Abstract
Background
Adequate medication adherence is critical for achieving sustained viral response (SVR) of hepatitis C virus (HCV) among people who inject drugs (PWID). However, it is less known which patterns of direct-acting antiviral (DAA) treatment adherence are associated with SVR in this population or what factors are associated with each pattern.
Methods
The randomized 3-arm PREVAIL study used electronic blister packs to obtain daily time frame adherence data in opiate agonist therapy program settings. Exact logistic regressions were applied to test the associations between SVR and 6 types of treatment adherence patterns.
Results
Of the 113 participants treated with combination DAAs, 109 (96.5%) achieved SVR. SVR was significantly associated with all pattern parameters except for number of switches between adherent and missed days: total adherent daily doses (exact adjusted odds ratio [AOR] = 1.12; 95% confidence interval [CI] = 1.04–1.22), percent total doses (1.09; 1.03–1.16), days on treatment (1.16; 1.05–1.32), maximum consecutive adherent days (1.34; 1.06–2.04), and maximum consecutive nonadherent days (0.85; .74–.95 = 0.003). SVR was significantly associated with total adherent doses in the first 2 months of treatment, it was not in the last month. While alcohol intoxication was significantly associated with frequent switches, drug use was not associated with any adherence pattern.
Conclusions
Consistent maintenance of adequate total dose adherence over the entire course of HCV treatment is important in achieving SVR among PWID. Additional integrative addiction and medical care may be warranted for treating PWID who experience alcohol intoxication.
Keywords: HCV, SVR, OAT, adherence pattern, PWID
Achieving sustained viral response (SVR) among hepatitis C virus–infected people who inject drugs critically hinges on treatment adherence. We proposed 6 adherence pattern parameterizations, and showed that SVR is associated with 5 pattern parameters that support adherence-promoting care models.
Hepatitis C virus (HCV) infection is a significant health burden worldwide [1–3] but particularly among people who inject drugs (PWID) [4, 5]. With the advent of highly effective direct-acting antiviral (DAA) medications [6] with shorter treatment periods, fewer side effects [7, 8], and decreased mortality [9–12], HCV cure (sustained viral response [SVR]) has increased from the era of the interferon-based treatments [13, 14]. Such effectiveness has also been reported in treating HCV among people who inject drugs (PWID) who account for a majority of the HCV-infected persons [15, 16]. Opioid agonist therapy (OAT) programs therefore provide natural opportunities for treating HCV and opioid use disorder simultaneously [17, 18] since about 60% of OAT program patients are infected with HCV [19].
Although HCV treatment is highly effective in OAT program settings, successful SVR hinges on adequate adherence to treatment regimens [20, 21]. Thus, intensive care models such as directly observed therapy have been implemented to increase adherence to HCV treatments. The PREVAIL randomized trial was conducted in an urban inner city OAT program setting to test the effectiveness of 3 intensive models of care on SVR and adherence [22]. The trial used electronic blister packs to rigorously measure adherence and showed that higher adherence is associated with successful SVR [23]. However, individual-level overall adherence was computed as a percentage of dispensed doses over the returned available blister packs regardless of length of prescribed treatment. This computation implicitly assumes that adherence rates would be the same for unreturned or missing blister packs.
Given the lack of consensus or research on identifying best parametrizations of adherence, it is critical to determine whether SVR is associated with adherence parametrized or summarized in different ways to account for patterns of adherence during treatment. For instance, Cunningham et al [20] showed that different pattern parameters of nonadherence did not impact SVR, suggesting a degree of forgiveness to nonadherence with the DAA regimens. To extend this research, we defined 6 adherence patterns using blister pack adherence data from the PREVAIL study and tested associations between each adherence parameter and SVR and identified patient characteristics associated with each parameter.
METHODS
Setting and Design
The present study is a secondary analysis of data collected from the 3-arm PREVAIL randomized clinical trial conducted at 3 OAT program sites in the Bronx, New York. This trial was designed to compare the effectiveness of HCV care models over a treatment period: standard individual therapy, group therapy, and directly observed therapy (DOT). The random allocations of these care models were stratified by OAT clinic site, human immunodeficiency virus (HIV) coinfection status, cirrhosis status, and IL28B genotype (TC/TT vs CC) with a 1:1:1 allocation ratio in each stratum. Eligible participants who refused to be randomized were given the opportunity to participate in separate trials, which allowed the participants to choose their preferred care models with consultation to providers. The PREVAIL study used electronic blister packs that accurately captured and recorded the time and date upon pop-up of a blister for a medication retrieval. Opt-out from the use of the blister packs was not allowed. A detailed description of the PREVAIL study design and settings, including inclusion and exclusion criteria, has been published [22].
