Healthy white matter development requires microglial modification of oligodendrocyte populations and myelin membrane.
Abstract
Interactions between microglia, the resident macrophages of the central nervous system (CNS), and myelin, the glial sheath on nerve fibers essential for rapid neural impulse transmission, are commonly studied in the context of neurotrauma and disease. However, interactions between microglia and myelin under normal physiological conditions have been largely overlooked. This review summarizes recent research indicating that the unique properties of microglia evident in disease states also enable microglia to regulate myelination during development and throughout life. This includes phagocytosis of cells and myelin membrane as well as the release of trophic factors, cytokines, and chemokines. The ability of microglia to sense neuronal activity and molecular features of the microenvironment enables them to optimize myelination by influencing early oligodendrogenesis, myelin formation, and removal of aberrantly targeted myelin. Understanding how microglia participate in myelination under normal conditions provides a new perspective that will increase understanding of developmental abnormalities.
INTRODUCTION
Microglia are the resident immune cells of the brain and spinal cord. They survey all regions of the brain including the fiber tracts, called white matter, that connect neurons into circuits. The white appearance derives from a multilayered, highly compacted cell membrane on nerve fibers (axons), called myelin, which is wrapped around axons by non-neuronal cells called oligodendrocytes. Severe dysfunction results when myelin is damaged because the myelin sheath is essential for normal conduction of nerve impulses.
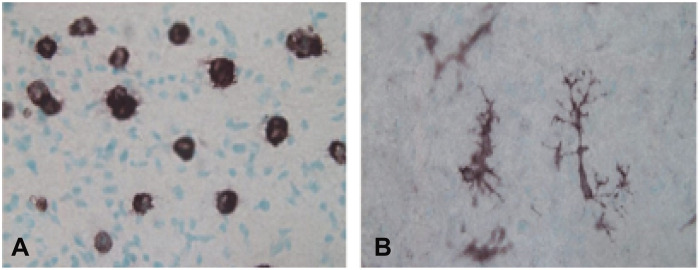
Microglia respond to neural injury and neurodegeneration by undergoing pronounced morphological and cell biological transformations to clear myelin debris and promote repair (1), but in autoimmune disorders, such as multiple sclerosis, microglia can contribute to disease progression by releasing inflammatory cytokines (2) and attacking the myelin sheath (3). The transition from a ramified “resting state” to an amoeboid morphology with high phagocytic activity is classically associated with microglia activation during disease (4), but as early as 1978, Imamoto and Leblond (5) observed that microglia in developing white matter take on an amoeboid cell morphology (Fig. 1). These early findings sparked a question—what functions might these specialized microglia serve in the development of white matter?
Fig. 1. Amoeboid white matter microglia.
Microglia in the white matter tracts such as the corpus callosum maintain an amoeboid morphology into early postnatal development (A), while microglia found in other regions of the brain rapidly transition into a ramified state (B). Reprinted with permission from Kaur et al. (78).
Microglia are mechanistically poised to sense and respond to the changing conditions in the developing brain through a plethora of signaling pathways and phagocytic activity, yet we are only beginning to understand the multifaceted functions that microglia have during early myelination events. Efficient and highly organized myelin deposition is necessary for effective brain function, and the temporal and mechanistic features of early microglia uniquely position the population to provide essential regulation of myelination during white matter development. These cells contribute to myelination through the phagocytosis of excess oligodendrocytes and myelin membrane that forms abnormally. In addition, microglia support oligodendrocyte survival and differentiation and regulate apoptosis of oligodendrocytes by secreting several trophic factors and other intercellular signaling molecules, including chemokines and cytokines. Collectively, this review summarizes the rapidly expanding body of evidence that begins to answer the nearly 50-year-old question as to what role microglia may be playing in the formation and organization of myelin during early white matter development.
EMERGENCE OF GLIAL POPULATIONS IN THE DEVELOPING WHITE MATTER
Infiltration, development, and diversification of microglia
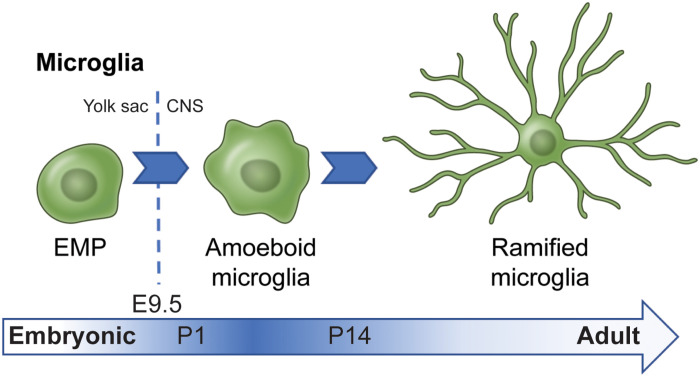
Cell lineage tracing reveals that microglia arise from a primitive erythromyeloid progenitor (EMP) population (Fig. 2) present in the yolk sac before embryonic day 8 (E8) in mice and reach the brain by E9.5 (6). Microgliogenesis and brain infiltration are dependent on a number of factors including expression of matrix metalloproteinases and the lineage-specific transcription factors Irf8 and Sfpi (7). In mice, these brain-resident macrophages proliferate steadily within the prenatal brain before sharply increasing their population size by approximately 10-fold during the first 2 weeks of postnatal development (8). It is widely accepted that the vast majority of adult microglia originate from EMP, but there is evidence of a perinatal wave of infiltrating peripheral monocytes that may contribute to the rapid expansion of the postnatal microglia population (9). Early primitive macrophage colonization is also observed in human development with cells expressing Iba1 (a calcium binding protein commonly used as a microglia marker) penetrating the cerebral wall at 4.5 weeks of gestation, followed by notable periods of prenatal proliferation (10).
Fig. 2. Microglia in developing white matter.
Microglia arise from the yolk sac EMP population. After EMP-derived cells are incorporated into the CNS around E9.5 and take on microglia-specific profile, microglia of the early white matter begin to exhibit an amoeboid morphology with retracted processes. This is similar to the morphology of “activated” microglia that respond to injury and disease. However, in normally developing white matter tracts, amoeboid microglia appear shortly after birth (observed as early as P1 in mice) (14) and remain prevalent in the postnatal brain until transitioning toward a ramified state later in development (approximately P14 in mice) (43). Under inflammatory conditions, monocytes from the bloodstream can also infiltrate the brain.
