Abstract
Introduction
Meta-analyses conducted so far on the association between diabetes mellitus (DM) and the tuberculosis (TB) development risk did not sufficiently take confounders into account in their estimates. The objective of this systematic review was to determine whether DM is associated with an increased risk of developing TB with a sensitivity analyses incorporating a wider range of confounders including age, gender, alcohol consumption, smoke exposure, and other comorbidities.
Methods
Pubmed, Embase, Web of Science and Global Index Medicus were queried from inception until October 2020. Without any restriction to time of study, geographical location, and DM and TB diagnosis approaches, all observational studies that presented data for associations between DM and TB were included. Studies with no abstract or complete text, duplicates, and studies with wrong designs (review, case report, case series, comment on an article, and editorial) or populations were excluded. The odds ratios (OR) and their 95% confidence intervals were estimated by a random-effect model.
Results
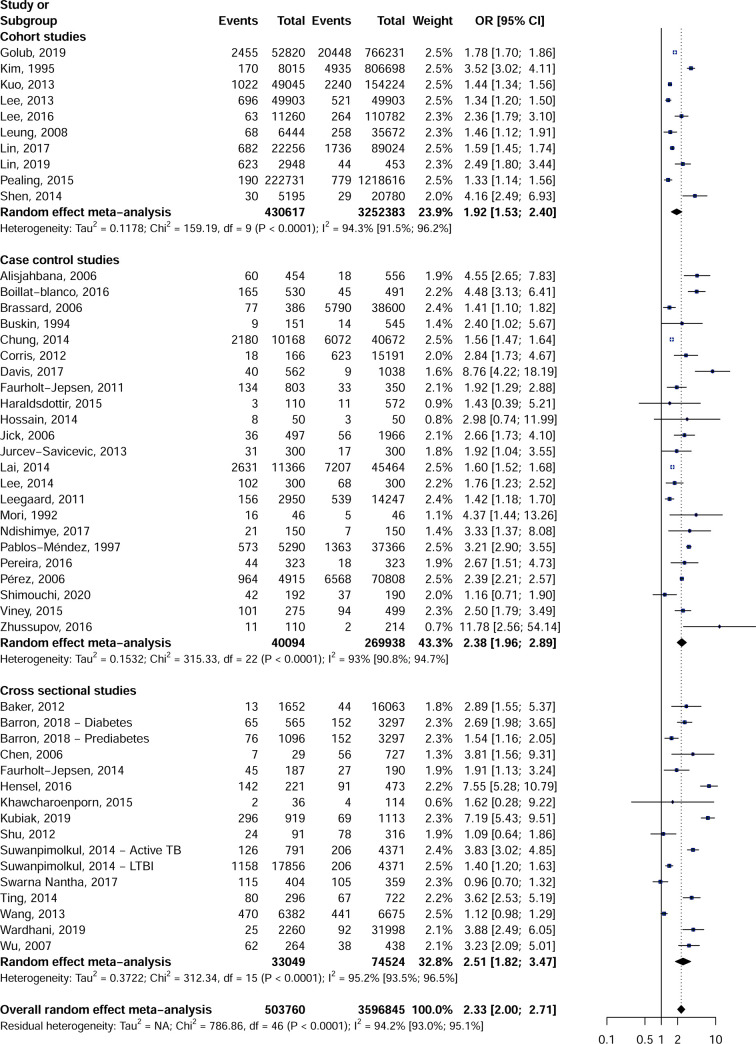
The electronic and manual searches yielded 12,796 articles of which 47 were used in our study (23 case control, 14 cross-sectional and 10 cohort studies) involving 503,760 cases (DM or TB patients) and 3,596,845 controls. The size of the combined effect of TB risk in the presence of DM was OR = 2.3, 95% CI = [2.0–2.7], I2 = 94.2%. This statistically significant association was maintained in cohort (OR = 2.0, CI 95% = [1.5–2.4], I2 = 94.3%), case control (OR = 2.4, CI 95% = [2.0–2.9], I2 = 93.0%) and cross-sectional studies (OR = 2.5, CI 95% = [1.8–3.5], I2 = 95.2%). The association between DM and TB was also maintained in the sensitivity analysis including only studies with similar proportions of confounders between cases and controls. The substantial heterogeneity observed was mainly explained by the differences between geographic regions.
Conclusions
DM is associated with an increased risk of developing latent and active TB. To further explore the role of DM in the development of TB, more investigations of the biological mechanisms by which DM increases the risk of TB are needed.
Review registration
PROSPERO, CRD42021216815.
Introduction
About 25% of the global population is infected with Mycobacterium tuberculosis (MTB) [1], including nearly 10 million new cases of active tuberculosis (TB) and 1.5 million deaths recorded each year [2]. These statistics have crowned TB as one of the leading causes of death from infectious diseases worldwide. MTB infections are more prevalent in developing regions of Southeast Asia (44%), Africa (25%) and the West Pacific (18%), with 2/3 of cases recorded in India, Indonesia, China, Philippines, Pakistan, Nigeria, Bangladesh and South Africa [2]. The International Diabetes Federation estimated that nearly half a billion people (about 10% of the global population) were living with diabetes mellitus (DM) each year, including more than 4 million deaths [3]. This incidence is predicted to increase by more than 10% by 2045, leading to about 700 million cases. The majority of people living with DM are registered in the urban areas of low-and middle-income countries where TB is also dominant. Five of 8 countries with the highest incidence of TB are among the 10 countries with the highest prevalence of DM [2, 3].
Compared to patients with TB only, patients with TB and DM are more likely to have more severe clinical pictures, greater infectivity, treatment failure for TB, relapses after recovery, and high mortality [4–8]. The global escalation of DM and TB epidemics is therefore detrimental and especially for low-resource countries where a very high proportion of DM remains undiagnosed or untreated due to poor resourced health systems [9, 10]. This high increase of DM patients in areas with high TB endemicity is of great concern to TB control efforts because numerous studies have suggested that DM increases the risk of developing latent and active TB [11, 12]. Diabetes mellitus is indeed a disease that can alter the host’s immunity and lead to increased susceptibility to several diseases including tuberculosis [13]. The association between DM and TB has been established in several systematic reviews including active TB [14, 15], latent TB [16] and multidrug-resistant TB [17, 18]. There are multiple confounding factors for the association between DM and TB, the main ones being: HIV infections [19, 20], undernutrition [21], smoking and alcoholism [22, 23]. Although all of these reviews have been devoted to the association between DM and TB, apart from adjusting analyses for age [14, 24], other major confounding factors such as HIV infection, alcohol or smoke exposure have received very little attention. In view of the increasing incidence of DM epidemic, further evidence of the association of DM and TB would be of crucial importance in the fight against the double DM-TB epidemic [25]. Furthering this knowledge could include implications such as the implementation of education, prevention, two-way early detection and co-management programs for MD and TB [26]. In this meta-analysis, including a sensitivity analysis with studies with similar proportions of confounders among cases and controls, we further assess the association between DM and TB.
Methods
Literature search
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for the preparation (PROSPERO ID = CRD42021216815, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021216815) and writing of this review (S1 Table). A comprehensive search strategy for relevant articles was applied in several electronic databases including Pubmed, Embase, Web of Science, and Global Index Medicus. We searched from the date the databases were created to October 2020. The search terms covered exposure (DM) and outcome (TB) (S2 Table). Beyond this electronic search, we performed an additional review of the bibliographic references of relevant works for additional inclusions.
Inclusion and non-inclusion criteria
We included in the present review, all observational studies (cohort, case-control and cross-sectional) which investigated the association between DM and TB without any restriction by geographic location, time and DM and TB diagnostic approaches. The studies included were those written in English or French. Excluded from this review were studies for which we did not have access to the abstract and/or full-text, duplicates, studies with designs or populations inappropriate for the purposes of the present work.
Study selection and data extraction
The results of the manual and electronic search were screened by two investigators (JETB and SK) using the Rayyan review application. Eligibility and data extraction from full texts were carried out by all investigators in this review. The following parameters were extracted from the included articles: first author, year of publication, study design, sampling approach, timing (retrospectively/prospectively) of (exposure follow up, timing of DM and TB testing), country, study period and duration, age range of participants, DM and TB testing approaches, DM and TB case definition, inclusion and exclusion criteria, pairing parameters, data on qualitative and quantitative confounding factors and data on the total numbers of cases (diabetic or TB) and controls. Qualitative confounders included gender, smoking, alcohol consumption, HIV infection, malignant diseases, chronic kidney diseases, and several other socio-demographic and co-morbidities. Quantitative confounders included age, body mass index, and several other blood components. Discussion and consensus among investigators were used if there were any disagreement.
