Abstract
The goal of these recommendations is to provide a framework to practitioners for implementing useful, evidence-based recommendations for the preparation of platelet-rich fibrin (PRF) and its use in various dermatological indications. The Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) assigned the task of preparing these recommendations to its taskforce on platelet-rich plasma. A comprehensive literature search was done in the English language on the PRF across multiple databases. The grade of evidence and strength of recommendation was evaluated on the GRADE framework (Grading of Recommendation, Assessment, Development and Evaluation). A draft of clinical recommendations was developed on the best available evidence which was also scrutinized and critically evaluated by the IADVL Academy of Dermatology. Based on the inputs received, this final consensus statement was prepared. A total of 40 articles (meta-analyses, prospective and retrospective studies, reviews [including chapters in books] and case series) were critically evaluated and the evidence thus gathered was used in the preparation of these recommendations. This expert group recommends use of A-PRF+ protocol, that is (200 g for 8 min) for preparation of solid PRF and C-PRF protocol (700 g for 8 min) for liquid PRF. Swing out bucket model of centrifuge or the horizontal centrifuge is recommended for preparation of both PRF, and liquid PRF. Centrifugation must begin within 90–120 s of drawing of blood. PRF can be used in various indications for skin rejuvenation and nonhealing ulcers as either monotherapy or in combination with other therapies.
Keywords: A-PRF, centrifuge, C-PRF, I-PRF, platelet-rich fibrin guidelines, platelet-rich plasma, preparation, RCF, recommendations, regenerative medicine, RPM, standardization
Introduction
The clinical uses of platelet concentrates were elucidated in the late 1990s by Marx et al.[1] and Anitua et al.[2] Platelet-rich plasma (PRP) has found various applications in different fields ranging from dentistry to dermatology. PRP was found to have tremendous growth potential and contained a supraphysiological dose of growth factors which induces faster healing.[3] The easy availability of growth factors, which simply requires drawing blood, popularized PRP as the go-to product in regenerative medicine.
One of the limitations of PRP that were reported were the presence of external anticoagulant. The final release of growth factors is intricately linked with the clotting mechanism and anticoagulants inhibit that mechanism. Anitua et al.,[2] the original authors that introduced the use of PRP in various indications, later in their research attempted to prepare PRP without the need for anticoagulant. Clearly, a need was felt to prepare the autologous platelet concentrates without any external anticoagulant.
A second limitation is the sudden release of growth factors by the liquid PRP on activation. Nearly 95% of growth factors were released following activation with calcium chloride or bovine thrombin.[1] These limitations later led to the development of the second generation of platelet concentrates without anticoagulant. The fibrin matrix traps the growth factors and cells and slowly releases them over time.[4]
Dr. Joseph Choukroun and Dr. David Dohan's original research led to the development of a platelet concentrate where blood was drawn without anticoagulant.[5] It was rapidly spun at 750 g for 12 min in a centrifuge. The RBC settled down, whereas the platelets and WBC got trapped in the fibrin clot as they descended. This formulation was termed as platelet-rich fibrin or PRF. As WBC is an essential part of wound healing, this was called L-PRF or Leukocyte rich PRF. The three-dimensional fibrin matrix provides the scaffold for healing and serves as a reservoir of growth factors that may be released up to 14 days after preparation.[6]
Later studies by Ghanaati et al.[7] and Fujioka-Kobayashi et al.[8] showed that the high centrifugal forces pushed the WBC and the platelets to the bottom of the tubes, whereas the PRF was taken from the upper part of the tube. They showed that decreasing the centrifugal speed to 200 g (1300 RPM) And increasing the duration of spin can increase the number of leukocytes and platelets in the PRF. This was termed as A-PRF or Advanced PRF. Later, a new protocol with further decrease in the duration of spin to 8 min and keeping the centrifugal speeds same (200 g) was developed. It was named “A-PRF +” and this was associated with even higher growth factor yield.[8]
The A-PRF clot produced using the above protocol can be compressed using a PRF box used in dentistry to prepare PRF membrane. A flat metal plate is placed on the clot which compresses it into a flat membrane. The device also has a cylindrical hole into which a clot can be placed. A small weight is placed on the clot which pushes out the water and makes the clot into a small plug-like structure. Both the PRF membrane and PRF plugs are useful in dentistry. When PRF is used for an ulcer or for wound healing, the PRF clot can be directly used and compressed onto the wound base to fit it in.
PRF has shown to produce a higher cumulative yield of growth factors than even PRP.[6] Also, this release is slow and over a few days, making it ideal for tissue regeneration and growth stimulation. However, the injection of solid PRF membrane is not possible. Following extensive basic research, Miron et al.[9] observed that by further reducing the centrifugal force (g force) and the time duration of spin, a liquid PRF can be prepared. This was termed as Injectable-PRF or I-PRF. The centrifugal speed was kept at 60 g for 3 min. This small centrifugation time allows separation to occur before the clot has had time to form and preparation remains liquid. The volume of I-PRF produced in a 10 mL tube is usually 1-1.5 mL only. It has been found to have a higher concentration of platelets and WBC than L-PRF and A-PRF. It remains a liquid for 15–20 min before it coagulates to form a clot. During this time, the I-PRF can be injected into the scalp or skin of the face or it can be mixed with bone grafting materials and molded into the required shape and allow it to clot into shape.
With so many terms representing just two forms of platelet concentrate, it is recommended to use the term PRF for the solid PRF (A-PRF, A-PRF + and L-PRF) and liquid PRF for the liquid or injectable forms of PRF (I-PRF and C-PRF) [Figure 1]. Generally PRFM or platelet-rich fibrin matrix term is used when PRP is prepared using anticoagulant and is later activated using activator and a clot is produced.[10,11]
Figure 1.
