Abstract
Background
Recurrent reports of suboptimal influenza vaccine effectiveness have renewed calls to develop improved, broadly cross-protective influenza vaccines. Here, we evaluated the safety and immunogenicity of a novel, saponin (Matrix-M)–adjuvanted, recombinant hemagglutinin (HA) quadrivalent nanoparticle influenza vaccine (qNIV).
Methods
We conducted a randomized, observer-blind, comparator-controlled (trivalent high-dose inactivated influenza vaccine [IIV3-HD] or quadrivalent recombinant influenza vaccine [RIV4]), safety and immunogenicity trial of qNIV (5 doses/formulations) in healthy adults ≥65 years. Vaccine immunogenicity was measured by hemagglutination-inhibition assays using reagents that express wild-type hemagglutination inhibition (wt-HAI) sequences and cell-mediated immune responses.
Results
A total of 1375 participants were randomized, immunized, and followed for safety and immunogenicity. Matrix-M–adjuvanted qNIV induced superior wt-HAI antibody responses against 5 of 6 homologous or drifted strains compared with unadjuvanted qNIV. Adjuvanted qNIV induced post-vaccination wt-HAI antibody responses at day 28 that were statistically higher than IIV3-HD against a panel of homologous or drifted A/H3N2 strains, similar to IIV3-HD against homologous A/H1N1 and B (Victoria) strains and similar to RIV4 against all homologous and drifted strains evaluated. The qNIV formulation with 75 µg Matrix-M adjuvant induced substantially higher post-vaccination geometric mean fold increases of influenza HA-specific polyfunctional CD4+ T cells compared with IIV3-HD or RIV4. Overall, similar frequencies of solicited and unsolicited adverse events were reported in all treatment groups.
Conclusions
qNIV with 75 µg Matrix-M adjuvant was well tolerated and induced robust antibody and cellular responses, notably against both homologous and drifted A/H3N2 viruses. Further investigation in a pivotal phase 3 trial is underway.
Clinical Trials Registration
Keywords: influenza, vaccination, hemagglutination inhibition, cell-mediated immunity
We compared multiple formulations of a recombinant Matrix-M–adjuvanted nanoparticle influenza vaccine with 2 licensed vaccines in older adults. The nanoparticle vaccine was well tolerated and induced hemagglutination-inhibition antibody and CD4+ T-cell responses to vaccine-homologous and drifted A/H3N2 influenza viruses.
Seasonal influenza vaccination has been the cornerstone of prevention efforts to address the substantial health and economic burdens of influenza [1, 2]. However, recent developments, including several severe A(H3N2)-predominant influenza seasons; recurrent reports of poor field vaccine effectiveness from Europe, Canada, and the United States; the increasingly recognized risk of antigenic mismatch arising from egg-based vaccine production; and the mounting challenge of predicting which viruses will circulate in the face of increasing strain diversity, have undermined confidence in available influenza vaccines and reignited calls for developing improved, broadly cross-protective influenza vaccines [3–19]. These challenges have been acutely represented by contemporary circulating influenza A(H3N2) viruses because of their rapid rate of genetic and antigenic evolution, increased susceptibility to egg-adaptive mutations, and because they account for the majority of influenza-attributable morbidity and mortality [17, 20–22]. Additional challenges to overcome include potential modulatory effects of early-life immunological imprinting on vaccine effectiveness, limited durability of vaccine-induced protective immune responses, and limited induction of cellular immunity [4, 7, 23–25].
We recently described the development of a novel saponin (Matrix-M)-adjuvanted recombinant hemagglutinin (HA) trivalent nanoparticle influenza vaccine (tNIV) produced in a Sf9 insect cell/recombinant baculovirus system that retains fidelity to wild-type (wt) circulating virus HA sequences and contains conserved epitopes that stimulate broadly neutralizing antibodies (bnAbs) [26, 27]. In a phase 1 study, tNIV demonstrated improved induction of wild-type hemagglutination inhibition (wt-HAI) antibody titers against A/H3N2 drift variants isolated over a 5-year period compared with an egg-derived, trivalent high-dose inactivated influenza vaccine (IIV3-HD) [28].
In the present phase 2 study, we further evaluated the safety and immunogenicity of various doses and formulations of quadrivalent NIV (qNIV) in adults aged ≥65 years, with or without Matrix-M adjuvant, compared with 2 currently licensed influenza vaccines for which enhanced efficacy relative to standard IIV has been reported (IIV3-HD and quadrivalent recombinant HA influenza vaccine [RIV4]) [25].
METHODS
Study Design
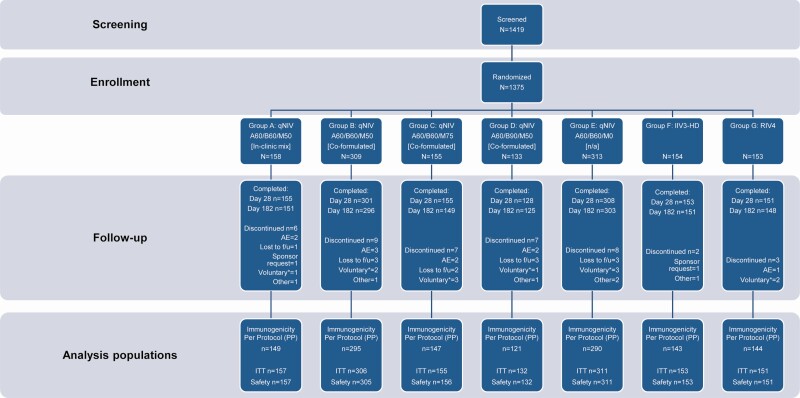
This randomized, observer-blind, comparator-controlled, dose and formulation optimization trial enrolled 1375 clinically stable adults aged ≥65 years across 14 US sites from 24 September 2018 to 19 October 2018. Eligible participants were randomized into 1 of 7 treatment groups, stratified by age (60 to <75 and ≥75 years), gender, and receipt of 2017–2018 seasonal influenza vaccine (Figure 1; Supplementary Table 1). Inclusion and exclusion criteria are detailed in the Supplementary Appendix.
Figure 1.
Flow diagram on screening, enrollment, and disposition of participants through the study. Safety population is defined as all participants who provided consent, were randomized, and received any investigational treatment; used for all descriptive safety analyses. Immunogenicity per protocol population is defined as all participants in the safety population who received the assigned investigational treatment according to the protocol, had wild-type hemagglutination inhibition (HAI) serology results for day 0 and day 28, and had no major protocol deviations that affected the primary immunogenicity outcomes as determined by the sponsor prior to database lock and unblinding; used for all immunogenicity analyses. The ITT population is defined as all participants in the safety population who provided any HAI serology data. Abbreviations: A, influenza A strain hemagglutinin (HA) antigen content in micrograms for each of A/H1N1 and A/H3N2 strains; AE, adverse event; B, influenza B strain HA antigen content in micrograms for each of B/Victoria and B/Yamagata lineage strains; f/u, follow-up; IIV3-HD, trivalent high-dose inactivated influenza vaccine (Fluzone High-Dose); ITT, intent-to-treat population; M, Matrix-M adjuvant content in micrograms; qNIV, quadrivalent recombinant nanoparticle influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine (Flublok Quadrivalent); voluntary*, voluntary withdrawal unrelated to an adverse event.
