Abstract
Objective:
To conduct a 9-month pilot Internet randomized controlled trial (RCT) of cherry extract and diet modification in gout to assess feasibility of an Internet study and obtain effect estimates.
Methods:
After providing online informed consent in response to Internet advertisements and social media or clinic flyers, 84 people with physician-confirmed gout were randomized to either to cherry extract 3,600 mg/day (n=41) or dietitian-assisted diet modification for gout (n=43). All study outcomes were collected via Internet and phone calls. The primary objective was feasibility of an Internet study and secondary objectives were to obtain effect estimates for gout flares, functional ability assessed with the health assessment questionnaire (HAQ) and adverse events (AEs) for future trials.
Results:
Of the 84 people randomized, overall completion rates were >80% for most study procedures up to 6 months and similar for the two active comparators. Improvements were seen in gout flares and HAQ scores in cherry extract and diet modification groups at 9-months compared to baseline: gout flares/month, 0.22 vs. 0.36 (p=0.049) and 0.28 vs. 0.31 (p=0.76); proportion with any gout flare, 56% vs. 98% (p<0.0001) and 65% vs. 98% (0.0002); and mean (±standard deviation) HAQ score, 0.28±0.54 vs. 0.55±0.68 (p=0.001) and 0.23±0.40 vs. 0.48±0.61 (p=0.06), respectively. Any AEs and gastrointestinal symptoms/AEs at 9-months in cherry extract and diet modification groups were 3% vs. 0% and 28% vs. 27%.
Conclusion:
An Internet gout RCT is feasible for non-pharmacological gout treatments. A hypothesis-testing, large Internet RCT of cherry extract vs. placebo is needed.
Background
Gout affects 8.3 million Americans and the associated societal burden is rising due to increasing prevalence.[1] Patients with gout are offered life-long treatment with effective urate-lowering therapy (ULT), usually allopurinol or febuxostat. Most patients (68%) do not take these medications regularly,[2] at least partly due to the concern of side effects, the intermittent nature of gout, and medication cost.[3–5] At least 40% of gout patients considered diet and/or dietary supplements such as cherry extract as acceptable treatments for gout.[6] Forty percent of the patients with gout used cherry extract or other cherry products for treatment of gout.[7, 8] Higher intake of purine-rich foods (meat, seafood etc.) increases the risk of gout flares,[9, 10] while skim or low-fat dairy products reduce the risk of gout flares. [11] Thus, patients with gout consider dietary modification and cherry extract as alternative non-pharmacological treatments.
Preliminary evidence suggests the efficacy of cherries and cherry extract in gout. In an observational study, one serving of cherries or any cherry extract intake over 2-days lowered the risk of gout flare by 35% and 45% compared with no intake, respectively.[7] Cherry juice concentrate decreased gout flares; however, sample size was small and study was observational.[12] Potential mechanisms of efficacy of cherry products include the inhibition of inflammatory cytokines and markers by cherry concentrate [13, 14] and/or associated antioxidant properties.[15–20]. While a dietary supplement does not require U.S. Food and Drug Administration (FDA) approval, it is important to establish effectiveness through rigorous trials rather than anecdotal evidence or testimonials.
The 2012 American College of Rheumatology (ACR) and the European League Against Rheumatism gout treatment guidelines emphasized the importance of diet and dietary supplements[21–23] yet there is a significant lack of randomized controlled trials (RCTs) assessing their efficacy.[21] Patients with gout consider studies of diet and supplements the highest priorities for future research.[24] Therefore, evaluating non-pharmacological gout treatments using a rigorously designed clinical trial, is a high priority.
The primary objective of our 9-month Internet pilot feasibility study, coMparative feasibility study IN GOUt: CHerry extract vs. diet modification (mini-GOUCH), was to demonstrate the feasibility of key Internet study procedures. Secondary objectives were to obtain preliminary effect size estimates for gout flares, function and adverse events for the two active interventions, cherry extract and diet modification for gout treatment.[25]
Methods
Study Sample, Patient Enrollment and Screening Using an Internet website
The study was approved by the Institutional Review Board (IRB) at the University of Alabama at Birmingham. Since cherry extract is not a drug, biologic, or device regulated by the U.S. Food and Drug Administration (FDA), study registration was not required on clinicaltrials.gov. All study procedures were done at patient’s convenience at their home.
We built an Internet website (www.cherries4gout.com) and modified VioScreen™, a reliable and user-friendly graphical NIH-funded Food Frequency Questionnaire (FFQ)[26–28] into an Internet tool, GoutWell, that sent gout education and adherence messages and 2-weekly gout flare survey. Iterative piloting and modification of GoutWell and key study procedures including website log in procedure, online patient consent, baseline and follow-up survey content, and 2-weekly gout flare assessments was done in 15 patients with gout. This led to the finalized study website content, GoutWell and study procedures including the choice of the study follow-up surveys to be 3-monthly and the gout flare assessments to be 2-weekly.
All potential participants were invited for study enrollment by providing an Internet link to the study website at the Gout and Uric Acid Education Society website, www.gouteducation.org, via a Google Ad, or via an IRB-approved flyer at the University of Alabama Health Services Foundation clinic waiting rooms from 2/2016 to 10/2016. The study website, www.cherries4gout.com, provided description of the study and study procedures to potential participants. All study participants were recruited online. Participants provided online informed consent, and completed a study screening form that included demographics, contact information (address/email/phone number), whether they had a physician-confirmed diagnosis of gout, self-reported 1977 American College of Rheumatology (ACR) classification criteria for gout,[29] current use of urate-lowering therapies (allopurinol, febuxostat, probenecid or pegloticase) or cherry extract, juice or concentrate and the number of gout flares in the last year.
Inclusions criteria were: (1) US adults ≥ 18 years; (2) a valid US mailing address and email address; (3) patient self-reported physician diagnosis of gout. Exclusion criteria were: self-reported presence of other types of inflammatory arthritis including rheumatoid arthritis or spondyloarthritis; and the current use of cherry extract, juice or concentrate.
For participants potentially meeting study eligibility criteria, a trained study coordinator (C.G.) contacted the physician office and requested the healthcare provider to confirm the diagnosis of gout and provide medical records ($50 incentive) and obtained physician-reported ACR gout classification criteria.[29] To build patient rapport, the study coordinator (C.G.) had a 15-minute informational call session with each patient informing them of study procedures.
Randomization and Study Intervention
Participants were randomized in a 1:1 ratio to one of 2 groups: cherry extract (3 capsules of 1,200 mg daily, each equivalent to 32 oz. of cherry juice or a pound of cherries[30]) or individualized diet modification. Randomization was done, using an online computerized permuted variable block design with simple randomization, programmed via the Research Electronic Data Capture (RedCap®; Nashville, TN) database. We sent either the 3-month supply of cherry capsules, or individualized diet recommendation (based on baseline FFQ data) to each study participant at 3-, 6- and 9-months, supplemented with study coordinator calls to encourage cherry extract adherence or dietitian calls to discuss specific recommendations (details below). Receipt was confirmed via email or phone conversation. Due to the nature of interventions, participants and the study team were aware of group assignments.
