Abstract
Preclinical research has demonstrated that exposure to acute stress is associated with attenuated pain perception, so called stress-induced analgesia (SIA). Mechanisms of SIA in humans have not been reliably demonstrated. This study examined the role of the endogenous opioid system in the impact of combined interpersonal and mental stressors on evoked pain responses in 84 participants (34 women). Using a within-subject, double-blinded, counterbalanced design, participants were administered either oral placebo or the opioid antagonist naltrexone (50 mg) across two testing sessions. In each session, they experienced two evoked pain stimuli (cold pressor test [CPT], heat pain) after an extended rest period (rest condition) and after exposure to an acute stressor (a combination of public speaking and mental arithmetic challenge; stress condition). Results showed that both stress and opioid blockade produced significant changes in hormonal and cardiovascular measures, consistent with successful induction of acute stress. Stress was associated with attenuated pain perception (SIA) as indicated by significantly increased CPT tolerance. These effects were particularly pronounced in individuals experiencing the stress condition first. More importantly, SIA effects on CPT tolerance were abolished by opioid blockade. There were no significant SIA effects on heat pain responses. This study demonstrates that the endogenous opioid system may mediate effects of acute stress on pain perception, although this effect seems to be qualified by the type of evoked pain stimuli experienced.
Keywords: Stress-induced analgesia, Pain, Stress, Endogenous opioid, Opioid blockade, Naltrexone
Introduction
Preclinical and human studies indicate that exposure to acute stress may produce a so-called stress induced analgesia (SIA) evidenced by decreased pain perception and increased pain tolerance (al’Absi and Rokke 1991; Ferdousi and Finn 2018; Janssen et al. 2001). SIA, however, has been less consistently observed in humans (Ahmad and Zakaria 2015; Butler and Finn 2009; Flor et al. 2002; Nilsen et al. 2006; Swaab et al. 2005) possibly due to differences in the type of evoked pain induction procedures, inconsistent protocols in terms of duration of stress exposure, and use of different pain-related dependent measures. This human literature on stress and pain has also been confused by studies focusing on clinical pain, for which stress has been found to contribute to increased perceived pain (Bansevicius et al. 1997; Caceres and Burns 1997; Lundberg 1999). Nevertheless, previous research using the cold pressor test in healthy humans has demonstrated the presence of SIA (al’Absi et al. 2013; al’Absi and Petersen 2003).
Existing work has examined several potential mechanisms of SIA, including cardiovascular, baroreceptor-related, and hormonal mechanisms (al’Absi and Petersen 2003; al’Absi et al. 2002; Edwards et al. 2007; Ghione 1996; Rau and Elbert 2001). The endogenous opioid system is directly involved in modulating both pain and the stress response (Akil et al. 1984; Drolet et al. 2001; Martin del Campo et al. 1994; Morris et al. 1990; Rushen et al. 1993; Wand and Schumann 1998), and has been proposed as a possible mechanism underlying SIA (Bandura et al. 1988; Fechir et al. 2012; Ferdousi and Finn 2018; Jungkunz et al. 1983; Willer et al. 1981; Willer and Ernst 1986). Opioid-mediated SIA, if present, may occur through activation of opioidrelevant structures in descending pain pathways (Millan 2002; Ossipov et al. 2010). Transmission of nociceptive information from sensory receptors (nociceptors) to brain structures processing pain including thalamus, hypothalamus, amygdala, and the cortex is known to be modulated by the endogenous opioid system. This is known as “top-down” modulation or the descending pain pathway. Neurons within the primary opioid-rich region of the brain, the periaqueductal gray, directly project and receive inputs from these pain-modulating regions of the brain. These interconnections combined with the extensive presence of opioid receptors and peptides at multiple levels of the descending pain pathway contribute to modulation of pain (Benarroch 2012; Ferdousi and Finn 2018; McNally and Akil 2002). These same brain regions are also involved in regulating the stress response (McEwen 2007), with opioidergic stress response inhibition evidenced by studies showing that opioid blockade increases stress responsiveness (McCubbin et al. 1998, 1996). Such findings support a potential role for the endogenous opioid system in SIA.
Preclinical research has demonstrated a role for the endogenous opioid system in SIA in animals (Parikh et al. 2011; Ferdousi and Finn 2018). Findings in humans regarding opioid-mediated SIA are less consistent. For example, several studies using stressors including threat of electric shock, burn injury, and memory tasks produced mixed results regarding opioid-mediated SIA in the context of electrical (Flor et al. 2002; Robertson et al. 2008; Willer and Ernst 1986). Other work using exposure to phobic stimuli as a stressor also failed to reveal evidence for opioid involvement in SIA in response to electrical pain stimuli (Janssen and Arntz 1997, 1999). In contrast, a small study (n = 14) using a cognitive stressor (Stroop task) found that naloxone reversed the analgesic effect of the stressful task on responses to electrical pain stimulation (Fechir et al. 2012). Other work using stressors, such as threat of electric shock (Willer and Albe-Fessard 1980; Willer and Ernst 1986) or combat-related stimuli (Pitman et al. 1990) has also reported reduction of SIA following opioid blockade in the context of electrical and heat pain stimuli (Werner et al. 2015).
The involvement of the opioid system in SIA appears to be influenced by both the nature of stressors (e.g., controllability and duration) and the pain induction procedures, with SIA in the context of prolonged and uncontrollable stressors most likely to be mediated by opioid mechanisms (Hyson et al. 1982; Maier 1986). In other stressful contexts, nonopioid mechanisms of SIA appear to predominate, potentially reflecting HPA (al’Absi et al. 2004) and endocannabinoid activity (Hohmann et al. 2005; Yesilyurt et al. 2015). To date, no research has directly examined the role of the endogenous opioid system in SIA occurring in the context of a social-evaluative stressor.
The existing literature is limited by the small number of prior human studies addressing opioid SIA issues, the dearth of studies examining opioid SIA in the context of interpersonal stressors most relevant to daily life, small sample sizes, focus on a single sex, and failure to verify the effects of the stressors evaluated (e.g., a variety of physiological measures and self-report). The current study was conducted to examine the role of the endogenous opioid system in SIA using a combination of social-evaluative and cognitive stressors, and employing a pain-induction procedure (cold pressor test; CPT) previously demonstrated to be sensitive to acute stress, as well as a thermal (heat) evoked pain stimulus. Using a double-blinded, counterbalanced, within-subject design, participants were administered either placebo or the opioid antagonist naltrexone, and then experienced two evoked pain stimuli after extended rest and after exposure to acute stressors. Because of previously documented sex differences in pain perception and endogenous opioid system function (al’Absi et al. 2004, 2006; Bruehl et al. 2007; Ceballos et al. 2007; Frew and Drummond 2007; Mogil and Bailey 2010; Smith et al. 2006; Zubieta et al. 2002), we also tested for possible sex differences in opioid SIA. We hypothesized that stress would elicit SIA for both evoked pain tasks, and that participants when receiving naltrexone would display less SIA than when receiving placebo if the SIA was opioid-mediated.
Methods
Participants
Participants were recruited via local newspapers and flyers placed in the community as well as from introductory psychology classes held at the University of Minnesota. Inclusion criteria were as follows: (a) 18–60 years of age; (b) biological parents available to confirm parental blood pressure history; (c) no current or past history of hypertension; (d) no history of renal or hepatic dysfunction, current cardiac or other chronic diseases (e.g., angina, coronary heart disease, arrhythmias, diabetes, hyperlipemia, neurological disorders, thyroid, respiratory disorders); (e) no current opiate dependence, recent daily opiate use, or use of any opiate medication within 3 days prior to the study; (f) no contraindications to naltrexone; (g) no history of a major psychiatric disorder (e.g., depression, schizophrenia, alcohol and drug abuse); (h) no routine use of prescription medications of any kind; and (i) not pregnant (females only). Participants received approximately $20/h for their participation. This study was approved by the Institutional Review Board of the University of Minnesota.
