Abstract
Background
In addition to traditional cardiovascular (CV) risk factors, antiretroviral therapy, lifestyle, and human immunodeficiency virus (HIV)-related factors may contribute to future CV events in persons with HIV (PWH).
Methods
Among participants in the global REPRIEVE randomized trial, we characterized demographics and HIV characteristics relative to ACC/AHA pooled cohort equations (PCE) for atherosclerotic CV disease predicted risk and CV health evaluated by Life’s Simple 7 (LS7; includes smoking, diet, physical activity, body mass index, blood pressure, total cholesterol, and glucose).
Results
Among 7382 REPRIEVE participants (31% women, 45% Black), the median PCE risk score was 4.5% (lower and upper quartiles Q1, Q3: 2.2, 7.2); 29% had a PCE score <2.5%, and 9% scored above 10%. PCE score was related closely to known CV risk factors and modestly (<1% difference in risk score) to immune function and HIV parameters. The median LS7 score was 9 (Q1, Q3: 7, 10) of a possible 14. Only 24 participants (0.3%) had 7/7 ideal components, and 36% had ≤2 ideal components; 90% had <5 ideal components. The distribution of LS7 did not vary by age or natal sex, although ideal health was more common in low sociodemographic index countries and among Asians. Poor dietary and physical activity patterns on LS7 were seen across all PCE scores, including the lowest risk categories.
Conclusions
Poor CV health by LS7 was common among REPRIEVE participants, regardless of PCE. This suggests a critical and independent role for lifestyle interventions in conjunction with conventional treatment to improve CV outcomes in PWH.
Clinical Trials Registration: NCT02344290.
AIDS Clinical Trials Group study number: A5332.
Keywords: atherosclerotic cardiovascular disease, cardiac prevention, cardiovascular health, cardiovascular risk, lifestyle modifications
Measures of cardiovascular (CV) risk and health are not closely related in persons with human immunodeficiency virus (HIV) in the REPRIEVE trial. Poor health scores among low-CV-risk persons with HIV suggest a critical role for lifestyle interventions regardless of CV risk prediction.
Persons with human immunodeficiency virus (HIV, PWH) are at increased risk for major adverse cardiovascular (CV) events, including myocardial infarction, heart failure, stroke, pulmonary hypertension, and sudden cardiac death [1-4], even after controlling for known risk factors. The associations between HIV and CV events are multifactorial and include inflammation and immune function changes related to chronic infection as well as metabolic dysregulation associated with HIV [2, 3, 5]. Furthermore, HIV is associated with adverse social determinants of health, with attendant increases in CV risk [6, 7]. Finally, both uncontrolled viremia and antiretroviral therapy (ART) can adversely affect CV risk factors and may increase CV risk [8].
As survival with HIV has improved, the relative impact of cardiovascular disease (CVD) has increased [6]. As a result, CVD is an important, potentially modifiable cause of morbidity and mortality [9] and an important challenge [10]. Although HIV is recognized in current guidelines [11] as a “risk enhancer” using the American College of Cardiology/American Heart Association Pooled Cohort Equations (PCE), optimal strategies for primary prevention in PWH remain unknown.
In addition to traditional risk factors and scores, the American Heart Association recommends evaluating Life’s Simple 7 (LS7) as a novel, comprehensive measure of CV health, both to characterize populations and to guide interventions [12]. LS7 was conceived as more actionable given its inclusion of 4 health behaviors—smoking, diet, physical activity, and body mass index (BMI)—and 3 health factors—blood pressure, total cholesterol, and glucose—with defined goals for improvement. Furthermore, LS7 is closely associated with CV outcomes in multiple cohorts [13]. However, to our knowledge, it has not been applied in a population of PWH.
The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) is the largest long-term randomized trial to assess statin therapy as a primary CVD prevention strategy among PWH [14]. Using baseline data from trial participants enrolled across 5 continents, we sought to describe CVD risk factor distributions, examine associations between CVD risk and HIV characteristics, and characterize CV health among middle-aged PWH without known CVD.
METHODS
Trial Design and Study Participants
REPRIEVE (NCT02344290) enrolled 7770 participants in a prospective, double-blind, placebo-controlled, multicenter, phase III efficacy study comparing pitavastatin calcium 4 mg daily versus placebo among PWH on ART [14]. The trial enrolled at >100 sites in 12 countries between March 2015 and July 2019. Primary entry criteria included PWH ≥40 and ≤75 years of age, on stable combination ART for at least 6 months, with a CD4 + T-cell count >100 cells/mm3 and low-to-intermediate 10-year atherosclerotic cardiovascular disease (ASCVD) risk of <15%, in combination with low-density lipoprotein (LDL) cholesterol level as previously described [15]. During the course of the study, the ASCVD eligibility criterion was modified to ensure adequate numbers across the desired spectrum of low to intermediate traditional risk [14]. Key exclusion criteria throughout enrollment included known CV disease, diabetes with LDL cholesterol ≥70 mg/dL, impaired renal function, decompensated cirrhosis, active cancer, and ongoing statin use. Details are available in the REPRIEVE rationale and design article [14].
At trial entry, detailed information on medical history and lifestyle behaviors was ascertained. Diet and physical activity assessments were conducted using the Rapid Eating and Activity Assessment for Patients Questionnaire [16]. In addition to lipids tested locally that were used to determine trial eligibility, fasting lipids were obtained at study entry and tested at a Quest Diagnostic lab (Baltimore, Maryland, USA). Further information on ascertainment methods of key data elements and their presentation is provided in the Supplementary materials.
The coordinating centers and sites obtained institutional review board and other applicable regulatory entity approvals. All participants provided informed consent.
CV Risk and Health Scores
PCE were used to predict 10-year CV risk [15]. Because the PCE risk score used to determine study eligibility may have been obtained with nonfasting lipids, we recalculated the score using centrally tested fasting lipids from study entry, and other inputs as recorded into the study database. The recalculated PCE score showed good agreement with site scores used for study eligibility (not shown).
CV health was evaluated with LS7 metrics adapted from Lloyd-Jones et al [12] with modifications for scoring diet, physical activity, and smoking (Supplementary Tables 1 and 2). Each individual component of LS7 was categorized as ideal, intermediate, and poor. A total of 5–7 ideal components out of 7 was considered ideal, 3–4 intermediate, and ≤2 poor overall CV health, according to prognostically validated cut points. An ordinal overall score was calculated as the sum of the 7 individual components as either poor (0 points), intermediate (1 point), or ideal (2 points), yielding a scale from 0 (worst) to 14 (best).
Further details on PCE and LS7 and their adaptation in REPRIEVE are provided in the Supplementary materials.