Baseline and Follow-up Assessments
Participants who met all criteria were invited to participate in the study within 4 weeks of the screening visit, by sending a link to participant’s preferred email address with patient’s unique ID with login and password. Participants completed assessments of gout flare (baseline 12-month recall for the number of gout flares), activity limitation with Health assessment questionnaire (HAQ), FFQ, self-reported comorbidity index, smoking and alcohol use, adverse events and ULT use. They also completed the baseline blood draw for baseline serum urate (SU) at a closest/most convenient Quest® laboratory site, scheduled by the study coordinator (C.G.) based on patient preference. Test results were sent to the UAB team in a HIPAA-compliant manner and recorded.
Participants completed brief online questionnaires (<30-minutes total) every 3 months at 3-, 6- and 9-months (HAQ, adverse events, gout medication use) after randomization, sent to their email address via a link, using their unique login and password. Two-weekly gout flare questionnaires were completed via email, followed by a study coordinator phone call for non-responders (<2-minutes). Automatic reminder emails were sent to non-responsive participants every 24 hours for 5 days. Serum urate blood draw were done at the nearest Quest® laboratory site only at 9-months. Each study participant was invited to join group teleconference call sessions lasting 30–60 minutes either at 0-, 1-, 4-months with study coordinators (cherry extract group) or 0-, 3- and 6-months with a registered dietitian (A.W., or B.D.; diet modification group).
Participants were mailed a $10 Visa gift card for the successful completion of each internet study visit (baseline, follow-up) and $40 for completion of baseline and 9-month SU blood draws. We emailed personalized birthday Ecards and thank you notes to keep patients engaged.
Study Outcomes
Feasibility of key procedures (physician office confirmation of gout diagnosis and provision of ACR gout classification criteria; 3-, 6-, and 9-month follow visits; 0- and 9-month serum urate blood draw; 2-weekly gout flare assessments; 0-, 6- and 9-month diet sessions; 0-, 1-, and 4-month cherry extract sessions) was assessed by completion rates of key study procedures among study participants. These feasibility rates were our primary outcome.
Patient-reported and Validated Gout Flares:
Patient self-reported gout flares with 2-weekly email/call were the main measure. Validated gout flares were assessed based on the presence of three of the following:[31, 32] patient self-reported gout flare, any patient-reported warm joint, any patient-reported swollen joint and patient-reported pain at rest score of >3 (0–10 scale). We assessed gout flares/month, the proportion with any gout flares and the time to the first gout flare. Gout flare rates (/month) were calculated during study follow-up based on the prospectively reported gout flares divided by the number of months of follow-up completed by each patient, e.g., if a patient reported 6 gout flares and completed 6.5 months of study follow-up, the gout flare rate was 0.92. Baseline gout flare rate (/month) was assessed by dividing the number of gout flares reported by each patient at the baseline visit for a prior 12-month baseline period (based on patient recall) by 12.
Activity limitation Assessment was done with Health Assessment Questionnaire (HAQ)[33–35], a validated measure, at 0-, 3-, 6- and 9-months. HAQ assesses difficulty in 20 items in 8 categories (dressing, arising, eating, walking, hygiene, reaching, gripping, and outside activity), the total score ranges from 0 (no disability) to 3 (complete disability). The minimal clinically meaningful improvement threshold is 0.22.[36]
Serum urate was assessed at 0- and 9-months determined by a standardized assay using an enzymatic uricase method (Stanbio Laboratory, Boerne, TX).[37]
Adverse Event (AEs) were captured using a standardized AE form with 78 listed potential AEs. An additional detailed form including a comprehensive list of gastrointestinal adverse events (since these are most common potential AEs with cherry extract) via Internet every 3-months.
Statistical Analyses
Outcomes within cherry extract and diet modification groups were compared to baseline values using paired t-tests for continuous outcomes (# gout flares, HAQ, SU) and McNemar’s test for categorical outcomes (HAQ MCID, proportion with any gout flares). Gout flare rates (/month) were compared during study follow-up to baseline using Poisson regression. Time free of gout flares during the study period was compared between treatment arms using Kaplan-Meier curve. We compared characteristics of study drop-outs and partial completers with completers using t-test and McNemar’s test. All analyses were intention to treat. A p-value <0.05 was considered statistically significant. Data are reported according to the CONSORT guidelines [38].
Results
Trial participant characteristics and Study Flow chart
One-hundred and fifty-four potential participants were identified, of whom 84 participants with gout were randomized, 41 to the cherry extract arm and 43 to the diet modification arm (Appendix 1; CONSORT flow chart). Reasons for 70 screen failures included the following: no consent for MD confirmation of gout diagnosis (n=16; 23%); diagnosis was not gout or gout diagnosis not confirmed by the healthcare provider (n=9; 13%); incomplete screening assessment completion either due to lack of time or interest (n=35; 50%); and non-US residence (n=10; 14%).
The mean age of study participants was 56 years (standard deviation (SD), 14), 72% were male, 68% were white, mean (SD) BMI was 33 (9) Kg/m2, mean (SD) gout flares in the last year were 4 (5.4), i.e. 0.33 gout flares/month. Eighty percent had ever had some sort of gout medication prescription, and 37% were currently on ULT, allopurinol, febuxostat or probenecid. ULT use and other characteristics of subjects randomized to the two active comparators, cherry extract vs. individualized diet modification, were similar (Table 1).
Table 1.