Quality assessment
The quality of the included observational studies was assessed according to the Joanna Briggs Institute scale (S3 Table) [27]. The cross-sectional, case-control and cohort studies consisted of 8, 10 and 11 questions respectively with the expected answers being (Yes, No, Unclear or Not applicable). We attributed 1 mark for the answers (Yes) and 0 for the other answers (No, Unclear and Not applicable). We rated studies as having low, moderate, and high risk of bias according to total marks per study. All investigators in this study independently collected answers to the Joanna Briggs Institute scale questions in duplicate. Disagreements were resolved by discussion and consensus.
Statistical analysis
We opted to group the results according to study designs (cross-sectional, control cases, and cohorts). We selected the data from the reference methods (culture for active TB, IGRA for latent TB, and OGTT for DM) in studies reporting multiple data on the relationship between DM and TB for the same population. The odds ratio (OR), its confidence interval (95% CI) and the prediction interval were calculated using random-effects models on the R software version 4.0.3 [28]. Egger’s test (< 0.1) and funnel charts (asymmetric distribution) were used to indicate the existence of publication bias [29]. The Chi-square test and the I2 and H indices were used to estimate heterogeneity in the estimates [30]. Subgroup analyses and metaregression were used to investigate the parameters responsible for the heterogeneity. Parameters included in these subgroup analyses included sampling method, number of study sites, timing of DM and TB testing, country, country income level [31], and study duration. P values < 0.05 indicated statistical significance. Sensitivity analyses that included only studies with a low risk of bias and studies comparable with regard to confounding factors were performed to enhance the accuracy of our results. We determined the comparability of studies with confounding factors using Chi-square, Fisher or Student’s T-test as reported previously [32].
Results
Study selection
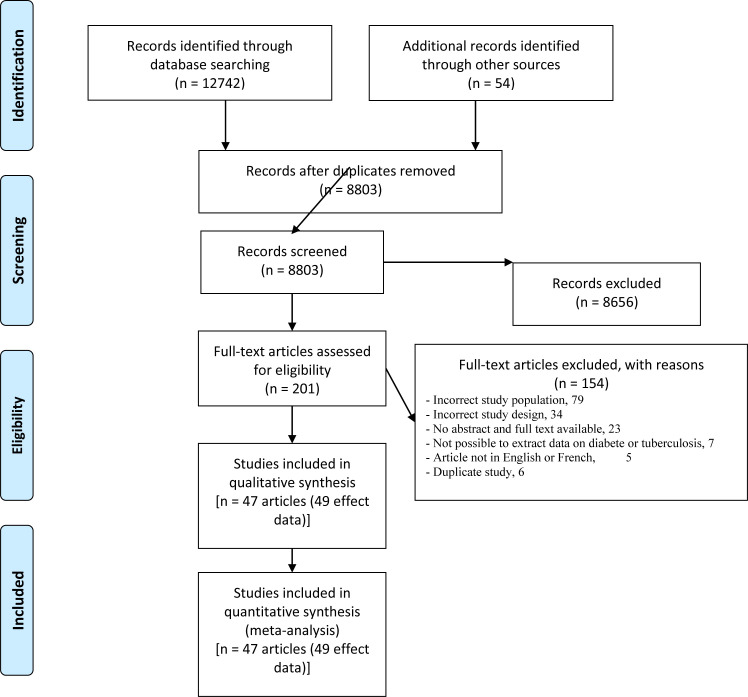
The electronic search yielded 12,742 articles from PubMed (6725), Web of Science (6123), Embase (693), and Global Index Medicus (201). Manual search yielded 54 additional articles (Fig 1). From these, the eligibility review resulted in 201 articles and the exclusion brought this number to 154 (S4 Table) and finally the inclusion resulted 47 articles used (49 effect estimates) in this review [33–79].
Fig 1. PRISMA flow-chart of studies selected for the meta-analysis.
Summary characteristics of included studies
The detailed description of the individual characteristics of the included studies is presented in Table 1. The included studies were published between 1992 and 2020. Cases (TB), controls (non-TB), exposed (diabetics) and unexposed (non-diabetics) were recruited from 1976 to 2018. The majority of studies had a case-control design (23/49), while 16 were cross-sectional and 10 cohort studies. No investigator of the included studies performed a prospective follow-up of exposed/unexposed subjects in the included studies. Five studies were representative of the national population. Included studies were performed in 18 countries spread across different regions of the world and more particularly in China (16/49) and the United States of America (11/49). High-income countries (26/49) were the most represented and only one study was conducted in low-income countries [46]. The vast majority of studies recruited adults over 15 years old. Apart from studies with multiple diagnostic methods, the International Classification of Diseases (ICD) code was the most widely used approach for DM (10/26) and TB (11/20). Nineteen included studies paired reference with controls with at least one parameter.
Table 1. Individual characteristics of included studies.
| Study Design | Country | Study period | TB stage | TB diagnosis approach | DM diagnosis approach | Controls | Matched parameters between cases and controls | Author, Year |
|---|---|---|---|---|---|---|---|---|
| Case control | Indonesia | Mar/2001-Mar/2005 | Active TB | Clinical, chest X-rays, Microscopy | Fasting blood glucose | Presumed healthy controls | Age, Gender, county of residence | Alisjahbana, 2006 [33] |
| Cross sectional | China | Aug/2001-Dec/2004 | Active TB | ICD code, Medical records | ICD code, Medical records | Non-DM | Unclear/ Not reported | Baker, 2012 [34] |
| Cross sectional | United States of America | 2011–2012 | Latent TB infection | IGRA Test, Tuberculin skin test | Doctor-diagnosed DM, Glycated hemoglobin A1c test | Non-TB diseases | Unclear/ Not reported | Barron, 2018 –DM [35] |
| Cross sectional | United States of America | 2011–2012 | Latent TB infection | IGRA Test, Tuberculin skin test | Doctor-diagnosed DM, Glycated hemoglobin A1c test | Non-TB diseases | Unclear/ Not reported | Barron, 2018 –PreDM [35] |
| Case control | Tanzania | Jun/2012-Dec/2013 | Active TB | Clinical, chest X-rays, Microscopy | Fasting blood glucose, Oral glucose tolerance test, Glycated hemoglobin A1c test | Presumed healthy controls | Age, Gender | Boillat-blanco, 2016 [36] |
| Case control | United States of America | Sep/1998-Dec/2003 | Active TB | ICD code | ICD code | Presumed healthy controls | Unclear/ Not reported | Brassard, 2006 [37] |
| Case control | United States of America | 1988–1990 | Active TB | Microscopy, Culture, PCR | Unclear/ Not reported | Non-TB diseases | Unclear/ Not reported | Buskin, 1994 [38] |
| Cross sectional | China | Jan/1983- Dec/2003 | Active TB | Clinical, chest X-rays, Culture | Unclear/ Not reported | Non-TB diseases | Unclear/ Not reported | Chen, 2006 [39] |
| Case control | China | 1997–2010 | Active TB | ICD code | ICD code | Presumed healthy controls | Age, Gender, Recruitment time | Chung, 2014 [40] |
| Case control | United States of America | 1976–1980 | Active TB | Doctor-diagnosed TB | Doctor-diagnosed DM, Oral glucose tolerance test | Non-TB diseases | Unclear/ Not reported | Corris, 2012 [41] |
| Case control | Kazakhstan | Jun/ 2012- May/ 2014 | Active TB | Clinical, chest X-rays, Culture | Doctor-diagnosed DM | Presumed healthy controls | County of residence | Davis, 2017 [42] |
| Case control | Tanzania | Apr/2006-Jan/2009 | Active TB | Microscopy, Culture | Fasting blood glucose, Oral glucose tolerance test | Presumed healthy controls | Age, Gender | Faurholt-Jepsen, 2011 [44] |
| Cross sectional | Tanzania | Apr/ 2006—Mar/ 2009 | Active TB | Culture | Fasting blood glucose, Oral glucose tolerance test | Presumed healthy controls | Age, Gender | Faurholt-Jepsen, 2014 [43] |
| Cohort | United States of America | Jan/ 2001—Dec/ 2011 | Active TB | chest X-rays | ICD code, Fasting blood glucose | Non-DM | Unclear/ Not reported | Golub, 2019 [45] |
| Case control | Guinea-Bissau | July/2010-July/2011 | Active TB | Clinical, chest X-rays, Microscopy | Fasting blood glucose, Random blood sugar test | Presumed healthy controls | Unclear/ Not reported | Haraldsdottir, 2015 [46] |
| Cross sectional | United States of America | October/2013-August/2014 | Latent TB infection | chest X-rays, IGRA Test | Glycated hemoglobin A1c test | Non-TB diseases | Unclear/ Not reported | Hensel, 2016 [47] |
| Case control | Bangladesh | Jan/2008-Jul/2008 | Active TB | Microscopy | Oral glucose tolerance test | Non-TB diseases | Unclear/ Not reported | Hossain, 2014 [48] |
| Case control | United Kingdom | 1990–2001 | Active TB | Medical records | Medical records | Presumed healthy controls | Age, Gender, County of residence | Jick, 2006 [49] |
| Case control | Croatia | 2006–2008 | Active TB | Culture | Unclear/ Not reported | Non-TB diseases | Age, Gender, county of residence | Jurcev-Savicevic, 2013 [50] |
| Cross sectional | Thailand | Mar/2012-Mar/2013 | Latent TB infection | Tuberculin skin test, IGRA Test | Unclear/ Not reported | Presumed healthy controls | Unclear/ Not reported | Khawcharoenporn, 2015 [51] |
