Abstract
The Special Interest Group (SIG) on leprosy thought it to be prudent to revisit its previous practice recommendations through this update. During this period, the pandemic course shifted to a 'second wave' riding on the 'delta variant'. While the number of cases increased manifold, so did the research on all aspects of the disease. Introduction of vaccination and data from various drug trials have an impact on current best practices on management of diseases including leprosy. The beneficial results of using steroids in management of COVID-19, gives elbow room regarding its usage in conditions like lepra reactions. On the other hand, the increase in cases of Mucormycosis again underlines applying due caution while recommending immunosuppressants to a patient already suffering from COVID-19. This recommendation update from SIG leprosy reflects current understanding about managing leprosy while the dynamic pandemic continues with its ebbs and flows.
Keywords: Guidelines, leprosy, pandemic
Background
The Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) Special Interest Group (SIG) on leprosy, under the aegis of the IADVL Academy, published recommendations for the management of leprosy in the context of the coronavirus disease (COVID-19) pandemic based on available evidence and expert opinion in 2020.[1] These were deliberated and brought out early in the pandemic to benefit dermatologists and leprologists, regarding the management of leprosy cases with the advent of this novel virus. A couple of factors, that is, the plethora of data published regarding COVID-19 in the past year and the unprecedented surge which India witnessed, the SIG-leprosy sought to revisit these recommendations. There is wide speculation about a third or maybe multiple waves in this pandemic, thus we expect that the new recommendation will remain relevant for some time. (Level of Evidence VII)
What is the significance of this surge for leprosy care in India?
The second wave of COVID-19 pandemic in India reported a peak of 4,01,993 on 30th April and the country needed to channel all health resources to cope with this surge.[2] Thus, all other diseases and neglected tropical diseases, including leprosy, would receive reduced attention. In a recently published multicentric (multicountry) questionnaire-based study during the first wave, the investigators found the suspension of the following health services like diagnostic (5%), reconstructive surgery (87%), active case detection (76.9%), and chemoprophylaxis (66.7%),[3] thus confirming the widespread adverse impact of coronavirus disease (COVID-19) on healthcare delivery of leprosy.
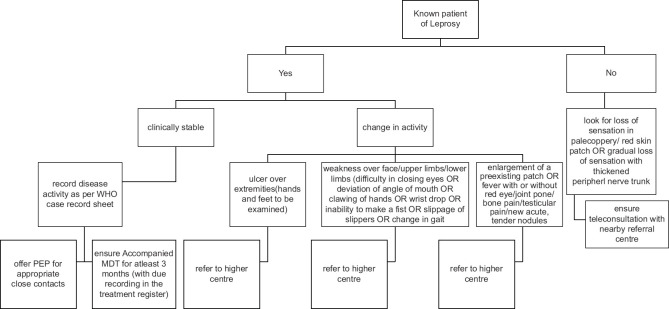
From a bird's eye view, the factors impacting leprosy care during this pandemic include poor access to care, susceptibility to COVID-19 due to use of steroids for management of reactions, unemployment, poverty, overcrowding, stretched healthcare systems, reinforced isolation, fewer funds for research and rehabilitation, and dual stigmatization.[4] With the identification of the value of systemic corticosteroids in the management of complications associated with COVID-19, it has been felt that all forms of oral corticosteroids are no more freely available to leprosy patients including those suffering from lepra reactions. It is obvious considering the above-mentioned constraints that we look beyond the traditional service delivery, to methods such as e-learning, telemedicine, mobile health, and other innovative approaches, to maintain the continuity of services, countering stigma, and ensuring the respect of human rights of people affected by leprosy. Another way is to train and empower healthcare workers using a simple algorithmic approach to reach out in the community and segregate patients requiring urgent referral to higher centers while continuing provision of multidrug therapy (MDT) directly to the patients to prevent defaulting (Figure 1 proposed algorithm for healthcare workers). (Level of Evidence VII)
Figure 1.
Algorithm for healthcare workers
It is a quirk of fate that the Spanish flu of 1918–1919 and the COVID-19 pandemic are separated almost exactly a century apart, but both teach us the same with regard to the importance of public health. We have progressed on various fronts in the interim like a better understanding of diseases, the development of new treatments, the discovery of vaccines, and easier dissemination of scientific information by social media, and yet the inequalities in the society dilute much of these modern achievements.[5]
Is there a new risk group?
