Abstract
Background
Yearly influenza immunization is recommended for immunocompromised (IC) individuals, although immune responses are lower than that for the nonimmunocompromised and the data on vaccine effectiveness (VE) in the IC is scarce. We evaluated VE against influenza-associated hospitalization among IC adults.
Methods
We analyzed data from adults ≥ 18 years hospitalized with acute respiratory illness (ARI) during the 2017–2018 influenza season at 10 hospitals in the United States. IC adults were identified using prespecified case definitions using electronic medical record data. VE was evaluated with a test-negative case-control design using multivariable logistic regression with polymerase chain reaction–confirmed influenza as the outcome and vaccination status as the exposure, adjusting for age, enrolling site, illness onset date, race, days from onset to specimen collection, self-reported health, and self-reported hospitalizations.
Results
Of 3524 adults hospitalized with ARI, 1210 (34.3%) had an immunocompromising condition. IC adults were more likely to be vaccinated than non-IC (69.5% vs 65.2%) and less likely to have influenza (22% vs 27.8%). The mean age did not differ among IC and non-IC (61.4 vs 60.8 years of age). The overall VE against influenza hospitalization, including immunocompetent adults, was 33% (95% confidence interval [CI], 21–44). VE among IC vs non-IC adults was lower at 5% (95% CI, –29% to 31%) vs 41% (95% CI, 27–52) (P < .05 for interaction term).
Conclusions
VE in 1 influenza season was very low among IC individuals. Future efforts should include evaluation of VE among the different immunocompromising conditions and whether enhanced vaccines improve the suboptimal effectiveness among the immunocompromised.
Keywords: influenza, vaccine, vaccine effectiveness, immunocompromised
Annual influenza vaccination is recommended for immunocompromised (IC), but its effectiveness is not well known. Using prespecified case definitions of immunocompromise, we demonstrated that in the 2017–2018 influenza season, influenza vaccine effectiveness was not significant among hospitalized IC adults.
The number of immunocompromised (IC) individuals has increased because of greater longevity of the population, increasing numbers of solid organ and stem cell transplants, advances in the treatment of hematologic and solid malignancies, increase in the number of individuals living with human immunodeficiency virus (HIV), and the use of steroids, immune-modulating agents, and other immunosuppressive drugs to treat autoimmune and inflammatory conditions [1, 2]. Immunosuppressive conditions are heterogeneous and the degree and type of immune deficiency caused by each one of these conditions vary, but a unifying consequence is an increased risk of many infectious diseases including influenza [3]. Influenza is a common cause of illness and death, with an estimated 140 000–810 000 influenza-associated hospitalizations and 12 000–61 000 influenza-associated deaths annually in the United States [4].
IC individuals are at higher risk for influenza-related complications, including increased frequency of hospitalization, intensive care unit admission, longer duration of hospitalization, and death [5–10]. Influenza vaccination is the best available intervention for preventing these complications, and annual influenza vaccination is recommended for IC individuals [11]. However, the data on protection afforded by influenza vaccines in IC adults are scarce. A recent study on cancer patients demonstrated a vaccine effectiveness (VE) of 20% against influenza hospitalization compared with 42% in the general population [12, 13]. Most studies of IC adults are small and evaluate immunogenicity as a surrogate of effectiveness [14]. These immunogenicity studies among various IC groups have demonstrated that antibody responses to inactivated influenza vaccines are suboptimal compared with those without immunosuppression [14, 15]. However, immune response to vaccine does not necessarily directly relate to vaccine effectiveness [16, 17]. Since the 2015–2016 influenza season, the Centers for Disease Control and Prevention–funded U.S. Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN) has estimated influenza VE among adults hospitalized for acute respiratory infections.
Understanding influenza VE in IC individuals is crucial to the development of appropriate vaccination and public health policies. The purpose of this study was to evaluate influenza VE among hospitalized immunocompromised adults enrolled in the HAIVEN study during the 2017–2018 influenza season, when specific efforts were made to identify immunocompromised patients using case definitions for immunocompromising conditions.
METHODS
Study Design and Enrollment
The HAIVEN study is a multicenter, prospective, test-negative, case-control study to determine an annual estimate of VE against influenza-associated hospitalizations among adults in the United States. Methods for the HAIVEN study have been described previously [18]. Briefly, adults ≥ 18 years of age with new or worsening cough or sputum production of ≤ 10 days’ duration and a respiratory specimen collected ≤ 10 days from illness onset and ≤ 72 hours after hospital admission at 1 of 10 hospitals in Pennsylvania, Michigan, Tennessee, and Texas were eligible. Inclusion criteria included age ≥18 years, admission for an acute respiratory illness, or worsening of a chronic respiratory illness with a new or worsening cough. During the 2017–2018 influenza season, details on demographics, symptoms, influenza vaccination status, number of recent hospitalizations, and history of organ or stem cell transplant, and chemotherapy or radiation therapy in the preceding year were collected through the enrollment interview. Information about the clinical course and disease severity was obtained from electronic medical records (EMRs). All International Classification of Diseases-10 Diagnosis Clinical Modification (ICD-10-CM) codes and current procedural terminology (CPT) codes from all encounters in the 12 months before enrollment were obtained from the EMRs and used to identify the high-risk conditions associated with an increased risk of serious influenza complications [11].
