Abstract
Stellate ganglion block (SGB) is a procedure involving the injection of a local anesthetic surrounding the stellate ganglion to inhibit sympathetic outflow. The objective of this review was to summarize existing evidence on the use of SGB in adults with psychiatric disorders. A systematic search identified 17 published studies and 4 registered clinical trials. Eighty-eight percent of published studies, including 2 randomized controlled trials (RCTs), used SGB for posttraumatic stress disorder (PTSD), although its use for schizophrenia spectrum disorders was also explored. Administration of 1 to 2 SGBs using right-sided laterality with 0.5% ropivacaine was most common. Preliminary evidence from clinical trials and case studies supports the feasibility of SGB for treating psychiatric disorders involving dysregulation of the sympathetic nervous system, although effectiveness evidence from RCTs is mixed. One RCT concluded that improvement in PTSD symptoms was significant, while the other concluded that it was nonsignificant. Improvements were noted within 5 minutes of SGB and lasted 1 month or longer. Registered clinical trials are exploring the use of SGB in new psychiatric disorders, including major depressive disorder and borderline personality disorder. More studies with larger sample sizes and alternate protocols are needed to further explore therapeutic potential of SGB for psychiatric disorders.
Keywords: psychiatric disorders, systematic review, stellate ganglion block, anesthesia, posttraumatic stress disorder, autonomic nerve block, stellate ganglion, sympathetic nervous system, sympatholytics
Introduction
Psychiatric disorders affect over 1 billion people worldwide, which is equivalent to approximately 16% of the global population. 1 In 2016, it was estimated that 162.5 million disability adjusted life years (DALYs) were lost due to these disorders, with depressive, anxiety, substance use, and alcohol use disorders being responsible for two-thirds of the 162.5 million. However, this burden is considered to be an underestimate, and it is speculated that mental illness in fact is responsible for the second highest percentage of DALYs rather than fifth, as was reported in 2013. 2
With a large proportion of the population with one or multiple psychiatric disorders, there is a great need for new treatment options that are more effective and tolerable. For instance, 50% to 60% of patients with depression do not respond to their first course of treatment. 3 Additionally, the adverse events experienced or long duration of time that it takes for patients to experience the benefits of therapies also hinders individuals from taking advantage of current treatment options. 4 Thus, it is becoming increasingly apparent that novel effective and tolerable therapeutic strategies are needed.
A stellate ganglion block (SGB) is a procedure involving injection of a local anesthetic surrounding the stellate ganglion, a sympathetic ganglion in the neck, to inhibit sympathetic outflow to the ipsilateral portion of the head, neck, thorax, and upper extremities. 5 The SGB is used as both a diagnostic and therapeutic technique for several sympathetically mediated conditions. Diagnostically, SGBs can be used to confirm the presence of sympathetically mediated pain. 6 SGBs are also currently used in the treatment of several conditions, including complex regional pain syndrome, phantom limb pain, peripheral vascular disease, Raynaud disease, and intractable angina. 6 More recently, research has begun examining the potential benefits of SGBs for psychiatric disorders, mainly posttraumatic stress disorder (PTSD).7,8
The first reported use of SGB for psychiatric disorders occurred in 1947 with the treatment of depression. 9 The authors noted that SGB produced improved mood, and occasionally a euphoric feeling, in these patients. 9 The first reported use for PTSD occurred in a 1990 case study. 10 This study reported on the use of SGB for a 15-year-old female with reflex sympathetic dystrophy and PTSD. A series of 13 SGBs resulted in reduced pain and markedly improved PTSD symptoms. 10 These early studies were pivotal, as they paved the way for later research on SGB for psychiatric disorders.
A 2017 review provided a breakdown of literature on SGB for PTSD from 1947 to 2016. 4 This review included case studies and case reports, systematic reviews, randomized controlled trials (RCTs), and all other relevant research including but not limited to books. The conclusion was that while more research is needed, SGB has a significant potential benefit in PTSD, particularly for individuals who have not responded to conventional treatment options. Additionally, another prior review explored the utility of SGB for PTSD, depression, and anxiety. 7 This review included relevant articles published from 2016 onwards, including 1 systematic review, 8 1 RCT, 11 and 1 evidence-based guideline. 12 Ultimately, this review was only able to comment on findings about PTSD, as no relevant evidence was identified for the use of SGB for anxiety or depression. It was concluded that evidence regarding the clinical effectiveness of SGB for PTSD treatment was mixed and that there were no significant differences in the rate of adverse effects between treatment and sham groups. Additionally, the Veterans Affairs and Department of Defense Clinical Practice Guideline for treatment of PTSD was unable to come to any recommendation for or against SGB due to lack of evidence. 12
Historically, systematic reviews have focused on published studies. Synthesizing the results of these studies can help us understand the findings of existing research in the field. However, when exploring new treatments or the use of existing treatments for novel purposes, as is the case of SGB for psychiatric disorders, only exploring published studies does not give a complete up-to-date picture as to what research is being conducted. Reviewing registered clinical trials helps to provide a more fulsome picture of the current study designs and treatment protocols that are being utilized globally. Thus, including both published and unpublished studies provides the most well-rounded possible view of the current research landscape.
The purpose of this systematic review was to summarize the existing body of literature on the use of SGB for psychiatric disorders, including both published studies and registered clinical trials, in order to understand both the procedures followed and existing evidence regarding the safety, feasibility, and effectiveness of this novel treatment intervention. To our knowledge, this is the first review to explore the use of SGB for a wide array of psychiatric disorders utilizing the evidence from both published studies and registered clinical trials.
Methods
Search Methods
Published Studies
A comprehensive search of 3 Ovid databases (Medical Literature Analysis and Retrieval System Online, PsycINFO, and Embase) was conducted using the following search terms: stellate ganglion block, SGB, autonomic nerve block, post traumatic stress disorder, PTSD, depress*, MDD, depressive disorder, anxiety, mental disorder*, psychiatric disorder*, mood disorder*, psychiatric condition*, psychological disorder*, and psychotic disorder*. The search was limited to only include articles available in English and studies with human participants. Additional articles were identified by searching the references of extracted articles.
Registered Clinical Trials
Two public clinical trial registries, the ClinicalTrials.gov (https://www.clinicaltrials.gov/) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; https://www.who.int/ictrp/en/), were also searched for relevant ongoing clinical trials using the aforementioned search terms.
All searches were conducted between June 2, 2021, and June 9, 2021. Two authors (JK and HL) independently screened the articles and assessed whether they met the eligibility criteria. Discrepancies were discussed and resolved by consensus (ID and VB). The full search strategies are provided in Supplement 1 of the Supplemental Material.
Eligibility Criteria
All search results were screened and assessed for eligibility. Primary research articles, including RCTs, open-label trials, surveys, and case studies, were included. Both published studies and registered clinical trials were accepted, and the inclusion criteria were the same for both the published and registered trial searches. Articles were included in the systematic review if they explored the effects of SGB in adult human participants (age 18 or older) with any psychiatric disorder. All animal studies were excluded. Only articles available in English were accepted, and there was no restriction on the year that the article was published.