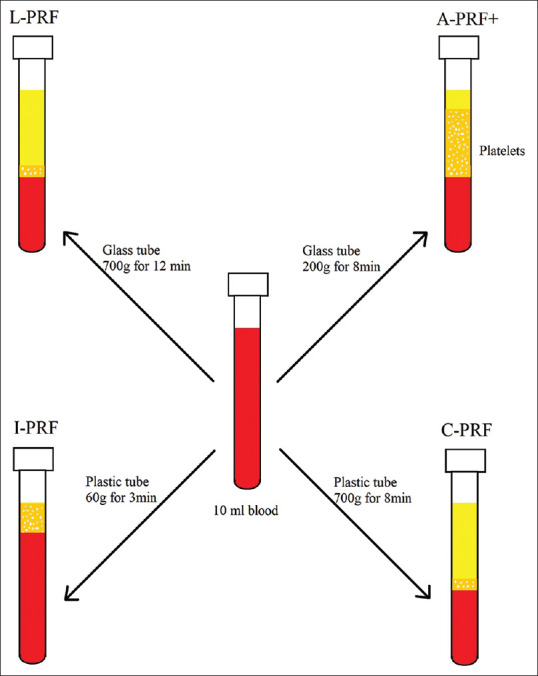
Shows schematic representation of different types of second generation platelet concentrates that can be prepared using different centrifugation protocols and tubes. Light yellow colour represents cell free plasma, orange colour represents plasma containing cells predominantly platelets and red colour represents RBC layer
Scope of recommendations
There is a confusion in the minds of clinicians regarding the ideal method of preparation of PRF. Various authors have used different types of centrifuges and different spin parameters. Literature is full of different types of PRF (L-PRF, A-PRF, A-PRF+, I-PRF, C-PRF, Alb-PRF, Bio-PRF® etc.). Some of the terms are synonyms and some are trademarks. There is a need for consensus on the various aspects of PRF preparation. These recommendations are intended for dermatologists who are involved in the preparation of PRF. The goal of these recommendations is to provide a framework to practitioners for implementing useful, evidence-based recommendations for the preparation of PRF and its use in dermatology.
Methodology of Preparation of Recommendations
A comprehensive literature search was done in the English language on the preparation of PRP and its use in androgenetic alopecia across multiple databases (PubMed, Embase, Medline, Google Scholar, and Cochrane). The search keywords used, alone or in combination, were “PRF”, “Platelet Rich Fibrin”, “Platelet Concentrate”, “ Platelet Rich Fibrin Matrix”, “Injectable PRF”, “I-PRF”, “Rejuvenation” and “Nonhealing ulcer”. The grade of evidence and strength of recommendation was evaluated on the GRADE framework.[12] The quality of evidence and the strength of recommendation are shown in Table 1.[13]
Table 1.
GRADE framework
| GRADE Framework | |
|---|---|
| A. Quality of evidence | |
| High quality | Well performed randomised control trials or clear evidence from multiple well conducted observational studies showing very large effect |
| Moderate quality | Randomised control trials with essential limitations |
| Low quality | Observational studies or controlled trial with severe limitations |
| Very-low quality | Non-systematic observations, biologic reasoning or observational studies with severe limitations |
| B. Strength of recommendation | |
| Strong | A strong recommendation was given when benefits distinctly outweighed the risks for nearly all patients. As practitioners, most patients must receive this course of action |
| Weak | A weak recommendation was given when risks and benefits were more closely balanced or were uncertain. As practitioners, patients must be explained about all the different options, and an option suitable for patients needs must be chosen |
A draft was prepared which was then sent for review to the members of IADVL taskforce for PRP, appointed by the IADVL Academy of Dermatology. It was also sent to the IADVL Academy members for critical comments. Based on the inputs, the final consensus statement was prepared. A total of 40 articles (meta-analyses, prospective and retrospective studies, reviews, chapters in books and case series) were critically evaluated and the evidence thus gathered was used in the preparation of these recommendations. The recommendations have discussed the following points.
-
Facets of preparation of PRF
Ideal centrifugation parameters for PRF preparation
Ideal centrifuge machine for the preparation of PRF
Blood collection tubes for preparation of PRF and Liquid PRF
Time of initiation of centrifugation
Preparation of PRF and Liquid PRF
-
PRF in dermatology
PRF in skin rejuvenation
PRF in wound healing
Use of second-generation platelet concentrates in hair disorders has been discussed in a different article; hence, it is not taken up here.
Facets of preparation of PRF
Ideal centrifugation parameters for PRF preparation
A-PRF + protocol, that is (200 g for 8 min), has been found to produce a fibrin clot with the highest platelet and WBC count and highest overall cumulative growth factor yield. C-PRF (700 g for 8 min) is the most optimum protocol for preparation of liquid PRF. Quality of evidence: High, Strength of recommendation: Strong.
Ghanaati et al.[7] in 2014 performed a histopathological analysis of PRF clot produced using the traditional L-PRF protocol and found that most of the cells were accumulated at junction of the clot and the RBC layer or in the RBC layer. Fujioka-Kobayashi et al.[8] showed that low-speed protocols of A-PRF and A-PRF + release much higher cumulative quantity of growth factors like (EGF, VEGF, TGF-b1, PDGF-AB etc.) over a period of 10 days.[8] Miron et al.[14] evaluated the distribution of platelets and WBC when PRF was prepared using different protocols. They found a much more even distribution of platelets and WBCs with A-PRF and A-PRF+ protocol. Fujioka-Kobayashi et al.[8] also studied the growth of human gingival fibroblast when cultured with different PRFs. A-PRF+ Protocol showed the highest cumulative growth factor release and the highest Levels of human fibroblast cellular migration, proliferation and the highest collagen type I production at days 3 and 7.
Miron et al.[9] showed that a liquid PRF could be produced by reducing the centrifugation time and speeds (60 g for 3 min). Authors named it I-PRF or injectable PRF. This new form of PRF was very versatile as it could now be injected before forming the clot. However, because of the smaller centrifugation time and speed in, only two- to three-fold increase in platelets and 1.5-fold increase in leukocyte concentration could be achieved in I-PRF.[15] Comparatively PRP could achieve a fivefold amplification of platelet concentration. A liquid formulation of PRF with much higher platelet concentration was required. Ghanaati et al.[7] and Fujioka-Kobayashi et al.[8] had previously shown that using the original L-PRF protocol, that is 700 g for 12 min, nearly all the WBCs and platelets were concentrated at the buffy coat layer, whereas almost no platelets in the layers above that. This could be used as a method to concentrate platelets in the form of liquid PRF. Miron et al.[15] showed that a nearly 10-fold increase in platelet concentration could be achieved if liquid PRF was prepared using the original L-PRF protocol. This was later termed Concentrated PRF or C-PRF. This method requires spinning the blood at 700 g for 8 min and 0.3–0.5 mL layer just above and including the buffy coat is taken. Later, the same authors studied and compared I-PRF and C-PRF on growth factor release and collagen production by cultured gingival fibroblasts. They found almost two- to threefold higher increase in growth factor released during 10 day period and a further fourfold increase in gingival fibroblast migration and collagen type I synthesis in the C-PRF arm when compared with the original I-PRF.[16] It is recommended to use C-PRF protocol for when using liquid PRF for various indications. On comparing I-PRF to PRP, I-PRF was found to have a higher long-term release of growth factors. Although PRP showed a significantly higher cellular proliferation, I-PRF showed higher cell migration and collagen 1 expression at day 3 and 7 when compared to PRP.[9]
Ideal centrifuge machine for the preparation of PRF
Swing out bucket model of centrifuge or the horizontal centrifuge is recommended for preparation of both PRF and Liquid PRF. Quality of Evidence: High, Strength of Recommendation: Strong.
