Abstract
The goal of these recommendations is to provide a framework to practitioners for implementing useful, evidence-based recommendations for the preparation of platelet-rich plasma (PRP) in various dermatological indications. The Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) assigned the task of preparing these recommendations to its task force on PRP. A comprehensive literature search was done in the English language on the preparation of PRP across multiple databases. The grade of evidence and strength of recommendation was evaluated on the GRADE framework (Grading of Recommendation, Assessment, Development and Evaluation). A draft of clinical recommendations was developed on the best available evidence which was also scrutinized and critically evaluated by the IADVL Academy of Dermatology. Based on the inputs received, this final consensus statement was prepared. A total of 45 articles (meta-analyses, prospective and retrospective studies, reviews [including chapters in books], and case series) were critically evaluated and the evidence thus gathered was used in the preparation of these recommendations. This expert group recommends the use of double-spin manual method for the preparation of PRP. The recommended parameters for centrifuge are 100–300 g for 5–10 min for the first spin and 400–700 g for 10–17 min for the second spin. The recommended platelet concentration in PRP for the treatment of various dermatological conditions is 1–1.5 million platelets/μL. The activation of PRP is not required when it is injected into soft tissues.
Keywords: Centrifuge, guidelines, platelet-rich plasma, preparation, PRP kits, RCF, recommendations, regenerative medicine, standardization, RPM
Introduction
Platelet-rich plasma (PRP) has made a steady transition from the fields of hematology, dentistry, and sports medicine into dermatology, aesthetics, and trichology. And even though a wealth of data are being reported, published, and accumulated, there exists a dearth of reproducible data. This transcends into indications of use, methods of administration, dosage to be delivered, expected outcomes, follow-up sessions, and even methods of preparation. Apart from a couple of review articles, there is a lack of consensus on standardizing preparation based selectively on the platelet biophysiology and the indication of use. Through this paper, we aim to delineate a preparation protocol based on data analysis of the existing scientific literature and a consensus of expert peers.
Scope of recommendations
There is a lack of standardization of preparation of PRP in the medical literature. The current reporting of the methodology of PRP preparation and the composition of the final PRP product is inconsistent and insufficient for comparison between studies.[1] The goal of these recommendations is to provide a framework to practitioners for implementing useful, evidence-based recommendations for the preparation of PRP and its use in various dermatological indications.
Methodology of Preparation of Recommendations
A comprehensive literature search was done in the English language on the preparation of PRP across multiple databases (PubMed, Embase, Medline, Google Scholar, and Cochrane). Medical Subject Headings (MeSH) items were added to the search like “PRP,” “Preparation,” “Recommendations,” “Method,” “Double spin,” “Centrifuge,” “Platelet concentrate,” “RPM,” “Ideal platelet concentration,” “Activation,” and “Number of spins.” The grade of evidence and strength of recommendation were evaluated on the GRADE framework (Grading of Recommendation, Assessment, Development and Evaluation).[2] The GRADE framework was chosen for these recommendations as it allows the strength of recommendation to be practice-based and relatively independent of the quality of evidence. The quality of evidence and strength of recommendation were graded as shown in Table 1.[3]
Table 1.
GRADE Framework consisting of four grades of quality of evidence and two grades of strength of recommendation
| GRADE Framework | |
|---|---|
|
| |
| A. Quality of evidence | |
| High quality | Well performed randomized control trials or clear evidence from multiple well-conducted observational studies showing very large effect |
| Moderate quality | Randomized control trials with essential limitations |
| Low quality | Observational studies or controlled trial with severe limitations |
| Very low quality | Non-systematic observations, biologic reasoning, or observational studies with severe limitations |
|
| |
| B. Strength of recommendation | |
|
| |
| Strong | A strong recommendation was given when benefits distinctly outweighed the risks for nearly all patients. As practitioners, most patients must receive this course of action |
| Weak | A weak recommendation was given when risks and benefits were more closely balanced or were uncertain. As practitioners, patients must be explained about all the different options, and an option suitable for patient needs must be chosen |
A draft was prepared which was then sent for review to the members of the IADVL task force for PRP, appointed by the IADVL Academy of Dermatology. It was also sent to the IADVL Academy members for critical comments. Based on the inputs, the final consensus statement was prepared. A total of 45 articles (meta-analyses, prospective and retrospective studies, reviews, chapters in books, and case series) were critically evaluated and the evidence thus gathered was used in the preparation of these recommendations. The recommendations have the following points.
-
Preparation of PRP
Open method
Closed method
-
Facets of PRP preparation
Blood draw
Number of spins
Anticoagulant
Ideal centrifugation parameters
Centrifuge characteristics—temperature control
Activation of PRP
Types of centrifuges
-
Mathematics in relation to PRP preparation
Basic formulas
Volume of blood to be drawn
Platelet purity vs. platelet yield
RPM vs. relative centrifugal force (RCF).