SARS-CoV-2 infects all ages, while high-risk groups were already identified mutants of the virus presumably resulted in increased infectivity with more cases in young adults and children.[6,7]
It is well established that India has the highest burden of leprosy in the world and has reported 1,14,451 new leprosy cases in 2019 including 9,227 new pediatric cases in 2018.[8] With COVID-19 increasingly affecting the younger population, its complex interplay with leprosy is likely to play out more during this surge and further.
Leprosy and COVID-19, a mercurial relationship
Expert opinions and some guidelines are suggesting that COVID-19 can influence the incidence and severity of lepra reactions. We must remain mindful that the concurrent stress of the pandemic may also play an important role in triggering or maintenance of reactions. To date, the data suggesting any change in rates of incidence, severity, or recurrence of reactions in leprosy are limited.[9]
Santos et al. (2021)[10] published a surveillance study of hospitalized cases of leprosy with COVID-19 coinfection from 17 March to 30 June 2020 at Aracaju in Brazil. Out of a total of 378 leprosy cases under treatment, only four (1%) were diagnosed with COVID-19 and hospitalized. All the four had the lepromatous disease and ominously all expired. Authors themselves point out that three of them had other comorbidities. In the recently held Biennial conference of Indian Association of Leprologists (IAL), Pai et al[11] presented a series of seven cases with leprosy and COVID-19 coinfection from Mumbai and they observed that despite the presence of comorbidities like diabetes, hypertension, asthma, and tuberculosis in four patients and use of prednisolone for reaction all the patients recovered and none of them developed severe disease. Therefore, the data outlining the impact of COVID-19 on leprosy are still conflicting.
Understanding about markers of prognosis
During the past year of the pandemic, our understanding of predictors of unfavorable outcomes in COVID-19 has improved. The most significant ones include advanced age, raised levels of AST, creatinine, BUN, bilirubin, and neutrophils. Likewise, a reduction in eosinophils, monocytes, basophils, and lymphocytes in peripheral blood smear also help predict the condition of a severely ill patient. Most of these investigations are routine and largely affordable.[12] However, in a study of nine patients with lepromatous leprosy with COVID-19 infection from Turkey, it was observed that all the patients recovered and were discharged despite having poor prognostic markers like low calcium levels, elevated procalcitonin, and interleukins-6 (IL-6) values, which are accepted as criteria of poor prognosis.[13] According to one hypothesis, the severity of the host response in infection with SARS-CoV-2 may be due to aberrant activation of neutrophils in peripheral blood. COVID-19 patients' blood cell counts show a progressive rise in white blood cells, neutrophil percentage, absolute neutrophil count, and neutrophil-to-lymphocyte ratio (NLR). Like COVID-19, cytokine storm and high levels of inflammatory serum mediators including c-reactive protein, neutrophilia, and high NLR have been described in patients with type 2 lepra reaction.[14] A recent case report on concomitant COVID-19 with type 2 lepra reaction has raised the possibility that elevated interferon-γ (INF-γ) levels in the reaction may have a favorable effect on COVID-19 co-infection.[15] That case was successfully managed by utilizing daily prednisolone and methotrexate.
Summary of recent advances and new recommendations
Salient changes in the recommendations between version 1[1] and the current version with level of evidence are summarized in Table 1.
Table 1.
