Abstract
Introduction: The Centers for Disease Control and Prevention (CDC) has listed primary immunodeficiency disorders as being predisposed to severe coronavirus disease 2019 (COVID-19). However, patients affected with X-linked agammaglobulinemia (XLA) have shown contrary results. In this study, we present 2 boys in late adolescence from south India with XLA who were infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as well as a review of cases reported in the literature.
Case Presentation: Two patients with XLA had been diagnosed late and were started on regular immunoglobulin prophylaxis only during adolescence. Both of them had developed bronchiectasis, an irreversible suppurative lung disease. However, both patients made an uneventful recovery without the need for artificial ventilation or convalescent plasma.
Conclusion: Successful outcomes of patients with XLA and COVID-19, except for delayed recovery, from our experience and from global reports are intriguing and the role of B cell depletion is being studied as well. Further research and clinical experience are necessary to fully elucidate the reasons for these observations.
Keywords: COVID-19, SARS-CoV-2, X-linked agammaglobulinemia, primary immunodeficiency
Introduction
Coronavirus disease 2019 (COVID-19) has caused a global crisis, infecting millions and mainly manifesting with mild-to-moderate symptoms. One of the highly vulnerable groups was thought to be patients with primary immunodeficiency disorders (PIDs).1 However, contrary to expectations, patients with humoral immunodeficiency, particularly X-linked agammaglobulinemia (XLA), have been reported to have less severe COVID-19.2 In fact, the role of acalabrutinib, an inhibitor of Bruton's tyrosine kinase in treating severe COVID-19, is being investigated.
We present 2 case reports of adolescents with XLA from south India who were affected with COVID-19 and recovered without the need for artificial ventilation or convalescent plasma. In both patients, the diagnosis of XLA had been confirmed with whole-exome sequencing. Rapid antigen tests for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remained positive for >2 weeks in both cases. However, no post-COVID-19 illness or reinfection has been reported in either patient. The clinical and laboratory parameters of patients are summarized in Table 1. We have also reviewed all the cases reported to date in Supplementary Table S1.
Table 1.
Summary of Clinical Features of Coronavirus Disease-2019 and X-Linked Agammaglobulinemia
| Present illness (COVID-19) | ||
| Parameter | Patient 1 | Patient 2 |
| Age in years (gender) | 18 (M) | 19 (M) |
| Duration of fever (days) | 5 | 10 |
| Other symptoms | Anosmia, ageusia | Expectoration, breathlessness |
| Lymphopenia | No | Yes |
| Chest X-ray | Not taken | Opacity and left perihilar opacity |
| Supplemental oxygen | No | At 2 l/min |
| Clinical investigation | TC-10,600 mm3 | TC-16,700 mm3 |
| Renal and liver function | Normal | Normal |
| sugar | Normal | Normal |
| Electrocardiogram | Normal | Normal |
| Medications given | Azithromycin, | Injection Ceftriaxone, |
| Duration of hospitalization | 11 days in first line treatment center | 22 days |
| Underlying disease (XLA) | ||
| Age at onset (months) | 6 | 2 |
| Age at diagnosis (years) | 8 | 17 |
| Hospitalizations (No.) | 20 | >30 |
| Infections | Diarrhea | Empyema, septic shock, sinusitis, rec |
| Complications | Nonsuppurative arthritis | Stunted growth |
| Immunoglobulin profile in g/L (N) | IgA—0.28 (0.68–3.78) | IgA—0.05 (0.68 – 3.78) |
| CD3 cells/mm3 (range) | 9,553 (570–2,400) | 525 (570–2,400) |
| Genetic mutation | BTK c.C862T:p.R288W (rs128621194) | (hg19) deletion. BTK Exon 3-5 deletion |
COVID-19, coronavirus disease 2019; rec, recurrent infections; XLA, X-linked agammaglobulinemia.