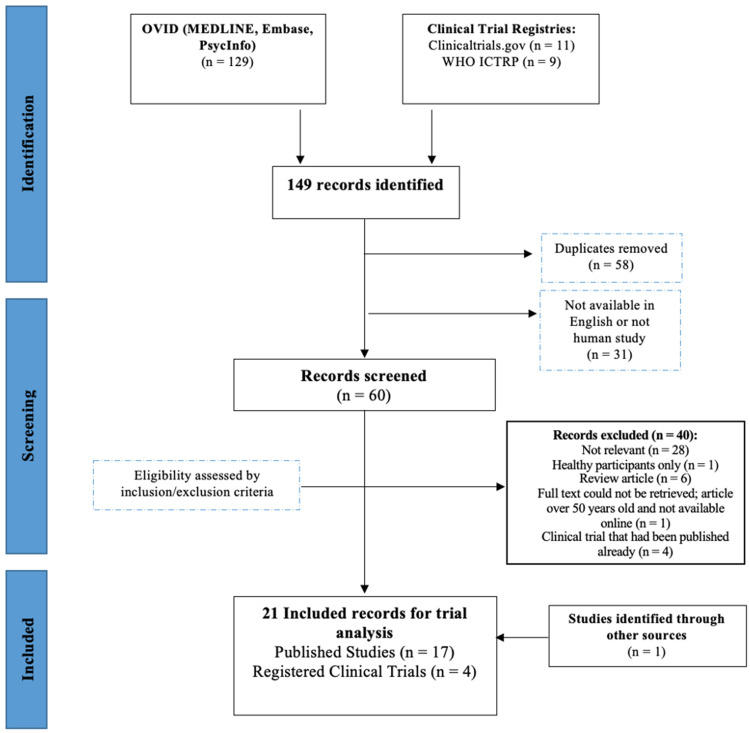
Published studies and registered trials were excluded if they enrolled or targeted to enroll healthy controls only or had any participants below the age of 18. Published studies were also excluded if they were narrative or systematic review articles, and registered studies were excluded and instead included as published studies if they had already been completed and published. Figure 1 displays the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart outlining the screening process.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
Variable Extraction
Published Studies
From each article, preliminary information about the country of origin, principal investigator(s), publication year, and primary sponsor(s) was extracted. Demographic data of study participants were also collected, including primary and secondary diagnoses (if applicable), age, and inclusion sex/gender.
Additionally, the following variables of interest were extracted: sample size, study design (ie, randomized vs. nonrandomized), presence of masking (ie, yes vs. no), use of additional treatments (eg, psychoeducation), comparison type (eg, active treatment vs. sham saline injection), interventional model (ie, parallel or single-group assignment), treatment duration (ie, number of sessions), follow-up (ie, yes vs. no and interval between follow-ups when applicable), study outcomes (eg, treatment effectiveness, safety/feasibility), anesthetic used (eg, ropivacaine 0.5%), volume injected (eg, 7 mL), laterality (ie, left, right, or bilateral), study results, adverse effects, and dropout rates.
Registered Clinical Trials
The same variables were extracted as from the published studies. Additionally, trial status, registration and projected completion dates, and minimum and maximum inclusion ages were noted.
All data extraction was conducted by 2 independent reviewers (JK and HL), and discrepancies were resolved by consensus. When necessary, a third party (ID and VB) resolved discrepancies.
Quality of Assessment
Published Studies
All published studies were assessed for quality using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) checklist. 13 Supplement 2 of the Supplemental Material summarizes the included studies in terms of the GRADE criteria. Two studies were RCTs,11,14 and thus had a low risk of selection, performance, and detection bias according to GRADE criteria. However, the use of saline in the sham condition may contribute to performance bias despite the blinding of participants. Individuals may have inferred that they were assigned to the sham condition due to the lack of Horner syndrome (symptoms such as miosis, ptosis, and scleral injection due to interruption of a sympathetic pathway), 11 and may have responded differently to outcome scales as a result. 7 Three studies were open-label trials,15–17 and are at higher risk of performance bias due to the lack of blinding, and selection bias, and detection bias cannot be adequately reported. However, all participants received the same treatments, likely minimizing selection bias. Selection, performance, and detection bias are not applicable to case studies and surveys. One study included less than 80% of participants in the analysis, 18 thus increasing risk of reporting bias. Four studies have a moderate to high risk of selective reporting,19–22 as variables were not reported consistently. All clinical trials ended as scheduled.
Results
In total, 149 records were identified using 3 databases and 2 clinical trials registries. After duplicates and articles not available in English or not studying human participants were removed automatically through Ovid, the remaining 60 articles were screened according to the inclusion and exclusion criteria. One additional article was included by examining the reference sections of eligible articles. Ultimately, 21 studies (17 published articles and 4 registered studies) met the aforementioned eligibility criteria and were included in the review. The first study exploring the use of SGB in psychiatric patients was not included as the full text could not be retrieved. 9 Additionally, the first study exploring the use of SGB in patients with PTSD was not included as it featured a pediatric patient. 10 However, the importance of these 2 articles to the field should not go unnoted.
Studies by Research Design
Published Studies
Among published studies, 5 clinical trials,11,14–17 1 survey, 23 and 11 case studies and case series,18–22,24–29 were included. Two of the 5 clinical trials were RCTs,11,14 whereas the other 3 followed an open-label design and were nonrandomized.15–17 Both published RCTs utilized masking and followed a parallel assignment interventional model, while all of the open-label trials followed a single-group interventional model. One open-label trial utilized pulse radiofrequency as an adjunctive treatment to SGB. 24
Registered Clinical Trials
Four registered clinical trials were included. Three of the 4 were RCTs,30–32 and 1 followed an open-label design. 33 Two of the 3 RCTs utilized masking,30,31 with 1 trial using double-blind masking, 31 and the other using quadruple-blind masking. 30 All RCTs followed a parallel assignment model, whereas the open-label trial utilized a single-group interventional model. Three of the 4 trials utilized additional treatments alongside SGB, including Massed Prolonged Exposure, 33 psychoeducation, 30 and dialectical behavior therapy. 32
Studies by Start Date and Status of Completion
Published Studies
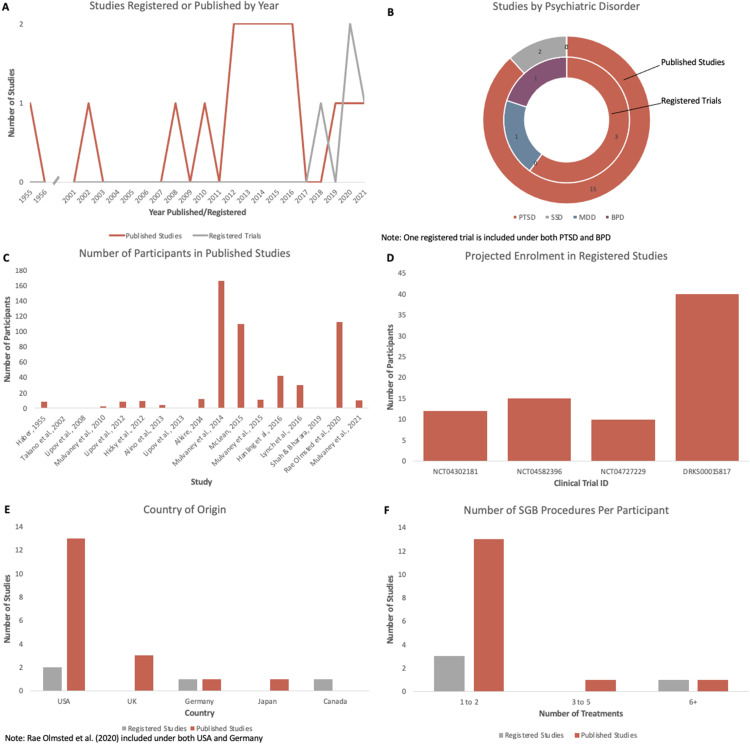
Aside from 1 study published in 1955, 19 16 of 17 articles were published from 2002 onwards. One RCT was published in 2016, 14 and the other was published in 2020. 11 The nonrandomized open-label trials were published in 2014, 15 2016, 16 and 2021. 17 One case study, series, or survey was published annually in each of 1955, 19 2002, 21 2008, 24 2010, 29 2014, 18 and 2019, 25 and 2 were published annually in each of 2012,26,28 2013,20,22 and 201523,27 (Figure 2a).
