Abstract
The growth response of Helicobacter pylori in broth was determined in the presence of ferrous sulfate, sodium pyruvate, and mucin (porcine stomach). The addition of either ferrous sulfate and sodium pyruvate or mucin to brain heart infusion broth with 7% horse serum (BHI-HS) enhanced the growth of H. pylori. The best growth of strain NB2-1, which was the slowest growing of 10 H. pylori strains evaluated, occurred in the presence of 0.05% ferrous sulfate and 0.05% sodium pyruvate. The addition of 0.3% mucin to BHI-HS reduced the lag time of H. pylori by 48 h and enhanced the growth. On the basis of the results for 10 H. pylori strains, the combination of ferrous sulfate (0.025%), sodium pyruvate (0.025%), and mucin (0.15%) in BHI-HS counteracted the inhibitory effects of the antibiotics used in culture media for selective growth of H. pylori. Results suggest that these supplements may be useful for enhancement of the growth of H. pylori in enrichment media.
Helicobacter pylori induces chronic gastric inflammation and gastric ulcer and may also be a contributory factor in human gastric carcinoma (10, 17). Besides its natural habitat in the human stomach, other sources of H. pylori and its mode of transmission are unknown (4). Identification of the reservoirs and routes of infection of H. pylori has been impeded by the difficulty of isolating the pathogen from foods and environmental sources. This is largely attributed to the fastidious nature of H. pylori and its relatively low growth rate, thereby limiting its growth in a competitive environment.
H. pylori requires for growth a nutrient-rich medium, serum, and a microaerobic environment. Under the best conditions, on a nonselective agar-containing medium, 2 to 3 days is generally required for the formation of small colonies, whereas the implementation of growth in a liquid medium can be more challenging (12). Some supplements such as whole blood, serum, yeast extract, lysed human erythrocytes, IsoVitaleX, hemin, cyclodextrin, and cholesterol have been determined to enhance H. pylori growth in culture broth (6, 9, 18). However, growth of H. pylori in liquid media is influenced by the initial inoculum concentration and also strain variation (12, 19). Generally, the lower the initial inoculum of H. pylori, the longer the lag time. To determine the occurrence of H. pylori in environmental and food sources, a selective enrichment medium is needed that will enable growth of a small population of helicobacters to detectable levels in an environment that may contain large populations of competitive microorganisms. The purpose of the study described here was to identify supplements to culture media that would enhance the growth of H. pylori under culture conditions with antibiotics that suppress the growth of competitive microorganisms.
Bacterial strains and culture conditions.
A total of 10 H. pylori strains were used for this study. Five H. pylori strains, strains NB2-1, 1324P-1, 26695, G2-1, and WV 99, were obtained from Douglas Berg (Washington University, St. Louis, Mo.), and five fresh clinical isolates of H. pylori, strains D5131, D5135, D5136, D5178, and D5251, were obtained from Ben Gold (Emory University and Centers for Disease Control and Prevention, Atlanta, Ga.). Bacteria were grown on plates of brain heart infusion (BHI) agar supplemented with 7% horse serum (HS). Cultures were incubated at 37°C for 3 days in a GasPak jar (BBL Microbiology Systems, Cockeysville, Md.) whose atmosphere was evacuated three times and replaced with a microaerobic gas mixture composed of 5% oxygen, 10% carbon dioxide, and 85% nitrogen.
Growth studies.
Cultures were prepared by the procedures described previously by Jiang and Doyle (8). Briefly, 3-day-old cultures were harvested from BHI agar-HS plates. A washed suspension of ca. 5 × 108 H. pylori cells per ml was prepared in fresh BHI broth-HS and was inoculated into fresh BHI broth-HS to provide a final inoculum of approximately 5 × 103 CFU per ml.
Prior to inoculation of H. pylori, appropriate volumes of filter-sterilized (pore size, 0.2 μm; Nalgene sterile syringe filter) ferrous sulfate and sodium pyruvate (FP; Sigma Chemical Co., St. Louis, Mo.) solutions (5%) were added aseptically to 100 ml of sterile BHI broth-HS to provide final concentrations of each component in FP of 0.025, 0.05, 0.075, and 0.10%. Porcine stomach mucin (Sigma Chemical Co.) was added to BHI broth at final concentrations of 0.15, 0.30, and 0.45% prior to heating in an autoclave. H. pylori-selective supplement (Dent) containing vancomycin (10 mg/liter), trimethroprim lactate (5 mg/liter), cefsulodin (5 mg/liter), and amphotericin B (5 mg/liter), purchased from Oxoid (Oxoid, Hampshire, England), and filter sterilized (pore size, 0.2 μm; Nalgene sterile syringe filter) polymyxin B solution (2,500 U/liter) were added to 100-ml volumes of BHI broth-HS for studies with antibiotics. A combination of ferrous sulfate (0.025%), sodium pyruvate (0.025%), and mucin (0.15%) was used with the antibiotics.
