Abstract
Background
Uncomplicated urinary tract infection (uUTI) is predominantly caused by Escherichia coli, which has increasing antimicrobial resistance (AMR) at the United States (US)-community level. As uUTI is often treated empirically, assessing AMR is challenging, and there are limited contemporary data characterizing period prevalence in the US.
Methods
This was a retrospective study of AMR using Becton, Dickinson and Company Insights Research Database (Franklin Lakes, New Jersey, US) data collected 2011–2019. Thirty-day, nonduplicate Escherichia coli urine isolates from US female outpatients (aged ≥12 years) were included. Isolates were evaluated for nonsusceptibility (intermediate/resistant) to trimethoprim-sulfamethoxazole, fluoroquinolones, or nitrofurantoin, and assessed for extended-spectrum β-lactamase production (ESBL+) and for ≥2 or ≥3 drug-resistance phenotypes. Generalized estimating equations were used to model AMR trends over time and by US census region.
Results
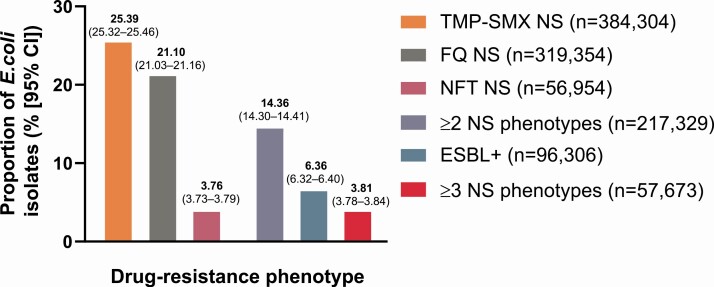
Among 1 513 882 E. coli isolates, the overall prevalence of isolates nonsusceptible to trimethoprim-sulfamethoxazole, fluoroquinolones, and nitrofurantoin was 25.4%, 21.1%, and 3.8%, respectively. Among the isolates, 6.4% were ESBL+, 14.4% had ≥2 drug-resistance phenotypes, and 3.8% had ≥3. Modeling demonstrated a relative average yearly increase of 7.7% (95% confidence interval [CI], 7.2–8.2%) for ESBL+ isolates and 2.7% (95% CI, 2.2–3.2%) for ≥3 drug-phenotypes (both P < .0001). Modeling also demonstrated significant variation in AMR prevalence between US census regions (P < .001).
Conclusions
Period prevalence of AMR among US outpatient urine-isolated E. coli was high, and for multidrug-resistance phenotypes increased during the study period with significant variation between census regions. Knowledge of regional AMR rates helps inform empiric treatment of community-onset uUTI and highlights the AMR burden to physicians.
Keywords: Escherichia coli, antimicrobial resistance, uncomplicated urinary tract infection, antimicrobial stewardship
This study provides contemporary data on the prevalence and trends of antimicrobial resistance among E. coli isolated from female outpatient urine cultures in the United States, 2011–2019. The prevalence of antimicrobial resistance is high and increasing for key phenotypes.
Urinary tract infections (UTIs) are among the most common community-onset bacterial infections [1] and are treated by a variety of healthcare professionals. Uncomplicated UTIs (uUTIs), those occurring in females in the absence of underlying abnormalities of the urinary tract or immunosuppression, affect 10–12% of adult women at least once per year in the United States (US), with 20–30% of those having a subsequent recurrent infection [2, 3]. The incidence of uUTI is bimodal, with peaks in young, sexually active women aged 15–24 years [4, 5] and in postmenopausal women [1]. The microbial etiology of uUTI is well characterized, with Escherichia coli being the predominant uropathogen isolated in community-onset uUTI [6, 7].
There has been a notable increase in antimicrobial resistance (AMR) among E. coli from community-onset uUTIs in the last 2 decades. A 2012 in vitro study of E. coli urine isolates from US outpatients (2001–2010) found significant increases in AMR to ciprofloxacin and trimethoprim-sulfamethoxazole (TMP-SMX) [8]. Similarly, in a study of urine isolates from 18 European countries in 2018, resistance to TMP-SMX was found in 32.7% (range: 23.1–56.2% across countries) of E. coli isolates from clinical urine samples and the prevalence of fluoroquinolone (FQ) resistance was >20% [9].
The acquisition of AMR genes is especially important among Enterobacterales, as these are associated with the potential to confer cross- or co-resistance to multiple drug classes, causing multidrug resistance (MDR) [10]. Production of extended-spectrum beta-lactamases (ESBLs) is one of the most clinically relevant MDR phenotypes [11]. The increasing prevalence of ESBL-producing (ESBL+) E. coli, which are often co-resistant to TMP-SMX and FQ [12], is of global concern and has implications for the empiric treatment of community-onset uUTI. The Centers for Disease Control and Prevention Threat Report (2019) classifies ESBL+ Enterobacterales as a serious health threat with $1.2 billion estimated attributable US healthcare costs in 2017 [11]. MDR phenotypes have historically been associated with nosocomial infections but have now emerged at the community level, limiting oral therapeutic options for uUTI [13, 14] and resulting in increased rates of treatment failure, patient morbidity, and healthcare costs, as well as higher rates of hospitalization and increased use of broad-spectrum antibiotics [15, 16].
