Abstract
There is little evidence regarding the effectiveness of procedural interventions for treatment of pain after an acute herpes zoster (AHZ) infection before the development of postherpetic neuralgia (PHN). In our case, a 61-year-old male presented with 1 month of left foot pain following an AHZ infection. After 1 month of pain refractory to treatment and admission to our hospital for acute pain management, a nerve stimulator was placed at the left first sacral (S1) dorsal root ganglion (DRG), which significantly decreased his pain despite his ongoing dermatologic manifestations of AHZ. In conclusion, we describe a case of nerve stimulator placement at the S1 DRG as a successful treatment for intractable pain following an AHZ infection.
Keywords: Anesthesia/pain, acute herpes zoster, dorsal root ganglion, stimulation, postherpetic neuralgia
Introduction
Postherpetic neuralgia (PHN) is the most common complication following acute herpes zoster (AHZ) infection. It is triggered by a reactivation of the varicella zoster virus (VZV; i.e. shingles), which lies dormant in the sensory dorsal root ganglia (DRG) following contraction of the chickenpox.1–4 PHN is suspected when the pain from AHZ lasts long after the rash has disappeared. The acute phase of herpes zoster is defined within 30 days of the viral infection with onset of rash. 3 If adequate reduction in pain is not achieved within the acute phase of herpes zoster, permanent damage in the affected neurons can occur, likely from neuronal inflammation followed by nociceptive sensitization and central hyperexcitability, resulting in PHN after 120 days.2,4–7 The pain is commonly described as a persistent burning pain that spreads along a single, unilateral dermatome, usually a thoracic dermatome or the ophthalmic division (V1) of the trigeminal nerve. Patients with zoster-related pain often have sensory abnormalities, including dysesthesia (unpleasant sensation), allodynia (painful response to a normally non-painful stimulus), and hyperalgesia (exaggerated response to a painful stimulus).2,5,8
PHN affects approximately 10% to 18% of patients following AHZ infection. 4 The incidence may be higher in patients with risk factors such as advanced age, greater severity of acute pain before onset of the rash, ophthalmic herpes compared to spinal segment type, cancer, diabetes, immunosuppression, and lymphoproliferative disorders.1,2 Because of the complex pathology of PHN, no definitive, disease-modifying treatment has been established. 2 For this reason, prevention of AHZ is the primary defense against latent VZV infection, and consequently, PHN. 1 The Advisory Committee of Immunization Practices (ACIP) offers recommendations for varicella vaccinations in children, as well as two licensed recombinant zoster vaccines to prevent shingles in healthy adults 50 years and older.1,2 For those who have contracted an AHZ infection, antiviral medications, including acyclovir, famciclovir, and valacyclovir, have shown to reduce the severity as well as hasten the resolution of the pain and rash.1,2 Otherwise, therapy is directed at symptom control.
Because the pain associated with AHZ is primarily neuropathic in nature, there are several pharmacologic options used for treatment. Topical treatments are reasonable for first-line therapy for mild pain. These include capsaicin (0.075% cream, 8% patch), lidocaine-prilocaine (2.5%–2.5% cream), and lidocaine (2.5% cream, 5% patch).2,8 First-line systemic treatments include a trial of anticonvulsant medications such as gabapentin or pregabalin.2,8 Antidepressants like amitriptyline, nortriptyline, trazodone, and duloxetine have also had varied success. Other medication options include acetaminophen and nonsteroidal anti-inflammatory medications. Opioids are cautiously used and are helpful in some well-selected patients. 2
Besides pharmacotherapy, noninvasive measures such as physical therapy, behavior therapy, and transcutaneous electrical nerve stimulation (TENS) have been used in the treatment of zoster-related pain. 8 Central, spinal, and sympathetic nerve blocks have also shown variable results in the treatment of PHN.2,4,6,8 After failing conservative treatment modalities, surgical interventions can include dorsal root entry zone lesioning, spinal nucleotractotomy, and stereotactic radiosurgery of the nerve root. 6 Other treatments include pulsed or routine radiofrequency denervation of DRG, and spinal cord and peripheral nerve stimulation.3,5,6
This case study demonstrates the use of DRG stimulation of the first sacral (S1) dorsal root ganglion (DRG) in a case of acute neuropathic pain associated with AHZ infection, before the development of PHN.
Written HIPAA authorization was obtained from the patient.