Although microglia share a common origin, there is substantial heterogeneity within the population. Microglia undergo coordinated transcriptional changes throughout neurodevelopment, and microglia found in the postnatal corpus callosum have distinct gene expression profiles that differ from adult microglia (11), as well as from microglia in other regions of the postnatal brain (12). Recent work by Hagemeyer et al. (13) identifies a population of microglia in the early postnatal corpus callosum and white matter tracts of cerebellum that display markers of microglial activation such as Mac3, and an up-regulation of genes related to activated microglia including Igf1 and Itgax (also known as Cd11c). The studies show that these cells arise from central nervous system (CNS) endogenous precursors and have no contribution from circulating blood monocytes. The cells retain an amoeboid morphology transiently from postnatal day 1 (P1) through P9 to P10 in mice. These amoeboid microglia then transition toward a ramified state later in development (14). In contrast, microglia outside white matter tracts quickly take on a ramified morphology after birth and do not show signs of activation (13). Separately, Wlodarczyk and colleagues (15) detect a subpopulation of microglia localized specifically to the corpus callosum and cerebellum white matter tracts of healthy mouse brains from P2 to P7, which expresses the integrin complement receptor CD11c. CD11c is a microglia marker rarely observed in the healthy adult CNS, but it is up-regulated in a TREM2-dependent manner in disease-associated microglia populations that arise during neuroinflammatory and neurodegenerative disease models such as the experimental autoimmune encephalomyelitis (16), aging, Alzheimer’s, and amyotrophic lateral sclerosis (17). Such disease-associated microglia have been shown to have a protective function by clearing degenerating neurons, myelin debris, and pathological tau accumulations (1,18,19). The discovery of this subpopulation of activated microglia within white matter regions during early postnatal development, coinciding with a period of extensive oligodendrogenesis and the initiation of myelination (20), has sparked in-depth investigation of the function of microglia in the development of white matter myelin.
Oligodendrogenesis and myelination
CNS myelination is an essential developmental process characterized by the production and organization of myelin membrane into multilayered ensheathments along axons (Fig. 3). The dense lipid membrane layers act as an electrical insulator for neurons, promoting rapid saltatory conduction along axons by reducing transmembrane current leakage and electrical capacitance (21) and constraining sodium channels to the gaps between myelin segments known as nodes of Ranvier (22). In addition to increasing the conduction velocity of neuronal signal transmission, myelin also provides metabolic support to neurons through lactate transport (23, 24). Furthermore, oligodendrogenesis and proper myelination are essential to the complex cognitive functions of a healthy brain. Mice with irregular myelination induced by oligodendrocyte-specific knockdown of cyclin-dependent kinase 5 (Cdk5) have significantly impaired long-term memory consolidation, fear-conditioned learning, and motor skill learning (25). Even in adults, when inhibition of de novo oligodendrogenesis is disrupted with no impairment of preexisting myelin, mice struggle to learn complex motor tasks (26) and to recall spatial and fear memories (27).
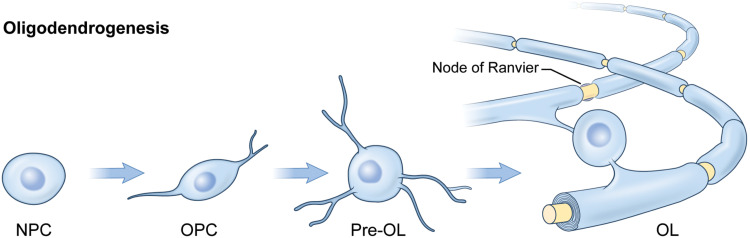
Fig. 3. Progression of oligodendrogenesis and myelination.
NPCs can give rise to a number of CNS cell types including the oligodendrocyte. NPCs differentiate into OPCs. OPCs exist as their own distinct population throughout the brain while also giving rise to oligodendrocyte (OL) cells, which, when mature, extend processes to ensheath neighboring axons in segments of insulatory myelin membrane.
Oligodendrocytes differentiate from a population of cells known as oligodendrocyte progenitor cells (OPCs) that initially populate the brain in three distinct waves beginning during embryogenesis (28). The first and second waves of OPCs (occurring at approximately E11.5 and E15 in mice) divide asymmetrically from neural progenitor cells (NPCs) in the telencephalon ventricular zones and migrate outward to disperse evenly throughout the developing cortex; the final wave of OPCs arises from within the cortex near the time of birth (28). As oligodendrocytes mature, each cell can extend 20 to 60 myelinating processes that contact individual axons and wrap multiple compacted layers of membrane around them (29). Although myelination in the CNS begins in late fetal development and is most active in the early postnatal period, it is a slow process that is not completed in some CNS regions until early adulthood. In adulthood, myelin remains plastic (30), and myelin in the cerebral cortex begins to decrease in later life (31).
The myelinating processes of a mature oligodendrocyte are guided by physical (32) and biochemical signals toward axons, selectively myelinating in a neuronal subtype–specific manner (33). Not all nerve axons are myelinated, and the extent of myelination varies greatly, even along an individual axon, within a neuronal subtype, or region of the brain (34). Targeting of axons for myelination is directed, in part, by electrical activity in axons. The activity-dependent release of the neurotransmitter glutamate promotes the formation of an axoglial signaling complex and stimulates local synthesis of myelin basic protein (MBP) in the adjacent oligodendrocyte cell process (35) to initiate myelination preferentially on electrically active axons (36). Once an axon has been targeted, the oligodendrocyte process wraps around the axon, leading with an inner tongue of myelin membrane that extends underneath previously deposited layers of membrane. Simultaneously, the membrane must expand laterally to define the length of the sheath, while membrane compaction and interlamellar proteins help stabilize external layers of myelin (37). The highly specific patterning of myelin deposition is believed to be integral to long-distance neuronal communication and the synchronization of spike-time arrivals across varied synaptic inputs (38, 39). The complex structural organization of myelin is not easily coordinated and is prone to errors. Recent studies have documented a number of ultrastructural myelin abnormalities, such as outfoldings, bulging, fragmentation, and splitting, that arise during early myelination but are rapidly resolved as development progresses. This research indicates that microglia are critical in removing abnormal myelination (40).
Myelination is a complex biological event, which is highly dependent on intercellular interactions. Oligodendrocytes must recognize the appropriate cells to myelinate and the correct cellular region to myelinate (only axons, rather than dendrites or neuronal cell bodies). Myelination requires specialized synthesis of enormous amounts of membrane and complex cellular motility and dynamics to wrap membrane around axons and exclude the cytoplasm from each concentric membrane layer. Microglia are particularly well suited to guide the process of oligodendrogenesis, modulate myelin organization, and correct errors in myelin formation. In the following sections, we will explore the functional importance of these mechanisms of microglia activity in the development of healthy white matter.
REGULATION OF OLIGODENDROCYTE-LINEAGE CELL POPULATIONS
Oligodendrocyte apoptosis and clearance by microglia
Phagocytosis by microglia is an important mechanism for the removal of cellular debris following injury, and recent studies are finding that microglia perform a similar function during development. Although we commonly think of development as a time of cell proliferation, a marked degree of cell death is observed in white matter tracts of the developing CNS. In the rat optic nerve, there is a steep increase in oligodendrogenesis after birth; however, this is closely followed by a spike in apoptosis of oligodendrocytes and late-stage OPCs (41). Similarly, as much as 20% of the immature oligodendrocyte population in the cerebral cortex shows signs of fragmentation and degeneration in young rats (42). Single-cell deep sequencing has identified a transient early postnatal subset of amoeboid microglia in the mouse corpus callosum and cerebellar white matter with a gene expression profile that is notably similar to that of microglia found in adult neurodegenerative disease states (43). These microglia are observed in the white matter from P4 to P14 in mice, and they demonstrate high phagocytic activity as compared to microglia from other brain regions (43). Microglia from early postnatal white matter contain fragments of the apoptotic marker cleaved caspase 3 (CC3) colocalized with MBP, a marker of mature oligodendrocytes (43). These results indicate that an early postnatal population of amoeboid microglia contribute to white matter development by actively phagocytosing dying oligodendrocyte-lineage cells. Li et al. (43) also observe amoeboid microglia in early white matter associated with nonapoptotic oligodendrocytes.