Preparation of PRP
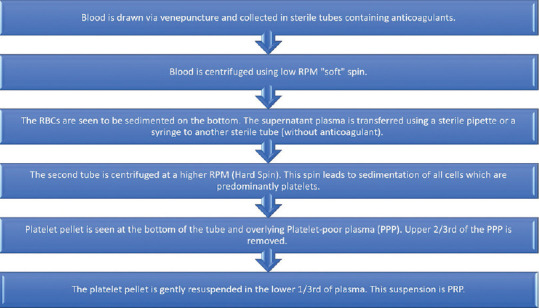
There are numerous methods of PRP preparation. However, all of them primarily involve differential centrifugation.[4] There are two primary methods of PRP preparation:
Open technique: This method involves the open preparation of PRP. The blood encounters the environment in the working area. Pipettes and tubes are sterilized separately and used in the process of preparation of PRP.[5]
Closed technique: This method involves the use of commercial devices or kits. Here the blood or the PRP is not exposed to the environment during the process of preparation of PRP.[5]
Open method
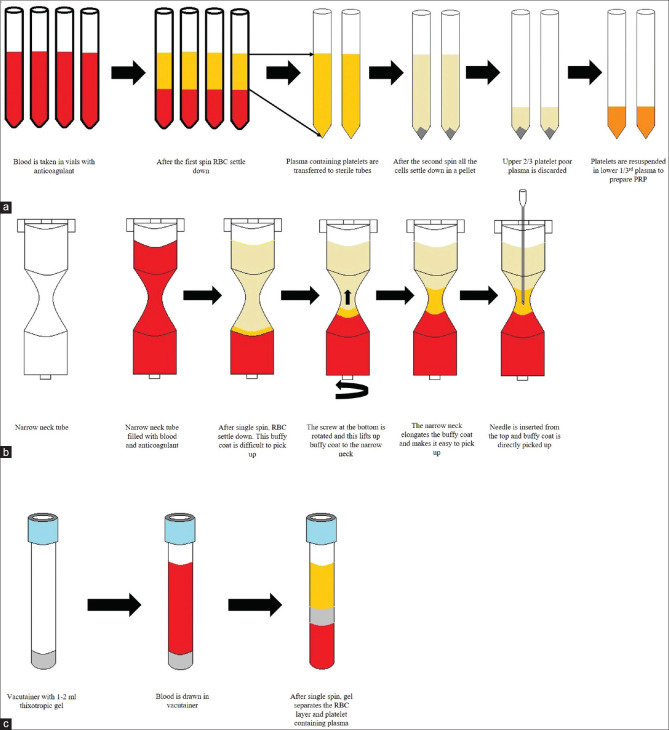
The double-spin open method of preparation of PRP has been shown in Figure 1. The first spin sediments the heavier Red blood cells (RBCs). The liquid supernatant is transferred to another tube and centrifuged again. The second spin is a higher RPM spin which pellets the platelets and the remaining cells. The top two-thirds of the cell-free supernatant after the second spin is discarded and the cell pellets are resuspended in a smaller volume of plasma.
Figure 1.
Step by step method of preparation of PRP using the double-spin open method
The double-spin open method is the preferred method of preparation for dermatologic needs due to its low cost, good platelet yield, and versatile volume of PRP production.
(Quality of Evidence: High; Strength of recommendation: Strong)
The position of supernatant transfer after the first spin determines the type of PRP produced. For the preparation of pure PRP (P-PRP), only the supernatant along with the top buffy coat is transferred to the second sterile tube for the second spin. Similarly, to prepare leukocyte-rich PRP (L-PRP), we need to transfer the complete supernatant along with the entire buffy coat and some RBCs to the second sterile tube.
Different equipment can be utilized to prepare PRP when using the open method. Conical bottom plastic tubes are commonly used to prepare PRP, Figure 2. These tubes are cost-effective and can be sterilized using ethylene oxide (ETO). These tubes can be used for both the first and second spin. Another method to prepare PRP is with the help of a 9 mL acid citrate dextrose-A (ACD-A) vacutainer, Figure 3. This vacutainer is used only for the first spin. There are some concerns about the vacutainers being sterile but not pyrogen or endotoxin-free as they are designed for diagnostic purposes and not for therapeutic purposes. Newhall et al.[6] tested vacutainers for the presence of endotoxin and found that some of the sodium heparin plastic vacutainers tested positive for endotoxin. They did not find any endotoxin in the glass tubes containing the same anticoagulant. These findings are corroborated by Aziz et al.[7] Hence, glass vacutainers must be preferred over plastic vacutainers for the preparation of PRP.
Figure 2.

Shows a screw top plastic tube which needs to be individually sterilized using ethylene oxide (ETO)
Figure 3.

Shows yellow top tube (vacutainer with ACD-A anticoagulant)
Another criticism of PRP produced using the open method is the risk of contamination of PRP. This can be minimized by preparing the PRP using all aseptic precautions with preparation preferably done under laminar airflow. A face mask, sterile disposable gloves, and a sterile gown are a must for the physician preparing the PRP as handling of blood products to prevent infection. PRP has been found to have strong antimicrobial activity. Drago et al.[8] and Li et al.[9] found a strong antimicrobial activity of PRP against methicillin-resistant Staphylococcus aureus (MRSA) and Group A Streptococci. Both these studies have used pure PRP and not L-PRP hence leukocytes did not play a role in the bactericidal activity of PRP.
Double spin is the standard method of the preparation of PRP as confirmed by various studies and the American Association of Blood Banks technical manual.[4,10] Single-spin method is not preferred as it leads to a lower platelet yield of 53% as measured by Harrison et al.[11]
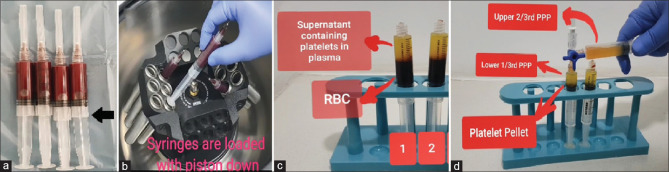
Fukaya et al.[12] suggested an innovative and economical method for the preparation of PRP—the syringe-only method. They recommend a simple modification of a 5 mL syringe that allows its insertion into the centrifuge. The syringe is inserted piston down into the centrifuge. After separation, the supernatant is transferred to a different syringe using a 3-way canula for the second spin. The advantage of this method is that it is a closed method where the blood product remains inside commonly available medical disposables like 3-way canula and syringes, which are even approved for intravenous injections [Figure 4].
Figure 4.
Shows the syringe-only method of PRP preparation. (a) Blood is drawn in 5 mL syringes and wings are cut as shown by black arrow. (b) Syringes are loaded in the centrifuge piston down. (c) RBC layer settles to the bottom of the syringe that is toward the piston. D. The supernatant is transferred to a different syringe using a 3-way canula
Another innovative method described in the literature is the turn-down turn-up method to prepare PRP. This method needs only one 10 mL syringe and two vacutainers, preferably 8.5 mL. It is a double-spin method where vacutainers are placed in an inverted position for the first spin and the correct position for the second spin. This method has the drawback of having a high hematocrit or RBC-containing PRP. Machado et al.[13] were able to produce a 4.17-folds increase in platelet concentration in their PRP using this method [Figure 5].
