Abstract
Background
It is imperative to identify new targets for improved vaccines and therapeutics against influenza. One such target is the relatively conserved stalk region of the influenza A hemagglutinin (HA) surface protein.
Methods
We conducted a randomized, double-blind, phase 2, placebo-controlled trial of a monoclonal antibody that targets the HA stalk (CR6261) in a H1N1pdm09 healthy volunteer human challenge model. A single 50 mg/kg dose of CR6261 was infused 24 hours after challenge. The primary efficacy outcome was area under the curve (AUC) of viral RNA detection over time.
Results
Ninety-one healthy volunteers were randomized and underwent influenza challenge; 49 received CR6261 and 42 received placebo. CR6261 had no statistically significant effect on AUC (AUC, 48.56 log [copies/mL] × days, interquartile range [IQR], 202 vs AUC, 25.53 log [copies/mL] × days, IQR, 155; P = .315) and no clinically significant effect on influenza disease measures including number of symptoms, duration of symptoms, or inFLUenza Patient-Reported Outcome (FLU-PRO) scores. Preexisting anti-NA antibody titers were most predictive of reduced influenza disease. CR6261 reached a mean peak serum concentration of 1 × 106 ng/mL 15 minutes after infusion and a mean peak of 5.97 × 102 ng/mL in the nasal mucosa 2–3 days after infusion.
Conclusions
The results of this study suggest that a monoclonal anti-stalk approach to prevent or treat influenza infection may be limited in efficacy. Future approaches should consider including and evaluating anti-stalk antibodies as part of a multifaceted strategy rather than as a stand-alone therapeutic.
Clinical Trials Registration
Keywords: influenza A, HA stalk, anti-HA stalk antibody, CHIM, challenge study
Administration of intravenous monoclonal antibody CR6261 24 hours after influenza challenge was found to be safe, although it had no effect on viral replication or influenza severity among healthy volunteers.
Influenza causes significant morbidity and mortality during seasonal epidemics and sporadic pandemics. Approved therapeutics for currently circulating strains include the neuraminidase inhibitors and Baloxavir, a cap-dependent endonuclease inhibitor. Rapid development of resistance has already been observed against this newest antiviral [1]. Adamantanes are no longer recommended due to high levels of resistance in circulating influenza strains, and none of these antivirals have demonstrated significant benefit in those with complicated or severe infection.
Improving vaccines and therapeutics for influenza has become a worldwide priority [2–4]. It is imperative to identify new targets such as the relatively conserved stalk region of the influenza A hemagglutinin (HA) surface protein. The HA stalk can be divided into group 1 and group 2 to include all of the HA subtypes. This makes the stalk an attractive target to potentially induce broadly protective antibodies against multiple influenza A subtypes. Much effort in making a universal vaccine over the last decade has focused on this promising strategy.
CR6261 is a monoclonal anti-HA stalk antibody that has demonstrated broad neutralization [5, 6] and protection in animals [7]. It stabilizes the prefusion HA structure and prevents pH-dependent fusion of cellular and viral membranes in endosomes [5]. In vitro, CR6261 exhibits neutralizing activity against group 1 influenza viruses, which include H1, H2, H5, H6, H8, and H9. It has also been shown to have therapeutic and prophylactic efficacy against H1N1 and H5N1 in animals [8, 9]. A phase 1 placebo-controlled study with escalating doses of CR6261 found CR6261 to be safe (NCT01406418).
Here, we conducted a randomized, double-blind, placebo-controlled trial of CR6261 in the validated National Institute of Allergy and Infectious Diseases (NIAID) H1N1pdm09 healthy volunteer human challenge model to assess the efficacy of an intravenous infusion of CR6261 24 hours after exposure to influenza.
METHODS
Challenge Virus
The A/California/04/2009/H1N1 passage 6 challenge virus is a live wild-type virus manufactured as previously described. A 107 50% tissue culture infectious dose (TCID50) was determined empirically previously to cause >60% mild to moderate influenza disease (MMID) [10].
Clinical Study
Healthy volunteers aged between 18 and 45 years were enrolled between March 2015 and February 2018. They were screened on a separate protocol (ClinicalTrials.gov NCT01386424) to be nonsmokers, healthy, with a body mass index ≥18 and ≤35, no influenza vaccine received within the past influenza season, and a serum hemagglutination inhibition (HAI) antibody titer of ≤1:10 within 60 days prior to enrollment. Participants were admitted to the National Institutes of Health (NIH) Clinical Center and administered a single intranasal dose of 107 TCID50 of challenge virus using the MAD Nasal TM https://www.teleflex.com/usa/en/product-areas/anesthesia/atomization/mad-nasal-device/index.html intranasal mucosal atomization device (Teleflex, Morrisville, NC).
Participants were randomized after challenge 1:1, double-blinded, to receive CR6261 monoclonal antibody or placebo 24 hours after influenza challenge. Participants received 50 mg/kg of CR6261 or placebo (5% dextrose in water) as a single intravenous (IV) infusion. Participants were isolated for a minimum of 10 days with challenge occurring on the second day (day 0) and infusion on the third day (day 1) of hospitalization. Isolation, evaluation, and testing were performed as previously described [10–12]. Participants were discharged after a minimum of 10 days and at least 2 negative nasal washes for influenza. After discharge, participants returned for 2 outpatients visits on day 29 and day 66.