Salient changes in the recommendations by IADVL SIG Leprosy & IADVL academy between First recommendation[1] & the updated version
| First Recommendation[1] | Updated Recommendation | Level of Evidence |
|---|---|---|
| Advocacy (by IADVL & IADVL SIG Leprosy) | Continue | VII |
| Continuation of WHO MB MDT in event of likely COVID-19 infection | Recommendation unchanged (continue MDT) | VII |
| Provision of accompanied MDT whenever possible | Recommendation unchanged | VII |
| Continuation of leprosy-related services by state and district leprosy units | Recommendation unchanged | VII |
| Use of corticosteroids for reactions | Continue optimal dose of corticosteroids, exercising caution during phase of viremia. | II |
| Limit use of corticosteroids below 20 mg/day | ||
| Use of other immunosuppressants | Avoid, when possible, can be given if indicated under supervision. | V |
| Methotrexate (clinical trials ongoing for possible benefit in cytokine storm due to COVID-19) | ||
| Colchicine (clinical trials ongoing for possible benefit in COVID-19) | ||
| Use of chloroquine & hydroxychloroquine | Not recommended in view of recent evidence of their non-benefit vis-à-vis co-infection with COVID-19 | II |
| Use of alternate MDT in case of nonavailability of WHO MDT | Recommendation unchanged | VII |
| Emphasis on self-care for prevention of ulcers and deformities | Recommendation unchanged | VII |
| Use of teledermatology and telecounseling services | Recommendation unchanged. | VII |
| A hybrid model utilizing trained healthcare workers can be oriented for taking leprosy care directly to the patient. (see algorithm) | ||
| Use of minocycline | Recommendation unchanged | VII |
| Elective reconstructive surgeries to be rescheduled | Can be performed as per need under due COVID protocol | VII |
| Leprosy post exposure prophylaxis (LPEP) | LPEP to be offered to all close contacts wherever the index case and family members consent. | VII |
| Role of Clofazimine | Clofazimine, impressive in-vitro efficacy in COVID-19, can be tried in traditional dosages (open market availability is a limiting factor) | VII |
| COVID vaccination | To be encouraged, patients under steroids to be informed about possible sub-optimal uptake. | VII |
| MIP can be offered to patients who are not yet eligible as per national COVID vaccination guidelines. | ||
| Emerging cases of mucormycosis | Exercise extra caution while starting steroids and educate the patient regarding early signs (see Table 2) | VII |
| Hypercoagulability and thromboembolic events | In possibly thromboembolic conditions like leprosy patient with reaction on steroids with or without thalidomide. It is recommend to | VII |
| a. add acetyl salicylic acid (100 mg daily) and | ||
| b. avoid concomitant use of steroid with thalidomide | ||
| Mental health | Mental health is an important concern and should be specifically enquired about and offered appropriate counseling on a case-to-case basis. | VII |
IADVL: The Indian Association of Dermatologists, Venereologists and Leprologists; SID: Special Interest Group; WHO: World Health Organization; MDT: multidrug therapy; Covid 19: coronavirus disease
Re-purposing of drugs traditionally used for management of leprosy to be used in COVID-19
The search for drugs useful in COVID-19, has brought the spotlight on a few which are utilized in the management of leprosy.
Anti-leprosy drugs
Clofazimine
Clofazimine exhibited antiviral activity in Vero E6 cells infected by SARS-CoV-2 in vitro, thereby prompting a clinical trial of the drug combined with IFN β-1b. Further, it was demonstrated that administration of clofazimine reduced viral load in the lungs and also fecal viral shedding.[16] Being a cheap drug, it may serve as an additional tool against the variants of coronavirus too, on which the vaccines are less effective. The downside is the unreliable supply of clofazimine in the open market, although it is available in the World Health Organization (WHO) MDT packs in government supply. Considering the dual advantage that it offers, regular availability of clofazimine in the open market should be ensured. (Level of Evidence VII)
Drugs given in reactions
Caution using steroids