A total of 94 participants were enrolled in the study. Ten individuals withdrew by the second laboratory session. Reasons for withdrawal varied, including conflicts in participants’ schedule and loss of interest in the study. Women participated during the same phase of the menstrual cycle in both sessions. No one in the sample had experienced menopause. A preliminary analysis using one-way ANOVAs and Chi-square tests found no differences in age, BMI, education, marital status, ethnicity, and tobacco use between those who completed the study and those who withdrew (p’s > 0.20). The final sample consisted of 84 participants (34 women and 50 men).
Apparatus and measures
Pain induction and measures
We used the cold pressor test (CPT) and thermal (heat) pain induction to assess sensitivity to evoked pain stimuli. The order of the two pain tasks was counterbalanced. In the CPT, participants were instructed to place their hand into a 1-gallon container filled with an ice-water slurry (0–1 °C), and remove the hand when they could no longer tolerate the pain (i.e., pain tolerance was assessed in seconds) or when the maximum time of 240 s was reached. In the heat pain task, a computer-operated 2 cm2 Peltier thermode was used (Medoc TSA 2001, Minneapolis, MN). Heat stimuli were applied to the skin on the left volar forearm, with a baseline temperature of 35 °C used between trials. Pain perception was assessed using an ascending method of limits protocol with a staircase ramp of 1 °C/s (Fillingim et al. 1998). Participants were instructed to press a button at the point when the stimulus first became painful (pain threshold) and once again when the stimulus became intolerable (pain tolerance). This procedure was repeated four times and the mean of the last three trials was calculated to determine pain threshold and tolerance. The position of the thermode was changed by one centimeter after each trial to control for habituation or sensitization. The thermal stimulation device had an automated maximum temperature cutoff of 52 °C. This latter value was used when the participant did not report reaching pain threshold or tolerance within a trial. The heat pain task lasted approximately 10 min. Immediately after CPT tolerance was reached and after the final heat pain tolerance trial, participants completed the short form of the McGill Pain Questionnaire (MPQ; Melzack 1987) to assess overall pain experience. It took less than 1 min to complete this questionnaire.
Stress tasks
A public speaking task and a mental arithmetic challenge were used in a fixed sequence to induce stress. The public speaking task was a modified version of the Trier Social Stress Test (al’Absi et al. 1997b; Kirschbaum et al. 1993), adapted to accommodate the need for repeated exposure to the public speaking task. Participants were asked to construct and deliver a 4-min speech while being videotaped. Participants were informed that they would be evaluated by judges on poise, articulation, and clarity. Participants were given 4 min to prepare before each speech. Two different versions of stressful scenarios were administered to minimize habituation effects between the two laboratory sessions. In the mental arithmetic challenge (8 min), participants were required to continuously add the digits of a three-digit number and add the sum to the original number. When a mistake was made, the participant was stopped to restart from the previous correct response (i.e., a mild form of interpersonal harassment). Participants were seated throughout both the speech and mental arithmetic stressors, and were instructed to minimize physical movement or gestures during the tasks. The comparison non-stress resting condition consisted of watching the nature-focused documentary television series Planet Earth for a similar length of time as the stressors.
Opioid blockade
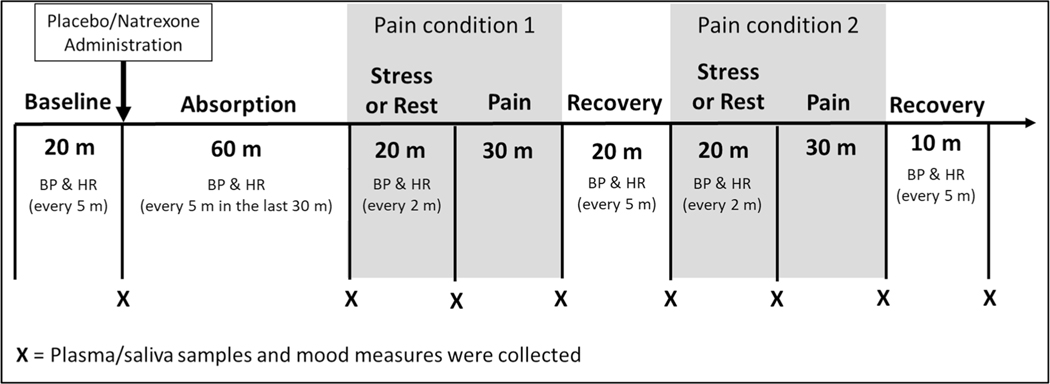
We used a standard oral dose (50 mg) of naltrexone to achieve opioid blockade. Naltrexone is a pure opioid antagonist that provides effective blockade at multiple opiate receptor subtypes. Research in humans (King et al. 2002) demonstrates the effectiveness of this dose in blocking the inhibitory effects of endogenous opioids on the hypothalamic–pituitary–adrenocortical (HPA) axis. Prior work indicates this dose is adequate to produce nearly complete blockade (95%) of μ-opioid receptors (Weerts et al. 2008). Naltrexone reaches peak plasma concentrations within 45–60 min of administration; we, therefore, had a post ingestion rest period of 60 min (Fig. 1).
Fig. 1.
Laboratory protocol. BP blood pressure, HR heart rate
Physiological and biological measures
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were measured using a Dinamap oscillometric monitor (Critikon, Tampa, FL). Blood samples were collected from an indwelling venous cannula according to the protocol described below to measure adrenocorticotropic hormone (ACTH) and plasma cortisol. ACTH was assayed by RIA kits (Nicols Institute, Bad Nauheim, Germany) with a lower sensitivity of 1.0 pg/ml. Plasma cortisol was assayed in duplicate using EIA (DSL, Sinsheim, Germany) with a lower sensitivity of 0.1 μg/dl. Inter- and intra-assay coefficients of variance for these assays were below 10%. Saliva samples were collected using Salivette tubes (Sarstedt, Rommelsdorf, Germany) for the measurement of salivary cortisol responses to stress and opioid blockade.
Mood measures
We assessed positive affect using items of cheerfulness, content, calmness, controllability, and interest. Distress was assessed using items of anxiety, irritability, impatience, and restlessness. These measures were included as a manipulation check for the stressor, with both factors shown to reliably track mood state changes in response to behavioral challenges (al’Absi et al. 1997a, 2003).
Procedure
Interested participants contacted the laboratory and an initial phone screening was conducted. Individuals who met the eligibility criteria were invited to an on-site medical screening. After providing informed consent, participants’ height and weight were measured for calculation of body mass index. They were also asked to complete a form assessing demographic information (age, education, ethnicity, marital status) and substance use. Participants were then scheduled for two laboratory sessions (i.e., two drug conditions). All laboratory sessions started at noon to control for diurnal fluctuations in hormones and lasted about 3.5 h. Prior to each session, research staff contacted the participants to ensure that they refrained from caffeine for 4 h; alcohol use, exercise, and any analgesic medication use for 24 h; and opioid analgesic medication for 72 h prior to their scheduled session.