Statistical Analysis
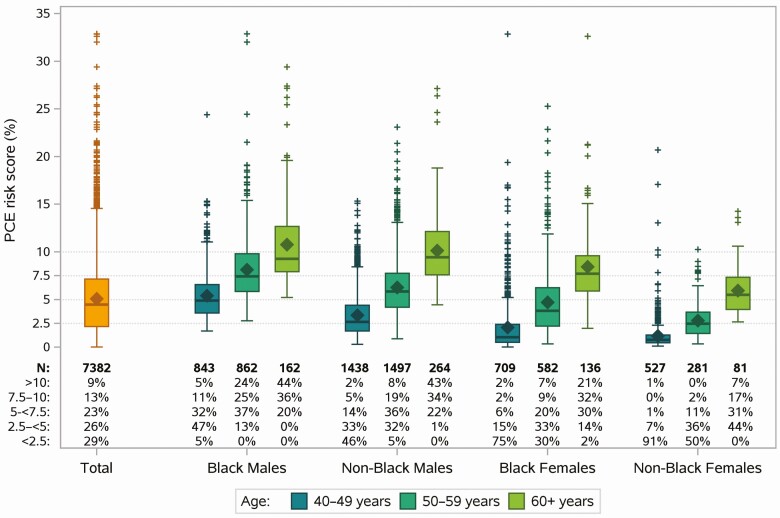
CVD risk prediction by PCE was described using summary statistics and risk categories (<2.5%, 2.5% to <5%, 5% to <7.5%, 7.5% to 10%, >10%), overall and within strata defined by unmodifiable PCE components: sex, race (Black, non-Black), and age (40–49 years, 50–59 years, ≥60 years). The contributions of modifiable PCE components (total cholesterol, high-density lipoprotein [HDL] cholesterol, systolic blood pressure, hypertension treatment, smoking, and diabetes) were examined by PCE risk score categories overall and stratified by sex, race, and age.
PCE risk score distributions in relation to HIV characteristics were examined graphically using box plots. Furthermore, linear regression models were used to estimate the effect size, adjusted for sex, race, age, and enrollment period (before and after enrollment closed to participants with lowest CVD risk). Evaluation of ART types was also adjusted by sociodemographic index (SDI) (high including US, Canada, and Europe vs middle/low) to account for regional differences in ART use known from previous analyses [17].
CV health by LS7 was summarized descriptively overall, by factors of interest, and in relation to PCE risk category overall and stratified by sex, race, and age.
Given the large sample size and high power to detect minimal effect sizes, formal statistical inference was guided by very low significance level (alpha = 0.001) and clinically meaningful estimated effect sizes (1% shift in PCE risk score). All analyses were conducted using SAS software, Version 9.4 (TS1M5, SAS/STAT 14.3, SAS Institute Inc., Cary, North Carolina, USA) on a Linux operating environment.
RESULTS
Population
Among the 7770 REPRIEVE participants, PCE risk score was calculated using centrally determined fasting lipids in 7382 participants, with a median age of 50 years (lower and upper quartiles Q1, Q3: 45, 55; minimum, maximum: 40, 74); 69% were natal males, and 45% Black, 35% White, and 13% Asian (Table 1). The 388 participants excluded from the analysis (380 due to no fasting lipids [69% of those due to participant not fasting] and 8 due to missing smoking status) included a higher proportion of participants from countries with low SDI (56% vs 10% among those included in the analysis) and non-Blacks (81% vs 55%); distributions of sex and age were similar (data not shown).
Table 1.
Demographics, Risk Factors, and Human Immunodeficiency Virus (HIV) Characteristics by Pooled Cohort Equations (PCE) Risk Score
| PCE Risk Score (%) | ||||||
|---|---|---|---|---|---|---|
| Characteristica | Total (N = 7382) |
0–<2.5 (N = 2113, 29%) |
2.5–<5 (N = 1956, 26%) |
5–<7.5 (N = 1676, 23%) |
7.5–10 (N = 954, 13%) |
>10 (N = 683, 9%) |
| Demographics | ||||||
| Sociodemographic index | ||||||
| High | 3 986 (54%) | 840 (40%) | 1075 (55%) | 1010 (60%) | 618 (65%) | 443 (65%) |
| Middle | 2 663 (36%) | 1087 (51%) | 677 (35%) | 477 (28%) | 239 (25%) | 183 (27%) |
| Low | 733 (10%) | 186 (9%) | 204 (10%) | 189 (11%) | 97 (10%) | 57 (8%) |
| Age, y | 50 (45, 55) | 45 (42, 48) | 49 (46, 53) | 53 (48, 56) | 55 (51, 59) | 57 (53, 61) |
| Min, Max | 40, 74 | 40, 61 | 40, 66 | 40, 69 | 40, 72 | 40, 74 |
| Natal sex | ||||||
| Male | 5066 (69%) | 779 (37%) | 1467 (75%) | 1418 (85%) | 816 (86%) | 586 (86%) |
| Female | 2316 (31%) | 1334 (63%) | 489 (25%) | 258 (15%) | 138 (14%) | 97 (14%) |
| Race | ||||||
| Black | 3294 (45%) | 755 (36%) | 825 (42%) | 821 (49%) | 485 (51%) | 408 (60%) |
| White | 2614 (35%) | 654 (31%) | 762 (39%) | 618 (37%) | 367 (38%) | 213 (31%) |
| Asian | 923 (13%) | 519 (25%) | 205 (10%) | 129 (8%) | 43 (5%) | 27 (4%) |
| Other | 551 (7%) | 185 (9%) | 164 (8%) | 108 (6%) | 59 (6%) | 35 (5%) |
| Cardiovascular risk factors | ||||||
| Smoking status | ||||||
| Current | 1852 (25%) | 147 (7%) | 361 (18%) | 568 (34%) | 398 (42%) | 378 (55%) |
| Former | 1845 (25%) | 458 (22%) | 559 (29%) | 460 (27%) | 238 (25%) | 130 (19%) |
| Never | 3685 (50%) | 1508 (71%) | 1036 (53%) | 648 (39%) | 318 (33%) | 175 (26%) |
| Family history of premature CVD | ||||||
| Yes | 1385 (19%) | 357 (17%) | 380 (19%) | 308 (18%) | 198 (21%) | 142 (21%) |
| No | 5763 (78%) | 1710 (81%) | 1514 (77%) | 1326 (79%) | 704 (74%) | 509 (75%) |
| Unknown | 230 (3%) | 44 (2%) | 61 (3%) | 42 (3%) | 51 (5%) | 32 (5%) |
| Hypertension | 2624 (36%) | 401 (19%) | 596 (30%) | 671 (40%) | 499 (52%) | 457 (67%) |
| History of diabetes | 63 (1%) | 2 (<0.5%) | 10 (1%) | 10 (1%) | 10 (1%) | 31 (5%) |
| BMI, kg/m2 | 25.9 (22.9, 29.5) | 25.6 (22.5, 29.6) | 26.0 (23.0, 29.6) | 25.9 (23.1, 29.1) | 26.0 (23.2, 29.4) | 26.2 (22.9, 29.9) |
| Waist circumference, cm | 92 (84, 101) | 90 (82, 99) | 92 (85, 101) | 93 (85, 101) | 94 (86, 103) | 95 (86, 103) |
| Metabolic syndrome | 2046 (28%) | 382 (18%) | 556 (29%) | 502 (30%) | 331 (35%) | 275 (41%) |
| History of depression treatment | 2149 (29%) | 518 (25%) | 573 (29%) | 513 (31%) | 309 (32%) | 236 (35%) |
| Entry lipids | ||||||
| Triglycerides, mg/dL | 112 (80, 166) | 98 (72, 141) | 116 (83, 171) | 120 (83, 179) | 123 (85, 177) | 127 (88, 199) |
| Total cholesterol, mg/dL | 183 (160, 208) | 180 (157, 204) | 182 (159, 208) | 185 (161, 212) | 188 (164, 213) | 182 (159, 206) |
| LDL-C, mg/dL | 106 (86, 128) | 103 (85, 123) | 106 (86, 128) | 108 (88, 131) | 112 (90, 132) | 105 (86, 125) |
| HDL-C, mg/dL | 47 (39, 59) | 51 (42, 63) | 47 (39, 57) | 46 (39, 57) | 45 (37, 57) | 44 (36, 53) |
| Cardiovascular medications | ||||||
| Ever been on a statin | 475 (6%) | 111 (5%) | 124 (6%) | 108 (6%) | 81 (8%) | 51 (7%) |
| Current use of antihypertensive medication | 1470 (20%) | 179 (8%) | 300 (15%) | 346 (21%) | 319 (33%) | 326 (48%) |
| Current use of antiplatelet therapy | 272 (4%) | 27 (1%) | 61 (3%) | 88 (5%) | 54 (6%) | 42 (6%) |