Investigational treatments comprised a single intramuscular injection of 1 of the following on day 0 (Table 1): group A, qNIV, with Matrix-M adjuvant and antigens mixed in the clinic just prior to administration; group B, qNIV, preformulated with Matrix-M; group C, qNIV, preformulated with high-dose Matrix-M; group D, qNIV, preformulated with high-dose B antigens and Matrix-M; group E, qNIV formulated without adjuvant; group F, licensed IIV3-HD (trivalent Fluzone High-Dose); or group G, licensed RIV4 (FluBlok Quadrivalent; Figure 1; Supplementary Table 1). On day 28, participants in group E were administered a rescue injection with a licensed seasonal influenza vaccine; all other participants were administered a placebo injection on day 28 to maintain trial blinding. Enrollment was divided into 3 stages to monitor safety (Supplementary Appendix).
Table 1.
Summary of Wild-type Hemagglutination Inhibition Antibody Responses by Treatment Group, Strain, and Time Point—Per Protocol Population
| Treatment Group | A | B | C | D | E | F | G | |
|---|---|---|---|---|---|---|---|---|
| Hemagglutinin and Matrix Content | qNIV A60/B60/M50 | qNIV A60/B60/M50 | qNIV A60/B60/M75 | qNIV A60/B90/M50 | qNIV A60/B60/M0 | IIV3-HD | RIV4 | |
| Formulation | In-Clinic Mix | Coformulated | NA | NA | NA | |||
| Participants in Group (N) | 149 | 295 | 147 | 121 | 290 | 143 | 144 | |
| Visit | Parameter | |||||||
| A/Michigan/45/2015 (H1N1) (homologous strain) | ||||||||
| Day 0 | GMT | 50.1 | 47.9 | 55.3 | 48.7 | 48.7 | 50.5 | 44.0 |
| 95% CI | (44.6–56.4) | (44.2–51.9) | (49.0–62.3) | (42.6–55.8) | (44.8–52.8) | (45.2–56.4) | (39.5–49.1) | |
| Day 28 | GMT | 98.9 | 91.3 | 99.1 | 79.5 | 90.3 | 96.9 | 82.1 |
| 95% CI | (86.4–113.1) | (82.5–101.0) | (86.2–114.0) | (68.6–92.2) | (81.0–100.5) | (84.5–111.1) | (71.6–94.2) | |
| GMFRPost/Pre | 2.0 | 1.9 | 1.8 | 1.6 | 1.9 | 1.9 | 1.9 | |
| 95% CI | (1.8–2.2) | (1.8–2.1) | (1.6–2.0) | (1.5–1.8) | (1.7–2.1) | (1.7–2.1) | (1.7–2.1) | |
| A/Singapore/INFIMH-16–0019/2016 (H3N2) (homologous strain) | ||||||||
| Day 0 | GMT | 21.4 | 22.0 | 22.8 | 23.4 | 21.3 | 23.2 | 19.5 |
| 95% CI | (18.9–24.3) | (20.0–24.1) | (19.7–26.3) | (20.2–27.1) | (19.3–23.5) | (20.4–26.4) | (17.1–22.2) | |
| Day 28 | GMT | 65.8 | 65.4 | 64.2 | 59.4 | 50.8 | 46.5 | 66.6 |
| 95% CI | (55.2–78.4) | (58.3–73.5) | (54.2–76.2) | (50.0–70.5) | (45.0–57.4) | (38.6–55.9) | (54.9–80.9) | |
| GMFRPost/Pre | 3.1 | 3.0 | 2.8 | 2.5 | 2.4 | 2.0 | 3.4 | |
| 95% CI | (2.6–3.6) | (2.7–3.3) | (2.4–3.3) | (2.2–3.0) | (2.1–2.7) | (1.8–2.3) | (2.8–4.1) | |
| B/Colorado/06/2017 (Victoria lineage) (homologous strain) | ||||||||
| Day 0 | GMT | 47.6 | 46.4 | 48.5 | 52.7 | 47.5 | 52.0 | 42.9 |
| 95% CI | (43.1–52.6) | (42.8–50.2) | (44.0–53.6) | (46.7–59.4) | (43.9–51.3) | (46.1–58.5) | (38.5–47.7) | |
| Day 28 | GMT | 86.8 | 83.2 | 89.6 | 95.5 | 73.2 | 93.2 | 83.3 |
| 95% CI | (77.1–97.7) | (76.0–91.0) | (79.3–101.2) | (82.8–110.3) | (67.6–79.3) | (81.6–106.5) | (73.2–94.9) | |
| GMFRPost/Pre | 1.8 | 1.8 | 1.8 | 1.8 | 1.5 | 1.8 | 1.9 | |
| 95% CI | (1.6–2.0) | (1.7–1.9) | (1.7–2.0) | (1.6–2.1) | (1.4–1.7) | (1.6–2.0) | (1.7–2.2) | |
| B/Phuket/3073/2013 (Yamagata lineage) (homologous strain) | ||||||||
| Day 0 | GMT | 50.4 | 47.7 | 47.6 | 53.6 | 48.8 | 52.9 | 46.9 |
| 95% CI | (44.7–56.7) | (44.4–51.3) | (42.9–52.9) | (46.9–61.2) | (45.2–52.7) | (47.3–59.1) | (42.1–52.2) | |
| Day 28 | GMT | 108.5 | 101.7 | 104.9 | 113.8 | 87.5 | 64.5 | 102.0 |
| 95% CI | (96.2–122.4) | (93.3–110.8) | (92.4–119.2) | (97.6–132.6) | (79.5–96.4) | (57.3–72.6) | (88.6–117.4) | |
| GMFRPost/Pre | 2.2 | 2.1 | 2.2 | 2.1 | 1.8 | 1.2 | 2.2 | |
| 95% CI | (1.9–2.4) | (2.0–2.3) | (2.0–2.5) | (1.8–2.5) | (1.6–2.0) | (1.1–1.3) | (1.9–2.5) | |
| A/Switzerland/9715293/2013 (H3N2) (drift strain) | ||||||||
| Day 0 | GMT | 54.6 | 60.0 | 60.9 | 60.6 | 58.6 | 62.3 | 54.8 |
| 95% CI | (47.8–62.4) | (54.3–66.2) | (52.7–70.3) | (52.1–70.5) | (52.8–65.1) | (54.3–71.6) | (47.8–62.9) | |
| Day 28 | GMT | 146.8 | 160.4 | 154.8 | 137.9 | 122.4 | 133.4 | 158.8 |
| 95% CI | (124.0–173.9) | (143.9–178.7) | (132.7–180.7) | (117.2–162.2) | (109.1–137.3) | (111.2–160.0) | (132.2–190.9) | |
| GMFRPost/Pre | 2.7 | 2.7 | 2.5 | 2.3 | 2.1 | 2.1 | 2.9 | |
| 95% CI | (2.3–3.1) | (2.4–3.0) | (2.2–2.9) | (2.0–2.7) | (1.9–2.3) | (1.9–2.4) | (2.5–3.4) | |
| A/Wisconsin/19/2017 (H3N2) (drift strain) | ||||||||
| Day 0 | GMT | 21.7 | 23.1 | 24.0 | 23.9 | 22.1 | 24.5 | 20.7 |
| 95% CI | (19.2–24.6) | (21.1–25.3) | (20.7–27.7) | (20.9–27.3) | (20.1–24.4) | (21.6–27.7) | (18.1–23.6) | |
| Day 28 | GMT | 61.1 | 63.2 | 63.0 | 58.2 | 50.1 | 46.1 | 64.3 |
| 95% CI | (51.8–72.0) | (56.3–70.9) | (53.5–74.2) | (48.9–69.2) | (44.4–56.5) | (38.7–55.1) | (53.5–77.2) | |
| GMFRPost/Pre | 2.8 | 2.7 | 2.6 | 2.4 | 2.3 | 1.9 | 3.1 | |
| 95% CI | (2.4–3.3) | (2.5–3.0) | (2.3–3.0) | (2.1–2.9) | (2.0–2.5) | (1.7–2.1) | (2.6–3.7) | |
GMT was defined as the antilog of the mean of the log-transformed titer values for a given treatment group and time point. Individual antibody values recorded as below the lower limit of quantitation (LLOQ) were set to half LLOQ. GMFRPost/Pre was defined as the ratio of 2 geometric mean titers within the treatment group at 2 time points between post-vaccination (day 28) and pre-vaccination (day 0). The 95% CI and P value were obtained by paired t test of GMR = 1. Individual antibody values recorded as below the LLOQ were set to half LLOQ.