Treatment for uUTI is often empiric, with varying practices regarding initial oral antibiotic choice [7, 17–19]. The Infectious Diseases Society of America (IDSA) recommends either a 3-day course of TMP-SMX or a 5-day course of nitrofurantoin (NFT), or fosfomycin (single dose) [7], whereas the European Association of Urology recommends fosfomycin (single dose), NFT (5-days), or pivmecillinam (3–5 days) for first-line therapy [17]. Urine cultures are generally not recommended for the first incident case of symptomatic uUTI; however, they are recommended if no improvement is seen within 48 hours of treatment in the case of recurrent uUTI or in postmenopausal women with intermittent uUTI symptoms [18]. Thus, evaluation of outpatient urine isolates often comprises a mixture of samples obtained at the time of initial presentation of uUTI and those obtained after failure of empiric therapy. The current IDSA guidelines for uUTI were published in 2011; as such, it would be informative to assess and characterize contemporary regional AMR burden among urinary isolates from outpatients.
This study investigated nonsusceptibility among E. coli urine isolates collected from adolescent and adult female outpatients in the US between 2011 and 2019 to characterize trends and the geographic distribution among E. coli urine isolates over the study period.
METHODS
Study Design
This was a retrospective, multicenter, cohort study among E. coli isolated from female urine cultures collected at US outpatient facilities included in the Becton, Dickinson and Company (BD) Insights Research Database (Franklin Lakes, New Jersey, USA; see Supplementary materials for additional information). The distribution of hospitals in the database is similar to that of the US as a whole [11], suggesting appropriate demographic coverage. Urine isolates collected from females ≥12 years of age who had a noncontaminant urine culture with reported identification of E. coli and a susceptibility result from the outpatient setting were included in this study. Isolates were collected between January 2011 and December 2019 (the study period). Eligible isolates were 30-day nonduplicate, defined as the first urine E. coli isolate per patient collected within 30 days [20]. Urine E. coli isolates from the same patient within 30 days were included if the E. coli isolate had different drug susceptibilities (>1 susceptibility interpretive criteria difference) and subsequent E. coli isolates were included if collected >30 days from the previous isolate. Data identified included: age sex; isolated uropathogens; tested antibiotic agents; susceptibility testing methods; and institution type, size, and geographic location. Individual laboratories in each institution performed their own antimicrobial susceptibility testing using Clinical and Laboratory Standards Institute (CLSI)-approved methods and interpreted results using Food and Drug Administration (FDA)/CLSI breakpoints and interpretive criteria [21].
Prevalence and Distribution of AMR (Primary Analysis)
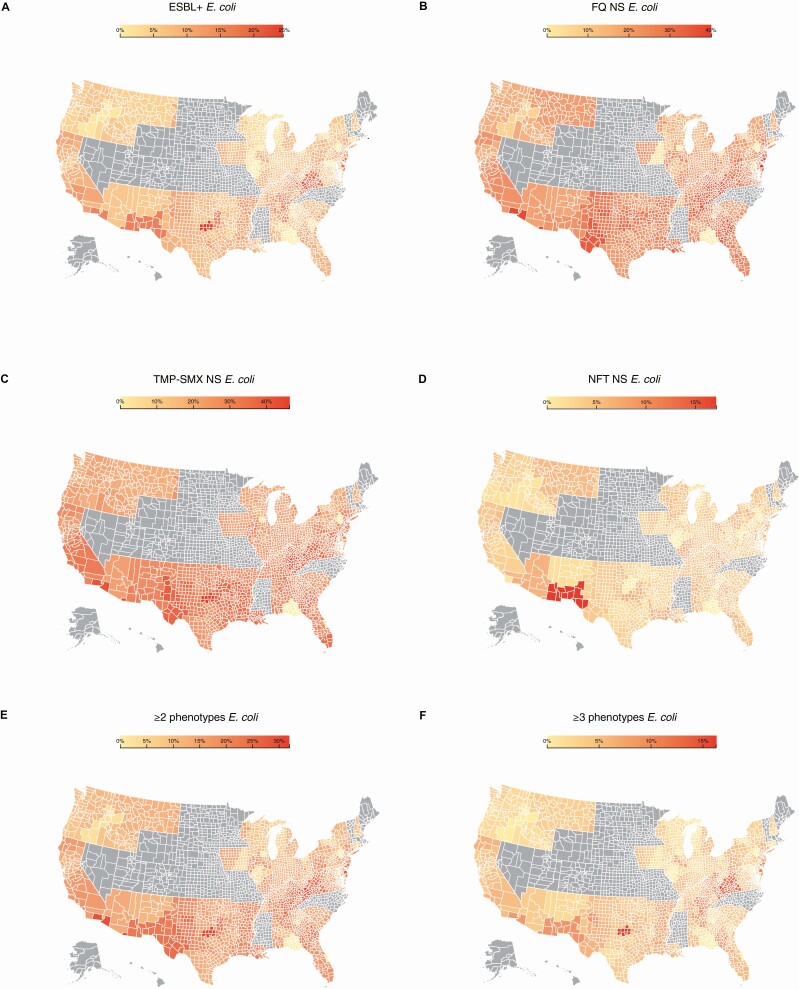
AMR among nonduplicate E. coli was defined as nonsusceptible (resistant or intermediate susceptibility results) to FQ, NFT, or TMP-SMX and also included isolates that were ESBL+ (confirmed by commercial laboratory panels or nonsusceptibility to ceftriaxone, cefotaxime, ceftazidime, or cefepime). The term “nonsusceptible” is used throughout per American Medical Association guidance, however, this aligns with the CLSI guidelines definition of not susceptible in this article; AMR and nonsusceptibility are also used interchangeably. The ≥2 and ≥3 nonsusceptible phenotype categories were defined as isolates with 2 or more and 3 or more drug-resistance phenotypes (nonsusceptible to FQ, NFT, TMP-SMX, or ESBL+, respectively). Geospatial heatmaps were developed using data-driven-documents (D3.js), a JavaScript visualization library, to geographically depict the proportion of isolates with each drug-resistance phenotype of interest. The heatmaps included data at the county level to understand differences in regional resistance in the US (Figure 3). For a county that did not have any isolates tested, the susceptibility results of the nearest county either within or across state lines were populated. Counties with insufficient isolates tested (<1% and <30 isolates tested) or states with no isolate results are marked in gray.