Participant characteristics
| All participants (N= 84) N (%)* | Cherry extract (N = 41) N (%)* | Diet modification (N=43) N (%)* | p-value | |
|---|---|---|---|---|
|
| ||||
| Age, Mean (± SD) | 55.8±13.9 | 58.2±15.5 | 53.6 ±11.9 | 0.13 |
| Gender, Male n (%) | 61 (72%) | 31 (76%) | 30 (70%) | 0.54 |
| Race, n (%) | 0.55 | |||
| White | 57 (68%) | 30 (73%) | 27 (63%) | |
| Black or African American | 21 (25%) | 9 (22%) | 12 (28%) | |
| Asian/Other1/mixed | 6 (7%) | 2 (5%) | 4 (9%) | |
| Body Mass Index, in Kg/m2, Mean (± SD) | 33.3±9.0 | 32.6±7.9 | 34.0±10.0 | 0.49 |
| Sangha Comorbidity Index Score, Mean (± SD) | 3.6±3.5 | 3.3±3.9 | 4.0±3.0 | 0.37 |
| Ever Smoked | 34 (49%) | 15 (37%) | 19 (44%) | 0.48 |
| Currently smoke | 3 (9%) | 2 (13%) | 1 (5%) | 0.41 |
| Alcohol intake, Mean (± SD) | ||||
| #days past week with drink | 1.9±3.0 | 2.0±2.3 | 1.7±3.5 | 0.68 |
| #days past month with drink | 6.9±9.5 | 7.9±9.9 | 5.9±9.1 | 0.35 |
| #drinks | 1.5±1.8 | 1.5±1.9 | 1.4±1.8 | 0.73 |
| #days with >5 drinks | 1.7±4.5 | 1.8±3.4 | 1.6±5.4 | 0.87 |
| Largest #drinks | 2.3±3.0 | 2.4±2.7 | 2.2±3.2 | 0.75 |
| Doctor ever prescribed a medication for gout | 67 (80%) | 31 (76%) | 36 (84%) | 0.35 |
| Currently taking allopurinol, febuxostat or probenecid2 | 30 (37%) | 13 (33%) | 17 (42%) | 0.40 |
| #gout flares in the last year, Mean (± SD) | 4.0±5.4 | 4.3±6.0 | 3.7±4.8 | 0.62 |
| Baseline HAQ-DI score, Mean (± SD) | 0.53±0.7 | 0.58±0.7 | 0.49±0.6 | 0.58 |
| Maximum pain Intensity in last 24 hrs (0–10), Mean (± SD) | 2.51±3.0 | 2.83±3.1 | 2.21±2.9 | 0.34 |
| Activity Level | 0.87 | |||
| Sedentary | 24 (32%) | 13 (33%) | 11 (31%) | |
| Low Active | 26 (35%) | 12 (31%) | 14 (39%) | |
| Active | 22 (29%) | 12 (31%) | 10 (28%) | |
| Very Active | 3 (4%) | 2 (5%) | 1 (3%) | |
| Extremely Active | 0 | 0 | 0 | |
| Took survey on 3 | 0.94 | |||
| Computer | 47 (62%) | 22 (62.9%) | 25 (61.0%) | |
| Touchscreen | 8 (11%) | 4 (11.4%) | 4 (9.8%) | |
| Smartphone | 21 (28%) | 9 (25.7%) | 12 (29.3%) | |
N (%), unless specified otherwise
Other race includes: Native Hawaiian or other pacific islander, American Indian or Alaskan native, Asian, other and mixed
Missing frequency, n=3
Missing frequency, n=8
Primary Outcome: Completion Rates of Key Internet Study Procedure
The completion rates for the key study procedures in our Internet pilot study were high up to 6-months: physician office confirmation of gout diagnosis and classification criteria, 100% and 94%; baseline, 3-, 6-, 9-month study follow-up assessments, 100%, 93%, 81% and 69%; gout flare surveys every 2-weeks, 75%; baseline and 9-month serum urate blood draw, 100% and 77%; telephone support sessions to support diet modification by a registered dietitian, baseline, 6- and 9-month, 83%, 87% and 86%; and telephone support sessions to support cherry extract adherence by the study coordinator, baseline, 1-, and 4-month, 97%, 90% and 81% (Table 2).
Table 2.
Study completion rates, overall and by treatment allocation
| All subjects (N=84) | Cherry extract (n=41) | Diet modification (n=43) | |
|---|---|---|---|
|
| |||
| Physician office communication | |||
| Gout diagnosis confirmation | 100% | 100% | 100% |
| ACR gout classification criteria | 94% | 93% | 95% |
| Internet study visits with survey assessments | |||
| Baseline assessment | 100% | 100% | 100% |
| 3-month assessment | 93% | 100% | 86% |
| 6-month assessment | 81% | 90% | 72% |
| 9-month assessment | 69% | 78% | 60% |
| Serum urate blood draw testing1 | |||
| Baseline | 100% | 100% | 100% |
| 9-month | 77% | 83% | 72% |
| 2-weekly Gout flare assessment2 | 75% | 82% | 69% |
| Telephone support sessions for diet modification (Dietitian) | |||
| Baseline | -- | -- | 83% |
| 6-month | -- | -- | 87% |
| 9-month | -- | -- | 86% |
| Telephone support sessions for cherry extract adherence (study coordinator) | |||
| Baseline | -- | 97% | -- |
| 1-month | -- | 90% | -- |
| 4-month | -- | 81% | -- |
All study procedures and visits were completed over the Internet by participants in the comfort of their homes, or on the phone
Serum urate blood draw was done at the Quest Laboratory site closest to the patient residence or by patient choice, and results were reported directly to the study team in a HIPAA-compliant method
Two- weekly Gout flare assessments were done via the Internet; non-responders received a follow-up phone call to complete the brief questionnaire on the phone lasting <2 minutes
The completion rates were higher in cherry extract group compared to diet modification group for 3-, 6-, 9-month study follow-up assessments, 100% vs. 86%, 90% vs. 72% and 78% vs 62% and 2-weekly gout flare surveys, 82% vs. 69%, respectively (Table 2).
Patient-reported Gout Flares and HAQ
Comparing the rates over 9-months to the recalled rates at baseline, within group differences for cherry extract and diet modification were as follows: gout flares/month, 0.22 vs. 0.36 at baseline (p=0.049) and 0.28 vs. 0.31 (p=0.76); and proportion with any gout flare, 56% vs. 98% (p<0.0001) and 65% vs. 98% (p=0.0002), respectively. The mean HAQ scores improved in cherry extract 0.28 vs. 0.55 (p=0.001), but not in the diet modification group, 0.23 vs. 0.48 (p=0.06; Table 3 and 4). Most improvements in gout flare rates were noted in people taking concurrent ULT in both groups (Table 3). Mean HAQ improvements exceeded the MCID threshold of 0.22 for both groups at 3-, 6- and 9-months; for example, 59% of cherry extract and 54% of diet modification groups had HAQ improvements exceeding MCID at 9-months (Table 4). The validated gout flare rate (a subset of all gout flares using a validated definition [31, 32]) over 9-months were 0.18/month in cherry extract and 0.20/month in diet modification group.
Table 3.