Case description
A pleasant 61-year-old male was referred to the Blaustein Pain Treatment Center for persistent, severe burning, aching, left foot pain following a herpes zoster infection. His medical history was significant for rheumatoid arthritis and multiple myeloma and was undergoing his fourth course of chemotherapy treatment (lenalidomide-dexamethasone-bortezomib) at the time of the eruption of a vesicular, erythematous rash on the dorsal aspect of his left foot. On examination, there was no motor weakness to the left foot, but his range of motion was mildly limited due to pain. The rash was quickly identified as a herpes zoster infection, and he was treated with valacyclovir for a 7-day course. He was then continued on oral acyclovir for herpes zoster viral prevention in the setting of his ongoing chemotherapy. However, his left foot pain persisted and intensified despite resolution of the rash. The patient failed to respond to medication management, including anticonvulsants (gabapentin), antidepressants (duloxetine and nortriptyline), opioids (oxycodone and hydromorphone), topical analgesics (capsaicin cream, EMLA cream, and lidocaine patch), and an NMDA-receptor antagonist infusion (ketamine via intravenous infusion) during a hospital admission. He also received Scrambler Therapy (CalmareTM) and later a left transforaminal epidural steroid injection (containing 0.25% bupivacaine and dexamethasone) at the level between the fourth and fifth lumbar vertebra (L4-5) without improvement. The L4-5 space was targeted given the ease of access and the expectation that the medications would spread to the adjacent levels.
Approximately 1 month after the acute zoster infection and ongoing severe pain despite multimodal management, the patient underwent a trial of a left S1 DRG stimulator while an inpatient. For placement of the stimulator, the patient was mildly sedated with intravenous midazolam and fentanyl. Using an anteroposterior (AP) fluoroscopic view, the skin over the left S1 foramen was topicalized. A 14-gauge Tuohy needle was then advanced under fluoroscopic guidance slightly into the posterior border of the S1 foramen. An Abbott DRG 4-contact lead and sheath complex was then advanced under live fluoroscopy to the caudal epidural space in the lateral projection and then the lead was advanced until all four electrodes were appropriately placed over the sacrum (Figure 1). The sheath was then retracted leaving the lead in place with the electrodes non-displaced. A strain relief loop was created in the epidural space via the lead and sheath complex and the lead was confirmed in place. 9 The lead was then stimulated resulting in good coverage of the patient’s left foot pain. The sheath and Tuohy needle were withdrawn using reverse Seldinger technique under live fluoroscopy to confirm lead stability. The site was secured in a sterile fashion. The device was kept on continuously (through wake/sleep cycle) based on the patient’s preference. Stimulation was paresthesia-based (frequency 20 Hz) with 300 us pulse width and 900 uA amplitude.
Figure 1.
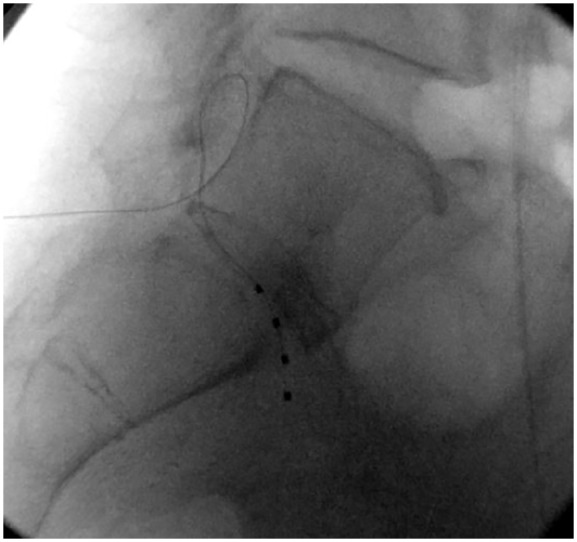
Lateral view of the lumbosacral region with Abbott lead in place.
The patient tolerated the procedure well, and his pain improved significantly with some decreased allodynia of the left foot. Since his trial had a positive result, surgical implantation was pursued. The S1 foramen was accessed similarly as in the trial. Because the patient reported some residual pain in the sole of his foot during the trial, and in the event of DRG electrode migration—which may occur more frequently with sacral electrodes—a second lead was advanced through the L5 S1 interspace and ultimately placed under the left pedicle of L5 (Figure 2). Together, these leads were tested intraoperatively and found to completely cover the painful regions of the left lower extremity and foot. The leads were tunneled to the left gluteal pocket and connected to the Abbott implantable pulse generator (IPG). Surgery was tolerated well. The stimulation device settings remained the same as those during the trial.
Figure 2.