Figure 3.
Regional distribution of E. coli AMR phenotypes in the US, 2019. (A) ESBL+; (B) FQ NS; (C) TMP-SMX NS; (D) NFT NS; (E) ≥2 nonsusceptible phenotypes; (F) ≥3 nonsusceptible phenotypes. For a county that did not have any isolates tested, the susceptibility results of the nearest county either within or across state lines were populated. Counties with insufficient isolates tested (<1% and <30 isolates tested) or states with no isolate results are marked in gray. Abbreviations: AMR, antimicrobial resistance; E. coli, Escherichia coli; ESBL+, extended-spectrum β-lactamase-producing; FQ, fluoroquinolone; NFT, nitrofurantoin; NS, nonsusceptible; TMP-SMX, trimethoprim-sulfamethoxazole.
Minimum Inhibitory Concentration Breakpoints (Secondary Analysis)
The primary analysis was based on susceptibility results reported by each institution; however, the CLSI minimum inhibitory concentration (MIC) interpretive breakpoints for several antibiotics changed during the study period (Supplementary Table 1). It was not clear when new breakpoints were adopted by laboratories for cefazolin, cefepime, ciprofloxacin, and levofloxacin; therefore, a secondary analysis was performed based on changes in the MIC interpretive standards for E. coli to cefazolin (2014 and 2019), cefepime (2014), levofloxacin (2019), and ciprofloxacin (2019) over the study period [21–25]. The secondary analysis was conducted only among isolates with available MIC values for these antibiotics, to further understand how the breakpoint changes and variation in implementation of new breakpoints potentially affected the primary analysis results.
Trends in resistance were assessed using the most recent MIC breakpoints for E. coli isolates (susceptible/intermediate/ resistant [SIR]) to: cefazolin (≤16/–/≥32), cefepime (≤2/4–8 [susceptible dose-dependent]/≥16), ciprofloxacin (≤0.25/0.5/≥1), and levofloxacin (≤0.5/1/≥2)[21]. Isolates were excluded from this analysis when the MIC breakpoint data were either not reported or when the current interpretive criteria could not be applied to the original MIC (eg, original MIC lying outside of the specific SIR category using current breakpoints and therefore deemed non-evaluable). Isolates were further excluded if they came from healthcare institutions where >50% of the isolates were deemed nonevaluable, in order to avoid the potential for overestimation of nonsusceptible prevalence.
Statistical Analyses
AMR prevalence among E. coli was examined using descriptive statistics to report prevalence (%) and modelling methods (generalized estimating equations [GEE]) to estimate prevalence with 95% confidence intervals (CI). GEE models also assessed AMR trends (average yearly change) over time and by US census region. GEE models were adjusted for geographic (urban vs rural) status, healthcare facility characteristics (bed size and teaching status), and for the number of facilities per year (Supplementary Table 2). In the secondary analysis, generalized linear modeling (GLM) was used to evaluate AMR rates across the study period and to compare AMR rates between the original and new interpretative criteria. Bonferroni corrections were used when comparing rates for multiple years. Trends in AMR for each antibiotic were evaluated by year, and the relative average annual percent change in resistance was estimated based on GLM. All analyses were conducted using the Statistical Analysis System (SAS) V9.4 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Overall Prevalence of AMR Among E. coli Isolates in the US
Overall, 1 513 882 nonduplicate urine E. coli isolates were included in the primary analyses. These were collected from 106 to 295 outpatient facilities distributed across the US between 2011 and 2019 (Supplementary Table 2). The age distribution of isolates included in the primary analysis was as follows: 12–17 (3.2%), 18–54 (46.3%), 55–64 (12.3%), 65–74 (14.7%), and ≥75 (23.5%) years. The overall prevalence of E. coli isolates nonsusceptible to TMP-SMX, FQ, and NFT was 25.39% (95% CI, 25.32–25.46), 21.10% (95% CI, 21.03–21.16), and 3.76% (95% CI, 3.73–3.79), respectively (Figure 1). ESBL+ was found in 6.36% (95% CI, 6.32–6.40) of the E. coli isolates, whereas 14.36% (95% CI, 14.30–14.41) and 3.81% (95% CI, 3.78–3.84), respectively, were in the ≥2 and ≥3 nonsusceptible phenotype categories (Figure 1).
Figure 1.