Abbreviations: CI, confidence interval; GMRPost/Pre, ratio of GMTs at point day 28/day 0; GMT, geometric mean titer; N, number of participants in per protocol population; n, number of participants with nonmissing hemagglutination-inhibition titer results at each visit; NA, not applicable; qNIV, quadrivalent recombinant nanoparticle influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine (Flublok Quadrivalent).
Participants had scheduled follow-up during up to 7 in-person clinic visits or telephone calls that spanned 182 days to measure vital signs, perform physical exams, report adverse events (AEs), record concomitant medication changes, and collect blood samples for immunogenicity analyses (Supplementary Table 2).
Ethics and Oversight
All participants provided written informed consent. Quorum Review (Seattle, WA) reviewed and approved the trial.
Study Objectives
The primary objectives were to describe the safety and tolerability of each test vaccine and to demonstrate a Matrix-M adjuvant effect by demonstrating the immunogenic superiority of qNIV (60 µg HA per A and B strain) formulated with 50 µg Matrix-M1 per dose compared with qNIV (60 µg HA per A and B strain) without adjuvant, based on day 28 post-vaccination wt-HAI antibody responses against 4 vaccine-homologous (2 influenza A and 2 influenza B strains) and/or 2 antigenically drifted A/H3N2 strains. Additional objectives included describing post-vaccination wt-HAI antibody response (secondary objective) and cellular immune response (exploratory objective) of qNIV relative to IIV3-HD and RIV4 at various time points (Supplementary Appendix).
Vaccines
Details of qNIV formulations, Matrix-M adjuvant, and comparators (IIV3-HD and RIV4) are provided in the Supplementary Appendix.
Immunogenicity Assessments
Blood samples were collected from participants on days 0, 28, 56, and 182 for serological analyses. To measure the most biologically relevant vaccine-induced HAI antibody responses, that is, those against circulating wt hemagglutinins without egg-adaptative antigenic changes, we previously developed the wt-HAI assay as a modification of the classic HAI method by using recombinant wt HA virus-like particles as the agglutinating agent (Supplementary Appendix) [28].
Peripheral blood mononuclear cells (PBMCs) were collected in a subset of 189 participants (comprising participants from 3 study sites [approximately 63 per site]) at days 0 and 7 for cell-mediated immunity (CMI) analyses. Due to limited availability of PBMCs per participant, only a subset of informative treatment groups and strains were tested for CMI based on the results of the wt-HAI data (Supplementary Appendix).
Safety Assessments
Safety follow-up consisted of collection of all solicited local and systemic AEs over 7 days post-day 0 dosing; all AEs through 28 days post-day 0 dosing; and all medically attended events (MAEs), serious AEs (SAEs), and significant new medical conditions (SNMCs; including immunologically mediated AEs of special interest) through day 182 post-day 0 dosing.
Statistical Analyses
Safety, immunogenicity per protocol, and intent-to-treat populations are described in Figure 1 and the Supplementary Appendix.
Data concerning wt-HAI titers were expressed as geometric mean titers (GMTs), geometric mean fold-rise (GMFRPost/Pre), between-group ratio of GMTs (GMTR), seroconversion rate (SCR), and seroprotection rate (SPR; Supplementary Appendix).
For CMI responses, measured by intracellular cytokine staining, peripheral blood CD4+ T-cell producing interleukin-2 (IL-2), interferon gamma (IFN-γ), and/or tumor necrosis factor alpha (TNF-α) cytokines following in vitro restimulation with influenza vaccine-homologous (A/Singapore/FIMH-16–0019/2016[H3N2], A/Michigan/45/2015[H1N1], B/Colorado/06/2017[Victoria]) or drifted (A/Wisconsin/19/2017[H3N2]) strain-specific HAs were reported as median cell counts, geometric mean cell counts (GMCs), and GMFRPost/Pre of double-cytokine (2 of 3: IL-2, IFN-γ, or TNF-α) or triple-cytokine producing (IL-2, IFN-γ, and TNF-α) influenza strain-specific CD4+ T cells (for each individual strain). Between-group differences were reported as the ratio of GMCs at day 7 of double- or triple-cytokine responses (and associated 90% confidence intervals [CIs]) [29].
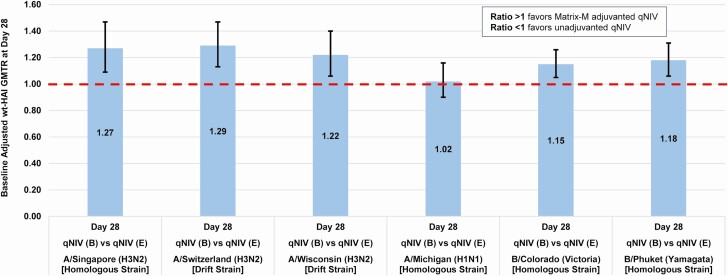
The success criterion for the primary immunogenicity objective is described in Figure 2 and the Supplementary Appendix. A total sample size of 1350 was selected to provide ≥80% power to achieve the primary immunogenicity objective of demonstrating an adjuvant effect.