Clinical outcomes were measured by clinician assessments and the inFLUenza Patient-Reported Outcome (FLU-PRO) tool, a standardized and validated questionnaire for evaluating influenza severity [13–15]. Daily nasal washes were collected for the presence of influenza and other respiratory pathogens. Nasal washes from day –1 and day 2 were used to evaluate anti-HA stalk immunoglobulin A (IgA). Minitip flocked swabs (Becton, Dickson and Company, Franklin Lakes, NJ) were used to collect nasal samples 3 times per day then placed in viral transport media for quantitative reverse-transcription polymerase chain reaction (qRT-PCR) assay. CR6261 pharmacokinetics was evaluated on serum samples collected prior to CR6261 infusion; 15 minutes after infusion; 24, 48, 96, and 168 hours after infusion; and on day 29 and day 66 after challenge. Nasal swabs obtained twice daily both before and 9 days after CR6261 infusion were also tested.
The primary objective was to determine if there was a reduction in the area under the curve (AUC) using 1-step real-time qRT-PCR assay. Secondary objectives included comparing clinical illness severity and evaluating safety and pharmacokinetics of CR6261. The sample size was 122 for a power of 90% to be able to detect a decrease in mean AUC of 50% and adjusting for interim analyses at 33% and 67% of the final sample size and allowing for 5% loss from final analysis.
CR6261 and Placebo
CR6261 was manufactured and provided by Janssen Infectious Diseases and Vaccines, Leiden, Netherlands. It is a human IgG1 monoclonal antibody produced in PER.C6 cells. It is directed at a conserved region of the HA stem. It was supplied as a sterile lyophilized cake (400 mg/vial) and reconstituted in 250 mL of 5% dextrose in water. Placebo was 5% dextrose in water. Infusions were administered over a 2-hour period. Two lots of CR6261 were used.
Virologic Assays
A multiplex test of 21 respiratory pathogens was performed daily from nasal washes using the FilmArray RespiratoryPanel (BioFire Diagnostics, Salt Lake City, UT) [16]. Quantitation of influenza virus was performed using a previously validated qRT-PCR assay for the influenza A virus matrix 1 gene [17]. An external standard was used to calculate copy number. Assays were performed in the Janssen laboratory initially and then the NIH laboratory due to the closure of the Janssen laboratory partway through the study. Results are presented by laboratory and combined, but stratified by laboratory.
Pharmacokinetics and Immunogenicity Assays
An MSD-based immunogenicity electrochemiluminescence immunoassay (ECLIA) (Meso Scale Discovery, Inc, Gaithersburg, MD) method was developed, optimized, and validated to measure CR6261 concentration. The validated immunoassay method had a lower limit of quantification (LLOQ) of 500.00 ng/mL with a minimum required dilution of 50. Streptavidin-coated 96-well plates were blocked for 30 minutes, and the standard curve calibrators, quality controls, and test samples were prepared using automated liquid handling. A biotinylated-C4G8 capture antibody and Sulfo-Tag C11A12 were used at a final concentration of 1.0 μg/mL. After a 2-hour incubation at room temperature, read buffer was added and the electrochemiluminescent signal was read. CR6261 concentrations were determined by interpolation from a standard curve using a 5PL curve fit with 1/y2 weighting. The standard curve range was 10.00 to 640.00 ng/mL to define the standard curve limits of quantification with anchoring points for curve fitting at 5 ng/mL and 1280 ng/mL.
Concentration of CR6261 from nasal swabs was also performed using MSD-ECLIA with a LLOQ of 0.05 μg/mL with a minimum required dilution of 5. The standard curve range was 0.01 to 0.64 μg/ml to define the standard curve limits of quantification with anchoring points for curve fitting at 0.005 μg/mL and 1.28 μg/mL.
Immunologic Assays
Standard methods were used to measure serum HAI and neuraminidase inhibition (NAI) antibody titers against the challenge virus [18–20]. Serum anti-HA stalk antibody and influenza-specific anti-HA stalk IgA were measured from nasal washes using enzyme-linked immunosorbent assay as previously described [21].
Deep Sequencing
Thirty-eight participants with the highest viral copy numbers by qRT-PCR assay in a single sample were chosen for deep sequencing to identify the A388V mutation in the HA stalk identified previously [22, 23]. RNA isolated from each patient’s nasal wash sample (10 μL) was amplified and sequenced on an Illumina MiSeq machine as previously described [23]. Generated reads were demultiplexed using Illumina software and were mapped to the HISAT2 (version 2.2.0) indexed A/California/04/2009/H1N1 genome using Hista2 [24–26]. SAMtools mpileup (version 2.1.0) [27] was used to make single-nucleotide polymorphism calls at HA nucleotide 1195 site with minimum base Phred quality score as 25.