Overall AMR results in non-duplicate urine E. coli isolates (N = 1 513 882) from US females, 2011–2019. Abbreviations: AMR, antimicrobial resistance; CI, confidence interval; E. coli, Escherichia coli; ESBL+, extended-spectrum β-lactamase-producing; FQ, fluoroquinolone; NFT, nitrofurantoin; NS, nonsusceptible; TMP-SMX, trimethoprim-sulfamethoxazole.
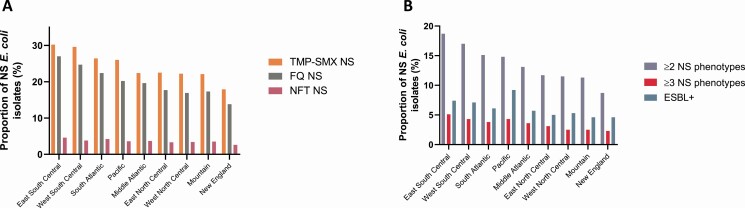
There was variation in the prevalence of nonsusceptible phenotypes across US census regions (Figure 2). The highest overall prevalence for most nonsusceptible phenotypes (except ESBL+) was in the East South Central census region, where the prevalence of TMP-SMX, FQ, and NFT nonsusceptible E. coli was 30.2%, 27.0%, and 4.6%, respectively. In this region, ESBL+ prevalence was 7.4% among E. coli isolates, with 18.7% and 5.1% of isolates, respectively, in the ≥2 and ≥3 nonsusceptible phenotype categories. The lowest prevalence of nonsusceptibility was consistently found in the New England region, where 17.9%, 13.8%, and 2.6% of E. coli isolates were TMP-SMX, FQ, and NFT nonsusceptible, respectively; 4.6% were ESBL+, 8.7% were in the ≥2 nonsusceptible phenotype category, and 2.3% were in the ≥3 nonsusceptible phenotype category. The Pacific region had the highest ESBL+ rate, where 9.2% of isolates had this phenotype overall. Adjusted results were broadly similar to the unadjusted results (Table 1).
Figure 2.
Overall proportion of (A) AMR and (B) ≥2 and ≥3 nonsusceptible phenotype category E. coli isolates by census region. New England: CT, MA, ME, NH, RI, VT; Middle Atlantic: NJ, NY, PA; East North Central: IL, IN, MI, OH, WI; West North Central: IA, KS, MN, MO, ND, NE, SD; South Atlantic: DE, DC, FL, GA, MD, NC, SC, VA, WV; East South Central: AL, KY, MS, TN; West South Central: AR, LA, OK, TX; Mountain: AZ, CO, ID, MT, NM, NV, UT, WY; Pacific: AK, CA, OR, WA. Abbreviations: AMR, antimicrobial resistance; E. coli, Escherichia coli; ESBL+, extended-spectrum β-lactamase-producing; FQ, fluoroquinolone; NFT, nitrofurantoin; NS, nonsusceptible; TMP-SMX, trimethoprim-sulfamethoxazole.
Table 1.
Model-Estimated Overall Prevalence of AMR for Each Phenotype, AMR Trend Over Year (2011–2019), and Regional Differences in Nonsusceptibility Among Outpatient Urine E. coli Isolates
| Phenotype Category (N = 1 513 882) | ||||||
|---|---|---|---|---|---|---|
| TMP-SMX NS (n = 384 304) | FQ NS (n = 319 354) | NFT NS (n = 56 954) | ESBL+ (n = 96 306) | NS ≥ 2 Drug Classes (n = 217 329) | NS ≥ 3 Drug Classes (n = 57 637) | |
| Overall (across years) estimate of AMR, % (95% CI) | 26.0 (25.9 to 26.1) | 23.0 (22.9 to 23.2) | 4.0 (4.0 to 4.1) | 6.8 (6.7 to 6.8) | 15.7 (15.5 to 15.8) | 4.2 (4.1 to 4.3) |
| Trend over year (2011–2019): average yearly change in NS, % (95% CI) | 0.0 (–0.2 to 0.1; p = 0.6737) | –0.6 (–0.8 to –0.4; P < .0001) | –6.1 (–6.5 to –5.6; P < .0001) | 7.7 (7.2 to 8.2; P < .0001) | –0.8 (–1.1 to –0.6; P < .0001) | 2.7 (2.2 to 3.2; P < .0001) |
| Variation in AMR by US Census Region (2011–2019), % (95% CI)* | ||||||
| East North Central (n = 354 353) | 22.3 (21.8 to 22.7) | 15.2 (14.8 to 15.7) | 3.4 (3.2 to 3.6) | 4.1 (3.9 to 4.4) | 10.7 (10.3 to 11.1) | 2.8 (2.6 to 3.0) |
| East South Central (n = 173 127) | 29.4 (28.9 to 30.1) | 22.8 (22.2 to 23.5) | 4.5 (4.2 to 4.7) | 6.5 (6.1 to 6.9) | 16.7 (16.1 to 17.3) | 4.4 (4.1 to 4.7) |