Figure 2.
Demonstration of adjuvant effect–baseline adjusted ratio of day 28 wt-HAI geometric mean titers (GMTs; GMTR) (Matrix-M–adjuvanted qNIV [group B]/unadjuvanted qNIV [group E]). Full strain names: A/Singapore/INFIMH-16–0019/2016 (H3N2); A/Switzerland/9715293/2013 (H3N2); A/Wisconsin/19/2017 (H3N2); A/Michigan/45/2015 (H1N1); B/Colorado/06/2017 (Victoria lineage); B/Phuket/3073/2013 (Yamagata lineage). The primary immunogenicity objective of demonstrating an adjuvant effect required establishing immunogenic superiority of group B (qNIV 60 µg hemagglutinin [HA] × 4 strains with 50 µg Matrix-M1 adjuvant) relative to group E (qNIV 60 µg HA × 4 strains without adjuvant) by excluding values ≤1.0 at the lower 95% confidence bound for the baseline-adjusted ratio of day 28 post-vaccination wt-HAI GMTs (ie, GMT of group B [adjuvant]/GMT of group E [no adjuvant] at day 28) for not less than 2 of 6 influenza strains (ie, any 2 of 4 vaccine-homologous strains and/or 2 antigenically drifted influenza strains), while no other strain(s) demonstrated GMTRs that were significantly <1.0. Abbreviations: qNIV, quadrivalent recombinant nanoparticle influenza vaccine; wt-HAI, wild-type sequenced hemagglutinin inhibition antibody.
All statistical analyses were performed using SAS statistical software (version 9.4).
RESULTS
Study Participants
A total of 1375 participants were enrolled and randomized into 1 of 7 treatment groups (Figure 1; Supplementary Table 1). Twelve (<1%) participants discontinued from the trial through day 28 (Figure 1). Baseline characteristics of participants were similar across treatment groups. Mean age ranged from 71.8 years to 72.9 years (Supplementary Table 3). The proportion of females in each treatment group varied between 49% and 65%. The majority of participants in each group were white and had received influenza vaccine during the previous season (85%–89%).
Safety
The safety and reactogenicity profile was comparable between treatments groups through day 182 (Table 2). All treatments were well tolerated. Rates of all solicited AEs were comparable (27.3%–38.9%) across treatment groups; severe solicited local or systemic AEs were infrequent (<3.5% in any group). The most common solicited local AEs were injection site pain (10.3%–19.3%), swelling (3.8%–10.2%), and redness (2.6%–7.6%); common solicited systemic AEs included headache (7.4%–14.7%), muscle pain (4.6%–13.1%), and fatigue (5.1%–10.8%; Supplementary Table 6). MAEs were reported in a similar proportion of participants across all groups (24%–32% qNIV, 22% IIV3-HD, 27% RIV4), with no apparent clustering by diagnosis or treatment group. Overall, SAE rates were as expected given the age of the enrolled population and reported in <10% of participants across the entire study. SAEs occurred in 5.1%–9.1% of participants in the adjuvanted qNIV groups (pooled rate, 5.9%) compared with 3.9% of IIV3-HD participants and 2.0% of RIV4 participants (Table 2; Supplementary Table 7). No SAEs were considered related to study treatment in any treatment group, among 63 SAEs reported in 59 participants. Of 59 participants, 7 died; all events were determined to be not related by the study investigators.
Table 2.
Safety Summary of Adverse Events Post-Vaccination Day 0 through Day 181—Safety Population
| Treatment Group | A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|---|
| Hemagglutinin and Matrix Content | qNIV A60/B60/M50 | qNIV A60/B60/M50 | qNIV A60/B60/M75 | qNIV A60/B90/M50 | qNIV A60/B60/M0 | IIV3-HD | RIV4 |
| Formulation | In-Clinic Mix | Coformulated | NA | NA | NA | ||
| Participants in Group (N) | 157 | 305 | 156 | 132 | 311 | 153 | 151 |
| n (% of participants) 95% CI | |||||||
| All AEs | 100 (63.7) | 189 (62.0) | 92 (59.0) | 71 (53.8) | 165 (53.1) | 93 (60.8) | 87 (57.6) |
| 95% CI | (55.7–71.2) | (56.3–67.4) | (50.8–66.8) | (44.9–62.5) | (47.3–58.7) | (52.6–68.6) | (49.3–65.6) |
| Solicited AEsa | 61 (38.9) | 99 (32.5) | 59 (37.8) | 39 (29.5) | 85 (27.3) | 58 (37.9) | 56 (37.1) |
| 95% CI | (31.2–46.9) | (27.2–38.0) | (30.2–45.9) | (21.9–38.1) | (22.5–32.6) | (30.2–46.1) | (29.4–45.3) |
| Severe solicited AEs | 3 (1.9) | 10 (3.3) | 5 (3.2) | 2 (1.5) | 4 (1.3) | 2 (1.3) | 4 (2.6) |
| 95% CI | (.4–5.5) | (1.6–5.9) | (1.0–7.3) | (.2–5.4) | (.4–3.3) | (.2–4.6) | (.7–6.6) |
| Solicited local AEs | 30 (19.1) | 74 (24.3) | 34 (21.8) | 22 (16.7) | 40 (12.9) | 40 (26.1) | 22 (14.6) |
| 95% CI | (13.3–26.1) | (19.6–29.5) | (15.6–29.1) | (10.7–24.1) | (9.3–17.1) | (19.4–33.9) | (9.4–21.2) |
| Severe local AEs | 1 (0.6) | 2 (0.7) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| 95% CI | (.0–3.5) | (.1–2.3) | (.0–2.3) | (.0–2.8) | (.0–1.8) | (.0–2.4) | (.0–2.4) |
| Solicited systemic AEs | 42 (26.8) | 63 (20.7) | 45 (28.8) | 27 (20.5) | 65 (20.9) | 37 (24.2) | 39 (25.8) |
| 95% CI | (20.0–34.4) | (16.3–25.6) | (21.9–36.6) | (13.9–28.3) | (16.5–25.8) | (17.6–31.8) | (19.1–33.6) |
| Severe systemic AEs | 2 (1.3) | 9 (3.0) | 5 (3.2) | 2 (1.5) | 3 (1.0) | 2 (1.3) | 4 (2.6) |
| 95% CI | (.2–4.5) | (1.4–5.5) | (1.0–7.3) | (.2–5.4) | (.2–2.8) | (.2–4.6) | (.7–6.6) |
| Unsolicited AEsb | 73 (46.5) | 144 (47.2) | 56 (35.9) | 52 (39.4) | 104 (33.4) | 59 (38.6) | 56 (37.1) |
| 95% CI | (38.5–54.6) | (41.5–53.0) | (28.4–44.0) | (31.0–48.3) | (28.2–39.0) | (30.8–46.8) | (29.4–45.3) |
| Related unsolicited AEs | 8 (5.1) | 18 (5.9) | 5 (3.2) | 5 (3.8) | 8 (2.6) | 7 (4.6) | 6 (4.0) |