Effect estimate for key outcomes within each treatment arm compared to baseline (pre to post comparisons)
| Cherry extract N (%) or mean (SD) (N = 41)† |
p-value | Diet modification N (%) or mean (SD) (N = 43)† |
p-value | |||
|---|---|---|---|---|---|---|
| Baseline | 9-month | Baseline | 9-month | |||
|
| ||||||
| Number of gout flares/month (patient-reported) * | 0.36 | 0.22 | 0.049 | 0.31 | 0.28 | 0.76 |
| Proportion with any gout flare (patient-reported) * | 40 (98%) | 23 (56%) | <0.0001 | 42 (98%) | 28 (65%) | 0.0002 |
| HAQ ** | 0.55 (0.68)1 | 0.28 (0.54) | 0.001 | 0.48 (0.61)2 | 0.23 (0.40) | 0.06 |
| Serum urate ** | 7.24 (1.98) | 7.16 (1.71) | 0.77 | 7.14 (1.99) | 7.00 (1.91) | 0.63 |
|
| ||||||
| Patients Reporting Taking ULT Medications at Baseline | ||||||
|
| ||||||
| N=13 | N=17 | |||||
|
| ||||||
| Number of gout flares/month (patient-reported) * | 0.57 | 0.21 | 0.04 | 0.30 | 0.19 | 0.18 |
| Proportion with any gout flare (patient-reported) * | 12 (92%) | 5 (38%) | 0.008 | 16 (94%) | 8 (47%) | 0.0047 |
| HAQ ** | 0.35 (0.42)3 | 0.18 (0.39) | 0.07 | 0.45 (0.28)4 | 0.23 (0.26) | 0.19 |
| Serum urate ** | 7.3 (1.7) | 7.3 (1.7) | 0.68 | 7.5 (1.9) | 7.7 (1.7) | 0.25 |
|
| ||||||
| Patients Reporting Not Taking ULT Medications at Baseline | ||||||
|
| ||||||
| N=27 | N=24 | |||||
|
| ||||||
| Number of gout flares/month (patient-reported) * | 0.27 | 0.23 | 0.42 | 0.28 | 0.33 | 0.61 |
| Proportion with any gout flare (patient-reported) * | 27 (100%) | 17 (63%) | NE | 24 (100%) | 20 (83%) | NE |
| HAQ ** | 0.61 (0.76)5 | 0.33 (0.60) | 0.01 | 0.49 (0.71)6 | 0.19 (0.43) | 0.08 |
| Serum urate ** | 7.0 (2.1) | 6.9 (1.7) | 0.84 | 6.9 (2.0) | 6.8 (2.0) | 0.64 |
NE, Not estimable; ULT, urate-lowering medication
Due to missing data, the denominators were smaller than the total sample, since these were for paired sample t-test:
N=32
N=26
N=9
N=7
N=22
N=18
There were three people with missing data for ULT use, therefore those with ULT (n=30) and without ULT at baseline (n=51) don’t add up to 84
Cumulative 9-month flares averaged to monthly gout flares during the study collected with 2-weekly gout flare questionnaire compared to average monthly baseline flares based on 12-month baseline report on patient recall (calculated by dividing baseline flares divided by 12)
Comparison of baseline to the 9-month study follow-up value
Bold represents significant p-value < 0.05
Table 4.
Secondary Outcomes over 9-months in both active treatment groups
| Baseline | 3-Month | 6-Month | 9-Month | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| N | Mean (SD) | p-value | N | Mean (SD) | p-value | N | Mean (SD) | p-value | N | Mean (SD) | p-value | |
|
|
||||||||||||
| HAQ Score (0–3; lower=better) | ||||||||||||
| Cherry extract | 41 | 0.58 (0.71) | 0.57 | 41 | 0.21 (0.38) | 0.75 | 37 | 0.21 (0.42) | 0.66 | 32 | 0.20 (0.40) | 0.90 |
| Diet modification | 43 | 0.49 (0.60) | 37 | 0.18 (0.29) | 31 | 0.17 (0.31) | 26 | 0.18 (0.36) | ||||
| Proportion with HAQ MCID1 | ||||||||||||
| Cherry extract | N/A | 41 | 58.5% | 0.82 | 37 | 64.9% | 0.22 | 32 | 59.4% | 0.79 | ||
| Diet modification | N.A | 37 | 54.1% | 31 | 48.4% | 26 | 53.9% | |||||
| Maximum Pain in last 24 hrs, 0–10 | ||||||||||||
| Cherry extract | 41 | 2.83 (3.07) | 0.34 | 41 | 1.46 (2.47) | 0.65 | 37 | 1.30 (2.31) | 0.81 | 32 | 1.16 (2.27) | 0.62 |
| Diet modification | 43 | 2.11 (2.89) | 37 | 1.24 (1.72) | 31 | 1.16 (2.34) | 26 | 0.88 (1.77) | ||||
| Any adverse event, % | ||||||||||||
| Cherry extract | N/A | 40 | 2.5 | 1.00 | 37 | 8.1 | 0.25 | 32 | 3.1 | 1.00 | ||
| Diet modification | N/A | 35 | 0.0 | 30 | 0.0 | 26 | 0.0 | |||||
| Specific gastrointestinal adverse event, % | ||||||||||||
| Cherry extract | N/A | 41 | 31.7 | 0.81 | 37 | 32.4 | 0.80 | 32 | 28.1 | 1.00 | ||
| Diet modification | N/A | 37 | 35.1 | 31 | 35.5 | 26 | 26.9 | |||||
HAQ MCID: HAQ score reduction of 0.22 or more during FU
N/A: not applicable
The time to the first patient-reported gout flare in cherry extract vs. diet modification, 101.6 (sd, 74.5) vs. 73.6 days (sd, 58.1; p=0.14; Figure 1), and the total number of gout flares, 67 vs. 75 (p=0.19; Appendix 2) were not significantly different.
Figure 1. Kaplan-Meier graph of the proportion of people who were gout flare free during the 9-month study follow-up.