| Current use of non-statin lipid-lowering therapy | 159 (2%) | 34 (2%) | 51 (3%) | 41 (2%) | 23 (2%) | 10 (1%) |
| HIV characteristics | ||||||
| Nadir CD4 count, cells/mm3 | ||||||
| <50 | 1342 (18%) | 319 (15%) | 361 (18%) | 338 (20%) | 193 (20%) | 131 (19%) |
| 50–199 | 2219 (30%) | 639 (30%) | 575 (29%) | 513 (31%) | 267 (28%) | 225 (33%) |
| 200–349 | 1960 (27%) | 611 (29%) | 518 (26%) | 434 (26%) | 245 (26%) | 152 (22%) |
| ≥350 | 1616 (22%) | 494 (23%) | 454 (23%) | 326 (19%) | 204 (21%) | 138 (20%) |
| Unknown | 245 (3%) | 50 (2%) | 48 (2%) | 65 (4%) | 45 (5%) | 37 (5%) |
| History of AIDS-defining diagnosis | 1724 (23%) | 504 (24%) | 430 (22%) | 417 (25%) | 225 (24%) | 148 (22%) |
| CD4 count, cells/mm³ | 626 (453, 832) | 646 (480, 840) | 629 (465, 839) | 616 (428, 814) | 597 (435, 823) | 623 (434, 842) |
| CD4:CD8 ratio | 0.83 (0.57, 1.16) | 0.88 (0.64, 1.22) | 0.83 (0.56, 1.17) | 0.80 (0.52, 1.13) | 0.78 (0.52, 1.09) | 0.75 (0.50, 1.05) |
| HIV-1 RNA, copies/mL | ||||||
| <LLQ | 5140 (88%) | 1448 (89%) | 1365 (87%) | 1160 (87%) | 688 (87%) | 479 (86%) |
| LLQ–< 400 | 593 (10%) | 144 (9%) | 158 (10%) | 136 (10%) | 88 (11%) | 67 (12%) |
| ≥400 | 128 (2%) | 27 (2%) | 39 (2%) | 36 (3%) | 15 (2%) | 11 (2%) |
| Antiretroviral treatment | ||||||
| Total ART use, y | 10 (5, 15) | 9 (5, 13) | 9 (5, 15) | 10 (6, 16) | 11 (6, 16) | 12 (7, 18) |
| Entry ART regimen duration, y | 2.3 (0.8, 5.1) | 2.5 (0.8, 5.1) | 2.4 (0.8, 5.3) | 2.3 (0.8, 5.3) | 2.1 (0.8, 4.9) | 1.9 (0.8, 4.3) |
| Entry ART regimen class | ||||||
| NRTI + NNRTI | 3409 (46%) | 1197 (57%) | 896 (46%) | 683 (41%) | 363 (38%) | 270 (40%) |
| NRTI + INSTI | 1911 (26%) | 394 (19%) | 524 (27%) | 468 (28%) | 314 (33%) | 211 (31%) |
| NRTI + PI | 1411 (19%) | 412 (19%) | 370 (19%) | 338 (20%) | 170 (18%) | 121 (18%) |
| NRTI-sparing | 195 (3%) | 34 (2%) | 55 (3%) | 55 (3%) | 32 (3%) | 19 (3%) |
| Other NRTI-containing | 456 (6%) | 76 (4%) | 111 (6%) | 132 (8%) | 75 (8%) | 62 (9%) |
| Entry NRTIb | ||||||
| ABC | 952 (13%) | 177 (8%) | 237 (12%) | 258 (15%) | 157 (16%) | 123 (18%) |
| TDF | 4514 (61%) | 1562 (74%) | 1211 (62%) | 940 (56%) | 474 (50%) | 327 (48%) |
| TAF | 1064 (14%) | 155 (7%) | 271 (14%) | 287 (17%) | 198 (21%) | 153 (22%) |
| Other | 852 (12%) | 219 (10%) | 237 (12%) | 191 (11%) | 125 (13%) | 80 (12%) |
Data shown are n (%) for categorical measures, median with lower and upper quartiles (Q1, Q3) for continuous measures. For age, minimum (min) and maximum (max) are also shown. All statistics are calculated out of participants with data collected. Missing data include family history of premature CVD (n = 4); BMI (n = 4); waist circumference (n = 97); metabolic syndrome (n = 34); LDL-C (n = 46); CD4:CD8 ratio (n = 1217); HIV-1 RNA (n = 1521); total ART use (n = 2); entry ART regimen duration (n = 1).
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; BMI, body mass index; CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; INSTI, integrase strand transfer inhibitor; LDL-C, low-density lipoprotein cholesterol; LLQ, lower limit of quantification; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
aRefer to Supplementary materials for definitions of characteristics and ascertainment of associated data elements.
bEntry NRTI is defined as any ABC use including use with TDF (n = 63) or TAF (n = 6), TDF without ABC, TAF without ABC, and Other.
Risk Factors
CV risk factors were common, with 50% being current or former smokers, 19% having a family history of premature CVD, 36% being hypertensive, a median BMI of 25.9 kg/m2, and 29% having a history of treatment for depression (Table 1). Trial entry criteria limited enrollment of people with diabetes to those with very low LDL cholesterol [14]. The overall median PCE risk score was 4.5% (Q1, Q3: 2.2, 7.2), with the proportion of participants decreasing from 29% in the lowest PCE risk category (<2.5%) to 9% in the highest category (>10%); 78% had a PCE risk score <7.5%. As expected, the prevalence of factors used to compute the PCE score increased across increasing PCE risk categories, including age, sex, smoking, diabetes, race, and elevated blood pressure. There were limited increases in LDL cholesterol across PCE risk categories, as expected based on trial entry criteria.
Risk factors not included in the PCE scoring algorithm, including depression, metabolic syndrome, and waist circumference, were more prevalent in the higher PCE risk categories. For example, across PCE risk categories, median waist circumference increased by 5 cm, metabolic syndrome prevalence increased from 18% to 41%, and the proportion of participants from high SDI countries increased from 40% to 65% (Table 1).
Relative Contribution of Risk Score Components
PCE risk score distributions stratified by unmodifiable PCE components (sex, race, and age) are shown in Figure 1. The distributions of modifiable PCE components across risk score categories suggested a strong contribution of hypertension treatment and blood pressure across all subgroups, greatest among Blacks (Supplementary Figure 1A). Likewise, the percentage of smokers also increased with increasing risk score categories with more smokers in the younger age groups (data not shown). In contrast, trends in the distributions of total cholesterol and HDL cholesterol were less clear, perhaps due to constraining eligibility criteria (Supplementary Figure 1B), although greater contributions of total cholesterol and HDL cholesterol were seen among nonsmokers (data not shown).
Figure 1.
Distribution of PCE risk score by natal sex, race, and age. Distribution of PCE risk score as a continuous measure (box plots) and according to risk categories (axis table). Abbreviation: PCE, pooled cohort equations.