| 95% CI | (2.2–9.8) | (3.5–9.2) | (1.0–7.3) | (1.2–8.6) | (1.1–5.0) | (1.9–9.2) | (1.5–8.4) |
| Severe unsolicited AEs | 10 (6.4) | 20 (6.6) | 11 (7.1) | 11 (8.3) | 13 (4.2) | 6 (3.9) | 7 (4.6) |
| 95% CI | (3.1–11.4) | (4.1–9.9) | (3.6–12.3) | (4.2–14.4) | (2.2–7.0) | (1.5–8.3) | (1.9–9.3) |
| Severe/related unsolicited AEs | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 95% CI | (.0–2.3) | (.0–1.8) | (.0–2.3) | (.0–2.8) | (.0–1.2) | (.0–2.4) | (.0–2.4) |
| Serious AEs | 8 (5.1) | 16 (5.2) | 8 (5.1) | 12 (9.1) | 6 (1.9) | 6 (3.9) | 3 (2.0) |
| 95% CI | (2.2–9.8) | (3.0–8.4) | (2.2–9.9) | (4.8–15.3) | (.7–4.2) | (1.5–8.3) | (.4–5.7) |
| Significant new medical conditions | 5 (3.2) | 18 (5.9) | 10 (6.4) | 10 (7.6) | 15 (4.8) | 6 (3.9) | 9 (6.0) |
| 95% CI | (1.0–7.3) | (3.5–9.2) | (3.1–11.5) | (3.7–13.5) | (2.7–7.8) | (1.5–8.3) | (2.8–11.0) |
| Medically attended events | 51 (32.5) | 87 (28.5) | 29 (18.6) | 38 (28.8) | 74 (23.8) | 34 (22.2) | 40 (26.5) |
| 95% CI | (25.2–40.4) | (23.5–33.9) | (12.8–25.6) | (21.2–37.3) | (19.2–28.9) | (15.9–29.6) | (19.6–34.3) |
% = (n/N) × 100. Percentages are based on the number of participants in each treatment group in the safety population. Treatment group in the safety population is based on the actual dose(s) received. An AE was considered treatment-emergent if it began on or after the study day 0 vaccination. The Clopper-Pearson method was applied to calculate the proportion CI. Participants with multiple events within a category were counted only once, using the event with the greatest severity and/or relationship (possible, probable, definite) as applicable. For the total number of treatment-emergent AEs for each respective category, counts were limited to those events that fulfill the AE category.
Abbreviations: AE, adverse event; CI, confidence interval; IIV3-HD, trivalent high-dose inactivated influenza vaccine (Fluzone High-Dose); N, number of participants who receive test article at day 0; n, number of participants in each specified category of adverse events; NA, not applicable; qNIV, quadrivalent recombinant nanoparticle influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine (Flublok Quadrivalent).
aIncludes solicited AEs reported by participants (via diary or spontaneously) with a recorded start date within the 7-day post-each vaccination window (ie, from study day 0 through study day 6).
bIncludes unsolicited AEs, significant new medical conditions (SNMCs), medically attended events (MAEs), and serious adverse events (SAEs) with an onset date on or after day 0 to day 27 post-vaccination and SAEs, SNMCs, and MAEs from post-vaccination on day 0 through day 181.
Immunogenicity
wt-HAI Antibody Responses
The primary objective of demonstrating an adjuvant effect was achieved, with statistically significant increases in wt-HAI antibody responses ranging from 15% to 29% on 5 of 6 strains evaluated for group B (qNIV 60 µg HA per strain preformulated with 50 µg Matrix-M adjuvant per dose) compared with group E (qNIV 60 µg HA per strain without adjuvant; Figure 2).
For all 6 influenza A and B homologous and drifted strains evaluated, groups A, B, and C (qNIV with 60 µg HA per strain, mixed in-clinic or preformulated, with either 50 µg or 75 µg Matrix-M per dose) showed comparable induction of wt-HAI antibody responses based on day 28 GMTs, geometric mean ratios (GMRs), SCRs, and SPRs (Table 1; Supplementary Table 4), indicating that extended preformulation of antigen and adjuvant was feasible as it yielded immunogenicity similar to in-clinic mixture immediately before administration. There was no apparent incremental advantage to an increased adjuvant dose of Matrix-M based on wt-HAI antibody responses alone. In contrast, group D (which received an increased dose of both B antigens, but similar in A antigen content to other groups) compared with other Matrix-M–adjuvanted qNIV groups (A, B, and C) did not show improvement in wt-HAI responses against B strains but instead showed a tendency to reduced wt-HAI antibody response against several A strains, suggesting that an asymmetric increase in the content of B antigens relative to A antigens was not beneficial and potentially interfering.
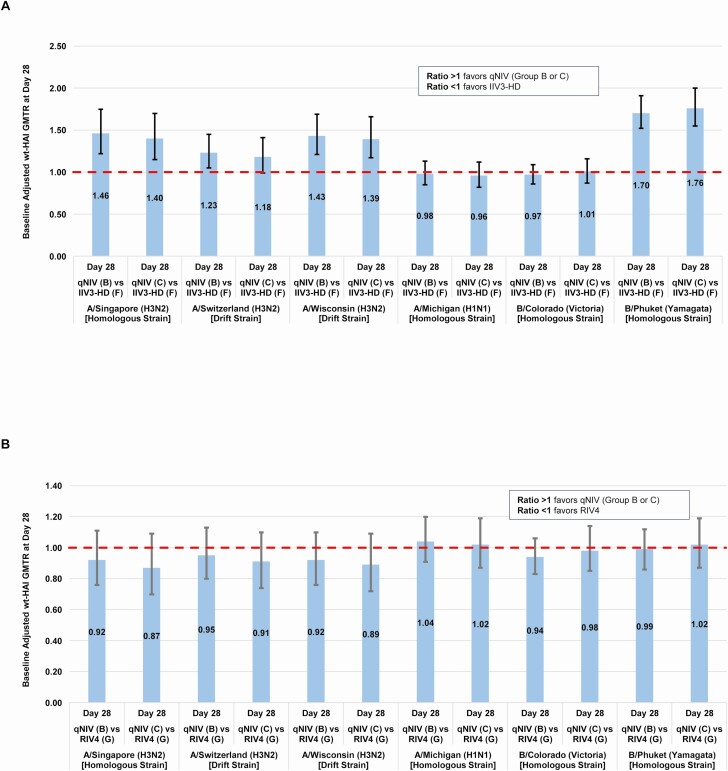
Based on the comparability of qNIV groups A, B, and C on wt-HAI responses, groups B and C were further compared with IIV3-HD and RIV4. Group A was not further considered due to the requirement of an in-clinic mix of antigen and adjuvant. At day 28, qNIV groups B and C, compared with IIV3-HD, showed increased wt-HAI antibody responses across a panel of A/H3N2 strains: 40%–46% increased against the vaccine-homologous A/H3N2 strain (A/Singapore/INFIMH-16–0019/2016), 18%–23% increased against a historically antigenically drifted A/H3N2 strain (A/Switzerland/9715293/2013), and 39%–43% increased against a contemporary, antigenically drifted A/H3N2 strain (A/Wisconsin/19/2017; Figure 3A). The wt-HAI responses to vaccine homologous A/H1N1 and B-Victoria lineage strains were comparable between qNIV groups B and C vs IIV3-HD. In contrast, across all 6 homologous or drifted strains evaluated, qNIV groups B and C showed wt-HAI antibody responses comparable to RIV4 (Table 1; Figure 3B). As expected, all treatment groups showed decay of wt-HAI antibody titers at later time points (day 56 and through day 182), although between-group differences were largely preserved (Supplementary Table 4).