HCV DAA Treatments
Although the PREVAIL study included interferon and ribavirin-based treatments such as telaprevir/pegylated interferon/ribavirin (TVR/PEG/RBV), sofosbuvir/pegylated interferon/ribavirin (SOF/PEG/RBV), and sofosbuvir/ribavirin (SOF/RBV), this analysis focused on adherence to combination DAAs (sofosbuvir/ledipasvir [SOF/LDV] or sofosbuvir/simeprevir [SOF/SIM]).
Participants
A total of 150 PWID living with HCV were enrolled in the PREVAIL study. A detailed description of baseline characteristics for study participants has been published [23]. This study analyzed data obtained from a subset of 113 PREVAIL participants treated with combination DAAs after excluding 2 participants deceased without determined SVR status.
Daily Time Frame Adherence Determination
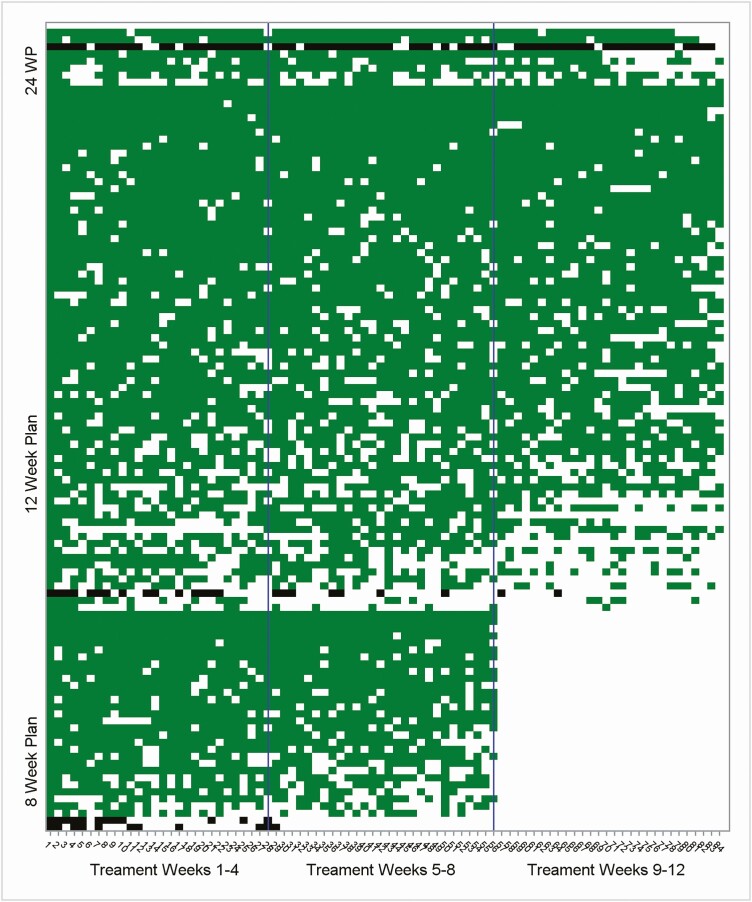
Individual level daily time frame (DTF) adherence was determined based on blister pack pop-up dates. The DTF window was between 12:00 am and 11:59 pm. The scale of DTF adherence was binary: DTF = 0 for no pop-up or missed dose and DTF = 1 for 1 or more pop-ups or adherent dose for a given treatment day window. Undetermined DTF adherence on the missing dates due to lost or unreturned blister pack were treated as a missed dose (ie, DTF = 0). Lengths of individual-level planned/prescribed treatment varied: 8 (N = 31), 12 (N = 74), and 24 (N = 8) weeks. As treatments were dispensed in electronic blister packs for up to 12 weeks, we computed DTF adherence only for 8 or 12 weeks even if planned treatment was 24 weeks. Figure 1 depicts DTF adherence over treatment weeks for all 113 participants treated with combination DAAs.
Figure 1.
Daily time frame adherence heat map across all 113 participants treated with combination direct-acting antivirals. The y-axis represents each participant. The order of the participants was sorted by planned weeks (24, 12, and 8) in a descending manner. Within each planned week, the participants axis is further sorted by total doses, again in a descending manner. The x-axis represents the treatment days, and the reference lines on the x-axis divide weeks 1–4, 5–8, and 9–12. The green areas represent days with adherent doses among participants with successful sustained viral response (SVR), whereas the black areas represent days with adherent doses among participants with failed SVR. The white areas represent days with missed doses or the nonapplicable doses for the 9–12 treatment weeks of the 8 planned weeks.