HIV-related Characteristics and PCE
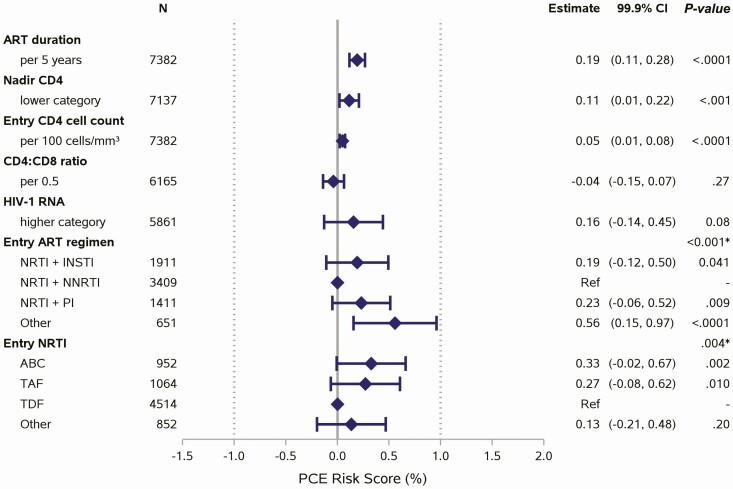
Median duration of ART use was 10 years (Q1, Q3: 5, 15), and 88% had viral suppression with a median CD4 count of 626 cells/mm3 (Table 1). ART regimens are shown in Table 1. Comparison of HIV characteristics by PCE risk category showed some trends in ART duration and clinical immunologic parameters. For example, lower nadir CD4 was associated with increased PCE risk score but was attenuated when stratified by sex, race, and age (Supplementary Figure 2). In models adjusted for sex, race, age, and enrollment period, longer ART duration, entry ART regimen, lower nadir CD4, and higher entry CD4 were each associated with higher PCE risk score (P < .001), but all effect sizes were small (upper bound of 99.9% confidence interval [CI] was <1% difference in score; Figure 2).
Figure 2.
Associations between HIV characteristics and PCE risk score. Each factor of interest was evaluated in a separate linear regression model, adjusted for natal sex, race, age, and enrollment period. The models for ART regimen and NRTI also adjusted for sociodemographic index. Nadir CD4 categories (<50, 50–199, 200–349, ≥350 cells/mm³) and HIV-1 RNA categories (<LLQ, LLQ –<400, ≥400 copies/mL) were treated as linear terms. Reference lines are shown at 0% (no effect) and at shift of 1% (minimum clinically meaningful effect). Other ART regimen includes other NRTI-containing and NRTI-sparing regimens. Other NRTI includes no NRTI. *Type 3 P-values for any difference between groups. Abbreviations: ABC, abacavir; ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HIV-1, human immunodeficiency virus type 1; INSTI, integrase strand transfer inhibitor; LLQ, lower limit of quantification; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PCE, pooled cohort equations; PI, protease inhibitor; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Life’s Simple 7
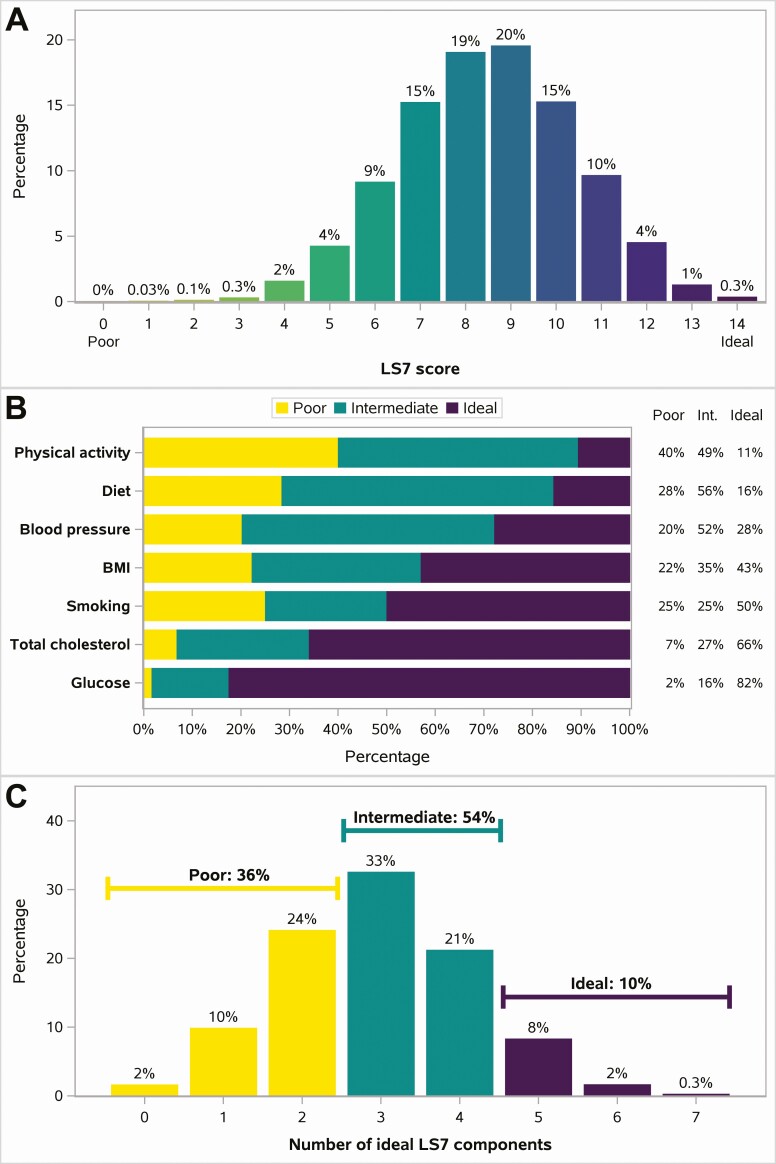
The population median LS7 score (out of an ideal score of 14) was 9 (Q1, Q3: 7, 10; Figure 3). Ten percent had overall ideal CV health (≥5/7 ideal components) including 24 participants (0.3%) with 7/7 ideal components, 2% with 6 out of 7, and 8% with 5 out of 7 ideal components. Table 2 shows distribution of overall CV health by demographics and select characteristics. Thirty-six percent (34% of women; 37% of men) met ideal targets in ≤2 components, considered poor CV health. LS7 distributions were similar by sex, age, most HIV characteristics, and ART use. Asians and those in low SDI regions had a higher proportion with overall ideal CV health, largely driven by lower BMI and less smoking (Supplementary Figure 3). Note that the majority of Asians were enrolled from low and middle SDI countries.
Figure 3.
Distribution of LS7. A, Overall LS7 score. B, Individual LS7 components. C, Overall ideal CV health categories based on number of components meeting ideal targets. Number of participants = 7382. Percentages are calculated out of participants with data collected. Missing data include: number of ideal LS7 components and LS7 score (n = 89); BMI (n = 4); physical activity (n = 25); diet (n = 18); glucose (n = 53). Abbreviations: BMI, body mass index; CV, cardiovascular; Int, intermediate; LS7, Life’s Simple 7.
Table 2.