Figure 5.
Shows the turn-down turn-up method of preparation of PRP. (a) Vacutainer is placed in the centrifuge top down. (b) RBC sediment toward the rubber stopper, extracted with 10 cc syringe. (c) Vacutainer is placed in a regular fashion in the centrifuge for the second spin. (d) PRP prepared
Closed preparation systems
Closed systems, as mentioned earlier, do not expose the platelets to the external environment after the process of blood collection. This system involves the use of a commercial kit paired mostly with a complementary centrifuge machine. And while most of these kits are marketed with Food and Drug Administration (FDA) 'labels,' it would be pertinent to understand that these approvals are simply for device safety (510 [k] approval) and not for efficacy. Magalon et al. (2016)[14] attempted to classify and compare these kits with 'homemade' PRP (PRP prepared using an open system). The classification takes the following parameters into account: Dose of injected platelets (yield), efficiency or recovery of platelets during the process, purity of platelets, and activation. The dose of platelets injected was the total number of platelets in the final PRP produced (concentration of platelets × volume of PRP) measured in billion platelets. The efficiency of platelet recovery was calculated as a percentage of original platelets that were recovered in the final PRP product. The purity of platelets measured the contamination of platelets by other cell types. It measured the percentage of cells that were platelets out of all the cells in the PRP and finally if the PRP was activated or not. The classification then gives ranks to the different methods of preparation of PRP from A to D, A being the best. An interesting observation that comes from this classification is that methods that have a higher dose of platelet, do not produce a pure PRP. This indicates that the purity of PRP may be inversely related to the number of platelets captured. The only system that has received an A rank in the dose of platelets (more than 5 billion platelets injected) is the Magellan system®. But the same system has received a C or D rank in the purity of platelets (only 30–70% of cells in PRP are platelets). Homemade PRP has received the next highest score average on the classification. Although the score is the same as the Arthrex system®, the dose of injected platelets was higher in homemade PRP. Double-spin open system (homemade in the classification) may be the recommended method of preparation of PRP which has received good scores on all parameters, is easy to prepare, and is cost-effective.
The most commonly used commercial kits involve the use of three basic methods—the narrow neck tube method, the gel separator method, or the automated cell separators.
The narrow-neck tube method uses a special tube that has four parts—an extended top, a constricted center (or the narrow neck), an expanded bottom, and a turn screw. Typically, the buffy coat is a very thin layer and hence challenging to pick up. The narrow neck elongates the buffy coat and makes it quite easy to pick up. Numerous advantages of this technique are that it requires only a single spin and a study by Harrison et al.[11] found a 2.5-folds increase in the platelet count using this kit. Gupta et al.[15] compared the double-spin manual method to Dr. PRP kit and found better amplification by the manual method which was 4.8-folds vs. Dr. PRP™ kit (narrow neck tube) which provided 2.8-folds keeping all other parameters constant.
One of the most commonly available commercial kits for the preparation of PRP utilizes the gel separation technique. These kits contain 1–2 mL of a thixotropic polymer gel in the blood collection tube. The gel has a specific gravity lower than RBC and White Blood Cells (WBC) but more than the platelets. On centrifugation, the gel settles between the plasma containing platelets and all other components below. These kits are easy to use and prepare almost a pure PRP which can be ideal for the face and other cosmetic areas. However, there exists a controversy associated with the platelet counts produced by many of these kits. Many studies have shown low platelet concentration with the PRP produced using these kits. Mazzucco et al.[16] compared four different methods of preparation of PRP and found a significantly lower platelet concentration as well as lower growth factor concentration in the gel separation kits when compared to the other methods. Franka Klatte-Shulz and colleagues compared platelet preparations by different methods and their effect on growth factor release.[17] They found that gel separator tubes did not increase platelet count but decreased it by 30% accompanied by a lower growth factor release. Arshdeep et al.[18] used PRP prepared by the gel separator method in male patients of androgenetic alopecia and found no significant hair growth in their study. A possible reason for this lower platelet count with gel separator methods may be due to the specific gravity of thixotropic gel. Karpatkin et al.[19] described two density populations of platelets. Lighter small platelets with a specific gravity less than 1.046 and large heavy platelets with a specific gravity more than 1.055. Smaller platelets are generally older platelets which are less metabolically active and the larger platelets are younger platelets that are more metabolically active. The specific gravity of WBC is 1.050, and the specific gravity of the gel is less than that of the WBCs. After centrifugation, the gel lies above the WBC layer or the buffy coat layer and the heavier platelets (specific gravity 1.055) are trapped even below the WBC layer. These platelets are not captured in the PRP. However, if PRP produced using gel separator tubes indeed contains smaller platelets only then the mean platelet volume (MPV) of platelets in PRP must be lower than MPV for whole blood, a point confirmed by a study by Berndt et al.[20] They found MPV of PRP to be 8.2 FL as compared to 8.7 FL for whole blood indicating a preferential separation of smaller platelets in PRP. Harrison et al.[11] also performed aliquot testing for platelet concentration in single-spin methods and found most of the platelets in the buffy coat, and upper RBC layer indicating that some of the platelets definitely have a slightly higher density and hence are trapped in the buffy coat layer.
Gkini et al. were able to produce 5.8 fold amplification of platelet concentration in their PRP produced using gel separator kits but they did so by modifying the manufacturer's instructions and performing a second spin on the plasma collected over the gel.[21] Doing so would void FDA approval of the kit.
Automated cell separators—another technology available commercially which are fully automatic, closed systems. These machines can give PRP in the required volume. They usually have a high RBC contamination, as high as 9.8%[22] in Magellan system™, and they can be expensive.
A schematic representation of different methods of preparation of PRP is shown in Figure 6.
Figure 6.
(a) Schematic representation of double-spin open method of preparation of PRP is shown (b) Schematic representation of narrow neck tube method of PRP preparation available in different types of commercial kits. (c) Schematic representation of gel separator tube method of preparation of PRP available as commercial kit
Facets of PRP Preparation
There are different facets of PRP preparation which are a source of confusion to dermatologists. Although the jury is still out on most of these factors, these recommendations are an attempt to provide a framework based on the current evidence.