| Middle Atlantic (n = 265 840) | 21.9 (21.4 to 22.3) | 15.8 (15.3 to 16.3) | 3.7 (3.5 to 4.0) | 4.7 (4.4 to 5.0) | 11.3 (10.9 to 11.8) | 3.1 (2.8 to 3.3) |
| Mountain (n = 58 407) | 21.6 (21.0 to 22.2) | 14.8 (14.3 to 15.4) | 3.8 (3.5 to 4.1) | 3.9 (3.6 to 4.2) | 10.3 (9.8 to 10.8) | 2.2 (2.0 to 2.4) |
| New England (n = 19 450) | 17.1 (16.3 to 17.9) | 10.6 (10.0 to 11.2) | 2.5 (2.2 to 2.9) | 3.0 (2.7 to 3.4) | 7.0 (6.5 to 7.5) | 1.6 (1.4 to 1.9) |
| Pacific (n = 173 228) | 25.3 (24.7 to 25.9) | 16.5 (16.0 to 17.0) | 3.6 (3.4 to 3.9) | 7.5 (7.0 to 8.0) | 12.9 (12.4 to 13.4) | 3.7 (3.4 to 4.0) |
| South Atlantic (n = 205 042) | 26.2 (25.6 to 26.8) | 19.3 (18.7 to 19.9) | 4.4 (4.2 to 4.7) | 5.1 (4.8 to 5.5) | 13.9 (13.4 to 14.4) | 3.5 (3.2 to 3.8) |
| West North Central (n = 18 448) | 21.3 (20.4 to 22.2) | 11.7 (11.1 to 12.4) | 3.1 (2.7 to 3.5) | 3.5 (3.2 to 3.9) | 8.5 (7.9 to 9.1) | 1.6 (1.4 to 1.8) |
| West South Central (n = 245 987) | 29.1 (28.5 to 29.7) | 20.1 (19.5 to 20.7) | 3.7 (3.5 to 3.9) | 5.8 (5.4 to 6.1) | 14.7 (14.2 to 15.3) | 3.5 (3.3 to 3.8) |
Models were adjusted for hospital characteristics (bed size, urban/rural status, and teaching status).
East North Central: IL, IN, MI, OH, WI; East South Central: AL, KY, MS, TN; Middle Atlantic: NJ, NY, PA; Mountain: AZ, CO, ID, MT, NM, NV, UT, WY; New England: CT, MA, ME, NH, RI, VT; Pacific: AK, CA, OR, WA; South Atlantic: DE, DC, FL, GA, MD, NC, SC, VA, WV; West North Central: IA, KS, MN, MO, ND, NE, SD; West South Central: AR, LA, OK, TX.
Abbreviations: AMR, antimicrobial resistance; CI, confidence interval; E. coli, Escherichia coli; ESBL+, extended-spectrum β-lactamase-producing; FQ, fluoroquinolone; NFT, nitrofurantoin; NS, nonsusceptible; TMP-SMX, trimethoprim-sulfamethoxazole.*Significant variation between regions and age groups was found, P < .0001.
Figure 3 shows heatmaps of the distribution of the E. coli nonsusceptible phenotypes at the US county level in 2019. These illustrate the high prevalence of nonsusceptibility in the East South Central and Pacific regions and also reveal concentrated areas of high nonsusceptibility within other regions, notably in the Southwestern states and on the Northeastern coast.
Trends in AMR Among E. coli Isolates in the US
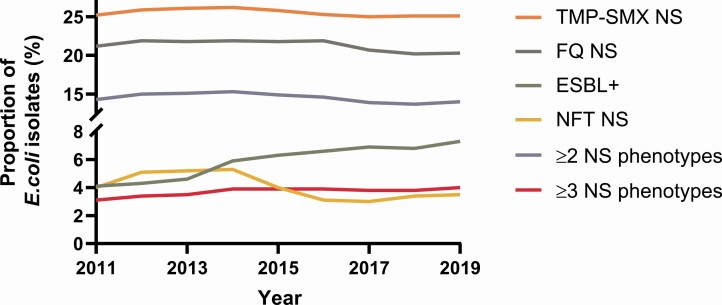
Trends in E. coli nonsusceptible phenotypes between 2011 and 2019 are shown in Figure 4. Over the 9 years included, the ESBL+ rate increased every year (except 2018), beginning at 4.1% and increasing to 7.3%. TMP-SMX nonsusceptible E. coli prevalence was consistently 25% or greater and ranged from 25.0% (2017) to 26.2% (2014). The prevalence of FQ nonsusceptible E. coli decreased over the study period from 21.9% to 20.3%, however, was consistently >20% (range: 20.2–21.9%). The prevalence of urine E. coli isolates in the ≥3 nonsusceptible phenotype category increased from 3.1% (2011) to 4.0% (2019) over the study period, while the prevalence of E. coli isolates in the ≥2 nonsusceptible phenotype category decreased by 1.0% and ranged from 13.7% (2018) to 15.3% (2014). Prevalence of isolates nonsusceptible to NFT decreased over the study period from 4.0% in 2011 to 3.5% in 2019 (range: 5.3% [2014] to 3.0% [2017]).
Figure 4.
Trends in AMR among E. coli isolates, 2011–2019. Abbreviations: AMR, antimicrobial resistance; E. coli, Escherichia coli; ESBL+, extended-spectrum β-lactamase-producing; FQ, fluoroquinolone; NFT, nitrofurantoin; NS, nonsusceptible; TMP-SMX, trimethoprim-sulfamethoxazole.