Life’s Simple 7 (LS7) by Demographics, Risk Factors and Human Immunodeficiency Virus (HIV) Characteristics
| Overall Cardiovascular Health by LS7a | |||||
|---|---|---|---|---|---|
| Characteristicb | Total (N = 7293) |
Poor (N = 2607, 36%) |
Intermediate (N = 3930, 54%) |
Ideal (N = 756, 10%) |
|
| Demographics | |||||
| Sociodemographic index | High | 3910 | 1727 (44%) | 1889 (48%) | 294 (8%) |
| Middle | 2652 | 776 (29%) | 1588 (60%) | 288 (11%) | |
| Low | 731 | 104 (14%) | 453 (62%) | 174 (24%) | |
| Age, y | 40–49 | 3481 | 1079 (31%) | 1967 (57%) | 435 (12%) |
| 50–59 | 3177 | 1294 (41%) | 1627 (51%) | 256 (8%) | |
| ≥60 | 635 | 234 (37%) | 336 (53%) | 65 (10%) | |
| Natal sex | Male | 5002 | 1838 (37%) | 2665 (53%) | 499 (10%) |
| Female | 2291 | 769 (34%) | 1265 (55%) | 257 (11%) | |
| Race | Black | 3256 | 1162 (36%) | 1831 (56%) | 263 (8%) |
| White | 2575 | 1056 (41%) | 1278 (50%) | 241 (9%) | |
| Asian | 921 | 163 (18%) | 546 (59%) | 212 (23%) | |
| Other | 541 | 226 (42%) | 275 (51%) | 40 (7%) | |
| Cardiovascular risk factors | |||||
| Family history of premature CVD | Yes | 1362 | 577 (42%) | 680 (50%) | 105 (8%) |
| No | 5700 | 1927 (34%) | 3143 (55%) | 630 (11%) | |
| Unknown | 227 | 103 (45%) | 103 (45%) | 21 (9%) | |
| History of depression treatment | Yes | 2107 | 941 (45%) | 1017 (48%) | 149 (7%) |
| No | 5184 | 1665 (32%) | 2912 (56%) | 607 (12%) | |
| Alcohol use | Rarely/Never | 5421 | 1842 (34%) | 2941 (54%) | 638 (12%) |
| Sometimes | 1479 | 597 (40%) | 782 (53%) | 100 (7%) | |
| Usually/Often | 393 | 168 (43%) | 207 (53%) | 18 (5%) | |
| Substance use | Current | 138 | 58 (42%) | 68 (49%) | 12 (9%) |
| Former | 2171 | 1021 (47%) | 1023 (47%) | 127 (6%) | |
| Never | 4983 | 1528 (31%) | 2838 (57%) | 617 (12%) | |
| HIV characteristics | |||||
| Nadir CD4 count (cells/mm³) | <50 | 1325 | 489 (37%) | 711 (54%) | 125 (9%) |
| 50–199 | 2198 | 746 (34%) | 1210 (55%) | 242 (11%) | |
| 200–349 | 1937 | 675 (35%) | 1046 (54%) | 216 (11%) | |
| ≥350 | 1593 | 599 (38%) | 836 (52%) | 158 (10%) | |
| Unknown | 240 | 98 (41%) | 127 (53%) | 15 (6%) | |
| History of AIDS-defining diagnosis | Yes | 1707 | 595 (35%) | 904 (53%) | 208 (12%) |
| No | 5586 | 2012 (36%) | 3026 (54%) | 548 (10%) | |
| CD4 count (cells/mm³) | <350 | 962 | 319 (33%) | 519 (54%) | 124 (13%) |
| 350–499 | 1350 | 440 (33%) | 765 (57%) | 145 (11%) | |
| ≥500 | 4981 | 1848 (37%) | 2646 (53%) | 487 (10%) | |
| CD4:CD8 ratio | <0.5 | 1194 | 417 (35%) | 665 (56%) | 112 (9%) |
| 0.5–<1 | 2720 | 901 (33%) | 1522 (56%) | 297 (11%) | |
| ≥1 | 2177 | 795 (37%) | 1143 (53%) | 239 (11%) | |
| HIV-1 RNA (copies/mL) | <LLQ | 5075 | 1971 (39%) | 2643 (52%) | 461 (9%) |
| LLQ–< 400 | 583 | 254 (44%) | 287 (49%) | 42 (7%) | |
| ≥400 | 127 | 42 (33%) | 74 (58%) | 11 (9%) | |
| Antiretroviral treatment | |||||
| Total ART use, y | <5 | 1582 | 539 (34%) | 884 (56%) | 159 (10%) |
| 5–10 | 2132 | 754 (35%) | 1156 (54%) | 222 (10%) | |
| ≥10 | 3577 | 1312 (37%) | 1890 (53%) | 375 (10%) | |
| Entry ART regimen class | NRTI + NNRTI | 3385 | 1032 (30%) | 1926 (57%) | 427 (13%) |
| NRTI + INSTI | 1872 | 827 (44%) | 911 (49%) | 134 (7%) | |
| NRTI + PI | 1394 | 461 (33%) | 790 (57%) | 143 (10%) | |
| NRTI-sparing | 192 | 88 (46%) | 88 (46%) | 16 (8%) | |
| Other NRTI-containing | 450 | 199 (44%) | 215 (48%) | 36 (8%) | |
| Entry NRTIc | ABC | 937 | 414 (44%) | 457 (49%) | 66 (7%) |
| TDF | 4468 | 1398 (31%) | 2559 (57%) | 511 (11%) | |
| TAF | 1040 | 522 (50%) | 455 (44%) | 63 (6%) | |
| Other | 848 | 273 (32%) | 459 (54%) | 116 (14%) | |
Data shown are n (%).
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; BMI, body mass index; CVD, cardiovascular disease; INSTI, integrase strand transfer inhibitor; LLQ, lower limit of quantification; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SDI, sociodemographic index; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
aOverall cardiovascular health defined by the number of ideal LS7 components: poor (0–2 ideal components), intermediate (3–4), and ideal (5–7).
bRefer to the Supplementary materials for definitions of characteristics and ascertainment of associated data elements.
cEntry NRTI is defined as any ABC use including use with TDF (n = 63) or TAF (n = 6), TDF without ABC, TAF without ABC and Other.
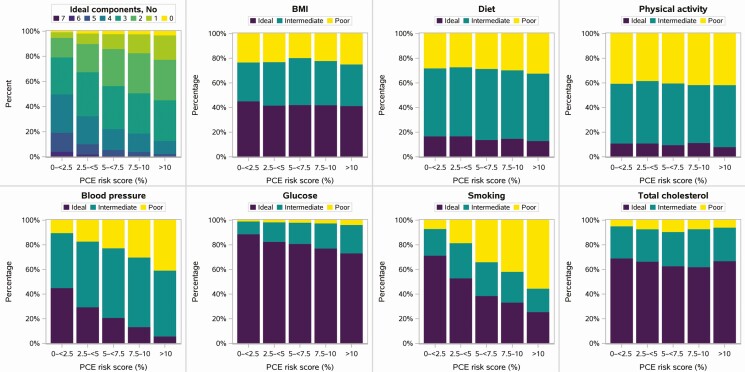
When assessed in relation to PCE CV risk, LS7 scores for smoking, blood pressure, and glucose all worsened with increasing PCE risk, as expected given their inclusion as PCE components. As a result, the number of components meeting ideal targets and LS7 score also tracked with PCE (Figure 4, Table 3). In contrast, diet quality, physical activity, total cholesterol, and BMI did not show clinically significant variation with increasing PCE overall, although trends with BMI were more apparent when stratified by sex, race, and age (Supplementary Figure 4). Importantly, the lifestyle-modifiable components of poor dietary and physical activity patterns and BMI were common even among those with low PCE score. Indeed across the entire cohort, only 111 participants (2%) had ideal lifestyle components (3/3 ideal health behaviors), 11% had 2 out of 3, and 87% (91% of women; 86% of men) had either 1 out of 3 ideal health behaviors (43%) or none (44%).