Participants were tested individually. Upon arrival to the laboratory, the participant was greeted and seated upright in a comfortable chair in a testing room. An IV cannula was inserted by a research nurse and the blood pressure cuff was attached. The protocol consisted of: (1) 20 min baseline; (2) 60 min absorption period; (3) rest or stress—pain; (4) 20 min rest; (5) rest or stress—pain; and (6) 10 min recovery (Fig. 1). During the initial baseline, the participant was asked to sit quietly and watch episodes of Planet Earth. Then, the participant consumed a tablet containing 50 mg of naltrexone or placebo. There were two evoked pain assessment conditions, with order of the conditions counterbalanced across participants and the order kept constant across the two test sessions (i.e., drug conditions). In one pain condition, the pain stimuli were experienced after a 20 min resting period (i.e., rest condition) and in the other, the pain stimuli were experienced after undergoing the two acute stressors (combined public speaking and mental arithmetic; stress condition). BP and HR readings were obtained every 5 min during baseline, absorption, and recovery periods, and every 2 min during the stress tasks. Blood and saliva samples and a self-report measure of mood were collected at the end of each period.
Data analysis
Data were managed in IBM SPSS Statistics for Windows, version 27 (IBM Corp., Armonk, NY, USA) and quality checks were performed (e.g., range checks and checks for inappropriate input). Frequency and descriptive statistics were calculated. Possible sex differences in demographic variables were examined using ANOVAs and Chi-square tests. Sex differences and drug effects on stress and pain measures were tested by MANCOVA using Wilks’ Lambda tests. Tobacco use and task order were included as covariates. To confirm effects of stress and opioid blockade, we conducted a series of 2 sex (female, male) × 2 drug (placebo, naltrexone) × 3 time (baseline, stress, recovery) MANCOVAs on distress, positive affect, SBP, DBP, and HR. ACTH and plasma and salivary cortisol were log transformed to meet the normality assumption. These hormonal measures were tested in 2 sex × 2 drug × 4 time (baseline, rest, stress, recovery) MANCOVAs to verify effects of stress and opioid blockade. A significant interaction that involved the within-subject time effect was followed-up by examination of change scores (the value of baseline was subtracted from the stress value). These change scores were analyzed using a sex × drug ANCOVA. A significant main effect of time was further examined by a multiple comparison test with the Bonferroni correction. For testing primary SIA hypotheses, a series of 2 sex × 2 drug × 2 stress condition (rest, stress) MANCOVAs were performed with evoked pain measures as the dependent variables (CPT tolerance, heat pain threshold and tolerance, MPQ scores after each pain task). Because of occasional missing data, variations existed in the analyses reported regarding sample size and degrees of freedom.
Results
Sample characteristics
The mean age of the sample was 20.8 (SD = 2.0) years. Most participants were White and never married. Females and males did not differ in demographic variables (p’s > 0.10; see Table 1).
Table 1.
Sample characteristics
| Range | Female (n = 34) | Male (n = 50) | |
|---|---|---|---|
| Age (years) | 18–33 | 20.2 (0.5) | 21.2 (0.4) |
| Body mass index (kg/ m2) | 18.7–36.9 | 24.1 (0.6) | 24.8 (0.5) |
| Education (years) | 3–22 | 14.7 (0.4) | 14.3 (0.4) |
| Ethnicity (n; %) | |||
| Single | 30 (88.2) | 46 (92.0) | |
| Other | 4 (11.8) | 4 (8.0) | |
| Marital status | |||
| Caucasian | 30 (88.2) | 40 (80.0) | |
| Other | 4 (11.8) | 10 (20.0) | |
| Tobacco use | |||
| No | 24 (70.6) | 27 (54.0) | |
| Yes | 10 (29.4) | 23 (46.0) |
Otherwise indicated, entries show mean and standard error of the mean
Manipulation checks on stressor effects
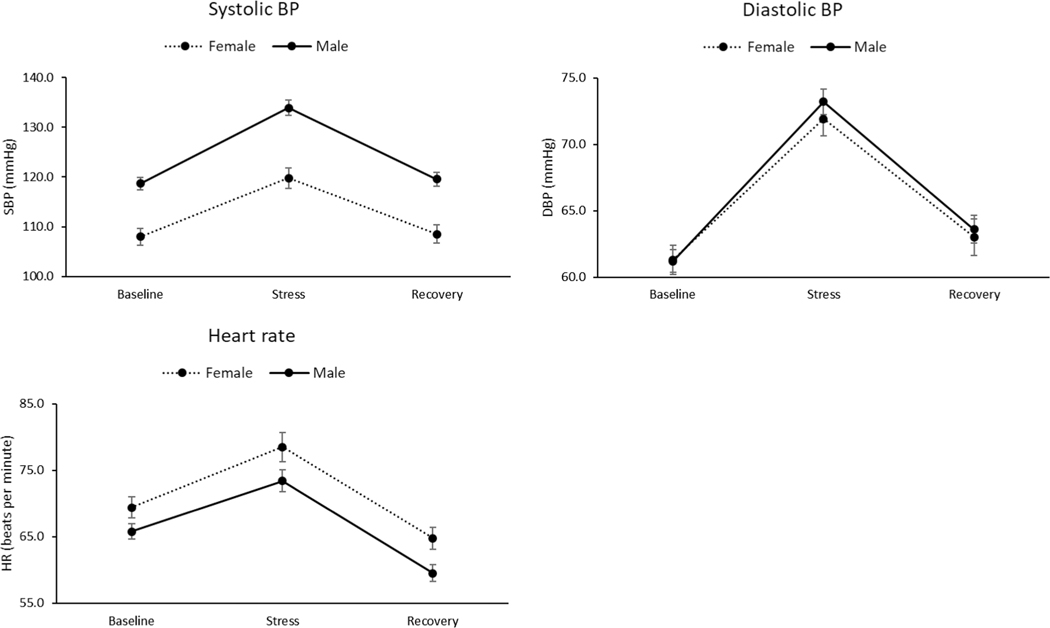
Distress was increased after exposure to the combined stressors in all participants, as indicated by a significant time effect (F (2, 76) = 17.0, p < 0.001, η2 = 0.31; see Table 2). This was further qualified by a sex × time interaction (F (2, 76) = 3.67, p = 0.03, η2 = 0.09). A change score analysis showed that females showed a greater stress-related increase in distress than males (F (1, 78) = 8.94, p = 0.004, η2 = 0.10). Conversely, positive affect decreased significantly after stress exposure (F (2, 76) = 75.0, p < 0.001, η2 = 0.66). SBP, DBP, and HR also increased significantly during the combined stressors (F (2, 77) = 110–252, p < 0.001, η2 = 0.74–88; see Fig. 2). Males displayed a greater stress-related SBP increase than females (F (1, 78) = 32.5, p < 0.001, η2 = 0.29), while females showed a greater stress-related HR increase than males (F (1, 78) = 5.16, p = 0.03, η2 = 0.06). There were also significant sex × time interactions for combined stressor effects on both ACTH (F (3, 39) = 4.86, p = 0.006, η2 = 0.27) and plasma cortisol (F (3, 47) = 3.02, p = 0.04, η2 = 0.16). Change score analyses indicated that, relative to males, females displayed greater stress-related increases in both ACTH (F (1, 46) = 4.39, p = 0.04, η2 = 0.09) and plasma cortisol (F (1, 54) = 3.93, p = 0.05, η2 = 0.07). The findings above indicate that the combined laboratory stressor protocol was successful in eliciting significantly increased physiological stress responses and perceived stress.
Table 2.
