Abstract
Delta-8-tetrahydrocannabinol (Δ8-THC) is chemically and functionally similar to delta-9-tetrahydrocannabinol (Δ9-THC) (the primary psychoactive cannabinoid in the cannabis plant) and is currently widely available “over-the-counter” across the United States due to unregulated sales. However, these products have a questionable legal status based on current U.S. laws, as Δ8-THC is considered a Schedule I drug by the federal Drug Enforcement Administration (DEA). Despite this designation, Δ8-THC products (e.g., gummies, edibles, oils, and vapes) are largely unregulated and are sold in gas stations, online, and other marketplaces (most often outside of authorized dispensaries) and are marketed as legal hemp products. This problem arises from a purposeful misinterpretation of the 2018 Farm Bill, which some interpret as legalization of non-Δ9-THC cannabinoids (notably, Δ8-THC). The widespread availability of Δ8-THC products has not been without health consequences. The lack of regulation means that there are no required warning labels or packaging protections in place and no mandated laboratory analysis to assure label accuracy or product purity. As Δ8-THC produces physiological and toxicological effects that are similar to Δ9-THC, high-dose exposure of Δ8-THC (e.g., consuming a full bag of Δ8-THC gummies) has resulted in recent reports of medical emergencies, including calls to poison control centers and presentations to emergency departments, with some pediatric patients arriving unconscious and unresponsive. Several states and regulatory agencies have called for legislation to regulate Δ8-THC, but little progress has occurred nationally thus far.
Keywords: Δ8-THC, delta-8-tetrahydrocannabinol, United States Farm Bill, Drug Enforcement Agency, Schedule I, regulatory
Delta-8-Tetrahydrocannabinol: Legal Hemp-Derived Product or Schedule I Drug?
In December 2018, Congress passed the Agricultural Improvement Act of 2018, commonly known as the 2018 Farm Bill, which amended the federal Controlled Substances Act to exclude hemp and hemp derivatives from the Schedule I definition of cannabis (with the intention of allowing farmers to produce industrial hemp).1 This bill indicated that Cannabis plants containing <0.3% Δ9-THC and their derived products were no longer scheduled substances. However, the statute is silent on the psychoactive compound delta-8-tetrahydrocannabinol (Δ8-THC) and other cannabinoids. Initially, this was not thought to be problematic because Δ8-THC occurs at very low levels in legal hemp plants. However, Δ8-THC can be readily chemically synthesized from cannabidiol (CBD)2—this has led to synthetic Δ8-THC being added in high concentrations to edible and inhaled products (e.g., gummies and vapes) that are marketed as “legal hemp” products. In addition, plant material with low Δ8- and Δ9-THC concentrations is being laced with synthetic Δ8-THC at concentrations far above what would naturally occur in the plant (similar to the process to create illicit K2/Spice products in which plant material is sprayed with illegal synthetic cannabinoids [e.g., JWH compounds]).
Importantly, in August 2020, the Drug Enforcement Administration (DEA) issued new regulations aimed at clarifying the Farm Bill and quite clearly indicated the Schedule I status of Δ8-THC.3 Entitled “Implementation of the Agriculture Improvement Act of 2018,” the DEA's Interim Final Rule states that “[a]ll synthetically derived tetrahydrocannabinols remain Schedule I controlled substances” as the Farm Bill “does not impact the control status of synthetically derived tetrahydrocannabinols.” The DEA also specifically lists Δ8-THC as a Schedule I substance under the category “tetrahydrocannabinols.”4
The perceived legal loophole in the 2018 Farm Bill has prompted the proliferation of Δ8-THC products, with sales occurring in the same marketplaces as hemp and CBD products throughout the United States (e.g., gas stations and bodegas). Despite widespread availability, clinical researchers cannot easily conduct studies in humans with Δ8-THC products (e.g., no USP/pharmaceutical-grade drug sources available for safe testing). However, data on the health consequences of these products are desperately needed.
Behavioral Pharmacology and Potential Therapeutic Efficacy
We are aware of one randomized double-blind study examining the abuse potential and physiological effects of Δ8-THC.5 This study, published in 1972, compared the effects of (1) oral doses of Δ8-THC (20, 40 mg) and Δ9-THC (20 mg) in a small sample (n=6), and (2) intravenous doses of Δ8-THC (n=3) and Δ9-THC (n=4). Intravenous doses began at 1 mg per injection; injections continued up to a total dose of 9 mg if tolerated (no placebo controls were implemented in either arm of the study). The results indicated that oral Δ8-THC and Δ9-THC produced a similar profile of typical cannabinoid agonist effects, including euphoria, dry mouth, reddened eyes, dizziness, blurred vision, relaxation, and small increases in heart rate (7–12 bpm); symptom onset occurred 30–90 min after administration, with peak effects at 2.5–3.5 h (with some effects still present at 5 h). The authors reported that composite subjective mood scores indicated that 40 mg Δ8-THC >20 mg Δ9-THC >20 mg Δ8-THC. Results from the IV study were comparable, with dose-dependent increases in tachycardia (9–41 bpm), faster onset (i.e., peak effects at 20–60 min), and global drug effect ratings that were slightly greater for Δ9-THC. The authors concluded that Δ8-THC is slightly less potent than Δ9-THC; however, a formal potency analysis was not conducted. Nonetheless, the clinically relevant profile of the two compounds suggests quite similar magnitude and profile of effects. However, anecdotal reports and product marketing advertisements suggest that Δ8-THC produces a more mellow and mild high than Δ9-THC. More research on the topic is necessary, particularly studies using highly controlled methods to further investigate the abuse potential, impairment, pharmacokinetic effects, and the overall risk profile of currently used products.