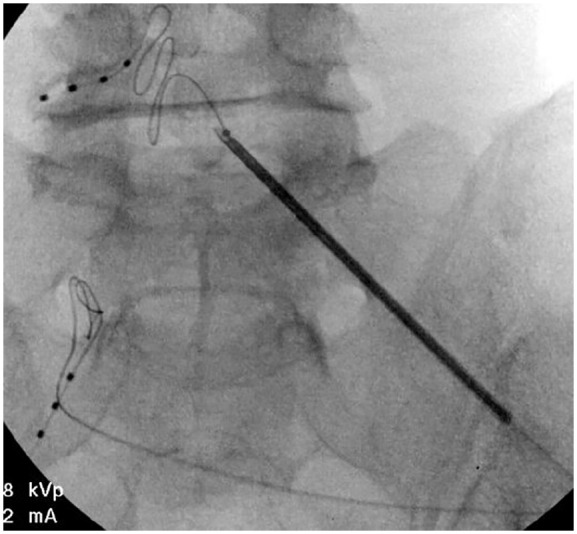
AP view of leads in place.
After 1-month follow-up, the patient reported continued pain relief and a return to his daily activities. While he continued to have mild pain at night (1 out of 10 in severity) after 2 months, he was able to wean off all medications except gabapentin and duloxetine, which he continued to take for pain associated with his multiple myeloma.
Discussion
There are approximately 1 million new cases of AHZ each year in the United States. 3 AHZ is characterized by a painful rash from reactivation of the VZV, which lies dormant in the dorsal root ganglia following initial infection with the chickenpox. Factors most associated with reactivation of the virus include advanced age, stress, and a compromised immune system. Despite resolution of the rash, as many as 18% of patients have persistent neuropathic pain, resulting in a chronic pain condition referred to as PHN.
Current treatment options for pain associated with AHZ may still be inadequate for many patients, which include pharmacologic management (anticonvulsants, antidepressants, acetaminophen, nonsteroidal anti-inflammatories, opioids, NMDA receptor-antagonists, and topical analgesics), TENS, behavioral therapy, physical therapy, nerve blocks, and steroid injections. The use of spinal cord stimulators are considered last-resort treatments for PHN because of varied success rates and potential complications.3,6 In contrast, peripheral nerve stimulation has been used to treat neuropathic pain for years, but has only recently been used for treatment of PHN. 7 Moreover, there is scarce evidence for the use of DRG stimulation in the acute phase of a herpes zoster infection.
Conclusion
Our case of a 61-year-old immunosuppressed gentleman with 1 month of persistent pain due to an AHZ infection is likely the first of its kind to demonstrate the use of DRG stimulation for intractable acute neuropathic pain following an acute zoster infection, but before the development of PHN. While well-designed clinical studies must be done to investigate the efficacy of DRG stimulation for pain associated with an AHZ infection, this case report suggests DRG stimulation to be a viable option for those who have failed conventional treatments in the acute setting.
Footnotes
Author contributions: Alexandra Roybal is the primary writer of the manuscript and conducted most of the literature research. Eellan Sivanesan contributed to the case description and editing of the manuscript. Yian Chen wrote part of the manuscript and is the primary editor.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iD: Alexandra Elyse Roybal  https://orcid.org/0000-0002-4079-4111
https://orcid.org/0000-0002-4079-4111
References
- 1. Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44: S1–S26. [DOI] [PubMed] [Google Scholar]
- 2. Johnson RW, Rice ASC. Postherpetic neuralgia. New Engl J Med 2014; 371: 1526–1533. [DOI] [PubMed] [Google Scholar]
- 3. Lynch P, McJunkin T, Erros E, et al. Case report: successful epiradicular peripheral nerve stimulation of the C2 dorsal root ganglion for postherpetic neuralgia. Neuromodulation 2001; 14: 58–61. [DOI] [PubMed] [Google Scholar]
- 4. Marin M, Güris D, Chaves S, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56(RR-4): 1–40. [PubMed] [Google Scholar]
- 5. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers 2017; 3: 17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim ED, Lee YI, Park HJ. Comparison of efficacy of continuous epidural block and pulsed radiofrequency to the dorsal root ganglion for management of pain persisting beyond the acute phase of herpes zoster. PLoS ONE 2017; 12(8): e0183559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li D, Sun G, Sun H, et al. Combined therapy of pulsed radiofrequency and nerve block in postherpetic neuralgia patients: a randomized clinical trial. PeerJ 2018; 6: e4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shrestha M, Chen A. Modalities in managing postherpetic neuralgia. Korean J Pain 2018; 31(4): 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velsen V, van Helmond N, van Chapman K. Creating a strain relief loop during S1 transforaminal lead placement for dorsal root ganglion stimulation for foot pain: a technical note. Pain Pract 2018; 18(4): 539–543. [DOI] [PubMed] [Google Scholar]