GEE modeling results were broadly similar to this descriptive data. Modeling demonstrated a significant yearly relative increase of 7.7% (95% CI, 7.2–8.2%; P < .0001) for the ESBL+ phenotype and a significant yearly relative decreasing trend for FQ nonsusceptible and ≥2 nonsusceptible phenotype category (P < .0001; Table 1). For the ≥3 nonsusceptible phenotype category, modelling demonstrated a significant relative increase of 2.7% annually (95% CI, 2.2–3.2%; P < .0001; Table 1). For NFT nonsusceptibility, GEE modeling demonstrated a significant yearly relative decrease of 6.1% (95% CI, –6.5% to –5.6%; P < .0001; Table 1). No significant trend for TMP-SMX nonsusceptibility was shown by GEE modeling (Table 1).
Secondary Analysis (MIC Breakpoints)
The secondary analysis assessed the resistance prevalence using the most recent MIC breakpoint values for cefazolin, cefepime, levofloxacin, and ciprofloxacin. Overall isolate prevalence determined to be nonsusceptible from 2011 to 2019 for cefazolin and cefepime was lower in the secondary versus the primary analysis. Cefazolin nonsusceptible isolate prevalence was 11.9% in the primary analysis and 8.6% in the secondary analysis with the most recent MIC breakpoints applied. For cefepime, nonsusceptible isolate prevalence was 4.4% in the primary analysis and 3.2% in the secondary analysis. However, for ciprofloxacin and levofloxacin, nonsusceptible isolate prevalence was higher when MICs were interpreted using secondary analysis criteria (Supplementary Table 1). The prevalence of ciprofloxacin nonsusceptibility increased from 20.3% to 25.4% and levofloxacin nonsusceptibility increased from 22.0% to 30.7%. GLM results are shown in Supplementary Table 3.
DISCUSSION
Between 2011 and 2019, FQ nonsusceptible and TMP-SMX nonsusceptible prevalence was strikingly high among urine E. coli isolates from adult and adolescent female outpatients in the US. For FQ nonsusceptible and TMP-SMX nonsusceptible isolates, prevalence was consistently above 20%; further, more than 14% of isolates were in the ≥2 nonsusceptible phenotype category.
The IDSA recommends that if AMR is >20% in a given region, the antibiotic should cease to be used for empiric treatment [7]. Although the overall annual prevalence of the TMP-SMX nonsusceptible phenotype remained >20% over the study, there was regional variation in prevalence by year, and GEE modeling did not identify a significant change in TMP-SMX nonsusceptible trends from 2011 to 2019. Despite a significant decreasing trend in FQ nonsusceptible E. coli, this phenotype was recorded for ~21% of included isolates examined over the study period. The FDA has issued a “black box” warning for the safety of FQ due to observed collagen-associated (tendons, muscles, joints) and neurological adverse effects [26, 27]. However, this warning was issued in 2016 and has not led to a significant reduction in FQ prescribing to date [28].
Perhaps the most concerning finding was the prevalence of ESBL+ E. coli isolates, which was 6.4% for the overall study period, with a significant relative annual increase of 7.7% demonstrated in GEE modelling (95% CI, 7.2–8.2%). This agrees with previous literature reporting increasing ESBL+ rates in the US [29–31]. The increasing prevalence of ESBL+ Enterobacterales is a global concern given that it limits effective empiric oral therapeutic options. Greater ESBL+ prevalence also has potential implications for the route of antibiotic administration and the care setting in which therapy can be provided. AMR in patients with outpatient uUTI is likely to create a greater burden on emergency departments and acute care settings, impacting healthcare costs and surge capacity [32].
NFT was the only agent that saw a reduction in the prevalence of nonsusceptible isolates over time. This may be due to changes in prescribing practices influenced by the American Geriatrics Society (AGS), which in 2015 highlighted the need to consider not using NFT in the presence of low creatinine clearance among elderly patients [33]. Additionally, the CDC’s 2016 Outpatient Antimicrobial Stewardship Guidelines championed retrospective outpatient antimicrobial use metrics and education to facilitate more appropriate antimicrobial prescribing [34]. Older patients with uUTI could be presumed to have inherent renal insufficiency, and this, coupled with the AGS recommendations, may have changed NFT prescribing patterns. Similarly, asymptomatic bacteriuria is more prevalent in older-age groups and initiatives to decrease unnecessary antimicrobial use in these groups may have shifted the aggregate use of NFT from 2016 onward.
The geographic distribution of nonsusceptible E. coli showed a high prevalence in the East South Central census region across the study period, with the lowest prevalence seen in the New England region albeit with a limited number of BD sites (Supplementary Table 2). Notably, ESBL+ prevalence was highest in the Pacific region (descriptive and model-estimated results). Additionally, there were areas of high nonsusceptibility at the county level, notably in states bordering Mexico. The high prevalence of nonsusceptible E. coli in US southern-border regions has long been recognized and is often attributed to the availability of over-the-counter antimicrobial treatments, compounded by under-trained staff, in pharmacies outside the US [35–37]. Furthermore, antibiotic prescribing rates have been shown to be high in the southern US, with several states prescribing at a rate of >850 per 1000 population [38]; these data serve to highlight the need to educate physicians on best prescribing practice.