Various studies have compared the centrifuge rotor characteristics and its effect on the PRF produced. Fixed-angle centrifuge has a major disadvantage. Due the backward centrifugal force, the cells are first pushed to the back of the tube and then they move up or down along the back end of the tube. This leads of smearing of cells along the back end of the tube and cell damage [Figures 2 and 3]. Another disadvantage of a fixed angle centrifuge is that RCF values cannot be copied from a different centrifuge. Figure 4 shows a photo of two fixed angle centrifuges which have tubes at different angles. At the same RCF-max, both these centrifuges will produce vastly different results. However, comparison can be easily done in a swinging bucket model. Tsujino et al.[17] performed in their study sliced up a PRF membrane into seven to eight different slices and performed a histopathological analysis on them. They found that in PRF produced in a fixed-angle centrifuge, almost all the cells are found in the distal surface and almost none in the proximal surface. [Figure 5]. This is critical when preparing PRF for wound healing. If the proximal surface is laid on the wound side almost none of the platelets would be in the contact with the wound wall showing poor results. Miron et al.[18] performed electron microscopic examination on PRF clots produced using three different centrifuges and found that A-PRF+ protocol could reliably produce high-quality PRF irrespective of the centrifuge machine used. They found that blood collection tubes were critical for preparation of good quality PRF.
Figure 2.
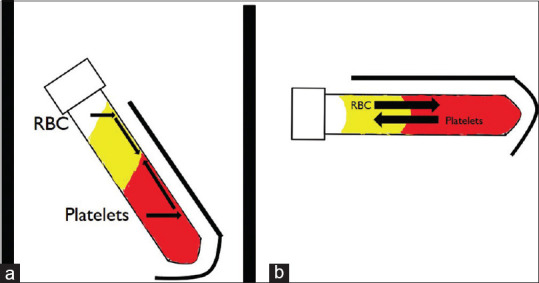
Black arrows show paths that cells take when tubes containing blood are centrifuged. (a) In a fixed angle centrifuge, the cells first hit the distal part of the tube and then start to creep up or down along the back wall of the tube. (b) In swinging bucket model or horizontal centrifuge, there is unhindered movement of platelets and RBC
Figure 3.

Shows the smearing of cells on the back wall of the tube in a fixed angle centrifuge indicating risk of cell damage
Figure 4.
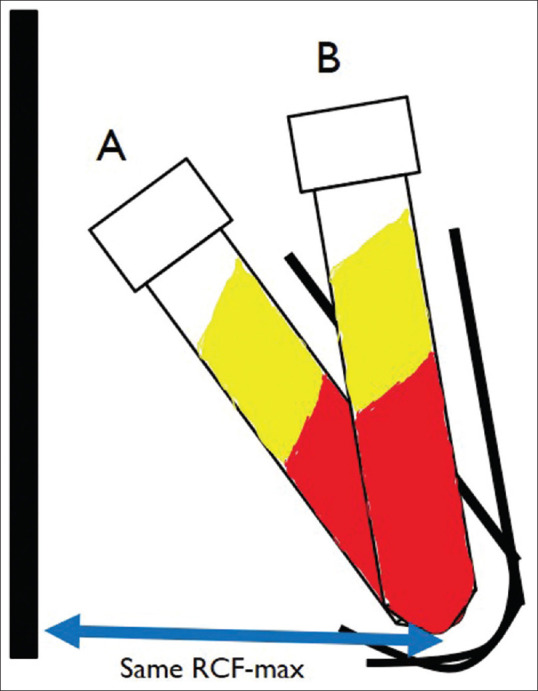
Schematic representation of fixed angle centrifuge with different angles. With the same Rmax (Maximum radius) these two centrifuges deliver different forces
Figure 5.
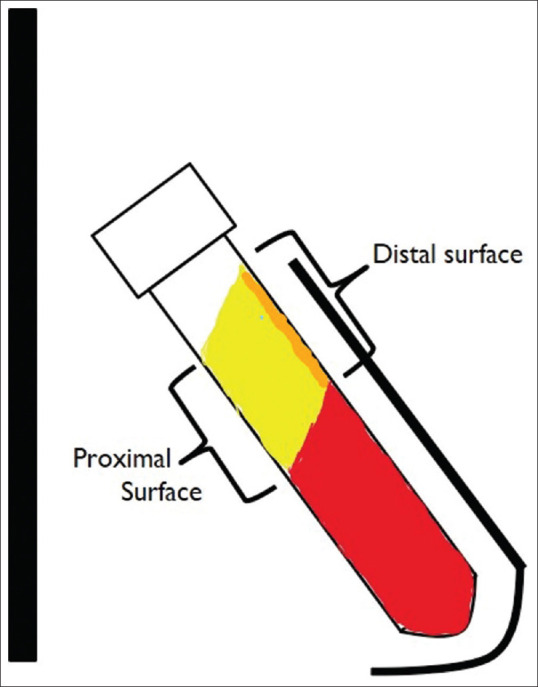
Schematic representation of the position of platelets in a PRF clot produced using a fixed angle centrifuge
Blood collection tubes for preparation of PRF and liquid PRF
No additive glass tubes are ideal to prepare PRF membrane, whereas a no additive Polyethylene Terephthalate PET plastic Vacutainer is ideal for preparation of liquid PRF. Quality of evidence: Low, Strength of recommendation: Strong.