Figure 3.
A, qNIV (group B or C) compared with IIV3-HD–baseline adjusted ratio of day 28 wt-HAI geometric mean titers (GMTs; GMTR) (qNIV [group B or C]/IIV3-HD [group F]). Full strain names: A/Singapore/INFIMH-16–0019/2016 (H3N2); A/Switzerland/9715293/2013 (H3N2); A/Wisconsin/19/2017 (H3N2); A/Michigan/45/2015 (H1N1); B/Colorado/06/2017 (Victoria lineage); B/Phuket/3073/2013 (Yamagata lineage). B, qNIV (group B or C) compared with RIV4–baseline adjusted ratio of day 28 wt-HAI GMTs (GMTR) (qNIV [group B or C]/RIV4 [group G]). Since day 56 samples were tested separately from day 0 and day 28 samples, day 56 titers were adjusted for the long-term assay variability. The adjustment was based on retesting of a randomly selected subset, 50 participants, of day 0 samples concurrently with day 56 samples. Abbreviations: B, group B; C, group C; F, group F; IIV3-HD, trivalent high-dose inactivated influenza vaccine; qNIV, quadrivalent recombinant nanoparticle influenza vaccine; wt-HAI, wild-type sequenced hemagglutinin inhibition antibody.
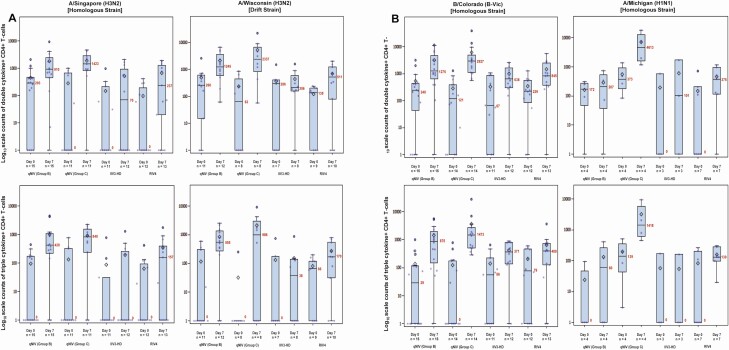
Cell-Mediated Immune Responses: Double- and Triple-Cytokine Producing Influenza Strain-Specific CD4+ T Cells
Pre- and post-vaccination distributions of strain-specific CD4+ T-cell responses for qNIV groups B and C and IIV3-HD and RIV4 are shown in Figure 4 and described in Supplementary Tables 5A–5C. At day 7 post-vaccination, polyfunctional phenotypes of double- and triple-cytokine producing influenza vaccine-homologous and drifted A/H3N2 strain-specific responses were induced in all Matrix-M adjuvant-containing qNIV formulations (groups A, B, C, and D; Supplementary Table 5A). Among the 4 Matrix-M–adjuvanted qNIV formulations, group C, with a higher dose of Matrix-M adjuvant (75 µg), showed the greatest induction of post-vaccination double- and triple-cytokine CD4+ T-cell responses across all strains evaluated and, when compared with the unadjuvanted formulation of qNIV, demonstrated 11.1- to 13.6-fold increases (all P < .01) in day 7 post-vaccination double-cytokine responses. Remarkably, and uniquely to qNIV group C, most participants had an influenza strain-specific double-cytokine CD4+ T-cell response across all homologous and drifted strains evaluated, indicating a relative absence of CMI “non-responders” (Figure 4A).
Figure 4.
Log10 scale counts of double- or triple-cytokine producing strain-specific CD4+ T cells by treatment group, time point, and strain. Cell-mediated immune (CMI) responses were measured by intracellular cytokine staining. Counts of peripheral blood CD4+ T cells producing interleukin-2 (IL-2), interferon gamma (IFN-γ), and/or tumor necrosis factor alpha (TNF-α) cytokines were measured following in vitro restimulation with vaccine-homologous (A/Singapore/FIMH-16–0019/2016 [H3N2]; A/Michigan/45/2015 [H1N1]; /Colorado/06/2017 [Victoria]), or drifted (A/Wisconsin/19/2017 [H3N2]) strain-specific recombinant wild-type sequence hemagglutinins (HAs). A, CMI responses against A/Singapore and A/Wisconsin. B, CMI responses against B/Colorado and A/Michigan. Box plots are shown for counts of double-cytokine producing (any 2 of IFN-γ, TNF-α, or IL-2) or triple-cytokine producing (all 3 of IFN-γ, TNF-α, and IL-2) strain-specific CD4+ T-cell responses across the 4 strains evaluated using peripheral blood mononuclear cells obtained from a subgroup of participants on day 0 (pre-vaccination) and day 7 (post-vaccination). The box plots represent the interquartile range (±3 standard deviations), the solid horizontal black line represents the median, the number in red indicates the median count of double- or triple-cytokine producing CD4+ T cells, respectively, and the open diamond represents the mean. Group B is qNIV 60 µg HA × 4 strains with 50 µg Matrix-M1 adjuvant; group C is qNIV 60 µg HA × 4 strains with 75 µg Matrix-M1 adjuvant; group F is IIV3-HD; and group G is RIV4. Note that the number of strains tested for a given participant’s sample was dependent on the number of cells available; thus, not all samples could be tested across all 4 strains. Abbreviations: IIV3-HD, trivalent high-dose inactivated influenza vaccine; qNIV, quadrivalent recombinant nanoparticle influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine.
Compared with IIV3-HD and RIV4, qNIV group C showed substantially higher post-vaccination fold rises in inductions of double- and triple-cytokine producing CD4+ T cells (Supplementary Table 5A). In terms of between-group differences, qNIV group C induced 4.1- to 30.8-fold and 6.6- to 31.5-fold higher double-cytokine influenza strain-specific responses at day 7 post-vaccination compared with IIV3-HD and RIV4 across strains, respectively, and corresponding 9.9- to 66.6-fold and 9.6- to 14.1-fold higher triple-cytokine influenza HA-specific responses at day 7 post-vaccination compared with IIV3-HD and RIV4 across strains, respectively (Supplementary Tables 5B and 5C).