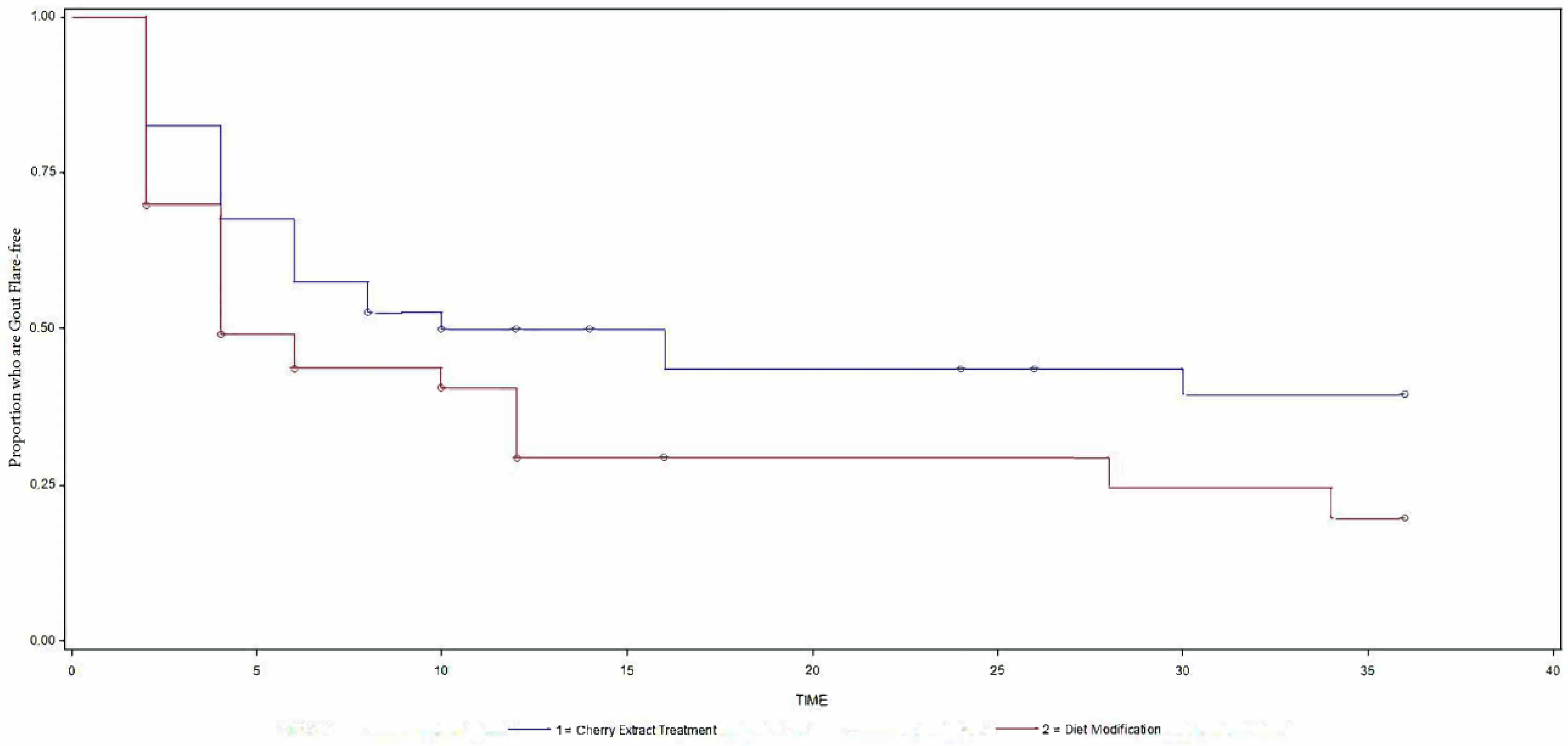
Y-axis shows proportion of patients who were gout flare free during the 9-month study follow-up (1 equates 100%) and x-axis the number of weeks of study follow-up. The top blue line represents the cherry extract and the bottom red line the diet modification group. More people in the cherry extract group (top survival curve) were gout flare free compared to the diet modification group (bottom survival curve).
Serum Urate (SU) and pain
There were no significant changes in serum urate from baseline to 9-months within either group (p=0.77 and 0.63; Table 3). No differences were noted between cherry extract vs. diet modification groups for serum urate or pain intensity (Table 3 and 4; Appendix 2).
Adverse Event (AEs) and specific gastrointestinal symptoms/AEs
At 3-, 6- and 9-months, respective rates of occurrence of any AE in cherry extract vs. diet modification were similar: 2% vs. 0%, 8% vs. 0%; and 3% vs. 0% (p>0.05 for all; Table 4). Respective rates of the occurrence of specific gastrointestinal AEs were also similar between the two groups: 32% vs. 35%; 32% vs. 36%; and 28% vs. 27% (Table 4; Appendix 3).
Non-responder characteristics
Study drop-outs and partial completers differed from study completers in having a higher number of gout flares in the last year, 3.5 and 6.9 vs. 3.5 (overall p-value =0.02); completers had lower baseline gout flare rates than non-completers (p=0.006; Table 5). Differences in age were not significant, 48 and 53 vs. 58 years (p=0.13). Other patient characteristics were similar.
Table 5.
Comparison of characteristics of study completers vs. drop-outs
| Dropouts after baseline (n=6) | Study completers (n=58) | Partial Completers* (n=20) | p-value completers vs. partial completers | Overall p-value | |
|---|---|---|---|---|---|
|
| |||||
| Age, mean (SD) | 47.6 (10.3) | 57.8 (14.7) | 52.9 (11.5) | 0.18 | 0.13 |
| Gender, Male | 6 (100%) | 42 (72%) | 13 (65%) | 0.53 | 0.24 |
| Select 1977 ACR gout classification criteria | |||||
| Hyperuricemia | 2 (33%) | 49 (85%) | 16 (80%) | 0.01 | |
| Joint subcortical cysts | 0 (0%) | 11 (19%) | 8 (40%) | 0.06 | |
| Urate crystals | 2 (33%) | 39 (67%) | 12 (60%) | 0.25 | |
| Race, n (%) | 0.75 | 0.82 | |||
| White | 4 (67%) | 40 (69%) | 13 (65%) | ||
| Black or African American | 1 (17%) | 15 (26%) | 5 (25%) | ||
| Other | 1 (17%) | 3 (5%) | 2 (10%) | ||
| #gout flares in the last year, mean (SD) | 3.5 (2.8) | 3.0 (2.6) | 6.9 (9.6) | 0.006 | 0.02 |
| Pt.-reported Last uric acid level at the first visit (mg/dl), mean (SD) | 9.0 (2.1) | 7.3 (3.2) | 7.8 (2.5) | 0.59 | 0.67 |
| BMI, mean (SD) | 29.1 (6.8) | 33.3 (9.6) | 34.5 (7.8) | 0.63 | 0.45 |
| Baseline HAQ-DI score, mean (SD) | 0.33 (0.57) | 0.55 (0.69) | 0.65 (0.73) | 0.57 | 0.59 |
| Residency Address in Alabama State, n (%) | 1 (17%) | 31 (53%) | 8 (40%) | 0.94 | 0.70 |
| Sangha Comorbidity Index Score, mean (SD) | 1.7 (1.5) | 3.8 (3.5) | 3.8 (3.9) | 0.99 | 0.36 |
| Ever Smoked | 1 (17%) | 23 (40%) | 10 (50%) | 0.41 | 0.34 |
| Currently smoke | 0 (0%) | 1 (4%) | 2 (20%) | 0.15 | 0.33 |
| Alcohol intake | |||||
| #days past week with drink | 1.7 (1.9) | 2.1 (3.4) | 1.3 (2.0) | 0.34 | 0.62 |
| #days past month with drink | 7.5 (9.3) | 7.3 (9.9) | 5.5 (8.6) | 0.46 | 0.75 |
| #drinks | 1.8 (1.0) | 1.4 (1.9) | 1.4 (1.9) | 0.94 | 0.87 |
| #days with >5 drinks | 0.8 (2.0) | 1.9 (5.2) | 1.3 (2.8) | 0.60 | 0.77 |
| Largest #drinks | 3.2 (4.5) | 2.2 (2.7) | 2.1 (3.1) | 0.90 | 0.75 |
| Doctor ever prescribed allopurinol, febuxostat or probenecid? | 6 (100%) | 46 (79%) | 15 (75%) | 0.68 | 0.40 |
| Prescribed allopurinol | 3 (50%) | 30 (52%) | 14 (70%) | 0.15 | 0.35 |
| Prescribed febuxostat | 1 (17%) | 1 (1%) | 0 (0%) | 0.55 | 0.05 |
| Current activity level | 0.48 | 0.81 | |||
| Sedentary | 2 (40%) | 14 (27%) | 8 (44%) | ||
| Low Active | 2 (40%) | 20 (39%) | 4 (22%) | ||
| Active | 1 (20%) | 16 (31%) | 5 (31%) | ||
| Very Active | 0 (0%) | 2 (4%) | 1 (4%) | ||
| Extremely Active | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Maximum pain in the last 24 hours | 2.8 (4.4) | 2.5 (3.0) | 2.5 (2.7) | 0.98 | 0.96 |
Partial completers: Completed at least one FU assessment, but not all three (3, 6- or 9-month)
Bold represents significant p-value < 0.05
Discussion
The primary objective of our pilot feasibility study was to demonstrate the feasibility of conducting a randomized trial of two active non-pharmacological treatments, cherry extract and individualized diet modification, for people with gout using an efficient Internet-study design. Secondary objective was to obtain effect estimates for each active intervention. The pilot study was not designed to test the superiority of one active intervention over the other active intervention.