STATISTICAL METHODS
The AUCs of the qRT-PCR assay across day 1 through day 8 were compared for the primary analysis. Since the qRT-PCR assay was completed in 2 laboratories, AUC analyses are presented by laboratory and then as a combined analysis stratified by laboratory via a nonparametric covariate adjustment, taking into account laboratory/assay variability. A 2-sided Wilcoxon ranked sum test was used to compare median AUC between CR6261 and placebo recipients. A Fisher exact test was used to compare demographics and clinical endpoints. A Student t test was used to compare mean ages. Geometric mean titers with 95% confidence intervals were calculated to compare HAI and NAI titers. The Wilcoxon ranked sum test was used for group comparisons in nonbinary endpoints including FLU-PRO scores, HAI, and NAI titers. Logistic regression and quasi-Poisson regression were used for multivariate analyses with interaction terms between treatment and each titer considered and removed if not deemed to be significantly different than 0. All analysis was 2-sided with P ≤ .05 considered significant. Statistical analyses were performed using R (R: A Language and Environment for Statistical Computing, Vienna, Austria) and GraphPad Prism 8 (GraphPad, La Jolla, CA).
This study was performed under US Food and Drug Administration investigational new drug numbers 124375 and 110697. It was approved by the NIAID Institutional Review Board and conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. All participants provided written informed consent prior to enrollment.
RESULTS
Efficacy of CR6261
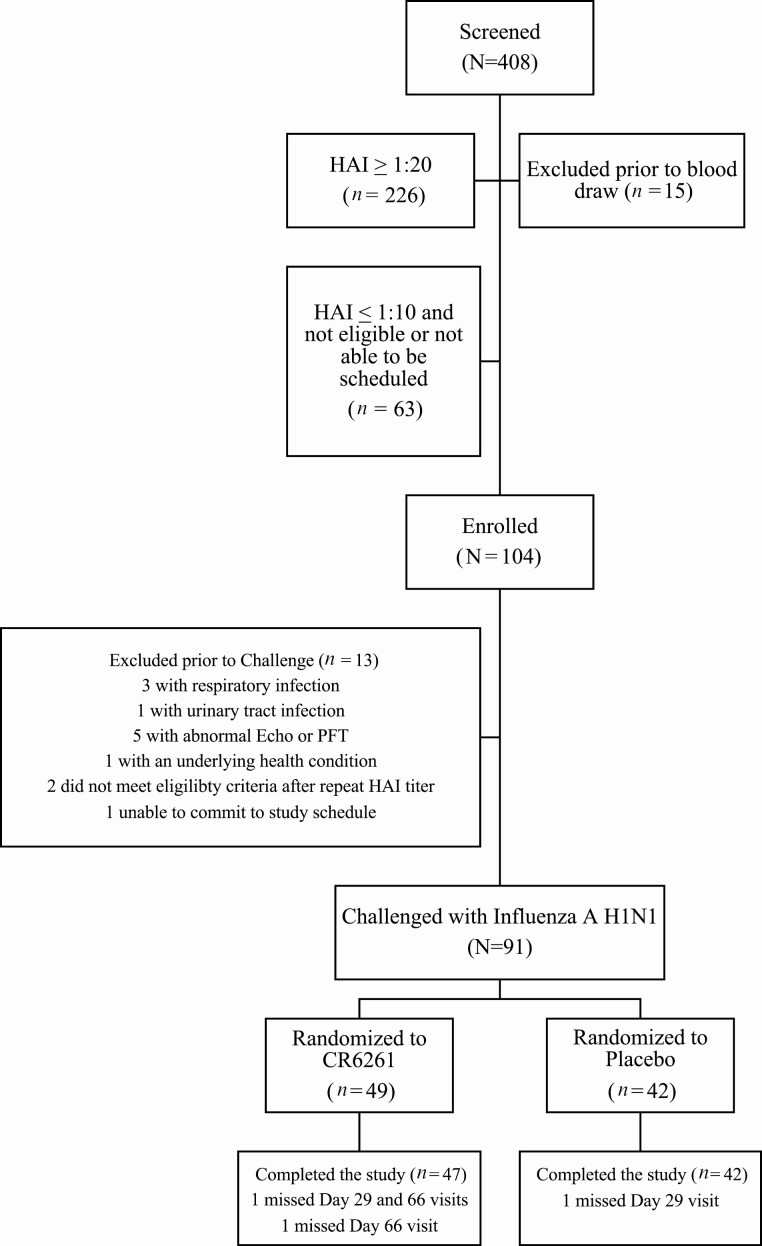
Between March 2015 and February 2018, 104 healthy volunteers were enrolled and 91 participants (Table 1) underwent challenge; 49 participants received treatment with CR6261 and 42 participants received placebo (Figure 1). Eight participants had at least 1 nasal wash that was positive for a noninfluenza respiratory virus during the quarantine. Of these, 5 received CR6261 and 3 received placebo.
Table 1.
Demographics of Study Participants
| Demographic | CR6261 (N = 49) | Placebo (N = 42) | P Value |
|---|---|---|---|
| Sex, N (%), female | 17 (34.7%) | 22 (52.4%) | .096 |
| Age, mean (standard deviation), years | 30.2 (5.54) | 31.8 (6.34) | .188 |
| Race, N (%), Black/African-American (vs White) |
21 (42.9%) | 16 (38.1%) | .674 |
| Hispanic (vs not Hispanic), N (%) | 5 (10.2%) | 3 (7.14%) | .721 |
Figure 1.
Study enrollment. A total of 408 participants were screened; 104 were enrolled, and 91 were randomized to CR6261 or placebo. A total of 91 participants underwent influenza challenge; 49 (54%) received CR6261, and 42 (46%) received placebo. Abbreviations: HAI, hemagglutination inhibition; PFT, pulmonary function test.