| Cohort | Korean | 1988–1990 | Active TB | chest X-rays, Microscopy, Culture | Glucose oxidase test | Non-DM | Unclear/ Not reported | Kim, 1995 [52] |
| Cross sectional | India | May/2014-Nov/2015 | Active TB | Tuberculin skin test, Microscopy, Culture | Clinical, Random blood sugar test | Presumed healthy controls | Unclear/ Not reported | Kubiak, 2019 [53] |
| Cohort | China | 2000–2011 | Active TB | ICD code | ICD code | Presumed healthy controls | Age, Gender | Kuo, 2013 [54] |
| Case control | China | 1998–2011 | Active TB | ICD code | ICD code | Non-TB diseases | Age, Gender | Lai, 2014 [55] |
| Cohort | China | 1997–2007 | Active TB | ICD code | ICD code | Non-DM | Age, Gender, Recruitment time | Lee, 2013 [56] |
| Case control | China | 2006–2017 | Active TB | Clinical, Medical records, chest X-rays, Microscopy, Culture | ICD code, Medical records, Fasting blood glucose, Glycated hemoglobin A1c test | Non-TB diseases | Unclear/ Not reported | Lee, 2014 [58] |
| Cohort | China | Mar/2005-Dec/2012 | Active TB | ICD code, Medical records | Fasting blood glucose | Non-DM | Unclear/ Not reported | Lee, 2016 [57] |
| Case control | Denmark | Jan/1980-Dec/2008 | Active TB | ICD code | Clinical, Medical records, Glycated hemoglobin A1c test | Non-TB diseases | Age, Gender, county of residence | Leegaard, 2011 [59] |
| Cohort | China | Jan/2000—Dec/2005 | Active TB | Clinical, Medical records, chest X-rays, Histopathology, Culture | Glycated hemoglobin A1c test | Non-DM | Unclear/ Not reported | Leung, 2008 [60] |
| Cohort | China | 2000–2009 | Active TB | ICD code | ICD code | Non-DM | Age, Gender, Recruitment time | Lin, 2017 [62] |
| Cohort | China | 2005–2013 | Latent TB infection | Clinical, chest X-rays, Tuberculin skin test, IGRA Test | Doctor-diagnosed DM | Non-DM | Unclear/ Not reported | Lin, 2019 [61] |
| Case control | India | Jan/1983-Dec/1989 | Active TB | Tuberculin skin test | Medical records, Fasting blood glucose, Any glucose level | Non-TB diseases | Unclear/ Not reported | Mori, 1992 [63] |
| Case control | Romania | Mar/2014—Mar/2015 | Active TB | Microscopy, Culture, PCR | Unclear/ Not reported | Non-TB diseases | Age, Gender, county of residence | Ndishimye, 2017 [64] |
| Case control | United States of America | 1991 | Active TB | ICD code | ICD code | Non-TB diseases | Unclear/ Not reported | Pablos-Méndez, 1997 [65] |
| Cohort | United Kingdom | Jan/1990-Dec/2012 | Active TB | ICD code | ICD code | Non-DM | Age, Gender | Pealing, 2015 [66] |
| Case control | Brazil | Aug/2008-Apr/2010 | Active TB | Clinical, Microscopy, Culture | Fasting blood glucose, Oral glucose tolerance test | Non-TB diseases | Age, Gender | Pereira, 2016 [67] |
| Case control | United States of America | 1999–2001 | Active TB | ICD code | ICD code | Non-TB diseases | Unclear/ Not reported | Pérez, 2006 [68] |
| Cohort | China | 2002–2011 | Active TB | ICD code | ICD code | Non-DM | Gender | Shen, 2014 [69] |
| Case control | Japan | Jan/2015-Dec/2018 | Active TB | Clinical, chest X-rays, IGRA Test, Microscopy, Culture, PCR | Doctor-diagnosed DM | Non-TB diseases | County of residence | Shimouchi, 2020 [70] |
| Cross sectional | China | Mar/2011-Feb/2012 | Latent TB infection | ELISA, Microscopy, Culture | Unclear/ Not reported | Non-TB diseases | Unclear/ Not reported | Shu, 2012 [71] |
| Cross sectional | United States of America | Apr/2005-Mar/2012 | Latent TB infection | Tuberculin skin test, IGRA Test | Medical records | Non-DM | Unclear/ Not reported | Suwanpimolkul, 2014 [72] |
| Cross sectional | United States of America | Apr/2005-Mar/2012 | Active TB | Tuberculin skin test, IGRA Test | Medical records | Non-DM | Unclear/ Not reported | Suwanpimolkul, 2014 [72] |
| Cross sectional | Malaysia | Oct/2014-Dec/2015 | Latent TB infection | Clinical, chest X-rays, Tuberculin skin test, Microscopy | Fasting blood glucose, Glycated hemoglobin A1c test, Random blood sugar test | Non-DM | Unclear/ Not reported | Swarna Nantha, 2017 [73] |
| Cross sectional | China | Jan/2011-Dec/2012 | Latent TB infection | Medical records, chest X-rays, IGRA Test | Unclear/ Not reported | Non-TB diseases | Unclear/ Not reported | Ting, 2014 [74] |
| Case control | Republic of Kiribati | Jun/2010-Mar/2012 | Latent TB infection | Clinical, Doctor-diagnosed DM, chest X-rays, Tuberculin skin test, Microscopy, Culture | Glycated hemoglobin A1c test | Non-TB diseases | Unclear/ Not reported | Viney, 2015 [75] |
| Cross sectional | China | Sep/2010-Dec/2012 | Active TB | Clinical, chest X-rays, Microscopy | Fasting blood glucose | Non-TB diseases | County of residence | Wang, 2013 [76] |
| Cross sectional | Indonesia | 2014–2015 | Active TB | Doctor-diagnosed DM | Doctor-diagnosed DM | Non-TB diseases | Unclear/ Not reported | Wardhani, 2019 [77] |
| Cross sectional | China | Jan/2002-Dec/2004 | Active TB | Culture | Medical records | Presumed healthy controls | Unclear/ Not reported | Wu, 2007 [78] |
| Case control | Kazakhstan | Jun/2012-Jan/2013 | Active TB | Clinical, chest X-rays, Microscopy, Culture, PCR | Unclear/ Not reported | Presumed healthy controls | Age | Zhussupov, 2016 [79] |
DM: Diabetes Mellitus; ICD: International Classification of Diseases; TB: Tuberculosis.
Risk of bias in included studies
The methodological quality of the included studies is shown in S5 Table. Overall, the included studies had a low risk of bias (32/49). Most of the included studies collected data and considered confounding factors in the analysis of the association between DM and the TB development risk. In cohort studies, diabetic and nondiabetic patients were generally recruited from the same population, diagnosed with DM and TB in the same way, tested for absence of TB at the start of the follow-up, and followed up with a completeness rate. TB and non-TB patients recruited from case control studies were generally comparable and diagnosed with TB and DM in the same way. In cross-sectional studies, the study context and inclusion criteria for participants were well defined.
Meta-analysis
In this meta-analysis, 503,760 cases (diabetic or TB) and 3,596,845 controls were considered to calculate the combined effect of the association between DM and the TB risk. Regarding the study design, the 49-effect estimate showed an increased risk of developing TB in diabetic patients (OR = 2.3, 95% CI = [2.0–2.7]) (Fig 2). This overall effect was associated with substantial heterogeneity (I2 = 94.2% [93.0–95.1]). The association between DM and the risk of developing TB was conserved in the 10 cohort (OR = 2.0, CI 95% = [1.5–2.4]), the 23 case-control (OR = 2.4, CI 95% = [2.0–2.9]) and 16 cross-sectional studies (OR = 2.5, CI 95% = [1.8–3.5]). A significant publication bias was recorded in the cross-sectional (p Egger = 0.058) and the case-control studies (p Egger = 0.093) unlike the cohort studies (p Egger = 0.417) which did not present any publication bias (Table 2, S1–S3 Figs). Considering only studies with low risk of bias sensitivity analysis did not reveal any difference from the overall results. The data collected for 81 qualitative variables and 16 quantitative variables considered to be confounding factors enabled us to select studies that had similar proportions in references and controls (S6 and S7 Tables). For cohort studies, sensitivity analyses including only comparable studies for confounding factors showed similar results to overall results, including factors such as HIV infection, malignancies and age. For the case-control studies, the same trend was observed for the sensitivity analysis including only comparable studies mainly for alcohol drinkers, chronic kidney disease, drug users, HIV infected patients, tobacco exposure, and age. For the cross-sectional studies, on the other hand, the overall effect observed was lost for the sensitivity analyses including only comparable studies for certain confounding factors including chronic kidney disease, patients with cirrhosis of the liver or malignant diseases.