One other study has been conducted on the therapeutic effects of Δ8-THC in pediatric cancer patients.6 In this study, eight children (ages 3–13 years) with hematologic cancer were given oral Δ8-THC at 18 mg/m2 to reduce vomiting. The authors reported a complete prevention of vomiting in patients, noted that no major side effects were observed, and the dose of Δ8-THC administered was much higher and better tolerated than Δ9-THC in adult patients. However, there was also no placebo or standard of care group for comparison (metoclopramide was used as a control at the start of the study; however, it was discontinued because Δ8-THC appeared more effective). Additional research is also needed to examine the potential therapeutic effects of Δ8-THC.
Clinical Management of Δ8-THC Intoxication
Clinically, distinguishing Δ8-THC ingestion from Δ9-THC ingestion by hospital toxicology laboratories is difficult and does not change medical management. Currently, symptomatic and supportive care without any antidotal therapy is indicated for acute intoxication. Signs and symptoms of Δ8- and Δ9-THC intoxication are identical5 (e.g., tachycardia, bradycardia, hypotension, dizziness, dryness of mucous membranes, paresthesia, incoordination, sedation, blurring/distortion of vision, and euphoria); respiratory depression and severe sedation are more common in children7 and after high-dose exposure in adults. However, given its nebulous legal status and ease of accessibility, it is reasonable to expect further ingestions to present to the emergency department.
Chemistry of Δ8-THC
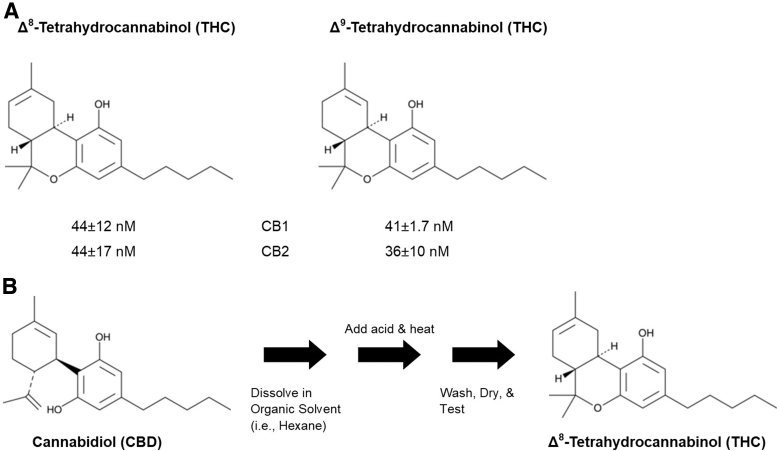
As noted in Figure 1A, Δ8-THC and Δ9-THC are almost identical in structure. The only difference is the location of the double bond in the upper left corner of each molecule (hence “delta-8” versus “delta-9” based on which carbon begins the double bond). Both compounds also display remarkably similar binding affinities and potencies at the two major cannabinoid receptors (CB1 and CB2). In the plant, both molecules are also synthesized from the common precursor cannabigerol. However, most cannabis plants express high concentrations of Δ9-THC, whereas Δ8-THC is detected at low concentrations. To circumvent this, large quantities of Δ8-THC are being chemically synthesized from excess CBD that has been derived from hemp plants (Fig. 1B). This chemical conversion process was patented and described in detail by Raphael Mechoulam and colleagues in 19662; this information can be easily accessed and various internet sites describe this conversion process in step-by-step detail. The resulting concentrated Δ8-THC (which may be contaminated and of unknown purity) can then be added to edible, plant, or inhaled products and sold to the public. These products are then sold as “legal hemp,” based on the manufacture's/seller's interpretation of the Farm Bill, due to the absence of Δ9-THC in the products.
FIG. 1.