Figure 2.
(A) Number of studies published or registered per year. Studies were registered between 2018 and 2021. Studies were published between 1955 and 2021, with 15 of 17 published from 2008 onwards. (B) Studies by psychiatric disorder. Fifteen of 17 published studies looked at PTSD, while 2 looked at SSDs. Registered trials have begun to explore PTSD, BPD, and MDD. (C) Number of participants per published study. Ten of 17 studies had 1 to 10 participants. (D) Projected total enrolment (all treatment arms) in registered trials. Enrollment for all studies is between 10 to 40 participants. (E) Country of origin for published and registered studies. Sixteen of 17 published studies and 2 of 4 registered trials are based in the United States. (F) Number of SGB procedures administered per participant in published studies and registered trials. One to 2 administrations is the most common procedure for both published and registered trials.
Abbreviations: BPD, borderline personality disorder; MDD, major depressive disorder; SGB, stellate ganglion block; SSD, schizophrenia spectrum disorder; PTSD, posttraumatic stress disorder.
Registered Clinical Trials
Four registered trials were included in the review. The included trials were either in the currently recruiting stage (50%),30,33 or were not yet recruiting (50%).31,32 One clinical trial was registered in 2018, 32 2 were registered in 2020,30,33 and 1 was registered in 2021 31 (Figure 2a).
Studies by Clinical Disorder
Published Studies
The published studies explored SGB for 2 types of psychiatric disorders. Both RCTs and all 3 nonrandomized trials examined SGB in patients with PTSD.11,14–17 Ten of the case studies, series, and surveys reported on the use of SGB for PTSD,18,20,22–29 whereas 2 reported on the use of SGB in individuals with schizophrenia spectrum disorders (SSDs)19,21 (Figure 2b).
Registered Clinical Trials
The registered trials explored the use of SGB for patients with PTSD, borderline personality disorder (BPD), and major depressive disorder (MDD). The 3 RCTs were examining the use of SGB for PTSD (exclusively among cardiac arrest survivors), 30 MDD, 31 and comorbid BPD and PTSD. 32 The nonrandomized open-label trial explored the use of SGB in PTSD 33 (Figure 2b).
Studies by Participant Characteristics
Published Studies
The mean age of participants in 1 RCT was 37.3 years old, 11 while the other RCT did not specify but had an inclusion age of 18 years or older. 14 Both RCTs included all sexes/genders, but had 88.5% and 81% male participants, respectively.11,14 The sample sizes in the RCTs were N = 113 and N = 42.11,14
Among the nonrandomized open-label trials that specified age, the mean age of participants were 36 and 53 years old.16,17 One trial had only male participants, 16 another had 60% female participants, 17 and the third did not specify. 15 One study had 1 to 10 participants, 17 while the others had 11 to 50.15,16
The mean age of participants in case studies, series, and surveys ranged from 36 to 67.8 years old, and most were male. One study had only 1 female participant, 25 2 had both sexes but primary males,26,27 6 had only males,19–22,24,28,29 and 3 did not report on sex breakdown.18,23,28 The number of participants ranged from N = 1 to 166. Nine of the 12 had 1 to 10 participants,19–22,24–26,28,29 1 had 11 to 50 participants, 27 and 2 had 101 or more participants18,23 (Figure 2c).
Registered Clinical Trials
All registered trials had a minimum inclusion age of 18. The maximum inclusion ages were 50, 32 65,31,33 and 85. 30 All trials were recruiting individuals of all sexes and genders. The estimated total enrolments for the RCTs were N = 15, 30 10, 31 and 40 participants, 32 and for the open-label trial was N = 12 33 (Figure 2d). The nonrandomized open-label trial was accepting healthy volunteers, 33 although the proportion of total enrolment was not specified and a separate treatment arm was not designated for these individuals.
Studies by Country of Origin
Published Studies
Both RCTs,11,14 and all 3 open-label trials,15–17 were conducted in the United States. One RCT, 11 while regulated in the United States, took place in both the United States and Germany. All case studies, series, and surveys took place in the United States, except for one, 21 which took place in Japan (Figure 2e).
Registered Clinical Trials
Two ongoing studies were taking place in the United States,30,33 with 1 RCT occurring in Canada, 31 and 1 in Germany 32 (Figure 2e).
Studies by Treatment Parameters
Published Studies
Both RCTs used 1 to 2 right-sided SGBs with 0.5% ropivacaine.11,14 One RCT used 5 mL of anesthetic and a fluoroscopic imaging approach, 14 whereas the other used 7 to 10 mL and an ultrasound-guided approach. 11 The RCT reported on time between SGBs gave treatments 2 weeks apart. 11
The 3 non-RCTs all used 1 to 2 SGBs.15–17 Right-sided laterality was more common (66.7%),15,16 as was 0.5% ropivacaine (66.7%).16,17 Seven and eight milliliter were the 2 volumes of anesthetic used, and an ultrasound-guided imaging approach was used in both of the studies that reported on this.16,17
Among case studies, case series, and surveys, 1 to 2 administrations was also the most common (80%),18,20,22,24,26–29 followed by 3 to 5 (10%), 25 and 6 or more (10%). 19 The most commonly used anesthetic was 0.5% ropivacaine (50%),18,20,23,28,29 followed by 0.5% bupivacaine (30%),22,24,26 and one study with each of 1% procaine (10%), 19 and an unreported concentration of lidocaine (10%). 25 The volume of anesthetic injected ranged from 3 to 12 mL, with the most common being 7 mL (50%).20,24,26,28,29 Most SGBs used right-sided laterality (81.8%),18,20,22–27,29 and a fluoroscopic imaging technique (77.8%).20,22,23,25,26,28,29 Among studies with multiple SGBs, the interval between treatments varied from 3 to 4 times weekly to 7 months apart. Two studies administered SGBs 1 week apart or less,19,21 2 studies administered SGBs 1 to 2 months apart,25,28 and 2 studies administered SGBs 3 or more months apart.22,29
Registered Clinical Trials
Three registered trials,30,31,33 including 2 RCTs and 1 nonrandomized trial reported a 1-time administration of SGB, whereas 1 RCT reported the use of 8 SGB procedures twice weekly 32 (Figure 2f). The most commonly used anesthetic was 0.5% ropivacaine which was used in 1 RCT and 1 nonrandomized trial,30,33 followed by 1 RCT each using 1% ropivacaine, 32 and 0.5% bupivacaine. 31 The volume of an anesthetic injected ranged from 3 to 8 mL. Only one of the ongoing studies provided information on the laterality and technique and is performing ultrasound-guided bilateral SGBs. 32
Table 1 outlines the breakdown of anesthetics used, concentration, and volume.
Table 1.