Case Presentation
Patient 1
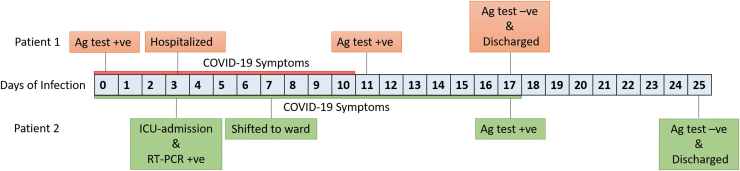
An 18-year-old boy with acute onset of fever, rhinitis, myalgia, and headache tested positive for COVID-19 on day 1. He had fever and rhinitis for 5 days and myalgia and headache for 1 week. He had decreased taste and smell sensation for 1 week after the fever subsided. Examination revealed stable vitals with no evidence of any system involvement. Investigations were unremarkable. He was totally asymptomatic from day 10 and was discharged on day 17 after the rapid antigen test was negative, as per recommendations (Fig. 1). He was employed as a salesman in a supermarket and could have come in contact with COVID-19-positive individuals. None of his family members were affected.
FIG. 1.
Timeline for SARS-CoV-2 infection in patients affected with XLA. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; XLA, X-linked agammaglobulinemia.
The patient had a history of recurrent pneumonia, pyoderma, and diarrhea with onset at 6 months of age. He required decortication for empyema thoracis and was mechanically ventilated once for pneumococcal meningitis with poor sensorium. Pseudomonas aeruginosa was grown in sputum culture on multiple occasions. Although a diagnosis of XLA was made at 8 years of age, he received regular intravenous immunoglobulin (IVIG) infusions only from 14.5 years of age. He developed bronchiectasis and suffered from recurrent arthritis. He had short stature and delayed puberty.
Patient 2
A 19-year-old boy presented with a high fever and cough with copious expectoration for 3 days and breathlessness for 2 days. On examination, he was conscious, oriented, tachypneic, and in hypotensive shock, and SpO2 in room air was 93%. He was pale, emaciated, had grade 3 clubbing, and pectus excavatum. Auscultation revealed harsh vesicular breath sounds with bilateral coarse crepitations. Other systems were within normal limits.
He was treated with parenteral fluids and antibiotics. Nasopharyngeal swab for SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) tested positive. He was commenced on injection dexamethasone and prophylactic IVIG, which was due. He was hospitalized for 22 days of which the initial 3 days were in intensive care. Rapid antigen test for COVID-19 became negative 21 days after the initial RT-PCR (Fig. 1). None of his family members developed COVID-19. He had contact with a COVID-19-positive case while traveling.
He was diagnosed to have XLA only at the age of 17 years. He had been hospitalized >30 times and had life-threatening infections including pneumococcal meningitis, empyema, septic shock, and recurrent pneumonia. Although IVIG had been started at 4 years of age with a diagnosis of hypogammaglobulinemia, it was discontinued after a year. He had arthritis of both knees, which subsided after IVIG prophylaxis was restarted. He had developed bilateral cystic bronchiectasis, but was not hypoxic and was on monthly IVIG infusions and daily azithromycin prophylaxis.
Discussion
We report the cases of 2 adolescent boys with XLA who were infected with SARS-CoV-2 and made an uneventful recovery despite the fact that they had been diagnosed late and had developed bronchiectasis, an irreversible lung disease.
The unexpected good outcomes of both our patients led us to review reports available globally in this group of patients. As of now, there are 11 patients with XLA who reported having COVID-19, all of whom have recovered. The clinical features are summarized in Supplementary Table 1.