Phagocytosis of living oligodendrocyte-lineage cells
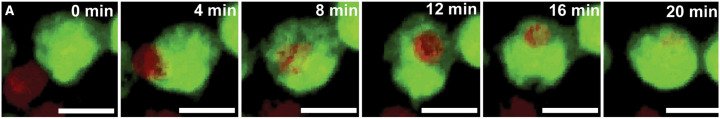
Microglia can also regulate the oligodendrocyte-lineage cell population through phagocytosis of living cells. Amoeboid microglia in the corpus callosum during early postnatal development have recently been shown to engulf NG2+ OPCs over a narrow window between P4 and P11, peaking at P7, in mice (Fig. 4) (44). Intriguingly, only 10 to 15% of the NG2+ OPC cells phagocytosed by these microglia express apoptotic markers, while the remaining 85 to 90% of phagocytosed OPCs are not apoptotic (44). Microglia are therefore not simply responding to apoptotic signals and removing the debris but rather actively modulating cell population dynamics in the developing white matter.
Fig. 4. Microglia phagocytose live OPCs during postnatal white matter development.
Time-lapse confocal imaging captures a CX3CR1+ amoeboid microglia (green) phagocytosing a live NG2+ OPC (red) in the corpus callosum of a P7 mouse brain. Reprinted from Nemes-Baran et al. (44) under Creative Commons licensing.
While the purpose of the overproduction of oligodendrocyte cells in developing white matter has yet to be determined conclusively, each of the three distinct waves of migrating OPCs identified across development are capable of compensating when other OPC waves are ablated (28). Kessaris et al. (28) conclude that prenatal overproduction of OPCs may provide a compensatory safety net to minimize the impact of an early insult on the healthy development of CNS white matter. Notably, oligodendrocytes are particularly vulnerable to hypoxia/ischemia at birth (45). In addition to microglia culling the excess oligodendrocytes that provide this reserve capacity, increased cellular competition over finite territory and trophic resources also reduces much of the initial oligodendrocyte-lineage overpopulation. Oligodendrocyte-lineage cells may be phagocytosed by microglia as live OPCs, while others may differentiate into mature oligodendrocytes, only to find that they lack necessary resources, undergo apoptosis, and eventually become targets of phagocytosis.
The need for such cellular elimination becomes evident when investigating the potential effects of an overcrowded oligodendrocyte-lineage population on overall myelin health. When microglial phagocytosis of live OPCs is inhibited experimentally in mice, the OPC population is increased, and there is a decrease in myelin thickness in studies on the corpus callosum. Inhibition of microglial phagocytosis was accomplished by knocking out the fractalkine receptor CX3CR1, an important receptor for microglial recognition of OPCs (44). This suggests an optimal number of OPCs for optimal myelin ensheathment of axons. Another study finds that pharmacological stimulation of excessive oligodendrogenesis increases the incidence of ectopic myelination, in which myelin sheaths were incorrectly targeted to wrap neuronal cell bodies (46). It is hypothesized that overcrowding of oligodendrocyte-lineage cells may lead to an imbalance in the ratio of oligodendrocytes to axons, resulting in aberrant myelin targeting and perhaps a reduction in the health of oligodendrocyte-lineage populations as competition over trophic resources increases. These findings support the conclusion that microglia help ensure correct myelin targeting by carefully maintaining a balanced oligodendrocyte-lineage cell population.
PHAGOCYTOSIS OF MYELIN MEMBRANE
In addition to engulfing whole cells, microglia have been observed to specifically phagocytose myelin sheaths. Several groups have documented the elimination of nascent myelin deposits during development. Live imaging of myelination initiation in the zebrafish spinal cord indicates that most of the myelin sheaths are rapidly deposited over a narrow period during early development; however, several of the initial sheaths are later retracted in a slower process (47). Similar studies report that early myelin retraction events occurred more frequently on smaller caliber axons (48) and axons with inhibited vesicular release (49). These early studies hypothesize that these retraction events may be evidence of corrective action following aberrant myelin targeting. While endogenous retraction and breakdown of myelin by oligodendrocytes do contribute to the removal of some myelin sheaths, in 2020, Hughes and Appel (50) reported that microglia assist in the removal of aberrantly deposited myelin. Furthermore, these studies found that myelin sheaths that were phagocytosed were longer than sheaths that were removed through endogenous retraction, leading the authors to hypothesize that phagocytic pruning functions extend the window for myelin refinement by allowing removal of segments that have already begun to bind to the axon (50). Microglia that populate developing white matter tracts of mice have also been observed with myelin MBP inclusions (40). Abnormalities in the ultrastructure of myelin membrane have been found in the optic nerve of healthy P10 mice, but these aberrations are resolved by adulthood, indicating that occasional myelin structural errors are likely a normal part of development (40). Both studies used methods of microglial inhibition and ablation to investigate the impact on myelination and found a marked increase in the prevalence of abnormal myelin structures and ectopic myelination events as compared to wild-type controls (40, 50). Together, these recent findings demonstrate a role for phagocytic microglia in refining and remodeling myelin structures during development.
MICROGLIA INTERACT WITH OLIGODENDROCYTES THROUGH RELEASE OF BIOCHEMICAL SIGNALING MOLECULES
Microglia do not act on the early CNS exclusively through their phagocytic function; they also participate in complicated intercellular signaling networks through the production and secretion of biochemical factors. Some molecules released by microglia can be inflammatory or cytotoxic to oligodendrocytes, while other factors are essential to oligodendrocyte survival, differentiation, and successful myelination.
Microglial trophic factors support oligodendrocyte-lineage cell survival, differentiation, and myelination
We have discussed the importance of microglia clearance of some oligodendrocytes to maintain population balance, given the myelination requirements and resources available in a specific region; however, microglia also act to fortify the remaining oligodendrocyte population. Oligodendrocyte-lineage cells die rapidly when cultured in isolation, but apoptosis is markedly reduced by the addition of growth factors, suggesting the need for intercellular support for sustained survival of oligodendrocyte-lineage cells (41). A plethora of factors have been shown to support healthy oligodendrocyte development and maturation, many of which cannot be produced by oligodendroglia but rather must be provided by other cells in the surrounding environment. Microglia-released soluble factors collected in conditioned media have been shown to support OPC survival and oligodendrocyte differentiation (51), increase the expression of the myelin markers MBP and proteolipid protein (PLP), and promote in vitro myelination (52). While astrocyte-conditioned media also support oligodendrocyte survival, microglia-released factors are particularly beneficial for oligodendrocyte differentiation and myelination (51).
One microglial factor that has received attention for its role in supporting oligodendrocyte health is insulin-like growth factor 1 (IGF-1). IGF-1 is found at high levels in microglia-conditioned media (51) and significantly reduces oligodendrocyte apoptosis in culture (41). IGF-1 has been localized to amoeboid microglia within the developing rat corpus callosum (53), and CD11c+ microglia from the corpus callosum produce IGF-1 at a level sevenfold greater than CD11c− microglia found elsewhere in the postnatal brain (15). Mice lacking IGF-1 have reduced OPC and oligodendrocyte populations as well as reduced myelination as compared to controls (54). Notably, the same population of microglia that display highly activated phagocytic activity in developing white matter also show elevated expression of IGF-1 (43). Specific knockout of IGF-1 from CD11c+ microglia results in irregular myelination, characterized by decreased Mbp, Plp, Mag, and Mog gene expression, and thinner myelin sheaths in the corpus callosum (15). Therefore, early white matter microglia that act to remove unhealthy and extraneous oligodendrocytes simultaneously contribute to the health of surviving oligodendrocyte-lineage population by providing trophic support. It is not yet clear from the available literature whether these dichotomous actions are taken on by separate subsets of the microglia or whether individual cells actively switch their activities based on localized needs.