Adherence Pattern Variables
Based on the daily DTF adherence, we defined the following adherence pattern variables: total number of adherent days/doses ranging from 0 to 84, computed as the sum of the DTF adherent days/doses over the 8- or 12-week treatment period; percent total doses/days, ranging from 0% to 100%, computed as total doses divided by prescribed treatment days (56 or 84 days); days on treatment ranging from 0 to 84, computed as number of days from first to last adherent day; maximum number of consecutive adherent doses ranging from 0 to 84; maximum number of consecutive missed doses ranging from 0 to 84; and number of days switched between adherent and missed doses ranging from 0 to 83. The total number of adherent doses was further broken down and computed in each of the following intervals of prescribed treatment weeks: 1–4 weeks ranging from 0 to 28, 5–8 weeks ranging from 0 to 28, and 9–12 weeks ranging from 0 to 28 doses. Figure 2 illustrates an example of how the pattern variable values were computed.
Figure 2.
Hypothetical adherence pattern over a 4-week treatment period for illustration of adherence pattern parameter computations. Total number of doses within the treatment period = 16; percent adherent doses = 16 of 28 = 57.1%; days on treatment = 27 (dotted arrow); longest/maximum days of consecutive adherence = 8 (solid arrow); longest/maximum days of consecutive missed adherence = 5 (dashed arrow); number of “switches” between on and off medication days = 9; total doses weeks 1–4 = 16; total doses weeks 5–8 = N/A; and total doses weeks 9–12 = N/A. The numbers in the cells represent days from a start day. Adherence days are indicated in green, and nonadherence days are indicated in blank. Abbreviation: N/A, not applicable.
Sustained Viral Response
SVR was declared successful if HCV RNA viral load at least 12 weeks after the patient-specific end of treatment was undetectable, lower than 43 mm/IU at the earlier stage of the trial, or lower than 15 mm/IU at the later stage. Otherwise, the SVR was declared unsuccessful or failed.
Baseline Characteristics
Demographic factors included age, race, sex, employment status, marital status, housing status, and education level. Clinical factors included HIV coinfection, cirrhosis status, HCV subtypes, IL28B genotypes, moderate/severe depression based on the Beck depression inventory score ≥20, psychiatric illness (any experience of a major depressive episode, psychotic disorder, generalized anxiety disorder, or current manic episode by medical chart review), and alcohol intoxication for 1 or more days within the past 30 days based on the Addiction Severity Index. Medication factors included prior experiences with HCV medications, planned treatment length, and combination DAA regimens. Drug use factors included self-reported drug use 6 months prior to baseline and urine toxicology positive at baseline and during the treatment period (including opiates, cocaine, oxycodone, benzodiazepine, or amphetamine).
Statistical Analyses
Descriptive statistics are presented in terms of mean, standard deviation (SD), range, frequency, and percentages. To test significance of associations between adherence pattern variables and SVR, we applied exact logistic regressions that included study arm and length of planned treatment in weeks as covariates. Exact adjusted odds ratios (AORs) along with 95% confidence intervals (CIs) were used to estimate effect size for each association. To identify factors associated with each adherence pattern, we applied general linear models in a form of multiway analysis of variance for all pattern parameters, again with study arm and length of planned treatment included as covariates. We summarized these results in forest plots depicting estimated means resulting from general linear model fitting, their 95% CIs, and P values. Last, comparisons of total doses for each 4-week interval were made using the Wilcoxon rank sum test. Statistical significance was declared if a 2-sided P value was < .05. All statistical analyses were conducted with SAS v9.4 (SAS Inc, Cary, NC).
RESULTS
Baseline Characteristics
Table 1 presents the baseline characteristics of the 113 participants. Mean (SD) age was 51.8 (10.1) years, 59.3% were male, the majority were Hispanic/Latino/a (54.9%), and 24.8% had unstable housing. Overall, 11.5% were also living with HIV, 27.4% had cirrhosis, 22.1% had experienced alcohol intoxication in the prior 30 days, and 71.7% used any drugs during the treatment period.
Table 1.
Baseline Characteristics of the 113 Participants Treated With Combination Direct-Acting Antivirals
| Baseline Characteristic | Mean (Standard Deviation), n (%) |
|---|---|
| Demographic factors | |
| Age, years | 51.8 (10.1) |
| Age ≥50 years | 70 (62.0) |
| Race/Ethnicity | |
| Black | 31 (27.4) |
| Hispanic/Latino/a | 62 (54.9) |
| White | 11 (9.7) |
| Other | 9 (8.0) |
| Male | 67 (59.3) |
| Employed | 22 (19.5) |
| Married/Cohabitation | 40 (35.4) |
| Unstable housing | 28 (24.8) |
| High school or higher | 69 (61.1) |
| Clinical factors | |
| Also living with human immunodeficiency virus | 13 (11.5) |
| Cirrhosis | 31 (27.4) |
| HCV subtype 1a | 94 (83.2) |
| IL28B TC/TT | 89 (78.8) |
| Depression (Beck depression inventory score ≥20) | 41 (36.3) |
| Psychiatric illness | 53 (46.9) |
| Alcohol intoxication 30 days prior | 25 (22.1) |
| Medication factors | |
| Length of planned treatment, weeks | |
| 8 | 31 (27.4) |
| 12 | 74 (65.5) |
| 24 | 8 (7.1) |
| HCV treatment-naive | 98 (86.7) |
| Combination direct-acting antiviral regimen | |
| Sofosbuvir/Ledipasvir | 102 (90.3) |
| Sofosbuvir/Simeprevir | 11 (9.7) |
| Any drug use | |
| 6 months prior to baseline | 72 (63.7) |
| At baseline | 55 (48.8) |
| During treatment | 81 (71.7) |
Abbreviation: HCV, hepatitis C virus.