Influenza Case Classification
Enrolled patients provided respiratory specimens for influenza testing by polymerase chain reaction (PCR). Specimens were either nasal and oropharyngeal swabs that were tested in research laboratories with Centers for Disease Control and Prevention PCR protocols or clinical nasopharyngeal specimens tested by PCR in hospital laboratories provided they were collected within 10 days of illness onset and 72 hours of admission. Enrolled patients who tested positive for influenza were classified as cases and those who tested negative for all influenza types were controls.
Influenza Vaccination Status
Self-reported current season influenza vaccination status was confirmed by medical record review, state immunization registry records, occupational health records, health insurance billing claims, and records from patients’ primary care providers. Information collected included date and route of administration and product name, manufacturer, and lot number. Self-reported vaccination was accepted if the patient provided a date and location for the vaccination. A participant was considered vaccinated if he or she received the 2017–2018 influenza vaccine ≥ 14 days before illness onset. Because up to 14 days is required to mount an immune response to vaccination, those vaccinated 0–13 days before illness onset were excluded becaue of indeterminate vaccination status.
Identification of Immunocompromising Conditions
All ICD-10-CM codes for all encounters and receipt of the biologic chemotherapeutic agents bortezomib, carfilzomib, daratumumab, dasatinib, gemtuzumab, and imatinib in the year before study enrollment were collected from EMR data. In the 2017–2018 influenza season, the enrollment questionnaire asked if the participant received chemotherapy or radiation therapy for cancer in the 12 months before enrollment. Eight groups of immunocompromising conditions were defined: organ transplantation, stem cell transplantation, underlying immunodeficiency (inborn errors of immunity), connective tissue disorder, receipt of chemotherapy or radiation therapy, hematologic conditions, chronic steroid use, and HIV. The basis for the groups was a previously described algorithm for identifying patients with active immunosuppression using ICD and CPT codes in a large database of patients with severe sepsis [19]. We slightly modified this algorithm in 2 aspects. For solid malignancies, we only included patients actively treated with chemotherapy or radiation to improve specificity of immunosuppression. We also included patients on chronic use of steroids (identified by ICD-10-CM codes). We considered the enrollment question on receipt of chemotherapy or radiation therapy as the gold standard and our data found that ICD-10-CM and CPT codes have low sensitivity to identify patients receiving chemotherapy or radiation therapy (Supplementary Table S1). Therefore, we identified patients with immunocompromising conditions based on ICD-10-CM codes listed (Supplementary Table S2), except for the receipt of chemotherapy or radiation therapy, which were determined from ICD-10-CM codes, or receipt of one of the biologic chemotherapeutic agents listed, or a positive answer to the enrollment question about the receipt of chemotherapy or radiation therapy.
The IC groups were mutually exclusive (ie, if a participant had more than 1 IC condition, the participant was classified based on a hierarchical algorithm as shown in Supplementary Figure S1 and counted only once). The different classification groups were defined based on the authors’ expert opinion. For example, if a participant had ICD-10-CM codes for organ transplant and chemotherapy, this participant was grouped in organ transplant.
Statistical Analysis
Demographic and other characteristics of the IC and non-IC groups were compared using Pearson χ 2 test or Fisher exact test for categorical variables and 2-sample t test for continuous variables.
VE was calculated by estimating the odds of influenza positivity among vaccinated patients compared with unvaccinated patients for the IC and non-IC groups using multivariable logistic regression using influenza positivity as the outcome and vaccination status as the exposure variable, with VE = (1 – adjusted odds ratio) × 100% [20].
In the primary analysis, we stratified the sample by immunocompromised status and estimated VE in each stratum:
where
flu = 1 if PCR-confirmed flu case (of specific type/subtype); 0 otherwise
vacc = 1 if received vaccine ≥14 days before symptom onset; 0 otherwise
Z = vector of adjustment variables including age (continuous), enrollment site, race, days from illness onset to specimen collection, date of illness onset (categorized as pre-peak, peak, or post-peak influenza periods [18], Supplement 1), self-reported health status (poor/fair and good/very good/excellent), and self-reported number of hospitalizations
and with VE defined as
To test if VE differed by immunocompromised status, we regressed flu status on vaccination status, immunocompromised status, and the pairwise multiplicative interaction between vaccination status and immunocompromised status:
where variables are defined as previously and
Effect modification of VE by immunocompromised status was assumed to be statistically significant if the test statistic for assessing if the coefficient for the interaction term, β 3, differed from zero had a P value < .05.
In secondary analyses, we stratified subjects by type-specific immunocompromised status and estimated VE within each stratum using a main effects model:
where variables are defined as previously except for models for immunodeficiency and HIV subgroups, in which Z = vector of adjustment variables including age (continuous), enrollment site, race, days from illness onset to specimen collection, date of illness onset (categorized as pre-peak, peak, or post-peak influenza periods [18]), self-reported health status (poor/fair and good/very good/excellent), and self-reported number of hospitalizations.
