J. Nutr. Biochem.) 2014;25(1):73–80. doi: 10.1016/j.jnutbio.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., et al. Luteolin, ellagic acid and punicic acid are natural products that inhibit prostate cancer metastasis. Carcinogenesis. 2014;35(10):2321–2330. doi: 10.1093/carcin/bgu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., et al. Genetics and biology of prostate cancer. Gene Dev. 2018;32(17–18):1105–1140. doi: 10.1101/gad.315739.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., et al. Resveratrol inhibits the tumor migration and invasion by upregulating TET1 and reducing TIMP2/3 methylation in prostate carcinoma cells. Prostate. 2020;80(12):977–985. doi: 10.1002/pros.24029. [DOI] [PubMed] [Google Scholar]
- Watkins Bruner D., et al. Relative risk of prostate cancer for men with affected relatives: systematic review and meta-analysis. Int. J. Canc. 2003;107(5):797–803. doi: 10.1002/ijc.11466. [DOI] [PubMed] [Google Scholar]
- Way T.-D., et al. Inhibition of epidermal growth factor receptor signaling by Saussurea involucrata, a rare traditional Chinese medicinal herb, in human hormone-resistant prostate cancer PC-3 cells. J. Agric. Food Chem. 2010;58(6):3356–3365. doi: 10.1021/jf903793p. [DOI] [PubMed] [Google Scholar]
- Wolk A. Diet, lifestyle and risk of prostate cancer. Acta Oncol. 2005;44(3):277–281. doi: 10.1080/02841860510029572. [DOI] [PubMed] [Google Scholar]
- Wu L., et al. ERG is a critical regulator of wnt/LEF1 signaling in prostate cancer. Canc. Res. 2013;73(19):6068–6079. doi: 10.1158/0008-5472.CAN-13-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., et al. Preclinical evaluation of the supercritical extract of Azadirachta indica (neem) leaves in vitro and in vivo on inhibition of prostate cancer tumor growth. Mol. Canc. Therapeut. 2014;13(5):1067–1077. doi: 10.1158/1535-7163.MCT-13-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., et al. Effects of RNase L mutations associated with prostate cancer on apoptosis induced by 2′, 5′-oligoadenylates. Canc. Res. 2003;63(20):6795–6801. [PubMed] [Google Scholar]
- Xu J., et al. Evidence for a prostate cancer susceptibility locus on the X chromosome. Nat. Genet. 1998;20(2):175–179. doi: 10.1038/2477. [DOI] [PubMed] [Google Scholar]
- Xu L., et al. (-)-Gossypol enhances response to radiation therapy and results in tumor regression of human prostate cancer. Mol. Canc. Therapeut. 2005;4(2):197–205. [PubMed] [Google Scholar]
- Xu D., et al. The wedelolactone derivative inhibits estrogen receptor-mediated breast, endometrial, and ovarian cancer cells growth. BioMed Res. Int. 2014;2014:713263. doi: 10.1155/2014/713263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., et al. pH-triggered charge-reversal and redox-sensitive drug-release polymer micelles codeliver doxorubicin and triptolide for prostate tumor therapy. Int. J. Nanomed. 2018;13:7229. doi: 10.2147/IJN.S182197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., et al. Quercetin inhibits angiogenesis through thrombospondin-1 upregulation to antagonize human prostate cancer PC-3 cell growth in vitro and in vivo. Oncol. Rep. 2016;35(3):1602–1610. doi: 10.3892/or.2015.4481. [DOI] [PubMed] [Google Scholar]
- Yatkin E., et al. Novel lignan and stilbenoid mixture shows anticarcinogenic efficacy in preclinical PC-3M-luc2 prostate cancer model. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., et al. Resveratrol inhibits proliferation and promotes apoptosis via the androgen receptor splicing variant 7 and PI3K/Akt signaling pathway in LNCaP prostate cancer cells. Oncology Letters. 2020;20(5) doi: 10.3892/ol.2020.12032. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M., et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat. Genet. 2007;39(5):645–649. doi: 10.1038/ng2022. [DOI] [PubMed] [Google Scholar]