This study represents (to the authors’ knowledge) the largest published sample of E. coli isolated from urine cultures among US outpatients and addresses a gap in the scientific literature, which currently lacks surveillance data for AMR in outpatient UTI. A further strength of the study was the secondary analysis addressing the change in certain MIC breakpoints over the study period. For most recent MIC breakpoints, overall lower rates of E. coli nonsusceptible to cefazolin and cefepime and higher nonsusceptible rates for ciprofloxacin and levofloxacin were observed. The secondary analysis excluded a large number of isolates that were not evaluable, although the directional rate of change in nonsusceptible within this evaluable cohort was similar to that of the overall cohort when including facilities where the majority of isolates were evaluable (>50%).
Study limitations include the fact that susceptibility testing and results are based on local laboratory practice. Although all laboratories in the US follow CLSI and American Society for Microbiology guidelines, susceptibility results were based on local laboratory practices and reporting. The data used in this study could not be definitively linked to clinically confirmed cases of uUTI due to lack of confirmation of clinical symptoms, diagnoses (International Classification of Diseases-9/10 diagnosis codes), and pharmacy claims. However, it is important to note that there have been no changes in the recommendations for culturing practices during the study period and even with the inability to conclusively link the data to uUTI, these are the best available real-world data pertaining to AMR in outpatient UTI [6]. Although the data set has ample coverage of the US and robust representation of the population, there were also under-represented areas in the US (Supplementary Table 2). Finally, the study methodology included only nonduplicate urine samples from outpatients without recent known hospitalization; however, the possibility of hospitalization in a non-affiliated healthcare center could not be ruled out. Additionally, more than one isolate from the same patient could have been included if collected >30 days from previous isolate, suggesting that the study population included patients with recurrent UTIs and potentially overestimated resistance.
CONCLUSION
Using a large data set of urine E. coli isolates from females attending outpatient facilities in the US, this study demonstrated a high prevalence of nonsusceptible E. coli, with significant variation in prevalence depending on geographic location within the US. Moreover, the prevalence of ESBL+ and E. coli isolates in the ≥3 nonsusceptible phenotype category increased annually between 2011 and 2019. High rates of AMR and MDR E. coli limit the effective empiric options for the treatment of uUTI. Raising awareness of current regional patterns of nonsusceptible E. coli isolates from outpatient UTIs can help to guide empiric treatment decisions of physicians and demonstrate the need for both antimicrobial stewardship efforts in outpatient settings, as well as new oral antibiotics to address the growing prevalence of resistant E. coli in the US.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors would like to thank Iain Gillespie, PhD (Senior Director, Infectious Diseases & Global Health Head, Epidemiology at GlaxoSmithKline plc.) for his careful review and insightful comments. The authors wish to thank John Murray, MPH (Manager, Decision Science and Visualization at Becton, Dickinson and Company) and Latha Vankeepuram, MS (Program Manager, Data Science and Analytics at Becton, Dickinson and Company) for their dedicated contribution of database management for this study. Medical writing support, under the direction of authors, was provided by Fraser Shearer, MSc, of Ashfield MedComms (Glasgow, UK), an Ashfield Health company, and was funded by GlaxoSmithKline plc. Trademarks are owned by or licensed to their respective owners (the GlaxoSmithKline group of companies or Becton, Dickinson and Company).
Financial support. This study was supported by GlaxoSmithKline plc. V. G., N. S.O., K. K., K. Y., G. Y., F. M. G., A. J., and A. M. report that GlaxoSmithKline plc. provided funding of study, funding of medical writing support (provided by Fraser Shearer, Ashfield MedComms, an Ashfield Health company), funding for article processing charges.
Potential conflicts of interest. F. M. G., A. J., N. S. O., and A. M. report stock and employment with GlaxoSmithKline plc. V. G., K. Y., and A. M. report having stock in and being employed by Becton, Dickinson and Company. G. Y. reports being employed by Becton, Dickinson and Company. K. K. also reports grants/contracts from Merck with University of Michigan. K. K. was paid consulting fees for consulting for GlaxoSmithKline plc and serving on the advisory board for Utility Therapeutics, Allecra, Shinoigi, Merck, and Spero. K. K. reports stock or stock options with Merck. K. Y. reports being on the AdvaMed Digital Health Committee and on the DiME Digital Health Steering Committee for the Digital Medicine Society and notes that both of these roles tangentially deals with safe harbor of large data sets and other related digital health information. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol 2019; 11:1756287219832172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am 2014; 28:1–13. [DOI] [PubMed] [Google Scholar]
- 3. Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health 1990; 80:331–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fihn SD. Clinical practice: acute uncomplicated urinary tract infection in women. N Engl J Med 2003; 349:259–66. [DOI] [PubMed] [Google Scholar]
- 5. Schmiemann G, Kniehl E, Gebhardt K, Matejczyk MM, Hummers-Pradier E. The diagnosis of urinary tract infection: a systematic review. Dtsch Arztebl Int 2010; 107:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonkat G, Bartoletti R, Bruyère F, et al. Urological infections. Arnhem, the Netherlands: EAU Guidelines Office, 2020. [Google Scholar]