Self-report mood, physiological, and biological measures by sex and drug condition
| Female |
Male |
|||
|---|---|---|---|---|
| Placebo | Naltrexone | Placebo | Naltrexone | |
| Distress | ||||
| Baseline | 4.4 (0.6) | 4.2 (0.6) | 4.2 (0.5) | 4.2 (0.4) |
| Stress | 7.4 (0.8) | 8.4 (1.0) | 5.4 (0.6) | 5.8 (0.7) |
| Recovery | 5.3 (0.8) | 5.5 (0.8) | 4.8 (0.6) | 5.0 (0.6) |
| Positive affect | ||||
| Baseline | 19.7 (1.2) | 19.2 (1.1) | 18.5 (0.9) | 19.0 (0.8) |
| Stress | 13.4 (1.2) | 12.5 (1.2) | 13.7 (1.0) | 14.5 (0.9) |
| Recovery | 14.7 (1.3) | 15.0 (1.3) | 16.1 (1.0) | 15.6 (1.0) |
| Systolic BP | ||||
| Baseline | 107.7 (1.9) | 108.2 (1.7) | 119.5 (1.4) | 117.8 (1.3) |
| Stress | 121.2 (2.3) | 118.4 (2.3) | 134.8 (1.7) | 133.3 (1.8) |
| Recovery | 108.4 (2.0) | 108.6 (1.9) | 120.5 (1.5) | 118.7 (1.5) |
| Diastolic BP | ||||
| Baseline | 61.5 (1.1) | 61.0 (1.3) | 61.6 (0.9) | 60.8 (1.0) |
| Stress | 73.1 (1.4) | 70.8 (1.4) | 73.5 (1.1) | 72.8 (1.1) |
| Recovery | 63.5 (1.5) | 62.4 (1.4) | 64.3 (1.2) | 62.9 (1.1) |
| Heart rate | ||||
| Baseline | 69.2 (1.6) | 69.8 (1.8) | 65.7 (1.2) | 65.9 (1.4) |
| Stress | 79.8 (2.1) | 77.3 (2.5) | 73.1 (1.6) | 73.7 (1.9) |
| Recovery | 65.4 (1.6) | 64.3 (1.8) | 60.2 (1.3) | 58.8 (1.4) |
| ACTH | ||||
| Baseline | 2.5 (0.1) | 2.3 (0.2) | 3.3 (0.1) | 3.2 (0.1) |
| Pain (rest) | 2.4 (0.1) | 2.7 (0.2) | 3.0 (0.1) | 3.2 (0.1) |
| Pain (stress) | 2.5 (0.1) | 2.8 (0.2) | 3.0 (0.1) | 3.2 (0.2) |
| Recovery | 2.3 (0.1) | 2.9 (0.2) | 2.8 (0.1) | 2.9 (0.1) |
| Plasma cortisol | ||||
| Baseline | 4.8 (0.1) | 4.8 (0.1) | 4.8 (0.1) | 4.7 (0.1) |
| Pain (rest) | 4.6 (0.1) | 4.8 (0.1) | 4.4 (0.1) | 4.6 (0.1) |
| Pain (stress) | 4.8 (0.1) | 4.9 (0.1) | 4.5 (0.1) | 4.6 (0.1) |
| Recovery | 4.6 (0.1) | 4.9 (0.1) | 4.3 (0.1) | 4.5 (0.1) |
| Saliva cortisol | ||||
| Baseline | 2.3 (0.2) | 2.3 (0.1) | 2.6 (0.1) | 2.5 (0.1) |
| Pain (rest) | 1.7 (0.2) | 2.3 (0.1) | 1.8 (0.1) | 2.1 (0.1) |
| Pain (stress) | 1.8 (0.2) | 2.3 (0.1) | 2.1 (0.1) | 2.3 (0.1) |
| Recovery | 1.7 (0.2) | 2.5 (0.1) | 1.8 (0.1) | 2.2 (0.1) |
Entries show mean and the standard error of the mean that are adjusted for smoking status and the order of task
Fig. 2.
Cardiovascular responses to stress as a function of sex. Line bars indicate standard error of the mean. BP blood pressure, SBP systolic blood pressure, DBP diastolic blood pressure, HR heart rate
Effects of naltrexone on stress responses
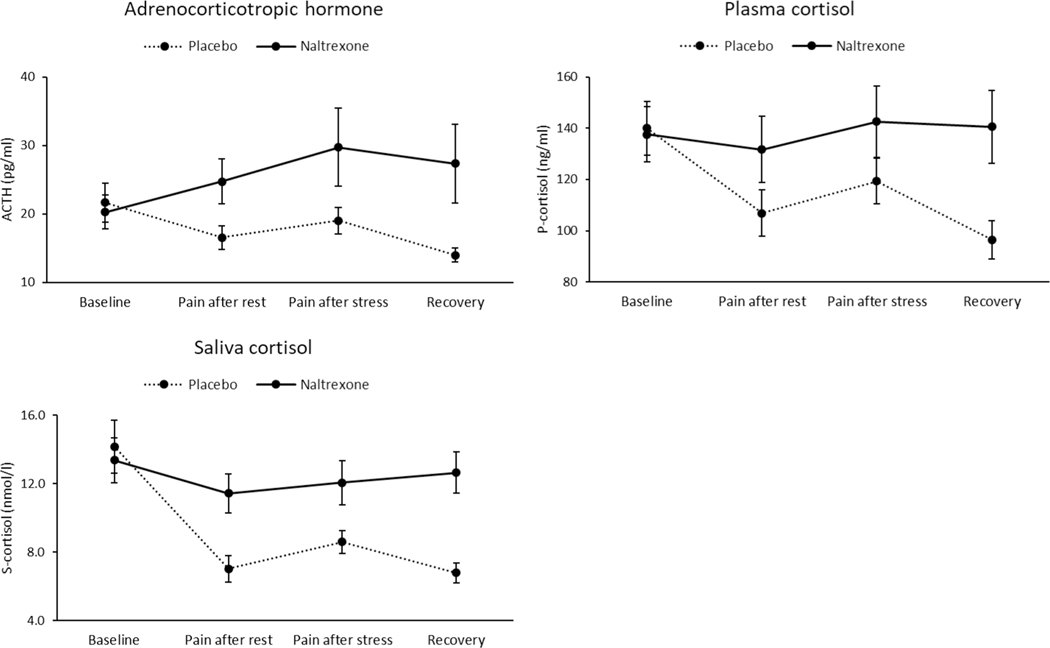
DBP was lower after administration of naltrexone than after placebo (F (1, 78) = 4.57, p = 0.04, η2 = 0.06). There was a drug × time interaction for ACTH (F (3, 39) = 3.98, p = 0.01, η2 = 0.23), plasma cortisol (F (3, 47) = 5.96, p = 0.002, η2 = 0.28), and salivary cortisol (F (3, 74) = 15.6, p < 0.001, η2 = 0.39; see Fig. 3). Change score analyses indicated that stress-related increases in these physiological stress markers in the naltrexone session were greater than in the placebo session (ACTH: F (1, 46) = 6.67, p = 0.01, η2 = 0.13; plasma cortisol: (F (1, 54) = 3.17, p = 0.08, η2 = 0.06; saliva cortisol: (F (1, 77) = 7.95, p = 0.006, η2 = 0.10). This suggest that opioid blockade enhanced physiological stress reactivity (i.e., changes from baseline to the combined stressors).
Fig. 3.