Breakdown of Anesthetics Used in Stellate Ganglion Block Studies for Psychiatric Disorders.
| Anesthetic Used | Concentration | Volume (mL) | No. of Studies | % of Studies |
|---|---|---|---|---|
| Lidocaine | Not specified | 3 | 1 | 5.26 |
| Ropivacaine | 0.50% | 5 | 2 | 10.53 |
| 6 | 1 | 5.26 | ||
| 6.5 | 1 | 5.26 | ||
| 7 | 4 | 21.05 | ||
| 7 to 8 | 1 | 5.26 | ||
| 7 to 10 | 1 | 5.26 | ||
| 8 | 1 | 5.26 | ||
| 1% | 3 | 1 | 5.26 | |
| Procaine | 1% | 12 | 1 | 5.26 |
| Bupivacaine | 0.50% | 6.5 | 1 | 5.26 |
| 7 | 2 | 10.53 | ||
| Not specified | 1 | 5.26 | ||
| 0.25% | Not specified | 1 | 5.26 | |
| Total | 19 | 100 |
Studies by Outcome Measures
Published Studies
Both RCTs reported change in Clinically Administered PTSD Scale (CAPS) score as a primary outcome measure.11,14 Secondary outcomes for both included change in PTSD checklist (PCL) score, as well as safety/feasibility (ie, adverse effects). CAPS is a validated and extensively used structured interview to determine PTSD diagnosis and symptom severity, 34 and PCL is a commonly used self-report measure to determine PTSD symptom severity, and thus infer diagnostic status. 35
Among the nonrandomized open-label trials,15–17 change in PTSD symptomatology was also a primary outcome. This was measured using CAPS in 1 study, 15 and PCL in the other 2.16,17 Safety/feasibility was not an outcome for these studies.
Of the case studies, case series, and surveys, improvement of PTSD symptomatology was the most common outcome reported (75%).18,20,22,24–29 This was most commonly measured using PCL score (77.8%).18,20,22,25–27,29 Safety and tolerability of SGB was the primary outcome in 1 study. 23 The case study in which the patient had schizophrenia used the Brief Psychiatric Rating Scale (BPRS) score to measure symptom improvement, 21 and the series in which patients had psychosis used symptoms of mental deterioration to measure symptom improvement. 19
Aside from 1 study that reported on electrophysiology (ie, electromyogram) and biological measures (ie, respiratory sinus arrhythmia and heart rate variability), 15 no neuroimaging, electrophysiology, laboratory tests, or biological measures were used as outcome measures. Most available literature was focused on clinical measures.
Registered Clinical Trials
Of the 4 registered trials, all reported treatment effectiveness
and 2 reported safety/feasibility as a primary or secondary outcome measure.30,31 All of the 3 trials that enrolled patients with PTSD used PCL score,30,32,33 and one also used CAPS score to measure SGB's effect. 33 Additionally, the RCT on MDD used the Montgomery-Åsberg Depression Rating Scale score to measure effectiveness, 31 and the trial on PTSD alongside BPD used a variety of scales to measure dissociative symptoms, 32 including Dissociations-Tension-Scale-4, Clinical Global Impression Scale, Beck Depression Inventory, Borderline Symptom List-23, State-Trait Anxiety Inventory, Fragebogen für dissoziative Symptome (the German version of the Dissociative Experiences Scale), and Symptom Checklist 90-R. Additionally, 2 of the 4 trials used biological measures (eg, wound healing) as an outcome.30,32
Review of Published Study Results
Published Studies
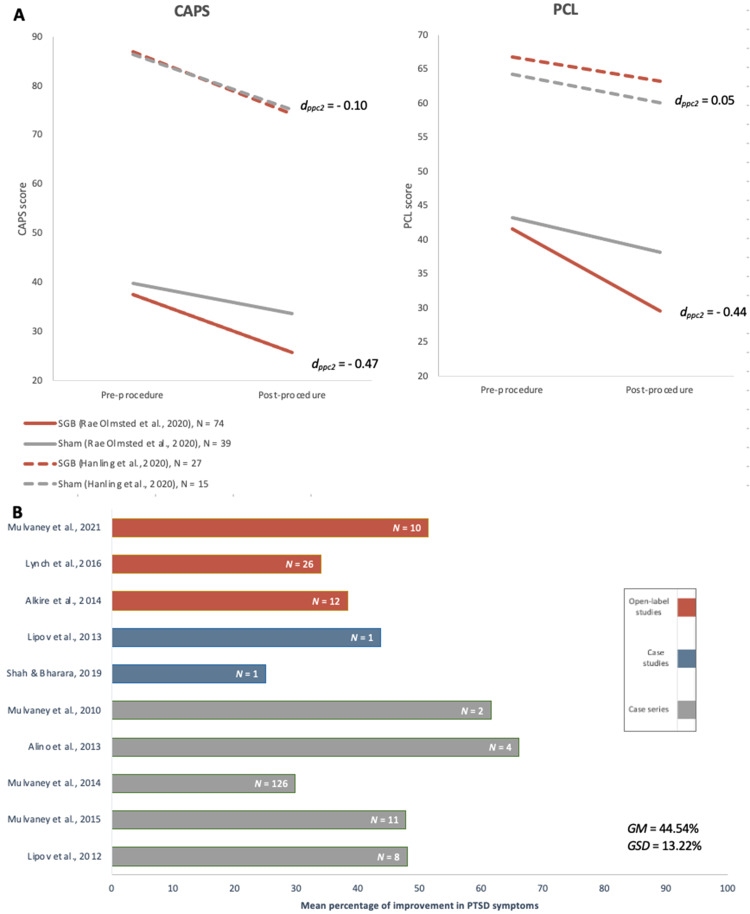
One of the 2 RCTs found significantly greater improvement in CAPS-5 symptom severity score and PCL scores in the experimental group at 8 weeks from baseline compared to the sham group (Figure 3a). 11 The authors did not define the clinical significance of these improvements. However, the other RCT found no clinically significant improvement in PCL score (ie, ≥10-point decrease and ≥30% improvement) or CAPS-4 score (ie, ≥15-point decrease and ≥30% improvement), and no significant difference between treatment and sham groups at 1 week or 1 month after the first SGB, or at 1 month after the second SGB. 14 At 1 week after the second SGB, the CAPS-4 score had ≥15-point decrease but did not have ≥30% improvement or a significant difference from the sham group.
Figure 3.
(A) mean change in CAPS (top left) and PCL (top right) scales for PTSD symptoms from baseline to the primary endpoint after 1 round of stellate ganglion block treatment reported by randomized-controlled trials. Effect size (dppc2) for mean differences of groups with unequal sample sizes within a pre-post-control design were calculated according to the Morris (2008) method. 36 Note: Posttreatment primary endpoint varied among studies. (B) Mean percentage of improvement in PTSD symptoms from baseline to the primary endpoint after 1 round of stellate ganglion block treatment reported by case studies, case series, and open-label studies.
Abbreviations: CAPS, Clinician-Administered Posttraumatic Stress Disorder Scale; GSD, Grand standard deviation estimate; GM, Grand mean estimate; PCL, posttraumatic stress disorder checklist; PTSD, posttraumatic stress disorder.
All non-RCTs, case studies, and case series on PTSD reported substantial improvements in PTSD symptomatology from baseline (Figure 3b). A breakdown of main findings from all published studies is outlined in Table 2. The RCTs did not measure when the response was first noted, but one case series reports PTSD symptom improvement as soon as 5 minutes post SGB. 29 In studies that reported the length of response after 1 single SGB treatment and had follow-up times of 1 month or greater,15,16,18–20,22,24,25,28,29 all studies had a sustained response at 1 month, and response in some cases was sustained for 6 months or longer after the initial procedure.18,19,29
Table 2.