H. pylori was inoculated as described above, and cultures were incubated at 37°C in a GasPak jar under microaerobic conditions with agitation at 150 rpm.
Enumeration of H. pylori.
At appropriate times, cultures were serially diluted (1:10) in 0.01 M phosphate-buffered saline (pH 7.2), and H. pylori was enumerated in duplicate by surface plating onto BHI agar-HS. The plates were then incubated at 37°C for 3 to 7 days in GasPak jars under microaerobic conditions. Generation times were derived from the exponential growth rates and were determined in duplicate.
Results.
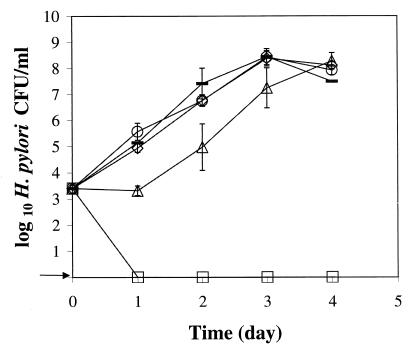
H. pylori strain NB2-1, the slowest growing of 10 H. pylori strains evaluated, did not grow within 4 days in BHI broth-HS at 37°C with agitation under microaerobic conditions. However, the bacteria grew well in BHI broth-HS supplemented with ferrous sulfate and sodium pyruvate and held in the same GasPak jar under the same environmental conditions (Fig. 1). Growth was visible at 24 h in the broths supplemented with 0.05, 0.075, and 0.1% FP, with generation times of 3.2 to 4.3 h during 72 to 96 h of growth. Growth was delayed about 24 h in BHI broth-HS supplemented with 0.025% FP, but thereafter, the generation time was 3.7 h.
FIG. 1.
Growth of H. pylori strain NB2-1 at 37°C with agitation under microaerobic conditions in BHI broth-HS with different concentrations of ferrous sulfate and sodium pyruvate (FP). Symbols: □, BHI broth-HS; ▵, BHI broth-HS with 0.025% FP; ◊, BHI broth-HS with 0.05% FP; ○, BHI broth-HS with 0.075% FP; —, BHI broth-HS with 0.1% FP. The arrow indicates that H. pylori was not detected at the minimum level of sensitivity (<10 CFU/ml).
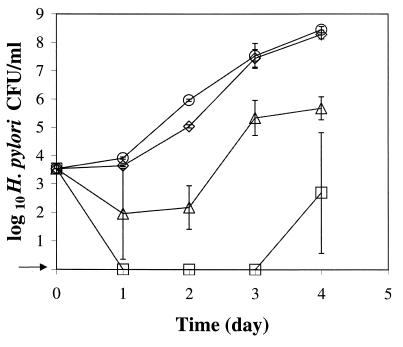
Growth of H. pylori strain NB2-1 was also enhanced by supplementing BHI broth-HS with mucin (Fig. 2). Porcine gastric mucin at concentrations of 0.15, 0.30, and 0.45% reduced the lag time by 24 to 48 h compared with that for growth in unsupplemented BHI broth-HS. The generation times for H. pylori in broths supplemented with mucin at 0.15, 0.30, and 0.45% were 4.3, 3.8, and 4.0 h, respectively. The addition of mucin at either 0.3 or 0.45% had a similar effect on enhancing the growth of strain NB2-1.
FIG. 2.
Growth of H. pylori strain NB2-1 at 37°C with agitation under microaerobic conditions in BHI broth-HS with different concentrations of mucin. Symbols: □, BHI broth-HS; ▵, BHI broth-HS with 0.15% mucin; ◊, BHI broth-HS with 0.3% mucin; ○, BHI broth-HS with 0.45% mucin. The arrow indicates that H. pylori was not detected at the minimum level of sensitivity (<10 CFU/ml).