Because we did not specifically calculate sample sizes for this study, we did a post hoc power analysis based on the observed number of cases (n = 900) and controls (2600), vaccination rate among controls (67%), power of 80%, and a significance level of 0.05. We determined a minimum detectable vaccine effectiveness of 20% in our overall study population during the 2017–2018 influenza season based on these assumptions.
Analyses were conducted with SAS, version 9.4, software. Statistical significance was defined as a P value < .05 or a 95% confidence interval (CI) excluding the null value. We interpreted differences in VE estimates by IC vs non-IC subgroups, considering P value <.15 as statistically significant, which is in line with guidance for interpreting interaction between 2 dichotomous variables when effect size is expected to be moderate to high [21, 22]. The study protocol was approved by the research ethics boards at the participating institutions.
RESULTS
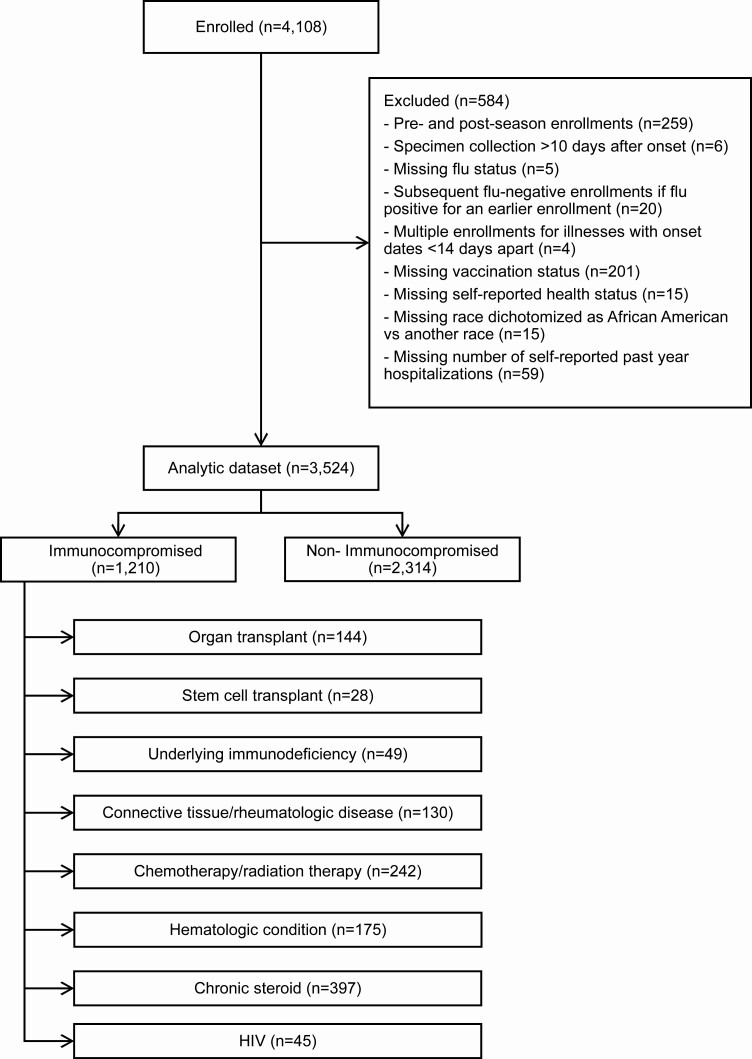
A total of 4108 hospitalized adults were enrolled in HAIVEN in the 2017–2018 influenza season. Of these, 584 were excluded because of enrollment earlier or later than the period of influenza circulation in the community (n = 259), missing vaccination status (n = 201), missing number of self-reported past year hospitalizations (n = 59), and other reasons (n = 65) (Figure 1). In the resulting dataset (n = 3524), 1210 (34.3%) adults were identified as having an immunocompromising condition: organ transplant (n = 144, 11.9%); stem cell transplant (n = 28, 2.3%); underlying immunodeficiency (n = 49, 4.0%); connective tissue and rheumatologic disease (n = 130, 10.7%); chemotherapy and radiation therapy (n = 242, 20%); hematologic condition (n = 175, 14.5%); chronic steroid use (n = 397, 32.8%); and HIV (n = 45, 3.7%).
Figure 1.
US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN) study population, 2017–2018. Immunocompromised groups were mutually exclusive and followed the order listed here. As an example, if an individual had an ICD-10 code that classified him/her as an organ transplant, this individual was grouped in this category, even if he/she also had an ICD-10 for chemotherapy. Abbreviations: HIV, human immunodeficiency virus; ICD, International Classification of Diseases.
Overall, participants were more likely to be female (56.9%) and white (62.2%). Mean age was 61 (standard deviation 17.1) years, 66.7% were vaccinated, 25.8% had influenza, and 84.2% had ≥ 3 high-risk conditions (Table 1). The IC and non-IC groups differed for several characteristics. IC participants were significantly more likely than non-IC to be of white race (67.9% vs 59.3%, P < .001), have a lower body mass index (30.1 vs 31.2, P = .003), be vaccinated (60.2% vs 54.6%, P = .002), have a longer hospital length of stay (4 vs 3 days, P < .001), have ≥ 3 high-risk conditions (94.2% vs 79%, P < .001), have had ≥ 4 hospitalizations in the previous year (25.5% vs 19.1%, P < .001), and present earlier in the pre-peak period (42.1% vs 37.4%, P = .02). IC participants were significantly less likely than non-IC participants to test positive for influenza (22% vs 27.8%, P < .001) and to self-report their health as fair or poor (45% vs 53.6%, P < .001) (Table 1).