Summary of Published Studies Exploring Stellate Ganglion Block for Psychiatric Disorders.
| Shah & Bharara, 201925 | McLean, 201523 | Alkire et al., 201415 | |
|---|---|---|---|
| Participants | 1 | 110 | 12 |
| Mean Age | 46 | Not reported | Not reported |
| Sex | Female | Not reported | Not reported |
| Psychiatric Disorder(s) | PTSD | PTSD | PTSD |
| Study Type & Design | Case study, open label design | Survey | Clinical trial, open label design, single-group assignment |
| SGB Procedure | 3 treatments, 2 months apart 3 mL lidocaine Right sided Fluoroscopic imaging |
Varying number of treatments 5 mL ropivacaine 0.5% Right sided Fluoroscopic imaging |
1 treatment Unreported dosage of bupivacaine 0.25% Right sided Imaging technique not reported |
| OUTCOMES | PTSD symptom improvement (PCL) | Safety and patient acceptability | PTSD symptom improvement (CAPS) Psychophysiological measures |
| Findings |
|
|
|
| Adverse Events | Not reported | None | Not reported |
Abbreviations: CAPS, Clinically Administered Posttraumatic Stress Disorder Scale; EMG, electromyography; PCL, posttraumatic stress disorder checklist; PCL-M, posttraumatic stress disorder checklist - military version; PTSD, posttraumatic stress disorder; RAVLT, Rey Auditory Verbal Learning Test; RCT, randomized clinical trial; SGB, stellate ganglion block.
One study examining the safety/feasibility determined that 100% of participants were satisfied with the procedure, and 95% would undergo repeat procedures due to the tolerability of side effects. 23
The case series examining patients with psychosis determined that patients in early stages showed a positive response, whereas those in advanced stages showed little improvement or rapid relapse of symptoms. 19 In the case study examining schizophrenia, the patient's reported final BPRS score was 19, indicating improvement in symptoms of schizophrenia. 21
Adverse Effects and Dropout Rates
Published Studies
Of the 2 RCTs, the completion rates were 95.6% and 42.9%, respectively.11,14 However, although only 42.9% of participants completed all follow-ups, 14 100% were used in the analysis. The nonrandomized open-label trials had completion rates of 86.7% and 90.9%.16,17 All case studies and case series except for one with a 54.8% completion rate, 18 reported no dropouts.
All adverse effects reported were minor and temporary. One RCT deemed 10 adverse effects as likely or possibly related to the study, with no significant difference between SGB and sham groups. 14 The other RCT reported 6 adverse effects, 11 with 3 being possibly or likely related to treatment. No statistical analysis was reported for this RCT. Some listed adverse effects included headache and soreness, stiffness, redness, or pain at the injection site.
A breakdown of study attributes for registered trials is outlined in Table 3.
Table 3.
Summary of Registered Clinical Trials Exploring Stellate Ganglion Block for Psychiatric Disorders.
| NCT04302181 33 | NCT04582396 30 | NCT04727229 31 | DRKS00015817 32 | |
|---|---|---|---|---|
| Number of participants (projected) | 12 | 15 | 10 | 40 |
| Inclusion age | 18 to 65 | 18 to 85 | 18 to 65 | 18 to 50 |
| Inclusion sex/gender | All | All | All | All |
| Psychiatric disorder(s) | PTSD | PTSD (following cardiac arrest) | MDD | PTSD, BPD |
| Study type and design | Clinical trial, open-label design, single-group assignment | RCT, quadruple masking, parallel assignment | RCT, double masking, parallel assignment | RCT, no masking, parallel assignment |
| Comparison type | None | Experimental (SGB and psychoeducation) versus placebo (saline injection and psychoeducation) | Experimental (SGB) versus placebo injection (saline) | Experimental (SGB and dialectical behavior therapy) versus control (dialectical behavior therapy only) |
| SGB procedure | 1 treatment 6.5 mL ropivacaine 0.5% Laterality not reported Imaging technique not reported |
1 treatment 7 to 8 mL ropivacaine 0.5% Laterality not reported Imaging technique not reported |
1 treatment Unreported dose of bupivacaine 0.5% Laterality not reported Imaging technique not reported |
8 treatments; 2 per wk 3 mL ropivacaine 1% Bilateral Ultrasound guided |
| Additional treatments | Massed PE for 10 90-min sessions along with out of session treatment assignments for the majority of the day | Psychoeducation | None | Dialectical behavior therapy |
| Outcomes | Treatment efficacy (CAPS, PCL, PHQ-9, Brief Inventory of Psychosocial Functioning, GAD-7, PTCI) | Feasibility Treatment efficacy (PCL) Proportion of patients with clinically significant symptoms of cardiac anxiety, GAD and depression, moderate-high levels of physical activity, reduced sleep duration |
Feasibility, Recruitment, Acceptability, Safety Treatment efficacy (MADRS) |
Treatment efficacy (DSS-4, CGI, BDI, BSL-23, STAI-S, PCL-5, FDS, SCL-90-R, POSAS) |
Abbreviations: BPD, borderline personality disorder; BDI, Beck Depression Inventory; BSL, Borderline Symptom List; CAPS, Clinically Administered Posttraumatic Stress Disorder Scale; CGI, Clinical Global Impression Scale; DRKS, Deutsches Register Klinischer Studien (German Clinical Trials Register); FDS, Fragebogen fur dissoziative Symptome (questionnaire for dissociative symptoms); GAD, Generalized Anxiety Disorder; MADRS, Montgomery-Asberg Depression Rating Scale; MDD, major depressive disorder; NCT, National Clinical Trial number; PCL, posttraumatic stress disorder checklist; PE, prolonged exposure; PHQ-9, Patient Health Questionnaire - 9; POSAS, Patient and Observer Scar Assessment Scale; PTCI, Posttraumatic Cognitions Inventory; PTSD, posttraumatic stress disorder; RCT, randomized clinical trial; SCL-90-R, Symptom Checklist 90-R; SGB, stellate ganglion block; STAI-S, State-Trait-Anxiety Inventory-S;
Discussion
Published Studies
This systematic review examined the use of SGB for the treatment of psychiatric disorders. Five published clinical trials for PTSD were included (3 open label and 2 RCTs), while the rest were case studies, series, and surveys. The comparison types differed among these studies (ie, no comparison in 2, SGB vs sham in 2, right-sided SGB vs left-sided SGB in 1), as well as the number of SGBs performed and the intervals between the last treatment and the follow-up visits. Thus, due to large methodological variation, these studies were deemed not suitable for a meta-analysis. For the use of SGB for other psychiatric disorders, no clinical trials have been published, so meta-analysis could not be done.
Synthesizing the data from included studies indicated that the most common treatment parameters involved administration of 1 to 2 SGB treatments with right-sided laterality, using 0.5% ropivacaine in a volume of 7 mL. Most studies, including both RCTs, utilized right-sided laterality. This may be due to the right hemisphere of the brain having a more significant involvement in the human stress response. 37 Alternatively, the right side of the body may have a higher incidence of sympathetic predominance, while the left side—a higher incidence of parasympathetic predominance, suggesting that the blockade of the right stellate ganglion might exert more rapid and profound functional efforts. 38 Interestingly, 1 clinical trial used left-sided SGB in patients for whom right-sided SGB failed, and this was found to result in clinically significant improvement. 17 The authors suggested that individuals who responded to a left- but not right-sided SGB had anatomical differences requiring contralateral rather than ipsilateral intervention. 17 However, the exact reason for this is unknown. Additionally, most studies used 7 mL of anesthetic. This concentration is deemed acceptable since imaging techniques employed in those studies allow better visualization and more precise targeting of the stellate ganglion, thus permitting lower volumes of local anesthetic to be used compared to historically done field blocks. 39 Feigl et al. 40 determined that 5 mL should be sufficient to cause an ipsilateral SGB, and that doses of 10 to 20 mL or higher may cause unintended consequences such as hoarseness or contralateral Horner syndrome. Most studies have followed this best practice by remaining within the 5 to 10 mL range. Finally, ropivacaine was likely the most commonly used anesthetic due to its properties as a long-acting local anesthetic. 41 More research needs to be done to determine if total dosage, volume, or choice of local anesthetic has an impact on the effectiveness and tolerability of the procedure.