Figure 4.
Life’s Simple 7 by PCE risk score. Abbreviations: BMI, body mass index; PCE, pooled cohort equations.
Table 3.
Life’s Simple 7 (LS7) by Pooled Cohort Equations (PCE) Risk Score
| PCE Risk Score (%) | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Total (N = 7382) |
0–<2.5 (N = 2113) |
2.5–<5 (N = 1956) |
5–<7.5 (N = 1676) |
7.5–10 (N = 954) |
>10 (N = 683) |
| LS7 overall | ||||||
| Number of ideal LS7 components | ||||||
| 0 | 122 (2%) | 13 (1%) | 32 (2%) | 35 (2%) | 21 (2%) | 21 (3%) |
| 1 | 723 (10%) | 94 (4%) | 161 (8%) | 196 (12%) | 142 (15%) | 130 (19%) |
| 2 | 1762 (24%) | 326 (16%) | 433 (22%) | 487 (29%) | 300 (32%) | 216 (32%) |
| 3 | 2379 (33%) | 619 (29%) | 676 (35%) | 566 (34%) | 301 (32%) | 217 (32%) |
| 4 | 1551 (21%) | 640 (30%) | 430 (22%) | 274 (17%) | 138 (15%) | 69 (10%) |
| 5 | 609 (8%) | 319 (15%) | 160 (8%) | 78 (5%) | 36 (4%) | 16 (2%) |
| 6 | 123 (2%) | 72 (3%) | 32 (2%) | 16 (1%) | 3 (<1%) | 0 (0%) |
| 7 | 24 (<1%) | 16 (1%) | 7 (<1%) | 0 (0%) | 0 (0%) | 1 (<1%) |
| LS7 scorea | ||||||
| Min-Max | 1–14 | 3–14 | 1–14 | 1–13 | 2–13 | 3–14 |
| Median (Q1, Q3) | 9 (7, 10) | 9 (8, 11) | 9 (7, 10) | 8 (7, 9) | 8 (7, 9) | 7 (6, 9) |
| 10th, 90th percentiles | 6–11 | 7–12 | 6–11 | 6–10 | 6–10 | 5–9 |
| LS7 components | ||||||
| LS7: Smoking | ||||||
| Ideal (2) | 3685 (50%) | 1508 (71%) | 1036 (53%) | 648 (39%) | 318 (33%) | 175 (26%) |
| Intermediate (1) | 1845 (25%) | 458 (22%) | 559 (29%) | 460 (27%) | 238 (25%) | 130 (19%) |
| Poor (0) | 1852 (25%) | 147 (7%) | 361 (18%) | 568 (34%) | 398 (42%) | 378 (55%) |
| LS7: BMI | ||||||
| Ideal | 3163 (43%) | 956 (45%) | 817 (42%) | 707 (42%) | 401 (42%) | 282 (41%) |
| Intermediate | 2567 (35%) | 666 (32%) | 690 (35%) | 639 (38%) | 342 (36%) | 230 (34%) |
| Poor | 1648 (22%) | 491 (23%) | 449 (23%) | 329 (20%) | 210 (22%) | 169 (25%) |
| LS7: Physical activity | ||||||
| Ideal | 773 (11%) | 232 (11%) | 215 (11%) | 162 (10%) | 109 (11%) | 55 (8%) |
| Intermediate | 3635 (49%) | 1022 (49%) | 987 (51%) | 836 (50%) | 448 (47%) | 342 (50%) |
| Poor | 2949 (40%) | 853 (40%) | 745 (38%) | 672 (40%) | 396 (42%) | 283 (42%) |
| LS7: Diet | ||||||
| Ideal | 1146 (16%) | 355 (17%) | 329 (17%) | 232 (14%) | 141 (15%) | 89 (13%) |
| Intermediate | 4121 (56%) | 1163 (55%) | 1093 (56%) | 964 (58%) | 528 (56%) | 373 (55%) |
| Poor | 2097 (28%) | 591 (28%) | 529 (27%) | 476 (28%) | 281 (30%) | 220 (32%) |
| LS7: Total cholesterol | ||||||
| Ideal | 4864 (66%) | 1461 (69%) | 1301 (67%) | 1053 (63%) | 592 (62%) | 457 (67%) |
| Intermediate | 2010 (27%) | 551 (26%) | 514 (26%) | 465 (28%) | 294 (31%) | 186 (27%) |
| Poor | 508 (7%) | 101 (5%) | 141 (7%) | 158 (9%) | 68 (7%) | 40 (6%) |
| LS7: Blood pressure | ||||||
| Ideal | 2048 (28%) | 953 (45%) | 577 (29%) | 350 (21%) | 128 (13%) | 40 (6%) |
| Intermediate | 3836 (52%) | 942 (45%) | 1043 (53%) | 947 (57%) | 539 (56%) | 365 (53%) |
| Poor | 1498 (20%) | 218 (10%) | 336 (17%) | 379 (23%) | 287 (30%) | 278 (41%) |
| LS7: Glucose | ||||||
| Ideal | 6040 (82%) | 1869 (89%) | 1602 (83%) | 1343 (81%) | 731 (77%) | 495 (73%) |
| Intermediate | 1162 (16%) | 219 (10%) | 308 (16%) | 286 (17%) | 193 (20%) | 156 (23%) |
| Poor | 127 (2%) | 18 (1%) | 30 (2%) | 31 (2%) | 23 (2%) | 25 (4%) |
Data shown are n (%) for categorical measures, median with lower and upper quartiles (Q1, Q3), 10th and 90th percentiles, minimum (min) and maximum (max) for continuous measures. All statistics are calculated out of participants with data collected. Missing data include number of ideal LS7 components and LS7 score (n = 89) due to no BMI (n = 4), Physical activity (n = 25), Diet (n = 18) or Glucose (n = 53).
Abbreviation: BMI, body mass index.
aOverall score was calculated as the sum of the 7 individual components as shown in the smoking component in the table yielding a scale from 0 (worst cardiovascular health) to 14 (best cardiovascular health).
DISCUSSION
Among 7382 participants in the global REPRIEVE trial, CV risk measured by the PCE risk score tracked with demographics and risk factors. HIV-related factors and immune function parameters were less strongly associated, but in a direction suggesting importance of immune dysfunction. The vast majority (90%) of this global population with low to moderate CVD risk demonstrated poor to intermediate health using a standardized assessment of CV health. Several components, including BMI, physical activity, and diet, did not relate to the PCE risk score, suggesting the critical need to assess and address these poor health characteristics to improve overall CVD outcomes in this population.
As expected, higher PCE score tracked well with its input components including age, sex, race, smoking, and systolic blood pressure [15]. Relationships with diabetes and especially hyperlipidemia were limited by trial entry requirements. Known risk factors not included in the PCE inputs also tracked with increasing scores, including family history of premature CVD, depression, and the metabolic abnormalities of obesity, metabolic syndrome, and waist circumference.
Several HIV parameters were modestly related to PCE risk score. However, the higher PCE risk among those with longer ART duration is potentially due to long-term adverse effects of HIV infection or various ART regimens on metabolic pathways [18], including lipids [19]. PCE risk also tended to be higher among those with entry NRTI-containing regimens that included ABC or TAF. A relationship between ABC and increased myocardial infarction rates has been found in some studies [20], possibly related to effects on platelet and endothelial function [21], but not in other studies [22]. In contrast, TAF was associated with higher PCE, possibly due to enrollment timing, as well as direct effects on lipids levels relative to TDF, or preferential use in higher risk groups [23]. Notably, neither integrase strand transfer inhibitor use nor BMI was associated with PCE risk. In this cross-sectional study, it is impossible to assess complex relationships between various ART regimens and CV risk, lipids and inflammation, or outcomes.