Adherence Patterns
Summary descriptive statistics are presented in Table 2. Mean (SD) total number of adherent doses declined as time passed: weeks 1–4, 22.5 (5.1); weeks 5–8, 21.4 (6.1); and week 9–12, 19.6 (7.8). Total doses over the first or second 4-week interval were not significantly different between participants with 8-week and 12- or 24-week planned treatment periods. Despite differences in planned treatment length, none of percent total dose, maximum consecutive dose, or maximum consecutive missed doses were significantly different. However, total dose, days on treatment, and number of switches were all significantly different as might have been expected due to the different treatment lengths between the 8-week and 12- or 24-week plans.
Table 2.
Summary Statistics for the Adherence Patterns Among 113 Participants Treated With Combination Direct-Acting Antivirals
| Planned Week | ||||
|---|---|---|---|---|
| All (N = 113) | 8 Week (N = 31) | 12–24 Week (N = 82) | ||
| Adherence Patterna | Mean (SD) | Mean (SD) | Mean (SD) | P Valueb |
| Total dose | 58.2 (17.6) | 42.7 (11.7) | 64.0 (15.9) | <.001 |
| Weeks 1–4 | 22.5 (5.1) | 22.7 (5.2) | 22.5 (5.1) | .820 |
| Weeks 5–8 | 21.4 (6.1) | 20.0 (7.3) | 22.0 (5.6) | .134 |
| Weeks 9–12 | 19.6 (7.8)c | … | 19.6 (7.8) | … |
| Percent total dose | 76.2 (19.4) | 76.3 (20.8) | 76.2 (18.9) | .980 |
| Days on treatment | 73.4 (14.1) | 53.2 (6.8) | 81.0 (6.5) | <.001 |
| Maximum consecutive adherent dose | 21.7 (17.6) | 19.6 (15.1) | 22.5 (18.5) | .437 |
| Maximum consecutive missed dose | 5.2 (6.6) | 4.3 (6.5) | 5.5 (12.1) | .397 |
| Switches between adherent and missed doses | 18.9 (11.5) | 14.1 (8.3) | 20.8 (12.1) | .001 |
Abbreviation: SD, standard deviation.
aAll adherence pattern measures were computed over the 8- or 12-week planned treatment period except that the totals were also further broken down by the three 4-week intervals.
b P values are based on 2-sample t tests.
c N = 82.
SVR and Adherence Patterns
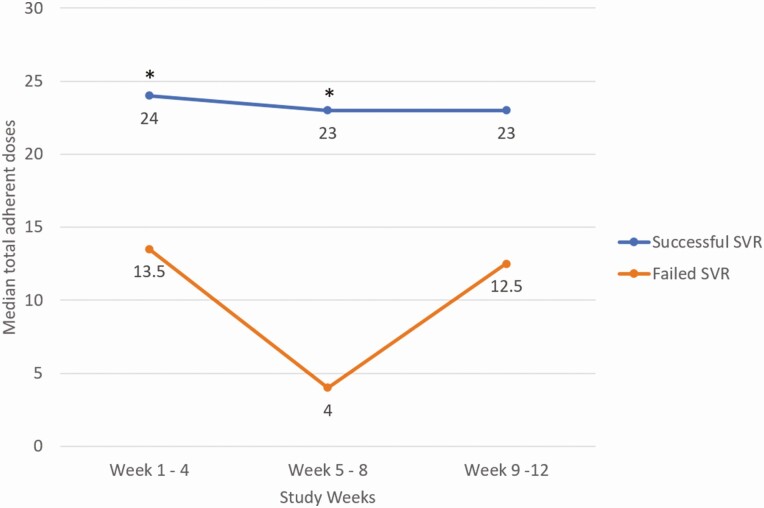
Four participants failed SVR, yielding a successful SVR rate of 96.5% (109 of 113, exact 95% CI = 91.2%–99.0%). Table 3 presents the association between SVR and each adherence pattern. Total number of daily doses was significantly associated with SVR (exact AOR = 1.12, 95% CI = 1.04–1.22, P = .002) as was percent total dose (exact AOR = 1.09, 95% CI = 1.03–1.16, P < .001). When the total adherent doses were broken down by 4-week intervals, both the first 4-week (exact AOR = 1.25, 95% CI = 1.04–1.59, P = .015) and middle 4-week (exact AOR = 1.31, 95% CI = 1.09–1.79, P = .001) total doses were significantly associated with SVR, whereas the last 4-week total doses were not (exact AOR = 1.12, 95% CI = .92–1.45, P = .293). The adjusted OR values for total doses represent the effect of a 1-dose increase on the odds of achieving SVR. This result was consistent when the interval-by-interval total doses were compared between participants who did and did not achieve SVR. Specifically, the medians of the first and the middle 4-week total doses were significantly different between those who had successful and failed SVR, whereas those of the last 4-week total doses were not (Figure 3). Days on treatment was also significantly associated with SVR (exact AOR = 1.16, 95% CI = 1.05–1.32, P = .002) as was maximum number of consecutive adherent doses (exact AOR = 1.34, 95% CI = 1.06–2.04, P = .004). On the other hand, maximum number of consecutive nonadherent/missed doses was significantly and inversely associated with SVR (exact AOR = 0.85, 95% CI = .74–.95, P = .003). However, number of days switched between adherent and missed doses was not associated with SVR (exact AOR = 1.01, 95% CI = .93–1.13, P = 1.000).