Additionally, it was determined that 1 single SGB procedure has effects that can appear within 5 minutes and last a minimum of 1 month. However, the 1 RCT that found significant results did not report on when the effects first appeared nor how long they lasted, as improvement was only measured at baseline and at 8 weeks post SGB. 11 Thus, case studies were used to infer this information, and more RCTs need to be done to determine the exact timeline of the treatment.
One of the 2 included RCTs on SGB for PTSD found statistically significant improvement in CAPS score following administration of 2 SGBs. 11 Nevertheless, the other RCT reported no significant effect after 1 or 2 administrations of SGB. 14 There are several differences in study design that could potentially account for these inconsistent findings. For instance, a nonexhaustive list of differences includes the sample sizes (N = 113 11 vs. 42, 14 ) follow-up time (8 11 vs 1 week and 1 month, 14 ) anesthetic volume (7-10 11 vs 5 mL, 14 ) and imaging techniques utilized (ultrasound-guided 11 vs fluoroscopic. 14 ) Additionally, the participant population in Hanling et al 14 included patients who were undergoing medical disability evaluation boards. This is potentially confounding, as participants may have been inclined to downplay their symptom improvement in order to obtain or retain benefits. Prior systematic reviews have also noted these mixed results between RCTs,7,8 further suggesting that more research must be done.
It has been proposed that the reason for SGB's benefit in PTSD is that SGB decreases nerve growth factor levels, thus reducing norepinephrine and the hyper-aroused state of the sympathetic nervous system present in PTSD. 5 While this hypothesis is one of several potential mechanisms, all follow the common theme of SGB inhibiting sympathetic nervous system activity and thus the sympathetic chronic stress response present in many psychiatric disorders.
To date, no RCTs have been conducted exploring the use of SGB for SSDs. However, case studies have begun to explore the potential utility of SGB for these psychiatric disorders. One included case series explored the use of several bilateral SGBs on patients with psychosis with cerebral arteriosclerosis or senile psychosis. 19 While the results collected were mainly qualitative and did not include any quantitative assessments, the tentative conclusion was that SGB may be beneficial in reducing mental deterioration in patients with early stages of psychosis. It is important to note that simultaneous bilateral SGBs in the absence of intubation and mechanical ventilation is not recommended and can be life-threatening, as this can lead to paralysis due to phrenic or recurrent laryngeal nerve blockade. 42 Another study 21 reported on a patient with schizophrenia who had an SGB for neck-shoulder pain. They observed a drastic decline in schizophrenia symptoms. However, given that this was a case study with only one participant, this result may not be replicable. Schizophrenia and other psychotic disorders are postulated to follow a similar model to PTSD, involving dysfunction of the autonomic nervous system and sympathetic dominance. 43 This is speculated to be due to reduced vagal modulation, and, hence, increased sympathetic activity and elevated cortisol levels due to lack of normal inhibition from the parasympathetic nervous system. 44 Such a mechanism may plausibly underlie the observed benefit of SGB for these psychiatric disorders.
Recently, development of new treatments for psychiatric disorders has emphasized improvement in specific symptom clusters often in a trans-diagnostic manner, rather than solely by the diagnosed disorder. 45 SGB reinforces this, as certain symptoms of PTSD show more improvement than others following the procedure. Specifically, SGB has been shown to primarily decrease hyperarousal and anxiety symptoms.11,15,16,20,24,26,29 Additionally, several studies report improvement in avoidance, pain and physical functioning, memory, and sleep.11,16,22,24,27,29 This targeting of specific symptoms may indicate the use of SGB for these symptom clusters across several psychiatric disorders.
McLean (2015) conducted a survey on the safety and tolerability of SGB in individuals with PTSD. 23 They found that 100% of individuals were satisfied with the process and procedure, 100% would recommend it to a friend, and 95% were willing to undergo repeat procedure due to tolerability of side effects. This is consistent with the included RCTs and other studies indicating that the adverse effects associated with SGB are minimal, and all are minor and temporary.11,14,17,18,28 No significant difference was found between rate of adverse effects in treatment and control groups, and all adverse effects were minor and self-resolving.11,14 Interestingly, the attitudes reflected in the aforementioned survey results parallel those of behavioral health clinicians. Of those surveyed, 100% of clinicians characterized SGB as “Very Beneficial” or “Somewhat Beneficial,” and 95% would recommend SGB to a colleague. 46 Thus, both patients and clinicians report generally positive experiences with SGB.
Registered Clinical Trials
The 4 registered trials provide important information about the changing landscape of the research on SGB for psychiatric disorders and address some of the current shortcomings. More RCTs are needed on this topic, and these studies begin to address this need. The trials also add to the existing literature on the study design, allowing a more fulsome picture to be formed on how SGBs should be conducted. Additionally, while 3 of the registered trials are examining patients with PTSD, new psychiatric disorders such as MDD and BPD are also being explored. This is important, since expanding the knowledge base on SGB can help us determine for which disorders SGB may be used in the clinical setting as a regular treatment regimen.
Based on the hypothesized mechanism of SGB as a down-regulator of sympathetic nervous system activity, it has the potential to show promise in the ongoing trials exploring SGB for BPD and MDD. It has been found that individuals with BPD have a dysregulated sympathetic stress response, involving increased sympathetic tone and decreased parasympathetic tone. 47 This results in elevated hypothalamic-pituitary axis activity and cortisol levels, leading to a stress response that is associated with emotional reactivity in BPD. 48 Likewise, MDD is associated with chronic stress, resulting in continuous activation of the sympathetic nervous system. 49 Thus, for similar reasons that SGB appears to work for PTSD and psychotic disorders, it may prove beneficial in these disorders through its ability to attenuate sympathetic outflow.
Strengths and Limitations
One strength of this review is that it is examined both unpublished and published literature. This gives a more well-rounded picture of the landscape of research on the use of SGB for psychiatric disorders. Additionally, 3 databases and 2 clinical trial registries were searched to attempt to explore as much of the existing research as possible. However, one limitation is that much of the research done so far, and thus much of the research included in the review, is in the form of case studies and case series. This limits the usefulness of the research, as not following a strict model as is done in clinical trials limits the data synthesis and potential points of comparison. Finally, the 2 published RCTs that were included in the review noted that the treating physicians could not be blinded. While their interaction with patients was limited, this introduces potential bias in establishing SGB's effectiveness and interpreting the results.
Future Directions and Conclusion
More RCTs with larger sample sizes and more diverse patient populations (including nonmilitary populations) are needed. More studies need be done for PTSD, since the few RCTs have inconsistent results, and more studies need be done for other psychiatric disorders to determine the potential breadth of SGB's effectiveness. Future RCTs should have extended follow-up times; the length of effect from one single procedure is not yet known, so this should be determined to inform best practices. Finally, research should aim to determine the exact mechanism of action of this procedure in specific psychiatric disorders in order to optimize the treatment.
