As vaccination policies for SARS-CoV-2 have evolved to include younger, active populations,1 evidence of vaccine hesitancy in some groups has emerged.2 For athletic individuals, a key concern relates to the potential effect of vaccine-associated side-effects on sporting participation;3, 4 even brief interruptions to training can affect athletic performance.5
To evaluate this concern, in the context of the Tokyo Olympic and Paralympic Games, we established a prospective monitoring system to characterise SARS-CoV-2 vaccine side-effects and their effects on sporting participation. Elite international athletes (UK athletes preparing for Olympic and Paralympic competition) completed a daily electronic questionnaire for 10 days following SARS-CoV-2 vaccination via a mobile phone application. Serious vaccine complications were logged on a separate electronic medical record system. Prior informed consent to use anonymised athlete data was obtained.
Complete data were available for 127 athletes (57 [45%] female, 70 [55%] male; mean age 27·5 years [SD 4·9]) who received two doses of the Pfizer-BioNTech vaccine tozinameran (BNT162b2), administered between May 12 and Sept 2, 2021, in line with licenced recommendations, from 435 athletes regularly using a COVID-19 monitoring application. Of these participants, 97 (76%) were Olympic sport athletes and 30 (24%) were Paralympic sport athletes, with 67 subsequently competing at the Tokyo Olympic Games and 22 competing at the Tokyo Paralympic Games.
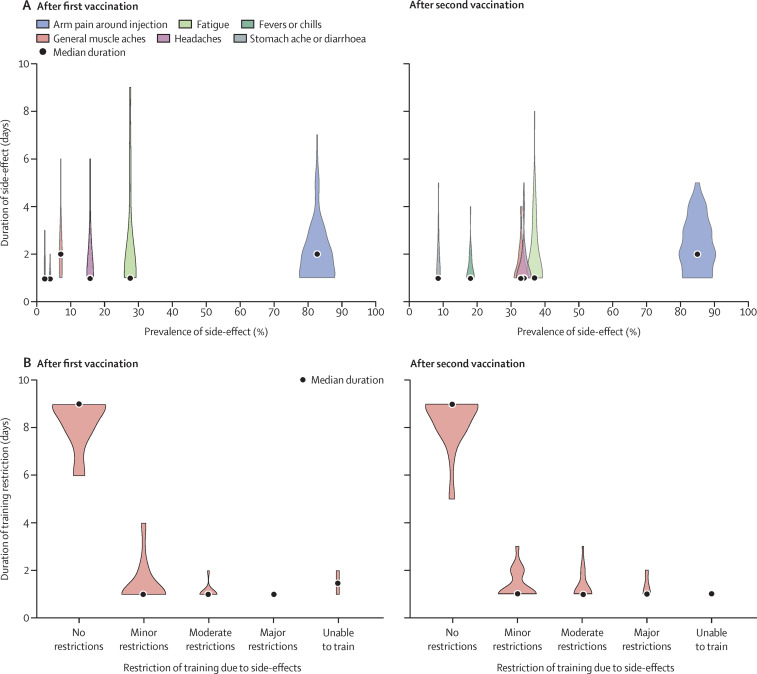
The most prevalent side-effect was arm pain around the injection site (in 94% of athletes), lasting a median of 2 days (IQR 1–3). Systemic side-effects were reported in 70% of participants, with generalised fatigue in 28% after the first vaccination (median 1 day [1–2]) and 37% after the second vaccination (1 day [1–3]; figure A ). Several side-effects were more commonly reported (p<0·01) after the second vaccination than after the first: fever or chills (2% after first vaccination vs 18% after second vaccination), muscle aches (7% vs 33%), and headache (16% vs 34%). Most athletes (93 [73%]) reported zero or only minor effect on their ability to train (figure B); eight (6%) athletes felt completely unable to train, of whom seven returned to training after 1 day. There was no difference in vaccine side-effect profiles between Paralympic and non-Paralympic athletes, nor between male and female athletes.
Figure.