There was no statistically significant difference in the primary outcome measure between the CR6261 group and placebo (median AUC, 48.6 log [copies/mL] × days and 25.5 log [copies/mL] × days, respectively; P = .315; Table 2). The incidence of shedding was also similar between the 2 groups (P = .646). Overall, 76% of individuals experienced symptoms in the CR6261 group, a statistically significant reduction compared with 93% in the placebo group (Table 3). However, this did not result in a significant reduction in incidence of MMID (presence of at least 1 symptom of influenza plus detectable shedding) or confirmed influenza infection (symptoms plus a 4-fold rise in convalescent HAI titer or MMID; Table 3).
Table 2.
Primary Outcome: Median Area Under the Curve Log(RNA Copies per Milliliter) of Influenza A Virus × Days (Interquartile Range)
| Laboratories | CR6261 (N = 49) | Placebo (N = 42) | P Value |
|---|---|---|---|
| Janssen laboratory (N = 69) | 29.7 (251) | 19.8 (178) | .396 |
| National Institutes of Health laboratory (N = 22) | 66.5 (144) | 32.4 (82.2) | .615 |
| Combined (N = 91) | 48.6 (202) | 25.5 (155) | .315a |
Area under the curve = Log(RNA copies per milliliter) of Influenza A virus x days. Interquartile range = 75th percentile–25th percentile.
aStratified for laboratory via nonparametric covariate adjustment.
Table 3.
Secondary Clinical Endpoints
| Influenza Severity | CR6261 (N = 49) |
Placebo (N = 42) |
P Value |
|---|---|---|---|
| Mild to moderate influenza disease,a N (%) | 26 (53%) | 29 (69%) | .137 |
| Confirmed influenza infection, N (%) | 36 (73%) | 37 (88%) | .114 |
| Any symptoms, N (%) | 37 (76%) | 39 (93%) | .045b |
| Number of symptoms, median (95% CI) | 3 (2–5) | 4 (3–5) | .244 |
| Duration of symptoms, median (95% CI) | 5 (3–7) | 6 (5–7) | .141 |
| Any shedding, N (%) | 33 (67%) | 31 (74%) | .646 |
| Duration of shedding, median (95% CI) | 2 (1–4) | 2.5 (1–5) | .498 |
| inFLUenza Patient-Reported Outcome (FLU-PRO) score, median (95% CI) | 0.038 (.013–.084) | 0.057 (.041–.084) | .230 |
Abbreviation: CI, confidence interval.
aMild to moderate influenza disease is defined as the presence of at least 1 symptom of influenza plus detectable shedding.
bStatistical significance of P < .05.
The severity of illness was compared between the 2 groups, and no significant difference was observed. Both groups had similar symptom severity by FLU-PRO scores (Table 3). Duration and number of symptoms experienced from influenza were also similar, ranging from 0 to 11 days of symptoms and 0 to 19 individual influenza symptoms. No statistically significant difference in the duration of detectable shedding was noted between groups, with a range of 0 to 9 days of shedding in all participants (Table 3).
Pharmacokinetics of CR6261
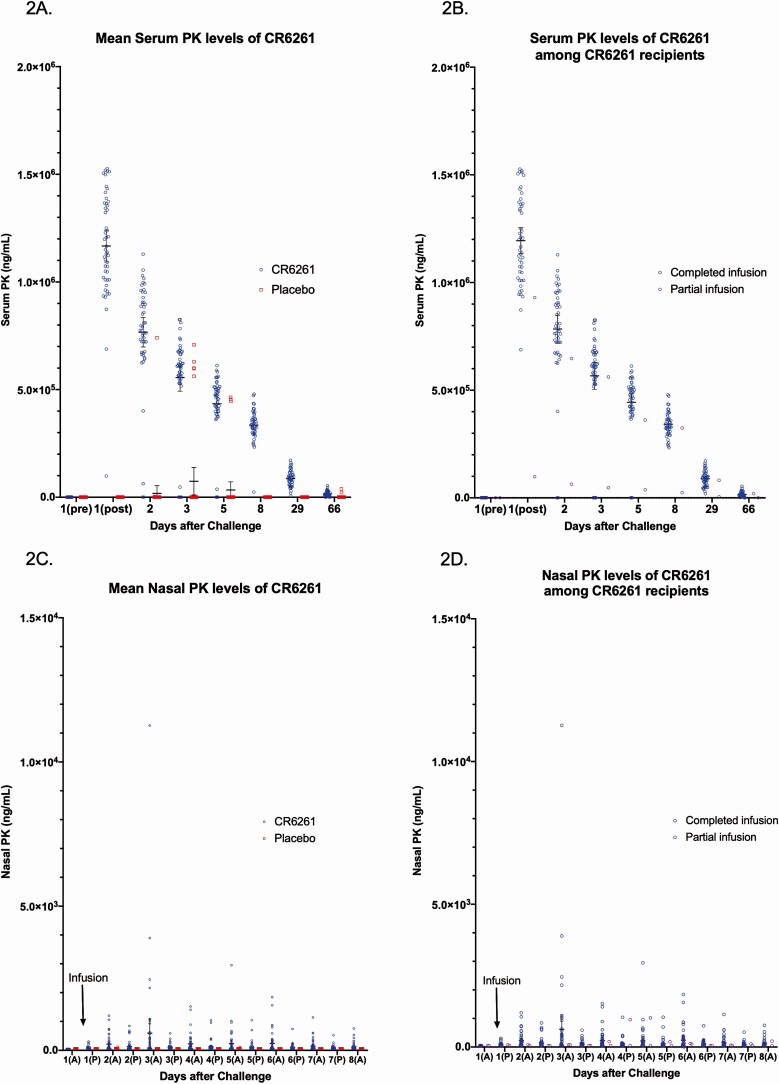
All participants in the CR6261 group had measurable levels of CR6261 in the serum and almost all in nasal swabs. A mean of more than 1 × 106 ng/mL of CR6261 was detected in the serum 15 minutes after infusion (Figure 2A, 2B). Levels steadily decreased over time but still maintained mean levels of 3 × 105 ng/mL of CR6261 1 week later. Levels returned to near predosing levels by day 66 (Figure 2A). Nasal swab levels of CR6261 reached a peak mean of 5.97 × 102 ng/mL 2–3 days after CR6261 infusion (Figure 2C, 2D). No anti-CR6261 antibodies were detected in any participant in either group.