Overall, preliminary evidence indicates that SGB is safe and feasible for patients with PTSD, although the evidence from RCTs is mixed. As the first review exploring the utility of SGB in all psychiatric disorders, this systematic review both outlines the results that SGB has had thus far, as well as informs future research in the field to have the potential to incorporate SGB as a mainstream clinical treatment for a variety of psychiatric disorders.
Supplemental Material
Supplemental material, sj-docx-1-css-10.1177_24705470211055176 for Stellate Ganglion Block for Psychiatric Disorders: A Systematic Review of the Clinical Research Landscape by Jaimie Kerzner, Helen Liu, Ilya Demchenko, David Sussman, Duminda N. Wijeysundera, Sidney H. Kennedy, Karim S. Ladha and Venkat Bhat in Chronic Stress
Supplemental material, sj-docx-2-css-10.1177_24705470211055176 for Stellate Ganglion Block for Psychiatric Disorders: A Systematic Review of the Clinical Research Landscape by Jaimie Kerzner, Helen Liu, Ilya Demchenko, David Sussman, Duminda N. Wijeysundera, Sidney H. Kennedy, Karim S. Ladha and Venkat Bhat in Chronic Stress
Supplemental material, sj-docx-3-css-10.1177_24705470211055176 for Stellate Ganglion Block for Psychiatric Disorders: A Systematic Review of the Clinical Research Landscape by Jaimie Kerzner, Helen Liu, Ilya Demchenko, David Sussman, Duminda N. Wijeysundera, Sidney H. Kennedy, Karim S. Ladha and Venkat Bhat in Chronic Stress
Footnotes
Author Contributions: Kerzner and Bhat conceptualized the study. Kerzner and Liu conducted a systematic literature search and performed the data extraction. Kerzner created the first version of the article. The study conceptualization, data analysis, and article preparation were overseen by Demchenko and Bhat. All authors provided critical revision of the article for important intellectual content. All authors read and approved the final version of the article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kerzner, Liu, Sussman, and Demchenko do not have any disclosures. Wijeysundera is supported in part by Merit Awards from the Department of Anesthesiology and Pain Medicine at the University of Toronto (Toronto, Canada), a New Investigator Award from the Canadian Institutes of Health Research (Ottawa, Canada) and the Endowed Chair in Translational Anesthesiology Research at St. Michael’s Hospital (Toronto, Canada) and the University of Toronto (Toronto, Canada). The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. Kennedy has received honoraria or research funds from Abbott, Alkermes, Allergan, Boehringer Ingelheim, Brain Canada, Canadian Institutes of Health Research, Janssen, Lundbeck, Lundbeck Institute, Ontario Brain Institute, Ontario Research Fund, Otsuka, Pfizer, Servier, Sunovion, Sun Pharmaceuticals, and holds stock in Field Trip Health. Ladha is supported in part by Merit Awards from the Department of Anesthesiology and Pain Medicine at the University of Toronto (Toronto, Canada), and is a Co-Principal Investigator of a study funded by Shoppers Drug Mart. Bhat is supported by an Academic Scholar Award from the University of Toronto Department of Psychiatry, and has received research support from CIHR, Brain & Behavior Foundation, Ministry of Health Innovation Funds, Royal College of Physicians and Surgeons of Canada, Department of Defense, Canada, and an investigator-initiated trial from Roche Canada.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
ORCID iDs: Ilya Demchenko https://orcid.org/0000-0001-7876-4981
Venkat Bhat https://orcid.org/0000-0002-8768-1173
Sidney H. Kennedy https://orcid.org/0000-0001-5339-7185
Karim S. Ladha https://orcid.org/0000-0001-7303-2458
Trial Registration: Not applicable, because this article does not contain any clinical trials.
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019;21(2):10. [DOI] [PubMed] [Google Scholar]
- 2.Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. The Lancet Psychiatry. 2016;3(2):171–178. [DOI] [PubMed] [Google Scholar]
- 3.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649–659. [DOI] [PubMed] [Google Scholar]
- 4.Summers MR, Nevin RL. Stellate ganglion block in the treatment of post-traumatic stress disorder: a review of historical and recent literature. Pain Pract. 2017;17(4):546–553. [DOI] [PubMed] [Google Scholar]
- 5.Lipov EG, Joshi JR, Sanders S, et al. A unifying theory linking the prolonged efficacy of the stellate ganglion block for the treatment of chronic regional pain syndrome (CRPS), hot flashes, and posttraumatic stress disorder (PTSD). Med Hypotheses. 2009;72(6):657–661. [DOI] [PubMed] [Google Scholar]
- 6.Piraccini E, Munakomi S, Chang K-V. Stellate ganglion blocks. In: StatPearls. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 7.Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Can J Heal Technol. 2021;1(3):1–30. [PubMed] [Google Scholar]
- 8.Peterson K, Bourne D, Anderson J, et al. Evidence Brief: Effectiveness of Stellate Ganglion Block for Treatment of Posttraumatic Stress Disorder (PTSD) . 2017. http://www.ncbi.nlm.nih.gov/pubmed/28742302.
- 9.Karnosh LJ, Gardner WJ. The effects of bilateral stellate ganglion block on mental depression; report of 3 cases. Cleve Clin Q. 1947;14(3):133–138. [DOI] [PubMed] [Google Scholar]
- 10.Lebovits AH, Yarmush J, Lefkowitz M. Reflex sympathetic dystrophy and posttraumatic stress disorder: multidisciplinary evaluation and treatment. Clinical Journal of Pain. 1990;6(2):153–157. [DOI] [PubMed] [Google Scholar]
- 11.Rae Olmsted KL R, Bartoszek M, Mulvaney S, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Department of Veterans Affairs, Department of Defense VA / DoD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder. 2016. http://discovery.ucl.ac.uk/164862/%0Ahttp://www.healthquality.va.gov/guidelines/MH/srb/OvercomingSuicidalThoughtsandFeelingsFINAL.pdf%0Ahttps://www.kcl.ac.uk/kcmhr/publications/Reports/Files/Parlimentry-Office-of-Science-&-Technology--- Psychological-Healt. Accessed 4 August 2021.