Soresina et al. reported 2 patients with XLA who developed interstitial pneumonia due to COVID-19 but recovered, without requiring supplemental oxygen or ICU care.2 In a case series by Ho et al. that included 9 patients with common variable immune deficiency (CVID) and 3 patients with XLA infected with SARS-CoV-2, 2 patients with CVID died, whereas none of those with XLA required ventilatory support and none died.3 Similarly, a recent study reported 60 patients with PID infected with SARS-CoV-2 from the United Kingdom, of whom 4 patients with XLA recovered.4 Mira et al. reported the remarkable recovery of a 39-year-old patient with XLA, after a stagnant clinical course, after infusion of convalescent plasma.5 Jin et al. also reported 3 patients with XLA, who had prolonged courses of COVID-19 with conventional treatment but recovered soon after infusion of donor plasma, followed by a decrease in inflammatory markers and an increase in titers of antibodies to SARS-CoV-2. In 8 of 11 patients with XLA and COVID-19, the recovery was delayed for >3 weeks.6 Guetl et al. reported a patient with viral shedding documented up to 7 weeks after disease onset. The patient had a prolonged hospital stay and recovered after infusion of convalescent plasma and interleukin (IL)-6 blockade with tocilizumab.7
Reports of COVID-19 in cohorts of patients with inborn errors of immunity afford an excellent opportunity to study immunological responses and disease biology. From our experience and the review of reported cases of XLA with SARS-CoV-2 infection, we conclude that the absence of B cells is not detrimental except in delayed recovery.8 There is speculation that in the setting of COVID-19, the lack of B cells could be protective in some instances. For instance, CVID patients with lower CD19 fared better than those with higher CD19, and patients with autoimmune diseases on rituximab seemed to have a milder disease despite total B cell depletion.9 These observations though surprising seem to make sense in the light of the following findings. In the context of SARS-CoV-2, all antibodies produced by B cells are not protective. Recently, pathogenic antibodies directed against immunomodulatory proteins including interferon and other cytokines have been implicated in severe COVID-19.10 B cell-derived cytokines could also contribute to hyperinflammation. Furthermore, the deficiency of Bruton tyrosine kinase (Btk) enzyme in XLA could be protective against cytokine storm as this enzyme is required for the activation of macrophages and IL-6 production.5 This is the rationale for the successful use of acalabrutinib in treating severe COVID-19 and the initiation of a randomized controlled trial.9
The role of B cells in evading SARS-CoV-2 is not completely understood. In the case of patients affected with XLA, the absence of B cells does not increase the severity of the disease. In fact, it may be protective but may lead to delayed recovery. Further research and clinical experience are still needed to elucidate the role of XLA plays in SARS-CoV-2 infections.
Supplementary Material
Acknowledgments
The authors thank all the health care workers and family members who participated in the study. We also thank Dr. Dhanasooraj Dhananjayan and the Multidisciplinary Research Unit at Government Medical College, Kozhikode.
Author Disclosure Statement
All the authors have agreed to the content of the article, and declare no conflicts of interest.
Funding Information
This study was supported by the Council of Scientific and Industrial Research (CSIR) India through grant number MLP1801 (RareGen-CSIR India), Science & Engineering Research Board (SERB) through grant number EMR/2016/006828/HS (SERB, DST), and Foundation for Primary Immunodeficiency Diseases (FPID).
Supplementary Material
References
- 1. Babaha F, Rezaei N. Primary immunodeficiency diseases in COVID-19 pandemic: a predisposing or protective factor? Am J Med Sci 2020; 360:740–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soresina A, Moratto D, Chiarini M, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover [Internet]. Pediatr Allergy Immunol 2020; 31:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ho H-E, Mathew S, Peluso MJ, et al. Clinical outcomes and features of COVID-19 in patients with primary immunodeficiencies in New York City. J Allergy Clin Immunol Pract 2020; 9:490–493.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shields AM, Burns SO, Savic S, et al. COVID-19 in patients with primary and secondary immunodeficiency: the United Kingdom experience [Internet]. Journal of Allergy and Clinical Immunology 2021; 147:870–875.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mira E, Yarce OA, Ortega C, et al. Rapid recovery of a SARS-CoV-2–infected X-linked agammaglobulinemia patient after infusion of COVID-19 convalescent plasma [Internet]. J Allergy Clin Immunol 2020; 8:2793–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jin H, Reed JC, Liu STH, et al. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J Allergy Clin Immunol Pract 2020; 8:3594–3596.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guetl K, Moazedi-Fuerst F, Rosskopf K, et al. SARS-CoV-2 positive virus culture 7 weeks after onset of COVID-19 in an immunocompromised patient suffering from X chromosome-linked agammaglobulinemia [Internet]. J Infect 2021; 82:414–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quinti I, Lougaris V, Milito C, et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146:211–213.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roschewski M, Lionakis MS, Sharman JP, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol 2020; 5:eabd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020; 370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.