Table 1.
Patient Characteristics Overall by Immunocompromising Condition, US Hospitalized Adult Influenza Vaccine Effectiveness (HAIVEN) Study, 2017–2018 (n = 3524)
| Total (n = 3524) | Nonimmunocompromised (n = 2314) | Immunocompromised (n = 1210) | P Value | |
|---|---|---|---|---|
| Enrollment site, n (%) | ||||
| Michigan | 943 (26.8) | 714 (30.9) | 229 (18.9) | <.001 |
| Pennsylvania | 834 (23.7) | 571 (24.7) | 263 (21.7) | |
| Tennessee | 589 (16.7) | 369 (16.0) | 220 (18.2) | |
| Texas | 1158 (32.9) | 660 (28.5) | 498 (41.2) | |
| Female, n (%) | 2004 (56.9) | 1317 (56.9) | 687 (56.8) | .94 |
| Age group, n (%), y | ||||
| 18–49 | 790 (22.4) | 534 (23.1) | 256 (21.2) | .37 |
| 50–64 | 1173 (33.3) | 753 (32.5) | 420 (34.7) | |
| 65–74 | 798 (22.6) | 517 (22.3) | 281 (23.2) | |
| 75+ | 763 (21.7) | 510 (22.0) | 253 (20.9) | |
| Age, mean ± SD | 61.0 ± 17.1 | 60.8 ± 17.7 | 61.4 ± 15.9 | .29 |
| Race, n (%) | ||||
| White, non-Hispanic | 2193 (62.2) | 1371 (59.3) | 822 (67.9) | <.001 |
| Non-white | 1331 (37.8) | 943 (40.8) | 388 (32.1) | |
| BMI, mean ± SD | 30.8 ± 9.5 | 31.2 ± 9.8 | 30.1 ± 8.9 | .001 |
| Any flu, n (%) | ||||
| Negative | 2614 (74.2) | 1670 (72.2) | 944 (78.0) | <.001 |
| Positive | 910 (25.8) | 644 (27.8) | 266 (22.0) | |
| Documented vaccination, n (%) | ||||
| No | 1174 (33.3) | 805 (34.8) | 369 (30.5) | .01 |
| Yes | 2350 (66.7) | 1509 (65.2) | 841 (69.5) | |
| Vaccine type, n (%) | ||||
| Standard dose | 1443 (61.4) | 921 (59.7) | 522 (62.1) | .02 |
| High dose | 523 (22.3) | 365 (23.4) | 158 (18.8) | |
| Unknown | 384 (16.3) | 223 (14.8) | 161 (19.1) | |
| Length of stay, median (IQR) | 3.0 (4.0) | 3.0 (3.0) | 4.0 (4.0) | <.001 |
| Influenza-like illness symptoms, n (%) | ||||
| No | 1208 (34.3) | 797 (34.4) | 411 (34.0) | .78 |
| Yes | 2316 (65.7) | 1517 (65.6) | 799 (66.0) | |
| Number of high-risk conditions, n (%) | ||||
| No high-risk conditions | 162 (4.6) | 141 (6.1) | 21 (1.7) | <.001 |
| 1–2 high-risk conditions | 394 (11.2) | 345 (14.9) | 49 (4.1) | |
| ≥3 high-risk conditions | 2968 (84.2) | 1828 (79.0) | 1140 (94.2) | |
| Self-reported hospitalizations in the prior year, n (%) | ||||
| 0–3 hospitalizations | 2773 (78.7) | 1871 (80.9) | 902 (74.6) | <.001 |
| ≥4 hospitalizations | 751 (21.3) | 443 (19.1) | 308 (25.5) | |
| Interval from illness onset and specimen collection, n (%) | ||||
| 0–1 d | 693 (19.7) | 435 (18.8) | 258 (21.3) | .14 |
| 2–4 d | 1621 (46.0) | 1065 (46.0) | 556 (46.0) | |
| 5–10 d | 1210 (34.3) | 814 (35.2) | 396 (32.7) | |
| Onset date | ||||
| Pre-peak | 1374 (39.0) | 865 (37.4) | 509 (42.1) | .02 |
| Peak | 844 (24.0) | 559 (24.2) | 285 (23.6) | |
| Post-peak | 1306 (37.1) | 890 (38.5) | 416 (34.4) | |
| Self-reported health status, n (%) | ||||
| Excellent/very good/good | 1739 (49.4) | 1074 (46.4) | 665 (55.0) | <.001 |
| Fair/poor | 1785 (50.7) | 1240 (53.6) | 545 (45.0) |
Abbreviations: BMI, body mass index; IQR, interquartile range; SD, standard deviation.