Lower nadir CD4 was associated with increased PCE risk score, suggesting that degree of initial immune dysfunction relates to traditional longer-term risk indices. These findings are consistent with current theories, supported by associations between coronary plaque and arterial inflammation in HIV [24, 25], that increased inflammation and immune activation may contribute to excess CVD risk in PWH [24–26]. The degree and directionality of these effects extends our knowledge of the potential biological impact of immunological dysfunction on risk in PWH and suggests that immunological effects may be reflected in the components of the PCE score, despite its lack of any HIV-specific elements. Future REPRIEVE analyses will compare more nuanced mechanistic measures of immune function [27] to PCE risk, providing further insights as to whether immunologic factors drive events through risk pathways not assessed in traditional scoring algorithms.
In contrast to scores predicting risk for future CV events, LS7 assesses CV health. It is inversely and closely related to CV outcomes in diverse populations even after adjustment for age, race, and sex and other characteristics, as shown in a meta-analysis of nine prospective cohort studies involving 12 878 participants [13]. The LS7 metrics include only potentially modifiable components, some of which overlap with PCE (health factors: blood pressure, total cholesterol, smoking), whereas others do not (lifestyle or health behaviors: diet, physical activity, BMI, glucose). To our knowledge, this is the first application of LS7 to a large PWH cohort. Overall, only 10% of individuals had ≥5 of 7 categories meeting criteria for ideal CV health, compared with nearly 17% in the 2011–12 National Health and Nutrition Examination Survey assessment, and over a third had poor CV health (≤2 of categories meeting ideal) [28]. Other cohorts show similar poor CV health, including Atherosclerosis Risk in Communities (ARIC), which showed that just 0.1% had all 7 categories meeting ideal criteria [29]. Of note, compared to the ARIC cohort, higher proportions of REPRIEVE participants had ideal status for BMI, diet, cholesterol, and glucose, but fewer met ideal criteria for smoking, physical activity, and blood pressure.
As expected, those characteristics common to LS7 and PCE showed progressive worsening with increasing risk category; however, the others did not. Thus, LS7 complements the PCE risk score for overall assessments of CV status and underscores its possible utility as a tool for guiding CV health improvement. Specifically, even among those with lowest PCE risk, there were many participants with poor diet, elevated BMI, and low physical activity. It is likely that CV health is poorer among the higher CVD risk PWH population not included in this trial. Our findings are congruent with previous data in a small cohort study showing PWH to be substantially more sedentary than demographically matched HIV-negative controls, although differences in healthy diet were not significant [30]. Ongoing research in this area is expected to shed more light on these modifiable components of CV health [31].
Although this study has considerable strengths, including use of a large sample size and global multiracial populations, it also has limitations in representing the overall population of PWH. Trial inclusion and exclusion criteria were related to risk scores, lipids, and other relevant factors which may have shaped some of the distributions. The study was not a randomized trial of ART, and thus our purpose was not to compare CVD risk/health between ART treatment groups but to examine whether such factors are associated with traditional risk/health algorithms. Ultimately, the ongoing REPRIEVE trial will examine CV risk and health estimates in relation to incident major adverse CV events.
CONCLUSION
Among 7382 REPRIEVE trial participants, the 10-year PCE-predicted CV risk score tracked well with demographics and other CV risk factors but was related less strongly to HIV characteristics or specific ART use. Ideal CV health was rare regardless of PCE risk score. Moreover, the PCE risk score did not capture common unhealthy behaviors, including poor diet, high BMI, and low physical activity. Our findings strongly suggest that key health behaviors should be assessed, in addition to standard risk, to better understand CV health in PWH. Furthermore, lifestyle interventions may be indicated regardless of CV risk estimate and/or conventional treatment. Ultimately, it will be important to assess how well the PCE and lifestyle-based behavior assessment algorithms can predict CVD risk over time in REPRIEVE and to determine the optimal risk/health stratification system in this population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants, site staff, and study-associated personnel for their ongoing participation in the trial. They are indebted to the NHLBI and especially the REPRIEVE Program Officer—Patrice Desvigne-Nickens, MD—for strategic guidance and exceptional support, as well as to the NIAID and Beverly L. Alston-Smith, MD. Additionally, the authors acknowledge the AIDS Clinical Trial Group (ACTG) for clinical site support, the ACTG Clinical Trials Specialists for regulatory support, the data management center (Frontier Science Foundation) for data support, and the Center of Biostatistics in AIDS Research for statistical support.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institute of Allergy and Infectious Diseases; the National Institutes of Health; or the U.S. Department of Health and Human Services. Anonymized data will be made publicly available after the conclusion of the REPRIEVE randomized trial, in keeping with NHLBI policies, at the BioLINCC repository (www.biolincc.nhlbi.nih.gov).
Financial support. The REPRIEVE trial and this work were supported by grant numbers U01 HL123336 and U01 HL123339 from the National Heart, Lung, and Blood Institute (NHLBI) with additional support from National Institute of Allergy and Infectious Diseases (NIAID) grant numbers UM1 AI068636 and UM1 AI106701. S. G. also receives support from grant number P30 DK 040561, G. S. B. receives support from grant number R01 MD013493, and U. H. receives support from Oregon Health and Science University (American Heart Association, grant number 13FTF16450001), Columbia University (National Institutes of Health [NIH], grant number 5R01-HL109711), and NIH/NHLBI grant numbers 5K24HL113128 and 5T32HL076136. Research funding was also provided by Kowa Pharmaceuticals America, Gilead Sciences, and ViiV Healthcare.
Potential conflicts of interest.