Fig 2. Association between diabetes mellitus and risk of tuberculosis in cohort, case control and cross-sectional studies.
Table 2. TB development in people with and without DM and influence of confounders.
| OR (95%CI) | 95% Prediction interval | N Studies | N LRTI cases | N controls | H (95%CI) | I2 (95%CI) | P heterogeneity | P Egger test | |
|---|---|---|---|---|---|---|---|---|---|
| Cohort studies | |||||||||
| Overall | 1.9 [1.5–2.4] | [0.8–4.4] | 10 | 430617 | 3252383 | 4.2 [3.4–5.2] | 94.3 [91.5–96.2] | < 0.001 | 0.417 |
| Low risk of bias | 1.6 [1.4–1.7] | [1.1–2.2] | 7 | 414459 | 2424452 | 2.9 [2.1–4] | 88.2 [78.2–93.7] | < 0.001 | 0.496 |
| Asbestosis | 1.6 [1.5–1.7] | NA | 1 | 22256 | 89024 | NA | NA | 1 | NA |
| Autoimmune disorders | 1.3 [1.2–1.5] | NA | 1 | 49903 | 49903 | NA | NA | 1 | NA |
| Bet nut use | 2.4 [1.8–3.1] | NA | 1 | 11260 | 110782 | NA | NA | 1 | NA |
| Chronic kidney disease | 1.8 [1.7–1.9] | NA | 1 | 52820 | 766231 | NA | NA | 1 | NA |
| HIV infection | 1.5 [1.3–1.7] | NA | 2 | 72159 | 138927 | 2.3 [1.1–4.7] | 81 [19.2–95.6] | 0.022 | NA |
| Male gender | 1.8 [1.2–2.5] | [0.3–9.4] | 4 | 83798 | 195379 | 2.6 [1.7–4.1] | 85.4 [63.9–94.1] | < 0.001 | 0.377 |
| Malignant disease | 1.8 [1.7–1.8] | NA | 2 | 59264 | 801903 | 1.4 | 49 | 0.161 | NA |
| Pneumoconiosis | 1.6 [1.5–1.7] | NA | 1 | 22256 | 89024 | NA | NA | 1 | NA |
| Age | 1.5 [1.3–1.7] | NA | 2 | 72159 | 138927 | 2.3 [1.1–4.7] | 81 [19.2–95.6] | 0.022 | NA |
| Case control studies | |||||||||
| Overall | 2.4 [2–2.9] | [1–5.5] | 23 | 40094 | 269938 | 3.8 [3.3–4.4] | 93 [90.8–94.7] | < 0.001 | 0.093 |
| Low risk of bias | 2.2 [1.7–2.9] | [0.8–6.2] | 13 | 28831 | 144497 | 2.6 [2.1–3.3] | 85.4 [76.6–90.9] | < 0.001 | 0.05 |
| Adenotonsillectomy | 1.6 [1.5–1.7] | NA | 1 | 11366 | 45464 | NA | NA | 1 | NA |
| Central sewage system | 1.9 [1–3.5] | NA | 1 | 300 | 300 | NA | NA | 1 | NA |
| Chronic kidney disease | 1.9 [1.4–2.6] | [0.3–14] | 3 | 710 | 814 | 1.7 [1–3.1] | 64.7 [0–89.9] | 0.059 | 0.271 |
| Co_morbidity | 1.9 [1–3.5] | NA | 1 | 300 | 300 | NA | NA | 1 | NA |
| Currently rent home | 11.8 [2.6–54.1] | NA | 1 | 110 | 214 | NA | NA | 1 | NA |
| Drinker | 3.2 [2.9–3.5] | [2.5–3.9] | 4 | 5850 | 38388 | 1.2 [1–1.9] | 26.3 [0–72.1] | 0.254 | 0.101 |
| Drug user | 3.8 [1.8–7.9] | [0–16592.3] | 3 | 1012 | 1488 | 2.2 [1.2–3.9] | 79.5 [34.7–93.5] | 0.008 | 0.916 |
| Ever injected heroin | 8.8 [4.2–18.2] | NA | 1 | 562 | 1038 | NA | NA | 1 | NA |
| Ever smoked heroin | 8.8 [4.2–18.2] | NA | 1 | 562 | 1038 | NA | NA | 1 | NA |
| Ever used opium | 8.8 [4.2–18.2] | NA | 1 | 562 | 1038 | NA | NA | 1 | NA |
| Extra pulmonary lesion | 1.8 [1.2–2.5] | NA | 1 | 300 | 300 | NA | NA | 1 | NA |
| Family history of diabetes mellitus | 3 [0.7–12] | NA | 1 | 50 | 50 | NA | NA | 1 | NA |
| Hepatitis C infection, Anti_HCV | 11.8 [2.6–54.1] | NA | 1 | 110 | 214 | NA | NA | 1 | NA |
| HIV infection | 1.5 [1.3–1.8] | [0.5–4.6] | 3 | 3360 | 14761 | 2 [1.1–3.6] | 74.9 [16.8–92.4] | 0.019 | 0.277 |
| Hyperlipidaemia | 1.6 [1.5–1.6] | NA | 1 | 10168 | 40672 | NA | NA | 1 | NA |
| Illicit drug use | 1.9 [1–3.5] | NA | 1 | 300 | 300 | NA | NA | 1 | NA |
| Immunosuppressive therapy | 1.9 [1–3.5] | NA | 1 | 300 | 300 | NA | NA | 1 | NA |
| Living in a crowded home | 4.6 [2.6–7.8] | NA | 1 | 454 | 556 | NA | NA | 1 | NA |
| Male gender | 2.1 [1.6–2.7] | [0.9–4.9] | 10 | 26090 | 117338 | 2.1 [1.5–2.8] | 77.2 [58.2–87.6] | < 0.001 | 0.036 |
| Malignant disease | 1.8 [1.2–2.5] | NA | 1 | 300 | 300 | NA | NA | 1 | NA |
| Marital status, Single | 2.1 [1.5–3.1] | NA | 2 | 953 | 500 | 1.1 | 17.3 | 0.272 | NA |
| Other chronic diseases | 1.9 [1–3.5] | NA | 1 | 300 | 300 | NA | NA | 1 | NA |
| Pancreatitis | 2.4 [1–5.7] | NA | 1 | 151 | 545 | NA | NA | 1 | NA |
| Physical activity | 3 [0.7–12] | NA | 1 | 50 | 50 | NA | NA | 1 | NA |
| Poly_drug resistant | 1.8 [1.2–2.5] | NA | 1 | 300 | 300 | NA | NA | 1 | NA |
| Previous hospitalizations | 1.9 [1–3.5] | NA | 1 | 300 | 300 | NA | NA | 1 | NA |
| Prisoners | 1.9 [1–3.5] | NA | 1 | 300 | 300 | NA | NA | 1 | NA |
| Smoke Exposure | 2.5 [2–3.3] | [1.4–4.4] | 4 | 702 | 16807 | 1 [1–1.5] | 0 [0–53.1] | 0.806 | 0.86 |
| Transplantation | 1.9 [1–3.5] | NA | 1 | 300 | 300 | NA | NA | 1 | NA |
| Age | 2.4 [1.6–3.7] | [0.6–10.1] | 5 | 4756 | 15561 | 2.9 [2–4.3] | 88.5 [75.8–94.5] | < 0.001 | 0.393 |
| Cigarettes smoked in a week | 8.8 [4.2–18.2] | NA | 1 | 562 | 1038 | NA | NA | 1 | NA |
| Cross sectional studies | |||||||||
| Overall | 2.5 [1.8–3.5] | [0.6–9.7] | 16 | 33049 | 74524 | 4.6 [3.9–5.3] | 95.2 [93.5–96.5] | < 0.001 | 0.058 |
| Low risk of bias | 2.4 [1.6–3.5] | [0.5–11.4] | 12 | 30309 | 41171 | 5.2 [4.4–6.1] | 96.3 [94.8–97.3] | < 0.001 | 0.15 |
| Anemia | 1.1 [0.6–1.9] | NA | 1 | 91 | 316 | NA | NA | 1 | NA |
| Atrial fibrillation | 1 [0.7–1.3] | NA | 1 | 404 | 359 | NA | NA | 1 | NA |
| Autoimmune disorders | 2.1 [0.9–4.7] | NA | 2 | 387 | 1038 | 3.7 [2–6.7] | 92.5 [74.7–97.8] | < 0.001 | NA |
| Bronchial asthma | 1 [0.7–1.3] | NA | 1 | 404 | 359 | NA | NA | 1 | NA |
| Bronchiectasis | 3.2 [2.1–5] | NA | 1 | 264 | 438 | NA | NA | 1 | NA |
| Chronic kidney disease | 1.9 [0.7–4.6] | NA | 2 | 700 | 1081 | 5.4 [3.3–8.9] | 96.6 [90.9–98.7] | < 0.001 | NA |
| Chronic liver disease | 1 [0.7–1.3] | NA | 1 | 404 | 359 | NA | NA | 1 | NA |
| Chronic obstructive pulmonary disease | 1 [0.7–1.3] | NA | 1 | 404 | 359 | NA | NA | 1 | NA |
| Drinker | 5 [2.6–9.6] | NA | 2 | 1873 | 16536 | 2.6 [1.3–5.3] | 85.5 [41.6–96.4] | 0.009 | NA |
| Gout | 1 [0.7–1.3] | NA | 1 | 404 | 359 | NA | NA | 1 | NA |
| Health care worker | 3.6 [2.5–5.2] | NA | 1 | 296 | 722 | NA | NA | 1 | NA |
| Hemodialysis patients | 1.9 [0.9–4.1] | NA | 2 | 355 | 754 | 3.1 [1.6–5.9] | 89.5 [60.9–97.2] | 0.002 | NA |
| Hepatitis B infection, HBsAg | 2.5 [1.3–4.8] | [0.2–29.5] | 5 | 2315 | 8153 | 4.5 [3.4–6] | 95.1 [91.2–97.3] | < 0.001 | 0.597 |