Patients with lepra reactions are often on systemic corticosteroids. These are essential drugs especially in managing neuritis where delay in their use can lead to permanent disability. There is an obvious apprehension that the usage of corticosteroids may immunosuppress the patient and thereby lead to a poor outcome in the event of co-infection with COVID-19. There is a trend noted for an increased risk of poor outcome in COVID-19 hospitalized patients in case of previous exposure to corticosteroid before hospital admission. However, in a recently published study, the authors conceded that after adjustment for potential confounders, they found no evidence for increased risk in patients who were on steroids before the infection.[17] The reports are stating that COVID-19 is a biphasic illness with an initial viremia and steroid usage at the time of viraemia may suppress protective innate response as well as result in delayed recovery.[18] Yet, the available data on this aspect of steroids in COVID-19 are equivocal. The preliminary results of the RECOVERY trial support use of dexamethasone for up to 10 days in patients who were receiving invasive mechanical ventilation, but report no benefit and possible harm in the subgroup of hospitalized patients who were not on respiratory support.[19] The studies in this regard to leprosy patients are lacking except for a few case reports and series, where patients were on prednisolone for management of reactions and no adverse outcomes were observed.[11,15]
There is a recent and important caveat though, prolonged steroid use or abuse may predispose the patients to the development of rhinocerebral and pulmonary mucormycosis. In a nutshell, each case needs to be considered on its merit, steroids should not be used indiscriminately and one should not refrain from utilizing systemic steroids on recommended indications. (Level of Evidence II)
Methotrexate and other immunomodulators
High-dose of methotrexate with leucovorin rescue has been postulated as an immune stabilization strategy for SARS-CoV-2 induced “PANIC” attack for severe COVID-19.[20] Recent work by Gisondi et al.[21] suggests that outcomes of patients receiving systemic immunomodulatory therapies in COVID-19 patients are similar to those of the general population. These findings may relate to the aberrant cytokine and inflammatory responses in severe COVID-19, which may be treated or partially blunted by cytokine-targeted therapy.[22] Holcomb et al.[23] have reported that the risk of both COVID-19 and poor outcomes is minimally affected by dermatologic immunomodulatory medications. Based on this evidence, we suggest that systemic immunomodulators can be used judiciously for the control of severe leprosy reactions. (Level of Evidence V)
Colchicine
An age-old drug colchicine is also utilized in type 2 lepra reactions.[24,25] There are suggestions about its favorable role in COVID-19 and especially in a resource-poor setting like ours. Colchicine was suggested to be useful for the treatment of some complications of COVID-19 infection, based on its action on IL-6 and inflammasomes, which may help in ameliorating the cytokine storm.[26,27]
Colchicine can be tried in the condition of coexistence of COVID-19 and type 2 lepra reaction.[28] The dose of colchicine is 0.5 to 2 mg/day in two to three divided doses. Monitoring for the adverse effects by regular complete blood counts, liver and renal function tests. The total daily dose should be ≤4 mg and in renal patients ≤2 mg.[29]
Vaccines
Vaccines against SARS-CoV-2 are still our best bet against the current pandemic. With patients of lepra reactions on steroids or immunosuppressants, doubts abound regarding the safety and reliable immunogenicity. The thumb rule should be that COVID vaccination should not be delayed and although such recommendations are somewhat general in nature but can be extrapolated to leprosy.[30] (Level of Evidence VII).
The vaccines are expected to be safe for patients on drugs affecting immunity with some diminution in efficacy. Such a diminution too is dependent on the dose of immunosuppressives, their mechanism of action, and the type of vaccine administered.[31]
Theoretically, BCG vaccine can confer protection in SARS-CoV-2 via two mechanisms: (i) induction of controlled pro-inflammatory pathways (“trained immunity” and early activation of CD4 + T-cells) and (ii) during hyperinflammatory conditions, BCG can also trigger anti-inflammatory or tolerogenic pathways to douse the excess inflammation.[32]
Mycobacterium indicus pranii (MIP) vaccine is a potent toll-like receptor 2 (TLR2) agonist and a poly TLR antagonist. In a retrospective observational study, 117 (84 males and 33 females) COVID-19 patients, the use of this vaccine was seen to be associated with rapid recovery in 116/117 patients from COVID-19 who were discharged from the hospital within 10 days.[33]
In a separate trial (ARMY-1), the use of the MIP vaccine as an immunomodulator in addition to standard care resulted in early clinical improvement compared to standard care alone.[34] Larger randomized controlled trials are recommended to assess the role of the MIP in COVID-19.