The material of the tubes plays a critical role in the preparation of PRF. The choice of material will depend on the type of platelet concentrate required. For solid PRF we need to clot the blood as soon as possible so that the platelets are trapped in the clot as they are being pushed down during centrifugation. This allows an even distribution of platelets in the PRF. Hydrophilic materials allow platelets to come in contact with the walls and promote clotting. Hence, the blood should be taken in hydrophilic material like glass. Plastic red top tubes (Clot activator tubes) imitate this effect of glass by having a silica coating inside. Miron et al.[18] compared three different PRF tubes using three different centrifuges. They found that the centrifuges did not make much difference in the quality of PRF produced but the plain glass tubes produced 250% bigger PRF membrane than silica-coated plastic tube. Furthermore a study by Masuki et al.[19] showed the presence of silica particles in the PRF produced using silica-coated plastic tubes and showed that these silica particles have an acute cytotoxic effect of human periosteal cells. A simple test designed by Miron et al.[18] to detect the presence of silica or other additives in the tubes is by simple filling the tubes half with water and then shaking it. The presence of froth or turbidity indicated presence of added materials. [Figure 6]. Another factor that promotes coagulation is contact with oxygen. Hence, when preparing PRF, it is advisable to pop open the lid of the tube after centrifugation and keep it in a test-tube stand for 5 min. This increases the size of the clot produced. Jianpiampoolpol et al.[20] showed that PRF can also be produced in additive-free screw cap plastic tubes but formation is delayed.
Figure 6.
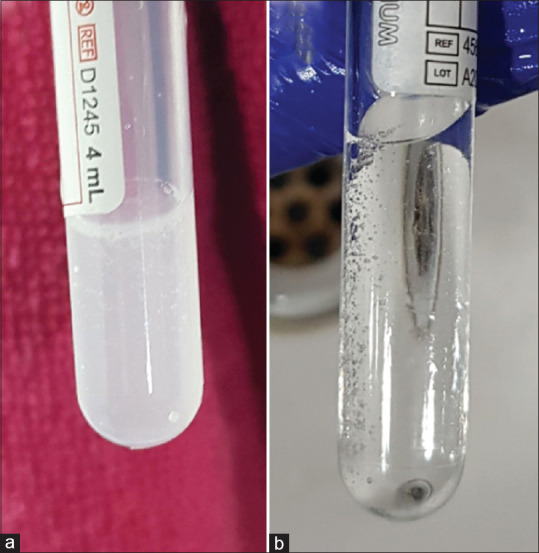
Shake test to test for the presence of additives in tubes. (a) Turbidity in the tube suggests presence of additives in the tubes. (b) Clear water in the tube suggests that there is no additive in the tube
Conversely, for preparation of liquid PRF, we need to delay coagulation so that the PRF stays liquid long enough for us to be able to inject it. For this, the best tubes are additive-free white-top polythene terephthalate (PET) plastic vacutainers. PET plastic is hydrophobic in nature and repels water, and hence the platelets. This prevents the activation of platelets during centrifugation and delays the start of clot formation by 15–20 min. This time is enough to collect the liquid PRF and inject it. Unlike PRF clot preparation, the top of the vacutainer should not be opened during the preparation and while withdrawing liquid PRF. Exposure to air may lead to initiation of clotting. It is best to use a 1.5 inch 18 gauge needle [Figure 7] to draw out the liquid PRF through the rubber seal itself. This will allow liquid PRF to stay liquid for some time.
Figure 7.

18 G 1.5 inch needle is used to pick up I-PRF by passing it through the rubber stopper. This allows I-PRF to stay in closed containers during the whole preparation process
Time of initiation of centrifugation
Centrifugation must begin within 90–120 s of drawing of blood. Quality of evidence: Low, Strength of Recommendation: Strong.
Miron et al.[21] has found a 23% reduction in the size of clot produced when start centrifugation was delayed for more than 120 s after blood draw. It is advisable to keep the centrifuge open and timer set before the blood draw is started.
Preparation of PRF and liquid PRF
There are various methods described in the literature for the preparation of PRF. The A-PRF+ (200 g for 8 min) protocol with additive-free glass tubes in a swing out bucket model of centrifuge (horizontal centrifugation) is the ideal method of preparation of PRF. Steps have been given in Figure 8. Ideal platelet yield, whereas preparing liquid PRF preparation can be achieved using the C-PRF protocol (700 g for 8 min) using PET plastic vacutainers in a horizontal centrifuge. Steps of preparation for the traditional I-PRF (60 g for 3 min) and the newer C-PRF (700 g for 8 min) have been shown in Figures 9 and 10 respectively. A distinct disadvantage of I-PRF and C-PRF is the small volumes produced from large volumes of blood drawn [Figure 11]. The platelet extraction efficiency of PRP preparation is generally higher. Another disadvantage of C-PRF is the lower position of buffy coat [Figure 12], the lid must be popped open to reach the buffy coat area. This may initiate clotting due to contact with oxygen. For, I-PRF, the buffy coat is much higher, and we can use 18G 1.5 inch needle to draw out the I-PRF [Figure 7]. The needle is inserted through the rubber top and the I-PRF does not need to come in contact with air.
Figure 8.

Step by step process for making Platelet Rich Fibrin using A-PRF + protocol
Figure 9.

Step by step process for making liquid Platelet Rich Fibrin using I-PRF protocol
Figure 10.

Step by step process for making liquid Platelet Rich Fibrin using C-PRF protocol
Figure 11.
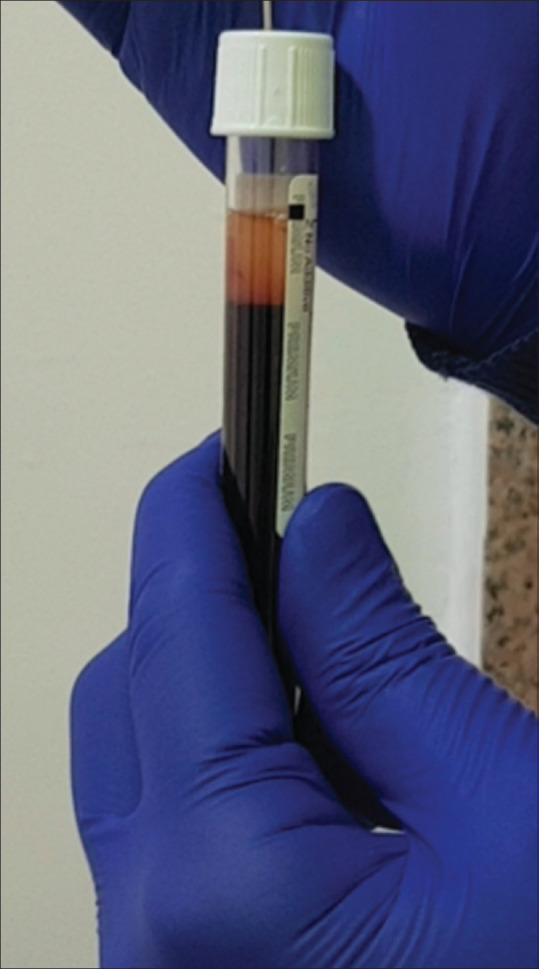
I-PRF prepared in white top additive free PET plastic vacutainer. 1-1.5 mL is produced in each tube
Figure 12.