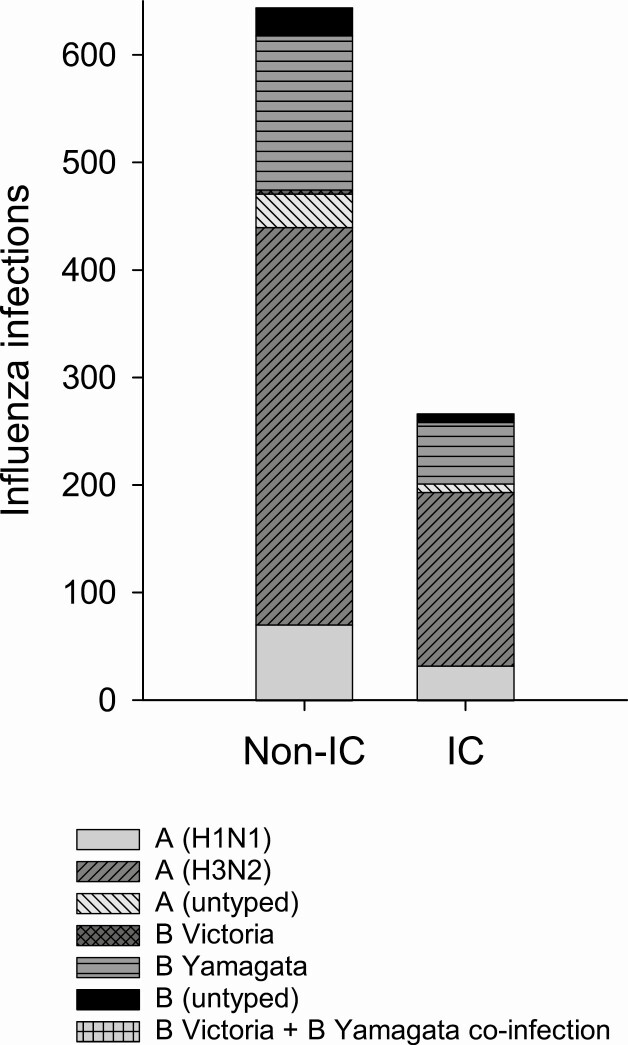
There were 266 influenza cases in the IC adults and 644 influenza cases in non-IC adults. Most influenza infections were caused by influenza A; 530 (78.8%) were A(H3N2) viruses. Of 238 influenza B infections, 200 (84%) were due to B Yamagata lineage viruses (Figure 2).
Figure 2.
Influenza virus type/subtype and lineage in the non-immunocompromised and immunocompromised groups. Abbreviations: IC, immunocompromised; non-IC, nonimmunocompromised.
The patients in the 8 immunocompromised groups differed in sex, enrollment site, age/age group, race, body mass index, influenza status, documented influenza vaccination, number of high-risk conditions, and self-reported health status, but not in the number of hospitalizations in the previous year, interval from illness onset to specimen collection, and date of illness onset (Supplementary Table S3).
Overall, vaccination was 33% (95% CI, 21–44) effective in preventing hospitalization. Among IC adults, VE was 5% and not significant (95% CI, –29% to 31%). VE in non-IC adults was 41% (95% CI, 27–52) (P < .05 for interaction term) (Table 2). VE for the different immunocompromised conditions varied widely, from –73% for individuals with underlying immunodeficiency to 84% for stem cell transplant; however, this study was not powered to look at these subgroups and the confidence intervals varied widely (Supplementary Figure S2).
Table 2.
Influenza Vaccine Effectiveness for Prevention of Influenza A or B–associated Hospitalizations in Immunocompromised and Nonimmunocompromised Adults, US Hospitalized Adult Influenza Vaccine Effectiveness (HAIVEN) Study, 2017–2018
| N Influenza Cases (% Vaccinated) | Unadjusted VE, % (95% CI) | Adjusted VE, % (95% CI)a | |
|---|---|---|---|
| All, n = 3524 | 910 (67) | 28 (16–38) | 33 (21–44) |
| Nonimmunocompromised, n = 2314 | 644 (65) | 36 (23–47) | 41 (27–52) |
| Immunocompromised, n = 1210 | 266 (70) | -0.3 (–35 to 25) | 5 (–29 to 31) |
| Nonimmunocompromised, influenza A | 471 (65) | 31 (15–44) | 31 (14–46) |
| Immunocompromised, influenza A | 202 (70) | 1 (–37 to 29) | 4 (–36 to 32) |
| Nonimmunocompromised, H1N1 | 71 (65) | 57 (32–74) | 52 (19–71) |
| Immunocompromised, H1N1 | 33 (70) | 60 (17–80) | 51 (–2 to 77) |
| Nonimmunocompromised, H3N2 | 369 (65) | 21 (0–37) | 23 (0–40) |
| Immunocompromised, H3N2 | 161 (70) | –28 (–87 to 12) | –18 (–75 to 20) |
| Nonimmunocompromised, influenza B | 173 (65) | 34 (10–52) | 45 (22–61) |
| Immunocompromised, influenza B | 65 (70) | 1 (–70 to 43) | 13 (–52 to 51) |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
aAdjusted for enrolling site, onset date (pre-peak, peak, post-peak), age, race, days from illness onset to specimen collection (0–1, 2–4, 5–10 days), self-reported health (poor/fair, good/very good/excellent), and self-reported hospitalizations.