Other factors produced by microglia have been found to support the health and maturation of OPCs and oligodendrocytes in the developing CNS. Microglia-derived IGF-2 has been shown to prevent tumor necrosis factor–α (TNF-α)–induced apoptosis of oligodendrocytes in vitro (55). In addition, IGF-2 was up-regulated following IGF-1 knockout, perhaps as compensation in the absence of the usual important trophic factor. Notably, however, this study did not investigate the source of IGF-2; therefore, any compensatory benefits for oligodendrogenesis in this experiment cannot be conclusively attributed to microglia (54). Furthermore, inhibition of microglial activation by minocycline treatment impairs maturation of oligodendrocytes in vivo, by reducing levels of the microglial cytokines interleukin-β (IL-1β) and IL-6 (56). A pathway dependent on the interaction between microglia-derived transglutaminase-2 (TG2) and the extracellular matrix protein laminin has been found to support OPC proliferation in the corpus callosum (57). In addition, neuropilin-1 (NP1), a transmembrane molecule expressed by activated microglia, has been recently shown to promote OPC proliferation in response to platelet-derived growth factor AA (PDGF AA) through its activation of the PDGFRα receptor (58). Collectively, these findings illustrate the complexity of microglial signals promoting the development of healthy oligodendrocyte populations and proper myelin formation.
Microglial signals involved in induction of apoptosis of mature oligodendrocytes
Disease-state microglia are well known cytotoxic actors in the CNS, capable of inducing cell death through a myriad of inflammatory cytokines, proteinases, and reactive oxygen intermediates, and microglia may use similar cytotoxic factors in regulation of oligodendrocyte populations in development. Microglia in the early postnatal white matter are transcriptionally similar to microglia found in disease states (43). These microglia have elevated proinflammatory cytokine and chemokine gene transcripts, including elevated Mif, Ccl3, Ccl4, CCl6, and Ccl9, as compared to other early microglia populations (43), suggesting that some of the mechanisms of microglial cytotoxicity may be used by microglia under physiologically normal developmental conditions. Although microglia can contribute to pathology, they also have known protective functions in neurodegenerative disease states (17, 19). Testing these hypotheses is an important direction for future research.
MULTIDIMENSIONAL SURVEILLANCE OF THE NEUROENVIRONMENT
The complex microglia-mediated intercellular interactions taking place during development are not entirely preprogrammed processes; rather, microglia acutely sense the need for specific mechanistic actions through a multitude of receptors and signaling molecules. These signals can be produced specifically by an individual cell to target microglia action, or these signals can be broader indicators of cellular population dynamics in the tissue and network functionality in developing neural circuits. This versatility allows microglia to sense and respond to a diverse array of needs and to efficiently provide support or regulation where required.
Sensing the health of cellular networks
The rapid motility of macrophage cells allows microglia to scour large portions of the CNS for signals indicative of the health and density of regional cell populations. Because microglia are responsible for maintaining balanced oligodendrogenesis while simultaneously regulating against overproliferation and crowding, they must have a mechanism to sense oligodendrocyte population dynamics. Chemoattractants are soluble signals that do not target specific cells for removal but serve to indicate need for microglia regulation. Following injury or insult, oligodendrocytes are known to release chemoattractant molecules, including CXCL10, CCL2, CCL3, and CCL5, which recruit microglia to the site of disruption and initiate cell removal (59). Inversely, oligodendrocytes experiencing cellular stress can also produce αB-crystallin, which attracts microglia that, in turn, act to promote improved health of the oligodendrocytes (60). While it is evident that microglia are capable of sensing oligodendrocyte health in response to disease, it is unknown whether similar recruitment molecules are released in response to nonpathological cellular stressors that can occur during development, such as overcrowding or mild resource scarcity due to competition. Furthermore, once microglia are alerted to a regional need for action, it would be important to determine how they target specific cells for elimination while selecting others for survival.
Signals driving cell-specific microglia action
Phagocytosis of apoptotic and degrading cells is a common function of microglia throughout life. Although signals uniquely targeting dying oligodendrocytes for phagocytosis have not been identified, phosphatidylserine translocation to the cell surface is a common apoptotic event across many cell types and functions as a phagocytosis signal for microglia (61). In the brain, externalized phosphatidylserine interacts with soluble TAM receptor ligands such as GAS6 and protein S to initiate microglial phagocytosis through the TAM receptor tyrosine kinases Mertk and Axl (62). This is one of the same signals that mark synapses for pruning during development (63), and abnormal myelin structures appearing during postnatal myelination also express phosphatidylserine on their surface to attract phagocytic microglia (40).
The accumulation of evidence supporting microglia phagocytosis of live oligodendrocyte-lineage cells and myelin has led investigators to question what signals might be directing microglia to phagocytose a specific target as compared to a neighboring cell or myelin sheath segment. The fractalkine ligand CX3CL1 is expressed by developing OPCs, and fractalkine receptor CX3CR1-deficient microglia are less efficient at OPC phagocytosis despite having an equivalent number of microglia and OPC-microglia contact events when compared with wild-type mice (44). This finding suggests that the CX3CL1-CX3CR1 signaling pathway is involved in targeting live OPCs for phagocytosis; however, the mechanisms driving OPC expression of CX3CL1 have yet to be elucidated.
Sensing neuronal activity changes and regulation through myelin modification
Microglia are capable of sensing neuronal activity both at the synapse (64) and along axons, allowing these cells to gather information about the neural circuit functionality at a broad and local level, and to control the level of neuronal activity through a negative feedback mechanism (65). A number of neurotransmitters and purinergic molecules act either directly or indirectly to initiate microglial responses to changes in neuronal activity (66). Neuron-derived CX3CL1 is an essential regulatory signal that interacts with the microglial receptor CX3CR1 to induce microglia-mediated synapse remodeling in response to changes in neuronal activity (67). Signaling through interaction between local axonal release of potassium and the microglia receptor THIK-1 also promotes microglia-neuron interaction. In experiments by Ronzano et al. (68), neuronal activity is reported to stabilize microglia activity and hold microglia at the node of Ranvier, while inhibition of neuronal activity or microglia-nodal interactions increases dynamic activity of microglia. As myelination alters the conduction velocity, synchronization, and overall functionality of neural networks, microglia modulation of myelination may be an indirect mechanism through which microglia can sense and respond to changes in neuronal activity.
Myelin targeting and thickness is influenced by neuronal activity such that active axons are preferentially myelinated over inactive axons (36) and increased neuronal activity stimulates thicker myelin (69). Recent findings open the possibility that selective myelination of active neurons may be refined after initial myelin deposition through a microglia-mediated mechanism. Neuronal activity may bias microglia selection of myelin segments for phagocytosis, as phagocytosis in zebrafish studies is elevated when neuronal activity is reduced in neighboring axons, while myelin elimination is reduced when regional neuronal activation is increased (50). This recent study examined the effects of shifts in neuronal activity across an entire neuroanatomical structure (optic tectum) however, microglia are also capable of sensing changes in activity at the level of individual axons and nodes. It remains unclear how such highly localized shifts in activity might alter microglia-myelin interactions. Such investigation is pertinent to our understanding of development because neuronal activity rarely undergoes marked changes under physiological conditions; rather, small local shifts often accumulate over time to drive the complex higher-order processes such as learning and memory.