The spectre of mucormycosis
Recent findings of increased cases of mucormycosis in SARS-CoV-2 and its relation with the use of immunosuppressives/corticosteroids raise the concern about the development of mucormycosis in leprosy. Neutrophil dysfunction, high and prolonged use of oral steroids and thalidomide in lepra reaction can increase the incidence of mucormycosis in leprosy. Basílio et al.[35] reported a case of cutaneous mucormycosis in an immunosuppressed patient with multibacillary leprosy, under prolonged corticosteroid and thalidomide therapy to control leprosy type 2 reaction. The judicious use of corticosteroids and immunosuppressives in leprosy is recommended. (Level of Evidence VII)
Additionally, the dermatologist needs to be aware of the dermatological manifestations of mucormycosis. These can be described as primary, secondary, and contiguous. Primary mucormycosis is further classified into acute necrotizing type and chronic granulomatous type. The acute necrotizing type has reddish-purple indurated plaques progressing to necrosis and eschar formation, while the chronic granulomatous type is identified by an erythematous plaque with a progressive margin and areas of healing and scar formation. While the management of acute necrotizing mucormycosis requires control of the predisposing condition, surgical debridement, and liposomal amphotericin B, the chronic type can be managed by liposomal amphotericin B.
The secondary cutaneous mucormycosis is also the most common and is seen in hospital and emergency settings. The cutaneous involvement is due to dissemination from an internal source. The cutaneous features are similar to that of the acute necrotizing type of primary mucormycosis. The contiguous type arises due to spread from underlying structures like sinuses. This type presents with oral ulcers, mucosal eschars, periorbital edema, periorbital cellulitis, ophthalmoplegia, loss of vision, and neurological deficits. Cutaneous features generally appear subsequent to sinus/nose involvement in the form of pain/loss of sensation over face, facial puffiness or necrosis of the skin over the cheek, eye, or lateral nasal wall area.[36]
The leprosy workers should educate the patient about these early signs of mucormycosis and advised them for immediate consultation with an expert if such a situation arises [Table 2].
Table 2.
Early Signs/Symptoms of Mucormycosis
| Signs and symptoms |
|---|
| Headache |
| Eye pain |
| Eye swelling |
| Eye congestion |
| Double vision |
| Diminution/Loss of vision |
| Nasal blockade |
| Abnormal black discharge (blood/crust) from nose |
| Facial pain |
| Facial redness |
| Facial swelling |
Thromboembolic events
COVID-19 may predispose patients to thrombotic disease, both in the venous and arterial circulations, because of excessive inflammation, platelet activation, endothelial dysfunction, and stasis.[37] It is biochemically characterized by the elevated D-dimer without remarkable changes of other global coagulation markers and is associated with various thrombotic complications and disease severity.[38] In leprosy, Mycobacterium leprae directly/indirectly affects the cutaneous vessels and sometimes mimics vasculitis. The concomitant use of thalidomide and oral corticosteroids in erythema nodosum leprosum (ENL) increases the risk of coagulopathy. It has been red-flagged and due vigilance is advised, although lack of robust evidence has also been acknowledged.[39,40] With COVID-19 infection, another pro- coagulatory variable gets added to the mix. It must be noted, that in the absence of routine assessment for ruling out thrombophilia, introducing aspirin (100 mg/day) was part of an existing recommendation for treating ENL.[41] So IADVL SIG leprosy recommends the addition of low-dose aspirin and timely initiation of antithrombotic therapy in COVID-19 positive leprosy cases considering the risk factors like steroid or thalidomide use, obesity, diabetes, and in nonambulatory patients. (Level of Evidence VII)
Status of reconstructive surgeries
Many major reconstructive surgeries have been performed during the COVID-19 pandemic all over the world including India. There are also published reports in which researchers found that patient safety and postoperative outcomes have been demonstrated to be equivalent to the pre-COVID era.[42] The major concern during the surgery is following strict COVID-19 protocol, avoidance of aerosol generation, and care of healthcare professionals to avoid COVID-19 infection. (Level of Evidence VII)
Leprosy post-exposure prophylaxis (LPEP)
LPEP with single-dose rifampicin (SDR) program helps in the feasibility of combining three key interventions: systematically tracing contacts of individuals newly diagnosed with leprosy, screening the traced contacts for leprosy, and administering SDR to eligible contacts. (Level of Evidence VII)
Though during the phase of lockdown, this program is operationally difficult but with proper training of healthcare workers on telemedicine [Figure 1], this can be implemented.