Successful recruitment of 11 patients/month and high study completion rates for most study procedures (primary study objective) up to 6 months suggest a strong ability to recruit larger samples of gout patients in a future hypothesis-testing trial. We optimized and finalized several key study procedures during our pilot study including, but not limited to, determining the optimal frequency of patient assessments (pre-pilot; see methods), obtaining records and gout diagnosis confirmation from the provider office staff, follow-up telephone sessions to improve adherence to study intervention and build good rapport with patients in an Internet study, the completion of “virtual” study follow-up visits at 3-, 6- and 9-months and implementation of 2-weekly gout flare emails to prospectively collect gout flare data.
We obtained preliminary effect size estimates for co-primary efficacy outcomes, validated gout flares [31, 32] and functional limitation using HAQ, in the active interventions (secondary study objective) to adequately power our next-step hypothesis-testing RCT comparing cherry extract and placebo in people with gout. We also obtained estimates of safety outcome, AEs and serum urate and pain intensity, secondary outcomes for our future RCT (Table 4). Our study addressed non-pharmacological treatment of gout, which addresses one of the national health care priorities in comparative effectiveness research.[25]
This pilot study was not powered for testing superiority of one active treatment over another. We provide comparative results of cherry extract vs diet modification for the interested reader. Given the small sample size of our study the absence of a difference between groups is at high risk of type II error, i.e., failing to reject null hypothesis when a true difference is present. Subgroup analyses of people with or without concurrent ULT use have even smaller sizes, and should be interpreted with caution.
Compared to the baseline, gout flares decreased both statistically significantly and clinically meaningfully in the cherry extract treatment arm. The direction of change was as expected for cherry extract arm. Reductions in the diet modification group were significant for proportion with any flares, but not for flare rates. Diet modification was chosen as the a priori weak active comparator due to well-known issues of dietary adherence over the long term. Potential reduction in gout flares in the diet modification group may be related to an increase in anti-oxidant diet content [39, 40] due to a shift towards more plant-based diet and reduction in animal-based diet and complex sugars. Gout flares were distributed evenly in the cherry extract vs. diet modification groups: 44% vs. 35% of the people had no gout flares; and 56% vs. 65% had one or more gout flares during the study follow-up, with majority reporting a total of 1–3 gout flares.
Mean HAQ scores improved by 50% in each group at 9-months, i.e. 0.27 in cherry and 0.24 in diet group, which exceeded the MCID of 0.22 and was statistically significant [36]; respectively, 59% and 54% patients achieved HAQ score improvement exceeding MCID of 0.22 at 9-months. Not surprisingly, a 39% reduction in gout flares with cherry extract in our study matches the 45% risk reduction for gout flares previously noted with cherry extract intake for two days compared with no intake in an observational case-crossover study.[7]
Both placebo effect and regression to the mean are viable alternate explanations for within group changes we noted in cherry extract and diet modification groups. We considered the possibility that people with more active gout may have enrolled in our study. However, demographics, comorbidity load and baseline gout flare rates in our patient sample were similar to people in previous RCTs in gout, which makes this less likely [41–46]. After accounting for a 20–30% placebo effect, a 39% reduction in gout flares and 50% reduction in HAQ scores in the cherry extract group indicates that some improvement (10–20%) in gout flares and function may be attributable to cherry extract. Study drop-outs did not differ from completers; partial completers had more baseline flares than completers. It is possible that the treatment effect could have been over-estimated if those without complete data were less likely to respond to cherry extract. Another possibility we considered was whether people enrolled in our study increased adherence to their ULT, but given no meaningful reduction in serum urate in either group, this possibility is unlikely to explain an improvement in outcomes in our study. Increased adherence to anti-inflammatory drugs by study subjects might also partially explain study findings. A detailed assessment of socioeconomic status and education could have informed us further about the study sample characteristics and generalizability of our findings, and will be done in the future trial.
Given the evidence from our pilot study, emerging data of its anti-inflammatory effect [13, 14] and its use by 40% or more gout patients [7, 8], a randomized placebo-controlled trial of cherry extract vs. placebo is needed to assess efficacy/effectiveness at this time. Some improvements may be due to the adoption of healthy behaviors including exercise during the study, rather than the intervention (cherry extract or diet modification). Since these were not measured and our pilot feasibility trial lacked a placebo arm, their contribution to observed effects cannot be determined. No changes in body mass index, diet, or ULT dose or adherence were noted during the study follow-up in the cherry extract arm, and therefore these factors are unlikely to explain these improved outcomes.
Several potential mechanisms for cherry extract’s beneficial effect on gout flare are hypothesized. Gout is associated with increased levels of inflammatory cytokines and oxidative stress [47], since xanthine oxidoreductase that converts hypoxanthine to xanthine to uric acid, also leads to the generation of reactive oxygen species [48]. Cherry concentrate lowered cytokines (interleukin-1-beta (IL-1-β), IL-6, IL-8, tumor necrosis factor-alpha (TNF-α), inflammatory marker, i.e., high-sensitivity C-reactive protein (hsCRP)) and sUA in healthy subjects.[13, 14] Anthocyanins and other phenolics that are found in cherries have antioxidant properties.[15–20] A future RCT will include these biomarkers to understand its mechanism of action.
Most improvements in gout flares in cherry extract arm were attributable to the subgroup that was concurrently using ULTs. As a subgroup analysis this should be interpreted with caution. Cherry extract may be providing anti-inflammatory prophylaxis in patients taking ULTs; only a quarter of our patients used colchicine, the usual choice for anti-inflammatory prophylaxis to prevent gout flares [21–23]. Other explanations may be higher cherry extract adherence and/or a less active disease in people taking concurrent ULT. This indicates that the future RCT should recruit patients with/without concurrent ULT and examine the effect of cherry extract by ULT use.