(A) Comparison of the chemical structures of Δ8-THC and Δ9-THC and the binding affinity (Ki) at the two cannabinoid receptors (showing near equal binding at both receptors). (B) Conversion of CBD into Δ8-THC. Note that altering the chemical conditions can produce more or less Δ9-THC (and even Δ10-THC). Moreover, the U.S. Cannabis Council report indicates that 94% of tested over-the-counter marketed Δ8-THC products contain Δ9-THC levels ≥0.3%, the legal limit designated by the 2018 Farm Bill.1 Δ8-THC, delta-8-tetrahydrocannabinol; Δ9-THC, delta-9-tetrahydrocannabinol; CBD, cannabidiol.
States' Response to Widespread Availability of Δ8-THC
Currently, individual states are scrambling to close the perceived legal loophole allowing unregulated sales of Δ8-THC, with several states defining Δ8-THC as a controlled substance or attempting to place outright bans on the production and sale of Δ8-THC (e.g., Michigan, Hawaii, and Oklahoma).8 In the absence of additional federal legislation, the likely result will be a patchwork set of state laws with no standardization. This fractured regulatory landscape coupled with the ease of manufacturing these products allows for the distribution of potentially dangerous products (e.g., inaccurate labels that over- or underestimate the cannabinoid content, contamination with toxic chemicals due to the manufacturing process, presence of Δ9-THC as either a by-product of the chemical synthesis or added for additional psychoactive effects).
Conclusion
Patients and consumers deserve to be able to make informed decisions about the products they are using. However, the cannabis market has far outpaced scientific understanding and doctors cannot advise their patients about the use, safety, and efficacy of many widely available cannabis products.
Overall, we have presented the public health challenges of widespread availability of Δ8-THC products due to the misinterpreted legal loopholes in the current legislation. We respectfully suggest a multipronged approach: (1) federal legislative statements that provide clear guidance on plant material composition (e.g., <0.3% of any form of THC, including Δ8- and Δ9-THC and all other Schedule I cannabinoids); (2) federal legislation should unilaterally address all synthetic cannabinoids, including the high concentrations of Δ8-THC used to adulterate legal products; (3) if high dose Δ8-THC products are permitted to be sold, they should be regulated for content and purity and be sufficiently labeled to warn of the side effects and potential for toxicity/overdose. Finally, it is critical for researchers to access and study the actual products being used in the community to provide controlled data for physicians and to better inform public health.
Abbreviations Used
- Δ8-THC
delta-8-tetrahydrocannabinol
- Δ9-THC
delta-9-tetrahydrocannabinol
- CBD
cannabidiol
- DEA
Drug Enforcement Administration
- JWH
John W. Huffman
- USP
United States Pharmacopeia
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by National Institute on Drug Abuse grants R21 DA045101 (S.B.) and R01 DA045700 (S.B.), the Penn State University Elliot S. Vesell Professorship (K.E.V.) and the Pennsylvania-designated Medical Marijuana Academic Clinical Research Center at Penn State (K.E.V.).
Cite this article as: Babalonis S, Raup-Konsavage WM, Akpunonu PD, Balla A, Vrana KE (2021) Δ8-THC: legal status, widespread availability and safety concerns, Cannabis and Cannabinoid Research 6:5, 362–365, DOI: 10.1089/can.2021.0097.
References
- 1. United States H.R.2, Agriculture Improvement Act of 2018.. In: 115th United States Congress. Available at: https://www.congress.gov/bill/115th-congress/house-bill/2 Accessed July 1, 2021.
- 2. Gaoni Y, Mechoulam R. The isomerization of cannabidiol to tetrahydrocannabinols. Tetrahedron. 1966;22:1481–1488. [Google Scholar]
- 3. United States Department of Justice, United States Drug Enforcement Administration. Implementation of the Agriculture Improvement Act of 2018. Available at: https://www.federalregister.gov/documents/2020/08/21/2020–17356/implementation-of-the-agriculture-improvement-act-of-2018 Accessed July 14, 2021.
- 4. United States Department of Justice, United States Drug Enforcement Administration. Controlled Substance Schedules. Available at: https://www.deadiversion.usdoj.gov/schedules/orangebook/c_cs_alpha.pdf Accessed July 14, 2021.
- 5. Hollister LE, Gillespie HK. Delta-8- and delta-9-tetrahydrocannabinol comparison in man by oral and intravenous administration. Clin Pharmacol Ther. 1973; 14:353–357. [DOI] [PubMed] [Google Scholar]
- 6. Abrahamov A, Mechoulam R. An efficient new cannabinoid antiemetic in pediatric oncology. Life Sci. 1995;56:2097–102. [DOI] [PubMed] [Google Scholar]
- 7. Richards JR, Smith NE, Moulin AK. Unintentional cannabis ingestion in children: a systematic review. J Pediatr. 2017;190:142–152. [DOI] [PubMed] [Google Scholar]
- 8. United States Cannabis Council. The unregulated distribution and sale of consumer products marketed as delta-8 THC, 2021. Available at: https://irp.cdn-website.com/6531d7ca/files/uploaded/USCC%20Delta-8%20Kit.pdf; 2021 Accessed July 10, 2021.