T. U. has received research funding from the NIH/NIA (grant number R01 AG054366) (subcontract from UC Denver) and the NIH/NIAID (grant number UM1 AI068634) (Statistical and Data Management Center (SDMC), AIDS Clinical Trials Group (ACTG)); reports grant number U01 HL123339 (REPRIEVE DCC) from the NIH/NHLBI, and subcontract from MGH in support of REPRIEVE-EU from Kowa Pharmaceuticals during the conduct of the study. G. S. B. has received research funding from the NIH (grant number R01 MD013493) and reports to the REPRIEVE parent grant from the NIH during the conduct of the study. C. J. F. has received research funding from Amgen, Cytodyn, ViiV Healthcare, Merck, Janssen, and Pfizer, and serves as Chair of the DSMB for the Intrepid Study (funded by Kowa Pharmaceuticals) and reports grants to institution for research from Gilead Sciences during the conduct of the study. M. V. Z. has received research funding from the NIH (grant numbers R01AI123001, R01HL137562, R01HL146267, U01HL123336, and R01HL151283) and the Nutrition Obesity Research Center at Harvard, has received honoraria for lectures at academic medical centers, received meeting/travel support as a speaker at CROI and the International Workshop on HIV and Women, and participates on the DSMB for 2 NIH grant-funded studies; reports being co-principal investigator (PI) on Investigator-Initiated Industry Grant from Gilead Sciences, paid to PI’s institution (Massachusetts General Hospital (MGH), during the conduct of the study. E. T. O. has received research funding to their institution from Gilead Sciences, ViiV Healthcare, and GSK, has been a paid consultant to Merck, ViiV Healthcare, and Theratechnologies, serves as chair of the Comorbidity Transformational Science Group for the NIH-funded ACTG, and is a member of the Scientific Review Committee for the NIH-funded HVTN; and reports funding to their institution from the NIH during the conduct of the study. K. V. F. has received support for travel from the Infectious Disease Society of America and the American College of Cardiology. J. A. A. has received research funding/institutional Support for Clinical Trials COVID and/or HIV from Gilead Sciences, Merck, Emergent Biosolutions, Glaxo-Smith-Kline, Janssen, Atea, Frontier Technologies, Pfizer, Viiv Healthcare, and Regeneron, and participates on the DSMB/Advisory Board for GSK (Shingles vaccine) and Merck (HIV new therapeutics scientific advisory); reports an MGH subcontract, paid to their institution, during the conduct of the study. J. C. has received consulting fees as a consultant to Merck, serves as a DSMB board member for Resverlogix, serves as an unpaid board member of IAS-USA, and serves as an unpaid foundation board member for CROI. C. A. S. is an employee and Chief Medical Officer of Kowa Pharmaceuticals. K. M. is an employee and stockholder of Gilead Sciences. V. E. has received payment from Gilead Sciences and ViiV Healthcare and participates on the DSMB/Advisory Board for Janssen and MSD. M. S. is completing a fellowship at Gilead Sciences, has received payment from Gilead Sciences, Janssen, Merck Sharp and Dohme for presentations and educational events, and has received travel assistance/CROI assistance from ViiV Healthcare. A. M. N has received research funding paid to their institution from Amarin, BMS, Esperion, Amgen, Sanofi, Regeneron, and Janssen and has been a consultant to/received consulting fees from Amarin, Amgen, Astra Zeneca, BI, CSL, Esperion, Janssen, Lilly, Sanofi, Regeneron, NovoNordisk, Novartis, The Medicines Company, New Amsterdam, Cerner, 89Bio, and Pfizer. U. H. reports grants on behalf of the institution from Kowa Pharmaceuticals and the NIH during the conduct of the study. H. J. R. has received research funding from the NIH/NIAID (grant numbers AI068634 and AI123001) and the NIH/NHLBI (grant numbers HL137562 and HL146199; reports NIH/NHLBI (grant number HL123339) and Kowa Pharmaceuticals grants during the conduct of the study. S. G. has received consulting fees as a consultant to ViiV Healthcare and Theratechnologies; reports support from the NIH, Kowa Pharmaceuticals Gilead Sciences, and ViiV Healthcare, during the conduct of the study. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 2019; 140:e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 2018; 138:1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zanni MV, Toribio M, Wilks MQ, et al. Application of a novel CD206+ macrophage-specific arterial imaging strategy in HIV-infected individuals. J Infect Dis 2017; 215:1264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feinstein MJ, Bogorodskaya M, Bloomfield GS, et al. Cardiovascular complications of HIV in endemic countries. Curr Cardiol Rep 2016; 18:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thienemann F, Sliwa K, Rockstroh JK. HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur Heart J 2013; 34:3538–46. [DOI] [PubMed] [Google Scholar]
- 8. Ballocca F, Gili S, D’Ascenzo F, et al. HIV infection and primary prevention of cardiovascular disease: lights and shadows in the HAART Era. Prog Cardiovasc Dis 2016; 58:565–76. [DOI] [PubMed] [Google Scholar]
- 9. Joint United Nations Programme on HIV/AIDS (UNAIDS). Ending AIDS: progress towards the 90-90-90 targets. 2017. Available at: https://www.unaids.org/en/resources/documents/2017/90-90-90. Accessed 7 July 2021.
- 10. Lerner AM, Eisinger RW, Fauci AS. Comorbidities in persons with HIV: the lingering challenge. JAMA 2020; 323:19–20. [DOI] [PubMed] [Google Scholar]
- 11. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2019; 74:1376–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lloyd-Jones DM, Hong Y, Labarthe D, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American heart association’s strategic impact goal through 2020 and beyond. Circulation 2010; 121:586–613. [DOI] [PubMed] [Google Scholar]
- 13. Fang N, Jiang M, Fan Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: a meta-analysis. Int J Cardiol 2016; 214:279–83. [DOI] [PubMed] [Google Scholar]
- 14. Grinspoon SK, Fitch KV, Overton ET, et al. ; REPRIEVE Investigators . Rationale and design of the randomized trial to prevent vascular events in HIV (REPRIEVE). Am Heart J 2019; 212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 2014; 129:S49–73. [DOI] [PubMed] [Google Scholar]
- 16. Gans KM, Risica PM, Wylie-Rosett J, et al. Development and evaluation of the nutrition component of the rapid eating and activity assessment for patients (REAP): a new tool for primary care providers. J Nutr Educ Behav 2006; 38:286–92. [DOI] [PubMed] [Google Scholar]
- 17. Fichtenbaum CJ, Ribaudo HJ, Leon-Cruz J, et al. ; REPRIEVE Investigators . Patterns of antiretroviral therapy use and immunologic profiles at enrollment in the REPRIEVE trial. J Infect Dis 2020; 222:S8–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS 2016; 30:1495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lv Z, Chu Y, Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV AIDS (Auckl) 2015; 7:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elion RA, Althoff KN, Zhang J, et al. ; North American AIDS Cohort Collaboration on Research and Design of IeDEA . Recent abacavir use increases risk of type 1 and type 2 myocardial infarctions among adults with HIV. J Acquir Immune Defic Syndr 2018; 78:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O’Halloran JA, Dunne E, Tinago W, Denieffe S, Kenny D, Mallon PWG. Switching from abacavir to tenofovir disoproxil fumarate is associated with rises in soluble glycoprotein VI, suggesting changes in platelet-collagen interactions. AIDS 2018; 32:861–6. [DOI] [PubMed] [Google Scholar]
- 22. Ding X, Andraca-Carrera E, Cooper C, et al. No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J Acquir Immune Defic Syndr 2012; 61:441–7. [DOI] [PubMed] [Google Scholar]
- 23. Huhn GD, Shamblaw DJ, Baril JG, et al. Atherosclerotic cardiovascular disease risk profile of tenofovir alafenamide versus tenofovir disoproxil fumarate. Open Forum Infect Dis 2020; 7:ofz472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zanni MV, Abbara S, Lo J, et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS 2013; 27:1263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diaz CM, Segura ER, Luz PM, et al. Traditional and HIV-specific risk factors for cardiovascular morbidity and mortality among HIV-infected adults in Brazil: a retrospective cohort study. BMC Infect Dis 2016; 16:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoffmann U, Lu MT, Olalere D, et al. ; REPRIEVE Investigators . Rationale and design of the mechanistic substudy of the randomized trial to prevent vascular events in HIV (REPRIEVE): effects of pitavastatin on coronary artery disease and inflammatory biomarkers. Am Heart J 2019; 212:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shay CM, Ning H, Allen NB, et al. Status of cardiovascular health in US adults: prevalence estimates from the national health and nutrition examination surveys (NHANES) 2003-2008. Circulation 2012; 125:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; ARIC Study Investigators . Community prevalence of ideal cardiovascular health, by the American heart association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011; 57:1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Webel A, Horvat Davey C, Schexnayder J, et al. Impact of perceived cardiovascular risk on cardiovascular disease prevention behaviors in people with and without HIV infection. J Acquir Immune Defic Syndr 2020; 83:513–21. [DOI] [PubMed] [Google Scholar]
- 31. Webel AR, Long D, Rodriguez B, et al. The PROSPER-HIV study: a research protocol to examine relationships among physical activity, diet intake, and symptoms in adults living with HIV. J Assoc Nurses AIDS Care 2020; 31:346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.