Blood draw
A wide bore needle must be used when drawing blood for preparing PRP, preferably 21G or larger.
(Quality of evidence: High; Strength of recommendation: Strong)
Mani et al.[23] in their study compared 21G needle and 21G butterfly cannula for the blood draw and did not find any evidence of platelet activation on either side in 25 volunteers. Lippi et al.[24] found a higher d-dimer level (sign of platelet activation) and a lower platelet count with the needles with smaller gauges of 23G and 25G. Although the difference was minimal, the results were still statistically significant. Hence, a needle of size 21G or more must be used to draw blood when preparing PRP. The time of blood draw is also an important parameter. In their experiment, Miron et al.[25] found that the size of PRF clot produced reduces by 23% if the time from blood draw to the start of centrifugation exceeds 120 s.
Number of spins
The double-spin method is the recommended method of preparation of PRP. (Quality of evidence: High; Strength of recommendation: Strong)
The American Association Blood Bank Technical Manual recommends the preparation of PRP by the double-spin method.[10] Tamimi et al.[26] found that the double-spin method was able to achieve a higher average platelet concentration in their PRP as compared to the single-spin method. Harrison et al.[11] compared six different single-spin methods of preparation of PRP. Manual methods and commercial kits, both were included in the study. They found that out of all the single-spin methods only one method was able to reach the ideal platelet concentration of 1 million platelets/μL. Franka Klatte-Schulz et al.[17] in their study observed that one of the commercial single-spin kits for the preparation of PRP actually had a 30% lower platelet concentration as compared to whole blood. This decrease in platelet count goes against the definition of PRP which is a plasma product with a platelet concentration that is higher than whole blood. Mazzucco et al.[16] compared four common methods of preparation of PRP and found that the single-spin method had significantly lower platelet concentration and growth factor concentration as compared to double-spin methods. One of the commercial single-spin kits was used for the treatment of androgenetic alopecia and the authors found no significant increase in the hair density or hair count.[18] This indicates that the double-spin method should be the recommended method of preparation of PRP.
Anticoagulant
Acid citrate dextrose (ACD-A) is the preferred anticoagulant for the preparation of PRP.
(Quality of evidence: High; Strength of recommendation: Low)
Choosing the right anticoagulant is a critical step in the preparation of PRP. Acid citrate dextrose-A (ACD-A, trisodium citrate (TSC) (3.8 or 3.2%), citrate phosphate dextrose adenine (CPDA), heparin, and EDTA are the common anticoagulants used for numerous purposes of preparation of blood components. ACD-A has a lower pH and lower extracellular calcium ion concentration than TSC. This environment allows for more reliable prevention of platelet aggregation.[4] The anticoagulant, CPDA was found to be 10% less effective at maintaining platelet viability as compared to ACD-A.[27] EDTA was also found to damage the platelet membrane and hence not advisable for use in PRP preparation.[28] Lei et al.[29] compared various aspects of anticoagulants used in the preparation of PRP. They found ACD-A to be superior to TSC and heparin in maintaining platelet membrane integrity and preventing inadvertent activation of platelets during centrifugation. On activation, PRP produced using ACD-A, released significantly more Transforming growth factor beta1 (TGF -β1) and produced a more enhanced proliferation of human marrow stromal cells as compared to heparin and TSC. do Amaral et al. observed a conflicting result in their study, which found TSC to be the superior anticoagulant in terms of platelet survival and platelet membrane integrity.[30] They also found a higher TGF-β1 release from PRP produced using TSC as compared to ACD-A. Mussbacher et al.[31] performed a study to assess the degree of platelet activation after storage of blood in different anticoagulants for 30 min. No increase in PF4 concentration was seen when citrate-theophylline-adenosine-dipyridamole (CTAD) and ACD-A were used as anticoagulants, indicating no platelet activation. Sodium citrate did not show any increase in PF4 concentration only when the blood was stored at 4°C. This finding indicates a slight superiority of ACD-A over sodium citrate in preventing platelet activation. These conflicting results have led to the low strength of recommendation. However, all studies found both ACD-A and TSC as acceptable anticoagulants for the preparation of PRP.
Ideal centrifuge parameters
Recommended parameters for centrifuge are 100–300 g for 5–10 min for the first spin and 400–750 g for 10–17 min for the second spin (3.5 –10 mL blood per tube).
(Quality of evidence: Medium; Strength of recommendation: Low)
Several studies have described the optimum protocol for the preparation of PRP. Each study reaches its unique conclusion depending on variables like the number of spins, the volume of blood taken, the RCF used, and the time for centrifugation. It is difficult to conclude the ideal centrifugation parameters from these studies. Various studies have shown the ideal parameters for the preparation of PRP. The studies that performed the double-spin method, kept the volume of blood draw less than 60 mL, and reported their parameters in RCF were evaluated.[32,33,34,35] The blood volume drawn ranged from 3.5 mL to 10 mL. The parameters for the first spin ranged from 100 to 300 g for 5–10 min, with a higher RCF spun for a lesser time and a lower RCF for a longer time. For the second spin, it ranged from 400 to 750 g for 10–15 min.[26,36,37,38] A critical point to note here is that increasing RCF beyond a certain limit might lead to activation of platelets and premature release of growth factors.[33] Perez et al.[33] in their study observed the highest platelet yield at 100 g for 10 min for the first spin and 400 g for 10 min for the second spin. They found signs of platelet activation at RCF of 800 g or more (increased sP selectin levels). This indicates that there might be spontaneous activation of platelets at higher RCFs and lower RCFs and longer time durations of spin are preferred than vice-versa.
Ideal platelet concentration of PRP for use in dermatological conditions
The recommended platelet concentration in PRP for treatment of various dermatological conditions is 1–1.5 million platelets/μL.