Our pilot study has several limitations. Lack of blinding likely contributed to a potential observer bias for patient-reported outcomes. Baseline gout flare frequency reporting is subject to recall bias; baseline flares may have been over-reported [49] or under-reported, and we are unable to predict the direction of recall bias; however similar gout flare rate has been reported in previous studies.[50] Gout flares were captured with 2-weekly gout flare questionnaire during the 9-month study follow-up, a robust method of flare assessment, but differing in method and duration (9-month vs. 1 year) from baseline flare assessment. To overcome this, we compared the monthly gout flare rates within each group. Our study provided estimates for both patient-reported and validated gout flares [31, 32]. Our short follow-up limits the ability to understand the long-term effects of cherry extract or diet modification, and whether patients can adhere to these interventions long-term. A future placebo-controlled RCT of 12–16 month duration can eliminate the issues of the recall bias and short study duration, by allowing for the occurrence of adequate number of gout flares. Whether a sample of people participating in Internet studies is more or less generalizable vs. large academic center gout studies in unclear to us.
In summary, we conducted one of the first pilot Internet gout studies of two active interventions, with high completion rates. Findings from this pilot feasibility study have allowed us to finalize key procedures, intervention, comparisons and outcomes for our next step large, hypothesis-testing trial of cherry extract vs. placebo in people with gout. The proposed RCT will answer the question whether cherry extract can be an adjunct treatment in the management of gout. Regardless of the outcome of the proposed RCT, the execution of an Internet RCT approach has the potential to make studies in rheumatology more affordable and easier to do for patients. It might even make samples more generalizable than the traditional RCTs that select samples from high-volume clinic research sites.
Supplementary Material
Acknowledgements:
We thank all the patients for participating in the study.
Funding: This study was funded by an intramural grant from the UAB Center for Outcomes and Effectiveness Research and Education (COERE)/Minority Health Research Center (PI, Singh) and an intramural grant from the UAB Center for Clinical and Translational Studies (CCTS; PI, Singh). The funding body did not play any role in design, in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
List of Abbreviations
- CONSORT
CONsolidated Standards of Reporting Trials
- IRB
Institutional Review Board
- UAB
University of Alabama at Birmingham
- RCT
randomized controlled trial
- HAQ
Health assessment questionnaire
- AEs
adverse events
- SU
serum urate
- ACR
American College of Rheumatology
- RedCap
Research Electronic Data Capture
Footnotes
Financial Conflict: JAS has received consultant fees from Crealta/Horizon, Fidia, UBM LLC, Medscape, WebMD, the National Institutes of Health and the American College of Rheumatology. JAS is a member of the Veterans Affairs Rheumatology Field Advisory Committee. JAS is the editor and the Director of the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis. JAS served as a member of the American College of Rheumatology’s (ACR) Annual Meeting Planning Committee (AMPC) and Quality of Care Committees, the Chair of the ACR Meet-the-Professor, Workshop and Study Group Subcommittee and the co-chair of the ACR Criteria and Response Criteria subcommittee. JAS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms-length funding from 36 companies. SM has no relevant disclosures. RW is the owner and CEO of Viocare, a company that markets online dietary assessment tools. KGS has received research grants from Amgen, Ironwood/AstraZeneca, Horizon, Merck, SOBI, and Takeda pharmaceuticals and consultant fees from Abbott, Amgen, Ironwood/AstraZeneca, Bayer, BMS, Horizon, Lilly, Merck, Pfizer, Radius, Roche/Genentech, SOBI, and Takeda pharmaceuticals. Other authors have no relevant disclosures.
Declarations
Ethics approval and consent to participate: The University of Alabama at Birmingham’s (UAB) Institutional Review Board (IRB) approved this study. Each patient participating in the study provided informed consent. All investigations were conducted in conformity with ethical principles of research.
Conflict to publish: Not applicable
Availability of data and materials:
We will make data available to colleagues, after appropriate approvals and permissions from the respective IRBs including the UAB IRB have been obtained, and UAB data security and data transfer requirements are met.
References
- 1.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–41. [DOI] [PubMed] [Google Scholar]
- 2.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer K, Carr A, Doherty M. Patient and provider barriers to effective management of gout in general practice: a qualitative study. Ann Rheum Dis. 2012;71(9):1490–5. [DOI] [PubMed] [Google Scholar]
- 4.Lindsay K, Gow P, Vanderpyl J, Logo P, Dalbeth N. The experience and impact of living with gout: a study of men with chronic gout using a qualitative grounded theory approach. J Clin Rheumatol. 2011;17(1):1–6. [DOI] [PubMed] [Google Scholar]
- 5.Harrold LR, Mazor KM, Velten S, Ockene IS, Yood RA. Patients and providers view gout differently: a qualitative study. Chronic Illn. 2010;6(4):263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh JA, Shah N, Edwards NL. A cross-sectional internet-based patient survey of the management strategies for gout. BMC Complement Altern Med. 2016;16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Neogi T, Chen C, Chaisson C, Hunter DJ, Choi HK. Cherry consumption and decreased risk of recurrent gout attacks. Arthritis Rheum. 2012;64(12):4004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh JA, Bharat A, Edwards NL. An internet survey of common treatments used by patients with gout including cherry extract and juice and other dietary supplements. J Clin Rheumatol. 2015;21(4):225–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Chen C, Choi H, Chaisson C, Hunter D, Niu J, et al. Purine-rich foods intake and recurrent gout attacks. Ann Rheum Dis. 2012;71(9):1448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Woods R, Chaisson CE, Neogi T, Niu J, McAlindon TE, et al. Alcohol consumption as a trigger of recurrent gout attacks. American Journal of Medicine. 2006;119(9):800.e13–8. [DOI] [PubMed] [Google Scholar]
- 11.Dalbeth N, Ames R, Gamble GD, Horne A, Wong S, Kuhn-Sherlock B, et al. Effects of skim milk powder enriched with glycomacropeptide and G600 milk fat extract on frequency of gout flares: a proof-of-concept randomised controlled trial. Ann Rheum Dis. 2012;71(6):929–34. [DOI] [PubMed] [Google Scholar]
- 12.Schlesinger N, Rabinowitz R, Schlesinger M. Pilot studies of cherry juice concentrate for gout flare prophylaxis. J Arthritis. 2012;1:1–5. [DOI] [PubMed] [Google Scholar]