DISCUSSION
During the high-severity 2017–2018 US influenza season, we found that influenza vaccination reduced the odds of influenza-associated hospitalization among adults by 33%. Overall, VE during the 2017–2018 season was lower than that estimated in previous seasons in this network (42%–54%) [18, 23]. That influenza A(H3N2) viruses circulating in 2017–2018 were antigenically different from the vaccine H3N2 strain because of suspected egg-adapted glycosylation in the antigenic epitopes of the vaccines may be responsible for the lower VE [24]. Compared with VE in non-IC adults (41%), VE in IC adults was substantially lower (5%) during this season. This lower VE among IC adults is unlikely to be an artifact, because the findings are consistent with the immunogenicity studies of inactivated influenza vaccines that have demonstrated significantly reduced humoral immune responses to standard inactivated influenza vaccines in immunosuppressed patients with HIV, organ transplants, cancer, and those receiving immunosuppressants [14]. In this network, influenza vaccination rate among controls was greater in the IC (60%) than in the non-IC group (54%), which is consistent with national US data in the insured population [25]. The higher vaccination rate among IC may be due to more frequent healthcare encounters and closer monitoring among IC patients offering more opportunities to vaccinate, or a heightened perception of risk for influenza complications by providers, leading to increased willingness to recommend influenza vaccine, and by patients, leading to greater willingness to receive vaccination.
Limited data exist on the prevention of influenza infection on IC adults by vaccination. Most studies have focused on the measurement of humoral antibody response among patients with particular immunocompromising conditions and have reported significantly reduced humoral immune responses [15, 26, 27]. However, this approach disregards the relationship between clinical outcomes and immune response, the levels of antibody titers from previous immunizations that may cause overestimations of response, and the role of cell-mediated immune response to vaccination in the prevention of influenza infection. Although studies of high-dose influenza vaccine have demonstrated improved antibody responses in adult organ transplant recipients and improved antibody responses and outcomes in adults older than 65 years of age compared with standard dose vaccine, it is unknown if enhanced vaccine options, such as high-dose and adjuvanted vaccines, could improve VE in immunocompromised groups [27–30]. Increasing the evidence base for informing the use of enhanced influenza vaccines in immunosuppressed populations is necessary for determining if these interventions might offer added value to standard influenza vaccines and potentially contribute to improving efficacy of these vaccines.
A primary challenge in the study of influenza VE in IC individuals is the definition of immunocompromise. Immunocompromising conditions are heterogenous and the degree of immunosuppression among groups is challenging to quantify. Additionally, within a defined IC group, differences in the degree of immunosuppression are difficult to assess, based on clinical records. We considered ~34% of the adults hospitalized with an acute respiratory illness during the influenza season as being immunocompromised by predefining groups of immunocompromising conditions that were identified by ICD-10-CM codes for all medical encounters in the preceding year. To complement our case definition, we also analyzed the addition of CPT codes for chemotherapy administration, chemotherapeutic drugs recorded in the EMRs, and a question at the time of enrollment about the receipt of chemotherapy or radiation therapy in the preceding 12 months. Although we did not collect other immunosuppressant and biological data, we identified a similar proportion of IC adults among those hospitalized with acute respiratory illness as identified in the study by Patel et al that used MarketScan data to estimate the prevalence of immunosuppressive conditions and risk for acute respiratory illnesses [25].
Findings of this study should be interpreted in the context of several limitations. Although we used an objective and systematic mechanism to identify the different IC groups, our identification of the IC groups accounts for only a rough measure of immunosuppression. We did not consider the presence of more than 1 immunocompromising condition, and we were unable to evaluate the effect of timing of vaccination in relation to the immunosuppression. We were unable to evaluate VE among different IC because of inadequate sample sizes. A study with a greater number of IC adults that allows for analyses of subgroups, virus subtypes, and different vaccine formulations is needed for definitive conclusions. Our study is also limited to a single season when vaccine was mismatched to the circulating A/H3N2 viruses and thus may not be applicable to other influenza viruses. Data are also from 10 hospitals in 4 US states and may not be generalizable.
Our study’s strengths include the use of a standardized protocol with symptom-based eligibility and comprehensive PCR testing to identify influenza cases and controls, a test-negative case control design, and recruitment in geographically diverse areas. Furthermore, our study shows that immunocompromising conditions can be identified based on EMR data, without the need for cumbersome medication reviews.
Proper identification of IC groups in future VE studies will have implications for public policy development, such as a recommendation for a different vaccine formulation for IC groups, or a consideration for chemoprophylaxis for those with immunocompromise.