(Quality of evidence: Low; Strength of recommendation: Strong)
Like any medicine, PRP works in a specific therapeutic range. The increased concentration of platelets is associated with a higher concentration of growth factors.[39] Sundman et al.[40] in their study found a significantly higher concentration of TGF-β1 and Dose Efficiency Purity Activation (DEPA) classification was seen in the PRP produced by the kit that amplified platelet count by 4.69-folds vs. a 1.99-fold kit. This result indicates a dose-dependent relationship between growth factor concentration and platelet concentration in PRP. In an in vivo animal study, bone regeneration was seen with platelet concentration ranging from 0.95 to 1.7 million platelets/μL.[41] Klatte-Schulz et al.[17] in their study on the effect of PRP on a culture of tenocytes found that the growth and survival of tenocytes and expression of type I collagen did not correlate with growth factor concentration linearly. However, higher platelet concentration was associated with higher growth and survival of tenocytes. Giusti et al.[42] found a dose-dependent proliferation of human-cultured umbilical vein endothelial cells (HUVECs) which peaked at 1.5 million/μL platelet concentration and cell proliferation was impaired at a higher platelet concentration. At 72 h post-incubation, HUVEC proliferation followed a bell-shaped distribution with the zone of highest proliferation lying between 1 million and 2 million and it fell precipitously below 1 million and above 2 million. The Dose Efficiency Purity Activation (DEPA) classification gives an 'A' rating to a dose of platelets more than 5 billion platelets. This is ideal as according to the definition of PRP—1 million platelets per μL is the same as 1 billion platelets per mL. When produced in 5 mL volume, it is 5 billion total platelets. However, if the same volume of platelets were concentrated in 1 mL volume, the concentration would be 5 million/μL (50 lakhs) and this would not be optimal.
Xiao et al.[43] studied the effect of PRP on cultured human hair dermal papilla (DP) cells and found 1.3 million/μL platelets in PRP to be the ideal concentration for DP cell proliferation. A higher concentration of platelets in PRP was found to be inhibitory in all the above studies. Low quality of evidence was given due to a lack of a direct study of hair growth and density compared with different concentrations of platelets in PRP.
Centrifuge characteristics—Temperature control during centrifugation
Temperature-controlled centrifuges may allow an enhanced platelet survival and recovery; however, they may not be necessary for the preparation of PRP.
(Quality of evidence: Low; Strength of recommendation: Low)
There have been conflicting reports regarding the effect of temperature on platelets. The American Association of Blood Banks Technical Manual advises not to chill the blood below 20°C before the start of PRP preparation.[10] An old study published in 1968 evaluated the effect of heat on platelets and found that platelets exposed to 42°C for 15 min showed no functional changes in the platelets. Exposure to temperatures above 42°C and for a longer duration led to irreversible damage to platelets.[44] Another study by Maurer-Spurej et al.[45] confirmed this result. They also found no change in platelet structure or morphology at 37 and 42°C. Surprisingly, they found a change in platelet morphology and signs of activation of platelets (spherical shape of platelets in place of resting discoid shape) when they were incubated at 22°C. Faraday and Rosenfeld showed an increase in P-selectin expression (a sign of platelet activation) and enhanced aggregation when activated by thrombin or collagen at 22°C as opposed to 37°C.[46] Michelson et al.[47] showed contradictory findings to this. They reported hemostatic failure and impaired platelet activation after hypothermic exposure to platelets during extracorporeal cooling of blood during cardiopulmonary bypass surgery. A study by Amable et al.[32] showed high platelet concentration in PRP, and high growth factor concentration (post-activation) in PRP produced using a temperature-controlled centrifuge. In comparison, Fukaya et al.[12] used a regular, non-temperature-controlled centrifuge (KOKUSAN H-19α centrifuge) and produced a PRP with high platelet concentration and high growth factor concentration (post-activation). These conflicting results indicate that further randomized controlled trials need to be performed to conclude the need for a temperature-controlled centrifuge for the preparation of PRP.
Activation of PRP
Activation of PRP is not required when it is injected into soft tissues.
(Quality of evidence: Medium; Strength of recommendation: Strong)
Activation of PRP primarily refers to two processes—Release of Growth Factors (GFs) from platelets following degranulation and cleavage of fibrinogen to form the matrix. This process turns liquid plasma into a solid clot or a membrane.[48] A study by Cavallo et al. compared the growth factor release from the platelets by different activators like thrombin, calcium chloride, a combination of thrombin and calcium chloride, and collagen type I.[48] They found the slowest activation with collagen type I. Sudden activation leads to the dumping of all the Growth Factors (GFs) immediately, which may not be ideal. When PRP is used for treatment of hard tissues like bone fractures, activation of PRP is a must.[49] But, when PRP is injected into a soft tissue, it does not need to be activated as the natural collagen type I acts as a natural activator.[50] When PRP is injected into a soft tissue, it does not need to be activated as the natural collagen type I acts as a natural activator.[50] Gentile et al.[51] compared the results of activated and non-activated PRP in its use in androgenetic alopecia. They found a more significant increase in hair count and hair density with non-activated PRP than with activated PRP. An identical PRP preparation device was used in both cases. This result indicates that PRP need not be activated when used for androgenetic alopecia and any other indication where PRP is injected in the dermis.
Type of centrifuge
A microprocessor-controlled centrifuge with a brushless motor and swing-out rotor is ideal for the preparation of PRP.
(Quality of evidence: Low; Strength of recommendation: Strong)
The centrifuges which only have a dial on them without a digital display must not be used for PRP preparation as we may not be able to set the required RPM. Also, these centrifuges are designed for laboratory use and have an electrical motor. The RPM may change if the voltage in the electrical line fluctuates. A centrifuge with a brushless motor is better than a carbon brush motor as the brushes deteriorate after some time and start damaging the armature of the motor. A brushless motor has a much longer life and requires less maintenance. The centrifuge machine must have an electronic display of RPM for accurate measurement. A swing-out rotor of the centrifuge allows a greater difference of centrifugal force between the top of the tube and the bottom of the tube allowing better separation of the cells. A fixed-angle centrifuge first pushes the cells on the back of the tube and then they are smeared to the back wall of the tube which may lead to their activation and damage as shown in Figures 7 and 8.
Figure 7.