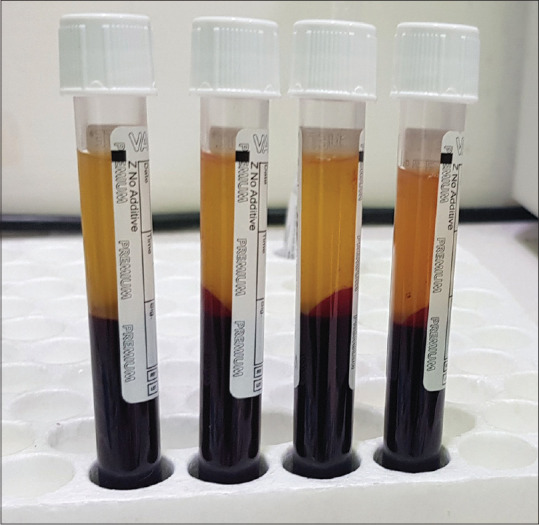
C-PRF prepared in white top additive free PET plastic vacutainer. 0.3-0.5 mL taken from the buffy coat region. The position of buffy coat is much lower in case of C-PRF as compared to I-PRF due to higher centrifugation speeds
Use of PRF in Dermatology
PRF in skin rejuvenation
Current Level of Evidence: Quality of evidence - low; strength of recommendation- weak.
I-PRF/C-PRF is emerging as a promising treatment modality for skin rejuvenation. These PRF products can be used as an anti-aging modality and for improving blemishes, acne scars and skin tone and texture. These can also be used for improvement of tear trough, nasolabial folds, marrionnete lines, peri-oral lines and skin of neck, chest and hands. Leukocytes play an important role, via a cluster of mesenchymal stem cells, with important regenerative functions, including stimulation of fibroblast propagation, improved anti-inflammatory effects, angiogenesis, and protein deposition (e.g., procollagen) for extracellular matrix remodeling.[22] Studies have also shown that skin fibroblasts migrate over 350% more in fluid-PRF when compared to control and PRP (200% increase). Fluid-PRF also significantly induced greater cell proliferation at 5 days. Although both PRP and fluid-PRF induced significantly elevated cell mRNA levels of PDGF, it was observed that TGF-beta, collagen 1, and fibronectin mRNA levels were all significantly highest in the fluid-PRF group. Lastly, fluid-PRF showed a significantly greater ability to induce collagen matrix synthesis when compared to PRP.[23,24] Hence PRF could offer superior results in skin rejuvenation than the conventional PRP. Currently much literature is not available for use of PRF in skin rejuvenation. In a recent study by Hassan et al.,[25] I-PRF prepared by PRF PROCESS™ system, was given by intradermal injections to 11 heathy females. 3 injections at monthly intervals were given and the efficacy was assessed by objective skin analysis (VISIA®) and a subjective patient-reported outcome (FACE-Q) assessment at baseline and after 3 months. Three facial regions- malar area, nasolabial folds and upper lip skin above the vermilion border was treated in all cases. 0.1 mL injection was given intradermally and 20 such injections were given on each side. Although a significant improvement in skin surface spots and pores was seen at 3-months follow-up (P = 0.01 and 0.03, respectively), other variables, such as skin texture, wrinkles, ultraviolet spots, and porphyrins, showed only a numerical improvement which was not significant. FACE-Q scales showed a significant improvement from baseline, including satisfaction with skin, satisfaction with facial appearance, satisfaction with cheeks, satisfaction with lower face and jawline, and satisfaction with lips. No major adverse effects were reported.
Cleopatra technique described by Nacopoulos and Vesala uses a combination of I-PRF and A-PRF matrices for facial rejuvenation.[26] They did a study on 34 patients where, 4 sessions of intradermal and subcutaneous PRF product was given at 2- to 3-week intervals. 10.5-13.5 mL of the PRF product was injected in each session. Clinical outcomes were assessed by 23 independent blinded reviewers and very encouraging results were reported. This technique is gaining popularity among physicians and patients alike. PRF combined with Nanofat derived stromal cells (NFSC) has been found to be an effective option for the efficacy facial skin rejuvenation.[27] NFSCs show excellent multipotential differentiation and paracrine function, and PRF promotes proliferation of NFSCs during the early stage after seeding. Both nanofat-PRF and HA injection improve facial skin status without serious complications, but the former was associated with greater patient satisfaction, implying that nanofat-PRF injection is a safe, highly effective, and long-lasting method for skin rejuvenation.
PRF is an excellent adjuvant for promoting the proliferation, differentiation, and paracrine function of adipose stem cells (ASCs). PRF has been widely used in clinical practice to improve the efficacy of cell-assisted lipotransfer (CAL) and wound repair by ASCs, due to its advantages in preventing immunologic rejection, simple production, and few complications.[28] PRF not only represents a rich source of growth factors but also provides a scaffold to support true tissue regeneration. Authors have suggested that a single session of PRF with nanofat would significantly improve skin hydration and for a longer duration compared to hyaluronic acid (HA) fillers that have short duration of efficacy due to micromolecular structure.
Addition of PRF to HA has been found to be an excellent candidate material for treating clinical signs of aging related to aging human dermal fibroblasts by increasing their responsiveness to transforming Growth Factor β 1 (TGF-β 1).[29] This indication needs to be further explored to increase the effectiveness of the commonly used HA fillers in aging skin.