| Hepatitis C infection, Anti_HCV | 3.5 [1.5–7.8] | [0–71340.3] | 3 | 1346 | 4497 | 4.8 [3.3–7.1] | 95.7 [90.7–98] | < 0.001 | 0.811 |
| HIV infection | 5.2 [3.1–8.7] | NA | 2 | 517 | 1195 | 2.8 [1.4–5.6] | 87.6 [51.8–96.8] | 0.005 | NA |
| Ischaemic heart disease | 1 [0.7–1.3] | NA | 1 | 404 | 359 | NA | NA | 1 | NA |
| Liver cirrhosis | 2.1 [0.9–4.7] | NA | 2 | 387 | 1038 | 3.7 [2–6.7] | 92.5 [74.7–97.8] | < 0.001 | NA |
| Living in a crowded home | 2.9 [1.6–5.4] | NA | 1 | 1652 | 16063 | NA | NA | 1 | NA |
| Male gender | 2.5 [1.6–4] | [0.5–11.9] | 7 | 3841 | 24363 | 3.1 [2.3–4.2] | 89.9 [81.7–94.4] | < 0.001 | 0.883 |
| Malignant disease | 2.6 [1.6–4.3] | [0.3–22.7] | 4 | 680 | 2203 | 2.2 [1.4–3.6] | 79.8 [46.4–92.4] | 0.002 | 0.962 |
| Osteoarthritis | 1 [0.7–1.3] | NA | 1 | 404 | 359 | NA | NA | 1 | NA |
| Residence in an indigenous community | 2.9 [1.6–5.4] | NA | 1 | 1652 | 16063 | NA | NA | 1 | NA |
| Self_reported history of renal failure | 7.2 [5.4–9.5] | NA | 1 | 919 | 1113 | NA | NA | 1 | NA |
| Smoke Exposure | 3 [1.8–5.2] | [0.4–22.9] | 5 | 2825 | 20871 | 3.1 [2.2–4.5] | 89.8 [79–95] | < 0.001 | 0.467 |
| Syphilis | 7.5 [5.3–10.8] | NA | 1 | 221 | 473 | NA | NA | 1 | NA |
| Thyroid disorder | 1 [0.7–1.3] | NA | 1 | 404 | 359 | NA | NA | 1 | NA |
| Total Bilirubin (mg_dL), Not Normal | 2 [1.4–3] | NA | 2 | 1661 | 6594 | 2.6 [1.3–5.3] | 85.4 [40.9–96.4] | 0.009 | NA |
| Age | 2.3 [1.5–3.6] | NA | 2 | 216 | 917 | 1.3 | 41.1 | 0.193 | NA |
| Body mass index | 7.5 [5.3–10.8] | NA | 1 | 221 | 473 | NA | NA | 1 | NA |
| Dialysis duration | 1.8 [0.8–4.2] | NA | 2 | 120 | 1043 | 2.4 [1.1–4.8] | 82 [23.9–95.7] | 0.018 | NA |
| Hemoglobin | 1.1 [1–1.3] | NA | 1 | 6382 | 6675 | NA | NA | 1 | NA |
Source of heterogeneity examination
The potential sources of heterogeneity were explored by the subgroup analyses. These sources included country, UNSD region, country income level, TB stage (active vs latent), and type of controls (S8 Table). In the cohort, control and cross-sectional designs only the geographic location (countries and UNSD regions) contributed to a source of heterogeneity (p subgroup difference <0.05). In cohort studies, however, all subcategories showed an association between DM and the risk of developing TB.
Discussion
This systematic review included 47 articles examining the association between DM and TB. Regardless of study design, region of origin, stage of TB (latent or active TB), type of controls (non-DM, non-TB, or presumed healthy), this meta-analysis suggests that DM increases the risk of developing TB. The overall effect observed suggests that patients with DM are two times more likely to develop TB than non-diabetics. This overall effect persisted in the sensitivity analysis including only studies with similar proportions of common confounders between cases and controls.
The statistically significant association between DM and TB observed in this review is consistent with those reported previously. A first qualitative review in 2007 with 9 included studies reported effect estimates ranging from 1.5 to 7.8 fold the risk of TB in DM patients [80]. Two other meta-analyses that included studies with patients with active TB and whose age-adjusted estimates were reported in 2008 and 2018 [14, 24]. One of these meta-analyses reported an estimated 3.1-fold effect for 3 cohort studies and the second an estimated 1.5-fold effect for 14 studies with low risk of bias. A final meta-analysis with studies recruiting patients with latent TB revealed no significant association for one cohort study and a weak association for 12 cross-sectional studies [16]. Compared to these previous systematic reviews, we included over 10 additional articles and used a very rigorous methodology including calculating effect estimates of primary data from included studies and taking into account a wide range of confounding factors of the association between DM and TB listed in the articles included [35, 43, 45, 48, 53, 58, 61, 62, 64, 67, 70, 73, 77, 79]. Little is known about the biological mechanisms that underlie a high risk of developing TB in patients with DM. Several hypotheses linked to an alteration of immune function in diabetics have however been suggested to explain this association between DM and TB [81–84]. These hypotheses include, but not limited to: depressed cellular immunity, alveolar macrophage dysfunction, low levels of interferon gamma, reduction of interleukin-12, and micronutrient deficiency. We recognize several potential limitations to this review. In addition to the fact that most of the included studies used multiple diagnostic approaches for TB and DM, other diagnostic methods including ICD codes, medical records and self-reported data may be associated with some inaccuracies. Different risk factors have been reported for pulmonary TB compared to extra-pulmonary TB. Very few included studies, however, differentiated pulmonary TB from extrapulmonary TB [85, 86]. Similarly, very few included studies reported information on DM types (1 or 2 and pre-DM or DM) and participant glycaemic control. However, these are conditions that influence susceptibility to TB [87]. Very few included studies reported treatment status for participant for TB. Normalization of glycaemic status has been established for TB patients receiving treatment [88, 89]. This could therefore have been the cause of the misclassification of cases and controls in the included studies. The above limitations would justify the substantial heterogeneity recorded in this meta-analysis. As previously reported [90], very few studies included in this meta-analysis were from Africa, thus compromising the generalizability of these results globally. It should also be noted that Africa has the highest rate of undiagnosed DM in the world and may therefore have a specific profile of the association between DM and TB [91].