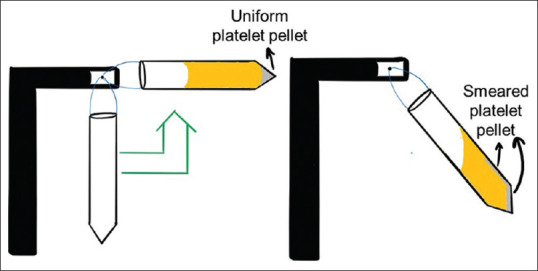
Diagrammatic representation of smearing of cells on the back wall of the tube in a fixed-angle centrifuge
Figure 8.

Shows smearing of cells on the back wall of the tube in a fixed-angle centrifuge
Mathematics in relation to PRP
Certain basic concepts of mathematics and physics are relevant to the preparation of PRP. These concepts will help us better understand the steps of the preparation of an ideal PRP.
Basic formulas
Platelet yield or Efficiency of PRP production or platelet recovery efficiency = ([Volume of PRP produced] × [Concentration of Platelets in PRP])/([Volume of whole blood drawn] × [Concentration of platelets in whole blood]).[14]
Dose of platelets = (Volume of PRP produced) × (Concentration of platelets in PRP)[14]
Platelet concentration amplification factor = Concentration of platelets in PRP/Concentration of PRP in whole blood.
We know that no process can have 100% efficiency. However, when deciding to choose a process, we must calculate the platelet yield for that process and choose a method with a high platelet yield. Even though a PRP method may boast of a high platelet concentration, if it is produced in a small volume, it may not be adequate to cover the entire treatment area. Similarly, if the PRP is produced in adequate volume but the concentration is not high, it will not provide the required growth factor concentration to reach the desired result.
The volume of whole blood that needs to be drawn
When producing PRP with the open or manual method, the volume of blood drawn can be varied depending on the volume of PRP required. The basic rule of physics states that if we reduce the volume of blood or plasma by half, we can maximally increase the concentration of platelets by a maximum of two folds (assuming 100% platelet recovery and platelets being indivisible). We know that platelet recovery cannot be 100%. Various studies give an average range of 50–80%.[14] So, to produce 5 mL PRP with at least 5-folds amplification of platelet concentration and assuming 80% platelet yield, we need to draw a minimum of 31.25 mL of blood. In the study by Dhurat et al.,[4] they produce a similar yield by taking 36 mL of blood.
Platelet yield versus Purity of PRP
Magalon et al.[14] in their study, compared different methods of PRP preparations on their platelet yields, purity of PRP, and dose of platelets. They found that none of the methods reached the A rank (highest rank) in all three categories. They found that methods that prepare high platelet yields were not pure and methods that prepare pure PRP often have poor yield. The reason behind this may be because of a different population of platelets as described by Karpatkin et al.[19] This population of heavier platelets may get trapped in the upper RBC layer and hence their retrieval may be difficult if purity is required. This may be a limitation of the differential centrifugation method of preparation of PRP.
Centrifuge characteristics—RPM versus RCF
RCF is the correct method of scientific communication as this parameter is the same irrespective of the type of centrifuge used to prepare the PRP. This is because different centrifuges have different sizes or different radii of rotors. This means that they produce different forces or different sedimentation rates even at the same RPM.
RCF is the centrifugal force produced by a centrifuge and is measured in the multiples of earth's gravity or the “g force” or “g's.” The formulas to convert RCF value to RPM and vice versa are given in Figure 9. Here, a critical point to note is the radius of the centrifuge. Three different radii can be measured—the minimum radius (R-min), the average radius (R-average), and the maximum radius (R-max), Figure 10. The R-min is the distance from the axis of rotation of the centrifuge to the top of the tube. R-average is the distance from the axis of rotation to the center of gravity of the blood column. R-max is the distance from the axis of rotation to the end of the tube. Different authors use different radii to calculate their RCF and hence when emulating their settings, we must be careful to use the same radius. A prominent study by Choukroun et al.[52] on the preparation of PRF uses R-min to calculate RCF in their research while Miron et al.[53] in their study to prepare I-PRF used R-max. The international standard for RCF is R-max, and it should be used when not specified.
Figure 9.
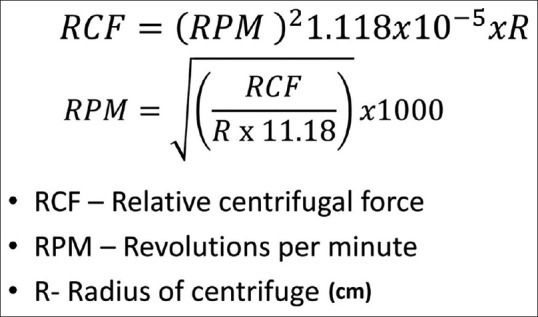
Shows the formulas to convert RCF to RPM and the reverse. Radius is measured in cm
Figure 10.
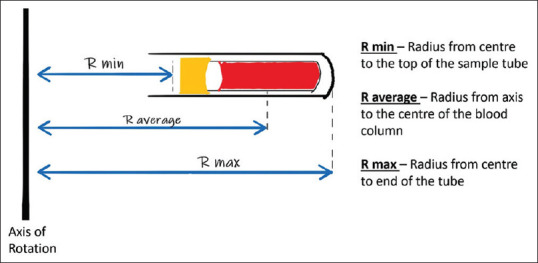
Shows the three different radii that can be measured in a swing-bucket type rotor centrifuge—R-min, R-average, R-max
Another important factor to be kept in mind while using a reference to standardize the PRP preparation is that along with using the same RCF, the tube length and diameter must also be the same. If we use a longer tube than the one described in the reference material, the top of the tube is closer to the center of the centrifuge and is acted on by a lower force and hence produces different results.