- 13.Meader N, King K, Llewellyn A, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Syst Rev. 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanling SR, Hickey A, Lesnik I, et al. Stellate ganglion block for the treatment of posttraumatic stress disorder: a randomized, double-blind. Controlled trial. Am Soc Reg Anesth Pain Med. 2016;41(4):494–500. [DOI] [PubMed] [Google Scholar]
- 15.Alkire MT, Hollifield M, Courtney C, et al. Stellate ganglion blockade for severe PTSD: clinical response and psychophysiological correlates. Biol Psychiatry. 2014;75(9):117S. [Google Scholar]
- 16.Lynch JH, Mulvaney SW, Kim EH, et al. Effect of stellate ganglion block on specific symptom clusters for treatment of post-traumatic stress disorder. Mil Med. 2016; 181(9):1135–1141. [DOI] [PubMed] [Google Scholar]
- 17.Mulvaney SW, Lynch JH, Curtis KE, et al. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;usab056:1–4. [DOI] [PubMed] [Google Scholar]
- 18.Mulvaney SW, Lynch JH, Hickey MJ, et al. Stellate ganglion block used to treat symptoms associated with combat-related post-traumatic stress disorder: a case series of 166 patients. Mil Med. 2014;179(10):1133–1140. [DOI] [PubMed] [Google Scholar]
- 19.Haber J. Stellate ganglion infiltration in organic psychoses of late life. Am J Psychiatry. 1955;111(10):751–755. [DOI] [PubMed] [Google Scholar]
- 20.Alino J, Kosatka D, McLean B, et al. Efficacy of stellate ganglion block in the treatment of anxiety symptoms from combat-related post-traumatic stress disorder: a case series. Mil Med. 2013;178(4):473–476. [DOI] [PubMed] [Google Scholar]
- 21.Takano M, Takano Y, Sato I. Unexpected beneficial effect of stellate ganglion block in a schizophrenic patient. Can J Anesth. 2002;49(7):758–759. [DOI] [PubMed] [Google Scholar]
- 22.Lipov EG, Navaie M, Brown PR, et al. Stellate ganglion block improves refractory post-traumatic stress disorder and associated memory dysfunction: a case report and systematic literature review. Mil Med. 2013;178(2):e260–264. [DOI] [PubMed] [Google Scholar]
- 23.McLean B. Safety and patient acceptability of stellate ganglion blockade as a treatment adjunct for combat-related post-traumatic stress disorder: a quality assurance initiative. Cureus. 2015;7(9):e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipov EG, Joshi JR, Lipov S, et al. Cervical sympathetic blockade in a patient with post-traumatic stress disorder: a case report. Ann Clin Psychiatry. 2008;20(4):227–228. [DOI] [PubMed] [Google Scholar]
- 25.Shah R, Shah R, Bharara N. Dramatic relief of non-combat PTSD after stellate ganglion block: a case report. Postgrad Med. 2019;131(suppl 1):94. [Google Scholar]
- 26.Lipov EG, Navaie M, Stedje-Larsen ET, et al. A novel application of stellate ganglion block: preliminary observations for the treatment of post-traumatic stress disorder. Mil Med. 2012;177(2):125–127. [DOI] [PubMed] [Google Scholar]
- 27.Mulvaney SW, Lynch JH, de Leeuw J, et al. Neurocognitive performance is not degraded after stellate ganglion block treatment for post-traumatic stress disorder: a case series. Mil Med. 2015;180(5):e601–e604. [DOI] [PubMed] [Google Scholar]
- 28.Hicky A, Hanling SR, Pevney E, et al. Stellate ganglion block for PTSD. Am J Psychiatry. 2012;169(7):760. [DOI] [PubMed] [Google Scholar]
- 29.Mulvaney SW, McLean B, de Leeuw J. The use of stellate ganglion block in the treatment of panic/anxiety symptoms with combat-related post-traumatic stress disorder; preliminary results of long-term follow-up: a case series. Pain Pract. 2010;10(4):359–365. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal S. Stellate Ganglion Blockade to Reduce Cardiac Anxiety and PTSD Symptoms in Cardiac Arrest Survivors (SGB-PsychoED). 2021. https://clinicaltrials.gov/ct2/show/NCT04582396. Accessed 9 June 2021.
- 31.Ladha K. Stellate Ganglion Block for Major Depressive Disorder. 2021. https://clinicaltrials.gov/ct2/show/NCT04727229. Accessed 9 June 2021. [Google Scholar]
- 32.Chung BY, Jörg C. Stellate ganglion block in patients with borderline personality disorder and posttraumatic stress disorder. 2018. https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00015817. Accessed 9 June 2021.
- 33.Peterson A. Augmenting Massed Prolonged Exposure With a Stellate Ganglion Block to Treat PTSD. 2020. https://clinicaltrials.gov/ct2/show/NCT04302181. Accessed 2 July 2021.
- 34.Weathers FW, Bovin MJ, Lee DJ, et al. The clinician-administered PTSD scale for DSM–5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blevins CA, Weathers FW, Davis MT, et al. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489–498. [DOI] [PubMed] [Google Scholar]
- 36.Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11(2):364–386. [Google Scholar]
- 37.Schore AN. Dysregulation of the right brain: a fundamental mechanism of traumatic attachment and the psychopathogenesis of posttraumatic stress disorder. Aust N Z J Psychiatry. 2002;36(1):9–30. [DOI] [PubMed] [Google Scholar]
- 38.Yokotoa S, Taneyama C, Goto H. Different effects of right and left stellate ganglion block on systolic blood pressure and heart rate. Open J Anesthesiol. 2013;3(3):143–147. [Google Scholar]
- 39.Kapral S, Krafft P, Gosch M, et al. Ultrasound imaging for stellate ganglion block: direct visualization of puncture site and local anesthetic spread: a pilot study. Reg Anesth J Neural Blockade Obstet Surgery. Pain Control. 1955;20(4):323–328. [PubMed] [Google Scholar]
- 40.Feigl GC, Rosmarin W, Stelzl A, et al. Comparison of different injectate volumes for stellate ganglion block: an anatomic and radiologic study. Reg Anesth Pain Med. 2007;32(3):203–208. [DOI] [PubMed] [Google Scholar]
- 41.Kuthiala G, Chaudhary G. Ropivacaine: a review of its pharmacology and clinical use. Indian J Anaesth. 2011;55(2):104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bataille B, Nucci B, Mora M, et al. Ultrasound-guided bilateral stellate ganglion blockade to treat digital ischemia in a patient with sepsis: a case report. Can J Anesth. 2016;63(1):56–60. [DOI] [PubMed] [Google Scholar]
- 43.Stogios N, Gdanski A, Gerretsen P, et al. Autonomic nervous system dysfunction in schizophrenia: impact on cognitive and metabolic health. npj Schizophr. 2021;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montaquila JM, Trachik BJ, Bedwell JS. Heart rate variability and vagal tone in schizophrenia: a review. J Psychiatr Res. 2015;69:57–66. [DOI] [PubMed] [Google Scholar]
- 45.Lynch JH. Stellate ganglion block treats posttraumatic stress: an example of precision mental health. Brain Behav. 2020;10(11):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynch JH, Muench PD, Okiishi JC, et al. Behavioral health clinicians endorse stellate ganglion block as a valuable intervention in the treatment of trauma-related disorders. J Investig Med. 2021;69(5):989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bourvis N, Aouidad A, Cabelguen C, et al. How do stress exposure and stress regulation relate to borderline personality disorder? Front Psychol. 2017;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Austin MA, Riniolo TC, Porges SW. Borderline personality disorder and emotion regulation: insights from the polyvagal theory. Brain Cogn. 2007;65(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Won E, Kim Y-K. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr Neuropharmacol. 2016;14(7):665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-css-10.1177_24705470211055176 for Stellate Ganglion Block for Psychiatric Disorders: A Systematic Review of the Clinical Research Landscape by Jaimie Kerzner, Helen Liu, Ilya Demchenko, David Sussman, Duminda N. Wijeysundera, Sidney H. Kennedy, Karim S. Ladha and Venkat Bhat in Chronic Stress
Supplemental material, sj-docx-2-css-10.1177_24705470211055176 for Stellate Ganglion Block for Psychiatric Disorders: A Systematic Review of the Clinical Research Landscape by Jaimie Kerzner, Helen Liu, Ilya Demchenko, David Sussman, Duminda N. Wijeysundera, Sidney H. Kennedy, Karim S. Ladha and Venkat Bhat in Chronic Stress
Supplemental material, sj-docx-3-css-10.1177_24705470211055176 for Stellate Ganglion Block for Psychiatric Disorders: A Systematic Review of the Clinical Research Landscape by Jaimie Kerzner, Helen Liu, Ilya Demchenko, David Sussman, Duminda N. Wijeysundera, Sidney H. Kennedy, Karim S. Ladha and Venkat Bhat in Chronic Stress