Prevalence and duration of SARS-CoV-2 vaccine side-effects in athletes and effect on sporting participation
(A) Prevalence and duration of Pfizer-BioNTech SARS-CoV-2 vaccine tozinameran (BNT162b2) side-effects in an elite international athlete cohort. (B) Severity and duration of training restrictions due to vaccine side-effects. The width of each shape indicates the number of athletes at that duration.
Our findings align with those reported in a UK community-based study; data from the Zoe COVID-19 tracking app revealed that 66% of individuals reported at least one local adverse effect and 25% had at least one systemic adverse effect, including fatigue and headache, lasting on average 1 day and being more prevalent following the second vaccination.6
Vaccination against a wide range of pathogens plays an important part in maintaining athlete health and is generally done without a need to interrupt training schedule.7 Our findings indicate that continuation of sport after SARS CoV-2 vaccination is also appropriate. Indeed, exercising in the peri-vaccination period might enhance the immune response,8 although further studies in this area are required. Protecting elite athletes against SARS-CoV-2 infection is important, with a 2021 report indicating that around one in four have not returned fully to sport a month after the onset of infection.9
Potential weaknesses of our study include the fact that the cohort was small and thus inadequately powered to detect infrequent adverse events. Our dataset also only provides insight regarding tozinameran; the UK vaccination strategy favoured use of this vaccine type in young individuals, and thus further work is needed to assess the impact of other SARS-CoV-2 vaccines in this population. Our methodological approach could also be used to evaluate tolerability and impact of non-SARS CoV-2 vaccines.
In conclusion, in elite athletes, vaccination against SARS-CoV-2 with the Pfizer-BioNTech vaccine tozinameran appears to be well tolerated and associated with few significant side-effects. When side-effects occurred, they were short-lived and did not affect sporting participation. This analysis should help to inform discussion regarding the risks and benefits of SARS-CoV-2 vaccination in the context of sport.
We declare no competing interests. We thank the athletes for their contribution to data collection and to the EIS Athlete Health, and Performance Data teams for developing the tracking app.
References
- 1.Narducci DM, Diamond AB, Bernhardt DT, Roberts WO. COVID vaccination in athletes and updated interim guidance on the preparticipation physical examination during the SARS-Cov-2 pandemic. Clin J Sport Med. 2021 doi: 10.1097/jsm.0000000000000981. published online Nov 1. [DOI] [PubMed] [Google Scholar]
- 2.Ainslie D, Ogwuru C, Sinclair R. Coronavirus and vaccine hesitancy, Great Britain: 9 August 2021. Aug 9, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandwellbeing/bulletins/coronavirusandvaccinehesitancygreatbritain/9august2021
- 3.Ingle S. Some GB Olympic athletes refusing COVID vaccine over side-effect fears. June 25, 2021. https://www.theguardian.com/sport/2021/jun/25/some-gb-olympic-athletes-still-refusing-to-have-covid-vaccine-boa-claims-athletics
- 4.Hull JH, Schwellnus MP, Pyne DB, Shah A. COVID-19 vaccination in athletes: ready, set, go…. Lancet Respir Med. 2021;9:455–456. doi: 10.1016/S2213-2600(21)00082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drew MK, Raysmith BP, Charlton PC. Injuries impair the chance of successful performance by sportspeople: a systematic review. Br J Sports Med. 2017;51:1209–1214. doi: 10.1136/bjsports-2016-096731. [DOI] [PubMed] [Google Scholar]
- 6.Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gärtner BC, Meyer T. Vaccination in elite athletes. Sports Med. 2014;44:1361–1376. doi: 10.1007/s40279-014-0217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker FL, Smith KA, Zúñiga TM, et al. Acute exercise increases immune responses to SARS CoV-2 in a previously infected man. Brain Behav Immun Health. 2021;18 doi: 10.1016/j.bbih.2021.100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hull JH, Wootten M, Moghal M, et al. Clinical patterns, recovery time and prolonged impact of COVID-19 illness in international athletes: the UK experience. Br J Sports Med. 2021 doi: 10.1136/bjsports-2021-104392. published online Aug 2. [DOI] [PubMed] [Google Scholar]