WHITE MATTER WITHOUT MICROGLIA
To further investigate the influence of microglia on developing white matter, many groups have used methods of pharmacological inhibition and transgenic knockdown of microglia in animal models. Colony-stimulating factor 1 receptor (CSF1R) is essential to microglia development and survival (70), and therefore, manipulation of this receptor is a popular method of reducing the microglia population. Mutation of the CSF1R gene has been observed in a clinical setting with similar detrimental effects on human microglia populations; white matter abnormalities were observed along with a history of seizure and developmental delays (71). Experimental methods exploring the impact of microglia on oligodendrocyte populations and myelin formation are summarized in Table 1.
Table 1. Effects of microglia ablation or inhibition on oligodendrogenesis and myelination.
CC, corpus callosum; DPF, days post-fertilization; OL, oligodendrocyte; SC, spinal cord; SVZ, subventricular zone; WM, white matter.
|
Method(s) for microglia
ablation/inhibition |
Organism
and ROI |
Effects on
oligodendrogenesis |
Effects on myelination | Reference |
| Csf1r−/− transgenic | P20 mouse CC and SVZ | High degree of OPC apoptosis; mature OL population reduced |
No noted effects on myelination |
(73) |
| Intraperitoneal administration of minocycline to prevent microglia activation |
P5 rat SVZ | Increased OPC population but reduced premyelinating and mature OL numbers; no difference in OL-lineage proliferation labeling |
Number of MBP+ cells decreased |
(56) |
| CSF1R inhibitor BLZ945 |
P20 and P40–P42 mouse WM | Reduced number of OLs in P20 CC |
P20: Reduced expression and labeling of myelin proteins in CC and cerebellum; decrease in % of myelinated axons; no difference in myelin thickness; P40–P42: no difference in PLP or MBP |
(13) |
| Transgenic deletion of Igf-1 gene in CD11c+ microglia |
P21 mouse CC | No noted effects on oligodendrogenesis |
Decreased expression on Plp, Mag, and Mog; reduced myelin staining; thinner myelin sheaths |
(15) |
| Csf1r−/− transgenic | 3-week rat CNS | Decrease of oligodendrocyte gene expression |
Reduced myelination in the CC; decrease of myelin- associated gene expression |
(72) |
| Pediatric-onset leukoencephalopathy patients (homozygous CSF1R mutations) |
Human brain tissue (10 months to 25 years) |
No noted effects on oligodendrogenesis |
Periventricular white matter abnormalities; cerebellar and CC hypoplasia/atrophy |
(71) |
| PLX5622 and PLX3397 (CSF1R inhibitors) |
8- to 10-week mouse CNS | Reduced OPC viability in vitro and ex vivo; regional reduction of OPCs in vivo |
No difference in mature OL populations or PLP labeling |
(74) |
| (i) Blockade of Irf8 translation (ii) CSF1R inhibitor GW2580 (iii) Microglia expression of nitroreductase + actuator |
4 DPF zebrafish optic tectum and SC |
No difference in number or distribution of OLs |
Oligodendrocytes produced more sheaths and sheaths were shorter in length; increased incidence of ectopic myelination |
(50) |
| Microglia ablation via Csf1r
double mutant |
10 DPF zebrafish SC | Reduced number of OLs | Reduced total myelinated area, increased number of myelin structure pathologies, and no difference in sheath length |
(40) |
Collectively, the results of these experiments indicate that microglia are significant regulators of oligodendrogenesis and myelination, but the results differ depending on the experimental approach. Multiple studies show changes in oligodendrogenesis following microglia ablation with abnormalities in myelination of specific regions of interest (13, 40, 56, 72). Other studies report myelination deficits in the absence of an effect on oligodendrocyte cell numbers (15, 50, 71). In contrast, some studies find no myelination deficits but do report that oligodendrogenesis is impaired (73, 74). The resilience of the microglia-oligodendrocyte relationship across a multiplicity of models can be expected given the importance of myelination to essential brain functions. For example, despite a decrease in oligodendrocyte density and myelin protein in microglia-deficient P20 corpus callosum, myelin protein levels are restored at a later time in development (13). Furthermore, microglia populations can recover from ablation rapidly (70), making the timing of experimental manipulation and tissue collection potential sources of variability. Many of these studies rely on global ablation of microglia; thus, indirect effects of ablating microglia beyond the white matter cannot be ruled out. Results differ, depending on the experimental approach, but as a whole, these experiments indicate that microglia are dynamic contributors to multiple processes involved in the formation and organization of healthy white matter.
SUMMARY AND CONCLUSION
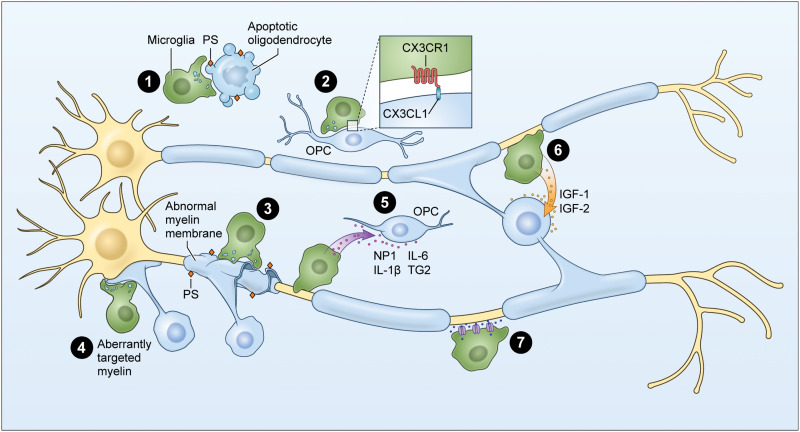
Microglia have been portrayed historically as passive surveyors of the healthy CNS, simply cleaning up cellular debris, unless activated by a pathogen or disease; however, microglia in the healthy early postnatal brain sense the surrounding microenvironment proactively, detecting neuronal activity levels and oligodendrocyte population dynamics to determine the need for modulation or stabilization of oligodendrogenesis and myelination. Recent experiments indicate that when microglia detect that oligodendrocyte population dynamics are unsustainable, or when aberrant myelin structures or changes in axons are sensed, microglia selectively target oligodendrocyte-lineage cells and myelin structures for removal. Alternatively, if microglia sense a need for increased or homeostatic maintenance of myelination of active axons, microglia play a supportive role by fortifying oligodendrocyte-lineage populations and promoting oligodendrogenesis and myelin formation (Fig. 5). In summary, studies examining the impact of microglia deficiency on oligodendrogenesis highlight the importance of microglia in proper myelin formation for normal brain function.
Fig. 5. Microglia actively regulate the oligodendrogenesis and myelination of the developing white matter.