- 7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 8. Sanchez GV, Master RN, Karlowsky JA, Bordon JM. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob Agents Chemother 2012; 56:2181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Critchley IA, Cotroneo N, Pucci MJ, Jain A, Mendes RE. Resistance among urinary tract pathogens collected in Europe during 2018. J Glob Antimicrob Resist 2020; 23:439–44. [DOI] [PubMed] [Google Scholar]
- 10. Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem 2009; 78:119–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. Atlanta, GA: U.S. Department of Health and Human Services, CDC, 2019. [Google Scholar]
- 12. Critchley IA, Cotroneo N, Pucci MJ, Mendes RE. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PLoS One 2019; 14:e0220265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frazee BW, Trivedi T, Montgomery M, Petrovic DF, Yamaji R, Riley L. Emergency department urinary tract infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae: many patients have no identifiable risk factor and discordant empiric therapy is common. Ann Emerg Med 2018; 72:449–56. [DOI] [PubMed] [Google Scholar]
- 14. Lob SH, Nicolle LE, Hoban DJ, et al. Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, smart 2010–2014. Diagn Microbiol Infect Dis 2016; 85:459–65. [DOI] [PubMed] [Google Scholar]
- 15. Hooton TM, Besser R, Foxman B, Fritsche TR, Nicolle LE. Acute uncomplicated cystitis in an era of increasing antibiotic resistance: a proposed approach to empirical therapy. Clin Infect Dis 2004; 39:75–80. [DOI] [PubMed] [Google Scholar]
- 16. Simmering JE, Tang F, Cavanaugh JE, Polgreen LA, Polgreen PM. The increase in hospitalizations for urinary tract infections and the associated costs in the united states, 1998–2011. Open Forum Infect Dis 2017; 4:ofw281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonkat G, Pickard R, Bartoletti R, et al. EAU guidelines on urological infections. Eur Assoc Urol 2020:22–6. Available at: https://uroweb.org/guideline/urological-infections/. [Google Scholar]
- 18. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 91: treatment of urinary tract infections in nonpregnant women. Obstet Gynecol 2008; 111:785–94. [DOI] [PubMed] [Google Scholar]
- 19. Hummers-Pradier E, Ohse AM, Koch M, Heizmann WR, Kochen MM. Management of urinary tract infections in female general practice patients. Fam Pract 2005; 22:71–7. [DOI] [PubMed] [Google Scholar]
- 20. Clinical and Laboratory Standards Institute. Clinical and laboratory standards institute (CLSI). Analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline—3rd ed., m39-a4. Wayne, PA: Clinical and Laboratory Standards Institute, 2009. [Google Scholar]
- 21. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 29th ed. CLSI supplement M100. Wayne, PA, USA: Clinical and Laboratory Standards Institute, 2019. [Google Scholar]
- 22. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 26th ed. CLSI supplement M100. Wayne, PA, USA: Clinical and Laboratory Standards Institute, 2016. [Google Scholar]
- 23. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 24th ed. CLSI supplement M100. Wayne, PA, USA, 2014. [Google Scholar]
- 24. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 21st ed. CLSI supplement M100. Wayne, PA, USA, 2011. [Google Scholar]
- 25. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 20th ed. CLSI supplement M100. Wayne, PA, USA, 2010. [Google Scholar]
- 26. U.S. Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Drug Safety Communications. White Oak, Maryland, USA, 2016. [Google Scholar]
- 27. Daneman N, Lu H, Redelmeier DA. Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ Open 2015; 5:e010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cowart K, Worley M, Rouby NE, Sando K. Evaluation of FDA boxed warning on prescribing patterns of fluoroquinolones for uncomplicated urinary tract infections. Ann Pharmacother 2019; 53:1192–9. [DOI] [PubMed] [Google Scholar]
- 29. Thaden JT, Fowler VG, Sexton DJ, Anderson DJ. Increasing incidence of extended-spectrum β-lactamase-producing Escherichia coli in community hospitals throughout the Southeastern United States. Infect Control Hosp Epidemiol 2016; 37:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gupta V, Ye G, Olesky M, et al. Trends in resistant Enterobacteriaceae and Acinetobacter species in hospitalized patients in the United States: 2013–2017. BMC Infect Dis 2019; 19:742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Talan DA, Takhar SS, Krishnadasan A, et al. Emergence of extended-spectrum β-lactamase urinary tract infections among hospitalized emergency department patients in the United States. Ann Emerg Med 2021; 77:32–43. [DOI] [PubMed] [Google Scholar]
- 32. May L, Mullins P, Pines J. Demographic and treatment patterns for infections in ambulatory settings in the United States, 2006–2010. Acad Emerg Med 2014; 21:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–46. [DOI] [PubMed] [Google Scholar]
- 34. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 2016; 65:1–12. [DOI] [PubMed] [Google Scholar]
- 35. Homedes N, Ugalde A. Mexican pharmacies and antibiotic consumption at the US-Mexico border. South Med Rev 2012; 5:9–19. [PMC free article] [PubMed] [Google Scholar]
- 36. de Guzman GC, Khaleghi M, Riffenberg RH, Clark RF. A survey of the use of foreign-purchased medications in a border community emergency department patient population. J Emerg Med 2007; 33:213–21. [DOI] [PubMed] [Google Scholar]
- 37. Vasquez Y, Hand WL. Antibiotic susceptibility patterns of community-acquired urinary tract infection isolates from female patients on the US (Texas)-Mexico border. J Appl Res 2004; 4:321–6. [Google Scholar]
- 38. Centers for Disease Control and Prevention. Antibiotic use in the United States, 2020 update: progress and opportunities. Available at: https://www.cdc.gov/antibiotic-use/stewardship-report/current.html. Accessed 24 February 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.