Hormonal responses to stress as a function of drug condition. Line bars indicate standard error of the mean
Stress and naltrexone effects on evoked pain responses
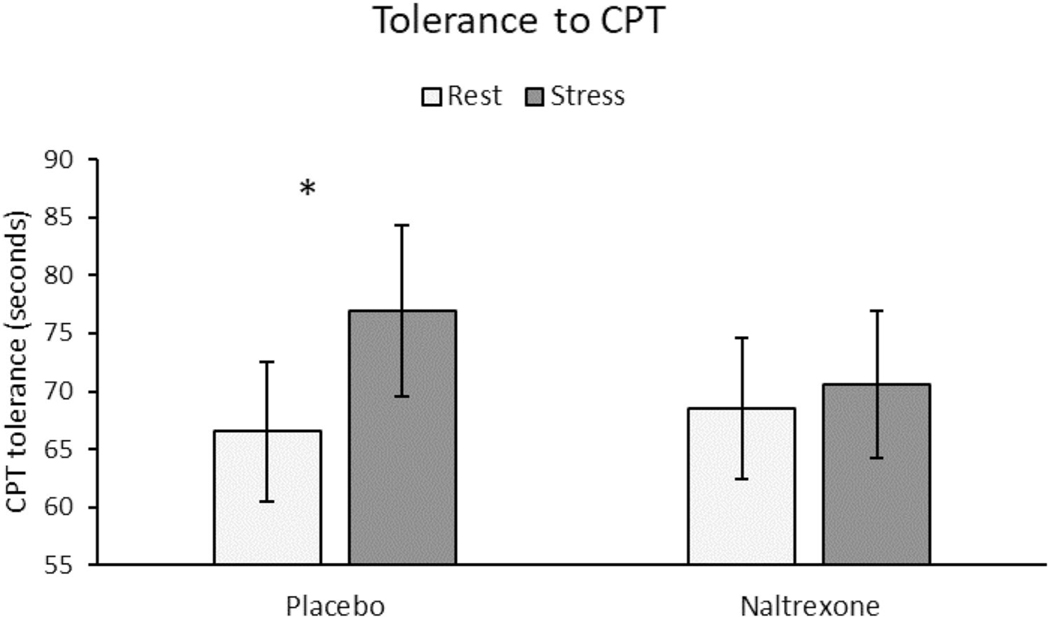
Females had a lower tolerance to the CPT than males (F (1, 72) = 3.87, p = 0.05, η2 = 0.05; see Table 3). Across both sexes, CPT tolerance was significantly higher after stress than rest (F (1, 72) = 4.17, p = 0.045, η2 = 0.06). This SIA effect in CPT tolerance was further qualified by a significant drug × stress interaction (F (1, 72) = 4.24, p = 0.04, η2 = 0.06). Analyses conducted separately within each drug condition (adjusted p value: 0.05/2 tests = 0.025) indicated that significant SIA in CPT tolerance was observed in the placebo condition (p = 0.015) but not in the naltrexone condition (p > 0.10; see Fig. 4). Females reported greater pain intensity based on total MPQ scores after the CPT than did males (F (1, 77) = 6.79, p = 0.01, η2 = 0.08). All other effects for CPT pain rating outcomes were nonsignificant (p’s > 0.10).
Table 3.
Pain measures by sex and drug condition
| Female |
Male |
|||
|---|---|---|---|---|
| Placebo | Naltrexone | Placebo | Naltrexone | |
| CPT tolerance (s) | ||||
| Rest | 55.1 (9.4) | 57.5 (9.4) | 78.0 (7.4) | 79.6 (7.5) |
| Stress | 64.2 (11.4) | 60.2 (9.8) | 89.6 (9.0) | 80.9 (7.8) |
| CPT MPQ-total | ||||
| Rest | 17.2 (1.4) | 19.0 (1.6) | 14.1 (1.1) | 13.6 (1.2) |
| Stress | 17.6 (1.3) | 18.2 (1.4) | 13.6 (1.0) | 13.8 (1.1) |
| Heat pain threshold (°C) | ||||
| Rest | 43.4 (0.6) | 42.7 (0.6) | 43.7 (0.5) | 43.6 (0.5) |
| Stress | 43.0 (0.6) | 43.2 (0.6) | 44.3 (0.4) | 43.8 (0.5) |
| Heat pain tolerance (°C) | ||||
| Rest | 48.0 (0.4) | 47.8 (0.4) | 49.1 (0.3) | 49.0 (0.3) |
| Stress | 47.9 (0.4) | 48.2 (0.4) | 49.3 (0.3) | 49.1 (0.3) |
| Heat pain MPQ-total | ||||
| Rest | 19.0 (1.3) | 18.9 (1.3) | 15.3 (1.0) | 15.2 (1.0) |
| Stress | 17.8 (2.1) | 19.6 (1.3) | 17.4 (1.6) | 14.9 (1.0) |
Entries show mean and the standard error of the mean that are adjusted for smoking status and the order of task
Fig. 4.
Tolerance to cold pressor test. Line bars indicate standard error of the mean. *A significant drug × stress interaction (p = 0.04) indicated a significant stress-induced analgesia in CPT tolerance in the placebo condition (p = 0.015) but not in the naltrexone condition (p > 0.10)
With respect to the heat pain task, no participants reached the 52 °C maximum prior to reporting heat pain threshold. For heat pain tolerance, 6 out of 83 participants reached 52 °C in both the stress and rest conditions after receiving placebo, and 6 (stress condition) and 5 (rest condition) out of 81 participants reached the 52 °C maximum after receiving naltrexone. Relative to males, females had a lower tolerance (F (1, 77) = 6.72, p = 0.01, η2 = 0.08) and higher MPQ scores (F (1, 78) = 4.14, p = 0.045, η2 = 0.05). There was no sex difference in pain threshold, nor were there any significant stress main effects or drug × stress interactions for heat pain tolerance, threshold, or pain ratings (all p’s > 0.10).
To explore possible sources underlying SIA and the effects of opioid blockade on SIA for the CPT, we conducted further analysis of the CPT tolerance outcome using univariate regressions. We found that greater SBP during stress was associated with a greater tolerance on the CPT task (r’s = 0.25–0.35, p’s < 0.01) in both drug conditions. In addition, SBP during stress was the only significant predictor of CPT tolerance in both the placebo (β = 0.52, t = 2.74, p = 0.009) and opioid blockade condition (β = 0.47, t = 2.36, p = 0.02), in a multiple regression including changes in distress, positive affect, DBP, HR, ACTH, and plasma and saliva cortisol during stress as predictors.
To evaluate whether differing pain task characteristics across modalities may have contributed to different SIA findings for the two tasks, we compared MPQ scores and standardized pain tolerance measures (Z-scores) across the two pain induction procedures. We found a difference in levels of perceived pain intensity on the MPQ across the two procedures (F (1, 77) = 7.75, p = 0.007, η2 = 0.09) and between men and women (F (1, 77) = 5.71, p = 0.02, η2 = 0.07). MPQ scores following the heat pain task were higher than after the CPT, and females reported greater pain intensity than males.
Discussion
The results of this study demonstrated SIA in response to combined cognitive (mental arithmetic) and social-evaluative (public speaking) stressors, evidenced by stress-induced increases in pain tolerance on the CPT. This SIA was abolished by opioid blockade, indicating that it was at least in part endogenous opioid-mediated. This finding supports research in preclinical animal models indicating a role of the endogenous opioid system in SIA (Ferdousi and Finn 2018), and builds on a small human literature indicating opioid-mediated SIA in healthy humans exposed to acute stressors, such as cognitive tasks (Fechir et al. 2012), threat of electric shock (Willer and Albe-Fessard 1980; Willer and Ernst 1986) or combat-related stimuli (Pitman et al. 1990).