Microglia sense and act on the early white matter by (1) clearing cellular debris from apoptotic oligodendrocytes through phosphatidylserine signaling, (2) phagocytosing live OPCs to regulate oligodendrocyte population dynamics, (3) removing abnormal myelin ultrastructure, (4) eliminating aberrantly targeted myelin, (5) promoting oligodendrogenesis, (6) fortifying mature oligodendrocytes and promoting myelination, and (7) sensing changes in neuronal activity to determine need for modification of myelin organization.
Currently, there is a rapidly expanding body of work investigating the microglia-oligodendrocyte relationship in development, but there is much that remains to be understood. Among these questions, it will be important to determine whether specialized types of microglia exist in white matter to influence myelination, or instead plasticity of microglia enables the same cells to carry out different functions as required. Notably, the extent to which white matter microglia are distinct from microglia in gray matter or differ from those that carry out immunological responses requires further investigation. The possible involvement of microglia in maintenance, remodeling, plasticity, and de novo myelination in the healthy adult brain, as distinct from during development, is a neglected but promising area of investigation. Cell-cell and intracellular signaling pathways in microglia and oligodendrocytes need to be identified and interpreted in the context of interactions among other brain cells, including neurons and astrocytes, to influence oligodendroglia and myelination. The mechanisms by which microglia are targeted to act on appropriate axons, oligodendrocytes, and subcellular domains to modify myelin must be further identified.
The growing awareness that microglia are critical in myelination in the healthy brain illuminates how disease and other nervous system insults that activate microglia during critical periods could impair myelination with long-term consequences. There is a large body of research indicating that viral infection during pregnancy, for example, can increase the probability of offspring developing cognitive impairments or mental illnesses, including schizophrenia, in later life (75). Other work confirms white matter abnormalities in such conditions (76). Similarly, substance abuse, including alcohol consumption during fetal development (77), and hypoxia/ischemia at birth result in white matter deficits (45), which could, in part, reflect disruption of microglia in regulating myelination when the cells become activated in an inflammatory state. There are currently no treatments for such white matter injuries from such insults in the perinatal period, but as knowledge of microglia-oligodendrocytes in the healthy brain increases, new understanding and new treatments may be forthcoming.
Acknowledgments
Funding: This work was supported by NIH intramural grant no. ZIAHD000713.
Author contributions: Both authors conceived and wrote the article together. Artwork by Alan Hoofring, NIH Medical Arts.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper. Additional data related to this paper may be requested from the authors.
REFERENCES AND NOTES
- 1.Safaiyan S., Besson-Girard S., Kaya T., Cantuti-Castelvetri L., Liu L., Ji H., Schifferer M., Gouna G., Usifo F., Kannaiyan N., Fitzner D., Xiang X., Rossner M. J., Brendel M., Gokce O., Simons M., White matter aging drives microglial diversity. Neuron 109, 1100–1117.e10 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Bsibsi M., Peferoen L. A. N., Holtman I. R., Nacken P. J., Gerritsen W. H., Witte M. E., Van Horssen J., Eggen B. J. L., Van Der Valk P., Amor S., Van Noort J. M., Demyelination during multiple sclerosis is associated with combined activation of microglia/macrophages by IFN-γ and alpha B-crystallin. Acta Neuropathol. 128, 215–229 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Prineas J. W., Kwon E. E., Cho E. S., Sharer L. R., Barnett M. H., Oleszak E. L., Hoffman B., Morgan B. P., Immunopathology of secondary-progressive multiple sclerosis. Ann. Neurol. 50, 646–657 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Stence N., Waite M., Dailey M. E., Dynamics of microglial activation: A confocal time-lapse analysis in hippocampal slices. Glia 33, 256–266 (2001). [PubMed] [Google Scholar]
- 5.Imamoto K., Leblond C. P., Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J. Comp. Neurol. 180, 139–163 (1978). [DOI] [PubMed] [Google Scholar]
- 6.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M. F., Conway S. J., Ng L. G., Stanley E. R., Samokhvalov I. M., Merad M., Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kierdorf K., Erny D., Goldmann T., Sander V., Schulz C., Perdiguero E. G., Wieghofer P., Heinrich A., Riemke P., Hölscher C., Müller D. N., Luckow B., Brocker T., Debowski K., Fritz G., Opdenakker G., Diefenbach A., Biber K., Heikenwalder M., Geissmann F., Rosenbauer F., Prinz M., Microglia emerge from erythromyeloid precursors via Pu.1-and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Alliot F., Godin I., Pessac B., Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Dev. Brain Res. 117, 145–152 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Askew K., Li K., Olmos-Alonso A., Garcia-Moreno F., Liang Y., Richardson P., Tipton T., Chapman M. A., Riecken K., Beccari S., Sierra A., Molnár Z., Cragg M. S., Garaschuk O., Perry V. H., Gomez-Nicola D., Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 18, 391–405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monier A., Adle-Biassette H., Delezoide A.-L., Evrard P., Gressens P., Verney C., Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J. Neuropathol. Exp. Neurol. 66, 372–382 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Matcovitch-Natan O., Winter D. R., Giladi A., Aguilar S. V., Spinrad A., Sarrazin S., Ben-Yehuda H., David E., González F. Z., Perrin P., Keren-Shaul H., Gury M., LaraAstaiso D., Thaiss C. A., Cohen M., Halpern K. B., Baruch K., Deczkowska A., LorenzoVivas E., Itzkovitz S., Elinav E., Sieweke M. H., Schwartz M., Amit I., Microglia development follows a stepwise program to regulate brain homeostasis. Science 353, aad8670 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Staszewski O., Hagemeyer N., Unique microglia expression profile in developing white matter. BMC Res. Notes. 12, 367 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagemeyer N., Hanft K. M., Akriditou M. A., Unger N., Park E. S., Stanley E. R., Staszewski O., Dimou L., Prinz M., Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 134, 441–458 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leong S. K., Ling E. A., Amoeboid and ramified microglia: Their interrelationship and response to brain injury. Glia 6, 39–47 (1992). [DOI] [PubMed] [Google Scholar]
- 15.Wlodarczyk A., Holtman I. R., Krueger M., Yogev N., Bruttger J., Khorooshi R., Benmamar-Badel A., Boer-Bergsma J. J., Martin N. A., Karram K., Kramer I., Boddeke E. W., Waisman A., Eggen B. J., Owens T., A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 36, 3292–3308 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wlodarczyk A., Løbner M., Cédile O., Owens T., Comparison of microglia and infiltrating CD11c+ cells as antigen presenting cells for T cell proliferation and cytokine response. J. Neuroinflammation 11, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T. K., David E., Baruch K., Lara-Astaiso D., Toth B., Itzkovitz S., Colonna M., Schwartz M., Amit I., A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Krasemann S., Madore C., Cialic R., Baufeld C., Calcagno N., El Fatimy R., Beckers L., O’Loughlin E., Xu Y., Fanek Z., Greco D. J., Smith S. T., Tweet G., Humulock Z., Zrzavy T., Conde-Sanroman P., Gacias M., Weng Z., Chen H., Tjon E., Mazaheri F., Hartmann K., Madi A., Ulrich J. D., Glatzel M., Worthmann A., Heeren J., Budnik B., Lemere C., Ikezu T., Heppner F. L., Litvak V., Holtzman D. M., Lassmann H., Weiner H. L., Ochando J., Haass C., Butovsky O., The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S. H., Meilandt W. J., Xie L., Gandham V. D., Ngu H., Barck K. H., Rezzonico M. G., Imperio J., Lalehzadeh G., Huntley M. A., Stark K. L., Foreman O., Carano R. A. D., Friedman B. A., Sheng M., Easton A., Bohlen C. J., Hansen D. V., Trem2 restrains the enhancement of tau accumulation and neurodegeneration by β-amyloid pathology. Neuron 109, 1283–1301.e6 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Sturrock R. R., Myelination of the mouse corpus callosum. Neuropathol. Appl. Neurobiol. 6, 415–420 (1980). [DOI] [PubMed] [Google Scholar]