- 13.Bell PG, Gaze DC, Davison GW, George TW, Scotter MJ, Howatson G. Montmorency tart cherry (Prunus cerasus L.) concentrate lowers uric acid, independent of plasma cyanidin-3-O-glucosiderutinoside. Journal of Functional Foods. 2014;11:82–90. [Google Scholar]
- 14.Bell PG, Walshe IH, Davison GW, Stevenson E, Howatson G. Montmorency Cherries Reduce the Oxidative Stress and Inflammatory Responses to Repeated Days High-Intensity Stochastic Cycling. Nutrients. 2014;6:829–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.C A, WR E. Total Anthocyanins and Total Phenolics of Fresh and Processed Cherries and Their Antioxidant Properties. Journal of Food Science. 2004;69(1):FCT67–FCT72. [Google Scholar]
- 16.Ou B, Bosak KN, Brickner PR, Iezzoni DG, Seymour EM. Processed tart cherry products--comparative phytochemical content, in vitro antioxidant capacity and in vitro anti-inflammatory activity. J Food Sci. 2012;77(5):H105–12. [DOI] [PubMed] [Google Scholar]
- 17.Piccolella S, Fiorentino A, Pacifico S, D’Abrosca B, Uzzo P, Monaco P. Antioxidant properties of sour cherries (Prunus cerasus L.): role of colorless phytochemicals from the methanolic extract of ripe fruits. J Agric Food Chem. 2008;56(6):1928–35. [DOI] [PubMed] [Google Scholar]
- 18.Seeram NP, Momin RA, Nair MG, Bourquin LD. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 2001;8(5):362–9. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Nair MG, Strasburg GM, Booren AM, Gray JI. Novel antioxidant compounds from tart cherries (Prunus cerasus). J Nat Prod. 1999;62(1):86–8. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Nair MG, Strasburg GM, Booren AM, Gray JI. Antioxidant polyphenols from tart cherries (Prunus cerasus). J Agric Food Chem. 1999;47(3):840–4. [DOI] [PubMed] [Google Scholar]
- 21.Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh JA. Research Priorities in Gout: The Patient Perspective (In press). J Rheumatol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institute of Medicine. 100 Initial Priority Topics for Comparative Effectiveness Research. http://www.iom.edu/~/media/Files/Report%20Files/2009/ComparativeEffectivenessResearchPriorities/Stand%20Alone%20List%20of%20100%20CER%20Priorities%20-%20for%20web.ashx. Institute of Medicine; 2009. Available at. Accessed 01/30/2014. [Google Scholar]
- 26.Kristal AK, Shattuck AL, Williams AE. Food frequency questionnaires for diet intervention research. Proceeding of the 17th National Nutrient Databank Conference, June 1992. Washington, DC: International Life Sciences Institute; 1992. p. 110–25. [Google Scholar]
- 27.Kristal AR, Kolar AS, Fisher JL, Plascak JJ, Stumbo PJ, Weiss R, et al. Evaluation of web-based, self-administered, graphical food frequency questionnaire. J Acad Nutr Diet. 2014;114(4):613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–87. [DOI] [PubMed] [Google Scholar]
- 29.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895–900. [DOI] [PubMed] [Google Scholar]
- 30.Knowledge Base. Cherry extract. What is it? http://www.wholehealthchicago.com/466/cherry-fruit-extract/. Chicago, IL: WholeHealth Chocago. The Center for Integrative Medicine. Available at. Accessed 06/21/2014. [Google Scholar]
- 31.Gaffo AL, Schumacher HR, Saag KG, Taylor WJ, Dinnella J, Outman R, et al. Developing a provisional definition of flare in patients with established gout. Arthritis Rheum. 2012;64(5):1508–17. [DOI] [PubMed] [Google Scholar]
- 32.Gaffo AL, Dalbeth N, Saag KG, Singh JA, Rahn EJ, Mudano AS, et al. Brief Report: Validation of a Definition of Flare in Patients With Established Gout. Arthritis Rheumatol. 2018;70(3):462–7. [DOI] [PubMed] [Google Scholar]
- 33.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23(2):137–45. [DOI] [PubMed] [Google Scholar]
- 34.Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9(5):789–93. [PubMed] [Google Scholar]
- 35.Singh JA, Taylor WJ, Simon LS, Khanna PP, Stamp LK, McQueen FM, et al. Patient-reported outcomes in chronic gout: a report from OMERACT 10. J Rheumatol. 2011;38(7):1452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient’s perspective. J Rheumatol. 1993;20(3):557–60. [PubMed] [Google Scholar]
- 37.Stanbio Laboratory. An EKF Diagnostics Company. Uric Acid LiquiColor® Test (Enzymatic). http://www.stanbio.com/products/chemistry? Boerne, TX: Stanbio Laboratory. Available at. Accessed 12/07/2012. [Google Scholar]
- 38.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–32. [DOI] [PubMed] [Google Scholar]
- 39.Carlsen MH, Halvorsen BL, Holte K, Bohn SK, Dragland S, Sampson L, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. International Journal of Food Science and Technology. 2002;37:153–61. [Google Scholar]
- 41.Becker MA, Schumacher HR Jr., Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450–61. [DOI] [PubMed] [Google Scholar]
- 42.Becker MA, Schumacher HR Jr., Wortmann RL, MacDonald PA, Palo WA, Eustace D, et al. Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with gout. Arthritis Rheum. 2005;52(3):916–23. [DOI] [PubMed] [Google Scholar]
- 43.Goldfien R, Pressman A, Jacobson A, Ng M, Avins A. A Pharmacist-Staffed, Virtual Gout Management Clinic for Achieving Target Serum Uric Acid Levels: A Randomized Clinical Trial. Perm J. 2016;20(3):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poiley J, Steinberg AS, Choi YJ, Davis CS, Martin RL, McWherter CA, et al. A Randomized, Double-Blind, Active- and Placebo-Controlled Efficacy and Safety Study of Arhalofenate for Reducing Flare in Patients With Gout. Arthritis Rheumatol. 2016;68(8):2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saag KG, Fitz-Patrick D, Kopicko J, Fung M, Bhakta N, Adler S, et al. Lesinurad Combined With Allopurinol: Randomized, Double-Blind, Placebo-Controlled Study in Gout Subjects With Inadequate Response to Standard of Care Allopurinol (A US-based Study). Arthritis Rheumatol. 2016. [DOI] [PubMed] [Google Scholar]
- 46.Schumacher HR Jr., Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59(11):1540–8. [DOI] [PubMed] [Google Scholar]
- 47.Zamudio-Cuevas Y, Hernandez-Diaz C, Pineda C, Reginato AM, Cerna-Cortes JF, Ventura-Rios L, et al. Molecular basis of oxidative stress in gouty arthropathy. Clinical rheumatology. 2015;34(10):1667–72. [DOI] [PubMed] [Google Scholar]
- 48.Battelli MG, Polito L, Bortolotti M, Bolognesi A. Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects. Oxidative medicine and cellular longevity. 2016;2016:3527579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg. 2010;126(2):619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khanna PP, Nuki G, Bardin T, Tausche AK, Forsythe A, Goren A, et al. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: Results from a cross-sectional survey. Health Qual Life Outcomes. 2012;10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We will make data available to colleagues, after appropriate approvals and permissions from the respective IRBs including the UAB IRB have been obtained, and UAB data security and data transfer requirements are met.