DISCUSSION
Seven of the last 9 US influenza seasons have been characterized by dominant or codominant circulation of A/H3N2 viruses, with frequent reports of suboptimal field vaccine effectiveness, driven principally by the underperformance of the A(H3N2) component of the vaccine [3, 8–11, 30]. The burden of A/H3N2-associated morbidity and mortality has been borne disproportionately among older adults [20–22]. Existing high vaccine coverage rates in this population suggest that new immunization strategies are urgently required [31]. We have developed qNIV, a novel recombinant HA nanoparticle vaccine formulated with Matrix-M adjuvant, to address limitations of currently licensed, predominantly egg-derived, seasonal influenza vaccines [26, 28]. We demonstrated in this study that qNIV induced cross-reactive humoral immune responses against drifted A/H3N2 viruses in a manner that IIV3-HD did not and cellular responses against both vaccine-homologous and drifted A/H3N2 viruses in a manner that neither IIV3-HD or RIV4 could. These results suggest that substantial quantitative and qualitative enhancements of the humoral and cellular immune response against seasonal influenza viruses are possible in an older adult population in whom the challenge of a senescent immune system has historically proven difficult for influenza vaccines to overcome, particularly induction of cellular immunity.
A fundamental problem that limits the performance of existing influenza vaccine technologies has been the induction of narrow, vaccine strain–specific immunity [32, 33]. This creates vulnerability to classic antigenic drift from a virus adapted to evolve rapidly in order to evade host immune pressure. The consequences of antigenic mismatch were illustrated by the 2 recent US influenza seasons, which were characterized by the emergence of A/H3N2 drift variants antigenically distinct from the A/H3N2 vaccine strains and produced estimates of A/H3N2-specific vaccine effectiveness in adults aged ≥65 years as low as 13% (95% CI, –46% to 48%) and 10% (95% CI, –32% to 39%) during the 2018–2019 and 2017–2018 seasons, respectively [8, 9]. Our previous phase 1/2 study of tNIV (conducted in advance of the 2017–2018 US influenza season) demonstrated a 60% improvement in wt-HAI antibody responses induced by tNIV compared with IIV3-HD against the then, newly emerged, antigenically advanced drift variant A/Singapore/INFIMH-16-0019/2016 (H3N2)–like clade 3C.2a1 [28]. In the present study, we showed that against both historic (A/Switzerland/9715293/2013 [H3N2] clade 3c.3a) and contemporary (A/Wisconsin/19/2017 [H3N2] clade 3C.2a2) A/H3N2 drift variants, qNIV demonstrated improved wt-HAI antibody responses relative to IIV3-HD. In contrast, qNIV appeared to induce similarly robust wt-HAI antibody as RIV4 against the same panel of vaccine-homologous and drifted A/H3N2 viruses. Based on previous work with tNIV in ferrets and vaccine-induced bnAbs isolated from mice [27], we posit that breadth of cross-reactivity induced against drifted influenza strains may, in part, be mediated by the induction of bnAbs that interact with conserved HA head epitopes, both near and distant to the receptor binding domain, as well as conserved HA stem epitopes [26, 27].
A second critical challenge that limits vaccine performance is the increasingly recognized problem of egg-adaptive antigenic changes that arise from traditional egg-based manufacturing methods [16, 18]. While not a new challenge, the consequences of this problem have gained focus with data characterizing specific egg-adapted HA antigenic site mutations as having deleterious effects on A(H3N2) vaccine immunogenicity and effectiveness [6, 12–15]. The potential adverse impact of egg-adaptive mutations on vaccine immunogenicity and the capacity of qNIV to overcome this problem by preserving wt HA sequences was illustrated in the phase 1/2 study by the substantially enhanced neutralizing antibody responses induced by tNIV relative to IIV3-HD against wt sequenced A/Singapore/INFIMH-16-0019/2016 (H3N2) virus. Whereas, when neutralizing antibody responses were assessed against egg-adapted A/Singapore/INFIMH-16-0019/2016 (H3N2) virus, both vaccines appeared to perform comparably (Supplementary Figure 1). These data highlight not only the problems of egg-derived influenza vaccines but also the corresponding problem of using egg-derived viral reagents in either HAI or neutralization assays, which may lead to a biased assessment of vaccine immunogenicity in favor of egg-derived vaccines and away from wt virus relevant immune responses that may better predict clinical protection against circulating viruses [34].
A third critical challenge, which is of particular importance for older adult immunization, has been the limited induction of cellular immunity by currently licensed influenza vaccines. A randomized clinical trial (RCT) from Canada in older adults evaluated CMI responses to 4 licensed inactivated seasonal influenza vaccines (standard subunit, MF-59 adjuvanted subunit, standard split virus, or intradermal split virus) and found that all 4 vaccines had similar but limited induction of CMI responses, including no meaningful post-vaccination increases in CD4+ and CD8+ T cells expressing IFN-γ, IL-2, or IL-10 [24]. Similarly, a recent RCT from Hong Kong in older adults that compared CMI responses of 3 “enhanced” influenza vaccines (IIV3-HD, RIV4, and MF-59-adjuvanted IIV3) relative to a standard-dose IIV3 also found only modest day 7 post-vaccination induction of strain-specific IFN-γ+ CD4+ T-cell responses (range, 1.0- to 2.6-fold increases for enhanced vaccines and 1.2- to 1.8-fold increases for standard-dose IIV) [25]. The reported magnitude of these post-vaccination CD4+ T-cell responses was in line with similar observations regarding IIV3-HD and RIV4 in our study. These findings come amid a growing recognition that CD4+ and CD8+ T-cell responses are important for modulating influenza disease severity and conferring a breadth of vaccine protection [35–37]. However, specific vaccine-induced CMI correlates of protection have yet to be identified in adult populations. Failure to induce potent cellular immunity to influenza may be a problem of increased consequence in older adults because age-related declines in T-cell function and concomitant age-related increases in frailty may converge to both diminish vaccine response and increase the risk of serious complications of influenza virus infection [38–40]. Notwithstanding the limited sample size for CMI assessments in this study, we observed statistically significant activation of influenza HA-specific polyfunctional CD4+ T-cell responses by qNIV in an older adult population, which could be restimulated by either vaccine-homologous or drifted HA antigens in a manner not previously reported for existing seasonal influenza vaccines.
In conclusion, this study showed that Matrix-M–adjuvanted recombinant qNIV was well tolerated and could markedly enhance both broadly cross-reactive antibody and cellular immune responses. Further development of qNIV is warranted.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants, the investigators from the clinical trial sites, members of the sponsor’s and contract research organization team, and the Clinical Immunology Laboratory staff for their contributions to the trial. Editorial assistance on the preparation of this manuscript was provided by Phase Five Communications, supported by Novavax, Inc.
Funding. This work was funded by the sponsor, Novavax Inc. (Gaithersburg, MD).