The absolute contraindications for PRF include Platelet dysfunction syndrome, critical thrombocytopenia, hemodynamic instability, septicemia and patients with unrealistic expectations. Relative contraindications include heavy smokers, drug or alcohol users, patients with chronic liver pathology, severe metabolic or systemic disorders, patients with cancer especially of the hematopoietic origin, patients having low hemoglobin (<10 g/mL) or platelet count (<1.5 lakh/μL) and patients having a history of recent fever or other illnesses. Also, patients on regular use of NSAIDS, prednisolone more than 20 mg per day and anticoagulant therapy should be avoided. A baseline hemoglobin, platelet count, HIV, HbsAg, HCV should be done for all cases. In the current Covid era, all precautions must be followed like sterile gown or personal protective equipment (PPE kit) with N95 mask and eye protection. Elective procedures must follow the latest local guidelines and ICMR guidelines for elective surgeries and procedures. Patients having a history of herpes simplex can be started on antiviral prophylaxis; acyclovir 400 mg twice a day or valacyclovir 500 mg once a day for 5-7 days started a day before the procedure.
The entire procedure should be carried out in a minor OT maintaining strict asepsis. Around 40 mL of blood needs to be drawn as only 1–1.5 mL of injectable PRF is obtained from 10 mL of blood. I-PRF/C-PRF has to be injected immediately. The product stays liquid for 15–20 min; hence, it cannot be stored. The skin is numbed with topical numbing creams, icing before, during and after the procedure or nerve blocks can be used. Deep dermal/subcutaneous injections are given. Around 3-4 mL is required for full-face injections, 1 mL per cheek, 1 mL for forehead, nose and chin and 1 mL for neck. Around 0.1 mL of product is delivered per prick. Slow injections are advocated to decrease the pain. Slight pain and redness are expected post procedure in all cases. Patient should also be aligned regarding the chances of bruising. Use of NSAIDS 2 weeks post procedure should be avoided. Patients are advised strict sun protection and liberal use of moisturizers for 2 weeks after the procedure. 3–6 sessions at a gap of 4–6 weeks are recommended. Results are usually visible after 4–6 weeks. This is not a volume filler but acts more at a cellular level.
PRF can have multiple indications for skin rejuvenation as monotherapy and adjunct therapy with fat transfer and HA fillers. More well-designed clinical studies are required to understand and standardize the full scope of this product. Figure 13 shows reduction in the nasolabial fold after single session of I-PRF. Intradermal injection in the nasolabial fold was done. Photographs were taken before and immediately after the procedure.
Figure 13.
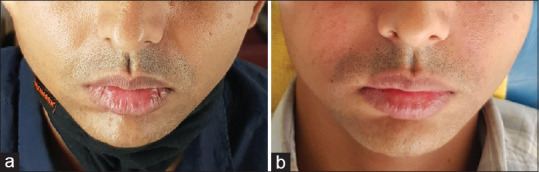
Intradermal injection of nasolabial fold with I-PRF. (a) Before treatment. (b) Immediately after treatment
Adverse effects with intradermal injection of PRF in the skin are less frequently reported. The predominant adverse effects noted are transient edema, pain, stinging at the time of injection, bleeding, swelling and bruising.[30] One of serious adverse effects reported after facial or periorbital injection of PRP was permanent blindness. It was reported mainly with glabellar and periocular injections of PRP, PRP + fat grafting and one case with PRF.[31,32,33] Various strategies advised by the Aesthetic Interventional Induced Visual Loss Consensus group (AIIVL) to prevent vision loss are – slow administration, low volume injection, applying occlusive pressure on the supraorbital notch when injecting in risky areas, injecting intradermally and not subdermally and use of large-bore cannulas (>25G).[31]
PRF in wound healing
Current Level of Evidence: Quality of evidence - High; Strength of recommendation- Strong.
PRF is used for indications varying from acne scars, facial rejuvenation, androgenetic alopecia (where an injectable version is used) and nonhealing ulcers of varying etiologies – trophic and neuropathic (leprous/diabetic), arterial, venous post-surgery and chronic 'hard-to-heal wounds' of any etiology.[34,35,36,37] The fibrin clot renders the growth factors (from platelets) and cytokines (from leukocytes) viable for a longer period of time by preventing proteolysis, functioning as a physiological bioscaffold synthesized by fibrin, fibronectin and vitronectin. These autologous growth factors include platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), transforming growth factor (TGF) and epidermal growth factor (EGF). And the gel-like fibrin is polymerized in a tetramolecular scaffold that houses circulating stem cells, cytokines, leukocytes and platelets.[38] PRF has distinct advantages over PRP – favorable healing properties owing to slow polymerization, higher efficiency of cell proliferation and migration, hemostatic effect, immune support, ease of preparation, single spin outcome, and the lack of anticoagulants and activators during preparation. As a result, there is a higher concentration of growth factors that engage in a controlled release over 7-10 days with fibrin acting as a drug delivery system.[39] Second-generation platelet concentrates also have antibacterial properties which may help in wound healing.[40]
Head-to-head clinicohistological studies have placed PRF superior to other biological membranes in terms of reduction of wound diameter and depth, including amniotic membrane, topical chlorhexidine gel, normal saline, topical metronidazole and conventional dressings including zinc oxide (Unna's paste) for various etiologies.[41,42,43,44,45] It is considered at par with other modalities like hyperbaric oxygen therapy, split skin and full thickness skin grafting, and vacuum-assisted negative pressure wound dressing.[46] The utility of PRF has extended into the field of wound healing by secondary intention via hemostasis, cellular chemotaxis and proliferation, angiogenesis, and extracellular matrix induction.[47] It may be used in an injectable form, as a matrix and most commonly, as a membrane.