Due to the inclusion of only observational studies in this meta-analysis, a causal link between DM and the risk of TB cannot be suggested. However, the results of this meta-analysis further strengthen the level of evidence for the association between DM and the risk of TB development. We therefore encourage specific studies on the association between DM and TB in the context of Africa. We advocated public health programs to prevent DM such as strengthening education on risk factors for DM and physical activities and sports. Patients with DM only and healthcare professionals should be educated about their increased risk of active or latent TB development. Two-way screening and management programs for DM and TB including latent TB would help reduce the incidence and burden associated with this double epidemic. Interventional studies to demonstrate the causal link between DM and TB are needed in the future. Further research on the biological mechanism by which DM increases the risk of TB are needed.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its S1–S3 Figs, S1–S8 Tables files.
Funding Statement
- Initials: SK - Grant number: VARIAFRICA-TMA2019PF-2705 - URL: http://www.edctp.org/projects-2/edctp2-projects/edctp-preparatory-fellowships-2019/ - The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This project is part of the EDCTP2 programme supported by the European Union under grant agreement TMA2019PF-2705.
References
- 1.Cohen A, Mathiasen VD. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. The European respiratory journal. 2019;54(3). doi: 10.1183/13993003.00655-2019 . [DOI] [PubMed] [Google Scholar]
- 2.World Health O. Global tuberculosis report 2020. Geneva: World Health Organization; 2020. 2016. [Google Scholar]
- 3.Foundation ID. IDF Diabetes Atlas 2019. 9th Edition ed2019.
- 4.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC medicine. 2011;9:81. Epub 2011/07/05. doi: 10.1186/1741-7015-9-81 ; PubMed Central PMCID: PMC3155828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lönnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. The lancet Diabetes & endocrinology. 2014;2(9):730–9. Epub 2014/09/10. doi: 10.1016/S2213-8587(14)70109-3 . [DOI] [PubMed] [Google Scholar]
- 6.Singla R, Khan N. Does diabetes predispose to the development of multidrug-resistant tuberculosis? Chest. 2003;123(1):308–9; author reply 9. Epub 2003/01/16. . [PubMed] [Google Scholar]
- 7.Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7(1):234. doi: 10.1186/1471-2458-7-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diabetes Dixon B. and tuberculosis: an unhealthy partnership. The Lancet Infectious diseases. 2007;7(7):444. Epub 2007/06/29. doi: 10.1016/S1473-3099(07)70144-5 . [DOI] [PubMed] [Google Scholar]
- 9.Beran D, Yudkin JS. Diabetes care in sub-Saharan Africa. Lancet (London, England). 2006;368(9548):1689–95. Epub 2006/11/14. doi: 10.1016/s0140-6736(06)69704-3 . [DOI] [PubMed] [Google Scholar]
- 10.Harries AD, Murray MB, Jeon CY, Ottmani SE, Lonnroth K, Barreto ML, et al. Defining the research agenda to reduce the joint burden of disease from diabetes mellitus and tuberculosis. Tropical medicine & international health: TM & IH. 2010;15(6):659–63. Epub 2010/04/22. doi: 10.1111/j.1365-3156.2010.02523.x ; PubMed Central PMCID: PMC4465347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odone A, Houben RM, White RG, Lönnroth K. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. The lancet Diabetes & endocrinology. 2014;2(9):754–64. Epub 2014/09/10. doi: 10.1016/S2213-8587(14)70164-0 . [DOI] [PubMed] [Google Scholar]
- 12.Restrepo BI. Convergence of the tuberculosis and diabetes epidemics: renewal of old acquaintances. Clin Infect Dis. 2007;45(4):436–8. Epub 2007/07/20. doi: 10.1086/519939 ; PubMed Central PMCID: PMC2900315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harries AD, Lin Y, Satyanarayana S, Lönnroth K, Li L, Wilson N, et al. The looming epidemic of diabetes-associated tuberculosis: learning lessons from HIV-associated tuberculosis. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2011;15(11):1436–44, i. Epub 2011/09/10. doi: 10.5588/ijtld.11.0503 . [DOI] [PubMed] [Google Scholar]
- 14.Jeon CY, Murray MB. Diabetes Mellitus Increases the Risk of Active Tuberculosis: A Systematic Review of 13 Observational Studies. PLoS Medicine. 2008;5(7):e152. doi: 10.1371/journal.pmed.0050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: A systematic review and meta-analysis. PLOS ONE. 2017;12(11):e0187967. doi: 10.1371/journal.pone.0187967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee M-R, Huang Y-P, Kuo Y-T, Luo C-H, Shih Y-J, Shu C-C, et al. Diabetes mellitus and latent tuberculosis infection: a systemic review and meta-analysis. Clinical Infectious Diseases. 2016:ciw836. doi: 10.1093/cid/ciw836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Li W, Xue M, Chen Y, Du X, Wang C, et al. Diabetes mellitus and the risk of multidrug resistant tuberculosis: a meta-analysis. Scientific Reports. 2017;7(1):1090. doi: 10.1038/s41598-017-01213-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tegegne BS, Mengesha MM, Teferra AA, Awoke MA, Habtewold TD. Association between diabetes mellitus and multi-drug-resistant tuberculosis: evidence from a systematic review and meta-analysis. Systematic reviews. 2018;7(1):161. doi: 10.1186/s13643-018-0828-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez L, Sekandi JN, Castellanos ME, Zalwango S, Whalen CC. Infectiousness of HIV-Seropositive Patients with Tuberculosis in a High-Burden African Setting. Am J Respir Crit Care Med. 2016;194(9):1152–63. Epub 2016/11/01. doi: 10.1164/rccm.201511-2146OC ; PubMed Central PMCID: PMC5114446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters JS, Andrews JR, Hatherill M, Hermans S, Martinez L, Schurr E, et al. Advances in the understanding of Mycobacterium tuberculosis transmission in HIV-endemic settings. The Lancet Infectious diseases. 2019;19(3):e65–e76. Epub 2018/12/18. doi: 10.1016/S1473-3099(18)30477-8 ; PubMed Central PMCID: PMC6401310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harries AD, Nkhoma WA, Thompson PJ, Nyangulu DS, Wirima JJ. Nutritional status in Malawian patients with pulmonary tuberculosis and response to chemotherapy. Eur J Clin Nutr. 1988;42(5):445–50. Epub 1988/05/01. . [PubMed] [Google Scholar]
- 22.Lin HH, Ezzati M, Chang HY, Murray M. Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Respir Crit Care Med. 2009;180(5):475–80. Epub 2009/06/23. doi: 10.1164/rccm.200904-0549OC . [DOI] [PubMed] [Google Scholar]
- 23.Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis—a systematic review. BMC Public Health. 2008;8:289. Epub 2008/08/16. doi: 10.1186/1471-2458-8-289 ; PubMed Central PMCID: PMC2533327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi S, Chandramohan D. Risk of active tuberculosis among people with diabetes mellitus: systematic review and meta-analysis. Tropical Medicine & International Health. 2018;23(10):1058–70. doi: 10.1111/tmi.13133 [DOI] [PubMed] [Google Scholar]
- 25.Executive B. Global strategy and targets for tuberculosis prevention, care and control after 2015: Report by the Secretariat. 2014. [Google Scholar]
- 26.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. The Lancet Infectious Diseases. 2009;9(12):737–46. doi: 10.1016/S1473-3099(09)70282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetc R, et al. Chapter 7: Systematic reviews of etiology and risk. Joanna Briggs Institute Reviewer’s Manual. Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute; ed: Aromataris E, Munn Z; 2017. [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemporary Clinical Trials. 2015;45(Pt A):139–45. doi: 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ: British Medical Journal. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 31.Nelson JF, Ship II. Intraoral carcinoma: predisposing factors and their frequency of incidence as related to age at onset. Journal of the American Dental Association (1939). 1971;82(3):564–8. Epub 1971/03/01. doi: 10.14219/jada.archive.1971.0080 . [DOI] [PubMed] [Google Scholar]