- 21.Waxman S. G., Pappas G. D., Bennett M. V. L., Morphological correlates of functional differentiation of nodes of Ranvier along single fibers in the neurogenic electric organ of the knife fish Sternarchus. J. Cell Biol. 53, 210–224 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan M., Meyer-Franke A., Lambertt S., Bennett V., Duncan I. D., Levinson S. R., Barres B. A., Induction of sodium channel clustering by oligodendrocytes. Nature 386, 724–728 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Lee Y., Morrison B. M., Li Y., Lengacher S., Farah M. H., Hoffman P. N., Liu Y., Tsingalia A., Jin L., Zhang P. W., Pellerin L., Magistretti P. J., Rothstein J. D., Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fünfschilling U., Supplie L. M., Mahad D., Boretius S., Saab A. S., Edgar J., Brinkmann B. G., Kassmann C. M., Tzvetanova I. D., Möbius W., Diaz F., Meijer D., Suter U., Hamprecht B., Sereda M. W., Moraes C. T., Frahm J., Goebbels S., Nave K. A., Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo F., Zhang J., Burke K., Romito-DiGiacomo R. R., Miller R. H., Yang Y., Oligodendrocyte-specific loss of Cdk5 disrupts the architecture of nodes of Ranvier as well as learning and memory. Exp. Neurol. 306, 92–104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenzie I. A., Ohayon D., Li H., De Faria J. P., Emery B., Tohyama K., Richardson W. D., Motor skill learning requires active central myelination. Science 346, 318–322 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steadman P. E., Xia F., Ahmed M., Mocle A. J., Penning A. R. A., Geraghty A. C., Steenland H. W., Monje M., Josselyn S. A., Frankland P. W., Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron 105, 150–164.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kessaris N., Fogarty M., Iannarelli P., Grist M., Wegner M., Richardson W. D., Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9, 173–179 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chong S. Y. C., Rosenberg S. S., Fancy S. P. J., Zhao C., Shen Y. A. A., Hahn A. T., McGee A. W., Xu X., Zheng B., Zhang L. I., Rowitch D. H., Franklin R. J. M., Lu Q. R., Chan J. R., Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc. Natl. Acad. Sci. U.S.A. 109, 1299–1304 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutta D. J., Woo D. H., Lee P. R., Pajevic S., Bukalo O., Huffman W. C., Wake H., Basser P. J., SheikhBahaei S., Lazarevic V., Smith J. C., Fields R. D., Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proc. Natl. Acad. Sci. U.S.A. 115, 11832–11837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.L. A. Yakovlev, A. R. Lecours, The myelogenic cycles of regional maturation of the brain, in Regional Development of the Brain in Early Life, A. Minkowski, Ed. (Blackwell Scientific Publications, 1967), pp. 3–70. [Google Scholar]
- 32.Lee S., Leach M. K., Redmond S. A., Chong S. Y. C., Mellon S. H., Tuck S. J., Feng Z. Q., Corey J. M., Chan J. R., A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917–922 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson H. N., Treichel A. J., Eggum E. N., Martell M. R., Kaiser A. J., Trudel A. G., Gronseth J. R., Maas S. T., Bergen S., Hines J. H., Individual neuronal subtypes control initial myelin sheath growth and stabilization. Neural Dev. 15, 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomassy G. S., Berger D. R., Chen H.-H., Kasthuri N., Hayworth K. J., Vercelli A., Seung H. S., Lichtman J. W., Arlotta P., Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wake H., Lee P. R., Fields R. D., Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wake H., Ortiz F. C., Woo D. H., Lee P. R., Angulo M. C., Fields R. D., Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat. Commun. 6, 7844 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snaidero N., Möbius W., Czopka T., Hekking L. H. P., Mathisen C., Verkleij D., Goebbels S., Edgar J., Merkler D., Lyons D. A., Nave K. A., Simons M., Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156, 277–290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fields R. D., A new mechanism of nervous system plasticity: Activity-dependent myelination. Nat. Rev. Neurosci. 16, 756–767 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato D., Wake H., Lee P. R., Tachibana Y., Ono R., Sugio S., Tsuji Y., Tanaka Y. H., Tanaka Y. R., Masamizu Y., Hira R., Moorhouse A. J., Tamamaki N., Ikenaka K., Matsukawa N., Fields R. D., Nabekura J., Matsuzaki M., Motor learning requires myelination to reduce asynchrony and spontaneity in neural activity. Glia 68, 193–210 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.M. Djannatian, U. Weikert, S. Safaiyan, C. Wrede, C. Deichsel, G. Kislinger, T. Ruhwedel, D. S. Campbell, T. van Ham, B. Schmid, J. Hegermann, W. Möbius, M. Schifferer, M. Simons, Myelin biogenesis is associated with pathological ultrastructure that is resolved by microglia during development. bioRxiv 2021.02.02.429485 [Preprint]. 4 February 2021. 10.1101/2021.02.02.429485. [DOI]
- 41.Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C., Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70, 31–46 (1992). [DOI] [PubMed] [Google Scholar]
- 42.Trapp B. D., Nishiyama A., Cheng D., Macklin W., Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J. Cell Biol. 137, 459–468 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q., Cheng Z., Zhou L., Darmanis S., Neff N. F., Okamoto J., Gulati G., Bennett M. L., Sun L. O., Clarke L. E., Marschallinger J., Yu G., Quake S. R., Wyss-Coray T., Barres B. A., Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101, 207–223.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nemes-Baran A. D., White D. R., DeSilva T. M., Fractalkine-dependent microglial pruning of viable oligodendrocyte progenitor cells regulates myelination. Cell Rep. 32, 108047 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Back S. A., Han B. H., Luo N. L., Chricton C. A., Xanthoudakis S., Tam J., Arvin K. L., Holtzman D. M., Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J. Neurosci. 22, 455–463 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almeida R. G., Pan S., Cole K. L. H., Williamson J. M., Early J. J., Czopka T., Klingseisen A., Chan J. R., Lyons D. A., Myelination of neuronal cell bodies when myelin supply exceeds axonal demand. Curr. Biol. 28, 1296–1305.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Czopka T., Ffrench-Constant C., Lyons D. A., Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev. Cell 25, 599–609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu P., Du J. L., He C., Developmental pruning of early-stage myelin segments during CNS myelination in vivo. Cell Res. 23, 962–964 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hines J. H., Ravanelli A. M., Schwindt R., Scott E. K., Appel B., Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hughes A. N., Appel B., Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 23, 1055–1066 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pang Y., Fan L. W., Tien L. T., Dai X., Zheng B., Cai Z., Lin R. C. S., Bhatt A., Differential roles of astrocyte and microglia in supporting oligodendrocyte development and myelination in vitro. Brain Behav. 3, 503–514 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamilton S. P., Rome L. H., Stimulation of in vitro myelin synthesis by microglia. Glia 11, 326–335 (1994). [DOI] [PubMed] [Google Scholar]