Vaccine effectiveness against influenza was not significant among hospitalized immunocompromised patients. In light of our findings, decreasing the burden of influenza in IC individuals may be less dependent on improving their vaccine coverage than on improving vaccination rates of close contacts of the IC individual, thereby creating a circle of protection around an IC individual. Mathematical modeling has shown that even small improvements in VE and vaccine coverage are associated with substantial reductions in influenza burden [31].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
HAIVEN Study Investigators. University of Pittsburgh Medical Center, Pennsylvania: Richard Zimmerman, Donald Middleton, Fernanda Silveira, Kailey Hughes, Heather Eng, Theresa Sax, Sean Saul, Charles Rinaldo, Balasubramani Goundappa, Mary Patricia Nowalk, Lori Steiffel, John Williams, Monika Johnson. Baylor Scott and White Health, Texas: Manjusha Gaglani, Kempapura Murthy, Tresa McNeal, Shekar Ghamande, Victor Escobedo, Anne Robertson, Lydia Clipper, Arundhati Rao, Kevin Chang, Marcus Volz, Kimberly Walker, Alejandro Arroliga. University of Michigan, Michigan: Arnold Monto, Emily Martin, Ryan Malosh, Joshua Petrie, Adam Lauring, Caroline Cheng, Hannah Segaloff, E J McSpadden, Emileigh Johnson, Rachel Truscon. Henry Ford Health System, Michigan: Lois Lamerato, Susan Davis, Marcus Zervos. Vanderbilt University Medical Center, Tennessee: H Keipp Talbot, Dayna Wyatt, Yuwei Zhu, Zhouwen Liu, Rendie McHenry, Natasha Halasa, Sandra Alvarez Calvillo, Stephanie Longmire, Laura Stewart. Centers for Disease Control and Prevention, Georgia: Jill Ferdinands, Alicia Fry, Elif Alyanak, Emily Smith, Courtney Strickland, Sarah Spencer, Brendan Flannery, Jessie Chung, Xiyan Xu, Stephen Lindstrom, LaShondra Berman, Wendy Sessions, Rebecca Kondor, Manish Patel.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of Centers for Disease Control and Prevention.
Financial support. This work was supported by the Centers for Disease Control and Prevention (cooperative agreement IP15-002). Vanderbilt also received support from CTSA award no. UL1 TR002243 from the National Center for Advancing Translational Sciences.
Potential conflicts of interest. D. B. M. has received personal fees from Sequris, Pfizer, Sanofi Pasteur, Moderna, Bavarian Nordik, and GlaxoSmithKline, and grants from Pfizer. M. P. N. has received grants from Merck. E. T. M. has received personal fees from Pfizer and grants from Merck. M. G. has received grants from Centers for Disease Control and Prevention-Abt Associates. J. F. reports nonfinancial support from the Institute for Influenza Epidemiology. R. K. Z. has received grants from Sanofi Pasteur. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
for the HAIVEN Study Investigators:
Richard Zimmerman, Donald Middleton, Fernanda Silveira, Kailey Hughes, Heather Eng, Theresa Sax, Sean Saul, Charles Rinaldo, Balasubramani Goundappa, Mary Patricia Nowalk, Lori Steiffel, John Williams, Monika Johnson, Manjusha Gaglani, Kempapura Murthy, Tresa McNeal, Shekar Ghamande, Victor Escobedo, Anne Robertson, Lydia Clipper, Arundhati Rao, Kevin Chang, Marcus Volz, Kimberly Walker, Alejandro Arroliga, Arnold Monto, Emily Martin, Ryan Malosh, Joshua Petrie, Adam Lauring, Caroline Cheng, Hannah Segaloff, E J McSpadden, Emileigh Johnson, Rachel Truscon, Lois Lamerato, Susan Davis, Marcus Zervos, H Keipp Talbot, Dayna Wyatt, Yuwei Zhu, Zhouwen Liu, Rendie McHenry, Natasha Halasa, Sandra Alvarez Calvillo, Stephanie Longmire, Laura Stewart, Jill Ferdinands, Alicia Fry, Elif Alyanak, Emily Smith, Courtney Strickland, Sarah Spencer, Brendan Flannery, Jessie Chung, Xiyan Xu, Stephen Lindstrom, LaShondra Berman, Wendy Sessions, Rebecca Kondor, and Manish Patel
References
- 1. Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA 2016; 316:2547–8. [DOI] [PubMed] [Google Scholar]
- 2. Rubin LG, Levin MJ, Ljungman P, et al. ; Infectious Diseases Society of America . 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58:e44–100. [DOI] [PubMed] [Google Scholar]
- 3. Dropulic LK, Lederman HM. Overview of infections in the immunocompromised host. Microbiol Spectr 2016; 4. doi: 10.1128/microbiolspec.DMIH2-0026-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Past seasons estimated influenza disease burden. Available at: https://www.cdc.gov/flu/about/burden/past-seasons.html. Accessed 30 July 2020.