The SIA effects noted for the CPT were not observed for the heat pain task in the current study. Consistent with absence of SIA on the heat pain task, standardized ratings of pain intensity were higher for this task than for the CPT. The discrepant findings across the two pain tasks in the current work are similar to the larger context of previous human studies of this issue, in which some studies support an opioid component of SIA (Fechir et al. 2012; Jungkunz et al. 1983; Willer and Albe-Fessard 1980; Willer et al. 1981; Willer and Ernst 1986) and other do not (Flor et al. 2002; Robertson et al. 2008; Willer and Ernst 1986). Reasons for the lack of consistency in findings supporting an opioid component of SIA in humans may in part be due to the different stressors used across studies (e.g., cognitive vs. physical vs. interpersonal). Another potential contributor relates to differences in specific pain task characteristics, such as the longer duration of the CPT pain stimulus (up to 240 s in this study) compared to the thermal task (each stimulus ≈ 15 s or less), or the relatively larger surface area of stimulus exposure in the CPT, which may impact level of engagement of endogenous pain pathways (Ruscheweyh et al. 2010). Animal research also suggests that stressor duration (Parikh et al. 2011) and perceived stressor controllability (Hyson et al. 1982; Maier 1986) are likely to impact the extent of opioid-mediated SIA. Potential impact of these factors on opioid-mediated SIA in the current work cannot be assessed.
The current findings also provide evidence supporting a role for cardiovascular-related mechanisms in SIA. Secondary analyses showed that greater systolic blood pressure during stress predicted the magnitude of the SIA observed for CPT tolerance. This finding is entirely consistent with prior work documenting links between the cardiovascular and pain regulatory systems (Bruehl and Chung 2004; Bruehl et al. 2002; Olsen et al. 2013), and more specifically, evidence suggesting that greater baroreceptor activation associated with stress-related SBP increases could account for these hypoalgesic effects (al’Absi et al. 2005; Angrilli et al. 1997; Bruehl and Chung 2004; Bruehl et al. 2008; Edwards et al. 2003, 2002; Rau and Elbert 2001). The fact that stressrelated BP hypoalgesic effects were observed in the current work even under opioid blockade adds to the small human literature suggesting that both opioid and nonopioid mechanisms may contribute to these associations (Bruehl et al. 2010, 2002; Ring et al. 2008).
Examination of physiological and self-report measures confirmed that the combined laboratory stressors effectively induced the stress response necessary to observe SIA. The stressors were associated with significant increases in blood pressure, heart rate, ACTH, and cortisol, as well as increased distress and reduced positive mood. Our findings also supported a role for endogenous opioids in inhibiting stress responsiveness, consistent with some prior work (e.g., McCubbin et al. 1996, 1998). Stress-related increases in ACTH and both blood and salivary cortisol were significantly larger in the naltrexone condition when endogenous opioids were blocked than during the placebo condition.
Beyond endogenous opioid mechanisms, stress response systems may also be involved in mediating the effects of stress on pain. For example, research has shown that increased adrenocortical activity, demonstrated by high basal cortisol levels, prior to exposure to pain may contribute to decreased pain sensitivity (al’Absi et al. 2002). This may reflect enhanced activity of both the opioid system and HPA axis, via increased corticotropin-releasing hormone (CRH) and subsequent increase of ACTH (Lariviere and Melzack 2000). CRH has also been suggested as possibly modulating pain pathways as indicated by pharmacological studies (Hargreaves et al. 1987), although this effect is not consistent (Lautenbacher et al. 1999; Miguel and Nunes-deSouza 2011; Mousa et al. 2007; Yarushkina and Filaretova 2018). Indirect evidence through assessing cortisol production in various chronic pain patients also suggests a role for the HPA axis in modulating pain (Morrison et al. 2000; Theorell et al. 2000), although specific mechanisms have not been directly examined.
Several potential limitations of this study should be highlighted. The within-subject design in which stress and rest conditions occurred in the same testing session could have led to carryover effects that impacted our findings, although this risk was mitigated by the counterbalanced order of stress/rest employed across participants, and also by having a resting period of at least 40 min between the stress and rest conditions. Another potential limitation is the relative homogeneity of the sample, which included primarily young and healthy participants. This homogeneity could, however, also be considered a strength in the context of optimizing the ability to test the mechanisms hypothesized in the study. Although we observed sex differences in pain, responses to stress, and opioid blockade effects consistent with earlier studies, we did not specifically address questions related to variability of sex hormones and its potential impact on pain outcomes. It should be noted, however, that the menstrual cycle phase was controlled for across the two sessions (opioid blockade vs. placebo condition). Finally, the sample had an overrepresentation of males. Future studies seeking to replicate the current results would benefit from testing a sample with equal sex distribution, and carrying out stress and resting condition procedures in separate sessions to address possible carryover effects. Notwithstanding its limitations, this study has several strengths including the comprehensive approach to evaluating outcomes measures related to stress and pain, and the use of a pharmacological challenge to tease out the role of the endogenous opioid system. The use of a well-established acute stress protocol, pain induction procedures, and the integration of multiple physiological, hormonal, and subjective stress measures lend credence to the robustness of the findings.
In conclusion, results from this study demonstrate that exposure to acute psychological stress leads to subsequent attenuated pain perception and that this SIA effect is mediated by endogenous opioid activity. These opioidergic SIA effects were noted for CPT tolerance but not heat pain stimuli. These findings also demonstrate significant hormonal and cardiovascular responses to both acute stress and endogenous opioid blockade. Results support a role for the endogenous opioid system in stress-related pain modulation, and also point out the importance of considering the nature and sources of pain induction when examining mechanisms of pain modulation in clinical research.
Acknowledgements
The authors would like to express their appreciation to Deanna Ellestad, and Angie Harju for their assistance on data collection and management of this study.
Funding This work was supported by grants R01DA16351 and R01DA027232 (MA), and R01AG048915 and R01DA050334 (SB) from the National Institute of Health, and an AHA-Grant-in-Aid (MA) from the American Heart Association—Minnesota.
Footnotes
Data availability Research data will be available upon request.
Declarations
Ethical approval This study was approved by the Institutional Review Board of the University of Minnesota. The procedures used in this study adhere to the principles of the Declaration of Helsinki.
Consent participate Written informed consent was obtained from each individual before participation to this study.
Conflict of interest The authors declare no conflict of interest regarding this study.