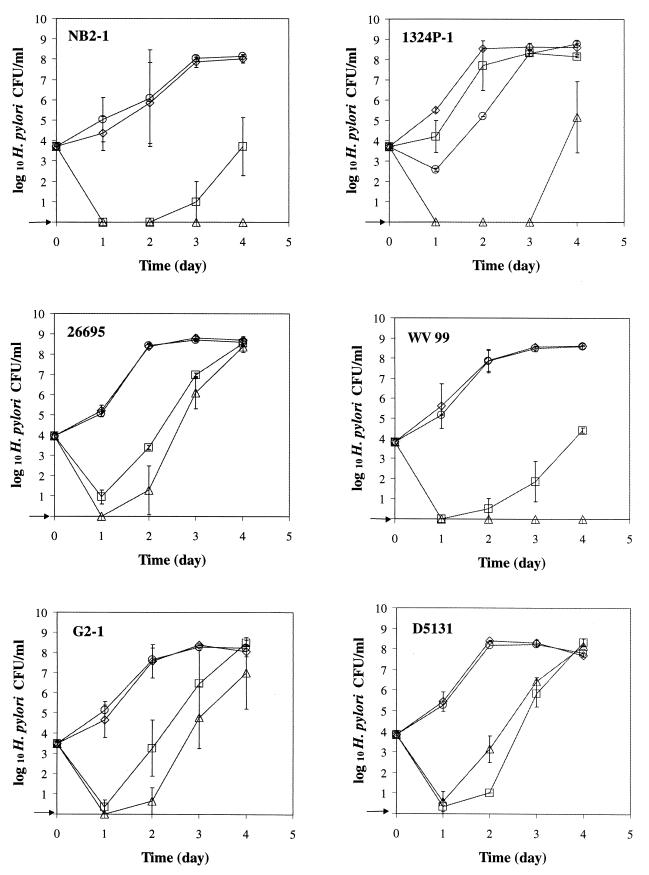
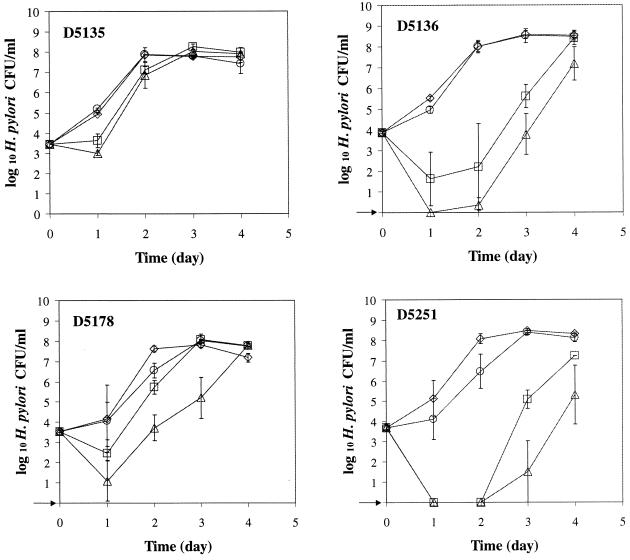
The five antibiotics used in this study had an inhibitory effect by delaying the growth of all strains except strain D5131 (Fig. 3). The addition of 0.15% mucin and 0.025% FP to BHI broth-HS with antibiotics reduced the lag time by 24 h for strains D5135 and D5178, by 48 h for strains 26695, G2-1, D5131, and D5136, and by at least 72 h for strains NB2-1, 1324P-1, WV 99, and D5251. Except for strains 1324P-1 and D5251, there was little difference in the growth rates of helicobacters in broth supplemented with mucin and FP or with mucin, FP, and antibiotics. With the low inoculum level (ca. 1 × 102 CFU/ml), the growth rates of H. pylori in the presence and absence of mucin, FP, and/or antibiotics were similar to that observed with a higher inoculum level (ca. 5 × 103 CFU/ml; data not shown). Overall, the counts for all H. pylori strains evaluated were substantially greater during the first 24 h of incubation when grown in BHI broth-HS supplemented with FP and mucin than in BHI broth-HS with no supplements.
FIG. 3.
Growth of 10 H. pylori strains at 37°C with agitation under microaerobic conditions. Symbols: □, BHI broth-HS; ▵, BHI broth-HS with antibiotics (vancomycin [10 mg/liter], trimethroprim lactate [5 mg/liter], cefsulodin [5 mg/liter], amphotericin B [5 mg/liter], and polymyxin B [2,500 U/liter]); ◊, BHI broth-HS with 0.025% FP and 0.15% mucin; ○, BHI broth-HS with 0.025% FP, 0.15% mucin, and antibiotics. The arrow indicates that H. pylori was not detected at the minimum level of sensitivity (<10 CFU/ml).