- Yeo C., et al. Epigallocatechin-3-Gallate suppresses vasculogenic mimicry through inhibiting the twist/VE-cadherin/Akt pathway in human prostate cancer PC-3 cells. Int. J. Mol. Sci. 2020;21(2):439. doi: 10.3390/ijms21020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T., et al. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Canc. Res. 2008;68(6):1843–1850. doi: 10.1158/0008-5472.CAN-07-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn D.-H., et al. Berberine improves benign prostatic hyperplasia via suppression of 5 alpha reductase and extracellular signal-regulated kinase in vivo and in vitro. Front. Pharmacol. 2018;9:773. doi: 10.3389/fphar.2018.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., et al. The effectiveness of intensity modulated radiation therapy versus three-dimensional radiation therapy in prostate cancer: a meta-analysis of the literatures. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., et al. Baicalin suppresses the cell cycle progression and proliferation of prostate cancer cells through the CDK6/FOXM1 axis. Mol. Cell. Biochem. 2020;469(1–2):169–178. doi: 10.1007/s11010-020-03739-1. [DOI] [PubMed] [Google Scholar]
- Yu-Rong W., et al. Triptolide reduces prostate size and androgen level on testosterone-induced benign prostatic hyperplasia in Sprague Dawley rats. Chin. J. Nat. Med. 2017;15(5):341–346. doi: 10.1016/S1875-5364(17)30054-7. [DOI] [PubMed] [Google Scholar]
- Yuan S., et al. Triptolide inhibits the migration and invasion of human prostate cancer cells via Caveolin-1/CD147/MMPs pathway. Biomed. Pharmacother. 2016;84:1776–1782. doi: 10.1016/j.biopha.2016.10.104. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Yang Y. Piperine depresses the migration progression via downregulating the Akt/mTOR/MMP-9 signaling pathway in DU145 cells. Mol. Med. Rep. 2018;17(5):6363–6370. doi: 10.3892/mmr.2018.8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.Q., et al. Inhibition of proliferation of prostate cancer cell line, PC-3, in vitro and in vivo using (-)-gossypol. Asian J. Androl. 2010;12(3):390–399. doi: 10.1038/aja.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., et al. Wnt/β-catenin signaling enhances hypoxia-induced epithelial–mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling. Carcinogenesis. 2013;34(5):962–973. doi: 10.1093/carcin/bgt027. [DOI] [PubMed] [Google Scholar]
- Zhang Q., et al. Berberine inhibits the expression of hypoxia induction factor-1alpha and increases the radiosensitivity of prostate cancer. Diagn. Pathol. 2014;9(1):1–7. doi: 10.1186/1746-1596-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., et al. Inhibition of prostatic cancer growth by ginsenoside Rh2. Tumour Biology. 2015;36(4):2377–2381. doi: 10.1007/s13277-014-2845-5. [DOI] [PubMed] [Google Scholar]
- Zhang H., et al. Genistein treatment duration effects biomarkers of cell motility in human prostate. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0214078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., et al. (−)-Epigallocatechin-3-gallate suppresses prostate cancer cell growth via activating miR-520a-3p. Revista Brasileira de Farmacognosia. 2020:1–9. [Google Scholar]
- Zhang X., et al. Quercetin enhanced paclitaxel therapeutic effects towards PC-3 prostate cancer through ER stress induction and ROS production. OncoTargets Ther. 2020;13:513. doi: 10.2147/OTT.S228453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., et al. Sulforaphane bioavailability and chemopreventive activity in men presenting for biopsy of the prostate gland: a randomized controlled trial. Nutr. Canc. 2020;72(1):74–87. doi: 10.1080/01635581.2019.1619783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., et al. Quercetin-loaded nanomicelles to circumvent human castration-resistant prostate cancer in vitro and in vivo. Journal of Nanoscale. 2016;8(9):5126–5138. doi: 10.1039/c5nr08966b. [DOI] [PubMed] [Google Scholar]
- Zhou A., et al. Interferon action and apoptosis are defective in mice devoid of 2′, 5′-oligoadenylate-dependent RNase L. EMBO J. 1997;16(21):6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., et al. Potential ameliorative effects of epigallocatechin-3-gallate against testosterone-induced benign prostatic hyperplasia and fibrosis in rats. Int. Immunopharm. 2018;64:162–169. doi: 10.1016/j.intimp.2018.08.038. [DOI] [PubMed] [Google Scholar]
- Zhou Y., et al. Sulforaphane metabolites cause apoptosis via microtubule disruption in cancer. Endocr. Relat. Canc. 2018;25(3):255–268. doi: 10.1530/ERC-17-0483. [DOI] [PubMed] [Google Scholar]