Figure 2.
A, Mean serum PK levels of CR6261 after influenza challenge for CR6261 and placebo recipients. Among CR6261 recipients, PK levels reached predosing levels by day 66. B, Serum PK levels of CR6261 after influenza challenge for CR6261recipients. Two participants developed hives during the infusion and so only received partial infusions (red). C, Nasal PK levels of CR6261 after influenza challenge for CR6261 and placebo recipients. CR6261 levels were identified from nasal swabs though at levels lower than serum. D, Mean nasal PK levels of CR6261 after influenza challenge for CR6261 recipients, of whom 2 developed hives during infusion and only received partial infusions (red). Lines represent means and 95% confidence interval. Abbreviation: PK, pharmacokinetic.
Antibody Responses to Influenza and Clinical Correlation
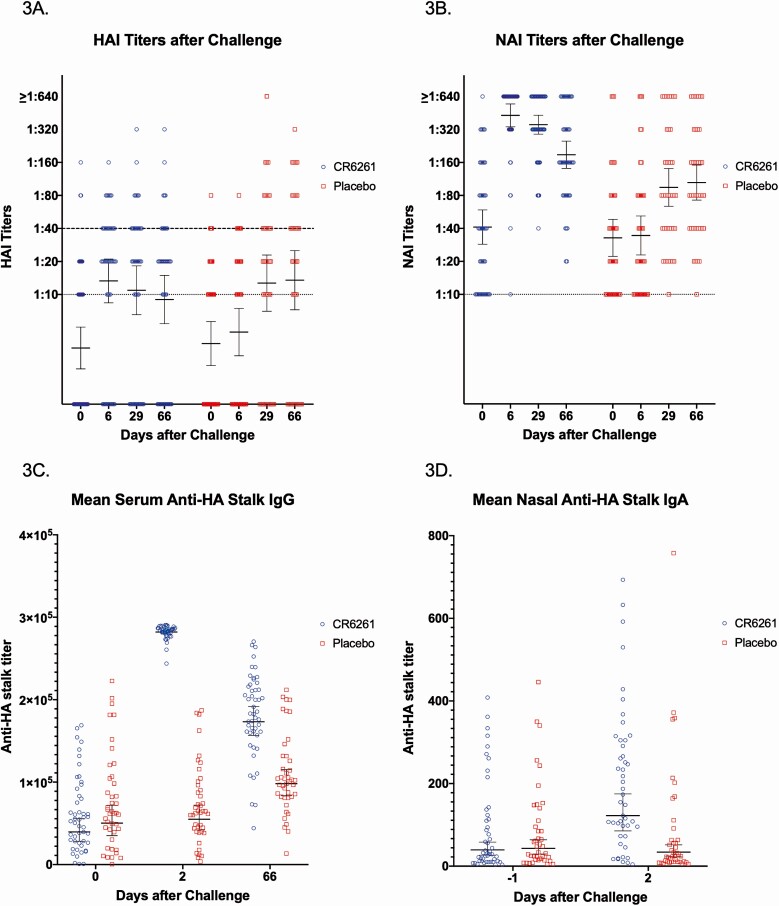
Participants in both groups experienced similar rises in titer after challenge (Figure 3A, 3B). Similar amounts of naturally occurring anti-HA stalk antibody were present in the serum and nasal wash prechallenge, but there was a statistically significant rise in serum anti-HA stalk IgG and nasal IgA 1 day after infusion that corresponded to the pharmacokinetic measurements (Figure 3C, 3D).
Figure 3.
Virus-specific antibody titers after influenza challenge for CR6261 and placebo recipients. A, Both treatment groups had a significant rise in HAI titer by day 29 and day 66 after influenza challenge. Dotted line indicates the lowest limit of detection (1:10). Dashed line indicates the level of protection (≥1:40). B, Both treatment groups had a significant rise in NAI titer by day 29 and day 66 after influenza challenge. Dotted line indicates the lowest limit of detection (1:10). C, One day after CR6261 infusion (day 2), there was a significant rise in anti-HA stalk IgG that remained elevated compared with placebo even to day 66. D, There was a significant rise in anti-HA stalk IgA in nasal samples 1 day after CR6261 infusion (day 2). In all plots, lines represent geometric mean titers and 95% confidence intervals. Abbreviations: HA, hemagglutinin; HAI, hemagglutination inhibition; Ig, immunoglobulin; NAI, neuraminidase inhibition.