Discussion.
Microaerophilic bacteria such as campylobacters are more sensitive than aerobic bacteria to toxic forms of oxygen derivatives such as hydrogen peroxide, singlet oxygen, and superoxide ions (7). A combination of ferrous sulfate, sodium metabisulfite, and sodium pyruvate (FBP) has successfully been used to enhance the growth and aerotolerance of Campylobacter spp. by neutralizing the toxic effect of oxygen (3). Even under optimal atmospheric conditions, the growth of Campylobacter jejuni was enhanced with iron supplementation (0.025% FeSO4) (15). Many selective media for isolation of campylobacters contain some or all of these compounds at concentrations in the range of 0.025 to 0.05% (2, 3). However, sodium metabisulfite is inhibitory to the growth of H. pylori (5). Therefore, in this study we chose FP as a medium supplement for H. pylori growth. Among the concentrations of FP studied, the best growth of H. pylori occurred in 0.05% FP. Theoretically, both ferrous iron and pyruvate undergo oxidative reactions under physiological conditions in the living cells. Hence, the function of FP for H. pylori is likely the same as that of FBP for Campylobacter spp.
Mucin, a high-molecular-weight glycoprotein, is the principal constituent of mucus, in which H. pylori occurs naturally in vivo. Studies have revealed that H. pylori binds to human gastric and salivary mucin in vitro (13, 16). The sulfated carbohydrates of mucin are responsible for this interaction. Namavar et al. (13) suggested that H. pylori uses its temporary attachment to mucin as a means of “tracking” toward the epithelium in vivo. Our observations by microscopic examination revealed that H. pylori cells often form clumps during the early stages of incubation in a liquid growth medium. Cellini et al. (1) also observed that the coccoid forms of H. pylori tended to aggregate in clusters. By attaching to mucin in the medium, H. pylori may form cell clumps that protect the cells from the harmful effects of the oxygen dissolved in the surrounding liquid. The increased viscosity provided by mucin in supplemented broth may also create a more favorable microaerobic environment for H. pylori growth initiation in a liquid environment. Slomiany et al. (14) reported that H. pylori can enzymatically degrade mucin, suggesting that mucin may serve as a nutrient for growth of H. pylori.
Antibiotics have been widely used as selective agents in enrichment and plating media for bacterial isolation. Most strains of H. pylori are inherently resistant to vancomycin, cefsulodin, trimethroprim lactate, polymyxins, and amphotericin B (11). However, the level of resistance to different antibiotics varies among strains of H. pylori, as well as among some injured bacterial cells that occur in the environment. Our studies have revealed that both FP and mucin can counteract the inhibitory effect of a combination of five antibiotics used to select for H. pylori. In this study, we tested five fresh clinical isolates of H. pylori, i.e., strains D5131, D5135, D5136, D5178, and D5251, in addition to five laboratory-adapted strains. Results revealed that FP and mucin have the same effect on enhancing the growth of both laboratory-adapted strains and clinical isolates recently obtained from infected patients.
In conclusion, we have determined that both FP and mucin can greatly reduce the growth lag time and enhance the growth of H. pylori in a liquid culture medium. The influence of these supplements in counteracting the inhibitory effects of antibiotics on H. pylori may be beneficial for the formulation of enrichment media for the isolation of H. pylori directly from the natural environment and foods.
Acknowledgments
This work was supported by a grant from the U.S. Department of Agriculture as part of the Alliance for Food Protection.
We thank Douglas Berg, Washington University, and Benjamin Gold, Centers for Disease Control and Prevention and Emory University, for providing isolates of H. pylori.