Conclusion
The science behind the preparation of PRP is still in its infancy. We are far from producing the 'ideal' PRP and using this autologous biological product to its fullest potential. There are multiple pitfalls that one must avoid while preparing PRP. It is critical for the dermatologists to be aware of the different facets of PRP preparation to ensure that they deliver the best therapeutic result to their patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kramer ME, Keaney TC. Systematic review of platelet-rich plasma (PRP) preparation and composition for the treatment of androgenetic alopecia. J Cosmet Dermatol. 2018;17:666–71. doi: 10.1111/jocd.12679. [DOI] [PubMed] [Google Scholar]
- 2.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ. 2008;336:1049–51. doi: 10.1136/bmj.39493.646875.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: A review and author's perspective. J Cutan Aesthetic Surg. 2014;7:189–97. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alves R, Grimalt R. A review of platelet-rich plasma: History, biology, mechanism of action, and classification. Skin Appendage Disord. 2018;4:18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newhall KJ, Diemer GS, Leshinsky N, Kerkof K, Chute HT, Russell CB, et al. Evidence for endotoxin contamination in plastic Na+-heparin blood collection tube lots. Clin Chem. 2010;56:1483–91. doi: 10.1373/clinchem.2006.144618. [DOI] [PubMed] [Google Scholar]
- 7.Aziz N, Irwin MR, Dickerson SS, Butch AW. Spurious tumor necrosis factor-α and interleukin-6 production by human monocytes from blood collected in endotoxin-contaminated vacutainer blood collection tubes. Clin Chem. 2004;50:2215–6. doi: 10.1373/clinchem.2004.040162. [DOI] [PubMed] [Google Scholar]
- 8.Drago L, Bortolin M, Vassena C, Taschieri S, Del Fabbro M. Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC Microbiol. 2013;13:47. doi: 10.1186/1471-2180-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Li B. PRP as a new approach to prevent infection: Preparation and in vitro antimicrobial properties of PRP. J Vis Exp. 2013:50351. doi: 10.3791/50351. doi: 10.3791/50351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweeny J, Grossman BJ. Blood collection, storage and component preparation methods. In: Brecher M, editor. Technical Manual. American Association of Blood Banks (AABB) 13th ed. Bethesda: S. Krager AG; 2002. pp. 955–8. [Google Scholar]
- 11.Harrison TE, Bowler J, Levins TN, Cheng A-L, Reeves KD. Platelet yield and yield consistency for six single-spin methods of platelet rich plasma preparation. Platelets. 2020;31:661–6. doi: 10.1080/09537104.2019.1663808. [DOI] [PubMed] [Google Scholar]
- 12.Fukaya M, Ito A. A new economic method for preparing platelet-rich plasma. Plast Reconstr Surg Glob Open. 2014;2:e162. doi: 10.1097/GOX.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machado ES, Leite R, Dos Santos CC, Artuso GL, Gluszczak F, de Jesus LG, et al. Turn down-turn up: Asimple and low-cost protocol for preparing platelet-rich plasma. Clin Sao Paulo Braz. 2019;74:e1132. doi: 10.6061/clinics/2019/e1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magalon J, Chateau AL, Bertrand B, Louis ML, Silvestre A, Giraudo L, et al. DEPA classification: A proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc Med. 2016;2:e000060. doi: 10.1136/bmjsem-2015-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta V, Parihar AS, Pathak M, Sharma VK. Comparison of platelet-rich plasma prepared using two methods: Manual double spin method versus a commercially available automated device. Indian Dermatol Online J. 2020;11:575–9. doi: 10.4103/idoj.IDOJ_653_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P. Not every PRP-gel is born equal.Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang. 2009;97:110–8. doi: 10.1111/j.1423-0410.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- 17.Klatte-Schulz F, Schmidt T, Uckert M, Scheffler S, Kalus U, Rojewski M, et al. Comparative analysis of different platelet lysates and platelet rich preparations to stimulate tendon cell biology: An in vitro study. Int J Mol Sci. 2018;19:212. doi: 10.3390/ijms19010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayatollahi A, Hosseini H, Shahdi M, AhmadNasrollahi S, NassiriKashani M, Yadangi S, et al. Platelet-rich plasma by single-spin process in male pattern androgenetic alopecia: Is it an effective treatment? Indian Dermatol Online J. 2017;8:460–4. doi: 10.4103/idoj.IDOJ_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karpatkin S, Charmatz A. Heterogeneity of human platelets. J Clin Invest. 1969;48:1073–82. doi: 10.1172/JCI106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berndt S, Turzi A, Pittet-Cuénod B, Modarressi A. Autologous platelet-rich plasma (CuteCell PRP) safely boosts in vitro human fibroblast expansion. Tissue Eng Part A. 2019;25:1550–63. doi: 10.1089/ten.TEA.2018.0335. [DOI] [PubMed] [Google Scholar]
- 21.Gkini M-A, Kouskoukis A-E, Tripsianis G, Rigopoulos D, Kouskoukis K. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthetic Surg. 2014;7:213–9. doi: 10.4103/0974-2077.150743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degen RM, Bernard JA, Oliver KS, Dines JS. Commercial separation systems designed for preparation of Platelet-Rich plasma yield differences in cellular composition. HSS J Musculoskelet J Hosp Spec Surg. 2017;13:75–80. doi: 10.1007/s11420-016-9519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mani H, Kirchmayr K, Kläffling C, Schindewolf M, Luxembourg B, Linnemann B, et al. Influence of blood collection techniques on platelet function. Platelets. 2004;15:315–8. doi: 10.1080/09537100410001711505. [DOI] [PubMed] [Google Scholar]
- 24.Lippi G, Salvagno GL, Montagnana M, Poli G, Guidi GC. Influence of the needle bore size on platelet count and routine coagulation testing. Blood Coagul Fibrinolysis Int J Haemost Thromb. 2006;17:557–61. doi: 10.1097/01.mbc.0000245300.10387.ca. [DOI] [PubMed] [Google Scholar]
- 25.Miron RJ, Dham A, Dham U, Zhang Y, Pikos MA, Sculean A. The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Investig. 2019;23:2179–85. doi: 10.1007/s00784-018-2673-x. [DOI] [PubMed] [Google Scholar]