Table 3.
Association Between Sustained Viral Response and Adherence Patterns Adjusted for Planned Treatment Weeks and Study Arms Among 113 Participants Treated With Combination Direct-Acting Antivirals
| Adherence Pattern | Exact Adjusted Odds Ratio for Sustained Viral Response | 95% Confidence Interval | P Value |
|---|---|---|---|
| Total dose | 1.12 | 1.04–1.22 | .002 |
| Weeks 1–4 | 1.25 | 1.04–1.59 | .015 |
| Weeks 5–8 | 1.31 | 1.09–1.79 | .001 |
| Weeks 9–12a | 1.12 | .92–1.45 | .293 |
| Percent total dose | 1.09 | 1.03–1.16 | <.001 |
| Days on treatment | 1.16 | 1.05–1.32 | .002 |
| Maximum consecutive adherent dose | 1.34 | 1.06–2.04 | .004 |
| Maximum consecutive missed dose | 0.85 | .74–.95 | .003 |
| Switches between adherent and missed doses | 1.01 | .91–1.13 | 1.000 |
aN = 82. The statistical results are obtained for applications of multivariable exact logistic regression models.
Figure 3.
Median total adherent doses between participants who had successful and failed SVR over the three 4-week intervals. *P values are based on Wilcoxon rank sum tests: P = .015 for weeks 1–4; P = .011 for weeks 5–8; and P = .337 for weeks 9–12. Abbreviation: SVR, sustained viral response.
Factors Associated With Adherence Patterns
Forest plots comparing all adherence patterns between categories of participant characteristics are presented in Supplementary Figures 1–6. Compared with White participants, Black participants (adjusted estimates 18.4 ± 7.8 (standard error) vs 30.7 ± 11.8, P = .048) and Hispanic/Latino/a participants (19.2 ± 6.1 vs 30.7 ± 11.8, P = .049) had significantly shorter maximum consecutive adherent doses (Supplementary Figure 4). Alcohol intoxication in the prior 30 days was associated with significantly higher frequency of the switches between adherent and missed doses (23.4 ± 5.0 vs 17.7 ± 3.2, P = .024; Supplementary Figure 6). Notably, drug use at any time point, that is, 6 months prior, at baseline, or during treatment, was not associated with any adherence pattern (Supplementary Figures 1–6).
DISCUSSION
Our most prominent finding was that higher adherence to combination DAA medications was associated with SVR even after adjustment for length of planned treatment and study arms that had different levels of intensity for HCV treatment. Specifically, greater total doses, greater percent total dose, more days on treatment, greater maximum consecutive doses, and smaller maximum consecutive missed doses were significantly associated with achieving SVR, whereas switches between adherent and missed doses were not associated with SVR. The maximum consecutive adherent dose had the greatest association with SVR in terms of ORs per unit changes, whereas the percent total dose had the strongest significant association in terms of P values. Given that former studies showed that adherence, albeit somewhat differently defined, was also significantly associated with SVR [20, 23], all of our findings collectively support that increased adherence in any pattern is critically important for achieving SVR among PWID treated with combination DAAs.