The relationships between the participants’ baseline immunity, treatment assignment, and clinical outcomes were assessed, and only baseline serum NAI titer was associated with a decreased probability of developing MMID and confirmed influenza in a logistic regression model (Table 4). In addition, baseline NAI titer was predicted to have –19% effect on duration of shedding/50-unit increase in titer using a quasi-Poisson regression model. CR6261 treatment and baseline HAI titer were not shown to have a statistically significant effect on clinical outcome measures in any of these models (Table 4).
Table 4.
Logistic Regression Models of Mild to Moderate Influenza Disease and Confirmed Influenza Infection
| Outcome | Covariate | Odds Ratioa (Confidence Interval) | P Value |
|---|---|---|---|
| Mild to moderate influenza disease | Baseline HAI | 0.77 (.27–2.20) | .63 |
| Baseline NAI | 0.66 (.49–.89) | .0070b | |
| Treatment (reference: placebo) | 0.52 (.20–1.36) | .18 | |
| Confirmed influenza infection | Baseline HAI | 1.06 (.32–3.52) | .93 |
| Baseline NAI | 0.82 (.70–.97) | .017b | |
| Treatment (reference: placebo) | 0.33 (.10–1.11) | .07 |
Abbreviations: HAI, hemagglutination inhibition; NAI, neuraminidase inhibition.
a Odds ratio defined in terms of 50-unit increase in baseline titers.
b Statistical significance of P < .05.
Effect of CR6261 on Intrahost Viral Evolution
Of the 38 participants whose viruses were deep sequenced, 31 sequenced with adequate coverage to be included. The mean day of shedding sequenced was similar between the placebo and CR6261 groups, 3.58 vs 3.24, respectively (P = .528). There was no statistically significant difference in the presence of the A388V mutation from viruses sequenced from the subset of participants, with 12 of 21 in the treatment group and 9 of 12 in the placebo group demonstrating more than 50% of reads with the 388V (P = .776).
Safety of CR6261
Overall, CR6261 was well tolerated. Thirty-five adverse events (AEs) were identified in all participants (Supplementary Table 1). One participant who received placebo was hospitalized for alcohol intoxication during follow-up, incurring a serious AE (SAE). No SAEs occurred related to any study intervention.
Two participants developed CR6261 infusion reactions, and both infusions were stopped early. One participant developed hives after 9 minutes that resolved (grade 2 AE), while the other developed a grade 3 AE of generalized hives and pruritis after receiving 99 minutes of CR6261 infusion that resolved after a single IV dose of diphenhydramine. Both incidents were reported to the Data Safety Monitoring Board (DSMB). An unblinded NIAID pharmacist investigated and reported to the DSMB after which that specific lot of CR6261 was removed, forcing a reduction in the study sample size from 122 to 91 due to limited availability of the remaining CR6261. No infusion reactions were noted thereafter.
Other AEs were mild, not clinically significant, and resolved without intervention. These were mostly laboratory findings that occurred similarly between the 2 treatment groups (Supplementary Table 1, Supplementary Figure 1). Other AEs possibly associated with CR6261 were all grade 1 symptoms that occurred infrequently (Supplementary Table 1).
DISCUSSION
The conserved HA stalk has generated much interest as a target for inducing broadly protective antibodies, both to serve as “universal” influenza vaccines and for the development of monoclonal antibodies as treatment [28–30]. Several early clinical trials were undertaken to assess its clinical efficacy [29]. Human trials of monoclonal antibody MHAA4549A found it to be safe and efficacious, particularly in the highest dose group, in an H3N2 challenge study; however, it had no treatment effect when evaluated in hospitalized patients with influenza [31–34]. Another monoclonal antibody, VIS410, was also found to be safe and efficacious in an H1N1 challenge and uncomplicated influenza infection [35–37]. This clinical study evaluated the use a monoclonal antibody CR6261 as a post-exposure prophylaxis treatment.
Given 24 hours after challenge with the H1N1pdm09 virus, CR6261 did not significantly reduce the number of individuals with viral shedding or MMID and did not reduce the duration or amount of viral shedding. CR6261 infusion did correlate with a reduction in the number of individuals with symptoms, leading to more asymptomatic shedders; however, those who developed symptoms seemed to suffer from similar number, duration, and severity of symptoms as those who received placebo.
Participants were given a high dose of anti-HA stalk antibody, achieving a high serum concentration (Figure 2A). However, a small amount of detectable antibody reached the nasal mucosa (Figure 2C). This reduced level of antibody at the respiratory mucosa may be a key factor limiting its effectiveness, given that influenza is typically a primarily mucosal infection. Viral replication at the respiratory mucosa was not affected by CR6261 as measured by either the AUC or incidence of shedding.
As demonstrated previously [10–12], baseline anti-NA serum immunity was the best predictor of reduced severity of illness in this study, correlating with reduced incidence of MMID, incidence of confirmed influenza infection, and duration of shedding. Those with the highest levels of anti-NA antibody in either the treatment or placebo group suffered from less severe disease, suggesting that anti-NA immunity is important to consider in vaccine development. It also suggests that anti-stalk immunity alone, whether naturally occurring or artificially induced, may not be enough to abrogate or prevent an influenza infection.