Mental health
Leprosy is a neglected tropical disease often associated with disability, stigma, and discrimination. This results in poor mental health, stress, and depression. The virus, SARS-CoV-2, is also associated with neuropsychiatric disorder, anxiety, fear, stress, and depression.[43,44,45]
It is recommended that mental issues among leprosy patients be directly enquired about, relaxation techniques communicated either by dermatologists themselves or by utilizing the services of trained counselors. (Level of Evidence VII)
Conclusion
A continued reassessment of existing practices and drug recommendations is required due to evolving and rapidly changing nature of the COVID 19 pandemic. A sustained and coordinated effort from the government, doctors, healthcare workers, NGOs, and self-help groups involved in leprosy care is needed to absorb the shock of the COVID-19 pandemic on marginalized patients of leprosy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rathod S, Suneetha S, Narang T, Bhardwaj A, Gupta SK, Kamoji SG, et al. Management of leprosy in the context of COVID-19 pandemic: Recommendations by SIG leprosy (IADVL Academy) Indian Dermatol Online J. 2020;11:345–8. doi: 10.4103/idoj.IDOJ_234_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.India's daily COVID-19 cases pass 400,000 for the first time as second wave worsens. [[Last accessed on 2021 Jul 25]]. Available from: https://www.reuters.com/world/asia-pacific/indi a-posts-record-daily-rise-covid-19-cases-401993-2021-05-01/
- 3.de Barros B, Lambert SM, Negera E, de Arquer GR, Sales AM, Darlong J, et al. An assessment of the reported impact of the COVID-19 pandemic on leprosy services using an online survey of practitioners in leprosy referral centres. Trans R Soc Trop Med Hyg. 2021:trab084. doi: 10.1093/trstmh/trab084. doi: 10.1093/trstmh/trab084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. COVID-19: WHO issues interim guidance for implementation of NTD programmes 2020, April 1. [[Last accessed on 2021 Jul 25]]. Available from: https://www.who.int/neglected_diseases/news/COVID19-WHO-interim-guidance-implementation-NTD-programmes/en/
- 5.Tomes N. “Destroyer and teacher”: Managing the masses during the 1918-1919 influenza pandemic. Public Health Rep. 2010;125((Suppl 3)):48–62. doi: 10.1177/00333549101250S308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021;76:428–55. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 7.Bhuiyan MU, Stiboy E, Hassan MZ, Chan M, Islam MS, Haider N, et al. Epidemiology of COVID-19 infection in young children under five years: A systematic review and meta-analysis. Vaccine. 2021;39:667–77. doi: 10.1016/j.vaccine.2020.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [[Last accessed on 2021 Jul 26]];World Health Organization. Weekly Epidemiological Record. 2020 95(36):417–440. Available at https://apps.who.int/iris/handle/10665/334140 . [Google Scholar]
- 9.Antunes DE, Goulart IMB, Goulart LR. Will cases of leprosy reaction increase with COVID-19 infection? PLoS Negl Trop Dis. 2020;14:e0008460. doi: 10.1371/journal.pntd.0008460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos VS, Quintans-Júnior LJ, Barboza WS, Araújo AAS, Martins-Filho PR. Clinical characteristics and outcomes in patients with COVID-19 and leprosy. J Eur Acad Dermatol Venereol. 2021;35:e1–2. doi: 10.1111/jdv.16899. [DOI] [PubMed] [Google Scholar]
- 11.Pai VV, Wakade A. Leprosy and COVID-19 Co-infection – Experience in a referral centre in Mumbai, India. Abstract in IAL Biennial Conference. 2021 [Google Scholar]
- 12.Ghaith MM, Albanghali MA, Aldairi AF, Iqbal MS, Almaimani RA, AlQuthami K, et al. Potential predictors of poor prognosis among severe COVID-19 patients: A single-center study. Can J Infect Dis Med Microbiol. 2021;2021:6656092. doi: 10.1155/2021/6656092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dertlioğlu SB, Karlıdağb GE, Ağlamış S. Clinical findings in patients with leprosy who are infected with COVID-19: A case series from Elazığ, Turkey. Lepr Rev. 2021;92:134–40. [Google Scholar]