- 32.Kenmoe S, Kengne-Nde C, Modiyinji AF, Bigna JJ, Njouom R. Association of early viral lower respiratory infections and subsequent development of atopy, a systematic review and meta-analysis of cohort studies. PLoS One. 2020;15(4):e0231816. doi: 10.1371/journal.pone.0231816 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alisjahbana B, van Crevel R, Sahiratmadja E, den Heijer M, Maya A, Istriana E, et al. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union Against Tuberculosis and Lung Disease. 2006;10(6):696–700. [PubMed] [Google Scholar]
- 34.Baker MA, Lin H-H, Chang H-Y, Murray MB. The risk of tuberculosis disease among persons with diabetes mellitus: a prospective cohort study. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2012;54(6):818–25. doi: 10.1093/cid/cir939 [DOI] [PubMed] [Google Scholar]
- 35.Barron MM, Shaw KM, Bullard KM, Ali MK, Magee MJ. Diabetes is associated with increased prevalence of latent tuberculosis infection: Findings from the National Health and Nutrition Examination Survey, 2011–2012. Diabetes Research and Clinical Practice. 2018;139:366–79. doi: 10.1016/j.diabres.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 36.Boillat-Blanco N, Ramaiya KL, Mganga M, Minja LT, Bovet P, Schindler C, et al. Transient Hyperglycemia in Patients With Tuberculosis in Tanzania: Implications for Diabetes Screening Algorithms. Journal of Infectious Diseases. 2016;213(7):1163–72. doi: 10.1093/infdis/jiv568 [DOI] [PubMed] [Google Scholar]
- 37.Brassard P, Kezouh A, Suissa S. Antirheumatic drugs and the risk of tuberculosis. Clin Infect Dis. 2006;43(6):717–22. Epub 2006/08/17. doi: 10.1086/506935 . [DOI] [PubMed] [Google Scholar]
- 38.Buskin SE, Gale JL, Weiss NS, Nolan CM. Tuberculosis risk factors in adults in King County, Washington, 1988 through 1990. American journal of public health. 1994;84(11):1750–6. doi: 10.2105/ajph.84.11.1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C-H, Lian J-D, Cheng C-H, Wu M-J, Lee W-C, Shu K-H. Mycobacterium tuberculosis infection following renal transplantation in Taiwan. Transplant Infectious Disease. 2006;8(3):148–56. doi: 10.1111/j.1399-3062.2006.00147.x [DOI] [PubMed] [Google Scholar]
- 40.Chung W-S, Lin C-L, Hung C-T, Chu Y-H, Sung F-C, Kao C-H, et al. Tuberculosis increases the subsequent risk of acute coronary syndrome: a nationwide population-based cohort study. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union Against Tuberculosis and Lung Disease. 2014;18(1):79–83. doi: 10.5588/ijtld.13.0288 [DOI] [PubMed] [Google Scholar]
- 41.Corris V, Unwin N, Critchley J. Quantifying the association between tuberculosis and diabetes in the US: a case-control analysis. Chronic Illness. 2012;8(2):121–34. doi: 10.1177/1742395312440294 [DOI] [PubMed] [Google Scholar]
- 42.Davis A, Terlikbayeva A, Aifah A, Hermosilla S, Zhumadilov Z, Berikova E, et al. Risks for tuberculosis in Kazakhstan: implications for prevention. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union Against Tuberculosis and Lung Disease. 2017;21(1):86–92. doi: 10.5588/ijtld.15.0838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faurholt-Jepsen D, Aabye MG, Jensen AV, Range N, Praygod G, Jeremiah K, et al. Diabetes is associated with lower tuberculosis antigen-specific interferon gamma release in Tanzanian tuberculosis patients and non-tuberculosis controls. Scandinavian Journal of Infectious Diseases. 2014;46(5):384–91. doi: 10.3109/00365548.2014.885657 [DOI] [PubMed] [Google Scholar]
- 44.Faurholt-Jepsen D, Range N, PrayGod G, Jeremiah K, Faurholt-Jepsen M, Aabye MG, et al. Diabetes Is a Risk Factor for Pulmonary Tuberculosis: A Case-Control Study from Mwanza, Tanzania. PLoS ONE. 2011;6(8):e24215. doi: 10.1371/journal.pone.0024215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golub JE, Mok Y, Hong S, Jung KJ, Jee SH, Samet JM. Diabetes mellitus and tuberculosis in Korean adults: impact on tuberculosis incidence, recurrence and mortality. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union Against Tuberculosis and Lung Disease. 2019;23(4):507–13. doi: 10.5588/ijtld.18.0103 [DOI] [PubMed] [Google Scholar]
- 46.Haraldsdottir TL, Rudolf F, Bjerregaard-Andersen M, Joaquím LC, Stochholm K, Gomes VF, et al. Diabetes mellitus prevalence in tuberculosis patients and the background population in Guinea-Bissau: a disease burden study from the capital Bissau. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2015;109(6):400–7. doi: 10.1093/trstmh/trv030 [DOI] [PubMed] [Google Scholar]
- 47.Hensel RL, Kempker RR, Tapia J, Oladele A, Blumberg HM, Magee MJ. Increased risk of latent tuberculous infection among persons with pre-diabetes and diabetes mellitus. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union Against Tuberculosis and Lung Disease. 2016;20(1):71–8. doi: 10.5588/ijtld.15.0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hossain M. Glucose Intolerance in Pulmonary Tuberculosis: A Case-Control Study. Chest. 2014;145(3):140A. doi: 10.1378/chest.1823698 [DOI] [Google Scholar]
- 49.Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis and rheumatism. 2006;55(1):19–26. Epub 2006/02/08. doi: 10.1002/art.21705 . [DOI] [PubMed] [Google Scholar]
- 50.Jurcev-Savicevic A, Mulic R, Ban B, Kozul K, Bacun-Ivcek L, Valic J, et al. Risk factors for pulmonary tuberculosis in Croatia: a matched case–control study. BMC Public Health. 2013;13(1):991. doi: 10.1186/1471-2458-13-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khawcharoenporn T, Apisarnthanarak A, Phetsuksiri B, Rudeeaneksin J, Srisungngam S, Mundy LM. Tuberculin skin test and QuantiFERON-TB Gold In-tube Test for latent tuberculosis in Thai HIV-infected adults. Respirology. 2015;20(2):340–7. Epub 2014/11/28. doi: 10.1111/resp.12442 . [DOI] [PubMed] [Google Scholar]
- 52.Kim SJ, Hong YP, Lew WJ, Yang SC, Lee EG. Incidence of pulmonary tuberculosis among diabetics. Tubercle and Lung Disease. 1995;76(6):529–33. doi: 10.1016/0962-8479(95)90529-4 [DOI] [PubMed] [Google Scholar]
- 53.Kubiak RW, Sarkar S, Horsburgh CR, Roy G, Kratz M, Reshma A, et al. Interaction of nutritional status and diabetes on active and latent tuberculosis: a cross-sectional analysis. BMC infectious diseases. 2019;19(1):627. doi: 10.1186/s12879-019-4244-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuo MC, Lin SH, Lin CH, Mao IC, Chang SJ, Hsieh MC. Type 2 diabetes: an independent risk factor for tuberculosis: a nationwide population-based study. PLoS One. 2013;8(11):e78924. Epub 2013/11/16. doi: 10.1371/journal.pone.0078924 ; PubMed Central PMCID: PMC3827305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai S-W, Lin C-L, Liao K-F, Tsai S-M. Increased risk of pulmonary tuberculosis among patients with appendectomy in Taiwan. European Journal of Clinical Microbiology & Infectious Diseases. 2014;33(9):1573–7. doi: 10.1007/s10096-014-2112-0 [DOI] [PubMed] [Google Scholar]
- 56.Lee M-C, Lee C-H, Shu C-C, Pong W-B, Lan C-C, Wang J-Y, et al. The impact of diabetes mellitus and its control on the development of tuberculosis: a nationwide longitudinal study in Taiwan: IMPACT OF DM TREATMENT ON RISK OF TB. Pharmacoepidemiology and Drug Safety. 2013;22(9):995–1003. doi: 10.1002/pds.3491 [DOI] [Google Scholar]
- 57.Lee P-H, Fu H, Lai T-C, Chiang C-Y, Chan C-C, Lin H-H. Glycemic Control and the Risk of Tuberculosis: A Cohort Study. PLoS medicine. 2016;13(8):e1002072. doi: 10.1371/journal.pmed.1002072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee P-H, Lin H-C, Huang AS-E, Wei S-H, Lai M-S, Lin H-H. Diabetes and risk of tuberculosis relapse: nationwide nested case-control study. PloS One. 2014;9(3):e92623. doi: 10.1371/journal.pone.0092623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leegaard A, Riis A, Kornum JB, Prahl JB, Thomsen VO, Sorensen HT, et al. Diabetes, Glycemic Control, and Risk of Tuberculosis: A population-based case-control study. Diabetes Care. 2011;34(12):2530–5. doi: 10.2337/dc11-0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leung CC, Lam TH, Chan WM, Yew WW, Ho KS, Leung GM, et al. Diabetic control and risk of tuberculosis: a cohort study. American journal of epidemiology. 2008;167(12):1486–94. doi: 10.1093/aje/kwn075 [DOI] [PubMed] [Google Scholar]