It is worth noting that timing of higher adherence would be critical based on our finding that both the first and the middle 4-week total adherent doses were significantly associated with SVR, whereas the last 4-week counterpart was not. This finding suggests that high adherence at the early stage of treatments can serve as an early indicator for SVR and thus should be encouraged. Additionally, the first 4-week total dose was highly correlated with overall total doses; specifically, correlations with overall total doses were 0.91 and 0.82 among participants prescribed for 8 weeks and for 12 or 24 weeks, respectively. However, lack of significance in the last 4-week period could be a type II error due to the reduced sample size during that period, given that effect sizes in terms of OR (Table 3) or median differences (Figure 3) during the last 4-week period were comparable with those during the 2 prior 4-week intervals. Furthermore, the mean total doses declined over the prescription week intervals (Table 2), which was also observed in the SIMPLIFY study [24], whereas the variation represented in the standard deviation increased over the intervals. This finding implies that participants tend to maintain higher adherence at the early stage of treatment, with smaller variation across the participants. However, the wider spread of the adherence in the later stage of the treatment period may differentiate between participants who continually maintained higher adherence and those who had declining adherence. Subsequently, the later lower adherence due to the declining adherence might also be associated with failed SVR. Collectively, consistently maintaining higher adherence over the entire treatment period would be critical for achieving SVR.
Our mean overall percent total dose was 76.2% with a median of 82.1%; this is lower than in other studies that assessed overall adherence to DAA regimens. Prior estimates of median overall adherence ranged from 94% to 96% based on electronic blister packs [24], wireless pill boxes or video-assisted DOT [25], ingestible sensors [26], or prescription records [27]. The discrepancies from our estimate may be due to a difference in settings, study population, or ways of calculating overall adherence. Regardless, despite a suboptimal adherence level, our high SVR rate (96.5%) is comparable to SVR rates of 87%–99% reported in the above studies with higher adherence rates. This finding supports the pharmacokinetically forgiving properties of DAAs and thus raises the question of whether shortened treatment length would result in comparably high SVR rates.
Black and Hispanic/Latino/a participants compared with White participants had significantly lower maximum consecutive adherent doses, which is consistent with other studies and demonstrates racial disparities in adherence [28, 29]. Participants with experiences of alcohol intoxication at baseline had significantly higher frequencies of switches between missed and adherence doses. The statistical significance of these findings may be a spurious result from multiple testing over multiple patterns. Despite this plausibility, alcohol intoxication might have interfered with consistently taking daily doses potentially due to impaired cognitive function associated with drinking. Patients who experience alcohol intoxication need to be carefully monitored to promote consistent adherence in addition to being provided with integrated addiction and medical care [30, 31]. Nonetheless, the finding that drug use at any time was not associated with any adherence patterns shows that treating HCV among PWID in the OAT setting is not only feasible but also successful without diminished adherence [31–33].
Although we found that all adherence patterns, except the switches, were associated with SVR, the optimal adherence patterns or their composites that would be most sensitive to SVR remain to be determined given that low adherence in any pattern could be associated not only with failed SVR but also possibly with development of medication resistance [34, 35]. In addition, determination of threshold levels of each adherence parameter associated with greater SVR, as attempted by Norton et al [36], would provide clinically important information. However, such information, albeit valuable, may not be useful in real practice where real-time monitoring of medication intake is often not possible. In this context, while electronic blister packs provide accurate adherence levels, their practical limitation and cost preclude general use. Use of a smartphone-based monitoring system [37–39] or self-report instruments such as visual analogue scales [40, 41] during treatment periods may be more useful for real-time monitoring.
The following limitations of this study should be considered. First, length of treatment days was truncated at 84 days or 12 weeks even though treatment may have been as long as 24 weeks. Second, lengths of actual treatments may not necessarily be the same as was planned. Third, the parent trial was conducted in inner city urban OAT program settings. Therefore, generalizability of findings to other settings such as rural or community-based clinics may be limited, and extendibility of our findings to HCV-infected patients who do not inject or use drugs is unknown. Fourth, lost or unreturned blister pack were treated as a missed dose, although a conservative estimated percentage of unreturned blister packs over all treatment weeks was less than 4% from all PREVAIL participants. As it is possible that participants still took doses but simply did not return blister packs, all pattern parameters might have been underestimated except for the consecutive missed dose, which might have been overestimated. Last, even if a blister was popped up, it was unknown whether the medication was retrieved and swallowed.
In conclusion, when treating HCV among PWID, consistently maintaining adequate adherence is important for achieving SVR. Although reducing the number of consecutive missed dose would also be effective, a certain level of minimum adherent doses would be required. To this end, identification of threshold levels of adherence patterns for SVR is warranted. More intensive care may also be warranted for promoting consistent adherence among patients who experience alcohol intoxication.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The parent PREVAIL trial is registered in ClinicalTrial.gov (NCT01857245). We are grateful for the collaborative support from the Addiction Research Center at Prisma Health in Greenville, South Carolina.