REFERENCES
- 1.Cellini L, Allocati N, Angelucci D, Lezzi T, Campli E D, Marzio L, Dainelli B. Coccoid Helicobacter pylori not culturable in vitro reverts in mice. Microbiol Immunol. 1994;38:843–850. doi: 10.1111/j.1348-0421.1994.tb02136.x. [DOI] [PubMed] [Google Scholar]
- 2.Corry J E L, Post D E, Colin P, Laisney M J. Culture media for the isolation of campylobacters. In: Corry J E L, Curtis G D W, Baird R M, editors. Culture media for food microbiology. Amsterdam, The Netherlands: Elsevier Science B. V.; 1995. pp. 129–163. [DOI] [PubMed] [Google Scholar]
- 3.George H A, Hoffman P S, Smibert R M, Krieg N R. Improved media for growth and aerotolerance of Campylobacter fetus. J Clin Microbiol. 1978;8:36–41. doi: 10.1128/jcm.8.1.36-41.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman K J, Correa P. The transmission of Helicobacter pylori. A critical review of the evidence. Int J Epidemiol. 1995;24:875–887. doi: 10.1093/ije/24.5.875. [DOI] [PubMed] [Google Scholar]
- 5.Hawrylik S J, Wasilko D J, Haskell S L, Gootz T D, Lee S E. Bisulfite or sulfite inhibits growth of Helicobacter pylori. J Clin Microbiol. 1994;32:790–792. doi: 10.1128/jcm.32.3.790-792.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henrisken T H, Broron O, Schoyen R, Thoresen T, Setegn D, Madebo T. Rapid growth of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1995;14:1008–1011. doi: 10.1007/BF01691385. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman P S, George H A, Krieg N R, Smibert R M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. II. Role of exogenous superoxide anions and hydrogen peroxide. Can J Microbiol. 1979;25:8–16. doi: 10.1139/m79-002. [DOI] [PubMed] [Google Scholar]
- 8.Jiang X P, Doyle M P. Effect of environmental and substrate factors on survival and growth of Helicobacter pylori. J Food Prot. 1998;61:929–933. doi: 10.4315/0362-028x-61.8.929. [DOI] [PubMed] [Google Scholar]
- 9.Kitsos C M, Stadtlander C T K-H. Helicobacter pylori in liquid culture: evaluation of growth rates and ultrastructure. Curr Microbiol. 1998;37:88–93. doi: 10.1007/s002849900344. [DOI] [PubMed] [Google Scholar]
- 10.McColl K E L. Helicobacter pylori: clinical aspects. J Infect. 1997;34:7–13. doi: 10.1016/s0163-4453(97)80003-5. [DOI] [PubMed] [Google Scholar]
- 11.Megraud F. Resistance of Helicobacter pylori to antibiotics. Aliment Pharmacol Ther. 1997;11(Suppl. 1):43–53. doi: 10.1046/j.1365-2036.11.s1.11.x. [DOI] [PubMed] [Google Scholar]
- 12.Morgan D R, Freedman R, Depew C E, Kraft W G. Growth of Campylobacter pylori in liquid media. J Clin Microbiol. 1987;25:2123–2125. doi: 10.1128/jcm.25.11.2123-2125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Namavar F, Sparrius M, Veerman E C, Appelmelk B J, Vandenbroucke-Grauls C M. Neutrophil-activating protein mediates adhesion of Helicobacter pylori to sulfated carbohydrates on high-molecular-weight salivary mucin. Infect Immun. 1998;66:444–447. doi: 10.1128/iai.66.2.444-447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slomiany B L, Bilski J, Serosisk J, Marty V L N, Dworkin H, VanHorn K, Zielenski J, Slomiany A. Campylobacter pylori degrades mucin and undermines gastric mucosal integrity. Biochem Biophys Res Commun. 1987;14:307–314. doi: 10.1016/s0006-291x(87)80511-9. [DOI] [PubMed] [Google Scholar]
- 15.Stern N J, Kazmi S U, Roberson B S, Ono K, Juven B J. Response of Campylobacter jejuni to combinations of ferrous sulphate and cadmium chloride. J Appl Bacteriol. 1988;64:247–256. doi: 10.1111/j.1365-2672.1988.tb03382.x. [DOI] [PubMed] [Google Scholar]
- 16.Tzouvelekis, L. S., A. F. Mentis, A. M. Makris, C. Spiliadis, C. Blackwell, and D. M. Weir. In vitro binding of Helicobacter pylori to human gastric mucin. Infect. Immun. 59:4252–4254. [DOI] [PMC free article] [PubMed]
- 17.Warren J R, Marshall B J. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 18.Wust J, Rutsch M, Stocker S. Enhanced growth of Helicobacter pylori using a liquid medium supplemented with human serum. Eur J Clin Microbiol Infect Dis. 1995;14:155–157. doi: 10.1007/BF02111882. [DOI] [PubMed] [Google Scholar]
- 19.Xia H X, English L, Keane C T, O'Morain C A. Enhanced cultivation of Helicobacter pylori in liquid media. J Clin Pathol. 1993;46:750–753. doi: 10.1136/jcp.46.8.750. [DOI] [PMC free article] [PubMed] [Google Scholar]