- 53.Kaur C., Sivakumar V., Dheen S. T., Ling E. A., Insulin-like growth factor I and II expression and modulation in amoeboid microglial cells by lipopolysaccharide and retinoic acid. Neuroscience 138, 1233–1244 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Ye P., Li L., Richards R. G., Diaugustine R. P., D’ercole A. J., Myelination is altered in insulin-like growth factor-I null mutant mice. J. Neurosci. 22, 6041–6051 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicholas R. S., Stevens S., Wing M. G., Compston D. A., Microglia-derived IGF-2 prevents TNFα induced death of mature oligodendrocytes in vitro. J. Neuroimmunol. 124, 36–44 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Shigemoto-Mogami Y., Hoshikawa K., Goldman J. E., Sekino Y., Sato K., Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 34, 2231–2243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giera S., Luo R., Ying Y., Ackerman S. D., Jeong S.-J., Stoveken H. M., Folts C. J., Welsh C. A., Tall G. G., Stevens B., Monk K. R., Piao X., Microglial transglutaminase-2 drives myelination and myelin repair via GPR56/ ADGRG1 in oligodendrocyte precursor cells. eLife 7, e33385 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sherafat A., Pfeiffer F., Reiss A. M., Wood W. M., Nishiyama A., Microglial neuropilin-1 promotes oligodendrocyte expansion during development and remyelination by transactivating platelet-derived growth factor receptor. Nat. Commun. 12, 2265 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balabanov R., Strand K., Goswami R., McMahon E., Begolka W., Miller S. D., Popko B., Interferon-gamma-oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. J. Neurosci. 27, 2013–2024 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Noort J. M., Bsibsi M., Gerritsen W. H., Van Der Valk P., Bajramovic J. J., Steinman L., Amor S., αB-crystallin is a target for adaptive immune responses and a trigger of innate responses in preactive multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 69, 694–703 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Martin S. J., Reutelingsperger C. P., McGahon A. J., Rader J. A., van Schie R. C., Laface D. M., Green D. R., Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182, 1545–1556 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fourgeaud L., Traves P. G., Tufail Y., Leal-Bailey H., Lew E. D., Burrola P. G., Callaway P., Zagorska A., Rothlin C. V., Nimmerjahn A., Lemke G., TAM receptors regulate multiple features of microglial physiology. Nature 532, 240–244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scott-Hewitt N., Perrucci F., Morini R., Erreni M., Mahoney M., Witkowska A., Carey A., Faggiani E., Schuetz L. T., Mason S., Tamborini M., Bizzotto M., Passoni L., Filipello F., Jahn R., Stevens B., Matteoli M., Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 39, e105380 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wake H., Moorhouse A. J., Jinno S., Kohsaka S., Nabekura J., Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29, 3974–3980 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Badimon A., Strasburger H. J., Ayata P., Chen X., Nair A., Ikegami A., Hwang P., Chan A. T., Graves S. M., Uweru J. O., Ledderose C., Kutlu M. G., Wheeler M. A., Kahan A., Ishikawa M., Wang Y. C., Loh Y. H. E., Jiang J. X., Surmeier D. J., Robson S. C., Junger W. G., Sebra R., Calipari E. S., Kenny P. J., Eyo U. B., Colonna M., Quintana F. J., Wake H., Gradinaru V., Schaefer A., Negative feedback control of neuronal activity by microglia. Nature 586, 417–423 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Umpierre A. D., Wu L. J., How microglia sense and regulate neuronal activity. Glia 69, 1637–1653 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gunner G., Cheadle L., Johnson K. M., Ayata P., Badimon A., Mondo E., Nagy M. A., Liu L., Bemiller S. M., Kim K. W., Lira S. A., Lamb B. T., Tapper A. R., Ransohoff R. M., Greenberg M. E., Schaefer A., Schafer D. P., Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat. Neurosci. 22, 1075–1088 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ronzano R., Roux T., Thetiot M., Aigrot M. S., Richard L., Lejeune F. X., Mazuir E., Vallat J. M., Lubetzki C., Desmazières A., Microglia-neuron interaction at nodes of Ranvier depends on neuronal activity through potassium release and contributes to remyelination. Nat. Commun. 12, 5219 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gibson E. M., Purger D., Mount C. W., Goldstein A. K., Lin G. L., Wood L. S., Inema I., Miller S. E., Bieri G., Zuchero J. B., Barres B. A., Woo P. J., Vogel H., Monje M., Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elmore M. R. P., Najafi A. R., Koike M. A., Dagher N. N., Spangenberg E. E., Rice R. A., Kitazawa M., Matusow B., Nguyen H., West B. L., Green K. N., Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oosterhof N., Chang I. J., Karimiani E. G., Kuil L. E., Jensen D. M., Daza R., Young E., Astle L., van der Linde H. C., Shivaram G. M., Demmers J., Latimer C. S., Keene C. D., Loter E., Maroofian R., van Ham T. J., Hevner R. F., Bennett J. T., Homozygous mutations in CSF1R cause a pediatric-onset leukoencephalopathy and can result in congenital absence of microglia. Am. J. Hum. Genet. 104, 936–947 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pridans C., Raper A., Davis G. M., Alves J., Sauter K. A., Lefevre L., Regan T., Meek S., Sutherland L., Thomson A. J., Clohisey S., Bush S. J., Rojo R., Lisowski Z. M., Wallace R., Grabert K., Upton K. R., Tsai Y. T., Brown D., Smith L. B., Summers K. M., Mabbott N. A., Piccardo P., Cheeseman M. T., Burdon T., Hume D. A., Pleiotropic impacts of macrophage and microglial deficiency on development in rats with targeted mutation of the Csf1r locus. J. Immunol. 201, 2683–2699 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nandi S., Gokhan S., Dai X. M., Wei S., Enikolopov G., Lin H., Mehler M. F., Richard Stanley E., The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev. Biol. 367, 100–113 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y., Given K. S., Dickson E. L., Owens G. P., Macklin W. B., Bennett J. L., Concentration-dependent effects of CSF1R inhibitors on oligodendrocyte progenitor cells ex vivo and in vivo. Exp. Neurol. 318, 32–41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown A. S., Schaefer C. A., Wyatt R. J., Qoetz R., Begg M. D., Qorman J. M., Susser E. S., Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: A prospective birth cohort study. Schizophr. Bull. 26, 287–295 (2000). [DOI] [PubMed] [Google Scholar]
- 76.Buchsbaum M. S., Tang C. Y., Peled S., Gudbjartsson H., Lu D., Hazlett E. A., Downhill J., Haznedar M., Fallon J. H., Atlas S. W., MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport 9, 425–430 (1998). [DOI] [PubMed] [Google Scholar]
- 77.Sowell E. R., Johnson A., Kan E., Lu L. H., Van Horn J. D., Toga A. W., O’Connor M. J., Bookheimer S. Y., Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J. Neurosci. 28, 1313–1319 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaur C., Rathnasamy G., Ling E. A., Biology of microglia in the developing brain. J. Neuropathol. Exp. Neurol. 76, 736–753 (2017). [DOI] [PubMed] [Google Scholar]