- 26.Tamimi FM, Montalvo S, Tresguerres I, Blanco Jerez L. A comparative study of 2 methods for obtaining platelet-rich plasma. J Oral Maxillofac Surg. 2007;65:1084–93. doi: 10.1016/j.joms.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Marx RE. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001;10:225–8. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Anitua E, Prado R, Sanchez M, Orive G. Platelet-rich plasma: Preparation and formulation. Oper Tech Orthop. 2012;22:25–32. [Google Scholar]
- 29.Lei H, Gui L, Xiao R. The effect of anticoagulants on the quality and biological efficacy of platelet-rich plasma. Clin Biochem. 2009;42:1452–60. doi: 10.1016/j.clinbiochem.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 30.do Amaral RJFC, da Silva NP, Haddad NF, Lopes LS, Ferreira FD, Filho RB, et al. Platelet-rich plasma obtained with different anticoagulants and their effect on platelet numbers and mesenchymal stromal cells behavior in vitro. Stem Cells Int. 2016;2016:7414036. doi: 10.1155/2016/7414036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mussbacher M, Schrottmaier WC, Salzmann M, Brostjan C, Schmid JA, Starlinger P, et al. Optimized plasma preparation is essential to monitor platelet-stored molecules in humans. PloS One. 2017;12:e0188921. doi: 10.1371/journal.pone.0188921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco I, Corrêa do Amaral RJ, Granjeiro JM, et al. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4:67. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez AG, Lana JF, Rodrigues AA, Luzo AC, Belangero WD, Santana MH. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematol. 2014;2014:176060. doi: 10.1155/2014/176060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297–301. doi: 10.1016/s0278-2391(00)90058-2. [DOI] [PubMed] [Google Scholar]
- 35.Jo CH, Roh YH, Kim JE, Shin S, Yoon KS. Optimizing platelet-rich plasma gel formation by varying time and gravitational forces during centrifugation. J Oral Implantol. 2013;39:525–32. doi: 10.1563/AAID-JOI-D-10-00155. [DOI] [PubMed] [Google Scholar]
- 36.Bausset O, Giraudo L, Veran J, Magalon J, Coudreuse JM, Magalon G, et al. Formulation and storage of platelet-rich plasma homemade product. Biores Open Access. 2012;1:115–23. doi: 10.1089/biores.2012.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araki J, Jona M, Eto H, Aoi N, Kato H, Suga H, et al. Optimized preparation method of platelet-concentrated plasma and noncoagulating platelet-derived factor concentrates: Maximization of platelet concentration and removal of fibrinogen. Tissue Eng Part C Methods. 2012;18:176–85. doi: 10.1089/ten.tec.2011.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kececi Y, Ozsu S, Bilgir O. A cost-effective method for obtaining standard platelet-rich plasma. Wounds. 2014;26:232–8. [PubMed] [Google Scholar]
- 39.Jungbluth P, Grassmann J-P, Thelen S, Wild M, Sager M, Windolf J, et al. Concentration of platelets and growth factors in platelet-rich plasma from Goettingen minipigs? GMS Interdiscip Plast Reconstr Surg DGPW. 2014;3:Doc11. doi: 10.3205/iprs000052. doi: 10.3205/iprs000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39:2135–40. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- 41.Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34:665–71. doi: 10.1016/j.bone.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Giusti I, Rughetti A, D'Ascenzo S, Millimaggi D, Pavan A, Dell'Orso L, et al. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009;49:771–8. doi: 10.1111/j.1537-2995.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 43.Xiao S-E, Miao Y, Wang J, Jiang W, Fan Z-X, Liu X-M, et al. As a carrier-transporter for hair follicle reconstitution, platelet-rich plasma promotes proliferation and induction of mouse dermal papilla cells. Sci Rep. 2017;7:1125. doi: 10.1038/s41598-017-01105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White JG. Effects of heat on platelet structure and function. Blood. 1968;32:324–35. [PubMed] [Google Scholar]
- 45.Maurer-Spurej E, Pfeiler G, Maurer N, Lindner H, Glatter O, Devine DV. Room temperature activates human blood platelets. Lab Invest. 2001;81:581–92. doi: 10.1038/labinvest.3780267. [DOI] [PubMed] [Google Scholar]
- 46.Faraday N, Rosenfeld BA. in vitro hypothermia enhances platelet GPIIb-IIIa activation and P-selectin expression. Anesthesiology. 1998;88:1579–85. doi: 10.1097/00000542-199806000-00022. [DOI] [PubMed] [Google Scholar]
- 47.Michelson AD, Barnard MR, Khuri SF, Rohrer MJ, MacGregor H, Valeri CR. The effects of aspirin and hypothermia on platelet function in vivo. Br J Haematol. 1999;104:64–8. doi: 10.1046/j.1365-2141.1999.01146.x. [DOI] [PubMed] [Google Scholar]
- 48.Cavallo C, Roffi A, Grigolo B, Mariani E, Pratelli L, Merli G, et al. Platelet-rich plasma: The choice of activation method affects the release of bioactive molecules. BioMed Res Int. 2016;2016:6591717. doi: 10.1155/2016/6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marlovits S, Mousavi M, Gäbler C, Erdös J, Vécsei V. A new simplified technique for producing platelet-rich plasma: A short technical note. Eur Spine J. 2004;13((Suppl 1)):S102–6. doi: 10.1007/s00586-004-0715-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gentile P, Cole JP, Cole MA, Garcovich S, Bielli A, Scioli MG, et al. Evaluation of not-activated and activated PRP in hair loss treatment: Role of growth factor and cytokine concentrations obtained by different collection systems. Int J Mol Sci. 2017;18:408. doi: 10.3390/ijms18020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cervantes J, Perper M, Wong LL, Eber AE, Villasante Fricke AC, Wikramanayake TC, et al. Effectiveness of platelet-rich plasma for androgenetic alopecia: A review of the literature. Skin Appendage Disord. 2018;4:1–11. doi: 10.1159/000477671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choukroun J, Diss A, Simonpieri A, Girard M-O, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate.Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56–60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 53.Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, et al. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin Oral Investig. 2017;21:2619–27. doi: 10.1007/s00784-017-2063-9. [DOI] [PubMed] [Google Scholar]