Of the many methods, the standardized preparation method commonly in use is by Fujioka-Kobayashi et al.[8] wherein 10 mL of whole venous blood is collected in a plain sterile tube. (with no added anticoagulant) Centrifugation was done at 200 g (Relative Centrifugal Force RCF) for 8 min. Two layers are obtained post-centrifugation – the upper PRF clot/matrix/gel and the lower fraction replete with red blood cells. This gel is extracted using a pair of forceps and the RBC base is cut off and discarded. Depending on the dimensions of the wound, the clot may be used directly or it may be compressed and flattened to form a membrane. Routine wound dressing is done. This may be repeated weekly or fortnightly till the wound surface heals. Other modalities may be used in combination or in a sequential manner. Nagaraju et al. in their case series performed weekly treatments and found 97.74% reduction in volume of the ulcer by the second sitting and complete clearance of all lesions in up to 5 weeks.[35] Similar results were obtained by Sarvajnamurthy et al. in the healing of chronic venous ulcers with complete healing in mean 5.1 weeks (SD 3.1).[36]
Conclusion
A diverse range of second-generation platelet products have shown promise in various indications in dermatology. There is a plethora of variation of each step preparation of PRF products which needs to be understood. These products are relatively underutilized by dermatologists. Further research is required to ascertain the utility of these products in dermatological practice.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Marx RE. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001;10:225–8. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Anitua E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–35. [PubMed] [Google Scholar]
- 3.Fijnheer R, Pietersz RN, de Korte D, Gouwerok CW, Dekker WJ, Reesink HW, et al. Platelet activation during preparation of platelet concentrates: A comparison of the platelet-rich plasma and the buffy coat methods. Transfusion (Paris) 1990;30:634–8. doi: 10.1046/j.1537-2995.1990.30790385523.x. [DOI] [PubMed] [Google Scholar]
- 4.Ehrenfest DM, Corso MD, Diss A, Mouhyi J, Charrier J-B. Three-dimensional architecture and cell composition of a choukroun's platelet-rich fibrin clot and membrane. J Periodontol. 2010;81:546–55. doi: 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]
- 5.Miron RJ, Fujioka-Kobayashi M, Bishara M, Zhang Y, Hernandez M, Choukroun J. Platelet-rich fibrin and soft tissue wound healing: A systematic review. Tissue Eng Part B Rev. 2017;23:83–99. doi: 10.1089/ten.TEB.2016.0233. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20:2353–60. doi: 10.1007/s00784-016-1719-1. [DOI] [PubMed] [Google Scholar]
- 7.Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, et al. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40:679–89. doi: 10.1563/aaid-joi-D-14-00138. [DOI] [PubMed] [Google Scholar]
- 8.Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88:112–21. doi: 10.1902/jop.2016.160443. [DOI] [PubMed] [Google Scholar]
- 9.Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, et al. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin Oral Investig. 2017;21:2619–27. doi: 10.1007/s00784-017-2063-9. [DOI] [PubMed] [Google Scholar]
- 10.Roy S, Driggs J, Elgharably H, Biswas S, Findley M, Khanna S, et al. Platelet-rich fibrin matrix improves wound angiogenesis via inducing endothelial cell proliferation. Wound Repair Regen. 2011;19:753–66. doi: 10.1111/j.1524-475X.2011.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee A, Debnath K. Comparative evaluation of growth factors from platelet concentrates: An in vitro study. J Indian Soc Periodontol. 2019;23:322–8. doi: 10.4103/jisp.jisp_678_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ. 2008;336:1049–51. doi: 10.1136/bmj.39493.646875.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miron RJ, Chai J, Zheng S, Feng M, Sculean A, Zhang Y. A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J Biomed Mater Res A. 2019;107:2257–71. doi: 10.1002/jbm.a.36734. [DOI] [PubMed] [Google Scholar]
- 15.Miron RJ, Chai J, Zhang P, Li Y, Wang Y, Mourão CF de AB, et al. A novel method for harvesting concentrated platelet-rich fibrin (C-PRF) with a 10-fold increase in platelet and leukocyte yields. Clin Oral Investig. 2020;24:2819–28. doi: 10.1007/s00784-019-03147-w. [DOI] [PubMed] [Google Scholar]
- 16.Fujioka-Kobayashi M, Katagiri H, Kono M, Schaller B, Zhang Y, Sculean A, et al. Improved growth factor delivery and cellular activity using concentrated platelet-rich fibrin (C-PRF) when compared with traditional injectable (i-PRF) protocols. Clin Oral Investig. 2020;24:4373–83. doi: 10.1007/s00784-020-03303-7. [DOI] [PubMed] [Google Scholar]
- 17.Tsujino T, Masuki H, Nakamura M, Isobe K, Kawabata H, Aizawa H, et al. Striking differences in platelet distribution between advanced-platelet-rich fibrin and concentrated growth factors: Effects of silica-containing plastic tubes. J Funct Biomater. 2019;10:43. doi: 10.3390/jfb10030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miron RJ, Xu H, Chai J, Wang J, Zheng S, Feng M, et al. Comparison of platelet-rich fibrin (PRF) produced using 3 commercially available centrifuges at both high (~700 g) and low (~200 g) relative centrifugation forces. Clin Oral Investig. 2020;24:1171–82. doi: 10.1007/s00784-019-02981-2. [DOI] [PubMed] [Google Scholar]
- 19.Masuki H, Isobe K, Kawabata H, Tsujino T, Yamaguchi S, Watanabe T, et al. Acute cytotoxic effects of silica microparticles used for coating of plastic blood-collection tubes on human periosteal cells. Odontology. 2020;108:545–52. doi: 10.1007/s10266-020-00486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jianpeampoolpol B, Phuminart S, Subbalekha K. Platelet-rich fibrin formation was delayed in plastic tubes. Br J Med Med Res. 2016;14:1–9. [Google Scholar]
- 21.Miron RJ, Dham A, Dham U, Zhang Y, Pikos MA, Sculean A. The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Investig. 2019;23:2179–85. doi: 10.1007/s00784-018-2673-x. [DOI] [PubMed] [Google Scholar]