- 14.Schmitz V, Dos Santos JB. COVID-19, leprosy, and neutrophils. PLoS Negl Trop Dis. 2021;15:e0009019. doi: 10.1371/journal.pntd.0009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxena S, Khurana A, B S, Sardana K, Agarwal A, Muddebihal A, et al. Severe type 2 leprosy reaction with COVID-19 with a favourable outcome despite continued use of corticosteroids and methotrexate and a hypothesis on the possible immunological consequences. Int J Infect Dis. 2021;103:549–51. doi: 10.1016/j.ijid.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan S, Yin X, Meng X, Chan JF, Ye ZW, Riva L, et al. Clofazimine broadly inhibits coronaviruses including SARS-CoV-2. Nature. 2021;593:418–23. doi: 10.1038/s41586-021-03431-4. [DOI] [PubMed] [Google Scholar]
- 17.Lafaurie M, Martin-Blondel G, Delobel P, Kamar N, Charpentier S, Sommet A, et al. Impact of previous exposure to systemic corticosteroids on unfavorable outcome in patients hospitalized for COVID-19. BMC Pharmacol Toxicol. 2021;22:14. doi: 10.1186/s40360-021-00480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arora K, Panda PK. Steroid harms if given early in COVID-19 viraemia. BMJ Case Rep. 2021;14:e241105. doi: 10.1136/bcr-2020-241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frohman EM, Villemarette-Pittman NR, Cruz RA, Longmuir R, Rowe V, Rowe ES, et al. Part II.high-dose methotrexate with leucovorin rescue for severe COVID-19: An immune stabilization strategy for SARS-CoV-2 induced 'PANIC' attack. J Neurol Sci. 2020;415:116935. doi: 10.1016/j.jns.2020.116935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gisondi P, Zaza G, Del Giglio M, Rossi M, Iacono V, Girolomoni G. Risk of hospitalization and death from COVID-19 infection in patients with chronic plaque psoriasis receiving a biologic treatment and renal transplant recipients in maintenance immunosuppressive treatment. J Am Acad Dermatol. 2020;83:285–7. doi: 10.1016/j.jaad.2020.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, et al. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020;27:1451–4. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holcomb ZE, Santillan MR, Morss-Walton PC, Salian P, Her MJ, Giannotti NM, et al. Risk of COVID-19 in dermatologic patients receiving long-term immunomodulatory therapy. J Am Acad Dermatol. 2020;83:1215–8. doi: 10.1016/j.jaad.2020.06.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarojini PA, Mshana RN. Use of colchicine in the management of erythema nodosum leprosum (ENL) Lepr Rev. 1983;54:151–3. [PubMed] [Google Scholar]
- 25.Sharma VK, Kumar B, Kaur I, Singh M, Kaur S. Colchicine in the treatment of type 2 lepra reaction. Indian J Lepr. 1986;58:43–7. [PubMed] [Google Scholar]
- 26.Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39:2085–94. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalili N, Kashefizadeh A, Nafar M, Poorrezagholi F, Firouzan A, Samadian F, et al. Adding colchicine to the antiretroviral medication-lopinavir/ritonavir (Kaletra) in hospitalized patients with non-severe Covid-19 pneumonia: A structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:489. doi: 10.1186/s13063-020-04455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyes AZ, Hu KA, Teperman J, Wampler Muskardin TL, Tardif JC, Shah B, et al. Anti-inflammatory therapy for COVID-19 infection: The case for colchicine. Ann Rheum Dis. 2021;80:550–7. doi: 10.1136/annrheumdis-2020-219174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sardana K, Sinha S, Sachdeva S. Colchicine in dermatology: Rediscovering an old drug with novel uses. Indian Dermatol Online J. 2020;11:693–700. doi: 10.4103/idoj.IDOJ_475_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erskine D. Using COVID-19 vaccines in a patient taking immunosuppressive medicines. 2021. [[Last acessed on 2021 May 18]]. Available from: https://www.sps.nhs.uk/articles/using-covid-19-vaccines-in-patient-taking-immunosuppressive-medicines/