- 5. Kumar D, Michaels MG, Morris MI, et al. ; American Society of Transplantation H1N1 Collaborative Study Group . Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect Dis 2010; 10:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ljungman P, de la Camara R, Perez-Bercoff L, et al. ; Infectious Diseases Working Party, European Group for Blood and Marrow Transplantation; Infectious Complications Subcommittee, Spanish Group of Haematopoietic Stem-cell Transplantation . Outcome of pandemic H1N1 infections in hematopoietic stem cell transplant recipients. Haematologica 2011; 96:1231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chemaly RF, Vigil KJ, Saad M, et al. A multicenter study of pandemic influenza A (H1N1) infection in patients with solid tumors in 3 countries: early therapy improves outcomes. Cancer 2012; 118:4627–33. [DOI] [PubMed] [Google Scholar]
- 8. Memoli MJ, Athota R, Reed S, et al. The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts. Clin Infect Dis 2014; 58:214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garnacho-Montero J, León-Moya C, Gutiérrez-Pizarraya A, et al. ; on Behalf GETGAG Study Group . Clinical characteristics, evolution, and treatment-related risk factors for mortality among immunosuppressed patients with influenza A (H1N1) virus admitted to the intensive care unit. J Crit Care 2018; 48:172–7. [DOI] [PubMed] [Google Scholar]
- 10. Collins JP, Campbell AP, Openo K, et al. Outcomes of immunocompromised adults hospitalized with laboratory-confirmed influenza in the United States, 2011–2015. Clin Infect Dis 2020; 70:2121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2020-21 influenza season. MMWR Recomm Rep 2020; 69:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blanchette PS, Chung H, Pritchard KI, et al. Influenza vaccine effectiveness among patients with cancer: a population-based study using health administrative and laboratory testing data from Ontario, Canada. J Clin Oncol 2019; 37:2795–804. [DOI] [PubMed] [Google Scholar]
- 13. Nichols MK, Andrew MK, Hatchette TF, et al. Influenza vaccine effectiveness to prevent influenza-related hospitalizations and serious outcomes in Canadian adults over the 2011/12 through 2013/14 influenza seasons: a pooled analysis from the Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS Network). Vaccine 2018; 36:2166–75. [DOI] [PubMed] [Google Scholar]
- 14. Beck CR, McKenzie BC, Hashim AB, Harris RC, Nguyen-Van-Tam JS; University of Nottingham Influenza and the ImmunoCompromised (UNIIC) Study Group, . Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. J Infect Dis 2012; 206:1250–9. [DOI] [PubMed] [Google Scholar]
- 15. Watcharananan SP, Thakkinstian A, Srichunrasmee C, Chuntratita W, Sumethkul V. Comparison of the immunogenicity of a monovalent influenza A/H1N1 2009 vaccine between healthy individuals, patients with chronic renal failure, and immunocompromised populations. Transplant Proc 2014; 46:328–31. [DOI] [PubMed] [Google Scholar]
- 16. Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol 2010; 10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004; 103:133–8. [DOI] [PubMed] [Google Scholar]
- 18. Ferdinands JM, Gaglani M, Martin ET, et al. Prevention of influenza hospitalization among adults in the United States, 2015–2016: results from the US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). J Infect Dis 2019; 220:1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greenberg JA, Hohmann SF, Hall JB, Kress JP, David MZ. Validation of a method to identify immunocompromised patients with severe sepsis in administrative databases. Ann Am Thorac Soc 2016; 13:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine 2013; 31:3104–9. [DOI] [PubMed] [Google Scholar]
- 21. Durand CP. Does raising type 1 error rate improve power to detect interactions in linear regression models? A simulation study. PLoS One 2013; 8:e71079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marshall SW. Power for tests of interaction: effect of raising the type I error rate. Epidemiol Perspect Innov 2007; 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tenforde MW, Chung J, Smith ER, et al. Influenza vaccine effectiveness in inpatient and outpatient settings in the United States, 2015 - 2018. Clin Infect Dis 2020; ciaa407. doi: 10.1093/cid/ciaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levine MZ, Martin ET, Petrie JG, et al. Antibodies against egg- and cell-grown influenza A(H3N2) viruses in adults hospitalized during the 2017–2018 influenza season. J Infect Dis 2019; 219:1904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel M, Chen J, Kim S, et al. Analysis of MarketScan data for immunosuppressive conditions and hospitalizations for acute respiratory illness, United States. Emerg Infect Dis 2020; 26:1720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Helanterä I, Janes R, Anttila VJ. Clinical efficacy of seasonal influenza vaccination: characteristics of two outbreaks of influenza A(H1N1) in immunocompromised patients. J Hosp Infect 2018; 99:169–74. [DOI] [PubMed] [Google Scholar]
- 27. Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66:1698–704. [DOI] [PubMed] [Google Scholar]
- 28. DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–45. [DOI] [PubMed] [Google Scholar]
- 29. Doyle JD, Beacham L, Martin ET, et al. Relative and absolute effectiveness of high-dose and standard-dose influenza vaccine against influenza-related hospitalization among older adults - United States, 2015–2017. Clin Infect Dis 2020; ciaa160. doi: 10.1093/cid/ciaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Young-Xu Y, Van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among veterans health administration patients. J Infect Dis 2018; 217:1718–27. [DOI] [PubMed] [Google Scholar]
- 31. Hughes MM, Reed C, Flannery B, et al. Projected population benefit of increased effectiveness and coverage of influenza vaccination on influenza burden in the United States. Clin Infect Dis 2020; 70:2496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.