- 61.Lin C-H, Kuo S-C, Hsieh M-C, Ho S-Y, Su I-J, Lin S-H, et al. Effect of diabetes mellitus on risk of latent TB infection in a high TB incidence area: a community-based study in Taiwan. BMJ Open. 2019;9(10):e029948. doi: 10.1136/bmjopen-2019-029948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin H-F, Liao K-F, Chang C-M, Lai S-W, Tsai P-Y, Sung F-C. Anti-Diabetic Medication Reduces Risk of Pulmonary Tuberculosis in Diabetic Patients: A Population-based Cohort Study in Taiwan. KUWAIT MEDICAL JOURNAL. 2017:7. [Google Scholar]
- 63.Mori MA, Leonardson G, Welty TK. The benefits of isoniazid chemoprophylaxis and risk factors for tuberculosis among Oglala Sioux Indians. Archives of internal medicine. 1992;152(3):547–50. [PubMed] [Google Scholar]
- 64.Ndishimye P, Domokos B, Stillo J, Seghrouchni F, Mrabet O, Homorodean D, et al. A case control study of risk factors associated with pulmonary tuberculosis in romania: experience at a clinical hospital of pneumology. Clujul medical (1957). 2017;90(1):54–9. doi: 10.15386/cjmed-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pablos-Méndez A, Blustein J, Knirsch CA. The role of diabetes mellitus in the higher prevalence of tuberculosis among Hispanics. American journal of public health. 1997;87(4):574–9. doi: 10.2105/ajph.87.4.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pealing L, Wing K, Mathur R, Prieto-Merino D, Smeeth L, Moore DAJ. Risk of tuberculosis in patients with diabetes: population based cohort study using the UK Clinical Practice Research Datalink. BMC medicine. 2015;13:135. doi: 10.1186/s12916-015-0381-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pereira SM, Araújo GSd , Santos CAdST , Oliveira MGd, Barreto ML. Association between diabetes and tuberculosis: case-control study. Revista de saude publica. 2016;50:82. doi: 10.1590/S1518-8787.2016050006374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pérez A, Brown HS, Restrepo BI. Association between tuberculosis and diabetes in the mexican border and non-border regions of texas. The American journal of tropical medicine and hygiene. 2006;74(4):604–11. doi: 10.4269/ajtmh.2006.74.604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen T-C, Lin C-L, Wei C-C, Liao W-C, Chen W-C, Chen C-H, et al. Increased Risk of Tuberculosis in Patients With Type 1 Diabetes Mellitus: Results From a Population-Based Cohort Study in Taiwan. Medicine. 2014;93(16):e96. doi: 10.1097/MD.0000000000000096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimouchi A, Tsuda Y, Komukai J, Matsumoto K, Yoshida H, Ohkado A. Characteristics of individuals with tuberculosis in an urban, poor population in Osaka City, Japan—a case-control study. Western Pacific surveillance and response journal: WPSAR. 2020;11(1):22–8. doi: 10.5365/wpsar.2018.9.1.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shu C-C, Wu V-C, Yang F-J, Pan S-C, Lai T-S, Wang J-Y, et al. Predictors and Prevalence of Latent Tuberculosis Infection in Patients Receiving Long-Term Hemodialysis and Peritoneal Dialysis. PLoS ONE. 2012;7(8):e42592. doi: 10.1371/journal.pone.0042592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suwanpimolkul G, Grinsdale JA, Jarlsberg LG, Higashi J, Osmond DH, Hopewell PC, et al. Association between diabetes mellitus and tuberculosis in United States-born and foreign-born populations in San Francisco. PloS One. 2014;9(12):e114442. doi: 10.1371/journal.pone.0114442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swarna Nantha Y, Puri A, Mohamad Ali SZ, Suppiah P, Che Ali SA, Ramasamy B, et al. Epidemiology of latent tuberculosis infection among patients with and without diabetes mellitus. Family practice. 2017;34(5):532–8. doi: 10.1093/fampra/cmx017 [DOI] [PubMed] [Google Scholar]
- 74.Ting W-Y, Huang S-F, Lee M-C, Lin Y-Y, Lee Y-C, Feng J-Y, et al. Gender disparities in latent tuberculosis infection in high-risk individuals: a cross-sectional study. PloS One. 2014;9(11):e110104. doi: 10.1371/journal.pone.0110104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Viney K, Cavanaugh J, Kienene T, Harley D, Kelly PM, Sleigh A, et al. Tuberculosis and diabetes mellitus in the Republic of Kiribati: a case-control study. Tropical Medicine & International Health. 2015;20(5):650–7. doi: 10.1111/tmi.12462 [DOI] [PubMed] [Google Scholar]
- 76.Wang Q, Ma A, Han X, Zhao S, Cai J, Ma Y, et al. Prevalence of Type 2 Diabetes among Newly Detected Pulmonary Tuberculosis Patients in China: A Community Based Cohort Study. PLoS ONE. 2013;8(12):e82660. doi: 10.1371/journal.pone.0082660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wardhani I, Sudaryo MK. Relationship between Diabetes Mellitus and Tuberculosis in Indonesia. Indian Journal of Public Health Research & Development. 2019;10(2):392. doi: 10.5958/0976-5506.2019.00321.8 [DOI] [Google Scholar]
- 78.Wu H-P, Pan Y-H, Hua C-C, Shieh W-B, Jiang B-Y, Yu T-J. Pneumoconiosis and liver cirrhosis are not risk factors for tuberculosis in patients with pulmonary infection. Respirology. 2007;12(3):416–9. doi: 10.1111/j.1440-1843.2007.01033.x [DOI] [PubMed] [Google Scholar]
- 79.Zhussupov B, Hermosilla S, Terlikbayeva A, Aifah A, Ma X, Zhumadilov Z, et al. Risk Factors for Primary Pulmonary TB in Almaty Region, Kazakhstan: A Matched Case-Control Study. Iranian journal of public health. 2016;45(4):441–50. [PMC free article] [PubMed] [Google Scholar]
- 80.Stevenson CR, Critchley JA, Forouhi NG, Roglic G, Williams BG, Dye C, et al. Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illness. 2007;3(3):228–45. doi: 10.1177/1742395307081502 [DOI] [PubMed] [Google Scholar]
- 81.Young F, Critchley JA, Johnstone LK, Unwin NC. A review of co-morbidity between infectious and chronic disease in Sub Saharan Africa: TB and diabetes mellitus, HIV and metabolic syndrome, and the impact of globalization. Globalization and health. 2009;5:9. Epub 2009/09/16. doi: 10.1186/1744-8603-5-9 ; PubMed Central PMCID: PMC2753337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ottmani SE, Murray MB, Jeon CY, Baker MA, Kapur A, Lönnroth K, et al. Consultation meeting on tuberculosis and diabetes mellitus: meeting summary and recommendations. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2010;14(12):1513–7. Epub 2010/12/25. . [PubMed] [Google Scholar]
- 83.Biochemistry Brownlee M. and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–20. Epub 2001/12/14. doi: 10.1038/414813a . [DOI] [PubMed] [Google Scholar]
- 84.Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44(3):617–26. Epub 2014/01/23. doi: 10.1002/eji.201344301 ; PubMed Central PMCID: PMC4213860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Antony SJ, Harrell V, Christie JD, Adams HG, Rumley RL. Clinical differences between pulmonary and extrapulmonary tuberculosis: a 5-year retrospective study. Journal of the National Medical Association. 1995;87(3):187–92. Epub 1995/03/01. ; PubMed Central PMCID: PMC2607831. [PMC free article] [PubMed] [Google Scholar]
- 86.Sreeramareddy CT, Panduru KV, Verma SC, Joshi HS, Bates MN. Comparison of pulmonary and extrapulmonary tuberculosis in Nepal- a hospital-based retrospective study. BMC Infect Dis. 2008;8:8. Epub 2008/01/26. doi: 10.1186/1471-2334-8-8 ; PubMed Central PMCID: PMC2245948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McMahon MM, Bistrian BR. Host defenses and susceptibility to infection in patients with diabetes mellitus. Infectious disease clinics of North America. 1995;9(1):1–9. Epub 1995/03/01. . [PubMed] [Google Scholar]
- 88.Oluboyo PO, Erasmus RT. The significance of glucose intolerance in pulmonary tuberculosis. Tubercle. 1990;71(2):135–8. Epub 1990/06/01. doi: 10.1016/0041-3879(90)90010-6 . [DOI] [PubMed] [Google Scholar]
- 89.Tabarsi P, Baghaei P, Marjani M, Vollmer WM, Masjedi MR, Harries AD. Changes in glycosylated haemoglobin and treatment outcomes in patients with tuberculosis in Iran: a cohort study. J Diabetes Metab Disord. 2014;13(1):123. Epub 2015/01/01. doi: 10.1186/s40200-014-0123-0 ; PubMed Central PMCID: PMC4280034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bailey SL, Ayles H. Association between diabetes mellitus and active tuberculosis in Africa and the effect of HIV. Tropical Medicine & International Health. 2017;22(3):261–8. doi: 10.1111/tmi.12822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. Epub 2009/11/10. doi: 10.1016/j.diabres.2009.10.007 . [DOI] [PubMed] [Google Scholar]