References
- Ahmad AH, Zakaria R (2015) Pain in times of stress. Malays J Med Sci 22:52–61 [PMC free article] [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM (1984) Endogenous opioids: biology and function. Annu Rev Neurosci 7:223–255 [DOI] [PubMed] [Google Scholar]
- al’Absi M, Petersen KL (2003) Blood pressure but not cortisol mediates stress effects on subsequent pain perception in healthy men and women. Pain 106:285–295 [DOI] [PubMed] [Google Scholar]
- al’Absi M, Rokke PD (1991) Can anxiety help us tolerate pain? Pain 46:43–51 [DOI] [PubMed] [Google Scholar]
- al’Absi M, Bongard S, Buchanan T, Pincomb G, Licinio J, Lovallo WR (1997a) Neuroendocrine and hemodynamic responses to extended mental and interpersonal stressors. Psychophysiology 34:266–275 [DOI] [PubMed] [Google Scholar]
- al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR (1997b) Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology 34:266–275 [DOI] [PubMed] [Google Scholar]
- al’Absi M, Petersen KL, Wittmers LE (2002) Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain 96:197–204 [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B (2003) Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav 74:401–410 [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, Grant JE (2004) Sex differences in pain and hypothalamicpituitary-adrenocortical responses to opioid blockade. Psychosom Med 66:198–206 [DOI] [PubMed] [Google Scholar]
- al’Absi M, France CR, Ring C, France J, Harju A, McIntyre D, Wittmers LE (2005) Nociception and baroreceptor stimulation in hypertension-prone men and women. Psychophysiology 42:83–91 [DOI] [PubMed] [Google Scholar]
- al’Absi M, France C, Harju A, France J, Wittmers L (2006) Adrenocortical and nociceptive responses to opioid blockade in hypertension-prone men and women. Psychosom Med 68:292–298 [DOI] [PubMed] [Google Scholar]
- al’Absi M, Nakajima M, Grabowski J (2013) Stress response dysregulation and stress-induced analgesia in nicotine dependent men and women. Biol Psychol 93:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrilli A, Mini A, Mucha RF, Rau H (1997) The influence of low blood pressure and baroreceptor activity on pain responses. Physiol Behav 62:391–397 [DOI] [PubMed] [Google Scholar]
- Bandura A, Cioffi D, Taylor CB, Brouillard ME (1988) Perceived self-efficacy in coping with cognitive stressors and opioid activation. J Pers Soc Psychol 55:479–488 [DOI] [PubMed] [Google Scholar]
- Bansevicius D, Westgaard RH, Jensen C (1997) Mental stress of long duration: EMG activity, perceived tension, fatigue, and pain development in pain-free subjects. Headache 37:499–510 [DOI] [PubMed] [Google Scholar]
- Benarroch EE (2012) Periaqueductal gray: an interface for behavioral control. Neurology 78:210–217 [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY (2004) Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neurosci Biobehav Rev 28:395–414 [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Ward P, Johnson B, McCubbin JA (2002) The relationship between resting blood pressure and acute pain sensitivity in healthy normotensives and chronic back pain sufferers: the effects of opioid blockade. Pain 100:191–201 [DOI] [PubMed] [Google Scholar]
- Bruehl S, al’Absi M, France CR, France J, Harju A, Burns JW, Chung OY (2007) Anger management style and endogenous opioid function: is gender a moderator? J Behav Med 30:209–219 [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Diedrich L, Diedrich A, Robertson D (2008) The relationship between resting blood pressure and acute pain sensitivity: effects of chronic pain and alpha-2 adrenergic blockade. J Behav Med 31:71–80 [DOI] [PubMed] [Google Scholar]
- Bruehl S, Burns JW, Chung OY, Magid E, Chont M, Gilliam W, Matsuura J, Somar K, Goodlad JK, Stone K, Cairl H (2010) Hypoalgesia associated with elevated resting blood pressure: evidence for endogenous opioid involvement. J Behav Med 33:168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RK, Finn DP (2009) Stress-induced analgesia. Prog Neurobiol 88:184–202 [DOI] [PubMed] [Google Scholar]
- Caceres C, Burns JW (1997) Cardiovascular reactivity to psychological stress may enhance subsequent pain sensitivity. Pain 69:237–244 [DOI] [PubMed] [Google Scholar]
- Ceballos NA, France CR, al’Absi M (2007) Influence of naltrexone administration on dehydroepiandrosterone sulfate levels in male and female participants. Biol Psychol 74:414–416 [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF (2001) Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry 25:729–741 [DOI] [PubMed] [Google Scholar]
- Edwards L, McIntyre D, Carroll D, Ring C, Martin U (2002) The human nociceptive flexion reflex threshold is higher during systole than diastole. Psychophysiology 39:678–681 [DOI] [PubMed] [Google Scholar]
- Edwards L, McIntyre D, Carroll D, Ring C, France CR, Martin U (2003) Effects of artificial and natural baroreceptor stimulation on nociceptive responding and pain. Psychophysiology 40:762–769 [DOI] [PubMed] [Google Scholar]
- Edwards L, Ring C, France CR, al’Absi M, McIntyre D, Carroll D, Martin U (2007) Nociceptive flexion reflex thresholds and pain during rest and computer game play in patients with hypertension and individuals at risk for hypertension. Biol Psychol 76:72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechir M, Breimhorst M, Kritzmann S, Geber C, Schlereth T, Baier B, Birklein F (2012) Naloxone inhibits not only stress-induced analgesia but also sympathetic activation and baroreceptor-reflex sensitivity. Eur J Pain 16:82–92 [DOI] [PubMed] [Google Scholar]
- Ferdousi M, Finn DP (2018) Stress-induced modulation of pain: Role of the endogenous opioid system. Prog Brain Res 239:121–177 [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Maixner W, Kincaid S, Silva S (1998) Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain 75:121–127 [DOI] [PubMed] [Google Scholar]
- Flor H, Birbaumer N, Schulz R, Grusser SM, Mucha RF (2002) Pavlovian conditioning of opioid and nonopioid pain inhibitory mechanisms in humans. Eur J Pain 6:395–402 [DOI] [PubMed] [Google Scholar]
- Frew AK, Drummond PD (2007) Negative affect, pain and sex: the role of endogenous opioids. Pain 132(Suppl 1):S77–S85 [DOI] [PubMed] [Google Scholar]
- Ghione S (1996) Hypertension-associated hypalgesia. Evidence in experimental animals and humans, pathophysiological mechanisms, and potential clinical consequences. Hypertension 28:494–504 [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Mueller GP, Dubner R, Goldstein D, Dionne RA (1987) Corticotropin-releasing factor (CRF) produces analgesia in humans and rats. Brain Res 422:154–157 [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D (2005) An endocannabinoid mechanism for stress-induced analgesia. Nature 435:1108–1112 [DOI] [PubMed] [Google Scholar]
- Hyson RL, Ashcraft LJ, Drugan RC, Grau JW, Maier SF (1982) Extent and control of shock affects naltrexone sensitivity of stressinduced analgesia and reactivity to morphine. Pharmacol Biochem Behav 17:1019–1025 [DOI] [PubMed] [Google Scholar]
- Janssen SA, Arntz A (1997) No evidence for opioid-mediated analgesia induced by phobic fear. Behav Res Ther 35:823–830 [DOI] [PubMed] [Google Scholar]
- Janssen SA, Arntz A (1999) No interactive effects of naltrexone and benzodiazepines on pain during phobic fear. Behav Res Ther 37:77–86 [DOI] [PubMed] [Google Scholar]
- Janssen SA, Spinhoven P, Brosschot JF (2001) Experimentally induced anger, cardiovascular reactivity, and pain sensitivity. J Psychosom Res 51:479–485 [DOI] [PubMed] [Google Scholar]
- Jungkunz G, Engel RR, King UG, Kuss HJ (1983) Endogenous opiates increase pain tolerance after stress in humans. Psychiatry Res 8:13–18 [DOI] [PubMed] [Google Scholar]