- 22.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate.Part III: leucocyte activation: A new feature for platelet concentrates?? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e51–5. doi: 10.1016/j.tripleo.2005.07.010. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Yang Y, Zhang Y, Miron RJ. Fluid platelet-rich fibrin stimulates greater dermal skin fibroblast cell migration, proliferation, and collagen synthesis when compared to platelet-rich plasma. J Cosmet Dermatol. 2019;18:2004–10. doi: 10.1111/jocd.12955. [DOI] [PubMed] [Google Scholar]
- 24.Karimi K, Rockwell H. The benefits of platelet-rich fibrin. Facial Plast Surg Clin N Am. 2019;27:331–40. doi: 10.1016/j.fsc.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Hassan H, Quinlan DJ, Ghanem A. Injectable platelet-rich fibrin for facial rejuvenation: A prospective, single-center study. J Cosmet Dermatol. 2020;19:3213–21. doi: 10.1111/jocd.13692. [DOI] [PubMed] [Google Scholar]
- 26.Nacopoulos C, Vesala A-M. Lower facial regeneration with a combination of platelet-rich fibrin liquid matrices based on the low speed centrifugation concept-Cleopatra technique. J Cosmet Dermatol. 2020;19:185–9. doi: 10.1111/jocd.13196. [DOI] [PubMed] [Google Scholar]
- 27.Liang ZJ, Lu X, Li DQ, Liang YD, Zhu DD, Wu FX, et al. Precise Intradermal injection of nanofat-derived stromal cells combined with platelet-rich fibrin improves the efficacy of facial skin rejuvenation. Cell Physiol Biochem. 2018;47:316–29. doi: 10.1159/000489809. [DOI] [PubMed] [Google Scholar]
- 28.Wei H, Gu SX, Liang YD, Liang ZJ, Chen H, Zhu MG, et al. Nanofat-derived stem cells with platelet-rich fibrin improve facial contour remodeling and skin rejuvenation after autologous structural fat transplantation. Oncotarget. 2017;8:68542–56. doi: 10.18632/oncotarget.19721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azyenela R, Julianto I, Wirohadidjojo Y. The addition of hyaluronic acid into platelet-rich fibrin lysate in restoration of senescent human dermal fibroblasts activities. Malays J Med Biol Res. 2018;5:85–92. [Google Scholar]
- 30.Mercuri S, Paolino G, Nicola MR, Vollono L. Investigating the safety and efficacy of platelet-rich plasma (PRP) treatment for female androgenetic alopecia: Review of the literature. Medicina (Kaunas) 2021;57:311. doi: 10.3390/medicina57040311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karam E, Gan A, Mendoza RM, Martinez E, Perez E. Visual loss after platelet-rich plasma injection into the face. Neuroophthalmology. 2020;44:371–8. doi: 10.1080/01658107.2020.1740936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhalla M, El-Housseini Z, Asaria R. Blindness associated with platelet-rich plasma temporomandibular joint injections. Br J Oral Maxillofac Surg. 2020;58:1197–9. doi: 10.1016/j.bjoms.2020.08.042. [DOI] [PubMed] [Google Scholar]
- 33.Kalyam K, Kavoussi SC, Ehrlich M, Teng CC, Chadha N, Khodadadeh S, et al. Irreversible blindness following periocular autologous platelet-rich plasma skin rejuvenation treatment. Ophthalmic Plast Reconstr Surg. 2017;33:S12–6. doi: 10.1097/IOP.0000000000000680. [DOI] [PubMed] [Google Scholar]
- 34.Shashank B, Bhushan M. Injectable platelet-rich fibrin (PRF): The newest biomaterial and its use in various dermatological conditions in our practice: A case series. J Cosmet Dermatol. 2021;20:1421–6. doi: 10.1111/jocd.13742. [DOI] [PubMed] [Google Scholar]
- 35.Nagaraju U, Sundar PK, Agarwal P, Raju BP, Kumar M. Autologous platelet-rich fibrin matrix in non-healing trophic ulcers in patients with Hansen's disease. J Cutan Aesthetic Surg. 2017;10:3–7. doi: 10.4103/JCAS.JCAS_17_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarvajnamurthy S, Suryanarayan S, Budamakuntala L, Suresh DH. Autologous platelet rich plasma in chronic venous ulcers: Study of 17 cases. J Cutan Aesthetic Surg. 2013;6:97–9. doi: 10.4103/0974-2077.112671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steenvoorde P, van Doorn LP, Naves C, Oskam J. Use of autologous platelet-rich fibrin on hard-to-heal wounds. J Wound Care. 2008;17:60–3. doi: 10.12968/jowc.2008.17.2.28179. [DOI] [PubMed] [Google Scholar]
- 38.Rani A, Mohanty S. Platelet-rich fibrin: A boon as healing and filling material. Indian J Multidiscip Dent. 2016;6:42. [Google Scholar]
- 39.Naik B, Karunakar P, Jayadev M, Marshal VR. Role of platelet rich fibrin in wound healing: A critical review. J Conserv Dent. 2013;16:284–93. doi: 10.4103/0972-0707.114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kour P, Pudakalkatti PS, Vas AM, Das S, Padmanabhan S. Comparative evaluation of antimicrobial efficacy of platelet-rich plasma, platelet-rich fibrin, and injectable platelet-rich fibrin on the standard strains of porphyromonas gingivalis and aggregatibacter actinomycetemcomitans. Contemp Clin Dent. 2018;9((Suppl 2)):S325–30. doi: 10.4103/ccd.ccd_367_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sari R, Larasati G, Kuncorowati N, Syaify A. Platelet-rich fibrin (PRF) membranes accelerate open wound healing better than amniotic membranes: A histological study on the proliferation phase. Wound Med. 2020;31:100190. [Google Scholar]
- 42.Rashed S. A comparative prospective clinical study of efficacy of platelet-rich fibrin (PRF) and chlorhexidine gel for wound healing enhancement and prevention of alveolar osteitis. Egypt Dent J. 2019;65:1035–45. [Google Scholar]
- 43.Somani A, Rai R. Comparison of efficacy of autologous platelet-rich fibrin versus saline dressing in chronic venous leg ulcers: A randomised controlled trial. J Cutan Aesthetic Surg. 2017;10:8–12. doi: 10.4103/JCAS.JCAS_137_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taneja N, Kudva P, Goswamy M, Bhat GK, Kudva HP. A comparative evaluation of platelet-rich fibrin with metronidazole and platelet-rich fibrin alone in the treatment of intrabony periodontal defects: A clinical and radiographical study. J Interdiscip Dent. 2017;7:101–10. [Google Scholar]
- 45.Yuvasri G, Rai R. Comparison of efficacy of autologous platelet-rich fibrin versus Unna's paste dressing in chronic venous leg ulcers: A comparative study. Indian Dermatol Online J. 2020;11:58–61. doi: 10.4103/idoj.IDOJ_119_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: A multicenter randomized controlled trial. Diabetes Care. 2008;31:631–6. doi: 10.2337/dc07-2196. [DOI] [PubMed] [Google Scholar]
- 47.Desai CB, Mahindra UR, Kini YK, Bakshi MK. Use of platelet-rich fibrin over skin wounds: Modified secondary intention healing. J Cutan Aesthetic Surg. 2013;6:35–7. doi: 10.4103/0974-2077.110096. [DOI] [PMC free article] [PubMed] [Google Scholar]