Disclaimer. The contents of the work are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies or the US government.
Financial support. This work was supported in part by the National Institute on Drug Abuse (R01DA034086) and Gilead Sciences (IN-337-1779) grants.
Potential conflicts of interest. A. H. L. has served on advisory boards for Merck Pharmaceuticals, AbbVie, and Gilead Sciences and has received research grants from Merck Pharmaceuticals and Gilead Sciences. M. H. reports personal fees from HealthCorps outside the submitted work. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014; 61:S45–57. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020; 5:245–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2:161–76. [DOI] [PubMed] [Google Scholar]
- 4. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011; 378:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis 2014; 59:1411–9. [DOI] [PubMed] [Google Scholar]
- 6. Soriano V, Vispo E, Poveda E, et al. Directly acting antivirals against hepatitis C virus. J Antimicrob Chemother 2011; 66:1673–86. [DOI] [PubMed] [Google Scholar]
- 7. Banerjee D, Reddy KR. Review article: safety and tolerability of direct-acting anti-viral agents in the new era of hepatitis C therapy. Aliment Pharmacol Ther 2016; 43:674–96. [DOI] [PubMed] [Google Scholar]
- 8. Lee LY, Tong CY, Wong T, Wilkinson M. New therapies for chronic hepatitis C infection: a systematic review of evidence from clinical trials. Int J Clin Pract 2012; 66:342–55. [DOI] [PubMed] [Google Scholar]
- 9. Dieperink E, Pocha C, Thuras P, Knott A, Colton S, Ho SB. All-cause mortality and liver-related outcomes following successful antiviral treatment for chronic hepatitis C. Dig Dis Sci 2014; 59:872–80. [DOI] [PubMed] [Google Scholar]
- 10. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308:2584–93. [DOI] [PubMed] [Google Scholar]
- 11. Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology 2019; 69:487–97. [DOI] [PubMed] [Google Scholar]
- 12. Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: impact on mortality in patients without advanced liver disease. Hepatology 2018; 68:827–38. [DOI] [PubMed] [Google Scholar]
- 13. Asselah T, Boyer N, Saadoun D, Martinot-Peignoux M, Marcellin P. Direct-acting antivirals for the treatment of hepatitis C virus infection: optimizing current IFN-free treatment and future perspectives. Liver Int 2016; 36:47–57. [DOI] [PubMed] [Google Scholar]
- 14. Welsch C, Jesudian A, Zeuzem S, Jacobson I. New direct-acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut 2012; 61:36–46. [DOI] [PubMed] [Google Scholar]
- 15. Bruggmann P, Grebely J. Prevention, treatment and care of hepatitis C virus infection among people who inject drugs. Int J Drug Policy 2015; 26:S22–6. [DOI] [PubMed] [Google Scholar]
- 16. Grebely J, Dore GJ. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral Res 2014; 104:62–72. [DOI] [PubMed] [Google Scholar]
- 17. Butner JL, Gupta N, Fabian C, Henry S, Shi JM, Tetrault JM. Onsite treatment of HCV infection with direct acting antivirals within an opioid treatment program. J Subst Abuse Treat 2017; 75:49–53. [DOI] [PubMed] [Google Scholar]
- 18. Rosenthal ES, Silk R, Mathur P, et al. Concurrent initiation of hepatitis C and opioid use disorder treatment in people who inject drugs. Clin Infect Dis 2020; 71:1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Novick DM, Kreek MJ. Critical issues in the treatment of hepatitis C virus infection in methadone maintenance patients. Addiction 2008; 103:905–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cunningham EB, Hajarizadeh B, Amin J, et al. ; SIMPLIFY and D3FEAT Study Groups . Adherence to once-daily and twice-daily direct-acting antiviral therapy for hepatitis C infection among people with recent injection drug use or current opioid agonist therapy. Clin Infect Dis 2020; 71:e115–24. [DOI] [PubMed] [Google Scholar]
- 21. Mason K, Dodd Z, Guyton M, et al. Understanding real-world adherence in the directly acting antiviral era: a prospective evaluation of adherence among people with a history of drug use at a community-based program in Toronto, Canada. Int J Drug Policy 2017; 47:202–8. [DOI] [PubMed] [Google Scholar]
- 22. Akiyama MJ, Agyemang L, Arnsten JH, et al. Rationale, design, and methodology of a trial evaluating three models of care for HCV treatment among injection drug users on opioid agonist therapy. BMC Infect Dis 2018; 18:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akiyama MJ, Norton BL, Arnsten JH, Agyemang L, Heo M, Litwin AH. Intensive models of hepatitis C care for people who inject drugs receiving opioid agonist therapy: a randomized controlled trial. Ann Intern Med 2019; 170:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cunningham EB, Amin J, Feld JJ, et al. ; SIMPLIFY Study Group . Adherence to sofosbuvir and velpatasvir among people with chronic HCV infection and recent injection drug use: the SIMPLIFY study. Int J Drug Policy 2018; 62:14–23. [DOI] [PubMed] [Google Scholar]