- 31.Gresham LM, Marzario B, Dutz J, Kirchhof MG. An evidence-based guide to SARS-CoV-2 vaccination of patients on immunotherapies in dermatology. J Am Acad Dermatol. 2021;84:1652–66. doi: 10.1016/j.jaad.2021.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basak P, Sachdeva N, Dayal D. Can BCG vaccine protect against COVID-19 via trained immunity and tolerogenesis? Bioessays. 2021;43:e2000200. doi: 10.1002/bies.202000200. [DOI] [PubMed] [Google Scholar]
- 33.Ingale A, Ingale F, Kunwar B, Ahmed S, Salvi K, Chavan V, et al. Role of mycobacterium w for the treatment of COVID-19: An observational study. J Assoc Physicians India. 2021;69:19–22. [PubMed] [Google Scholar]
- 34.Sehgal IS, Guleria R, Singh S, Siddiqui MS, Agarwal R. A randomised trial of Mycobacterium w in critically ill patients with COVID-19 (ARMY-1) ERJ Open Res. 2021:00059–2021. doi: 10.1183/23120541.00059-2021. doi: 10.1183/23120541.00059-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basílio FM, Hammerschmidt M, Mukai MM, Werner B, Pinheiro RL, Moritz S. Mucormycosis and chromoblastomycosis occurring in a patient with leprosy type 2 reaction under prolonged corticosteroid and thalidomide therapy. An Bras Dermatol. 2012;87:767–71. doi: 10.1590/s0365-05962012000500017. [DOI] [PubMed] [Google Scholar]
- 36.Vinay K, Rudramurthy SM, Dogra S. Emergence of Mucormycosis during COVID-19 pandemic and dermatological manifestations. Indian Dermatol Online J. 2021;12:493–6. doi: 10.4103/idoj.idoj_406_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chhabria BA, Pannu AK, Bhalla A. Venous thrombo-embolism: Thalidomide and leprosy. QJM. 2017;110:383–4. doi: 10.1093/qjmed/hcx063. [DOI] [PubMed] [Google Scholar]
- 38.Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69:1181–9. doi: 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bishnoi A, Singh V, Handa S, Vinay K. Thalidomide and thromboprophylaxis for dermatologic indications: An unmet need for more evidence. J Am Acad Dermatol. 2018;79:e45–6. doi: 10.1016/j.jaad.2018.04.048. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi S, Yamamoto Y, Hosokawa A, Hagiwara K, Uezato H, Takahashi K. Deep venous thrombosis and pulmonary embolism secondary to co-administration of thalidomide and oral corticosteroid in a patient with leprosy. J Dermatol. 2012;39:711–4. doi: 10.1111/j.1346-8138.2011.01484.x. [DOI] [PubMed] [Google Scholar]
- 41.Vernal S, Brochado MJF, Bueno-Filho R, Louzada-Junior P, Roselino AM. Anti-phospholipid syndrome in seven leprosy patients with thrombotic events on corticosteroid and/or thalidomide regimen: Insights on genetic and laboratory profiles. Rev Soc Bras Med Trop. 2018;51:99–104. doi: 10.1590/0037-8682-0216-2017. [DOI] [PubMed] [Google Scholar]
- 42.Patel NG, Reissis D, Mair M, Hart A, Ragbir M, Giele H, et al. Safety of major reconstructive surgery during the peak of the COVID-19 pandemic in the United Kingdom and Ireland-multicentre national cohort study. J Plast Reconstr Aesthet Surg. 2020;74:1161–72. doi: 10.1016/j.bjps.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serrano-Castro PJ, Estivill-Torrús G, Cabezudo-García P, Reyes-Bueno JA, Ciano Petersen N, Aguilar-Castillo MJ, et al. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: A delayed pandemic? Neurologia (Engl Ed) 2020;35:245–51. doi: 10.1016/j.nrl.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debnath M, Berk M, Maes M. Changing dynamics of psychoneuroimmunology during the COVID-19 pandemic. Brain Behav Immun Health. 2020;5:100096. doi: 10.1016/j.bbih.2020.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner-Musa J, Ajayi O, Kemp L. Examining social determinants of health, stigma, and COVID-19 disparities. Healthcare (Basel) 2020;8:168. doi: 10.3390/healthcare8020168. [DOI] [PMC free article] [PubMed] [Google Scholar]