Potential conflicts of interest. V. S., J. P., I. C., J. F., M. Z., S. C.-C., H. Z., B. Z., N. P., M. J. M., G. S., L. F., and G. M. G. are current employees of Novavax, Inc. A. F., D. N. T., M. S., R. C., N. W., and X. P. are former employees of Novavax, Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paget J, Spreeuwenberg P, Charu V, et al. ; Global Seasonal Influenza-associated Mortality Collaborator Network and GLaMOR Collaborating Teams . Global mortality associated with seasonal influenza epidemics: new burden estimates and predictors from the GLaMOR Project. J Glob Health 2019; 9:020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Past weekly surveillance reports. Available at: https://www.cdc.gov/flu/weekly/pastreports.htm. Accessed 7 February 2020.
- 4. Kissling E, Pozo F, Buda S, et al. Low 2018/19 vaccine effectiveness against influenza A(H3N2) among 15–64-year-olds in Europe: exploration by birth cohort. Euro Surveill 2019; 24:1900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kissling E, Pozo F, Buda S, et al. ; I-MOVE/I-MOVE+ Study Team . Effectiveness of influenza vaccine against influenza A in Europe in seasons of different A(H1N1)pdm09 and the same A(H3N2) vaccine components (2016–17 and 2017–18). Vaccine X 2019; 3:100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skowronski DM, Chambers C, De Serres G, et al. Early season co-circulation of influenza A(H3N2) and B(Yamagata): interim estimates of 2017/18 vaccine effectiveness, Canada, January 2018. Euro Surveill 2018; 23:18-00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skowronski DM, Sabaiduc S, Leir S, et al. Paradoxical clade- and age-specific vaccine effectiveness during the 2018/19 influenza A(H3N2) epidemic in Canada: potential imprint-regulated effect of vaccine (I-REV). Euro Surveill 2019; 24:1900585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flannery B, Kondor RJG, Chung JR, et al. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis 2020; 221:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rolfes MA, Flannery B, Chung JR, et al. Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis 2019; 69:1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flannery B, Chung JR, Monto AS, et al. Influenza vaccine effectiveness in the United States during the 2016–2017 season. Clin Infect Dis 2019; 68:1798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russell K, Chung JR, Monto AS, et al. Influenza vaccine effectiveness in older adults compared with younger adults over five seasons. Vaccine 2018; 36:1272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zost SJ, Parkhouse K, Gumina ME, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A 2017; 114:12578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu NC, Zost SJ, Thompson AJ, et al. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog 2017; 13:e1006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One 2014; 9:e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levine MZ, Martin ET, Petrie JG, et al. Antibodies against egg- and cell-grown influenza A(H3N2) viruses in adults hospitalized during the 2017–2018 influenza season. J Infect Dis 2019; 219:1904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Subbarao K, Barr I. A tale of two mutations: beginning to understand the problems with egg-based influenza vaccines? Cell Host Microbe 2019; 25:773–5. [DOI] [PubMed] [Google Scholar]
- 17. Allen JD, Ross TM. H3N2 influenza viruses in humans: viral mechanisms, evolution, and evaluation. Hum Vaccin Immunother 2018; 14:1840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378:7–9. [DOI] [PubMed] [Google Scholar]
- 19. Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belongia EA, McLean HQ. Influenza vaccine effectiveness: defining the H3N2 problem. Clin Infect Dis 2019; 69:1817–23. [DOI] [PubMed] [Google Scholar]
- 21. Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of mortality attributable to influenza and RSV in the United States during 1997–2009 by influenza type or subtype, age, cause of death, and risk status. Influenza Other Respir Viruses 2014; 8:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of hospitalization attributable to influenza and RSV in the US during 1997–2009, by age and risk status. BMC Public Health 2017; 17:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferdinands JM, Fry AM, Reynolds S, et al. Intraseason waning of influenza vaccine protection: evidence from the US Influenza Vaccine Effectiveness Network, 2011-12 through 2014-15. Clin Infect Dis 2017; 64:544–50. [DOI] [PubMed] [Google Scholar]
- 24. Kumar A, McElhaney JE, Walrond L, et al. Cellular immune responses of older adults to four influenza vaccines: results of a randomized, controlled comparison. Hum Vaccin Immunother 2017; 13:2048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cowling BJ, Perera RAPM, Valkenburg SA, et al. Comparative immunogenicity of several enhanced influenza vaccine options for older adults: a randomized, controlled trial. Clin Infect Dis 2020; 71:1704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith G, Liu Y, Flyer D, et al. Novel hemagglutinin nanoparticle influenza vaccine with Matrix-M™ adjuvant induces hemagglutination inhibition, neutralizing, and protective responses in ferrets against homologous and drifted A(H3N2) subtypes. Vaccine 2017; 35:5366–72. [DOI] [PubMed] [Google Scholar]
- 27. Portnoff AD, Patel N, Massare MJ, et al. Influenza hemagglutinin nanoparticle vaccine elicits broadly neutralizing antibodies against structurally distinct domains of H3N2 HA. Vaccines (Basel) 2020; 8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378:2346–8. [DOI] [PubMed] [Google Scholar]
- 29. Couch RB, Bayas JM, Caso C, et al. Superior antigen-specific CD4+ T-cell response with AS03-adjuvantation of a trivalent influenza vaccine in a randomised trial of adults aged 65 and older. BMC Infect Dis 2014; 14:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–51. [DOI] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention. 2010–11 through 2018–19 influenza seasons vaccination coverage trend report. Available at: https://www.cdc.gov/flu/fluvaxview/reportshtml/trends/index.html. Accessed 7 February 2020.
- 32. Belongia EA, Kieke BA, Donahue JG, et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis 2009; 199:159–67. [DOI] [PubMed] [Google Scholar]
- 33. Coughlan L, Palese P. Overcoming barriers in the path to a universal influenza virus vaccine. Cell Host Microbe 2018; 24:18–24. [DOI] [PubMed] [Google Scholar]
- 34. Ward BJ, Pillet S, Charland N, Trepanier S, Couillard J, Landry N. The establishment of surrogates and correlates of protection: useful tools for the licensure of effective influenza vaccines? Hum Vaccin Immunother 2018; 14:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilkinson TM, Li CKF, Chui CSC, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 2012; 18:274–80. [DOI] [PubMed] [Google Scholar]
- 36. Sridhar S, Begom S, Bermingham A, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 2013; 19:1305–12. [DOI] [PubMed] [Google Scholar]
- 37. Clemens EB, Van De Sandt C, Wong SS, Wakim LM, Valkenburg SA. Harnessing the power of T cells: the promising hope for a universal influenza vaccine. Vaccines (Basel) 2018; 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine 2007; 25:599–604. [DOI] [PubMed] [Google Scholar]
- 39. Andrew MK, Shinde V, Ye L, et al. ; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network and the Toronto Invasive Bacterial Diseases Network . The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shahid Z, Kleppinger A, Gentleman B, Falsey AR, McElhaney JE. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine 2010; 28:6145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.