- 25. Brooks KM, Castillo-Mancilla JR, Morrow M, et al. Adherence to direct-acting antiviral therapy in people actively using drugs and alcohol: the INCLUD Study. Open Forum Infect Dis 2021; 8:ofaa564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sulkowski M, Luetkemeyer AF, Wyles DL, et al. Impact of a digital medicine programme on hepatitis C treatment adherence and efficacy in adults at high risk for non-adherence. Aliment Pharmacol Ther 2020; 51:1384–96. [DOI] [PubMed] [Google Scholar]
- 27. Aas CF, Vold JH, Skurtveit S, et al. On the path towards universal coverage of hepatitis C treatment among people receiving opioid agonist therapy (OAT) in Norway: a prospective cohort study from 2013 to 2017. BMJ Open 2020; 10:e036355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slevin AR, Hart MJ, Van Horn C, et al. Hepatitis C virus direct-acting antiviral nonadherence: relationship to sustained virologic response and identification of at-risk patients. J Am Pharm Assoc (2003) 2019; 59:51–6. [DOI] [PubMed] [Google Scholar]
- 29. Serper M, Evon DM, Stewart PW, et al. Medication non-adherence in a prospective, multi-center cohort treated with hepatitis C direct-acting antivirals. J Gen Intern Med 2020; 35:1011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knott A, Dieperink E, Willenbring ML, et al. Integrated psychiatric/medical care in a chronic hepatitis C clinic: effect on antiviral treatment evaluation and outcomes. Am J Gastroenterol 2006; 101:2254–62. [DOI] [PubMed] [Google Scholar]
- 31. Read P, Lothian R, Chronister K, et al. Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. Int J Drug Policy 2017; 47:209–15. [DOI] [PubMed] [Google Scholar]
- 32. Elsherif O, Bannan C, Keating S, McKiernan S, Bergin C, Norris S. Outcomes from a large 10 year hepatitis C treatment programme in people who inject drugs: no effect of recent or former injecting drug use on treatment adherence or therapeutic response. PLoS One 2017; 12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bielen R, Moreno C, Van Vlierberghe H, et al. Belgian experience with direct acting antivirals in people who inject drugs. Drug Alcohol Depend 2017; 177:214–20. [DOI] [PubMed] [Google Scholar]
- 34. Akiyama MJ, Lipsey D, Ganova-Raeva L, et al. A phylogenetic analysis of hepatitis C virus transmission, relapse, and reinfection among people who inject drugs receiving opioid agonist therapy. J Infect Dis 2020; 222:488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dietz J, Susser S, Vermehren J, et al. Patterns of resistance-associated substitutions in patients with chronic HCV infection following treatment with direct-acting antivirals. Gastroenterology 2018; 154:976–88.e4. [DOI] [PubMed] [Google Scholar]
- 36. Norton BL, Akiyama MJ, Agyemang L, Heo M, Pericot-Valverde I, Litwin AH. Low adherence achieves high HCV cure rates among people who inject drugs treated with direct-acting antiviral agents. Open Forum Infect Dis 2020; 7:ofaa377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Molton JS, Pang Y, Wang Z, et al. Prospective single-arm interventional pilot study to assess a smartphone-based system for measuring and supporting adherence to medication. BMJ Open 2016; 6:e014194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bommakanti KK, Smith LL, Liu L, et al. Requiring smartphone ownership for mHealth interventions: who could be left out? BMC Public Health 2020; 20:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Litwin AH, Shafner L, Norton B, et al. Artificial intelligence platform demonstrates high adherence in patients receiving fixed-dose ledipasvir and sofosbuvir: a pilot study. Open Forum Infectious Diseases 2020; 7: ofaa290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burton MJ, Voluse AC, Patel AB, Konkle-Parker D. Measuring adherence to hepatitis C direct-acting antiviral medications: using the VAS in an HCV treatment clinic. South Med J 2018; 111:45–50. [DOI] [PubMed] [Google Scholar]
- 41. Pericot-Valverde I, Rennert L, Heo M, et al. Rates of perfect self-reported adherence to direct-acting antiviral therapy and its correlates among people who inject drugs on medications for opioid use disorder: the PREVAIL study. J Viral Hepat 2021; 28:548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.