- King AC, Schluger J, Gunduz M, Borg L, Perret G, Ho A, Kreek MJ (2002) Hypothalamic-pituitary-adrenocortical (HPA) axis response and biotransformation of oral naltrexone. Preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology 26:778–788 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH (1993) The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81 [DOI] [PubMed] [Google Scholar]
- Lariviere WR, Melzack R (2000) The role of corticotropin-releasing factor in pain and analgesia. Pain 84:1–12 [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Roscher S, Kohl G, Vedder H, Krieg J (1999) Corticotropin-releasing-hormone lacks analgesic properties: an experimental study in humans, using non-inflammatory pain. Pain 83:1–7 [DOI] [PubMed] [Google Scholar]
- Lundberg U (1999) Stress responses in low-status jobs and their relationship to health risks: musculoskeletal disorders. Ann N Y Acad Sci 896:162–172 [DOI] [PubMed] [Google Scholar]
- Maier SF (1986) Stressor controllability and stress-induced analgesia. Ann N Y Acad Sci 467:55–72 [DOI] [PubMed] [Google Scholar]
- Martin del Campo AF, Dowson JH, Herbert J, Paykel ES (1994) Effects of naloxone on diurnal rhythms in mood and endocrine function: a dose-response study in man. Psychopharmacology 114:583–590 [DOI] [PubMed] [Google Scholar]
- McCubbin JA, Wilson JF, Carlson CR, Bruehl S, Ibarra P, Norton JA, Colclough GW (1996) Relaxation training and opioid inhibition of blood pressure response to stress. Consult Clin Psychol 64:593–601 [DOI] [PubMed] [Google Scholar]
- McCubbin JA, Bruehl S, Wilson JF, Sherman JJ, Norton JA, Colclough G (1998) Endogenous opiods inhibit ambulatory blood pressure during naturally occurring stress. Psychosom Med 60:227–231 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904 [DOI] [PubMed] [Google Scholar]
- McNally GP, Akil H (2002) Role of corticotropin-releasing hormone in the amygdala and bed nucleus of the stria terminalis in the behavioral, pain modulatory, and endocrine consequences of opiate withdrawal. Neuroscience 112:605–617 [DOI] [PubMed] [Google Scholar]
- Melzack R (1987) The short-form McGill Pain Questionnaire. Pain 30(2):191–197 [DOI] [PubMed] [Google Scholar]
- Miguel TT, Nunes-de-Souza RL (2011) Anxiogenic and antinociceptive effects induced by corticotropin-releasing factor (CRF) injections into the periaqueductal gray are modulated by CRF1 receptor in mice. Horm Behav 60:292–300 [DOI] [PubMed] [Google Scholar]
- Millan MJ (2002) Descending control of pain. Prog Neurobiol 66:355–474 [DOI] [PubMed] [Google Scholar]
- Mogil JS, Bailey AL (2010) Sex and gender differences in pain and analgesia. Prog Brain Res 186:141–157 [DOI] [PubMed] [Google Scholar]
- Morris M, Salmon P, Steinberg H, Sykes EA, Bouloux P, Newbould E, McLoughlin L, Besser GM, Grossman A (1990) Endogenous opioids modulate the cardiovascular response to mental stress. Psychoneuroendocrinology 15:185–192 [DOI] [PubMed] [Google Scholar]
- Morrison MF, Redei E, TenHave T, Parmelee P, Boyce AA, Sinha PS, Katz IR (2000) Dehydroepiandrosterone sulfate and psychiatric measures in a frail, elderly residential care population. Biol Psychiatry 47:144–150 [DOI] [PubMed] [Google Scholar]
- Mousa SA, Bopaiah CP, Richter JF, Yamdeu RS, Schäfer M (2007) Inhibition of inflammatory pain by CRF at peripheral, spinal and supraspinal sites: involvement of areas coexpressing CRF receptors and opioid peptides. Neuropsychopharmacology 32:2530–2542 [DOI] [PubMed] [Google Scholar]
- Nilsen KB, Westgaard RH, Stovner LJ, Helde G, Rø M, Sand TH (2006) Pain induced by low-grade stress in patients with fibromyalgia and chronic shoulder/neck pain, relation to surface electromyography. Eur J Pain 10:615–627 [DOI] [PubMed] [Google Scholar]
- Olsen RB, Bruehl S, Nielsen CS, Rosseland LA, Eggen AE, Stubhaug A (2013) Hypertension prevalence and diminished blood pressurerelated hypoalgesia in individuals reporting chronic pain in a general population: the Tromsø study. Pain 154:257–262 [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F (2010) Central modulation of pain. J Clin Investig 120:3779–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh D, Hamid A, Friedman TC, Nguyen K, Tseng A, Marquez P, Lutfy K (2011) Stress-induced analgesia and endogenous opioid peptides: the importance of stress duration. Eur J Pharmacol 650:563–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, van der Kolk BA, Orr SP, Greenberg MS (1990) Naloxone-reversible analgesic response to combat-related stimuli in posttraumatic stress disorder. A pilot study. Arch Gen Psychiatry 47:541–544 [DOI] [PubMed] [Google Scholar]
- Rau H, Elbert T (2001) Psychophysiology of arterial baroreceptors and the etiology of hypertension. Biol Psychol 57:179–201 [DOI] [PubMed] [Google Scholar]
- Ring C, France CR, al’Absi M, Edwards L, McIntyre D, Carroll D, Martin U (2008) Effects of naltrexone on electrocutaneous pain in patients with hypertension compared to normotensive individuals. Biol Psychol 77:191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LJ, Hammond GR, Drummond PD (2008) The effect of subcutaneous naloxone on experimentally induced pain. J Pain 9:79–87 [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Stumpenhorst F, Knecht S, Marziniak M (2010) Comparison of the cold pressor test and contact thermode-delivered cold stimuli for the assessment of cold pain sensitivity. J Pain 11:728–736 [DOI] [PubMed] [Google Scholar]
- Rushen J, Schwartze N, Ladewig J, Foxcroft G (1993) Opioid modulation of the effects of repeated stress on A.C.T.H., cortisol, prolactin, and growth hormone in pigs. Physiol Behav 53:923–928 [DOI] [PubMed] [Google Scholar]
- Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK (2006) Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci 26:5777–5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ (2005) The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 4:141–194 [DOI] [PubMed] [Google Scholar]
- Theorell T, Hasselhorn HM, Vingrd E, Andersson B (2000) Interleukin 6 and cortisol in acute musculoskeletal disorders: results from a case-referent study in Sweden. Stress Med 16:27–35 [Google Scholar]
- Wand GS, Schumann H (1998) Relationship between plasma adrenocorticotropin, hypothalamic opioid tone, and plasma leptin. J Clin Endocrinol Metab 83:2138–2142 [DOI] [PubMed] [Google Scholar]
- Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, McCaul ME (2008) Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology 33:653–665 [DOI] [PubMed] [Google Scholar]
- Werner MU, Pereira MP, Andersen LP, Dahl JB (2015) Endogenous opioid antagonism in physiological experimental pain models: a systematic review. PLoS One 10:e0125887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer JC, Albe-Fessard D (1980) Electrophysiological evidence for a release of endogenous opiates in stress-induced ‘analgesia’ in man. Brain Res 198:419–426 [DOI] [PubMed] [Google Scholar]
- Willer JC, Ernst M (1986) Somatovegetative changes in stress-induced analgesia in man: an electrophysiological and pharmacological study. Ann N Y Acad Sci 467:256–272 [DOI] [PubMed] [Google Scholar]
- Willer JC, Dehen H, Cambier J (1981) Stress-induced analgesia in humans: endogenous opioids and naloxone-reversible depression of pain reflexes. Science 212:689–691 [DOI] [PubMed] [Google Scholar]
- Yarushkina NI, Filaretova LP (2018) The peripheral corticotropin-releasing factor (CRF)-induced analgesic effect on somatic pain sensitivity in conscious rats: involving CRF, opioid and glucocorticoid receptors. Inflammopharmacology 26:305–318 [DOI] [PubMed] [Google Scholar]
- Yesilyurt O, Seyrek M, Tasdemir S, Kahraman S, Deveci MS, Karakus E, Halici Z, Dogrul A (2015) The critical role of spinal 5-HT7 receptors in opioid and non-opioid type stress-induced analgesia. Eur J Pharmacol 762:402–410 [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS (2002) mu-opioid receptormediated antinociceptive responses differ in men and women. J Neurosci 22:5100–5107 [DOI] [PMC free article] [PubMed] [Google Scholar]