Overall, CR6261 infusion was safe. The cause of hives in the 2 CR6261 participants was not identified, but no other participant experienced this reaction, including after a new lot of CR6261 was used. The cause may have been lot-specific or due to problems with dose preparation. There was no association with other AEs. No evidence of antibody-dependent enhancement was observed.
Antigenic drift of the H1N1 stalk under pressure of monoclonal stalk antibodies was observed previously [38]. Recently, we demonstrated that the A388V mutation was seen in individuals challenged with the H1N1pdm09 [23] and that this mutation could interfere with the binding of CR6261 and other anti-stalk antibodies [22]. Naturally occurring stalk antibodies may drive selection for this mutation, but no evidence that the infusion led to selection for A388V was observed in this study.
The biggest limitation of the study was sample size. After removal of the specific lot of CR6261 due to the 2 infusion reactions, the sample size was readjusted to account for the limited remaining CR6261 available. With this reduction in sample size (from 122), the power was reduced from 90% to 83%, possibly affecting study results. The other major limitation was that the model did not replicate the natural infection route and had a limited population of participants as they must be healthy and young. However, we have demonstrated that this model does induce disease consistent with what is observed in natural infection in our previous studies [10, 12], and the challenge studies have been used to evaluate therapeutics historically [39].
CONCLUSIONS
When administered 24 hours after influenza challenge, CR6261 had no effect on viral replication in healthy volunteers. It had no meaningful efficacy in reducing influenza-induced disease but was safe with no evidence of antibody-dependent enhancement. Efficacy may be limited due to the low penetration of CR6261 at the mucosal level, while levels of naturally occurring anti-NA antibody appeared to be the best predictor of disease severity. Our study suggests that a monoclonal anti-stalk approach to prevent or treat influenza infection may offer limited efficacy and may perform better if used in conjunction with other strategies as opposed to stand-alone therapeutics or vaccines.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. H. and M. M. wrote the initial draft. M. M., A. H., K. L., and S. H. performed data analyses. L. A. R., A. C., Y. X., and M. G. performed laboratory testing and analyses. All authors contributed to editing, revising, and finalizing the manuscript.
Acknowledgments. The authors acknowledge the National Institutes of Health (NIH) Clinical Center (CC) staff involved in this study, especially the staff of the Special Clinical Studies Unit, the Microbiology Laboratory in Department of Laboratory Medicine, and the NIH CC Pharmacy. We express special thanks to Dr Richard Davey for his support of this work and the participants for their time and inconvenience.
Financial support. This study was funded in part by the intramural program of the National Institute of Allergy and Infectious Diseases, NIH, with federal funds from the National Cancer Institute, NIH under contract 75N9109D00024, task order 75N9109F00130, and through a Cooperative Research and Development Agreement with Janssen Infectious Diseases and Vaccines.
Potential conflicts of interest. A. L. and J. S. are employees of Janssen Infectious Diseases and Vaccines. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hayden FG, Sugaya N, Hirotsu N, et al. ; Baloxavir Marboxil Investigators Group . Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379:913–23. [DOI] [PubMed] [Google Scholar]
- 2. Bresee JS, McKinlay MA, Abramson J, Klugman KP, Wairagkar N; Global Funders Consortium for Universal Influenza Vaccine Development . Global funders consortium for universal influenza vaccine development. Vaccine 2019; 37: 211–3. [DOI] [PubMed] [Google Scholar]
- 3. Dyer O. Gates Foundation challenges researchers to create universal flu vaccine with $12m offer. BMJ 2018; 361:k1922. [DOI] [PubMed] [Google Scholar]
- 4. Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ekiert DC, Bhabha G, Elsliger MA, et al. Antibody recognition of a highly conserved influenza virus epitope. Science 2009; 324:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Throsby M, van den Brink E, Jongeneelen M, et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 2008; 3:e3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sutton TC, Lamirande EW, Bock KW, et al. In vitro neutralization is not predictive of prophylactic efficacy of broadly neutralizing monoclonal antibodies CR6261 and CR9114 against lethal H2 influenza virus challenge in mice. J Virol 2017; 91:e01603–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friesen RH, Koudstaal W, Koldijk MH, et al. New class of monoclonal antibodies against severe influenza: prophylactic and therapeutic efficacy in ferrets. PLoS One 2010; 5:e9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koudstaal W, Koldijk MH, Brakenhoff JP, et al. Pre- and postexposure use of human monoclonal antibody against H5N1 and H1N1 influenza virus in mice: viable alternative to oseltamivir. J Infect Dis 2009; 200:1870–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Memoli MJ, Czajkowski L, Reed S, et al. Validation of the wild-type influenza A human challenge model H1N1pdMIST: an A(H1N1)pdm09 dose-finding investigational new drug study. Clin Infect Dis 2015; 60:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han A, Czajkowski LM, Donaldson A, et al. A dose-finding study of a wild-type influenza A(H3N2) virus in a healthy volunteer human challenge model. Clin Infect Dis 2019; 69:2082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Memoli MJ, Shaw PA, Han A, et al. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. mBio 2016; 7:e00417–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han A, Poon JL, Powers JH 3rd, Leidy NK, Yu R, Memoli MJ. Using the inFLUenza Patient-Reported Outcome (FLU-PRO) diary to evaluate symptoms of influenza viral infection in a healthy human challenge model. BMC Infect Dis 2018; 18:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Powers JH 3rd, Bacci ED, Leidy NK, et al. Performance of the inFLUenza Patient-Reported Outcome (FLU-PRO) diary in patients with influenza-like illness (ILI). PLoS One 2018; 13:e0194180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Powers JH, Guerrero ML, Leidy NK, et al. Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza. BMC Infect Dis 2016; 16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poritz MA, Blaschke AJ, Byington CL, et al. FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection. PLoS One 2011; 6:e26047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krafft AE, Russell KL, Hawksworth AW, et al. Evaluation of PCR testing of ethanol-fixed nasal swab specimens as an augmented surveillance strategy for influenza virus and adenovirus identification. J Clin Microbiol 2005; 43: 1768–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cottey R, Rowe CA, Bender BS. Influenza virus. Curr Protoc Immunol 2001; Chapter 19: Unit 19.1. John Wiley & Sons, Inc. Available at: https://currentprotocols.onlinelibrary.wiley.com/doi/full/10.1002/0471142735.im1911s42. [DOI] [PubMed] [Google Scholar]
- 19. Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull 1979; 35:69–75. [DOI] [PubMed] [Google Scholar]
- 20. Wan H, Gao J, Xu K, et al. Molecular basis for broad neuraminidase immunity: conserved epitopes in seasonal and pandemic H1N1 as well as H5N1 influenza viruses. J Virol 2013; 87:9290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park JK, Han A, Czajkowski L, et al. Evaluation of preexisting anti-hemagglutinin stalk antibody as a correlate of protection in a healthy volunteer challenge with influenza A/H1N1pdm virus. mBio 2018; 9:e02284–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park JK, Xiao Y, Ramuta MD, et al. Pre-existing immunity to influenza virus hemagglutinin stalk might drive selection for antibody-escape mutant viruses in a human challenge model. Nat Med 2020; 26:1240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiao Y, Park JK, Williams S, et al. Deep sequencing of 2009 influenza A/H1N1 virus isolated from volunteer human challenge study participants and natural infections. Virology 2019; 534:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 2015; 12:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 2019; 37:907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc 2016; 11:1650–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li H, Handsaker B, Wysoker A, et al. ; 1000 Genome Project Data Processing Subgroup . The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009; 25:2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nabel GJ, Fauci AS. Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nat Med 2010; 16:1389–91. [DOI] [PubMed] [Google Scholar]
- 29. Sedeyn K, Saelens X. New antibody-based prevention and treatment options for influenza. Antiviral Res 2019; 170:104562. [DOI] [PubMed] [Google Scholar]
- 30. Wei CJ, Crank MC, Shiver J, Graham BS, Mascola JR, Nabel GJ. Next-generation influenza vaccines: opportunities and challenges. Nat Rev Drug Discov 2020; 19:239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deng R, Lee AP, Maia M, et al. Pharmacokinetics of MHAA4549A, an anti-influenza A monoclonal antibody, in healthy subjects challenged with influenza A virus in a phase IIa randomized trial. Clin Pharmacokinet 2018; 57: 367–77. [DOI] [PubMed] [Google Scholar]
- 32. Lim JJ, Deng R, Derby MA, et al. Two phase 1, randomized, double-blind, placebo-controlled, single-ascending-dose studies to investigate the safety, tolerability, and pharmacokinetics of an anti-influenza A virus monoclonal antibody, MHAA4549A, in healthy volunteers. Antimicrob Agents Chemother 2016; 60:5437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lim JJ, Nilsson AC, Silverman M, et al. A phase 2 randomized, double-blind, placebo-controlled trial of MHAA4549A, a monoclonal antibody, plus oseltamivir in patients hospitalized with severe influenza A virus infection. Antimicrob Agents Chemother 2020; 64:e00352–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McBride JM, Lim JJ, Burgess T, et al. Phase 2 randomized trial of the safety and efficacy of MHAA4549A, a broadly neutralizing monoclonal antibody, in a human influenza A virus challenge model. Antimicrob Agents Chemother 2017; 61:e01154–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hershberger E, Sloan S, Narayan K, et al. Safety and efficacy of monoclonal antibody VIS410 in adults with uncomplicated influenza A infection: results from a randomized, double-blind, phase-2, placebo-controlled study. EBioMedicine 2019; 40:574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sloan SE, Szretter KJ, Sundaresh B, et al. Clinical and virological responses to a broad-spectrum human monoclonal antibody in an influenza virus challenge study. Antiviral Res 2020; 184:104763. [DOI] [PubMed] [Google Scholar]
- 37. Wollacott AM, Boni MF, Szretter KJ, et al. Safety and upper respiratory pharmacokinetics of the hemagglutinin stalk-binding antibody VIS410 support treatment and prophylaxis based on population modeling of seasonal influenza A outbreaks. EBioMedicine 2016; 5:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson CS, Ortega S, Chaves FA, et al. Natural and directed antigenic drift of the H1 influenza virus hemagglutinin stalk domain. Sci Rep 2017; 7:14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hayden FG. Experimental human influenza: observations from studies of influenza antivirals. Antivir Ther 2012; 17:133–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.